Abstract
There is some evidence that Mycoplasma species may be associated with lower airway disease in cats. Retrospective and prospective studies were carried out on a total population of 76 cats but failed to identify any cases of Mycoplasma species infection by bacterial culture alone. The overall prevalence of bacterial infection (15.8%) was also lower than that identified in previous studies. When a molecular detection technique, the PCR-DGGE, was employed the prevalence of Mycoplasma species detected was 15.4%, with M felis, M gateae and M feliminutum species identified, although the significance of these Mycoplasma species in feline lower airway disease remains in question. However, the PCR-DGGE technique allowed species identification and indicated the presence of M feliminutum, a species not previously isolated from the lower airways of cats.
Introduction
Mycoplasmas are prokaryotic organisms within the class Mollicutes. 1 The absence of a cell wall can affect their survival outwith the host environment and special culture techniques are often required to identify these slow-growing organisms. Few laboratories regularly identify the species involved as, until recently, this required laborious and subjective biochemical and immunological testing of cultured organisms.2–4 In addition, appropriate antibacterial susceptibility testing is not widely available for Mycoplasma species. 2
Mycoplasma species are often considered commensal organisms of mucosal membranes, with a number of species identified from the upper respiratory tracts of healthy cats, including Mycoplasma felis, Mycoplasma gateae, Mycoplasma feliminutum and Mycoplasma arginini. 2 Mycoplasma species are not generally considered to be present in the lower airways of healthy cats.5,6 Studies in the USA and Australia have suggested that approximately 22% of cats with lower airway disease may have a concurrent Mycoplasma species infection.5,7 The species of Mycoplasma involved in both studies was not identified.
Prior to this study, clinical perception at our establishment was that Mycoplasma species were rarely identified in lavage fluid from cats with lower airway disease. The primary aim of this study was to assess whether the prevalence of Mycoplasma species infection was, indeed, lower in cats from Scotland and Northern England than in Australia and the USA by means of a retrospective study. Results of this study subsequently led to a prospective study to investigate whether alternative methods of sample collection and assay could improve identification of this organism. The hypothesis was that use of a Mycoplasma species transport medium and/or a polymerase chain reaction (PCR) test to identify Mycoplasma species would enable better detection of these organisms in bronchoalveolar lavage (BAL) fluid.
Materials and methods
Retrospective study — case selection
The Veterinary Database at the University of Edinburgh Hospital for Small Animals (UoE-HFSA) was searched for feline patients that had undergone investigation of lower airway disease between 1 July 1999 and 1 July 2005. Inclusion criteria for the study were performance of BAL with culture of the lavage fluid. Exclusion criteria were missing or incomplete records. Records were evaluated for signalment, presenting problem(s) relating to the lower respiratory tract (cough, wheeze and/or dyspnoea), radiological findings, lavage fluid culture and cytology, and primary diagnosis. A diagnosis of asthma was based on a normal or predominantly bronchial radiographic lung pattern, BAL fluid where eosinophils comprised >25% of the nucleated cells, and response to treatment with corticosteroids and/or bronchodilators. A diagnosis of chronic bronchitis was based on a normal or predominantly bronchial radiographic lung pattern, BAL fluid containing predominantly neutrophils, and response to treatment with corticosteroids and/or bronchodilators. Bronchopneumonia was diagnosed on the basis of a bronchial or alveolar radiographic lung pattern, a BAL fluid containing predominantly neutrophils, positive bacterial culture or identification of an infectious agent, and response to therapy with antibacterials. The cases with positive BAL culture results were chosen for further review.
Prospective study — case selection
Feline patients referred to the UoE-HFSA for investigation of lower airway disease were recruited into the study from 1 July 2005 to 1 July 2008. Inclusion criteria included history of cough, wheeze and/or dyspnoea. Cases were excluded if they had received antibiotics within 1 week of presentation or where the case file could not be retrieved. Diagnostic procedures comprised those normally undertaken for clinical evaluation of these cases, namely: routine haematology and serum biochemistry, thoracic radiography and BAL (± bronchoscopy at the attending clinician’s discretion). Additional tests performed in the prospective study comprised evaluation of each patient for feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV), and extended testing of the BAL fluid for Mycoplasma species by means of transport media and a PCR assay.
Diagnostic investigations — both studies
Samples were submitted to the University of Edinburgh Veterinary Pathology Unit (VPU) and Veterinary Microbiology Laboratory (VML) for further evaluation.
Haematology analysis was performed using a Pentra 60 haematology analyser (ABX Diagnostics). Biochemistry was performed on an opeRA autoanalyser (Bayer Diagnostics). Electrolytes were measured using an AVL 9180 Electrolyte Analyser (AVL Scientific Corporation).
