Abstract
In the central nervous system, embryonic and adult neural stem/progenitor cells (NSCs) generate the enormous variety and huge numbers of neuronal and glial cells that provide structural and functional support in the brain and spinal cord. Over the last decades, nuclear receptors and their natural ligands have emerged as critical regulators of NSC homeostasis during embryonic development and adult life. Furthermore, substantial progress has been achieved towards elucidating the molecular mechanisms of nuclear receptors action in proliferative and differentiation capacities of NSCs. Aberrant expression or function of nuclear receptors in NSCs also contributes to the pathogenesis of various nervous system diseases. Here, we review recent advances in our understanding of the regulatory roles of steroid, non-steroid, and orphan nuclear receptors in NSC fate decisions. These studies establish nuclear receptors as key therapeutic targets in brain diseases.
Keywords: Brain development, Neurogenesis, Astrogliogenesis, Agonists/antagonists, Drug targets, Neurological diseases, Glucocorticoid, Retinoic acid
Introduction
The mammalian central nervous system (CNS) is one of the most complex tissues. Generation of this complexity requires a well-synchronized action of extrinsic morphogenetic cues and intrinsic gene regulatory networks in neural stem/progenitor cell (NSC) fate decisions. NSCs are characterized by the ability to proliferate and generate neurons or macroglial cells, such as oligodendrocytes and astrocytes, during development. However, one of the breakthrough discoveries in neuroscience, a few decades ago, was that NSCs are not only restricted to embryonic development, but they are maintained throughout the whole adult life generating neural cells in many mammalian species including humans [1–4]. Abberant specification choices of NSCs during development or adulthood can result in severe developmental defects, abnormalities, neurological diseases, or cancers. Therefore, elucidation of regulatory factors that control NSC properties could offer significant insights into the treatment of CNS-related diseases and disorders. Towards this end, many lines of research strongly implicate nuclear receptors (NRs) in NSC homeostasis during embryonic and adult life. Most importantly, there is a wide spectrum of pharmacological compounds targeting NRs activity [5–11], raising the possibility of using these molecules in novel therapeutic approaches for neural diseases.
In general, NRs constitute a large superfamily of transcriptional regulators, including 49 members in mouse and 48 in human. Through their ability to regulate gene expression of many downstream effector genes, NRs are involved in various physiological functions, controlling development of several organs, as well as metabolism, reproduction, stem cell pluripotency, tissue, and cell homeostasis. The canonical structure of NRs is consisted of four distinct functional domains: (a) the ligand-independent AF-1 transactivation domain (also known as A/B domain); (b) the DNA-binding domain, a zinc-finger domain required for binding to response elements (DBD or C domain); (c) the hinge region (D domain) which contains the nuclear localization signal (NLS); and (d) the ligand-binding domain (LBD or E domain), a ligand-dependent AF-2 transactivation domain. Regarding their molecular mechanism of action, NRs bind to specific DNA response elements in the regulatory regions of target genes [7, 12]. In most cases, their ability to regulate transcription is initiated by the binding of specific ligands to LBD of the corresponding NRs.
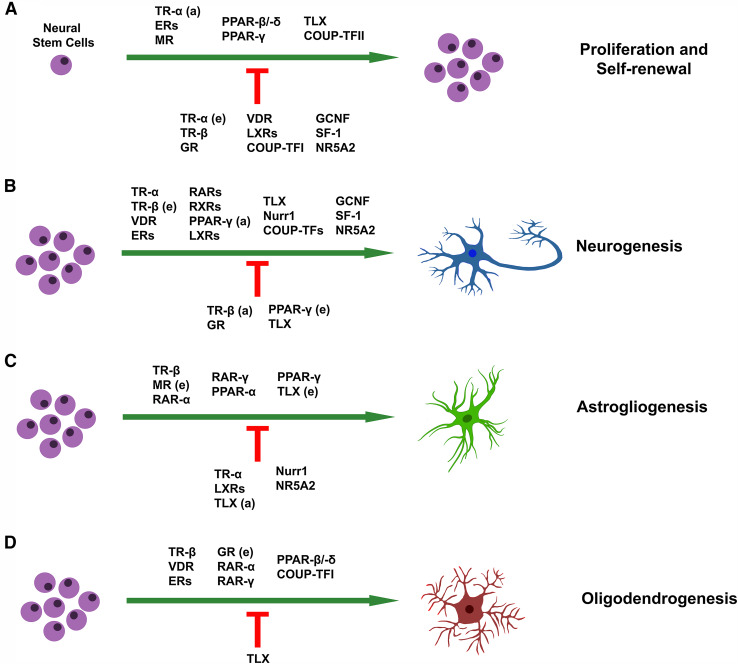
Most NRs are expressed in the mammalian brain and have important roles in brain organogenesis and function. In addition, many of these NRs have been implicated in neurological, neurodegenerative, and psychiatric diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, amyotrophic lateral sclerosis (ALS), and schizophrenia [11, 13–16]. Despite their importance in nervous system and the fact that could be utilized as potential pharmacological targets for these diseases, there is only a limited number of articles that review the role of NRs in brain development and adult neurogenesis [17–21]. Here, we thoroughly discuss and present an updated view of the specific roles of steroid, non-steroid, and orphan NRs in NSC biology (Fig. 1; Table 1).
Fig. 1.
Schematic illustration of how specific NRs control NSC homeostasis. NRs affect the ability of NSCs to proliferate and self-renew (a), to differentiate into neurons (b) or glial cells, namely astrocytes (c) or oligodendrocytes (d). NRs that promote these fate decisions are indicated above the green arrows, while NRs that suppress them are below the red repression symbols. e embryonic NSCs, a adult NSCs
Table 1.
Overview of the roles of NRs in proliferation and differentiation properties in embryonic and adult NSCs
| Members of nuclear receptors family | Nuclear receptor symbol | Functions | References | |
|---|---|---|---|---|
| Embryonic NSCs | Adult NSCs | |||
| Steroid hormone receptors | ||||
| Thyroid hormone receptor α (TR-α) | NR1A1 | Inhibition of proliferation and astrogliogenesis; induction of neuronal differentiation; regulation of oligodendrogenesis | Promotion of cell survival; induction of neuronal differentiation | [39–46, 49, 51–53] |
| Thyroid hormone receptor β (TR-β) | NR1A2 | Regulation of neuronal differentiation; regulation of oligodendrogenesis | Inhibition of proliferation; regulation of gliogenesis | [38, 43–46, 54] |
| Vitamin D receptor (VDR) | NR1I1 | Suppression of proliferation; induction of neuronal differentiation and maturation; specification of DA neurons | Regulation of proliferation; induction of differentiation into neurons and oligodendrocytes; enhancement of neuronal survival | [63–67, 70, 71] |
| Estrogen receptor α (ERα) | NR3A1 | Regulation of proliferation; promotion of differentiation into DA neurons; induction of oligodendrogenesis | Enhancement of proliferation | [79–82, 85, 87] |
| Estrogen receptor β (ERβ) | NR3A2 | Regulation of proliferation; promotion of neurogenesis and differentiation into DA neurons; induction of oligodendrogenesis; enhancement of neuronal survival | Enhancement of proliferation; stimulation of cerebellar Purkinje cells maturation | [79–83, 85, 87, 91] |
| Glucocorticoid receptor (GR) | NR3C1 | Inhibition of proliferation; repression of neurogenesis; induction of apoptosis | Inhibition of proliferation; repression of neurogenesis; induction of apoptosis; induction of oligodendrogenesis | [103–112, 115–128] |
| Mineralocorticoid receptor (MR) | NR3C2 | Promotion of cell survival; regulation of neurogenesis | Promotion of cell survival; regulation of neurogenesis; induction of astrogliogenesis | [112, 135–140] |
| Non-steroid hormone receptors | ||||
| Retinoic acid receptor α (RAR-α) | NR1B1 | Induction of gliogenesis; neuronal maturation | Neuronal maturation; regulation of neurogenesis; inhibition of proliferation; induction of gliogenesis | [155, 162, 164] |
| Retinoic acid receptor β (RAR-β) | NR1B2 | Induction of proliferation and neuronal differentiation; neuronal maturation | Induction of proliferation and neuronal differentiation; neuronal maturation | [150–155, 162] |
| Retinoic acid receptor γ (RAR-γ) | NR1B3 | Induction of gliogenesis | [155] | |
| Retinoic X receptor α (RXR-α) | NR2B1 | Regulation of neurogenesis | Regulation of neurogenesis | [173–176] |
| Retinoic X receptor β (RXR-β) | NR2B2 | Regulation of neurogenesis | Regulation of neurogenesis | [173–176] |
| Retinoic X receptor γ (RXR-γ) | NR2B3 | Regulation of neurogenesis | Maturation of oligodendrocytes; regulation of neurogenesis | [144, 171–176] |
| Peroxisome-proliferator-activated receptor α (PPAR-α) | NR1C1 | Induction of astrogliogenesis; astrocyte maturation | [189] | |
| Peroxisome-proliferator-activated receptor β/δ (PPAR-β/-δ) | NR1C2 | Induction of gliogenesis; maturation of oligodendrocytes | Induction of proliferation; promotion of neuronal differentiation | [189, 190, 196] |
| Peroxisome-proliferator-activated receptor γ (PPAR-γ) | NR1C3 | Induction of proliferation; promotion of cell survival; regulation of neurogenesis | Induction of proliferation; promotion of neuronal differentiation; neuronal maturation | [181–184, 186, 187, 195, 198] |
| Liver X receptor α (LXR-α) | NR1H3 | Inhibition of proliferation; induction of neurogenesis | Promotion of cell survival; induction of neurogenesis | [9, 15, 218–221] |
| Liver X receptor β (LXR-β) | NR1H2 | Inhibition of proliferation; induction of neurogenesis | Promotion of cell survival; induction of neurogenesis | [9, 15, 218–221] |
| Orphan receptors | ||||
| TLX | NR2E1 | Induction of proliferation; suppression of neuronal differentiation; generation and maturation of astrocytes | Induction of proliferation; promotion of neurogenesis; suppression of gliogenesis | [225–227, 231, 234–241, 243] |
| Chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) | NR2F1 | Suppression of proliferation; induction of neurogenesis and neuronal maturation; promotion of oligodendrocyte differentiation | Suppression of proliferation; regulation of differentiation (retina); enhancement of neuronal survival | [275–282] |
| Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) | NR2F2 | Promotion of proliferation; induction of neuronal differentiation | Suppression of proliferation; regulation of differentiation (retina); enhancement of neuronal survival | [276, 277, 281, 282] |
| Nuclear receptor-related 1 protein (Nurr1) | NR4A2 | Promotion of differentiation into DA neurons; survival of DA neurons; suppression of astrogliogenesis | Promotion of differentiation into DA neurons; survival of DA neurons | [292–298, 304] |
| Steroidogenic factor 1 (SF-1) | NR5A1 | Promotion of cell-cycle exit; induction of neuronal differentiation | Induction of neuronal differentiation | [319, 320] |
| Liver receptor homolog 1 (LRH-1) | NR5A2 | Suppression of proliferation; induction of neuronal differentiation; inhibition of astrogliogenesis | [329–331] | |
| Germ cell nuclear factor (GCNF) | NR6A1 | Suppression of proliferation; promotion of neuronal differentiation and maturation | [334, 341–343, 347] | |
Steroid hormone nuclear receptors
Thyroid hormone receptors (TRs)
Thyroid hormone receptors are ligand-dependent nuclear receptors with two members, TR-α (NR1A1) and TR-β (NR1A2) [22]. The natural ligands of TRs are the thyroid hormones, 3,5,39-triiodo-l-thyronine (T3) and 3,5,39,59-tetraiodo-l-thyronine or thyroxine (T4), which are synthesized in thyroid gland [23, 24]. After activation, TRs bind to specific DNA regions that are called thyroid hormone response elements (TRE) as monomers, homodimers, heterodimers of TR-α with TR-β, or heterodimers with other factors such as Retinoic X Receptors (RXRs) [25–27]. TRs are expressed in almost every tissue [28, 29] and have great implication in metabolism, development, growth and function of many organs and tissues including liver, heart, bones, adipose tissue and CNS [30].
In CNS, TRs are expressed both in fetal and adult brain. In particular, TR-α is the major TR of the brain and is expressed in many areas of embryonic as well as adult CNS including hypothalamus, cerebellum, olfactory bulb, cerebral cortex, striatum and hippocampus. It is expressed in neurons and in some sub-populations of astrocytes and oligodendrocytes [31–33]. The expression of TR-β mainly begins in the post-natal period and is restricted in cerebral cortex, cerebellum, hypothalamus and hippocampus [32, 34, 35]. TRs have a significant role in fetal brain development. The thyroid hormone deficiency during development results in cognitive problems, hyperactivity, anxious behavior and locomotor disorders both in humans and rodents [36]. These defects are the result of an underdeveloped brain. In particular, the absence of thyroid hormones in the developing fetal brain of rats caused low proliferation, reduced cortical thickness and attenuated neurogenesis [37, 38]. Accordingly, the loss of TRs exhibits similar effects with the thyroid hormone deficiency. Loss of TR-α leads to dwarfism, metabolic disorders, underdeveloped brain and lethality in the neonates. In addition, TR-β deficiency affects learning, memory and development [39].
TRs are important regulators of NSC homeostasis (Fig. 1). Specifically, treatment of mouse embryonic telencephalic NSCs from E13.5 with T3, suppressed proliferation and induced neuronal differentiation via negative regulation of STAT3 signaling pathway. Knock-down of TR-α1 isoform counteracted the neurogenic effects of T3 on these NSCs [40]. In agreement, T3 is sufficient to induce dopaminergic (DA) neurogenesis in ex vivo cultured mouse embryonic NSCs (E13.5) from ventral midbrain. TR-α1 activation increases the expression levels of Otx-2, an important transcription factor in DA neurogenesis. Thyroid hormone deficiency leads to reduced DA neurons in ventral midbrain area, causing motor disorders [41]. Moreover, TR-α1 activation promotes retinoic acid (RA)-inducible neuronal differentiation of mouse embryonic stem cells (ESCs), a phenotype which is abolished in ESCs deficient for TR-α1 [42]. Furthermore, TR-α1 is necessary for proper post-natal cerebellum development in rodents. Dominant negative mutation of this receptor reduced proliferation and migration of granule cells and negatively affected the development of Purkinje cells as well as maturation of Bergman glia [43]. Interestingly, TRs have also been implicated in the regulation of gliogenesis. Treatment with T3 of rat embryonic NSCs from E16 induced a gliogenic phenotype [44]. In addition, both TR-α and TR-β are implicated in differentiation of neonatal rat NSCs into oligodendrocyte progenitors and their subsequent maturation into oligodendrocytes through the regulation of aTf (apotransferrin), a key regulator of oligodendrogenesis. On the other hand, TR-α1 antagonist decreased the number of mature oligodendrocytes [45, 46]. Furthermore, a TR-β agonist, GC-1, promoted the maturation of oligodendrocyte precursor cells into mature and functional oligodendrocytes both in mice and human [47].
