Abstract
Metabolomics is an analytical technique that investigates the small biochemical molecules present within a biological sample isolated from a plant, animal, or cultured cells. It can be an extremely powerful tool in elucidating the specific metabolic changes within a biological system in response to an environmental challenge such as disease, infection, drugs, or toxins. A historically difficult step in the metabolomics pipeline is in data interpretation to a meaningful biological context, for such high-variability biological samples and in untargeted metabolomics studies that are hypothesis-generating by design. One way to achieve stronger biological context of metabolomic data is via the use of cultured cell models, particularly for mammalian biological systems. The benefits of in vitro metabolomics include a much greater control of external variables and no ethical concerns. The current concerns are with inconsistencies in experimental procedures and level of reporting standards between different studies. This review discusses some of these discrepancies between recent studies, such as metabolite extraction and data normalisation. The aim of this review is to highlight the importance of a standardised experimental approach to any cultured cell metabolomics study and suggests an example procedure fully inclusive of information that should be disclosed in regard to the cell type/s used and their culture conditions. Metabolomics of cultured cells has the potential to uncover previously unknown information about cell biology, functions and response mechanisms, and so the accurate biological interpretation of the data produced and its ability to be compared to other studies should be considered vitally important.
Keywords: Metabolomics, Cell culture, In vitro, Methods, Standardisation, Experimental design
What is metabolomics?
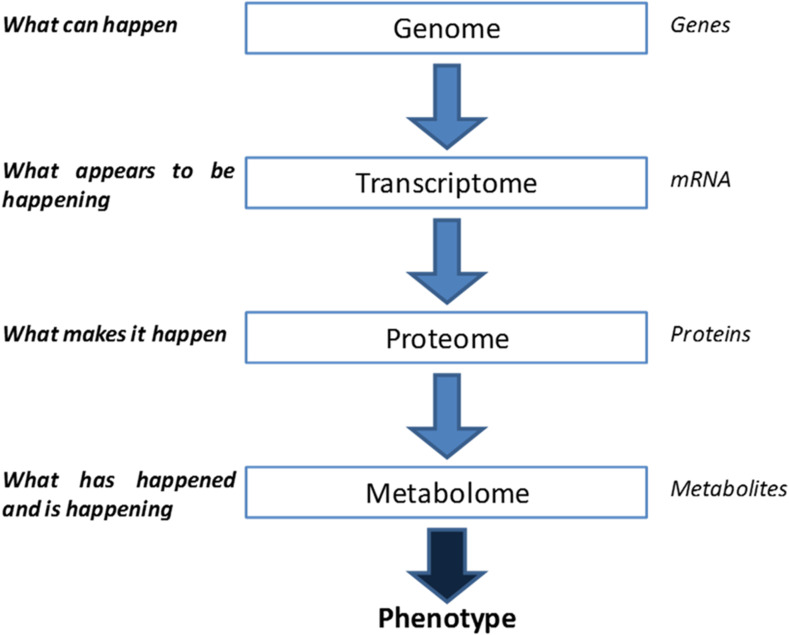
Metabolomics studies frequently state that the metabolome is a closer reflection of the phenotype of an organism, tissue or cell than the other ‘omics analyses of proteomics, transcriptomics and genomics [1–4]. When taking the ‘omics cascade (Fig. 1) into consideration, it is easy to see why this is a widely accepted view. Within a biological system the genome, transcriptome and proteome lead into the many biochemical reactions that occur inside different compartments within a cell. These chemical reactions that produce one or more small molecules are important in maintaining cellular homeostasis and are essential for metabolism. The small molecules that are shuffled around the vast network of metabolic pathways are termed metabolites. There are many thousands of metabolites within a single-cell-type system, the whole collection of which is referred to as the metabolome. The composition of metabolites in the metabolome dictates the status of the cell’s function directly related to its purpose and response to its environment. Metabolomics attempts to measure changes in the metabolome of a given biological system in response to a challenge to normal cellular homeostasis. These challenges can be from physiological or infectious disease, changes in environment, exposure to toxins, interactions by drugs or other external stressors.
Fig. 1.
The ‘omics cascade.
Adapted from Dettmer et al. [5]
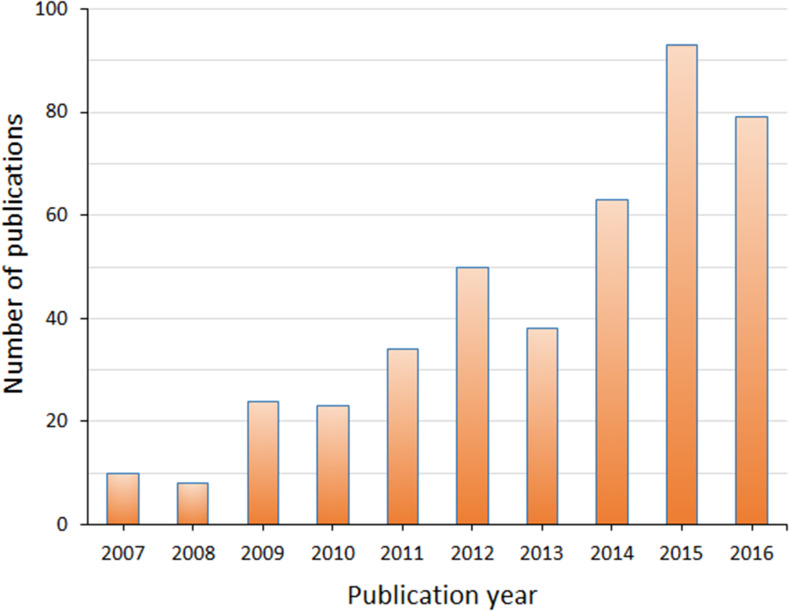
Metabolomic analyses rely on the latest advances in the field of separation sciences: nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). Reviews of both techniques are available [5–10], especially on the use of MS in metabolomics. The development of new MS techniques has provided a multitude of different platforms for the analysis of metabolites in biological samples, and the use of MS in metabolomics continues to grow in popularity due to its flexibility of application to different types of samples, relatively low set-up cost compared to NMR, robust reproducibility, and extremely high sensitivity. A search of the literature on the US National Centre for Biotechnology Information (NCBI)’s ‘PubMed’ citations database (www.ncbi.nlm.nih.gov/pubmed) with the search terms in “All Fields” defined as “cell culture OR in vitro AND metabolomics AND mass spectrometry” resulted in 422 citations listed in the past decade of 2007–2016 inclusive: 99 citations in the first half of the decade (years 2007–2011) and 323 citations in the second half (2012–2016). Figure 2 clearly displays the increasing popularity of research in this area, especially in the most recent half of the past decade, and so it is important to validate the methods currently used by the many different applications of cell culture metabolomics, so that results may be compared and meaningfully interpreted. This search was inclusive of both targeted or ‘specific monitoring’ and untargeted or ‘scanning’ approaches to sample analysis by MS. Metabolomic studies can consist of either of these approaches; however, it is the untargeted style of analyses that is the most exciting in terms of discovering ‘biomarkers’ or elucidating metabolic profiles, especially when used in a well-defined biological system, such as cultured mammalian cells. This review focuses on the use of MS-based, untargeted metabolomic analyses in cell culture studies and the importance of interpreting biological insights from the vast amounts of data that are collected.
Fig. 2.
Number of publications each year in the past decade of 2007–2016 inclusive, for the subject area of cell culture metabolomics by mass spectrometry
Biological insights from metabolomics data
Metabolomics is well regarded in the scientific community for its potential to discover new information or previously unknown ‘biomarkers’, and provide a huge amount of data which can be interpreted in many areas of investigation [7]. It is the ability of these data to provide valuable biological insights that have come into inquiry in recent reviews, which state that the major current bottleneck in metabolomic data handling is biological interpretation [6, 11, 12]. This is a logical argument when considering the ever-changing and constantly updated knowledge-bases on metabolic pathways, intracellular signalling pathways and the vast network of control mechanisms. It is also important to acknowledge that metabolomics is currently unable to detect every form of every known metabolic intermediate involved in all biochemical pathways, from a single analysis using a single platform. This is a well-documented issue that has been somewhat accounted for in well-designed metabolomic experiments, but the concern of accurate biological interpretation of data remains. One suggestion to provide a more streamlined approach to the interpretation of metabolomic data is to attempt to link the expected metabolome to the phenotype of the biological system under investigation, before any metabolomic experiments and analysis are carried out. Web-based databases such as MetaboAnalyst (http://www.metaboanalyst.ca/) and the Kyoto Encyclopaedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg) are useful tools in exploring metabolic pathways for comprehensive interpretation of metabolomic data [13].
Untargeted metabolomics studies may expose a group of metabolites that have unexpectedly changed from the normal metabolome, and from there they can be further investigated and the pathways they might be involved in can be teased apart—but this might not always be the case. There might not be any vast differences in abundance of metabolites, but rather subtler changes in accumulation. Attempting to biologically interpret these subtle changes can raise many challenging questions. As metabolic pathways are highly regulated and controlled via many different mechanisms, it is widely accepted that a fully integrative analysis combining metabolomics with transcriptomics and proteomics will provide a fuller understanding of the processes taking place that result in a change to the metabolome. There is also acceptance that any such study would be a substantial investment of resources and time, and therefore it is reasonable that this cannot be undertaken with every study with currently available technology. There are several ways to justify the use of metabolomics-only investigations, primarily to do with experimental design and careful choice of what type of sample to analyse [6, 12].
The benefits of untargeted, cultured cell metabolomics
It is not always possible in metabolomic studies for the researcher to have strict control over the number and type of samples that are available for analysis, such as in clinical or animal studies. One increasingly popular application of metabolomics, where the samples are more easily controlled and experiments can be more carefully designed specifically for metabolomic interpretation is the use of cultured mammalian cells [14, 15]. Using established cell lines, whether animal or human-sourced, typically has no ethical concerns which may limit control groups or numbers of replicates. There are more opportunities to control variables in the growth and sampling stages using cell culture, compared to animal studies or clinical samples. This feature of cell culture adds strength to metabolomic data, as the number of external variables that may contribute to a change in the metabolome (other than the variable of interest) can be adequately controlled and essentially eliminated from analyses of the data.