Further investigations were performed with the patients under general anaesthesia. Orthogonal radiographs (right lateral and ventro-dorsal views) were taken with positive pressure ventilation to facilitate optimal inspiratory views. Bronchoscopy was performed, dependent on the clinician’s assessment. Bronchoalveolar lavage was performed by passing a sterile 172 cm end-opening endoscopy catheter (Portex) down the lumen of the sterile endotracheal tube until it lodged blindly within a bronchus. Three aliquots of 3 ml warmed, sterile saline were used to lavage the airway, with recovery by syringe aspiration, following brief coupage of the thorax. Quantification of recovery volume was not performed. The recovered BAL fluid was pooled within a plain, sterile tube, before decanting 0.5–1 ml aliquots for further assessment.
One 0.5 ml sample of the BAL fluid was submitted in an ethylenediamine tetra-acetic acid (EDTA) tube for cytological assessment by the VPU. Following centrifugation at 1500 rpm (224g) for 5 mins (Thermo Fisher Cytospin III), the cytospin was stained with May-Grunwald Giemsa stain. The smear was evaluated for the presence of oral contamination (eg, squames ± Simonsiella species) and evidence of bacterial infection (eg, intracellular bacteria), and the percentage of cell populations was calculated.
A second 1 ml aliquot was submitted in a plain tube for bacteriological culture. An anaerobic environment was not maintained, but samples were submitted to the VML within 4 h of collection. In addition to routine aerobic and anaerobic culture, the BAL fluid was inoculated on to a blood agar plate and incubated at 37ºC with 10% CO2 for 5 days for the culture of Mycoplasma species. While this would identify Mycoplasma species within 72 h if organisms were present in large numbers, the fluid was also inoculated into mycoplasma broth (CM0403; Oxoid) supplemented with penicillin, thallous acetate, yeast extract and horse serum (SR0059; Oxoid). The broth was incubated at 37ºC with 10% CO2 for 5 days after which it was transferred to mycoplasma agar (CM0401; Oxoid) supplemented as above and incubated as before for a further 5 days. This was to ensure that small numbers of organisms could be isolated. Positive cultures would be checked microscopically using Diene’s Stain.
Diagnostic investigations — prospective study only
Blood was tested for FeLV antigen and FIV antibody by an in-house enzyme-linked immunosorbent assay (SNAP FIV/FeLV Combo; Idexx Laboratories).
A 1 ml aliquot of the BAL fluid was submitted for bacterial culture to the VML in a transport medium appropriate for Mycoplasma species culture (M4 Microtest tube; Remel). A further 1 ml aliquot of this fluid was submitted to the Animal Health and Veterinary Laboratories Agency, Weybridge, for identification of Mycoplasma species by PCR and denaturing gradient gel electrophoresis, as previously reported by McAuliffe et al. 8 Briefly, DNA was extracted from the BAL fluid using a Genelute genomic DNA kit (Sigma), according to the manufacturer’s instructions. Amplification of DNA was achieved by means of a universal bacterial primer GC-341F and a specific reverse primer for Mollicutes (R543). Cycling conditions comprised denaturation at 94°C for 5 mins, then 30 cycles of 95°C for 1 min, 56°C for 45 s and 72°C for 1 min, followed by an extension step of 72°C for 10 mins, using a gradient thermocycler (iCycler; Bio-Rad). Samples were then transferred to polyacrylamide/bis (37.5:1) gels with denaturing gradients from 30% to 60% [where 100% is 7M urea and 40% (v/v) deionised formamide] in 1 × Tris-acetate-EDTA electrophoresis buffer. Following electrophoresis, gels were stained with SBYR Gold (Cambridge BioScience) and visualised with ultraviolet illumination. Bands identified on the gel were compared with reference species to enable identification of species identified from the clinical cases (see Figure 1).
Figure 1.
