Abstract
Hematopoiesis takes place in the bone marrow of adult mammals and is the process by which blood cells are replenished every day throughout life. Differentiation of hematopoietic cells occurs in a stepwise manner through intermediates of differentiation that could be phenotypically identified. This has allowed establishing hematopoietic cell classification with hematopoietic stem cells (HSCs) at the top of the hierarchy. HSCs are mostly quiescent and serve as a reservoir for maintenance of lifelong hematopoiesis. Over recent years, it has become increasingly clear that HSC quiescence is not only due to intrinsic properties, but is also mediated by cognate interactions between HSCs and surrounding cells within micro-anatomical sites called “niches”. This hematopoietic/stromal crosstalk model also applies to more mature progenitors such as B cell progenitors, which are thought to reside in distinct “niches”. This prompted many research teams to search for specific molecular mechanisms supporting leuko-stromal crosstalk in the bone marrow and acting at specific stage of differentiation to regulate hematopoietic homeostasis. Here, we review recent data on adhesion mechanisms involved in HSCs and B cell progenitors interactions with surrounding bone marrow stromal cells.
Keywords: Adhesion, Niche, Hematopoietic stem cell, B cell
Introduction
In the bone marrow (BM) of adult mammals, hematopoietic stem and progenitor cells (HSPC) are in close contact with specialized stromal cells forming micro-anatomical units called niches. The niche is a complex three-dimensional structure consisting in different types of components: local supporting stromal cells, extracellular matrix (ECM) and soluble factors. BM stromal cells encompass a variety of cell populations such as fibroblasts, reticular cells, endothelial cells, adipocytes and osteoblasts. ECM provides structural integrity, anchorage for the cell, initiates many signal transduction events and acts as a store for cytokines and growth factors present in the niche, making them readily available to the cells [1, 2]. Fibronectin, collagens I, III, IV, tenascin, osteopontin and laminin are major components of BM ECM [3–5]. HSC and B cell progenitors are not randomly distributed in the BM but are arranged in a positional hierarchy relative to mesenchymal and osteoblastic progenitors, endothelial cells and blood perfusion according to their maturation stage (Fig. 1). This indicates that adhesion of HSPC to BM microenvironment must be tightly controlled according to the stage of hematopoietic differentiation and to the composition of the niches.
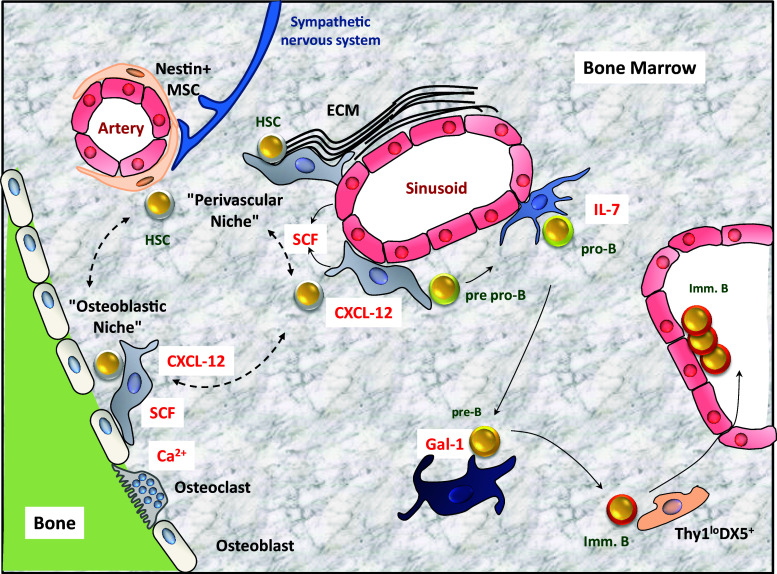
Fig. 1.
Cellular composition of adult bone marrow niches. Cells forming the different niches of the BM in contact with hematopoietic stem cells (HSC) and cells of the B cell lineage are shown. Soluble factors secreted by stromal cells are indicated in red. Arrows and dashed arrows depict the presumed migration of hematopoietic stem and progenitor cells from one niche to another. ECM extracellular matrix
Cellular composition of HSCs niches
The endosteal niche
Hematopoietic stem cells with slow-dividing/quiescent potential are localized close to endosteal lining of BM cavities in trabecular regions of long bones, whereas more differentiated hematopoietic progenitors are found mainly in the central BM region [6]. The endosteal niche provides HSC anchorage through cell–cell interactions but also growth factors and cytokines that regulate HSC self-renewal and maintain cells in the slow-cycling state inhibiting their differentiation [7]. HSCs isolated from endosteal regions by flow-cytometry show higher reconstitution activity and in vitro hematopoiesis as compared to HSCs isolated from the centre of the BM [8]. Osteoblasts (OBs) are the main contributors to the endosteal niche and are organized in a layer along the endosteum at the interface between bone and marrow [9]. The major function of osteoblasts in bone remodelling is the secretion of non-mineralized bone matrix proteins. Osteoblasts are derived from mesenchymal stem cells (MSC) and display a CD45−CD31−TER119−Sca1−CD51+ phenotype. They produce soluble factors that regulate HSC retention and quiescence, such as CXC-chemokine ligand 12 (CXCL-12), osteopontin (OPN), angiopoietin 1 (Ang-1), stem-cell factor (SCF) or thrombopoietin (TPO) [10–12]. This is in agreement with findings showing that OBs can expand the number of HSCs by two- to fourfold in vitro and that increased numbers of OBs in Mx1-cre, Bmpr1fl/fl mice is correlated to increased pools of HSCs [13, 14]. However, since targeted depletion of CXCL-12 or SCF in OBs does not affect HSCs, it is likely that they rather represent a secondary source for niche factors [15, 16]. In addition to specific cellular composition, endosteal niches present particular physico-chemical properties as compared to central BM. In steady state, the endosteal region of the BM is hypoxic with less than 2 % O2. The low perfusion of this niche is not related to the distance from blood vessels, but is rather due to low blood velocity, which results in poor supply of nutrients and oxygen to the osteoblastic niche. Hypoxia-inducible transcription factor (HIF-1α) signalling has recently been identified as determinant regulators of HSC quiescence in the endosteal niche [17]. The second characteristic of this niche is the elevated Ca2+ concentration due to active bone remodelling with coupled bone formation and degradation. Soluble Ca2+ ions are released by osteoclasts degrading the bone and are re-deposited in the neo-formed bone matrix. HSCs express calcium-sensing receptor (CaR) and it has been reported that cells lacking this receptor fail to localize in endosteal niche [18].
The vascular niche
Immunolocalization of HSCs in BM sections has allowed the discovery of a second specialized HSC niche: the vascular niche [19]. Since only a fraction of HSCs are localized close to BM sinusoids and because quiescent HSCs have been found in osteoblastic niches, it is thought that the vascular niche maintain HSCs which are in active cell cycle phases and is replenished from dormant cells localized in osteoblastic niches [20]. The blood vessels that define the vascular niche are thin-walled sinusoids with a wall consisting of a single endothelial cell layer with intercellular gaps that are endowed with specific adhesive properties [21–23]. Mesenchymal stromal cells, CXCL-12-abundant reticular (CAR) cells and sympathetic neurons together with Schwann cells constitute the perivascular niche surrounding endothelial cells that are organized around HSCs in a specific manner as individual units called hemospheres [24]. BM endothelial cells (BMECs) are functionally and phenotypically distinct from microvasculature-endothelial cells from other organs [25]. They express cytokines such as CXCL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), SCF and adhesion molecules such as E-selectin and vascular cell-adhesion molecule 1 (VCAM-1). The earliest functional in vivo evidence supporting BMECs role in the vascular niche was the observation that the gp130 cytokine receptor deletion in ECs led to BM hypocellularity and HSC decreased number [26]. Furthermore, conditional deletion of vascular endothelial growth factor receptor 2 (VEGFR-2) in adult mice inhibits regeneration of sinusoidal ECs and prevents hematopoietic reconstitution in irradiated animals [27]. This paracrine effect of BMECs on HSC self-renewal has been confirmed in vitro using serum/cytokine free EC cultures and has allowed identification of endothelial Jagged-1 as a key player in HSC self-renewal factor in vivo [28–30]. These results are in agreement with the fact that transplantation of endothelial progenitor cells accelerates recovery of BM sinusoidal vessels after irradiation and correlates with higher recoveries in HSCs numbers [31]. More recently, Méndez-Ferrer et al. have identified a nestin-expressing MSC population (Nestin+ MSC) with adipogenic, chrondrogenic and osteogenic potential derived from neural crest and located around sinusoids [32, 33]. These MSCs express high levels of HSC maintenance factors, including CXCL-12, SCF, Ang-1, IL-7, VCAM-1 and OPN. In vivo depletion of Nestin+ MSCs results in 50 % reduction in MPP and HSC [33]. Finally, CAR cells have also been shown to be necessary for maintenance of BM HSC content in vivo [34]. CAR cells are tightly associated with sinusoidal endothelium and have a similar morphology to vascular pericytes. They express HSC maintenance factors such as CXCL-12 and SCF and in vivo ablation of CAR cells results in 50 % reduction in the repopulation unit frequency in the BM. [35]. It has thus been suggested that CAR cells and Nestin+ MSCs represent two highly overlapping CXCL-12-expressing cell populations [36]. However, because Nestin+ MSCs are approximately four times less abundant than CAR cells, and contain all colony-forming-unit fibroblast activity within the marrow, it may well be that Nestin+ MSCs represent a CAR cell subset or that Nestin+ cells are still heterogeneous in nature.