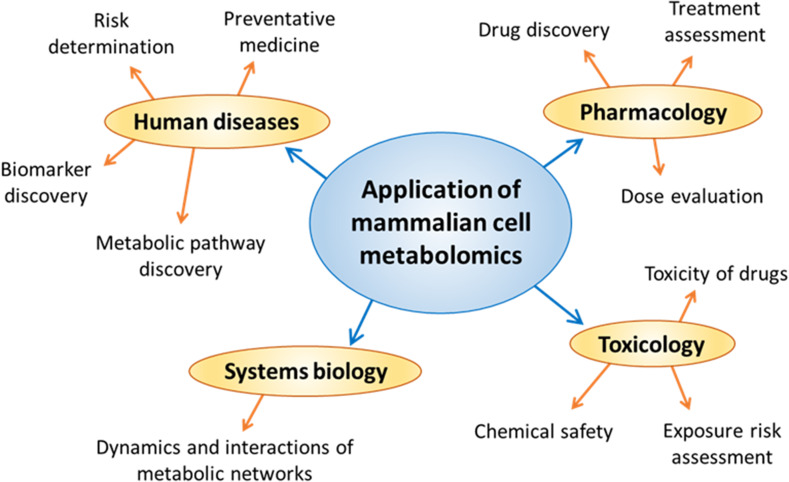
Metabolomics of cultured cells has the potential to produce information about cell biology, functions and response mechanisms [14]. Areas of research where mammalian cell metabolomics has been used are presented in Fig. 3, showing that it is central to a number of different biological applications. Cell culture metabolomics has already provided unique biological insights in specific applications such as energy metabolism dysfunctions [16, 17], metabolic flux between cells and tissues [15, 18], metabolic pathways involved in cancer cell development and response to treatment [19–21], and cellular response to chemical toxins for mechanism of action studies or to test the toxicology of unknown compounds [22–26]. Very recently, metabolomics of single-cells has become possible with a huge increase in sensitivity capabilities of instrumentation and constantly advancing technologies. Single-cell metabolomics is a unique application within the greater field of in vitro metabolomics, with specific protocols for culturing and extracting metabolites from single-cells [27, 28]. In cell culture metabolomics, it is possible to measure both intracellular metabolites from isolated cells, as well as the extracellular metabolites from the cell culture medium. This makes cell culture metabolomics unique in that the release of metabolites from the cells can also be studied. Extracellular metabolite analysis can potentially give a better understanding of the metabolic state inside the cell and so provides a more complete biological interpretation of metabolic data [29].
Fig. 3.
Research areas where metabolomic analysis of cultured mammalian cells has been used
Cell culture metabolomics has become an attractive application for untargeted, screening-type analysis. Due to the hypothesis-generating style of untargeted metabolomics, it is understandable that such an approach might not be attractive to the researcher, if only a limited number of clinical or animal samples are available. Cultured cell or in vitro samples can more easily accommodate re-visiting the sample-set, if any interesting or previously unknown metabolites are highlighted by an untargeted study. Untargeted metabolomics has been suggested to be the future of all metabolomic investigations [12], as the technological platforms used and the potential to collect vast amounts of data in a short time window continue to advance. Handling the vast amounts of data that untargeted studies can produce has presented problems with the strategy which are important to acknowledge, such as distinguishing ‘right’ and ‘wrong’ features, and how to deal with missing values. These issues have been thoroughly investigated and useful approaches have been developed to fairly correct and adjust untargeted metabolomic data so that it can be meaningfully interpreted [10, 30–33]. It has been postulated that targeted metabolomics studies will be made redundant, as the same information might be collected at the same time as vast amounts of unknown information, potentially unearthing previously unknown trends. Using cultured cell models where the number of controls and replicates can be easily manipulated in the experimental design will be a forefront in the development, validation and standardisation of untargeted metabolomics studies in the future.
Standardisation of cell culture protocols for metabolomics analysis
The need for standardisation of a procedure for in vitro metabolomics experiments has been well documented [34–36]. Interestingly, since these first requests for a standardised technique, there have been many published cell culture metabolomics studies that follow their own direction despite this need being identified. Many recent papers state in their concluding comments that a standardised procedure is still needed, some even referencing these earlier published articles. And so the argument remains, should there be more effort in the metabolomics community to undertake the task of developing an effective, robust standard procedure to generate cultured cell samples for metabolomic analysis? With the use of cultured cell models in metabolomics becoming more widespread and applicable to many areas of medical research, and the new applications continuing to be identified, the answer is clearly ‘yes’. The application of metabolomics to cultured cell studies has been thoroughly discussed in a number of proceedings, perhaps most notably when applied to toxicity testing of existing and newly developed chemicals [37–40], where the potential for cultured cell metabolomics has been suggested as a viable alternative to the use of animals in toxicology testing which are the current standard for establishing reference doses that are considered safe to human health. In a potentially high-impacting area of research such as this, it would be necessary for efforts to be focused on a standard procedure for cultured mammalian cell metabolomics that can be easily followed, used and interpreted by research bodies worldwide.
van der Werf et al. [35] identified the “need for a minimal set of reporting standards that allow the scientific community to evaluate, understand, repeat, compare and re-investigate metabolomics studies” in the context of microbial and in vitro experiments. This study was supported by a subgroup of the Metabolomics Standards Initiative (MSI) (http://www.metabolomics-msi.org/), a program set up in 2005 by the Metabolomics Society, with the aim to standardise multiple aspects of metabolomic studies including chemical analysis, metabolite identification, data processing, ontology, as well as providing a clear description of the biological system studied in order to provide biological context. The subgroup was given the task of producing a document of recommended minimal reporting standards specifically for in vitro-based metabolomic experiments. The document titled “Core Information for Metabolomics Reporting (CIMR): In vitro Biology/Microbiology Context” (http://cosmos-fp7.eu/system/files/presentation/invitro.pdf) contains considerable information to guide experimental design, as well as descriptive reporting standards. The subsequent publication [35] was an effort to share this information with the greater metabolomics community, with the aim of generating feedback for future editions. The authors stress that the most important aim of metabolomic studies is not data generation, but translating that data into biologically relevant information. To do this with cultured cell studies, it is of utmost importance to generate a snapshot of the metabolome at a given point in time, and so metabolism quenching, adequate harvesting and storage procedures for cells are important in preserving the composition of metabolites in the metabolome. The report covers all aspects of a biological experiment starting from defining the exact biological question through experimental design, sample generation and preparation, up until the stages immediately preceding chemical analysis by NMR or MS. The reporting standards for chemical analysis procedures in metabolomic studies have already been well established in a partnering document provided by the MSI (available at http://cosmos-fp7.eu/msi), and also by other reporting standards efforts by multiple associations.
In this review, recent mammalian cell culture metabolomics studies will be compared for their cell culture and sample preparation procedures, prior to metabolite analysis by NMR or MS. Both targeted and untargeted studies are incorporated in this comparison, so that multiple classes of metabolites are included. These studies will also be compared to the suggested minimal reporting standards presented by van der Werf et al. [35], so that a combined, optimal procedure of cell culture practices and sample preparation specific for untargeted metabolomics studies can be suggested. It is anticipated that this suggested standard procedure be applicable to a wide variety of different cell types and be easily manageable for varying quantities of cells required, and for multiple types of predominantly MS-based analytical platforms.
Area for improvement 1: reporting of cell culture procedures
This review presents a summary of mammalian cell culture metabolomics studies published in the period 2011–2016. Table 1 summarises these studies that are selected specifically for their application of metabolomics to mammalian cell lines, and so includes different analytical platforms utilised (i.e., NMR, GC– and LC–MS). A general observation from this comparison is that reporting standards are not conserved in current cell culture metabolomics studies, and many call for such a strategy to be established. This is understandable as there are some aspects of cell culture which would potentially affect the results of detected metabolites. The minimum reported conditions for any cell culture study should be growth environment, medium constituents, passage numbers used and number of cells seeded or level of growth confluence reached. However, these basic components are absent from some published metabolomics studies. The use of cell culture models in biology has traditionally involved either recombinant DNA experiments to assess expression levels, protein extraction to identify function, or fractionation of cells to isolate specific components. The importance of passage numbers used has not been demonstrated in these studies, an observation which may have simply been extended to untargeted metabolomics experiments. The screening approach of untargeted metabolomics means that there is potential for differences associated with passage number to be detected in the metabolome. In order to assess whether this is the case there is the need for a study that specifically addresses the effect (if any) of passage number on the metabolome. This would allow for more accurate interpretation of the data when analysing the true response of the metabolome to a particular challenge, by minimising any variability of the data that may be due to passage number. This is one aspect of cell culture metabolomics where there is evidence for further development into a standardised protocol, which would benefit the data and allow for an easier comparison not only between groups within a single study, but between multiple studies of a similar application.
Table 1.
