Abstract
Skin is an organ that is susceptible to damage by external injury, chronic inflammation, and autoimmunity. Tissue damage causes alterations in both the configuration and type of cells in lesional skin. This phenomenon, called tissue remodeling, is a universal biological response elicited by programmed cell death, inflammation, immune disorders, and tumorigenic, tumor proliferative, and cytoreductive activity. In this process, changes in the components of the extracellular matrix are required to provide an environment that facilitates tissue remodeling. Among these extracellular matrix components, periostin, a glycoprotein that is predominantly secreted from dermal fibroblasts, has attracted attention. Periostin localizes in the papillary dermis of normal skin, and is aberrantly expressed in the dermis of lesional skin in atopic dermatitis, scar, systemic/limited scleroderma, melanoma, cutaneous T cell lymphoma, and skin damage caused by allergic/autoimmune responses. Periostin induces processes that result in the development of dermal fibrosis, and activate or protract the immune response. The aim of this review was to summarize recent knowledge of the role of periostin in the pathogenesis of dermatoses, and to explore whether periostin is a potential therapeutic target for skin diseases.
Keywords: Skin diseases, Periostin, Scleroderma, Atopic dermatitis, Melanoma, Scar, Mycosis fungoides
Introduction
Matricellular proteins (MPs) belong to the non-structural cellular matrix, and are involved in development, pathology, and wound healing [1, 2]. MPs are expressed during and after birth, and are required for proper growth and development. Their expression is regulated depending on post-natal conditions. Expressed MPs exert biochemical effects on cells via cell surface receptors such as integrins [1, 2]. The roles of many MPs have been confirmed based on studies of skin wound healing. Periostin is a unique MP whose function was first analyzed in studies on cardiovascular diseases [3, 4]. Periostin is an N-glycoprotein that was initially identified as osteoblast specific factor-2 [5]. Subsequently, based on findings of that its expression was confined to the periosteum and periodontal ligament, this MP was given the name periostin [6]. Periostin has several functional domains, including a cysteine-rich EMI domain and four tandem-fasciclin-like domains. The EMI domain is important for association between proteins, and the tandem-fasciclin-like domain is important for binding to integrins αvβ3 and αvβ5 [1]. Periostin produces variants as a result of mRNA splicing in the C-terminal domain [1]. The type and number of splicing variants are reported to vary in each organ, but the characteristic variant in skin is not clearly known at the present time. As with other MPs, periostin is induced when tissue damage results in tissue repair. For example, mast cells play an important role in tissue repair, and histamine derived from mast cells has been reported to induce periostin from fibroblasts [7, 8]. This review outlines the function of periostin in the skin and introduces recent findings on the involvement of periostin in the pathology of skin diseases.
Role of periostin in wound healing and scar formation in skin
Skin is frequently damaged by trauma, inflammation, and tumor progression. Skin damage initiates a series of physiological processes including the production of humoral factors, mediators, cytokines, growth factors, and extracellular matrix (ECM) components, and the recruitment of various cells related to repair [9]. The tissue repair process is divided into the inflammatory response, proliferation, and remodeling/regenerative phases [2, 9]. The inflammatory phase begins after cessation of bleeding due to the formation of blood clots. During the inflammatory phase, cells necessary for repair are recruited into the damaged tissue, to the site of the lesion. The gathered cells proliferate and differentiate in the lesion and form “granulation tissue”, characterized by angiogenesis, tissue balls, and the invasion of inflammatory cells [2, 9]. The emergency matrix fibrin and fibronectin form a temporary scaffold that is required for tissue remodeling [10]. The macrophages infiltrated in the granulation tissue produce TGFβ to promote wound healing process [11] (Fig. 1).
Fig. 1.
