Abstract
Case details A 14-year-old female cat presented with signs of respiratory distress. Pleural fluid was found on radiographic assessment. Cytologic evaluation of the fluid revealed malignant melanocytosis. The cat had a previous history of a recurrent malignant melanoma near the base of the right ear. Due to declining clinical condition, the cat was euthanized.
Clinical significance Cutaneous malignant melanomas (or melanosarcomas) are uncommon neoplasms in cats, and knowledge is limited. As far as the authors are aware, there are no previous reports in the veterinary literature of malignant melanocytes being identified in pleural effusion in cats, as they have in dogs. This report suggests that, despite conflicting information in the literature regarding the clinical behavior of cutaneous melanomas in cats, these tumors are capable of recurrence and metastasis. Aggressive treatment may be necessary even, as in this case, if the tumor is well differentiated on histopathology.
Clinical report
A 4.6 kg, 14-year-old spayed female Maine Coon presented as an emergency with a 1-day history of increased respiratory effort which progressed to dyspnea, along with anorexia of 2 days' duration. The cat was an indoor/outdoor cat of unknown retroviral status.
Physical examination and initial diagnostics
On presentation, the cat was dyspneic with pale to cyanotic mucous membranes and mild serous nasal discharge. Heart rate was 200 beats/min and respiratory rate was 16 breaths/min. A temperature was not obtained. The cat was moderately dehydrated. Thoracic auscultation revealed increased breath sounds on inspiration. An open wound with granulation tissue was noted rostral to the right ear base. The remainder of the physical examination was unremarkable.
Initial diagnostics included a complete blood count, a biochemical profile (Antech Diagnostics, Irvine, CA), in-house venous blood gas analysis and thoracic radiographs. Abnormalities on the blood panel included a leukocytosis of 21,300 cells/μl (reference interval [RI] 3500–16,000), hypercapnea (PCO2 55 mmHg), elevated alanine aminotransferase (370 IU/l; RI 10–100), elevated aspartate aminotransferase (321 IU/l; RI 10–100), elevated creatinine kinase (1070 IU/l; RI 56–529), hypertriglyceridemia (173 mg/dl; RI 25–160), elevated magnesium (3.0 mEq/l; RI 1.5–2.5) and severe hypoglycemia (17 mg/dl; RI 64–170). The last was assumed to be a spurious result, reflecting in vitro consumption, as the sample had been transported and the duration of time to evaluation was prolonged.
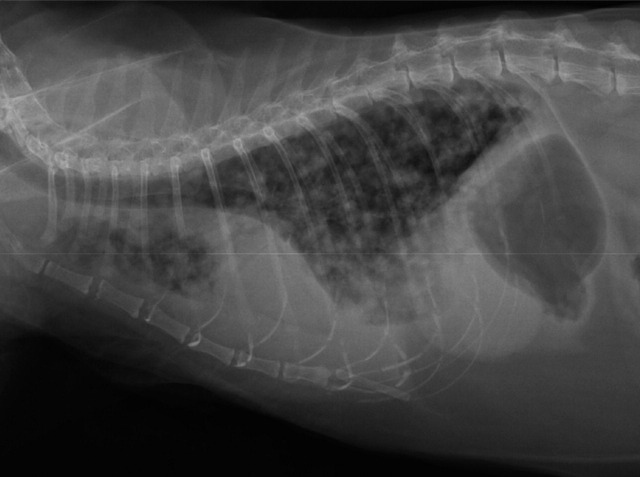
Thoracic radiographs revealed mild pleural effusion and a diffuse miliary interstitial pattern (Fig 1). Ultrasound-guided thoracocentesis yielded 140 ml of serosanguinous fluid.
FIG 1.
