Abstract
A successful, euthyroid outcome after radioiodine therapy in hyperthyroid cats ranges from 83% to 95%. Thyroid volume has been reported as one of the factors influencing radioiodine therapy outcome in man and cats. The goal of this study was to describe the most reliable and practically applicable formula to determine thyroid volume using scintigraphy. The volume of each thyroid lobe of 32 hyperthyroid cats was determined by ultrasound and scintigraphy. The ultrasonographically determined volume (ellipsoid formula) for each thyroid lobe was compared with the scintigraphic volume that was calculated using eight different formulas: F1 [(π/6) × L × H × W], F2 [(π /2) × L × W2], F3 [0.33 × (area cm2)3/2], F4 [1.08 × (π /6) × L × W2], F5 (area × H), F6 (0.27 × area × L), F7 (π × L × W2) and F8 [π × (4/3) × W3]. F1, F3, F4 and F6 did not differ statistically from the volumes measured on ultrasound, while F2, F5, F7 and F8 did. Subjective shape assessment of the thyroid lobes, assigned as cylindrical or spherical, and the use of corresponding formulas, did not appear to be useful.
Introduction
Treatment of hyperthyroidism in cats with radioactive iodine is the therapy of choice whenever this technique is available and the patient is a suitable candidate. Medicinal and surgical treatment have considerable disadvantages, eg, adverse side effects, daily pilling, and the risk of anaesthesia in older cats and postsurgical complications.1,2 A disadvantage of radioactive iodine treatment is that it requires hospitalisation and specialised infrastructure. Whether a fixed dose, a scoring system or individual dose calculations using kinetic studies are applied seems to have no effect on the outcome. In most cases therapy success or failure is determined based on the combination of total T4 (TT4) blood concentration and the presence of clinical signs. Some patients show a TT4 value just outside the normal limit, but without any clinical signs of hypo- or hyperthyroidism. A euthyroid outcome varies in literature between 83% and 95%.3–10 The exact causes for therapy failure, either resulting in hypothyroidism or persistent hyperthyroidism, remain unclear to this day. In human medicine a large thyroid volume has been suggested to be one of the predisposing factors for persistent hyperthyroidism,11–14 although no consensus exists. 15 Extrapolation of data from human medicine has to be considered with care as the aetiology of hyperthyroidism in humans is different from that in cats. Furthermore, hypothyroidism is often accepted as a successful outcome in human medicine.11,13,15 The influence of thyroid volume on a hypothyroid outcome specifically has also been described in humans 16 and argued.17,18 An autoimmune cause has been proposed in the latter case. 17 Follow-up ultrasound volume estimation after radioiodine therapy has also been reported to be a reliable prognostic factor of therapy outcome in human medical literature. 12 In veterinary medicine, one study reported the possible influence of a large thyroid volume on persistent hyperthyroidism following radioiodine treatment in cats. 8 Determination of predisposing factors for therapy failure (persistent hyperthyroidism or hypothyroidism necessitating hormone replacement) may lead to new protocols and individual dose calculations to improve radioiodine therapy outcome. If thyroid volume influences therapy results, this may be a parameter to take into account. Calculation of thyroid volume on a planar scintigram could be incorporated easily in the existing diagnostic procedure prior to radioiodine therapy, requiring no additional handling of the patient. The aim of this study was to describe different formulas for scintigraphic thyroid volume measurement, based on the planar scintigraphic acquisition used for diagnosis of hyperthyroidism in cats and to determine the most applicable formula for thyroid volume. Because of its accessibility and non-invasiveness, ultrasound was chosen as the reference method.19,20
Materials and methods
Patients
Data from 32 cats presented to our clinic between October 2010 and October 2011 for a diagnostic 99mTc-pertechnetate (TcO4-) scan and radioiodine treatment were collected [mean age 12.2 years (range 8.9–17.6); 18 female/14 male]. The cats were diagnosed with hyperthyroidism by referring veterinarians based on clinical signs and serum TT4. Inclusion criteria were unilateral or bilateral disease where one lobe was obviously larger than the other one, and overlap of lobes on both ventral and lateral planar scan was not an issue. In this latter case, only the measurements of the largest lobe were used in the study, as the smaller focus could not be recognised on the lateral scan. Overlapping lobes were excluded as this could interfere with correct measurements, as well as ectopic intrathoracic thyroid tissue that was not reachable with ultrasound. Cats that received anti-thyroid medication were off this medication for a minimum of 3 days prior to radioiodine treatment. All cats were kept fasted for at least 12 h to avoid complications with anaesthesia.