Cellular composition of B cell niches
While cellular composition of HSC niches has been extensively studied, BM stromal cells supporting committed hematopoietic lineages are still poorly understood and concern essentially B cell lineage. Following the work initiated by Dexter and colleagues showing the existence of adherent cells from the BM supporting long-term growth of HSC and myeloid cells [37], protocols have been adapted to establish long-term cultures of B cells [38]. Close contacts between B cells and stromal cells with characteristics of macrophage and reticular cells have been reported in vitro and in vivo [39, 40]. However, the questions of how these stromal cells could influence B cell differentiation and where are they localized in the BM were still remaining. The identification of cells producing CXCL-12 and IL-7 as main extrinsic factors involved in B cell differentiation and proliferation has been the first demonstration of a direct influence of BM microenvironment on B cells. Indeed, the chemokine CXCL-12 has been isolated from a stromal cell line, which sustains B cell development in vitro and stimulates growth of pre-B cell clones [41]. Soon after, it has been shown that B cell differentiation is affected from the earliest pre-pro-B cell stage in mice deficient for CXCL-12 or its receptor CXCR4 [42, 43]. Finally, CXCL-12 has been shown to be implicated in the retention of pro-B and pre-B cells in the BM as well as in the homing of plasma cells to the BM [44, 45]. Similar to CXCL-12, the IL-7 cytokine has been identified based on its property to induce proliferation of B cell progenitors [46]. Hardy and colleagues have then shown that pro-B cells proliferate in presence of IL-7 [47]. In addition, mice deficient for IL-7 or IL-7 receptor α chain (IL7Rα) present an arrest of differentiation at the pre-pro-B cell stage, and IL-7 controls EBF1 expression for B cell commitment [48–50]. Finally, in addition to its role in B cell differentiation and proliferation, IL-7 is also implicated in pro-B cell survival and in the control of IgH gene recombination [51]. In situ visualization and identification of stromal cells expressing CXCL-12 has been possible by the use of reporter mice in which one allele of CXCL-12 was replaced with the gene coding for GFP [52]. Pre-pro-B cells are in contact with CAR cells mainly located away from the endosteum [34]. IL-7, which is necessary at the transition between the pre-pro-B and pro-B cell stage, has been shown to be expressed by stromal cells distinct from the CAR cells and pro-B cells are localized in the vicinity of IL-7 expressing cells [52]. Once pro-B cells express a functional Igµ chain as part of a pre-BCR and become pre-B cells, they are relocalized away from the IL-7 expressing stromal cells. These results are consistent with previous reports showing that cooperation between IL-7R and pre-BCR signals allows a better proliferation in a low concentration of IL-7 and therefore a more potent selection of pre-BCR expressing cells [53, 54]. More recently, we have shown that pre-B cells are in contact with stromal cells expressing the pre-BCR ligand Galectin-1 (Gal-1) [55]. Such Gal-1 expressing stromal cells are distinct from IL-7 expressing cells and are scattered throughout the BM parenchyma. At the immature B cell stage, cells expressing a self-reactive BCR are negatively selected by a process called receptor editing, which consists in reinitiating rearrangement to replace autoreactive IgL chains [56]. DX5 expressing stromal cells have been shown to be in close contact with immature B cells and to protect them from BCR-mediated apoptosis [57, 58]. Altogether, these studies suggest that differentiating B cells have to encounter different stromal cells expressing, respectively, CXCL-12, IL-7, Gal-1 and DX5 to complete differentiation.
Adhesion molecules involved in bone marrow leuko-stromal interactions
The precise localization of different hematopoietic cells near different stromal cells in the BM is not the result of random distribution indicating that hematopoietic cells at different stages of differentiation are endowed with specific adhesive and migrative properties. Alternatively and according to instructive model, it may well be that stromal cells acquire specific properties when in contact with hematopoietic cells at precise stage of differentiation. In the following part, we will focus on adhesion mechanisms that are involved in retention of hematopoietic cells at different stages of differentiation within specific niches. Cell–cell or cell–matrix interactions involve multiple ligands and cell-adhesion molecules (CAMs), which comprise several groups of integral membrane proteins. The CAMs mediate homophilic adhesion between cells of a single type or heterophilic adhesion between cells of different types. They are distributed along the plasma membranes and the cytosol-facing domains of these proteins are usually connected to elements of the cytoskeleton. CAMs interactions mediate mechanical adhesion between cells and transduce intracellular signals. These adhesive proteins not only maintain tissue integrity but also serve as biosensors that modulate cell behaviour in response to surrounding microenvironment. Cell to cell adhesion is initiated by one or more CAMs and then reinforced by clustering of certain adhesion molecules in specialized junctions. There are five major classes of junctions: tight junctions, gap junctions, adherens junctions, desmosomes and hemidesmosomes. They perform a structural role to hold cells together but they also connect the internal cytoskeleton directly to the extracellular space, either to another cell or to the ECM. Cytoskeleton-associated junctions are organized into three parts: cell-adhesion molecules; adapter proteins, which connect the CAMs to cytoskeletal filaments; and the bundle of cytoskeletal filaments itself. Cytoskeleton is essential not only for stabilization of cell adhesion but also for cell shape, polarity and migration. Remodelling of cytoskeleton according to the cellular needs is mediated by members of the Rho subfamily of small GTP-binding proteins (Rho, Rac, Cdc42). Cell adhesion is regulated by multiple mechanisms: specific adhesion molecules expression, regulation of CAMs surface density, receptor clustering and receptor activation state. CAMs fall into six main classes: cadherins, selectins, immunoglobulin superfamily (Ig-Sf), mucin-like family, CD44 family and integrins (Fig. 2). Adhesive properties of cadherins, selectins and integrins depend on Ca2+ ions, whereas adhesion mediated by Ig-superfamily CAMs does not.
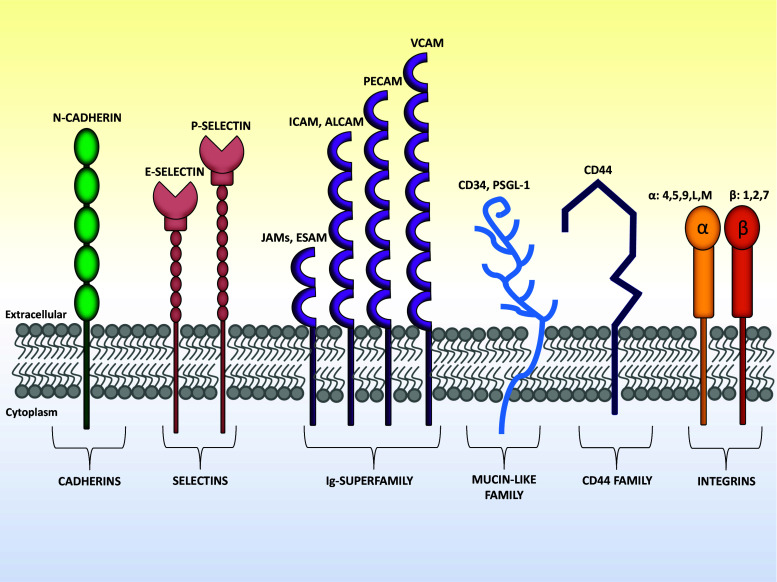
Fig. 2.
