Abstract
Retinoid X receptors (RXRs) form a unique subclass within the nuclear receptor (NR) superfamily of ligand-dependent transcription factors. RXRs are obligatory partners for a number of other NRs, placing RXRs in a coordinating role at the crossroads of multiple signaling pathways. In addition, RXRs can function as self-sufficient homodimers. Recent advances have revealed RXRs as novel regulators of osteoclastogenesis and bone remodeling. This review outlines the versatility of RXR action in the control of transcription of bone-forming osteoblasts and bone-resorbing osteoclasts, both through heterodimerization with other NRs and through RXR homodimerization. RXR signaling is currently a major therapeutic target and, therefore, knowledge of how RXR signaling affects bone remodeling creates enormous potential for the translation of basic research findings into successful clinical therapies to increase bone mass and improve bone quality.
Keywords: Rexinoids, 9-cis retinoic acid, Bexarotene, Agonists, Antagonists, Homodimers, Heterodimers, Permissive, MAFB, Skeleton, Bone resorption, Osteoporosis
Introduction
Bone is a specialized connective tissue that also serves as an organ system in higher vertebrates. Basic bone functions include protection of internal organs and locomotion, and additionally the storage of minerals and lipids and the production of hematopoietic cells that nourish the body and play a vital role in protecting against infection. Recent studies have demonstrated that bone also produces hormones that control energy balance and mineral homeostasis, giving rise to the idea of the skeleton as a true endocrine organ [1]. Bone undergoes constant remodeling through a dynamic process of skeletal dissolution followed by bone formation. This cycle is maintained in a tight balance by highly regulated differentiation, activity, and apoptosis of two main cell types: bone-forming osteoblasts and bone resorbing osteoclasts [2–4]. These and other cells of the osteoblast family (osteocytes and lining cells) contribute to bone turnover by producing or responding to hormones, cytokines, and growth factors implicated in bone development, remodeling, and repair [4, 5]. Differentiation and function of osteoblasts and osteoclasts is highly controlled at the transcriptional level by changes in the expression of numerous regulatory genes. Variations in the expression of these genes can result in altered bone homeostasis and the development of many bone diseases, such as osteoporosis [3].
Nuclear receptors (NRs) are transcription factors that are important effectors of signals regulating bone development and homeostasis. Many members of the NR superfamily respond to ligands known to affect bone homeostasis, including thyroxine and sex hormones, vitamins D and A, metabolites (e.g., dietary lipids), and drugs. To act as functional transcription factors, receptors for some of these molecules need to heterodimerize with retinoid X receptors (RXRs). About a third of the 48 human NR superfamily members serve as RXR heterodimerization partners, leading to RXRs being described as the central NRs [6]. RXRs were initially described as silent partners in these heterodimers, thought to have no transcriptional activity; however, RXRs are now known to actively contribute to the transcriptional activity of dimers in which they participate. In addition, RXRs control their own specific signaling pathways by acting as self-sufficient homodimers [7, 8]. Because of the important roles of RXRs in diseases including cancer and metabolic, autoimmune, and neurodegenerative disorders, they have become major targets for drug discovery [9–11]. A number of potent synthetic RXR ligands (called rexinoids) have been described [12]. Rexinoids activate several heterodimers and also RXR homodimers, suggesting potential utility against multiple therapeutic targets. However, RXR-targeting drugs also have adverse effects in different organs. The design of effective treatment strategies, therefore, requires a thorough understanding of how RXRs regulate bone remodeling.
The retinoid X receptors
RXRs, much more than silent partners of other nuclear receptors
Mammals have three RXR isotypes encoded by distinct genes: RXRα (NR2B1/RXRA), RXRβ (NR2B2/ RXRB), and RXRγ (NR2B3/RXRG). The three RXR isotypes show tissue-specific expression, with partially overlapping functions [13]. RXRs are master regulators of gene expression, integrating and modulating multiple functions through their ability to form obligate heterodimers with many other NRs [14]. RXRs can also regulate gene expression as self-sufficient homodimers or homotetramers, generating a so-far poorly explored complexity of RXR-dependent gene regulation. This versatility permits RXRs to exert pleiotropic transcriptional control over a wide range of biological processes, including cell differentiation, cell death, development, immune responses, and lipid and glucose metabolism.
Based on in vitro transfection experiments, RXR heterodimers are classified as permissive or non-permissive, depending on whether they can be activated by RXR ligands or not [15, 16]. Permissive heterodimers are formed with peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs), pregnane X receptor (PXR), farnesoid X receptor (FXR), and the constitutive androstane receptor (CAR). These receptors are lipid-activated NRs with low affinity for their ligands. Also considered permissive are heterodimers formed by RXR and the orphan receptors Nurr1 and Nur77, because they activate transcription in response to RXR agonists [17]. Although Nur factors have no known endogenous ligands, several synthetic compounds act as Nur agonists [18–20] (Table 1). An important regulatory feature of permissive heterodimers is that binding by agonists for both partners could have a synergistic effect relative to that resulting from binding of a single receptor ligand [21]. Dual-ligand regulation of permissive heterodimers can result in robust transcriptional activity and thus achieve profound biological responses.
Table 1.
RXRs and heterodimer partners expressed in the osteoblast and osteoclast lineages
| NR | Isotypes | Osteoblast | Osteoclast | Natural ligands | Synthetic ligands | DR |
|---|---|---|---|---|---|---|
| RXR | α(NR2B1) | E [51] | E [57] |
Retinoids Fatty acids |
Rexinoids Tributyltin |
DR-1 |
| β(NR2B2) | E [51] | E [57] | ||||
| γ (NR2B3) | E [51] | NE [57] | ||||
| PPAR | α(NR1C1) | E [51] | E [58] |
Arachidonic acid metabolites Linoleic acid |
GW7647 Fibrate derivatives |
DR-1 |
| β/δ (NR1C2) | E [51] | E [45, 57] |
Prostaglandin I2 Carbaprostacyclin |
GW0742 L165041 |
||
| γ(NR1C3) | E [51] | E [57, 59] | Fatty acids |
TZD GW9662 THR0921 CLX-090717 Tributyltin |
||
| LXR | α(NR1H1) | E [57, 60] | E [57, 60] | Oxysterols |
GW3965 T0901317 |
DR-4 |
| β(NR1H2) | E [57, 60] | E [57, 60] | ||||
| FXR | NR1H4 | E [51, 56] | E [61] |
Farnesol Bile acids |
Fexaramine 6E-CDCA GW4064 |
IR-1 |
| PXR | NR1I2 | E [109] | E [62] |
Xenobiotics Vitamin K2 Sterols and their metabolites |
Rifampicin Hyperforin Taxol Phenobarbital Ritonavir Carbamazipine |
DR-3–5 IR-6 ER-6,8 |
| CAR | NR1I3 | E [52] | E [52] |
Xenobiotics Endobiotics Steroid hormones |
TCPOBOP CITCO |
DR-4 DR-5 |
| Nur77 | NR4A1 | E [51] | E [57, 63] | Not known |
DIMs [17] Citosporone B [18] |
DR-5 |
| Nurr1 | NR4A2 | E [51] | NE [63] | Not known | DIMs [19] | DR-5 |
| RAR | α(NR1B1) | E [51] | E [57] | Retinoids |
AM580 TTNPB |
DR-2 DR-5 |
| β(NR1B2) | E [51] | E [57] | ||||
| γ(NR1B3) | E [51] | E [57] | ||||
| TR | α(NR1A1) | E [51, 110] | E [57] | Thyroid hormones |
GC-1 KB141 GC-24 |
DR-4 |
| β(NR1A2) | E [51, 110] | E [57] | ||||
| VDR | NR1I1 | E [54] | NE [54] | 1,25(OH)2 VD 3 |
MC903 EB1089 KH1060 |
DR-3 |
Bold: permissive RXR heterodimer partners; italics: non-permissive RXR heterodimer partners; E: expressed; NE: not expressed
Non-permissive RXR heterodimers are formed with endocrine receptors with high affinity for their cognate ligands. These partners include retinoic acid receptors (RARs), vitamin D receptor (VDR), and thyroid receptors (TRs) [22]. Purely non-permissive heterodimers have intrinsic repressive activity in the unliganded state and are activated only by ligands specific for the partner NR, with the RXR normally acting as an obligatory but silent partner. RXRs are generally silent in VDR and TR heterodimers although exceptions to this rule have been described in specific conditions [14]. RAR/RXR heterodimers have been termed conditionally non-permissive. These heterodimers can be further activated by specific RXR ligands in addition to the RAR ligand, and in a few cases RXR ligands have been observed to promote RAR/RXR function even when an RAR ligand is absent [23].
