Abstract
Background
As part of efforts to monitor the impact of vector control strategies so that they can be improved and more targeted, we collected baseline data on aspects of the bionomics of endophilic anophelines in southern Ghana.
Materials and Methods
Indoor resting anophelines were collected using mouth aspirators and pyrethroid spray catch. Anopheles females were identified to species level using morphological characteristics and sibling species were distinguished by PCR. The presence of the L1014F mutation, conferring resistance to insecticides, was determined in An. gambiae s.s. and An. coluzzii samples using TaqMan real-time PCR. Host blood meal sources were determined by PCR, and the presence of Plasmodium falciparum circumsporozoite proteins determined by ELISA.
Results
A total of 892 female Anopheles (31% An. gambiae, 41% An. coluzzii and 28% An. funestus) were collected from six villages. The L1014F mutation was almost fixed in all populations studied (allele frequencies: 0.87-1.00). Both An. gambiae s.l. and An. funestus fed mainly on humans, with a human blood index of 1, although some animal feeding was recorded in An. gambiae. P. falciparum was detected in all ecological zones and in all three major vector species, being 4.9% in An. funestus, 3.8% in An. gambiae s.s. and 1.1% in An. coluzzii.
Conclusions
These findings suggest that the three major vectors of malaria are present in all ecological zones of southern Ghana and contribute to disease transmission. The near fixation of the L1014F mutation in southern Ghana poses a great threat to vector control, thus highlighting the urgent need to implement measures to maintain the efficacy of current control tools and to develop novel control strategies.
1 Introduction
In Ghana, four dominant malaria vector species are responsible for malaria transmission: Anopheles funestus s.s., and three members of the An. gambiae complex: An. gambiae s.s., An. arabiensis and An. coluzzii [1]. An. gambiae s.l. and members of the An. funestus group are sympatric over much of their range in Africa [2]. Studies in southern Ghana have found that An. gambiae s.s., An. coluzzii and An. funestus are the dominant malaria vector species [3-6]. These species are sympatric across much of Ghana but An. gambiae s.s. predominates in the middle belt, characterised by forest type vegetation, whereas An. coluzzii predominates in the northern and coastal savannah regions [7,8]. However, this information alone is too general and cannot be used to extrapolate effective vector control strategies against these species under the diverse ecological conditions experienced in Ghana. It has been established that occurrence, population density and vector competence of An. gambiae s.l. and An. funestus vary geographically and are influenced by a number of environmental conditions, such as climate, rainfall and vegetation, as well as activities which influence their breeding [2,3,7,9,10]. It is therefore essential to understand the distribution and population dynamics of these anophelines in the various ecological settings before implementing appropriate malaria vector control interventions.
In Ghana, the national vector control programme, spearheaded by the Ghana National Malaria Control Program (NMCP), among other malaria control initiatives, prioritises use of insecticide-treated bednets (ITNs) and indoor residual spraying (IRS) as the main malaria vector control tools [1]. These strategies rely heavily on few insecticides recommended by WHO [11]. Unfortunately, there is strong evidence that mosquito populations in Ghana have developed resistance to insecticides used in malaria vector control [4-8,12]. This development calls for a shift towards integrated vector management (IVM). There are several approaches available, including, but not limited to: insecticide-treated wall linings (ITWL), entomopathogenic fungi, larviciding, and the development and implementation of novel strategies to counter this problem. In addition, insecticide resistance management needs to be implemented to prolong effectiveness of the insecticides currently available for malaria vector control [1]. It is against this background that the studies reported in this article focused on understanding the bionomics and susceptibility levels of vector populations from southern Ghana as an essential step towards the development and implementation of effective IVM with a strong insecticide resistance management component.
2 Materials and methods
2.1 Study area
The study was carried out in six villages in southern Ghana, covering three ecological zones: forest ecological zone (FEZ), forest transition ecological zone (FTEZ) and coastal savannah ecological zone (CSEZ) (Figure 1).
Figure 1.
