Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Sacituzumab govitecan (SG), a first-in-class anti–trophoblast cell surface antigen 2 (Trop-2) antibody-drug conjugate, demonstrated superior efficacy over single-agent chemotherapy (treatment of physician's choice [TPC]) in patients with metastatic triple-negative breast cancer (mTNBC) in the international, multicenter, phase III ASCENT study.
Patients were randomly assigned 1:1 to receive SG or TPC until unacceptable toxicity/progression. Final efficacy secondary end point analyses and post hoc analyses of outcomes stratified by Trop-2 expression and human epidermal growth factor receptor 2 status are reported. Updated safety analyses are provided.
In this final analysis, SG (n = 267) improved median progression-free survival (PFS; 4.8 v 1.7 months; hazard ratio (HR), 0.41 [95% CI, 0.33 to 0.52]) and median overall survival (OS; 11.8 v 6.9 months; HR, 0.51 [95% CI, 0.42 to 0.63]) over TPC (n = 262). SG improved PFS over TPC in each Trop-2 expression quartile (n = 168); a trend was observed for improved OS across quartiles. Overall, SG had a manageable safety profile, with ≤5% of treatment-related discontinuations because of adverse events and no treatment-related deaths. The safety profile was consistent across all subgroups.
These data confirm the clinical benefit of SG over chemotherapy, reinforcing SG as an effective treatment option in patients with mTNBC in the second line or later.
INTRODUCTION
Metastatic triple-negative breast cancer (mTNBC) is typified by an aggressive clinical course and poor outcomes, despite chemotherapy treatment.1-5 Trophoblast cell surface antigen 2 (Trop-2), an epithelial antigen overexpressed in 80%-90% of all triple-negative breast cancers (TNBCs), is associated with poor prognosis, increased tumor growth, and decreased survival, representing a potential treatment target.6-8 Sacituzumab govitecan (SG), an antibody-drug conjugate (ADC) with Trop-2 antibodies coupled to a cytotoxic SN-38 payload via a proprietary hydrolysable linker,9-11 was the first ADC approved in multiple countries (including the United States) for patients with mTNBC12-14 on the basis of results from the phase III ASCENT clinical trial.15 Presented are the final preplanned efficacy and safety secondary outcomes of SG versus treatment of physician's choice (TPC) in the intention-to-treat (ITT) population and post hoc biomarker analyses.
METHODS
Study design and patient eligibility were previously described15 (Data Supplement, Fig S1, online only).
Secondary end points included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), duration of response (DOR), time to response, and safety in the ITT population.16 Median follow-up was calculated by time from random assignment to death or last known date alive. Post hoc subgroup analyses were by Trop-2 expression and human epidermal growth factor receptor 2 (HER2) status in the ITT population. Membrane histochemical score (H-score) and percentage of membrane cells were used for Trop-2 membrane expression. Expression was determined using a validated immunohistochemistry (IHC) assay,17 categorized on the basis of numerical scores, and then divided into equally sized quartiles. Interaction between treatment and Trop-2 expression was assessed using the type III Wald chi-square test.
For the post hoc HER2 subgroup analysis, local IHC and in-situ hybridization (ISH) results for the ITT population of ASCENT were analyzed retrospectively to associate SG efficacy with HER2 status as HER2 IHC0 or HER2-low, defined as HER2 IHC1+, or IHC2+, and ISH-negative. Thus, among the patients in the ITT population, only patients with known Trop-2 expression or HER2 IHC status were included in these subgroup analyses.
Efficacy was evaluated in the ITT population. Kaplan-Meier estimates were used to analyze PFS and OS; benefit was measured using Cox proportional hazards models. Response rates were compared using the stratified Cochran-Mantel-Haenszel method. Safety was assessed in patients receiving ≥1 dose of study drug.15 Statistical subgroup analysis methods are presented in the Data Supplement; nominal P values are reported.
Trial conduct was in accordance with Good Clinical Practice and Principles in the Declaration of Helsinki. An independent ethics committee provided approval at each site. All patients signed written informed consent.15
RESULTS
Patients
Patients (N = 529; SG, n = 267; TPC, n = 262; Table 118-20; Data Supplement, Figs S1 and S2) had median follow-ups of 11.2 months (SG; range, 0.3-30.8) and 6.3 months (TPC; range, 0-29.4). The most common reason for discontinuation was disease progression (SG, 85%; TPC, 70%).
TABLE 1.
