Abstract
Neutrophil extracellular trap (NET) formation is a hallmark of many disorders that involve neutrophil recruitment, tissue damage, and inflammation. As NET formation is often associated with neutrophil death, the term “NETosis” has become popular. Upon discovery that neutrophils may survive NET release, apparent misnomers, such as “vital NETosis,” have been proposed. Meanwhile, it has become obvious that certain stimuli can trigger neutrophil necroptosis, a process associated with NET-like chromatin release. Here, we discuss the relationship between NET release and neutrophil death in view highlighting that many assays used in the field do not properly distinguish between the two. An updated nomenclature is needed replacing the term “NETosis” to meet the growing variety of settings leading to chromatin release with and without neutrophil death. Dissecting which triggers of NET release involve which signaling pathway will help to define drugable molecular targets that inhibit NET release and/or neutrophil necrosis in specific disorders.
Keywords: Sterile inflammation, Infection, Host defense, Gout, Regulated necrosis, Histones
Introduction
Neutrophils are important effector cells of the innate immune system [1]. Neutrophils are continuously generated in large numbers from myeloid precursors in the bone marrow. They have a short lifespan of around 5.4 days during which they continue a process of maturation and senescence [2]. Neutrophil senescence involves upregulation of CXCR4, facilitating homing back to the bone marrow and other organs where neutrophils undergo apoptosis and phagocytic clearance by macrophages [3]. By contrast, neutrophil activation in disease can shorten or prolong their lifespan depending on the disease context [4, 5]. Activated neutrophils are found in all sorts of tissue inflammation, which set off research efforts to unravel the molecular mechanisms of neutrophil homing, patrolling, or chemotaxis during the last two decades [1, 6].
In 2004, the formation of neutrophil extracellular traps (NETs) was first described as an alternative route of bacterial killing [7], implying that beyond apoptosis, there is a necrosis-like form of neutrophil death since then referred to as NETosis. Confusing input came from other domains. The cell biology domain reclassified the classical cell death categories of apoptosis and necrosis into a myriad of novel categories defined by distinct causative signaling pathways [8]. Whether NETosis is one of those or a distinct category has remained unclear. The immunology domain observed that leukocyte-death-upon-activation is a common phenomenon not only for effector T cells but also for inflammasome-activated macrophages and dendritic cells [9, 10]. Finally, NET formation was described to occur also without immediate neutrophil death [11], which was quickly referred to as “vital NETosis” [11, 12], although this term is an obvious contradiction in itself (Table 1). Such nomenclatures in part mirror and potentially account for the general confusion about the evolving spectrum of evidence on NET formation. Here, we try to address this problem by carefully dissecting NET formation from neutrophil death, which often but obviously not always go together. A clear distinction is often difficult, because many bioassays used in the field do not clearly distinguish between neutrophil death and NET formation. However, based on the data available, we propose a working model toward a better understanding of the role of NET release and neutrophil death in inflammation and tissue injury.
Table 1.
Terms frequently used in the context of NET formation
| Term | Definition |
|---|---|
| NET | Neutrophil extracellular traps are chromatin expulsed from neutrophils decorated with nuclear and cytosolic components such as proteolytic enzymes |
| NETting | The process of NET formation by groups of neutrophils, e.g., in pus, tophus, or thrombus formation |
| NETosing | The neutrophils/PMNs capable for forming NETs |
| NETosis | NET formation in association with death of the neutrophil, common in pus, tophi, or thrombosis, but the term does not specify the mode of cell death |
| Suicidal NETosis | NET formation in association with the death of the neutrophil, but the term does not specify the mode of cell death. “Suicidal” implies that the trigger for death is intrinsic, which is usually not the case. Imprecise term that is to be avoided |
