Abstract
CFTR biogenesis starts with its co-translational insertion into the membrane of endoplasmic reticulum and folding of the cytosolic domains, towards the acquisition of a fully folded compact native structure. Efficiency of this process is assessed by the ER quality control system that allows the exit of folded proteins but targets unfolded/misfolded CFTR to degradation. If allowed to leave the ER, CFTR is modified at the Golgi and reaches the post-Golgi compartments to be delivered to the plasma membrane where it functions as a cAMP- and phosphorylation-regulated chloride/bicarbonate channel. CFTR residence at the membrane is a balance of membrane delivery, endocytosis, and recycling. Several adaptors, motor, and scaffold proteins contribute to the regulation of CFTR stability and are involved in continuously assessing its structure through peripheral quality control systems. Regulation of CFTR biogenesis and traffic (and its dysregulation by mutations, such as the most common F508del) determine its overall activity and thus contribute to the fine modulation of chloride secretion and hydration of epithelial surfaces. This review covers old and recent knowledge on CFTR folding and trafficking from its synthesis to the regulation of its stability at the plasma membrane and highlights how several of these steps can be modulated to promote the rescue of mutant CFTR.
Keywords: CFTR, Cystic fibrosis, Endoplasmic reticulum quality control, Folding, Trafficking, Membrane stability
Introduction
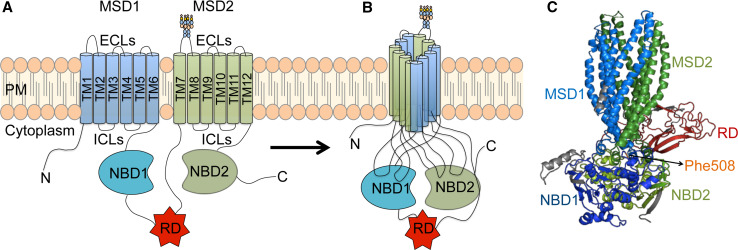
Cystic fibrosis (CF) is the most common autosomal recessive lethal disorder in the Caucasian population and results from mutations in the cystic fibrosis transmembrane conductance Regulator (CFTR) gene [1]. This gene encodes the CFTR protein that functions as a cAMP-regulated chloride (Cl−) channel at the apical surface of epithelial cells. CFTR, or ABCC7, as it is designated for being a member of the ATP-binding cassette (ABC) transporter superfamily, is composed of five domains: two membrane-spanning domains (MSD1 and MSD2), each one composed of six transmembrane segments (TM1-6 and TM7-TM12), that form the pore of the channel allowing Cl− (and also bicarbonate HCO3 −) to flow across the membrane and two cytosolic nucleotide binding domains (NBD1 and NBD2), where adenosine triphosphate (ATP) is hydrolysed, regulating channel gating—plus a fifth CFTR-exclusive regulatory domain (RD) that contains multiple phosphorylation sites, also relevant for channel activity [2] (Fig. 1).
Fig. 1.
CFTR protein structure. a CFTR protein is composed of five domains—two membrane-spanning domains (MSD1 and MSD2), each one composed of six transmembrane segments (TM1–6 and 7–12) forming the channel pore, two cytosolic nucleotide binding domains (NBD1 and NBD2), and a regulatory domain (RD). b Five domains pack together with a swap structure involving transmembrane segments from the two MSDs and close contacts of intracellular loops with sequences within the NBDs. c Such intramolecular interactions lead to a compact structure of the native protein, such as the one depicted in this structural model for CFTR (figure prepared using the open source program PyMOL distributed by Schrödinger (https://www.pymol.org/) and the PDB coordinates for CFTR reported by [16])
Since CFTR is a complex protein with multiple domains, its processing and trafficking are highly regulated allowing the correct delivery of newly synthesized proteins to the plasma membrane (PM). CFTR biogenesis starts with co-translational folding of the nascent polypeptide as it is translocated, inserted in the endoplasmic reticulum (ER) membrane, and core-glycosylated. After proper folding, CFTR travels from the ER to the Golgi apparatus, where the glycan moieties are processed, and finally, mature CFTR is carried in vesicles from the trans-Golgi network (TGN) to the PM [3]. At the PM, CFTR levels result from a balance between membrane delivery (anterograde trafficking), endocytosis, and recycling [4].
To date, more than 2000 mutations have been reported in the CFTR gene and ultimately lead to CFTR channel dysfunction resulting in a wide spectrum of clinical manifestations in CF patients [5]. The F508del mutation, a deletion of phenylalanine 508 in NBD1, is the most common cause of CF, occurring in approximately 85 % of CF patients in at least one allele, and leads to CFTR misfolding and ER retention. As a result, the mutant protein is prematurely degraded, thus precluding its delivery to the cell surface [3]. This occurs as the misfolded protein is recognized by the ER quality control (ERQC) machinery—that monitors proper protein folding and domain assembly to prevent the accumulation of abnormal and non-functional proteins that would greatly impair the secretory pathway. Proteins that fail to fold properly are retained, retro-translocated, and ultimately degraded [4, 6, 7]. Many studies have focused on CFTR folding and trafficking to the PM, as well as on the intervening quality control mechanisms so as to find targets that can modulate and improve the efficacy of mutant CFTR rescue.
This review covers the most relevant knowledge about CFTR biogenesis, folding, and trafficking from its place of synthesis to its place of function. We also address the impact of the F508del mutation in these events and summarize the current approaches to promote its rescue.
CFTR biogenesis and folding in the endoplasmic reticulum
CFTR membrane insertion and domain assembly
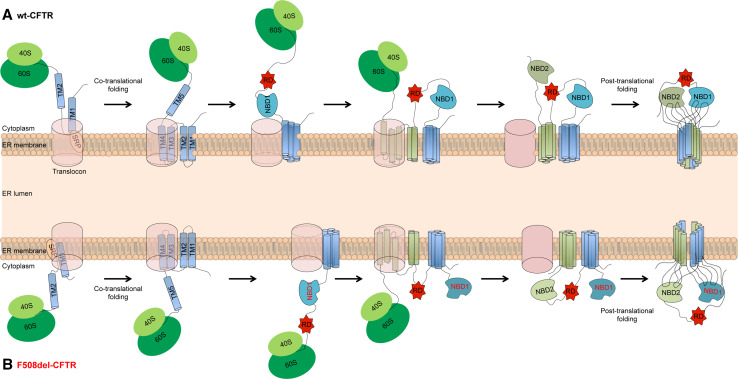
Folding of CFTR is achieved through co- and post-translational mechanisms [8] (Fig. 2) that cooperate as early as the synthesis of MSD1, the first domain to be translated [9]. The early studies on the topogenesis of TM1 and TM2 segments from MSD1 proposed that the TM1 segment contains a signal sequence important for defining CFTR topology and folding at the ER membrane. Once the TM1 segment emerges from the ribosome, the signal sequence is recognized by the signal recognition particle (SRP), promoting the insertion of C-terminal flanking sequences into the ER membrane through the Sec61 translocon to establish the C-trans orientation of the protein [10]. Then, the TM2 segment emerges and the CFTR topology at the ER membrane is defined by a co-translational mechanism. However, the signal sequence found in TM1 appears to be inefficient in promoting the ribosome-mediated translocation of CFTR. This is due to the presence of two charged residues, Glu92 and Lys95, in the otherwise hydrophobic core of TM1. An additional and complementary signal sequence is thereby present in TM2 [8]. An alternative mechanism for CFTR ER insertion was then proposed, whereby TM1 segments which fail to bind SRP emerge directly into the cytosol, eventually leading to the TM2 signal sequence being recognized by the SRP and initiating the translocation of its own N-terminal flanking sequence, thereby establishing a N-trans orientation of CFTR at the ER membrane [8]. Consequently, the TM1 segment will achieve a proper membrane-spanning topology at the ER membrane by a post-translational mechanism mediated by the peptide loop between TM1 and 2—extracellular loop 1 (ECL1). Thus, the integration of TM1 and TM2 segments at the ER membrane occurs mainly when both are already present [8]. TM3 and TM4 also seem to function together when inserting into the translocon, probably because the TM3 segment of MSD1 also contains an inefficient signal sequence. This suggests a mechanism similar to TM1, in which TM3 arises from the ribosome directly to the cytosol and the peptide loop created between TM3-4 (ECL2) leads its post-translational insertion at the ER membrane. Subsequently, TM5 arises from the ribosome, creating a peptide loop with TM6 (ECL3) before insertion into the ER membrane [11]. The fact that both ECL2 and ECL3 are very short results in this segment pair being a single topogenic determinant when inserting into the membrane, in what appears to be a common characteristic of many membrane proteins [12]. Additional studies demonstrated that several TM segments of the MSD1-2 domains of CFTR (TM3–7 and TM9–11) can also be inserted independently into the ER membrane [13].
Fig. 2.
Model for the co- and post-translational folding of CFTR. a When nascent wt-CFTR emerges from the ribosome, the signal(s) in the TM segments are recognized by the signal recognition particle (SRP) anchoring the nascent protein to the translocon. The domains are inserted in the ER membrane and translocated or remain in the cytosol as CFTR is translated. Post-translational interactions involving the intracellular loops are essential for a proper protein conformation. b F508del (represented in red) impairs CFTR folding and domain–domain interactions, leading to its ER retention and degradation
This post-translational folding of at least some of the TM pairs was observed by in vitro translation approaches coupled to limited proteolysis that evidenced that CFTR folding is almost completed during its synthesis and the most pronounced post-translational conformational change is observed mainly for the MSDs [14]. These post-translational changes are responsible for the complex domain swap structure in which the transmembrane segments are organized around the channel pore in two subgroups that are formed by TM1–2, 9–12 and by TM3–6, 7–8.