Scintigraphy
A catheter was placed in a cephalic vein and each cat was injected intravenously with TcO4- (mean activity 97.68 ± 28.49 MBq). Approximately 40 mins after injection the diagnostic scans were performed on the gamma camera (GCA 7200 A; Toshiba) equipped with a low energy high resolution (LEHR) collimator. For this procedure patients were anaesthetised with propofol intravenously (IV) (4–8 mg/kg to effect, Propovet; Ecuphar, 10 mg/ml). Two planar static images were made, one in ventral recumbency and one in right or left lateral recumbency, depending on the side of the affected thyroid lobe (eg, left-sided unilateral affected animals would be positioned in left lateral recumbency). The acquisition parameters were 200 kcounts, 256 × 256 matrix size and 0.1 cm pixel size yielding a 25.6 × 25.6 cm field-of-view. All images were processed using multimodality software (Hermes V5.0; Nuclear Diagnostics AB). Regions of interest (ROI) were drawn over the affected thyroid lobes using a 30% threshold ROI tool. This threshold value was established previously using 99mTc-labelled phantoms with a known volume and known dimensions (mimicking an enlarged thyroid gland), scanned identically to the cats. A 30% threshold ROI was found to give measurements most comparable with the phantom’s real dimensions. Length, height and width of each thyroid lobe were measured manually using this ROI delineation. The area (cm2) of each lobe was calculated automatically by the thyroid volume function of the Hermes software.
For volume estimation, eight formulas that were described previously in human and veterinary literature or incorporated in the Hermes software were tested using these four parameters.8,21–25 The following formulas were tested. Formula 1 to calculate the volume of an ellipsoid [F1 = (π/6) × L × H × W], formula 2 [F2 = (π /2) × L × W2], formula 3 [F3 = 0.33 × (area cm2)3/2], formula 5 (F5 = area × H) and formula 6 (F6 = 0.27 × area × L). Formula 4 is incorporated in the Hermes software [F4 = 1.08 × (π /6) × L × W2]. Formula 7 is the formula of the volume of a cylinder (F7 = π × L × W2) and formula 8 is the formula of the volume of a sphere [F8 = π x (4/3) × W3]. Additional to the application of both formulas to all thyroids, formulas 7 and 8 were also tested on the respective shapes (F7 to cylindrical-shaped thyroid lobes and F8 to spherical-shaped lobes) to examine a potential influence of the shape on the usefulness of these formulas. All measurements and shape evaluation were performed by the same clinician (VV).
Ultrasound
Ultrasound was performed immediately following scintigraphy, while the patients were still sedated. The cats were placed in dorsal recumbency in a cushion with the head extended. B-mode ultrasound was performed using a 10–15 MHz, multi-frequency linear array probe (CnTI Mylab 30; Esaote). The highest frequency (15 MHz) was used for all patients; gain and position of the focal spot were optimised for each patient individually.
The presence of any cystic lesions that could interfere with therapy efficacy was evaluated, as well as the thyroid parenchyma, and measurements were made for volume determination. For this purpose the thyroid lobes were scanned in three planes (transverse, sagittal and dorsal) (Figure 1). Maximal dimensions in each plane were searched for before freezing the image and making measurements with the electronic caliper. The sagittal view provided length and height, the transverse view provided height and width, and the dorsal view provided length and width parameters. Of these six measurements the maximal length (L), height (H) and width (W) values were chosen and thyroid volume was calculated by the ellipsoid formula (volume = π/6 × length × height × width).19,20 Ultrasounds were performed by two of the authors (AC, VV).
Figure 1.