Schematic representation of adhesion molecules involved in bone marrow leuko-stromal interactions. Representative members of the six adhesion molecular families involved in cell/cell and cell/ECM interactions within BM niches are shown
Cadherins
Cadherins are the major CAMs responsible for Ca2+-dependent cell–cell adhesion. The cadherin superfamily encompasses classical (E-cadherin, N-cadherin, P-cadherin and VE-cadherin) and non-classical cadherins (desmocollin, desmoglein, T-cadherin and proto-cadherin). Cadherins are homodimers, with the extracellular part of each polypeptide folded into five extracellular cadherin repeats with Ca2+-binding sites between each pair of repeats. As the amount of Ca2+ increases, the extracellular parts of the cadherin chains become more rigid. When enough Ca2+ is bound, the cadherin dimer extends from the surface, where it can bind to a cadherin dimer on a neighbouring cell. Cadherin–cadherin interactions occur in adherens junctions in which cadherins are clustered laterally to form zipper-like structures. If Ca2+ is removed, the extracellular part of the protein becomes floppy and accessible to proteolytic enzymes [59]. Cadherins function as transmembrane adhesion proteins that indirectly link the actin cytoskeletons of neighbouring cells. Cadherin cytoplasmic tail interacts indirectly with actin filaments by a group of intracellular anchor proteins called catenins. β-Catenin binds directly to the cadherin cytoplasmic domain; subsequently α-catenin binds to β-catenin and serves to link the complex to the actin cytoskeleton by direct interaction with actin. These interactions are essential for efficient cell–cell adhesion, as cadherins that lack their cytoplasmic domain cannot hold cells strongly together. The non-classical cadherins, involved in the desmosomes formation, interact with intermediate filaments by a different set of intracellular anchor proteins. Cells can regulate the adhesive activity of cadherin proteins by the phosphorylation of proteins anchored to the cadherin cytoplasmic tail. Cadherins can transmit signals to the cell interior. p120–catenin complex, which binds to the membrane proximal domain of the cadherin, is implicated in signalling to Rho GTPases. Vascular endothelial cadherin (VE-cadherin) not only mediates adhesion between endothelial cells but also required for endothelial cell survival. Indeed, deletion of VE-cadherin impairs vascular remodelling and endothelial cell survival while endothelial cells still adhere one to each other via N-cadherin [60, 61].
Selectins
Selectins are cell-surface carbohydrate-binding proteins that mediate a variety of transient, Ca2+-dependent, cell–cell adhesion interactions in the bloodstream. Each selectin is a transmembrane protein with a highly conserved lectin domain that binds to a specific oligosaccharide on another cell. The cell–cell adhesions mediated by selectins are heterophilic: selectins bind to specific oligosaccharides on glycoproteins and glycolipids. The three main selectin types are: L-selectin expressed by Leukocytes, P-selectin first identified in Platelets but also found in activated endothelial cells and E-selectin specifically expressed by activated endothelial cells. Selectins have an important role in binding leukocytes to endothelial cells lining blood vessels, thereby enabling the blood cells to migrate out of the bloodstream into a tissue. For example in lymphoid organs, the endothelial cells express oligosaccharides that are recognized by L-selectin on lymphocytes, causing their rolling on endothelial cells [62]. In inflammatory condition, local chemical mediators induce endothelial E- and P-selectin expression, which allows recruitment of leukocytes expressing oligosaccharide on membrane glycolipids and glycoproteins. Selectins do not act alone; they collaborate with integrins, which strengthen the binding of the blood cells to the endothelium. Selectins and integrins act in sequence to let white blood cells leave the bloodstream and enter tissues (recently reviewed in [63]). The selectins mediate a weak adhesion because the binding of the lectin domain of the selectin to its carbohydrate ligand is of low affinity. This allows the white blood cell to adhere weakly and reversibly to the endothelium, rolling along the surface of the blood vessel propelled by the flow of blood. The rolling continues until the blood cell activates integrins, causing the cell to bind strongly to the endothelial cell surface and to crawl out of the blood vessel between adjacent endothelial cells.
Ig-superfamily
The molecules responsible for Ca2+-independent cell–cell adhesion belong to the large immunoglobulin superfamily (Ig-Sf) of proteins. These proteins contain one or more Ig-like domains that are classified as V or C according to their similarity with the variable or constant domains of immunoglobulins. Ig CAMs have a large amino-terminal extracellular domain containing Ig folds, a single transmembrane helical segment and a cytoplasmic tail. The latter interacts with cytoskeletal protein such as actin, ankyrins or spectrins and activates intracellular signalling pathways [64]. Outside the cell Ig CAMs mediate primarily homophilic cell–cell adhesion but also some heterophilic interactions. Indeed, in the context of antigen presentation, leuko-stromal or leuko-endothelial interactions, Ig CAMs are major counter-receptors for integrins. Members of the Ig CAM family function in a wide variety of cell types and are involved in many different biological processes. The most well characterized CAMs of this family in the context of BM homeostasis are ICAM-1 (CD54), ICAM-2 (CD102), ICAM-3 (CD50), PECAM-1 (CD31), VCAM-1 (CD106), Junctional Adhesion Molecules (JAMs), endothelial cell-selective adhesion molecule (ESAM) and ALCAM (CD166).
The mucin-like family
Mucin-like family or sialomucins represent an emerging adhesion molecular family consisting in a group of heavily glycosylated proteins rich in serine and threonine residues [65]. The CD34 antigen was the first sialomucin to be described as cell surface marker of human hematopoietic stem and progenitor cells (HSPCs) [66]. Additional members expressed by HSCs include CD45RA, PSGL-1, CD43 or CD164 [67]. Mucin-like molecules have been successively reported as anti-adhesive receptors endowed with cytoprotective functions or as pro-adhesive molecules interacting with selectins. For example, CD34 and GlyCAM-1 expressed on certain endothelial cells of lymph nodes bind to L-selectin on leukocytes. Conversely, PSGL-1 expressed on leukocytes interacts with endothelial E-selectin and P-selectin in inflammatory conditions.
CD44
CD44 is a highly elongated molecule composed of three distinct regions: N-terminal, middle and C-terminal domains. CD44 is encoded by a single gene, but exists in more than 20 isoforms because of alternative splicing and different post-translational modifications [68, 69]. The extracellular part is responsible for binding hyaluronic acid but CD44 can also interact with other ligands, such as OPN, collagens, matrix metalloproteinases (MMPs) or E-selectin. The cytoplasmic tail is associated with actin filaments of cytoskeleton through an ankyrin-like molecule. CD44 has been implicated in thymus homing of BM prothymocyte [70].
Integrins
Integrins are heterodimers of α and β subunits and each subunit has a large extracellular domain, a single membrane-spanning region and a short cytoplasmic domain. Both subunits contribute to the ligand-binding site and 18 types of α subunits can pair in various combinations with 8 types of β subunits. Integrins bind to ECM proteins such as fibronectin, laminin or collagen [71] and to Ig CAMs such as ICAMs, JAMs or VCAM-1. Integrins containing β2 chain are exclusively expressed by hematopoietic cells and can bind Ig Sf molecules in addition to seric factors such as iC3b, Factor X or fibrinogen. The main ligands of integrin α4β1 are VCAM-1 and the alternatively spliced CS-1 segment of fibronectin [72, 73], although α4β1 can bind to alternative ligands such as OPN, thrombospondin or JAM-B [74–77]. Integrins typically exhibit relatively low affinities for their ligands. However, the multiple weak interactions generated by binding of hundreds or thousands of integrin molecules to their ligand allow a cell to remain firmly anchored providing the so-called “velcro effect”. Alternatively, in situations where cells are migrating, specific contacts must be highly dynamic, a phenomenon which is facilitated if individual interactions are of weak affinity. Integrins undergo dynamic changes during the ligand binding process, including relative movements of subunits and conformational changes within domains [78]. Integrins can exist in various affinity states for their ligands; these affinity states can be regulated either by extracellular factors such as divalent cations or by intracellular signalling involving small GTPases [79, 80]. Cells attach to the ECM through two types of integrin-dependent structures: focal adhesions and hemidesmosomes [81, 82]. Intracellular integrin domain interacts with a variety of signalling and structural proteins: the adapter proteins talin and vinculin to form focal adhesion, the adaptor protein paxillin to activate focal adhesion kinase (FAK) or the related kinase Pyk-2, actin or intermediate filaments. Because integrins do not have intrinsic kinase activity, the recruitment of these non-receptor kinases is essential for activation of intracellular signalling pathway. Intracellular adaptors recruited to integrin cytoplasmic tails activate a number of Src-homology domain (SH)2 and SH3 containing adaptor proteins which will result in activation of phosphoinositide 3 kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways.
Adhesion molecule expression by hematopoietic stem and progenitor cells
BM HSPCs express a large panel of adhesion molecules. Indeed, in human, they are identified by expression of the mucin-like molecule CD34, but they express other molecules of the same family such as CD43, PSGL-1 and CD164. Moreover, number of studies has shown that HSPC express ICAM-1, PECAM-1, L-selectin, CD44, αLβ2, αVβ3, α4β7, or the β1 integrin subfamily [83–86]. More recently, others and we have reported that HSCs express high levels of diverse Ig-Sf adhesion molecules such as ALCAM [87, 88], ESAM [89, 90], JAM-A [91] or JAM-C [92–94].