Both NBD1 and RD domains were found to emerge and fold in the cytosol by a co-translational mechanism [14]. NBD1 folding progresses through folding of the individual subdomains: first, the N-terminal ATP binding, followed the alpha-helical, and, then, the alpha/beta-core subdomains [15]. After NBD1 and RD domains emerge into the cytosol, MSD2 is inserted into the ER membrane. Correct insertion of TM8 in the ER membrane is dependent on TM7, with the process relying on physical interaction through ECL4 [10, 11]. TM8 terminates the translocation of the ECL4 loop into the ER lumen and prevents the insertion of intracellular loop 4 (ICL4), acting as a stop transfer sequence. TM7 and TM8 apparently work in a cooperative manner. For the TM9–10 and TM11–12 segments of MSD2 domain, the mechanism is similar to that observed for TM3–4 and 5–6 in MSD1 [11, 13]. Taken together, the six pairs of transmembrane segments seem to work together as topogenic signals, with TM1–2 and TM7–8 exhibiting more complex integration patterns, whereas TM3–4, 5–6, 9–10, and 11–12 insert as helical hairpins, probably due to the presence of very short ECLs. Finally, for NBD2, the last domain to be translated, evidence supports the idea that its folding is mainly co-translational [14].
Although some steps of the CFTR folding remain unclear, the available data support an idea that each CFTR domain folds independently and CFTR achieves its native conformation primarily through a co-translational mechanism followed and/or complemented by a post-translational process for the acquisition of compactly folded domains. These processes require domain–domain interactions that begin in the early steps of CFTR folding to achieve a proper conformation. Domain–domain interactions are thus critical for the acquisition of the native conformation and these involve specific interaction of NBD1 with MSD2 through ICL4 and NBD2 with MSD1 through ICL2 [16]. Interestingly, when expressed alone or even through various incomplete combinations, CFTR domains are recognized in vivo as non-native. This suggests that interdomain interactions are critical to achieve the full native state by a cooperative mechanism. This hypothesis is supported by the observation that CF-causing mutations residing in one domain lead to conformational defects in other domains [17], namely MSD mutations affecting NBD folding and NBD mutations affecting the acquisition of a compact MSD structure [18].
Impact of F508del mutation on CFTR structure and folding
The most common CF-causing mutation, F508del, results in a conformational defect in CFTR [19]. Although located in NBD1, studies with the isolated domain revealed little or no structural changes apart from the surface topography close to the F508 position [20, 21].
Nonetheless, several reports have identified an intrinsic NBD1 folding problem, initially reported as a delayed folding kinetics of F508del-NBD1 when compared with wt-NBD1 [22], and later confirmed by the observation that some amino-acid changes within NBD1 can not only contribute to the solubilization of the purified domain, but also to the rescue of full-length F508del-CFTR to the PM [23] and increased binding affinity of F508del-NBD1 to Hsp70 [24].
The overall defect has also been attributed to interferences with domain–domain interactions, leading to impaired CFTR folding [16, 25] and enhanced protein degradation rate [26]. The contacts between NBD1 and ICL4 appear to be compromised by the absence of F508del. These interdomain contacts were reported to be corrected by suppressor mutations that reverse the F508del defect by restoring the NBD1-ICL4 interface [25]. Revertant R1070W was shown to substantially improve CFTR maturation [25, 27, 28], and the bulky side chain of the tryptophan residue suggested to fill in the space left empty by the absence of F508 at the NBD1-ICL4 interface [16, 27, 28]. Similarly, V510D also restores this contact by promoting the formation of a salt bridge between the aspartate residue at position 510 and R1070 [29]. The second site mutation G550E was also shown to restore F508del-CFTR function. This mutation lies in the LSGGQ motif of NBD1, suggesting that this change promotes ATP binding, in turn, leading to NBD1 stabilization [30] and to the intramolecular dimerization of NBD1 and NBD2 [28], which is critical not only for the compactness of CFTR but also for its gating mechanism.
ERQC folding checkpoints
CFTR folding is tightly regulated to allow correct insertion of the protein in the ER membrane and proper maturation. Thus, mutant proteins which fail to achieve a native conformation are retained in the ER. This retention is promoted by the ER quality control (ERQC) machinery, which recognizes non-native proteins and directs them for degradation via the ubiquitin–proteasome pathway [6, 7, 28].
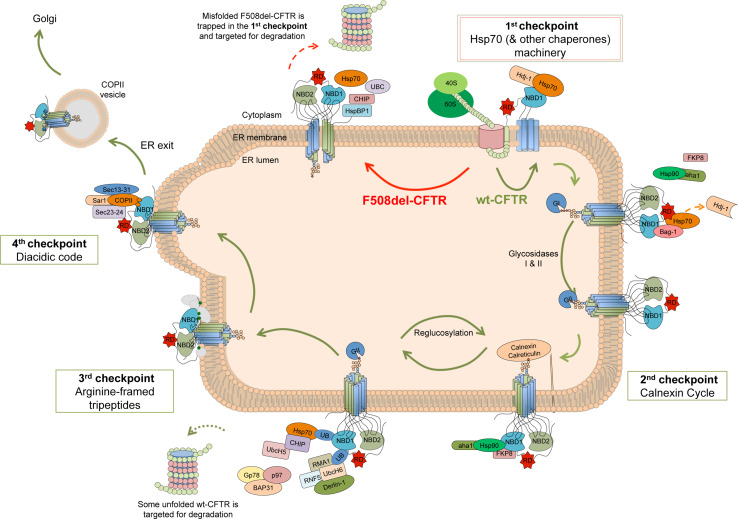
The ERQC machinery is comprised of cytosolic and ER chaperones and lectins that interact with immature CFTR, assisting its folding and assembly. Although it is not completely understood how the ERQC distinguishes a native protein conformation from a misfolded one, for CFTR, at least four checkpoints are known to be involved (Fig. 3) [4, 7, 30].
Fig. 3.
ERQC machinery regulating CFTR folding and trafficking. Schematic model of ERQC checkpoints for CFTR. When the nascent polypeptide emerges from the ribosome, it interacts with chaperones/co-chaperones that assist its folding (first checkpoint). Then, the immature protein is glycosylated and interacts with the calnexin/calreticulin system (second checkpoint). CFTR retention in the ER is modulated through the exposure of retention/retrieval motifs (AFTs) that form a third checkpoint at the ER exit sites. As CFTR achieves a correct folding, these AFTs are internalized and export motifs mediate its inclusion into COPII vesicles (fourth checkpoint). Misfolded F508del-CFTR is retained in the ER being trapped at the first checkpoint (dependent on the Hsp70 machinery) and subsequently degraded at the proteasome
The first CFTR checkpoint occurs as nascent polypeptide chains emerge from the ribosome and interact the cytosolic chaperone Hsp70 and its co-chaperones of the Hsp40 family [31, 32]. Hsc70 and its co-chaperone Hdj-2 are involved in the early steps of wt-CFTR biogenesis, facilitating its folding process [32]. Therefore, Hsp70 and Hdj-1 co-expression stabilizes wt-CFTR. However, overexpressing this chaperone pair does not contribute to the escape of F508del-CFTR from the ER [31], probably due to a strong recognition of the folding defect, which has been extensively reported in terms of increased binding both in vivo [33] and in vitro [24]. This checkpoint seems to be very early in CFTR biogenesis, thus accounting for most of the retention of F508del-CFTR [7]. The chaperone system Hsp90 was also reported to regulate CFTR folding, although at a later stage of its biogenesis [34]. Interestingly, the Hsp90 co-chaperone Aha1 has a stronger interaction with F508del-CFTR than with wt-CFTR and its downregulation rescues the mutant’s traffic and function, suggesting that this chaperone/co-chaperone complex has a crucial role in trapping F508del-CFTR at the ER based on its folding state [35, 36]. FKP8, an additional Hsp90 co-chaperone with peptidylprolyl isomerase activity, also assists CFTR folding.
Altogether, the Hsp70/40/90 chaperone systems appear to form a chaperone trap [37] responsible, at least partially, for the partitioning of mutant CFTR between the folding and degradative pathways. In fact, when CFTR is retained for too long in this trap [38], pro-degradative co-chaperones are thought to replace productive ones thus targeting CFTR to degradation. The E3 ubiquitin ligase CHIP (Carboxy-Terminal of Hsp70 Interacting Protein) has been proposed to have a key role in this process by working together with the E2 enzyme UbcH5 [39, 40]. In addition, a complex formed by RMA1 and RNF5 associates with Derlin-1 and the E2 ligase Ubc6e to ubiquitinate CFTR [41], an event that is followed by recruitment of other partners, such as Gp78, BAP31, and p97, that deliver ubiquitinated CFTR to the proteasome [42, 43]. Bag-1, another co-chaperone of Hsp70, was shown to have a twofold role on F508del-CFTR stabilization: on one side contributing to the release of the mutant protein from the Hsp70 machinery [39] and on the other competing with ubiquitin for binding to F508del-CFTR, thus preventing its proteasomal proteolysis [44]. J-domain proteins—the family of co-chaperones that potentiate Hsp70 ATPase activity and to which Hsp40 belongs—also have a role in this pro-degradation pathway, as reported for cysteine string proteins (Csp). Overexpression of Csp blocks CFTR maturation, suggesting a role in regulating CFTR release from the Hsp70 machinery [45].
The second CFTR checkpoint involves the close association between folding and N-glycosylation in the ER. As it occurs for secretory pathway proteins, nascent CFTR undergoes co-translational core glycosylation, which consists in the addition of a 14-unit oligosaccharide by ER membrane-resident oligosaccharyltransferase: two N-acetylglucosamine, nine mannose, and three glucose residues. These glycan moieties decorate residues Asn894 and Asn900, both lying within glycosylation consensus sequences (Asn-X-Ser/Thr) in ECL4 [9, 13].