A summary of recent studies in metabolomics analyses of mammalian cell lines, the conditions reported for sample preparation, quenching and extraction, normalisation procedures carried out and the study application
| Cell line | Cell type | Passage number/s | Samples | Quenching | Extraction | n a | Sample normalisationb | Analysisc | No. of metabolitesd | Application | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | |||||||||||
| SH-SY5Y | Neuroblastoma | 28 and 29 | Cells and culture media | Ice-cold PBS | Deuterated water and homogenisation | 5 |
Cell number Cells counted following scraping |
HR-MAS NMR |
28 Untargeted, identified metabolites |
Profile the metabolic effects of the neuroinflammatory cytokine interferon-α2 | [41] |
| H4, U251 and U87 | Astrocytoma | – | Cells | Wash 5% mannitol solution | 1 mL MeOH:water (21:79) added to 2 mL chloroform followed by sonication | 3 | Cell protein dry weight |
CE-MS QC pooled samples |
123 Untargeted, identified metabolites |
Profile metabolic fluctuations in astrocytes of the neuroprotectant methionine enkephalin | [42] |
| L02 | Hepatocytes | – | Cells | Ice-cold PBS | Dried cell pellet, MeOH:water:chloroform extraction, only polar layer was collected | 8 | Weight of dried cell pellets before extraction | 1H NMR |
26 Untargeted, identified metabolites |
Dose–response effects of the chemotherapeutic agent, cisplatin | [43] |
| HepG2 | Hepatocarcinoma | – | Cells | Ice-cold 0.9% NaCl | Polar and non-polar extracts, does not specify details of extraction solution | 5 | – | 1H NMR |
26 Untargeted plus targeted (bile acids) |
Hepatotoxicity of the immunosuppressant drug, cyclosporin A | [25] |
| – | Culture media | – | Did not specify | 3 | Cell number | 1H NMR |
44 Untargeted, identified metabolites |
Drug toxicity of the anticancer pro-drug flutamide, and its active metabolite hydroxyflutamide | [44] | ||
| “Within 20 passages” | Culture media | – | Did not specify | 3 | Cell number | 1H NMR |
34 Untargeted, identified metabolites |
Metabolic effects of the protective therapeutic agent resveratrol | [45] | ||
| 7, 11a, 11b, 17 and 30 | Cells | Ice-cold 0.9% NaCl | Ultrasonication, polar and non-polar extracts, does not specify details of extraction solution, both polar and non-polar fractions were freeze-dried | 4 | Detected phospholipid levels as an internal measure for the number of cells | 1H NMR, LC–MS, GC–MS |
Does not specify Untargeted, identified and unknown metabolites |
Test hepatotoxicity of the carcinogen compound TCDD | [46] | ||
| – | Cells | Liquid N2 | Biphasic extraction with water:methanol:chlorofom | 3 | – |
UPLC–QTOF MS QC pooled samples |
Varied for each analysis Untargeted |
Develop a predictive hepatotoxicity model and potential mechanisms of action for several hepatotoxins | [47] | ||
| OVCAR-8 | Ovarian cancer | – | Cells and culture media | Dry ice-cooled MeOH + 0.85% ammonium bicarbonate | MeOH:chloroform:water (7:2:1) | 3 | DNA quantification of cell pellets | HPLC–MS/MS |
29 Targeted amino acids and metabolites |
Effects to amino acid metabolism with treatment of l-asparaginase | [48] |
| HeLa | Cervical cancer | – | Cells | −80 °C MeOH | 80% MeOH/20% water | 8 | Cell number |
Ion-trap GC–MS QC pooled samples |
353 ion peaks Untargeted |
Metabolic profiling of the anticancer properties of celastrol | [49] |
| MCF-7 and COC1/DDP | Breast cancer | – | Cells | Wash with cooled PBS | Cell trypsinisation, followed by multiple freeze–thaw cycles for cell lysis and addition of methanol:chloroform | 3 | – | UPLC–MS/MS |
Two groups of 24 and 15 Targeted biomarker metabolites |
Investigating the metabolic bases of drug resistance in tumour cells | [50] |
| RPTEC/TERT1 | Renal epithelial | – | Cells and culture media | Ice cold MeOH | Ammonia bicarbonate wash, 100% MeOH lysing reagent | 3 | – | 1H NMR, infusion Orbitrap-MS |
192 significant features Untargeted |
Metabolic effects of drug-induced cell stress by cyclosporine A | [51] |
| – | Cells and culture media | MeOH | MeOH:chloroform:water, only aqueous layer was analysed | 3 | Median fold change post analysis | 1H NMR | 166 | Profile the metabolic effects of low-level toxicant exposure | [26] | ||
| MRC-5 | Foetal lung fibroblasts | – | Cells | Liquid N2 | MeOH with sonication | 4 | Sample dry weight | GC–MS, LC–QQQ–MS |
28 Untargeted, identified metabolites |
To assess the dose–response toxicity of SiO2 nanoparticles | [52] |
| HL-1 | Cardiomyocytes | – | Culture media | – | Centrifugation of culture media, filtration of proteins | 3 | Cell number | HPLC |
22 Targeted metabolites |
Assessment of drug-induced toxicity | [53] |
| Animal | |||||||||||
| CHO | Chinese hamster ovary | – | Culture media | – | Centrifugation of culture media, filtration of proteins | – | Cell number | HPLC, GC–MS |
8 Targeted metabolites |
Quantify the flux of glucose, organic acids and amino acids over a time course | [54] |
| – | Culture media | – | Minimal detail included, dilution of culture media only for LC analysis | 3 | Cell number | HPLC–ESI–MS/MS |
1 Targeted metabolite |
Quantify L-alanyl-l-glutamine in culture media to optimise conditions for cell growth | [55] | ||
| – | Cells and culture media | PBS wash, frozen at −70 °C | Centrifugation of culture media, dilution with buffered deuterated water. Addition of dry-ice cold MeOH to cell extracts, followed by bead mill homogenisation. Two-phase separation by addition of water and chloroform | 2 or 1 | Cell number | 1H NMR |
32 Identified metabolites |
Quantify metabolites involved in energy utilisation to optimise conditions for cell growth | [56] | ||
| – | Cells and culture media | Ice-cold NaCl | Filter centrifugation, diluted with 20:80% MeOH:water. Cells were centrifuged and extracted with MeOH and chloroform | 3 | Cell number | UPLC–MS/MS |
100 Untargeted features, 19 identified metabolites |
Characterise important metabolites for cell growth optimisation | [57] | ||
| – | Cells and culture media | Ice-cold NaCl (0.9% w/v) | Cell suspension centrifuged, extracted in 50% aqueous acetonitrile incubated for 10 min on ice, supernatant collected and freeze-dried | 3 | Cell number | HPLC, GC–MS |
87 intracellular, and 49 extracellular Untargeted metabolites |
Profiling metabolites in different growth conditions at different time points | [58] | ||
| L929 | Mouse fibroblasts | – | Cells and culture media | NaCl/H2O (0.9% w/v) | Cells scraped into 1:1 MeOH:water, followed by ultrasonic homogenisation, and same extraction solution added to culture media and incubated for 10 min. Both extracts were then dried under nitrogen | 10 | – | GC–TOF MS |
25 Identified metabolites |
Cytotoxicity assessment of TiO2 nanoparticles | [59] |
| BRIN-BD11 and INS-1E | Rat insulinoma, pancreatic beta cells | – | Cells and buffered culture media | Ice-cold PBS | Cells scraped into PBS, centrifuged and 2:1 MeOH:chloroform added, vortexed, water added, organic layer analysed only | 8–15 | Protein concentration | GC–MS, NMR |
31 Identified metabolites |
Metabolite profiling of hyperglycaemia | [60] |
Each line represents an individual published study. Information is only included in the table if it was reported in the publication
a‘n’ number of biological replicates used in metabolomic analysis
bNormalisation strategies to cell number were counted from separate samples set up in parallel with samples for metabolomic analysis
cThe use of QC samples in instrumental analysis was not reported unless mentioned under ‘Analysis’
d‘No. of metabolites’ is what was reported in the publication and noted as identified if reported
Area for improvement 2: metabolite extraction procedures
Most recent cell culture metabolomics studies share the same emphasis on the importance of the metabolite extraction procedure. However, there are many variations of the process which do not seem to be dependent on any characteristic of the cells themselves, but are often directed towards specific metabolites. For example, a specific extraction technique may not be reproducible for all cell types in terms of the level of metabolite recovery. It would be reasonable to assume that cells from membranous or fibrous tissues, such as epithelial cells or keratinocytes, or cells that produce lots of extracellular matrix such as fibroblasts or hepatocytes, may be more difficult to extract small metabolite molecules from than other cell types. Current metabolite extraction techniques appear to adopt something of a ‘one-size-fits-all’ approach regardless of the cell line used. By comparing multiple cultured cell metabolomics studies, the optimal techniques for specific types of cells may be determined. An ideal extraction procedure for metabolomics of cultured cells should immediately quench metabolism and quantitatively collect and extract all metabolites. However, this is a significant challenge given the range of classes of metabolite compounds and their varying stability. Recent studies have attempted to optimise and validate extraction protocols for the metabolomics of cultured cells. Although these extraction processes are diverse in their methodologies, some have conserved or similar steps. These processes have been extensively reviewed [1, 61, 62]. A common conclusion from the attempts to review cell metabolomics extraction procedures is that there now needs to be an attempt to standardise the procedure, and for those standardised operating protocols to be made widely available. This will allow future cell culture metabolomics studies to be more easily compared.
Table 2 provides a comparison of recent studies with the specific aim of optimising a quenching, extraction and sample preparation procedure for metabolomic analysis of cultured mammalian cells. The most common extraction protocol summarised from these studies is as follows: following the discard of culture medium, cells are washed with cold PBS to both quench metabolism and wash the cells of any presence of metabolites carried over from the culture medium, an important process for the effective detection and separation of intracellular from extracellular metabolites and other medium components. Some optimisation studies recommend against the use of 100% methanol as a quenching solution, due to potential membrane leakage and thus decreased metabolite recovery compared to buffered, isotonic solutions [63–65]. Despite this, there are still studies that used methanol in the quenching step for cells [66–69]. This is one step of the extraction procedure that could be relatively easily standardised. Following washing and quenching, the cells are collected for extraction in either the quenching solution or directly in extraction solvent. A recent trend for adherent cells is to add the extraction solution directly to the cells on the surface of the culture plate or flask immediately after removing the culture medium, and to scrape the cells into the solvent for collection. Cells that are collected in a buffered solution are centrifuged or concentrated, before having extraction solvent added to them. It is generally now accepted amongst most studies that trypsinisation of adherent cells is not recommended for metabolomic analysis, due to the poor recovery of some metabolites that leak through cell membranes during the trypsinisation process [63, 69–72]. Cell scraping into quenching or extraction solutions is an accepted alternative, as it has been shown to have far superior metabolite recovery rates than trypsinisation.
Table 2.
