Abstract
A 4-year-old castrated male Russian Blue cat was evaluated for acute right hind limb lameness 18 months after receiving a renal transplant. Radiographs showed a subluxated right femoral head and lysis of the acetabulum and femoral neck. A femoral head and neck ostectomy was performed on the right coxofemoral joint. Histologic evaluation of the right femoral head revealed lesions indicative of a chronic, granulomatous osteomyelitis and periostitis associated with an intralesional Mycobacterium species. However, the cat’s clinical condition declined despite treatment and the owner elected humane euthanasia. All renal transplant recipients receive immunosuppressive therapy to prevent allograft rejection. The non-tuberculous mycobacterial infection of the coxofemoral joint was thought to develop secondary to long-term immunosuppressive treatment. This report illustrates the need to consider these rare opportunistic infections even many months to years following renal transplantation. Early awareness, stringent immunosuppressive drug monitoring and targeted treatment once a diagnosis has been made may be important in the successful management and prevention of mycobacterial infections in this population of patients.
Case Report
A 4-year-old castrated male Russian Blue cat was referred to Matthew J Ryan Veterinary Hospital at the University of Pennsylvania as a candidate for renal transplantation. Prior to transplantation, bloodwork revealed an elevated blood urea nitrogen [(BUN) 70 mg/dl; reference interval (RI) 15–32 mg/dl], creatinine [(Creat) 4.9 mg/dl; RI 1–2 mg/dl], phosphorus [(Ph) 7.8 mg/dl; RI 3.0–6.6 mg/dl], anemia (19%; RI 31–48%) and thrombocytopenia (32.8 x 103/μl, RI 200–500 x 103/μl). Because of the anemia and thrombocytopenia, a bone marrow aspirate was performed, which was normal. Urine specific gravity was 1.016 with no bacterial growth and a normal urine protein:creatinine ratio. Cardiac evaluation revealed a grade III/VI systolic murmur and a hyperdynamic left ventricular enlargement with mild mitral regurgitation. Abdominal ultrasound showed small polycystic kidneys. All other diagnostics [thoracic/abdominal radiography, feline leukemia virus (FeLV), feline immunodeficiency virus (FIV) and toxoplasmosis titer] were normal. Renal transplantation was performed according to a technique described previously.1,2 The azotemia resolved within 24 h postoperatively. The cat was discharged from the hospital after 4 days.
Immunosuppressive therapy included oral cyclosporine (CyA; Neoral, Novartis Pharmaceutical Corporation (2–5 mg/kg q12h) and prednisolone (Watson Laboratories) (0.5 mg/kg q12h). Cyclosporine administration was initiated 72 h prior to surgery and prednisolone administration was started the morning of surgery. The cyclosporine dose was adjusted to maintain a 12 h trough blood level of 300–500 ng/ml using high-performance liquid chromatography (HPLC) or 300–800 ng/ml using monoclonal specific radioimmunoassay (mRIA-sp). The prednisolone dosage was decreased to 0.5 mg/kg q24h at 6 months after transplantation. Five months after surgery, cyclosporine levels were elevated (1161 ng/ml; mRIA-sp). This corresponded to the owner changing the cyclosporine brand from Neoral to Cyclosporine Oral Solution, USP (Sidmak Laboratories). Although the dose was decreased from 5.6 mg/kg q12h to 0.4 mg/kg q12h, the cyclosporine level remained elevated (>1500 ng/ml; HPLC). The oral dose for Cyclosporine was again decreased (0.4 mg/kg q24h) and the levels became sub-therapeutic (92 ng/ml; HPLC). At 8.5 months following transplantation, bloodwork revealed anemia (packed cell volume 23.4%), thrombocytopenia (55 × 103/μl; platelet clumping present), mild leukocytosis (18.5 × 103/μl, RI 3.5–18 × 103/μl), hyperproteinemia (9.9 g/dl; RI 5.2–8.8 g/dl) with hyperglobulinemia (6.7 g/dl; RI 2.3–5.3 g/dl)) and normal BUN and Creat. A Toxoplasma gondii IgG and IgM antibody test was negative. Abdominal ultrasound showed changes consistent with chronic renal disease in the native kidneys and a normal renal allograft. At this time, the owner changed the cyclosporine brand back to Neoral (2 mg/kg q12h) and the cyclosporine levels stabilized within the therapeutic range. Although the cat was stable clinically, bloodwork performed 11 months postoperatively revealed persistent hyperproteinemia, hyperglobulinemia, hypernatremia and recurrence of azotemia. No diagnostics were pursued at this time.