This core oligosaccharide is processed in the ER, initially with the removal of the first two glucose units by glucosidase I (GI). This modification results in a monoglucosylated structure that is recognized by the chaperone lectins calnexin (CNX) and calreticulin, which assist CFTR folding. Upon removal of the last glucose residue by glucosidase II (GII), affinity for CNX decreases. If folding is unproductive, the protein becomes a target of UDP-glycoprotein glucosyltransferase (UGGT), which promotes its re-glucosylation. Hereupon, a new round of chaperone binding, de-glucosylation, and folding assessment begins [46]. When retained for too long in this cycle, unfolded CFTR may became a substrate for mannosidase activity elicited by EDEM proteins, creating a Man8B-determinant that is a signal for glycoprotein ER-associated degradation (GERAD) [7].
CNX binds both wt-CFTR and F508del-CFTR, the latter with increased affinity [7, 47, 48]. This interaction is not essential for CFTR trafficking through the ER, since unglycosylated wt-CFTR, which cannot bind to calnexin, still progresses from the ER to the plasma membrane in a functional state [7, 48]. However, it was reported that upon compromising the CFTR-calnexin interaction, wt-CFTR processing is decreased, while F508del-CFTR is not affected. In contrast, when this interaction is forced (by calnexin overexpression), immature wt-CFTR is stabilized [7]. Thus, N-glycosylation and calnexin appear to have a relevant role in the ERQC of CFTR, being important for the folding of immature wt-CFTR. Most F508del-CFTR appears to be excluded from this calnexin/calreticulin checkpoint, being directed to degradation in the earlier Hsp70-dependent checkpoint. As GII removes the third glucose residue, folded CFTR dissociates from calnexin/calreticulin and traffics out of the ER to achieve a complex glycosylation pattern in the Golgi apparatus [7, 9].
ERQC trafficking checkpoints
Assessment of CFTR folding is coupled to its export from the ER to the Golgi—a step at which the trafficking machinery recognizes specific export/retention signals in the protein. One such process is mediated by arginine-framed tripeptides (AFTs) that mediate CFTR retention by the ER machinery [49] and constitute the third CFTR ERQC checkpoint. AFTs are transient retention/retrieval motifs with the consensus arginine/any amino-acid/arginine (Arg/X/Arg—RXR) [49]. CFTR possesses four such motifs, distributed across the N-terminal tail (Arg29GlnArg31), NBD1 (Arg516TyrArg518 and Arg553AlaArg555), and RD (Arg764ArgArg766). F508del-CFTR fails to exit the ER due to the exposure of such motifs [50]. In fact, it was reported that simultaneous mutation of all four AFTs in F508del-CFTR by the substitution of arginine by lysine at positions 29, 516, 555, and 766 (that is F508del-R29K-R516K-R555K-R766K-CFTR, or simply F508del-4RK-CFTR) rescues F508del-CFTR processing by promoting its escape from the ERQC [50]. This rescue does not correspond to a folding correction but rather to overcoming specific traffic factors that may be involved in the retention [28, 30].
A fourth checkpoint is in place when CFTR is packaged into coat protein (COP) II-coated vesicles at ER exit sites. This process relies on a specific export motif, the di-acidic exit code (Asp565AlaAsp567—DAD) located in NBD1. This sequence is required for Sec24-mediated packing into the vesicles. Previously, it was shown that alanine substitution of the second Asp residue in DAD does not affect CFTR folding, but it reduces its association with Sec24, and exit from the ER [51, 52]. However, simultaneous mutation of both Asp residues totally abrogates CFTR processing [28].
After successfully overcoming all four checkpoints, CFTR is packed into the aforementioned COPII vesicles en route to the Golgi apparatus, a process that involves general traffic machinery proteins, such as Sar1 GTPase and the heterodimeric Sec23–24 and Sec13–31 complexes [53].
Cellular response to misfolded F508del-CFTR protein in the ER
Unfolded or misfolded proteins in the ER are either refolded or degraded through ERAD. When ER folding capacity is exceeded, such proteins accumulate disturbing the cellular proteostasis and generating ER stress. In this case, cellular mechanisms are activated to attenuate translation, decrease the protein load, enhance the ERQC machinery, facilitate folding, and increase degradation. Altogether, these mechanisms are termed unfolded protein response (UPR), which influences cell fate to restore ER homeostasis under stress. The classic UPR consists of three signalling pathways initiated by the stress sensor proteins inositol-requiring enzyme 1α (IRE-1α), eukaryotic translation initiation factor 2-α kinase 3 (PERK), and activating transcription factor 6 (ATF6) [54].
There is still some controversy about whether or not the UPR is induced in CF, because the rapid degradation of the mutant protein does not lead to a significant ER accumulation. Some studies have even reported inefficient activation of UPR response in CF [54, 55]. The active form of XBP-1 (XBP-1s) was found to be over-expressed in other studies, suggesting that the classic UPR IRE-1α signalling pathway is activated in CF primary cells and CF system models [54, 55]. This signalling activates ER stress responsive genes, regulates ER biogenesis, facilitating the entry of nascent polypeptides into the ER as well as protein folding and assembly [56]. The activation of PERK-eIF2α signalling occurs through the phosphorylation of PERK and eIF2α which were reported to be important in blocking mRNA translation and entry of nascent polypeptides into the ER. In CF, decreased phosphorylation of both proteins has been reported, suggesting once again a defect in the activation of this UPR branch [54, 55]. However, an increased association of BiP with ATF6 was reported in cells expressing F508del-CFTR [57].
Interestingly, it was shown that endogenous CFTR mRNA levels but also protein processing efficiency are decreased when the UPR is activated. This inhibition of CFTR expression is the main cause for a reduction of cAMP-activated chloride secretion, a direct outcome of CFTR function [58], and may partially account for the overall reduction of mutant F508del-CFTR expression that has been reported in different models and patient-derived materials [59, 60].
From the ER to the PM: the Golgi and alternative routes
After exiting the ER, CFTR is transported through the Golgi. Proteins that follow the secretory pathway are packed into COPII vesicles at ER exit sites (ERES). As CFTR is transported in COPI vesicles from the early cis-Golgi to the medial and then trans-Golgi cisternae (a process dependent on small GTPase Arf1), the ER-characteristic high-mannose oligosaccharide structures (linked at Asn894 and Asn900—see above) are modified rendering the protein resistant to the activity of endoglycosidase H (a biochemical tool commonly used to assess traffic of CFTR and other glycoproteins) and leading to the acquisition of a complex structure that includes removal of glycan units and addition of new ones, including the ones characteristic of trans Golgi, such as fucose, neuraminic acid, or sialic acid. Overall, these changes increase the apparent molecular weight of CFTR, producing the characteristic mature form (post-Golgi), known as band C, which is never detected in ER-retained variants, such as F508del-CFTR [61].
Besides this so-called conventional trafficking, an increasing number of secretory pathway proteins have been described to deviate from this traffic “dogma” [62], by either exiting the ER using COPII-independent mechanisms or bypassing the Golgi. CFTR is, in fact, an example of such alternative processes. Initial reports using quantitative immunoelectron microscopy documented very low levels of wt-CFTR in the Golgi region due to a limiting step in recruitment at ERES [63] and raising the hypothesis of a novel mode of anterograde (and also retrograde) trafficking between the ER and the Golgi. This was later confirmed by the description that, in some cell types, CFTR follows a distinct pathway that is independent of typical Golgi trafficking adaptors, such as Arf1, Rab1a/Rab2, or syntaxin 5 (a Golgi SNARE), but dependent on the late-endosomal target-SNARE syntaxin 13 [53]. These observations suggest a route from the ER to the plasma membrane, with a possible recycling back to the Golgi, as evidenced by the acquisition of the complex oligosaccharide structure.
More recent studies have also identified a role for Golgi reassembly stacking proteins (GRASPs) in mediating unconventional CFTR trafficking. This route allows the delivery of core-glycosylated (ER-characteristic) wt- and rescued F508del-CFTR to the PM [64] and requires monomerization and ER relocalization of GRASPs allowing them to access ER-localized CFTR [65].
Although very little is known about this Golgi bypass of CFTR, it may involve a peri-centriolar intermediate compartment where CFTR can accumulate upon blockage of the secretory pathway but from where it can be rapidly delivered to the PM upon release of blockage [62].
CFTR in post-Golgi compartments
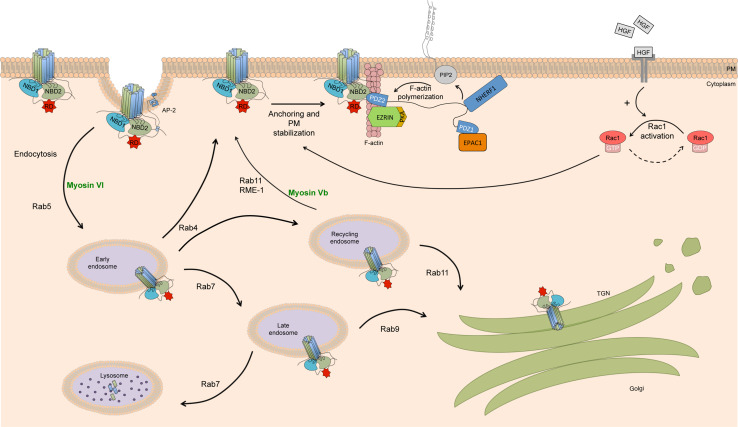
CFTR levels at the plasma membrane (PM) (Fig. 4) result from a balance of three distinct processes: anterograde trafficking (as CFTR is delivered from the trans-Golgi network to the PM), endocytosis, and recycling. Whereas anterograde trafficking of CFTR has not been reported as extensively regulated (although some reports describe a role for PKA in promoting CFTR insertion in the PM [66]), both endocytosis and recycling are finely tuned to control the amount of the protein that is at the PM.
Fig. 4.
