Abstract
Background
S100A8 is a melanoma biomarker expressed in the melanoma-associated epidermal keratinocytes, but its diagnostic utility has not been compared with other biomarkers, including PRAME.
Objectives
To compare the utility of S100A8 and PRAME immunohistochemistry (IHC) in the differential diagnosis of melanoma and naevi in a case–control study.
Methods
A previously described cohort of 209 melanomas (case samples) and naevi (control samples) dual-immunostained for S100A8 and PRAME were included. For S100A8, previously reported scores indicating the proportion of tumour-associated epidermis stained (0 = indeterminate; 1 = 0–4%; 2 = 5–25%; 3 = 26–50%; 4 = 51–75%; 5 = > 75%) were utilized. PRAME IHC was reviewed by at least two reviewers and a consensus score assigned, with score indicating the proportion of tumour stained (0 = indeterminate; 1 = 0%; 2 = 1–50%; 3 = > 50%). A positive test was defined as > 50% staining.
Results
The area under the receiver operating characteristic curves for S100A8 (0.833) and PRAME (0.874) were not significantly different from each other (P = 0.22). The diagnostic sensitivity and specificity were 42.4% [95% confidence interval (CI) 32.6–52.8%] and 98.2% (95% CI 93.6–99.8%) for S100A8, and 79.8% (95% CI 70.5–87.2%) and 87.3% (95% CI 79.6–92.9%) for PRAME, respectively. A combined test requiring both S100A8 and PRAME IHC positivity had a sensitivity of 39.4% (95% CI 29.7–49.7%) and specificity of 99.1% (95% CI 95.0–100.0%).
Conclusions
S100A8 and PRAME have utility in the diagnostic workup of melanoma, with S100A8 being more specific and PRAME being more sensitive when using this threshold. Our findings suggest that these two immunohistochemical markers may favourably complement one another to improve the detection of melanoma.
Early diagnosis of melanoma is critical for improved survival, with a 99% 5-year relative survival rate for thin melanomas vs. a 25% 5-year relative survival rate for metastatic tumours.1 Histological examination is the gold standard for diagnosis of melanoma, but a subset of melanocytic tumours can be difficult to diagnose, lacking interobserver agreement in up to 15–35%.1 Subjectivity in the interpretation and reporting of these lesions can lead to overtreatment or undertreatment.2
PRAME (PRAME nuclear receptor transcriptional regulator or preferentially expressed antigen in melanoma) is a tumour-associated antigen overexpressed in melanomas, making it a helpful tool in differentiating between benign vs. malignant melanocytic neoplasms. In a study by Lezcano et al., 87% of metastatic melanomas and 90% of primary cutaneous melanomas expressed PRAME, whereas most melanocytic naevi were negative for PRAME.3 Additionally, when the threshold for staining is set to > 76%, PRAME immunohistochemistry (IHC) has a 100% specificity and a 67% sensitivity for distinguishing malignant from benign melanocytic neoplasms.4 Therefore, PRAME IHC is commonly used in routine dermatopathology practice in the diagnostic workup of melanoma. However, ancillary tests such as PRAME IHC should be interpreted alongside other histopathological features and, in some cases, other supplementary IHC stains.
S100A8 is a calcium-binding protein highly expressed in certain inflammatory conditions, including psoriasis and various human cancers such as breast, lung, gastric, colorectal, pancreatic and prostate cancer, as well as in melanoma.5,6 S100A8, complexed with S100A9, forms calprotectin, which is released during tissue damage. S100A8 is expressed by immune cells in metastatic melanomas and serum levels of S100A8 inversely correlate with survival after immunotherapy.7 Additionally, S100A8 is 1 of the 23 genes included in a gene expression-based diagnostic test for melanoma.8
We previously showed that S100A8 is also expressed by the epidermal keratinocyte microenvironment of melanoma, but not in the interfollicular keratinocyte microenvironment of melanocytic naevi.9 Owing to its potential role as a marker of the keratinocyte microenvironment of melanoma, we aimed to compare the utility of S100A8 vs. PRAME IHC for distinguishing naevi from melanoma.
Materials and methods
Sample selection
A cohort of common melanocytic naevi, dysplastic melanocytic naevi, melanoma in situ (MIS) and melanoma were previously identified by Kiuru et al.9 Slides that were previously dual-stained for S100A8 and PRAME were retrieved from the University of California, Davis Dermatopathology archive under IRB-approved protocol. In total, 209 samples were included (case samples: invasive melanoma, n = 41; MIS, n = 58; control samples: dysplastic naevus, n = 54; common naevus, n = 56).
Immunohistochemistry scoring
Previously stained slides were reviewed by two to four reviewers (S.L.W., M.K., J.H. and M.A.F.) and a consensus PRAME IHC staining score was assigned to blinded tumour samples, where score indicated the proportion of tumour stained (0 = indeterminate; 1 = absent or 0%; 2 = present, focal or ≤ 50%; and 3 = present, diffuse or > 50%).
Results of S100A8 IHC staining score were previously reported in Kiuru et al.9 These scores were similarly assigned by consensus, with the score indicating the proportion of tumour-associated epidermis stained (0 = indeterminate; 1 = 0–4%; 2 = 5–25%; 3 = 26–50%; 4 = 51–75%; 5 = > 75%). Staining patterns of S100A8 (nuclear vs. cytoplasmic) were documented for samples with a staining score of ≥ 2.