Adhesion mechanisms involved in HSC/Niche interactions
In addition to their well-documented role in initiation and maintenance of contact between HSCs and hematopoietic microenvironment, CAMs act as bona fide signalling molecules and directly participate to haematopoietic regulation. Coupling cell-adhesion with signalling allows integration of cell localization within a given microenvironment with signals responsible for survival, growth and differentiation. Haematopoiesis is sustained by a renewable pool of stem cells that interacts with distinct, sequential and specific microenvironments during normal development and throughout adult life. Indeed, HSC location changes during development. Initially, haematopoiesis occurs in the yolk sac and the aorta-gonad-mesonephros (AGM) region before moving to the foetal liver and finally to the BM near birth. Since foetal trafficking of HSCs has been recently reviewed [95], we will focus on adhesion mechanisms used by adult HSC in several key biological processes: homing (selective movement of HSCs to BM), transmigration (movement of HSCs across the endothelial barrier), lodgement (restricted settling of the cells in the niche), engraftment (proliferation, self-renewal and differentiation of HSCs) and mobilization (induced relocalization of HSCs in the bloodstream) (Fig. 3).
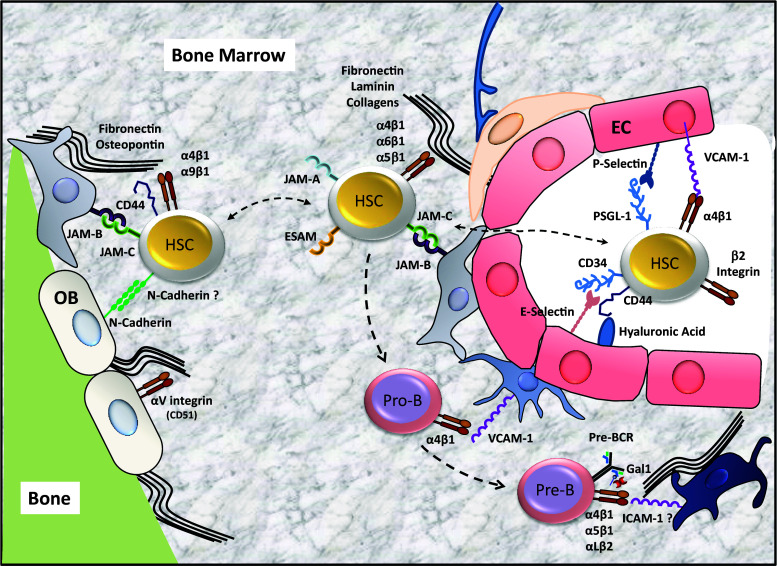
Fig. 3.
Leuko-stromal adhesion interactions within BM niches. Adhesion receptors interactions between hematopoietic stem cells (HSC), pro B cells (pro-B) or pre-B cells (pre-B) with stromal cells and extracellular matrix components of the bone marrow are shown. EC endothelial cells, OB osteoblasts
Homing
Strictly speaking, homing to the BM refers to specific leuko-endothelial interactions of circulating HSCs with BMECs allowing specific trans-endothelial migration of HSCs in BM parenchyma. Extravasation of circulating HSCs within the BM requires a set of molecular interactions that support the recognition of BM sinusoidal endothelium by circulating HSCs. HSCs are captured by and roll on E-selectin, P-selectins and VCAM-1 which are constitutively expressed by endothelial cells of the BM. In 1995, Papayannopoulou et al. have developed a progenitor homing assay in adult mice and shown that homing was significantly reduced (~50 %) by the inhibition of VCAM-1 or its ligand α4β1 (VLA-4)—using function blocking antibodies [96]. Three years later, Frenette et al. have added P- and E-selectins to the picture by demonstrating that homing of HSCs to the BM of E- and P-selectin-deficient mice is severely impaired and can be further compromised using a function blocking VCAM-1 antibody [97]. Intravital microscopy of adult BM vasculature has confirmed the specific contribution of α4β1/VCAM-1 and P- and E-selectins to rolling interactions of progenitors on BM sinusoids and venules [98]. PSGL-1 has been shown to be the major selectin ligand on HSCs [99]. Analyses of selectin ligand expression and function have uncovered developmental regulation of key glycosylation enzymes that synthesize selectin ligands. CD34+ cord blood-derived cells have reduced selectin mediated rolling compared to adult CD34+ cells obtained from the BM or peripheral blood. This deficiency is due to reduced fucosyltransferase activity in neonatal CD34+ cells [100]. Conversely, in vitro fucosylation of CD44 in mesenchymal progenitor cells increased BM homing ability by converting CD44 into a bona fide E-selectin ligand [101]. CD44 is expressed on HSPCs but is unable to bind to E-selectin unless properly fucosylated [102]. CD44 may also contribute to progenitor homing by interacting with hyaluronic acid [103]. HSC express high levels of α4 integrins that mediate rolling on VCAM-1. It has been shown that α4 integrin synergize with β2 integrins during the initial endothelial capture of HSCs to the BM [104]. This was confirmed in another study in which the function of α4 integrin in BM homing has been extended to α4β7/MadCAM interaction [84]. Although results are contradictory, α6 integrin has also been involved in HSC migration to the BM either as positive or negative regulator of homing [105, 106]. Early on, it has been thought that integrin activation converting rolling HSC in firmly adhered cells could be controlled by CXCL-12 signalling through the seven-transmembrane G protein-coupled receptor CXCR4 because of the central role of this axis in hematopoiesis and in foetal BM colonization [107, 108]. Later on, blockade of CXCR4 was shown to inhibit human CD34+CD38− immature human progenitor engraftment in NOD/SCID mice while CXCR4 overexpression increased their abilities to engraft [109, 110]. In another study, CXCL-12 was shown to mediate activation of αLβ2, α4 and α5β1 integrins [111]. However, engraftment does not only reflect homing and a more recent study using short term homing assays has demonstrated that CXCR4 is dispensable for HSC homing to the BM [112]. Rho family proteins Rac1 and Rac2 are activated in response to CXCL-12/CXCR4 signalling. Downstream signals from CXCR4 involve the activation of PI3K, aPKC-ζ and ERK signalling, although aPKC-ζ is dispensable for hematopoietic activity as demonstrated using conditional knock-out mice [113–115]. The combination of Flt3 ligand and CXCL-12 acts synergistically in the migration of CD34+ cells while prolonged exposure to Flt3 ligand may down-regulate CXCR4 expression and impair the migration of CD34+ cells toward CXCL-12 [116]. Following firm adhesion, CXCL-12 induces an integrin-dependent transmigration of progenitors across the endothelial lining cells, a phenomenon referred to as diapedesis. Various adhesion molecules and associated proteins such as CD9 [117], CD99 [118] or PECAM-1 [119] have been shown to contribute to HSCs trans-endothelial migration. Trans-endothelial migration is also facilitated by CXCL-12 dependent modulation of CD44 cell adhesion by increasing its avidity to hyaluronan in the BM sinusoidal endothelium [103].
Lodgement
Lodgement has often been used interchangeably with homing, but it implies a more definitive settling of HSCs in their niche. Within the BM, HSCs are attracted to CXCL-12 rich regions near blood vessels and the endosteum where they lodge into special microenvironments, respectively, the vascular/endothelial and the endosteal/osteoblastic niches. HSCs take advantage of selectively expressed cell surface molecules capable of mediating strong niche interactions. To retain HSCs, the niches express cell adhesion molecules, matrix proteins, growth factors and chemokines that regulate stem cell quiescence and attraction to the niche. Differential engraftment of human CD34+CD38− stem cells injected either directly in the marrow or intravenously has allowed demonstrating that the CXCR4 receptor is necessary for SCID-repopulating cell engraftment even when the homing step is bypassed by intra-marrow injection [120]. HSCs have been localized near spindle-shaped N-cadherin expressing osteoblasts in the endosteal niche [13], however this was not due to homophilic N-cadherin-mediated retention since HSC activity is restricted to cells lacking N-cadherin expression [121]. This has been confirmed in follow-up studies showing that low levels of N-cadherin distinguish the HSC population, which is primed to migrate out of the niche [122]. Parathyroid hormone (PTH) has been reported to increase osteoblasts CXCL-12 expression reinforcing HSCs retention in the endosteal niche [123] and increase the number of HSCs in BM [6]. Other studies suggest that the transmembrane form of stem cell factor (tm-SCF) plays an important role in the lodgement into the endosteal niche. Mice lacking tm-SCF showed a significant impairment in the lodgement of transplanted cells within the endosteal region. The important role of tm-SCF in this process was confirmed by analyzing the spatial distribution of HSC isolated using a neutralizing antibody to c-kit, the SCF receptor [124]. Studies by Kiel and collaborators have shown that the majority of HSCs were in the perivascular region with only a minority (~16 %) at the periendosteal region. Histologic analysis revealed that 70 % of HSCs identified based on the SLAM markers (CD48−CD150+LSK+) are near blood vessels [19]. Interaction with sinusoidal endothelium at the vascular niche has been suggested to induce hematopoietic cell expansion, differentiation and egress of mature progenitors [21]. BMECs produce a large set of soluble factors including cytokines and hematopoietic growth factors, such as CXCL-12, IL-6, G- and GM-CSF but also growth inhibitor factors such as transforming growth factor-β (TGF-β) and nitric oxide that all together regulate HSC activity in vascular niche [125]. It has also been shown that inhibition of the tyrosine kinase Tie2 receptor, expressed on endothelium and on hematopoietic cells, leads to a delay in hematopoietic recovery [21]. In addition, basic fibroblast growth factor-2 (FGF-2), which is involved in vascular development, has also been shown to be a major mediator of HSC niche remodelling [126]. From an adhesive point of view, the remarkable finding that HSC quiescence and self-renewal are enhanced in E-Selectin knock-out mice indicate a central role for BMECs [127], although the exact mechanism through which E-Selectin influences HSC remains to be determined.
