Abstract
A 14-year-old male domestic shorthair cat presented with an acute onset of aggressive behaviour, fear and hypersalivation. Neurological examination revealed bilateral mydriasis and left-sided facial twitching and hemiparesis. Magnetic resonance imaging (MRI) showed moderate bilateral symmetrical T2-hyperintensity along the entire hippocampus and bilateral asymmetric T2-hyperintensity in the pyriform lobes. Marked bilateral contrast enhancement of the hippocampus was evident on post-contrast T1-weighted images. The partial complex seizures were refractory to medical treatment and the cat was euthanased 4 days after admission. The clinical and MRI findings were consistent with feline hippocampal necrosis (FHN). On histopathology, neuronal necrosis and astrocytosis were present in the hippocampi and pyriform lobes. In addition, an oligodendroglioma was detected in the right pyriform lobe. Contrary to previous reports of FHN in which no underlying cause could be identified, we believe that in this case the seizure focus arose from a neoplastic lesion within the right pyriform lobe. This unique case report represents the so-called ‘dual pathology’ of temporal lobe epilepsy in humans, in which an extrahippocampal lesion within the temporal lobe results in hippocampal sclerosis.
Case Report
A 14-year-old male neutered, indoor, British Shorthair cat presented with a 4-day progressive history of uncoordinated gait and behavioural changes, including distress, aggression and continuous growling. The owner also reported tachypnoea, anorexia and hypersalivation. No history of possible poisoning or recent trauma was reported. The cat received a commercial feline diet. During the last weeks the owner had noticed an occasional cough.
All vital parameters were normal on physical examination. On neurological examination the cat showed an aggressive behaviour. Moreover, it appeared confused and hypersensitive to environmental stimuli. The gait was ataxic and tetraparetic. Proprioceptive positioning was normal, but hopping reactions were reduced on the left thoracic and pelvic limb. Hypersalivation, bilateral mydriasis and occasional left-sided facial twitching were noticed (Figure 1). During hospitalisation the cat developed polyphagia and showed continuous left-sided facial twitching and occasional clonic movements of the left front paw. Tachycardia was recorded (170–200 bpm).
Figure 1.

Cat with temporal lobe epilepsy. Note the bilateral mydriasis and left-sided facial twitching (ie, clonic contraction of the left lip and eyelids)
Based on the behavioural changes and partial seizures, the lesion was localised mainly to the forebrain — in particular a lesion involving the limbic system or diencephalon. The most likely differential diagnoses included brain neoplasia, brain infarct, encephalitis, feline hippocampal necrosis (FHN) and thiamine deficiency.
Complete blood cell count and extended serum biochemistry, including bile acid stimulation test and protein electrophoresis, were unremarkable. Serum testing for feline immunodeficiency virus and feline leukaemia virus was negative. Serology for Toxoplasma gondii was negative. Feline T4 concentration was normal. Serial blood pressure measurements were within normal limits. Thoracic radiographs were unremarkable.