Summary of recent optimisation efforts of metabolite extraction procedures for cell culture metabolomics
| References | Cell line/s | Samples | Cell washing | Quenching | Extraction | Analytical platform | Comments |
|---|---|---|---|---|---|---|---|
| 2017 | |||||||
| Kapoore et al. [65] | MDA-MB-231 | Cells | Comparison of no wash, to one wash or two washes with ice-cold PBS or distilled water | Comparison of no quenching, to 100% methanol, 60% methanol both unbuffered and buffered with 0.85% AMBIC or 70 mM HEPES, and direct quenching with LN2 | Culture media discarded → cells washed for 60 s → cells quenched (−50 °C) and scraped into quenching solution → addition of 100% methanol and freeze–thaw cycles for metabolite extraction → samples lyophilized prior to derivatisation for analysis |
GC–MS Selected 11 metabolite classes |
Compared five washing protocols and five quenching protocols for minimum metabolite leakage from a single breast cancer adherent mammalian cell line Optimised protocol was a single PBS wash and quenching with 60% methanol supplemented with 70 mM HEPES |
| 2016 | |||||||
| Muschet et al. [71] | THLE-2, HK-2, HepG2, SGBS | Cells | Washed twice with warmed PBS | 88% methanol precooled on dry ice | Trypsinisation and cell scraping were compared. Extraction solution was the quenching methanol plus additional 88% methanol → homogenisation with 80 mg glass beads → centrifugation → stored at −80 °C |
FIA-MS/MS, LC–MS/MS Targeted assay of 188 preselected metabolites |
Cell harvesting methods of trypsinisation or cell scraping are tested with the same extraction procedure across four different human cell lines The effect of harvesting technique on metabolite extraction was dependant on the cell lines used, and the specific metabolites. Cell scraping better correlated to metabolite concentrations and cell numbers, than trypsinisation |
| Peterson et al. [73] | MDA-MB-231 | Cells | Washed three times with ice-cold 0.9% saline | Ice-cold saline (quenching considered to occur during washing steps) | Chloroform:methanol:water (1:3:1) extraction solution, either added directly to cells, or following collection by scraping into saline → extraction by vortex mixer for 30 min → centrifugation → evaporated to dryness → stored at −80 °C |
HPLC-QE Orbitrap MS, normal phase LC, positive and negative ESI, 32 min acquisition at 0.3 mL/min, full scan range 85–1275 m/z Untargeted analysis |
Recommends the direct extraction of metabolites from adherent cells in the culture dish, as it was found to have a higher recovery of both polar and non-polar metabolites than removing cells from the dish before metabolite extraction |
| Garcia-Canaveras et al. [72] | HepG2 | Cells | Single wash with cold PBS | Direct addition of LN2 | Comparison of five extraction solutions: HEPES-EDTA; 100% methanol; and water:methanol:chloroform in a monophasic, biphasic and sequential phase extraction → cold extraction solution added directly to cells or to collected trypsinised cells and subject to three freeze–thaw cycles → centrifugation → evaporated to dryness → reconstitution of sample for appropriate analytical technique |
LC–QTOF MS, reverse and normal phase LC, positive and negative ESI, full scan range 50–1200 m/z Untargeted analysis |
Optimisation study for several steps of adherent mammalian cell metabolite extraction and analysis by LC–MS Optimal cell harvest and metabolite extraction procedure was found to be direct addition of extraction solution and scraping of cells over trypsinisation |
| 2015 | |||||||
| Kapoore et al. [66] | MDA-MS-436, MCF-7, HMEC-1 | Cells | Washed twice with ice-cold PBS | 60% methanol at −50 °C | Trypsinisation and cell scraping were compared. Extraction of metabolites from cell mass was via addition of 100% methanol followed by three freeze–thaw cycles: snap freezing in liquid nitrogen → thawing on dry ice → vortexing → centrifugation. Extraction procedure was repeated and the two extracts combined and freeze-dried (lyophilised) | GC–MS | Metabolite leakage is influenced by the cell line used. An extra protein precipitation step in the extraction procedure did not show any significant improvement in metabolite recoveries. Recommends the use of rapid, combined quenching and extraction approaches to minimise metabolite leakage occurring from cells |
| Madji Hounoum et al. [74] | NSC-34 | Cells | Washed twice with either PBS at 37 °C or 0.9% (w/v) NaCl | 100% methanol at −40 °C | Cells were scraped into quenching methanol → four different extraction solvents were compared: methanol:water (1:1), acetonitrile:water (1:1), dichloromethane:methanol:water (3:3:2), and dichloromethane:acetonitrile:water (3:3:2) → vortexing and sonication → centrifugation → dried in vacuum concentrator |
NMR, GC–MS, LC–HRMS GC–MS: ZB-5 column, full scan range 50–500 m/z, untargeted analysis with compound matching to in-house standards library LC–HRMS: reverse phase LC, positive and negative ESI, full scan range 60–900 m/z |
Optimisation study that focuses on simultaneous, combined-analysis-based metabolomics studies, and suitable extraction and sample preparation methods that best fit all different platforms A dual-phase extraction (methanol:dichloromethane:water) was selected, as the different phases could be analysed differently by multiple analytical platforms |
| Ser et al. [75] | HCT116 | Cells and culture medium | Comparison of PBS, Millipore water or no wash | Comparison of cells placed on ice (4 °C) or dry ice (−80 °C) | Comparison of 80% methanol:20% water and 80%methanol:20%water + 5% formic acid → cells scraped → centrifugation → dried in vacuum concentrator → stored at −80 °C |
LC–QEMS Reverse-phase LC, positive and negative ESI, full scan range 50–900 m/z |
Different metabolite extraction procedure for medium samples: methanol:water (20:3) → vigorous vortexing → centrifugation → dried in vacuum centrifuge Extraction temperature (either −80 °C or 4 °C) does not change metabolite quantification. Recommends use of formic acid in extraction solvent, and the exclusion of a cell washing step |
| 2014 | |||||||
| Matheus et al. [70] | ECC1, HeLa | Cells | Cold PBS | Kept on ice | Cells were either trypsinised or scraped into PBS → centrifugation → resuspension in D2O → cells disrupted either by probe sonication or bath sonication → centrifugation → stored at −80 °C | NMR | Sonication of cells suspended in water was the only metabolite extraction procedure tested in this study, and was deemed suitable for NMR analysis |
| Rahman et al. [76] | HeLa, Hek293, MEF | Cells | Warmed PBS | Snap-frozen in liquid nitrogen | 40% acetonitrile:40% methanol:20% water → cells scraped → agitation → centrifugation → dried in vacuum concentrator → stored at −80 °C |
LC–MS/MS Reverse-phase LC, positive and negative ESI, 20 min acquisition at 0.5 mL/min flow rate |
Does not compare several attempted extraction techniques, rather reports on one technique and the optimisation of MS parameters for the chosen procedure |
| 2013 | |||||||
| Bi et al. [77] | Panc-1 | Cells | Isotonic saline at 37 °C | Liquid nitrogen | Trypsin detachment of cells or cell scraping into water → four different extraction solvents: acetonitrile, methanol, methanol: chloroform: water, and methanol: chloroform: acetonitrile → vortexing → centrifugation → dried under nitrogen | LC–QTOF-MS | Trypsinisation found to be inadequate due to substantial metabolite leakage. Flash quenching with liquid nitrogen, followed by methanol/chloroform/water extraction was optimal for analysis by LC–MS |
| 2012 | |||||||
| Hutschenreuther et al. [78] | MCF-7 | Cells | PBS | Placed in −80 °C | Trypsin detachment of cells or cell scraping → centrifugation → comparison of different extraction solvents: ethanolic potassium hydroxide; water:methanol:chloroform; and 100% methanol → extract purification using 3 kDa membrane filters → dried in vacuum concentrator |
GC–MS 5-MS column, full scan range 78–600 m/z |
Recommends methanol as an extraction solvent, with direct quenching and cell scraping |
| 2011 | |||||||
| Dettmer et al. [63] | SW480 | Cells | PBS or no wash | PBS or direct methanol | Seven different extraction solvents added to cell pellets or direct to cells; methanol:chloroform: water (1:1:0.1); 100% methanol; 80% methanol:20% water; 100% acetone; 80% acetone:20% water; methanol:isopropanol: water (1:1:0.1); and acid–base methanol. Three different cell disruption techniques were compared using methanol:water (80:20) as extraction solvent; freeze/thaw; ultrasonication; and homogenisation |
GC–MS 5-MS column, full scan range 50–600 m/z, untargeted analysis with compound matching to in-house standards library |
Extensive comparison of several extraction procedures; trypsinisation vs. cell scraping in buffer or directly into extraction solvent; seven different extraction solvents; freeze/thaw cycles vs. ultrasonication or homogenisation. Direct scraping of cells into methanol/water extraction solvent was chosen as the method of choice |
| Lorenz et al. [79] | INS-1 | Cells | Deionised water at 37 °C or Krebs–Ringer-HEPES buffer at 37 °C | Direct addition of liquid nitrogen to culture dish | Different extraction solvents added to plate; 90% methanol:10% chloroform; 100% ethanol; 100% acetonitrile; 100% methanol → cells scraped → centrifugation | HPLC–MS | Extraction solvent 90% methanol:10% chloroform found to be superior for metabolite recovery and stability |
| Martineau et al. [67] | MCF-7, MDA-MB-468, SKBr3, ZR75-1 | Cells | PBS | 100% methanol | Cells scraped into methanol used for quenching → comparison of five different extraction solvents; acetonitrile:water (4:1); methanol:water (4:1); 100% methanol; perchloric acid (2% v/v in water); or methanol:dichloromethane:water (2:2:1.8) → vortexed → centrifuged → dried by nitrogen gas evaporation | NMR | Concludes that methanol:dichloromethane:water extraction solvent is the most suitable for NMR analysis of four different breast cancer cell lines |
| Sheikh et al. [80] | MCF-7 | Cells | Ice-cold PBS | Kept on ice | Cell pellets suspended in water and lysed by freeze–thaw cycles → ultrasonication → methanol:chloroform:acetonitrile → dried in vacuum concentrator |
UPLC–QTOF-MS Positive and negative ESI |
Study states that a variety of extraction procedures were tested, but reports on only the one deemed most successful, which is then verified using seven other cancer cell lines. Conclusion is that this method “can be successfully used for cell metabolomics” |
| 2010 | |||||||
| Danielsson et al. [68] | INS-1 832/13 | Cells | Ice-cold PBS | Methanol at −80 °C | Compared extraction solvents of 100% methanol or 82% methanol:18% water → Comparison of three cell disruption methods: stainless steel ball mill; snap-freezing in liquid nitrogen; and vortexing → centrifugation → dried in vacuum concentrator |
GC–MS 5-MS column, full scan range 50–800 m/z |
Optimised cell disruption, metabolite extraction and GC–MS settings with the aim of an unbiased, high-throughput method. Concluded that extraction with 82% methanol:18% water with cell disruption using the ball-mill was the most effective extraction procedure |
| Dietmair et al. [64] | CHO | Cells | PBS or no wash | Comparison of cold, isotonic saline (0.9% NaCl), cold 60% methanol, and cold 60% methanol buffered with 0.9% ammonium bicarbonate | Several different extraction solvents added to cell suspensions, a mixture of hot (>70 °C) or cold (<4 °C) solvents consisting: 50% acetonitrile:50% water; 100% methanol; 50% methanol:50% water; 50% methanol:50% chloroform; 80% methanol:20% water; 75% ethanol; 75% ethanol buffered with HEPES; 100% water; potassium hydroxide; and perchloric acid → dried in vacuum concentrator → resuspended in water and stored at −80 °C | HPLC | Extensive study that compares several quenching and extraction procedures that were chosen from literature. Concluded that quenching with cold saline effectively halted metabolism, and 50% acetonitrile:50% water was the superior extraction solvent |
| 2009 | |||||||
| Teng et al. [69] | MCF-7, MDA-MB-231 | Cells | Ice-cold PBS | 100% methanol | Cells scraped into quenching methanol → added extraction solvent methanol:chloroform:water (4:4:2.85) → only aqueous phase collected → dried in vacuum concentrator | NMR | Compares a direct quenching and collection method with trypsinisation before quenching and extraction. Concludes that direct quenching and collection of samples exhibits much greater recovery of metabolites than the conventional trypsinisation method (approx. 50-fold higher) |
The composition of extraction solvent used is varied amongst many studies, and can be the most difficult component to optimise due to the many variations that are available to extract different classes of metabolite. Many studies state that the choice of extraction solvent should be study-dependent with regard to the cell line being used, and whether the metabolomic analysis targets specific metabolites or metabolite classes, or is an unbiased (as far as possible), untargeted analysis. Despite this aspiration, many studies used the same extraction solvent, methanol and water in an 80:20% ratio, respectively. This gives a single-phase extraction supernatant and allows for the whole extraction to be collected. Also common is the use of a dual-phase (polar and non-polar) extraction using methanol, chloroform and water (or acetonitrile). The ratio of these components is not as conserved, but is most commonly used at 1:1 methanol and chloroform, with a smaller proportion of water or acetonitrile. This results in a separation of (more) polar and non-polar extraction phases, which can be collected separately. Many studies collect only the aqueous or polar fraction of the extraction and discard (or at least do not report the analysis of) the non-polar fraction.