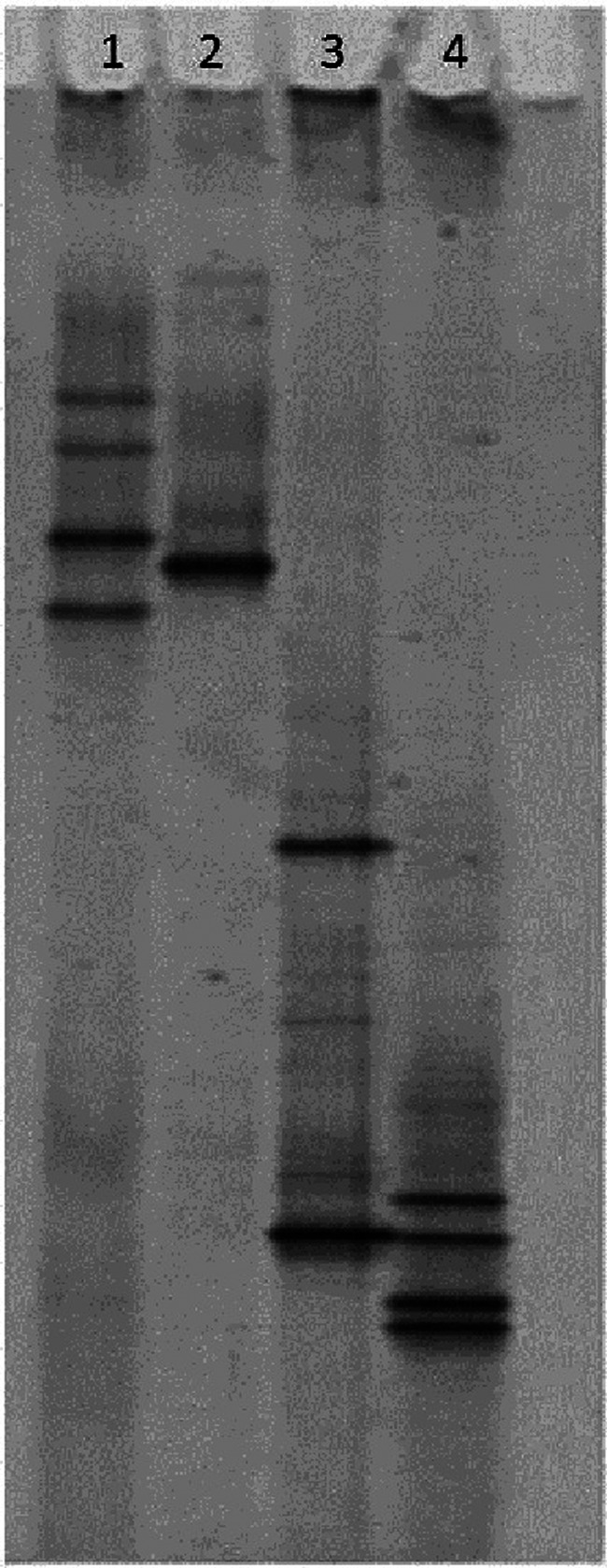
PCR-DGGE gel from feline control samples. Lane 1 - Mycoplasma felis; Lane 2 - Mycoplasma feliminutum; Lane 3 - Mycoplasma gateae; Lane 4 - Mycoplasma arginini
Statistical analysis
Findings were tabulated in a spreadsheet (Microsoft Excel for Windows). Statistical analysis was performed with a commercial software programme (Minitab 15; Minitab). Continuous data were analysed for normality and expressed as mean (± standard deviation). Prevalence data are given as percentage population and confidence intervals (CI) were generated by means of a test for one proportion, with the α-level set at 0.05. This test was also used to investigate the alternate hypothesis that disease prevalence within this study differed from previous studies, with statistical significance set at P <0.05.
Results
Retrospective study
From the UoE-HFSA database, 55 feline cases were identified as having had a BAL and culture performed. Of these 55 cases, the records could not be found for five cases, and these cases were thus excluded. The remaining 50 cases comprised 28 neutered males and 22 neutered females. Ages ranged from 6 months to 16 years with a mean age of 6.39 years (SD ± 4.31). The majority of cats were domestic shorthair cats (n = 28), with the following breeds also represented: Siamese (n = 7), Bengal (n = 3), Maine Coon (n = 2), Persian (n = 2), Burmese (n = 2) and one each of Abyssinian, Balinese, British Shorthair, Korat, Oriental and Somali.
A diagnosis of asthma was made in 23 cases and bronchitis in one case, giving an overall prevalence of 48% for lower airway disease. Concurrent disease was identified in seven of these cases, comprising infection (n = 3), hypertrophic cardiomyopathy (n = 2), laryngeal lymphoma (n = 1) and hyperthyroidism (n = 1). Although four cases had positive bacterial cultures from the BAL fluid, in one case, where low numbers of mixed bacteria were cultured, this was considered contamination (see Table 1). Bronchopneumonia was diagnosed in 13 cases, but positive cultures only obtained in four BAL samples (see Table 1). Mycobacterial infection was confirmed in two cases and suspected in a third, herpesvirus and toxoplasmosis comprised one case each and no organism was identified in four cases, including one case of lobar pneumonia. The remaining cases comprised rhinitis (n = 5), pulmonary adenocarcinoma (n = 2) idiopathic pulmonary fibrosis (n = 1), nasal adenocarcinoma (n = 1) nasopharyngeal stenosis (n = 1) and retropharyngeal mass (n = 1). The cases with primary upper airway disease also had radiographic changes suggestive of lower airway disease, hence their inclusion in the study. No diagnosis was given in two cases.