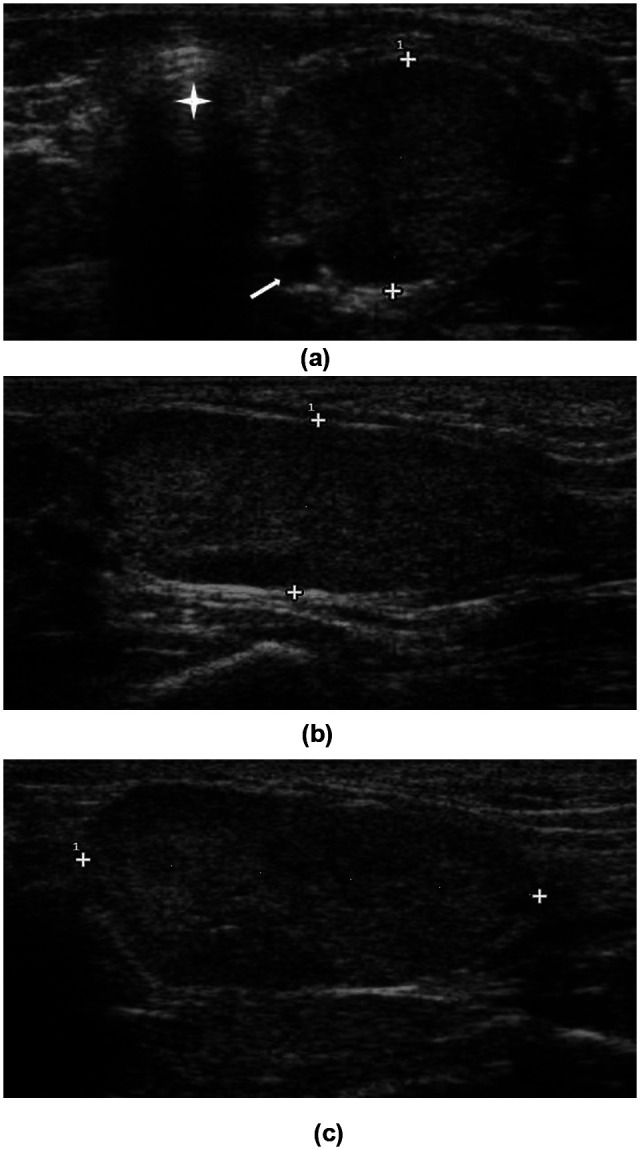
Transverse (a), sagittal (b) and dorsal (c) images of the left thyroid lobe of a unilaterally affected hyperthyroid cat. The caliper indicates the height of the lobe in (a) and (b), and the length in (c). Common carotid artery (white arrow), trachea (star)
Statistics
A paired t-test was used to compare the volume measured on ultrasound with the volume obtained by scintigraphy for each thyroid lobe using the different formulas. Thyroid shapes on planar scintigraphy were divided into two groups: cylinder- and sphere-shaped. Both groups were tested separately for applicability of the cylinder and sphere formula using a paired t-test.
Results
Table 1 summarises the mean L, H and W as measured on scintigram, as well as the same measurements on ultrasound. The mean area on the scintigram was 2.56 cm2 (SD = 1.26; range 0.61–4.92 cm2). The mean thyroid volumes calculated on ultrasound and scintigraphy are summarised in Table 2. Thirteen cats (40.6%) had cystic lesions of variable size in the affected thyroid lobe. This ranged from small diffuse lesions to cystic lesions that occupied nearly the entire lobe in two cats. In another cat both thyroid lobes had an abnormal ultrasonographic appearance, although on the scintigram only one lobe showed TcO4- uptake.
Table 1.
Mean length, height and width (mm), range and standard deviation, measured on ultrasound and scintigraphy
| Length | Height | Width | |
|---|---|---|---|
| Ultrasound | 26.7 (13.3–46; ± 6.6) | 10.5 (5.4–25.4; ± 4.2) | 13.3 (5.1–35.1; ± 5.6) |
| Scintigraphy | 20.7 (8–35; ± 6.3) | 13.2 (6–21.9; ± 3.8) | 13.1 (6–25.8; ± 3.7) |
Table 2.