Statistical analysis
We used receiver operating characteristic (ROC) curve analysis to evaluate the diagnostic accuracy of S100A8 and PRAME IHC staining for identifying malignant from benign samples, and to assess their accuracy in distinguishing early melanomas, specifically MIS from dysplastic naevi. Using frequency tables, we summarized the variables used for analysis: sample type, S100A8 staining score and PRAME staining score. For binary classification of the sample type, we defined common naevus and dysplastic naevus control samples as not having disease, and MIS and invasive melanoma case samples as having disease. The area under the ROC curve (AUC) for S100A8 staining score and PRAME staining score were compared separately to chance, which has an AUC of 0.5, and to each other.
To evaluate the impact of age, sex and anatomic location on the scores among samples that were malignant (invasive melanoma and MIS), adjacent-category logit models were fitted on S100A8 and PRAME ordinal staining scores, separately for each score and covariate. We used univariable models to fit the data. All the analyses were performed using SAS statistical software 9.4. (SAS Institute, Cary, NC, USA)
Results
In total, 209 melanocytic lesions were analysed, including 56 common naevi, 54 dysplastic naevi, 58 MIS and 41 invasive melanomas. For binary classification of the sample type, 47.4% (99/209) were classified as having disease (melanoma or MIS, the case group) whereas 52.6% (110/209) were classified as not having disease (common naevus or dysplastic naevus, the control group). Demographics and characteristics of the patients can be found in Table 1. This cohort was previously described by Kiuru et al.9
Table 1.
Patient demographics and melanocytic lesion characteristics.1
| Characteristics, n (%) | Common nevus (n=56) | Dysplastic nevus (n=54) | Melanoma in situ (n=58) | Melanoma (n=41) |
|---|---|---|---|---|
| Sex of patient | ||||
| Male | 23 (41.1) | 28 (51.9) | 33 (56.9) | 27 (65.9) |
| Female | 33 (58.9) | 26 (48.1) | 25(43.1) | 14 (34.1) |
| Age of patient | ||||
| < 35 yrs old | 19 (33.9) | 7 (13.0) | 1 (1.7) | 4 (9.8) |
| 35–70 yrs old | 35 (62.5) | 40 (74.1) | 40 (69.0) | 20 (48.8) |
| >70 yrs old | 2 (3.6) | 7 (13.0) | 17 (29.3) | 17 (35.4) |
| Location of tumor | ||||
| Face | 5 (8.9) | 1 (1.9) | 10 (17.2) | 7 (17.1) |
| Scalp, Neck | 9 (16.1) | 0 (0.0) | 5 (8.6) | 5 (9.8) |
| Trunk | 29 (51.8) | 41 (75.9) | 19 (32.8) | 10 (24.4) |
| Upper extremities | 5 (8.9) | 7 (13.0) | 20 (34.5) | 10 (24.4) |
| Lower extremities | 8 (14.3) | 5 (9.3) | 4 (6.9) | 7 (17.1) |
| Histologic growth pattern | ||||
| Junctional | 7 (12.5) | 26 (48.1) | N/A | N/A |
| Compound | 24 (42.9) | 28 (51.9) | N/A | N/A |
| Intradermal | 25 (44.6) | 0 (0.0) | N/A | N/A |
| Tumor thickness, avg. (range) | N/A | N/A | N/A | 2.31 (0.2–50) |
| Ulcerations | ||||
| Present | N/A | N/A | N/A | 4 (9.8) |
| Absent | N/A | N/A | N/A | 29 (70.7) |
| Indeterminate | N/A | N/A | N/A | 8 (19.5) |
| Primary tumor stage | ||||
| T1a | N/A | N/A | N/A | 23 (56.1) |
| T1b | N/A | N/A | N/A | 1 (2.4) |
| T1 indeterminate2 | N/A | N/A | N/A | 5 (12.2) |
| T2a | N/A | N/A | N/A | 1 (2.4) |
| T2b | N/A | N/A | N/A | 1 (2.4) |
| T2 indeterminate | N/A | N/A | N/A | 0 (0.0) |
| T3a | N/A | N/A | N/A | 4 (9.8) |
| T3b | N/A | N/A | N/A | 1 (2.4) |
| T3 indeterminate | N/A | N/A | N/A | 2 (4.9) |
| T4a | N/A | N/A | N/A | 1 (2.4) |
| T4b | N/A | N/A | N/A | 1 (2.4) |
| T4 indeterminate | N/A | N/A | N/A | 1 (2.4) |
| Melanoma subtype | ||||
| Superficial spreading | N/A | N/A | N/A | 1 (2.4) |
| Nodular | N/A | N/A | N/A | 1 (2.4) |
| Lentigo maligna3 | N/A | N/A | N/A | 1 (2.4) |
| Acral lentiginous | N/A | N/A | N/A | 0 (0.0) |
| Indeterminate | N/A | N/A | N/A | 36 (87.8) |
| Other | N/A | N/A | N/A | 3 (7.3) |
S100A8 staining reported in: Kiuru M, Kriner MA, Wong S, et al. High-Plex Spatial RNA Profiling Reveals Cell Type–Specific Biomarker Expression during Melanoma Development. J Invest Dermatol. May 2022;142(5):1401–1412.e20. doi:10.1016/j.jid.2021.06.041
Cases were indeterminate if the presence of ulceration was unknown.
