Abstract
A diverse range of drug resistance mechanisms in cancer cells and their microenvironment significantly reduces the effectiveness of anti-cancer therapies. Growing evidence suggests that transcriptional effectors of the Hippo pathway, YAP and TAZ, promote resistance to various anti-cancer therapies, including cytotoxic chemotherapy, molecular targeted therapy, and radiation therapy. Here, we overview the role of YAP and TAZ as drug resistance mediators, and also discuss potential upstream regulators and downstream targets of YAP/TAZ in cancer. The widespread involvement of YAP and TAZ in resistance mechanisms suggests that therapeutic targeting of YAP and TAZ may expedite the development of effective anti-resistance therapies.
Keywords: Drug, Resistance, YAP, TAZ, Hippo pathway, Biomarker
Introduction
Advances in cancer therapies have improved patient survival in many types of cancer, but resistance against therapies crucially limits the opportunity of complete tumor remission or further survival improvement. Malignant tumors are based on complex, redundant, and heterogeneous survival mechanisms that prevent tumor death by single pathway blockade [1–3]. Moreover, the initial response to anti-cancer therapy varies from patients to patients according to biologic properties of their tumors. Researchers are trying to identify clinical and biological markers predicting therapeutic responses, and to block innate resistance mechanisms to enhance drug efficiency. Anti-cancer therapy is also hampered by acquired resistance where tumors initially respond to therapies, but become resistant during treatment through various genetic and epigenetic changes. Several mechanisms have been implicated in acquired resistance, including amplification of therapeutic targets, emergence of gatekeeper mutations in targeted kinases, acquired mutations in upstream and downstream genes of the same pathway, activation of alternative signaling pathways, and changes in drug uptake or efflux [1, 4]. Acquired resistance in cancer cells also comes from adaptive responses such as epigenetic activation of prosurvival signaling and epithelial–mesenchymal transition (EMT), as well as from their interactions with the tumor microenvironment [5–8]. Surviving cancer cells upon anti-cancer therapies are often originated from intratumoral genetic heterogeneity and clonal selection, which make the distinction between innate and acquired resistance somewhat blurred.
It is clear that overcoming therapy resistance will benefit the majority of cancer patients, and thus developing anti-cancer agents that are free of resistance is one of the most important goals in current cancer research. A recent clinical trial showed a promising result in treating non-small cell lung cancer (NSCLC) exhibiting acquired resistant to epithelial growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs), using a new compound specific for EGFR with the T790M gatekeeper mutation [9]. In addition, a combination of an MEK inhibitor and a BRAF inhibitor has been shown to be effective for overcoming BRAF inhibitor resistance in melanoma [10], and various new drug combinations are now tested by clinical trials. These results demonstrate that targeting drug resistance is clinically feasible and effective in improving patient survival. However, although specific resistance mechanisms have been revealed for selected population of cancer patients, major mechanisms to explain widespread emergence of resistance are still unknown and efficient strategies to overcome resistance have not been developed for majority of anti-cancer agents.
Yes-associated protein (YAP) and its paralog transcriptional co-activator with a PDZ-binding domain (TAZ) are transcriptional regulators that play key roles in embryonic development and tissue regeneration [11, 12]. Recent findings indicate that YAP and TAZ are deeply implicated in cancer pathogenesis across multiple cancer types [13–15]. This review will highlight the role of YAP/TAZ in cancer with a special focus on their involvement in anti-cancer therapy resistance.
The Hippo-YAP/TAZ pathway
Components of the Hippo pathway were first discovered as organ size regulators in Drosophila [16, 17]. Inactivation of Hippo pathway genes Warts, Hippo, Salvador, and Mats has been shown to cause significant increases in the size of multiple organs as a result of excessive cell proliferation and defective cell death [16, 18–23]. A screen for Warts-interacting proteins also identified Yorkie (Yki), the Drosophila ortholog of the mammalian YAP, as a key link between the Hippo pathway and transcriptional control [24]. Subsequent studies have revealed analogous composition and function of the mammalian Hippo-YAP/TAZ pathway.
Detailed regulatory mechanisms and updated findings on the Hippo-YAP/TAZ pathway have been thoroughly reviewed in recent articles [12, 13, 25]. Briefly, the central components of the mammalian Hippo pathway comprise serine/threonine kinases MST1/2 (Hippo orthologs) and LATS1/2 (Warts orthologs), and scaffold proteins SAV1 (Salvador ortholog) and MOB1A/B (Mats orthologs). Upon activation by MST1/2, LATS1/2 phosphorylate serine residues in multiple HXRXXS motifs of YAP and TAZ, leading to their cytoplasmic retention and degradation [26, 27]. When the Hippo pathway is inactivated, YAP/TAZ translocate into the nucleus and upregulate the transcription of genes associated with the cell cycle, cell proliferation, and cell survival. YAP/TAZ do not bind to DNA by themselves, but they are potent transcriptional co-activators that mainly support the activity of TEA domain family member (TEAD) transcription factors [28]. In addition, YAP/TAZ interact with AP-1 [29], RUNX2 [30] and SMADs [31], and can also suppress gene transcription via their interaction with the NuRD complex [32]. Transcriptional targets of YAP/TAZ include CTGF, ANKRD1, CYR61, ITGB2 and AXL, which have been identified by YAP/TAZ overexpression studies [28, 33]. Chromatin immunoprecipitation followed by DNA sequencing (ChIP-seq) analyses further identified a group of YAP/TAZ target genes whose major functional categories are cell proliferation, RNA metabolism, extracellular matrix (ECM) and actin cytoskeleton organization, and cell migration [29, 34]. Remarkably, it appears that specific ligands and designated transmembrane receptors are not involved in the mammalian Hippo pathway. Instead, Hippo pathway activity is controlled by various intrinsic and environmental inputs including cell polarity [35], cell–cell and cell–matrix adhesion [36, 37], matrix stiffness [38, 39], actin cytoskeleton architecture and contractility [36–38, 40], mechanical stress, and cell metabolism [41–43]. Moreover, the Hippo-YAP/TAZ pathway crosstalks with multiple other signaling pathways such as GPCR [44], EGF [45, 46], and TGFβ pathways [47], as well as canonical and non-canonical WNT pathways [48–50].
Studies using genetically engineered mouse models have demonstrated functional conservation between the Drosophila Hippo–Warts–Yki pathway and the mammalian Hippo-YAP/TAZ pathway. Loss of Hippo components or hyperactivation of YAP in mice causes organ size enlargement, although they may not drive growth in every tissue. For example, liver-specific knockout of MST1/2 or overexpression of YAP results in a significant increase in liver size with increased cell numbers [51–53]. Similarly, conditional SAV knockout or YAP hyperactivation promotes heart growth through cardiomyocyte proliferation [54]. Notably, YAP activation is also involved in maintaining undifferentiated status of progenitor and stem cells. Knockout of Hippo components or hyperactivation of YAP in mouse skin epidermis causes an expansion of basal progenitor cells and epidermal thickening [51, 55]. Moreover, maintenance of pluripotency in embryonic stem (ES) cells depends on YAP activity, and transcriptional targets of YAP in ES cells include a group of genes known to promote stemness [56]. In line with its role in stemness control, the Hippo-YAP/TAZ pathway has been shown to modulate the regeneration of multiple tissues [57–61].
Role of YAP/TAZ in cancer pathogenesis
Growing evidence indicates that YAP and TAZ play important roles in pathogenic mechanisms of cancer including tumorigenesis, metastasis, and drug resistance [13–15]. It has been shown that overexpression of YAP in mammary epithelial cells induces multiple hallmarks of cancers such as anchorage- and growth factor-independent growth, suppression of apoptosis, and EMT [62]. In vivo tumorigenic potential of YAP/TAZ activation has been validated in a number of mouse models. Long-term YAP activation or MST1/2 double knockout in the liver causes not only the enlargement of the organ but also the development of hepatocellular carcinoma [51–53]. MST1/2 double knockout also causes epithelial hyperplasia and adenoma in the colon [63]. Moreover, MOB1-depleted (Mob1a null; Mob1b het or Mob1a het; Mob1b null) mice are susceptible to developing cancers of various origins including skin cancer, osteosarcoma, fibrosarcoma, liver cancer, and breast cancer [64]. A number of xenograft studies in mice further demonstrated that inactivation of YAP or TAZ decreases tumor mass in YAP/TAZ-dependent cancers [65–69]. Together, these evidences indicate that activation of YAP/TAZ can induce cellular transformation that gives features of malignancy to non-transformed cells.