Following addition of an extraction solvent, it is now common practice for cells to undergo a tissue-homogenisation step, to ensure that all cells are lysed and the metabolites released into solution, maximising the metabolite recovery rate. Homogenisation is typically carried out either by mechanical lysis (tissue lysis mill) or sonication (probe or bath). Studies that have compared cell and tissue homogenisation techniques have found that any homogenisation step greatly increases the recovery of metabolites compared to no homogenisation [63, 68]. Following this, the cellular debris needs to be separated from the metabolites now in solution. This is achieved through centrifugation and the supernatant, which is now the sample extract, is collected. The pellet of cells may be subject to a repeat extraction and homogenisation step, to ensure maximum recovery of metabolites [65, 66]. To increase detection by the instrument, the extract needs to be concentrated so that the maximum amount of metabolites can be analysed in a small volume. This can be done by vacuum concentration, or freeze-drying in an aqueous solution. This is the last step in the metabolite extraction procedure of cultured cells, and from here the dried samples can be prepared for the chosen analytical platform.
Area for improvement 3: normalisation strategies for metabolomics data
Data sets collected from untargeted metabolomics studies are typically very large, potentially containing thousands of metabolite ‘features’ across hundreds of samples. The resulting data matrix is typically constructed using a peak-picking (deconvolution) software package. Prior to statistical data analysis, the data in the matrix can undergo various normalisation, scaling and transformation processes in order to improve the statistical functionality of the data [81–83]. Normalisation of the data matrix occurs when it is required to correct for any variation in the data set that may have occurred due to non-biological variables that cannot be controlled, such as variation in instrumental performance, and differences in the amount of material being analysed. In the case of instrumental variability, the addition of an internal standard compound to each sample to normalise all other peak abundances is a common step in untargeted metabolomic analysis.
In cell culture studies, especially when testing the response of a system to a physical challenge, the amount of material available for metabolomic analysis will likely differ between sample groups. Only a handful of publications have focused on the importance of normalising metabolomics data to the physical amount of the sample from cell culture studies. A number of differing techniques have been used, including protein or DNA concentration, tissue weight or cell number, and these options have been compared [71, 78, 84, 85]. However, there are still many cell culture metabolomics studies that fail to report the normalisation techniques used in data analysis, and so it remains an issue that should be addressed in the standardisation of cell culture metabolomics protocols. When reported, the most common method of normalisation of sample variation (pre-instrumental analysis) is to the cell number upon sample harvesting. Despite the need for a separate, parallel sample to be set up specifically for cell counting due to the destructive nature of harvesting for metabolomics, the method of cell number normalisation is widely considered to be the most appropriate for data normalisation, and should be considered as a standard approach when possible.
A study that specifically investigated the use of multiple measures to normalise metabolomic data from adherent cell lines recommended the use of DNA quantification of the usually discarded cell pellet (post metabolite extraction) in place of other methods [85]. This study compared total protein concentration, DNA concentration, and cell number at the time of harvest, and presented their correlation with the number of cells first seeded. Specific metabolites were selected and quantified over a range of seeding densities which were then normalised using all four strategies. The study concluded that DNA concentration was the best strategy, based on the lowest deviation from the mean for the normalised peak areas and the ease of sample collection compared to other methods. When considering the outcomes of this study, it is important to note that the study collected the data for other normalisation methods at the time of cell harvest and compared only to the number of cells at the time of seeding, rather than at the time of harvest. Also, if normalising to DNA concentration was applied to a study that investigated the cellular response to a cytotoxic agent that affects the rate of growth of cells or directly damages DNA, then the correlation to seeding density interpretations suddenly becomes questionable, a fact acknowledged by Silva et al. [85]. The authors also stated that cell counting was the preferred normalisation strategy when dealing with low cell numbers, specifically under 500,000 cells, something which is possible using many current analytical platforms.
Silva et al. [85] concluded that the measurement of protein concentration was highly variable from metabolite-extracted samples of the same seeding density, and did not recommend protein concentration as a normalisation strategy for metabolomic data of cell culture. A subsequent study by Rahman et al. [76] reported contradictory results to this study, supporting the use of protein concentration to normalise metabolomics data. This later study conducted both cell number and protein content normalisation of peak areas to develop a MS method for targeted metabolomics of three different adherent cell lines. They concluded that cell number and protein content correlated well, and so comparable normalisation could be carried out by either measures. These contradicting recommendations on what is suitable for data normalisation highlight the problem in generating consensus for standardisation efforts.
A different strategy for data normalisation presented by Hutschenreuther et al. [78] compared the cell number with the measured total ion chromatogram (TIC) area of a sample to normalise individual peak areas, and concluded that either could be used as they had a similar number of resulting “false significants”, as well as an equal correlation with a linear range of sample loadings. The study suggests that using the TIC area to normalise peak area would be a suitable replacement for cell number, and therefore the need to set up replicate cell counting samples and include a counting step could be omitted from cell harvesting protocols. Rather than use the area of the TIC, Cao et al. [84] used the intensities of several, consistently detected intracellular metabolites to normalise remaining peak intensities, following analysis by GC–time of flight (TOF)-MS. The study compared two cell lines using this method and chose metabolites for normalisation that did not significantly differ between the two cell lines. The strategy was implemented to correct the data for variation between the two cell lines, and was successful under principal component analysis. This is an important result in validating the use of multiple different cell lines for the same experimental tests and being able to compare the data and effectively interpret the results. However, concern remains in regard to application in a treatment-response scenario. The response of these particular metabolites to an interfering agent may be different between control and treatment groups, thus ruling out the use of this normalisation strategy in toxin-response studies, at least between the treatment groups within the same cell line.
Summary of standardised reporting and optimised extraction methods for cultured cell metabolomics studies
This review has compared the CIMR report [35] with recent studies in metabolomics of cultured mammalian cells (Tables 1, 2) in order to provide a summary of a standard reporting procedure for untargeted mammalian cell culture metabolomics studies. The specific steps, outlined in Table 3, represent the currently accepted best-practice for maintaining quality control and sample integrity during a cell culture metabolomics study. As with any other metabolomics experiment, a full schedule of the sample processing and analysis protocol that follow these steps would also be included, relative of course to the specific analytical platform used in the study.
Table 3.