Peripheral quality control regulating CFTR stability at the PM. Schematic representation of the processes that monitor CFTR internalization from PM. Several protein partners regulate CFTR endocytosis, recycling, and membrane anchoring. This includes adaptors proteins (Dab2, AP-2), PDZ domain-containing and interacting proteins (Ezrin, EPAC1, and NHERF1), the cytoskeleton, and motor proteins (myosins) that altogether have a crucial role in regulating CFTR PM levels
CFTR is endocytosed in clathrin-coated vesicles [66] and accumulates in the early endosomes [67, 68]. After this, about 50 % of internalized wt-CFTR is rapidly recycled back to the PM, whereas the remainder accumulates in a distinct recycling compartment, from where it still goes back to the PM although at a slower rate [68, 69]. Using a confocal microscopy-based internalization assay, it was shown that 15–20 % of CFTR present at the post-Golgi and PM compartments resides in the recycling pool at steady state [70]. Recycling of internalized CFTR to the PM has been considered to be the main mechanism for sustaining a functional pool of CFTR at the membrane (when compared with CFTR delivery to the PM), albeit some evidence suggest that up to 50 % of surface CFTR in airway epithelial cells exist in an immobile pool, tethered to filamentous actin (F-actin) [4, 71].
CFTR endocytosis and recycling: sorting motifs and interacting proteins
The endocytic and recycling pathways for CFTR, as well as its stability at the membrane, are regulated by a variety of different protein interactors, with a particular role being played by Rab family small GTPases, PDZ domain-containing proteins and myosins, and also by the presence of specific trafficking motifs in CFTR structure/sequence.
The incorporation of CFTR into clathrin-coated vesicles is dependent on the interaction of CFTR sequence motifs (mainly at the C-terminal tail) with endocytic adaptor proteins. These short sequence motifs (usually four to seven residues long) which are termed endocytic signals occur in the cytoplasmic domains of membrane proteins with bulky and hydrophobic residues playing an important role. Two major classes of endocytic motifs have been identified: tyrosine-based (NPXY or YXXΦ, where X stands for a variable amino acid and Φ for a bulky hydrophobic amino acid) and dileucine-based (D/EXXXLL/I and DXXLL). The use of chimeric proteins containing both the N- and C-termini of CFTR fused to the transmembrane regions of the transferrin receptor (TfR) [72] prompted the identification of specific motifs in those regions of CFTR. Alignment of the amino-acid sequences of CFTR C-termini from a variety of species reveals the presence of a conserved YXXΦ motif—1424YDSI, which was shown to be a real tyrosine internalization motif [72, 73]. Besides this motif, it was also proposed that two additional ones are present in the same region—1413FVLI and 1430LL. Mutagenesis strategies revealed that disruption of the 1424YDSI and 430LL motifs results in similar levels of inhibition of CFTR endocytosis [73, 74]. The 1413FVLI signal is not, however, a proper endocytic motif, but it is located within a patch that is needed for CFTR maturation [75].
These endocytic signals are recognized by adaptor proteins that mediate the interaction between CFTR and clathrin in the vesicle coat. AP-2 (assembly polypeptide-2) is a heterotetrameric complex that contains α, β2, and µ2 adaptins [76] and functions as an adaptor protein at the PM. The µ2 subunit was shown to interact with the Tyr1424 motif [77, 78] and the β2 subunit binds to clathrin forming a complex that promotes the internalization of CFTR from PM. After internalization, AP-2 and clathrin dissociate and recycle back to the PM as soluble proteins, whereas the uncoated vesicles traffic through the early endosomes [79].
Intracellular membrane trafficking requires the interaction of coat proteins and their adaptors with a complex protein network, including the cytoskeleton, motor proteins and protein kinases [80]. Sorting of CFTR from the PM into different routes is regulated by small GTPases of the Rab family. Rab5 regulates the initial entrance to the early endosomes, from where CFTR can be recycled back to the PM, a process that depends on Rab4, or sent to the recycling endosomes. From the latter, Rab11 regulates an alternative pathway of recycling to the PM and Rab7 promotes targeting to late endosomes. At this, CFTR can also be sent to the trans-Golgi network, a process mediated by Rab9 [68, 81]. In addition, RME-1 facilitates exit of CFTR from the recycling endosome [82].
Cytoskeletal motor proteins also participate in CFTR traffic through interactions with Rab GTPases and adaptor proteins. Myosin Vb and myosin VI are part of the myosin superfamily which consists of 18 different classes of myosin motors capable of using ATP hydrolysis to move on actin filaments [79, 83]. Myosin VI is most likely to participate in the apical membrane endocytosis in epithelial cells, because it moves toward the F-actin minus end, which is oriented away from the PM. Myosin VI regulates early steps of TfR endocytosis, including uptake of TfR into clathrin-coated pits and formation of clathrin-coated vesicles, as well as the later stages of TfR endocytosis, including movement of uncoated vesicles toward the early endosomes on actin filaments. Myosin VI is relevant at the early endocytic events, such as clustering of CFTR in clathrin-coated pits and formation of clathrin-coated vesicles, in a complex involving the adaptor Disabled-2 (Dab-2) [79]. Dab2 forms a complex with myosin VI and AP-2 at the cell surface facilitating CFTR endocytosis by an actin-dependent mechanism, a process which also depends on the tyrosine- and dileucine-based motifs present in the C-terminal tail of CFTR [78, 79, 84–86].
Another motor protein, myosin Vb, which specifically interacts with Rab11a, regulates CFTR-mediated chloride secretion across human airway epithelial cells by facilitating the apical membrane recycling of both wild-type and F508del-CFTR [83]. The interaction between Rab11a and myosin Vb was shown to be dependent on Rab-binding sites in the myosin Vb tail domain (amino acids 1231–1818) [83]. Interestingly, it has been shown that Rab11b also regulates the apical recycling of CFTR in polarized intestinal epithelial cells. The functional differences between these two similar Rab11 isoforms may, in fact, account for tissue-specific traffic [87].
CFTR membrane anchoring
The stability of CFTR at the PM is dependent on the interaction with several proteins, among which PDZ domain-containing proteins (PDZ proteins) are most relevant. PDZ domains are protein–protein interaction domains 80–90 amino-acid residues long which typically anchor appropriate targets to the cytoskeleton [88, 89]. The C-terminus of CFTR (residues DTRL) is a consensus PDZ binding motif (C-terminal X-[S/T]-X-[V/I/L]) binding several PDZ proteins [71, 90], including Na+/H+-exchanger regulatory factor isoform-1 (NHERF-1, also known as EBP50, ezrin-binding protein, 50 kDa), NHERF-2, NHERF-3 (also known as CFTR-associated protein 70KDa, or CAP70), NHERF-4, and CAL (CFTR-associated ligand) [91].
NHERF-1 anchors CFTR to the actin cytoskeleton through a multiprotein complex. In the PDZ-dependent CFTR–NHERF-1 complex, NHERF-1 interacts with ezrin, a member of the ezrin/radixin/moesin (ERM) family. This locks CFTR in an immobile and actin-tethered complex that prevents its endocytosis [92]. NHERF-1 targets exosome- and endosome-associated CFTR to the apical membrane of epithelial cells [93, 94], increases its chloride channel activity, and has also been suggested to induce CFTR dimerization and facilitate CFTR intermolecular interactions altering channel conformation and activity [69, 71, 93].
The role of NHERF-1 in CFTR stabilization involves interaction with small GTPases of the Rho family (reviewed in [4]). These GTPases, found in all eukaryotic organisms, are divided into three subfamilies, grouped according to their functional and structural similarity to their three founding members, RhoA, Rac1, and Cdc42. The members of the Rho family are key regulators of actin cytoskeleton dynamics, cell polarity and membrane trafficking through F-actin remodelling [95, 96]. Consistently, NHERF-1 overexpression stimulates the activation of endogenous RhoA and RhoA-activated kinase (ROCK), thus leading to reorganization of the actin cytoskeleton and stabilization of the multiprotein CFTR–NHERF-1–ezrin–actin complex at the apical PM [97].
PDZ protein binding also regulates CFTR’s fate. Whereas NHERF-1 and NHERF-2 stabilize CFTR apical membrane expression, overexpression of CAL shortens the half-life of mature CFTR by promoting its endocytosis and lysosomal degradation [98]. Solution-state binding assays revealed that the affinity of the CFTR C terminus is much lower for CAL than for NHERF-1/2. Therefore, this affinity profile encourages the identification of pharmaceuticals that displace CAL but not NHERF-1/2 [88].
Several other pathways regulating CFTR PM stability involve NHERF proteins. Stimulation with vasoactive intestinal peptide (VIP) increases CFTR PM localization through VIP–NHERF-1 interaction [99]. More recently, it was also shown that activation of EPAC1 (exchange protein directly activated by cAMP 1) by cAMP promotes its interaction with NHERF-1 and CFTR, leading to an increase in its plasma membrane levels through reduced endocytosis [100].
F508del-CFTR at the PM and peripheral quality control
F508del-CFTR can be rescued to the PM by different strategies (reviewed in [101]). The pathway followed by the mutant protein to the PM is, in general, the same described above. Different studies reported, however, reduced stability of rescued F508del-CFTR at the PM [68, 81, 102, 103], showing PM half-life (t 1/2) of 1 h, while wt-CFTR reached 3 h in polarized human airway epithelial cells. This reduction has been attributed to either an increased endocytosis rate [81] or a decrease in recycling back to the PM [103]. Such changes suggest that the CFTR structure is also assessed at the PM by a peripheral quality control (PQC) system [103, 104]. Thus, depending on its folding state, after endocytosis, rescued proteins are recycled back to the membrane or degraded [66, 68]. The general folding machinery is a major player in the PQC. Co-chaperones Bag-1, Hsp40, HOP, and Aha1 were shown to be required for removing rescued F508del-CFTR from PM, facilitating non-native CFTR internalization and lysosomal targeting through the interaction with ubiquitination machinery [105].