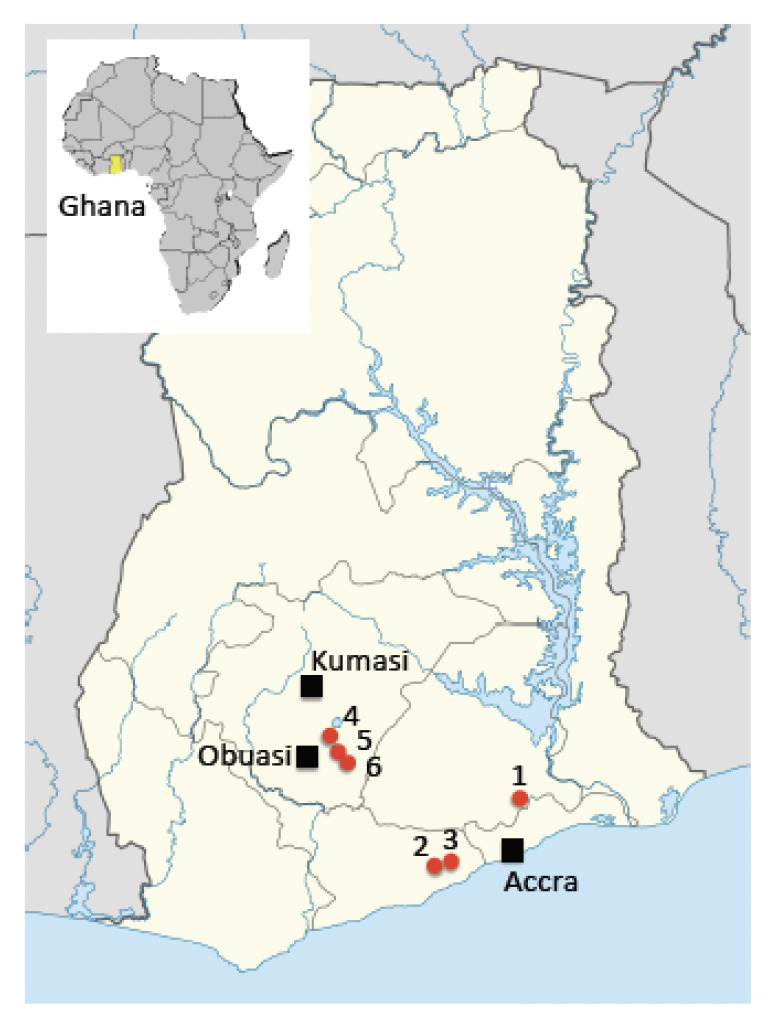
Map of Ghana showing the geographical location of study villages. 1: Osorongma, 2: Okyereko, 3: Adawukwa, 4: Kusa Dinkyeae, 5: Atatam, 6: Agyenkwaso.
In the FEZ, mosquitoes were collected from three villages: Atatam (06° 17.377”N, 001o 27.545”W), Agyenkwaso (06° 15.547”N, 001° 26.689”W) and Kusa Dinkyeae (06° 18.894”N, 001° 29.622”W), all located in the Adansi North District of the Ashanti Region (Figure 1). Atatam lies 257 m above sea level, is surrounded by mountains, from which several streams reach the village, and has 1,220 inhabitants. Agyenkwaso lies 223 m above sea level, with a population of about 1,300, while Kusa Dinkyeae has only 800 inhabitants and lies 268 m above sea level. Atatam and Agyenkwaso are proximal to each other while Kusa Dinhyeae is more isolated in the forest. A mountain separates Atatam and Kusa Dinkyeae. The main economic activity in these three villages is cocoa farming, interspersed with coffee, oil palm and subsistent farming. All three villages have been targeted by expansion of the Obuasi malaria control programme run by the AngloGold Ashanti Gold Mine because of their proximity to Obuasi municipality.
From the CSEZ, two villages, Gomoa Okyereko (05° 24.828”N, 000° 36.284”W) and Gomoa Adawukwa (05° 24.960N 000o 36.729”), were sampled. Okyereko and Adawukwa are located in the Gomoa East District of the Central Region of Ghana, with an elevation of 19 and 22 meters above sea level, respectively. Okyereko is an irrigated rice farming village with about 1,500 inhabitants. Water from a dam about 500 m away from the village flows through canals to irrigate the rice fields, which are less than 100 m from the village. Flooded rice fields provide active breeding sites for different species of mosquitoes, including anophelines. Cement block houses with corrugated iron sheet roofing dominate, and eaves are either open or closed. Adawukwa is located about 500 m from Okyereko, and the two villages are separated by a river. About 500 m upstream the river, is a swamp where sugarcane cultivation, the main occupation of the inhabitants, takes place. There are about 1,200 people in the village, who, besides sugarcane farming, are involved in maize farming and sugarcane alcohol distillation. There is a brick and roofing tile factory in the village and, as a result, most of the houses, which are either made of brick or cement blocks, are roofed with brick tiles and often have open eaves. Apart from a few households with ITNs and the use by individuals of mosquito-repelling coils, there is no active malaria control programme running in any of the six villages. Domestic animals, such as goat, sheep, chicken, dogs, cats and pigs, are present in all villages. Cattle were not seen in any of the villages sampled.
The sixth village where mosquito sampling was carried out, Osorongma (05° 52.757”N, 000° 06.563”W), lies 94 m above sea level and is located in the FTEZ. It is a peri-urban village close to Dodowa in the Dangme West District of the Greater Accra Region of Ghana, with about 200 inhabitants, who are mainly farming cassava and mango. Houses in the village are a mix of cement block and mud houses, all with corrugated iron sheet roofing, usually with open eaves.