| Lytic NETosis | NET formation by pathogen-induced lysis of neutrophils, e.g., S. aureus |
| Vital NETosis | NET formation without the death of the neutrophil. As “Osis” implies death and “Vital” implies alive the term is a contradiction in itself and should be avoided |
| Neutrophil necroptosis | Neutrophil death that can be blocked by inhibitors of the necroptosis pathway (RIPK3–MLKL) |
| Neutrophil apoptosis | Neutrophil death that can be blocked by inhibitors of caspase 3, 8, and 9 |
RIPK receptor interacting protein kinase, MLKL mixed lineage kinase domain-like
“NETosis”. NET formation in association with neutrophil death
NETs were first described using extensive cell imaging techniques after stimulation of human neutrophils with phorbol 12-myristate 13-acetate (PMA) or interleukin (IL)-8 [7]. Three years later, the same group reconfirmed an observation made in 1996 that neutrophils undergo a distinct form of cell death following PMA stimulation, which is neither apoptosis nor necrosis, and named it “NETosis” [13, 14]. “NETosis,” unlike apoptosis or necrosis, was described to involve expansion of the nuclear material, chromatin decondensation, nuclear envelope disintegration, subsequent mixing of cytoplasmic, and nuclear components followed by plasma membrane rupture and release of NETs [14, 15] (Fig. 1). Since then, NET release was considered to imply neutrophil death and the term “NETosis” established in the literature. In the last decade, researchers extensively studied “NETosis” mostly using conventional bioassays, which do not distinguish the two phenomena NET release and neutrophil death (Table 2). For example, the most widely used assays for NET release, Picogreen and Sytox assays, involve detection of cell-free DNA as the main principle. However, since these methods also detect necrosis-related passive release of chromatin, it is difficult to distinguish this process from a proactive chromatin expulsion [16, 17]. Simultaneously, some researchers used lactose dehydrogenase (LDH) assay to measure NET formation in vitro [18]. However, cells and tissues release LDH upon toxic or injury-related damage [19], making this assay highly unspecific for distinguishing NET formation and cell death. Furthermore, detection of histone deimination (citrullination of histones) by immunoblots or immunohistology was considered as an indicator for NET formation [20], since histone deimination induces chromatin decondensation, which is an essential step during NET release [21]. Several studies implicated the involvement of peptidyl-arginine deiminase 4 (PAD4) in NET formation [20–22]. PAD4 is the enzyme required for citrullination of histones and chromatin decondensation during NET formation [21]. Accordingly, chemical inhibition of PAD4 using Cl-amidine impaired NETosis in animal models of anti-GBM disease or lupus nephritis [23, 24]. However, the requirement of PAD4 in NET formation is a debated question owing to the unspecific effects of Cl-amidine for PAD4 [25, 26], as well as the inconsistencies observed in NET formation in Pad4-deficient mice. For example, Pad4-/- mice displayed impaired NET formation during necrotizing fasciitis [20] but succumbed to influenza pneumonitis [27], which involves influenza virus-induced NETs in the lung [28]. These disparities suggest that the involvement of PAD4 in NET formation depends on the stimulus. Indeed, it is shown that some stimuli, e.g., calcium ionophores, activate PKCζ, and thus PAD4; whereas PMA activates PKCα and, thus, inhibits PAD4 [29], while both stimuli still induce NET release. Another widely used technique for assaying NET formation is ‘microscopy.’ Researchers have used immunofluorescence (IF), confocal, and electron microscopy techniques to characterize either the presence of NETs, by detecting the co-localization of neutrophil-specific proteins and DNA [14], or the morphological appearances of NETs [7, 14]. However, the main drawback of using these techniques is the need for cell fixation prior to microscopic examinations. Therefore, this technique cannot really distinguish the process of NET formation and cell death. Researchers also used enzyme-linked immunosorbent assay (ELISA), a technique to detect complexes of neutrophil-specific proteins and DNA, e.g., myeloperoxidase (MPO)-DNA or neutrophil elastase (NE)-DNA complexes [30–32], as an indicator of the NET formation. However, although these assays confirm the presence of NETs, they fail to distinguish the NET formation and cell death.