TRs are also expressed in adult hippocampal NSCs and are correlated with the regulation of adult neurogenesis. In particular, chemically-induced thyroid hormone deficiency in rats results in decreased survival in newborn cells, attenuated neuronal differentiation and delayed neuronal maturation in the hippocampal area [35, 48]. Similarly, expression of mutant TR-α1 with low ligand affinity decreased adult hippocampal neurogenesis and cell survival in mice [49]. In contrast, knock-out of the other member of TRs, TR-β, induced hippocampal NSC proliferation without affecting differentiation [50]. TR-α1 is also expressed in the NSCs of subventricular zone (SVZ) in mice and is implicated in neurogenesis. The lack of TR-α1 mimics the antineurogenic effects of hypothyroidism on NSCs of SVZ [51]. Mechanistically, TR-α1 directly represses the expression of Sox2, a stemness marker of NSCs and induces neuronal differentiation. Conversely, there is an increase in Sox2 expression in the NSCs lacking TR-α1 [52]. Furthermore, TRs exert an important role in adult gliogenesis, both astrogliogenesis and oligendrodrogenesis. Ablation of TR-α1 in mice causes deficient astrocyte maturation in various regions of cerebellum, suggesting that TR-α1 is required to mediate the actions of thyroid hormones in cerebellar astrocyte maturation [53]. In addition, hyperthyroidism in rats leads NSCs to oligodendrogenic fate and proper oligodendrocyte precursor cells maturation compared to normal or hypothyroidic rats [54]. In conclusion, TRs exert their fundamental role in CNS in various developmental stages and cell populations. Based on their implication in proliferation, survival and differentiation of embryonic and adult NSCs, TRs are promising targets for treatment of neuropathological diseases.
Vitamin D receptor (VDR)
The last few years increasing evidence implicate Vitamin D receptor (VDR; NR1I1), a steroid hormone receptor, in NSC homeostasis and brain development. VDR is activated by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the biologically most active metabolite of vitamin D3, leading to its heterodimerization with RXR. Subsequently, the heterodimer binds to the VDR response element (VDRE) on the promoters of target genes and regulates their transcription. This action is facilitated by the recruitment of co-activators and co-repressors in the targeted promoters [55, 56]. Interestingly, VDR is widely distributed in CNS. It is detected in developing brain from E11.5 in mouse and E12 in rats. In adult brain, VDR is localized in several areas including substantia nigra, cortex, hippocampus, hypothalamus, cerebellum, olfactory system and basal ganglia, while it is also expressed in peripheral nervous system. In particular, VDR is found in both neurons and glia in adult rat and human brains [57–60]. Remarkably, vitamin D acting through VDR is important in several processes, such as calcium homeostasis, bone integrity and brain development. Its deficiency has been linked with schizophrenia, Parkinson’s disease, Alzheimer’s disease, autism and depression [60, 61].
During rat brain development, high levels of VDR are detected from E15 to E22, a period that cell proliferation is reduced and apoptosis is increased, suggesting the involvement of VDR in these processes [62]. In agreement, an animal model for developmental vitamin D (DVD) deficiency in rodents displayed increased mitosis and reduced apoptosis both in embryonic and neonatal brain [63, 64]. Consequently, brain morphology of these neonatal rats was also altered, exhibiting larger and longer hemispheres, thinner cortex and increased volume of lateral ventricles [63]. Furthermore, NSCs isolated from SVZ of DVD-deficient neonatal brain exhibited increased proliferating properties as compared to controls [65]. In addition, these DVD-deficient neonates demonstrated decreased levels of the neurotrophic factors, Glial-derived neurotrophic factor (GDNF) and Nerve growth factor (NGF), as well as neurotrophin receptor p75NTR, all of which are important in growth and survival of developing neurons in the brain, further indicating the neurotrophic and neuroprotective role of vitamin D [63]. Vitamin D3 via VDR is also involved in differentiation and maturation of neuronal cells during brain development. In particular, in an embryonic hippocampal cell line, vitamin D3 promotes differentiation through down-regulation of proliferation-associated genes (cyclin D1 and PCNA) and induction of NGF and STAT-3 that stimulate axonal and neurite outgrowth [66]. Moreover, in DVD-deficient embryos Nurr1 and p57Kip2 which are markers for DA phenotype, were significantly reduced, suggesting a crucial role of vitamin D3 in specification of DA neurons [67].
VDR is also involved in adult neurogenesis (Fig. 1). Adult brain of DVD-deficient rats displays larger lateral ventricles, while reintroduction of vitamin D in their diet at birth is able to ameliorate this effect [68]. These adult rats are characterized by altered behavior, including spontaneous hyperlocomotion, a symptom analogous to schizophrenia in humans, as well as deficits in learning and memory [60]. Significantly, adult hippocampal neurogenesis is impaired in DVD-deficient adult rats and this defect can be reversed by haloperidol, an inverse agonist of dopamine [69]. In addition, 1α-hydroxylase knock-out mice that develop deficiency in 1,25(OH)2D3 due to inability to synthesize this active metabolite of vitamin D3, exhibit increased proliferation of progenitors cells in adult dentate gyrus (DG), increased apoptosis and decreased survival of newly born neurons. In this study, it was also found that the enhanced proliferation in the absence of 1,25(OH)2D3 was mediated by increased Ca2+ influx via L-VGCC, whereas reduced survival of neurons was due to down-regulation of NGF [70]. Recently, a study in adult murine NSCs showed that these cells express VDR and its levels were increased after 1,25(OH)2D3 treatment. Through this action, 1,25(OH)2D3 was able to promote proliferation of NSCs and enhance their differentiation into neurons and oligodendrocytes but not astrocytes. In these cells the expression of neurotrophic factors, Neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF) and GDNF, was elevated, which may explain why vitamin D3 is sufficient to regulate NSC homeostasis [71].
Taken together, these data highlight the key role of VDR and vitamin D3 in developing and adult brain via regulation of proliferation and differentiation of NSCs as well as neurotrophic and neuroprotective actions that sustain the survival of neuronal cells. Furthermore, deficiency in vitamin D experiments have demonstrated its association with neuropsychiatric disorders and neurodegenerative diseases [60, 61], suggesting the necessity for further investigation to fully understand its functions.
Estrogen receptors (ERs)
Estrogen receptors (ERs) are members of NR3A family of nuclear receptors and also of steroid hormone receptors. The nuclear ERs subtypes are ERα (NR3A1) and ERβ (NR3A2), which are also detected in membrane and cytoplasm. In particular, estrogen hormones activate ERs to regulate the transcription of downstream target genes, either directly via their dimerization and binding to estrogen response element (ERE) of DNA or indirectly through interaction with co-factors such as AP-1 and Sp-1. Some of the effects of estrogen via ERs are so rapid that cannot be explained by activation of transcription and protein synthesis. These effects are referred as non genomic and are mediated through membrane associated ERs [72, 73]. ERs are expressed in many tissues including uterus, breast, prostate, cardiovascular system and brain. These NRs are found in the developing as well as adult brain, specifically in pituitary, hypothalamus, hippocampus, cerebral cortex, cerebellum, amygdala and spinal cord. ERα and ERβ exhibit distinct differences in their expression patterns in CNS [74, 75].
ERs have an important role in reproduction, breast cancer and cognition [76–78]. They have also been implicated in the development of CNS. Significantly, Brannvall and co-workers showed that activated ERs by 17β-estradiol (E2) regulate proliferation of embryonic and adult NSCs. Their effect on proliferation of embryonic NSCs depends on the presence of EGF and is mediated via upregulation of cyclin-dependent kinase inhibitor, p21Cip1. In addition, E2 increases the differentiation of embryonic NSCs to neurons at the expense of glial cell differentiation [79]. Further studies have shown that E2 or bisphenol A (BPA), an estrogenic-like endocrine-disrupting chemical, promote the proliferation and oligodendroglia differentiation of rat embryonic NSCs in the absence of mitogens (EGF, FGF-2) or differentiation factors [80, 81]. Moreover, it was found that E2 acts through ERs in human embryonic NSCs to induce their differentiation into DA neurons [82]. Regarding cortical morphogenesis during brain development, ERβ knock-out mice display thinner cortex at E18.5, due to abnormal migration of neurons and increased apoptosis, suggesting a significant role of ERβ in neuronal differentiation and survival in late corticogenesis [83]. On the contrary, ERα was detected in early stages of corticogenesis, indicating a potential role in brain development [84].
ERs also influence adult hippocampal neurogenesis through their activation by E2 [75]. It has been demonstrated that ERα and ERβ enhance proliferation and possibly affect differentiation of adult NSCs in the DG [85–87]. In addition, ERβ has been found to regulate hippocampal-dependent spatial learning, while ERβ knock-out mice failed to perform hippocampus-dependent spatial tasks. ERβ regulates this process by modulating synaptic plasticity and adult neurogenesis of hippocampus [75, 86–88]. Both ERs, upon activation, exert neuroprotective functions in CA1 neurons against global ischemia in rodents, via their ability to inhibit apoptosis [75, 89]. Furthermore, it is reported that ERs may have a role in cerebellum development. Kim and co-workers have shown that C17.2 cells (immortalized neural progenitor cells from neonatal mouse cerebellum) treated with BPA, displayed a significant decrease in their proliferation, indicating a role of ERs in early neonatal cerebellum formation [90]. In vivo and in vitro studies suggest that ERβ affects differentiation and maturation of cerebellar Purkinje cells through stimulation of dendritic growth and spine [91]. To sum up, nuclear ERs are involved in CNS development and function but further studies are essential for clarifying their specific mechanisms of action.
Glucocorticoid receptor (GR)
Glucocorticoid receptor (NR3C1) is a member of the steroid hormone nuclear receptors which includes the isoforms GRα, GRβ, GRγ, GRA, and GRP which are the result of alternative splicing of the same gene [92, 93]. Its physical ligands are cortisol in humans and corticosterone in rodents [94]. The release of glucocorticoids in blood circulation after their production in adrenal glands is under the control of inflammatory signals and stress-induced hypothalamus–pituitary–adrenals (HPA) axis activation [95]. GR activation involves glucocorticoid (GC) binding, dimerization of the complex and binding to a glucocorticoid response element (GRE) [96]. GR can also bind DNA elements as monomer after protein–protein interactions with other transcription factors. GR also exerts its actions via non genomic mechanisms [97]. GR is globally expressed in all organs, tissues and cell types [98, 99], and has major role in many biological functions such as development, reproduction, metabolism, immune response and cardiovascular functions [100–102].
GR has a significant role in CNS development. High dose of a synthetic GR agonist in pregnancy leads to nervous system-related pathology such as cognitive and motor disorders in humans [103, 104]. GR is expressed from E11.5 in NSCs of dorsal and ventral telencephalon in rodents. In particular, GR is detected in radial glial cells and intermediate progenitor cells [105]. GR activation negatively regulates fetal brain development. The synthetic GR agonist dexamethasone (DEX), represses the migration of cortical post-mitotic neurons in rodents [106]. In embryonic NSCs, GR exerts an antiproliferative role. In particular, DEX or corticosterone was able to suppress proliferation via reduction of cyclin D1 levels in mouse NSCs. This phenotype was abolished by the GR antagonist mifepristone [107]. Furthermore, DEX is able to induce the expression of senescence markers and negative regulators of cell cycle in mouse embryonic NSCs by GR activation [108]. Beyond the regulation of cell cycle, GR activation induces apoptosis in NSCs. Specifically, the use of DEX in rat neonatal hippocampal NSCs shows an apoptotic phenotype which is repressed by pretreatment of the cells with mifepristone [109]. It has also been implicated in the suppression of Gap Junction Intracellular Communication (GJIC), which is pivotal for NSCs’ survival [110]. In mouse NSCs, the GR-dependent apoptotic effect is mediated through the regulation of the Puma pro-apoptotic gene of Bcl-2 protein family [111]. Along these lines, in human NSCs, activation of GR leads to low proliferative activity and suppression of neurogenesis. Particularly, DEX treatment in human embryonic NSCs reduces proliferation and neuronal differentiation by inducing the expression of Dkk1, a direct suppressor of Wnt signaling. Furthermore, in human fetus derived hippocampal NSC cell line, HPC03A/07, GR negatively affects Shh and TGFb-SMAD2/3 signaling pathways, through which it exerts its antiproliferative and antineurogenic actions [112].
Although GR is a negative regulator of NSCs’ survival, there is evidence that some substances promote NSCs’ survival and neurogenesis via GR activation mechanisms. In particular, co-treatment with the antidepressant sertraline and DEX enhanced the proliferative and neurogenic capacities in human-derived multipotent NSC cell line. On the other hand, sertraline had no effect when NSCs were treated with GR antagonist [8]. Furthermore, ginsenoside Rg1, a steroidal saponin, induces neuronal differentiation via GR signal transduction in mouse ESCs, while the blockade of GR with an antagonist did not promote the initial effects of Rg1 [113]. These data indicate that GR function in embryonic NSCs is not totally understood and more mechanistic insights are required towards this direction.
In addition, GR is expressed in adult brain and has a significant role in its function [114, 115]. As a major effector in stress response, GR has a pivotal role in mood, cognition, and behavior [96, 116]. It is significant that chronic administration of corticosterone is characterized by a depression-like phenotype in rodents [117]. GR is also expressed in hippocampal NSCs and its activation suppresses proliferation and neurogenesis [118]. More specifically, adult rodents treated with corticosterone and DEX showed reduced proliferative activity in the DG of hippocampus. A very important observation was that after adrenalectomy or chemical removal of glucocorticoids, there was a significant increase in the number of newborn neurons in the hippocampal area [119–122]. Moreover, there was increased neurogenesis in the hippocampus after the knock-down of GR in mice, indicating that antineurogenic effects of glucocorticoids are mediated by GR activation [123]. Similar to embryonic NSCs, GR promotes cell death in adult NSCs via the induction of another member of pro-apoptotic Bcl-2 protein family, namely, Bax [124]. In addition, DEX induces the expression of p21Cip1, a critical cell-cycle regulator, in mouse hippocampal NSCs [125]. Another important observation is that GR activation has neurodegenerative effects on spinal cord. Treatment of adult mice with methylprednisolone, a GR agonist, reduced the number of NSCs in the spinal cord, both in vitro and in vivo [126].