There was one melanoma classified as being both superficial spreading type and lentigo maligna.
For all samples (n = 209), patient ages ranged from to 13 to 91 years [median 57 years, mean 54.5 years (SD = 17.2)]. In total, 111 (53.1%) patients were male and 98 (46.9%) were female.
Most of the common and dysplastic naevi were located on the trunk (63.6%, 70/110), followed by the lower (11.8%, 13/110) and upper extremities (10.9%, 12/110). MIS and invasive melanomas were most commonly located on the upper extremities (30%, 30/99) or trunk (29%, 29/99), followed by the face (17%, 17/99) and lower extremities (11%, 11/99).
The common naevi had either junctional (n = 7), compound (n = 24) or intradermal growth (n = 25); the dysplastic naevi had either junctional (n = 26) or compound (n = 28) growth.
Melanoma tumour thickness ranged from 0.2 mm to 50.0 mm [median thickness 0.5 mm, mean thickness 2.3 mm (SD = 7.7)]. Of the invasive melanoma, 71% (29/41) were T1, 5% (2/41) were T2, 17% (7/41) were T3 and 7% (3/41) were T4.
The mean staining scores for benign vs. malignant samples were 1.25 (SD = 0.63) vs. 2.98 (SD = 1.47) for S100A8 and 1.48 (SD = 0.71) vs. 2.74 (SD = 0.56) for PRAME, respectively. In the ROC analysis for distinguishing malignant from benign samples, the AUC was 0.833 for S100A8 [95% confidence interval (CI) 0.780–0.885] and 0.874 for PRAME (95% CI 0.828–0.920), resulting in a combined AUC of 0.926 (Figure 1). Both S100A8 and PRAME demonstrated AUC values significantly greater than chance alone (P < 0.001 for both) and did not exhibit a significant difference between each other (P = 0.22).
Figure 1.
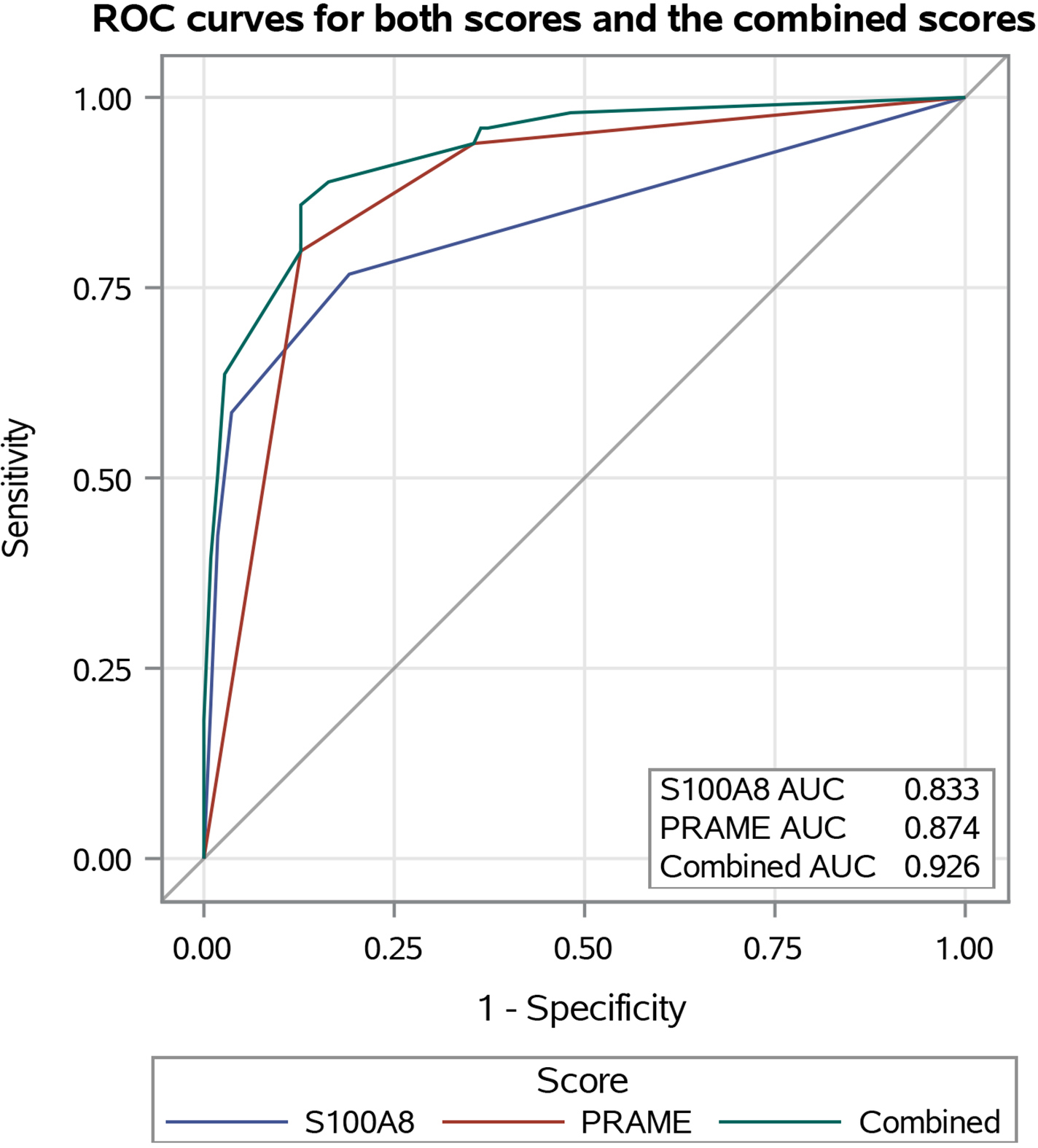
Receiver operating characteristic (ROC) curves comparing the diagnostic accuracy of S100A8 immunohistochemistry (IHC) (blue) to PRAME IHC (red) in distinguishing melanoma and melanoma in situ samples from the case group from the benign common and dysplastic naevi samples from the control group. The combined ROC curve for S100A8 and PRAME IHC is in green. Both curves are compared with the grey curve for chance (under the ROC curve = 0.5). AUC, area under the ROC curve.
