Abstract
Nicotinamide adenine dinucleotide (NAD+) is a vital molecule found in all living cells. NAD+ intracellular levels are dictated by its synthesis, using the de novo and/or salvage pathway, and through its catabolic use as co-enzyme or co-substrate. The regulation of NAD+ metabolism has proven to be an adequate drug target for several diseases, including cancer, neurodegenerative or inflammatory diseases. Increasing interest has been given to NAD+ metabolism during innate and adaptive immune responses suggesting that its modulation could also be relevant during host–pathogen interactions. While the maintenance of NAD+ homeostatic levels assures an adequate environment for host cell survival and proliferation, fluctuations in NAD+ or biosynthetic precursors bioavailability have been described during host–pathogen interactions, which will interfere with pathogen persistence or clearance. Here, we review the double-edged sword of NAD+ metabolism during host–pathogen interactions emphasizing its potential for treatment of infectious diseases.
Keywords: Nicotinamide adenine dinucleotide (NAD+), Host-pathogen interaction, NAD+/NADH ratio, NADPH, Sirtuins, l-tryptophan
Nicotinamide adenine dinucleotide (NAD+) was initially discovered by Sir Arthur Harden as a ‘cozymase’ for yeast fermentation over 100 years ago. The succeeding work contributed to the identification of NAD+ as a player in hundreds of biochemical reactions through its role in redox reactions. NAD+ is either consumed as a co-substrate by NAD+-consuming enzymes or used as an electron carrier in redox reactions. Yet, the intracellular NAD+/NADH ratio is key to the maintenance of an adequate metabolic status and cell survival. Growing evidences indicate that NAD+ biosynthetic pathways and metabolism are playing a major role in host–pathogen interactions. In this review, we overview these mechanisms highlighting the role of NAD+ metabolism as an attractive therapeutic target for microbe infections.
NAD+ biosynthesis: where the tale begins
NAD+ biosynthesis in mammalian cells
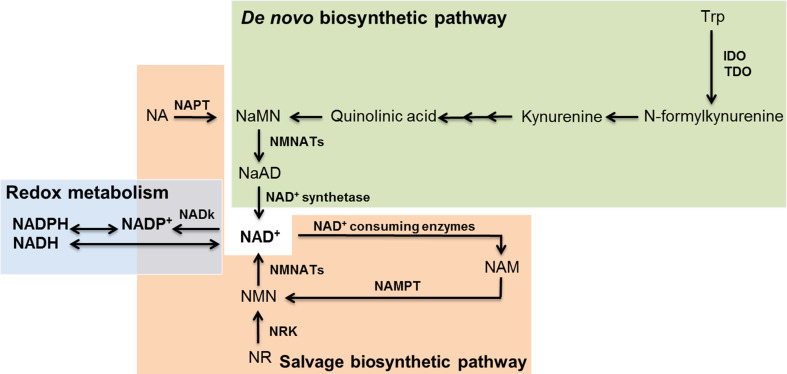
The biosynthesis of NAD+ in mammals occurs through two different pathways: the de novo and the salvage pathways (Fig. 1). The de novo pathway begins with the uptake and conversion of dietary l-tryptophan in N-formylkynurenine, which is mediated by the rate-limiting indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO). After several subsequent enzymatic reactions, quinolinic acid is formed and further condensed in nicotinic acid mononucleotide (NaMN). NaMN is converted in nicotinic acid adenine dinucleotide (NaAD) by the activity of nicotinamide mononucleotide adenylyltransferase isoforms (NMNATs 1–3). The completion of the de novo pathway occurs with the production of NAD+ by the glutamine-dependent NAD+ synthase [50].
Fig. 1.