Despite the antiproliferative role of GR in adult CNS and NSCs, a correlation between GR activation and oligodendrogenesis has also been reported. GR is expressed in oligodendrocyte precursor cells of many brain regions including hippocampus, somatosensory cortex, basolateral amygdala, corpus callosum and external capsule. The same expression levels were observed in mature oligodendrocytes in these regions [127]. In agreement with these observations, another study revealed a role of GR in oligodendrogenesis. It was observed that acute stress in adult rats increased the number of oligodendrocytes and decreased the number of neurons in DG of hippocampus. The same effect was observed with the administration of corticosterone. Consistently, when these NSCs where cultured and treated with corticosterone, there was an increase in the Olig2 positive cells [128]. Collectively, these experiments render GR one of the most critical regulators of embryonic and adult neurogenesis as well as CNS function.
Mineralocorticoid receptor (MR)
Mineralocorticoid receptor (NR3C2) is a steroid hormone nuclear receptor with Mineralocorticoid aldosterone as its specific natural ligand. The mechanism of MR actions shares similarities with GR actions. MR binds to the same DNA response elements with GR and is also activated by glucocorticoids. In fact, MR has higher affinity for glucocorticoids than GR [129]. Therefore, the conversion of cortisol or corticosterone into inactive steroid cortisone is essential for MR to exert its mineralocorticoid-dependent actions [130]. MR controls many physiological functions [131] and is expressed in many tissues and cell types including kidney, adipose tissue, endothelium, macrophages, and CNS [132].
In CNS, MR shows a restricted expression in the area of hippocampus and amygdala [133]. The concentration of mineralocorticoids (aldosterone) is very low in these regions and in brain generally, and thus, MR activation is instead mediated by glucocorticoids [134]. Activation of MR in these brain regions has a neuroprotective role, in contrast to the neurodegenerative effects of GR activation [135]. This neuroprotective activity directly affects mood, behavior, and cognitive functions [136]. In particular, inactivation of MR in the limbic regions of the mouse brain caused learning and behavioral disorders, probably due to hippocampal dysfunction [137]. In addition, treatment of ex vivo cultured hippocampal neurons with aldosterone was able to counteract the apoptotic effect of DEX-induced GR activation. On the other hand, the use of MR antagonist was able to mimic the apoptotic action of GR activation [138].
The expression of MR in hippocampus raises the possibility of a potential role for this NR in adult NSC homeostasis. Indeed, although MR is poorly expressed in NSCs at proliferative state, it is significantly increased in differentiation state [18]. Furthermore, administration of aldosterone in adrenalectomized mice increased the proliferation of adult NSCs in the dentate gyrus of hippocampus [139]. In addition, genetic deletion of MR, caused a significant impairment of adult neurogenesis and decreased size of granule layer in dentate gyrus of hippocampus, followed by neuropathological phenotypes [140]. Besides the involvement of MR in regulating neurogenesis, recent data indicate an additional correlation with astrogliogenesis. Activation of MR was able to induce an astrogliogenic phenotype in human embryonic NSCs. This is the first indication that associates MR activation with astrogliogenesis [112].
Non-steroid hormone nuclear receptors
Retinoic acid receptors (RARs)
Retinoic acid receptors are ligand-dependent nuclear receptors and their natural ligand is retinoic acid (RA), a metabolite of vitamin A. This NR family is comprised of three members, RAR-α (NR1B1), RAR-β (NR1B2), and RAR-γ (NR1B3). After activation, RARs form heterodimers with RXRs and bind to specific DNA sequences that are called Retinoic Acid Response Elements (RAREs). RA signaling exerts a fundamental role in development, cell growth, and differentiation in many organs and tissues, including CNS. Knock-out mice models for RARs exhibit growth retardation, malformations in many organs, sterility, and early post-natal lethality [141]. RA signaling is correlated with CNS development and function. RARs exert a critical role in anteroposterior and dorsoventral patterning of neural plate and neural tube. RA signaling is involved in the early development of hindbrain and spinal cord. Moreover, RARs have great implication in differentiation of many neural progenitor cell populations [142]. Recent studies also associate RA signaling with blood brain barrier (BBB) formation. In particular, activation of RAR-β induces the expression of specific markers for BBB in endothelial cells that are responsible for its formation [143]. Beyond CNS development, RARs have a significant role in CNS functions. Specifically, RA signaling regulates synaptic plasticity and consequently behavioral and learning abilities [144]. Furthermore, RAR impairments are correlated with anxiety, schizophrenia, locomotor disorders, and neurodegenerative disorders such as Parkinson's and Alzheimer's disease [13, 145, 146]. Along these lines, amyloid beta (Αβ) reduces RA synthesis and specifically represses RAR-α signaling. On the other hand, specific RAR-α agonist ameliorates cognitive deficits in Alzheimer's disease mouse models via the induction of anti-inflammatory signals, amyloid beta (Aβ) clearance, and tau protein dephosphorylation [147].
All members of RARs are expressed in the embryonic CNS and their activation significantly controls proliferation and differentiation of NSCs. RA signaling is necessary for the proper development of embryonic forebrain. In particular, RA deficiency in mice causes reduction in the expression of genes involved in ventral forebrain development, impaired response to Sonic hedgehog (Shh) signal, and attenuated FGF signaling. Consistently, it is observed defective telencephalic vesicle outgrowth and overall thinning of the forebrain neuroepithelium [148]. From mechanistic point of view, RAR activation induces the expression of JMJD3, a member of the jumonji C family of putative histone demethylases, and thus promotes the neuronal fate of forebrain NSCs [149]. In striatal area, ablation of RAR-β reduces proliferation and differentiation of embryonic progenitors via negative regulation of cyclin E2 and proneural marker Ascl1 (Mash1), resulting in abnormal development and psychomotor disorders [150]. In agreement, Rarb −/− mice exhibit low proliferative activity and premature neuronal differentiation in striatal NSCs due to repression of fgf3 and induction of Meis1 expression, an important regulator of neurogenesis [151]. Moreover, RA is essential for the maintenance of NSC population in the olfactory bulb of chick and mouse embryo. RA deficiency reduced the expression of neuronal precursor markers such as Ascl1 and Ngn1 [152]. Embryonic spinal cord is another CNS region that RARs exert a pivotal role in differentiation and especially in motor neuronal fate of NSCs [153, 154]. RAR-β activation by specific agonists promotes differentiation of NSCs into motor neuron progenitors via induction of islet-1. Although, RAR-α induces gliogenesis of NSCs, it is also essential for the maintenance of motor neuron progenitors and their maturation into functional motor neurons. Finally, RAR-γ activation triggers NSCs differentiation in astrocytes and oligodendrocytes. Interestingly, treatment with RA mimics the effects of RAR-β activation [155]. The inextricable correlation between RA signaling and neural development is revealed by its action on ESC neuronal differentiation. In particular, treatment of mouse ESCs with RA induces the proneural marker Pax6 and thus leads these cells to a neuronal fate [156]. Recent studies define Protein Arginine Methyl transferase 1 (PRMT1) and 8 (PRMT8) as critical rheostats of RA response. Both PRMT1 and PRMT8 are key regulators of ESC differentiation into neurons [157]. Moreover, RA and Shh agonist promote ESC differentiation into spinal cord motor neurons. RARs negatively regulate histone 3 methylation status and lead to further activation of Hox1–Hox5 genes domain in these cells [158]. Human ESCs are also affected by RA-induced neuronal differentiation. Treatment of human ESCs and iPSCs with RA induces a motor neuron phenotype via repression of GLI3, a negative regulator of Shh signaling pathway [159]. RAR activation is also implicated in the neuronal fate of many pluripotent embryonic carcinoma cell lines [160].
RARs are key players in adult neurogenesis. Specifically, both RAR-α and RAR-β are expressed in the adult forebrain [161]. Activation of RAR-β induces Shh signaling and synergistically promote proliferation and neuronal precursor identity of adult SVZ NSCs. Furthermore, RAR-α, via FGF signaling, promotes these neural precursor into migrating neuroblasts. Interestingly, RAR-α and Shh signaling decrease NSC proliferation and promote a gliogenic phenotype in vitro [162]. RA signaling also controls adult hippocampal neurogenesis. NSCs treated with RA exhibited a neurogenic phenotype, via induction of the negative cell-cycle regulator p21Cip1 and the neuronal precursor marker NeuroD [163]. Recent studies reveal a fundamental role of RAR-α in neurogenesis. More specifically, RAR-α agonist promoted the neuronal fate of adult hippocampal NSCs, whereas antagonist attenuated RA-induced neuronal differentiation [164]. RA signaling has also neuroprotective action during aging. In particular, RAR agonist Am80 induces proliferation and cell survival in the hippocampus of aged mice. Am80 promotes the expression of ADAM10, which facilitates Notch1 cleavage, resulting in increased levels of Hes5, which is important for the maintenance of adult NSC identity. ADAM10 also inhibits the formation of Αβ via generation of amyloid precursor protein, suggesting a neuroprotective function. Consequently, Am80 administration in these mice ameliorated the age-related cognitive deficits [165]. Furthermore, RAR activation exerts a determinant role in neuronal fate of adult pluripotent cells. RAR-β induces neuronal markers such as Nestin, beta tubulin III, and tau in mesenchymal stem cells (MSCs), which is a bone-marrow-derived cell population [166]. In conclusion, RARs regulate many developmental and functional processes in CNS and are promising tools in regenerative medicine.
Retinoic X receptors (RXRs)
Retinoic X receptors share a significant homology with RARs; however, they have critical differences in structure and ligand binding. Similar to RARs, RXR family includes three members, RXR-α (NR2B1), RXR-β (NR2B2), and RXR-γ (NR2B3). The natural ligands of RXRs are the isoform of all-trans RA, 9-cis RA, docosahexaenoic acid (DHA), oleic acid, and phytanic acid. RXRs exert their actions as heterodimer partners of many NR families. They mainly form heterodimers with RARs and bind to RAREs. These NRs are also able to form heterodimers with TRs, PPARs, LXRs, Nurr1, and Nurr77. They have pronounced implications in development, growth, function, and homeostasis of many organs [167–169]. RXRs are expressed in almost every tissue of developing and adult mammals. In particular, RXR-α and RXR-β exhibit ubiquitous expression, whereas RXR-γ expression is restricted in muscle tissues and brain [170].
One of the major target tissues of RXR action is CNS. RXRs are implicated in the proper development and function of specific neural populations. Mice lacking RXR-γ1 displayed reduced choline acetyltransferase activity in the striatum and altered response to dopamine antagonists [171]. Moreover, genetic ablation of RXR-γ in mice depleted hippocampal long-term depression, which affects synaptic plasticity and exerts an essential role in cognitive functions [144]. RXR-γ is also implicated in maturation of oligodendrocytes. Specifically, knock-down or pharmacological inhibition of this member of RXRs repressed differentiation of precursor cells into adult oligodendrocytes, whereas 9-cis RA increased remyelination in cerebral cells [172]. RXRs exert a neuroprotective role in neurodegenerative diseases. Particularly, bexarotene (Bxt), a specific RXR agonist, attenuates neurodegenerative phenotype in animal models of Amyotrophic Lateral Sclerosis, Parkinson’s and Alzheimer’s diseases [173–175]. Besides the neuroprotective role of RXRs, recent data correlate RXR activation with neurogenesis. Bxt increases proliferation of DG NSCs in a mouse model for Alzheimer’s disease. RXR activation also promotes the expression of neuronal markers in embryonic and adult NSCs, suggesting a positive effect on neurogenesis [176].
Peroxisome-proliferator-activated receptors (PPARs)
Peroxisome-proliferator-activated receptors belong to nuclear receptors superfamily and include three members, PPAR-α (NR1C1), PPAR-β/-δ (NR1C2), and PPAR-γ (NR1C3). PPARs are expressed in many tissues and organs including heart, kidney, pancreas, spleen and CNS. These NRs have been implicated in metabolic, developmental and other functional processes [177]. The mechanism that PPARs exert their actions is initiated via ligand binding. These NRs have various ligands such as fatty acids, arachidonic acid metabolites (eicosanoids) and hypolipidemic drugs [178]. The next step in the activation cascade is the formation of heterodimers with RXRs and binding to peroxisome-proliferator DNA response elements (PPREs) [179].
All three members of PPARs are expressed in the developing CNS. In rodents, PPAR-β and PPAR-γ expression in the CNS starts from E11.5 and E13.5, respectively. PPAR-α is detected at E13.5, but its expression is decreased until E15.5 [180]. The best studied member of PPARs is PPAR-γ, and its activation exerts a protective role in NSCs. PPAR-γ induces growth of mouse embryonic NSCs via EGF-ERK pathway activation. Conversely, genetic ablation of this NR inhibited cell growth in NSCs, a phenotype that was mimicked by PPAR-γ antagonist [181]. In addition, PPAR-γ counteracts the inhibitory effect of advanced glycated ends (AGEs) on NSC growth. It has been shown that AGEs are associated with the pathogenesis of diabetic cognitive deficits and neurodegeneration. Interestingly, under conditions of PPAR-γ knock-down, AGEs were not able to inhibit growth of NSCs, suggesting that PPAR-γ could be a potential pharmacological target for AGE-related neurodegenerative conditions [182, 183]. PPAR-γ is also correlated with the neuronal differentiation of embryonic NSCs. PPAR-γ activation, mediated by 15-deoxy-PGJ2, leads to an excessive neuronal phenotype in mouse embryonic NSCs through the regulation of MAP kinase, JNK [184]. In agreement, the PPAR-γ activation by pioglitazone rescued the antineurogenic effect of Pex11β (peroxisomal membrane elongation factor) knock-down on mouse ESCs [185]. The protective actions of PPAR-γ activation were also observed in human NSCs. In particular, rosiglitazone was able to inhibit the apoptotic phenotype in human NSCs treated with TNFα. This effect was abolished by PPAR-γ antagonist, indicating that this NR is required for NSC survival. More specifically, PPAR-γ activation induces the expression of important regulators of mitochondrial biogenesis and function, such as AMPK, SIRT1, and PGC-1α. Rosiglitazone is also able to abolish the apoptotic phenotype of Αβ in human NSCs by normalization of oxidative stress and mitochondrial function [186, 187]. PPAR-γ is further implicated in the neuronal fate of human ESCs. PPAR-γ inactivation in RA-treated human ESCs resulted in reduced expression levels for neural precursor markers, Sox1 and Pax6 [188]. Although PPAR-γ is the best characterized member of PPARs in NSC homeostasis, the other two members have also been implicated in CNS development. Particularly, PPAR-β and PPAR-α are expressed during early astroglial differentiation, while the expression of PPAR-α remains in the mature astrocytes and might be involved in the regulation of metabolism [189]. In addition, PPAR-β promotes maturation of oligodendrocytes. Agonists for this NR induce the differentiation of oligodendrocytes both in mixed glial cultures and in oligodendrocytes-enriched cultures, through the induction of myelin basic protein and proteolipid protein [190]. Interestingly, activation of PPAR-β also promotes the neuronal differentiation of mouse ESCs, indicating the major role of PPARs in CNS development [191]. Beyond NSCs, PPARs exert a protective role in mature neurons. Specifically, PPAR-γ activation in embryonic hippocampal neurons inhibits the oxidative stress-mediated apoptosis via the induction of anti-apoptotic protein Bcl-2 [192].