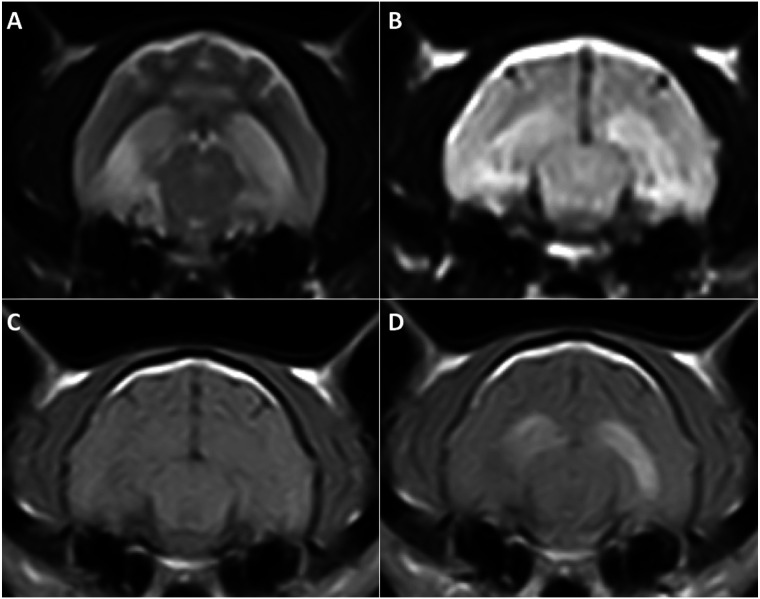
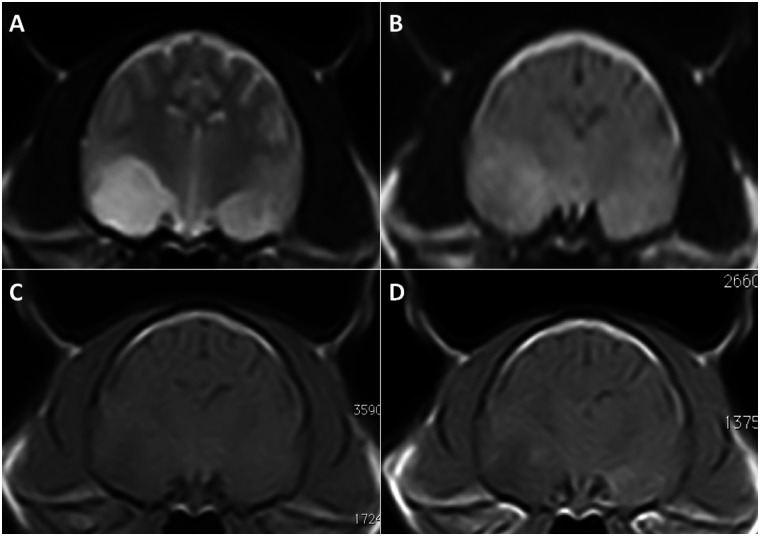
Magnetic resonance imaging (MRI) of the brain (Esaote VetMR 0.2T) was performed under general anaesthesia. T2-weighted (T2W) and pre- and post-contrast T1-weighted (T1W) images, following a gadobenate-dimeglumine bolus (0.1 mmol/kg IV: MultiHance; Bracco), were acquired in transverse and sagittal planes. Fluid attenuated inversion recovery (FLAIR) images were acquired in the transverse plane only. Bilateral hyperintensity along both entire hippocampi (2a and b) and within the pyriform lobes, on the right side in particular (Figure 3a and b), were present on both T2W and FLAIR sequences. A poorly defined area in the right pyriform lobe was hypointense on pre- and post-contrast T1W images (Figure 3c and d). The left pyriform lobe (Figure 3c and d) and both hippocampi (Figure 2c and d) enhanced strongly after contrast medium administration.
Figure 2.
Transverse T2W (a) and FLAIR (b) images, showing a moderate hyperintense signal along both entire hippocampi. Both hippocampi were isointense on the pre-constrast T1W images (c). They strongly enhanced on post-contrast T1W images (d)
Figure 3.
Transverse T2W (a) and FLAIR (b) images, showing a moderate hyperintense signal in the area of both pyriform lobes. This hyperintensity affected a much larger area within the right pyriform lobe in comparison to the left. Transverse pre- (c) and post-contrast T1W images (d) demonstrate a similar hypointense area in the right pyriform lobe. The left pyriform lobe enhanced mildly on post-contrast T1W images (d)
Cisternal cerebrospinal fluid (CSF) showed a mild increase in total protein level (0.35 g/l, reference < 0.25 g/l), with a normal white blood cell count. Polymerase chain reaction in the CSF for T gondii and feline infectious peritonitis virus was negative.
The clinical signs and MRI findings were compatible with previous descriptions of FHN.1,2
Supportive fluid therapy and anti-convulsant therapy was initiated soon after admission using phenobarbitone (2.5 mg/kg slow IV q12h after an initial loading dose of 10 mg/kg slow IV over 30 mins: Phenobarbital; Martindale Pharmaceuticals), followed by a constant rate infusion of midazolam (0.02 mg/kg/h: Hypnovel; Roche Products). Additional medical therapy involved anti-inflammatory dosage of corticosteroids (dexamethasone 0.1 mg/kg SC q24h: Dexadreson; Intervet) and thiamine supplementation (20 mg slow IV q24h: Combivit; Norbrook Laboratories). As soon as the midazolam was reduced, facial twitching returned. The neurological deficits, including the abnormal aggressive behaviour, persisted despite medical therapy. After 4 days of treatment, the owner elected euthanasia.