The PQC operates through regulation of F508del-CFTR PM anchoring by the PDZ machinery. Previously, it was reported that activation of Rac1 signalling improves F508del-CFTR stability at the PM [96]. This was demonstrated to occur through activation of ezrin by phosphorylation. This modification sustains ezrin in an active form enabling it to interact with F-actin and NHERF-1. These interactions promote PIP2 (4,5-bisphosphate)-dependent increase in F-actin polymerization at PM, linking PM F508del-CFTR to the actin cytoskeleton thus promoting its stabilization at the PM [97, 104, 106]. NHERF-1 interacts with ezrin through its ERM-binding domain [107], and whereas in cells expressing wt-CFTR, it locates at the apical membrane, in cells expressing F508del-CFTR, NHERF-1 is almost absent from the PM showing a diffuse distribution in the cytoplasm [107]. NHERF-1 appears to be a key factor to increase F508del-CFTR stability at PM by protecting it from lysosomal degradation [108]. In agreement with this finding, it was also shown that overexpression of NHERF-1 rescues F508del-CFTR functional expression in CF cells through a mechanism that involves increase of cytoskeleton organization [97, 107].
Modulation of other key players was also reported as critical in stabilizing F508del-CFTR at the PM. It was shown that F508del-CFTR PM localization can be rescued by overexpression of Rab11, by inhibition of Rab-5 dependent endocytosis [68, 81], by overexpression of NHERF-2, or by suppression of endogenous CAL [109].
Post-translational modifications as regulators of CFTR biogenesis and trafficking
During its biogenesis and life-cycle, CFTR undergoes several post-translational modifications which can modify its fate in the cellular context. Post-translational modifications can have a role in protein processing and folding, achievement of a mature and functional conformation, and regulation of its overall stability. CFTR is glycosylated cotranslationally and the glycan moiety plays a role in helping in the acquisition of a stable mature conformation as well as with trafficking through the secretory pathway (see above “ERQC folding checkpoints”).
Another post-translational modification that affects CFTR is ubiquitination. This process consists in the covalent attachment of ubiquitin and its polymerization on the substrate, representing a signal for the recognition for degradation by the 26S proteasome but also for the activation of signalling pathways. CFTR folding is assisted by chaperones and co-chaperones that also recruit and interact with members of the ubiquitination system, such as E3 ubiquitin ligases (CHIP, c-Cbl, and Nedd4-2) [40, 84, 110–112]. During CFTR maturation, cross talk between folding and degradation pathways occurs and influences its fate (see above “ERQC folding checkpoints”). Prolonged interaction of unfolded CFTR intermediates with Hsc70 recruits CHIP and UbcH5a which shifts the activity of Hsc70 from folding to degradation [39, 40, 105]. The interaction of CFTR with Hsc70 was also demonstrated to be destabilized in the presence of Bag-1 reducing the amount of CFTR available for CHIP and, thus, decreasing CFTR ubiquitination and degradation [44, 113]. RMA1/RNF5 is an ER-associated E3 ubiquitin ligase complex containing Ubc6e and Derlin-1. It was found to be involved in the regulation of CFTR, acting before the synthesis of NBD2 by sensing the folding state of CFTR’s amino-terminal region [41]. The ubiquitin system appears to operate not only in the early steps of CFTR folding in ER, but also in post-Golgi compartments [105]. CHIP was reported to promote post-endocytic ubiquitination of F508del-CFTR, also regulating the PQC of CFTR [84, 105]. c-Cbl is another ubiquitin ligase that was also implicated in the regulation of CFTR by two distinct mechanisms. First, it uses an ubiquitination-independent mechanism, which facilitates CFTR endocytosis, decreasing its PM stability by acting as a scaffolding protein [110, 114]. Second, c-Cbl targets CFTR for degradation by an ubiquitination-dependent mechanism [110]. In addition, several lines of evidence reported that Nedd4-2 also regulates the membrane trafficking of F508del-CFTR but not wt-CFTR by promoting its ubiquitination [111, 112].
More recently, sumoylation was also described to regulate CFTR fate. Sumoylation consists in the conjugation of small ubiquitin-like modifiers (SUMO), which regulate protein transport and degradation. This modification was reported to promote both wt-CFTR and F508del-CFTR degradation through interaction with Hsp27 and the E2 enzyme UBC9, both involved in SUMO conjugation. In vitro assays with isolated wt- and F508del-NBD1 showed increased sumoylation in the latter, suggesting a mechanism for discrimination between folded and misfolded regions in CFTR [115, 116].
Increasing evidence has also accumulated towards a role for phosphorylation in the regulation of CFTR trafficking (reviewed in [117]). Casein Kinase 2 (CK2), Spleen Tyrosine (SYK), Lemur Tyrosine Kinase 2 (LMTK2), and With-No-Lysine Kinase 4 (WNK4) have all been implicated in different steps of CFTR biogenesis and stability. Phosphorylation of CFTR at Tyr512 by SYK decreases its PM stability through a process that involves SYK regulation by WNK4 [60, 118]. In addition, LMTK2 (Lemur tyrosine kinase-2), a membrane-anchored Serine/Threonine (Ser/Thr) kinase, was shown to phosphorylate CFTR at Ser737, regulating its levels at the PM by facilitating its endocytosis [119, 120]. Like Dab2, LMTK2 is also a myosin VI-binding partner, forming a complex through its interaction with the Trp/Trp/Tyr site (Trp-Tryptophan and Y-tyrosine) in the C-terminal tail of myosin VI, most likely promoting CFTR inclusion into early endosomes.
Targeting the folding and trafficking defects for CFTR rescue
Understanding CFTR folding and trafficking has played an important role in the design of strategies to overcome the basic defects in the mutant protein(s). The rescue of F508del-CFTR from its ER retention has been achieved by three main strategies: physical (low temperature incubation), genetic (second site mutations), and chemical (low-molecular weight compounds with unspecific—chemical chaperones—or specific targets—pharmacological chaperones/correctors) (reviewed in [101]).
Low temperature, although not amenable to clinical use, is known to rescue F508del-CFTR trafficking to the PM and to partially restore its function [121]. A recent study assessing the F508del-CFTR interactome reported that the temperature shift reduces interaction of the mutant with several proteins involved in ERQC and in lysosomal targeting, suggesting that low temperature slows down protein activity leading to F508del-CFTR escape from the ERQC [28, 122] and thus prompting the identification of novel therapeutic targets among these interactors.
Simultaneously with the search for targets to modulate CFTR biosynthesis/trafficking, great effort has been put forward in the identification of small molecule compounds to rescue F508del-CFTR. Correctors and potentiators have been developed to rescue the trafficking and functional defects of F508del-CFTR (and other CFTR mutants), respectively (reviewed in [101, 123].
The most successful corrector was VX-809 (Lumacaftor), found to rescue F508del-CFTR traffic to the PM and function [124]. Structure-based docking studies proposed that VX-809 binds to the putative pocket formed at the NBD1/ICL4 interface [28, 125, 126], leading to the stabilization of F508del-CFTR at the early stages of its biogenesis [127]. However, in clinical trials, VX-809 showed modest results and did not improve lung function in F508del-homozygous patients [128]. This was suggested to occur due to the interference of in vivo conditions, such as inflammation and infection, that interfere with the mechanism of action of the compound. In fact, both transforming growth factor beta 1 (TGF-β1) whose levels are higher in CF patients [129] and presence of Pseudomonas aeruginosa [130] were reported to decrease the efficiency of VX-809. The quest for better correctors continues, even if a combination of VX-809 with the approved potentiator VX-770 is now available to F508del homozygous patients under the brand name Orkambi. The modest, although significant, effect of this combination [131] and the possible negative impact of VX-770 on VX-809 effect [132, 133] prompt the search for better compounds.
The defective PM stability has also raised interest leading to the identification of possible therapeutic targets (see above “F508del-CFTR at the PM and peripheral quality control”). Activation of the Rac1 signalling pathway with hepatocyte-growth factor [96] or activation of EPAC1 by the cAMP analogue 007-AM [100] have been shown to increase F508del-CFTR rescue by VX-809. Although translation of these findings into the clinic needs to be taken with caution, they validate PM stabilization as a relevant goal in rescuing F508del-CFTR.
Conclusion
Extensive work in the last decades has increased our knowledge on the complex mechanisms of CFTR biogenesis, processing, trafficking, and membrane stability. Characterization of such pathways together with the identification of key players (both CFTR-interacting proteins and CFTR intrinsic sequence motifs and patches) prompted the understanding of the molecular basis of the disease, particularly in what concerns the several defects exhibited in the most common disease-causing variant of the protein, F508del-CFTR. The current approaches targeting the basic defect focus on CFTR itself. All the evidences accumulated throughout the years have now identified targets that can promote correction/modulation of the proteostasis network that is largely responsible for discriminating between native and non-native proteins. This has now culminated in the introduction of novel strategies—including combinatory ones—to improve the outcome of current treatments.
Acknowledgments
Work supported by centre Grant (to BioISI, Centre Reference: UID/MULTI/04046/2013), Grant PGG-039-2014 (Gilead Genése Portugal), and Romain Pauwels Research Award to C.M.F. SC is recipient of SFRH/BD/52491/2014 PhD fellowship (FCT, Portugal). The authors also want to thank Dr. Luka Clarke and Dr. Hugo M Botelho (Faculty of Sciences, University of Lisboa, Portugal) for reviewing this manuscript and for helpful suggestions.