In the ROC analysis for distinguishing MIS samples from dysplastic naevi, the AUC for S100A8 and PRAME was 0.757 (95% CI 0.677–0.837) and 0.781 (95% CI 0.700–0.861), respectively, and there was no statistically significant difference between them (P = 0.67) (Figure 2).
Figure 2.
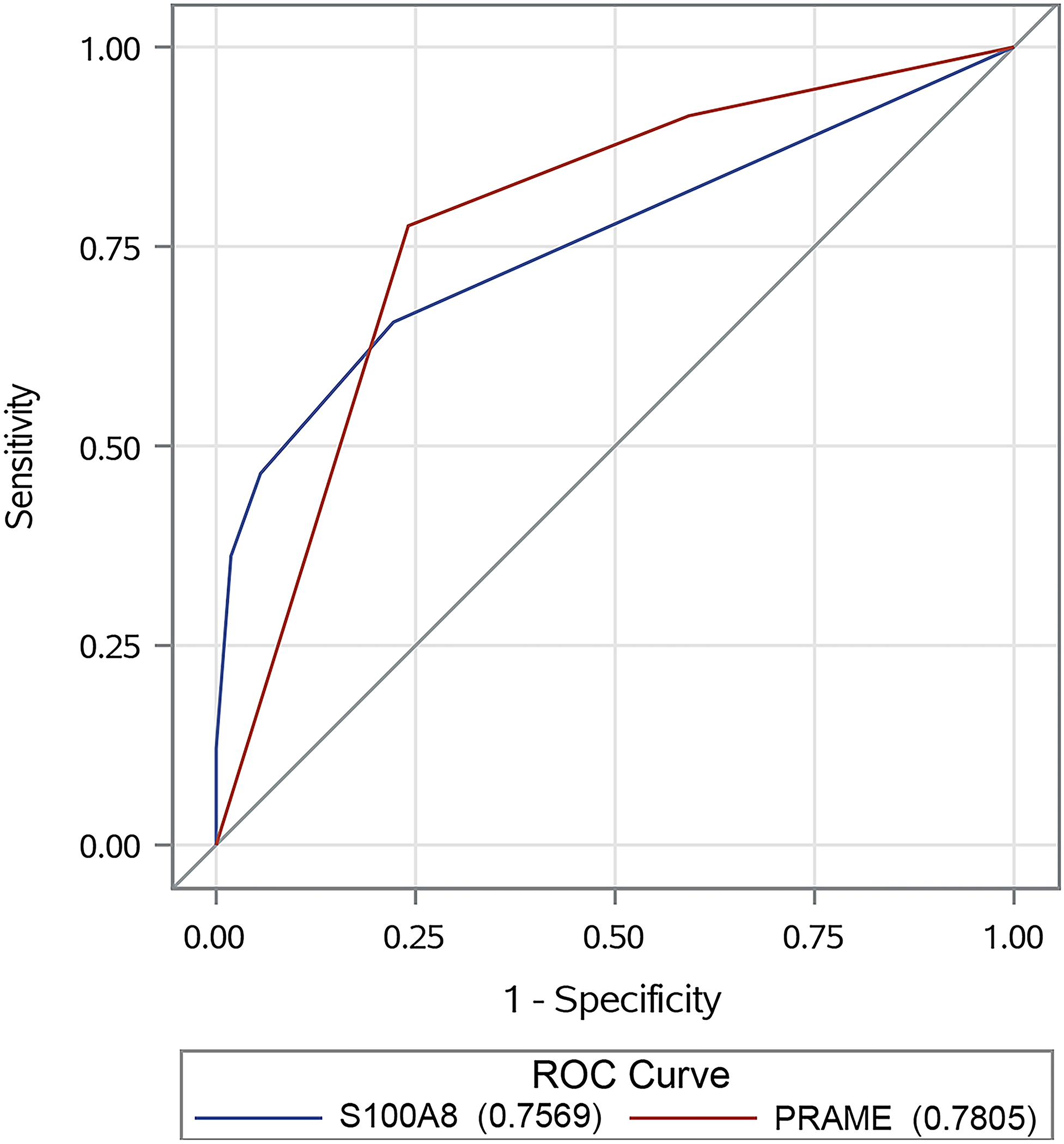
Receiver operating characteristic (ROC) curves comparing the diagnostic accuracy of S100A8 immunohistochemistry (IHC) (blue) to PRAME IHC (red) in distinguishing melanoma in situ samples from the case group from dysplastic naevi samples from the control group. Both curves are compared with the grey curve for chance (under the ROC curve = 0.5).