Table 1.
Retrospective study: cases with a positive bronchoalveolar lavage fluid culture
| Breed | Age (years) | Gender | Diagnosis | Organism cultured |
|---|---|---|---|---|
| Korat | 10 | FN | Bronchopneumonia | Actinomyces |
| Siamese | 3 | FN | Asthma | Chryseobacterium indolgenes |
| Siamese | 12 | MN | Asthma | Pasteurella species |
| DSH | 11 | FN | Asthma; laryngeal lymphoma | Gram-negative bacilli |
| DSH | 9 | MN | Not obtained | Pasteurella multocida |
| Persian | 8 | FN | Nasopharyngeal stenosis | Staphylococcus aureus |
| DSH | 8 | MN | Bronchopneumonia | Pasteurella multocida |
| DSH | 3 | FN | Asthma | Mixed growth; small numbers |
| DSH | 11 | MN | Bronchopneumonia; renal lymphoma | Corynebacterium |
| DSH | 6 | MN | Bronchopneumonia | Pasteurella multocida |
DSH = domestic shorthair, FN = female neutered, MN = male neutered
Bacteria were cultured from 10 of the cases (see Table 1), giving a prevalence of 20% (CI: 10.0–33.7%). In one of these cases, a small number of a mixed population was suggestive of contamination and, in a second cat, the identification of Staphylococcus aureus was suggestive of extension of nasopharyngeal disease. Pasteurella species was the most commonly identified infectious agent (four cases), and Mycoplasma species were not identified in any case (0%: CI 0.0–5.8%).
Prospective study
During the prospective study, 30 cases presented to the UoE-HFSA with a history of cough, wheeze and/or dyspnoea. For two of these cases, the files could not be found, and for a further two, the cats had received antibiotics in the week prior to presentation; these four cases were excluded. One cat presented for investigation on three occasions; therefore, although 26 cases were assessed, this represented only 24 cats. Eleven cats were neutered females, with one entire male and 12 neutered males. Ages ranged from 6 months to 18 years with a mean age of 8.86 years (SD ± 4.20). This was significantly older (P = 0.02) than the population in the retrospective study. As before, the majority of cats were domestic shorthair cats (n = 13), with other breeds represented as follows: Siamese (n = 3), Oriental (n = 2) and one each of domestic longhair, Devon Rex, Maine coon, Persian, Ragdoll and Russian Blue.
The majority of cases (15/26; 57.7%) were diagnosed with lower airway disease [bronchitis (n = 5) or asthma (n = 9)] based on a diffuse bronchointerstitial radiographic pattern, negative culture and predominance of eosinophils (asthma) or non-degenerate neutrophils (bronchitis) on BAL fluid. Pneumonia or bronchopneumonia was diagnosed in six cases and pulmonary neoplasia in one and suspected in a second. Four cats presented with signs of upper airway disease (sneeze or stertor) in addition to coughing and wheezing. In two cases this was associated with a final diagnosis of asthma. One cat was diagnosed with lipoid pneumonia following inhalation of liquid paraffin.
Lymphopenia was the most commonly identified haematological abnormality (13/26), followed by neutrophilia (4/26) and eosinophilia (3/26). All three cats with peripheral eosinophilia were diagnosed with asthma. Three cats also had neutropenia, which is an unusual finding in respiratory disease. In one case it was attributed to increased tissue demand associated with bronchopneumonia and, in a second case, the value was only marginally below the reference interval and thus clinically insignificant. For the third case that exhibited a more significant neutropenia, no explanation was found. Mild thrombocytopenia and monocytosis were each documented in one case. Elevations in blood glucose (<15 mmol/l) were present in 50% of cats and consistent with stress hyperglycaemia. Elevated urea was documented in 8/26 cases and elevated creatinine in 5/26 cases. Hypokalaemia was also identified infrequently (4/26). No cats were positive for FeLV antigen or FIV antibody.