Recommended experimental design, procedures and minimum reporting standards of a mammalian cell culture metabolomics study, adapted from van der Werf et al. [35], with examples given of each step
| Category | Detail | Example |
|---|---|---|
| 1. Cell culture general growth | Cell line/s used and their source | SH-SY5Y human neuroblastoma, sourced from ECACC |
| Configuration of cellular growth | Adherent layer | |
| Medium and substrates used inclusive of type, supplier, concentrations, additions, supplementations, serum percentage, etc. | DMEM:Ham’s F-12 (1:1) supplemented with 1% v/v 2 mM l-glutamine, 1% v/v 10,000 U/mL penicillin and streptomycin, and 10% v/v FCS, all sourced from Sigma Aldrich | |
| Growth containers used inclusive of type, supplier, size and geometry | Cells were grown in 75 cm2 tissue culture flasks (sourced from Corning) with 10 mL volume of medium | |
| Inoculation procedures used, splitting procedures, seeding densities and levels of confluence achieved | For passaging confluent cells and cell counting during experimentation, cells were detached with addition of 1× trypsin/EDTA after first removing the medium and washing the cells with pre-warmed 1× PBS | |
| Environmental conditions of growth | Cells were kept in a humidified incubator at 37 °C and 5% CO2 | |
| 2. Characteristics of cells during experimental growth | Growth containers, growth phase of differentiated state | Cells were cultivated in six-well tissue culture plates (sourced from Corning) |
| Numbers of culture passage/s and biological replicates | Cell culture passage numbers 28–32 were used for experimentation with four biological replicates per treatment group | |
| Cell density (optical density or cell number) | Cells seeded at a density of 4 × 105 cells/well in 2 mL of medium | |
| Depletion of nutrients at treatment time | Plates were left for 24 h to allow for cell adhesion before treatment | |
| Any phenotypic characteristics specific to the question under study | If carried out, such as differentiation | |
| 3. Particulars of the treatment conditions | Nature of the treatment itself e.g. physical stressor, chemical, etc. | Chemical treatment was the neurotoxin insecticide, permethrin |
| Dose/s of treatment and vehicle | Permethrin was added as a solution in 100% methanol to a final concentration of 100 µg/mL per well, or 0.25 mM | |
| Treatment times or intervals | Exposure to permethrin was for 6 and 24 h | |
| Purity concentration of treatment agent | Permethrin (mixture of cis- and trans-isomers) was purchased at 99.5% purity | |
| Details of any pre-treatment procedure | Following 24 h post seeding, cells were left for 24 h in 1 mL of serum-free medium before treatment was added | |
| 4. Quenching details | The time of sample removal, temperature, and time until metabolic activity ceased | Immediately following exposure time, cell number and viability were determined using the cell number samples, and samples for metabolomics analysis were removed from incubation and cellular activity halted by placing the plates directly onto ice |
| Description of quenching technique | Addition of 1 mL 4 °C 1× PBS to each well | |
| 5. Sample collection | Discrimination of extracellular from intracellular metabolites | 1 mL of medium from each well was collected into one microcentrifuge tube for analysis of extracellular metabolites. Remaining medium removed and adhered cell washed with PBS, before collecting for analysis of intracellular metabolites |
| Method of collection and storage | Quenching PBS removed as a wash step, adhered cells were then collected by scraping into 100 µL of additional PBS and transferring into one microcentrifuge tube. All collected samples were immediately snap-frozen and freeze-dried and stored at −80 °C | |
| 6. Metabolite extraction procedure | Composition of extraction solution | 80% methanol, 20% water solution containing 2.6 µg/mL of 13C6-sorbitol as an internal standard compound |
| Description of extraction process | 500 µL of extraction solution added to each freeze-dried sample and homogenised in a tissue lyser for 40 s. Extracts were centrifuged and supernatant collected into a fresh microcentrifuge tube. Extracted metabolites were concentrated by evaporating the methanol in a vacuum concentrator | |
| If present, details of multiple phases collected | Single-phase (methanol/water) extraction was collected | |
| If known, knowledge of the expected recovery rate and stability of extracted metabolites | If known, should include recovery rate of the metabolites, either targeted for or untargeted, dependant on class of compound, stability in the sample preparation procedure, etc. | |
| 7. Biomass normalisation | Details of parallel samples set up next to experimental samples for determination of cell number to use for data normalisation | One extra well per treatment was seeded parallel along with metabolomics samples, for cell counting and viability |
| 8. Sample handling | Any additional clean-up steps undertaken for purification or to protect from degradation | Extracted metabolites were protected from degradation by addition of water and further snap-freezing and freeze-drying |
| How the sample are stored when not in use | Once completely dry, metabolite extracts were stored at −80 °C | |
| 9. Quality control samples | Details of constituents of QC samples and their use in the analysis | One extra sample from all treatment groups was pooled during extraction procedure and separated into equal volumes for QC samples |
| 10. Expected metabolite detection information | If known, any information on the detection limits, or stability of metabolites expected in the samples | Any expected features in the cell samples (e.g. specific sugars, lipids etc.) that are in relatively high abundance or trace levels, and if it is known if there will be endogenous metabolites that are easily degraded or converted into structurally different features during the analytical process |
ECACC European Collection of Cell Cultures, DMEM Dulbecco’s Modified Eagle Medium, FCS foetal calf serum, EDTA ethylenediaminetetraacetic acid, PBS phosphate buffered saline
Conclusion
This review has provided a summary of the current optimisation and standardisation efforts for the sample growth and preparation stages of cultured mammalian cell metabolomics studies. In spite of these efforts, there remain many individual published studies that conclude with a request for a standardised approach to be developed. The reasons for this disconnect are not clear. It is possible that the relative ease of application of the technologies used in metabolomics to a wide variety of different biological systems has meant that researchers have overlooked the suggested reporting standards. This in itself is an issue—that these standards are still seen as only a ‘suggestion’. It is difficult to suggest exactly how an accepted procedure could be established across a global scientific community. Such an initiative begins with the conscious effort of individual studies to conduct experiments with a focus on quality of reporting. There is also a need for all cultured cell-based metabolomics studies to be published with full disclosure of information. Both of these approaches would lead to a greater quantity of greater quality metabolomics studies in the literature. Repeatability of studies, including use of the same procedures in different biological models is another key aspect of this initiative. This combination of steps would help move towards the validation of a standard procedure, which when carried out effectively, across numerous times and different groups could become an accepted standard procedure within the wider cultured cell metabolomics community.
This review highlights some of the current areas for improvement of recent standardisation efforts, including improving the detail of reported culture conditions, specifying metabolite extraction procedures, and normalising data to tissue weight/cell number. With these areas of improvement in mind, and adapting from the multitude of past optimisation studies, this review suggests a procedure of mammalian cell culture to follow for specific untargeted metabolomics by mass spectrometry-based experiments. There may very well be a requirement for specific modifications to the procedure to include further optimised steps, e.g. extraction procedures specific to the type of cell line used, or to include specific details of any cell lines that are modified in their expression behaviour, etc. The overall aim in suggesting a standardised reporting procedure is to allow cultured mammalian cell metabolomics studies to be compared, interpreted, and used to decipher a ‘whole-system’ response to variety of challenges. A key advantage of cultured cell metabolomics is its ability to directly profile the metabolism of specific cell types with minimal interference from other factors, compared to animal models or clinical samples. There is currently no other experimental design that can provide as much detailed information directly related to the phenotypical behaviour of a biological system captured as a single snapshot. Cellular biochemistry has been extensively studied and much is known about the mechanisms of cellular metabolism, but there remain countless interactions that are only theoretical, particularly in response to a change in environment. The application of metabolomics to mammalian cell culture presents the opportunity to uncover these interactions, and potentially identify new target areas for therapies or intervention. It is an exciting, new field of cellular biochemistry that deserves to be fully explored, and for the data it generates to be accurately interpreted. With greater focus on these efforts, there is no question of the untold benefits and potential of cultured cell metabolomics in enhancing our knowledge of cellular biochemistry.
Acknowledgements
This review was supported in part by the Australian Government Research Training Program Scholarship.
Compliance with ethical standards
Conflict of interest
The author declares that they have no conflict of interest.
References
- 1.León Z, García-Cañaveras JC, Donato MT, Lahoz A. Mammalian cell metabolomics: experimental design and sample preparation. Electrophoresis. 2013;34(19):2762–2775. doi: 10.1002/elps.201200605. [DOI] [PubMed] [Google Scholar]
- 2.Idle JR, Gonzalez FJ. Metabolomics. Cell Metab. 2007;6:348–351. doi: 10.1016/j.cmet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blow N. Biochemistry’s new look. Nature. 2008;455:697–700. doi: 10.1038/455697a. [DOI] [PubMed] [Google Scholar]
