Abstract
Hailey–Hailey disease (HHD) is a rare autosomal dominant acantholytic dermatosis, characterized by a chronic course of repeated and exacerbated skin lesions in friction regions. The pathogenic gene of HHD was reported to be the ATPase calcium-transporting type 2C member 1 gene (ATP2C1) located on chromosome 3q21–q24. Its function is to maintain normal intracellular concentrations of Ca2+/Mn2+ by transporting Ca2+/Mn2+ into the Golgi apparatus. ATP2C1 gene mutations are reportedly responsible for abnormal cytosolic Ca2+/Mn2+ levels and the clinical manifestations of HHD. Environmental factors and genetic modifiers may also affect the clinical variability of HHD. This article aims to critically discuss the clinical and pathological features of HHD, differential diagnoses, and genetic and functional studies of the ATP2C1 gene in HHD. Further understanding the role of the ATP2C1 gene in the pathogenesis of HHD by genetic, molecular, and animal studies may contribute to a better clinical diagnosis and provide new strategies for the treatment and prevention of HHD.
Keywords: Clinical manifestation, Gene function, Genetics, Mutation analysis, Pathogenesis, Animal model
Introduction
Hailey–Hailey disease (HHD, OMIM 169600), also known as familial benign chronic pemphigus, is an autosomal dominant acantholytic genodermatosis with complete penetrance [1–3]. It was first reported by the Hailey brothers in 1939 [4]. The disease usually develops in the third or fourth decade with an incidence estimated to be 1:50,000–1:40,000 [2, 5, 6]. Two-thirds of all HHD patients have family histories [1], and both genders are affected equally [7]. Genetic studies on HHD have been comparatively productive over the past two decades. The ATPase calcium-transporting type 2C member 1 gene (ATP2C1, OMIM 604384) was discovered to be the disease-causing gene responsible for HHD [8, 9]. To date, at least 177 mutations in the ATP2C1 gene have been identified as the causes of HHD (Human Gene Mutation Database and Leiden Open Variation Database v.3.0) [10]. In vitro experiments and animal models promote the understanding of the relationship between the ATP2C1 gene and HHD [11–16]. This review provides an overview of HHD clinical and pathological features, differential diagnoses, and genetic and functional studies of the ATP2C1 gene in HHD, including causative mutations and genetically modified animal models.
Clinical and pathological features and differential diagnoses of Hailey–Hailey disease
The typical skin lesions of HHD usually occur in friction or intertriginous regions, including the neck, axillae, groin, perineum, and the submammary area. It may form blisters, erosions or scales with a foul-smelling exudate [2, 17]. Approximately 71% of HHD patients have longitudinal white bands in their fingernails [2]. The typical distribution of this genodermatosis usually occurs in a comparatively symmetrical pattern, exhibiting a generalized and bilateral involvement (Fig. 1a) [18]. In a few unusual cases, two distinct segmental patterns of mosaic manifestations can be distinguished from typical HHD (Table 1) [18–21]. A case report of type 1 segmental manifestation of HHD described skin lesions that always occurred in a segmental pattern on the left side of the body, but nowhere on the right. No family history of skin disorders was observed, and the patient’s daughters had no skin diseases (Fig. 1b) [19]. A case of type 2 segmental manifestation of HHD reported that the unilateral linear lesions occurred initially at the age of three months and persisted into adulthood with frequent aggravations. The eruption then evolved and nonsegmental bilateral skin lesions of HHD developed in body folds (Fig. 1c). Affected family members had nonsegmental HHD [22, 23]. The distinction between type 1 and type 2 segmental mosaics is of the utmost importance in genetic counseling. Type 1 segmental HHD patients have a slightly increased risk of having children with nonsegmental HHD. For type 2, this risk is 50% [24]. Type 2 segmental skin lesions are notoriously difficult to treat [25]. The three types of HHD have a chronic course of repeated remissions and aggravations [17]. Lesions are often triggered or aggravated by minor trauma, friction, heating, humidity, secondary infections, etc. [2, 17]. Squamous cell carcinomas have been observed in several HHD cases [26–29].
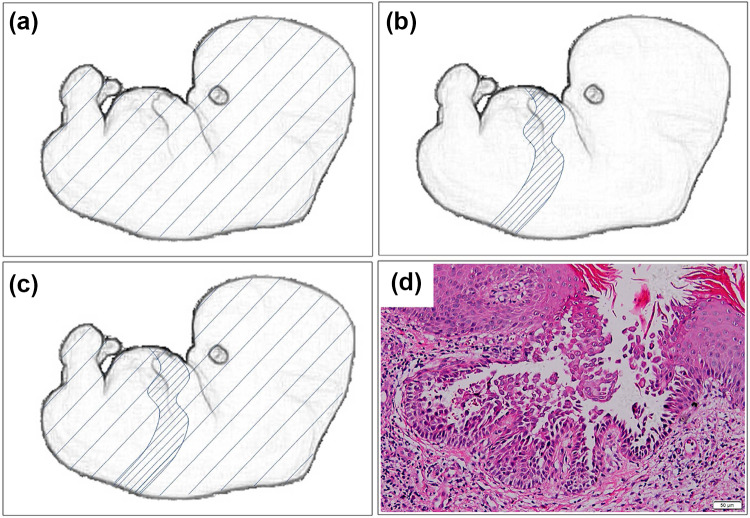
Fig. 1.
Schematic representation of Hailey–Hailey disease. a The distribution of typical HHD usually occurs in a comparatively symmetrical pattern, exhibiting a generalized and bilateral involvement. b The type 1 segmental HHD reflects a localized postzygotic mutation that occurs at an early stage of embryogenesis, resulting in heterozygosity and a segmental cutaneous disease. c The type 2 segmental HHD reflects a germline mutation in combination with somatic loss of heterozygosity in embryos, which develops a nonsegmental, diffuse distribution of skin lesions with a homozygous or hemizygous state of the underlying mutation in more severely affected segmental areas. d Histopathology section of HHD, showing acantholysis resulted from disruption of cell–cell contacts, which appears like a dilapidated brick wall (H&E stain; ×100; Olympus BX53, Japan)
Table 1.