In adult CNS, all members of PPARs are expressed in many brain areas, both in neuronal and glial cell populations [193]. PPARs are also expressed in adult NSCs exerting an important role in the regulation of adult neurogenesis [194]. In particular, PPAR-γ activation by pioglitazone increased the size of SVZ in adult rats by inducing proliferation and neuronal differentiation. Similar effects were also reproduced in ex vivo cultured adult NSCs [195]. In addition, activation of PPAR-β/-δ in the DG of adult mice was sufficient to increase the proliferative activity and neuronal differentiation of NSCs. Consequently, it was also able to ameliorate the depressive behavior induced by chronic mild stress [196]. Furthermore, activation of PPAR-β/-δ counteracted the defects of MPTP-induced Parkinsonic phenotype and brain ischemic injury [197]. It was also observed that activated PPAR-γ promoted the survival of neuronal precursors after spinal cord injury [198]. To sum up, these data and the fact that functional impairment of PPARs is correlated with defects in CNS development and function [199–201], render PPARs as important pharmacological targets for the treatment of neurodegenerative diseases.
Liver X receptors (LXRs)
The liver X receptors (LXRs) are ligand-dependent transcription factors and include two members, LXR-α (NR1H3) and LXR-β (NR1H2) which have 78% similarity within their LBDs [202–204]. Their natural ligands are derivatives of cholesterol and are called oxysterols [205, 206]. Their mechanism of action involves the binding of the ligand and the formation of heterodimers with the RXR. The heterodimer binds to specific response elements within promoters of target genes and regulates their expression [207]. The best characterized roles of LXRs are the cholesterol homeostasis [208–211] and the immune response regulation [212–214], despite the fact that LXRs and their natural ligands are detected in many other tissues, including CNS [215–217]. The deletion of both LXRs in mice led to an increase of self-renewal properties of NSCs and consequently a reduction in neurogenesis, resulting in decreased DA neurons at birth. Oxysterol ligands, through the activation of LXRs, increased the number of DA neurons in mouse ESCs, whereas the opposite effect took place in the absence of LXRs. Similarly, oxysterol treatment of human ESCs during DA differentiation increased neurogenesis as well as the number of mature DA neurons and reduced proliferating progenitor cells [218]. In addition, two endogenous midbrain ligands of LXRs, 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid (CA), a cholic acid that activates LXRs, and 24,25-EC, seem to selectively regulate the development of distinct midbrain neuronal populations. CA increased survival and neurogenesis of Brn3a-positive red nucleus neurons, whereas 24,25-EC promoted DA neurogenesis [219]. Another interesting observation is that the administration of GW3965 (LXR agonist) in an adult mouse model for Alzheimer’s disease promoted the survival of NSCs in the subgranular zone (SGZ) of the DG [15]. Furthermore, deletion of LXRs in adult mice showed accumulation of lipids in the brain and loss of adult spinal cord motor neurons and ventral midbrain DA neurons [220]. Lack of Lxrα or Lxrβ genes increased amyloid deposition in the amyloid precursor protein (APP) transgenic mice model of Alzheimer’s disease [221]. These neurodegenerative effects may be the result of destabilization of cholesterol homeostasis in CNS due to the lack of LXRs. Moreover, use of synthetic agonists of LXRs in Alzheimer’s disease mouse model improved the pathophysiology of the disease [9]. These results make LXRs highly important regulators of neurogenesis and neuronal survival.
Orphan nuclear receptors
TLX
TLX is an orphan nuclear receptor, also known as NR2E1 with no identified ligand. This NR is found in both vertebrates and invertebrates and its homologue in Drosophila is tailless (tll) gene [222, 223]. Mouse TLX is detected in the developing brain from E8.5 until E13.5 and also in the developing retina from E11.5 until E17.5 [224–226]. TLX has high expression levels in adult brain and specifically in cortex, SGZ of the DG, and SVZ of lateral ventricle [227, 228]. Significantly, TLX has critical functions in regulating the proliferation and differentiation of NSCs and hence brain and retina development as well as behavioral phenotypes (Fig. 1; Table 1) [19].
In particular, TLX mutant mice have no obvious defects during early development, though adult mice display reduced thickness of their cortical layers, reduced cerebral hemispheres, defects in the eye, olfactory bulb and hippocampal DG [229, 230]. Roy and co-workers have shown that these defects are connected with altered proliferation versus differentiation decisions of neural progenitors during embryonic development, mainly via its key role in controlling timing of these processes in the embryonic cortex [231]. Accordingly, in TLX-null mice, the cyclin-dependent kinase inhibitor p21Cip1 was upregulated, whereas cyclin D1 was downregulated [232]. These regulatory actions may explain the TLX role in controlling the timing of differentiation. In addition, mouse TLX has a crucial role in retinogenesis. TLX-deficient mice exhibited retinal and optic nerve degeneration resulting in visual impairment [226, 233]. Specifically, TLX regulates the proliferation of retina progenitor cells via PTEN-cyclin D1 pathway and is significant for astrocyte development through its interaction with Shh and BMP signaling pathway which activate Pax2 expression [225, 226, 234].
Apart from TLX functions in embryonic development, TLX is also a pivotal regulator of NSCs in adult neurogenesis. This orphan receptor is essential for the proliferation and self-renewal of adult NSCs, for the repression of glial differentiation, and also for activation of neuronal differentiation [227, 235–238]. In particular, TLX silencing in adult mouse brain displayed reduction of NSC proliferation and defects in spatial learning [239]. In molecular terms, TLX controls NSC proliferation through p53 and via interaction with histone deacetylases (HDACs) which repress p21Cip1 and Pten expression [237, 240]. In addition, TLX activates Wnt/b-catenin signaling and binds to Oct3/4 upon hypoxia to stimulate the proliferation of adult NSCs [241, 242]. Regarding differentiation of NSCs in adult brain, TLX contributes to the generation of new neurons. Ιt promotes the expression of Ascl1 and DCX proneural genes, resulting in neuronal differentiation of hippocampal NSCs and suppresses BMP-SMAD pathway to regulate the post-natal astrogliogenesis [236, 243]. Interestingly, TLX through promotion of hippocampal neurogenesis enhances learning and memory [244].
Although TLX function in brain development is well characterized, until recently there was little evidence about its regulation. Recent studies have shown that Sox2 binds to TLX and enhances its expression and activity [245, 246]. In addition, IL-1β negatively regulates TLX, causing the decrease of proliferation of hippocampal neural precursor cells and the reduction of neurogenesis [247, 248]. It was also found that miRNAs regulate post-transcriptionally various neurogenic genes, including TLX. MiR-9, miR-137, miR-219, miR-378, let-7b and let-7d were shown to modulate TLX expression in embryonic and adult brain, thus regulating proliferation and differentiation of NSCs [249–253].
Significantly, mutations in mouse TLX caused aggressive behavior, lack of normal maternal instincts, fearfulness, and learning disabilities, suggesting TLX connection with human neurological disorders [229, 239, 254]. In fact, mutations in human TLX are associated with bipolar disorder, microcephaly, and schizophrenia [16, 255]. Furthermore, TLX plays an important role in CNS tumorigenesis. Increased TLX expression was observed in several types of human brain tumors, such as glioblastoma, neuroblastoma, astrocytoma, and ependymomas [256–261]. In particular, TLX was found to induce the genesis and expansion of glioblastoma cells and is correlated with poor prognosis of glioblastoma patients [256, 257, 262, 263]. Furthermore, TLX contributes to the progression of neuroblastoma by increasing the self-renewal and migration of neuroblastoma cells and it is also linked with poor survival [258, 264]. Collectively, these observations render TLX as an ideal candidate target gene for therapeutic purposes.
Chicken ovalbumin upstream promoter-transcription factors (COUP-TFs)
Chicken ovalbumin upstream promoter-transcription factors (COUP-TFs) are orphan nuclear receptors and members of the steroid/thyroid hormone receptor superfamily. The most studied members of this family are COUP-TFI (or NR2F1) and COUP-TFII (or NR2F2) [265]. COUP-TFs were first found to bind to the promoter of ovalbumin and activate its transcription. In general, they activate or repress the transcription of several genes either directly through binding to specific response elements or indirectly via competition or interaction with other receptors or co-repressors [266]. Notably, it was reported to repress the transcription of TRs, RARs, RXRs, and VDRs which are important for brain development, implying a regulatory role of COUP-TFs in this developmental process [267, 268]. In fact, COUP-TFs have been established to be crucial for differentiation of NSCs, neuronal migration, and eye morphogenesis. Moreover, COUP-TF homologues exhibit distinct and overlapping expression patterns in developing nervous system [269]. In adult, COUP-TFI is detected in cerebrum, striatum, olfactory bulb, hypothalamus, and spinal cord and its functions are well characterized. On the other hand, COUP-TFII is expressed in midbrain, hypothalamus, eye, cerebellum, and spinal cord, and although COUP-TFII knock-out mice die in E9.5, its role in development is poorly understood [270–273].
COUP-TFs are significantly involved in neurogenesis. Naka and co-workers showed that COUP-TFs induce differentiation of embryonic NSCs and promote gliogenesis via epigenetic modifications [274]. With gain- and loss-of-function experiments in mouse embryos, it was shown that COUP-TFI represses proliferation and induces neural differentiation of cortical ventricular zone’s progenitors and SVZ’s basal progenitors through repression of MEK/ERK, PI3K/Akt, and Wnt/beta-catenin signaling [275]. Recently, Zhou and co-workers found that both COUP-TFI and COUP-TFII decrease the proliferation of mouse SVZ progenitors through the regulation of Asc11 proneural gene and are responsible for migration of neuroblasts and survival of the SVZ cells [276]. In addition, COUP-TFII was found to regulate cerebellum development. In mouse post-natal cerebellum, it promotes proliferation of granule cell progenitors and protects them from apoptosis through binding and activation of the IGF-1 promoter [277]. Furthermore, COUP-TFI mutant mice display defects in oligodendrocyte differentiation as well as axon myelination. Additional observations indicate that the involvement of COUP-TFI in these processes could be explained by the positive regulation of SCIP/Oct-6/Tst-1 transcription factor [278]. COUP-TFI also contributes significantly to maturation of differentiated neural cells. COUP-TFI-deficient mice die 2 days after birth and display atypical neuronal axonal projection, guidance, and arborization [279]. It is also involved in the development of forebrain via its role in neuronal migration and neurite outgrowth [280]. Importantly, COUP-TFs are critical for mouse eye development. COUP-TF knock-out mice exhibit congenital ocular coloboma and microphthalmia. Apparently, these NRs are required for the proper differentiation of mouse neural progenitor cells in the developing eye through direct regulation of Pax6 and Otx2 genes [281, 282]. Concluding, COUP-TFs expression in specific areas of developing or adult brain contributes to the formation of these areas through regulation of proliferation and cell fate determination of NSCs.
Nuclear receptor-related 1 protein (Nurr1)
The orphan nuclear receptor Nurr1, also known as NR4A2, is a member of nuclear receptor superfamily. The other two members of NR4A family are Nur77 (NR4A1) and Nor1 (NR4A3). They are all encoded by immediate-early genes and their transcription can be induced by physiological cell-specific stimuli such as cAMP, inflammatory signals, hormones, calcium, growth factors, and neurotransmitters [283]. In particular, Nurr1 activates the transcription of specific genes through binding to their regulatory DNA elements. Nurr1 binds to NGFI-B response element (NBRE) as monomer or homodimer or to Nur response element as homodimer and promotes the TH (tyrosine hydroxylase) and DAT (dopamine transporter) expression [284–286]. It has also been found to bind as monomer, homodimer, or heterodimer to RXR [287]. In mice, Nurr1 expression appears at E10.5 in the ventral midbrain. In adult brain, Nurr1 expression is reduced, but is detected in high levels in substantia nigra, cerebrum, cerebellum, olfactory bulb, and hypothalamus [288–290]. Remarkably, Nurr1 has been reported to have a significant role in CNS development and precisely in the differentiation of neural progenitor cells, especially in DA system.
Nurr1 mutant mice die soon after birth and are not able to develop DA neurons in midbrain, while heterozygous mice display decreased levels of dopamine [291]. Significantly, Nurr1 overexpression in rat neural progenitor cells promotes their differentiation and generates DA neurons, whereas mouse and human neural progenitor cells require the co-overexpression of Nurr1 and Fox2 to differentiate into functional DA neurons [292, 293]. Further studies have shown that Nurr1 determines the DA phenotype of neurons by transcriptionally regulating genes responsible for synthesis, packaging and transport of dopamine such as TH, DAT, AADC (aromatic l-amino acid decarboxylase), and VMAT2 (vesicular monoamine transporter-2) [294, 295]. In particular, Nurr1 recruits Pitx3 (pituitary homeobox 3) to reduce its association with co-repressor SMRT, leading to activation of Nurr1 target genes that are pivotal for complete determination of DA neuronal fate [296]. Another study in mouse NSC line has showed that Nurr1 combined with FGF-8 and Shh are able to specify the DA neuronal fate of these cells [297]. Moreover, Nurr1 is essential for axonal growth, mitochondrial function, maintenance, and survival of DA neurons in adult midbrain [298–300]. In mice, Nurr1 deletion in late maturing or adult striatal DA neurons has led to loss of these neurons and neuronal degeneration which associate Nurr1 with neurodegenerative diseases [298]. Specifically, Nurr1 regulates mitochondrial survival through repression of p53 tumor suppressor and reduction of pro-apoptotic Bax gene [301]. Furthermore, this NR regulates the expression of Ret, a receptor of GDNF which protects midbrain DA neurons from apoptosis, cell death and neurotoxicity [302, 303]. Finally, Nurr1 might have a role in the suppression of astrocytic differentiation. Overexpression of Nurr1 in rat embryonic cortical NSCs induced neuronal differentiation and repression of astrogliogenesis. This effect of Nurr1 was accompanied with the upregulation of neurogenic neurotrophins BDNF, GDNF and NT-3 as well as down-regulation of astrogenic factors LIF (leukemia inhibitory factor) and CNTF [304].