RXR, a promiscuous dimerization partner
The heterodimerization capacity of RXRs, together with the diversity of their ligands, suggests that RXRs can regulate a wide range of cellular pathways. This is possible due to the complex and highly regulated mechanism by which RXRs regulate the transcriptional activity of multiple genes. Although RXRs can establish heterodimers with other NRs spontaneously [24], dimerization is normally ligand-dependent. RXRs form transcriptionally inactive unliganded homotetramers that upon ligand binding dissociate to allow the formation of homo- and heterodimers [25]. Thus, one level of regulation of RXR activity depends on the local availability of ligands for RXRs and their heterodimeric partners. Another level of regulation relies on the binding of RXRs and their heterodimer partners to consensus DNA motifs in target genes [26]. These consensus motifs consist of two copies of the hexamer AGGTCA, or derivatives of this, arranged as tandem repeats. The specificity of each of these NR/DNA interactions is encoded by the orientation of the repeats (direct- (DR), inverted- (IR), or everted-repeats (ER)), as well as the number of nucleotides separating the two half-sites of the repeat (normally 1–5 nucleotides). Many homodimers and heterodimers compete for the same response elements (Table 1). For instance, PPARα-independent activation of DR1 response elements has been described, suggesting that RXRα homodimers can bind on PPAR response elements and provides a molecular basis for the activation of some PPARα target genes by rexinoids in vivo [27]. Which genes are expressed in a specific tissue may thus be determined by the local abundance of RXRs and their partners. Promoter-bound RXRs moreover recruit various co-factors, including histone-modifying enzymes and chromatin-remodeling complexes [13]. RXRs generally bind corepressors that suppress their transcriptional activity in the absence of agonists [14]. The presence of agonists shifts the equilibrium from RXR-corepressor complexes to RXR-co-activator complexes, stimulating transcriptional activity. The dynamics and recruitment of co-factor complexes thus constitute another level of RXR regulation.
Therapeutic potential of RXR modulation
RXRs were first described in 1990 by Mangelsdorf et al. as NRs able to respond to vitamin A derivatives, with a remarkable ligand specificity for 9-cis retinoic acid (9-cisRA) [28, 29]. However, the identity of the physiological RXR ligands is still debated, and there is particular uncertainty about the status of 9-cisRA as an endogenous RXR agonist because many groups have been unable to detect endogenous 9-cisRA in vivo [30, 31]. More recently, other vitamin A metabolites, such as all-trans-retinaldehyde [32], b-apo-14′-carotenal [33], dihydroretinoids [34], and 9-cis-13,14-dihydroretinoic acid [35], have been demonstrated to act as endogenous RXR ligands in several tissues. Even all-trans retinoic acid (ATRA), which was always considered an RAR-specific ligand, has been suggested to directly bind and activate RXRs [36]. In addition, RXR may function as a fatty acid receptor in vivo, since some flexible unsaturated fatty acids, either endogenously produced or derived from the diet, were recently shown to act as natural RXR ligands. These compounds include methoprenic acid, phytanic acid, docosahexaenoic acid (DHA), docosatetraenoic acid, and arachidonic acid (reviewed in [31]). These findings suggest that RXRs can directly act as intracellular sensors that regulate cell and tissue homeostasis.
Interest in the pharmacological potential of RXR ligands arose from their strong apoptotic effect in vitro [37]. The subsequent development of rexinoids has revealed the potential of RXR synthetic ligands as chemotherapeutic agents and metabolic regulators. The rexinoid bexarotene (Targretin®), a pan-RXR agonist, is already used to treat refractory or persistent cutaneous T-cell lymphoma cancer [38], and others are being tested in preclinical settings to treat insulin resistance [39]. The anticarcinogenic and metabolic effects of rexinoids are proposed to derive from the ability of RXRs to form homodimers and homotetramers [27, 40]. However, the clinical use of rexinoids is limited by its secondary effects that include a rise in triglyceride levels, the suppression of the thyroid hormone axis, and the induction of hepatomegaly [41]. There is, therefore, much interest in the design of selective RXR modulators (SRXRMs) that target RXR homodimers or specific RXR heterodimers [11].
Bone turnover and remodeling
Bone density increases rapidly during adolescence, peaking approximately 10 years after the completion of skeletal growth [42]. In the adult skeleton, bone mass is homeostatically maintained by continuous replacement of old tissue with new bone tissue, which is the basis of the dynamic process of bone turnover and remodeling. An increase in bone resorption unbalanced by bone formation leads to bone loss [3]. Bone loss is a natural consequence of aging, but can also be accelerated by numerous conditions, leading to skeletal damage at a relatively young age. These conditions include postmenopausal osteoporosis, autoimmune diseases, periodontal infection, hyperparathyroidism resulting from cancer or impaired calcium absorption, infection with human immunodeficiency virus, and type 1 diabetes [43, 44]. Bone loss is also an adverse secondary effect of some drugs, including the PPARγ-specific agonists thiazolidinediones (TZDs), which increase fracture risk and cause secondary osteoporosis [45].
The main protagonists in the complex process of bone remodeling are bone-resorbing osteoclasts and bone-forming osteoblasts [3]. These two cell types are regulated by a multitude of systemic and local factors, including cytokines, growth factors, hormones, the immune system, and mechanical load. Although osteoblast and osteoclast activities are closely integrated, these cell types originate from different lineages and have opposing functions within the bone remodeling cascade.
Bone resorption and osteoclasts
Bone resorption is initiated by the proliferation and fusion of osteoclast precursors, monocyte/macrophage hematopoietic cells that give rise to multinucleated specialized osteoclasts. Pre-osteoblastic stromal cells produce two factors that together are necessary and sufficient for bone resorption: receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor-1 (M-CSF) [46]. Activation of M-CSF receptor in osteoclast progenitors triggers precursor proliferation, whereas RANK activation promotes osteoclast differentiation. Osteoclast differentiation and cell fusion are promoted by the dynamic expression of pro-osteoclastogenic transcription factors, such as c-fos and NFATc1 (nuclear factor of activated T cells, cytoplasmic 1). These factors up-regulate expression of genes encoding osteoclast function molecules, including cathepsin K (CTSK), tartrate-resistant acid phosphatase (TRAP), calcitonin receptor (CalR), dendritic cell-specific transmembrane protein, and b3-integrin7 [2]. Thereafter, the mature osteoclasts form polarized actin filaments and migrate to the bone to form a sealing zone between osteoclasts and the bone matrix. Osteoclasts then secrete proteolytic enzymes and acids, forming a low pH local environment and degrading organic and inorganic bone components. After bone matrix degradation, the osteoclasts detach from the site and migrate to a new resorption site. Osteoblasts then move into the area to replace the resorbed bone.
Bone formation and osteoblasts
Osteoblasts are bone-forming cells derived from mesenchymal stem cells [47]. Like osteoclastogenesis, osteoblast differentiation is regulated by several transcription factors, in this case including runt-related transcription factor-2 (Runx2), osterix (Osx), and activating transcription factor-4 (ATF4). Osteoblasts secrete bone matrix proteins such as alkaline phosphatase (ALP), type I collagen (Col1), and other non-collagen proteins such as osteocalcin (OC) and osteopontin (OP). The osteoblast lineage regulates osteoclastogenesis in the bone microenvironment through the release of RANKL and M-CSF. A further level of regulation is provided by the osteoblast production of the RANKL decoy receptor osteoprotegerin (OPG) [48]. OPG binds to RANKL, blocking its interaction with RANK on osteoclast precursors and thus inhibiting osteoclast differentiation and activity. Once osteoblasts have formed the bone they become quiescent bone-lining cells on the newly formed bone surface. In the adult skeleton, bone-lining cells cover most bone surfaces not undergoing formation or resorption.
Role of RXRs in bone remodeling
Evidence accumulated of several decades points to a role for RXR heterodimers in skeletal homeostasis (reviewed in [49–51]). However, these studies have generally focused on the RXR-partner, relegating RXRs to the status of silent NR in these heterodimers. Our recent study combining genetic loss-of-function and pharmacological gain-of-function strategies revealed a direct anti-osteoclastogenic function of RXRs in mice [8]. Additionally, epigenetic marker alterations in specific regions of the RXRα promoter in umbilical cord have been shown to predict size-corrected bone mineral content in childhood, identifying RXRα as a novel biomarker in early life for adverse bone outcomes [52]. In this review we discuss the importance of RXRs in bone remodeling, beyond their subordinate role as heterodimer partners of other NRs.
Osteoblasts and osteoclasts express RXRs and RXR-partner NRs
The RXRα, RXRβ, and RXRγ isotypes are involved in a plethora of tissue- and isotype-specific biological responses [13], and all cells in the body express at least one RXR isotype [22]. Of the 49 NRs found in rodents, around one-third has been shown to form heterodimers with RXRs [13, 14]. Many of these NRs are expressed in the osteoblast and osteoclast lineages, and a number of them can modulate their differentiation and activation, as we discuss below. Expression studies in calvarial osteoblast primary cultures and mesenchymal and osteoblastic cell lines show that the three RXR isotypes and their heterodimer partners are widely expressed in the osteoblast lineage [53–58] (Table 1). Osteoclast progenitors, which are bone marrow myeloid cells, express RXRα and RXRβ, but not RXRγ [8, 59]. Several RXR heterodimer partners, including PPARs, RARs, and LXRs, are expressed at several stages of osteoclast differentiation, and are found in bone marrow derived macrophages, osteoclast cell lines, and cultured osteoclasts [49, 54, 59–65] (Table 1).
