Abstract
Ovarian remnant syndrome (ORS) is the presence of functional ovarian tissue with signs of oestrus as a complication after ovariohysterectomy (OHE) or ovariectomy. Stump pyometra is another complication that can be observed after OHE. However, there are few reports about ORS and stump pyometra in queens. In this report, three queens with recurrent oestrous behaviours after OHE are described. In two queens, ORS with stump pyometra was diagnosed and in one queen ORS alone was diagnosed by physical examination, medical history, vaginal cytology and ultrasonography. Remnant ovarian and uterine tissues were removed by laparotomy. Two queens recovered without any complications; however, one queen died 2 days after surgery. This study reveals that ORS and stump pyometra can result in severe disease and can be fatal.
Introduction
Spaying is performed to avoid over-population of unwanted queens and to eliminate unwanted behaviours associated with oestrus. Spaying is also recommended to reduce the risk of pathological conditions of the reproductive organs. 1 Thus, spaying is the most common conservative and medical procedure in queens. 2 However, some complications may develop after ovariohysterectomy (OHE), such as ovarian remnant syndrome (ORS). This syndrome may develop because of the failure to totally remove both ovaries (most commonly the right ovary) at OHE, or the presence of a partial or complete separation of a portion of normal ovary (the fragment may be located near the ovary or in the broad ligament) that is not detected at OHE.2–4 In some cases, uterine stump pyometra may occur because of ovarian remnants 3 and this situation may be fatal in affected queens. Worldwide, fatal complications occur as a result of surgical errors in routine OHE. In this article, we report and discuss the importance of ORS and also stump pyometra associated with ORS in queens.
Case 1
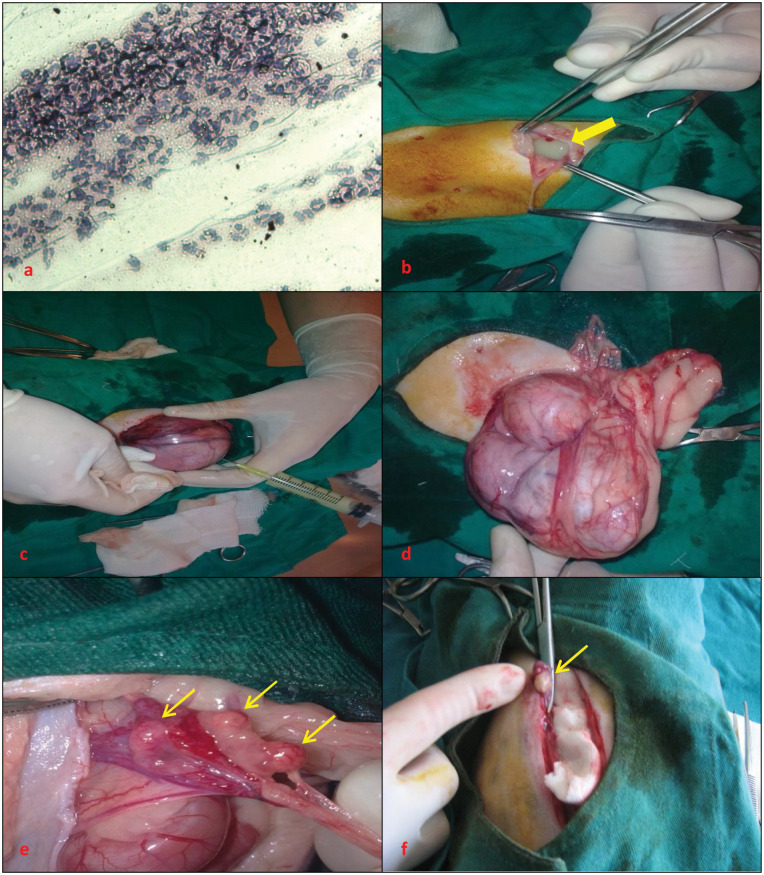
A 28-month-old spayed queen presented with a history of reduced appetite, lethargy and purulent vaginal discharge. OHE had been performed via a left flank approach about 18 months earlier. Reappearance of oestrous behaviours was noted 4 months before presentation. Vaginal cytology revealed abundant neutrophils and purulent debris consistent with stump pyometra, and cornified superficial cells consistent with the presence of oestrogen from remnant ovarian tissue (Figure 1a). Ultrasonographic examination revealed a ruptured stump pyometra on the basis of the presence of an echogenic (scattered in the form of snow flakes) structure, measuring 11.5 × 6.8 cm, and a small amount of free fluid in the abdominal cavity. Increased body temperature, increased heart and respiratory rate, and significant deviations of laboratory findings were found on physical and clinical examination (Table 1). Intravenous (IV) fluid (0.9 % NaCl and balanced electrolyte solution) administration and amoxicillin–clavulanic acid (Synulox 8.75 mg/kg/day, IM; Pfizer) therapy were started before surgery to improve the general condition of the queen. Surgery was performed to remove the remnant uterine tissue (Figure 1d) and completely flush the abdomen. Free purulent fluid was observed in the abdomen immediately after incision of the linea alba (Figure 1b). The uterine stump was visible after removal of the purulent fluid; however, the uterine stump contained purulent debris which was aspirated with