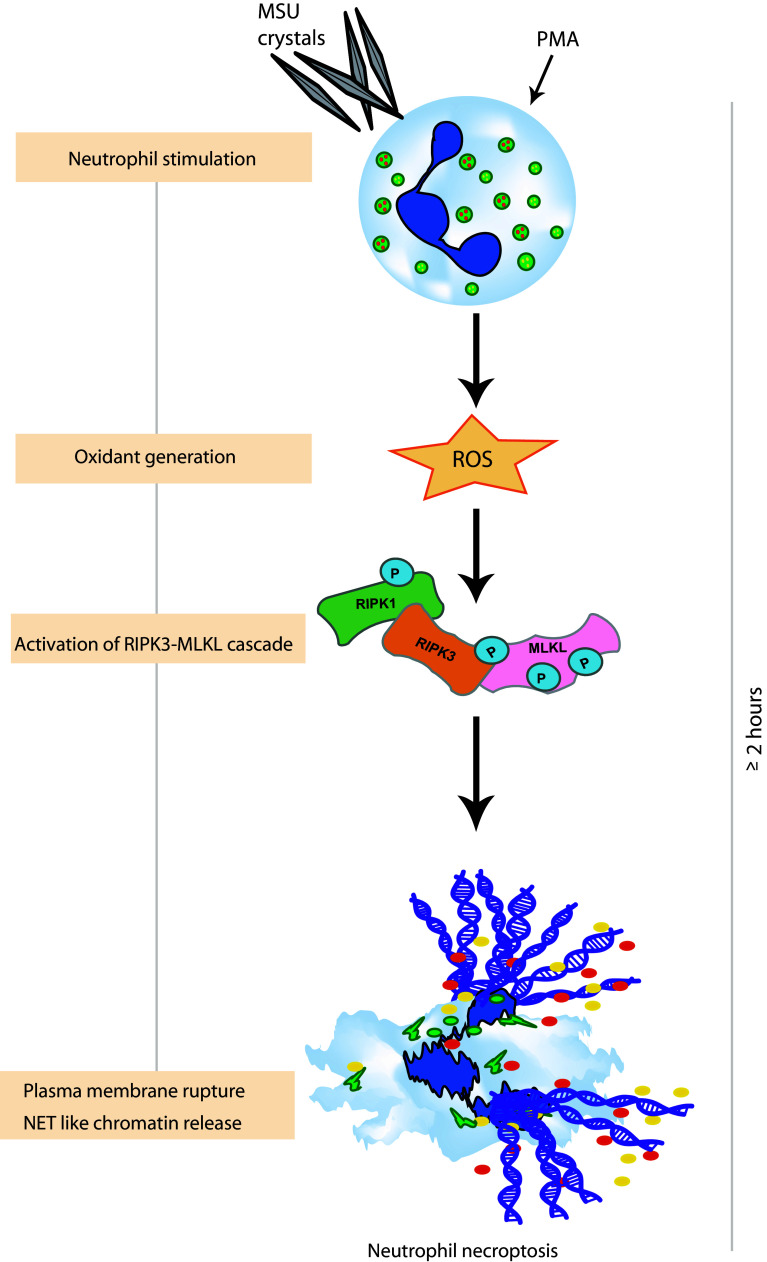
Fig. 1.
Chromatin release as a consequence of neutrophil necroptosis. Stimuli-like monosodium urate (MSU) crystals and phorbol 12-myristate 13-acetate (PMA) induce activation of a receptor interacting protein kinase (RIPK)3- and mixed lineage kinase domain-like (MLKL)-dependent signaling pathway downstream of reactive oxygen species (ROS). This leads to plasma membrane rupture and NET-like chromatin release together with granular enzymes as a consequence at around 2 h of stimulation
Table 2.
NET evaluation and bioassay characteristics
| Method | Target | Identifies NETs | Distinguishes NET release and cell death | Refs. |
|---|---|---|---|---|
|
PicoGreen/Sytox assay (spectrofluorometry) |
Extracellular and dead cell DNA | No | No | [16, 17] |
|
LDH assay (spectrometry) |
LDH release | No | No | [18] |
|
MPO-DNA complexes (capture ELISA) |
MPO and DNA | Yes | No | [30, 31] |
|
NE-DNA complexes (capture ELISA) |
NE and DNA | Yes | No | [32] |
|
Histone deimination (IF microscopy, WB) |
Citrullinated histones | Yes | No | [10, 20] |
|
Morphology (IF and confocal microscopy) |
DNA (DAPI, Sytox) Granule protein (MPO, NE, CitH3) |
Yes | No | [7, 14, 29] |
|
Micromorphology (electron microscopy) |
Ultrastructure of nuclei/cytoplasm | Yes | No | [7, 14] |
|
Live cell imaging (time lapse microscopy) |
DNA (Sytox, Hoechst), cytoplasm (cell tracking dye) | Yes | Yes | [14] |
| Combination of microscopy and flow cytometry | Subcellular morphology | Yes | Yes | [12] |
LDH lactate dehydrogenase, MPO myeloperoxidase, NE neutrophil elastase, WB western blot, IF immunofluorescence, DAPI 4′,6-diamidino-2-phenylindole, CitH3 citrullinated histones etc
In contrast, time-lapse video microscopy allowed observing the NET formation [14]. Neutrophils are imaged using a combination of nuclear (Sytox, Hoechet, PICO), cytoplasmic (calcien, granular dyes, e.g., NE), and cell death dyes (propidium iodide, annexin V), making it feasible to identify different components of NET formation process and cell death, in a manner dependent on each stimulus and time course [14]. Moreover, Zhao W et al. reported the use of a combination of microscopy and flow cytometry for simultaneous detection and quantification of NET formation [12]. Interestingly, this technique also claimed to distinguish between the NET formation with and without cell death [12]. Together, as a few methods are suitable to clearly distinguish NET release from neutrophil death, these two phenomena often seem connected and are referred to as “NETosis.” However, as neutrophils surviving NET release have been documented and since NET release upon certain stimuli can be inhibited with `conventional` cell death inhibitors, it seems obvious that the term “NETosis” is no longer universally appropriate.