Demographics and Baseline Characteristics
| Characteristic | ITT Population | HER2-Evaluable Population | Trop-2–Evaluable Population | |||
|---|---|---|---|---|---|---|
| SG (n = 267) | TPC (n = 262) | SG (n = 211) | TPC (n = 204) | SG (n = 168) | TPC (n = 150) | |
| Female, No. (%) | 265 (99) | 262 (100) | 209 (99) | 204 (100) | 166 (99) | 150 (100) |
| Age at study entry, years, median (range) | 54 (27-82) | 53 (27-81) | 54 (27-82) | 53 (27-81) | 53 (30-82) | 53 (30-81) |
| Race, No. (%)a | ||||||
| White | 215 (81) | 203 (77) | 169 (80) | 163 (80) | 135 (80) | 115 (77) |
| Black | 28 (10) | 34 (13) | 24 (11) | 25 (12) | 18 (11) | 23 (15) |
| Asian | 13 (5) | 9 (3) | 12 (6) | 5 (2) | 8 (5) | 5 (3) |
| Other | 11 (4) | 16 (6) | 6 (3) | 11 (5) | 7 (4) | 7 (5) |
| ECOG performance status, No. (%) | ||||||
| 0 | 121 (45) | 108 (41) | 91 (43) | 92 (45) | 73 (43) | 63 (42) |
| 1 | 146 (55) | 154 (59) | 120 (57) | 112 (55) | 95 (57) | 87 (58) |
| TNBC at initial breast cancer diagnosis, No. (%) | 192 (72) | 180 (69) | 155 (73) | 142 (70) | 120 (71) | 104 (69) |
| No. of previous chemotherapies, No. (%) | ||||||
| 2-3 | 184 (69) | 181 (69) | 145 (69) | 139 (68) | 126 (75) | 109 (73) |
| >3 | 83 (31) | 81 (31) | 66 (31) | 65 (32) | 42 (25) | 41 (27) |
| Previous systemic regimens, No., median (range)b | 4 (2-17) | 4 (2-14) | 4 (2-11) | 4 (2-14) | 4 (2-11) | 4 (2-11) |
| Previous use of checkpoint inhibitors, No. (%) | 79 (30) | 74 (28) | 58 (27) | 57 (28) | 44 (26) | 33 (22) |
| Setting of previous systemic therapies, No. (%) | ||||||
| Adjuvant | 161 (60) | 148 (56) | 127 (60) | 112 (55) | 101 (60) | 84 (56) |
| Neoadjuvant | 124 (46) | 125 (48) | 98 (46) | 101 (50) | 78 (46) | 76 (51) |
| Metastatic | 258 (97) | 260 (99) | 203 (96) | 203 (100) | 163 (97) | 149 (99) |
| Locally advanced disease | 10 (4) | 5 (2) | 7 (3) | 5 (2) | 5 (3) | 3 (2) |
| BRCA1/2 mutational status, No. (%)c | ||||||
| Negative | 150 (56) | 146 (56) | 121 (57) | 117 (57) | 92 (55) | 86 (57) |
| Positive | 20 (7) | 23 (9) | 14 (7) | 17 (8) | 9 (5) | 10 (7) |
NOTE. Patients (relapsed/refractory mTNBC [HER2 IHC0, 1, or 2/ISH-negative; ER/PR <1%]18-20 after ≥2 previous standard chemotherapy regimens for unresectable, locally advanced, or metastatic disease) were randomly assigned 1:1 to either SG or TPC until disease progression, unacceptable toxicity, study withdrawal, or death, whichever occurred first. Patients with known brain metastases were capped at 15%.
Abbreviations: BRCA, breast cancer gene; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ITT, intention-to-treat; mTNBC, metastatic triple-negative breast cancer; PR, progesterone receptor; SG, sacituzumab govitecan; TNBC, triple-negative breast cancer; TPC, treatment of physician's choice; Trop-2, trophoblast cell surface antigen 2.
Race was self-reported. Other includes American Indian or Alaska Native, Native Hawaiian, or other Pacific Islander.
Anticancer regimens refer to any treatment regimen that was used to treat breast cancer in any setting.
Positive denotes that the patient is either BRCA1-positive or BRCA2-positive. Negative denotes that the patient is both BRCA1-negative and BRCA2-negative.
Efficacy
ITT Population
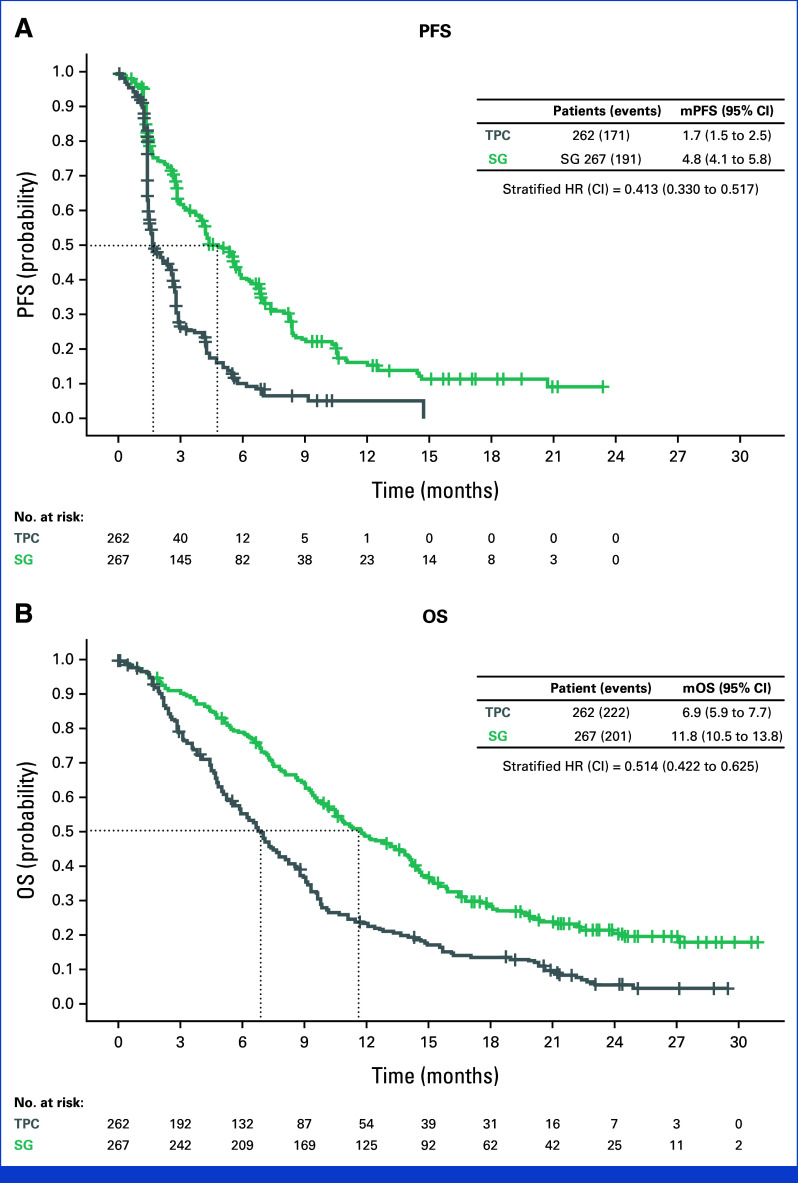
Consistent with the final efficacy analysis in the primary outcome population (patients without baseline brain metastases), SG improved median PFS (SG, 4.8 months; TPC, 1.7 months; hazard ratio (HR), 0.41 [95% CI, 0.33 to 0.52]) and median OS (SG, 11.8 months; TPC, 6.9 months; HR, 0.51 [95% CI, 0.42 to 0.63]) over TPC (Fig 1).15 For ORR and DOR, see the Data Supplement (Table S1).