a sterile syringe and needle (Figure 1c). Uterine stump tissue was enclosed in a gauze pad and surgically resected. Schmieden’s suture method with 3/0 polyglycolic acid sutures (Vicryl; Ethicon) was used for the remaining stump tissue. Cystic ovarian tissue was detected near the caudal pole of the right kidney (Figure 1e) and was resected. The ovarian and uterine stump tissues were not submitted for histopathology owing to the high cost. Finally, the abdominal cavity was flushed with warm 0.9 % NaCl solution. The queen died on day 2 after surgery, despite postoperative amoxicillin–clavulanic acid (Synulox 8.75 mg/kg/day, IM) and IV fluid administration.
Figure 1.
(a) Image of vaginal cytology in case 1. (b) Free purulent fluid (thick arrow) in the abdomen of case 1. (c) Aspirating the contents of the remnant uterine tissue in case 1. (d, e) Removal of remnant uterine and ovarian tissue (thin arrows) in case 1. (f) Removal of remnant ovarian tissue (thin arrow) in case 2
Table 1.
Values of pre-surgical vital, blood biochemical and haematological parameters before surgery in all cases
| Parameters | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Temperature (°C) | 40.6 | 38.3 | 38.1 |
| Heart rate (bpm) | 132 | 112 | 108 |
| Respiratory rate (breaths/min) | 26 | 22 | 20 |
| Erythrocyte (106 µl) | 4.5 | 6.0 | 6.5 |
| Leukocyte (103 µl) | 20.5 | 12 | 14 |
| Haematocrit (%) | 20 | 25 | 27 |
| Haemoglobin (g/dl) | 6 | 10 | 12 |
| Band neutrophil (%) | 21 | 2 | 3 |
| Segmented neutrophil (%) | 10 | 12.5 | 12 |
| Lymphocyte (%) | 1.5 | 4 | 7 |
| Monocyte (%) | 2 | 3 | 3 |
| Eosinophil (%) | 3 | 4 | 6 |
| Basophil (%) | 1 | 1 | 0 |
| ALT (IU/l) | 34 | 42 | 38 |
| AST (IU/l) | 35 | 25 | 22 |
| ALP (IU/l) | 38 | 22 | 18 |
| Creatinine (mg/dl) | 2.3 | 1.5 | 1.6 |
| BUN (mg/dl) | 38 | 26 | 20 |
ALT = alanine aminotransferase, AST = aspartate transaminase, ALP = alkaline phosphatase, BUN = blood urea nitrogen
Case 2
A 5-year-old spayed queen presented because of recurrent oestrous behaviour and aggression. OHE had been performed via a left flank approach about 4 years previously and oestrous behaviours had been observed 8 months after OHE. The cat had been treated previously (at another veterinary clinic) with a progesterone analogue three times after OHE in order to suppress signs of oestrus. Therefore, the name, dose or routes of progesterone analogue were unknown. Each treatment of progesterone was administered at an interval of 1 year. All examinations were carried out under sedation because of the aggressive nature of the queen. Vaginal cytology revealed the presence of cornified superficial epithelial cells, consistent with oestrus. A hyperechoic structure was found behind the right kidney on ultrasound examination. No evidence of an abnormal uterine stump was found. The queen’s vital parameters, complete blood cell (CBC) count and biochemical parameters were within normal limits (Table 1). A median ventral laparotomy revealed the presence of remnant ovarian tissue with cystic structures in the region of the right ovary. The ovarian tissue was resected (Figure 1f). Histopathology of the ovarian remnant tissue was not performed owing to the high cost. After surgery, the queen was treated with amoxicillin–clavulanic acid (Synulox 8.75 mg/kg/day, IM) for 5 days. The queen recovered well from surgery and, at the time of writing, has remained healthy without aggressive behaviour.