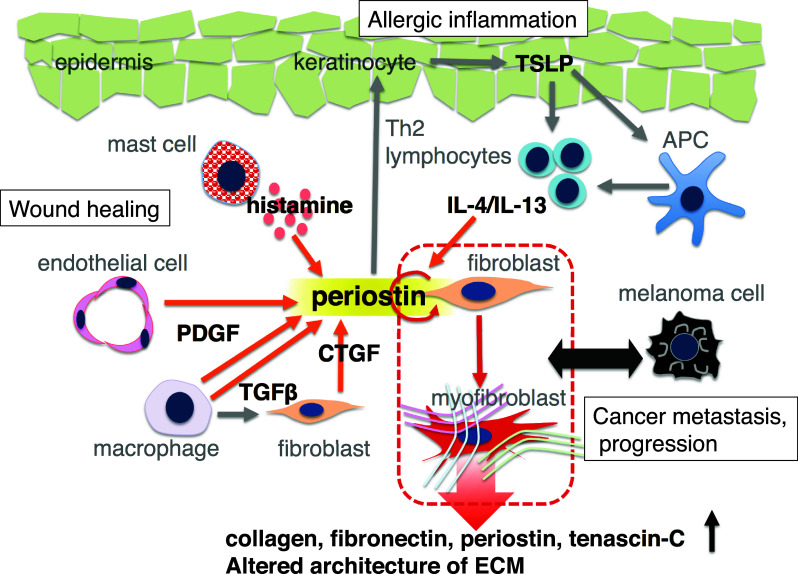
Schema illustrating the relationships between periostin and dermal fibroblasts, immune cells, endothelial cells, and keratinocytes. Fibroblast and endothelial cells secrete periostin upon stimulation due to a traumatic wound, histamines derived from degranulation of mast cell, Th2 cytokines, and fibrogenic cytokines (e.g., TGFβ, CTGF, and PDGF). Periostin transforms dermal fibroblasts into myofibroblasts, and modifies the structure of extracellular matrix
TGFβ activates dermal fibroblasts, and promotes their migration into granulation tissue, where they transform into myofibroblasts, capable of producing extracellular matrix [12]. Myofibroblasts produce type I collagen, which is the major component of dermal ECM among fibrillar collagen types. Type I and III collagens contribute to maintaining the elasticity and strength of skin. In hypertrophic scars, overproduction of ECM components (e.g., type I and III collagen, periostin, and tenascin) occurs, and the ratio of I/III collagen is two to three times that seen in normal scar tissue [13]. To date, ECM has been shown to be involved in scar formation after skin wound healing. The major sources of ECM production are dermal fibroblasts and myofibroblasts. TGFβ, bone morphogenetic proteins, proteoglycans (e.g., laminin, decorin and fibronectin), matrix metalloproteinases, and periostin are known to regulate ECM production [2]. Periostin induces the proliferation of fibroblasts and is involved in the TGFβ-provoked induction of myofibroblasts (Fig. 1).
Because the expression of periostin peaks after the completion of inflammatory phase, periostin has been thought to be involved in tissue repair after the proliferation stage, as Postn knockout mice exhibit delays in wound healing, and re-epithelialization in the proliferative phase [2, 14]. On another front, from the inflammatory to the proliferative stages, it is well known that mast cells and Th2 cytokine skewing promote fibrosis during the tissue remodeling process [15] (Fig. 1). Histamine derived from mast cells induces periostin by the activation of extracellular signal-regulated kinase 1/2 via the H1 receptor on fibroblasts [8]. Furthermore, comprehensive analysis using DNA microarrays revealed that Th2 type cytokine (IL-4/IL-13) induces periostin, and causes the binding of periostin with other matrix molecules such as tenascin-C, fibronectin, and collagen V [16]. In the remodeling/regenerative phase, which is the final process of wound healing, the surface of the granulation tissue on the skin ulcer is covered with a newly produced epidermis. This process is called re-epithelialization. Periostin induces the proliferation and differentiation of epithelial cells, resulting in appropriate re-epithelialization. Periostin is localized in the dermis just under the epidermis, termed the papillary dermis, in normal skin, but is widely distributed in all dermal layers in the scar lesion after wound healing [17]. Hypertrophic scar and keloid tissue are pathologically characterized by both acanthosis of the epidermis and thickened dermal scar tissue, and are found to show extensive and intense staining for periostin in abnormal scarring [17]. Thus, periostin plays an important role in wound healing processes in the proliferative and remodeling/regenerative phase, and its aberrant expression would contribute to abnormal scarring after wounding of skin.
Periostin and atopic dermatitis
Atopic dermatitis presents characteristic clinical manifestations for each age of onset and disease duration. The clinical picture of childhood atopic dermatitis is mainly eczematous lesion. Eczema is accompanied by strong itching, and scratching will lead to the development of further eczema. If disease duration is prolonged from childhood to adolescence, pruritus develops into dermatitis, and results in chronic dermatitis and/or lichenification of skin [18, 19].