NAD+ biosynthetic pathways and redox metabolism of mammals. The de novo pathway for NAD+ biosynthesis is indicated in green, the salvage pathway is indicated in orange and the redox metabolism of NAD/NADP is indicated in blue. IDO indoleamine 2,3-dioxygenase, NAD + nicotinamide adenine dinucleotide, NaAD nicotinic acid dinucleotide, NADP nicotinamide adenine dinucleotide phosphate, NADK NAD+ kinase, NAM nicotinamide; NaMN nicotinic acid mononucleotide, NAMPT nicotinamide phosphoribosyltransferase, NAPT nicotinic acid phosphoribosyltransferase, NMNAT nicotinamide mononucleotide adenylyltransferase, NMN nicotinamide mononucleotide, NR nicotinamide riboside, NRK nicotinamide riboside kinase, TDO tryptophan 2,3-dioxygenase, Trp tryptophan
NAD+ levels can also be restored through the import or recycling of nicotinic acid (NA), nicotinamide (NAM) or nicotinamide riboside (NR) in the salvage pathway. NA is converted to NaMN by the NA phosphoribosyltransferase (NAPT) to generate NAD+ through the Preiss–Handler pathway converging with the de novo pathway. NAM is used as a source of NAD+ through the activity of NAM phosphoribosyltransferase (NAMPT), which is the rate-limiting enzyme of the salvage pathway [77]. In addition, nicotinamide ribose kinase (NRK) induces the phosphorylation of NR in NAM mononucleotide (NMN), which is the converging point of the salvage pathway culminating in the production of NAD+ through the adenylation of NMN by NMNAT. Finally, NAD+ is further phosphorylated to NADP+ by the cytosolic NAD kinase (NADk) to enter redox metabolism.
NAD+ pools: a dynamic regulation for achieving ideal distribution
Metabolic functions and the modulation of several biological processes are dependent on the cellular homeostasis of NAD+/NADH ratio. Eukaryotic cells maintain an independent mitochondrial and cytosolic NAD+ pool, which allows coping with distinct stimuli and challenges [103]. NAD+ pools are used for protein post-translational modifications, such as protein mono- or poly(ADP ribosyl)ation or deacetylation, or for the production of second messengers, such as the Ca2+-mobilizing compounds cyclic adenosine diphosphoribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP). Nonetheless, the contribution of NAD+ fluxes between compartments and its consequent homeostasis is yet to be completely understood.
As different processes require different intermediates and, therefore different NAD+ levels, it has become clearer that this molecule is stored in a compartment-specific fashion. Cytosol and mitochondria represent the main intracellular NAD+ pools [19]. The NAD+/NADH ratio is about 100-fold lower in mitochondria when compared with the cytosolic compartment. Indeed, it has been estimated that the cytosolic compartment display NAD+/NADH ratios ranging between 60 and 700 whilst mitochondrial are set at 7 to 8 [100]. This ratio reflects the overall redox state of the cell and its maintenance is crucial for cell survival through the control of energy metabolism. The defined compartmentalization allows a preservation of oxidative phosphorylation in the event of a massive cytosolic NAD+ depletion with maintenance of cell viability and ATP stores for a short period of time. The “Mitochondrial Oasis Effect” refers to the capacity of mitochondria to dictate cell survival through maintenance of specific NAD+ pools, even after depletion of nuclear and cytosolic ones. This protection was shown to be dependent on NAMPT activity via mitochondrial SIRT3 and SIRT4 [103].
The compartmentalization of NAD+ synthesis is achieved by tightly controlled localization of the three NMNAT isoforms; nuclear NMNAT1, cytosolic NMNAT2 and mitochondrial NMNAT3 [58]. The rigorous subcellular localization of mammalian NMNAT isoforms suggests a predominant role for this enzyme in determining the subcellular NAD+ pool distribution. NMNAT1 is the most efficient enzyme involved in the forward and reverse equilibrium reaction that originates adenylyltransference or pyrophosphorylysis, respectively. Although NMNAT1 utilizes nicotinamide mononucleotide (NMN) as a major precursor for NAD+ synthesis, it was shown that NMNAT2 displays a high affinity for nicotinic acid mononucleotide (NaMN) that originates nicotinic acid adenine dinucleotide (NAAD), whilst hNMNAT3 was demonstrated to be the isoform with lower selectivity for purine nucleotides [46]. Although it is still debated whether NMNAT1-synthesized NAD+ may be exchanged between the nucleus and cytoplasm via nuclear pores, it is known that NAD+ synthesis is independently regulated in these compartments and the cytoplasmic NAD+ pool is maintained primarily by NMNAT2. In mammals, mitochondrial NAD+ is not exchangeable with the cytosol and thus NMNAT3 presumably participates in the maintenance of the organelle nucleotide pool [18, 45]. Indeed, it has been reported that FK866, a well-known inhibitor of rate-limiting NAMPT, does not affect mitochondrial NAD+ pool, possibly indicating that this enzyme is not a major regulator of NAD+ in this organelle [70].