Engraftment
Engraftment refers to the ability of HSCs to proliferate, to self-renew and to generate multilineage progeny. Following the lodgement into a suitable niche, HSCs expand their numbers while maintaining a balance between quiescence, self-renewal and lineage commitment. The physical interaction between stem cells and their niche components is a critical regulator of this balance. The retention of HSCs in the niche is regulated by adhesion molecules expressed by BM stromal cells and HSC itself. Cell adhesion molecules are not only required for HSC anchoring to the niche, but also involved in the regulation of cell cycle status of HSCs. During adult life, a proportion of HSCs are kept in a quiescent/dormant state (G0 phase of the cell cycle), which contributes to their long-term maintenance, thus preventing stem cell exhaustion and accumulation of mutations. The dormant cells represent about 15 % of HSCs and divide only about every 145 day, they represent a reserve stem cell pool endowed with long-term reconstitution properties [128]. This is thought to be due to differential adhesion of dormant and cycling HSC to stromal cells of the niches, such that when division occurs and adhesion is lost, HSCs engage differentiation and/or proliferation. The matrix glycoprotein OPN produced by osteoblasts interacts with CD44 and β1 integrins on HSCs and can regulate negatively the HSC proliferation and differentiation. OPN restricts HSCs from excessive expansion, limiting the size of HSC pool in the endosteal niche. OPN deficient mice exhibit markedly enhanced cycling of HSCs [4]. Interaction of the receptor tyrosine kinase Tie2, expressed on HSCs, with its ligand Ang-1, expressed by OBs, upregulates β1 integrin, which in turn promotes their interactions with ECM and cellular components of the niche. Moreover, Tie2/Ang-1 pathway is involved in the maintenance of long-term repopulating activity of HSCs in vivo inducing HSCs quiescence [10]. Similarly, c-kit/SCF interactions hold HSCs anchored to the endosteal niche, thus promoting their quiescence [124]. The class III receptor tyrosine kinase c-Kit is expressed on all HSCs and its ligand, SCF, is constitutively produced by BM endothelial cells and perivascular stromal cells as well as by other stromal cells. SCF/c-Kit interaction has been shown to regulate integrin activation and HSC adhesion to BM stromal cells [129–131]. TPO secreted by OBs is an important mediator in supporting thrombopoietin receptor (MPL)-dependent HSC/stromal cell adhesion which results in increased HSCs quiescence as demonstrated by the reduced pools of HSCs in Thpo and Mpl deficient mice [11, 132]. The role of α4β1 integrin in HSC retention has been evaluated using conditionally deficient mice for which a rapid increase in the number of circulating progenitors in blood was observed [133]. Other integrins may also participate to the engraftment. αMβ2 expressed on progenitors and activated HSCs appear to retain progenitors in situations where egress is enforced [134]. It has also been shown that α9β1 is involved in HSC/osteoblast adhesion and that anti-α9 and anti-β1 antibodies inhibit this interaction [135]. However, the function of integrins in leuko/stromal interactions could not be dissociated from their activation state, which is finely tuned by cytokines and chemokine receptors signalling [136]. This is well illustrated by apparent contradictory results obtained from the study of two independent CXCR4 conditional knock-out strains of mice. Gene deletions were, respectively, induced using Mx1-Cre or ROSA-Cre ERT2 systems and resulted in increased numbers of circulating progenitor cells in both cases. However, conclusions with respect of HSCs retention and expansion were radically different with a marked depletion in HSC numbers when deletion was induced using poly(I:C) [34], while HSC numbers were expanded when Tamoxifen was used for CXCR4 deletion [137]. This is likely due to interference of poly(I:C) response with CXCL-12/CXCR4 signalling within BM microenvironment. Rho GTPases relay the signals delivered by CXCR4 or c-Kit engagement. Rho GTPases encompass a class of intracellular signalling enzymes such as Rho, Rac or cdc42 that serve as crucial regulators of the actin cytoskeleton, cell polarity and proliferation. Rac1−/−, but not Rac2−/−, HSCs fail to engraft in the BM of irradiated recipient mice. Rac1−/− HSCs show impaired spatial localization to the endosteum and interaction with the BM microenvironment in vitro is markedly altered [138]. More recently, it has been shown that Rac1 activity leads to reversible conformational changes in human CXCR4 that potentiates CXCL-12/CXCR4 BM retention mechanisms of HSCs [139]. An important component of CXCL-12/CXCR4 pathway is the atypical protein kinase C ζ (aPKC-ζ), which translocates to the cell membrane upon CXCL-12 stimulation. Accordingly, inhibition of aPKC-ζ inhibits engraftment but not homing of human CD34+ cell in NOD/SCID mice, although this essential function was not confirmed in a study using hematopoietic-specific aPKC-ζ deficient mice [114, 115]. Finally, our laboratory has recently shown that JAM-B/JAM-C adhesion axis is also implicated in retention of HSCs within niches. Indeed, HSCs interact with, and adhere to, JAM-B-expressing BM stromal cells [94, 140]. The binding of JAM-B to HSCs is dependent on JAM-C expression by hematopoietic stem cells, which is down-regulated during transition from LT-HSC to ST-HSC [92]. Accordingly, imbalanced pools of HSC and myeloid progenitors in Jam-b and Jam-c-deficient animals are likely due to increased HSC cell cycle as a result of reduced adhesion of HSC to stromal cells. Nevertheless, reduced numbers of available niches for HSCs could not be excluded since decreased proportion of endothelial cells, osteoblasts, and mesenchymal stem cells has been observed in Jam-b-deficient mice [94]. On the opposite to JAM-C, ESAM expression by HSC is associated to cell cycling, but appears mandatory for re-establishment of homeostatic hematopoiesis after a myeloablative treatment [141]. This would be in agreement with the idea that cycling HSCs endowed with long-term reconstitution potential contribute to hematopoietic homeostasis in unperturbed hematopoiesis [142]. In summary, homing and engraftment are partially regulated by common mechanisms including cytokine signalling and adhesive interactions, but homing is a rapid process, which leads to transient retention and does not require cell division while engraftment does. Identification of further adhesion interactions that are functionally important to regulate HSC quiescence and cell cycling remain essential to further manipulate the hematopoietic system in order to improve outcome in patients treated with BM grafts after conditioning.