Lichenification of the skin is the major skin manifestation of chronic atopic dermatitis [18] (Fig. 2). Chronic inflammation and addictive scratching result in the development of acanthosis of the epidermis, prolongation of dermal papilla, proliferation of fibroblasts, and an increase in thickened collagen fibers (Fig. 2). These are typical observations of remodeling in protracted atopic dermatitis. Skin remodeling therefore contributes to the homing of inflammatory cells in the skin, and also leads to the prolongation of chronic inflammation by inhibiting drug delivery into the lesional skin. This is not limited to atopic dermatitis but is also seen in allergic inflammation in other organs such as asthma and allergic rhinitis: chronic intractable lesions are formed with changes such as basement membrane thickening and increased extracellular matrix. In the management of chronic inflammatory diseases, it is necessary to take measures to pay attention to remodeling in addition to symptomatic treatment. Mast cells are known to play an important role in the process of tissue remodeling, and their specific actions have been elucidated [20].
Fig. 2.
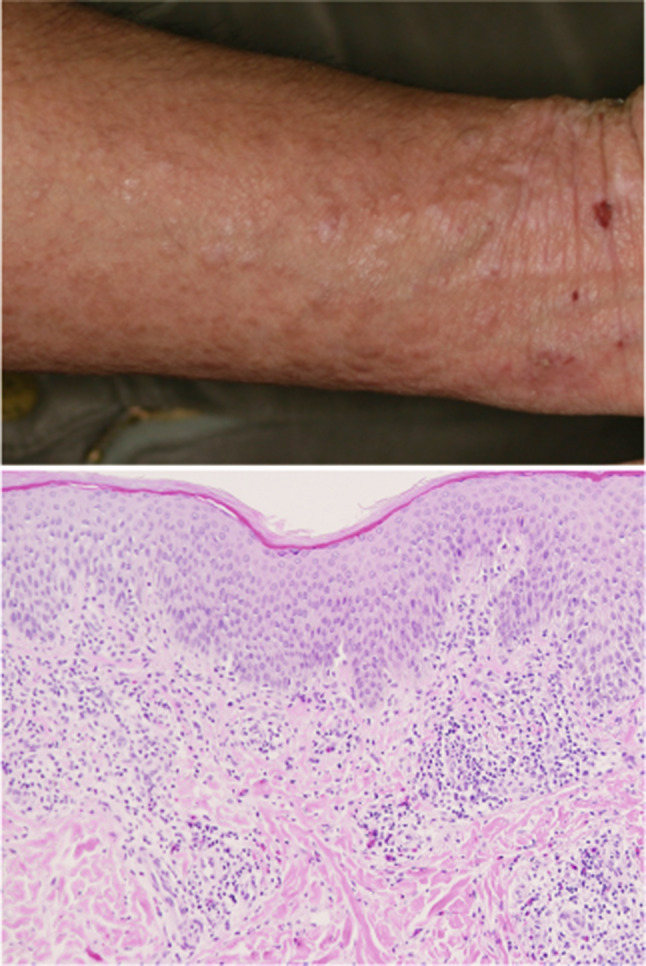
Clinical and pathological features of lichenification of skin in atopic dermatitis. a Clinical picture of lichenification of skin. b Pathological findings in lesional skin include acanthosis, increased number of blood vessels, perivascular inflammatory infiltrate, and thickened collagen bundles. Magnification, ×200
In atopic dermatitis lesions, an increase in the number of mast cells has been confirmed [20]. Mast cells are degranulated by antigen challenge, infection, and scratching of the skin surface. As a result, various mediators are released into the tissues to cause inflammation, and when acting on the constituent cells of the skin, they enhance cell proliferation ability [21]. Among these, histamine acts on cells expressing histamine receptors such as epidermal cells, fibroblasts, vascular endothelial cells, antigen-presenting cells (e.g., Langerhans cells, dendritic cells, and macrophages) and neurons, and results in an itching sensation, inflammatory cell recruitment, vasodilation, and leakage of plasma into the tissues [21].