In conclusion, Nurr1 nuclear receptor is associated with determination of cell fate and survival of DA neurons and its deletion or mutations lead to loss of DA neurons. For that reason, it has been linked to Parkinson’s disease (PD). A number of human genetic studies have identified mutations of Nurr1 gene that reduce its expression in PD patients [305, 306]. In addition, postmortem expression of Nurr1 in PD patient midbrains was found to be reduced, especially in Lewy body-containing neurons, indicating a protective role of Nurr1 [307, 308]. In addition, Nurr1 when overexpressed in mouse NSCs has a neuroprotective role against neurotoxic stimuli and can generate functional dopamine neurons [292, 309]. Taken together, these data and considering the recently discovered Nurr1-activating chemical compounds, suggest Nurr1 as a very promising therapeutic target for PD [11].
Steroidogenic factor 1 (SF-1)
Steroidogenic factor 1 (SF-1), also known as NR5A1 or adrenal 4-binding protein (Ad4BP), is an orphan NR that is homologous to Drosophila Fushi tarazu factor 1 (Ftz-F1). SF-1 binds to DNA of target genes as a monomer and regulates their transcription. In addition, the transcriptional activity of this NR is modulated by protein–protein interactions or post-translational modifications [310]. SF-1 is considered as an orphan NR, due to the fact that there is no ligand for SF-1 identified in vivo. However, it is found that hydroxysterols and phospholipids can function as ligands of SF-1 [311, 312]. It is expressed in adrenal cortex, hypothalamus and pituitary in developing brain [313, 314]. Interestingly, SF-1 is significant for development and function of endocrine system, as well as of hypothalamus–pituitary–gonad/adrenal axis [315]. Its functions are inferred from experiments with SF-1 knock-out mice which bear defects in adrenal gland, gonads, and pituitary gonadotropes [313, 316, 317]. They also exhibit disorganized ventromedial hypothalamic (VMH) neurons and increased anxiety-like behavior [313, 318]. Specifically, SF-1 regulates terminal differentiation of ventromedial hypothalamic nucleus (VMN) precursors, while SF-1 deletion leads to inhibition of differentiation, altered expression of early and late neuronal markers, and loss of neuronal projections [319]. Moreover, a recent study was focused on the role of SF-1 in the developing neocortex. SF-1 knock-out mouse embryos display significant impairments in neurogenesis. In particular, the deletion of SF-1 causes inhibition of cell-cycle exit of NSCs and increased numbers of apical progenitor cells. SF-1 modulates these processes via direct or indirect regulation of estrogen synthetase Cyp19a1 and ER [320]. To sum up, until now, SF-1 is found to be involved in the development of VMH and neocortex through regulation of proliferation and differentiation of NSCs and further investigation may unravel new functions of this orphan receptor.
NR5A2/LRH-1
NR5A2 is the other member of the NR5A subfamily, also known as LRH-1 (liver receptor homolog 1) with homology to Drosophila Ftz-F1. Similarly to SF-1, NR5A2 binds as monomer to DNA and activates or represses transcription of its target genes [321]. Although it is a constitutively active orphan NR, it was discovered that specific phospholipids can act as ligands of NR5A2 [10, 312]. In addition, NR5A2 has a predominant role in development of several tissues, steroidogenesis, embryogenesis, and neurogenesis. It is expressed during mouse embryogenesis and detected in several areas of adult mouse brain, such as cortex, hippocampus, and hypothalamus [322–324].
In mice, deletion of NR5A2 results in embryonic lethality by E6.5–E7.5, suggesting its significant role in early embryogenesis [325]. Furthermore, NR5A2 is required for the maintenance of pluripotency of ESCs in mouse epiblast through binding and activation of Oct4 [326]. Subsequently, Wagner and co-workers found that Wnt/beta-catenin signaling through the direct regulation of NR5A2 receptor is able to control the expression of pluripotency genes in ESCs [327]. Moreover, it was shown that it can replace Oct4 in the reprogramming of murine somatic cells into iPSCs (induced pluripotent stem cells) [328]. On the other hand, little was known about the role of NR5A2 in NSC homeostasis and CNS development. We have recently unraveled its fundamental role in embryonic neurogenesis. Accordingly, we demonstrated that NR5A2 expression is correlated with the neuronal lineage. It is detected in high levels in differentiated neuronal cells (bIII-Tubulin+ and NeuN+) and is poorly expressed in NSCs (Nestin+, Pax6+) at several stages of developing mouse spinal cord and telencephalon. Gain- and loss-of-function experiments in vitro and in vivo reveal that NR5A2 suppresses proliferation of embryonic NSCs, induces their neuronal differentiation, and inhibits astrogliogenesis. In particular, NR5A2 induces cell-cycle exit via binding and activation of cyclin-dependent kinase inhibitors p16Ink4a and p15Ink4b, promotes neuronal differentiation through direct regulation of Prox1, and blocks astrogliogenesis by negatively regulating Notch1 and JAK/STAT signaling pathways [329, 330]. In addition, upstream regulation of NR5A2 expression is mediated by proneural genes Neurog2 and Ascl1 (Mash1) that promote its expression, as well as by Notch1 and JAK/STAT signaling that repress NR5A2 to permit initiation of astrogliogenesis. In agreement with our observations, it was recently shown that NR5A2 together with RAR-γ or their agonists facilitate the capacity of neurogenic factors, Ascl1, Brn2, and Neurog2, to reprogram mouse fibroblasts into functional and mature neurons [331]. Collectively, these data manifest the critical function of NR5A2 in embryonic neural differentiation and raise the question whether it also plays any role in adult brain neurogenesis. The discovery of small molecules as potent ligands and pharmacological agonists of NR5A2 together with its action in NSC homeostasis suggest that NR5A2 could be a promising target gene for NSC-based treatments of CNS-related diseases.
Germ cell nuclear factor (GCNF)
Germ cell nuclear factor (GCNF), also called RTR (retinoid receptor-related testis-associated receptor), or NR6A1 is a member of the nuclear receptor family. GCNF binds to DNA as a homodimer or oligomer and represses transcription of target genes through recruitment of co-regulators, such as N-CoR (nuclear receptor co-repressor) [332–335]. GCNF is expressed in mouse embryos and in the developing nervous system, although in adults is mostly expressed during gametogenesis [336–340]. GCNF has a critical role in embryonic survival and development, since GCNF knock-out mice embryos die around E10.5 [337]. This orphan NR is also important for neuronal differentiation. It represses the expression of the pluripotent genes Oct4 and Nanog in embryonic and adult NSCs and in RA-induced murine ESCs, leading to the induction of neuronal fate [334, 341–344]. Mechanistically, GCNF recruits DNA methylation complexes to the promoter of Oct4 to silence its expression [345, 346]. Moreover, global analysis of gene expression in undifferentiated and differentiated human ESCs with RA revealed that this orphan receptor regulates a significant number of extra pluripotency genes [344]. These experiments may indicate a general role of GCNF in suppressing stemness and induction of differentiation in NSCs via the regulation of pluripotency genes. In addition, another study showed that the expression levels of GCNF are significant for the expression of MAP2 (microtubule-associated protein 2) and Syp (synaptic vesicle protein synaptophysin) proteins and, consequently, have a critical role in the differentiation and maturation of NSCs [347]. Furthermore, ablation of GCNF in mouse embryos causes abnormal forebrain and midbrain development [348]. Finally, a recent study highlighted the negative role of this NR in proliferation of ESCs by showing that GCNF regulates indirect the activation of cyclin D1 expression in ESCs through the inhibition of its suppressor, Mir302a [349]. It would be interesting to investigate whether GCNF regulates also cyclin D1 in NSCs, considering the key function of this cyclin in promoting NSC proliferation and inhibiting neuronal differentiation.
Concluding remarks and future prospects
NRs have fundamental roles in NSC homeostasis. They critically regulate NSCs proliferation and self-renewal properties as well as neural cell fate acquisition. In particular, many NRs control the maintenance of NSCs identity and their differentiation via induction or repression of neurogenesis or gliogenesis. Apart from NRs actions in differentiation of NSCs, they are also involved in cell maturation, leading to functional neural cell subtypes. Interestingly, some members of NR family display distinct and sometimes opposing roles in embryonic and adult NSCs, indicating their differential association with diverse signaling pathways in a context-dependent manner (Fig. 1; Table 1). Considering that NRs activity can be modulated by specific agonists/antagonists and that a large subset of FDA-approved drugs target NRs, elucidation of their tissue- and time-specific molecular mechanisms in NSCs will significantly advance novel therapeutic strategies for the treatment of neuro-developmental and adult CNS-related disorders. Therefore, it is of paramount importance to further investigate the utilization of these compounds in enhancing brain’s regenerative potential in many pathological conditions, including dementia, depression, CNS lesions, and other neurological diseases.
Acknowledgements
We would like to apologize for studies that were not cited due to space limitations. We thank Daphne Antoniou, Elpinickie Ninou, Valeria Kaltezioti, Artemis Michail, and Athanasios Stergiopoulos for helpful discussions and suggestions. The authors work was supported by the Fondation Santé Grant scheme, the Greek State Scholarships Foundation (IKY) and ARISTEIA-II (NeuroNetwk, No. 4786) Grant from General Secretariat of Research and Technology (GSRT), Athens, Greece.
Abbreviations
- AADC
Aromatic l-amino acid decarboxylase
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- AGE
Advanced glycated end
- ALS
Amyotrophic lateral sclerosis
- APP
Amyloid precursor protein
- BBB
Blood-brain barrier
- BDNF
Brain-derived neurotrophic factor
- BPA
Bisphenol A
- Bxt
Bexarotene
- CA
3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid
- CNS
Central nervous system
- CNTF
Ciliary neurotrophic factor
- COUP-TF
Chicken ovalbumin upstream promoter-transcription factor
- DA
Dopaminergic
- DAT
Dopamine transporter
- DBD
DNA binding domain
- DEX
Dexamethasone
- DG
Dentate gyrus
- DHA
Docosahexaenoic acid
- DVD
Developmental vitamin D
- E2
17β-Estradiol
- ER
Estrogen receptor
- ERE
Estrogen response element
- ESCs
Embryonic stem cells
- Ftz-F1
Fushi tarazu factor 1
- GC
Glucocorticoid
- GCNF
Germ cell nuclear factor
- GDNF
Glial-derived neurotrophic factor
- GJIC
Gap junction intracellular communication
- GR
Glucocorticoid receptor
- GRE
Glucocorticoid response element
- HDAC
Histone deacetylase
- HPA
Hypothalamus–pituitary–adrenals
- iPSCs
Induced pluripotent stem cells
- LBD
Ligand-binding domain
- LRH-1
Liver receptor homolog 1
- LXR
Liver X receptor
- MAP2
Microtubule-associated protein 2
- MR
Minerolocorticoid receptor
- MSCs
Mesenchymal stem cells
- NBRE
NGFI-B response element
- N-CoR
Nuclear receptor co-repressor
- NGF
Nerve growth factor
- NLS
Nuclear localization signal
- NR
Nuclear receptor
- NSCs
Neural stem/progenitor cells
- NT-3
Neurotrophin-3
- Nurr1
Nuclear receptor-related 1 protein
- PD
Parkinson’s disease
- Pex11β
Peroxisomal membrane elongation factor
- Pitx3
Pituitary homeobox 3
- PPAR
Peroxisome-proliferator-activated receptor
- PPRE
Peroxisome-proliferator DNA response element
- PRMT1
Protein arginine methyl transferase 1
- PRMT8
Protein arginine methyl transferase 8
- RA
Retinoic acid
- RAR
Retinoic acid receptor
- RARE
Retinoic acid response element
- RXR
Retinoic X receptor
- SF-1
Steroidogenic factor 1
- SGZ
Subgranular zone
- Shh
Sonic hedgehog
- SVZ
Subventricular zone
- Syp
Synaptic vesicle protein synaptophysin
- T3
3,5,39-Triiodo-l-thyronine
- T4
3,5,39,59-Tetraiodo-l-thyronine
- TH
Tyrosine hydroxylase
- TR
Thyroid hormone receptor
- TRE
Thyroid hormone response element
- VDR
Vitamin D receptor
- VDRE
VDR response element
- VMAT2
Vesicular monoamine transporter-2
- VMH
Ventromedial hypothalamic
- VMN
Ventromedial hypothalamic nucleus
Footnotes
Dimitrios Gkikas and Matina Tsampoula contributed equally to this work.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17(4):385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman IG. Nuclear receptors as drug targets for metabolic disease. Adv Drug Deliv Rev. 2010;62(13):1307–1315. doi: 10.1016/j.addr.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 7.Moore JT, Collins JL, Pearce KH. The nuclear receptor superfamily and drug discovery. ChemMedChem. 2006;1(5):504–523. doi: 10.1002/cmdc.200600006. [DOI] [PubMed] [Google Scholar]
- 8.Anacker C, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16(7):738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitz NF, et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci. 2010;30(20):6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474(7352):506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J, et al. Nurr1-based therapies for Parkinson’s disease. CNS Neurosci Ther. 2016;22(5):351–359. doi: 10.1111/cns.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116(Pt 4):585–586. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 13.Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10(5):409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- 14.Skerrett R, Malm T, Landreth G. Nuclear receptors in neurodegenerative diseases. Neurobiol Dis. 2014;72 Pt A:104–116. doi: 10.1016/j.nbd.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval-Hernandez AG, et al. Role of liver X receptor in AD pathophysiology. PLoS One. 2015;10(12):e0145467. doi: 10.1371/journal.pone.0145467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar RA, et al. Initial association of NR2E1 with bipolar disorder and identification of candidate mutations in bipolar disorder, schizophrenia, and aggression through resequencing. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):880–889. doi: 10.1002/ajmg.b.30696. [DOI] [PubMed] [Google Scholar]
- 17.Stergiopoulos A, Politis PK. The role of nuclear receptors in controlling the fine balance between proliferation and differentiation of neural stem cells. Arch Biochem Biophys. 2013;534(1–2):27–37. doi: 10.1016/j.abb.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Androutsellis-Theotokis A, et al. Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells. Horm Metab Res. 2013;45(2):159–168. doi: 10.1055/s-0032-1321789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Xiong JQ. The orphan nuclear receptor TLX/NR2E1 in neural stem cells and diseases. Neurosci Bull. 2016;32(1):108–114. doi: 10.1007/s12264-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maden M. Role and distribution of retinoic acid during CNS development. Int Rev Cytol. 2001;209:1–77. doi: 10.1016/S0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- 21.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3(3):249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- 22.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 23.Konig S, Moura Neto V. Thyroid hormone actions on neural cells. Cell Mol Neurobiol. 2002;22(5–6):517–544. doi: 10.1023/A:1021828218454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Germain DL, Galton VA, Hernandez A. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150(3):1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazar MA, Berrodin TJ, Harding HP. Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol. 1991;11(10):5005–5015. doi: 10.1128/MCB.11.10.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman BM, et al. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- 27.Hsu JH, et al. Retinoid-X receptor (RXR) differentially augments thyroid hormone response in cell lines as a function of the response element and endogenous RXR content. Endocrinology. 1995;136(2):421–430. doi: 10.1210/endo.136.2.7835272. [DOI] [PubMed] [Google Scholar]
- 28.Falcone M, et al. Antipeptide polyclonal antibodies specifically recognize each human thyroid hormone receptor isoform. Endocrinology. 1992;131(5):2419–2429. doi: 10.1210/endo.131.5.1425440. [DOI] [PubMed] [Google Scholar]
- 29.Hodin RA, Lazar MA, Chin WW. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Investig. 1990;85(1):101–105. doi: 10.1172/JCI114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 31.Wallis K, et al. The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24(10):1904–1916. doi: 10.1210/me.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz HL, et al. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. J Biol Chem. 1992;267(17):11794–11799. [PubMed] [Google Scholar]
- 33.Heuer H, Mason CA. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor alpha1. J Neurosci. 2003;23(33):10604–10612. doi: 10.1523/JNEUROSCI.23-33-10604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechan RM, et al. Immunocytochemical delineation of thyroid hormone receptor beta 2-like immunoreactivity in the rat central nervous system. Endocrinology. 1993;132(6):2461–2469. doi: 10.1210/endo.132.6.7684976. [DOI] [PubMed] [Google Scholar]