Two types of mosaic manifestations of Hailey–Hailey disease
| Clinical manifestation | Family history | Mutation type | References | |
|---|---|---|---|---|
| Type 1 segmental HHD | Segmental distribution of skin lesions | No | Postzygotic de novo mutation | [19] |
| Type 2 segmental HHD | Rather severely affected segmental lesions being superimposed on typical nonsegmental skin lesions | Yes | A germline mutation in combination with somatic loss of heterozygosity | [18, 21] |
Histopathology of skin lesions in HHD shows an intercellular split of the epidermal suprabasal layers (acantholysis) resulting from disruption of cell–cell contacts, with an appearance like a dilapidated brick wall (Fig. 1d) [3, 17, 30]. Ultrastructural studies show perinuclear aggregation of keratin filaments, and keratinocytes remain loosely linked by adhesion structures [3, 31]. Desmosomal proteins (i.e., desmoplakin I and II) lose their peripheral, dotted patterns and stain diffusely in the cytoplasm of most acantholytic cells [31, 32].
The diagnoses of HHD are usually not difficult being based on family histories, clinical manifestations, histologic and immunofluorescent examinations, and genetic analyses. The differential diagnosis of HHD often includes conditions such as Darier disease (DD, OMIM 124200), pemphigus vulgaris (PV, OMIM 169610), and relapsing linear acantholytic dermatosis (Table 2). DD is an autosomal dominant genodermatosis caused by the ATP2A2 gene (OMIM 108740), which encodes sarcoplasmic/endoplasmic reticulum Ca2+-ATPases (SERCA2) [33]. Typical clinical features of DD include warty papules and plaques in seborrheic and flexural regions, notches at the distal end of the nails, and palmoplantar pits [34, 35]. DD may also present as type 1 segmental disease caused by a localized postzygotic mutation, or type 2 segmental disease caused by a germline mutation in combination with postzygotic loss of heterozygosity [36, 37]. The symptoms of DD usually present in childhood and peak at puberty [38]. It may be accompanied by neuropsychiatric abnormalities, such as depression, mental retardation, bipolar disorder, and epilepsy [39, 40]. Sweating, heating, stress, and ultraviolent radiation may exacerbate DD [35]. Histologically, it is characterized by hyperkeratosis, suprabasal acantholysis, and eosinophilic dyskeratotic cells, known as corps ronds and grains [38, 39]. In PV, initial lesions usually present on the oral mucosa [41]. Histologically, it is characterized by suprabasal acantholysis and intraepithelial blisters. Direct immunofluorescent examination often shows IgG deposits in the epithelium [41]. Relapsing linear acantholytic dermatosis is clinically and histopathologically similar to HHD, and the skin lesions are along the lines of Blaschko [42, 43].
Table 2.
Differential diagnoses of Hailey–Hailey disease
| Responsible or related gene(s) | Age of onset | Skin lesions | Accompanied by neuropsychiatric disorders | Histologic examination | References | |
|---|---|---|---|---|---|---|
| HHD | ATP2C1 | The third or fourth decade | Blisters, erosions, or scale in friction or intertriginous regions | Occasionally reported | Dilapidated brick wall | [8, 90] |
| DD | ATP2A2 | Childhood and peak at puberty | Warty papules in seborrheic and flexural regions, notches at the distal end of the nails, and palmoplantar pits | More prevalent | Hyperkeratosis, suprabasal cleavage, and eosinophilic dyskeratotic cells | [38, 40] |
| PV | HLA, ST18 | The fifth or sixth decade | Flaccid mucosal bullae leading to erosions | High prevalence in a small sample study from India | Suprabasal acantholysis and intraepithelial blisters | [91–93] |
| Relapsing linear acantholytic dermatosis | N/A | 4–81 years old | Similar to HHD and along the lines of Blaschko | N/A | Similar to HHD | [42, 43] |
HLA Human leukocyte antigen, ST18 Suppression of tumorigenicity 18, N/A Not available
The ATP2C1 gene and protein
In 1994, the disease gene for HHD was first mapped to a 14-cM region on chromosome 3q21–q24 by linkage analysis [44]. Gene mapping was subsequently narrowed to a 5-cM region flanked by D3S1589 and D3S1290 [45]. In 2000, mutations in the ATP2C1 gene encoding secretory pathway Ca2+/Mn2+-ATPase (SPCA1) were reported to be responsible for HHD [8, 9].
The ATP2C1 gene contains 27 exons [46]. In addition to the four distinct splice isoforms (SPCA1 1a–d, corresponding to ATP2C1 1a–d) produced by alternative processing of the ATP2C1 pre-mRNA reported in most literature [11, 40], five other splice isoforms (SPCA1 1e–f and SPCA1 2a–c, corresponding to ATP2C1 1e–f and ATP2C1 2a–c) are also recorded in NCBI (https://www.ncbi.nlm.nih.gov/gene/27032; gene ID 27032) and UniProt (http://www.uniprot.org/uniprot/P98194; UniProt ID P98194). Of these nine splice isoforms, SPCA1 1a (NP_055197.2) is the “canonical” sequence containing 919 amino acids. SPCA1 2a (NP_001186109.1) is the longest isoform containing 973 amino acids. SPCA1 1c is an aberrant Ca2+ pump with limited functional capability due to the absence of exon 27, which results in the disruption of transmembrane 10 (M10) [11, 46]. SPCA1 is one of three type II phosphorylation (P)-type Ca2+ transport ATPases, consisting of ten hydrophobic transmembrane segments (M1–10) and three cytosolic domains, which are the actuator domain (A), the phosphorylation domain (P), and the nucleotide-binding domain (N) [47].
SPCAs include isoforms SPCA1 and SPCA2 [48]. SPCA1 is ubiquitously expressed in mammalian cells [49], and localized to the Golgi apparatus in keratinocytes with a high expression level and at variable levels in other human tissues [9, 14]. The precise location of SPCA1 is in tubular noncompact zones that interconnect Golgi stacks and within tubular parts of the trans–Golgi network [50]. SPCA1 is critical for maintenance of the Golgi ribbon and regulation of Ca2+ in the Golgi [50]. It accounts for 67% of Ca2+ transportation in the Golgi of keratinocytes [51].
SPCA2 is mainly located in the brain and testis [48]. SPCA2 transports Ca2+ with much poorer affinity compared with SPCA1. It is believed to preferentially transport Mn2+ and confers robust tolerance to Mn2+ toxicity [48].