FIG 1.
mPFS and mOS from Kaplan-Meier estimates in the ITT population. CIs for mPFS and mOS were computed using the Brookmeyer-Crowley method. Stratified log-rank test and stratified Cox regression were adjusted for the following stratification factors: number of previous chemotherapies and region. HR, hazard ratio; ITT, intention to treat; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival; SG, sacituzumab govitecan; TPC, treatment of physician's choice.
Trop-2–Evaluable Population
Staining was available for 60% of the ITT population, with the majority of patients having a medium or high Trop-2 expression (defined as H-score ≥130; Table 1). PFS and OS outcomes were comparable with ITT population outcomes (Data Supplement, Fig S3).
SG improved clinical outcomes over TPC in Trop-2 subgroups measured by H-score and percentage of membrane cells. SG improved median PFS across all subgroups over TPC, with a trend to improved median OS across all subgroups (Fig 2, Data Supplement, Table S2, Fig S4). ORR for SG was improved (Data Supplement, Table S3). ORR was higher with SG over TPC in all Trop-2–low groups (Data Supplement, Table S4). With higher Trop-2 levels, this improvement appeared to be greater. No significant interaction between treatment and Trop-2 expression was observed for PFS (range, P = .24 to 0.73). Interaction between treatment and continuous Trop-2 expression for OS was marginally significant (range, P = .04 to 0.05); interaction between treatment and Trop-2 H-score quartiles for OS was trending toward significance (P = .054; Data Supplement, Table S5). Importantly, SG improved outcomes compared with TPC across all levels of Trop-2 expression.
FIG 2.
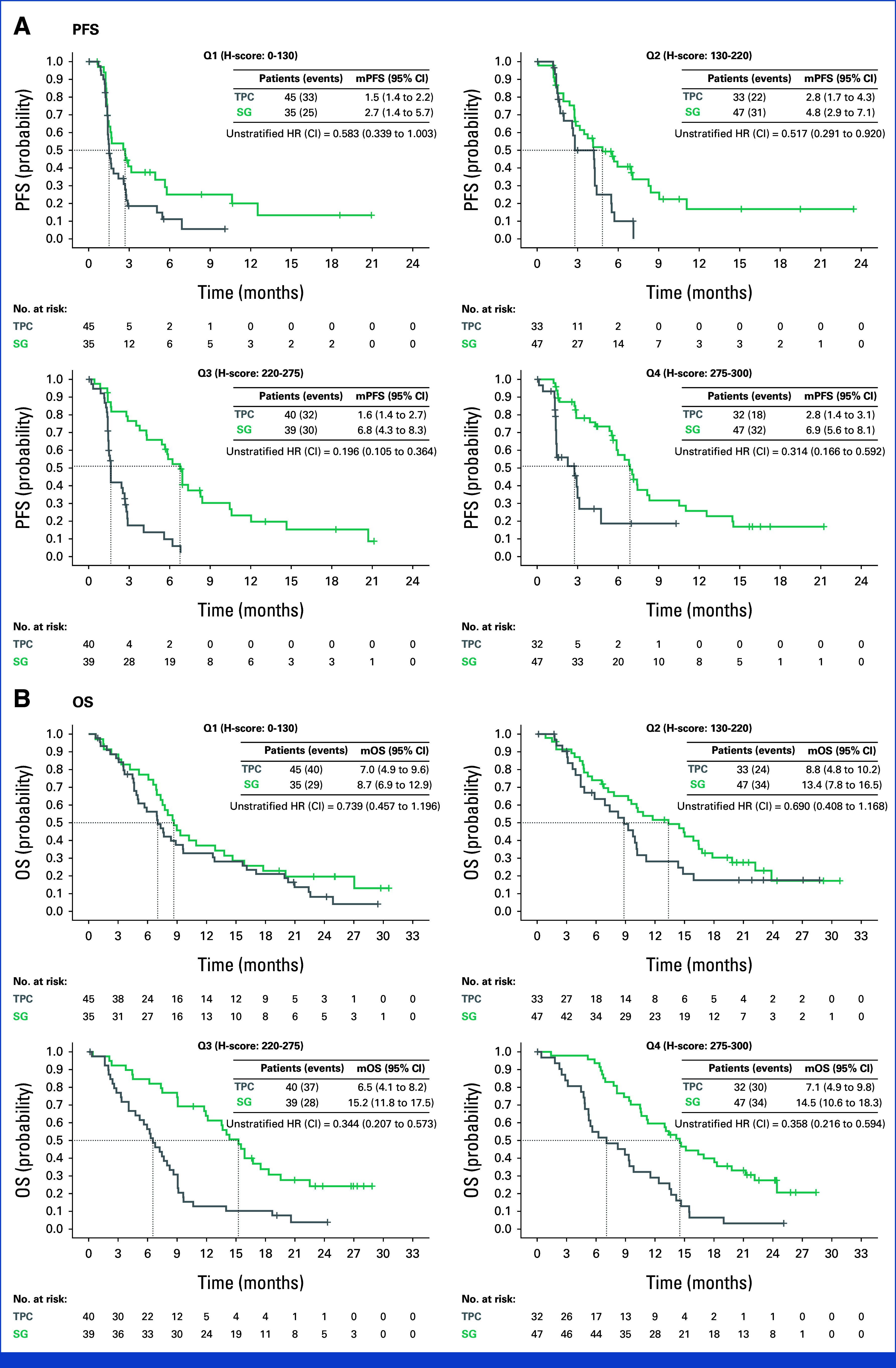
mPFS and mOS from Kaplan-Meier estimates in the Trop-2 expression–evaluable population by H-score. CIs for mPFS and mOS were computed using the Brookmeyer-Crowley method. HER2, human epidermal growth factor receptor 2; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival; Q, quartile; SG, sacituzumab govitecan; TPC, treatment of physician's choice; Trop-2, trophoblast cell surface antigen 2.