Signaling components in NET release
The process of NET release requires the activation of NADPH oxidase through the Raf–MEK–ERK pathway, reactive oxygen species (ROS) production, and upregulation of antiapoptotic proteins [14, 33]. Accordingly, neutrophils from humans or mice deficient in NADPH-oxidase cannot execute NET release [14, 34, 35]. But how do ROS exactly mediate NET formation? Several theories emerged to describe the involvement of ROS in “NETosis,” e.g., through NE, MPO, and histone deimination. Upon activation of neutrophils, ROS triggers the MPO-dependent proteolytic activity of NE [15]. In the cytosol, NE degrades F-actin to arrest the actin dynamics of neutrophils before translocating to the nucleus where it degrades core histones, e.g., H1 and then H4, and promotes chromatin decondensation [15, 36]. MPO further synergizes with NE to induce chromatin decondensation independent of its enzymatic activity [36]. Accordingly, humans deficient in MPO as well as mice deficient in NE cannot form NETs [36, 37]. However, the molecular mechanisms downstream to ROS production and upstream to NET formation and NETosis are not clear.
“NETosis”? chromatin release during neutrophil necroptosis
Cell death categories are no longer defined by morphological features but by executing biochemical pathways. Vice versa, the involved route of cell death is evidenced by preventing death through specific inhibition of the causative pathway [8]. Alike apoptosis, necrosis is often a regulated form of cell death, referred to regulated cell necrosis [38]. Based on the molecular mechanisms involved, these regulated necrosis pathways were named receptor interacting protein kinase (RIPK)-mediated necroptosis, iron-mediated ferroptosis, Poly (ADP-ribose) polymerase (PARP)-mediated parthanatos, mitochondrial permeability transition-related regulated necrosis, caspase-1/11-mediated pyroptosis, podoptosis, and catastrophic mitosis [8, 39]. However, as none of the aforementioned signaling elements is specific for neutrophils, the underlying route of “NETosis” remained elusive. Indeed, it remained unclear if NETosis is at all a unique cell death category, also because none of the other cell death categories is cell type-specific. In addition, NET release has been reported in cells other than neutrophils, referred to as “ETosis” [40].
We observed that only chemical inhibitors of the necroptosis pathway, such as RIPK1 stabilizers necrostatin (Nec)-1 and Nec-1s or the mixed lineage kinase domain-like (MLKL) inhibitor necrosulfonamide (NSA) inhibited NET release and neutrophil necrosis upon 2 h of PMA or monosodium urate (MSU) crystals stimulation of human neutrophils [41]. These stimuli also increased the expression of RIPK3 and phosphorylated MLKL (pMLKL), two core proteins of necroptosis signaling, in a time-dependent manner, suggesting the involvement of the necroptosis signaling pathway in PMA- and crystal-induced NET release [41]. These findings were corroborated by similar observations in Ripk3-deficient murine neutrophils. Furthermore, Nec-1 or NSA did not affect MSU crystals- or PMA-induced ROS production in neutrophils, and neutrophils of patients with chronic granulomatous disease (non-functional NADPH oxidase) did not express pMLKL after PMA stimulation [41], suggesting that pMLKL is a downstream event to ROS. Obviously, PMA and MSU crystals trigger NET-like chromatin release in the context of neutrophil necroptosis.