Although the overall frequency is not dramatic, recurrent mutations in genes encoding Hippo-YAP/TAZ pathway components have been reported in several types of human cancers. Germline loss-of-function mutations in NF2 (encoding Merlin, which facilitates activation of LATS1/2 by MST1/2) are common in neurofibromatosis type 2 [70], and germline and somatic mutations of NF2 are also reported in mesothelioma [71, 72]. Germline YAP R331W mutation has been reported to increase NSCLC risk [73]. YAP gene amplification is observed in a subset of hepatocellular carcinomas [74], esophageal squamous cell carcinomas [75], and medulloblastomas [76]. Gene fusions that occurred in YAP or TAZ are also commonly observed in epithelioid hemangioendotheliomas [77, 78]. Gene fusion and deletion in LATS2 have been found in mesothelioma [79], and a recent cancer genome analysis identified recurrent mutations in LATS1 gene in skin basal cell carcinoma [80]. In addition, YAP hyperactivation induced by activating mutations in GNAQ or GNA11 (encoding GPCR signaling components Gαq and Gα11, respectively) is the major oncogenic driver of uveal melanomas [81, 82].
Notably, when compared with gene mutation rate, elevations of YAP/TAZ expression and their nuclear enrichment are detected much more frequently in human cancers, including liver, ovary, breast, lung, and colon cancers [83–87]. Because the Hippo-YAP/TAZ pathway receives signaling inputs from diverse sources, it is likely that multiple genetic and epigenetic perturbations in upstream regulators of YAP/TAZ may exist in various cancers independent of Hippo pathway genetic alterations, comprehensively supporting tumor development. Moreover, recent studies also found that YAP activity is required in colon and pancreatic cancer tumorigenesis induced by KRAS and APC mutation, suggesting that YAP/TAZ activity cooperates with other oncogenic pathways in the tumorigenic process [88, 89]. Therefore, YAP and TAZ may contribute to a broad range of pathogenic mechanisms in cancer with various genetic and epigenetic alterations.
A recent genome-wide chromatin occupancy analysis done in human cholangiocarcinoma cells revealed that YAP binds to a subset of enhancers with high transcriptional outputs and recruits the mediator complex, an integrator of signals from multiple transcriptional regulators [90]. In this way, YAP plays a major role in defining transcriptional state and cell identity, and YAP/TAZ-driven transcriptional program in cancer cells appears to impose stem cell-like properties as well as to support cell proliferation [35, 91, 92]. It has been demonstrated that high levels of TAZ expression and activity are associated with higher grade histology and poor prognosis of breast cancer [35]. Loss of cell polarity protein Scribble in breast cancer stem cells enhances sustained self-renewal and tumor-initiation capacities by releasing TAZ from tumor suppressive Hippo kinases. Another recent study also demonstrated that increased interactions between YAP and transcription factor SRF in breast cancer cells induce a gene expression signature specific for mammary stem cells, and YAP-induced stemness acquisition involves IL-6 upregulation [91]. SRF–YAP–IL6 signaling is activated in basal-like breast cancer, and YAP/TAZ expression levels inversely correlate with patient survival.
YAP/TAZ activation also promotes invasion and metastasis of cancer cells. YAP/TAZ activation induces EMT, and rescues cancer cells from anoikis to promote their survival during circulation and spread from the primary sites [37, 62, 93]. YAP or TAZ knockdown in melanoma cells interferes with anchorage-independent growth, matrix invasion, and lung metastasis in xenograft models [94]. Conversely, overexpression of constitutively active YAP increases metastatic and invasive potential of breast cancer and melanoma cells, and the metastasis activity of breast cancer and melanoma cells is highly dependent on YAP-TEAD transcriptional activity [95]. Moreover, Lats1/2 expression levels are significantly lower in metastatic prostate cancer when compared with benign prostate tissues or localized prostate cancer, implying that Hippo downregulation and YAP/TAZ upregulation may play a role in prostate cancer metastasis [37].
Although oncogenic properties of YAP/TAZ have been revealed in a number of cancer types, studies on hematologic malignancies and breast cancer suggest tumor-suppressive roles of YAP/TAZ. YAP has been shown to bind and positively regulate p73, a tumor suppressive and pro-apoptotic protein, and this interaction is countered by AKT activation [96–98]. In addition, a study reported downregulation of YAP protein levels in breast cancer tissues, and demonstrated enhanced tumor growth after YAP knockdown [99]. Moreover, YAP expression levels are significantly reduced in hematologic cancers, and YAP downregulation prevents ABL1-induced YAP-p73 interaction which promotes DNA damage-induced apoptosis in hematologic cancers [100]. It is likely that YAP/TAZ behave either as oncogenic drivers or tumor suppressors, depending on the cellular context such as the abundance of transcriptional regulators interacting with YAP/TAZ in the nucleus.
Altogether, the evidence strongly supports critical involvement of the Hippo-YAP/TAZ pathway in cancer pathogenesis. The expanded knowledge on the Hippo-YAP/TAZ pathway allows us to understand previously unrecognized mechanisms of cancer behaviors, including stem cell-like properties, EMT, and resistance to therapies.
Role of YAP/TAZ in resistance to anti-cancer therapies
A series of studies has demonstrated YAP/TAZ activation and concordant induction of anti-cancer therapy resistance in various tumor models, eliciting intense interest in recent years. Here, we overview recent updates on the involvement of YAP/TAZ in resistance to multiple anti-cancer treatment modalities (Table 1).
Table 1.
Published studies on YAP/TAZ-dependent anti-cancer therapy resistance
| Type | Evidence | Proposed mechanism | References |
|---|---|---|---|
| Cytotoxic chemotherapy | Overexpression of TAZ in breast cancer cells promotes taxol and doxorubicin resistance | TAZ induces stem cell-related traits | [35, 92] |
| Overexpression of TAZ in breast cancer cells promotes taxol resistance | TAZ activation induces CYR61/CTGF expression, and CYR61/CTGF knockdown blocks taxol-resistance | [101] | |
| YAP mediates resistance to various anti-tubulin drugs in HeLa and other cancer cell lines | Anti-tubulin drug treatment activates Cdk1 to phosphorylate and inactivate YAP | [102] | |
| 5-FU treated colon cancer liver metastasis shows increased YAP expression, and YAP expression levels predict patient survival | c-Yes activates YAP in 5-FU resistant quiescent colon cancer cells | [103] | |
| YAP activation causes resistance to 5-FU and docetaxel. YAP is overexpressed in resistant esophageal cancer tumors and 5-FU resistant cell lines. | YAP increases EGFR expression by binding to its promoter | [104] | |
| Cisplatin-resistant oral squamous cancer cell lines show decreased phospho-YAP and increased nuclear YAP. YAP knockdown causes increased sensitivity to cisplatin | [106] | ||
| YAP activity correlates with cisplatin sensitivity of urothelial carcinoma cells. Nuclear YAP expression predicts poor outcome in urothelial carcinoma patients with chemotherapy | YAP knockdown causes increased DNA damage response and ATM activation upon cisplatin, accumulating DNA damages | [107] | |
| YAP and target gene expression are increased in cisplatin-resistant ovarian cancer cells, and YAP knockdown suppresses resistant cell viability | YAP induces increased autophagy by enhancing autolysosome degradation | [108] | |
| Molecular targeted therapy | YAP amplification drives tumor maintenance against KRAS withdrawal in inducible KRAS mutant pancreatic ductal adenocarcinoma model | The YAP/TEAD complex cooperates with E2F to activate cell cycle and DNA replication programs | [109] |
| YAP overexpression rescues KRAS mutant colon cancer cell lines from KRAS suppression | YAP induces epithelial-mesenchymal transition by interaction with FOS | [110] | |
| Combined YAP and RAF/MEK inhibition is synthetically lethal to BRAF or RAS-mutant tumors. Increased YAP expression is a biomarker of worse response to RAF/MEK inhibition in BRAF-mutant tumors | YAP induces the expression of Bcl-xL to antagonize anti-apoptotic signal | [113] | |
| BRAF inhibitor resistance of melanoma cells is dependent on YAP/TAZ activity, and inhibition of YAP/TAZ activation by actin modulation suppresses the resistance | Increased YAP/TAZ activity induces expression of E2F1-related cell cycle genes and activates EGFR, MYC, AKT | [114] | |
| YAP activation induces resistance to EGFR gefitinib in NSCLC cells. EGFR inhibitor resistant human NSCLC tumors show increased nuclear YAP staining compared with their pre-treatment tumors | YAP induces EMT, AXL expression, and ERK activation | [117] | |
| TAZ is upregulated in lung adenocarcinoma cells with EGFR T790 M mutation, and TAZ depletion sensitizes their response to gefitinib | TAZ depletion inhibits tumorigenicity, EMT, migration, and invasion of gefitinib-resistant NSCLC cells | [118] | |
| YAP activation signature is associated with poor response to cetuximab treatment in colorectal cancer patients | [119] | ||
| High TAZ expression level is associated with poor response to trastuzumab in HER2-positive breast cancer patients | [120] | ||
| YAP/TAZ inhibition diminishes matrix stiffness-dependent lapatinib resistance. Inhibition of YAP in tumor xenograft model slows the growth of HER2-amplified tumors and increases sensitivity to lapatinib | YAP and TAZ transduce resistant signals from rigid microenvironments | [121] | |
| Knockdown of YAP sensitizes ovarian cancer cells to cisplatin, EGFR inhibitor, and survivin inhibitor | PTPN14 downregulates YAP activity. PPXY motif containing PTPN14 fragment can sensitizes cancer cells to anti-cancer agents | [122] | |
| Radiotherapy | YAP expression level in pre-treatment tumor tissue correlates with poor survival of head and neck squamous cell carcinoma patients | [123] | |
| YAP overexpression promotes medulloblastoma tumorigenesis, and promotes survival of cerebellar granule neuron precursors cells after irradiation | YAP enables cells to enter mitosis with un-repaired DNA through driving IGF2/AKT activation, resulting in ATM/Chk2 inactivation | [124] | |
| YAP knockdown in urothelial carcinoma cells increases DNA damage response induced by γ-irradiation | YAP knockdown causes increased DNA damage response and ATM activation, promoting accumulation of DNA damage | [107] |
Cytotoxic chemotherapy
Cytotoxic chemotherapy agents interfere with cell division and survival by disturbing essential cellular processes, such as mitotic spindle formation, DNA replication, and the maintenance of DNA integrity. Despite widespread application of cytotoxic chemotherapy for many cancer types, complete cure of patients by cytotoxic agents is exceptional in many solid cancers. Once cancer cells acquire resistance to a certain regimen, they no longer respond to the specific regimen used. Recent studies have consistently suggested that YAP/TAZ upregulation confers resistance against a diverse range of cytotoxic agents such as anti-tubulin, anti-metabolite, and DNA-damaging agents.