Lateral thoracic radiograph demonstrating the presence of pleural effusion and a diffuse milliary interstitial pattern in all lung fields
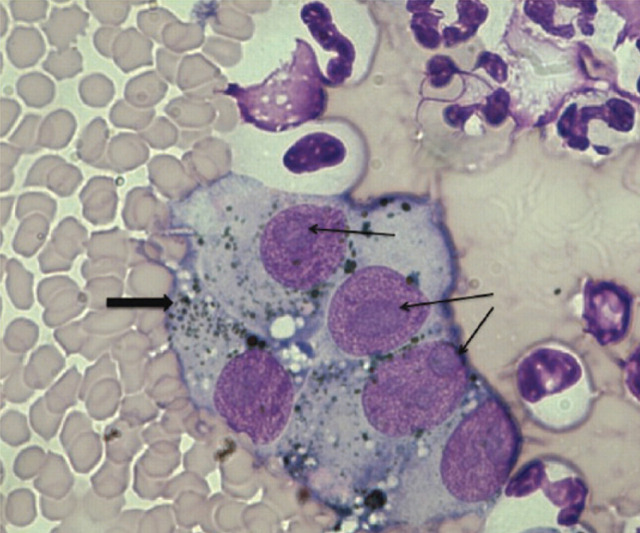
Fluid analysis (Antech Diagnostics, Irvine, CA) demonstrated a specific gravity of 1.021, white blood cell count of 920 cells/μl and a protein level of 3.1 g/dl, consistent with a modified transudate. Cytologic evaluation by a board-certified pathologist revealed the presence of round to spindle-shaped cells with abundant basophilic cytoplasm. Many of these cells contained green intracytoplasmic granules. The cells had round to oval nuclei with a fine chromatin pattern and one to two prominent, large nucleoli. The cells described were consistent with malignant melanocytes, with scattered mitotic figures reported. A few neutrophils and macrophages were also present. Many of the macrophages contained phagocytized melanin granules and were consistent with melanophages. The cytology slides were reviewed by a second board-certified pathologist who also confirmed a diagnosis of metastatic melanoma.
Initial treatment
Initial treatment consisted of furosemide 2.2 mg/kg SC. This was repeated 30 mins later at 1 mg/kg, with no changes in respiratory rate. The cat was started on crystalloid fluids at 15 ml/kg/h, terbutaline 1.25 mg SC, doxycycline 23 mg PO and cyproheptadine 2 mg PO. At this time the owner elected euthanasia.
Review of medical history
At the time of manuscript preparation, the patient's medical records were obtained. The cat's only previous health history was a slow-growing mass that had been removed from the right ear base 7 months prior to presentation at the emergency clinic. Histopathology of the mass was consistent with a well-differentiated melanosarcoma with a mitotic rate of 5 mitoses per 10 high power fields (HPFs) and adequate histologic margins. The mass recurred at a more rostral site 2 months after the first procedure, requiring a second resection. At that time, resection of the parotid salivary gland and regional lymph node was also performed. Histopathology of the mass was again consistent with malignant melanoma; a mitotic rate of 1–5 per 10 HPFs was reported. Tumor cells were seen at the margins of the sections. The lymph node contained neoplastic melanocytes, indicating metastasis to this tissue. No follow-up treatment had been pursued by the owner.
Immunohistochemical staining
At the time of manuscript preparation the cytology slides from the pleural effusion were re-stained with Melan-A (Ventana Medical Systems, Tuscon, AZ). Briefly, previously fixed and stained tissue aspirate slides were obtained. Heat-induced epitope retrieval with citrate buffer, pH 6.0, for 1 min at 125°C was followed by endogenous peroxidase blocking with 3% hydrogen peroxide and incubation with the primary antibody at room temperature for 10 h. The primary antibody used was a predilute monoclonal mouse anti-MART-1/ Melan-A. A prediluted, dual link polymer horseradish peroxidase secondary antibody and 3,3′-diaminobenzidine kit were used to detect the immunoreactive complexes. The slides then were counterstained with Mayer's hematoxylin.
The results of these stains were negative (Fig 2). As cutaneous melanomas are uncommon in cats, cytology from a confirmed melanoma was unavailable for the positive control. The positive control was instead performed on formalin-fixed tissue from a canine melanoma. These slides were de-paraffinized and then stained using the same methodology. For the negative control, the immuno-histochemistry was run with omission of the primary antibody.
FIG 2.
Photomicrograph of pleural effusion; magnification x 1000. Note the prominent nucleoli, which are often associated with melanocytes (thin arrows). Note also the green/black granules, consistent with melanin (thick arrow). Neutrophils, apoptotic cells and small lymphocytes can also be seen
Discussion
Melanomas are tumors of melanoblasts and melanocytes which can have a benign or malignant clinical course. They may occur on the skin, in the oral cavity or may be associated with the eye. They are common tumors in humans, horses and dogs, and occur less commonly in the cat. In humans, they are associated with ultraviolet light exposure, 1 but this relationship has not been shown in domestic animals as the majority of melanomas arise in haired skin or in the mouth.