NAD+ metabolism in pathogens: evolution towards auxotrophy
NAD+ metabolic networks present a remarkable intrinsic complexity and evolutionary variability [91]. Notably, the biosynthesis of NAD+ has evolved in several pathogenic organisms towards auxotrophy or to a restriction in the capacity to use a biosynthetic precursor. Several microorganisms encoded enzymes ae capable to utilize NAD+ from infected hosts. Candida glabrata, a fungus that lacks the genes for de novo synthesis, is a NAD+ auxotroph possessing a functioning salvage pathway that requires the uptake of external sources of NAD+ or precursors from the host cell milieu [20]. Haemophilus influenzae is a gram-negative bacterium that possesses an absolute need for NAD+ due to a lack of de novo biosynthetic enzymes or of salvaging NAM, niacin or other intermediates of the Preiss–Handler pathway. As NAD+ cannot be taken up into the bacterium cytosolic compartment as an intact molecule, previous studies have established that NMN and NR are the biochemical sources for NAD+ in H. influenzae. Two proteins were identified to play a key role in the uptake of NAD+; the outer membrane lipoprotein e(P4) and a periplasmic NAD nucleotidase (NadN). The e(P4) outer membrane protein and the NadN periplasmic enzyme convert NAD+ to NMN and NR [38]. The latter is able to cross the inner membrane to the cytoplasm, where NadR recycles it back to NAD+ by phosphorylating NR to NMN that is further adenylated to NAD+ [44, 87]. Shigella spp., the pathological agent of bacillary dysentery, lacks a de novo pathway for the synthesis of NAD+ and therefore requires nicotinic acid for growth [51]. As most prokaryotes, Shigella converts l-aspartate into the precursor for NAD+ synthesis quinolinic acid depending on the enzyme complex composed by quinolate synthase (NadA) and l-aspartate oxidase (NadB). Quinolinic acid is subsequently converted into nicotinic acid mononucleotide by quinolinate quinolinic acid concentration decreases the intracellular spreading of Shigella, which confirmed the occurrence of a selective pressure towards the inactivation of the nadA and nadB genes during evolution [72, 73]. Therefore, the available intracellular concentration of NA is not limiting for bacterial growth and in fact the reintroduction of functional copies of nadA and nadB into this strain restored the ability to synthesize quinolate, but resulted in strong attenuation of virulence, thus defining the nadA and nadB as an anti-virulent loci [73]. Comparative genomic studies have established that Leishmania protozoan parasites are also NAD+ auxotrophic organisms. Exogenous supplementation of NA, NAM or NR precursors increase the intracellular NAD+ content in Leishmania parasites [26]. In the case of Mycobacterium tuberculosis, NAD+ synthesis relies on both pathways, with the common enzyme being NAD+ synthase [99].