TAZ overexpression in breast cancer cells has been shown to induce resistance to taxol and doxorubicin, and upregulation of YAP/TAZ target genes CYR61 and CTGF appears to contribute to resistance development [35, 92, 101]. Similarly, YAP induces resistance to various anti-tubulin drugs, and CDK1-mediated inhibitory phosphorylation on YAP elevates sensitivity to anti-tubulin drugs [102]. In addition, cancer cell lines resistant to 5-fluorouracil (5-FU) exhibit increased YAP expression and activity, and YAP is also upregulated in therapy-resistant colon and esophageal cancers [103, 104]. Notably, either knockdown or pharmacological inhibition of YAP sensitizes esophageal cancer cells towards 5-FU and docetaxel by suppressing YAP-mediated upregulation of EGFR [104]. Cisplatin and other platinum compounds are widely used DNA-damaging cytotoxic agents, comprising the backbone of chemotherapeutics against many cancers, including ovary, cervix, bladder, and testis cancer [105]. YAP has been reported to promote resistance to cisplatin in oral squamous cell carcinoma, urothelial cell carcinoma, and ovarian cancer [106–108]. Considering pervasive usage of cytotoxic agents in cancer treatment, YAP/TAZ-driven resistance to multiple cytotoxic agents is an issue that deserves intense attention.
Molecular targeted therapy
As molecular alterations of cancer have been extensively profiled by recent genomic and proteomic technologies, researchers now focus on the development of anti-cancer drugs targeting recurrently mutated oncogenic drivers. Although several targeted therapy agents against major oncogenic drivers have been developed, the effectiveness of the agents varies widely because of innate and acquired resistance. Remarkably, recent studies have demonstrated that YAP can functionally substitute for oncogenic KRAS, which is one of the most common drivers of human cancer. When the expression of oncogenic KRASG12D was withdrawn in inducible KRASG12D-driven mouse pancreatic ductal adenocarcinoma (PDAC), tumor regression occurred at first, but a major fraction of tumors relapsed [109]. The relapsed tumors acquired YAP amplification, and elevated YAP-TEAD2 activity was shown to enable bypass of oncogenic KRAS activity through upregulation of cell cycle and DNA replication genes. An independent study also showed that YAP activation can rescue KRAS-dependent colon cancer cells from loss of viability induced by shRNA-mediated suppression of KRAS [110]. These findings imply that YAP/TAZ activation may induce resistance to targeted anti-cancer agents specific for RAS signaling pathways.
The MAPK pathway (RAF–MEK–ERK) is a central node in regulating cell proliferation and survival, and oncogenic mutations in MAPK components, including BRAF, constitutively activate cell proliferation. BRAF inhibitors and MEK inhibitors have proved effective in treating BRAF mutant melanoma and NSCLC. However, acquired resistance occurs in the majority of patients in a short time period after initial tumor shrinkage [111, 112]. Lin et al. recently reported that YAP can mediate resistance to RAF and MEK inhibitor therapies [113]. They demonstrated that YAP serves as a parallel survival input to inhibit apoptosis upon RAF and MEK inhibitor treatment, and YAP suppression significantly enhances sensitivity to RAF or MEK inhibitors. In addition, they supported their findings by confirming an increase of YAP protein levels in clinical samples of melanoma and NSCLC resistant to a BRAF inhibitor. Our group added another layer upon this discovery, using an in vitro model of BRAF inhibitor resistance [114]. BRAF inhibitor-resistant melanoma cells generated by chronic drug administration exhibited increased nuclear accumulation of YAP/TAZ when compared with parental melanoma cell lines. Concordant with Lin et al.’s findings, the viability of resistant melanoma cells was highly dependent on YAP/TAZ activity. We further found that the upregulation of YAP/TAZ activity in resistant melanoma cells is associated with marked actin cytoskeleton remodeling induced by BRAF inhibitor treatment. Oncogenic mutations in MAPK pathways frequently occur throughout cancer types [115], and MAPK inhibition appears to be effective in several cancers beyond melanoma [116]. Therefore, we speculate that combined YAP and MAPK inhibition may yield fruitful outcome in preventing resistance development in multiple cancer types.
Activating EGFR mutations are an important oncogenic driver in a subset of lung adenocarcinoma, and EGFR-TKIs are effective in treating tumors with EGFR mutations. It has been shown that EGFR inhibitor-resistant NSCLC cells exhibit both increased expression and nuclear enrichment of YAP [117]. In addition, YAP overexpression decreases EGFR-TKI sensitivity. Similarly, another study proposed TAZ as a mediator of intrinsic EGFR-TKI resistance in lung adenocarcinoma with EGFR T790M mutation [118]. Monoclonal antibody cetuximab is another important EGFR targeting modality, and YAP activation signature predicts fast tumor progression and poor disease control in colorectal cancer patients treated with cetuximab [119]. Moreover, in HER2-positive breast cancer, high TAZ expression levels were associated with poor therapeutic response to HER2 monoclonal antibody, trastuzumab, as well as chemotherapy combinations [120]. Concordantly, a study also demonstrated that the anti-proliferative effect of HER2 inhibitor lapatinib is influenced by YAP activity in vitro and in vivo [121]. Reduction of YAP activity suppresses the growth of HER2-positive tumors, and a trend of increasing sensitivity to lapatinib is observed as YAP activity decrease. Knockdown of YAP has been also shown to sensitize cancer cells to EGFR-TKI erlotinib as well as to a small-molecule antagonist of survivin [122]. These studies collectively suggest that YAP and TAZ mediate resistance to a variety of molecular-targeted agents against oncogenic EGFR–RAS–MAPK signaling pathways, which are central targets of recent cancer drug development.