A general necropsy was performed, with no gross abnormalities observed. The brain was fixed in 10 % formalin and coronal sections (3 µm) were stained with haematoxylin and eosin (H&E). Selected slides were stained for glial acidic fibrillary protein (GFAP). A moderately demarcated, densely cellular, neoplastic mass was found in the right pyriform lobe (Figure 4). The neoplastic cells were uniformly round, surrounded by a clear halo (‘honeycomb’ appearance). Mitotic figures were less than 1 per 10 high-power fields. The mass was consistent with an oligodendroglioma. Ischaemic cell change affected neurons in the left hippocampus (Figure 5), accompanied by vascular hyperplasia and proliferation of microglia. Early ischaemic changes were also present in the right hippocampus. GFAP identified reactive and normal astrocytes, but was immunonegative in neoplastic cells. Retrospectively, the asymmetric T2-hyperintensity and ill-defined T1-hypointensity of the right pyriform lobe on MRI corresponded with the histopathological detected oligodendroglioma.
Figure 4.
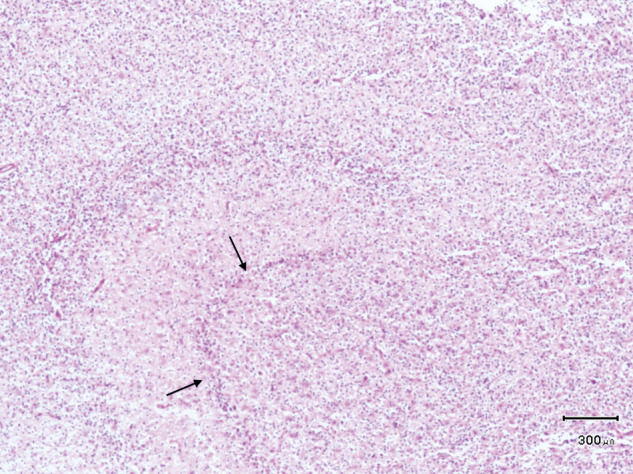
Histological section of the right hippocampus, showing marked infiltration of the grey and white matter with large numbers of neoplastic oligodendrocytes. The remaining hippocampus is hardly visible (arrows) (H&E)
Figure 5.
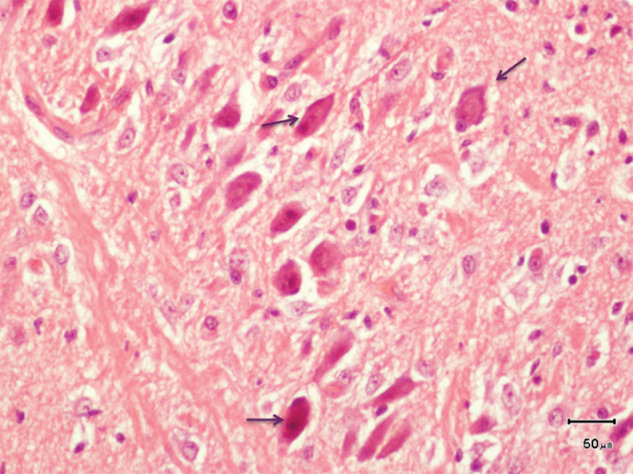
Histological section of the left hippocampus, showing shrunken neuron cell bodies (arrows) with brightly eosinophilic cytoplasm and angular cytoplasmic margins (H&E)
This report describes a cat with classical clinical signs and typical imaging characteristics of so-called FHN. Previously, FHN has been recognised as a disease entity by itself. However, in this case, an oligodendroglioma in the right pyriform lobe was found and believed to be the trigger of the hippocampal necrosis.
Like the Pakozdy,3,4 we believe that this case report and previous descriptions of FHN are strikingly similar with ‘temporal lobe epilepsy’ (TLE) in humans.1–5 Similar signs to FHN have been reproduced experimentally by injection of kainic injection in the hippocampus of cats. 6 As in cats, TLE manifests typically as complex partial seizures, characterised by sensations of fear or rage, and oro-alimentary automatism. 7 Additionally, a large set of autonomic signs has been reported to occur in TLE, such as mydriasis, hypersalivation, heart rate changes, hyperventilation and, rarely, coughing. 8 Of note, coughing preceded the partial complex seizures in this cat.