Several pathogens encode a nicotinamidase to convert NAM in NA for NAD+ synthesis. Leishmania nicotinamidase deletion led to a reduction of 70 % in NAD+ content, affecting both promastigote growth and the establishment of infection in mice [26]. This enzyme should further prevent the accumulation of anti-leishmanial NAM [85] by recycling it to NAD+. In addition, Borrelia burgdorferi and Brucella abortus nicotinamidases were shown to be essential for bacteria replication and infectivity [40]. These examples support that the inhibition of nicotinamidase may drive specific microbicidal effects towards intracellular pathogens. NMNAT encoded by Plasmodium (PfNMNAT) is quite divergent from the human homologs but share significant homology with bacterial counterparts. The inhibition of PfNMNAT results in the arrest of parasite growth in earlier events, thus indicating an importance of this biosynthetic enzyme in the development of Plasmodium parasites [63]. Therefore, NAM, nicotinamidase and NMNAT activities are determinants for pathogen survival in its mammalian host. These observations highlight that NAD+ is essential for parasite growth being required for the activity of several key substrates.
The importance of NAD+ in host–pathogen interactions
Modulation of host NAD+ levels by intracellular pathogens
Fluctuations in NAD+ levels in infected cells have been described for different classes of intracellular pathogens. Peripheral blood lymphocytes from HIV-infected individuals display a decrease of intracellular NAD+ levels, which may be reverted by exogenous administration of NAM [62]. In contrast, Plasmodium-infected erythrocytes display higher NAD+ levels than uninfected ones. This increase appears to be mediated by an increase in NAMPT and NAPT activity in infected cells, which allows the production of NAD+ through NAM and NA salvage, respectively [107]. Leishmania infantum induced a transitory NADH increase immediately after infection that was posteriorly reverted to higher NAD+/NADH ratio once the infection is established [56]. Therefore, the modulation of host NAD+ levels may vary accordingly to the infectious agent and probably the type of host cell due to their intrinsic metabolic requirements. Group A streptococci (Streptococcus pyogenes or GAS) represent a remarkable case of intracellular NAD+ modulation. GAS NAD+ glycohydrolase has the ability to cleave NAD+ producing nicotinamide and ADP-ribose but also cyclic ADP-ribose (cADPR) upon being injected in the cytosol of an infected host cell [95]. This results in a profound depletion of cellular NAD+ and ATP levels, leading to growth arrest and cell death [54]. As a consequence of depletion of host cell energy stores through the enzymatic action of NADase, GAS has proven to disrupt several innate processes of immune defense. As such, NAD+ glycohydrolase activity modifies several NAD+-dependent host cell responses including poly (ADP-ribose) polymerase (PARP)-1 activity [13], preventing phagolysosome acidification [3] and autophagy killing [64], which contributes to treatment failure, relapse and chronic persistence. Further studies in different types of pathogens are required to fully understand if the modulation of host NAD+ levels by intracellular pathogens is imperative for successful colonization.
Enzymes involved in NAD+ synthesis contribute to the immune response against pathogens
Tryptophan catabolism, which ultimately results in NAD+ production, has been shown to have a major role in the regulation of immune responses. The immunosuppressive effects of IDO have been vastly associated with impaired proliferation, induction of apoptosis and induction of regulatory T cells [71]. However, several pathogenic species are tryptophan auxotrophs, such as Chlamydia, Leishmania or Toxoplasma gondii. Thus, tryptophan depletion by IDO may further impact their intracellular survival [5]. IFN-γ-nduced IDO was also demonstrated to be responsible for inhibition of Staphylococcus aureus replication, the major causative agent of cerebral abscesses [83]. Recently, CD4 T cells were shown to contain M. tuberculosis growth by starving out of tryptophan [108]. Although M. tuberculosis can synthesize tryptophan under immune stress, blocking the bacterial tryptophan synthesis restored the efficacy of the immune system to kill the mycobacteria. Tryptophan catabolism by IDO is also a central mechanism for limiting tissue damage. The blockage of IDO was shown to attenuate T. gondii replication in the lung due to decreased inflammatory tissue damage [60]. Distinctively, in a Clostridium difficile infection model, IDO−/− mice showed increased immunopathology, as evidenced by increased mucosal destruction, cecal hemorrhage and higher levels of neutrophil-driven IFN-γ production. Therefore, tryptophan catabolism by IDO is a central mechanism for limiting tissue damage and for decreasing C. difficile bacterial burden, consequently restricting the observed pathology [22]. The absence of IDO was also correlated with a suppression of LP-BM5 murine leukemia virus replication via upregulation of type I IFNs [35]. IDO was found to be increased during HIV infection, which was associated with the dysfunction of CD8 immune T cells in controlling pathogens, the loss of Th22 cells and a consequent shift to Treg cells [4, 16]. Several other viral infections, including hepatitis B and C as well as influenza infections display an increased expression and activation of IDO, where tryptophan metabolism appears to have a crucial role in the fight against pathogens [81]. If in one hand the depletion of tryptophan may alter the phenotype of immune cells driving them towards immunosuppression, on the other hand it may have a severe impact on the growth of intracellular pathogens.