2.2 Mosquito collections and field processing
Sampling was carried out between September 2011 and January 2012, spanning the minor rainy season (September - November) and the dry season (December - March). Prior to mosquito sampling, permission to gain access to each village was sought from an opinion leader, such as the chief or assemblyman. In all cases, verbal informed consent was obtained from the head of each household before mosquito collections were carried out. Mosquitoes were collected early in the morning between 6 and 10 am inside houses, using standard indoor resting collection methods (mouth aspirators and pyrethroid spray collection). Immediately after collection, mosquitoes were killed in a killing bottle containing ethyl ether, individually placed in 1.5 ml microcentrifuge tubes containing silica gel and labelled. All specimens were transported to the laboratory where they were morphologically identified and sorted into species groups using the keys by Gillies and Coetzee [13], and stored at −20°C for further processing.
2.3 Laboratory analysis
A sub-sample of 30 or all (where less than 30 mosquitoes were collected) for each round of collection from every village was selected randomly for molecular assays. The head and thorax of each specimen was dissected and placed in labelled microcentrifuge tubes for enzyme-linked immunosorbent assay (ELISA) to determine the presence of P. falciparum sporozoites. DNA from the rest of the specimen (legs, wings and abdomen) was extracted using standard procedures described by Collins et al. [14]. The extracted DNA was used for species identification, kdr mutation determination and blood meal analysis by polymerase chain reaction (PCR).
Specimens identified morphologically as An. gambiae s.l. were identified to species level using the PCR assay described by Scott et al. [15]. Those specimens identified as An. gambiae s.l. were further identified as An. gambiae s.s. or An. coluzzii using the method described by Favia et al. [16]. Specimens identified morphologically as An. funestus were identified to species-specific level using the PCR assays of Koekemoer et al. [17], and those that failed to amplify after a single repeat were subjected to the IGS-based real-time PCR protocol for identification of An. funestus subgroup developed by Vezenegho et al. [18].
The West African knockdown resistance mutation (L1014F) was assayed in samples identified as An. gambiae s.s. and An. coluzzii using the TaqMan real time PCR (RT-PCR) assay described by Bass et al. [19]. The presence of P. falciparum parasites was determined in all specimens identified to the species-specific level using the ELISA protocol of Wirtz et al. [20]. To determine the blood meal source in blood-fed mosquitoes, the multiplex PCR method developed by Kent and Norris [21] was used. The assay was used to test for the presence of human, cow, pig, goat or dog blood in the specimens. Any specimens that gave multiple blood meals were repeated for confirmation. To confirm blood meal type, PCR products for each blood type were sequenced and checked for alignment with the respective host blood sequence.
2.4 Data analysis
Mosquito species density was calculated as the number of each species divided by the total number of mosquitoes collected per room per collection date. Knockdown resistance (kdr) data were analysed using a Chi-square test for goodness of fit, from which the allele frequencies were generated. Human blood index (HBI) was calculated as number of specimen positive for human blood divided by the total number of specimens tested. Sporozoite rate was calculated as number of specimen positive for P. falciparum divided by total number tested.
Data for indoor resting density and kdr allele frequencies for each species were compared across villages using ANOVA and Tukey’s test used for means separation. Student's t-test was used to compare percentage prevalence of An. gambiae s.s. and An. coluzzii in each village. All statistical analyses were performed using SPSS version 16, except for Chi-square tests, which were performed online using the Hardy-Weinberg equilibrium calculator [22].
2.5 Ethical clearance
This study was exempt from ethical approval by the Institutional Review Board of the Noguchi Memorial Institute for Medical Research, University of Ghana on the basis that it did not involve human participants.
3 Results
3.1 Indoor resting mosquito density
A total of 925 indoor resting mosquitoes, comprising 640 (68%) female An. gambiae s.l., 252 (27%) female An. funestus, 34 (4%) male anophelines and 9 (1%) Culex spp. were collected from the six villages (Table 1). The indoor resting densities of An. gambiae s.l. were generally higher (P<0.05) than those of An. funestus in all villages except Atatam and Agyenkwaso, where there was no significant differences in their abundance (P>0.05).
Table 1.