For S100A8, a positive test (> 50% tumour-associated epidermis stained, score ≥ 4) demonstrated 42.4% sensitivity (95% CI 32.6–52.8%) and 98.2% specificity (95% CI, 93.6–99.8%), resulting in a positive predictive value (PPV) of 95.5% (95% CI 83.9–98.8%) and a negative predictive value (NPV) of 65.5% (95% CI 61.5–69.2%). Specifically, 36.2% (21/58) of MIS tumours and 51.2% (21/41) of invasive melanomas stained positively for S100A8, compared with 1.8% (2/110) of naevi (common and dysplastic).9
For PRAME, a positive test (> 50% tumour staining, score ≥ 3) demonstrated 79.8% sensitivity (95% CI 70.5–87.2%) and 87.3% specificity (95% CI 79.6–92.9%), with a PPV of 84.8% (95% CI 77.4–90.3%) and a NPV of 82.1% (95% CI 76.3–87.7%). Positive PRAME staining was observed in 78% (45/58) of MIS tumours and 83% (34/41) of melanomas compared with 2% (1/56) and 24% (13/54) in common naevi and dysplastic naevi, respectively. S100A8 and PRAME staining score frequencies are summarized in Table 2.
Table 2.
S100A81 and PRAME immunohistochemistry staining results in nevi and melanoma cases.
| Tumor type, n (%) | Common nevi (n=56) | Dysplastic nevi (n=54) | Melanoma in situ (n=58) | Melanoma (n=41) |
|---|---|---|---|---|
| S100A8 staining score: percentage of tumor-associated epidermis stained2 | ||||
| 1: 0–4% | 47 (83.9) | 42 (77.8) | 20 (34.5) | 3 (7.3) |
| 2: 5–25% | 8 (14.3) | 9 (16.7) | 11 (19.0) | 7 (17.1) |
| 3: 26–50% | 0 (0.0) | 2 (3.7) | 6 (10.3) | 10 (24.4) |
| 4: 51%–75% | 0 (0.0) | 1 (19) | 14 (24.1) | 8 (19.5) |
| 5: >75% | 1 (1.8) | 0 (0.0) | 7 (12.1) | 13 (31.7) |
| PRAME staining score: percentage of tumor stained2 | ||||
| Absent; 0% | 49 (87.5) | 22 (40.7) | 5 (8.6) | 1 (2.4) |
| Present, focal; <50% | 6 (10.7) | 19 (35.2) | 8 (13.8) | 6 (14.6) |
| Present, diffuse; >50% | 1 (1.8) | 13 (24.1) | 45 (77.6) | 34 (82.9) |
| S100A8 staining pattern for cases with staining scores ≥2 | ||||
| Predominately nuclear | 9 (16.1) | 11 (20.4) | 23 (39.7) | 20 (48.8) |
| Nuclear + cytoplasmic | 0 (0.0) | 1 (1.9) | 15 (25.9) | 18 (43.9) |
| Not applicable | 47 (83.9) | 42 (77.8) | 20 (34.5) | 3 (7.3) |
S100A8 IHC staining reported in: Kiuru M, Kriner MA, Wong S, et al. High-Plex Spatial RNA Profiling Reveals Cell Type–Specific Biomarker Expression during Melanoma Development. J Invest Dermatol. May 2022;142(5):1401–1412.e20. doi:10.1016/j.jid.2021.06.041
A positive staining score was defined as greater than 50% staining.
To assess the combined diagnostic accuracy of S100A8 and PRAME, a positive test was defined as > 50% of the tumour staining positive for both, resulting in 39.4% sensitivity (95% CI, 29.7–49.7%) and 99.1% specificity (95% CI 95.0–100.0%). This produced a PPV of 97.5% (95% CI 84.5–99.6%) and an NPV of 64.5% (95% CI 60.8–68.1%). Alternatively, a positive test requiring > 50% of the tumour staining positive for either S100A8 or PRAME yielded 82.8% sensitivity (95% CI 73.9–89.7%) and 86.4% specificity (95% CI 78.5–92.2%), resulting in a PPV of 84.5% (95% CI 77.2–89.8%) and an NPV of 84.8% (95% CI 78.3–89.7%).
Of the 13/58 (22%) MIS and 7/41 (17%) invasive melanomas that stained negatively for PRAME (score of ≤ 2 or ≤ 50% stained), 2 (15%) and 1 (14%) of these, respectively, stained positively for S100A8. Conversely, of the 37/58 (64%) MIS and 20/41 (49%) invasive melanomas that stained negatively for S100A8 (score of ≤ 3 or ≤ 50% stained), 27 (73%) and 14 (70%) of these, respectively, stained positively for PRAME. Additionally, of the 110 common and dysplastic naevi, 108 (98.2%) stained negatively for S100A8 and 96 (87.3%) stained negatively for PRAME. Examples of these and their corresponding haematoxylin and eosin stains are presented in Figures 3 and 4.
Figure 3.