Melanomas account for 4–20% of all skin tumors in dog. 2,3 Of these, 80% are benign and 20% are malignant. 2 Melanocytic tumors are most common in older dogs, with the average age being approximately 9 years. 2 The most common sites in the dog include the oral cavity, mucocutaneous junction of the lip, haired skin, eyes, eyelids, and subungual locations. 2,4 Benign melanomas (melanocytomas) have a predilection for haired skin, the eyelid and forelimb, while malignant melanomas are most commonly found at mucocutaneous junctions, in the oral cavity and in subungual locations. 2,4 In the dog, the mitotic rate can be highly predictive of the degree of malignancy. A mitotic rate of less than 3 per 10 HPFs has been shown in some studies to strongly suggest benign biologic behavior. 2,3 Other prognostic factors described in the dog include breed, tumor size/volume, tumor cell proliferation rate and the presence/absence of nuclear atypica, deep inflammation, lymphatic invasion, intralesional necrosis and metastasis. 2–5 A report by Spangler and Kass suggests that prognostic factors in the dog vary based on tumor location. 5 The most common metastatic sites in the dog include the regional lymph node, lung and viscera. 2,3
This report supports the observation that cutaneous melanomas located on or near the ear may demonstrate a malignant course and should be monitored and treated aggressively.
Melanocytic neoplasms are infrequently described in the cat and knowledge is limited (see box). The cat described in this report had a documented malignant melanoma of the ear base. The initial histopathologic report suggested a well-differentiated tumor with relatively few mitotic figures. However, the tumor recurred within 2 months. The histopathology from the second surgery demonstrated a more aggressive tumor with regional metastasis. The discrepancy between the two reports could be due to many factors. It could reflect an ongoing malignant transformation; alternatively, it could reflect sampling of the lesion, or a different assessment of histologic criteria for melanomas in cats. The lack of standard criteria for assessing these tumors can result in discrepant interpretations between pathologists. Ideally, both sections should have been reviewed by the same pathologist, but the original biopsy was, unfortunately, not available for review.
The case outlined in this report is unique in that the cat had pleural effusion that contained malignant melanocytes, consistent with metastatic spread. To the authors' knowledge, this has not been previously described. A necropsy was not available so it is unknown if there was gross metastasis to the pleura, pulmonary parenchyma and/or thoracic lymph nodes.
Melanoma in the cat — what do we know?
The most common presentation is intraocular, with anterior uveal melanomas being the most common primary intraocular tumor of cats. 6 These tumors may be locally invasive or develop distant metastases. 6 Conjunctival melanocytic tumors have also been described in the cat. 7
Cutaneous melanomas in the cat account for less than 3% of feline skin tumors. 2,8,9 The mean age of cats with cutaneous malignant melanomas ranges from 9.8–12 years. 2,10,11 In a retrospective study of cutaneous neoplasms in 340 cats, melanomas were found to account for 0.8% of tumors, with a mean age of 10 years. 8 A sex or breed predilection has not been shown. 10 A more recent retrospective study showed that most cutaneous melanomas occurred on the head and neck, with the pinna being the most common site. 9 Other sites of occurrence reported in the literature include the nose and digit. 6,10 The etiology is unknown, although there has been speculation that the tumors may be induced by feline fibrosarcoma virus. 8 Cutaneous malignant melanomas in cats have been documented to metastasize to lymph nodes, pulmonary parenchyma, liver, spleen, skin and muscle. 6,10,11
Cutaneous malignant melanomas in cats have been documented to metastasize to lymph nodes, pulmonary parenchyma, liver, spleen, skin and muscle.
Prognostic factors related to melanomas are not established in the cat. While some reports have shown little correlation between histologic characteristics such as mitotic rate and clinical behavior, 6,11 others have demonstrated predictability. 9,10 Luna et al showed a difference in size between malignant and benign non-ocular melanomas; benign tumors ranged from 0.1–0.6 cm, while malignant tumors exceeded 0.6 cm. 10 Miller et al found a 93.3% correlation between histologic diagnosis and clinical behavior. 9 Intraocular melanomas are thought to be more clinically malignant than both oral melanomas and cutaneous melanomas. 6
The recommended treatment for cutaneous malignant melanoma in cats is wide surgical excision. Recurrence and metastatic rates range from 5–50%. 10,11 Ancillary treatments (radiation, chemotherapy) have not been critically evaluated in cats.