Indoor resting mosquito densities in six villages in southern Ghana.
| Mean number of mosquitoes/room ± S.E. | ||||
|---|---|---|---|---|
| Village | An. gambiae s.l. | An. Funestus group | Culex spp. | Total |
| Atatam | 7.20 ± 1.59b | 7.45 ± 1.46b | 0.00 ± 0.00c | 14.65 ± 1.90a |
| Agyenkwaso | 4.36 ± 1.15b | 5.18 ± 1.33ab | 0.27 ± 0.14c | 9.81 ± 1.08a |
| Kusa Dinkyeae | 8.63 ± 2.82a | 1.50 ± 0.76b | 0.00 ± 0.00c | 10.13 ± 2.57a |
| Osorongma | 10.00 ± 2.49a | 0.00 ± 0.00b | 0.00 ± 0.00b | 10.00 ± 2.49a |
| Adawukwa | 14.75 ± 2.60a | 4.38 ± 2.78b | 0.38 ± 0.38c | 19.51 ± 1.15a |
| Okyereko | 12.13 ± 2.19a | 0.13 ± 0.13b | 0.33 ± 0.33b | 12.39 ± 2.13a |
| Total | 9.18 ± 0.91b | 3.54 ± 0.65c | 0.11 ± 0.05d | 12.83 ± 0.90a |
Means in the same row with different letters are significantly different at P<0.05. S.E.: standard error of mean.
From a total of 308 female An. gambiae s.l. processed, 176 (57.71%) were identified as An. coluzzii and 132 (42.86%) as An. gambiae s.s. (Table 2). An. gambiae s.s. was predominant in Atatam, Agyenkwaso, and Osorongma, and was the only member of the species complex collected from Kusa Dinkyeae. On the other hand, collections from Adawukwa and Okyereko, which are very close to each other, were exclusively An. coluzzii.
Table 2.
Relative prevalence (%) of An. gambiae s.s. and An. coluzzii in six villages in southern Ghana.
| Village | N | An. coluzzii | An. gambiae s.s. |
|---|---|---|---|
| Atatam | 47 | 1.96 ± 1.96b | 98.04 ± 1.96a |
| Agyenkwaso | 22 | 23.61 ± 1.39b | 76.39 ± 1.39a |
| Kusa Dinkyeae | 29 | 0.00b | 100.00a |
| Osorongma | 43 | 6.00 ± 3.24b | 94.00 ± 3.24a |
| Adawukwa | 79 | 100.00a | 0.00b |
| Okyereko | 88 | 100.00a | 0.00b |
| Total | 308 | 57.71 ± 11.76a | 42.86 ± 11.69a |
Means in the same row with different letters are significantly different at P<0.05. S.E.: standard error of mean.
3.2 Prevalence of kdr mutations
The L1014F mutation was present in both An. gambiae s.s. and An. coluzzii (Table 3). The allele frequencies were slightly lower in An. coluzzii, ranging from 0.75 in the Osorongma population to 0.90 in the Agyenkwaso population. In Adawukwa and Okyereko, the allele frequencies were 0.87 and 0.86, respectively. In An. gambiae s.s., it was almost fixed in all the populations, being 0.97 in Atatam, 0.99 in Osorongma and 1.00 in Agyenkwaso and Kusa Dinkyeae.
Table 3.
Allele distribution of the west African kdr mutation in An. gambiae s.l. from six villages in southern Ghana.
| kdr Allele frequencies (n) | ||
|---|---|---|
| Village | An. coluzzii | An. gambiae s.s. |
| Atatam | −* | 0.97 (48)a |
| Agyenkwaso | 0.90 (5)a | 1.00 (17)a |
| Kusa Dinkyeae | − | 1.00 (29)a |
| Osorongma | 0.75 (2)a | 0.99 (39)a |
| Adawukwa | 0.87 (79)a | − |
| Okyereko | 0.86 (88)a | − |
* Insufficient specimens available for analysis. Values in the same column with same superscript letters are not significantly different at P<0.05.
3.3 Blood meal source and sporozoite rates
Results for blood meal source identification are shown in Table 4. The PCR method could not identify the source of blood meal in some specimens despite physical evidence of blood in the abdomen. A total of 414 specimens were assayed, with success rates ranging from 50 to 100%. A high level of anthropophagy was recorded for the three endophilic anophelines in all villages sampled. An. funestus populations from the three ecological zones sampled were exclusively anthropophagic (HBI = 1), i.e. no animal feeding was observed. An. gambiae s.s. and An. coluzzii populations were similarly highly anthropophagic; however, evidence of animal blood feeding was recorded in both species. All An. gambiae s.s. and An. coluzzii specimens that fed on animals had also fed on humans, except for one An. coluzzii specimen from Okyereko that had exclusively fed on animal blood (cow).
Table 4.