Mouse models of RXR and RXR-partner function in the skeleton
The generation of mice carrying RXR gene deletions or mutations has been a valuable tool for investigating the impact of RXRs on biological functions. Animals with systemic deletions have been generated for each of the three RXR isotypes. However, these deletions result in lethality, or cause systemic abnormalities that mask cell- and tissue-specific effects. For examples, ubiquitous inactivation of RXRα is embryonically lethal at mid-gestation due to hypoplastic development of the ventricular myocardium [66]. RXRβ knockout mice show reduced spermatid formation, whereas RXRγ knockout induces metabolic defects [67, 68]; however, these mice show no evident bone alterations (Table 2).
Table 2.
Adult bone phenotypes of knockout mice for RXR and RXR-partners
| NR | Mouse model | Cell type | Bone phenotype | Bone mineral density | Bone resorption | Bone formation |
|---|---|---|---|---|---|---|
| RXR | aRxra−/− [64] | Systemic | – | – | – | – |
| Rxrb−/− [65] | Systemic | Normal | NA | NA | NA | |
| Rxrg−/− [66] | Systemic | Normal | NA | NA | NA | |
| Mx1-Cre/Rxrafl/flbfl/fl [8] | Osteoclast lineage | Increased bone mass |
Male ↑ Female → |
↓ | → | |
| PPAR | Ppara−/− [110] | Systemic | Normal | → | → | → |
| PpardSox2-cKO [74] | Systemic (except placenta) | Osteopenia | ↓ | ↑ | → | |
| Pparg+/− [81] | Systemic |
Increased bone mass Osteopetrosis |
↑ | → | ↑ | |
| Tie2Cre/Ppargfl/fl [82] | Osteoclast lineage | Increased bone mass | ↑ | ↓ | → | |
| LXR | Lxra−/− [60] | Systemic | Increased bone mass | Male → Female ↑ | ↓ | → |
| Lxrb−/− [60] | Systemic | Normal | → | ↓ | ↑ | |
| Lxrab−/− [60] | Systemic | Normal | → | → | → | |
| FXR | Fxr−/− [61] | Systemic | Osteopenia |
Male ↓ Female → |
↑ | ↓ |
| PXR | Pxr−/− [62] | Systemic | Osteopenia |
Male ? Female ↓ |
↑ | ↓ |
| CAR | Car−/− [52] | Systemic | Increased bone mass |
Male ↑ Female → |
→ | ↑? |
| Nur77 | Nurr77−/− [63] | Systemic | Osteopenia | ↓ | ↑ | → |
| Nurr1 | Nurr1+/− [111] | Systemic | Normal | NA | NA | NA |
| RAR | Rara−/− [75] | Systemic | Normal | → | → | → |
| Rarb−/− | Systemic | Normal | NA | NA | NA | |
| Rarg−/− [75] | Systemic | Osteopenia | ↓ | ↑ | ? | |
| TR | TRα−/− [112] | Systemic | Osteosclerosis | ↑ | ↓ | NA |
| TRb−/− [112] | Systemic | Osteoporosis | ↓ | ↑ | NA | |
| VDR | VDR−/− [113, 114] | Systemic | Rickets Osteomalacia | ↓ | → | ↑ |
| Col2Cre+/−VDRfl/fl [83] | Chondrocyte | Increased bone mass | ↑ | ↓ | → |
Arrows indicate increase, decrease, or no change
NA not assessed
aEmbryonically lethal
The physiological functions of RXRs in specific tissues, including the bone, have been investigated in cell-type-specific RXR gene knockout mice (reviewed in [69]). The MxCre-mediated loxP recombination system was used to specifically delete RXR isotypes expressed in murine osteoclast progenitors. The lack of hematologic effects in mice lacking RXRα suggested that RXRβ might compensate for the loss of RXRα in bone marrow cells [70]. To address the possibility of compensation, mice were generated with double deletion of RXRα and RXRβ. These mice had complete deletion of the RXRα and RXRβ genes in all hematopoietic organs, including whole bone marrow, undifferentiated lineage marker-negative and mature lineage marker-positive cells, and osteoclasts [8]. Our examination of this mouse model revealed an important role for RXR homodimers in bone homeostasis and bone remodeling through the control of differentiating osteoclasts [8] (Table 2). Loss of RXR function in osteoclast progenitors resulted in formation of non-resorbing osteoclasts. Although these osteoclasts were larger than those from wild-type animals, they had defects in bone resorption due to their deficient expression of activity genes and failure to form cytoskeletal structures necessary for resorption of the bone matrix. This resulted in increased bone mass in male mice and protection from bone loss in an experimental model of postmenopausal osteoporosis. The increase in bone mass was due to lack of expression in osteoclast progenitors of the transcription factor MAFB (v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B), which was shown to be necessary for their proper M-CSF-dependent proliferation and further differentiation into functional osteoclasts. These studies demonstrated that in the absence of exogenous RXR ligand, RXR homodimers directly target and bind the Mafb promoter. These results suggest that RXR endogenous ligands might be produced during osteoclastogenesis. Supporting this idea, the fatty acid RXR ligands arachidonic acid and DHA have been found to play a role in osteoclastogenesis [71, 72]. However, the physiological role of RXR homodimers will remain an open question until the endogenous RXR ligands in the bone environment are revealed.
The promiscuity of RXRs, arising from their capacity to form heterodimers with many other NRs, makes it difficult to define the functions of RXR homodimers because in most cases the phenotypes observed in RXR mutant mice can be linked to alterations in pathways regulated by one or many RXR heterodimer partners. To unambiguously determine the specific dimer responsible for the bone phenotype of mice lacking RXRs, it is necessary to compare this phenotype with those presented by mice lacking RXR heterodimer partners. Systemic or cell-specific deficiency models for several RXR heterodimeric partners have revealed the importance of many of these NRs in the control of bone remodeling (Table 2). General deletion of VDR and TR, long established as regulators of bone and mineral homeostasis, has profound skeletal effects (for detailed reviews see [73–75]). VDR knockout mice present a general phenotype that mimics human vitamin D-dependent hereditary rickets type II, whose main clinical manifestations are low bone mineral density, rachitic malformation, growth retardation and short stature, hypocalcemia, hypophosphatemia, and hyperparathyroidism. Studies in VDR knockout mice reveal that the main role of VDR is to maintain serum calcium and phosphate homeostasis. VDR signaling additionally plays a direct role in osteoblast differentiation and an osteoblast-mediated role in osteoclastogenesis. The action of thyroid hormone on bone is mediated principally by TRα, which is expressed in the skeleton at much higher levels than TRβ. Mutation of TRβ disrupts the hypothalamic–pituitary axis, leading to systemic hyperthyroidism and overstimulation of intact TRα in bone. Thus, deletion or mutation of each of these receptors has opposite effects. TRα mutant mice have elevated bone mass as a result of reduced osteoclast activity resulting from the impaired thyroid hormone action in bone. In contrast, TRβ mutant mice have osteoporosis as a result of accelerated bone resorption due to the effects of systemic hyperthyroidism.
Studies reported in the past 5 years provide new insight into the role in bone remodeling of other RXR heterodimeric partners. General deletion of PPARβ/δ [76], RARγ [77], PXR [64], FXR [63], or Nur77 [65] in mice provokes osteopenia. In all these models, the systemic lack of the RXR heterodimeric partner increases osteoclastogenesis, due to the loss of either a direct or a paracrine effect of the NR on osteoclastic cells. Mice conditionally lacking PPARβ/δ in all tissues except the placenta (PpardSox2-cKO) have an above-normal osteoclast count and osteopenia due to decreased Wnt signaling in bone-lining osteoblasts [76]. Wnt/β-catenin signaling is required to direct mesenchymal progenitor cells toward the osteoblast lineage, and its inactivation imbalances bone formation and resorption due to a decline in the level of osteoblast-secreted OPG [78]. PPARβ/δ-deficient mice have an elevated RANKL-to-OPG ratio, thereby influencing the rate of osteoclastogenesis. Among RAR deletion mutants, only RARγ knockouts show increased osteoclastogenesis and osteopenia [77]. The authors of this study suggested that the increased bone resorption was in part due to an increase in osteoclast size but not in osteoclast numbers. Notably, both RARγ and RXR-double knockout mice have abnormally large osteoclasts [8, 77]. However, whereas in the RARγ knockout model this results in apparently elevated lytic activity, RXR-double knockout mice have bone resorption defects. Increases in osteoclast size in vivo have been linked either to reduced osteoclast resorptive activity [79] or to increased osteoclast activity [80, 81]. The authors did not further explore the mechanism underlying this RAR-mediated osteoclast phenotype, but speculated that it must involve M-CSF-mediated increased osteoclast progenitor fusion. In the case of RXR-deficient osteoclasts, our work excluded changes in the expression of cell fusion and adhesion molecules as the cause of the giant and resorption-deficient osteoclast phenotype [8]. Rather, we found that RXR-dependent expression of MAFB was involved in M-CSF-dependent proliferation of osteoclast progenitors and that this affected osteoclastogenesis. The reduced bone mass in female mice lacking PXR [64] and male mice lacking FXR [63] was proposed to be due both to decreased bone formation and increased bone resorption. Histomorphometrical differences observed in the trabecular bones of female Pxr−/− mice accounted for the osteopenic phenotype of these mice, but the underlying mechanism remains unexplored [64]. Calvarial cells from Fxr−/− mice show reduced expression of the osteoblast-specific transcription factors Runx2 and Osx and osteoblast marker genes, including Col1, ALP, and OC. The increased osteoclast activity of Fxr−/− mice was shown to be mediated both by osteoblasts and by osteoclasts. On the one hand, augmented expression of RANKL by FXR-deficient osteoblasts increased RANKL/OPG ratio, which could account for the increased bone resorption status. On the other hand, the lack of FXR in osteoclast precursors resulted in increased expression of the NFATc1 protein. NFATc1 is the key transcriptional factor for osteoclastogenesis [82], and its induction could, therefore, account for the elevated bone resorption in Fxr−/− mice. Similarly, increased expression of NFATc1 is involved in the osteopenic phenotype of general Nur77 knockout mice [65]. This study identified Nur77 as both regulator and transcriptional target of NFATc1: NFATc1 induces Nur77 expression at late stages of osteoclast differentiation, and in turn Nur77 transcriptionally upregulates E3 ubiquitin ligase Cbl-b, which triggers NFATc1 protein degradation. Interestingly, Nurr77−/− differentiating cultures contain larger and more active mature osteoclasts. However, it remains unclear whether this phenomenon is due to increased proliferation or to osteoclast progenitor fusion.