Radiation therapy
Radiation therapy accounts for another important axis of cancer therapy. Ionizing radiation to cancer cells causes DNA double-strand break, inducing cell cycle exit and apoptosis. Recent studies indicate that YAP mediates radiotherapy resistance, in addition to chemotherapy resistance, in several cancers. Microarray analyses of head and neck squamous cell carcinoma, comparing tumors with complete response and incomplete response to radiotherapy, revealed that high levels of YAP expression predict poor response to radiation therapy [123]. Concordant with this clinical finding, in vitro and in vivo YAP modulation in cancer models has also shown to affect radiation sensitivity. YAP knockdown in urothelial cell carcinoma potentiates DNA damage response and apoptosis by γ-irradiation, as well as by other DNA-damaging agents [107]. By contrast, YAP activation in medulloblastoma induces resistance to radiation [124]. In Shh-induced mouse medulloblastoma model, YAP overexpression maintains cell proliferation and prevents apoptosis after irradiation. Moreover, YAP acts to override cell cycle checkpoints in irradiated cerebellar granule neuron precursors, which are the origin of medulloblastoma. In another study on endometrial cancer, which is an important clinical target of radiation therapy, YAP nuclear expression levels were shown to be correlated with poor prognosis of patients, and YAP suppression in endometrial cancer cells increased sensitivity to radiation treatment [125]. Together, these results indicate that YAP/TAZ activity is implicated in the promotion of cell survival and growth in response to a wide range of anti-cancer therapies.
Molecular mechanisms of YAP/TAZ-driven resistance
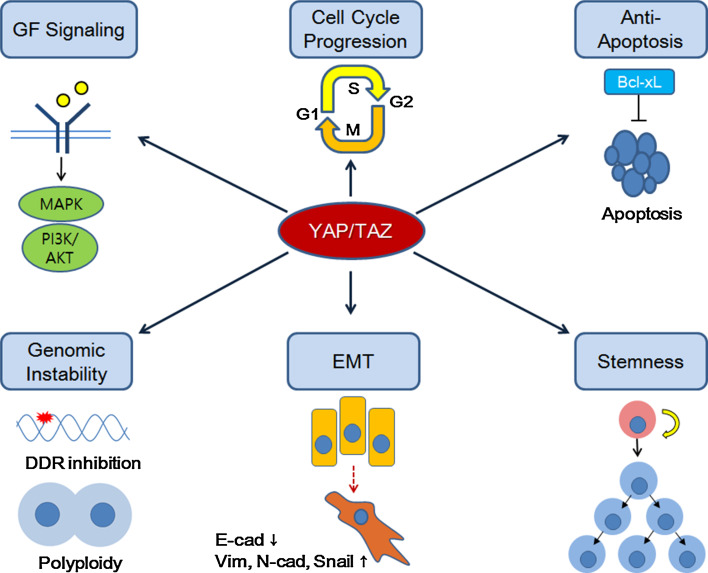
The biologic effects of YAP/TAZ mainly originate from their function in transcriptional activation of genes governing cell proliferation, survival, and stemness. The binding of Yki to Drosophila genome is enriched in proximal promoter regions [126]. However, YAP and TAZ in mammalian cells interact with a number of TEAD-binding enhancers and superenhancers in addition to gene promoters, driving high levels of transcriptional activity of a group of genes determining cellular state [90]. A genome-wide ChIP-seq study also revealed that YAP/TAZ/TEAD and AP-1 form a complex which binds to enhancers, activating transcription of target genes required for cell cycle entry and mitosis [29]. YAP/TAZ/TEAD complex not only upregulates genes expression, but can also repress gene expression by recruiting the NuRD complex which deacetylates histones at target genes [32]. Therefore, it is likely that YAP and TAZ may orchestrate pro-resistant gene transcription by regulating a complex repertoire of enhancer and promoter activity. Here, we highlight six molecular mechanisms as candidate downstream effectors of YAP/TAZ in the development of anti-cancer therapy resistance (Fig. 1).
Fig. 1.
Downstream effector mechanisms of YAP/TAZ-dependent anti-cancer therapy resistance
Activating growth factor signaling
Activation of YAP and TAZ upregulates genes encoding components of growth factor signaling which contributes to anti-cancer therapy resistance. YAP/TAZ have been shown to upregulate EGFR expression in chemoresistant esophageal squamous cell carcinoma and BRAF inhibitor-resistant melanoma [104, 114]. YAP directly binds to the promoter region of EGFR to increase its expression [104]. Moreover, YAP can induce the expression of EGFR ligand Amphiregulin to promote EGF-independent cell survival and migration [46]. Therefore, YAP/TAZ-driven drug resistance may involve potentiation of EGFR signaling and the activation of downstream MAPK and PI3K/AKT pathways. Activation of the insulin-like growth factor (IGF) pathway has been linked to drug resistance in many types of cancers [127], and a recent study indicated that YAP/TAZ upregulates the IGF pathway to promote cardiomyocyte proliferation [128]. Consistently, YAP-induced IGF2/AKT pathway activation in medulloblastoma promotes cancer cell survival upon irradiation, whereas IGF2 suppression abrogates the effect of YAP-dependent resistance [124]. These results further indicate that upregulation of growth factor signaling is a key mechanism of YAP/TAZ-driven anti-cancer therapy resistance. Inversely, EGFR and IGF signaling have been shown to enhance YAP/TAZ activity [45, 129, 130]. Thus, it is conceivable that a positive feedback loop between YAP/TAZ and EGFR/IGF pathways may further intensify drug resistance process.
Promoting cell cycle progression
The Hippo-YAP/TAZ pathway is known to mediate contact inhibition of cell proliferation and determine organ size through its role in the regulation of cell cycle entry and exit [26, 131]. The YAP/TAZ/TEAD complex directly targets enhancers of genes encoding DNA replication/repair machineries and transcriptional regulators required for cell cycle progression and mitosis [29]. In KRAS mutant PDAC model, the cooperation between YAP/TEAD and E2F1 transcription factor is essential for upregulating cell cycle machinery genes to bypass KRAS deprivation [109]. Our group also reported that depletion of YAP/TAZ in melanoma cells resistant to a BRAF inhibitor results in a significant downregulation of cell cycle/mitosis-associated genes, as well as genes with E2F motifs in their promoter [114]. These results suggest that transcriptional upregulation of cell cycle progression genes by YAP/TAZ makes cancer cells resistant to anti-proliferative effect of therapies. It is also noteworthy that LATS1/2 and YAP are involved in the regulation of p53 function. LATS2 has been shown to inhibit E3 ligase activity of MDM2 to stabilize p53 upon mitotic checkpoint activation or cytokinesis failure [132]. Consistently, LATS2 depletion blocks p53 activation in response to cytokinesis defects, allowing the propagation of tetraploid cells [133]. Moreover, LATS1/2 downregulation not only reduces tumor-suppressive role of p53, but also shifts the phosphorylation status and conformation of wild-type p53 to induce transcriptional activity similar to that of oncogenic p53 mutants [134]. YAP has also been shown to cooperate with oncogenic p53 mutants to induce transcriptional activation of cyclins and CDK1, promoting cell cycle progression [135]. Therefore, it is likely that YAP activation or LATS1/2 downregulation can interfere with growth inhibitory effect of anti-cancer therapy by disrupting the tumor-suppressor activity of wild-type p53 or potentiating the oncogenic activity of p53 mutants.
Suppressing apoptosis
YAP binds to the promoters of anti-apoptosis genes BCL2L1 and BIRC5, and upregulate their expression levels [136]. Moreover, YAP overexpression protects cells from apoptosis upon various agents, including taxol, cisplatin, staurosporine, and UV irradiation [62, 137]. In BRAF and RAS mutated cancer cell lines, the expression of BCL-xL, the anti-apoptotic isoform of BCL2L1, is dependent on YAP activity, and BCL-xL serves as a downstream effector of YAP in the development of resistance to RAF/MEK inhibitors [113]. Pharmacological inhibition of BCL-xL overcomes YAP-dependent resistance to RAF/MEK inhibitors. Therefore, YAP/TAZ activity may decrease apoptotic sensitivity to anti-cancer therapy agents by upregulating the expression of anti-apoptotic genes.
Modulating DNA damage response
Activation of YAP/TAZ has been shown to allow cancer cells to override DNA damage response induced by DNA-damaging agents and radiotherapy. Medulloblastoma cells in which YAP is activated bypass G1/S and G2/M cell cycle checkpoints induced by DNA damages, and the consequence is persistent DNA breaks and genomic instability [124]. This checkpoint bypass is ascribed to YAP-mediated activation of the IGF2-AKT pathway, which inactivates DNA damage response transducers ATM and CHK2. By contrast, YAP suppression causes increased DNA damage response and ATM activation upon cisplatin treatment, causing apoptosis of urothelial cancer cells through accumulation of DNA damages [107]. Interestingly, cytokinesis inhibition and resulting polyploidy cause an activation of Hippo kinase LATS2, which is followed by YAP/TAZ suppression and p53 activation in non-transformed cells [133]. By contrast, aberrant YAP/TAZ activation results in cytokinesis defect and polyploidy. In addition, knockout of LATS1 or LATS2 in mouse embryonic fibroblast increases cytokinesis failure and polyploidy [138]. Therefore, we speculate that elevated YAP/TAZ activity in tumors may cause genomic instability by impeding both DNA damage response and polyploidy suppression, and thus contribute to the emergence of drug resistance through mutations or copy number changes of pro-resistance genes.