Recently, Schauer et al. demonstrated that MSU crystals can induce aggregates of NETs, and the tophi, pathognomonic structures of chronic gout, share characteristics of aggregated NETs [35]. We observed that the deficiency of Ripk3, as well as inhibitors of the necroptosis pathway, inhibited MSU crystal-induced NET formation and gout-like tophus formation in vivo [41], confirming the involvement of neutrophil necroptosis along NET release. However, we also showed in an independent study that crystals of calcium oxalate, MSU, calcium pyrophosphate dehydrate, and cystine induce necroptosis in different cell types [42]. Obviously, crystals are potent inducers of necroptosis in immune and non-immune cells, and NET-like chromatin release is a consequence or a secondary event, following necroptotic neutrophil death.
NET release without neutrophil death
Neutrophils can form NETs upon certain kinds of bacterial infections in vivo without dying [11]. Pilsczeck et al. reported that upon infection with Staphylococcus aureus, neutrophils formed NETs within 5–60 min without dying and were independent of ROS production. These early NETs were also observed in vivo using spinning disk microscopy within 10 min after subcutaneous injection of S. aureus [43]. Obviously, during this process, the neutrophil`s plasma membrane remained intact, and the chromatin was released from the nucleus through intracellular vesicles that fused with the outer membrane to release NETs in the extracellular space [44]. NET release without neutrophil death was also observed within 30 min after stimulation of neutrophils by bacteria, fungi, or LPS [43–46]. This rapid NET formation is mediated by the complement system, TLR2 or fibronectin [44, 46]. Importantly, neutrophils releasing such NETs rapidly remained motile in vivo, retaining the possibility to multitask during the early infection phase [44]. This rapid NET release indicates the dynamic functions of neutrophil to trap bacteria in NETs, while the anuclear neutrophils are still able to contribute to bacterial killing by phagocytosis [44, 47].
Furthermore, S. aureus-induced rapid NETs are composed of histones, confirming that the NETs are originated from the nuclei, without involving mitochondrial DNA [43]. In contrast, other stimuli-like LPS and complement factor C5a induce NET release from mitochondrial DNA together with granular enzymes after GM-CSF priming in an ROS-dependent manner [48]. Interestingly, S. aureus rapidly induced NET release even before ROS was generated, whereas Aspergillus-induced NETs were independent of ROS [43, 46]. Moreover, statins (cholesterol-lowering drugs) have been reported to block the oxidative burst of polymorphonuclear neutrophils (PMNs), still enhancing NET formation against S. aureus [49]. Growing evidence demonstrated that NET formation with cell death involves oxidant generation, whereas rapid NET formation without cell death may or may not involve oxidant generation. For example, stimuli-like PMA or bacteria induced ROS-dependent NET formation and cell death, while stimuli-like ionomycin or certain bacterial/fungal products induced ROS-independent rapid NET formation without cell death [50].
Unlike RIPK3–MLKL-mediated neutrophil necroptosis [41], the rapid NET formation without cell death does not involve perforation of the plasma membrane [43]. Accordingly, the independency of the RIPK3–MLKL pathway for this rapid NET formation (45 min after neutrophil stimulation) was also recently demonstrated by Amini et al. using stimuli-like E. coli, GM-CSF-primed LPS, or complement factor C5a [51]. On first view, these observations seem contradictory to the involvement of necroptosis in PMA- and MSU crystal-induced NET release during neutrophil necrosis [41]. However, obviously, the nature of NET formation can differ in terms of timing, type of stimuli, or context of disease (Fig. 2). The term ‘NETosis’ is inappropriate not only to describe a vital process but also when NET release comes as a passive process secondary to plasma membrane rupture of neutrophils undergoing necroptosis or even other forms of regulated cell death. On the other hand, NET formation from neutrophils that remain vital is a primary event and a particular feature of host defense unique to neutrophils. In addition, it becomes important to carefully design future studies related to NETs and carefully distinguish the process of NET formation from that of neutrophil cell death.