HER2-Evaluable Population
In the ITT population, 78% of the ITT population were HER2-evaluable by IHC (SG, n = 211; TPC, n = 204); overall, 71% were HER2 IHC0 and 29% were HER2-low. PFS and OS in the HER2-evaluable population were comparable with those in the ITT population (Data Supplement, Fig S3). SG improved PFS and OS for both HER2 expression subgroups (Data Supplement, Table S2, Fig S5). ORR was improved for SG over TPC (Data Supplement, Table S3). Trop-2 was highly expressed regardless of HER2 status (Data Supplement, Fig S6).
Safety
The most frequent treatment-emergent adverse events (TEAEs), any grade, were the same as those in the previous analysis (safety population, N = 482; SG, n = 258; TPC, n = 224).15 Discontinuation rates (≤5%) were similar in both the groups.15 Subgroup safety was consistent with overall safety (Data Supplement, Tables S6 and S7).
DISCUSSION
This follow-up analysis of the ASCENT trial, which included patients with and without baseline brain metastases, validates the clinical benefit of SG over single-agent chemotherapy in PFS and OS15; SG efficacy was established across Trop-2 expression subgroups. There was a significant benefit with SG for patients in the two highest Trop-2–expressing quartiles in PFS, OS, and ORR. In the lowest two quartiles of Trop-2 expression, there were favorable HR point estimates for SG. These post hoc analyses were exploratory in nature and not powered to evaluate the impact of Trop-2 expression on the benefit of SG versus TPC.
SG efficacy in low–Trop-2-level tumors may be due to SG's bystander17 effect, high binding affinity, and high drug-antibody ratio, leading to effective antitumor activity even with low antigen expression. Thus, Trop-2 testing is not required for SG treatment. As reported,15 the most common SG grade ≥3 TEAEs were neutropenia (52%), diarrhea (12%), and leukopenia (11%). Patients treated with SG reported greater clinically meaningful improvements in primary health-related quality of life compared with TPC.16
Trastuzumab deruxtecan (T-DXd), an HER2-targeted ADC, is approved for patients with HER2-low mBC.21 In the phase III DESTINY-Breast04 trial, T-DXd demonstrated a significant improvement in PFS and OS outcomes compared with TPC in patients with mBC with confirmed HER2-low status including in a small cohort of patients with TNBC (T-DXd, n = 40; TPC, n = 18 in the HR-negative/HER2-low group) who received a median of one line of therapy for mBC.15,22 SG efficacy was observed in both HER2 IHC0 and HER2-low subgroups. Further research is needed on the optimal treatment sequencing of ADCs.
Limitations include the exploratory nature of the subgroup analyses, the relatively small number of patients in each subgroup, the use of archival tissue, and the lack of a prespecified analysis.
SG improved clinical outcomes over TPC in patients with pretreated mTNBC, including in subgroup analyses, confirming that Trop-2 IHC testing is unnecessary for SG treatment. These data reinforce SG as a standard-of-care treatment option in pretreated mTNBC. SG is currently recommended in major guidelines for treating patients with mTNBC who received at least two previous therapies (≥1 in the metastatic setting) owing to the significant clinical benefit observed in the phase III ASCENT study.13,14,23-25
ACKNOWLEDGMENT
We thank the patients, their caregivers, and families for their participation and commitment to clinical research. Thanks to the clinical trial investigators and their team members, without whom this work would not have been possible. The study was sponsored by Gilead Sciences, Inc, and was designed through a collaboration of the sponsor and the lead investigators. Medical writing and editorial assistance were provided by Gwendolyn F. Elphick, PhD, at Parexel, Inc, and was funded by Gilead Sciences, Inc.