Eighteen months after transplantation, the owner noted that the cat developed an acute right hind limb lameness. An orthopedic examination revealed muscle atrophy of the right thigh, and pain and crepitus on palpation of the right hip. Because the cat was fractious, pelvic radiographs were scheduled with general anesthesia.
Ten days prior to the scheduled radiographs and possible surgery, the cat became acutely lethargic, anorexic and was febrile. Bloodwork showed anemia (28.6%), severe leukocytosis (56.1 × 103/μl) with left shift (54% neutrophils, 8% lymphocytes, 28% bands), thrombocytopenia (126 × 103/μl) and azotemia (BUN 45.5 mg/dl; Creat 2.7 mg/dl). The urine specific gravity was 1.017 with a negative culture. The cat was discharged by the referring veterinarian on oral antibiotic therapy [enrofloxacin (Baytril; Bayer Healthcare) 5 mg/kg q24h and amoxicillin and clavulanic acid (Clavamox; Pfizer Animal Health) 13.75 mg/kg q12h]. Following 4 days of antibiotic therapy, the leukocytosis improved (27.7 × 103 μl; 8% bands) and the cat seemed brighter.
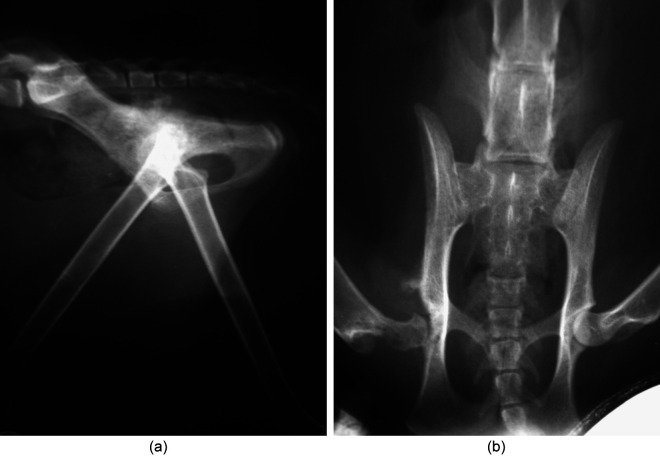
Pre-operatively, pelvic radiographs showed a subluxated right femoral head, lysis of the acetabulum and femoral neck most consistent with osteomyelitis or neoplasia (Figure 1). Thoracic radiographs were unremarkable. At surgery, the right coxofemoral joint capsule appeared thickened, irregular, friable and caseous. A femoral head and neck ostectomy was performed without complications. Bloodwork 1 week later revealed only an anemia (22.9%) and elevated BUN (49 mg/dl); however, the cat deteriorated clinically and the owner elected humane euthanasia with no necropsy.
Figure 1.
Lateral view (a) and (b) ventrodorsal pelvic radiograph obtained prior to femoral head and neck ostectomy. Notice the right subluxated femoral head, lysis of the acetabulum and femoral neck most consistent with osteomyelitis or neoplasia
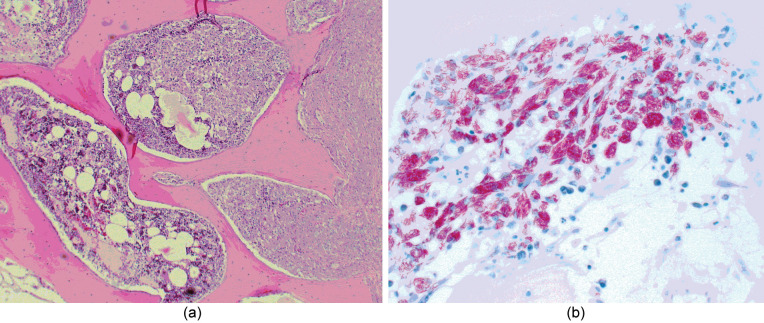
Cultures from the coxofemoral joint revealed Gram-positive cocci (Staphylococcus epidermis) and no anaerobic growth. Histopathology of the femoral head revealed severe, chronic, granulomatous osteomyelitis (Figure 2). Within the thickened periosteum and areas of bone lysis were diffuse sheets of epithelioid macrophages and neutrophils filled with a granular material suggestive of a mycobacterial infection. Acid-fast staining revealed many mycobacterial organisms packed within the macrophages (Figure 2).