Mobilization
Hematopoietic stem cells egress from the BM to the circulation occurs under homeostatic conditions but is dramatically increased when danger signals such as blood loss or inflammation are present. Loss of BM retention is mediated by disruption of adhesion interactions and differential sensing of promigratory signals. Under homeostatic conditions, HSCs egress follows a physiologically regulated circadian rhythm orchestrated by the central nervous system acting on β-adrenoreceptors expressed on non-hematopoietic cells that control levels of CXCL-12 [143, 144]. It has been proposed that the role of homeostatic HSC recirculation through blood, peripheral tissue and lymph is to regenerate peripheral pool of tissue-resident cells such as dendritic cells [145]. Nevertheless, the primary function of constitutive HSC recirculation consists essentially in niche regeneration and reseeding hematopoiesis at distant BM site in order to maintain constant number of HSC in the organism [146]. HSC recruitment into the blood is enhanced during stress situations such as inflammation or bleeding, which induce up-regulation of proteolytic activity, with concomitant down-regulation of CXCL-12 and adhesion interactions, paving the way for egressing HSCs [147, 148]. The major source of cells for clinical transplantation protocols is via peripheral blood (PB) mobilization of BM derived HSCs. G-CSF is the most commonly used agent in the clinical area that elicits robust mobilization in 5–10 days. Accordingly, mice deficient for the G-CSF receptor (encoded by Csf3r) are unresponsive to G-CSF treatment however Csf3r −/− HSCs can be mobilized by G-CSF in chimeric mice harbouring mixtures of Csf3r +/+ and Csf3r −/− hematopoietic cells. This suggests that expression of the CSF3R on HSCs is not required for G-CSF-mediated mobilization suggesting that CSF3R-dependent signals act in trans [149]. In a similar manner, down-regulation of CXCL-12/CXCR4 and VCAM-1/α4β1 retention signals following G-CSF-induced mobilization is indirect and likely due to proteolytic enzyme release from activated myeloid cells [150, 151]. However, studies using proteases-deficient mice revealed that mobilization does not only rely on VCAM-1 or CXCL-12 down-regulation since HSC release may occur in a stimulus specific manner or in absence of VCAM-1 down-regulation [152, 153]. This suggests that mobilization relies on an intricated response of BM microenvironment rather than a cell specific response [154]. Along this line, multiple proteases such as elastase, cathepsin-G, MMP-2, MMP-9 or CD26 have been shown to cleave CXCL-12 and several anti-proteases such as Serpin1 and Serpin3 have been shown to dramatically drop following G-CSF administration, shifting the balance between proteases and their inhibitors [155]. G-CSF stimulation has also been shown to induce osteoclast bone resorption and calcium release, which causes detachment of HSCs from fibronectin [156]. However, more recently Miyamoto et al. showed that mobilization after G-CSF injection in osteopetrotic mouse models is comparable or even higher compared with wild-type mice, suggesting that osteoclasts are dispensable for HSC mobilization [157]. In addition to G-CSF, several other compounds or drugs have been shown to trigger HSC egress from the marrow. Several cytokines have been suggested to increase the number of circulating progenitors in blood, including IL-1, IL-3, IL-6, IL-7, IL-8, IL-11, IL-12, GM-CSF and SCF. The administration of CXCR4 antagonist AMD3100 disrupts CXCL-12/CXCR4 interactions in the BM, leading to the loss of HSC retention and enhanced HSCs mobilization with synergistic effect with G-CSF [158]. Similarly, blocking α4 integrin with natalizumab in multiple sclerosis patients results in a six fold increase in circulating CD34+ cells one day after the first dose [159]. Finally, disruption of CD44 interaction using blocking antibody has also been shown to induce HSPC mobilization and CD44 is known to be down-regulated on mobilized immature human CD34+ cells [160, 161]. This is similar to what we recently reported for the adhesion molecule JAM-C, suggesting that coordinated regulation of some common adhesive mechanism occurring at leuko-stromal and leuko-endothelial cell–cell contacts may be essential for dynamic migration of HSCs in and out of the BM. In addition to these micro-environmental controls, cell-intrinsic mechanisms contribute to HSCs recruitment to the circulation upon demand. Cell autonomous mechanisms include signalling cascades that potentiate HSC motility, such as activation of Rho GTPases and generation of ROS [162]. Moreover, during mobilization, OPN-expressing osteoblasts are depleted through a reversible mechanism dependent on trophic endosteal macrophages that support osteoblast function [163, 164]. Altogether, these data support a model in which HSC retention in the BM is dependent on multiple signalling networks occurring into the BM microenvironment. Perturbation of this network results in mobilization of HSCs to the blood and loss of certain characteristic features of HSC residing in the BM. Mobilized HSCs express lower levels of c-Kit, integrin α4β1 and CXCR4 but higher levels of proapoptotic proteins such as caspase 3 and 4, and metalloproteinase enzymes. Moreover, mobilized HSCs show significantly higher percentage of non-cycling quiescent cells than their BM counterparts [165]. Although we learned a lot about adhesion interactions and cytokines that regulate dynamic HSC interactions with the niche over the last two decades, efforts must be done to better translate the findings to pathological situations in which niche interactions of sick hematopoietic cells are rather deleterious.
Adhesion mechanisms involved in B Cell/Niche interactions
Adhesion of developing B cells with stromal cells has been shown to strongly rely on α4β1 integrin both in mouse and in human. First, long term cultures of pre-B cells were inhibited by the addition of anti-α4β1 antibody that blocked interaction with VCAM-1 expressed by the stromal cells [166]. In addition, the level of VCAM-1 expression by established BM stromal cell cultures was shown to be important for adhesion and development of human B cell precursors [167]. Most importantly, B cell differentiation is severely compromised from the earliest stages in the BM of integrin α4 deficient chimeric mice demonstrating the crucial role played by this integrin for the interaction with B cell niches [168]. Silberstein and colleagues then demonstrated that sustained adhesion of pro-B and pre-B cells to stromal cell niches was dependent on a prolonged activation by CXCL-12 and relied on α4β1 integrin adhesion to VCAM-1 [169]. The increase in integrin-dependent adhesion was associated with a sustained activation of focal adhesion kinase (FAK) in pro-B and pre-B cells, but not in more mature B cells. Furthermore, the specific deletion of FAK in B cells induced a decrease in B cell development from the pro-B cell stage and an increase of pro-B cell egress to the periphery [170].
At the transition between pro-B and pre-B cell stage, cells are selected based on the expression of the pre-BCR. It was first suggested that pre-BCR activation was ligand-independent and that a tonic signal could be induced thanks to the localization of the pre-BCR in lipid rafts at the cell surface [171]. It has also been proposed that the pre-BCR was able to self-aggregate through the interaction of positively charged amino acids from the extra loop of λ5 (λ5-EL) either with negatively charges residues of the extra loop of VpreB on the adjacent pre-BCR or with the asparagine N46 from the Igµ CH1 domain [172, 173]. Other studies have shown the existence of a ligand-dependent activation. The λ5-EL interacts with heparan sulphate proteoglycans present at the surface of BM stromal cells [174]. The λ5-EL is also able to interact with Gal-1, inducing pre-BCR signalling [175]. Gal-1 is an S-type lectin, which binds β-galactoside glycoconjugates through a carbohydrate recognition domain (CRD). Gal-1 is secreted by BM stromal cells, binds λ5 through direct protein–protein contacts [175, 176] and is anchored to the pre-B cell and stromal cell surface by interacting with the glycosylated chains of integrins including α4β1, α4β7, α5β1 and αLβ2 [177, 178]. Tripartite interaction between pre-BCR, Gal-1 and integrins induce pre-BCR clustering and then signalling when pre-B cell integrins bind to their ligands present at the stromal cell surface. Finally, in the case of mice deficient for the alpha 1,6-fucosyltransferase, the core fucosylation of α4β1 was affected leading to an impaired interaction with VCAM-1 and a block at the pro-B to pre-B cell transition [179]. Altogether these results demonstrate the importance of glycosylated integrins and Gal-1 at the pre-BCR checkpoint and therefore in pre-B cell differentiation. While the early-B cells need prolonged contacts with stromal cells in the BM to accomplish their maturation, immature B cells have to egress to the periphery where they will terminate their selection. Immature B cell retention in the parenchyma is dependent on α4β1 integrin adhesion to VCAM-1 [180, 181]. Most importantly, their egress from the BM and then detachment from the stromal cells is controlled by Gαi protein-coupled receptors including CXCR4 and the receptor to Sphingosine-1 phosphate (S1PR) [182–184]. While S1PR expression is important for the export of immature B cells, a decrease in the level of CXCR4 is on the opposite required. To finish, when they leave the BM, many immature B cells stay in the sinusoids. This retention was shown to be dependent on the expression of the integrin α4β1 and the cannabinoid receptor 2 (CB2) and was proposed to facilitate receptor editing by increasing the time spent by B cells in the BM [182]. This concept is further supported by the fact that a high BCR signal, which is reminiscent of self-reactive B cells, antagonizes the decrease in CXCR4 expression by immature B cells [183].
Outlook
An incredible amount of information about hematopoietic cell crosstalk with BM microenvironment has been accumulated over the last 25 years. Recent studies have established a major role for endothelial cells in the regulation and the maintenance of HSCs during adult life, merging together two research fields: vascular biology and haematology. However, this could have been anticipated since definitive HSCs emerge from the haemangiogenic endothelium in the dorsal aorta during embryonic life. Whether maintenance of adult HSC share more in common with embryonic development than promiscuous interactions between HSC and endothelial cells remains to be determined, but we will likely get new insights from comparative studies of hematopoietic niches during embryogenesis, adult life and ageing. Indeed, it is becoming increasingly clear that haematological malignancies result from expression of a genetic alteration in a permissive microenvironment. However, we do not know a lot about the differential adhesive signals that make or convert a non-permissive microenvironment into a permissive one. Future studies aiming at identification of such pathways will help defining the best-suited therapeutic approaches to target leuko-stromal interactions in haematological malignancies while maintaining a supportive microenvironment for normal hematopoietic cells.
Acknowledgments
The authors apologize to those whose work has not been cited due to space limitation. MDG is supported by a grant from “Fondation de France”. Our laboratory is supported by Inca (# 5940), ARC Foundation (PJA# 20141201990 to MAL; PJA# 20131200298 to SM), SIRIC (# INCa-DGOS-Inserm 6038), PACA Cancéropôle and PACA Region.