None of the BAL fluid samples showed evidence of oral contamination. Routine culture was positive in only two cases of bronchopneumonia (7.69%; CI 0.94–25.1%), yielding Moraxella species in one cat and Pasteurella species in a second. Mycoplasma species were not cultured, either from direct culture, or when the transport medium was employed (0%; CI 0.0–10.9%). Mycoplasma species PCR was positive on four occasions (15.4%; CI 4.36–34.9%), but this was not statistically significant compared with prevalence obtained by culture (P = 0.11). In the four cases that were found to be positive by PCR, three different species of Mycoplasma species were identified (see Table 2). The case in which M feliminutum species was identified was also the case from which Moraxella species was cultured. Two cases occurred in the same cat, with different species (M gateae and M felis) being isolated on two separate occasions, 4 months apart. On its third presentation, Pasteurella species was isolated from this cat. In the fourth case, M felis was identified by PCR (see Figure 2).
Table 2.
Prospective study: cases with a positive bronchoalveolar lavage fluid Mycoplasma PCR
| Breed | Age (years) | Gender | Diagnosis | Mycoplasma species | Antibacterial |
|---|---|---|---|---|---|
| DSH | 6 | FN | Bronchopneumonia | M feliminutum | Doxycycline |
| Oriental | 9 | MN | Bronchopneumonia | M gateae | Marbofloxacin |
| Oriental | 9 | MN | Bronchopneumonia | M felis | Azithromycin Doxycycline |
| Devon rex | 10 | MN | Eosinophilic rhinitis Asthma |
M felis | Doxycycline |
DSH = domestic shorthair, FN = female neutered, MN = male neutered
Figure 2.
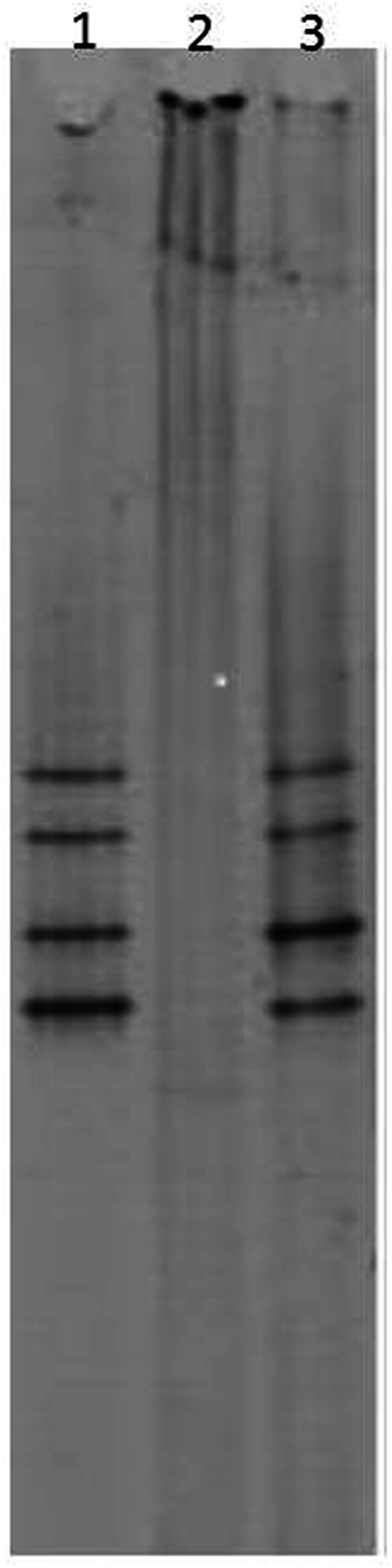
PCR-DGGE gel from case positive for Mycoplasma felis. Lane 1 - sample from case; Lane 2 - negative sample; Lane 3 - reference sample Mycoplasma felis
In total (combining the results from the retrospective and prospective studies), culture for bacteria was positive in 12/76 cases (15.79%; CI 8.43–25.96%) and Mycoplasma species culture was negative in all cases (0%; CI 0.00–3.68%). When comparison was made between the population in the retrospective study and the prospective study, there was no significant difference in the number of BAL cultures that were positive for bacteria (P = 0.107) or Mycoplasma species (P = 1.000).
Discussion
Mycoplasma species have been reported as a cause of feline pneumonia and pyothorax.9–13 Randolph et al 5 recovered Mycoplasma species in 21.4% (6/28) of tracheobronchial lavage samples from cats with pulmonary disease, using both a Hayflick medium with 15% horse serum and 20% porcine serum agar plates, and broth incubated at 35.5°C at 8% CO2 and 100% humidity. 14 Foster et al 7 identified Mycoplasma species in 22.4% (15/67) of cases, based on negative Gram-staining of BAL cytology and colonial morphology in aerobic culture of sheep blood agar at 37°C. In a study by Moise et al, 15 Mycoplasma species were present in 4/9 (44%) of cases tested, but the culture method employed was not described. In marked contrast, the current study failed to identify Mycoplasma species infection in 76 cases of feline lower airway disease when bacterial culture was employed, even when, in 30 cases, a mycoplasma transport medium was included. There are a number of possible explanations for this discrepancy in prevalence between the studies.