Abbreviations
- ABC
ATP-binding cassette
- ATF6
Activating transcription factor 6
- ATP
Adenosine triphosphate
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CHIP
Carboxy-terminal of Hsp70 interacting protein
- CK2
Casein kinase 2
- Cl−
Chloride
- CNX
Calnexin
- COP
Coat protein
- Csp
Cysteine string proteins
- Dab2
Disabled-2
- ECL
Extracellular loop
- EPAC
Exchange protein directly activated by cAMP
- ER
Endoplasmic reticulum
- ERES
ER exit sites
- ERQC
Endoplasmic reticulum quality control
- GI
Glucosidase I
- GII
Glucosidase II
- GERAD
Glycoprotein ER-associated degradation
- GRASP
Golgi reassembly stacking protein
- HCO3−
Bicarbonate
- IRE-1α
Inositol-requiring enzyme 1α
- LMTK2
Lemur tyrosine kinase 2
- MSD
Membrane-spanning domain
- NBD
Nucleotide-binding domain
- NHERF
Na+/H+-exchanger regulatory factor
- PERK
Protein kinase R-like ER kinase
- PM
Plasma membrane
- PQC
Peripheral quality control
- QC
Quality control
- RD
Regulatory domain
- SRP
Signal recognition particle
- SUMO
Small ubiquitin-like modifiers
- SYK
Spleen tyrosine kinase
- TfR
Transferrin receptor
- TGF-β
Transforming growth factor beta
- TGN
Trans-Golgi network
- TM
Transmembrane
- UGGT
UDP-glycoprotein glucosyltransferase
- UPR
Unfolded protein response
- VIP
Vasoactive intestinal peptide
- wt
Wild type
References
- 1.Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 2.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan JR, Tsui LC, Collins FS. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 3.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 4.Farinha CM, Matos P, Amaral MD. Control of CFTR membrane trafficking: not just from the ER to the Golgi. FEBS J. 2013 doi: 10.1111/febs.12392. [DOI] [PubMed] [Google Scholar]
- 5.The CFTR mutation database (2016). http://www.genet.sickkids.on.ca/
- 6.Amaral MD. Processing of CFTR: traversing the cellular maze–how much CFTR needs to go through to avoid cystic fibrosis? Pediatr Pulm. 2005;39:479–491. doi: 10.1002/ppul.20168. [DOI] [PubMed] [Google Scholar]
- 7.Farinha CM, Amaral MD. Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol. 2005;25:5242–5252. doi: 10.1128/MCB.25.12.5242-5252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Xiong X, Helm A, Kimani K, Bragin A, Skach WR. Co- and posttranslational translocation mechanisms direct cystic fibrosis transmembrane conductance regulator N terminus transmembrane assembly. J Biol Chem. 1998;273:568–576. doi: 10.1074/jbc.273.1.568. [DOI] [PubMed] [Google Scholar]
- 9.Patrick AE, Karamyshev AL, Millen L, Thomas PJ. Alteration of CFTR transmembrane span integration by disease-causing mutations. Mol Biol Cell. 2011;22:4461–4471. doi: 10.1091/mbc.E11-05-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitonzo D, Yang Z, Matsumura Y, Johnson AE, Skach WR. Sequence-specific retention and regulated integration of a nascent membrane protein by the endoplasmic reticulum Sec61 translocon. Mol Biol Cell. 2009;20:685–698. doi: 10.1091/mbc.E08-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carveth K, Buck T, Anthony V, Skach WR. Cooperativity and flexibility of cystic fibrosis transmembrane conductance regulator transmembrane segments participate in membrane localization of a charged residue. J Biol Chem. 2002;277:39507–39514. doi: 10.1074/jbc.M205759200. [DOI] [PubMed] [Google Scholar]
- 12.Sadlish H, Pitonzo D, Johnson AE, Skach WR. Sequential triage of transmembrane segments by Sec61alpha during biogenesis of a native multispanning membrane protein. Nat Struct Mol Biol. 2005;12:870–878. doi: 10.1038/nsmb994. [DOI] [PubMed] [Google Scholar]
- 13.Enquist K, Fransson M, Boekel C, Bengtsson I, Geiger K, Lang L, Pettersson A, Johansson S, von Heijne G, Nilsson I. Membrane-integration characteristics of two ABC transporters, CFTR and P-glycoprotein. J Mol Biol. 2009;387:1153–1164. doi: 10.1016/j.jmb.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Kleizen B, van Vlijmen T, de Jonge HR, Braakman I. Folding of CFTR is predominantly cotranslational. Mol Cell. 2005;20:277–287. doi: 10.1016/j.molcel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Khushoo A, Yang Z, Johnson AE, Skach WR. Ligand-driven vectorial folding of ribosome-bound human CFTR NBD1. Mol Cell. 2011;41:682–692. doi: 10.1016/j.molcel.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serohijos AW, Hegedus T, Aleksandrov AA, He L, Cui L, Dokholyan NV, Riordan JR. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du K, Lukacs GL. Cooperative assembly and misfolding of CFTR domains in vivo. Mol Biol Cell. 2009;20:1903–1915. doi: 10.1091/mbc.E08-09-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo TW, Bartlett MC, Clarke DM. Processing mutations disrupt interactions between the nucleotide binding and transmembrane domains of P-glycoprotein and the cystic fibrosis transmembrane conductance regulator (CFTR) J Biol Chem. 2008;283:28190–28197. doi: 10.1074/jbc.M805834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 20.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, Lorimer D, Kearins MC, Conners K, Condon B, Maloney PC, Guggino WB, Hunt JF, Emtage S. Impact of the deltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- 22.Qu BH, Thomas PJ. Alteration of the cystic fibrosis transmembrane conductance regulator folding pathway. J Biol Chem. 1996;271:7261–7264. doi: 10.1074/jbc.271.18.10577. [DOI] [PubMed] [Google Scholar]
- 23.Pissarra LS, Farinha CM, Xu Z, Schmidt A, Thibodeau PH, Cai Z, Thomas PJ, Sheppard DN, Amaral MD. Solubilizing mutations used to crystallize one CFTR domain attenuate the trafficking and channel defects caused by the major cystic fibrosis mutation. Chem Biol. 2008;15:62–69. doi: 10.1016/j.chembiol.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Scott-Ward TS, Amaral MD. Deletion of Phe508 in the first nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator increases its affinity for the heat shock cognate 70 chaperone. FEBS J. 2009;276:7097–7109. doi: 10.1111/j.1742-4658.2009.07421.x. [DOI] [PubMed] [Google Scholar]
- 25.He L, Aleksandrov AA, An J, Cui L, Yang Z, Brouillette CG, Riordan JR. Restoration of NBD1 thermal stability is necessary and sufficient to correct F508 CFTR folding and assembly. J Mol Biol. 2015;427:106–120. doi: 10.1016/j.jmb.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoelen H, Kleizen B, Schmidt A, Richardson J, Charitou P, Thomas PJ, Braakman I. The primary folding defect and rescue of DeltaF508 CFTR emerge during translation of the mutant domain. PLoS One. 2010;5:e15458. doi: 10.1371/journal.pone.0015458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibodeau PH, Richardson JM, Wang W, Millen L, Watson JM, Mendoza JL, Du K, Fischman S, Senderowitz H, Lukacs GL, Kirk K, Thomas PJ. The cystic fibrosis-causing mutation deltaF508 affects multiple steps in cystic fibrosis transmembrane conductance regulator biogenesis. J Biol Chem. 2010;285:35825–35835. doi: 10.1074/jbc.M110.131623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farinha CM, King-Underwood J, Sousa M, Correia AR, Henriques BJ, Roxo-Rosa M, Da Paula AC, Williams J, Hirst S, Gomes CM, Amaral MD. Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem Biol. 2013;20:943–955. doi: 10.1016/j.chembiol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Loo TW, Bartlett MC, Clarke DM. The V510D suppressor mutation stabilizes DeltaF508-CFTR at the cell surface. Biochemistry. 2010;49:6352–6357. doi: 10.1021/bi100807h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roxo-Rosa M, Xu Z, Schmidt A, Neto M, Cai Z, Soares CM, Sheppard DN, Amaral MD. Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide-binding domain of CFTR by different mechanisms. Proc Natl Acad Sci USA. 2006;103:17891–17896. doi: 10.1073/pnas.0608312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farinha CM, Nogueira P, Mendes F, Penque D, Amaral MD. The human DnaJ homologue (Hdj)-1/heat-shock protein (Hsp) 40 co-chaperone is required for the in vivo stabilization of the cystic fibrosis transmembrane conductance regulator by Hsp70. Biochem J. 2002;366:797–806. doi: 10.1042/bj20011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meacham GC, Lu Z, King S, Sorscher E, Tousson A, Cyr DM. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 1999;18:1492–1505. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Janich S, Cohn JA, Wilson JM. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA. 1993;90:9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Hutt DM, Roth DM, Chalfant MA, Youker RT, Matteson J, Brodsky JL, Balch WE. FK506 binding protein 8 peptidylprolyl isomerase activity manages a late stage of cystic fibrosis transmembrane conductance regulator (CFTR) folding and stability. J Biol Chem. 2012;287:21914–21925. doi: 10.1074/jbc.M112.339788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coppinger JA, Hutt DM, Razvi A, Koulov AV, Pankow S, Yates JR, 3rd, Balch WE. A chaperone trap contributes to the onset of cystic fibrosis. PLoS One. 2012;7:e37682. doi: 10.1371/journal.pone.0037682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumura Y, Sakai J, Skach WR. Endoplasmic reticulum protein quality control is determined by cooperative interactions between Hsp/c70 protein and the CHIP E3 ligase. J Biol Chem. 2013;288:31069–31079. doi: 10.1074/jbc.M113.479345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 40.Younger JM, Ren HY, Chen L, Fan CY, Fields A, Patterson C, Cyr DM. A foldable CFTR{Delta}F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol. 2004;167:1075–1085. doi: 10.1083/jcb.200410065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, Cyr DM. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Morito D, Hirao K, Oda Y, Hosokawa N, Tokunaga F, Cyr DM, Tanaka K, Iwai K, Nagata K. Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRDeltaF508. Mol Biol Cell. 2008;19:1328–1336. doi: 10.1091/mbc.E07-06-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Heath-Engel H, Zhang D, Nguyen N, Thomas DY, Hanrahan JW, Shore GC. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell. 2008;133:1080–1092. doi: 10.1016/j.cell.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 44.Mendes F, Farinha CM, Felicio V, Alves PC, Vieira I, Amaral MD. BAG-1 stabilizes mutant F508del-CFTR in a ubiquitin-like-domain-dependent manner. Cell Physiol Biochem. 2012;30:1120–1133. doi: 10.1159/000343303. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Peters KW, Sun F, Marino CR, Lang J, Burgoyne RD, Frizzell RA. Cysteine string protein interacts with and modulates the maturation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:28948–28958. doi: 10.1074/jbc.M111706200. [DOI] [PubMed] [Google Scholar]