Together these studies indicate that none of PPARβ/δ, RARγ, PXR, FXR, or Nur77 could be involved in the osteopetrotic phenotype of RXR-double knockout mice. Among the mouse models of deficiency for RXR heterodimer partners, the only ones to show increased bone mass are systemic or osteoclast-specific PPARγ knockouts [83, 84], systemic LXRα knockouts [62], systemic CAR knockouts [54], and chondrocyte-specific VDR knockouts [85] (Table 2). However, the mechanisms underlying this phenotype are different from that described in RXR-double knockout mice. For example, mice with general deletion of PPARγ (Pparg+/−) have high bone mass due to increased osteoblast number and bone formation rather than bone resorption [83]. The authors observed that PPARγ insufficiency favored differentiation of osteoblasts over adipocytes from their shared mesenchymal progenitor, a finding consistent with the role of PPARγ in adipogenesis [86]. The mechanism for this action remained unclear, since the authors observed an upregulation of key molecules for osteoblast differentiation (Runx2, Osx, and LRP5, the LDL receptor-related protein 5), but could not demonstrate direct binding of PPARγ to the promoter of these genes. More recently, PPARγ was revealed to play a role not only in osteoblastogenesis, but also in osteoclastogenesis [84]. Loss of PPARγ function in mouse hematopoietic lineages (Tie2Cre/Ppargfl/fl) causes osteoclast defects and impaired bone resorption. The lack of osteoclast bone-resorbing activity in these mice is due to complete blockade of osteoclast differentiation and reduction of c-fos expression [84]. Osteoclasts from mice with general deletion of LXRα (Lxra−/−) are unable to effectively resorb bone in the cortical compartment [62]. The authors speculated that LXRα might regulate late stages of osteoclast function rather than their differentiation from progenitor cells; however, the underlying mechanism was not defined. In male mice with systemic deletion of CAR (Car−/−), the increased bone mass observed was attributed to decreased hepatic expression of Cyp2b and a consequent reduction in testosterone metabolism [54]. Positive correlations between testosterone concentration and bone mass had been reported previously in animals and humans [87]. The mechanism driving the testosterone effect in bone is increased osteoprotegerin mRNA expression in mouse osteoblast cells [88]. Finally, the specific absence of VDR in chondrocytes (Col2Cre+/−VDRfl/fl) impairs osteoclastogenesis during early postnatal life [85]. Although the authors aimed to elucidate the function of VDR during growth-plate development and endochondral bone formation, growth plate morphology was normal in these mice. Unexpectedly, the authors demonstrated a VDR-mediated paracrine loop between chondrocytes, osteoblasts/osteocytes, and osteoclasts. The decrease in osteoclastogenesis in these mice is secondary to a decrease in chondrocyte RANKL production. These mice also have elevated levels of circulating phosphate and the VDR ligand 1,25-dihydroxyvitamin D3 (1,25(OH)2D) before weaning. This was thought to be due to decreased osteoblast expression of the phosphaturic hormone fibroblast growth factor 23 (FGF23) [85], which plays a direct role in skeletal remodeling through the control of osteoclastogenesis [89].
These studies together indicate that RXRs play pleiotropic functions as heterodimeric partners of other NRs with important roles in bone remodeling. Remarkably, because none of the RXR heterodimer partner-knockout mice recapitulate the mechanism leading to increased bone mass in RXR-deficient mice, these studies also support a previously unrecognized pro-osteoclastogenic function of RXR homodimers in physiological conditions [8].
Protective effect of pharmacological RXR activation on bone loss
The role of retinoids in bone biology has been studied extensively (for detailed reviews see [90, 91]). Numerous clinical studies have assessed the link between vitamin A intake and fracture risk; however, it remains unclear whether vitamin A is deleterious or protective to bone. The transcriptional effects of retinoid signaling are mediated through RAR/RXR heterodimers. RARs can be activated by all-trans retinoic acid (ATRA) and by 9-cisRA, whereas RXRs are activated only by 9-cisRA. Additionally, 9-cisRA induces RXR homodimer formation in vitro, suggesting the existence of a retinoid response pathway distinct from that activated by the heterodimer RAR/RXR [92]. Here we summarize the effects on bone remodeling of 9-cisRA and rexinoids, as well as several RXR permissive heterodimeric partner agonists (Table 3). However, it is important to note that in specific conditions even the non-permissive heterodimers might be directly activated by RXR agonists, depending on factors such as tissue specificity, the cellular environment, and the ability of various RXR ligands to recruit coactivator or corepressor complexes [14].
Table 3.
Effects of agonists of RXRs and their permissive heterodimer partners on osteoclast and osteoblast differentiation and activity
| NR | Ligand | Osteoclast differentiation or activity | Osteoblast differentiation or activity |
|---|---|---|---|
| RXRs | LG100268 [8, 95] | ↓a | ↓a |
| Bexarotene [8, 95] | ↓b | →b ↓a | |
| 9-cisRA [92–94] | ↑a ↓a | ||
| PPARα | Bezafibrate [102, 103] | ↑a | ↑a |
| Fenobbrate [103] | ↑a | ||
| Linoleic acid [102] | ↑a | ||
| PPARδ/β | Carbaprostacyclin [104] | ↑a | |
| L165041 [103] | ↑a | ||
| GW501516 [74] | ↑a | ||
| PPARγ | Rosiglitazone [82, 115, 116–118] | ↑a,b | ↓a,b |
| Ciglitizone [103] | ↑a | ||
| LXRs | GW3965 [99] | ↓a → b | → b |
| T0901317 [8, 100, 101] | ↓a → a,b | ↓a,b → b | |
| FXR | Chenodeoxycholic acid [56] | ↑a | |
| Farnesol [56] | ↑a | ||
| Bile acids [61] | ↓a | ↑a,b | |
| GW4064 [61] | ↓a | ↑a | |
| Fexaramine [61] | ↓a | ↑a | |
| PXR | Vitamin K2 [53] | ↑a | |
| Rifampicin [53] | ↑a | ||
| Hyperforin [53] | ↑a |
Arrows indicate increase, decrease, or no change
aIn vitro
bIn vivo
Our recent study demonstrated that in vitro RXR activation of bone marrow osteoclast progenitors with the rexinoid LG100268 inhibits osteoclastogenesis [8]. This effect was unexpected, given the anti-osteoclastogenic effect of RXR deficiency under baseline conditions. Treatment with LG100268 upregulates MAFB expression during in vitro osteoclast differentiation, blocking RANKL signaling and consequently the formation of mature active osteoclasts. This result is in agreement with a previously described anti-osteoclastogenic role of MAFB, which when overexpressed in osteoclast progenitors attenuates the expression of NFATc1 during RANKL-mediated osteoclastogenesis [93]. Our studies demonstrated that in vivo pharmacological activation of RXRs with bexarotene significantly diminished osteoclast activation and bone resorption in ovariectomized mice, but had no significant effect on steady-state bone turnover. The effect of bexarotene in steady-state conditions was recently tested in male Wistar rats [94]. Although the authors reported variations in the levels of OC and plasma TRAP, as well as in bone parameters, these changes were mild [94]. These results suggested that pharmacological RXR activation has no major effect in physiological conditions, but might be protective against bone loss after the menopause Whether RXR activation affects osteoblast differentiation and activation is debated. Recent studies report that 9-cisRA induces osteogenic differentiation of mesenchymal progenitor cells [95] and promotes osteogenic markers in mesenchymal progenitor cells [95] and human osteosarcoma cell lines [96]. However, long-term treatment with 9-cisRA inhibits differentiation of primary calvarial osteoblast cultures [97]. Decreases in ALP activity, mineralization, and expression of osteoblast-related genes are achieved in primary mouse mesenchymal stem cells upon RXR activation with bexarotene, LG100268, and the organotin contaminant tributyltin, which is also a known PPARγ agonist [98]. However, we found no changes in osteoblast number or activity in mice treated with bexarotene [8]. We concluded that RXR activation in mice provokes defects in osteoclastogenesis as a consequence of MAFB regulation, but other RXR-modulated signaling pathways could be involved in this effect. For instance, RXR agonists induce proteasome-mediated β-catenin degradation [99]. RXR-mediated β-catenin degradation is inhibited by cellular retinol-binding protein 1 (CRBP-1), a factor implicated in vitamin A metabolism and intracellular retinoid transport [100]. Wnt/β-catenin signaling influences progenitor cell differentiation toward osteoblasts or osteoclasts [78] and, therefore, modifications to Wnt/β-catenin signaling could account for the skeletal phenotype of bexarotene-treated mice. In addition, agonists of diverse RXR permissive heterodimers modulate other genes regulating osteoclast and osteoblast differentiation and activity, as discussed below.