- 35.Desouza LA, et al. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29(3):414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev. 1993;14(1):94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- 37.Mohan V, et al. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol. 2012;237(2):477–488. doi: 10.1016/j.expneurol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Pathak A, et al. Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb Cortex. 2011;21(1):11–21. doi: 10.1093/cercor/bhq052. [DOI] [PubMed] [Google Scholar]
- 39.Cheng SY. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab. 2005;16(4):176–182. doi: 10.1016/j.tem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, et al. Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRalpha1. Stem Cells Dev. 2012;21(14):2667–2681. doi: 10.1089/scd.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C, et al. Thyroid hormone-Otx2 signaling is required for embryonic ventral midbrain neural stem cells differentiated into dopamine neurons. Stem Cells Dev. 2015;24(15):1751–1765. doi: 10.1089/scd.2014.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee LR, et al. Thyroid hormone receptor-alpha inhibits retinoic acid-responsive gene expression and modulates retinoic acid-stimulated neural differentiation in mouse embryonic stem cells. Mol Endocrinol. 1994;8(6):746–756. doi: 10.1210/mend.8.6.7935490. [DOI] [PubMed] [Google Scholar]
- 43.Fauquier T, et al. Purkinje cells and Bergmann glia are primary targets of the TRalpha1 thyroid hormone receptor during mouse cerebellum postnatal development. Development. 2014;141(1):166–175. doi: 10.1242/dev.103226. [DOI] [PubMed] [Google Scholar]
- 44.Johe KK, et al. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10(24):3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 45.Marziali LN, Garcia CI, Pasquini JM. Transferrin and thyroid hormone converge in the control of myelinogenesis. Exp Neurol. 2015;265:129–141. doi: 10.1016/j.expneurol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Marziali LN, et al. Combined effects of transferrin and thyroid hormone during oligodendrogenesis in vitro. Glia. 2016;64(11):1879–1891. doi: 10.1002/glia.23029. [DOI] [PubMed] [Google Scholar]
- 47.Baxi EG, et al. A selective thyroid hormone beta receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia. 2014;62(9):1513–1529. doi: 10.1002/glia.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrogini P, et al. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81(4):244–253. doi: 10.1159/000087648. [DOI] [PubMed] [Google Scholar]
- 49.Kapoor R, et al. Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J. 2010;24(12):4793–4805. doi: 10.1096/fj.10-161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapoor R, et al. Loss of thyroid hormone receptor beta is associated with increased progenitor proliferation and NeuroD positive cell number in the adult hippocampus. Neurosci Lett. 2011;487(2):199–203. doi: 10.1016/j.neulet.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemkine GF, et al. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 2005;19(7):863–865. doi: 10.1096/fj.04-2916fje. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Juarez A, et al. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10(5):531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Morte B, et al. Aberrant maturation of astrocytes in thyroid hormone receptor alpha 1 knockout mice reveals an interplay between thyroid hormone receptor isoforms. Endocrinology. 2004;145(3):1386–1391. doi: 10.1210/en.2003-1123. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez M, et al. Thyroid hormone participates in the regulation of neural stem cells and oligodendrocyte precursor cells in the central nervous system of adult rat. Eur J Neurosci. 2004;20(8):2059–2070. doi: 10.1111/j.1460-9568.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald PN, et al. Vitamin D receptor and nuclear receptor coactivators: crucial interactions in vitamin D-mediated transcription. Steroids. 2001;66(3–5):171–176. doi: 10.1016/S0039-128X(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 56.Whitfield GK, et al. A highly conserved region in the hormone-binding domain of the human vitamin D receptor contains residues vital for heterodimerization with retinoid X receptor and for transcriptional activation. Mol Endocrinol. 1995;9(9):1166–1179. doi: 10.1210/mend.9.9.7491109. [DOI] [PubMed] [Google Scholar]
- 57.Eyles DW, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Prufer K, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135–145. doi: 10.1016/S0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 59.Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat. 2011;41(1):1–12. doi: 10.1016/j.jchemneu.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Kesby JP, et al. The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol. 2011;347(1–2):121–127. doi: 10.1016/j.mce.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Burkert R, McGrath J, Eyles D. Vitamin D receptor expression in the embryonic rat brain. Neurosci Res Commun. 2003;33(1):63–71. doi: 10.1002/nrc.10081. [DOI] [Google Scholar]
- 63.Eyles D, et al. Vitamin D3 and brain development. Neuroscience. 2003;118(3):641–653. doi: 10.1016/S0306-4522(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 64.Ko P, et al. Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cycle during rat brain development. Brain Res Dev Brain Res. 2004;153(1):61–68. doi: 10.1016/j.devbrainres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Cui X, et al. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci. 2007;25(4):227–232. doi: 10.1016/j.ijdevneu.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Marini F, et al. Effect of 1alpha, 25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus. 2010;20(6):696–705. doi: 10.1002/hipo.20670. [DOI] [PubMed] [Google Scholar]
- 67.Cui X, et al. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci Lett. 2010;486(3):220–223. doi: 10.1016/j.neulet.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 68.Feron F, et al. Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65(2):141–148. doi: 10.1016/j.brainresbull.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Keilhoff G, Grecksch G, Becker A. Haloperidol normalized prenatal vitamin D depletion-induced reduction of hippocampal cell proliferation in adult rats. Neurosci Lett. 2010;476(2):94–98. doi: 10.1016/j.neulet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y, et al. Abnormal neurogenesis in the dentate gyrus of adult mice lacking 1,25-dihydroxy vitamin D3 (1,25-(OH)2 D3) Hippocampus. 2012;22(3):421–433. doi: 10.1002/hipo.20908. [DOI] [PubMed] [Google Scholar]
- 71.Shirazi HA, et al. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98(2):240–245. doi: 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 73.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rollerova E, Urbancikova M. Intracellular estrogen receptors, their characterization and function (Review) Endocr Regul. 2000;34(4):203–218. [PubMed] [Google Scholar]
- 75.Fan X, et al. ERbeta in CNS: new roles in development and function. Prog Brain Res. 2010;181:233–250. doi: 10.1016/S0079-6123(08)81013-8. [DOI] [PubMed] [Google Scholar]
- 76.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamilton KJ, et al. Estrogen hormone biology. Curr Topics Dev Biol. 2017;125:109–146. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas C, Gustafsson JA. Estrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol Metab. 2015;26(9):467–476. doi: 10.1016/j.tem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 79.Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21(3):512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 80.Okada M, et al. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008;29(3):163–170. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- 81.Okada M, et al. Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. Int J Mol Sci. 2010;11(10):4114–4123. doi: 10.3390/ijms11104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kishi Y, et al. Estrogen promotes differentiation and survival of dopaminergic neurons derived from human neural stem cells. J Neurosci Res. 2005;79(3):279–286. doi: 10.1002/jnr.20362. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, et al. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA. 2003;100(2):703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, et al. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc Natl Acad Sci USA. 2001;98(5):2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanapat P, et al. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isgor C, Watson SJ. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134(3):847–856. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Mazzucco CA, et al. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141(4):1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 88.Liu F, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 89.Jover T, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22(6):2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim K, et al. Suppressive effects of bisphenol A on the proliferation of neural progenitor cells. J Toxicol Environ Health A. 2007;70(15–16):1288–1295. doi: 10.1080/15287390701434216. [DOI] [PubMed] [Google Scholar]
- 91.Sakamoto H, et al. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144(10):4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 92.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286(5):3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hollenberg SM, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 95.Quax RA, et al. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9(11):670–686. doi: 10.1038/nrendo.2013.183. [DOI] [PubMed] [Google Scholar]
- 96.Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat Rev Neurosci. 2015;16(4):189–200. doi: 10.1038/nrn3855. [DOI] [PubMed] [Google Scholar]
- 97.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130(2):289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goodwin JE, et al. Endothelial glucocorticoid receptor suppresses atherogenesis—brief report. Arterioscler Thromb Vasc Biol. 2015;35(4):779–782. doi: 10.1161/ATVBAHA.114.304525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kino T (2000) Glucocorticoid Receptor. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. Available via: https://www.ncbi.nlm.nih.gov/books/NBK279171/. Accessed 13 June 2017
- 100.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 101.Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks—an introduction to systems biology. J Clin Endocrinol Metab. 2004;89(2):563–564. doi: 10.1210/jc.2003-032026. [DOI] [PubMed] [Google Scholar]
- 102.Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med. 2004;117(3):204–207. doi: 10.1016/j.amjmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Yeh TF, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350(13):1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 104.Modi N, et al. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res. 2001;50(5):581–585. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Tsiarli MA, Paula A. Monaghan, and D.B. Defranco, Differential subcellular localization of the glucocorticoid receptor in distinct neural stem and progenitor populations of the mouse telencephalon in vivo. Brain Res. 2013;1523:10–27. doi: 10.1016/j.brainres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fukumoto K, et al. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14(12):1119–1131. doi: 10.1038/mp.2009.60. [DOI] [PubMed] [Google Scholar]
- 107.Sundberg M, et al. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci. 2006;26(20):5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bose R, et al. Glucocorticoids induce long-lasting effects in neural stem cells resulting in senescence-related alterations. Cell Death Dis. 2010;1:e92. doi: 10.1038/cddis.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sze CI, et al. The role of glucocorticoid receptors in dexamethasone-induced apoptosis of neuroprogenitor cells in the hippocampus of rat pups. Mediat Inflamm. 2013;2013:628094. doi: 10.1155/2013/628094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Samarasinghe RA, et al. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci USA. 2011;108(40):16657–16662. doi: 10.1073/pnas.1102821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noguchi KK, et al. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15(10):1582–1592. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anacker C, et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology. 2013;38(5):872–883. doi: 10.1038/npp.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J, et al. Ginsenoside Rg1 facilitates neural differentiation of mouse embryonic stem cells via GR-dependent signaling pathway. Neurochem Int. 2013;62(1):92–102. doi: 10.1016/j.neuint.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 114.van Eekelen JA, Bohn MC, de Kloet ER. Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Brain Res Dev Brain Res. 1991;61(1):33–43. doi: 10.1016/0165-3806(91)90111-U. [DOI] [PubMed] [Google Scholar]
- 115.Fuxe K, et al. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117(5):1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- 116.Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583(1):115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 118.Reimer R, et al. Nestin modulates glucocorticoid receptor function by cytoplasmic anchoring. PLoS One. 2009;4(6):e6084. doi: 10.1371/journal.pone.0006084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 120.Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137(1):83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brummelte S, Galea LA. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168(3):680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 122.Kim JB, et al. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027(1–2):1–10. doi: 10.1016/j.brainres.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 123.Fitzsimons CP, et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry. 2013;18(9):993–1005. doi: 10.1038/mp.2012.123. [DOI] [PubMed] [Google Scholar]
- 124.Almeida OF, et al. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14(5):779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- 125.Wagner K, et al. Prolactin induces MAPK signaling in neural progenitors without alleviating glucocorticoid-induced inhibition of in vitro neurogenesis. Cell Physiol Biochem. 2009;24(5–6):397–406. doi: 10.1159/000257432. [DOI] [PubMed] [Google Scholar]
- 126.Schroter A, et al. High-dose corticosteroids after spinal cord injury reduce neural progenitor cell proliferation. Neuroscience. 2009;161(3):753–763. doi: 10.1016/j.neuroscience.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 127.Matsusue Y, et al. Distribution of corticosteroid receptors in mature oligodendrocytes and oligodendrocyte progenitors of the adult mouse brain. J Histochem Cytochem. 2014;62(3):211–226. doi: 10.1369/0022155413517700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chetty S, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19(12):1275–1283. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Kloet ER, et al. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000;57(4):1329–1336. doi: 10.1046/j.1523-1755.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 130.Farman N, Bocchi B. Mineralocorticoid selectivity: molecular and cellular aspects. Kidney Int. 2000;57(4):1364–1369. doi: 10.1046/j.1523-1755.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- 131.Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol. 2005;19(9):2211–2221. doi: 10.1210/me.2005-0089. [DOI] [PubMed] [Google Scholar]
- 132.Martinerie L, et al. The mineralocorticoid signaling pathway throughout development: expression, regulation and pathophysiological implications. Biochimie. 2013;95(2):148–157. doi: 10.1016/j.biochi.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 133.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 134.Marzolla V, et al. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol. 2012;350(2):281–288. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 135.ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110. doi: 10.1016/j.psyneuen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 136.Le Menuet D, Lombes M. The neuronal mineralocorticoid receptor: from cell survival to neurogenesis. Steroids. 2014;91:11–19. doi: 10.1016/j.steroids.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 137.Berger S, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA. 2006;103(1):195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crochemore C, et al. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10(8):790–798. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 139.Fischer AK, et al. The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat. Brain Res. 2002;947(2):290–293. doi: 10.1016/S0006-8993(02)03042-1. [DOI] [PubMed] [Google Scholar]