Mutations of the ATP2C1 gene
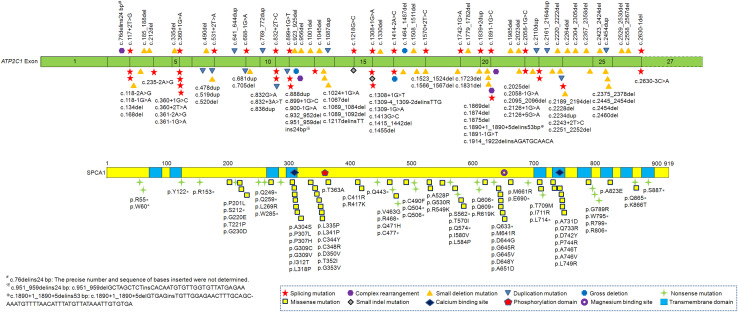
HHD was reported to be caused by haploinsufficiency of the ATP2C1 mutants [52]. There are at least 177 identified mutations distributed throughout the ATP2C1 gene with no apparent clustering (Fig. 2) [40, 53]. The numbers and types of the ATP2C1 gene mutations are as follows: 51 missense mutations (28.8%), 44 small deletion mutations (24.9%), 36 splicing mutations (20.3%), 25 nonsense mutations (14.1%), 12 duplication mutations (6.8%), four complex rearrangements (2.3%), three gross deletions (1.7%), and two small indel mutations (1.1%). About 37.2% (66/177) of the ATP2C1 mutations localize to only six exons (exons 12, 13, 21, 23, 24, and 25). The amino acids located at exons 12 and 23 are critical for Ca2+ binding, at exon 13 for phosphorylation, and at exon 21 for Mn2+ binding (UniProt ID P98194). M7 is encoded by sequence of exon 24 and M8 is encoded by exon 25. It is reasonable to conclude that mutations occurring in these regions may severely affect the functionality of SPCA1 [53]. Point mutations (base substitutions) account for 62.1% (110/177) of all mutations. However, no ATP2C1 mutations have been identified in a few patients with classical HHD, which may be explained by the limitations of current detection methods [54, 55].
Fig. 2.
Schematic representation of the reported ATP2C1 gene mutations in Hailey–Hailey disease. SPCA1 consists of ten transmembrane domains, a phosphorylation domain, a calcium binding site, and a magnesium binding site. All ATP2C1 mutations are described according to the ATP2C1 isoform 1a (NM_014382.3, NP_055197.2). The exon 27 of the ATP2C1 gene is not drawn to scale (as indicated by dashed lines)
More than 50% of all ATP2C1 mutations may generate premature termination codons (PTCs), leading to mRNA degradation via nonsense-mediated mRNA decay pathway or the synthesis of a truncated SPCA1 [56, 57]. Truncated mutations result in functional hemizygosity and produce a disease phenotype [7]. The high proportion of PTCs supports the argument that haploinsufficiency is the main pathogenetic mechanism for HHD [7, 58].
Hu et al. firstly identified 21 disease-causing mutations in the ATP2C1 gene in 61 unrelated kindreds, all with classical clinical and histological results [9]. Of these, the c.457C>T (p.R153*) and c.2375_2378del (p.Phe792Serfs*10) mutations were also identified in other four and five investigations, respectively [56, 59–64]. Shortly afterwards, two studies from the Welcome Trust Center for Human Genetics described 22 mutations in 20 HHD families and three sporadic cases with HHD [8, 56]. Haplotype analysis revealed a founder effect for a c.76delins24 bp mutation in two families [56]. In vitro studies showed that HHD mutant proteins L341P, C344Y, C411R, A528P, T570I, and G789R caused low expression levels of SPCA1. Normal mRNA levels and correct targeting to the Golgi suggest abnormal folding or instability of the mutated SPCA1 polypeptides, making the mutated SPCA1 sensitive to endoplasmic reticulum-mediated degradation [11, 56]. The P201L mutant had little functional effect on the enzymatic cycle of SPCA1, while I580V impeded the high-energy E1~P state to the lower energy E2-P state conformational transition [11, 65]. In the E1~P state, the enzyme binds Ca2+ with high affinity from the cytoplasmic side. In the E2-P state, the enzyme releases Ca2+ at the opposite side of the membrane as its affinity for Ca2+ decreases [65, 66]. SPCA1 possesses a single high-affinity ion binding and transport site, which is formed by E308 in M4 and N738 and D742 in M6 [58]. This site in SPCA1 corresponds to site II residues in SERCA2 [11, 47, 67]. Work on Pmr1, a yeast homolog of the ATP2C1 gene, revealed that N774 and D778 in M6 play vital roles in related ion pumps [67]. Yeast D51A and D53A mutations in an N-terminal EF hand-like Ca2+ binding motif caused a marked decreased affinity for Ca2+ transport, even though the pump activity was retained [12]. D742Y and G309C mutations blocked Ca2+- and Mn2+-dependent phosphoenzyme formation from ATP, highlighting the vital role of D742 (equivalent to D778 in Pmr1) in the structure of SPCA1 ion binding site. It also revealed a role for G309 in Mn2+ transport selectivity [11]. Mutations in the Ca2+ binding and translocation sites, formed by the precise juxtaposition of Ca2+ binding residues located in M4, M5, and M6, may block Ca2+ pump activity, in a manner similar to SERCAs [68]. In the type 1 segmental HHD cases, the segmental skin lesions were similarly severe to that of nonsegmental phenotype [19]. No molecular study was performed, and it was hypothesized that this type may be caused by a localized postzygotic mutation that occurs at an early stage of embryogenesis, resulting in heterozygosity and a segmental cutaneous disease [19]. In the type 2 segmental HHD cases, pronounced segmental skin lesions were superimposed on the typical nonsegmental phenotype [18]. Molecular and genetic studies revealed that the reported type 2 manifestation of HHD was caused by a germline mutation in combination with somatic loss of heterozygosity in embryos. This developed into a nonsegmental, diffuse distribution of skin lesions with a homozygous or hemizygous state of the underlying mutation in more severely affected segmental areas [18]. In addition to the identification of a splice site mutation (c.2146+1G>A) in the ATP2C1 gene within the heterozygous skin areas, the paternal wild-type allele harboring the ATP2C1 gene was consistently lost in more severely affected segmental skin areas [18].
Mutations in the ATP2C1 gene may lead to the Golgi apparatus fragmentation [50, 69], dysfunction of intra-Golgi transport [50], inefficient protein sorting [69], defective cell proliferation [50], impaired lipid handling [15], defects in post-translational processing of thyroglobulin, and endoplasmic reticulum-associated degradation of mutant thyroglobulin [70].