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Sanofi, Daiichi Sankyo/Astra Zeneca, Lilly, Lilly (Inst), Gilead Sciences, Gilead Sciences (Inst), Menarini, Menarini (Inst), Mersana
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Hope S. Rugo
Consulting or Advisory Role: Napo Pharmaceuticals, Puma Biotechnology, Mylan, Eisai, Daiichi Sankyo
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Merck (Inst), Daiichi Sankyo (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Pionyr (Inst), Taiho Oncology (Inst), Veru (Inst), GlaxoSmithKline (Inst), Hoffmann-La Roche AG/Genentech, Inc (Inst), Stemline Therapeutics (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca, Gilead Sciences, Pfizer
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183398
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Myovant Sciences, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Ellipses Pharma, OncoSec, Infinity Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi
Delphine Loirat
Honoraria: MSD, Gilead Sciences, Gilead Sciences, AstraZeneca, Lilly
Consulting or Advisory Role: Roche, MSD Oncology, AstraZeneca, Novartis, Pfizer, Immunomedics, Gilead Sciences, 4D Pharma, Lilly
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD, Pfizer, Gilead Sciences
Kevin Punie
Honoraria: Pfizer, Pfizer (Inst), Lilly (Inst), Roche (Inst), Novartis (Inst), Mundipharma (Inst), Gilead Sciences (Inst), Seagen (Inst), AstraZeneca, Lilly, Medscape/Exact Sciences, Focus Patient, Gilead Sciences, MSD, Novartis, Roche, Seagen, MSD (Inst)
Consulting or Advisory Role: AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Novartis, Pierre Fabre (Inst), Roche (Inst), Vifor Pharma (Inst), Teva (Inst), MSD (Inst), AstraZeneca, Lilly, Medscape/Exact Sciences, Gilead Sciences, Pfizer, Roche, Seagen, Sanofi, AstraZeneca
Research Funding: Sanofi (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Novartis, AstraZeneca, PharmaMar, Pfizer, Roche
Mafalda Oliveira
Honoraria: Roche, Pfizer, Eisai Europe, Seagen, Gilead Sciences, AstraZeneca, Lilly, Medscape
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Seagen, ITeos Therapeutics, Daiichi Sankyo/AstraZeneca, Gilead Sciences, Relay Therapeutics, Cureo Science, iOne, Lilly, MSD, Pfizer
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Zenith Epigenetics (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Eisai, Pierre Fabre, Gilead Sciences, AstraZeneca Spain
Uncompensated Relationships: Head of the SOLTI Breast Cancer Research Group
Adam Brufsky
Consulting or Advisory Role: Pfizer, Genentech/Roche, Agendia, Novartis, Lilly, Puma Biotechnology, Merck, Myriad Pharmaceuticals, Eisai, Seagen, Daiichi Sankyo/Lilly, Onc Live, Michael J. Hennessy Associates, Gilead Sciences, General Electric
Research Funding: Roche/Genentech (Inst), AstraZeneca/Daiichi Sankyo (Inst), Merck (Inst), Novartis (Inst), Gilead Sciences (Inst), Lilly (Inst), Puma Biotechnology (Inst)
Expert Testimony: Pfizer
Kevin Kalinsky
Employment: GRAIL, EQRx
Stock and Other Ownership Interests: GRAIL, EQRx
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Genentech/Roche, Immunomedics, Merck, Seagen, Oncosec, 4D Pharma, Daiichi Sankyo/AstraZeneca, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, Prelude Therapeutics, RayzeBio
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Lilly (Inst), Seagen (Inst), AstraZeneca (Inst), Daichi Sankyo (Inst), Ascentage Pharma (Inst)
Other Relationship: Immunomedics, Genentech
Javier Cortés
Stock and Other Ownership Interests: Leuko, MAJ3 Capital
Honoraria: Novartis, Eisai, Celgene, Pfizer, Roche, Samsung, Lilly, Merck Sharp & Dohme, Daiichi Sankyo, AstraZeneca, Gilead Sciences, Steamline Therapeutics
Consulting or Advisory Role: Celgene, Cellestia Biotech, AstraZeneca, Roche, Seagen, Daiichi Sankyo, ERYTECH Pharma, Polyphor, Athenex, Lilly, SERVIER, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim, Ellipses Pharma, HiberCell, Bioinvent, GEMoaB, Gilead Sciences, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Bridgebio
Research Funding: ARIAD (Inst), AstraZeneca (Inst), Baxalta (Inst), Bayer (Inst), Eisai (Inst), Guardant Health (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Puma Biotechnology (Inst), Queen Mary University of London (Inst), Roche (Inst), Piqur (Inst)
Patents, Royalties, Other Intellectual Property: Pharmaceutical combinations of a Pi3k inhibitor and a microtubule destabilizing agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A, HER2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/0338368 A1
Travel, Accommodations, Expenses: Roche, Pfizer, Eisai, Novartis, Daiichi Sankyo, Gilead Sciences, AstraZeneca, Merck Sharp & Dohme Steamline Therapeutics
Joyce O' Shaughnessy
Honoraria: AstraZeneca, Lilly, AbbVie, Celgene, Eisai, Novartis, Pfizer, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Consulting or Advisory Role: Novartis, Pfizer, Lilly, AbbVie, AstraZeneca, Celgene, Eisai, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Speakers' Bureau: AstraZeneca, Novartis, Lilly, Pfizer, Seagen
Research Funding: Seagen (Inst)
Travel, Accommodations, Expenses: Celgene, Lilly, Novartis, Pfizer, AbbVie, Agendia, Amgen, Eisai, GRAIL, Ipsen, Myriad Pharmaceuticals, Puma Biotechnology, Seagen, AstraZeneca, Sanofi, Roche
Véronique Diéras
Honoraria: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, AbbVie/Abbott, MSD Oncology, Daiichi Sankyo, Seagen, Gilead Sciences, Eisai Europe, Pierre Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Pfizer, AstraZeneca, AbbVie/Abbott, MSD Oncology, Daiichi Sankyo Europe GmbH, Seagen, Gilead Sciences, Eisai Europe, Pierre Fabre, Medac, Menarini Group
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer, Lilly, AstraZeneca, Daiichi Sankyo Europe GmbH, Seagen, Gilead Sciences
Lisa A. Carey