Mean volume (mm3), range and standard deviation for the different formulas by measurements made on the scintigraphic acquisitions
| Ultrasound | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| (π/6) × L × H × W | (π/6) × L × H × W | (π/2) × L × W2 | 0.33 × (area cm2)3/2 | 1.08 × 0.523 × L × W2 | Area × H | 0.27 × area × L | π × L × W2 | π × (4/3) × W3 |
| 2012 (191.8–10550; ± 2116.6) | 2223 (188–8250; ± 1753) | 6680 (565–29157; ± 5707) | 1472 (157–4705; ± 1059) | 2403 (203–10490; ± 2053) | 3771 (366–12877; ± 2794) | 1622 (160–4429; ± 1171) | 3340 (283–14579; ± 2854) | 1482 (113–8987; ± 1615) |
L = length, H = height, W = width
F1 (P = 0.426), F3 (P = 0.072), F4 (P = 0.142) and F6 (P = 0.220) showed no significant difference with the volume calculated from ultrasound measurements. On the contrary, F2 (P <0.00001), F5 (P = 0.00002), F7 (P = 0.0004) and F8 (P = 0.021) did show a significant difference. F4, F6 and F1, in particular, showed best agreement with ultrasonographically-determined volumes (Figure 2).
Figure 2.
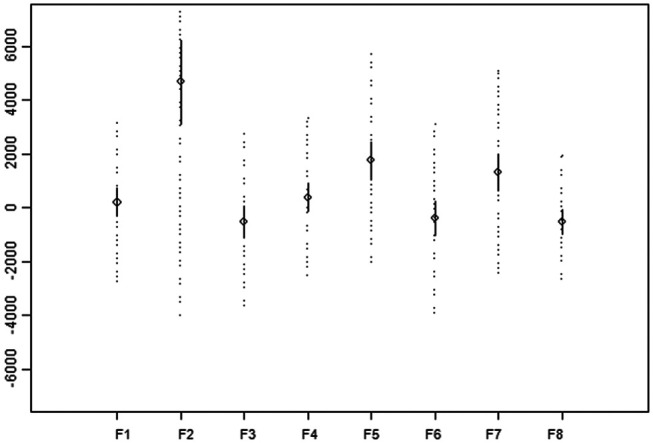
Mean difference between volumes measured on ultrasound (y-axis) and on scintigraphy (x-axis) for each of the eight formulas. Full line = 95% confidence interval, dotted line = 95% reference interval
Twenty-three of 32 thyroid lobes (72%) were defined as being cylinder-shaped and 9/32 lobes (28%) were defined as sphere-shaped (Figure 3). Small variations in shape, such as bean-shaped or bi-lobated, were recognized. Overall, the differences between the volume measured on ultrasound and scintigraphy were smaller for both shapes when F8 was used, making this formula more applicable than F7. F8 performed significantly (P = 0.003) better to calculate the volume of a sphere shaped lobe, ie it differed less from the ultrasound volume.
Figure 3.
A cylinder-shaped (a) and sphere-shaped (b) thyroid lobe with a 30% threshold ROI in two unilaterally affected hyperthyroid cats
Discussion
Although little is known about the influence of thyroid volume on radioiodine therapy outcome in veterinary medicine or about the most suitable method to determine this volume, it is one of the parameters that has been implicated in therapy failure both in humans and in cats.8,11–14 In this study threshold-dependent automatic ROI drawing (exclusion of pixels containing activity below a preset threshold) on planar thyroid scintigrams was used to minimise operator-dependent inaccuracies. The threshold has inevitable influence on the size of the ROI and, consequently, on the determined volumes. The ROI threshold for thyroid volume calculation in humans varies from 5% to 30%.24–26 This is comparable to the 30% threshold value we found most suitable for quantitative scan processing.
Forrest et al calculated thyroid volume with the sphere and cylinder formula (applied according to the visual evaluation of the lobes) in 80 cats and found a mean thyroid volume of 10400 mm3 (range 2100–28400 mm3). 8 This volume is more than five times the mean volume determined by the formulas with best agreement with ultrasound in this study. However, in this latter study, measurements of multiple thyroid foci were added up to calculate total active thyroid volume for dosimetric purposes. This does not allow a direct comparison with our results as volumes were calculated for well-delineated single lobes. Moreover, they did not compare the volume with the real volume or a volume measured with other imaging modalities; also no ROI threshold was mentioned.