No obvious genotype–phenotype relationships in HHD were reported. Patients with the same mutation type in the ATP2C1 gene had various clinical features, indicating that the appearance of HHD may also be affected by intrinsic and/or extrinsic factors, such as modifier genes, high temperature, minor trauma, and pathogens [61, 71–75]. Exome sequencing of a Greek family suggested that other ATPase gene variants may affect the expressivity of HHD via increasing Golgi stress and ionic imbalance, making certain individuals more susceptible to environmental triggers [76]. A few studies reported that the more severely the SPCA1 structure or production was impaired, the more severe the clinical phenotypes were [16, 58].
The ATP2C1 gene dysfunction and its mechanism in HHD
No differences in ATP2C1 mRNA levels were found between HHD patient skin taken from the axilla and the buttock (regions prone versus resistant to blistering). The Ca2+ gradient in epidermis of HHD was significantly decreased compared with normal skin [9, 14]. Ca2+ gradient impairment in the HHD upper epidermis correlates well with acantholysis in the suprabasal layer [14]. SPCA1 dysfunction may result in an inefficient increase of external Ca2+ in the granular layer, failing to stabilize desmosome integrity and activate Ca2+-sensing receptors. Ca2+-sensing receptors are responsible for triggering cell-to-cell adhesion, cell differentiation in the granular layer, and reconstituting the Ca2+ gradient [47].
In the skin of HHD patients, Golgi Ca2+ uptake rate of keratinocytes slowed, and the maximum Ca2+ level in the Golgi was notably lower [14]. Reduced Ca2+ concentration in the Golgi may impair the glycosylation of desmosomes [46]. Desmosome formation was delayed in SPCA1-deficient keratinocytes, but its assembly may be re-established by being cultured in an elevated Ca2+ concentration solution [5, 77]. Defective desmosomes may lead to an inability to form the intact structure of desmosomes and the split of epidermal cells [46]. The resting free cytoplasmic Ca2+ concentration in HHD skin was normal or higher [78, 79]. Keratinocytes responded less to increased extracellular Ca2+ concentrations [9]. High cytoplasmic Ca2+ concentrations could lead to decreased adenosine triphosphate (ATP) production by overloading mitochondria [80], followed by a failure of Ca2+-induced actin reorganization in keratinocytes of HHD [79]. Aging may reduce mitochondrial Ca2+ handling ability and ATP production capacity and increase mitochondrial vulnerability to Ca2+ concentration overload. This is in accordance with the relatively late-onset age of HHD [46, 80]. Other intrinsic and extrinsic factors, such as genetic modifiers, gene background, heating, and minor trauma, may also influence the onset age of HHD [40, 60].
Intriguingly, the main affected organ of HHD is skin, but no other organs. One reasonable explanation may be that a partial functional overlap of SPCA1 and other Ca2+ pumps may compensate for decreased pump function in these unaffected organs, which maintains a subtle, but steady state [57, 61]. The comparatively large percentage (67%) of Ca2+ uptake in the Golgi apparatus contributed by SPCA1 in keratinocytes may partially explain why skin is the main affected organ of HHD [51, 81]. However, a case report of a fatal liver injury was speculated to have been caused by HHD [17]. The predominant involvement of skin folds may be caused by exposure to external stimulations, such as friction, sweating, and secondary infections, and be unable to be compensated by other Ca2+ regulatory mechanisms [46].
In a gene-targeted mouse model for SPCA1, squamous cell tumors were observed in Spca1 +/− mouse models, while Spca1 −/− embryos showed growth retardation and neural tube closure failure and did not survive beyond gestation day 10.5 [15]. The mouse model phenotypes were different from the main characteristic of HHD in humans, which is acantholysis, although squamous cell tumors were occasionally observed in HHD patients [26–29]. A Caenorhabditis elegans (C. elegans) pmr-1 mutant showed wrong migration directions as well as shorter migration distances of ectodermal cells [16]. The defective migration of certain ectodermal cells in mouse and C. elegans models is superficially similar to HHD, suggesting that ATP2C1 gene mutations may lead to inefficient migration of epidermal cells in HHD [16]. The different responses in HHD patients and Spca1-deficient mouse models may result from Ca2+ pump dysfunction, causing chronic Golgi stress and/or ER stress, with the predominance of pro-apoptotic pathways in HHD patients and the predominance of pro-survival ones in mice, respectively [82]. In other words, mutations in the same gene may favor development of tumors in mice and acantholytic skin diseases in humans [15]. Another study suggested that apoptosis may not be the initial factor related to the pathogenetic mechanisms of HHD, but be the result of HHD acantholysis [83].
The splicing and expression of sense genes may be regulated by antisense transcription via certain mechanisms, for instance, RNA masking, increased RNA polymerase II occupancy, transcriptional interference, and DNA replication interference [84, 85]. The ATP2C1 gene transcription site is partially overlapped with the ASTE1 gene (asteroid homolog 1) on the opposing strand in the human but not mouse genome. Therefore, the different SPCA1 deficiency responses between humans and mouse models may be caused by a negative regulatory effect of the ASTE1 gene on splicing and expression of the ATP2C1 gene through antisense transcription [72].
The SPCA1 pump is crucial for Mn2+ detoxification by transporting Mn2+ from the cytoplasm into the Golgi [47]. Cytosolic overload and depletion of Golgi-hosted Mn2+ may lead to the loss of cell cycle control, genetic instability, and multinucleation [86]. Deficient Mn2+ homeostasis caused by ATP2C1 mutations may be the initial event in tumorigenesis in some HHD patients and Spca1 +/− mouse models [15, 86].
Conclusion
Acknowledging the three different classifications of HHD may help in understanding the clinical presentations and prognosis of HHD [24]. A close study of family history, clinical manifestations, and histologic and immunofluorescent examinations simplifies attempts to distinguish HHD from DD, PV, and other skin diseases. Mutation analysis provides valuable information to confirm clinical diagnoses [40]. Genetic studies, animal models, and functional analyses have supported the view that the ATP2C1 gene plays a vital role in the pathogenesis of HHD by regulating Ca2+/Mn2+ homeostasis [8, 9, 14–16]. Further investigations into the roles of Mn2+ in HHD with tumors [86], possible compensatory Ca2+ pumps for SPCA1 in nonlesional skin and noncutaneous tissues [46, 87], ATP2C1 mRNA expression levels in peripheral blood [71], genetic modifiers and environmental factors [2, 17, 76] may also help shed light on the pathogenetic mechanisms of HHD. Patients with atypical presentations and prenatal examinations may benefit from discovering novel ATP2C1 gene mutations and revealing the pathogenetic mechanisms of HHD [88, 89]. Therapies for this chronic and stubborn disease would include not only routine treatments such as corticosteroids, antibiotics, and surgical treatments [1], but also gene-targeted therapy.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2016YFC1306604), the National Natural Science Foundation of China (81670216), the Natural Science Foundation of Hunan Province (2015JJ4088 and 2016JJ2166), the grant for the Foster Key Subject of the Third Xiangya Hospital of Central South University (Clinical Laboratory Diagnostics), and the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (20150301), China.