In humans TLE is classified in three categories depending of the site of origin of the seizure focus in the temporal lobe.9,10 In ‘mesial TLE’, also called ‘hippocampal TLE’, seizures originate from the medial part of the temporal lobe, primarily in the hippocampus.9,10 Mesial TLE is the most common form of TLE and is characterised by hippocampal changes on MRI and neuronal cell loss on histopathology. In most human cases of mesial TLE, a structural lesion within the brain cannot be identified. 9 In early-onset TLE an association with febrile seizures in childhood or birth trauma has been reported. 9 In adult-onset mesial TLE an immune-mediated mechanism (limbic encephalitis) is proposed. 11 On the contrary, in ‘lateral temporal neocortical epilepsy’, also sometimes called ‘extra-hippocampal TLE’, seizures originate from a structural lesion within the lateral temporal neocortex.9,10 Hippocampal changes are lacking. Finally, a third category, called ‘dual pathology’, is defined as an extrahippocampal structural lesion within the temporal lobe (eg, neoplasia) in addition to hippocampal changes.12,13
In order to differentiate these three categories further, neuro-imaging or pathology is needed. Indeed, neither clinical features nor scalp-electroencephalography (EEG) have been proven to differentiate epilepsy arising from the lateral temporal cortex or from the hippocampus in an individual human patient.9,10 Seizures beginning in either the medial or lateral temporal areas often spread to involve both.
In this case, the MRI was characterised by bilateral symmetrical T2- and FLAIR-hyperintensity of the pyriform lobes and both hippocampi. Findings were identical to previous reports of FHN and strikingly similar to humans with the mesial form of TLE.2,14 In contrast to previous cases of FHN in which hippocampal necrosis was the only detected abnormality,1–5 an oligodendroglioma was found retrospectively and confirmed by histopathology in the right pyriform lobe. Therefore, we believe that this case should be classified within the dual pathology group, contrary to previous reports of FHN, which are comparable with (late-onset) mesial TLE. 3 Similar to previous reports of FHN neuronal degeneration gliosis was observed in both hippocampi.1,2,5
In humans there is controversy over whether the hippocampal sclerosis is really the ‘cause’ or ‘consequence’ of seizures. 15 Several imaging and pathological studies in humans and dogs have shown that prolonged seizures may cause hippocampal damage.16–20 The causes of the selective vulnerability of the hippocampus (pathoclisis) have been thought historically to be caused by repeated ischaemia provoked during seizure attacks. 21 However, the pathogenesis is far more complex and still the focus of intense research. 21 Today, it is concluded that hippocampal sclerosis is, presumably, both the cause and effect of seizures.
In this case, the presence of an extra-hippocampal structural lesion, ie, the oligodendroglioma in the right pyriform lobe, strongly suggests an extrahippocampal ictal origin of the temporal lobe seizures. 10 We hypothesise that ‘kindling’ of the right hippocampus by the adjacent temporal lobe lesion resulted in the seizure spreading to the other side. 22 Of note, in humans unilateral clonic movements of the face and legs and hemiparesis indicate a lesion contralateral to the signs. 23 This strengthens the theory that the seizure focus in this case was located in the right temporal lobe. Moreover, some reports associate tachycardia, cough and hypersalivation with a trend toward the right-sided temporal lobe.24–26 However, these symptoms can potentially be a manifestation of ictal spread patterns rather than of the trigger zones.22,27,28
So far, the prognosis has been considered poor for FHN.1,2,5 However, recent evidence suggests that if treatment is sufficiently long persevered, clinical recovery might occur.3,4 Seizures associated with TLE in humans are also typically resistant to antiepileptic drugs, similar to the case presented, but can be abolished by surgical treatment. Patients with dual pathology undergo resection of the hippocampus and the lesion. 29 In future, when EEG and/or functional imaging techniques (single-photon emission computed tomography) become more widely available in veterinary medicine to map the seizure focus, temporal lobectomy should probably be considered in medical refractory cases of FHN, similar to humans.30–33
We believe that the description of this one case contributes to, and may change significantly, our current understanding of FHN in cats. The existence of TLE in dogs and cats has been questioned in the past. But, the striking similarities of previous reports of FHN and this case report with mesial TLE and dual pathology, respectively, suggest strongly that the human classification also applies to animals.3,34–37 Moreover, this case report also shows that a careful search for an associated brain lesion is indicated when clinicians are presented with a cat with typical clinical and imaging signs of FHN.