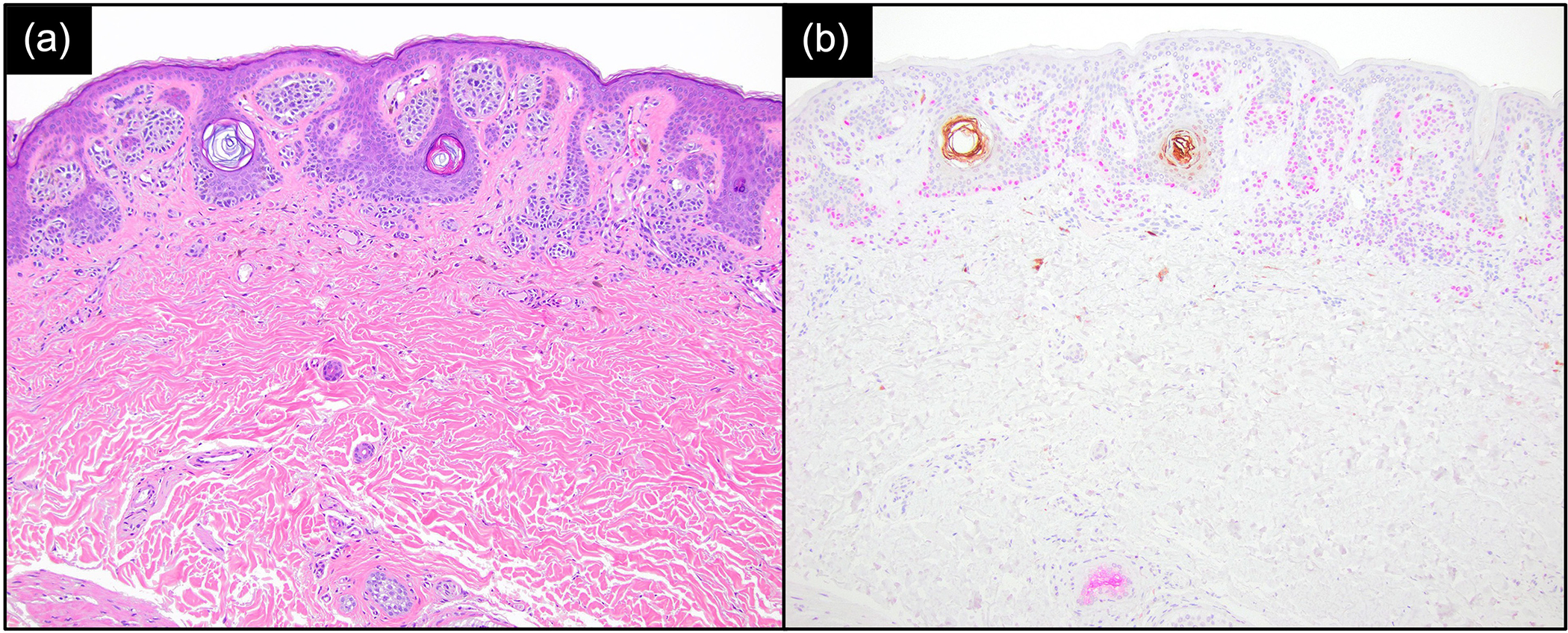
Common naevus showing positive PRAME expression (nuclear staining, magenta) and negative S100A8 expression (brown), with S100A8 staining the epidermal follicular structures. (a) Haematoxylin and eosin × 100; (b) S100A8/PRAME dual stain × 100.
Figure 4.
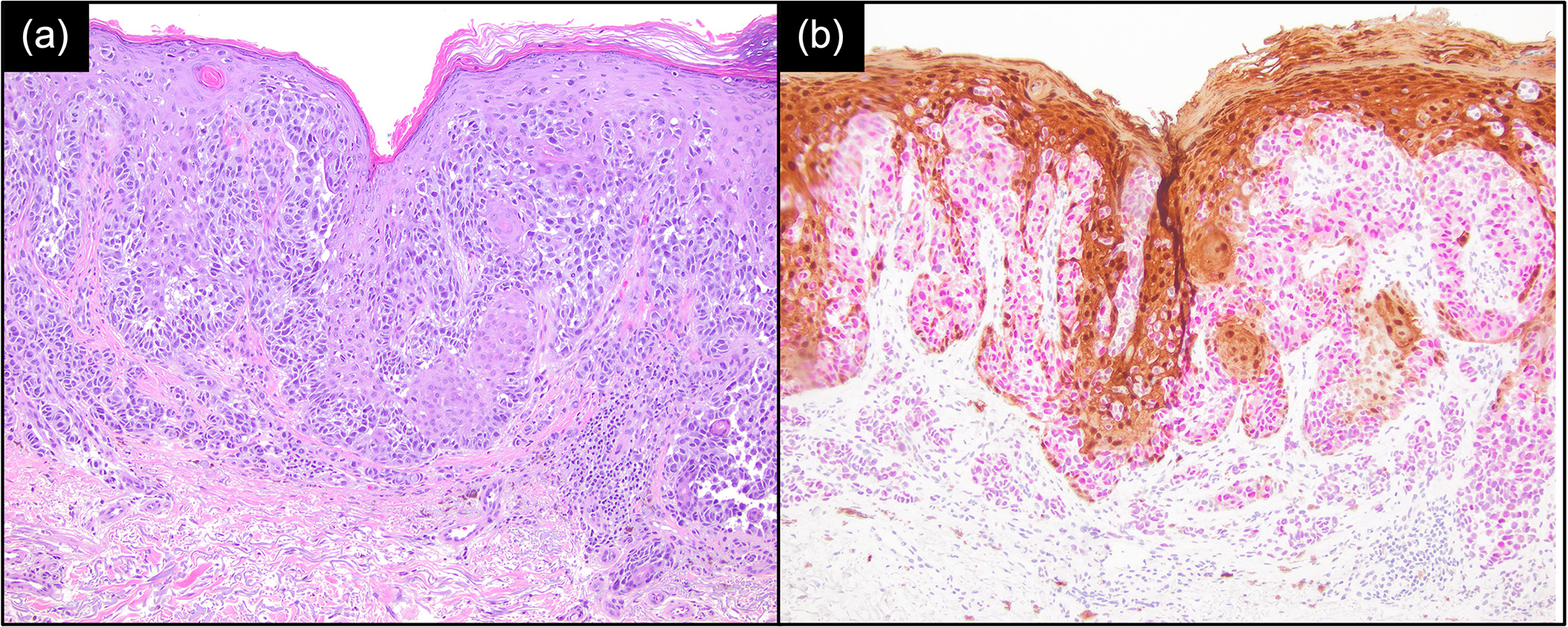
Melanoma showing positive PRAME expression (nuclear staining, magenta) and positive S100A8 expression (brown). (a) Haematoxylin and eosin × 100; (b) S100A8/PRAME dual stain × 100.