The first is that Mycoplasma species are genuinely infrequently present in the lower airways of cats in our referral population from Scotland and the North of England. Geographic discrepancies for Mycoplasma infections affecting other species, such as farm animals, have not been documented; however, to our knowledge, prevalence studies for Mycoplasma species infection in cats (other than the haemotropic Mycoplasma species) have not been performed in the UK.
Different techniques employed in the various studies could have contributed to the different outcomes documented — the highest detection levels were in a study employing cytological brush technique rather than BAL. 15 Direct comparison of techniques among the three BAL studies is limited by the absence of detail of some aspects in the previous studies; that said, some potential differences are considered below.
Contamination of BAL fluid from the upper airways could lead to increased recovery of Mycoplasma species, as these organisms are considered commensals of the upper airways. 6 In one study 5 an intravenous catheter was used for sampling, as described by Moise and Blue. 16 This was extended blindly beyond the endotracheal tube and may, therefore, have resulted in sampling from the trachea or tip of the endotracheal tube rather than the lower airways. However, the technique employed in the second study 7 was similar to the current study, making the BAL technique unlikely to account for the different results.
The death of viable organisms in transit prior to culture is potentially supported by the finding of Mycoplasma species DNA in the samples tested by PCR, with a prevalence of 15.4% — not dissimilar to the earlier studies. Mycoplasma species organisms can be short-lived outside the host 17 and failure to ensure an anaerobic environment by incompletely filling sample tubes may have resulted in the loss of viability of organisms. However, incorporating a mycoplasma transport medium into the prospective study did not improve recovery of these organisms, suggesting that death in transit to the microbiology laboratory was unlikely to be a factor. Transit times to the microbiology laboratories appeared similar in all three studies and are likely to be considerably less than those achievable in the general practice situation where postage to an external laboratory is likely to be required.
Suboptimal culture technique was considered as a possible explanation for the lack of recovery of Mycoplasma species organisms. Direct comparison with culture media employed in the previous studies was not possible, although one study 7 did not use a specific Mycoplasma species culture medium yet still cultured Mycoplasma species more frequently than in the present study. The culture medium used in the current study was a modified Hayflick medium (B); however, different Mycoplasma species media are available and there is no ideal medium that suits all Mycoplasma species. A different modified Hayflick medium (N) may be more suitable for culture of M felis; M feliminutum is a more fastidious organism that may grow better on SP4 medium. 18 Our results may reflect different requirements by different species of Mycoplasma and could explain why the species identified in the current study may have grown poorly on the media employed. Suboptimal culture media may result in low numbers of small colonies that may be difficult to detect. Incorporation of an indicator such as glucose or arginine that is fermented and gives rise to a colour change with phenol may also be beneficial. Other factors affecting culture could be variation in media components and preparation, environmental growth conditions and the duration of incubation. 18
Treatment of the cats with antibacterial agents prior to referral for examination is, perhaps, the most likely explanation for the inability to culture viable organisms, while still being able to detect residual Mycoplasma species DNA. Although cats that had received antibacterial therapy within the previous week were excluded from the study, further review of the cases identified that 17 cases (65%) had received antibacterial drugs in the 3 months prior to investigation. The antibacterial agents most frequently used were doxycycline (seven cases), amoxicillin-clavulanate (five cases), enrofloxacin (four cases) and one each of cephalosporin, metronidazole and clindamycin. Some cats had received more than one antibiotic.
The use of doxycycline and enrofloxacin could have compromised our ability to culture Mycoplasma species; the high number of cats that had received antibacterial drugs may also explain the identification of bacterial infection in only 2/26 cases. This prevalence (7.6%) is much less than the 24–48% reported previously in a number of studies7,15,19,20 and also less than the 33% reported in a study in healthy cats. 6 This may reflect increased awareness of the potential role of bacterial organisms in feline respiratory disease by general practitioners and increased use of antibacterial agents compared with when the earlier studies were performed.