Blood meal source for three endophilic anophelines from six villages in southern Ghana.
| Blood Meal Source (%) | ||||||
|---|---|---|---|---|---|---|
| Species | N | NIS (%) | Human | Animal | Mixed | HBI |
| An. gambiae s.s. | 128 | 89 (70) | 89 (100) | 10 (11) | 10(11) | 1 |
| An. coluzzii | 166 | 115 (60) | 114 (99) | 4 (3) | 3 (3) | 0.99 |
| An. funestus | 120 | 101 (84) | 101 (100) | 0 (0) | 0 (0) | 1 |
| Total | 414 | 305 (74) | 304 (99.7) | 14 (5) | 13 (5) | 0.99 |
NIS: Number identified successfully. HBI: human blood index. Percentages in blood meal source column may not add up to 100, as some specimens contained mixed blood meals.
Plasmodium falciparum infection rates were determined for 429 specimens, comprising 131 An. gambiae s.s., 175 An. coluzzii and 123 An. funestus (Table 5). There was evidence of infection in all the villages except Okyereko, where P. falciparum was not present in any of the 90 specimens processed. After pooling the data from all villages, infectivity was highest in An. funestus, followed by An. gambiae s.s. and lowest in An. coluzzii.
Table 5.
Plasmodium falciparum sporozoite infection rates of indoor resting anophelines from six villages in southern Ghana
| % Infected (n) | |||
|---|---|---|---|
| Village | An. funestus | An. coluzzii An. | gambiae s.s. |
| Atatam | 4.76 (63) | 0 (1) | 2.17 (46) |
| Agyenkwaso | 2.86 (35) | 20.00 (5) | 11.76 (17) |
| Kusa Dinkyeae | 8.33 (12) | - | 0 (29) |
| Osorongma | 0 (1) | 0 (3) | 5.13 (39) |
| Adawukwa | 10 (10) | 1.27 (79) | - |
| Okyereko | 0 (2) | 0 (88) | - |
| Total | 4.88 (123) | 1.14 (176) | 3.8 (131) |
4 Discussion
4.1 Indoor resting mosquito diversity
Anopheles gambiae s.l. and An. funestus s.s. were the only anophelines collected from the villages sampled. The in-door resting densities of these species from the FEZ did not differ, except for Kusa Dinkyeae where the indoor resting density of An. gambiae s.l. was higher than that of An. funestus. By contrast, more An. gambiae s.l. were collected indoors compared to An. funestus in Adawukwa and Okyereko from the CSEZ. Only one An. funestus specimen was collected from Osorongma in the FTEZ. Members of the An. gambiae complex showed varied distribution across the study area, with collections from the FEZ and FTEZ being predominantly An. gambiae s.s. with very few An. coluzzii, except in Kusa Dinkyeae, where no An. coluzzii was collected. An. gambiae s.l. from the CSEZ were, however, exclusively An. coluzzii. These results are similar to other findings from southern Ghana [4,6,7]. All collections from Obuasi in 2011 [6], a location closest to the three FEZ sampling sites, were An. gambiae s.s. Similarly, 100% of all collections from Kumasi, a locality in the FEZ, were An. gambiae s.s., and 98% from Osorongma in the FTEZ [4]. Results from Okyereko and Adawukwa (100% An. coluzzii from each site) also confirm findings dating back to 2004 [4]. The relative abundance of these two species, which until recently were considered molecular forms of the same species (An. gambiae s.s.), has been associated with breeding site characteristics. An. coluzzii tends to be associated with flooded or irrigated sites that provide permanent breeding conditions, whereas An. gambiae s.s. is associated with rain-dependent temporary sites [23].
The results obtained in the current study are in accordance with several reports across Africa, where the three species occur in sympatry over much of their range [2,24]. Prevalence of anophelines in any locality is influenced by ecological factors, such as availability of preferred hosts, breeding sites and environmental variables, such as temperature and relative humidity [7,25,26]. In this study, mosquitoes were collected using indoor collection techniques; therefore, availability of host and resting behaviour were the main expected determinants of occurrence. As all species collected in this study are highly anthropophilic, endophagic and endophilic [2], it was expected that they would be equally represented in the collections. However, this was not the case. The differences in the distribution observed could be attributed to the availability of breeding sites around the villages where mosquitoes were sampled. Presence of breeding sites suitable for proliferation of An. gambiae s.s. and An. funestus, i.e. availability of temporary water puddles during the rainy season due to poorly permeable clay soils and several rivers around the villages of Atatam and Agyenkwaso, could be the underlying factors for the presence of both species in equal densities. Since there were no permanent water bodies in Kusa Dinkyeae this may explain the predominance of An. gambiae s.s.