The S100A8 staining patterns in malignant samples (n = 99) demonstrated nuclear staining in 43% (43/99), a combined nuclear and cytoplasmic pattern in 33% (33/99) and 23% (23/99) were indeterminate with staining scores < 2 (Table 2). Among benign samples (n = 110), S100A8 displayed a predominantly nuclear staining pattern in 18.2% (20/110) of naevi, a combined nuclear and cytoplasmic pattern in 0.1% (1/110) and were indeterminate in 80.9% (89/110).
In evaluating age, sex and anatomic location of tumour as predictors of the ordinal staining scores among the patients’ samples using adjacent-category logit models, we observed that only sex (P = 0.002) and location (P = 0.04) had significant effects on S100A8 staining, whereas age did not (P = 0.79). Female patients had 1.63fold higher odds (95% CI 1.20–2.21) of having the next lowest staining score when compared with males; that is, females were more likely to have scores such as 1 vs. 2, 2 vs. 3, 3 vs. 4, and 4 vs. 5.
For location, tumours on the upper extremities and lower extremities were more likely to have lower S100A8 staining scores than tumours on the face. Specifically, tumours in these locations, respectively, had a 1.89 (95% CI 1.18–3.00) and 1.96 (95% CI 1.12–3.41) times higher odds for having the next lowest staining score when compared with tumours on the face. No significant differences were observed in S100A8 staining scores between tumours on the scalp/neck [odds ratio (OR) = 1.10, 95% CI 0.61–2.00] and those located on the trunk (OR = 1.50, 95% CI 0.95–2.37) when compared with tumours on the face. PRAME staining was not significantly affected by age (P = 0.90), sex (P = 0.42) or tumour location (P = 0.10).
Discussion
This study evaluated and compared the efficacy of S100A8 IHC in diagnosing melanomas to that of PRAME, a commonly used IHC stain for melanoma diagnosis. Although both S100A8 and PRAME demonstrated comparable high accuracy in distinguishing melanomas (case samples) from naevi (control samples), as evidenced by their AUC scores of 0.833 for S100A8 and 0.874 for PRAME, we found that S100A8 was more specific (98.2% vs. 87.3%) yet less sensitive (42.4% vs. 79.8%) than PRAME using a > 50% threshold for positivity. However, both markers were less accurate in differentiating MIS from dysplastic naevi, with AUC scores of 0.757 and 0.781 for S100A8 and PRAME, respectively. We also explored the combined use of these tests, necessitating positive staining for both IHC markers or positive staining for either marker. The former yielded a highly specific test (99.1%), although sensitivity was lower (39.4%), producing a PPV and NPV of 97.5% and 64.5%, respectively. Conversely, the latter demonstrated high sensitivity (82.8%) and specificity (86.4%), with a PPV of 84.5% and NPV of 84.8%, highlighting the collective value of S100A8 and PRAME in diagnosing and ruling out melanoma in clinical practice.
Additionally, we found that these markers can help detect samples that were malignant that the other has missed, especially because not every melanoma will stain positive for PRAME and vice versa. This is demonstrated in the 3/20 (15%) melanoma and MIS tumours that were missed by PRAME but detected by S100A8, and similarly, 40/57 (70%) melanoma and MIS tumours that were missed by S100A8 but were detected by PRAME. Together, our results validate how S100A8 can be a useful ancillary study in detecting melanomas, especially when it is interpreted alongside other histopathological features and possibly other ancillary tests such as PRAME.
Our findings of PRAME IHC are also consistent with prior reports. Rawson et al. found that 64% of all melanomas, and 70% of nondesmoplastic melanomas showed > 50% PRAME staining with an 84% sensitivity and 85% specificity.10 This is comparable with the sensitivity and specificity we found for PRAME using the same threshold for positivity. Conversely, Lezcano et al., who first described the use of PRAME in detecting melanomas, reported similar findings only when using a > 75% threshold for positivity, which may be explained by the interobserver subjectivity in judging staining patterns.3 They found that PRAME had an 83.2% sensitivity and 99.3% specificity for detecting primary melanomas (n = 155) vs. melanocytic naevi (n = 145).3 Moreover, our findings of PRAME positivity in 12.7% (14/110) of the benign melanocytic naevi corroborate the findings of Rawson et al. and Lezcano et al. who reported PRAME immunoreactivity in 14.6% and 13.1% of naevi, respectively.3,10 These findings further support PRAME expression as an effective IHC marker for distinguishing melanomas, specifically of nondesmoplastic subtypes.