There have been varying reports in the literature regarding the clinical behavior of cutaneous melanomas in cats. While some reports suggest a benign course, 4 others have demonstrated that lesions originating in this location are capable of recurrence and metastasis. 9,10 This report further supports the observation that cutaneous melanomas located on or near the ear may demonstrate a malignant course and should be monitored and treated aggressively.
Immunohistochemical staining has been used in both veterinary and human medicine to confirm a diagnosis of melanoma. Melan-A is an immunohistochemical marker that has been evaluated in cats on formalin-fixed tissue; it was shown in one report to be positive in 67% of feline melanomas. 12 The formalin-fixed tissue from this cat was not available for immunohistochemical staining. The authors attempted to confirm the cytologic diagnosis by applying the Melan-A stain to the cytology slides. Several reasons could explain the negative results. Although Melan-A has shown good sensitivity and specificity when used on cytologic specimens in dogs, to the authors' knowledge Melan-A staining on cytologic specimens from cats has not been investigated. 13 The slides were originally stained with a Wright-Geimsa stain. Melan-A was applied over this stain, possibly causing a false-negative result. Finally, a negative Melan-A stain does not rule out malignant melanocytosis as its sensitivity is not 100%.
Based on the results of biopsies and cytolological appearance of the pleural fluid, this case demonstrates that malignant melanoma can metastasize to pleural fluid. This information adds to the limited knowledge of cutaneous malignant melanoma in cats and suggests that these tumors are indeed capable of recurrence and metastasis, and aggressive treatment (wide surgical excision followed by systemic chemotherapy) may be necessary even if histopathology suggests a benign tumor. Melan-A staining may be helpful but further studies would be necessary to evaluate the use of Melan-A on cytologic slides of melanoma in cats.
Acknowledgments
Thanks go to Dr E J Ehrhart and The Molecular Pathology Laboratory at Colorado State University.
References
- 1. . Miller AJ, Mihm MC. Melanoma [review]. N Engl J Med 2006; 6: 51–65. [DOI] [PubMed] [Google Scholar]
- 2. . Goldschmidt MH. Pigmented lesions of the skin. Clin Dermatol 1994; 12: 507–14. [DOI] [PubMed] [Google Scholar]
- 3. . Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Vet Pathol 2002; 39: 651–87. [DOI] [PubMed] [Google Scholar]
- 4. . Goldschmidt MH. Benign and malignant melanocytic neoplasms of domestic animals. Am J Dermatopathol 1985; 7 (suppl): 203–12. [DOI] [PubMed] [Google Scholar]
- 5. . Spangler WL, Kass PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol 2006; 43: 136–49. [DOI] [PubMed] [Google Scholar]
- 6. . Day MJ, Lucke VM. Melanocytic neoplasia in the cat. J Small Anim Pract 1995; 36: 207–13. [DOI] [PubMed] [Google Scholar]
- 7. . Schobert CS, Labelle P, Dubielzig RR. Feline conjunctival melanoma: histopathological characteristics and clinical outcomes. Vet Ophthalmol 2010; 13: 43–46. [DOI] [PubMed] [Google Scholar]
- 8. . Miller MA, Nelson SL, Turk JR, et al. Cutaneous neoplasia in 340 cats. Vet Pathol 1991; 28: 389–95. [DOI] [PubMed] [Google Scholar]
- 9. . Miller WH, Scott DW, Andersone WL. Feline cutaneous melanocytic neoplasms: a retrospective analysis of 43 cases (1979–1991). Vet Dermatol 1993; 4: 19–26. [Google Scholar]
- 10. . Luna LD, Higginbotham ML, Henry CJ, et al. Feline non-ocular melanoma: a retrospective study of 23 cases (1991–1999). J Feline Med Surg 2000; 2: 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Patnaik AK, Mooney S. Feline melanoma: a comparative study of ocular, oral, and dermal neoplasms. Vet Pathol 1988; 25: 105–12. [DOI] [PubMed] [Google Scholar]
- 12. . Ramos-Vara JA, Miller MA, Johnson GC, et al. Melan A and S100 Protein immunohistochemistry in feline melanomas: 48 cases. Vet Pathol 2002; 39: 127–32. [DOI] [PubMed] [Google Scholar]
- 13. . Hoinghaus R, Hewicker-Trautwein M, Mischke R. Immunocytochemical differentiation of canine mesenchymal tumors in cytologic imprint preparations. Vet Clin Pathol 2008; 37: 104–11. [DOI] [PubMed] [Google Scholar]