Abbreviations
- A
Actuator domain
- ASTE1
Asteroid homolog 1
- ATP
Adenosine triphosphate
- ATP2C1
ATPase calcium–transporting type 2C member 1
- C. elegans
Caenorhabditis elegans
- DD
Darier disease
- HHD
Hailey–Hailey disease
- M
Transmembrane
- N
Nucleotide–binding domain
- P
Phosphorylation domain
- PTCs
Premature termination codons
- PV
Pemphigus vulgaris
- SERCA
Sarcoplasmic/endoplasmic reticulum Ca2+–ATPase
- SPCA
Secretory pathway Ca2+/Mn2+–ATPase
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Hao Deng and Heng Xiao contributed equally to this work.
References
- 1.Engin B, Kutlubay Z, Çelik U, Serdaroğlu S, Tüzün Y. Hailey–Hailey disease: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:452–455. doi: 10.1016/j.clindermatol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Burge SM. Hailey–Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275–282. doi: 10.1111/j.1365-2133.1992.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilgram GF, Caulfield JB, Lever WF. An electron-microscopic study of acantholysis and dyskeratosis in Hailey and Hailey’s disease. J Invest Dermatol. 1962;39:373–381. doi: 10.1038/jid.1962.127. [DOI] [PubMed] [Google Scholar]
- 4.Hailey H, Hailey H. Familial benign chronic pemphigus. Report of 13 cases in 4 generations of a family and report of 9 additional cases in 4 generations of a family. Arch Dermatol Syphilol. 1939;39:679–685. doi: 10.1001/archderm.1939.01480220064005. [DOI] [Google Scholar]
- 5.Raiko L, Siljamäki E, Mahoney MG, Putaala H, Suominen E, Peltonen J, Peltonen S. Hailey–Hailey disease and tight junctions: claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca2+/Mn2+ ATPase SPCA1 in cultured keratinocytes. Exp Dermatol. 2012;21:586–591. doi: 10.1111/j.1600-0625.2012.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellermayer R. Hailey–Hailey disease as an orthodisease of PMR1 deficiency in Saccharomyces cerevisiae . FEBS Lett. 2005;579:2021–2025. doi: 10.1016/j.febslet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Chao SC, Tsai YM, Yang MH. Mutation analysis of ATP2C1 gene in Taiwanese patients with Hailey–Hailey disease. Br J Dermatol. 2002;146:595–600. doi: 10.1046/j.1365-2133.2002.04697.x. [DOI] [PubMed] [Google Scholar]
- 8.Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, White J, Healy E, Dissanayake M, Larrègue M, Perrussel M, Lehrach H, Munro CS, Strachan T, Burge S, Hovnanian A, Monaco AP. Hailey–Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum Mol Genet. 2000;9:1131–1140. doi: 10.1093/hmg/9.7.1131. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EJ., Jr Mutations in ATP2C1, encoding a calcium pump, cause Hailey–Hailey disease. Nat Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 10.Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, Hussain M, Phillips AD, Cooper DN. The human gene mutation database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet. 2017;136:665–667. doi: 10.1007/s00439-017-1779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairclough RJ, Dode L, Vanoevelen J, Andersen JP, Missiaen L, Raeymaekers L, Wuytack F, Hovnanian A. Effect of Hailey–Hailey disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1) J Biol Chem. 2003;278:24721–24730. doi: 10.1074/jbc.M300509200. [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Marchi V, Wang R, Rao R. An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca2+/Mn2+-ATPase. Biochemistry. 1999;38:14534–14541. doi: 10.1021/bi9911233. [DOI] [PubMed] [Google Scholar]
- 13.Sepúlveda MR, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J Neurosci. 2009;29:12174–12182. doi: 10.1523/JNEUROSCI.2014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, Pozzan T, Mauro TM. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol. 2003;121:688–694. doi: 10.1046/j.1523-1747.2003.12528.x. [DOI] [PubMed] [Google Scholar]
- 15.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- 16.Praitis V, Simske J, Kniss S, Mandt R, Imlay L, Feddersen C, Miller MB, Mushi J, Liszewski W, Weinstein R, Chakravorty A, Ha DG, Schacht Farrell A, Sullivan-Wilson A, Stock T. The secretory pathway calcium ATPase PMR-1/SPCA1 has essential roles in cell migration during Caenorhabditis elegans embryonic development. PLoS Genet. 2013;9:e1003506. doi: 10.1371/journal.pgen.1003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amagai M, Kobayashi M, Wakabayashi K, Hakuno M, Hashiguchi A, Nishikawa T, Hata J. A case of generalized Hailey–Hailey disease with fatal liver injury. Keio J Med. 2001;50:109–116. doi: 10.2302/kjm.50.supplement2_109. [DOI] [PubMed] [Google Scholar]
- 18.Poblete-Gutiérrez P, Wiederholt T, König A, Jugert FK, Marquardt Y, Rübben A, Merk HF, Happle R, Frank J. Allelic loss underlies type 2 segmental Hailey–Hailey disease, providing molecular confirmation of a novel genetic concept. J Clin Invest. 2004;114:1467–1474. doi: 10.1172/JCI21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang LY, Lee JB, Richard G, Uitto J, Hsu S. Type 1 segmental manifestation of Hailey–Hailey disease. J Am Acad Dermatol. 2003;49:712–714. doi: 10.1067/S0190-9622(03)00847-8. [DOI] [PubMed] [Google Scholar]
- 20.Happle R. A rule concerning the segmental manifestation of autosomal dominant skin disorders. Review of clinical examples providing evidence for dichotomous types of severity. Arch Dermatol. 1997;133:1505–1509. doi: 10.1001/archderm.1997.03890480025004. [DOI] [PubMed] [Google Scholar]
- 21.Nanda A, Khawaja F, Al-Sabah H, Happle R. Type 2 segmental Hailey–Hailey disease with systematized bilateral arrangement. Int J Dermatol. 2014;53:476–478. doi: 10.1111/j.1365-4632.2012.05586.x. [DOI] [PubMed] [Google Scholar]