Adjacent-category logit modelling in our study demonstrated that patient sex and the anatomic location of the tumours significantly affected S100A8 staining scores among those patients with malignant samples, whereas age did not. Melanomas were more likely to have lower S100A8 staining scores if the patient was female rather than male, or if the tumours were located on the upper or lower extremities rather than the face. Interestingly, the prognosis of melanoma is often worse in males than in females, and in tumours located on the head/neck than in other locations,11,12 showing a similar pattern as S100A8 staining scores, although additional studies are needed to investigate these possible associations. On the other hand, neither patient age, sex nor location significantly affected PRAME staining scores among those patients with malignant samples.
Given the difficulty in diagnosing a subset of melanomas based on the histopathological features alone, reliable adjuvant tests are occasionally required. Therefore, the addition of S100A8 as a biomarker may be valuable in the diagnosis of melanoma.
Our study was limited to a single institution, suggesting the potential value of larger studies comparing S100A8 to other established melanoma biomarkers. Nonetheless, our findings support the utility of both S100A8 and PRAME as effective IHC markers in distinguishing melanomas from naevi, especially when evaluated in conjunction with clinical and histopathological features. With S100A8’s higher specificity and PRAME’s greater sensitivity, the combined use of these two IHC markers may favourably improve the detection of melanoma.
What is already known about this topic?
S100A8 is a biomarker expressed in melanoma-associated epidermal keratinocytes, serving as a potentially useful tool for differentiating benign and malignant melanocytic neoplasms.
What does this study add?
This study compares the diagnostic utility of S100A8 and PRAME in distinguishing melanoma from benign naevi.
S100A8 is more specific (98.2% vs. 87.3%) yet less sensitive (42.4% vs. 79.8%) than PRAME in identifying melanoma from naevi when using a > 50% threshold for positivity.
With S100A8’s higher specificity and PRAME’s greater sensitivity, the combined use of these two immunohistochemistry markers may favourably improve the detection of melanoma.
Acknowledgements
We would like to thank Lan Yu, Aubrey Gasper and Daniel Gong for laboratory and administrative assistance with this study.
Funding sources
This article was supported, in part, by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of National Institutes of Health through grant number K23AR074530 (M.K.), and by the Biostatistics Shared Resource, funded by the UC Davis Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (NCI P30CA093373).
Footnotes
Conflicts of interest
M.K. reports a pending patent application. The other authors declare no conflicts of interest.
Ethics statement
Ethical approval: The institutional review board at the University of California Davis approved this study (#756049–24). Informed consent: Informed consent was waived because of the use of de-identified samples.
Data availability
The data that support the findings of this study is not publicly available but can be made available upon reasonable request from the corresponding author.
References
- 1.American Cancer Society. Survival rates for melanoma skin cancer Available at: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html (last accessed 28 January 2024).
- 2.Lodha S, Saggar S, Celebi JT, Silvers DN. Discordance in the histopathologic diagnosis of difficult melanocytic neoplasms in the clinical setting. J Cutan Pathol 2008; 35:349–52. [DOI] [PubMed] [Google Scholar]
- 3.Lezcano C, Jungbluth AA, Nehal KS et al. PRAME Expression in melanocytic tumors. Am J Surg Pathol 2018; 42:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohman ME, Steen AJ, Grekin RC, North JP. The utility of PRAME staining in identifying malignant transformation of melanocytic nevi. J Cutan Pathol 2021; 48:856–62. [DOI] [PubMed] [Google Scholar]
- 5.Ryckman C, Vandal K, Rouleau P et al. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 2003; 170:3233–42. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 2006; 72:1622–31. [DOI] [PubMed] [Google Scholar]
- 7.Wagner NB, Weide B, Gries M et al. Tumor microenvironment-derived S100A8/A9 is a novel prognostic biomarker for advanced melanoma patients and during immunotherapy with anti-PD-1 antibodies. J Immunother Cancer 2019; 7:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke LE, Flake DD 2nd, Busam K et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer 2017; 123:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiuru M, Kriner MA, Wong S et al. High-Plex spatial rna profiling reveals cell type-specific biomarker expression during melanoma development. J Invest Dermatol 2022; 142:1401–12.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawson RV, Shteinman ER, Ansar S et al. Diagnostic utility of PRAME, p53 and 5-hmC immunostaining for distinguishing melanomas from naevi, neurofibromas, scars and other histological mimics. Pathology 2022; 54:863–73. [DOI] [PubMed] [Google Scholar]
- 11.Joosse A, de Vries E, Eckel R et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol 2011; 131:719–26. [DOI] [PubMed] [Google Scholar]
- 12.Callender GG, Egger ME, Burton AL et al. Prognostic implications of anatomic location of primary cutaneous melanoma of 1 mm or thicker. Am J Surg 2011; 202:659–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study is not publicly available but can be made available upon reasonable request from the corresponding author.