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 5.Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh Migr. 2014;8:563–577. doi: 10.4161/19336918.2014.968501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 7.Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassinger J, Haylock DN, Williams B, Olsen GH, Nilsson SK. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116:3185–3196. doi: 10.1182/blood-2009-12-260703. [DOI] [PubMed] [Google Scholar]
- 9.Shiozawa Y, Taichman RS. Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol. 2012;40:685–694. doi: 10.1016/j.exphem.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, Antonchuk J, Jacobsen SE. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 14.El-Badri NS, Wang BY, Cherry, Good RA. Osteoblasts promote engraftment of allogeneic hematopoietic stem cells. Exp Hematol. 1998;26:110–116. [PubMed] [Google Scholar]
- 15.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 19.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 21.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 22.Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- 23.Schweitzer KM, Vicart P, Delouis C, Paulin D, Drager AM, Langenhuijsen MM, Weksler BB. Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest. 1997;76:25–36. [PubMed] [Google Scholar]
- 24.Wang L, Benedito R, Bixel MG, Zeuschner D, Stehling M, Savendahl L, Haigh JJ, Snippert H, Clevers H, Breier G, Kiefer F, Adams RH. Identification of a clonally expanding haematopoietic compartment in bone marrow. EMBO J. 2013;32:219–230. doi: 10.1038/emboj.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–386. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 26.Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106:4093–4101. doi: 10.1182/blood-2005-02-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, Aifantis I, Frenette PS, Kitajewski J, Rafii S, Butler JM. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, Russell L, Chen B, Chao NJ, Chute JP. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Isern J, Mendez-Ferrer S. Stem cell interactions in a bone marrow niche. Curr Osteoporos Rep. 2011;9:210–218. doi: 10.1007/s11914-011-0075-y. [DOI] [PubMed] [Google Scholar]
- 37.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 38.Whitlock CA, Witte ON. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci USA. 1982;79:3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorshkind K, Schouest L, Fletcher WH. Morphologic analysis of long-term bone marrow cultures that support B-lymphopoiesis or myelopoiesis. Cell Tissue Res. 1985;239:375–382. doi: 10.1007/BF00218018. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen K, Osmond DG. Microenvironmental organization and stromal cell associations of B lymphocyte precursor cells in mouse bone marrow. Eur J Immunol. 1990;20:2395–2404. doi: 10.1002/eji.1830201106. [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/S1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 43.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/S1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 44.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 47.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- 54.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/S1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 55.Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, Mancini SJ. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117:6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 56.Pelanda R, Torres RM. Receptor editing for better or for worse. Curr Opin Immunol. 2006;18:184–190. doi: 10.1016/j.coi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–5944. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- 58.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289–299. doi: 10.1016/S1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]
- 59.Hyafil F, Babinet C, Jacob F. Cell–cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- 60.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 61.Giampietro C, Taddei A, Corada M, Sarra-Ferraris GM, Alcalay M, Cavallaro U, Orsenigo F, Lampugnani MG, Dejana E. Overlapping and divergent signaling pathways of N-cadherin and VE-cadherin in endothelial cells. Blood. 2012;119:2159–2170. doi: 10.1182/blood-2011-09-381012. [DOI] [PubMed] [Google Scholar]
- 62.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol. 2007;27:1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 65.Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 66.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 67.Watt SM, Chan JY. CD164–a novel sialomucin on CD34+ cells. Leuk Lymphoma. 2000;37:1–25. doi: 10.3109/10428190009057625. [DOI] [PubMed] [Google Scholar]
- 68.Hertweck MK, Erdfelder F, Kreuzer KA. CD44 in hematological neoplasias. Ann Hematol. 2011;90:493–508. doi: 10.1007/s00277-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 69.Ghaffari S, Smadja-Joffe F, Oostendorp R, Levesque JP, Dougherty G, Eaves A, Eaves C. CD44 isoforms in normal and leukemic hematopoiesis. Exp Hematol. 1999;27:978–993. doi: 10.1016/S0301-472X(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 70.Lesley J, Hyman R, Schulte R. Evidence that the Pgp-1 glycoprotein is expressed on thymus-homing progenitor cells of the thymus. Cell Immunol. 1985;91:397–403. doi: 10.1016/0008-8749(85)90237-0. [DOI] [PubMed] [Google Scholar]
- 71.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-W. [DOI] [PubMed] [Google Scholar]
- 73.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-Q. [DOI] [PubMed] [Google Scholar]
- 74.Bayless KJ, Meininger GA, Scholtz JM, Davis GE. Osteopontin is a ligand for the alpha4beta1 integrin. J Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 75.Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279:42393–42402. doi: 10.1074/jbc.M407631200. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig RJ, Hardt K, Hatting M, Bistrian R, Diehl S, Radeke HH, Podda M, Schon MP, Kaufmann R, Henschler R, Pfeilschifter JM, Santoso S, Boehncke WH. Junctional adhesion molecule (JAM)-B supports lymphocyte rolling and adhesion through interaction with alpha4beta1 integrin. Immunology. 2009;128:196–205. doi: 10.1111/j.1365-2567.2009.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr Opin Cell Biol. 2004;16:544–551. doi: 10.1016/j.ceb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Huveneers S, Danen EH. Adhesion signaling—crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 80.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 81.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012;24:116–124. doi: 10.1016/j.ceb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Osmani N, Labouesse M. Remodeling of keratin-coupled cell adhesion complexes. Curr Opin Cell Biol. 2015;32C:30–38. doi: 10.1016/j.ceb.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Prosper F, Verfaillie CM. Regulation of hematopoiesis through adhesion receptors. J Leukoc Biol. 2001;69:307–316. [PubMed] [Google Scholar]
- 84.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–2026. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 85.Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(−/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol. 2002;30:176–185. doi: 10.1016/S0301-472X(01)00777-9. [DOI] [PubMed] [Google Scholar]
- 86.Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, Tsukui H, Terasawa M, Shibata T, Nishida K, Kobayashi Y, Petrich BG, Nakauchi H, Eto K, Okano T. Integrin-alphavbeta3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeannet R, Cai Q, Liu H, Vu H, Kuo YH. Alcam regulates long-term hematopoietic stem cell engraftment and self-renewal. Stem Cells. 2013;31:560–571. doi: 10.1002/stem.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chitteti BR, Kobayashi M, Cheng Y, Zhang H, Poteat BA, Broxmeyer HE, Pelus LM, Hanenberg H, Zollman A, Kamocka MM, Carlesso N, Cardoso AA, Kacena MA, Srour EF. CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood. 2014;124:519–529. doi: 10.1182/blood-2014-03-565721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ooi AG, Karsunky H, Majeti R, Butz S, Vestweber D, Ishida T, Quertermous T, Weissman IL, Forsberg EC. The adhesion molecule esam1 is a novel hematopoietic stem cell marker. Stem Cells. 2009;27:653–661. doi: 10.1634/stemcells.2008-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yokota T, Oritani K, Butz S, Kokame K, Kincade PW, Miyata T, Vestweber D, Kanakura Y. The endothelial antigen ESAM marks primitive hematopoietic progenitors throughout life in mice. Blood. 2009;113:2914–2923. doi: 10.1182/blood-2008-07-167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugano Y, Takeuchi M, Hirata A, Matsushita H, Kitamura T, Tanaka M, Miyajima A. Junctional adhesion molecule-A, JAM-A, is a novel cell-surface marker for long-term repopulating hematopoietic stem cells. Blood. 2008;111:1167–1172. doi: 10.1182/blood-2007-03-081554. [DOI] [PubMed] [Google Scholar]
- 92.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Praetor A, McBride JM, Chiu H, Rangell L, Cabote L, Lee WP, Cupp J, Danilenko DM, Fong S. Genetic deletion of JAM-C reveals a role in myeloid progenitor generation. Blood. 2009;113:1919–1928. doi: 10.1182/blood-2008-06-159574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arcangeli ML, Frontera V, Bardin F, Obrados E, Adams S, Chabannon C, Schiff C, Mancini SJ, Adams RH, Aurrand-Lions M. JAM-B regulates maintenance of hematopoietic stem cells in the bone marrow. Blood. 2011;118:4609–4619. doi: 10.1182/blood-2010-12-323972. [DOI] [PubMed] [Google Scholar]
- 95.Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;32:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 100.Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110:559–569. doi: 10.1172/JCI0214047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 102.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 104.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, Harlan JM. Synergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood. 2001;97:1282–1288. doi: 10.1182/blood.V97.5.1282. [DOI] [PubMed] [Google Scholar]