- 22.Happle R. Superimposed segmental manifestation of both rare and common cutaneous disorders: a new paradigm. Actas Dermosifiliogr. 2009;100(Suppl 1):77–85. doi: 10.1016/S0001-7310(09)73171-0. [DOI] [PubMed] [Google Scholar]
- 23.König A, Hörster S, Vakilzadeh F, Happle R. Type 2 segmental manifestation of Hailey–Hailey disease: poor therapeutic response to dermabrasion is due to severe involvement of adnexal structures. Eur J Dermatol. 2000;10:265–268. [PubMed] [Google Scholar]
- 24.Happle R. The categories of cutaneous mosaicism: a proposed classification. Am J Med Genet A. 2016;170A:452–459. doi: 10.1002/ajmg.a.37439. [DOI] [PubMed] [Google Scholar]
- 25.Happle R. Superimposed segmental manifestation of polygenic skin disorders. J Am Acad Dermatol. 2007;57:690–699. doi: 10.1016/j.jaad.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 26.von Felbert V, Hampl M, Talhari C, Engers R, Megahed M. Squamous cell carcinoma arising from a localized vulval lesion of Hailey–Hailey disease after tacrolimus therapy. Am J Obstet Gynecol. 2010;203:e5–e7. doi: 10.1016/j.ajog.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Cockayne SE, Rassl DM, Thomas SE. Squamous cell carcinoma arising in Hailey–Hailey disease of the vulva. Br J Dermatol. 2000;142:540–542. doi: 10.1046/j.1365-2133.2000.03374.x. [DOI] [PubMed] [Google Scholar]
- 28.Holst VA, Fair KP, Wilson BB, Patterson JW. Squamous cell carcinoma arising in Hailey–Hailey disease. J Am Acad Dermatol. 2000;43:368–371. doi: 10.1067/mjd.2000.100542. [DOI] [PubMed] [Google Scholar]
- 29.Chun SI, Whang KC, Su WP. Squamous cell carcinoma arising in Hailey–Hailey disease. J Cutan Pathol. 1988;15:234–237. doi: 10.1111/j.1600-0560.1988.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 30.Wick MR. Bullous, pseudobullous, & pustular dermatoses. Semin Diagn Pathol. 2017;34:250–260. doi: 10.1053/j.semdp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Metze D, Hamm H, Schorat A, Luger T. Involvement of the adherens junction-actin filament system in acantholytic dyskeratosis of Hailey–Hailey disease. A histological, ultrastructural, and histochemical study of lesional and non-lesional skin. J Cutan Pathol. 1996;23:211–222. doi: 10.1111/j.1600-0560.1996.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 32.Harada M, Hashimoto K, Fujiwara K. Immunohistochemical distribution of CD44 and desmoplakin I & II in Hailey–Hailey’s disease and Darier’s disease. J Dermatol. 1994;21:389–393. doi: 10.1111/j.1346-8138.1994.tb01760.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O’Donovan M, Craddock N, Kucherlapati R, Rees JL, Owen M, Lathrop GM, Monaco AP, Strachan T, Hovnanian A. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- 34.Buteică E, Burada F, Stoicescu I, Stănoiu B, Georgescu CV. Darier disease and Hailey–Hailey disease. Rom J Morphol Embryol. 2007;48:423–426. [PubMed] [Google Scholar]
- 35.Burge SM, Wilkinson JD. Darier–White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40–50. doi: 10.1016/0190-9622(92)70154-8. [DOI] [PubMed] [Google Scholar]
- 36.Sakuntabhai A, Dhitavat J, Burge S, Hovnanian A. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol. 2000;115:1144–1147. doi: 10.1046/j.1523-1747.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- 37.Fölster-Holst R, Nellen RG, Jensen JM, Poblete-Gutiérrez P, Steijlen PM, Schwarz T, Happle R, Van Geel M, Frank J. Molecular genetic support for the rule of dichotomy in type 2 segmental Darier disease. Br J Dermatol. 2012;166:464–466. doi: 10.1111/j.1365-2133.2011.10593.x. [DOI] [PubMed] [Google Scholar]
- 38.Engin B, Kutlubay Z, Erkan E, Tüzün Y. Darier disease: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:448–451. doi: 10.1016/j.clindermatol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Takagi A, Kamijo M, Ikeda S. Darier disease. J Dermatol. 2016;43:275–279. doi: 10.1111/1346-8138.13230. [DOI] [PubMed] [Google Scholar]
- 40.Nellen RG, Steijlen PM, van Steensel MA, Vreeburg M, European Professional Contributors. Frank J, van Geel M. Mendelian disorders of cornification caused by defects in intracellular calcium pumps: mutation update and database for variants in ATP2A2 and ATP2C1 associated with Darier disease and Hailey–Hailey disease. Hum Mutat. 2017;38:343–356. doi: 10.1002/humu.23164. [DOI] [PubMed] [Google Scholar]
- 41.Cholera M, Chainani-Wu N. Management of pemphigus vulgaris. Adv Ther. 2016;33:910–958. doi: 10.1007/s12325-016-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arora S, Arora G, Ranjan P. Relapsing linear acantholytic dermatosis in a four-year-old boy. Indian J Dermatol Venereol Leprol. 2005;71:351–353. doi: 10.4103/0378-6323.16789. [DOI] [PubMed] [Google Scholar]
- 43.Duschet P, Happle R, Schwarz T, Gschnait F. Relapsing linear acantholytic dermatosis. J Am Acad Dermatol. 1995;33:920–922. doi: 10.1016/0190-9622(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda S, Welsh EA, Peluso AM, Leyden W, Duvic M, Woodley DT, Epstein EJ., Jr Localization of the gene whose mutations underlie Hailey–Hailey disease to chromosome 3q. Hum Mol Genet. 1994;3:1147–1150. doi: 10.1093/hmg/3.7.1147. [DOI] [PubMed] [Google Scholar]
- 45.Richard G, Korge BP, Wright AR, Mazzanti C, Harth W, Annicchiarico-Petruzzelli M, Compton JG, Bale SJ. Hailey–Hailey disease maps to a 5 cM interval on chromosome 3q21–q24. J Invest Dermatol. 1995;105:357–360. doi: 10.1111/1523-1747.ep12320741. [DOI] [PubMed] [Google Scholar]