Case 3
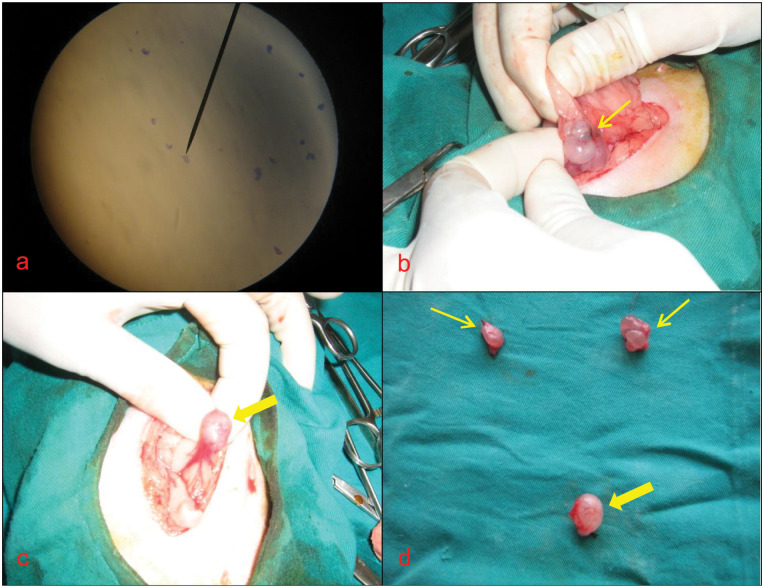
A 16-month-old spayed queen presented with a history of increased aggressiveness and mild signs of upper respiratory tract infection. The queen had been spayed via a left flank approach about 8 months previously. During the general examination, it was apparent the queen was displaying signs of oestrus. Evaluation of the medical history established that the signs of oestrus began 3 months after OHE. Vaginal cytology performed under sedation because of the queen’s aggressive nature revealed cornified superficial cells consistent with a source of oestrogen, such as an ovarian remnant (Figure 2a). The queen’s vital parameters, blood biochemical parameters and CBC count were within normal limits (Table 1). Abdominal ultrasound revealed the presence of an anechoic structure, measuring 0.7 × 1.1 cm, behind the bladder, as well as hypoechoic fields on the caudal aspects of both kidneys suggestive of ovarian structures. During exploratory laparotomy, two ovarian remnants were found on the caudal aspect of both kidneys (Figure 2b), and an enlarged uterine stump (Figure 2c) was found behind the bladder (Figure 2d). The ovarian remnants and uterine stump were resected. The ovarian and uterine stump tissues were not submitted for histopathology owing to the high cost. The queen was treated with amoxicillin–clavulanic acid (Synulox 8.75 mg/kg/day, IM) for 5 days after surgery and was discharged the following day without any complications. Follow-up revealed that the queen’s aggressiveness had resolved.
Figure 2.