Driving mesenchymal transition
In mammary epithelial cells, YAP/TAZ activation induces EMT phenotypes: loss of cell–cell contact, emergence of spindled morphology, and upregulation of mesenchymal markers, such as fibronectin, vimentin, and N-cadherin [62, 131]. EMT process of tumor cells not only underlies increased metastasis, but has also been implicated in resistance to anti-cancer therapies [139–141]. Notably, in KRAS-dependent colon cancer cell lines and a mouse lung cancer model, YAP rescues a reduction of tumor cell viability after KRAS suppression through its effect on EMT induction [110]. YAP appears to cooperate with c-FOS to activate a transcriptional program governing EMT [110]. This study suggests that YAP activity facilitates bypass of oncogenic drivers by promoting EMT of cancer cells. Although a direct causal link between YAP/TAZ-induced EMT and anti-cancer therapy resistance has not been reported yet, we propose that EMT induction may play a role in YAP/TAZ-mediated drug resistance.
Inducing stem cell-like properties
Transcriptional activation by YAP/TAZ promotes sustained self-renewal and pluripotency of stem cells, and affects expansion and lineage determination of progenitor cells in vivo [56, 142–145]. YAP/TAZ activation also induces stem cell-like properties in cancer cells. Importantly, stemness in cancer cells have been associated with properties enabling resistance to anti-cancer therapy, such as upregulation of drug transporter expression, elevated DNA repair capacity, and higher anti-apoptotic potential [146, 147]. Poorly differentiated breast cancer exhibits higher levels of YAP/TAZ target gene expression, and TAZ activity in breast cancer induces resistance to taxol and doxorubicin through elevation of stemness marker and tumor-initiating potential [35]. In addition, a study showed that Protease-Activated Receptor1 (PAR1) signaling promotes multidrug resistance in stem-like gastric cancer cells by inducing dephosphorylation and nuclear translocation of YAP through Rho GTPase activation [148]. PAR1-induced YAP activity significantly influences stem cell-like properties as well as drug efflux potential of gastric cancer cells. These results support the idea that YAP/TAZ may promote anti-cancer therapy resistance by inducing stem cell-like properties in cancer cells.
What drives YAP/TAZ activation against anti-cancer therapies?
Understanding how YAP and TAZ are activated in malignant tumors upon anti-cancer treatment would be imperative to overcome YAP/TAZ-driven resistance. Currently, molecular mechanisms of YAP/TAZ regulation during the development of dug resistance are largely unexplored for many cancer types. Although YAP/TAZ upregulation and nuclear enrichment are frequently observed in clinical samples from cancer patients, genetic alterations in Hippo-YAP/TAZ pathway components are rare. Moreover, acquired mutation in Hippo-YAP/TAZ pathway components has not yet been reported from analyses of therapy-resistant tumors. Notably, YAP/TAZ activation itself does not induce tumor formation in several tissues, but YAP/TAZ cooperate with other oncogenic pathways in the tumorigenic process [88, 89]. Thus, it is possible that elevated YAP/TAZ activity in resistant tumors might be a consequence of crosstalk with other oncogenic drivers. Importantly, recent studies have demonstrated that mutations in RAS, LKB1, or APC can activate YAP/TAZ [49, 65, 88, 149]. Oncogenic RAS promotes YAP stability by inhibiting ubiquitin ligase substrate recognition factors SOC5/6 [49, 65, 88, 149]. YAP activity is also enhanced by homozygous loss of LKB1 or APC [49, 65, 88, 149]. The emergence of additional mutations is a frequent event in therapy resistance, and thus acquired mutations in RAS, APC, and LKB1 genes, as well as in GPCR, TGFβ, and Wnt signaling components in resistant tumors may contribute to the activation of YAP/TAZ during resistance development.
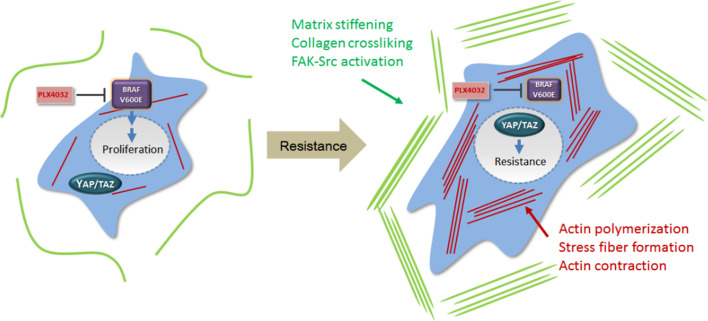
Although underlying mechanisms are not fully elucidated, it is clear that increases in intracellular tension, matrix stiffness, actin polymerization, and actin-myosin contractility result in increased YAP/TAZ nuclear localization and activity [36, 38, 150]. Thus, it is likely that mechanical stimuli and cytoskeletal architecture may influence YAP/TAZ-dependent therapy resistance in certain cancers. Our group reported actin cytoskeletal remodeling in melanoma cells, which promotes YAP/TAZ nuclear localization and consequential BRAF inhibitor resistance [114]. The actin cytoskeletal remodeling was apparently induced by changes in expression levels of multiple actin regulators in response to BRAF inhibitor treatment [114]. Moreover, another study demonstrated that lapatinib resistance in HER2-positive breast cancer cells depends on stroma rigidity-induced YAP/TAZ activation [121]. In line with these findings, BRAF inhibitor treatment makes melanoma tumor stroma stiffer through promotion of cancer-associated fibroblast activation and ECM production/remodeling [151]. Therefore, we speculate that cancer cells can adapt themselves to anti-cancer therapy by changing their mechanical properties in two ways: increasing intracellular actin cytoskeletal tension and stiffening tumor stroma by ECM remodeling (Fig. 2). These two mechanisms may synergize to upregulate YAP/TAZ activity as an adaptive response to anti-cancer therapies. Matrix stiffening and YAP/TAZ activation have also been reported to maintain cancer-associated fibroblasts [39], and a clinical study reported that combined high YAP/TAZ expression levels in non-lymphoid stromal cells and cancer cells correlate with poor neoadjuvant chemotherapy response in triple-negative breast cancer [152]. Altogether, these findings suggest that YAP/TAZ activation mechanisms associated with the tumor microenvironment play an important role in the therapy resistance development. Other studies have identified CDK1, PAR-1, and YES as potential YAP/TAZ regulators in drug resistance models [102, 103, 148]. Further research will clarify the relationship between therapy-induced adaptive responses and YAP/TAZ regulators.
Fig. 2.
YAP/TAZ activation by mechanotransduction in BRAF inhibitor resistance
YAP/TAZ as predictive biomarkers
Although preclinical studies have demonstrated that YAP/TAZ are involved in a wide range of therapeutic resistance, clinical application of YAP/TAZ as a predictive biomarker and a drug target is in its early stages. Systematic monitoring of YAP/TAZ activity in pre- and post-therapy tumor biopsy samples will fully establish YAP/TAZ as a useful clinical biomarker for predicting therapy resistance in many cancer types. YAP/TAZ activity in tumor tissues can be measured by YAP/TAZ immunohistochemical (IHC) staining. Because YAP/TAZ activity is regulated by both nuclear translocation and proteosomal degradation, YAP/TAZ scoring by IHC needs to be determined by combining subcellular localization and staining intensity. Indeed, high levels of nuclear YAP/TAZ staining have been reported to correlate with poor prognosis in breast cancer, NSCLC, and bladder cancer [85, 92, 153]. Elevated nuclear TAZ staining in HER2-positive breast cancer was associated with lower rates of pathologic complete response to trastuzumab [120]. In addition, high levels of nuclear YAP expression correlated with poor survival of urothelial cell carcinoma patients [107], and poor response to a BRAF inhibitor in melanoma and NSCLC patients [113]. Table 2 summarizes published studies that reported YAP/TAZ as therapy response markers.
Table 2.