Inevitably, differences in shape will have a considerable influence on the volume calculations and the formula to be used for these. For this reason the volumetric formulas of a sphere and a cylinder were tested in this study for the respective shapes. Surprisingly, agreement between volume measurements with ultrasound and F8 for cylinder-shaped thyroid lobes was higher than for sphere-shaped lobes, while for sphere-shaped thyroids F8 was not superior to F7. The reason for this is unclear.
A limitation of this study is that most formulas that were tested are derived from human medical publications; therefore, the correction factors may not be applicable to cats. Even in human medicine, there is a discrepancy between different publications concerning the formulas for volumetric calculations. Human medicine also faces the additional difficulty that thyroid pathology is more diverse compared with feline hyperthyroidism, with an inevitable influence on shape and volume calculations. F1 has been reported to be applicable for volume calculation of diffuse goitres in humans. 24 F3 was found to be an acceptable formula to estimate thyroid gland volume in patients with non-specified thyrotoxicosis, especially in combination with ultrasound and the use of the correction factor 0.419 instead of 0.33. 22 Other authors reported F3 and a formula similar to F3 (0.326 × area3/2) not to be useful in multinodular goiters or Graves’ disease.23–25 A formula very similar to F6 (0.323 × area × length) showed a reasonable correlation to ultrasound in patients with Graves’ disease. 26
In this study scintigraphic volume measurements were compared with ultrasound. However, in human medicine it was reported that the volume, as measured ultrasonographically prior to thyroidectomy, was underestimated in comparison with the real volume (after thyroid excision) for all pathologies. 27 Despite this the standard technique in human and veterinary medicine to determine thyroid volume is the use of ultrasound and the ellipsoid formula.8,19,20,22,26,28 To our knowledge no other formulas than the ellipsoid model have been used for routine ultrasonographic thyroid volume determination.
Ideally, computed tomography (CT) or magnetic resonance imaging (MRI) should be performed for volume calculations. Measurements and volumes calculated on CT data have already been reported for clinically healthy cats, but not in hyperthyroid cats. 29 The need for prolonged anaesthesia in a geriatric population often suffering from concurrent cardiac and/or renal disease and the additional cost hamper the use of these modalities in clinical settings. Moreover, studies in human medicine comparing MRI and ultrasound volume determination proposed ultrasound as a reliable alternative technique, provided that a correction factor is used.25,30
The mean volume of 2012 mm3 per hyperthyroid lobe was remarkably larger than the volume reported in previous publications. Some thyroid lobes were ill-defined on ultrasound, which possibly impeded reliable measurements. Barberet et al 20 described a mean volume of 572 mm3 for the left lobe and 552 mm3 for the right lobe, while Wisner et al 19 described a mean lobe volume of 578 mm3. When we have a closer look at the separate parameters we can conclude that the mean length of 26.7 mm is rather comparable with the mean length of Barberet et al 20 and Wisner et al 19 with 21.4 mm for the left lobe and 19.7 mm for the right lobe, and 20.2 ± 3.6 mm for the left lobe and 21.9 ± 4.4 mm for the right lobe, respectively. However, the mean height (10.5 mm) and width (13.3 mm) in this study are approximately double those measured by Wisner et al, 19 who determined the mean height for the left lobe to be 5.5 ± 2.4 mm and the mean height for the right lobe to be 8.1 ± 3.0 mm. The mean width was 5.7 ± 2.1 mm for the left lobe and 7.7 ± 2.4 mm for the right lobe. Mean values for height and width were not mentioned in the study of Barberet et al. 20 The reason for this difference is unclear. Most likely, the discrepancy between this study and the two others19,20 is owing to simple coincidence, as the degree of clinical hyperthyroidism in cats is also very variable. Furthermore, the group of cats in this study was approximately twice as large as in the other two studies.