We recently reported that rexinoids inhibit osteoclastogenesis via indirect regulation of Mafb expression, through LXR/RXR-induced expression of the master lipid homeostasis regulator SREBP-1c (sterol regulatory element binding protein-1c) [8, 101]. This is supported by an earlier report showing that LXR-specific ligand GW3965 induces Mafb expression and blocks in vitro osteoclastogenesis of human and murine osteoclast progenitors [102]. The authors concluded that the effect of GW3965 on osteoclastogenesis was NFATc1/p38/MITF-dependent and c-Fos or RANK-independent. Moreover, abolition of these effects in LXRβ−/− osteoclast precursors suggested that the LXR-specific ligand acts via an LXRβ-dependent mechanism [102]. Also consistent with the bexarotene data [8], treatment of ovariectomized mice with the LXR-specific agonist T0901317 provides protection from estrogen-depletion-induced bone loss [103]. The authors of this study concluded that the effects of LXR on osteoclastogenesis are osteoblast-mediated: in osteoblast/osteoclast co-cultures, LXR activation reduced the RANKL/OPG ratio, but when osteoblasts were absent no changes were observed in the number of in vitro differentiated osteoclasts, despite clear reductions in osteoclast size and activity [103]. However, our work shows that T0901317 blocks in vitro differentiation of osteoblast-free wild-type osteoclast cultures but not of their RXR-deficient or LXR-deficient counterparts [8], demonstrating an osteoblast-independent role of LXR activation in osteoclastogenesis. Another study claimed that the effects of LXR activation on murine osteoblasts depend on the duration of ligand exposure [104]. In this study, short T0901317 exposure decreased OC levels in primary murine osteoblasts and in male mice. However, long-term oral administration of T0901317 or GW3965 did not alter trabecular and cortical bone structure or bone turnover in female [104]. The gender effect in this study suggests that LXR activation effects are not exclusively dependent on treatment duration. Indeed, our work with RXR double-knockout mice demonstrated that the physiological role of RXRs in osteoclastogenesis is gender-dependent, affecting the bone phenotype of males but not females [8]. Gender differences were also assessed in PXR, FXR and CAR deficient mice [54, 63, 64]. Although the cause of these gender-specific bone phenotypes is unclear, it might involve crosstalk between RXRs and estrogen receptors [105], leading to sexually dimorphic expression of some RXR target genes. For instance, the expression of Cyp2b, the major metabolizing enzyme and major target gene of CAR/RXR [54], is gender-independent before puberty, but declines specifically in male mice during maturation [106]. In addition, estrogens reduce osteoclastogenesis and bone resorption [107, 108] and thus might influence the bone phenotypes of female mice.
Agonists for the three PPAR isotypes, α, δ/β and γ, have diverse effects on bone remodeling. PPARα activation with bezafibrate has anabolic effects on bone in vitro, stimulating osteoblast differentiation (shown by increased ALP activity, collagen production, and calcification) [109] and inhibiting the formation of human multinucleated osteoclasts [110]. The effect of PPARβ/δ activation in bone is more confusing. Activation of PPARβ/δ with GW501516 induces Wnt-dependent expression of osteoblast marker genes in osteoblasts and mesenchymal cells and has an osteoblast-mediated inhibitory effect on osteoclastogenesis [76]. However, two independent reports showed that PPARδ/β activation can induce bone resorption. In one report, carbaprostacyclin-mediated PPARδ/β activation upregulated CTSK and TRAP expression and potently induced the bone-resorbing activity of isolated mature rabbit osteoclasts [111]. Accordingly, in cultured human osteoclasts derived from peripheral blood mononuclear cells, PPARδ/β activation with L165041 stimulated the resorptive activity of mature osteoclasts [110]. The role of PPARγ activation in bone biology has been extensively studied due to its detrimental effects on bone anabolism in mice and humans (for reviews see [112, 113]). Recent trials report that long-term treatment of insulin resistance with the synthetic PPARγ agonist rosiglitazone increases fracture rates among diabetes patients [114]. PPARγ activation both suppresses osteoblastogenesis and activates osteoclastogenesis, thereby decreasing bone mass as a net effect.
Agonists of FXR increase bone formation while decreasing bone resorption. In vitro FXR activation with bile acids or synthetic FXR agonists enhances osteoblast differentiation through the transactivation of Runx2 [58] and enhanced signaling via extracellular signal-regulated kinase (ERK) and β-catenin [63]. As a consequence, FXR agonists stimulate the expression of osteoblast marker genes such as bone sialoprotein, OC, OP, and ALP [58]. FXR agonists also suppress RANKL-induced osteoclast differentiation from bone marrow macrophages and inhibit the expression of c-fos and NFATc1; however, the molecular mechanism driving these effects was not dissected [63]. Accordingly, a farnesol-enriched diet marginally protects against ovariectomy-induced bone loss and enhances bone mass gain in growing mice [63]. Finally, bone formation is potentiated by PXR activation with the anti-osteoporotic agent menaquinone-4 (Vitamin K2) [115], which induces the expression of key osteoblastic marker genes like ALP, OPG, OC, and OP [55] and extracellular matrix-related genes involved in collagen assembly [116].
RXR activation by rexinoids thus blocks osteoclast differentiation and protects against bone loss in postmenopausal conditions. This protective effect is driven by the activation of LXR/RXR and probably PPARα/RXR and FXR/RXR in cells of the osteoclast lineage. Bone anabolic effects might also be achieved by the activation of PPARα/RXR, PPARβ/δ/RXR, FXR/RXR, or PXR/RXR in osteoblastic cells.
Concluding remarks
Despite the growing body of literature on the roles of NRs in bone biology, few studies have examined the key role of RXRs, which were historically studied as subordinate partners of other NRs. However, we and others have demonstrated that RXRs can be directly activated by specific ligands and modulate multiple signaling pathways in vivo both as heterodimers with other NRs and as homodimers.
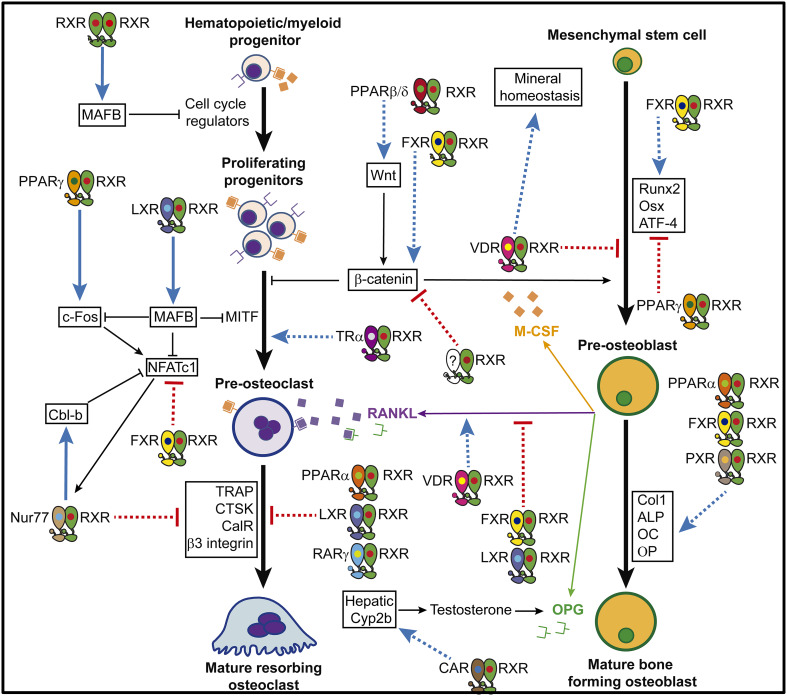
The studies summarized in this review indicate that RXRs can modulate osteoclast and osteoblast formation and function at several levels of cell differentiation and activation (Fig. 1). Remarkably, under physiological conditions RXRs control osteoclast progenitor proliferation and osteoclast activation independently of RXR heterodimeric partners with known roles in bone physiology. This finding supports an in vivo role for RXR homodimers, indicating that they can function as biologically relevant transcription units. Pharmacological RXR activation blocks osteoclast differentiation and protects female mice from estrogen-depletion-induced bone loss. Notably, the effects of RXR activation on bone remodeling are independent of PPARγ. This finding is important because PPARγ ligands have negative effects on bone anabolism in mice and humans, challenging their therapeutic benefits as insulin sensitizers.