- 4.Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1–2):155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 5.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26(1):51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn WB, Mamas M, Heazell A. Metabolomics in practice: successful strategies to generate and analyze metabolic data. Weinheim: Wiley-VCH; 2013. Metabolomics and its role in the study of mammalian systems; pp. 345–377. [Google Scholar]
- 7.Zhang A, Sun H, Wang P, Han Y, Wang X. Modern analytical techniques in metabolomics analysis. Analyst. 2012;137:293–300. doi: 10.1039/C1AN15605E. [DOI] [PubMed] [Google Scholar]
- 8.Wishart DS. Quantitative metabolomics using NMR. Trends Anal Chem. 2008;27:228–237. doi: 10.1016/j.trac.2007.12.001. [DOI] [Google Scholar]
- 9.Alonso A, Marsal S, Julia A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front Bioeng Biotechnol. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, Brown M, Knowles JD, Halsall A, Haselden JN, Nicholls AW, Wilson ID, Kell DB, Goodacre R, Consortium THSMH Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protocols. 2011;6(7):1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrer T, Zamboni N. High-throughput discovery metabolomics. Curr Opin Biotechnol. 2015;31:73–78. doi: 10.1016/j.copbio.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Sévin DC, Kuehne A, Zamboni N, Sauer U. Biological insights through nontargeted metabolomics. Curr Opin Biotechnol. 2015;34:1–8. doi: 10.1016/j.copbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinform. 2016;55:14.10.11–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 14.Zhang A, Sun H, Xu H, Qiu S, Wang X. Cell metabolomics. OMICS. 2013;17(10):495–501. doi: 10.1089/omi.2012.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sims JK, Manteiger S, Lee K. Towards high resolution analysis of metabolic flux in cells and tissues. Curr Opin Biotechnol. 2013;24:933–939. doi: 10.1016/j.copbio.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balcke GU, Kolle SN, Kamp H, Bethan B, Looser R, Wagner S, Landsiedel R, van Ravenzwaay B. Linking energy metabolism in mitochondrial respiration—a metabolomics in vitro approach. Toxicol Lett. 2011;203(3):200–209. doi: 10.1016/j.toxlet.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Veyrat-Durebex C, Corcia P, Piver E, Devos D, Dangoumau A, Gouel F, Vourc’h P, Emond P, Laumonnier F, Nadal-Desbarats L, Gordon PH, Andres CR, Blasco H. Disruption of TCA cycle and glutamate metabolism identified by metabolomics in an in vitro model of amyotrophic lateral sclerosis. Mol Neurobiol. 2016;53(10):6910–6924. doi: 10.1007/s12035-015-9567-6. [DOI] [PubMed] [Google Scholar]
- 18.Wegner A, Meiser J, Weindl D, Hiller K. How metabolites modulate metabolic flux. Curr Opin Biotechnol. 2015;34:16–22. doi: 10.1016/j.copbio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Kalluri U, Naiker M, Myers MA. Cell culture metabolomics in the diagnosis of lung cancer—the influence of cell culture conditions. J Breath Res. 2014;8(2):027109. doi: 10.1088/1752-7155/8/2/027109. [DOI] [PubMed] [Google Scholar]
- 20.Halama A. Metabolomics in cell culture—a strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch Biochem Biophys. 2014;564:100–109. doi: 10.1016/j.abb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Halama A, Riesen N, Moller G, Hrabě de Angelis M, Adamski J. Identification of biomarkers for apoptosis in cancer cell lines using metabolomics: tools for individualized medicine. J Intern Med. 2013;274(5):425–439. doi: 10.1111/joim.12117. [DOI] [PubMed] [Google Scholar]
- 22.Bouhifd M, Hartung T, Hogberg HT, Kleensang A, Zhao L. Toxicometabolomics. J Appl Toxicol. 2013;33(12):1365–1383. doi: 10.1002/jat.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol Toxicol. 2012;52:37–56. doi: 10.1146/annurev-pharmtox-010611-134748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vliet E, Morath S, Eskes C, Linge J, Rappsilber J, Honegger P, Hartung T, Coecke S. A novel in vitro metabolomics approach for neurotoxicity testing, proof of principle for methyl mercury chloride and caffeine. Neurotoxicology. 2008;29(1):1–12. doi: 10.1016/j.neuro.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Van den Hof WFPM, Ruiz-Aracama A, Van Summeren A, Jennen DGJ, Gaj S, Coonen MLJ, Brauers K, Wodzig WKWH, van Delft JHM, Kleinjans JCS. Integrating multiple omics to unravel mechanisms of cyclosporin A induced hepatotoxicity in vitro. Toxicol In Vitro. 2015;29(3):489–501. doi: 10.1016/j.tiv.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Ellis JK, Athersuch TJ, Cavill R, Radford R, Slattery C, Jennings P, McMorrow T, Ryan MP, Ebbels TMD, Keun HC. Metabolic response to low-level toxicant exposure in a novel renal tubule epithelial cell system. Mol Biosyst. 2011;7:247–257. doi: 10.1039/C0MB00146E. [DOI] [PubMed] [Google Scholar]
- 27.Rubakhin SS, Lanni EJ, Sweedler JV. Progress towards single cell metabolomics. Curr Opin Biotechnol. 2013;24:95–104. doi: 10.1016/j.copbio.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zenobi R. Single-cell metabolomics: analytical and biological perspectives. Science. 2013;342:1243259. doi: 10.1126/science.1243259. [DOI] [PubMed] [Google Scholar]
- 29.Aurich MK, Paglia G, Rolfsson Ó, Hrafnsdóttir S, Magnúsdóttir M, Stefaniak MM, Palsson BØ, Fleming RMT, Thiele I. Prediction of intracellular metabolic states from extracellular metabolomic data. Metabolomics. 2015;11:603–619. doi: 10.1007/s11306-014-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broadhurst DI, Kell DB. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2006;2(4):171–196. doi: 10.1007/s11306-006-0037-z. [DOI] [Google Scholar]
- 31.Hrydziuszko O, Viant MR. Missing values in mass spectrometry based metabolomics: an undervalued step in the data processing pipeline. Metabolomics. 2012;8(1):S161–S174. doi: 10.1007/s11306-011-0366-4. [DOI] [Google Scholar]
- 32.Grissa D, Petera M, Brandolini M, Napoli A, Comte B, Pujos-Guillot E. Feature selection methods for early predictive biomarker discovery using untargeted metabolomic data. Front Mol Biosci. 2016;3:30. doi: 10.3389/fmolb.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gromski PS, Xu Y, Kotze HL, Correa E, Ellis DI, Armitage EG, Turner ML, Goodacre R. Influence of missing values substitutes on multivariate analysis of metabolomics data. Metabolites. 2014;4(2):433–452. doi: 10.3390/metabo4020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villas-Boas SG, Koulmann A, Lane GA. Analytical methods from the perspective of method standardization. In: Nielsen J, Jewett MC, editors. Metabolomics: a powerful tool in systems biology, Topics in Current Genetics. Berlin: Springer; 2007. pp. 11–52. [Google Scholar]
- 35.van der Werf MJ, Takors R, Smedsgaard J, Nielsen J, Ferenci T, Portais JC, Wittmann C, Hooks M, Tomassini A, Oldiges M, Fostel J, Sauer U. Standard reporting requirements for biological samples in metabolomics experiments: microbial and in vitro biology experiments. Metabolomics. 2007;3:189–194. doi: 10.1007/s11306-007-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindon J, Nicholson J, Holmes E, Keun H, Craig A, Pearce J, Bruce S, Hardy N, Sansone S, Antti H, Jonsson P, Daykin C, Navarange M, Beger R, Verheij E, Amberg A, Baunsgaard D, Cantor G, Lehman-McKeeman L, Earll M, Wold S, Johansson E, Haselden J, Kramer K, Thomas C, Lindberg J, Schuppe-Koistinen I, Wilson I, Reily M, Robertson D, Senn H, Krotzky A, Kockhar S, Powell J, van der Ouderaa F, Plumb R, Schaefer H, Spraul M. Summary recommendations for standardization and reporting of metabolic analysis. Nat Biotechnol. 2005;23:833–838. doi: 10.1038/nbt0705-833. [DOI] [PubMed] [Google Scholar]
- 37.National Research Council (NRC) Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington DC, USA: National Academies Press; 2007. [Google Scholar]
- 38.Davis M, Boekelheide K, Boverhof DR, Eichenbaum G, Hartung T, Holsapple MP, Jones TW, Richard AM, Watkins PB. The new revolution in toxicology: the good, the bad, and the ugly. Ann N Y Acad Sci. 2013;1278:11–24. doi: 10.1111/nyas.12086. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez T, Daneshian M, Kamp H, Bois FY, Clench MR, Coen M, Donley B, Fischer SM, Ekman DR, Fabian E, Guillou C, Heuer J, Hogberg HT, Jungnickel H, Keun HC, Krennrich G, Krupp E, Luch A, Noor F, Peter E, Riefke B, Seymour M, Skinner N, Smirnova L, Verheij E, Wagner S, Hartung T, van-Ravenzwaay B, Leist M. Metabolomics in toxicology and preclinical research. Altex. 2013;30(2):209–225. doi: 10.14573/altex.2013.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prot JM, Leclerc E. The current status of alternatives to animal testing and predictive toxicology methods using liver microfluidic biochips. Ann Biomed Eng. 2012;40(6):1228–1243. doi: 10.1007/s10439-011-0480-5. [DOI] [PubMed] [Google Scholar]
- 41.Valeria R, Luisa S, Adele M, Stefania B, Fabio T, Nicoletta B, Carmine PM, Silvia A. Changes in the NMR metabolic profile of live human neuron-like SH-SY5Y cells exposed to interferon-α2. J Neuroimmune Pharmacol. 2016;11(1):142–152. doi: 10.1007/s11481-015-9641-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhaoa C, Dub H, Xuc L, Wange J, Tange L, Caog Y, Lig C, Wangg Q, Liue Y, Shanh F, Fenga J, Xub F, Gaoe P. Metabolomic analysis revealed glycylglycine accumulation in astrocytes after methionine enkephalin administration exhibiting neuron protective effects. J Pharm Biomed Anal. 2015;115:48–54. doi: 10.1016/j.jpba.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Wang W, Zhou X, Gu R, Ding Z. Dose responsive effects of cisplatin in L02 cells using NMR-based metabolomics. Environ Toxicol Pharmacol. 2014;37:150–157. doi: 10.1016/j.etap.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Snouber LC, Bunescu A, Naudot M, Legallais C, Brochot C, Dumas ME, Elena-Herrmann B, Leclerc E. Metabolomics-on-a-chip of hepatotoxicity induced by anticancer drug flutamide and its active metabolite hydroxyflutamide using HepG2/C3a microfluidic biochips. Toxicol Sci. 2013;132(1):8–20. doi: 10.1093/toxsci/kfs230. [DOI] [PubMed] [Google Scholar]