Image of vaginal cytology (a) and remnant ovarian (thin arrows) tissue (b, d), and remnant uterine (thick arrow) tissue (c, d) in case 3
Discussion
The most common cause of behavioural signs of oestrus after OHE is remnant ovarian tissue that has regained follicular activity. Usually, this is due to errors in surgical technique, where parts of one or both of the ovaries have been left inside the abdomen. Whether entrapped in a ligature or accidentally dropped into the peritoneal cavity, revascularisation of remnants by the omental blood supply can occur. Remnant ovarian tissue can produce hormones and even ovulate. 5 If the incision is too small or the patient is obese, the surgeon may not have adequate visualisation of the ovaries, increasing the risk of ORS. 6 In the present study, the reasons for the development of ORS are similar to those found in other published studies. In case 1 and case 2, it was concluded that performing OHE via the left flank may have limited access to the right ovary, thereby increasing the risk that the ovary was incompletely resected. In a retrospective study in both queens and bitches, 5 unilateral remnants were found slightly more often on the right side, near the kidney, though nearly half the cases were bilateral. In one study, 6 and also in case 3 of this report, it is possible that bilateral ovarian remnants were related to a shorter incision and/or an incision that was placed too caudally. It is possible that OHE via the ventral midline approach may reduce the risk of ORS.
The behavioural signs of oestrus include increased vocalisation, lordosis or crouching, rolling, treading motions with the hind limbs, holding the tail to one side, being attractive to tomcats and allowing copulation.5–8 In this study, recurrence of signs of oestrus after OHE was noted by two of the owners. In the third case, aggressive behaviour was a presenting complaint. Vaginal discharge was the presenting complaint in only one of the two cases with stump pyometra. ORS was suspected after detailed medical history and clinical examination in all cases. Recurrent oestrous signs after OHE were accepted as normal behaviour by the owners, which delayed presentation. Therefore, it is important that the veterinarian informs owners of female cats that oestrous signs should disappear completely after OHE.
An experimental model of ORS was performed by excision of the ovarian cortex from its vascular supply and subsequent suturing of the cortex to the lateral abdominal wall. This remnant tissue caused recurrent signs of oestrus 4 months after the surgery and even after ligation of the ovarian artery. 3 The interval between OHE and the occurrence of signs of oestrus is highly variable.6,7,9 Signs of oestrus have been reported from a few weeks to several years after OHE.5,6,7,9 In the cases reported here, signs of oestrus occurred from 3 months to 8 months after OHE.
Uterine stump pyometra may be associated with ovarian remnants or with administration of progestagens.3,8,10 In one report, uterine stump pyometra was associated with the presence of functional ovarian luteal tissue, which represented the remnant of old corpora lutea. 5 The luteal tissue may have maintained endometrial hyperplasia and the secretory activity of the endometrial glands which allowed the persistence or recurrence of uterine infection. Other authors have reported pyometra in neutered queens without any remnant ovarian tissue.8,11 In this report, stump pyometra was associated with remnant ovarian tissue; this situation was similar to the findings of a recently published case. 8 In case 1 and case 3 of this report, the severity of stump pyometra may have been related to the duration between OHE and the occurrence of ORS signs . In case 2 (spayed 4 years previously), abnormal uterine tissue was not found despite the presence of remnant ovarian tissue and administration of exogenous progesterone. It is possible that the development of stump pyometra requires the presence of remnant ovarian tissue, as well as incomplete removal of the uterus below the bifurcation. 11
The diagnosis of ORS in queens is based on behavioural signs, vaginal cytology, hormone assays, ultrasonography and exploratory laparotomy.7,12 Applying only one or two of these parameters may support the presence of a remnant. 12 Vaginal cytology is a very important diagnostic test for ORS and it is the least expensive and easiest method. In an ovariectomized queen, vaginal cytology findings may support the presence of an ovarian remnant, although a smear with normal anoestrus characteristics (low cellularity and mainly small basal cells) does not rule out ORS.7,13 In one study, 5 vaginal cytology revealed many superficial epithelial cells as indicative of oestrogenic influence. In this report, the vaginal cytology findings in case 1 and case 2 were similar and supported the presence of remnant ovarian tissue. Remnant ovarian tissues and uterine stump tissues were seen on ultrasound examinations and were confirmed at laparotomy. Physical examination, vaginal cytology and ultrasound findings were suggestive of the presence of ovarian remnant tissues; therefore, blood hormone levels were not evaluated.