Research Funding: NanoString Technologies (Inst), Seagen (Inst), Veracyte (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Novartis (Inst), Lilly (Inst), Genentech/Roche (Inst)
Uncompensated Relationships: Novartis (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Lilly, Seagen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Luca Gianni
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Synaffix, Revolution Medicines, Zymeworks, Menarini, Amgen, BioMedical Insights, Artemida Pharma, Denali Therapeutics, Daiichi Sankyo/Astra Zeneca
Patents, Royalties, Other Intellectual Property: Roche
Martine Piccart-Gebhart
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Menarini, Seagen, Camel-IDS, Immunomedics, Roche/Genentech, Immutep, NBE Therapeutics, Frame Therapeutics, Gilead Sciences
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Roche/Genentech (Inst), Radius Health (Inst), Synthon (Inst), Servier (Inst), Immunomedics/Gilead (Inst), Menarini (Inst)
Other Relationship: Oncolytics
Sibylle Loibl
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), Novartis (Inst), Seagen (Inst), Celgene (Inst), Lilly (Inst), AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Merck KGaA (Inst), AbbVie (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Pierre Fabre (Inst), GlaxoSmithKline (Inst), EirGenix (Inst), Eisai Europe (Inst), Relay Therapeutics (Inst), Sanofi (Inst), Olema Pharmaceuticals (Inst), Menarini Group (Inst), MSD Oncology (Inst)
Speakers' Bureau: AstraZeneca (Inst), Daiichi Sankyo Europe GmbH (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Gilead Sciences (Inst), Seagen (Inst)
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Celgene (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Daiichi Sankyo (Inst), Gilead Sciences (Inst), MolecularHealth (Inst), Menarini Group (Inst)
Patents, Royalties, Other Intellectual Property: Patent Pending EP14153692.0 (Inst), Patent Pending EP21152186.9 (Inst), Patent Issued EP15702464.7 (Inst), Patent Pending EP19808852.8 (Inst), Digital Ki67 Evaluator, VM Scope GmbH (Inst)
Oh Kyu Yoon
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Yang Pan
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Scott Hofsess
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences
See-Chun Phan
Employment: Gilead Sciences, Flatiron Health
Stock and Other Ownership Interests: Roche, Roche, Gilead Sciences
Research Funding: Gilead Sciences, Flatiron Health
Patents, Royalties, Other Intellectual Property: Use of Avastin for the treatment of metastatic breast cancer (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Flatiron Health
Sara A. Hurvitz
Stock and Other Ownership Interests: ROM Tech
Research Funding: Genentech/Roche (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Pfizer (Inst), Amgen (Inst), OBI Pharma (Inst), Puma Biotechnology (Inst), Dignitana (Inst), Bayer (Inst), Biomarin (Inst), Lilly (Inst), Merrimack (Inst), Cascadian Therapeutics (Inst), Seagen (Inst), Daiichi Sankyo (Inst), MacroGenics (Inst), Ambryx (Inst), Immunomedics (Inst), Pieris Pharmaceuticals (Inst), Radius Health (Inst), Arvinas (Inst), Zymeworks (Inst), Gilead Sciences (Inst), Phoenix Molecular Designs (Inst), CytomX Therapeutics (Inst), Samumed (Inst), Dantari (Inst), Orinove (Inst), Greenwich LifeSciences (Inst), AstraZeneca/Daiichi Sankyo (Inst), G1 Therapeutics (Inst), Orum (Inst)
Travel, Accommodations, Expenses: Lilly
Other Relationship: Roche, Pfizer
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ASCO Annual Meeting, Chicago, IL, and online, June 3-7, 2022.
SUPPORT
Supported by Gilead Sciences, Inc.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Gilead policy on data sharing may be found at https://www.gileadclinicaltrials.com/transparency-policy/.
AUTHOR CONTRIBUTIONS
Conception and design: Aditya Bardia, Hope S. Rugo, Adam Brufsky, Javier Cortés, Véronique Diéras, Luca Gianni, Oh Kyu Yoon, See-Chun Phan, Sara A. Hurvitz
Provision of study materials or patients: Aditya Bardia, Hope S. Rugo, Sara M. Tolaney, Adam Brufsky, Javier Cortés, Joyce O' Shaughnessy, Véronique Diéras, Sibylle Loibl, Sara A. Hurvitz
Collection and assembly of data: Aditya Bardia, Sara M. Tolaney, Delphine Loirat, Kevin Punie, Mafalda Oliveira, Adam Brufsky, Kevin Kalinsky, Véronique Diéras, Luca Gianni, Scott Hofsess, See-Chun Phan, Sara A. Hurvitz
Data analysis and interpretation: Aditya Bardia, Hope S. Rugo, Sara M. Tolaney, Delphine Loirat, Mafalda Oliveira, Adam Brufsky, Kevin Kalinsky, Javier Cortés, Joyce O' Shaughnessy, Véronique Diéras, Lisa A. Carey, Luca Gianni, Martine Piccart-Gebhart, Sibylle Loibl, Oh Kyu Yoon, Yang Pan, Scott Hofsess, See-Chun Phan, Sara A. Hurvitz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Sanofi, Daiichi Sankyo/Astra Zeneca, Lilly, Lilly (Inst), Gilead Sciences, Gilead Sciences (Inst), Menarini, Menarini (Inst), Mersana
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Hope S. Rugo
Consulting or Advisory Role: Napo Pharmaceuticals, Puma Biotechnology, Mylan, Eisai, Daiichi Sankyo
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Merck (Inst), Daiichi Sankyo (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Pionyr (Inst), Taiho Oncology (Inst), Veru (Inst), GlaxoSmithKline (Inst), Hoffmann-La Roche AG/Genentech, Inc (Inst), Stemline Therapeutics (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca, Gilead Sciences, Pfizer
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183398
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Myovant Sciences, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Ellipses Pharma, OncoSec, Infinity Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi
Delphine Loirat
Honoraria: MSD, Gilead Sciences, Gilead Sciences, AstraZeneca, Lilly
Consulting or Advisory Role: Roche, MSD Oncology, AstraZeneca, Novartis, Pfizer, Immunomedics, Gilead Sciences, 4D Pharma, Lilly