Histamine elicits innate immune system activation and tissue remodeling via H1 receptor activation [22]. Histamine stimulation results in the production of inflammatory mediators by skin fibroblasts, vascular endothelial cells, Langerhans cells, and eosinophils, in addition to causing the adhesion of inflammatory cells to vascular endothelial cells, and changes in the constitution of extracellular matrix, including collagen. This modification of ECM is involved in the pathogenesis of chronic inflammatory disease by promoting infiltration of leukocytes [21, 22]. As histamine stimulation induces collagen synthesis from fibroblasts [20], hardening of the skin is frequently observed in lichenified lesions [19]. This was also reproduced in vitro, and because histamine causes an increase in type 1 collagen synthesis 48 h after stimulation, it is thought that this response might involve one or more second messengers [8, 20].
In searching for atopic disease-related genes using genome-wide association studies and quantitative mRNA expression analysis, factors that induce periostin, which are extracellular matrix proteins (IL-4, IL-13, TGF-β, etc.), were confirmed to be strongly expressed at the lesion site [23, 24]. Atopic dermatitis-like skin inflammation, evoked by topical application of mite extract, was developed in wild-type and Postn knockout (KO) mice, and their skin manifestations were compared and examined. Unlike wild-type mice, Postn KO mice showed a relatively slight skin phenotype in the acanthosis and infiltration of inflammatory cells [25].
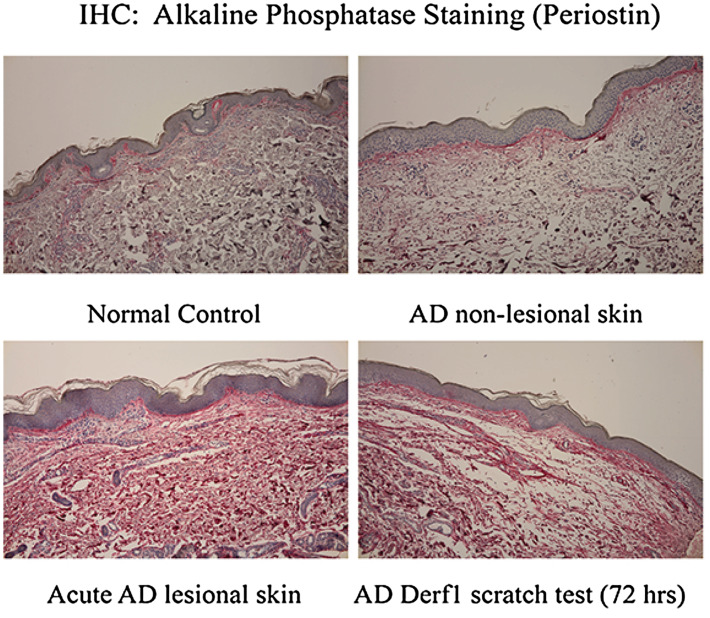
Izuhara et al. reported that periostin is involved in the chronicity of allergic skin inflammation by inducing Th2 chemokines, such as TSLP, from fibroblasts and keratinocytes [25, 26]. These results suggest that the enhancement of periostin expression is not simply the result of inflammation but is partly involved in the amplification process of pathogenesis. Our own studies have confirmed that periostin is strongly deposited in lesions in patients with atopic dermatitis [8] (Fig. 3). It is thought that periostin is involved in tissue remodeling of skin, including the chronic pathology of atopic dermatitis and lichenification.
Fig. 3.
Localization of periostin in lesional skin in atopic dermatitis (AD). Skin specimen derived from normal control (non-AD) (upper left), AD non-lesional skin (upper right), AD acute lesion (lower left), and 72 h after scratch test with Derf1 in AD patients (lower right) are presented. Periostin was stained with alkaline phosphatase (red). Magnification, ×100.
This figure is reused from Ref. [8] with permission from Elsevier Publishing Group
As described above, we confirmed that histamine induces periostin directly from fibroblasts via the H1 receptor, and type 1 collagen expression occurs via its autocrine action [8]. New findings on the relationship between mast cells and tissue remodeling have expanded our understanding of the mechanism of lichenification. Thus, therapeutic strategies targeting mast cells could contribute to regulating the excessively expressed periostin.