- 140.Gass P, et al. Genetic Disruption of Mineralocorticoid Receptor Leads to Impaired Neurogenesis and Granule Cell Degeneration in the Hippocampus of Adult Mice. EMBO Rep. 2000;1(5):447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.di Masi A, et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 142.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8(10):755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 143.Mizee MR, et al. Retinoic acid induces blood–brain barrier development. J Neurosci. 2013;33(4):1660–1671. doi: 10.1523/JNEUROSCI.1338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiang MY, et al. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 1998;21(6):1353–1361. doi: 10.1016/S0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 145.Jokic N, et al. Retinoid receptors in chronic degeneration of the spinal cord: observations in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2007;103(5):1821–1833. doi: 10.1111/j.1471-4159.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- 146.van Neerven S, Kampmann E, Mey J. RAR/RXR and PPAR/RXR signaling in neurological and psychiatric diseases. Prog Neurobiol. 2008;85(4):433–451. doi: 10.1016/j.pneurobio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Goncalves MB, et al. Amyloid beta inhibits retinoic acid synthesis exacerbating Alzheimer disease pathology which can be attenuated by an retinoic acid receptor alpha agonist. Eur J Neurosci. 2013;37(7):1182–1192. doi: 10.1111/ejn.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ribes V, et al. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133(2):351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- 149.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 150.Liao WL, et al. Modular patterning of structure and function of the striatum by retinoid receptor signaling. Proc Natl Acad Sci USA. 2008;105(18):6765–6770. doi: 10.1073/pnas.0802109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rataj-Baniowska M, et al. Retinoic acid receptor beta controls development of striatonigral projection neurons through FGF-dependent and Meis1-dependent mechanisms. J Neurosci. 2015;35(43):14467–14475. doi: 10.1523/JNEUROSCI.1278-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paschaki M, et al. Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev. 2013;8:13. doi: 10.1186/1749-8104-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94(4):503–514. doi: 10.1016/S0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 154.Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40(1):97–111. doi: 10.1016/S0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- 155.Goncalves MB, et al. Timing of the retinoid-signalling pathway determines the expression of neuronal markers in neural progenitor cells. Dev Biol. 2005;278(1):60–70. doi: 10.1016/j.ydbio.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 156.Bibel M, et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7(9):1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- 157.Simandi Z, et al. PRMT1 and PRMT8 regulate retinoic acid-dependent neuronal differentiation with implications to neuropathology. Stem Cells. 2015;33(3):726–741. doi: 10.1002/stem.1894. [DOI] [PubMed] [Google Scholar]
- 158.Mazzoni EO, et al. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat Neurosci. 2013;16(9):1191–1198. doi: 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calder EL, et al. Retinoic acid-mediated regulation of GLI3 enables efficient motoneuron derivation from human ESCs in the absence of extrinsic SHH activation. J Neurosci. 2015;35(33):11462–11481. doi: 10.1523/JNEUROSCI.3046-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mey J. New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. J Neurobiol. 2006;66(7):757–779. doi: 10.1002/neu.20238. [DOI] [PubMed] [Google Scholar]
- 161.Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25(33):7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goncalves MB, et al. Sequential RARbeta and alpha signalling in vivo can induce adult forebrain neural progenitor cells to differentiate into neurons through Shh and FGF signalling pathways. Dev Biol. 2009;326(2):305–313. doi: 10.1016/j.ydbio.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi J, Palmer TD, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol. 1999;38(1):65–81. doi: 10.1002/(SICI)1097-4695(199901)38:1<65::AID-NEU5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 164.Boku S, et al. Neonatal maternal separation alters the capacity of adult neural precursor cells to differentiate into neurons via methylation of retinoic acid receptor gene promoter. Biol Psychiatry. 2015;77(4):335–344. doi: 10.1016/j.biopsych.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kitaoka K, et al. The retinoic acid receptor agonist Am 80 increases hippocampal ADAM10 in aged SAMP8 mice. Neuropharmacology. 2013;72:58–65. doi: 10.1016/j.neuropharm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 166.Gong M, et al. Retinoic acid receptor beta mediates all-trans retinoic acid facilitation of mesenchymal stem cells neuronal differentiation. Int J Biochem Cell Biol. 2013;45(4):866–875. doi: 10.1016/j.biocel.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 167.Mangelsdorf DJ, et al. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 168.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Szanto A, et al. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl 2):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 170.Dolle P, et al. Developmental expression of murine retinoid X receptor (RXR) genes. Mech Dev. 1994;45(2):91–104. doi: 10.1016/0925-4773(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 171.Saga Y, et al. Impaired extrapyramidal function caused by the targeted disruption of retinoid X receptor RXRgamma1 isoform. Genes Cells. 1999;4(4):219–228. doi: 10.1046/j.1365-2443.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 172.Huang JK, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14(1):45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Riancho J, et al. Neuroprotective effect of bexarotene in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Front Cell Neurosci. 2015;9:250. doi: 10.3389/fncel.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bachmeier C, et al. Stimulation of the retinoid X receptor facilitates beta-amyloid clearance across the blood–brain barrier. J Mol Neurosci. 2013;49(2):270–276. doi: 10.1007/s12031-012-9866-6. [DOI] [PubMed] [Google Scholar]
- 175.McFarland K, et al. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem Neurosci. 2013;4(11):1430–1438. doi: 10.1021/cn400100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mounier A, et al. Bexarotene-activated retinoid X receptors regulate neuronal differentiation and dendritic complexity. J Neurosci. 2015;35(34):11862–11876. doi: 10.1523/JNEUROSCI.1001-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 178.Krey G, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11(6):779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 179.Kliewer SA, et al. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139(6):2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 181.Wada K, et al. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281(18):12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- 182.Wang SH, et al. PPARgamma-mediated advanced glycation end products regulation of neural stem cells. Mol Cell Endocrinol. 2009;307(1–2):176–184. doi: 10.1016/j.mce.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 183.Wang SH, et al. PPARgamma-mediated advanced glycation end products regulate neural stem cell proliferation but not neural differentiation through the BDNF-CREB pathway. Toxicol Lett. 2011;206(3):339–346. doi: 10.1016/j.toxlet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 184.Park KS, et al. Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp Cell Res. 2004;297(2):424–433. doi: 10.1016/j.yexcr.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 185.Esmaeili M, et al. Pioglitazone significantly prevented decreased rate of neural differentiation of mouse embryonic stem cells which was reduced by Pex11beta knock-down. Neuroscience. 2016;312:35–47. doi: 10.1016/j.neuroscience.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 186.Chiang MC, et al. PPARgamma regulates the mitochondrial dysfunction in human neural stem cells with tumor necrosis factor alpha. Neuroscience. 2013;229:118–129. doi: 10.1016/j.neuroscience.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 187.Chiang MC, et al. Rosiglitazone activation of PPARgamma-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction and oxidative stress. Neurobiol Aging. 2016;40:181–190. doi: 10.1016/j.neurobiolaging.2016.01.132. [DOI] [PubMed] [Google Scholar]
- 188.Taheri M, et al. A ground state of PPARgamma activity and expression is required for appropriate neural differentiation of hESCs. Pharmacol Rep. 2015;67(6):1103–1114. doi: 10.1016/j.pharep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 189.Cristiano L, et al. Peroxisome proliferator-activated receptors (PPARs) and related transcription factors in differentiating astrocyte cultures. Neuroscience. 2005;131(3):577–587. doi: 10.1016/j.neuroscience.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 190.Saluja I, Granneman JG, Skoff RP. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia. 2001;33(3):191–204. doi: 10.1002/1098-1136(200103)33:3<191::AID-GLIA1018>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 191.Mei YQ, et al. A flavonoid compound promotes neuronal differentiation of embryonic stem cells via PPAR-beta modulating mitochondrial energy metabolism. PLoS One. 2016;11(6):e0157747. doi: 10.1371/journal.pone.0157747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fuenzalida K, et al. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282(51):37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- 193.Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 194.Cimini A, et al. PPARs expression in adult mouse neural stem cells: modulation of PPARs during astroglial differentiaton of NSC. PPAR Res. 2007;2007:48242. doi: 10.1155/2007/48242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 195.Morales-Garcia JA, et al. Peroxisome proliferator-activated receptor gamma ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia. 2011;59(2):293–307. doi: 10.1002/glia.21101. [DOI] [PubMed] [Google Scholar]
- 196.Ji MJ, et al. Hippocampal PPARdelta overexpression or activation represses stress-induced depressive behaviors and enhances neurogenesis. Int J Neuropsychopharmacol. 2015;19(1):083. doi: 10.1093/ijnp/pyv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iwashita A, et al. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. J Pharmacol Exp Ther. 2007;320(3):1087–1096. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- 198.Meng QQ, et al. Rosiglitazone enhances the proliferation of neural progenitor cells and inhibits inflammation response after spinal cord injury. Neurosci Lett. 2011;503(3):191–195. doi: 10.1016/j.neulet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 199.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 200.Peters JM, et al. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta) Mol Cell Biol. 2000;20(14):5119–5128. doi: 10.1128/MCB.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zandi PP, et al. Association study of Wnt signaling pathway genes in bipolar disorder. Arch Gen Psychiatry. 2008;65(7):785–793. doi: 10.1001/archpsyc.65.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chawla A, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 203.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 204.Alberti S, Steffensen KR, Gustafsson JA. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene. 2000;243(1–2):93–103. doi: 10.1016/S0378-1119(99)00555-7. [DOI] [PubMed] [Google Scholar]
- 205.Janowski BA, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 206.Janowski BA, et al. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci USA. 1999;96(1):266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Edwards PA, Kennedy MA, Mak PA. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vasc Pharmacol. 2002;38(4):249–256. doi: 10.1016/S1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 208.Fu X, et al. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276(42):38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 209.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204(3):233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 210.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80(1):361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 211.Venkateswaran A, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97(22):12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Investig. 2006;116(3):607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hannedouche S, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475(7357):524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Valledor AF, et al. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101(51):17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Viennois E, et al. Targeting liver X receptors in human health: deadlock or promising trail? Expert Opin Ther Targets. 2011;15(2):219–232. doi: 10.1517/14728222.2011.547853. [DOI] [PubMed] [Google Scholar]
- 216.Bjorkhem I. Are side-chain oxidized oxysterols regulators also in vivo? J Lipid Res. 2009;50(Suppl):S213–S218. doi: 10.1194/jlr.R800025-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang L, et al. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci USA. 2002;99(21):13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sacchetti P, et al. Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell. 2009;5(4):409–419. doi: 10.1016/j.stem.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 219.Theofilopoulos S, et al. Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nat Chem Biol. 2013;9(2):126–133. doi: 10.1038/nchembio.1156. [DOI] [PubMed] [Google Scholar]
- 220.Andersson S, et al. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102(10):3857–3862. doi: 10.1073/pnas.0500634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zelcer N, et al. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver X receptors. Proc Natl Acad Sci USA. 2007;104(25):10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yu RT, et al. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 1994;370(6488):375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]
- 223.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Monaghan AP, et al. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121(3):839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 225.Miyawaki T, et al. Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci. 2004;24(37):8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang CL, et al. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 2006;20(10):1308–1320. doi: 10.1101/gad.1413606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shi Y, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427(6969):78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 228.Li S, et al. Characterization of TLX expression in neural stem cells and progenitor cells in adult brains. PLoS One. 2012;7(8):e43324. doi: 10.1371/journal.pone.0043324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Monaghan AP, et al. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390(6659):515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- 230.Land PW, Monaghan AP. Abnormal development of zinc-containing cortical circuits in the absence of the transcription factor Tailless. Brain Res Dev Brain Res. 2005;158(1–2):97–101. doi: 10.1016/j.devbrainres.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Roy K, et al. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24(38):8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li W, et al. Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol. 2008;22(1):56–64. doi: 10.1210/me.2007-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yu RT, et al. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci USA. 2000;97(6):2621–2625. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sehgal R, et al. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332(2):429–443. doi: 10.1016/j.ydbio.2009.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu HK, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22(18):2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Elmi M, et al. TLX activates MASH1 for induction of neuronal lineage commitment of adult hippocampal neuroprogenitors. Mol Cell Neurosci. 2010;45(2):121–131. doi: 10.1016/j.mcn.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 237.Niu W, et al. Activation of postnatal neural stem cells requires nuclear receptor TLX. J Neurosci. 2011;31(39):13816–13828. doi: 10.1523/JNEUROSCI.1038-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Obernier K, et al. Expression of Tlx in both stem cells and transit amplifying progenitors regulates stem cell activation and differentiation in the neonatal lateral subependymal zone. Stem Cells. 2011;29(9):1415–1426. doi: 10.1002/stem.682. [DOI] [PubMed] [Google Scholar]