It is of note that during the prospective study, one positive case of Mycoplasma species was cultured, although this case had been excluded from the study as it had received prior antibacterial therapy (marbofloxacin). Both the direct sample and the transport media sample cultured positive, indicating that the Mycoplasma species culture technique was appropriate in this case, but possibly dependent on bacterial numbers or Mycoplasma species present. Unfortunately, PCR testing was not performed on this sample to identify which species was involved. The ability to only culture Mycoplasma species in one case that had been excluded is a severe limitation of this study. Concurrent submission of samples to more than one bacteriology laboratory for culture would have helped to address some of the questions raised about the importance of different culture techniques.
As a result of the problems identified with culture requirements for Mycoplasma species, this study suggests that assessment of Mycoplasma species infection by PCR may be a more sensitive method of detection of these organisms. This is in contrast to the study by Johnson et al 17 that identified good agreement between culture and PCR for detection of Mycoplasma species in samples obtained from the nasal cavities of cats. Use of species PCR has a number of advantages: it is able to detect fastidious or non-cultivable organisms, and can do so more rapidly than standard culture techniques; it is not affected by death of organisms in transit, as may occur with submissions from general practice; and it allows the species involved to be identified. However, if the organism is not cultured, and only DNA is detected, the significance of that organism in causing infection at the site from which it is isolated is questionable. Low levels of contamination from the oral cavity, for example, may have been detected by PCR, but not by culture. This could have been addressed by performing Mycoplasma species PCR on BAL fluid from cats that were not suffering from lower airway disease to try to evaluate further the role of these organisms in lower airway disease. However, performance of BAL on healthy cats would be considered an experimental procedure in the UK necessitating a Home Office Licence.
Antimicrobial choices for Mycoplasma species infections are limited by difficulties in performing culture and sensitivity analysis, although guidelines have been produced for minimal inhibitory concentration testing. 18 Generally, Mycoplasma species are considered sensitive to macrolides, tetracyclines, chloramphenicol, fluoroquinolones, lincosamides and aminoglycosides, but are resistant to beta-lactam antibiotics, as they do not possess a cell wall. 1 Different antibacterial choices were employed to manage the cases positive for Mycoplasma species by PCR. The first case with Moraxella species and M feliminutum was prescribed doxycycline for 21 days, but required on-going management with salbutamol and fluticasone inhalers for lower airway disease. The second case, with M gateae infection, was prescribed a 21-day course of marbofloxacin initially and although clinical signs initially improved, recurrence of signs 4 months later was associated with isolation of M felis. This did not respond initially to 2 weeks of treatment with azithromycin, but improved when doxycycline was prescribed. As the latter medication was prescribed by the referring veterinary surgeon, it is unclear how long a course was prescribed. The fourth case was associated with M felis infection and responded to treatment with doxycycline for 30 days, although on-going problems with eosinophilic rhinitis remained. While these cases improved with antibacterial therapy, concurrent treatment with other drugs, such as bronchodilators or corticosteroids, makes it impossible to be conclusive about the role of infection in these cases.
Two cases demonstrated a lack of response to certain antibacterials. In addition to the M felis case not responding to azithromycin, the culture-positive case had been excluded because it had received marbofloxacin within 1 week of being evaluated. This is consistent with findings in a previous study, 21 where doxycycline was ineffective in one case, two cases failed to respond to enrofloxacin and one case failed to respond to clindamycin. Interestingly, two cases in that study responded well to azithromycin, whereas in the current study this antibiotic was found to be ineffective in the one case in which it was used. Unfortunately, too few positive cases were identified in this study to speculate on associations between antibacterial resistance and the species of Mycoplasma. This is an area that warrants further exploration.
No association with Mycoplasma species infection and positive retroviral status was identified despite an association between immunocompromise and Mycoplasma species infection having been identified in humans. 22
Conclusions
This study highlights the difficulties encountered in the recovery of Mycoplasma species from within the lower airways of cats. The prevalence of Mycoplasma species infection detected by PCR was 15.4%, which is lower than that detected in previous studies where bacterial culture was employed. This may reflect more frequent use of antibacterial agents by general practitioners in the management of feline lower airway disease. While the clinical significance of identifying Mycoplasma species DNA alone remains in doubt, the use of PCR will allow for future studies to investigate more fully the species of Mycoplasma involved in feline respiratory disease and will help to identify species variation in susceptibility to antibacterial drugs. To our knowledge this is the first time M feliminutum has been documented in association with lower respiratory tract disease in the cat.
Acknowledgments
The authors acknowledge the support of the veterinary and nursing staff at the Hospital for Small Animals for their assistance in the management of the patients in this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
The authors do not have any potential conflicts of interest to declare.