The breeding sites around villages in the CSEZ are different from those in the FEZ. Okyereko is an irrigated rice-cultivating community, and Adawukwa has a vast swamp and a nearby stream. The rice fields close to these two villages are ideal breeding sites for An. coluzzii and, to a lesser extent, An. funestus, while the edges of the stream and swamps are ideal sites for An. funestus breeding. This probably explains the presence of both species in Okyereko and Adawukwa. The highest density of An. funestus was recorded in Adawukwa, which is located closer to a swamp, suitable for An. funestus breeding. Our findings agree with those by Charlwood and Edoh [26], who reported that anopheline adult density is negatively correlated with the distance between larval habitats and houses. The complete absence of An. funestus in Osorongma could be explained by the absence of suitable breeding sites, as there are no permanent water bodies suitable for An. funestus breeding near this village. Furthermore, according to de Souza et al. [7], the distribution of the members of the An. gambiae complex appears to be driven by environmental factors, with An. gambiae s.s. more predominant in the forested areas characterised by lower mean daily temperatures, whereas An. coluzzii is predominant in the northern and coastal savannah areas, where mean daily temperatures are slightly higher.
The densities reported here may not represent the actual annual anopheline distribution in the study areas. Sampling was only limited to two seasons: the minor rainy season (September to November, 2011) and the dry season (December 2011 - January 2012). Species composition and abundance varies with time of year [6]. Yawson et al. [4] collected 58 An. funestus out of 341 specimens from 10 collections in Osorongma during the major rainy season (June to September), whereas in the present study, only one specimen of An. funestus was collected from the same village during the dry season. In this study, more An. gambiae was collected during the minor rainy season than the dry season and vice versa for An. funestus (data not shown).
4.2 Prevalence of the kdr mutation
We observed high variability in the prevalence of the L1014F mutation in An. gambiae s.s. and An. coluzzii. The near fixation of the L1014F mutation in the populations from the areas sampled is understandable, since this mutation is widespread and predominant in West Africa [27]. High frequencies of the L1014F mutation have been reported from same location or locations close to the sampled villages across southern Ghana [4-6,27]. Results from the current study show that the mutation is prevalent in both An. coluzzii (90%) and An. gambiae s.s. (98%). The L1014F mutation has shown a steady increase in An. coluzzii from different locations in southern Ghana since 2002 [4,8,28]. Around 2002-2004, the frequency was 1-3%, and increased to 54-79% by 2007/2008 [4,8,28]. The frequency recorded in this study for collections made in late 2011 and early 2012 ranged from 75 to 90% showing a further increase. This is not the first report of a temporal increase of the mutation in An. coluzzii in West Africa. Djegbe et al. [29] reported a near fixation of the allele in this species from Cotonou, and a significant temporal increase over a two-year period at Bohicon and Malanville in Benin. Santolamazza et al. [27] concluded that the distribution of the kdr mutation is non-uniform on the African continent and that its origin and spread in An. gambiae s.l. is an ongoing process.
The spread of the kdr mutation on the African continent has been attributed to introgression [30] and, to some extent, migration [29], driven mainly by widespread use of insecticides in agriculture and possibly for domestic purposes [31]. Insecticide usage is widespread in southern Ghana where this study was carried out [32]. In the forested areas where Atatam, Agyenkwaso and Kusa Dinkyeae are located, cocoa farming is the major agricultural activity, which has been and is still reliant on usage of insecticides of all classes, including organochlorines for capsids (Distantiella theobroma and Sahlbergella singularis) control [32]. Irrigated rice production around Okyereko and Adawukwa in the coastal savannah areas, and mango plantations of the eastern mango enclave of Ghana, around Osorongma, rely heavily on insecticide usage. Akogbeto et al. [33] demonstrated that insecticide-resistant An. gambiae populations are able to survive and proliferate in insecticide-contaminated soils and water from vegetable and cotton farms. Insecticide residues have been detected in water and soil samples from different parts of Ghana [32], suggesting the presence of heavy selection pressure on anopheline populations. Thus, whether the mutation enters the population by independent mutation events, introgression or by migration, it will easily be sustained and is likely to spread further in vector populations.
4.3 Vector competence
The success rate of the PCR method in identifying blood meal source was generally high (84% for An. funestus, 70% for An. gambiae s.s. and 60% for An. coluzzii), although it did not include primers for all the possible hosts found in the villages where mosquitoes were sampled, such as chicken, sheep, bats or rodents. It could be possible that specimens for which the blood meal source could not be determined had fed on such animals. It is important to note all possible hosts within a locality where samples are being collected and to include primers specific for as many hosts as possible in the PCR testing. Alternatively, a technique involving screening of mosquito blood meals with avian- and mammalian-specific primer pairs followed by sequencing could be used [34]. Analysis of the sequences can be used to determine the actual species each mosquito specimen fed on.