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD, Pfizer, Gilead Sciences
Kevin Punie
Honoraria: Pfizer, Pfizer (Inst), Lilly (Inst), Roche (Inst), Novartis (Inst), Mundipharma (Inst), Gilead Sciences (Inst), Seagen (Inst), AstraZeneca, Lilly, Medscape/Exact Sciences, Focus Patient, Gilead Sciences, MSD, Novartis, Roche, Seagen, MSD (Inst)
Consulting or Advisory Role: AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Novartis, Pierre Fabre (Inst), Roche (Inst), Vifor Pharma (Inst), Teva (Inst), MSD (Inst), AstraZeneca, Lilly, Medscape/Exact Sciences, Gilead Sciences, Pfizer, Roche, Seagen, Sanofi, AstraZeneca
Research Funding: Sanofi (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Novartis, AstraZeneca, PharmaMar, Pfizer, Roche
Mafalda Oliveira
Honoraria: Roche, Pfizer, Eisai Europe, Seagen, Gilead Sciences, AstraZeneca, Lilly, Medscape
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Seagen, ITeos Therapeutics, Daiichi Sankyo/AstraZeneca, Gilead Sciences, Relay Therapeutics, Cureo Science, iOne, Lilly, MSD, Pfizer
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Zenith Epigenetics (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Eisai, Pierre Fabre, Gilead Sciences, AstraZeneca Spain
Uncompensated Relationships: Head of the SOLTI Breast Cancer Research Group
Adam Brufsky
Consulting or Advisory Role: Pfizer, Genentech/Roche, Agendia, Novartis, Lilly, Puma Biotechnology, Merck, Myriad Pharmaceuticals, Eisai, Seagen, Daiichi Sankyo/Lilly, Onc Live, Michael J. Hennessy Associates, Gilead Sciences, General Electric
Research Funding: Roche/Genentech (Inst), AstraZeneca/Daiichi Sankyo (Inst), Merck (Inst), Novartis (Inst), Gilead Sciences (Inst), Lilly (Inst), Puma Biotechnology (Inst)
Expert Testimony: Pfizer
Kevin Kalinsky
Employment: GRAIL, EQRx
Stock and Other Ownership Interests: GRAIL, EQRx
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Genentech/Roche, Immunomedics, Merck, Seagen, Oncosec, 4D Pharma, Daiichi Sankyo/AstraZeneca, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, Prelude Therapeutics, RayzeBio
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Lilly (Inst), Seagen (Inst), AstraZeneca (Inst), Daichi Sankyo (Inst), Ascentage Pharma (Inst)
Other Relationship: Immunomedics, Genentech
Javier Cortés
Stock and Other Ownership Interests: Leuko, MAJ3 Capital
Honoraria: Novartis, Eisai, Celgene, Pfizer, Roche, Samsung, Lilly, Merck Sharp & Dohme, Daiichi Sankyo, AstraZeneca, Gilead Sciences, Steamline Therapeutics
Consulting or Advisory Role: Celgene, Cellestia Biotech, AstraZeneca, Roche, Seagen, Daiichi Sankyo, ERYTECH Pharma, Polyphor, Athenex, Lilly, SERVIER, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim, Ellipses Pharma, HiberCell, Bioinvent, GEMoaB, Gilead Sciences, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Bridgebio
Research Funding: ARIAD (Inst), AstraZeneca (Inst), Baxalta (Inst), Bayer (Inst), Eisai (Inst), Guardant Health (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Puma Biotechnology (Inst), Queen Mary University of London (Inst), Roche (Inst), Piqur (Inst)
Patents, Royalties, Other Intellectual Property: Pharmaceutical combinations of a Pi3k inhibitor and a microtubule destabilizing agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A, HER2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/0338368 A1
Travel, Accommodations, Expenses: Roche, Pfizer, Eisai, Novartis, Daiichi Sankyo, Gilead Sciences, AstraZeneca, Merck Sharp & Dohme Steamline Therapeutics
Joyce O' Shaughnessy
Honoraria: AstraZeneca, Lilly, AbbVie, Celgene, Eisai, Novartis, Pfizer, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Consulting or Advisory Role: Novartis, Pfizer, Lilly, AbbVie, AstraZeneca, Celgene, Eisai, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Speakers' Bureau: AstraZeneca, Novartis, Lilly, Pfizer, Seagen
Research Funding: Seagen (Inst)
Travel, Accommodations, Expenses: Celgene, Lilly, Novartis, Pfizer, AbbVie, Agendia, Amgen, Eisai, GRAIL, Ipsen, Myriad Pharmaceuticals, Puma Biotechnology, Seagen, AstraZeneca, Sanofi, Roche
Véronique Diéras
Honoraria: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, AbbVie/Abbott, MSD Oncology, Daiichi Sankyo, Seagen, Gilead Sciences, Eisai Europe, Pierre Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Pfizer, AstraZeneca, AbbVie/Abbott, MSD Oncology, Daiichi Sankyo Europe GmbH, Seagen, Gilead Sciences, Eisai Europe, Pierre Fabre, Medac, Menarini Group
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer, Lilly, AstraZeneca, Daiichi Sankyo Europe GmbH, Seagen, Gilead Sciences
Lisa A. Carey
Research Funding: NanoString Technologies (Inst), Seagen (Inst), Veracyte (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Novartis (Inst), Lilly (Inst), Genentech/Roche (Inst)
Uncompensated Relationships: Novartis (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Lilly, Seagen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Luca Gianni
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Synaffix, Revolution Medicines, Zymeworks, Menarini, Amgen, BioMedical Insights, Artemida Pharma, Denali Therapeutics, Daiichi Sankyo/Astra Zeneca
Patents, Royalties, Other Intellectual Property: Roche
Martine Piccart-Gebhart
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Menarini, Seagen, Camel-IDS, Immunomedics, Roche/Genentech, Immutep, NBE Therapeutics, Frame Therapeutics, Gilead Sciences
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Roche/Genentech (Inst), Radius Health (Inst), Synthon (Inst), Servier (Inst), Immunomedics/Gilead (Inst), Menarini (Inst)
Other Relationship: Oncolytics
Sibylle Loibl
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), Novartis (Inst), Seagen (Inst), Celgene (Inst), Lilly (Inst), AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Merck KGaA (Inst), AbbVie (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Pierre Fabre (Inst), GlaxoSmithKline (Inst), EirGenix (Inst), Eisai Europe (Inst), Relay Therapeutics (Inst), Sanofi (Inst), Olema Pharmaceuticals (Inst), Menarini Group (Inst), MSD Oncology (Inst)