Our aim was to define the most reliable formula to determine thyroid volume on scintigraphy. Given that thyroid volume is suggested to be a factor influencing therapy outcome, the determination of volume on planar scintigrams may provide an easy method in clinical surroundings where a scintigram is often used as a diagnostic tool prior to radioiodine therapy. Statistical analysis showed three formulas to be promising. F1, showing the best agreement with ultrasound, has the disadvantage that two scans, one in ventral and one in lateral recumbency, are necessary to obtain all parameters needed. On the contrary, F4 only requires one image. F6 requires determination of the thyroid area, which is a function specific to the Hermes software and may not be available in all systems. Applying specific formulas based on subjective assessment of thyroid shape on the planar scintigram does not seem to be of any use as the shape of the thyroid and its corresponding formula do not appear to improve volume calculations. The advantage of volume measurements on the scintigram is that only one examination has to be performed for information on thyroid activity and volume. Additional studies are needed to determine whether a correction factor, more applicable to cats, has to be introduced.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 20 July 2012
References
- 1. Kintzer PP. Considerations in the treatment of feline hyperthyroidism. Vet Clin North Am Small Anim Pract 1994; 24: 577–585. [DOI] [PubMed] [Google Scholar]
- 2. van Hoek I, Peremans K, Waelbers T, et al. Non-surgical treatment of feline hyperthyroidism: options and considerations. Flem Vet J 2007; 76: 69–80. [Google Scholar]
- 3. Meric SM, Hawkins EC, Washabau RJ, et al. Serum thyroxine concentrations after radioactive iodine therapy in cats with hyperthyroidism. J Am Vet Med Assoc 1986; 188: 1038–1040. [PubMed] [Google Scholar]
- 4. Meric SM, Rubin SI. Serum thyroxine concentrations following fixed-dose radioactive iodine treatment in hyperthyroid cats: 62 cases (1986–1989). J Am Vet Med Assoc 1990; 197: 621–623. [PubMed] [Google Scholar]
- 5. Slater MR, Komkov A, Robinson LE, et al. Long-term follow-up of hyperthyroid cats treated with iodine-131. Vet Radiol Ultrasound 1994; 35: 204–209. [Google Scholar]
- 6. Théon AP, Van Vechten MK, Feldman E. Prospective randomized comparison of intravenous versus subcutaneous administration of radioiodine for treatment of hyperthyroidism in cats. Am J Vet Res 1994; 55: 1734–1738. [PubMed] [Google Scholar]
- 7. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995; 207: 1422–1428. [PubMed] [Google Scholar]
- 8. Forrest LJ, Baty CJ, Metcalf MR, et al. Feline hyperthyroidism: efficacy of treatment using volumetric analysis for radioiodine dose calculation. Vet Radiol Ultrasound 1996; 37: 141–145. [Google Scholar]
- 9. Chun R, Garrett LD, Sargeant J, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound 2002; 43: 587–591. [DOI] [PubMed] [Google Scholar]
- 10. Wallack S, Metcalf M, Skidmore A, et al. Calculation and usage of the thyroid to background ratio on the pertechnetate thyroid scan. Vet Radiol Ultrasound 2010; 51: 554–560. [DOI] [PubMed] [Google Scholar]
- 11. Andrade VA, Gross JL, Luiza Maia A. The effect of methimazole pretreatment on the efficacy of radioactive iodine therapy in Graves’ hyperthyroidism: one-year follow-up of a prospective randomized study. J Clin Endocrinol Metab 2001; 86: 3488–3493. [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Arnaiz N, Andia E, Guma A, et al. Ultrasonographic thyroid volume as a reliable prognostic index of radioiodine-131 treatment outcome in Graves’ disease hyperthyroidism. Horm Metab Res 2003; 35: 492–497. [DOI] [PubMed] [Google Scholar]