- 45.Massimi M, Tomassini A, Sciubba F, Sobolev AP, Devirgiliis LC, Miccheli A. Effects of resveratrol on HepG2 cells as revealed by 1H-NMR based metabolic profiling. Biochim Biophys Acta. 2012;1820:1–8. doi: 10.1016/j.bbagen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Aracama A, Peijnenburg A, Kleinjans J, Jennen D, vanDelft J, Hellfrisch C, Lommen A. An untargeted mulit-technique metabolomics approach to studying intracellular metabolites of HepG2 cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genom. 2011;12:251–270. doi: 10.1186/1471-2164-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Canaveras JC, Castell JV, Donato MT, Lahoz A. A metabolomics cell-based approach for anticipating and investigating drug-induced liver injury. Sci Rep. 2016;6:27239. doi: 10.1038/srep27239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purwaha P, Lorenzi PL, Silva LP, Hawke DH, Weinstein JN. Targeted metabolomic analysis of amino acid response to l-asparaginase in adherent cells. Metabolomics. 2014;10:909–919. doi: 10.1007/s11306-014-0634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y, Qi Y, Liu H, Fan G, Chai Y. Effects of celastrol on human cervical cancer cells as revealed by ion-trap gas chromatography-mass spectrometry based metabolic profiling. Biochim Biophys Acta. 2013;1830(3):2779–2789. doi: 10.1016/j.bbagen.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Zhuang X, Zong L, Liu S, Liu Z, Song F. Investigations on the cell metabolomics basis of multidrug resistance from tumor cells by ultra-performance liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2016;408:5843–5854. doi: 10.1007/s00216-016-9696-4. [DOI] [PubMed] [Google Scholar]
- 51.Wilmes A, Limonciel A, Aschauer L, Moenks K, Bielow C, Leonard MO, Hamon J, Carpi D, Ruzek S, Handler A, Schmal O, Herrgen K, Bellwon P, Burek C, Truisi GL, Hewitt P, Consiglio ED, Testai E, Blaauboer BJ, Guillou C, Huber CG, Lukas A, Pfaller W, Mueller SO, Bois FY, Dekant W, Jennings P. Application of integrated transcriptomic, proteomic and metabolomic profiling for the delineation of mechanisms of drug induced cell stress. J Proteomics. 2013;79:180–194. doi: 10.1016/j.jprot.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Huang S-M, Zuo X, Li JJE, Li SFY, Bay BH, Ong CN. Metabolomics studies show dose-dependent toxicity induced by SiO2 nanoparticles in MRC-5 human fetal lung fibroblasts. Adv Healthc Mater. 2012;1(6):779–784. doi: 10.1002/adhm.201200114. [DOI] [PubMed] [Google Scholar]
- 53.Strigun A, Wahrheit J, Beckers S, Heinzle E, Noor F. Metabolic profiling using HPLC allows classification of drugs according to their mechanisms of action in HL-1 cardiomyocytes. Toxicol Appl Pharmacol. 2011;252:183–191. doi: 10.1016/j.taap.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Nicolae A, Wahrheit J, Bahnemann J, Zeng A-P, Heinzle E. Non-stationary 13C metabolic flux analysis of Chinese hamster ovary cells in batch culture using extracellular labeling highlights metabolic reversibility and compartmentation. BMC Syst Biol. 2014;8:50–64. doi: 10.1186/1752-0509-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krömer JO, Dietmair S, Jacob SS, Nielsen LK. Quantification of l-alanyl-l-glutamine in mammalian cell culture broth: evaluation of different detectors. Anal Biochem. 2011;416:129–131. doi: 10.1016/j.ab.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 56.Aranibar N, Borys M, Mackin NA, Ly V, Abu-Absi N, Abu-Absi S, Niemitz M, Schilling B, Li ZJ, Brock B, Russell-II RJ, Tymiak A, Reily MD. NMR-based metabolomics of mammalian cell and tissue cultures. J Biomol NMR. 2011;49:195–206. doi: 10.1007/s10858-011-9490-8. [DOI] [PubMed] [Google Scholar]
- 57.Selvarasu S, Ho YS, Chong WPK, Wong NSC, Yusufi FNK, Lee YY, Yap MGS, Lee D-Y. Comgined in silico modeling and metabolomics analysis to characterize fed-batch CHO cell culture. Biotechnol Bioeng. 2012;109(6):1415–1429. doi: 10.1002/bit.24445. [DOI] [PubMed] [Google Scholar]
- 58.Dietmair S, Hodson MP, Quek L-E, Timmins NE, Chrysanthopoulos P, Jacob SS, Gray P, Nielsen LK. Metabolite profiling of CHO cells with different growth characteristics. Biotechnol Bioeng. 2012;109(6):1404–1414. doi: 10.1002/bit.24496. [DOI] [PubMed] [Google Scholar]
- 59.Jin C, Liu Y, Sun L, Chen T, Zhang Y, Zhao A, Wang X, Cristau M, Wang K, Jia W. Metabolic profiling reveals disorder of carbohydrate metabolism in mouse fibroblast cells induced by titanium dioxide nanoparticles. J Appl Toxicol. 2012;33:1442–1450. doi: 10.1002/jat.2808. [DOI] [PubMed] [Google Scholar]
- 60.Wallace M, Whelan H, Brennan L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim Biophys Acta. 2013;1830:2583–2590. doi: 10.1016/j.bbagen.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 61.Čuperlović-Culf M, Barnett DA, Culf AS, Chute I. Cell culture metabolomics: applications and future directions. Drug Discov Today. 2010;15(15/16):610–621. doi: 10.1016/j.drudis.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Álvarez-Sánchez B, Priego-Capote F, Luque de Castro MD. Metabolomics analysis II: preparation of biological samples prior to detection. Trends Anal Chem. 2010;29(2):120–127. doi: 10.1016/j.trac.2009.12.004. [DOI] [Google Scholar]
- 63.Dettmer K, Nürnberger N, Kaspar H, Gruber MA, Almstetter MF, Oefner PJ. Metabolite extraction from adherently growing mammalian cells for metabolomics studies: optimization of harvesting and extraction protocols. Anal Bioanal Chem. 2011;399(3):1127–1139. doi: 10.1007/s00216-010-4425-x. [DOI] [PubMed] [Google Scholar]
- 64.Dietmair S, Timmins NE, Gray PP, Nielsen LK, Kromer JO. Towards quantitative metabolomics of mammalian cells: development of a metabolite extraction protocol. Anal Biochem. 2010;404(2):155–164. doi: 10.1016/j.ab.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 65.Kapoore RV, Coyle R, Staton CA, Brown NJ, Vaidyanathan S. Influence of washing and quenching in profiling the metabolome of adherent mammalian cells: a case study with the metastatic breast cancer cell line MDA-MB-231. Analyst. 2017;142:2038–2049. doi: 10.1039/C7AN00207F. [DOI] [PubMed] [Google Scholar]
- 66.Kapoore RV, Coyle R, Staton CA, Brown NJ, Vaidyanathan S. Cell line dependence of metabolite leakage in metabolome analyses of adherent normal and cancer cell lines. Metabolomics. 2015;11:1743–1755. doi: 10.1007/s11306-015-0833-4. [DOI] [Google Scholar]
- 67.Martineau E, Tea I, Loaec G, Giraudeau P, Akoka S. Strategy for choosing extraction procedures for NMR-based metabolomic analysis of mammalian cells. Anal Bioanal Chem. 2011;401:2133–2142. doi: 10.1007/s00216-011-5310-y. [DOI] [PubMed] [Google Scholar]
- 68.Danielsson APH, Moritz T, Mulder H, Spégel P. Development and optimization of a metabolomic method for analysis of adherent cell cultures. Anal Biochem. 2010;404(1):30–39. doi: 10.1016/j.ab.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Teng Q, Huang W, Collette TW, Ekman DR, Tan C. A direct cell quenching method for cell-culture based metabolomics. Metabolomics. 2009;5:199–208. doi: 10.1007/s11306-008-0137-z. [DOI] [Google Scholar]
- 70.Matheus M, Hansen S, Rozet E, Peixoto P, Maquoi E, Lambert V, Noel A, Frederich M, Mottet D, deTullio P. An easy, convenient cell and tissue extraction protocol for nuclear magnetic resonance metabolomics. Phytochem Anal. 2014;25:342–349. doi: 10.1002/pca.2498. [DOI] [PubMed] [Google Scholar]
- 71.Muschet C, Möller G, Prehn C, Hrabē de Angelis M, Adamski J, Tokarz J. Removing the bottlenecks of cell culture metabolomics: fast normalization procedure, correlation of metabolites to cell number, and impact of the cell harvesting method. Metabolomics. 2016;12(10):151. doi: 10.1007/s11306-016-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Canaveras JC, Lopez S, Castell JV, Donato MT, Laboz A. Extending metabolome coverage for untargeted metabolite profiling of ahderent cultured hepatic cells. Anal Bioanal Chem. 2016;408:1217–1230. doi: 10.1007/s00216-015-9227-8. [DOI] [PubMed] [Google Scholar]
- 73.Peterson AL, Walker AK, Sloan EK, Creek DJ. Optimized method for untargeted metabolomics analysis of MDA-MB-231 breast cancer cells. Metabolites. 2016;6(4):30–46. doi: 10.3390/metabo6040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madji Hounoum B, Blasco H, Nadal-Desbarats L, Diémé B, Montigny F, Andres CR, Emond P, Mavel S. Analytical methodology for metabolomics study of adherent mammalian cells using NMR, C-MS and LC–HRMS. Anal Bioanal Chem. 2015;407:8861–8872. doi: 10.1007/s00216-015-9047-x. [DOI] [PubMed] [Google Scholar]
- 75.Ser Z, Liu X, Tang NN, Locasale JW. Extraction parameters for metabolomics from cultured cells. Anal Biochem. 2015;475:22–28. doi: 10.1016/j.ab.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahman AMA, Pawling J, Ryczko M, Caudy AA. Targeted metabolomics in cultured cells and tissues by mass spectrometry: method development and validation. Anal Chim Acta. 2014;845:53–61. doi: 10.1016/j.aca.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Bi H, Krausz KW, Manna SK, Li F, Johnson CH, Gonzalez FJ. Optimization of harvesting, extraction, and analytical protocols for UPLC–ESI–MS-based metabolomics analysis of adherent mammalian cancer cells. Anal Bioanal Chem. 2013;405:5279–5289. doi: 10.1007/s00216-013-6927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hutschenreuther A, Kiontke A, Birkenmeier G, Birkemeyer C. Comparison of extraction conditions and normalization approaches for cellular metabolomics of adherent growing cells with GC–MS. Anal Methods. 2012;4:1953–1963. doi: 10.1039/c2ay25046b. [DOI] [Google Scholar]
- 79.Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal Chem. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheikh KD, Khanna S, Byers SW, Fornace A, Cheema AK. Small molecule metabolite extraction strategy for improving LC/MS detection of cancer cell metabolome. J Biomol Tech. 2011;22(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 81.Guida RD, Engel J, Allwood JW, Weber RJM, Jones MR, Sommer U, Viant MR, Dunn WB. Non-targeted UHPLC–MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics. 2016;12:93. doi: 10.1007/s11306-016-1030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xi B, Gu H, Baniasadi H, Raftery D. Statistical analysis and modeling of mass spectrometry-based metabolomics data. Methods Mol Biol. 2014;1198:333–353. doi: 10.1007/978-1-4939-1258-2_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boccard J, Veuthey JL, Rudaz S. Knowledge discovery in metabolomics: an overview of MS data handling. J Sep Sci. 2010;33(3):290–304. doi: 10.1002/jssc.200900609. [DOI] [PubMed] [Google Scholar]
- 84.Cao B, Aa J, Wang G, Wu X, Liu L, Li M, Shi J, Wang X, Zhao C, Zheng T, Guo S, Duan J. GC–TOFMS analysis of metabolites in adherent MDCK cells and a novel strategy for identifying intracellular metabolic markers for use as cell amount indicators in data normalization. Anal Bioanal Chem. 2011;400(9):2983–2993. doi: 10.1007/s00216-011-4981-8. [DOI] [PubMed] [Google Scholar]
- 85.Silva LP, Lorenzi PL, Purwaha P, Yong V, Hawke DH, Weinstein JN. Measurement of DNA concentration as a normalization strategy for metabolomic data from adherent cell lines. Anal Chem. 2013;85(20):9536–9542. doi: 10.1021/ac401559v. [DOI] [PMC free article] [PubMed] [Google Scholar]