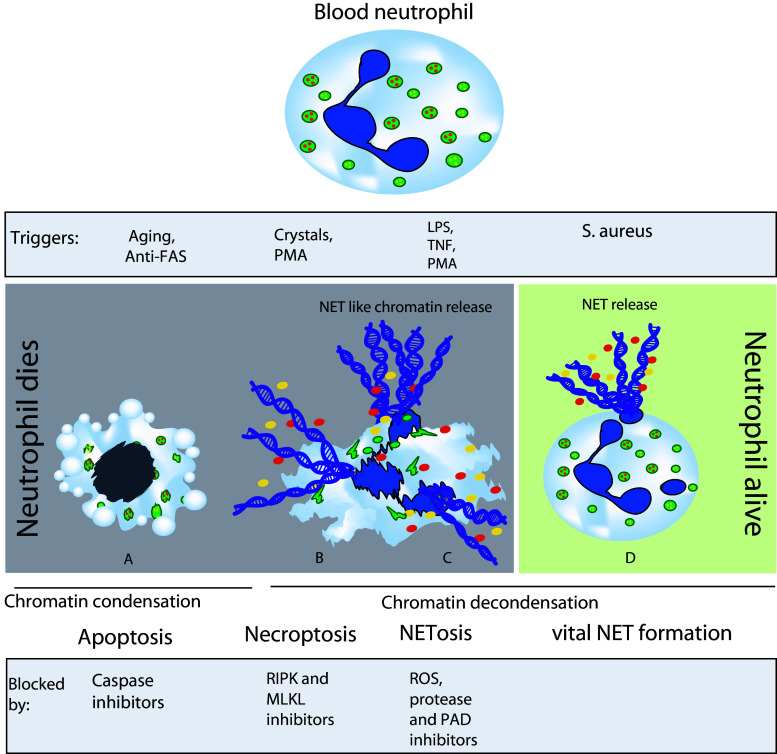
Fig. 2.
Neutrophil death and NET formation. Aging neutrophils die by apoptosis, whereas stimuli-like crystals, PMA, LPS, and TNF induce NET release associated with neutrophil death, referred to as necroptosis. Neutrophils are also known to release NETs without death, referred to as vital NET formation, upon certain bacterial stimuli, e.g., S. aureus. Inhibitors of necroptosis, e.g., RIPK1 and MLKL inhibitors as well as inhibitors of ROS, proteases, and PAD can block NET release associated with neutrophil death
Summary
NET release is a common phenomenon in infectious and non-infectious diseases involving neutrophil-related tissue injury and inflammation. The term “NETosis” has become popular, but the discovery of neutrophils surviving NET release has led to obvious misnomers, such as “vital NETosis.” Now, that also NET formation can be a consequence of neutrophil necroptosis, it is time to revise the nomenclature in this field. Neutrophil regulated necrosis can involve NET-like chromatin release, which involves completely different molecular pathways as compared with the NET release of vital neutrophils. “NETosis” implies NET formation together with neutrophil death, which involves ROS but may or may not involve downstream RIPK3–MLKL signaling. This distinction is not only semantic but essential to define drugable molecular targets that inhibit NET release and/or neutrophil necrosis in specific disease contexts. While at this point, many questions remain unanswered (Table 3), dissecting the routes of neutrophil death from those of NET release should help to better understand this fascinating phenomenon in host defense and sterile inflammation.
Table 3.
NETosis: open questions?