Speakers' Bureau: AstraZeneca (Inst), Daiichi Sankyo Europe GmbH (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Gilead Sciences (Inst), Seagen (Inst)
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Celgene (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Daiichi Sankyo (Inst), Gilead Sciences (Inst), MolecularHealth (Inst), Menarini Group (Inst)
Patents, Royalties, Other Intellectual Property: Patent Pending EP14153692.0 (Inst), Patent Pending EP21152186.9 (Inst), Patent Issued EP15702464.7 (Inst), Patent Pending EP19808852.8 (Inst), Digital Ki67 Evaluator, VM Scope GmbH (Inst)
Oh Kyu Yoon
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Yang Pan
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Scott Hofsess
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences
See-Chun Phan
Employment: Gilead Sciences, Flatiron Health
Stock and Other Ownership Interests: Roche, Roche, Gilead Sciences
Research Funding: Gilead Sciences, Flatiron Health
Patents, Royalties, Other Intellectual Property: Use of Avastin for the treatment of metastatic breast cancer (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Flatiron Health
Sara A. Hurvitz
Stock and Other Ownership Interests: ROM Tech
Research Funding: Genentech/Roche (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Pfizer (Inst), Amgen (Inst), OBI Pharma (Inst), Puma Biotechnology (Inst), Dignitana (Inst), Bayer (Inst), Biomarin (Inst), Lilly (Inst), Merrimack (Inst), Cascadian Therapeutics (Inst), Seagen (Inst), Daiichi Sankyo (Inst), MacroGenics (Inst), Ambryx (Inst), Immunomedics (Inst), Pieris Pharmaceuticals (Inst), Radius Health (Inst), Arvinas (Inst), Zymeworks (Inst), Gilead Sciences (Inst), Phoenix Molecular Designs (Inst), CytomX Therapeutics (Inst), Samumed (Inst), Dantari (Inst), Orinove (Inst), Greenwich LifeSciences (Inst), AstraZeneca/Daiichi Sankyo (Inst), G1 Therapeutics (Inst), Orum (Inst)
Travel, Accommodations, Expenses: Lilly
Other Relationship: Roche, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 3. Li CH, Karantza V, Aktan G, et al. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: A systematic literature review. Breast Cancer Res. 2019;21:143. doi: 10.1186/s13058-019-1210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Won KA, Spruck C. Triple negative breast cancer therapy: Current and future perspectives (review) Int J Oncol. 2020;57:1245–1261. doi: 10.3892/ijo.2020.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin L, Duan JJ, Bian XW, et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ambrogi F, Fornili M, Boracchi P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9:e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9:28989–29006. doi: 10.18632/oncotarget.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trerotola M, Cantanelli P, Guerra E, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–233. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 9. Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab govitecan (IMMU-132), an anti-trop-2/SN-38 antibody-drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26:919–931. doi: 10.1021/acs.bioconjchem.5b00223. [DOI] [PubMed] [Google Scholar]
- 10. Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther. 2020;20:871–885. doi: 10.1080/14712598.2020.1757067. [DOI] [PubMed] [Google Scholar]
- 11. Nagayama A, Vidula N, Ellisen L, et al. Novel antibody-drug conjugates for triple negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835920915980. doi: 10.1177/1758835920915980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency . Trodelvy (summary of product characteristics) https://www.ema.europa.eu/en/documents/product-information/trodelvy-epar-product-information_en.pdf [Google Scholar]
- 13. Michaleas S, Moreno Oliver A, Mueller-Berghaus J, et al. The European Medicines Agency review of sacituzumab govitecan for the treatment of triple-negative breast cancer. ESMO Open. 2022;7:100497. doi: 10.1016/j.esmoop.2022.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wahby S, Fashoyin-Aje L, Osgood CL, et al. FDA approval summary: Accelerated approval of sacituzumab govitecan-hziy for third-line treatment of metastatic triple-negative breast cancer. Clin Cancer Res. 2021;27:1850–1854. doi: 10.1158/1078-0432.CCR-20-3119. [DOI] [PubMed] [Google Scholar]
- 15. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 16. Loibl S, Loirat D, Tolaney SM, et al. Health-related quality of life in the phase III ASCENT trial of sacituzumab govitecan versus standard chemotherapy in metastatic triple-negative breast cancer. Eur J Cancer. 2023;178:23–33. doi: 10.1016/j.ejca.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 18. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 19. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 21. Narayan P, Osgood CL, Singh H, et al. FDA approval summary: Fam-trastuzumab deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27:4478–4485. doi: 10.1158/1078-0432.CCR-20-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in oncology: (NCCN Guidelines): Breast cancer v.04.2022. https://NCCN.org 2022.
- 24.European Society for Medical Oncology . ESMO Metastatic Breast Cancer Living Guidelines: Triple-Negative Breast Cancer. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guidelines/triple-negative-breast-cancer [Google Scholar]
- 25. Moy B, Rumble RB, Come SE, et al. Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline update. J Clin Oncol. 2021;39:3938–3958. doi: 10.1200/JCO.21.01374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gilead policy on data sharing may be found at https://www.gileadclinicaltrials.com/transparency-policy/.