Accepted: 21 May 2012
References
- 1. Greene C, Chalker VJ. Nonhemotropic mycoplasmal, ureaplasmal an L-form infections. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier, 2012, pp 319–325. [Google Scholar]
- 2. Bemis DA. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet Clin North Am Small Anim Pract 1992; 22: 1173–1186. [DOI] [PubMed] [Google Scholar]
- 3. Cole BC, Golightly L, Ward JR. Characterization of Mycoplasma strains from cats. J Bacteriol 1967; 94: 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalker VJ, Owen WMA, Paterson C, Barker E, Brooks H, Rycroft AN, Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology 2004; 150: 3491–3497. [DOI] [PubMed] [Google Scholar]
- 5. Randolph JF, Moise NS, Scarlett JM, Shin SJ, Blue JT, Corbett JR. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. Am J Vet Res 1993; 54: 897–900. [PubMed] [Google Scholar]
- 6. Padrid PA, Feldman BF, Funk K, Samitz EM, Reil D, Cross CE. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats. Am J Vet Res 1991; 52: 1300–1307. [PubMed] [Google Scholar]
- 7. Foster SF, Martin P, Braddock JA, Malik R. A retrospective analysis of feline bronchoalveolar lavage fluid cytology and microbiology (1995–2000). J Feline Med Surg 2004; 6: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McAuliffe L, Ellis RJ, Lawes JR, Ayling RD, Nicholas RAJ. 16S rDNA PCR and denaturing gradient gel electrophoresis; a single generic test for detecting and differentiating Mycoplasma species. J Med Microbiol 2005; 54: 731–739. [DOI] [PubMed] [Google Scholar]
- 9. Malik R, Love DN, Hunt GB, Canfield PJ, Taylor V. Pyothorax associated with a Mycoplasma species in a kitten. J Small Anim Pract 1991; 32: 31–34. [Google Scholar]
- 10. Foster SF, Barrs VR, Martin P, Malik R. Pneumonia associated with Mycoplasma spp in three cats. Aust Vet J 1998; 76: 460–464. [DOI] [PubMed] [Google Scholar]
- 11. Bart M, Guscetti F, Zurbriggen A, Pospischil A, Schiller I. Feline infectious pneumonia: A short literature review and a retrospective immunohistological study on the involvement of Chlamydia spp and distemper virus. Vet J 2000; 159: 220–230. [DOI] [PubMed] [Google Scholar]
- 12. Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc 2002; 38: 111–119. [DOI] [PubMed] [Google Scholar]
- 13. Trow AV, Rozanski EA, Tidwell AS. Primary mycoplasma pneumonia associated with reversible respiratory failure in a cat. J Feline Med Surg 2008; 10: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Randolph JF, Moise NS, Shin SJ, Blue JT, Bookbinder PR. Prevalence of mycoplasmal and ureaplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am J Vet Res 1993; 54: 387–391. [PubMed] [Google Scholar]
- 15. Moise NS, Wiedenkeller D, Yeager AE, Blue JT, Scarlett J. Clinical, radiographic and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986). J Am Vet Med Assoc 1989; 194: 1467–1473. [PubMed] [Google Scholar]
- 16. Moise NS, Blue J. Bronchial washings in the cat: procedure and cytologic evaluation. Compend Contin Educ Pract Vet 1983; 5: 621–630. [Google Scholar]
- 17. Johnson LR, Drazenovich NL, Foley JE. A comparison of routine culture with polymerase chain reaction technology for the detection of Mycoplasma species in feline nasal samples. J Vet Diagn Invest 2004; 16: 347–351. [DOI] [PubMed] [Google Scholar]
- 18. Hannan PCT. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Vet Res 2000; 31: 373–395. [DOI] [PubMed] [Google Scholar]
- 19. Dye JA, McKiernana C, Rozanski EA, Hoffmann WE, Losonsky JM, Homco LD, et al. Bronchopulmonary disease in the cat: Historical, physical, radiographic, clinicopathologic and pulmonary functional evaluation of 24 affected and 15 healthy cats. J Vet Int Med 1996; 10: 385–400. [DOI] [PubMed] [Google Scholar]
- 20. Schulz BS, Wolf G, Hartmann K. Bacteriological and antibiotic sensitivity test results in 271 cats with respiratory tract infections. Vet Rec 2006; 158: 269–270. [DOI] [PubMed] [Google Scholar]
- 21. Foster SF, Martin P, Allan GS, Barrs VR, Malik R. Lower respiratory tract infections in cats: 21 cases (1995–2000). J Feline Med Surg 2004; 6: 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitcher DG, Nicholas RAJ. Review: Mycoplasma host specificity: fact or fiction? Vet J 2005; 170: 300–306. [DOI] [PubMed] [Google Scholar]