YAP/TAZ as clinical biomarkers in predicting response to anti-cancer therapy
| Cancer type | Measurement | Predictive value of elevated YAP or TAZ expression/activity | References |
|---|---|---|---|
| Colon cancer | YAP transcript level detected by qRT-PCR | Shorter OS and DFS in colon cancer patients with liver metastasis who received neoadjuvant chemotherapy | [103] |
| Colon cancer | YAP target gene expression signature | Low response rate and shorter PFS on cetuximab treatment in colon cancer patients | [119] |
| Breast cancer | TAZ IHC staining scored by combining intensity and cellular localization | Low pCR in HER2-positive breast cancer patients who received trastuzumab-based neoadjuvant therapy | [120] |
| Urothelial cell carcinoma | Nuclear YAP IHC staining | Shorter OS and RFS in urothelial cell carcinoma patients who received perioperative chemotherapy | [107] |
| Melanoma and NSCLC | YAP IHC staining | Incomplete response to BRAF inhibitor in BRAF mutant melanoma and NSCLC | [113] |
| Head and neck squamous cell carcinoma | YAP IHC staining | Incomplete response, shorter RFS, and shorter CSS of head and neck squamous cell carcinoma patients who received primary radiotherapy or chemoradiation | [123] |
qRT-PCR quantitative reverse transcriptase polymerase chain reaction, IHC immunohistochemistry, OS overall survival, DFS disease-free survival, PFS progression-free survival, pCR pathologic complete response, RFS recurrence-free survival, CSS cause-specific survival
YAP/TAZ activity in non-cancerous cells in the tumor microenvironment also needs to be analyzed, because YAP/TAZ staining in cancer-associated fibroblasts or tumor-infiltrating lymphocytes has been reported to affect therapy response [152, 154].
Recent studies have analyzed genome-wide transcriptional signatures of YAP/TAZ activation, and demonstrated that the signatures are associated with therapy responses. Therefore, YAP/TAZ target gene signatures identified by microarray or RNA-seq analyses can be used as a surrogate marker for YAP/TAZ activation. In colon cancer, a YAP/TAZ target gene signature predicted progression-free survival after cetuximab treatment in KRAS-wild-type colon cancer [119], and YAP activation signature has also been associated with shorter progression-free survival after radiation therapy in head and neck cancer and squamous cell carcinoma [123]. Further studies will be required to establish reliable consensus signatures of YAP/TAZ activation in cancer cells and to confirm the significance of the association between the signature and therapy resistance.
Therapeutic targeting of YAP/TAZ for overcoming drug resistance
The crucial role of YAP/TAZ in resistance mechanisms proposes that therapeutic targeting of YAP/TAZ will facilitate the development of effective anti-resistance therapies. YAP mainly exerts their transcriptional activity by interaction with TEADs, and YAP–TEAD interaction is an important target for suppressing YAP-induced tumorigenesis and resistance. A screen for pharmacological inhibitors of YAP–TEAD interaction identified three porphyrin compounds [155]. Among them, verteporfin showed the strongest inhibition of the interaction between YAP and TEAD. Importantly, verteporfin suppresses YAP-induced hepatomegaly and hepatocellular carcinoma in vivo, and shows anti-tumor activity in GNAQ-mutated uveal melanoma [82]. Verteporfin has been used in photodynamic therapy for macular degeneration and pancreatic cancer, and further clinical indications need to be evaluated in a set of YAP-dependent tumors. Vestigial-like family member 4 (VGLL4) competes with YAP/TAZ for TEAD binding, suppressing transcriptional activity of YAP/TAZ [156]. VGLL4 expression levels in gastric cancer are lower than normal gastric mucosa, and inversely correlate with patient survival [156]. VGLL4 also suppresses cell proliferation and cancer progression in lung cancer [157]. A peptide drug which mimics the TEAD-interacting domain of VGLL4 (super-TDU) has been generated as an inhibitor of YAP/TAZ activity [156]. Super-TDU inhibits YAP-TEAD interaction and significantly decreases tumor growth in Helicobacter pylori-induced gastric cancer in mice.
Although kinases are generally considered druggable, Hippo pathway kinases MST1/2 and LATS1/2 are not valid targets for YAP/TAZ inhibitor development because they suppress YAP/TAZ and are tumor suppressors. YAP was originally recognized as a YES1-associated protein. SRC family kinase YES1 phosphorylates 357 tyrosine residue of YAP to promote YAP–β-Catenin–TBX5 complex activity in β-Catenin active cancers, and YES1 inhibition by dasatinib significantly decreases colon cancer cell proliferation [136]. Therefore, YES1 may be an effective target for suppressing β-Catenin/YAP-dependent tumor growth. HIPK2 has been also suggested to be a potential target for YAP/TAZ inhibition [158, 159]. Discovery of additional kinases necessary for YAP/TAZ activity will facilitate the development of pharmacological agents blocking YAP/TAZ-dependent resistance as well as tumorigenesis.
Because the actin cytoskeleton provides important inputs for Hippo-YAP/TAZ activity regulation, actin-modulating drugs would be effective in suppressing YAP/TAZ-mediated therapy resistance. Actin polymerization inhibitor cytochalasin D, actin–myosin contraction inhibitor blebbistatin, and Rho-actin pathway inhibitors C3 transferase and Y27632 have been shown to inhibit YAP/TAZ nuclear localization and their transcriptional activity [38]. Moreover, our group also showed that cytochalasin D and blebbistatin can suppress YAP-dependent BRAF inhibitor resistance [114]. However, the actin cytoskeleton is involved in a wide variety of cellular activities in addition to YAP/TAZ regulation, and thus actin-modulating drugs commonly present general toxicity. Therefore, it will be necessary to target specific actin regulators that play a direct role in resistance development. Our RNAi-based screening identified TESK1 as a candidate for druggable actin regulators acting in BRAF inhibitor-resistant melanoma [114]. Increased matrix stiffness also upregulates YAP/TAZ activity. Notably, inhibitors for lysyl oxidase-mediated collagen crosslinking have been reported to exert anti-tumor activity [160, 161], suggesting that targeting ECM stiffness would be a feasible strategy for inhibiting YAP/TAZ activity in resistant tumors.
Recent studies have shown that YAP/TAZ activity is modulated by cell metabolism. Inhibition of HMG-CoA reductase in the mevalonate pathway by statin or bisphosphonate suppresses nuclear localization and function of YAP/TAZ [43]. Statin and bisphosphonate block the generation of geranylgeranyl pyrophosphate by inhibiting mevalonic acid catabolism, and geranylgeranyl pyrophosphorylation of Rho GTPase is essential for full activation of Rho, which promotes YAP/TAZ activity [43]. Statin treatment decreases proliferation and stemness of breast cancer cells, and bisphosphonate decreases tumor size in breast cancer xenograft model. Cellular energy stress, such as glucose starvation, also regulates YAP/TAZ activity by AMPK-dependent phosphorylation of LATS and YAP [41, 42]. Thus, altering cellular energy status or AMPK pathway modulation may provide an alternative strategy to suppress YAP/TAZ in cancer cells.
Future clinical trials will test the effectiveness of YAP/TAZ inhibitors in suppressing anti-cancer therapy resistance. Either initial combination treatment or combination at the time of drug re-challenge after progression can be considered for the trials, depending on YAP/TAZ activation status of tumor tissue. Reduction of both YAP/TAZ nuclear staining and target gene expression levels in post-therapy tissues will confirm the actual blockade of YAP/TAZ activity by YAP/TAZ inhibitors administered. Because YAP/TAZ play important roles in normal physiologic activities in the human body, drug dosing and schedule as well as administration modality should be carefully optimized to minimize drug toxicity. Interestingly, YAP/TAZ inhibiting compounds metformin [41], statins [43], and bisphosphonates [43], which have been prescribed for diabetes, hypercholesterolemia and osteoporosis, respectively, are associated with lower risk of cancer occurrence [162]. Observational studies of comorbidity that investigate the clinical outcomes of cancer patients who take these agents can be designed to estimate the influence of YAP/TAZ blockade on drug resistance and patient survival.