Figure 2.
(a) Photomicrograph of a histological section from the femoral head revealing severe, chronic, granulomatous osteomyelitis and periostitis. Wright Giemsa stain. 4x (b) Photomicrograph of the same area showing a large numbers of macrophages packed with acid-fast filamentous bacteria. Ziehl-Neelsen stain, 20 x
As renal transplantation has become a more common therapeutic of end-stage renal failure in cats, stringent patient selection and improved postoperative management, including effective immunosuppressive therapy, have resulted in improved success rates.3,4 Although adequate immunosuppression is needed to maintain function of the renal allograft and prevent rejection, it can also lead to a higher risk of developing serious infectious complications.5–9
In humans receiving a renal transplant, infection remains one of the major causes of morbidity (15–44%) and mortality (<5%)10,11 and this has not declined in recent years.7,11 During the first month following surgery, infections result most commonly from nosocomial pathogens; 7 2–6 months following surgery, opportunistic infections are most common; after 6 months, community infections, including pulmonary infections, are more prevalent. 5
In the feline renal transplant recipient, postoperative infections have been reported to occur in 25% (43/169) and 36.7% (22/60) of patients in two recent retrospective studies.4,12 During the first year following transplantation, the most common cause of death was acute or chronic transplant rejection, followed by infection.4,12 Death associated with these infections was higher than that of the human equivalent at 14% (17/122). 12 In the same retrospective study, the most common infections were bacterial 53% (25/47), followed by viral 28% (13/47), fungal 13% (6/47) and protozoal 6.4% (3/47). Of these bacterial infections, 5/25 infections were mycobacterial infections determined by histological studies and of these, two were disseminated infections. 12
Mycobacterial organisms are aerobic, non-spore-forming, non-motile bacteria that are classified typically into three clinical syndromes in cats: classic tuberculosis, feline leprosy and atypical mycobacteria. 13 There are few clinical reports describing mycobacterial infections in cats,14–20 and only one other published report in a renal transplant patient. 21 In this report, Griffin et al describe a feline renal transplant patient that acquired a disseminated Mycobacterium avium complex infection 18 months after surgery. Similar to the patient described in this report, the cat received cyclosporine and prednisolone for immunosuppression to prevent allograft rejection. This non-tuberculous mycobacterial infection was though to develop secondary to long-term immunosuppressive treatment. These rare opportunistic infections should be considered months-to-years following renal transplantation.
Mycobacterial infections in human patients are variable with exposure history and area of endemicity. In solid-organ transplant recipients, the infection rate is 0.35–15% worldwide for Mycobacterium tuberculosis 22 and 0.16–2.8% for non-tuberculosis mycobacterial infections in the USA. 23 Non-tuberculosis mycobacterial infections in renal transplant patients due to Mycobacterium kansaii, Mycobacterium chelonae, Mycobacterium fortuitum and Mycobacterium haemophilium have been reported and usually present as cutaneous lesions of the extremities and, less commonly, tenosynovitis and arthritis. These infections of the bone and joints are extremely rare and involve patients given corticosteroids, organ transplantations or those with autoimmune disorders. 24 In the case of bone and joint infections, the source of the non-tuberculosis mycobacterial infection is most likely due to trauma, surgical incisions, puncture wounds, injections or hematogenous spread, 25 though no identifiable origin of infection is found in most cases. There are very few reported cases involving joint or bone infections in human renal transplant patients. In one report, multifocal spinal and extraspinal osteomyelitis developed after 15 years of postoperative immunosuppression secondary to M chelonae. 24 This is the first reported case of localized mycobacterial osteomyelitis infection in a feline renal transplant patient and occurring after a period of intermittent excessive immunosuppression over 8–10 months. Because there is no reported history of trauma, puncture wounds or injections, hematological spread or latent infection may be a more likely cause of the infection in this cat.