In one study, 5 the packed cell volume, serum total protein, blood glucose and blood urea nitrogen concentrations were within normal limits in a queen with ORS. In the present study, haematological and blood biochemical parameters were within normal reference intervals in case 2. ORS may not be expected to cause changes in haematological and blood biochemical parameters. In another study, 8 CBC count, biochemical parameters and total serum protein were within normal limits in a queen with stump pyometra. Haematological and blood biochemical parameters were within normal limits in case 3 of this report. In case 1, owing to the ruptured uterine remnant, haematological and blood biochemical parameters deviated from normal ranges. In case 1 the queen was in poor body condition and had a very large uterine stump, while, in case 3, the queen was in good general condition and had a small amount of uterine stump tissue. These findings and those of another published report 10 suggest that the size and condition of remnant uterine tissue affects haematological and biochemical blood parameters.
The treatment choices for ORSs are medical or surgical management. However, it is recommended that ovarian tissue be surgically removed. A thorough exploration of the peritoneal cavity is essential, checking the caudal poles of both kidneys, the omentum and the peritoneal walls, as these are commonly affected sites.4,5
In conclusion, remnant ovarian and uterine tissues may be due to surgical error and the outcome of stump pyometra may be fatal. Therefore, it is recommended that OHE should be performed via midline laparotomy with an incision of appropriate size (~1–3 cm). It should be explained to owners of female cats that the signs of oestrus will disappear completely after OHE. The owner should be informed about the signs of oestrus, such as vocalisation, lordosis or crouching, rolling, treading motions with the hindlimbs, holding the tail to one side and being attractive to tomcats. In case of occurrence of any of these signs after OHE, the owner should consult a veterinarian.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 17 May 2012
References
- 1. Howe LM. Surgical methods of contraception and sterilization. Theriogenology 2006; 66: 500–509. [DOI] [PubMed] [Google Scholar]
- 2. England GCW. Genital surgery in the bitch and queen cat. In: Noakes DE, Parkinson TJ, England GCW. (eds). Veterinary reproduction and obstetrics. 8th ed. Philadelphia, PA: WB Saunders, 2001, pp 367–380. [Google Scholar]
- 3. Johnston SD, Kustrıtz MVR, Olson PNS. Disorders of the feline ovaries. In: Johnston SD, Root Kustritz MV, Olson PNS. (eds.) Canine and feline theriogenology. 1st ed. Philadelphia, PA: Saunders, 2001, pp 453–462. [Google Scholar]
- 4. Little S. Feline reproduction. Problems and clinical challenges. J Feline Med Surg 2011; 13: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heffelfinger DJ. Ovarian remnant in a 2-year-old queen. Can Vet J 2006; 47: 165–167. [PMC free article] [PubMed] [Google Scholar]
- 6. Miller DM. Ovarian remnant syndrome in dogs and cats: 46 cases (1988–1992). J Vet Diagn Invest 1995; 7: 572–574. [DOI] [PubMed] [Google Scholar]
- 7. Wallace MS. The ovarian remnant syndrome in the bitch and queen. Vet Clin North Am 1991; 21: 501–507. [DOI] [PubMed] [Google Scholar]
- 8. Rota A, Pregel P, Cannizzo FT, Sereno A, Appino S. Unusual case of uterine stump pyometra in a cat. J Feline Med Surg 2011; 13: 448–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson CA. Reproductive system disorders. In: Nelson RW, Couto CG. (eds). Small animal internal medicine. 4th ed. St Louis, MO: Mosby, 2009, p 907. [Google Scholar]
- 10. Agudelo CF. Cystic endometrial hyperplasia–pyometra complex in cats. A review. Vet Quart 2005; 27: 173–182. [PubMed] [Google Scholar]
- 11. de Faria VP, Norsworthy GD. Pyometra in a 13-year-old neutered queen. J Feline Med Surg 2008; 10: 185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeNardo GA, Becker K, Brown NO, Dobbins S. Ovarian remnant syndrome – revascularization of free-floating ovarian tissue in the feline abdominal cavity. J Am Anim Hosp Assoc 2001; 37: 290–296. [DOI] [PubMed] [Google Scholar]
- 13. Pineda MH. Reproductive patterns of cats. In: Pineda MH, Dooley M. (eds). McDonald’s veterinary endocrinology and reproduction. 5th ed. Ames, IA: Iowa State Press, 2003, pp 505–522. [Google Scholar]