- 46.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pind S, Riordan JR, Williams DB. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- 48.Chang XB, Mengos A, Hou YX, Cui L, Jensen TJ, Aleksandrov A, Riordan JR, Gentzsch M. Role of N-linked oligosaccharides in the biosynthetic processing of the cystic fibrosis membrane conductance regulator. J Cell Sci. 2008;121:2814–2823. doi: 10.1242/jcs.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelsen K, Yuan H, Schwappach B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 2005;6:717–722. doi: 10.1038/sj.embor.7400480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang X, Cui L, Hou Y, Jensen TJ, Aleksandrov AA, Mengos A, Riordan JR. Removal of multiple arginine-framed trafficking signals overcomes misprocessing of delta F508 CFTR present in most patients with cystic fibrosis. Mol Cell. 1999;4:137–142. doi: 10.1016/S1097-2765(00)80196-3. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE. COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol. 2004;167:65–74. doi: 10.1083/jcb.200401035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoo JS, Moyer BD, Bannykh S, Yoo HM, Riordan JR, Balch WE. Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J Biol Chem. 2002;277:11401–11409. doi: 10.1074/jbc.M110263200. [DOI] [PubMed] [Google Scholar]
- 54.Blohmke CJ, Mayer ML, Tang AC, Hirschfeld AF, Fjell CD, Sze MA, Falsafi R, Wang S, Hsu K, Chilvers MA, Hogg JC, Hancock RE, Turvey SE. Atypical activation of the unfolded protein response in cystic fibrosis airway cells contributes to p38 MAPK-mediated innate immune responses. J Immunol. 2012;189:5467–5475. doi: 10.4049/jimmunol.1103661. [DOI] [PubMed] [Google Scholar]
- 55.Nanua S, Sajjan U, Keshavjee S, Hershenson MB. Absence of typical unfolded protein response in primary cultured cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;343:135–143. doi: 10.1016/j.bbrc.2006.02.137. [DOI] [PubMed] [Google Scholar]
- 56.Brewer JW. Regulatory crosstalk within the mammalian unfolded protein response. Cell Mol Life Sci. 2014;71:1067–1079. doi: 10.1007/s00018-013-1490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerbiriou M, Le Drevo MA, Ferec C, Trouve P. Coupling cystic fibrosis to endoplasmic reticulum stress: differential role of Grp78 and ATF6. Biochim Biophys Acta. 2007;1772:1236–1249. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Bartoszewski R, Rab A, Twitty G, Stevenson L, Fortenberry J, Piotrowski A, Dumanski JP, Bebok Z. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem. 2008;283:12154–12165. doi: 10.1074/jbc.M707610200. [DOI] [PubMed] [Google Scholar]
- 59.Ramalho AS, Beck S, Meyer M, Penque D, Cutting G, Amaral MD. 5 % of normal CFTR mRNA ameliorates the severity of pulmonary disease in Cystic Fibrosis. Am J Respir Cell Mol Biol. 2002;27:619–627. doi: 10.1165/rcmb.2001-0004OC. [DOI] [PubMed] [Google Scholar]
- 60.Luz S, Kongsuphol P, Mendes AI, Romeiras F, Sousa M, Schreiber R, Matos P, Jordan P, Mehta A, Amaral MD, Kunzelmann K, Farinha CM. Contribution of casein kinase 2 and spleen tyrosine kinase to CFTR trafficking and protein kinase A-induced activity. Mol Cell Biol. 2011;31:4392–4404. doi: 10.1128/MCB.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amaral MD, Farinha CM, Matos P, Botelho HM (2016) Investigating alternative transport of integral plasma membrane proteins from the er to the golgi: lessons from the cystic fibrosis transmembrane conductance regulator (CFTR). Methods Mol Biol (in press) [DOI] [PubMed]
- 62.Grieve AG, Rabouille C. Golgi bypass: skirting around the heart of classical secretion. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bannykh SI, Bannykh GI, Fish KN, Moyer BD, Riordan JR, Balch WE. Traffic pattern of cystic fibrosis transmembrane regulator through the early exocytic pathway. Traffic. 2000;1:852–870. doi: 10.1034/j.1600-0854.2000.011105.x. [DOI] [PubMed] [Google Scholar]
- 64.Gee HY, Noh SH, Tang BL, Kim KH, Lee MG. Rescue of DeltaF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell. 2011;146:746–760. doi: 10.1016/j.cell.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Kim J, Noh SH, Piao H, Kim DH, Kim K, Cha JS, Chung WY, Cho HS, Kim JY, Lee MG. Monomerization and ER relocalization of GRASP Is a requisite for unconventional secretion of CFTR. Traffic. 2016;17:733–753. doi: 10.1111/tra.12403. [DOI] [PubMed] [Google Scholar]
- 66.Bradbury NA. Intracellular CFTR: localization and function. Physiol Rev. 1999;79:S175–S191. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- 67.Cholon DM, O’Neal WK, Randell SH, Riordan JR, Gentzsch M. Modulation of endocytic trafficking and apical stability of CFTR in primary human airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2010;298:L304–L314. doi: 10.1152/ajplung.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gentzsch M, Chang XB, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:2684–2696. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:40099–40105. doi: 10.1074/jbc.M206964200. [DOI] [PubMed] [Google Scholar]
- 70.Holleran JP, Zeng J, Frizzell RA, Watkins SC. Regulated recycling of mutant CFTR is partially restored by pharmacological treatment. J Cell Sci. 2013;126:2692–2703. doi: 10.1242/jcs.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haggie PM, Kim JK, Lukacs GL, Verkman AS. Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol Biol Cell. 2006;17:4937–4945. doi: 10.1091/mbc.E06-08-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, Collawn JF. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J Biol Chem. 1999;274:3602–3609. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- 73.Hu W, Howard M, Lukacs GL. Multiple endocytic signals in the C-terminal tail of the cystic fibrosis transmembrane conductance regulator. Biochem J. 2001;354:561–572. doi: 10.1042/bj3540561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peter K, Varga K, Bebok Z, McNicholas-Bevensee CM, Schwiebert L, Sorscher EJ, Schwiebert EM, Collawn JF. Ablation of internalization signals in the carboxyl-terminal tail of the cystic fibrosis transmembrane conductance regulator enhances cell surface expression. J Biol Chem. 2002;277:49952–49957. doi: 10.1074/jbc.M209275200. [DOI] [PubMed] [Google Scholar]
- 75.Gentzsch M, Aleksandrov A, Aleksandrov L, Riordan JR. Functional analysis of the C-terminal boundary of the second nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator and structural implications. Biochem J. 2002;366:541–548. doi: 10.1042/bj20020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madden DR, Swiatecka-Urban A. Tissue-specific control of CFTR endocytosis by Dab2: cargo recruitment as a therapeutic target. Commun Integr Biol. 2012;5:473–476. doi: 10.4161/cib.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weixel KM, Bradbury NA. The carboxyl terminus of the cystic fibrosis transmembrane conductance regulator binds to AP-2 clathrin adaptors. J Biol Chem. 2000;275:3655–3660. doi: 10.1074/jbc.275.5.3655. [DOI] [PubMed] [Google Scholar]
- 78.Fu L, Rab A, Tang LP, Rowe SM, Bebok Z, Collawn JF. Dab2 is a key regulator of endocytosis and post-endocytic trafficking of the cystic fibrosis transmembrane conductance regulator. Biochem J. 2012;441:633–643. doi: 10.1042/BJ20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swiatecka-Urban A, Boyd C, Coutermarsh B, Karlson KH, Barnaby R, Aschenbrenner L, Langford GM, Hasson T, Stanton BA. Myosin VI regulates endocytosis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2004;279:38025–38031. doi: 10.1074/jbc.M403141200. [DOI] [PubMed] [Google Scholar]
- 80.Kravtsov DV, Ameen NA. Molecular motors and apical CFTR traffic in epithelia. Int J Mol Sci. 2013;14:9628–9642. doi: 10.3390/ijms14059628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, Stanton BA. The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J Biol Chem. 2005;280:36762–36772. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- 82.Picciano JA, Ameen N, Grant B, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol. 2003;285:C1009–C1018. doi: 10.1152/ajpcell.00140.2003. [DOI] [PubMed] [Google Scholar]
- 83.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem. 2007;282:23725–23736. doi: 10.1074/jbc.M608531200. [DOI] [PubMed] [Google Scholar]