All three major vector species from southern Ghana show high levels of anthropophagy, with An. funestus being exclusively anthropophagic consistent with their feeding behaviour. According to Sinka et al. [2] An. funestus shows fairly consistent behaviour (generally anthropophilic and endophilic) throughout its range, whereas members of the An. gambiae complex appear to exhibit greater phenotypic plasticity and opportunism in blood feeding than commonly thought. In the present study, An. gambiae s.s. and An. coluzzii demonstrated low levels of animal feeding, but all except a single specimen that fed on animals also fed on humans. This could imply that, in the absence of humans, they will likely feed on animals, but given the chance, will supplement with human blood.
With the high HBI recorded for both species in the two ecological zones, the sporozoite rate should reflect the prevalence of the parasite within the populations. This is evident in the variable sporozoite rates recorded for the three species. Thus, the sporozoite rate only reflects the prevalence of parasites in the population and indicates the competence of the species as vectors. The sporozoite rates recorded in the current study fall within the range of those recorded by other workers [3,5,6,12,35] in Ghana, confirming these areas as malaria endemic. However, a caveat on these data is that the ELISA method is known to often give false positive results, especially for P. falciparum and for zoophilic vector species [36]. The highest sporozoite rate of 20% in this study was recorded from Agyenkwaso, where some zoophily was also observed. Furthermore, the small sample size could also affect the sporozoite rate as all the sites with high rates had small sample sizes. Nevertheless, all three vector species are actively transmitting malaria in the villages sampled.
5 Conclusions
Anopheles gambiae s.s., An. coluzzii and An. funestus are the major indoor resting malaria vectors in all the three ecological zones where this study was carried out, but showing variations in their distribution. An. gambiae s.s. predominates in the forested areas, whereas An. coluzzii predominates in the coastal savannah and An. funestus shows a wider distribution across southern Ghana. This distribution is highly influenced by breeding site availability and environmental factors. Thus, any research and development activity that focuses on developing control tools targeting these major malaria vectors might have to be carried out across all the ecological zones to be relevant for area-wide implementation. Similarly, to carry out any control programme across these ecological zones, the tools must target all three species.
The L1014F kdr mutation is present at very high frequencies in all three ecological zones, with its spread in the population nearing fixation in both An. gambiae s.s. and An. coluzzii. This implies that control programmes must include resistance management strategies and incorporate novel tools that are not affected by the kdr mutation. There should also be extensive resistance monitoring in the country and further research into the impact of this and other resistance mechanisms on vector control activities in Ghana.
6 Acknowledgements
The authors would like to thank the chiefs and people of all six communities where the study was carried out. Many thanks to the International Atomic Energy Agency (IAEA) for the Technical Cooperation Fellowship to M.O. through the Graduate School of Nuclear and Allied Science, University of Ghana, and to the Management and staff of the Vector Control Reference Laboratory of the National Institute for Communicable Diseases in South Africa for hosting and assisting M.O. with all laboratory work. Thanks also to the management and staff of the Biotechnology and Nuclear Agriculture Research Institute of Ghana Atomic Energy Commission for logistical support during field collections.
References
- 1.World Health Organization: World Malaria Report. World Health Organization,; Geneva, Switzerland.: 2012.. 2012. [Google Scholar]
- 2.Sinka ME, Bangs MJ, Manguin S, Coetzee M et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appawu MA, Baffoe-Wilmot A, Afari EA, Dunyo S et al. Malaria vector studies in two ecological zones in Southern Ghana. Afr. Entomol. 2001;9:59–65. [Google Scholar]
- 4.Yawson AE, McCall PJ, Wilson MD, Donnelly MJ: Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med. Vet. Entomol. 2004;18:372–377. doi: 10.1111/j.0269-283X.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 5.Coetzee M. Van Wyk P, Booman M, Koekemoer LL et al. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bull. Soc. Pathol. Exot. 2006;99:400–403. [PubMed] [Google Scholar]
- 6.Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J et al. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasit. Vectors. 2011;4:107. doi: 10.1186/1756-3305-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Souza D,, Kelly-Hope L,, Lawson B, Wilson M et al. Environmental factors associated with the distribution of Anopheles gambiae s.s. in Ghana: an important vector of lymphatic filariasis and malaria. PLoS ONE. 2010;5:e9927. doi: 10.1371/journal.pone.0009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson CS, Wheetman D, Essandoh J, Yawson AE et al. Adaptive introgression between Anopheles sibling species eliminates a major genomic island but not reproductive isolation. Nat. Commun. 2014;5:4248. doi: 10.1038/ncomms5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi GP, Service MW, Pradhan GD: A survey of species A and B of the Anopheles gambiae Giles complex in the Kisumu area of Kenya prior to insecticidal spraying with OMS-43 (fenitrothion). Ann. Trop. Med. Parasitol. 1975;69:91–103. doi: 10.1080/00034983.1975.11686988. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay SW, Parson L, Thomas CJ: Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc. Biol. Sci. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization: Pesticides and their application for the control of vectors and pests of public health importance. WHO/CDS/NTD.WHOPES/GCDPP/2006.1.