Current immunosuppression used in the cat consists of cyclosporine in combination with prednisolone. CyA inhibits calcineurin, which is responsible for normally activation of the transcription of interleukin (IL)-2 and leads to a reduced function of T-effector cells. To what extent the disturbance of cell-mediated immunity contributed to this cat’s infection is unclear, but both steroids and CyA have been reported previously to exhibit immunomodulatory effects and inhibit T-cell activation in cats. 9 The cytokine interferon(IFN)-γ is important in acquired immunity to defend against mycobacterial infections. A study showing the addition of CyA, as well as the combination of CyA plus dexamethasone, to in vitro cultures of feline lymphocytes resulted in significant suppression of the production of many cytokines, including IFN-γ. 26 Recently, a disseminated M avium complex infection was described in a human patient with auto-antibody to IFN-γ. 27 It is unclear whether better control of CyA blood levels would decrease the incidence of opportunistic infections, as many infectious complications have been identified in feline patients with CyA levels maintained within therapeutic range (personal communication). 12 Because this treatment modality impairs host defense mechanisms allowing opportunistic infections, current research in the human and veterinary field focuses on investigating novel immunosuppressive drugs that are more specific in their mechanisms of action, resulting in less global immunosuppression. 9
Antimicrobial therapy combined with surgical correction was chosen for the treatment of the focal mycobacterial osteomyelitis. Though the special stains on histopathology of the coxofemoral joint revealed mycobacteria, the exact species defined via polymerase chain reaction followed by restriction analysis and nucleic hybridization would give additional insight into management and prevention of these cases. 13 Furthermore, growing the organism in a culture to obtain the sensitivity may also give insight into treatment options, as some mycobacterial antibiotic treatments may also interfere with cyclosporine levels. In the future, earlier awareness, more aggressive and targeted treatment, and more stringent immunosuppressive drug regimen monitoring may be very important in successful management and prevention of mycobacterial infections of renal transplant patients.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Accepted: 12 June 2012
References
- 1. Gregory CR, Bernsteen L. Organ transplantation in clinical veterinary practice. In: Slatter D. (ed). Textbook of small animal surgery. 3rd ed, vol 1. Philadelphia, PA: WB Saunders, 2003, pp 122–136. [Google Scholar]
- 2. Bernsteen L, Gregory CR, Pollard RE, et al. Comparison of two surgical techniques for renal transplantation in cats. Vet Surg 1999; 28: 417–420. [DOI] [PubMed] [Google Scholar]
- 3. Mathews KG, Gregory CR. Renal transplants in cats: 66 cases (1987–1996). J Am Vet Med Assoc 1997; 211: 1432–1436. [PubMed] [Google Scholar]
- 4. Schmiedt CW, Holzman G, Schwarz T, McAnulty JF. Survival, complications, and analysis of risk factors after renal transplantation in cats. Vet Surg 2008; 37: 683–695. [DOI] [PubMed] [Google Scholar]
- 5. Splendiani G, Cipriani S, Tisone G, et al. Infectious complications in renal transplant recipients. Transplant Proc 2005; 37: 2497–2499. [DOI] [PubMed] [Google Scholar]
- 6. Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009; 69: 2227–2743. [DOI] [PubMed] [Google Scholar]
- 7. Silkensen J. Long-term complications in renal transplantation. J Am Soc Nephrol 2000; 11: 582–588. [DOI] [PubMed] [Google Scholar]
- 8. Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients-an analysis of USRDS data. Am J Transplant 2007; 7: 653–661. [DOI] [PubMed] [Google Scholar]