- 105.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 106.Bonig H, Priestley GV, Wohlfahrt M, Kiem HP, Papayannopoulou T. Blockade of alpha6-integrin reveals diversity in homing patterns among human, baboon, and murine cells. Stem Cells Dev. 2009;18:839–844. doi: 10.1089/scd.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 108.Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 2000;72:408–411. [PubMed] [Google Scholar]
- 109.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 110.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 111.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 112.Foudi A, Jarrier P, Zhang Y, Wittner M, Geay JF, Lecluse Y, Nagasawa T, Vainchenker W, Louache F. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- 113.Bonig H, Priestley GV, Nilsson LM, Jiang Y, Papayannopoulou T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104:2299–2306. doi: 10.1182/blood-2004-04-1605. [DOI] [PubMed] [Google Scholar]
- 114.Petit I, Goichberg P, Spiegel A, Peled A, Brodie C, Seger R, Nagler A, Alon R, Lapidot T. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115:168–176. doi: 10.1172/JCI200521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, Cancelas JA. Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci USA. 2011;108:9957–9962. doi: 10.1073/pnas.1103132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood. 2005;105:3117–3126. doi: 10.1182/blood-2004-04-1440. [DOI] [PubMed] [Google Scholar]
- 117.Leung KT, Chan KY, Ng PC, Lau TK, Chiu WM, Tsang KS, Li CK, Kong CK, Li K. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117:1840–1850. doi: 10.1182/blood-2010-04-281329. [DOI] [PubMed] [Google Scholar]
- 118.Imbert AM, Belaaloui G, Bardin F, Tonnelle C, Lopez M, Chabannon C. CD99 expressed on human mobilized peripheral blood CD34+ cells is involved in transendothelial migration. Blood. 2006;108:2578–2586. doi: 10.1182/blood-2005-12-010827. [DOI] [PubMed] [Google Scholar]
- 119.Ross EA, Freeman S, Zhao Y, Dhanjal TS, Ross EJ, Lax S, Ahmed Z, Hou TZ, Kalia N, Egginton S, Nash G, Watson SP, Frampton J, Buckley CD. A novel role for PECAM-1 (CD31) in regulating haematopoietic progenitor cell compartmentalization between the peripheral blood and bone marrow. PLoS ONE. 2008;3:e2338. doi: 10.1371/journal.pone.0002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yahata T, Ando K, Sato T, Miyatake H, Nakamura Y, Muguruma Y, Kato S, Hotta T. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101:2905–2913. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 121.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Haug JS, He XC, Grindley JC, Wunderlich JP, Gaudenz K, Ross JT, Paulson A, Wagner KP, Xie Y, Zhu R, Yin T, Perry JM, Hembree MJ, Redenbaugh EP, Radice GL, Seidel C, Li L. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2:367–379. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 123.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, McCauley LK, Taichman RS. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 125.Li WM, Huang WQ, Huang YH, Jiang DZ, Wang QR. Positive and negative hematopoietic cytokines produced by bone marrow endothelial cells. Cytokine. 2000;12:1017–1023. doi: 10.1006/cyto.1999.0678. [DOI] [PubMed] [Google Scholar]
- 126.Itkin T, Kaufmann KB, Gur-Cohen S, Ludin A, Lapidot T. Fibroblast growth factor signaling promotes physiological bone remodeling and stem cell self-renewal. Curr Opin Hematol. 2013;20:237–244. doi: 10.1097/MOH.0b013e3283606162. [DOI] [PubMed] [Google Scholar]
- 127.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 128.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 129.Kinashi T, Springer TA. Steel factor and c-kit regulate cell–matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- 130.Kodama H, Nose M, Niida S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 131.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 132.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 133.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hidalgo A, Peired AJ, Weiss LA, Katayama Y, Frenette PS. The integrin alphaMbeta2 anchors hematopoietic progenitors in the bone marrow during enforced mobilization. Blood. 2004;104:993–1001. doi: 10.1182/blood-2003-10-3702. [DOI] [PubMed] [Google Scholar]
- 135.Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Muller CA, Aicher WK, Klein G. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 139.Zoughlami Y, Voermans C, Brussen K, van Dort KA, Kootstra NA, Maussang D, Smit MJ, Hordijk PL, van Hennik PB. Regulation of CXCR4 conformation by the small GTPase Rac1: implications for HIV infection. Blood. 2012;119:2024–2032. doi: 10.1182/blood-2011-06-364828. [DOI] [PubMed] [Google Scholar]
- 140.Arcangeli ML, Bardin F, Frontera V, Bidaut G, Obrados E, Adams RH, Chabannon C, Aurrand-Lions M. Function of Jam-B/Jam-C interaction in homing and mobilization of human and mouse hematopoietic stem and progenitor cells. Stem Cells. 2014;32:1043–1054. doi: 10.1002/stem.1624. [DOI] [PubMed] [Google Scholar]
- 141.Sudo T, Yokota T, Oritani K, Satoh Y, Sugiyama T, Ishida T, Shibayama H, Ezoe S, Fujita N, Tanaka H, Maeda T, Nagasawa T, Kanakura Y. The endothelial antigen ESAM monitors hematopoietic stem cell status between quiescence and self-renewal. J Immunol. 2012;189:200–210. doi: 10.4049/jimmunol.1200056. [DOI] [PubMed] [Google Scholar]
- 142.Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 143.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 144.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 147.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 148.Levesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 149.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95:3025–3031. [PubMed] [Google Scholar]
- 150.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.V98.5.1289. [DOI] [PubMed] [Google Scholar]
- 152.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 154.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 155.Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med. 2005;201:1077–1088. doi: 10.1084/jem.20042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Levesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465–3473. [PubMed] [Google Scholar]
- 157.Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, Miyamoto H, Hao W, Morioka H, Chiba K, Yasuda H, Penninger JM, Toyama Y, Suda T, Miyamoto T. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–3441. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 161.Lee S, Im SA, Yoo ES, Nam EM, Lee MA, Ahn JY, Huh JW, Kim DY, Lee SN, Kim MJ, Lee SJ, Chung WS, Seong CM. Mobilization kinetics of CD34(+) cells in association with modulation of CD44 and CD31 expression during continuous intravenous administration of G-CSF in normal donors. Stem Cells. 2000;18:281–286. doi: 10.1634/stemcells.18-4-281. [DOI] [PubMed] [Google Scholar]
- 162.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, Shivtiel S, Kalinkovich A, Caione L, Gammaitoni L, Laurenti E, Buss EC, Shezen E, Itkin T, Kollet O, Petit I, Trumpp A, Christensen J, Aglietta M, Piacibello W, Lapidot T. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117:419–428. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 163.Mayack SR, Wagers AJ. Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood. 2008;112:519–531. doi: 10.1182/blood-2008-01-133710. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 165.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 166.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dittel BN, LeBien TW. Reduced expression of vascular cell adhesion molecule-1 on bone marrow stromal cells isolated from marrow transplant recipients correlates with a reduced capacity to support human B lymphopoiesis in vitro. Blood. 1995;86:2833–2841. [PubMed] [Google Scholar]
- 168.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/S0092-8674(00)81301-X. [DOI] [PubMed] [Google Scholar]
- 169.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Park SY, Wolfram P, Canty K, Harley B, Nombela-Arrieta C, Pivarnik G, Manis J, Beggs HE, Silberstein LE. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol. 2013;190:1094–1102. doi: 10.4049/jimmunol.1202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243–253. doi: 10.1016/S1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 172.Ohnishi K, Melchers F. The nonimmunoglobulin portion of lambda5 mediates cell-autonomous pre-B cell receptor signaling. Nat Immunol. 2003;4:849–856. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- 173.Ubelhart R, Bach MP, Eschbach C, Wossning T, Reth M, Jumaa H. N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol. 2010;11:759–765. doi: 10.1038/ni.1903. [DOI] [PubMed] [Google Scholar]
- 174.Bradl H, Wittmann J, Milius D, Vettermann C, Jack HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of lambda 5 and stroma cell-associated heparan sulfate. J Immunol. 2003;171:2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- 175.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Elantak L, Espeli M, Boned A, Bornet O, Bonzi J, Gauthier L, Feracci M, Roche P, Guerlesquin F, Schiff C. Structural basis for galectin-1-dependent pre-B cell receptor (pre-BCR) activation. J Biol Chem. 2012;287:44703–44713. doi: 10.1074/jbc.M112.395152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Espeli M, Mancini SJ, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood. 2009;113:5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- 178.Rossi B, Espeli M, Schiff C, Gauthier L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol. 2006;177:796–803. doi: 10.4049/jimmunol.177.2.796. [DOI] [PubMed] [Google Scholar]
- 179.Li W, Ishihara K, Yokota T, Nakagawa T, Koyama N, Jin J, Mizuno-Horikawa Y, Wang X, Miyoshi E, Taniguchi N, Kondo A. Reduced alpha4beta1 integrin/VCAM-1 interactions lead to impaired pre-B cell repopulation in alpha 1,6-fucosyltransferase deficient mice. Glycobiology. 2008;18:114–124. doi: 10.1093/glycob/cwm107. [DOI] [PubMed] [Google Scholar]
- 180.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beck TC, Gomes AC, Cyster JG, Pereira JP. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J Exp Med. 2014;211:2567–2581. doi: 10.1084/jem.20140457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]