- 13. Zantut-Wittmann DE, Ramos CD, Santos AO, et al. High pre-therapy [99mTc] pertechnetate thyroid uptake, thyroid size and thyrostatic drugs: predictive factors of failure in [131I] iodide therapy in Graves’ disease. Nucl Med Commun 2005; 26: 957–963. [DOI] [PubMed] [Google Scholar]
- 14. Markovic V, Eterovic D. Thyroid echogenicity predicts outcome of radioiodine therapy in patients with Graves’ disease. J Clin Endocrinol Metab 2007; 92: 3547–3552. [DOI] [PubMed] [Google Scholar]
- 15. Sabri O, Zimny M, Schulz G, et al. Success rate of radioiodine therapy in Graves’ disease: the influence of thyrostatic medication. J Clin Endocrinol Metab 1999; 84: 1229–1233. [DOI] [PubMed] [Google Scholar]
- 16. Reinhardt MJ, Brink I, Joe AY, et al. Radioiodine therapy in Graves’ disease based on tissue-absorbed dose calculations: effect of pre-treatment thyroid volume on clinical outcome. Eur J Nucl Med 2002; 29: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 17. Tsuruta M, Nagayama Y, Yokoyama N, et al. Long-term follow-up studies on iodine-131 treatment of hyperthyroid Graves’ disease based on the measurement of thyroid volume by ultrasonography. Ann Nucl Med 1993; 7: 193–197. [DOI] [PubMed] [Google Scholar]
- 18. Ceccarelli C, Bencivelli W, Vitti P, et al. Outcome of radioiodine-131 therapy in hyperfunctioning thyroid nodules: a 20 years’ retrospective study. Clin Endocrinol 2005; 62: 331–335. [DOI] [PubMed] [Google Scholar]
- 19. Wisner ER, Théon AP, Nyland TG, et al. Ultrasonographic examination of the thyroid gland of hyperthyroid cats: comparison to 99mTcO4- scintigraphy. Vet Radiol Ultrasound 1994; 35: 53–58. [Google Scholar]
- 20. Barberet V, Baeumlin Y, Taeymans O, et al. Pre-and posttreatment ultrasonography of the thyroid gland in hyperthyroid cats. Vet Radiol Ultrasound 2010; 51: 324–330. [DOI] [PubMed] [Google Scholar]
- 21. Himanka E, Larsson LG. Estimation of thyroid volume: An anatomic study of the correlation between the frontal silhouette and the volume of the gland. Acta Radiol 1955; 43: 125–131. [PubMed] [Google Scholar]
- 22. Brown MC, Spencer R. Thyroid gland volume estimated by use of ultrasound in addition to scintigraphy. Acta Radiol Oncol 1978; 17: 337–341. [DOI] [PubMed] [Google Scholar]
- 23. Huysmans DAKC, De Haas MM, Van Den, Broeck WJM, et al. Magnetic resonance imaging for volume estimation of large multinodular goitres: a comparison with scintigraphy. Brit J Radiol 1994; 67: 519–523. [DOI] [PubMed] [Google Scholar]
- 24. Wesche MFT, Tiel-v.Buul MM, Smits NJ, et al. Ultrasonographic versus scintigraphic measurement of thyroid volume in patients referred for 131I therapy. Nucl Med Comm 1998; 19: 341–346. [DOI] [PubMed] [Google Scholar]
- 25. Van Isselt JW, de Klerk JMH, van Rijk PP, et al. Comparison of methods for thyroid volume estimation in patients with Graves’ disease. Eur J Nucl Med Mol Imaging 2003; 30: 525–531. [DOI] [PubMed] [Google Scholar]
- 26. Pant GS, Kumar R, Gupta AK, et al. Estimation of thyroid mass in Graves’ disease by a scintigraphic method. Nucl Med Commun 2003; 24: 743–748. [DOI] [PubMed] [Google Scholar]
- 27. Miccoli P, Minuto MN, Orlandini C, et al. Ultrasonography estimated thyroid volume: a prospective study about its reliability. Thyroid 2006; 16: 37–39. [DOI] [PubMed] [Google Scholar]
- 28. Stokkel MPM, Handkiewicz Junak D, Lassmann M, et al. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging 2010; 37: 2218–2228. [DOI] [PubMed] [Google Scholar]
- 29. Drost WT, Mattoon JS, Weisbrode SE. Use of helical computed tomography for measurement of thyroid glands in clinically normal cats. Am J Vet Res 2006; 67: 467–471. [DOI] [PubMed] [Google Scholar]
- 30. Reinartz P, Sabri O, Zimny M, et al. Thyroid volume measurement in patients prior to radioiodine therapy: comparison between three-dimensional magnetic resonance imaging and ultrasonography. Thyroid 2002; 122: 713–717. [DOI] [PubMed] [Google Scholar]