Periostin and scleroderma
Scleroderma is a disease in which various organs, including the skin, become fibrotic and sclerotic [27]. Sclerosis usually begins in the deep dermis of the skin, and is defined as an accumulation of ECM. This arises from a vicious cycle between excessive synthesis and degradation of ECM components, and changes with disease progression [27]. Deposition of ECM components, such as collagen, hyaluronic acid, glycosaminoglycan, and fibronectin, destroys the original structure and impairs the proper function of skin [28]. In addition, activation of myofibroblasts and resistance to apoptosis in fibroblasts are observed. Although the precursor of myofibroblasts in lesional skin in scleroderma has not been determined, interstitial fibroblasts or other cells such as pericytes, endothelial cells, and bone marrow-derived fibroblast-progenitor cells are known to differentiate into myofibroblasts [29]. Adipocytic progenitor cells have also been identified as a source of myofibroblasts [30].
Previous reports studying the pathogenesis of scleroderma have revealed that several mediators contribute to the activation of fibroblasts in skin lesion. TGFβ is considered to play a central role in the sclerosis process [31]. Normally, the harmful effects of over-exposure to TGFβ are suppressed by the negative feedback function provided by the orphan nuclear receptor, NR4A1 [32]. However, in scleroderma, continuous activation of TGFβ, mediated by abnormalities in its transcriptional and posttranscriptional regulation, or possibly due to a reduced feedback loop via NR4A1, may be involved in a mechanism that results in high susceptibility to fibrosis [31, 32]. Connective tissue growth factor (CTGF, also known as CCN2), which is induced by endothelial cells stimulated with TGFβ, endothelin-1, and angiotensin II, belongs to the CCN matrix protein family, and also promotes skin fibrosis process cooperatively with TGFβ [33–35]. Platelet-derived growth factor (PDGF), a potent mitogen for mesenchymal cells, is also known to be involved in fibrosis in scleroderma [36]. PDGF is produced by endothelial cells, platelets, macrophages and fibroblasts. Its receptors are highly expressed in the skin and lungs of patients with scleroderma.
The involvement of periostin in the pathology of scleroderma has been confirmed in animal models of scleroderma. Scleroderma-like skin sclerosis occurs following subcutaneous administration of bleomycin for several weeks. However, Postn KO mice do not develop scleroderma-like skin sclerosis and show no increase in type 1 collagen expression, despite increases in the expression levels of TGFβ and CTGF similar to that seen in the wild type [37]. In other words, these results indicate that the presence of periostin is necessary for the induction of type 1 collagen expression by TGFβ and CTGF in the pathogenesis of scleroderma. Furthermore, the induction of myofibroblasts by TGFβ does not occur in Postn KO mice [37]. Periostin acts on αv integrin of fibroblasts and induces the expression of type 1 collagen via the PI3K/Akt signaling pathway [37]. These results suggest that periostin creates an environment that is susceptible to fibrosis. Regarding the relationship between periostin and PDGF, a recent in vitro study investigated the effect of crenolanib, an inhibitor of PDGF receptor signaling, on the fibrotic activity of TGFβ-stimulated cultured dermal fibroblasts derived from scleroderma, and found attenuated expression of CTGF and periostin [38]. Thus, it could be said that periostin orchestrates the direction of the fibrotic response mediated by TGFβ, CTGF, and PDGF.
In the dermis of the scleroderma lesion, periostin is localized almost throughout the dermis, and its immunostaining intensity in the scleroderma is stronger than keloid and hypertrophic scars [37]. The finding that periostin is stained in a whole dermis has also been confirmed in morphea [39, 40]. Another report indicated the usefulness of serum periostin level as a biomarker for severe scleroderma disease [41]. Periostin is involved in both the pathogenesis and pathology of scleroderma and is a possible molecular target for the treatment of scleroderma.
One candidate therapeutic agent is a vitamin D analog, maxacalcitol, which has been confirmed to suppress periostin expression that is induced by stimulation with Th2 cytokine or TGFβ, and can be said to be a candidate for therapeutic drugs [42].
Periostin and melanoma
Melanoma is a life-threatening malignant skin tumor. Once melanoma gains metastatic potential and spreads to other organs, patient prognosis is adversely affected. Thus, it is imperative to understand how melanoma will gain metastatic and invasive capacity. To elucidate the mechanism of progression of melanoma, studying the cytoskeletal structure of melanoma cells and their relationship to the surrounding ECM is helpful to understand their motility and invasiveness [43]. In addition, changes in the interactions of melanoma cells with keratinocytes and fibroblasts allow survival and proliferation outside the normal epidermis [43]. Proteome and genome initiatives greatly increase our knowledge of which gene products are deregulated in invasive and metastatic melanomas.