- 239.Zhang CL, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 240.Sun G, et al. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci USA. 2007;104(39):15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Qu Q, et al. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12(1):31–40. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chavali PL, et al. Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J Biol Chem. 2011;286(11):9393–9404. doi: 10.1074/jbc.M110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Qin S, et al. Orphan nuclear receptor TLX regulates astrogenesis by modulating BMP signaling. Front Neurosci. 2014;8:74. doi: 10.3389/fnins.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Murai K, et al. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc Natl Acad Sci USA. 2014;111(25):9115–9120. doi: 10.1073/pnas.1406779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shimozaki K, et al. SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem. 2012;287(8):5969–5978. doi: 10.1074/jbc.M111.290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Islam MM, et al. Enhancer analysis unveils genetic interactions between TLX and SOX2 in neural stem cells and in vivo reprogramming. Stem Cell Rep. 2015;5(5):805–815. doi: 10.1016/j.stemcr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ryan SM, et al. Negative regulation of TLX by IL-1beta correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain Behav Immun. 2013;33:7–13. doi: 10.1016/j.bbi.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 248.Green HF, Nolan YM. Unlocking mechanisms in interleukin-1beta-induced changes in hippocampal neurogenesis—a role for GSK-3beta and TLX. Transl Psychiatry. 2012;2:e194. doi: 10.1038/tp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao C, et al. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sun G, et al. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huang Y, Liu X, Wang Y. MicroRNA-378 regulates neural stem cell proliferation and differentiation in vitro by modulating Tailless expression. Biochem Biophys Res Commun. 2015;466(2):214–220. doi: 10.1016/j.bbrc.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 252.Ni N, et al. Effects of let-7b and TLX on the proliferation and differentiation of retinal progenitor cells in vitro. Sci Rep. 2014;4:6671. doi: 10.1038/srep06671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhao C, et al. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Juarez P, et al. Serotonin(2)A/C receptors mediate the aggressive phenotype of TLX gene knockout mice. Behav Brain Res. 2013;256:354–361. doi: 10.1016/j.bbr.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 255.Kumar RA, et al. Absence of mutations in NR2E1 and SNX3 in five patients with MMEP (microcephaly, microphthalmia, ectrodactyly, and prognathism) and related phenotypes. BMC Med Genet. 2007;8:48. doi: 10.1186/1471-2350-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Park HJ, et al. The neural stem cell fate determinant TLX promotes tumorigenesis and genesis of cells resembling glioma stem cells. Mol Cells. 2010;30(5):403–408. doi: 10.1007/s10059-010-0122-z. [DOI] [PubMed] [Google Scholar]
- 257.Liu HK, et al. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24(7):683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chavali PL, et al. TLX activates MMP-2, promotes self-renewal of tumor spheres in neuroblastoma and correlates with poor patient survival. Cell Death Dis. 2014;5:e1502. doi: 10.1038/cddis.2014.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Taylor MD, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 260.Sharma MK, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67(3):890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 261.Sobhan PK, Funa K. TLX—its emerging role for neurogenesis in health and disease. Mol Neurobiol. 2017;54(1):272–280. doi: 10.1007/s12035-015-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zou Y, et al. The nuclear receptor TLX is required for gliomagenesis within the adult neurogenic niche. Mol Cell Biol. 2012;32(23):4811–4820. doi: 10.1128/MCB.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Johansson E, et al. Nuclear receptor TLX inhibits TGF-beta signaling in glioblastoma. Exp Cell Res. 2016;343(2):118–125. doi: 10.1016/j.yexcr.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 264.Sobhan PK, et al. ASK1 regulates the survival of neuroblastoma cells by interacting with TLX and stabilizing HIF-1alpha. Cell Signal. 2017;30:104–117. doi: 10.1016/j.cellsig.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 265.Wang LH, et al. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- 266.Park JI, Tsai SY, Tsai MJ. Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J Med. 2003;52(3):174–181. doi: 10.2302/kjm.52.174. [DOI] [PubMed] [Google Scholar]
- 267.Cooney AJ, et al. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993;268(6):4152–4160. [PubMed] [Google Scholar]
- 268.Tran P, et al. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992;12(10):4666–4676. doi: 10.1128/MCB.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Qiu Y, et al. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci USA. 1994;91(10):4451–4455. doi: 10.1073/pnas.91.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhou C, et al. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24(4):847–859. doi: 10.1016/S0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 271.Tomassy GS, et al. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci USA. 2010;107(8):3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lin FJ, et al. Coup d’Etat: an orphan takes control. Endocr Rev. 2011;32(3):404–421. doi: 10.1210/er.2010-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pereira FA, et al. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13(8):1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Naka H, et al. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11(9):1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- 275.Faedo A, et al. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18(9):2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhou X, et al. Transcription factors COUP-TFI and COUP-TFII are required for the production of granule cells in the mouse olfactory bulb. Development. 2015;142(9):1593–1605. doi: 10.1242/dev.115279. [DOI] [PubMed] [Google Scholar]
- 277.Kim BJ, et al. Chicken Ovalbumin Upstream Promoter-Transcription Factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol. 2009;326(2):378–391. doi: 10.1016/j.ydbio.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yamaguchi H, et al. The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev Biol. 2004;266(2):238–251. doi: 10.1016/j.ydbio.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 279.Qiu Y, et al. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11(15):1925–1937. doi: 10.1101/gad.11.15.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Armentano M, et al. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development. 2006;133(21):4151–4162. doi: 10.1242/dev.02600. [DOI] [PubMed] [Google Scholar]
- 281.Inoue M, et al. COUP-TFI and -TFII nuclear receptors are expressed in amacrine cells and play roles in regulating the differentiation of retinal progenitor cells. Exp Eye Res. 2010;90(1):49–56. doi: 10.1016/j.exer.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 282.Tang K, et al. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development. 2010;137(5):725–734. doi: 10.1242/dev.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sakurada K, et al. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126(18):4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 285.Sacchetti P, et al. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76(5):1565–1572. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 286.Ichinose H, et al. Molecular cloning of the human Nurr1 gene: characterization of the human gene and cDNAs. Gene. 1999;230(2):233–239. doi: 10.1016/S0378-1119(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 287.Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9(7):769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- 288.Zetterstrom RH, et al. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10(12):1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- 289.Le W, et al. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol. 1999;159(2):451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- 290.Alavian KN, et al. The lifelong maintenance of mesencephalic dopaminergic neurons by Nurr1 and engrailed. J Biomed Sci. 2014;21:27. doi: 10.1186/1423-0127-21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zetterstrom RH, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 292.Shim JW, et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007;25(5):1252–1262. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 293.Lee HS, et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28(3):501–512. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- 294.Flames N, Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annu Rev Neurosci. 2011;34:153–184. doi: 10.1146/annurev-neuro-061010-113824. [DOI] [PubMed] [Google Scholar]
- 295.Smits SM, et al. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18(7):1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 296.Jacobs FM, et al. Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development. 2009;136(4):531–540. doi: 10.1242/dev.029769. [DOI] [PubMed] [Google Scholar]
- 297.Kim TE, et al. Sonic hedgehog and FGF8 collaborate to induce dopaminergic phenotypes in the Nurr1-overexpressing neural stem cell. Biochem Biophys Res Commun. 2003;305(4):1040–1048. doi: 10.1016/S0006-291X(03)00879-9. [DOI] [PubMed] [Google Scholar]
- 298.Kadkhodaei B, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29(50):15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Heng X, et al. Nurr1 regulates Top IIbeta and functions in axon genesis of mesencephalic dopaminergic neurons. Mol Neurodegener. 2012;7:4. doi: 10.1186/1750-1326-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kadkhodaei B, et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci USA. 2013;110(6):2360–2365. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhang T, et al. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7(8):1408–1415. doi: 10.1158/1541-7786.MCR-08-0533. [DOI] [PubMed] [Google Scholar]
- 302.Wallen AA, et al. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci. 2001;18(6):649–663. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- 303.Tomac A, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373(6512):335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 304.Bae EJ, et al. Orphan nuclear receptor Nurr1 induces neuron differentiation from embryonic cortical precursor cells via an extrinsic paracrine mechanism. FEBS Lett. 2009;583(9):1505–1510. doi: 10.1016/j.febslet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 305.Le WD, et al. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33(1):85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 306.Wu Y, et al. Association of the polymorphisms in NURR1 gene with Parkinson’s disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25(6):693–696. [PubMed] [Google Scholar]
- 307.Chu Y, et al. Nurr1 in Parkinson’s disease and related disorders. J Comp Neurol. 2006;494(3):495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol. 2005;77(1–2):128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 309.Lee MA, et al. Overexpression of midbrain-specific transcription factor Nurr1 modifies susceptibility of mouse neural stem cells to neurotoxins. Neurosci Lett. 2002;333(1):74–78. doi: 10.1016/S0304-3940(02)00981-3. [DOI] [PubMed] [Google Scholar]
- 310.Hoivik EA, et al. Molecular aspects of steroidogenic factor 1 (SF-1) Mol Cell Endocrinol. 2010;315(1–2):27–39. doi: 10.1016/j.mce.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 311.Lala DS, et al. Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci USA. 1997;94(10):4895–4900. doi: 10.1073/pnas.94.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Krylova IN, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120(3):343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 313.Shinoda K, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204(1):22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 314.Ikeda Y, et al. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8(5):654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 315.Budefeld T, Tobet SA, Majdic G. Steroidogenic factor 1 and the central nervous system. J Neuroendocrinol. 2012;24(1):225–235. doi: 10.1111/j.1365-2826.2011.02174.x. [DOI] [PubMed] [Google Scholar]
- 316.Ingraham HA, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8(19):2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 317.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 318.Zhao L, et al. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22(6):1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tran PV, et al. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci. 2003;22(4):441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Komada M, Takahashi M, Ikeda Y. Involvement of SF-1 in neurogenesis and neuronal migration in the developing neocortex. Neurosci Lett. 2015;600:85–90. doi: 10.1016/j.neulet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 321.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14(5):250–260. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 322.Gofflot F, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131(2):405–418. doi: 10.1016/j.cell.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 323.Bookout AL, et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Grgurevic N, Tobet S, Majdic G. Widespread expression of liver receptor homolog 1 in mouse brain. Neuro Endocrinol Lett. 2005;26(5):541–547. [PubMed] [Google Scholar]
- 325.Pare JF, et al. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J Biol Chem. 2004;279(20):21206–21216. doi: 10.1074/jbc.M401523200. [DOI] [PubMed] [Google Scholar]
- 326.Gu P, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25(9):3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wagner RT, et al. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28(10):1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Heng JC, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 329.Kaltezioti V, et al. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. 2010;8(12):e1000565. doi: 10.1371/journal.pbio.1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Stergiopoulos A, Politis PK. Nuclear receptor NR5A2 controls neural stem cell fate decisions during development. Nat Commun. 2016;7:12230. doi: 10.1038/ncomms12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shi Z, et al. Retinoic acid receptor gamma (Rarg) and nuclear receptor subfamily 5, group A, member 2 (Nr5a2) promote conversion of fibroblasts to functional neurons. J Biol Chem. 2014;289(10):6415–6428. doi: 10.1074/jbc.M113.515601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Greschik H, Schule R. Germ cell nuclear factor: an orphan receptor with unexpected properties. J Mol Med (Berl) 1998;76(12):800–810. doi: 10.1007/s001090050284. [DOI] [PubMed] [Google Scholar]
- 333.Hummelke GC, Cooney AJ. Germ cell nuclear factor is a transcriptional repressor essential for embryonic development. Front Biosci. 2001;6:D1186–D1191. doi: 10.2741/Hummelke. [DOI] [PubMed] [Google Scholar]
- 334.Gu P, et al. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25(19):8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 335.Yan Z, Jetten AM. Characterization of the repressor function of the nuclear orphan receptor retinoid receptor-related testis-associated receptor/germ cell nuclear factor. J Biol Chem. 2000;275(45):35077–35085. doi: 10.1074/jbc.M005566200. [DOI] [PubMed] [Google Scholar]
- 336.Susens U, et al. The germ cell nuclear factor mGCNF is expressed in the developing nervous system. Dev Neurosci. 1997;19(5):410–420. doi: 10.1159/000111238. [DOI] [PubMed] [Google Scholar]
- 337.Chung AC, et al. Loss of orphan receptor germ cell nuclear factor function results in ectopic development of the tail bud and a novel posterior truncation. Mol Cell Biol. 2001;21(2):663–677. doi: 10.1128/MCB.21.2.663-677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chen F, et al. Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Mol Endocrinol. 1994;8(10):1434–1444. doi: 10.1210/mend.8.10.7854358. [DOI] [PubMed] [Google Scholar]
- 339.Zhang YL, et al. Expression of germ cell nuclear factor (GCNF/RTR) during spermatogenesis. Mol Reprod Dev. 1998;50(1):93–102. doi: 10.1002/(SICI)1098-2795(199805)50:1<93::AID-MRD12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 340.Lan ZJ, et al. Expression of the orphan nuclear receptor, germ cell nuclear factor, in mouse gonads and preimplantation embryos. Biol Reprod. 2003;68(1):282–289. doi: 10.1095/biolreprod.102.008151. [DOI] [PubMed] [Google Scholar]
- 341.Jeong Y, Mangelsdorf DJ. Nuclear receptor regulation of stemness and stem cell differentiation. Exp Mol Med. 2009;41(8):525–537. doi: 10.3858/emm.2009.41.8.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Akamatsu W, et al. Suppression of Oct4 by germ cell nuclear factor restricts pluripotency and promotes neural stem cell development in the early neural lineage. J Neurosci. 2009;29(7):2113–2124. doi: 10.1523/JNEUROSCI.4527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mullen EM, Gu P, Cooney AJ. Nuclear receptors in regulation of mouse ES cell pluripotency and differentiation. PPAR Res. 2007;2007:61563. doi: 10.1155/2007/61563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang H, et al. Germ cell nuclear factor (GCNF) represses Oct4 expression and globally modulates gene expression in human embryonic stem (hES) cells. J Biol Chem. 2016;291(16):8644–8652. doi: 10.1074/jbc.M115.694208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Gu P, et al. Differential recruitment of methylated CpG binding domains by the orphan receptor GCNF initiates the repression and silencing of Oct4 expression. Mol Cell Biol. 2009;29(7):1987. doi: 10.1128/MCB.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gu P, et al. Differential recruitment of methyl CpG-binding domain factors and DNA methyltransferases by the orphan receptor germ cell nuclear factor initiates the repression and silencing of Oct4. Stem Cells. 2011;29(7):1041–1051. doi: 10.1002/stem.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sattler U, et al. The expression level of the orphan nuclear receptor GCNF (germ cell nuclear factor) is critical for neuronal differentiation. Mol Endocrinol. 2004;18(11):2714–2726. doi: 10.1210/me.2004-0251. [DOI] [PubMed] [Google Scholar]
- 348.Chung AC, et al. Loss of orphan nuclear receptor GCNF function disrupts forebrain development and the establishment of the isthmic organizer. Dev Biol. 2006;293(1):13–24. doi: 10.1016/j.ydbio.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 349.Wang H, et al. GCNF-dependent activation of cyclin D1 expression via repression of Mir302a during ESC differentiation. Stem Cells. 2014;32(6):1527–1537. doi: 10.1002/stem.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]