- 46.Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 48.Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+, Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- 49.Gunteski-Hamblin AM, Clarke DM, Shull GE. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- 50.Micaroni M, Perinetti G, Berrie CP, Mironov AA. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic. 2010;11:1315–1333. doi: 10.1111/j.1600-0854.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 51.Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Wuytack F, Vanoevelen J, Van Baelen K, Simoni A, Rizzuto R, Missiaen L. Similar Ca2+-signaling properties in keratinocytes and in COS-1 cells overexpressing the secretory-pathway Ca2+-ATPase SPCA1. Cell Calcium. 2003;34:157–162. doi: 10.1016/S0143-4160(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 52.Ton VK, Rao R. Expression of Hailey–Hailey disease mutations in yeast. J Invest Dermatol. 2004;123:1192–1194. doi: 10.1111/j.0022-202X.2004.23437.x. [DOI] [PubMed] [Google Scholar]
- 53.Micaroni M, Giacchetti G, Plebani R, Xiao GG, Federici L. ATP2C1 gene mutations in Hailey–Hailey disease and possible roles of SPCA1 isoforms in membrane trafficking. Cell Death Dis. 2016;7:e2259. doi: 10.1038/cddis.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Sun XK, Zhu XJ. Four novel mutations in ATP2C1 found in Chinese patients with Hailey–Hailey disease. Br J Dermatol. 2003;149:471–474. doi: 10.1046/j.1365-2133.2003.05495.x. [DOI] [PubMed] [Google Scholar]
- 55.Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey–Hailey disease. Br J Dermatol. 2004;150:821–828. doi: 10.1111/j.1365-2133.2004.05904.x. [DOI] [PubMed] [Google Scholar]
- 56.Fairclough RJ, Lonie L, Van Baelen K, Haftek M, Munro CS, Burge SM, Hovnanian A. Hailey–Hailey disease: identification of novel mutations in ATP2C1 and effect of missense mutation A528P on protein expression levels. J Invest Dermatol. 2004;123:67–71. doi: 10.1111/j.0022-202X.2004.22713.x. [DOI] [PubMed] [Google Scholar]
- 57.Rácz E, Csikós M, Kárpáti S. Novel mutations in the ATP2C1 gene in two patients with Hailey–Hailey disease. Clin Exp Dermatol. 2005;30:575–577. doi: 10.1111/j.1365-2230.2005.01879.x. [DOI] [PubMed] [Google Scholar]
- 58.Matsuda M, Hamada T, Numata S, Teye K, Okazawa H, Imafuku S, Ohata C, Furumura M, Hashimoto T. Mutation-dependent effects on mRNA and protein expressions in cultured keratinocytes of Hailey–Hailey disease. Exp Dermatol. 2014;23:514–516. doi: 10.1111/exd.12410. [DOI] [PubMed] [Google Scholar]
- 59.Hamada T, Fukuda S, Sakaguchi S, Yasumoto S, Kim SC, Hashimoto T. Molecular and clinical characterization in Japanese and Korean patients with Hailey–Hailey disease: six new mutations in the ATP2C1 gene. J Dermatol Sci. 2008;51:31–36. doi: 10.1016/j.jdermsci.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda S, Shigihara T, Mayuzumi N, Yu X, Ogawa H. Mutations of ATP2C1 in Japanese patients with Hailey–Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654–1656. doi: 10.1046/j.0022-202x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 61.Dobson-Stone C, Fairclough R, Dunne E, Brown J, Dissanayake M, Munro CS, Strachan T, Burge S, Sudbrak R, Monaco AP, Hovnanian A. Hailey–Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338–343. doi: 10.1046/j.0022-202x.2001.01675.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang HZ, Tian HQ, Du DH, Wang GJ, Yan XX, Liu H, Zhou GZ, Fu XA, Yu YX, Yu GQ, Liu HX, Zhang FR. Analysis of ATP2C1 gene mutations in Chinese patients with Hailey–Hailey disease. Clin Exp Dermatol. 2012;37:190–193. doi: 10.1111/j.1365-2230.2011.04155.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang F, Yan X, Jiang D, Tian H, Wang C, Yu L. Eight novel mutations of ATP2C1 identified in 17 Chinese families with Hailey–Hailey disease. Dermatology. 2007;215:277–283. doi: 10.1159/000107620. [DOI] [PubMed] [Google Scholar]
- 64.Yokota K, Yasukawa K, Shimizu H. Analysis of ATP2C1 gene mutation in 10 unrelated Japanese families with Hailey–Hailey disease. J Invest Dermatol. 2002;118:550–551. doi: 10.1046/j.0022-202x.2001.01686.x. [DOI] [PubMed] [Google Scholar]
- 65.Brini M, Calì T, Ottolini D, Carafoli E. Calcium pumps: why so many? Compr Physiol. 2012;2:1045–1060. doi: 10.1002/cphy.c110034. [DOI] [PubMed] [Google Scholar]
- 66.Dang D, Rao R. Calcium-ATPases: gene disorders and dysregulation in cancer. Biochim Biophys Acta. 2016;1863:1344–1350. doi: 10.1016/j.bbamcr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y, Chen J, Rosas G, Tompkins DA, Holt PA, Rao R. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J Biol Chem. 2000;275:23927–23932. doi: 10.1074/jbc.M002618200. [DOI] [PubMed] [Google Scholar]
- 68.MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J Biol Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 69.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramos-Castañeda J, Park YN, Liu M, Hauser K, Rudolph H, Shull GE, Jonkman MF, Mori K, Ikeda S, Ogawa H, Arvan P. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J Biol Chem. 2005;280:9467–9473. doi: 10.1074/jbc.M413243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D, Li X, Xiao S, Huo J, Wang S, Zhou P. Detection and comparison of two types of ATP2C1 gene mutations in Chinese patients with Hailey–Hailey disease. Arch Dermatol Res. 2012;304:163–170. doi: 10.1007/s00403-011-1185-1. [DOI] [PubMed] [Google Scholar]
- 72.Micaroni M, Malquori L. Overlapping ATP2C1 and ASTE1 genes in human genome: implications for SPCA1 expression? Int J Mol Sci. 2013;14:674–683. doi: 10.3390/ijms14010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng TS, Ho KM, Lam CW. Heterogeneous mutations of the ATP2C1 gene causing Hailey–Hailey disease in Hong Kong Chinese. J Eur Acad Dermatol Venereol. 2010;24:1202–1206. doi: 10.1111/j.1468-3083.2010.03623.x. [DOI] [PubMed] [Google Scholar]