- 84.Fu L, Rab A, Tang L, Bebok Z, Rowe SM, Bartoszewski R, Collawn JF. DeltaF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments. PLoS One. 2015;10:e0123131. doi: 10.1371/journal.pone.0123131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cihil KM, Ellinger P, Fellows A, Stolz DB, Madden DR, Swiatecka-Urban A. Disabled-2 protein facilitates assembly polypeptide-2-independent recruitment of cystic fibrosis transmembrane conductance regulator to endocytic vesicles in polarized human airway epithelial cells. J Biol Chem. 2012;287:15087–15099. doi: 10.1074/jbc.M112.341875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collaco A, Jakab R, Hegan P, Mooseker M, Ameen N. Alpha-AP-2 directs myosin VI-dependent endocytosis of cystic fibrosis transmembrane conductance regulator chloride channels in the intestine. J Biol Chem. 2010;285:17177–17187. doi: 10.1074/jbc.M110.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell. 2009;20:2337–2350. doi: 10.1091/mbc.E08-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cushing PR, Fellows A, Villone D, Boisguerin P, Madden DR. The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry. 2008;47:10084–10098. doi: 10.1021/bi8003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ranganathan R, Ross EM. PDZ domain proteins: scaffolds for signaling complexes. Curr Biol. 1997;7:R770–R773. doi: 10.1016/S0960-9822(06)00401-5. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C- terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/S0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 91.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 92.Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- 93.Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 94.Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 96.Moniz S, Sousa M, Moraes BJ, Mendes AI, Palma M, Barreto C, Fragata JI, Amaral MD, Matos P. HGF stimulation of Rac1 signaling enhances pharmacological correction of the most prevalent cystic fibrosis mutant F508del-CFTR. ACS Chem Biol. 2013;8:432–442. doi: 10.1021/cb300484r. [DOI] [PubMed] [Google Scholar]
- 97.Favia M, Guerra L, Fanelli T, Cardone RA, Monterisi S, Di Sole F, Castellani S, Chen M, Seidler U, Reshkin SJ, Conese M, Casavola V. Na+/H+ exchanger regulatory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o- cells. Mol Biol Cell. 2010;21:73–86. doi: 10.1091/mbc.E09-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem. 2004;279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 99.Alshafie W, Chappe FG, Li M, Anini Y, Chappe VM. VIP regulates CFTR membrane expression and function in Calu-3 cells by increasing its interaction with NHERF1 and P-ERM in a VPAC1- and PKCepsilon-dependent manner. Am J Physiol Cell Physiol. 2014;307:C107–C119. doi: 10.1152/ajpcell.00296.2013. [DOI] [PubMed] [Google Scholar]
- 100.Lobo MJ, Amaral MD, Zaccolo M, Farinha CM. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J Cell Sci. 2016;129:2599–2612. doi: 10.1242/jcs.185629. [DOI] [PubMed] [Google Scholar]
- 101.Amaral MD, Farinha CM. Rescuing mutant CFTR: a multi-task approach to a better outcome in treating cystic fibrosis. Curr Pharm Des. 2013;19:3497–3508. doi: 10.2174/13816128113199990318. [DOI] [PubMed] [Google Scholar]
- 102.Penque D, Mendes F, Beck S, Farinha C, Pacheco P, Nogueira P, Lavinha J, Malho R, Amaral MD. Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Lab Invest. 2000;80:857–868. doi: 10.1038/labinvest.3780090. [DOI] [PubMed] [Google Scholar]
- 103.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loureiro CA, Matos AM, Dias-Alves A, Pereira JF, Uliyakina I, Barros P, Amaral MD, Matos P. A molecular switch in the scaffold NHERF1 enables misfolded CFTR to evade the peripheral quality control checkpoint. Sci Signal. 2015;8:ra48. doi: 10.1126/scisignal.aaa1580. [DOI] [PubMed] [Google Scholar]
- 105.Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abbattiscianni AC, Favia M, Mancini MT, Cardone RA, Guerra L, Monterisi S, Castellani S, Laselva O, Di Sole F, Conese M, Zaccolo M, Casavola V. Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. J Cell Sci. 2016;129:1128–1140. doi: 10.1242/jcs.177907. [DOI] [PubMed] [Google Scholar]
- 107.Guerra L, Fanelli T, Favia M, Riccardi SM, Busco G, Cardone RA, Carrabino S, Weinman EJ, Reshkin SJ, Conese M, Casavola V. Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues DeltaF508 CFTR functional expression in cystic fibrosis cells. J Biol Chem. 2005;280:40925–40933. doi: 10.1074/jbc.M505103200. [DOI] [PubMed] [Google Scholar]
- 108.Kwon SH, Pollard H, Guggino WB. Knockdown of NHERF1 enhances degradation of temperature rescued DeltaF508 CFTR from the cell surface of human airway cells. Cell Physiol Biochem. 2007;20:763–772. doi: 10.1159/000110436. [DOI] [PubMed] [Google Scholar]
- 109.Cushing PR, Vouilleme L, Pellegrini M, Boisguerin P, Madden DR. A stabilizing influence: CAL PDZ inhibition extends the half-life of DeltaF508-CFTR. Angew Chem Int Ed Engl. 2010;49:9907–9911. doi: 10.1002/anie.201005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ye S, Cihil K, Beer Stolz D, Pilewski JM, Stanton BA, Swiatecka-Urban A. C-CBL facilitates endocytosis and lysosomal degradation of CFTR in human airway epithelial cells. J Biol Chem. 2010;285:27008–27018. doi: 10.1074/jbc.M110.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem. 2009;284:25241–25253. doi: 10.1074/jbc.M109.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koeppen K, Chapline C, Sato JD, Stanton BA. Nedd4-2 does not regulate wt-CFTR in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L720–L727. doi: 10.1152/ajplung.00409.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsumura Y, David LL, Skach WR. Role of Hsc70 binding cycle in CFTR folding and endoplasmic reticulum-associated degradation. Mol Biol Cell. 2011;22:2797–2809. doi: 10.1091/mbc.E11-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cihil KM, Zimnik A, Swiatecka-Urban A. c-Cbl reduces stability of rescued F508-CFTR in human airway epithelial cells: implications for cystic fibrosis treatment. Commun Integr Biol. 2013;6:e23094. doi: 10.4161/cib.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahner A, Gong X, Schmidt BZ, Peters KW, Rabeh WM, Thibodeau PH, Lukacs GL, Frizzell RA. Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol Biol Cell. 2013;24:74–84. doi: 10.1091/mbc.E12-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gong X, Ahner A, Roldan A, Lukacs GL, Thibodeau PH, Frizzell RA. Non-native conformers of cystic fibrosis transmembrane conductance regulator NBD1 are recognized by Hsp27 and conjugated to SUMO-2 for degradation. J Biol Chem. 2016;291:2004–2017. doi: 10.1074/jbc.M115.685628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farinha CM, Swiatecka-Urban A, Brautigan DL, Jordan P. Regulatory crosstalk by protein kinases on CFTR trafficking and activity. Front Chem. 2016;4:1. doi: 10.3389/fchem.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendes AI, Matos P, Moniz S, Luz S, Amaral MD, Farinha CM, Jordan P. Antagonistic regulation of cftr cell surface expression by protein kinases WNK4 and spleen tyrosine kinase. Mol Cell Biol. 2011;31:4076–4086. doi: 10.1128/MCB.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luz S, Cihil KM, Brautigan DL, Amaral MD, Farinha CM, Swiatecka-Urban A. LMTK2 mediated phosphorylation regulates CFTR endocytosis in human airway epithelial cells. J Biol Chem. 2014;289:15080–15093. doi: 10.1074/jbc.M114.563742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H, Brautigan DL. Peptide microarray analysis of substrate specificity of the transmembrane Ser/Thr kinase KPI-2 reveals reactivity with cystic fibrosis transmembrane conductance regulator and phosphorylase. Mol Cell Proteom. 2006;5:2124–2130. doi: 10.1074/mcp.M600188-MCP200. [DOI] [PubMed] [Google Scholar]
- 121.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 122.Pankow S, Bamberger C, Calzolari D, Martinez-Bartolome S, Lavallee-Adam M, Balch WE, Yates JR., 3rd F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature. 2015;528:510–516. doi: 10.1038/nature15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Farinha CM, Matos P. Repairing the basic defect in cystic fibrosis—one approach is not enough. FEBS J. 2016;283:246–264. doi: 10.1111/febs.13531. [DOI] [PubMed] [Google Scholar]
- 124.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He L, Kota P, Aleksandrov AA, Cui L, Jensen T, Dokholyan NV, Riordan JR. Correctors of {Delta}F508 CFTR restore global conformational maturation without thermally stabilizing the mutant protein. FASEB J. 2013;27:536–545. doi: 10.1096/fj.12-216119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, Lukacs GL. Mechanism-based corrector combination restores DeltaF508-CFTR folding and function. Nat Chem Biol. 2013;9:444–454. doi: 10.1038/nchembio.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farinha CM, Sousa M, Canato S, Schmidt A, Uliyakina I, Amaral MD. Increased efficacy of VX-809 in different cellular systems results from an early stabilization effect of F508del-CFTR. Pharmacol Res Perspect. 2015;3:e00152. doi: 10.1002/prp2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, Deboeck K, Donaldson SH, Dorkin HL, Dunitz JM, Durie PR, Jain M, Leonard A, McCoy KS, Moss RB, Pilewski JM, Rosenbluth DB, Rubenstein RC, Schechter MS, Botfield M, Ordonez CL, Spencer-Green GT, Vernillet L, Wisseh S, Yen K, Konstan MW. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snodgrass SM, Cihil KM, Cornuet PK, Myerburg MM, Swiatecka-Urban A. Tgf-beta1 inhibits Cftr biogenesis and prevents functional rescue of DeltaF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS One. 2013;8:e63167. doi: 10.1371/journal.pone.0063167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stanton BA, Coutermarsh B, Barnaby R, Hogan D. Pseudomonas aeruginosa reduces VX-809 stimulated F508del-CFTR chloride secretion by airway epithelial cells. PLoS One. 2015;10:e0127742. doi: 10.1371/journal.pone.0127742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP, TRAFFIC Sutdy Group. TRANSPORT Study Group Lumacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cholon DM, Quinney NL, Fulcher ML, Esther CR, Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Science translational medicine. 2014;6:246ra296. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med. 2014;6:246ra297. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim SJ, Skach WR. Mechanisms of CFTR folding at the endoplasmic reticulum. Front Pharmacol. 2012;3:201. doi: 10.3389/fphar.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]