- 9. Aronson LR, Drobatz KJ, Hunter CA, Mason N. Effects of CD28 blockade on subsets of naïve T cells in cats. Am J Vet Res 2005; 66: 483–492. [DOI] [PubMed] [Google Scholar]
- 10. Sia IG, Paya CV. Infectious complications following renal transplantation. Surg Clin North Am 1998; 78: 95–112. [DOI] [PubMed] [Google Scholar]
- 11. Lim W, Chadban S, Campbell S, et al. Effect of interleukin-2 receptor antibody therapy on acute rejection risk and severity, long-term renal function, infection and malignancy-related mortality in renal transplant recipients. Transpl Int 2010; 12: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 12. Kadar E, Sykes JE, Kass PH, et al. Evaluation of the prevalence of infections in cats after renal transplantation: 169 cases (1987–2003). J Am Vet Med Assoc 2005; 227: 948–953. [DOI] [PubMed] [Google Scholar]
- 13. Greene C, Gunn-Moore D. Mycobacterial infections. In: Greene CE, CE (ed). Infectious diseases of the dog and cat. 3rd ed. Philadelphia, PA: WB Saunders, 2006, pp 462–477. [Google Scholar]
- 14. Knippel A, Hetzel U, Baumgärtner W. Disseminated Mycobacterium avium-intracellulare infection in a Persian cat. J Vet Med B Infect Dis Vet Public Health 2004; 51: 464–466. [DOI] [PubMed] [Google Scholar]
- 15. Horne KS, Kunkle GA. Clinical outcome of cutaneous rapidly growing mycobacterial infections in cats in the south-eastern United States: a review of 10 cases (1996–2006). J Feline Med Surg 2009; 11: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beccati M, Peano A, Gallo MG. Pyogranulomatous panniculitis caused by Mycobacterium alvei in a cat. J Small Anim Pract 2007; 48: 664. [DOI] [PubMed] [Google Scholar]
- 17. Couto SS, Artacho CA. Mycobacterium fortuitum pneumonia in a cat and the role of lipid in the pathogenesis of atypical mycobacterial infections. Vet Pathol 2007; 44: 543–546. [DOI] [PubMed] [Google Scholar]
- 18. Sieber-Ruckstuhl NS, Sessions JK, Sanchez S, et al. Long-term cure of disseminated Mycobacterium avium infection in a cat. Vet Rec 2007; 160: 131–132. [DOI] [PubMed] [Google Scholar]
- 19. Baral RM, Metcalfe SS, Krockenberger MB, et al. Disseminated Mycobacterium avium infection in young cats: overrepresentation of Abyssinian cats. J Feline Med Surg 2006; 8: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barry M, Taylor J, Woods JP. Disseminated Mycobacterium avium infection in a cat. Can Vet J 2002; 43: 369–371. [PMC free article] [PubMed] [Google Scholar]
- 21. Griffin A, Newton AL, Aronson LR, et al. Disseminated Mycobacterium avium complex infection following renal transplantation in a cat. J Am Vet Med Assoc 2003; 222: 1097–1101, 1077–1078. [DOI] [PubMed] [Google Scholar]
- 22. Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis 2008; 10L 358–363. [DOI] [PubMed] [Google Scholar]
- 23. Doucette K, Fishman JA. Non-tuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis 2004; 38: 1428–1439. [DOI] [PubMed] [Google Scholar]
- 24. Korres DS, Papagelopoulos PJ, Zahos KA, et al. Multifocal spinal and extra-spinal Mycobacterium chelonae osteomyelitis in a renal transplant recipient. Transpl Infect Dis 2007; 9: 62–65. [DOI] [PubMed] [Google Scholar]
- 25. Nagy GS, Rubin RH. Disseminated Mycobacterium avium-intracellulare in a kidney transplant recipient. Transpl Infect Dis 2001; 3: 220–230. [DOI] [PubMed] [Google Scholar]
- 26. Aronson LR, Stumhofer JS, Drobatz KJ, Hunter CA. Affect of cyclosporine, dexamethasone and (hu)CTLA4-Ig on production of cytokines in normal cats and those undergoing renal transplantation. Am J Vet Res 2011; 4: 541–549. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka Y, Hori T, Ito K, et al. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-gamma. Intern Med 2007; 46: 1005–1009. [DOI] [PubMed] [Google Scholar]