Naka and colleagues explored factors related to the development of melanoma by subtraction in quantitative proteomic analysis, called “isobaric tags for relative and absolute quantitation (iTRAQ)” [44]. These studies showed that periostin was highly expressed in the tissues of invasive melanomas. A relationship between melanoma and periostin was observed by Tilman and her colleagues, with their finding of increased Postn transcription in some melanoma cell lines [45]. Naka and colleagues observed increased expression of periostin when melanoma cells were co-cultured with normal human dermal fibroblasts in vitro [44]. Periostin derived from fibroblasts promote proliferation via its action on integrin in melanoma cells [44]. This tumor growth effect of periostin has been confirmed by in vivo experiments. Postn/Rag2 double knockout mice inoculated with melanoma developed significantly smaller sized tumor masses compared to those in Rag2 knockout mice [44]. Saya and colleagues reported the role of periostin as a chemotactic factor in melanoma cell metastasis [46]. Their findings confirmed that periostin derived from wounded skin promotes the migration of melanoma cells into the lesion in periostin-secreting wounded skin [46]. In this experiment, periostin did not affect the proliferation of melanoma cells [46]. Although the impact of periostin on the proliferation of melanoma cells remains unclear, these results indicated that an increase in periostin in the tumor microenvironment causes the progression of melanoma to a more serious disease stage. The periostin expressed in the melanoma microenvironment is thought to be derived from fibroblasts stimulated with CTGF produced from melanoma cells [47]. In summary, it is suggested that periostin derived from the stroma is greatly involved in the invasion and proliferation of melanoma.
The next task is to understand how MPs promote the invasion of melanoma cells. Recently, a new model has been developed to more closely reproduce the conditions of melanoma invasion in vivo. These models are developed to better understand how MPs involved in melanoma progression affect the motility of melanoma cells and their interaction with ECM, stromal cells, and blood vessels.
Periostin and other dermatoses
Even in the absence of dermatoses, periostin expression in skin decreases with skin aging, and affects collagen production [48]. Several reports indicate the possible association of periostin with the pathogenesis of certain dermatoses. The serum level of periostin was measured in subjects with chronic spontaneous urticaria, because periostin is a downstream signaling molecule for Th2 cytokines (e.g., IL-4 and IL-13) [49]. However, serum periostin levels were significantly lower in severe chronic spontaneous urticaria cases with increased serum IL-13 [49].
Although the role of periostin in the pathogenesis of dermatoses remains obscure, abnormal findings in immunohistochemical staining for periostin have been reported in pemphigus vulgaris, bullous pemphigoid, mycosis fungoides, and lichen sclerosus et atrophicus [40, 50, 51].
Mycosis fungoides (MF) comprises the majority of cutaneous lymphoma, and accounts for up to 40% of all cutaneous lymphoma [52]. Patients with MF ordinarily exhibit a chronic clinical course, and suffer from persistent symptoms. Most cases with MF remain at the early patch stage; however, some cases are at risk for gradual progression from the patch stage to the plaque and/or tumor stage [53]. In histopathological findings, the intensity of periostin-positive staining was more prominent in the early stage of this disease. Recent reports have pointed out an apparent increase in the number of tissue-infiltrating M2 macrophages in lesional skin. Monocyte-derived macrophages stimulated with periostin showed an increase in the phenotype characteristic of tumor-associated macrophages in the early stage of MF, and expressed significantly higher levels of CXCL5 and CXCL10 [51]. As these chemokines are known to affect the formation of cutaneous T cell lymphoma, increased periostin in ECM contributes to the pathogenesis of MF by increasing tissue-infiltrating macrophages.