Footnotes
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors declare that there is no conflict of interest.
Accepted: 16 June 2012
References
- 1. Fatzer R, Gandini G, Jaggy A, et al. Necrosis of hippocampus and piriform lobe in 38 domestic cats with seizures: a retrospective study on clinical and pathologic findings. J Vet Intern Med 2000; 14: 100–104. [DOI] [PubMed] [Google Scholar]
- 2. Schmied O, Scharf G, Hilbe M, et al. Magnetic resonance imaging of feline hippocampal necrosis. Vet Radiol Ultrasound 2008; 49: 343–349. [DOI] [PubMed] [Google Scholar]
- 3. Pakozdy A, Gruber A, Kneissl S, et al. Complex partial cluster seizures in cats with orofacial involvement. J Feline Med Surg 2011; 13: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pakozdy A, Leschnik M, Sarchahi AA, et al. Clinical comparison of primary versus secondary epilepsy in 125 cats. J Feline Med Surg 2010; 12: 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brini E, Gandini G, Crescio I, et al. Necrosis of hippocampus and piriform lobe: clinical and neuropathological findings in two Italian cats. J Feline Med Surg 2004; 6: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka T, Tanaka S, Fujita T, et al. Experimental complex partial seizures induced by a microinjection of kainic acid into limbic structures. Prog Neurobiol 1992; 38: 317–334. [DOI] [PubMed] [Google Scholar]
- 7. Engel J., Jr. Introduction to temporal lobe epilepsy. Epilepsy Res 1996; 26: 141–150. [DOI] [PubMed] [Google Scholar]
- 8. Janszky J, Fogarasi A, Toth V, et al. Peri-ictal vegetative symptoms in temporal lobe epilepsy. Epilepsy Behav 2007; 11: 125–129. [DOI] [PubMed] [Google Scholar]
- 9. O’Brien TJ, Kilpatrick C, Murrie V, et al. Temporal lobe epilepsy caused by mesial temporal sclerosis and temporal neocortical lesions. A clinical and electroencephalographic study of 46 pathologically proven cases. Brain 1996; 119: 2133–2141. [DOI] [PubMed] [Google Scholar]
- 10. Gil-Nagel A, Risinger MW. Ictal semiology in hippocampal versus extrahippocampal temporal lobe epilepsy. Brain 1997; 120: 183–192. [DOI] [PubMed] [Google Scholar]
- 11. Bien CG, Elger CE. Limbic encephalitis: a cause of temporal lobe epilepsy with onset in adult life. Epilepsy Behav 2007; 10: 529–538. [DOI] [PubMed] [Google Scholar]
- 12. Levesque MF, Nakasato N, Vinters HV, Babb TL. Surgical treatment of limbic epilepsy associated with extrahippocampal lesions: the problem of dual pathology. J Neurosurg 1991; 75: 364–370. [DOI] [PubMed] [Google Scholar]
- 13. Zentner J, Hufnagel A, Wolf HK, et al. Surgical treatment of temporal lobe epilepsy: clinical, radiological, and histopathological findings in 178 patients. J Neurol Neurosurg Psychiatry 1995; 58: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meiners LC, van Gils A, Jansen GH, et al. Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. Am J Neuroradiol 1994; 15: 1547–1555. [PMC free article] [PubMed] [Google Scholar]
- 15. Jefferys JG. Hippocampal sclerosis and temporal lobe epilepsy: cause or consequence? Brain 1999; 122: 1007–1008. [DOI] [PubMed] [Google Scholar]
- 16. Mellema LM, Koblik PD, Kortz GD, et al. Reversible magnetic resonance imaging abnormalities in dogs following seizures. Vet Radiol Ultrasound 1999; 40: 588–595. [DOI] [PubMed] [Google Scholar]