- 74.Nemoto-Hasebe I, Akiyama M, Osawa R, Nakamura H, Shimizu H. Diagnosis of Hailey–Hailey disease facilitated by DNA testing: a novel mutation in ATP2C1. Acta Derm Venereol. 2008;88:399–400. doi: 10.2340/00015555-0459. [DOI] [PubMed] [Google Scholar]
- 75.Wang CC, Chao SC, Tsai TH. Hailey–Hailey disease: a novel mutation of the ATP2C1 gene in a Taiwanese family with divergent clinical presentation. J Eur Acad Dermatol Venereol. 2008;22:1145–1146. doi: 10.1111/j.1468-3083.2007.02562.x. [DOI] [PubMed] [Google Scholar]
- 76.van Beek N, Patsatsi A, Gupta Y, Möller S, Freitag M, Lemcke S, Recke A, Zillikens D, Schmidt E, Ibrahim S. A family with atypical Hailey Hailey disease–is there more to the underlying genetics than ATP2C1? PLoS One. 2015;10:e0121253. doi: 10.1371/journal.pone.0121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hennings H, Holbrook KA. Calcium regulation of cell–cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983;143:127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- 78.Leinonen PT, Myllylä RM, Hägg PM, Tuukkanen J, Koivunen J, Peltonen S, Oikarinen A, Korkiamäki T, Peltonen J. Keratinocytes cultured from patients with Hailey–Hailey disease and Darier disease display distinct patterns of calcium regulation. Br J Dermatol. 2005;153:113–117. doi: 10.1111/j.1365-2133.2005.06623.x. [DOI] [PubMed] [Google Scholar]
- 79.Aronchik I, Behne MJ, Leypoldt L, Crumrine D, Epstein E, Ikeda S, Mizoguchi M, Bench G, Pozzan T, Mauro T. Actin reorganization is abnormal and cellular ATP is decreased in Hailey–Hailey keratinocytes. J Invest Dermatol. 2003;121:681–687. doi: 10.1046/j.1523-1747.2003.12472.x. [DOI] [PubMed] [Google Scholar]
- 80.Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/S0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 81.Missiaen L, Raeymaekers L, Dode L, Vanoevelen J, Van Baelen K, Parys JB, Callewaert G, De Smedt H, Segaert S, Wuytack F. SPCA1 pumps and Hailey–Hailey disease. Biochem Biophys Res Commun. 2004;322:1204–1213. doi: 10.1016/j.bbrc.2004.07.128. [DOI] [PubMed] [Google Scholar]
- 82.Shull GE, Miller ML, Prasad V. Secretory pathway stress responses as possible mechanisms of disease involving Golgi Ca2+ pump dysfunction. Biofactors. 2011;37:150–158. doi: 10.1002/biof.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Btadini W, Abou Hassan OK, Saadeh D, Abbas O, Ballout F, Kibbi AG, Dbaibo G, Darwiche N, Nemer G, Kurban M. Identification of several mutations in ATP2C1 in Lebanese families: insight into the pathogenesis of Hailey–Hailey disease. PLoS One. 2015;10:e0115530. doi: 10.1371/journal.pone.0115530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrissy AS, Griffith M, Marra MA. Extensive relationship between antisense transcription and alternative splicing in the human genome. Genome Res. 2011;21:1203–1212. doi: 10.1101/gr.113431.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li K, Ramchandran R. Natural antisense transcript: a concomitant engagement with protein-coding transcript. Oncotarget. 2010;1:447–452. doi: 10.18632/oncotarget.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.García-Rodríguez N, Díaz de la Loza Mdel C, Andreson B, Monje-Casas F, Rothstein R, Wellinger RE. Impaired manganese metabolism causes mitotic misregulation. J Biol Chem. 2012;287:18717–18729. doi: 10.1074/jbc.M112.358309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang D, Li X, Wang Z, Zhang Y, Guo K, Wang S, Tu C, Huo J, Xiao S. Hailey–Hailey disease: investigation of a possible compensatory SERCA2 up-regulation and analysis of SPCA1, p63, and IRF6 expression. Arch Dermatol Res. 2015;307:143–149. doi: 10.1007/s00403-014-1506-2. [DOI] [PubMed] [Google Scholar]
- 88.Iino Y, Kano T, Adachi F, Suzuki M, Nishikawa R, Ishii N, Ohata C, Furumura M, Hamada T, Hashimoto T. A case of bullous pemphigoid associated with psoriasis vulgaris showing Hailey–Hailey disease-like histopathological changes in regenerated epidermis without genomic mutation in ATP2C1 or ATP2A2 gene. J Eur Acad Dermatol Venereol. 2015;29:1646–1648. doi: 10.1111/jdv.12521. [DOI] [PubMed] [Google Scholar]
- 89.Shi BJ, Xiao S, Zhang Z, Lü J, Xue M, Jiang Y, Liu Y, Hao J, Diao QC. The ATP2C1 gene in Hailey–Hailey disease patients: one novel deletion and one novel splicing mutation. J Eur Acad Dermatol Venereol. 2015;29:2495–2497. doi: 10.1111/jdv.12603. [DOI] [PubMed] [Google Scholar]
- 90.Yokota K, Sawamura D. Hailey–Hailey disease with affective disorder: report of a case with novel ATP2C1 gene mutation. J Dermatol Sci. 2006;43:150–151. doi: 10.1016/j.jdermsci.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 91.Gil JM, Weber R, Rosales CB, Rodrigues H, Sennes LU, Kalil J, Chagury A, Miziara ID. Study of the association between human leukocyte antigens (HLA) and pemphigus vulgaris in Brazilian patients. Int J Dermatol. 2017;56:557–562. doi: 10.1111/ijd.13577. [DOI] [PubMed] [Google Scholar]
- 92.Sarig O, Bercovici S, Zoller L, Goldberg I, Indelman M, Nahum S, Israeli S, Sagiv N, Martinez de Morentin H, Katz O, Baum S, Barzilai A, Trau H, Murrell DF, Bergman R, Hertl M, Rosenberg S, Nöthen MM, Skorecki K, Schmidt E, Zillikens D, Darvasi A, Geiger D, Rosset S, Ibrahim SM, Sprecher E. Population-specific association between a polymorphic variant in ST18, encoding a pro-apoptotic molecule, and pemphigus vulgaris. J Invest Dermatol. 2012;132:1798–1805. doi: 10.1038/jid.2012.46. [DOI] [PubMed] [Google Scholar]
- 93.Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151–156. doi: 10.1016/j.ajp.2012.10.005. [DOI] [PubMed] [Google Scholar]