Fig. 1.
RXR-mediated regulation of bone remodeling. Blue arrows indicate genes or processes activated by RXR dimers, and red arrows represent repressive actions of RXR dimers (continuous line bone fide target gene; dotted line undefined mechanism)
The studies reviewed here highlight the potential utility of RXR ligands for the treatment of low bone mass disorders. However, medical use of currently available pan-RXR modulators is limited by the pleiotropic effects of RXR activation. This issue could be solved by the design of selective RXR modulators (SRXRMs), which are new designed rexinoids with heterodimer-selective and cell-specific activities. For instance, protection against bone loss might be achieved without undesirable secondary effects through the use of specific RXR homodimer antagonists in bone marrow progenitors or of specific LXR/RXR heterodimer agonists in differentiating osteoclasts. Advances in SRXRM development and the bone-specific delivery of these agents have the potential to overcome the current limitations to RXR targeting.
Acknowledgements
We thank S. Bartlett (CNIC) for editorial assistance. Some of the work reported in this review was supported by a grant from the Spanish Ministry of Economy and Competitiveness (SAF2015-64287) to M. Ricote. The CNIC is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505). We apologize to our many colleagues for not being able to cite all relevant references owing to space limitations.
Abbreviations
- ALP
Alkaline phosphatase
- ATF4
Activating transcription factor-4
- ATRA
All-trans retinoic acid
- CalR
Calcitonin receptor
- CAR
Constitutive androstane receptor
- Col1
Type I collagen
- CRBP-1
Cellular retinol-binding protein 1
- CTSK
Cathepsin K
- DHA
Docosahexaenoic acid
- DR
Direct repeat
- ER
Everted repeat
- ERK
Extracellular signal-regulated kinase
- FXR
Farnesoid X receptor
- IR
Inverted repeat
- LRP5
LDL receptor-related protein 5
- LXR
Liver X receptor
- MAFB
v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B
- M-CSF
Macrophage colony-stimulating factor-1
- NFATc1
Nuclear factor of activated T cells, cytoplasmic 1
- NR
Nuclear receptor
- Nurr1
Nuclear receptor related 1
- Nur77
Nerve growth factor IB
- OC
Osteocalcin
- OP
Osteopontin
- OPG
Osteoprotegerin
- PPAR
Peroxisome proliferator-activated receptor
- PXR
Pregnane X receptor
- RANKL
Receptor activator of NF-κB ligand
- RAR
Retinoid acid receptor
- Runx2
Runt-related transcription factor-2
- RXR
Retinoid X receptor
- SRXRMs
Selective retinoid X receptor modulators
- TRAP
Tartrate-resistant acid phosphatase
- TR
Thyroid hormone receptor
- TZD
Thiazolidinedione
- VDR
Vitamin D receptor
- 1,25(OH)2D
1,25-Dihidroxyvitamin D3
- 9-cRA
9-cis retinoic acid
References
- 1.Digirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012;8(11):674–683. doi: 10.1038/nrrheum.2012.157. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 3.Feng X, Mcdonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45(12):863–873. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahoum V, Perez E, Germain P, Rodriguez-Barrios F, Manzo F, Kammerer S, Lemaire G, Hirsch O, Royer CA, Gronemeyer H, De Lera AR, Bourguet W. Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc Natl Acad Sci USA. 2007;104(44):17323–17328. doi: 10.1073/pnas.0705356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunez V, Alameda D, Rico D, Mota R, Gonzalo P, Cedenilla M, Fischer T, Bosca L, Glass CK, Arroyo AG, Ricote M. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc Natl Acad Sci USA. 2010;107(23):10626–10631. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menendez-Gutierrez MP, Roszer T, Fuentes L, Nunez V, Escolano A, Redondo JM, De Clerck N, Metzger D, Valledor AF, Ricote M. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J Clin Invest. 2015;125(2):809–823. doi: 10.1172/JCI77186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas M, Sukhai MA, Kamel-Reid S. An emerging role for retinoid X receptor alpha in malignant hematopoiesis. Leuk Res. 2012;36(9):1075–1081. doi: 10.1016/j.leukres.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Yamada S, Kakuta H. Retinoid X receptor ligands: a patent review (2007–2013) Expert Opin Ther Patents. 2014;24(4):443–452. doi: 10.1517/13543776.2014.880692. [DOI] [PubMed] [Google Scholar]
- 11.Vaz B, De Lera AR. Advances in drug design with RXR modulators. Expert Opin Drug Discov. 2012;7(11):1003–1016. doi: 10.1517/17460441.2012.722992. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386(6623):407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 2010;21(11):676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta. 2012;1821(1):21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa R, Yu V, Näär A, Kyakumoto S, Han Z, Silverman S, Rosenfeld MG, Glass CK. Differential orientations of the DNA binding domain and C-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993;7:1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- 17.Campos-Melo D, Galleguillos D, Sanchez N, Gysling K, Andres ME. Nur transcription factors in stress and addiction. Front Mol Neurosci. 2013;6:44. doi: 10.3389/fnmol.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67(2):674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 19.Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, Li G, Xu Q, Zhang M, Weimer BC, Chen D, Cheng Z, Zhang L, Li Q, Li S, Zheng Z, Song S, Huang Y, Ye Z, Su W, Lin SC, Shen Y, Wu Q. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. 2008;4(9):548–556. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 20.De Miranda BR, Popichak KA, Hammond SL, Jorgensen BA, Phillips AT, Safe S, Tjalkens RB. The Nurr1 activator 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane blocks inflammatory gene expression in BV-2 microglial cells by inhibiting nuclear factor kappaB. Mol Pharmacol. 2015;87(6):1021–1034. doi: 10.1124/mol.114.095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leblanc BP, Stunnenberg HG. 0-Cis retinoic acid signaling: changing partners causes some excitement. Genes Dev. 1995;9:1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- 22.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lala DS, Mukherjee R, Schulman IG, Koch SS, Dardashti LJ, Nadzan AM, Croston GE, Evans RM, Heyman RA. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature. 1996;383(6599):450–453. doi: 10.1038/383450a0. [DOI] [PubMed] [Google Scholar]
- 24.Feige JN, Gelman L, Tudor C, Engelborghs Y, Wahli W, Desvergne B. Fluorescence imaging reveals the nuclear behavior of peroxisome proliferator-activated receptor/retinoid X receptor heterodimers in the absence and presence of ligand. J Biol Chem. 2005;280(18):17880–17890. doi: 10.1074/jbc.M500786200. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZP, Iyer J, Bourguet W, Held P, Mioskowski C, Lebeau L, Noy N, Chambon P, Gronemeyer H. Ligand- and DNA-induced dissociation of RXR tetramers. J Mol Biol. 1998;275(1):55–65. doi: 10.1006/jmbi.1997.1413. [DOI] [PubMed] [Google Scholar]
- 26.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–954. [PubMed] [Google Scholar]
- 27.Ijpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B (2004) In vivo activation of PPAR target genes by RXR homodimers. EMBO J 23(10):2083–2091 [DOI] [PMC free article] [PubMed]
- 28.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 29.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-V. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev. 2006;64(12):532–538. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 31.Perez E, Bourguet W, Gronemeyer H, De Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta. 2012;1821(1):57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13(6):695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol. 2007;21(1):77–88. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]
- 34.Moise AR, Alvarez S, Dominguez M, Alvarez R, Golczak M, Lobo GP, Von Lintig J, De Lera AR, Palczewski K. Activation of retinoic acid receptors by dihydroretinoids. Mol Pharmacol. 2009;76(6):1228–1237. doi: 10.1124/mol.109.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhl R, Krzyzosiak A, Niewiadomska-Cimicka A, Rochel N, Szeles L, Vaz B, Wietrzych-Schindler M, Alvarez S, Szklenar M, Nagy L, De Lera AR, Krezel W. 9-cis-13,14-dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet. 2015;11(6):e1005213. doi: 10.1371/journal.pgen.1005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuji M, Shudo K, Kagechika H. Docking simulations suggest that all-trans retinoic acid could bind to retinoid X receptors. J Comput Aided Mol Des. 2015;29(10):975–988. doi: 10.1007/s10822-015-9869-9. [DOI] [PubMed] [Google Scholar]