- 12.Owusu-Agyei S, Asante KP, Adjuik M, Adjei G et al. Epidemiology of malaria in the forest-savannah transitional zone of Ghana. Malar. J. 2009;8:220. doi: 10.1186/1475-2875-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillies MT, Coetzee M: A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region): The South African Institute for Medical Research,; Johannesburg: 1987. [Google Scholar]
- 14.Collins FH, Mendez MA, Rasmussen MO, Meheffey PC et al. A ribosomal RNA gene probe differentiates members of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 15.Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 16.Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I. et al. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol. Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 17.Koekemoer LL, Kamau L, Hunt RH, Coetzee M: A cocktail polymerase chain reaction (PCR) assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 18.Vezenegho SB, Bass C, Puinean M, Williamson MS et al. Development of multiplex real-time PCR assays for identification of members of the Anopheles funestus species group. Malar. J. 2009;8:282. doi: 10.1186/1475-2875-8-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bass C, Nikou D, Donnelly MJ, Williamson MS et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar. J. 2007;6:111. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirtz RA, Sattabonkot J, Hall T, Burkot TR et al. Development and evaluation of an ELISA for plasmodium vivax-VK247 sporozoites. J. Med. Entomol. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- 21.Kent RJ, Norris DE: Identification of mammalian blood meals in mosquitoes by a multiplex polymerase chain reaction targeting cytochrome b. Am. J. Trop. Med. Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez S, Gunt TR, Day INM: Hardy-Weinberg equilibrium testing of biological ascertainment for mendelian randomization studies. Am. J. Epidemiol. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y et al. A global map of dominant malaria vectors. Parasit. Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minakawa N, Seda P, Yan G: Influence of host and larval habitat distribution on the abundance of African malaria vectors in Western Kenya. Am. J. Trop. Med. Hyg. 2002;67:32–38. doi: 10.4269/ajtmh.2002.67.32. [DOI] [PubMed] [Google Scholar]
- 25.Kipyab PC, Khaemba BM, Mwangangi JM, Mbogo CM: The bionomics of Anopheles merus (Diptera: Culicidae) along the Kenyan Coast. Parasit. Vectors. 2013;6:37. doi: 10.1186/1756-3305-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlwood JD, Edoh D: Polymerase chain reaction used to describe larval habitat use by Anopheles gambiae complex (Diptera: Culicidae) in the environs of Ifakara, Tanzania. J. Med. Entomol. 1996;33:202–204. doi: 10.1093/jmedent/33.2.202. [DOI] [PubMed] [Google Scholar]
- 27.Santolamazza F, Calzetta M, Etang J, Barrese E et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and westcentral Africa. Malar. J. 2008;7:74. doi: 10.1186/1475-2875-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynd A, Wheetman D, Barbosa S, Yawson AE et al. Field, genetic and modelling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol. Biol. Evol. 2010;27:1117–1125. doi: 10.1093/molbev/msq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djegbe I, Boussari O, Sidick A, Martin T et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014Skdr mutation in Anopheles gambiae from West Africa. Malar. J. 2011;10:261. doi: 10.1186/1475-2875-10-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weill M, Chandre F, Brengues C, Manguin S et al. The kdr mutation occurs in the Mopti form of Anopheles gambiae s.s. through introgression. Insect Mol. Biol. 2000;9:451–455. doi: 10.1046/j.1365-2583.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- 31.Pinto J, Lynd A, Vicente JL, Santolamazza F et al. Multiple origins of knockdown resistance mutations in the afrotropical mosquito vector Anopheles gambiae. PLoS ONE. 2007;2:e1243. doi: 10.1371/journal.pone.0001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fianko JR, Donkor A, Lowor ST, Yeboah PO: Agrochemicals and the Ghanaian environment, a review. J. Environ. Prot. (Irvine, Calif.). 2011;2:221–230. [Google Scholar]
- 33.Akogbeto MC, Djouaka R, Noukpo H: [Use of agricultural insecticides in Benin] (L’utilisation des insecticides en agriculture au Benin). Bull. Soc. Pathol. Exot. 2005;98:400–405. [PubMed] [Google Scholar]
- 34.Molaei G, Oliver J, Andreadis TG, Armstrong PM et al. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am. J. Trop. Med. Hyg. 2006;75:1140–1147. [PubMed] [Google Scholar]
- 35.Badu K, Brenya RC, Timmann C, Garms R et al. Malaria transmission intensity and dynamics of clinical malaria incidence in a mountainous forest region of Ghana. MalariaWorld J. 2013;4:14. [Google Scholar]
- 36.Durnez L. Van Bortel W, Denis L, Roelants P et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 2011;10:95. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]