Conclusion
Knowledge derived from studies of periostin in the pathogenesis of skin diseases offers important indications of the importance of ECM in the lesional microenvironment. Studies of the role of periostin are just being initiated. It will not be long before these findings about periostin can be applied clinically in dermatology.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68(19):3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker JT, McLeod K, Kim S, Conway SJ, Hamilton DW. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res. 2016;365(3):453–465. doi: 10.1007/s00441-016-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genom. 2008;9(8):548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn GW., 2nd Periostin and myocardial repair, regeneration, and recovery. N Engl J Med. 2007;357(15):1552–1554. doi: 10.1056/NEJMcibr074816. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294(Pt 1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 7.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20(13):2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Murota H, Serada S, Fujimoto M, Kudo A, Naka T, Katayama I. Histamine contributes to tissue remodeling via periostin expression. J Investig Dermatol. 2014;134(8):2105–2113. doi: 10.1038/jid.2014.120. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol. 2001;28(10):521–534. doi: 10.1111/j.1346-8138.2001.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 10.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110(Pt 7):861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 11.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14(5):538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol. 2012;26(2):141–152. doi: 10.1111/j.1468-3083.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6(4):e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 17.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21(5):331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 18.Murota H, Katayama I. Exacerbating factors of itch in atopic dermatitis. Allergol Int. 2017;66(1):8–13. doi: 10.1016/j.alit.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Katayama I, Yokozeki H, Nishioka K. Mast-cell-derived mediators induce epidermal cell proliferation: clue for lichenified skin lesion formation in atopic dermatitis. Int Arch Allergy Immunol. 1992;98(4):410–414. doi: 10.1159/000236218. [DOI] [PubMed] [Google Scholar]
- 20.Murota H, Bae S, Hamasaki Y, Maruyama R, Katayama I. Emedastine difumarate inhibits histamine-induced collagen synthesis in dermal fibroblasts. J Investig Allergol Clin Immunol. 2008;18(4):245–252. [PubMed] [Google Scholar]
- 21.Murota H, Katayama I. Assessment of antihistamines in the treatment of skin allergies. Curr Opin Allergy Clin Immunol. 2011;11(5):428–437. doi: 10.1097/ACI.0b013e32834a96e9. [DOI] [PubMed] [Google Scholar]
- 22.Murota H, Katayama I. Emedastine difumarate: a review of its potential ameliorating effect for tissue remodeling in allergic diseases. Expert Opin Pharmacother. 2009;10(11):1859–1867. doi: 10.1517/14656560903078410. [DOI] [PubMed] [Google Scholar]
- 23.Hoffjan S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med (Berl) 2005;83(9):682–692. doi: 10.1007/s00109-005-0672-2. [DOI] [PubMed] [Google Scholar]
- 24.Wood SH, Ke X, Nuttall T, McEwan N, Ollier WE, Carter SD. Genome-wide association analysis of canine atopic dermatitis and identification of disease related SNPs. Immunogenetics. 2009;61(11–12):765–772. doi: 10.1007/s00251-009-0402-y. [DOI] [PubMed] [Google Scholar]
- 25.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Investig. 2012;122(7):2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi H, Masuoka M, Ohta S, Suzuki S, Arima K, Taniguchi K, Aoki S, Toda S, Yoshimoto T, Inagaki N, Conway SJ, Narisawa Y, Izuhara K. Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes. Allergol Int. 2012;61(4):563–572. doi: 10.2332/allergolint.10-OA-0297. [DOI] [PubMed] [Google Scholar]
- 27.Nishioka K, Katayama I, Kondo H, Shinkai H, Ueki H, Tamaki K, Takehara K, Tajima S, Maeda M, Hayashi S, Kodama H, Miyachi Y, Mizutani H, Fujisaku A, Sasaki T, Shimizu M, Kaburagi J. Epidemiological analysis of prognosis of 496 Japanese patients with progressive systemic sclerosis (SSc). Scleroderma Research Committee Japan. J Dermatol. 1996;23(10):677–682. doi: 10.1111/j.1346-8138.1996.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 28.Varga J, Rudnicka L, Uitto J. Connective tissue alterations in systemic sclerosis. Clin Dermatol. 1994;12(3):387–396. doi: 10.1016/0738-081X(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 29.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol. 2015;11(4):233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 30.Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafyatis R. Transforming growth factor beta—at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10(12):706–719. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, Mielenz D, Tomcik M, Furnrohr BG, Scholtysek C, Dees C, Beyer C, Kronke G, Metzger D, Distler O, Schett G, Distler JH. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21(2):150–158. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 33.Weng CM, Yu CC, Kuo ML, Chen BC, Lin CH. Endothelin-1 induces connective tissue growth factor expression in human lung fibroblasts by ETAR-dependent JNK/AP-1 pathway. Biochem Pharmacol. 2014;88(3):402–411. doi: 10.1016/j.bcp.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 2008;47(Suppl 5):v8–v9. doi: 10.1093/rheumatology/ken278. [DOI] [PubMed] [Google Scholar]