- 37.Mehta K, Mcqueen T, Neamati N, Collins S, Andreeff M. Activation of retinoid receptors RAR alpha and RXR alpha induces differentiation and apoptosis, respectively, in HL-60 cells. Cell Growth Differ. 1996;7(2):179–186. [PubMed] [Google Scholar]
- 38.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19(9):2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 39.Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl 2):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 40.Yasmin R, Kannan-Thulasiraman P, Kagechika H, Dawson MI, Noy N. Inhibition of mammary carcinoma cell growth by RXR is mediated by the receptor’s oligomeric switch. J Mol Biol. 2010;397(5):1121–1131. doi: 10.1016/j.jmb.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall PA, Jurutka PW, Wagner CE, Van Der Vaart A, Kaneko I, Chavez PI, Ma N, Bhogal JS, Shahani P, Swierski JC, Macneill M. Analysis of differential secondary effects of novel rexinoids: select rexinoid X receptor ligands demonstrate differentiated side effect profiles. Pharmacol Res Perspect. 2015;3(2):e00122. doi: 10.1002/prp2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine MA. Assessing bone health in children and adolescents. Indian J Endocrinol Metab. 2012;16(Suppl 2):S205–S212. doi: 10.4103/2230-8210.104040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover - role of the immune system. Nat Rev Endocrinol. 2016;12(9):518–532. doi: 10.1038/nrendo.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krakauer JC, Mckenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44(7):775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 45.Lecka-Czernik B. Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr Osteoporos Rep. 2010;8(4):178–184. doi: 10.1007/s11914-010-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 47.Titorencu I, Pruna V, Jinga VV, Simionescu M. Osteoblast ontogeny and implications for bone pathology: an overview. Cell Tissue Res. 2014;355(1):23–33. doi: 10.1007/s00441-013-1750-3. [DOI] [PubMed] [Google Scholar]
- 48.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imai Y, Youn MY, Inoue K, Takada I, Kouzmenko A, Kato S. Nuclear receptors in bone physiology and diseases. Physiol Rev. 2013;93(2):481–523. doi: 10.1152/physrev.00008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Z, Li X, Wan Y. Minireview: nuclear receptor regulation of osteoclast and bone remodeling. Mol Endocrinol. 2015;29(2):172–186. doi: 10.1210/me.2014-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato S, Suzawa M, Takada I, Takeyama K, Yanagizawa J, Fujiki R, Kitagawa H. The function of nuclear receptors in bone tissues. J Bone Miner Metab. 2003;21(6):323–336. doi: 10.1007/s00774-003-0453-3. [DOI] [PubMed] [Google Scholar]
- 52.Harvey NC, Sheppard A, Godfrey KM, Mclean C, Garratt E, Ntani G, Davies L, Murray R, Inskip HM, Gluckman PD, Hanson MA, Lillycrop KA, Cooper C. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res. 2014;29(3):600–607. doi: 10.1002/jbmr.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pirih FQ, Abayahoudian R, Elashoff D, Parhami F, Nervina JM, Tetradis S. Nuclear receptor profile in calvarial bone cells undergoing osteogenic versus adipogenic differentiation. J Cell Biochem. 2008;105(5):1316–1326. doi: 10.1002/jcb.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho HY, Jung JY, Park H, Yang JY, Jung S, An JH, Cho SW, Kim SW, Kim SY, Kim JE, Park YJ, Shin CS. In vivo deletion of CAR resulted in high bone mass phenotypes in male mice. J Cell Physiol. 2014;229(5):561–571. doi: 10.1002/jcp.24478. [DOI] [PubMed] [Google Scholar]
- 55.Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278(45):43919–43927. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Zhu J, Deluca HF. Identification of the vitamin D receptor in osteoblasts and chondrocytes but not osteoclasts in mouse bone. J Bone Miner Res. 2014;29(3):685–692. doi: 10.1002/jbmr.2081. [DOI] [PubMed] [Google Scholar]
- 57.Roforth MM, Liu G, Khosla S, Monroe DG. Examination of nuclear receptor expression in osteoblasts reveals Rorbeta as an important regulator of osteogenesis. J Bone Miner Res. 2012;27(4):891–901. doi: 10.1002/jbmr.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Id Boufker H, Lagneaux L, Fayyad-Kazan H, Badran B, Najar M, Wiedig M, Ghanem G, Laurent G, Body JJ, Journe F. Role of farnesoid X receptor (FXR) in the process of differentiation of bone marrow stromal cells into osteoblasts. Bone. 2011;49(6):1219–1231. doi: 10.1016/j.bone.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Rőszer T, Menendez-Gutierrez MP, Cedenilla M, Ricote M. Retinoid X receptors in macrophage biology. Trends Endocrinol Metab. 2013 doi: 10.1016/j.tem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol. 2007;21(3):231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 61.Wei W, Zeve D, Wang X, Du Y, Tang W, Dechow PC, Graff JM, Wan Y. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor gamma-expressing bone marrow cell population. Mol Cell Biol. 2011;31(23):4692–4705. doi: 10.1128/MCB.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res. 2006;21(8):1276–1287. doi: 10.1359/jbmr.060503. [DOI] [PubMed] [Google Scholar]
- 63.Cho SW, An JH, Park H, Yang JY, Choi HJ, Kim SW, Park YJ, Kim SY, Yim M, Baek WY, Kim JE, Shin CS. Positive regulation of osteogenesis by bile acid through FXR. J Bone Miner Res. 2013;28(10):2109–2121. doi: 10.1002/jbmr.1961. [DOI] [PubMed] [Google Scholar]
- 64.Azuma K, Casey SC, Ito M, Urano T, Horie K, Ouchi Y, Kirchner S, Blumberg B, Inoue S. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. J Endocrinol. 2010;207(3):257–263. doi: 10.1677/JOE-10-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Wei W, Huynh H, Zuo H, Wang X, Wan Y. Nur77 prevents excessive osteoclastogenesis by inducing ubiquitin ligase Cbl-b to mediate NFATc1 self-limitat. eLife. 2015;4:e07217. doi: 10.7554/eLife.07217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kastner P, Grondona JM, Mark M, Gansmuller A, Lemeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 67.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10(1):80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 68.Haugen BR, Jensen DR, Sharma V, Pulawa LK, Hays WR, Krezel W, Chambon P, Eckel RH. Retinoid X receptor gamma-deficient mice have increased skeletal muscle lipoprotein lipase activity and less weight gain when fed a high-fat diet. Endocrinology. 2004;145(8):3679–3685. doi: 10.1210/en.2003-1401. [DOI] [PubMed] [Google Scholar]
- 69.Gilardi F, Desvergne B. RXRs: collegial partners. Sub-cell Biochem. 2014;70:75–102. doi: 10.1007/978-94-017-9050-5_5. [DOI] [PubMed] [Google Scholar]
- 70.Ricote M, Snyder CS, Leung HY, Chen J, Chien KR, Glass CK. Normal hematopoiesis after conditional targeting of RXRalpha in murine hematopoietic stem/progenitor cells. J Leukoc Biol. 2006;80(4):850–861. doi: 10.1189/jlb.0206097. [DOI] [PubMed] [Google Scholar]
- 71.Rahman MM, Bhattacharya A, Banu J, Kang JX, Fernandes G. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J Cell Mol Med. 2009;13(8B):1833–1844. doi: 10.1111/j.1582-4934.2008.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly OJ, Gilman JC, Kim Y, Ilich JZ. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res. 2013;33(7):521–533. doi: 10.1016/j.nutres.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Bell TD, Demay MB, Burnett-Bowie SA The biology and pathology of vitamin D control in bone. J Cell Biochem 111(1):7–13. doi:10.1002/jcb.22661 [DOI] [PMC free article] [PubMed]
- 74.Cardoso LF, Maciel LM, Paula FJ. The multiple effects of thyroid disorders on bone and mineral metabolism. Arq Bras Endocrinol Metabol. 2014;58(5):452–463. doi: 10.1590/0004-2730000003311. [DOI] [PubMed] [Google Scholar]
- 75.Williams GR. Thyroid hormone actions in cartilage and bone. Eur Thyroid J. 2013;2(1):3–13. doi: 10.1159/000345548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scholtysek C, Katzenbeisser J, Fu H, Uderhardt S, Ipseiz N, Stoll C, Zaiss MM, Stock M, Donhauser L, Bohm C, Kleyer A, Hess A, Engelke K, David JP, Djouad F, Tuckermann JP, Desvergne B, Schett G, Kronke G. PPARbeta/delta governs Wnt signaling and bone turnover. Nat Med. 2013;19(5):608–613. doi: 10.1038/nm.3146. [DOI] [PubMed] [Google Scholar]
- 77.Green AC, Poulton IJ, Vrahnas C, Hausler KD, Walkley CR, Wu JY, Martin TJ, Gillespie MT, Chandraratna RA, Quinn JM, Sims NA, Purton LE. RARgamma is a negative regulator of osteoclastogenesis. J Steroid Biochem Mol Biol. 2015;150:46–53. doi: 10.1016/j.jsbmb.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Malhotra D, Yang Y. Wnts’ fashion statement: from body stature to dysplasia. BoneKEy Rep. 2014;3:541. doi: 10.1038/bonekey.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker EC, Mcgregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, Gillespie MT, Martin TJ, Sims NA. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23(12):2025–2032. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- 80.Lees RL, Sabharwal VK, Heersche JN. Resorptive state and cell size influence intracellular pH regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone. 2001;28(2):187–194. doi: 10.1016/S8756-3282(00)00433-6. [DOI] [PubMed] [Google Scholar]