- 17. Chan S, Chin SS, Kartha K, et al. Reversible signal abnormalities in the hippocampus and neocortex after prolonged seizures. Am J Neuroradiol 1996; 17: 1725–1731. [PMC free article] [PubMed] [Google Scholar]
- 18. Yamasaki H, Furuoka H, Takechi M, Itakura C. Neuronal loss and gliosis in limbic system in an epileptic dog. Vet Pathol 1991; 28: 540–542. [DOI] [PubMed] [Google Scholar]
- 19. Montgomery DL, Lee AC. Brain damage in the epileptic Beagle dog. Vet Pathol 1983; 20: 160–169. [DOI] [PubMed] [Google Scholar]
- 20. Palmer AC. Pathological changes in brain associated with fits in dogs. Vet Rec 1972; 90: 167–173. [DOI] [PubMed] [Google Scholar]
- 21. Thom M. Hippocampal sclerosis: Progress since Sommer. Brain Path 2009; 19: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertram EH. Temporal lobe epilepsy: where do the seizures really begin? Epilepsy Behav 2009; 14 (Suppl 1): 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marks WJ, Jr., Laxer KD. Semiology of temporal lobe seizures: value in lateralizing the seizure focus. Epilepsia 1998; 39: 721–726. [DOI] [PubMed] [Google Scholar]
- 24. Shah J, Zhai H, Fuerst D, Watson C. Hypersalivation in temporal lobe epilepsy. Epilepsia 2006; 47: 644–651. [DOI] [PubMed] [Google Scholar]
- 25. Wennberg R. Postictal coughing and noserubbing coexist in temporal lobe epilepsy. Neurology 2001; 56: 133–134. [DOI] [PubMed] [Google Scholar]
- 26. Mayer H, Benninger F, Urak L, et al. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 2004; 63: 324–328. [DOI] [PubMed] [Google Scholar]
- 27. Garcia M, D’Giano C, Estelles S, et al. Ictal tachycardia: its discriminating potential between temporal and extratemporal seizure foci. Seizure 2001; 10: 415–419. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann JM, Elger CE, Kleefuss-Lie AA. The localizing value of hypersalivation and postictal coughing in temporal lobe epilepsy. Epilepsy Res 2009; 87: 144–147. [DOI] [PubMed] [Google Scholar]
- 29. Li LM, Cendes F, Andermann F, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain 1999; 122: 799–805. [DOI] [PubMed] [Google Scholar]
- 30. Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia 2008; 49: 1296–1307. [DOI] [PubMed] [Google Scholar]
- 31. So EL. Integration of EEG, MRI, and SPECT in localizing the seizure focus for epilepsy surgery. Epilepsia 2000; 41 (Suppl 3): S48–S54. [DOI] [PubMed] [Google Scholar]
- 32. Martle V, Peremans K, Audenaert K, et al. Regional brain perfusion in epileptic dogs evaluated by technetium-99m-ethyl cysteinate dimer SPECT. Vet Radiol Ultrasound 2009; 50: 655–659. [DOI] [PubMed] [Google Scholar]
- 33. Jeserevics J, Viitmaa R, Cizinauskas S, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med 2007; 21: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 34. Kuwabara T, Hasegawa D, Kobayashi M, et al. Clinical magnetic resonance volumetry of the hippocampus in 58 epileptic dogs. Vet Radiol Ultrasound 51: 485–490. [DOI] [PubMed] [Google Scholar]
- 35. Holland CT. Successful long term treatment of a dog with psychomotor seizures using carbamazepine. Aust Vet J 1988; 65: 389–392. [DOI] [PubMed] [Google Scholar]
- 36. Kuwabara T, Hasegawa D, Ogawa F, et al. A familial spontaneous epileptic feline strain: a novel model of idiopathic/genetic epilepsy. Epilepsy Res 92: 85–88. [DOI] [PubMed] [Google Scholar]
- 37. Chandler K. Canine epilepsy: what can we learn from human seizure disorders? Vet J 2006; 172: 207–217. [DOI] [PubMed] [Google Scholar]