Conclusions and perspectives
A wealth of experimental evidence now supports that YAP and TAZ constitute a baseline of resistance to multiple anti-cancer therapies. Increased nuclear localization of YAP/TAZ and higher transcriptional activities of YAP/TAZ target genes have been observed in therapy-resistant tumors. Modulation of YAP/TAZ activity in cancer cells clearly influences sensitivities to anti-cancer therapies, and this phenomenon has been consistently reproduced in many resistance models. However, the majority of studies are based on RNAi-mediated knockdown or overexpression of YAP/TAZ, and clinical evidence for the link between YAP/TAZ and drug resistance is convincing in only a few cases. YAP/TAZ activation mechanisms during resistance development in vivo are largely unexplored. Moreover, because downstream effects of YAP/TAZ activity are complex, the resistance mechanism dominant upon each therapy modality remains obscure. It is also an unresolved issue whether YAP/TAZ activation indeed confers universal resistance regardless of the type of anti-cancer therapy. Notably, a recent study has demonstrated that YAP activation in prostate cancer cells recruits CXCR2-expressing myeloid-derived suppressor cells around cancer tissues by upregulating CXCL5 chemokine [163]. It will be intriguing to test whether YAP/TAZ can also induce resistance to immunotherapy agents by recruiting immune suppressive cells into tumor microenvironment. However, elevated expression of YAP/TAZ in tumor-infiltrating lymphocytes predicts a higher response rate to chemotherapy in cervical cancer [154]. Besides, the non-canonical Hippo/MST pathway is involved in the regulation of T cell development and migration in the thymus [164]. Therefore, both cancer cells and immune cells as well as their interactions should be considered when investigating the effect of pharmacological targeting of YAP/TAZ or Hippo kinases in vivo. Even though the overall portrait of YAP/TAZ-mediated resistance has not been completed yet, it is evident that YAP/TAZ are crucially implicated in the emergence of anti-cancer therapy resistance, and targeting YAP/TAZ will yield valuable source for developing novel anti-resistance strategies.
Acknowledgements
This study was supported by the Basic Core Technology Development Program of the National Research Foundation funded by the Korean Ministry of Science, ICT and Future Planning (2016903757).
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Lavi O. Redundancy: a critical obstacle to improving cancer therapy. Cancer Res. 2015;75:808–812. doi: 10.1158/0008-5472.CAN-14-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders NA, Simpson F, Thompson EW, Hill MM, Endo-Munoz L, Leggatt G, Minchin RF, Guminski A. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO Mol Med. 2012;4:675–684. doi: 10.1002/emmm.201101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lackner MR, Wilson TR, Settleman J. Mechanisms of acquired resistance to targeted cancer therapies. Future Oncol. 2012;8:999–1014. doi: 10.2217/fon.12.86. [DOI] [PubMed] [Google Scholar]
- 5.Taylor ST, Hickman JA, Dive C. Epigenetic determinants of resistance to etoposide regulation of Bcl-X(L) and Bax by tumor microenvironmental factors. J Natl Cancer Inst. 2000;92:18–23. doi: 10.1093/jnci/92.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, Haanen J, Blank C, Wesseling J, Willems SM, Zecchin D, Hobor S, Bajpe PK, Lieftink C, Mateus C, Vagner S, Grernrum W, Hofland I, Schlicker A, Wessels LF, Beijersbergen RL, Bardelli A, Di Nicolantonio F, Eggermont AM, Bernards R. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118–122. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 7.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 9.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 10.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandala M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey KF, Zhang X, Thomas DM. The hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 16.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/S0092-8674(03)00549-X. [DOI] [PubMed] [Google Scholar]
- 18.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 19.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 21.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 22.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 23.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Kim M, Kim T, Johnson RL, Lim DS. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11:270–282. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, Agarinis C, Schmelzle T, Bouwmeester T, Schubeler D, Bauer A. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 36.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 37.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 39.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 41.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, Bardeesy N, Liu J, Wu X. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 44.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 52.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, Lim DS. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G, Vakili K. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–G204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 59.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, Morikawa T, Hikasa H, Eggenschwiler J, Yabuta N, Nojima H, Nakagawa K, Hata Y, Nishina H, Mimori K, Mori M, Sasaki T, Mak TW, Nakano T, Itami S, Suzuki A. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, Kim C, Camargo FD. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SE, Lee JU, Lee MH, Ryu MJ, Kim SJ, Kim YK, Choi MJ, Kim KS, Kim JM, Kim JW, Koh YW, Lim DS, Jo YS, Shong M. RAF kinase inhibitor-independent constitutive activation of Yes-associated protein 1 promotes tumor progression in thyroid cancer. Oncogenesis. 2013;2:e55. doi: 10.1038/oncsis.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, Bolouri H, Kutyavin VI, Morrissey C, True LD, Nelson PS, Vasioukhin V. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, Lambert PF, Yang P, Davis JS, Wang C. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7:1426–1449. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eisinger-Mathason TS, Mucaj V, Biju KM, Nakazawa MS, Gohil M, Cash TP, Yoon SS, Skuli N, Park KM, Gerecht S, Simon MC. Deregulation of the Hippo pathway in soft-tissue sarcoma promotes FOXM1 expression and tumorigenesis. Proc Natl Acad Sci USA. 2015;112:E3402–E3411. doi: 10.1073/pnas.1420005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruttledge MH, Andermann AA, Phelan CM, Claudio JO, Han FY, Chretien N, Rangaratnam S, MacCollin M, Short P, Parry D, Michels V, Riccardi VM, Weksberg R, Kitamura K, Bradburn JM, Hall BD, Propping P, Rouleau GA. Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet. 1996;59:331–342. [PMC free article] [PubMed] [Google Scholar]
- 71.Baser ME, Rai H, Wallace AJ, Evans DG. Neurofibromatosis 2 (NF2) and malignant mesothelioma in a man with a constitutional NF2 missense mutation. Fam Cancer. 2005;4:321–322. doi: 10.1007/s10689-005-0659-8. [DOI] [PubMed] [Google Scholar]
- 72.Sekido Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int. 2011;61:331–344. doi: 10.1111/j.1440-1827.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H, Chang YH, Chen CY, Yang HI, Hsu CP, Yang TY, Chen KC, Hsu KH, Tseng JS, Hsia JY, Chuang CY, Yuan S, Lee MH, Liu CH, Wu GI, Hsiung CA, Chen YM, Wang CL, Huang MS, Yu CJ, Chen KY, Tsai YH, Su WC, Chen HW, Chen JJ, Chen CJ, Chang GC, Yang PC, Li KC. R331W missense mutation of oncogene YAP1 Is a Germline risk allele for lung adenocarcinoma with medical actionability. J Clin Oncol. 2015;33:2303–2310. doi: 10.1200/JCO.2014.59.3590. [DOI] [PubMed] [Google Scholar]
- 74.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, Chen HW, Pathan N, Krausz T, Dickson BC, Weinreb I, Rubin MA, Hameed M, Fletcher CD. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T, Akatsuka S, Horio Y, Hida T, Kondo Y, Toyokuni S, Osada H, Sekido Y. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 80.Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, Seplyarskiy VB, Sharpe HJ, McKee T, Letourneau A, Ribaux PG, Popadin K, Basset-Seguin N, Chaabene RB, Santoni FA, Andrianova MA, Guipponi M, Garieri M, Verdan C, Grosdemange K, Sumara O, Eilers M, Aifantis I, Michielin O, de Sauvage FJ, Antonarakis SE, Nikolaev SI. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 81.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, Chen Q, Gutkind JS. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, group AS. Bowtell DD, Harvey KF. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 87.Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 88.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, Chetram M, Joshi M, Wang F, Kallakury B, Toretsky J, Wellstein A, Yi C. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 90.Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, Pepe-Mooney B, Zhang T, Geeven G, Gray NS, de Laat W, Calogero RA, Camargo FD. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim T, Yang SJ, Hwang D, Song J, Kim M, Kyum Kim S, Kang K, Ahn J, Lee D, Kim MY, Kim S, Seung Koo J, Seok Koh S, Kim SY, Lim DS. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. doi: 10.1038/ncomms10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, Amabile MI, Pilozzi E, Patrizii M, Biffoni M, Maugeri-Sacca M, Piccolo S, De Maria R. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 93.Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498. doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nallet-Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, Sudol M, Herlyn M, Mauviel A. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J Invest Dermatol. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 97.Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 98.Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O’Neill E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 100.Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri A, Ponzoni M, Marcatti M, Richardson PG, Carrasco R, Kimmelman AC, Wong KK, Caligaris-Cappio F, Blandino G, Kuehl WM, Anderson KC, Tonon G. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y, Khanal P, Savage P, She YM, Cyr TD, Yang X. YAP-induced resistance of cancer cells to antitubulin drugs is modulated by a Hippo-independent pathway. Cancer Res. 2014;74:4493–4503. doi: 10.1158/0008-5472.CAN-13-2712. [DOI] [PubMed] [Google Scholar]