- 35.Stawski L, Han R, Bujor AM, Trojanowska M. Angiotensin II induces skin fibrosis: a novel mouse model of dermal fibrosis. Arthritis Res Ther. 2012;14(4):R194. doi: 10.1186/ar4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwayama T, Olson LE. Involvement of PDGF in fibrosis and scleroderma: recent insights from animal models and potential therapeutic opportunities. Curr Rheumatol Rep. 2013;15(2):304. doi: 10.1007/s11926-012-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, Katayama I. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012;7(7):e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makino K, Makino T, Stawski L, Mantero JC, Lafyatis R, Simms R, Trojanowska M. Blockade of PDGF receptors by crenolanib has therapeutic effect in patient fibroblasts and in preclinical models of systemic sclerosis. J Investig Dermatol. 2017 doi: 10.1016/j.jid.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MW, Park JT, Kim JH, Koh SJ, Yoon HS, Cho S, Park HS. Periostin in mature stage localized scleroderma. Ann Dermatol. 2017;29(3):268–275. doi: 10.5021/ad.2017.29.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakizaki A, Fujimura T, Furudate S, Kambayashi Y, Aiba S. Immunohistochemical similarities between lichen sclerosus et atrophicus and morphea: a case study. Case Rep Dermatol. 2015;7(1):39–45. doi: 10.1159/000381010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013;168(4):717–725. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- 42.Terao M, Yang L, Matsumura S, Yutani M, Murota H, Katayama I. A vitamin D analog inhibits Th2 cytokine- and TGF beta-induced periostin production in fibroblasts: a potential role for vitamin D in skin sclerosis. Dermatoendocrinology. 2015;7(1):e1010983. doi: 10.1080/19381980.2015.1010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaggioli C, Sahai E. Melanoma invasion—current knowledge and future directions. Pigment Cell Res. 2007;20(3):161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 44.Kotobuki Y, Yang L, Serada S, Tanemura A, Yang F, Nomura S, Kudo A, Izuhara K, Murota H, Fujimoto M, Katayama I, Naka T. Periostin accelerates human malignant melanoma progression by modifying the melanoma microenvironment. Pigment Cell Melanoma Res. 2014;27(4):630–639. doi: 10.1111/pcmr.12245. [DOI] [PubMed] [Google Scholar]
- 45.Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A. Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer. 2007;6:80. doi: 10.1186/1476-4598-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukuda K, Sugihara E, Ohta S, Izuhara K, Funakoshi T, Amagai M, Saya H. Periostin is a key niche component for wound metastasis of melanoma. PLoS One. 2015;10(6):e0129704. doi: 10.1371/journal.pone.0129704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchenreuther J, Vincent KM, Carter DE, Postovit LM, Leask A. CCN2 expression by tumor stroma is required for melanoma metastasis. J Investig Dermatol. 2015;135(11):2805–2813. doi: 10.1038/jid.2015.279. [DOI] [PubMed] [Google Scholar]
- 48.Egbert M, Ruetze M, Sattler M, Wenck H, Gallinat S, Lucius R, Weise JM. The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J Dermatol Sci. 2014;73(1):40–48. doi: 10.1016/j.jdermsci.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Bae Y, Izuhara K, Ohta S, Ono J, Hong GU, Ro JY, Park GH, Choi JH. Periostin and interleukin-13 are independently related to chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2016;8(5):457–460. doi: 10.4168/aair.2016.8.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujimura T, Kakizaki A, Furudate S, Aiba S. A possible interaction between periostin and CD163+ skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid. Exp Dermatol. 2016 doi: 10.1111/exd.13157. [DOI] [PubMed] [Google Scholar]
- 51.Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, Aiba S. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol. 2016;25(2):107–112. doi: 10.1111/exd.12873. [DOI] [PubMed] [Google Scholar]
- 52.Kempf W, Sander CA. Classification of cutaneous lymphomas—an update. Histopathology. 2010;56(1):57–70. doi: 10.1111/j.1365-2559.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 53.Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM, Wilkins B, Fields PA, Dean A, Webb K, Scarisbrick J, Morris S, Whittaker SJ. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]