- 81.Fujita K, Iwasaki M, Ochi H, Fukuda T, Ma C, Miyamoto T, Takitani K, Negishi-Koga T, Sunamura S, Kodama T, Takayanagi H, Tamai H, Kato S, Arai H, Shinomiya K, Itoh H, Okawa A, Takeda S. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med. 2012;18(4):589–594. doi: 10.1038/nm.2659. [DOI] [PubMed] [Google Scholar]
- 82.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 83.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–855. doi: 10.1172/JCI200419900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13(12):1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 85.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116(12):3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siersbaek R, Nielsen R, Mandrup S. PPARgamma in adipocyte differentiation and metabolism–novel insights from genome-wide studies. FEBS Lett. 2010;584(15):3242–3249. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 87.Murphy S, Khaw KT, Cassidy A, Compston JE. Sex hormones and bone mineral density in elderly men. Bone Miner. 1993;20(2):133–140. doi: 10.1016/S0169-6009(08)80022-0. [DOI] [PubMed] [Google Scholar]
- 88.Chen Q, Kaji H, Kanatani M, Sugimoto T, Chihara K. Testosterone increases osteoprotegerin mRNA expression in mouse osteoblast cells. Horm Metab Res. 2004;36(10):674–678. doi: 10.1055/s-2004-826013. [DOI] [PubMed] [Google Scholar]
- 89.Allard L, Demoncheaux N, Machuca-Gayet I, Georgess D, Coury-Lucas F, Jurdic P, Bacchetta J. Biphasic effects of vitamin D and FGF23 on human osteoclast biology. Calcif Tissue Int. 2015;97(1):69–79. doi: 10.1007/s00223-015-0013-6. [DOI] [PubMed] [Google Scholar]
- 90.Green AC, Martin TJ, Purton LE. The role of vitamin A and retinoic acid receptor signaling in post-natal maintenance of bone. J Steroid Biochem Mol Biol. 2016;155(Pt A):135–146. doi: 10.1016/j.jsbmb.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 91.Henning P, Conaway HH, Lerner UH. Retinoid receptors in bone and their role in bone remodeling. Front Endocrinol. 2015;6:31. doi: 10.3389/fendo.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X-K, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992;358:587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- 93.Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109(8):3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- 94.Nowak B, Matuszewska A, Filipiak J, Nikodem A, Merwid-Lad A, Piesniewska M, Fereniec-Golebiewska L, Kwiatkowska J, Szelag A. The influence of bexarotene, a selective agonist of the retinoid receptor X (RXR), and tazarotene, a selective agonist of the retinoid acid receptor (RAR), on bone metabolism in rats. Adv Med Sci. 2016;61(1):85–89. doi: 10.1016/j.advms.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, Zhang BQ, Wagner ER, Rastegar F, Kim SH, Jiang W, Shen J, Huang E, Gao Y, Gao JL, Zhou JZ, Luo J, Huang J, Luo X, Bi Y, Su Y, Yang K, Liu H, Luu HH, Haydon RC, He TC, He BC. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5(7):e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, Wang Y, Jiang W, Luo Q, Shi Q, Zhang BQ, Liu B, Lei X, Luo J, Luo X, Wagner ER, Kim SH, He CJ, Hu Y, Shen J, Zhou Q, Rastegar F, Deng ZL, Luu HH, He TC, Haydon RC. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res. 2010;16(8):2235–2245. doi: 10.1158/1078-0432.CCR-09-2499. [DOI] [PubMed] [Google Scholar]
- 97.Kneissel M, Studer A, Cortesi R, Susa M. Retinoid-induced bone thinning is caused by subperiosteal osteoclast activity in adult rodents. Bone. 2005;36(2):202–214. doi: 10.1016/j.bone.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Baker AH, Watt J, Huang CK, Gerstenfeld LC, Schlezinger JJ. Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem Res Toxicol. 2015;28(6):1156–1166. doi: 10.1021/tx500433r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao JH, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N, Cordrey A, Zhao Y, Chandraratna RA. Adenomatous polyposis coli (APC)-independent regulation of beta-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem. 2003;278(32):29954–29962. doi: 10.1074/jbc.M304761200. [DOI] [PubMed] [Google Scholar]
- 100.Xu L, Song C, Ni M, Meng F, Xie H, Li G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRalpha-induced beta-catenin degradation. Int J Biochem Cell Biol. 2012;44(4):612–619. doi: 10.1016/j.biocel.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 101.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 102.Remen KM, Henning P, Lerner UH, Gustafsson JA, Andersson G. Activation of liver X receptor (LXR) inhibits receptor activator of nuclear factor kappaB ligand (RANKL)-induced osteoclast differentiation in an LXRbeta-dependent mechanism. J Biol Chem. 2011;286(38):33084–33094. doi: 10.1074/jbc.M111.235937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleyer A, Scholtysek C, Bottesch E, Hillienhof U, Beyer C, Distler JH, Tuckermann JP, Schett G, Kronke G. Liver X receptors orchestrate osteoblast/osteoclast crosstalk and counteract pathologic bone loss. J Bone Miner Res. 2012;27(12):2442–2451. doi: 10.1002/jbmr.1702. [DOI] [PubMed] [Google Scholar]
- 104.Prawitt J, Beil FT, Marshall RP, Bartelt A, Ruether W, Heeren J, Amling M, Staels B, Niemeier A Short-term activation of liver X receptors inhibits osteoblasts but long-term activation does not have an impact on murine bone in vivo. Bone 48(2):339–346. doi:10.1016/j.bone.2010.08.018 [DOI] [PubMed]
- 105.Song MR, Lee SK, Seo YW, Choi HS, Lee JW, Lee MO. Differential modulation of transcriptional activity of oestrogen receptors by direct protein-protein interactions with retinoid receptors. Biochem J. 1998;336(Pt 3):711–717. doi: 10.1042/bj3360711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jarukamjorn K, Sakuma T, Nemoto N. Sexual dimorphic expression of mouse hepatic CYP2B: alterations during development or after hypophysectomy. Biochem Pharmacol. 2002;63(11):2037–2041. doi: 10.1016/S0006-2952(02)00989-9. [DOI] [PubMed] [Google Scholar]
- 107.Liu CC, Howard GA. Bone-cell changes in estrogen-induced bone-mass increase in mice: dissociation of osteoclasts from bone surfaces. Anat Rec. 1991;229(2):240–250. doi: 10.1002/ar.1092290211. [DOI] [PubMed] [Google Scholar]
- 108.Takano-Yamamoto T, Rodan GA. Direct effects of 17 beta-estradiol on trabecular bone in ovariectomized rats. Proc Natl Acad Sci USA. 1990;87(6):2172–2176. doi: 10.1073/pnas.87.6.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Still K, Grabowski P, Mackie I, Perry M, Bishop N. The peroxisome proliferator activator receptor alpha/delta agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int. 2008;83(4):285–292. doi: 10.1007/s00223-008-9175-9. [DOI] [PubMed] [Google Scholar]
- 110.Chan BY, Gartland A, Wilson PJ, Buckley KA, Dillon JP, Fraser WD, Gallagher JA. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40(1):149–159. doi: 10.1016/j.bone.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 111.Mano H, Kimura C, Fujisawa Y, Kameda T, Watanabe-Mano M, Kaneko H, Kaneda T, Hakeda Y, Kumegawa M. Cloning and function of rabbit peroxisome proliferator-activated receptor delta/beta in mature osteoclasts. J Biol Chem. 2000;275(11):8126–8132. doi: 10.1074/jbc.275.11.8126. [DOI] [PubMed] [Google Scholar]
- 112.Kawai M, Sousa KM, Macdougald OA, Rosen CJ. The many facets of PPARgamma: novel insights for the skeleton. Am J Physiol Endocrinol Metab. 2010;299(1):E3–E9. doi: 10.1152/ajpendo.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21(12):722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 114.Falchetti A, Masi L, Brandia ML. Thiazolidinediones and bone. Clin Cases Miner Bone Metab. 2007;4(2):103–107. [PMC free article] [PubMed] [Google Scholar]
- 115.Miki T, Nakatsuka K, Naka H, Kitatani K, Saito S, Masaki H, Tomiyoshi Y, Morii H, Nishizawa Y. Vitamin K(2) (menaquinone 4) reduces serum undercarboxylated osteocalcin level as early as 2 weeks in elderly women with established osteoporosis. J Bone Miner Metab. 2003;21(3):161–165. doi: 10.1007/s007740300025. [DOI] [PubMed] [Google Scholar]
- 116.Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281(25):16927–16934. doi: 10.1074/jbc.M600896200. [DOI] [PubMed] [Google Scholar]
- 117.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho ES, Kim MK, Son YO, Lee KS, Park SM, Lee JC. The effects of rosiglitazone on osteoblastic differentiation, osteoclast formation and bone resorption. Mol Cells. 2012;33:173–181. doi: 10.1007/s10059-012-2240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]