- 103.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monte D, Stechly L, Skrypek N, Langlois C, Grard G, Millet G, Leteurtre E, Dumont P, Truant S, Pruvot FR, Hebbar M, Fan F, Ellis LM, Formstecher P, Van Seuningen I, Gespach C, Polakowska R, Huet G. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, Wu TT, Johnson RL, Hung MC, Ajani JA (2015) The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res [DOI] [PMC free article] [PubMed]
- 105.Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 106.Yoshikawa K, Noguchi K, Nakano Y, Yamamura M, Takaoka K, Hashimoto-Tamaoki T, Kishimoto H. The Hippo pathway transcriptional co-activator, YAP, confers resistance to cisplatin in human oral squamous cell carcinoma. Int J Oncol. 2015;46:2364–2370. doi: 10.3892/ijo.2015.2948. [DOI] [PubMed] [Google Scholar]
- 107.Ciamporcero E, Shen H, Ramakrishnan S, Yu KuS, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti S, Daga M, Azabdaftari G, Attwood K, Johnson C, Zhang J, Barrera G, Pili R. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35:1541–1553. doi: 10.1038/onc.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao L, Shi XY, Zhang Y, Zhu Y, Zhu L, Tian W, Zhu BK, Wei ZL. YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. Onco Targets Ther. 2016;9:1105–1114. doi: 10.2147/OTT.S112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, Zwang Y, Roberts TM, Root DE, Jacks T, Hahn WC. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 113.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, Pham L, Wang MM, Karachaliou N, Cao MG, Manzano JL, Ramirez JL, Torres JM, Buttitta F, Rudin CM, Collisson EA, Algazi A, Robinson E, Osman I, Munoz-Couselo E, Cortes J, Frederick DT, Cooper ZA, McMahon M, Marchetti A, Rosell R, Flaherty KT, Wargo JA, Bivona TG. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim MH, Kim J, Hong H, Lee SH, Lee JK, Jung E, Kim J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016;35:462–478. doi: 10.15252/embj.201592081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 116.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee JE, Park HS, Lee D, Yoo G, Kim T, Jeon H, Yeo MK, Lee CS, Moon JY, Jung SS, Kim JO, Kim SY, Park DI, Park YH, Lee JC, Oh IJ, Lim DS, Chung C. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem Biophys Res Commun. 2016;474:154–160. doi: 10.1016/j.bbrc.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 118.Xu W, Wei Y, Wu S, Wang Y, Wang Z, Sun Y, Cheng SY, Wu J. Up-regulation of the Hippo pathway effector TAZ renders lung adenocarcinoma cells harboring EGFR-T790 M mutation resistant to gefitinib. Cell Biosci. 2015;5:7. doi: 10.1186/2045-3701-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS, Jang HJ, Park YY, Kopetz S, Kim SS, Oh SC, Lee JS. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21:357–364. doi: 10.1158/1078-0432.CCR-14-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, Di Benedetto A, Natoli C, Gamucci T, Angelucci D, Ramieri MT, Di Lauro L, Sergi D, Bartucci M, Dattilo R, Pagliuca A, De Maria R, Maugeri-Sacca M. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget. 2014;5:9619–9625. doi: 10.18632/oncotarget.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, LaBarge MA. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI, Wang Q. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–2229. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akervall J, Nandalur S, Zhang J, Qian CN, Goldstein N, Gyllerup P, Gardinger Y, Alm J, Lorenc K, Nilsson K, Resau J, Wilson G, Teh B. A novel panel of biomarkers predicts radioresistance in patients with squamous cell carcinoma of the head and neck. Eur J Cancer. 2014;50:570–581. doi: 10.1016/j.ejca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Fernandez LA, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, Nahle Z, Kenney AM. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsujiura M, Mazack V, Sudol M, Kaspar HG, Nash J, Carey DJ, Gogoi R. Yes-associated protein (YAP) modulates oncogenic features and radiation sensitivity in endometrial cancer. PLoS ONE. 2014;9:e100974. doi: 10.1371/journal.pone.0100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oh H, Slattery M, Ma L, Crofts A, White KP, Mann RS, Irvine KD. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 2013;3:309–318. doi: 10.1016/j.celrep.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L, Luu HH. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 131.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Furth N, Bossel Ben-Moshe N, Pozniak Y, Porat Z, Geiger T, Domany E, Aylon Y, Oren M. Down-regulation of LATS kinases alters p53 to promote cell migration. Genes Dev. 2015;29:2325–2330. doi: 10.1101/gad.268185.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Agostino S, Sorrentino G, Ingallina E, Valenti F, Ferraiuolo M, Bicciato S, Piazza S, Strano S, Del Sal G, Blandino G. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2016;17:188–201. doi: 10.15252/embr.201540488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee KK, Yonehara S. Identification of mechanism that couples multisite phosphorylation of Yes-associated protein (YAP) with transcriptional coactivation and regulation of apoptosis. J Biol Chem. 2012;287:9568–9578. doi: 10.1074/jbc.M111.296954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yabuta N, Okada N, Ito A, Hosomi T, Nishihara S, Sasayama Y, Fujimori A, Okuzaki D, Zhao H, Ikawa M, Okabe M, Nojima H. Lats2 is an essential mitotic regulator required for the coordination of cell division. J Biol Chem. 2007;282:19259–19271. doi: 10.1074/jbc.M608562200. [DOI] [PubMed] [Google Scholar]
- 139.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita M, Chan Z, Baron A, Franklin W, Drabkin HA, Girard L, Gazdar AF, Minna JD, Bunn PA., Jr Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 141.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64:489–500. [PMC free article] [PubMed] [Google Scholar]
- 142.Beverdam A, Claxton C, Zhang X, James G, Harvey KF, Key B. Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J Invest Dermatol. 2013;133:1497–1505. doi: 10.1038/jid.2012.430. [DOI] [PubMed] [Google Scholar]
- 143.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 144.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Skibinski A, Breindel JL, Prat A, Galvan P, Smith E, Rolfs A, Gupta PB, Labaer J, Kuperwasser C. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep. 2014;6:1059–1072. doi: 10.1016/j.celrep.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 147.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujimoto D, Ueda Y, Hirono Y, Goi T, Yamaguchi A. PAR1 participates in the ability of multidrug resistance and tumorigenesis by controlling Hippo-YAP pathway. Oncotarget. 2015;6:34788–34799. doi: 10.18632/oncotarget.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, Voorhoeve PM, Cohen SM. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. EMBO J. 2014;33:2447–2457. doi: 10.15252/embj.201489385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 151.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vici P, Ercolani C, Di Benedetto A, Pizzuti L, Di Lauro L, Sperati F, Terrenato I, Gamucci T, Natoli C, Di Filippo F, Botti C, Barba M, Mottolese M, De Maria R, Maugeri-Sacca M. Topographic expression of the Hippo transducers TAZ and YAP in triple-negative breast cancer treated with neoadjuvant chemotherapy. J Exp Clin Cancer Res. 2016;35:62. doi: 10.1186/s13046-016-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ, Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, Zeng YX, Zhou FJ, Xie D. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buglioni S, Vici P, Sergi D, Pizzuti L, Di Lauro L, Antoniani B, Sperati F, Terrenato I, Carosi M, Gamucci T, Vincenzoni C, Mariani L, Vizza E, Venuti A, Sanguineti G, Gadducci A, Barba M, Natoli C, Vitale I, Mottolese M, De Maria R, Maugeri-Sacca M. Analysis of the hippo transducers TAZ and YAP in cervical cancer and its microenvironment. Oncoimmunology. 2016;5:e1160187. doi: 10.1080/2162402X.2016.1160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, Wang X, Guo T, Li P, Zhao Y, Ji H, Zhang L, Zhou Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 157.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, Li F, Chen H, Zhou Z, Zhang L, Ji H. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poon CL, Zhang X, Lin JI, Manning SA, Harvey KF. Homeodomain-interacting protein kinase regulates Hippo pathway-dependent tissue growth. Curr Biol. 2012;22:1587–1594. doi: 10.1016/j.cub.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 159.Chen J, Verheyen EM. Homeodomain-interacting protein kinase regulates Yorkie activity to promote tissue growth. Curr Biol. 2012;22:1582–1586. doi: 10.1016/j.cub.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 160.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen YY, Weaver VM. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10:625–642. doi: 10.1038/nrclinonc.2013.169. [DOI] [PubMed] [Google Scholar]
- 163.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Du X, Yu A, Tao W. The non-canonical Hippo/Mst pathway in lymphocyte development and functions. Acta Biochim Biophys Sin. 2015;47:60–64. doi: 10.1093/abbs/gmu112. [DOI] [PubMed] [Google Scholar]