| Do different stimuli activate different molecular mechanisms for NET formation? |
|---|
| What are the molecular mechanisms downstream to ROS production and upstream to NET formation? |
| Which molecular mechanisms operate during ROS-independent NET formation? |
| Is PAD4 (and citrullination of histones) required for NET formation? |
| Are suicidal and vital NET formation different processes or is formation of vital NETs just an initial event in suicidal NETosis? |
| Is RIPK3–MLKL signaling involved in the vital NET formation process? |
| Is RIPK3–MLKL signaling involved in pathogen-induced NETosis? |
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 3.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–593. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 4.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80(8):2012–2020. [PubMed] [Google Scholar]
- 5.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 11.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Fogg DK, Kaplan MJ. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods. 2015;423:104–110. doi: 10.1016/j.jim.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59(2):229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köcktritz-Blickwede MV, Chow O, Ghochani M, Nizet V (2010) Visualization and functional evaluation of phagocyte extracellular traps. In: Kabelitz D, Kaufmann SHE (eds) Methods in microbiology, vol 37. Elsevier Ltd., pp I39–I60. doi:10.1016/S0580-9517(10)37007-3
- 17.Arai Y, Yamashita K, Mizugishi K, Watanabe T, Sakamoto S, Kitano T, Kondo T, Kawabata H, Kadowaki N, Takaori-Kondo A. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19(12):1683–1689. doi: 10.1016/j.bbmt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Yamaguchi M, Terao Y, Hamada S, Ooshima T, Kawabata S. alpha-Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J Biol Chem. 2012;287(13):10472–10481. doi: 10.1074/jbc.M111.280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francois M, Le Cabec V, Dupont MA, Sansonetti PJ, Maridonneau-Parini I. Induction of necrosis in human neutrophils by Shigella flexneri requires type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect Immun. 2000;68(3):1289–1296. doi: 10.1128/IAI.68.3.1289-1296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, Wang Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar SV, Kulkarni OP, Mulay SR, Darisipudi MN, Romoli S, Thomasova D, Scherbaum CR, Hohenstein B, Hugo C, Muller S, Liapis H, Anders HJ. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J Am Soc Nephrol JASN. 2015;26(10):2399–2413. doi: 10.1681/ASN.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Investig. 2013;123(7):2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, Takahara H, Thompson PR. Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry. 2010;49(23):4852–4863. doi: 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, Knuckley B, Ebrahimi P, Chumanevich AA, Luo Y, Hashimoto H, Sato M, Hofseth LJ, Thompson PR. The development of N-alpha-(2-carboxyl)benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-alpha-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem. 2011;54(19):6919–6935. doi: 10.1021/jm2008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6(7):e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neeli I, Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 2013;4:38. doi: 10.3389/fimmu.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazawa D, Shida H, Tomaru U, Yoshida M, Nishio S, Atsumi T, Ishizu A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol JASN. 2014;25(5):990–997. doi: 10.1681/ASN.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayah DM, Mallavia B, Liu F, Ortiz-Munoz G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP, 3rd, Saggar R, Ardehali A, Ware LB, Christie JD, Belperio JA, Looney MR. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191(4):455–463. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7(2):75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 34.Rohm M, Grimm MJ, D’Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82(5):1766–1777. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 36.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linkermann A, Green DR. Necroptosis. New England J Med. 2014;370(5):455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebrahim M, Mulay SR, Anders HJ, Thomasova D. MDM2 beyond cancer: podoptosis, development, inflammation, and tissue regeneration. Histol Histopathol. 2015;30(11):1271–1282. doi: 10.14670/HH-11-636. [DOI] [PubMed] [Google Scholar]
- 40.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1(21):pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 41.Desai J, Vr SK, Mulay SR, Konrad L, Romoli S, Schauer C, Herrmann M, Bilyy R, Muller S, Popper B, Nakazawa D, Weidenbusch M, Thomasova D, Krautwald S, Linkermann A, Anders HJ. Neutrophil extracellular trap formation can involve RIPK1-RIPK3-MLKL signalling. Eur J Immunol. 2015 doi: 10.1002/eji.201545605. [DOI] [PubMed] [Google Scholar]
- 42.Mulay SR, Desai J, Kumar VRS, Eberhard JN, Thomasova D, Romoli S, Grigorescu M, Kulkarni OP, Popper B, Vielhauer V, Zuchtriegel G, Reichel C, Bräsen JH, Romagnani P, Bilyy R, Munoz LE, Herrmann M, Liapis H, Krautwald S, Linkermann A, Anders HJ. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus . J Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 44.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 46.Byrd AS, O’Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol. 2013;190(8):4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peschel A, Hartl D. Anuclear neutrophils keep hunting. Nat Med. 2012;18(9):1336–1338. doi: 10.1038/nm.2918. [DOI] [PubMed] [Google Scholar]
- 48.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 49.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8(5):445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92(4):841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 51.Amini P, Stojkov D, Wang X, Wicki S, Kaufmann T, Wong WW, Simon HU, Yousefi S. NET formation can occur independently of RIPK3 and MLKL signaling. Eur J Immunol. 2015 doi: 10.1002/eji.201545615. [DOI] [PMC free article] [PubMed] [Google Scholar]