Besides IDO, other enzymes in the biosynthetic pathway of NAD+ synthesis have been demonstrated to play a role during infection. NAMPT was shown to inhibit HIV replication at an early step through abrogation of the integration of proviral DNA [97]. However, an increase in NAMPT expression could prevent HIV-1 replication rather than inhibiting it [14]. NAMPT targeting could provide strong anti-inflammatory effects leading to a decrease of the inflammatory tissue damage, without compromising host defense as exemplified during S. aureus infection [78].
Overall, a special attention should be paid to the dual effect of targeting host enzymes involved in NAD+ synthesis, especially now that novel IDO and NAMPT inhibitors are being tested for cancer chemotherapy [102], which may increase the potential immunosuppression of the patients.
The importance of NAD+-consuming proteins in infected cells
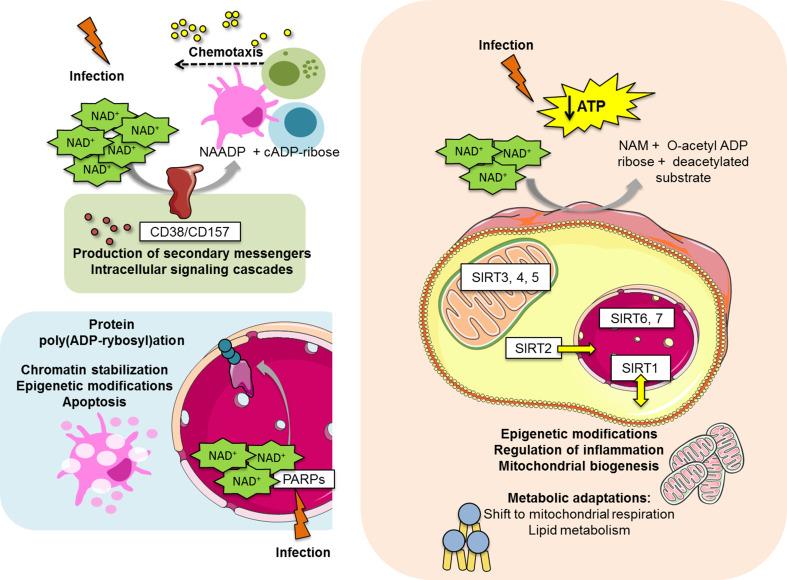
NAD+ is a cofactor for three classes of proteins: sirtuins, PARPs and membrane proteins CD38/CD157, where it contributes as a source of ADP-ribose. These NAD+-consuming proteins are considered metabolic sensors with a vital role in energy metabolism, cell survival, proliferation and effector functions. The mechanistic action of NAD+-consuming proteins during host–pathogen interaction is illustrated in Fig. 2.
Fig. 2.
Mechanistic action of NAD+-consuming proteins during host–pathogen interaction. The three major classes of NAD+-consuming proteins are involved in NAD+ breakdown and utilization, which originates functional and metabolic alterations upon challenge. NAD+ cleavage in NAADP and cADP-ribose is catalyzed by the ectoenzymes CD38 and CD157. When extracellular NAD+ content is increased, a high NAD+ turnover results in increased concentration of NAADP and cADP-ribose, which contribute for Ca2+ mobilization. This phenomenon leads to the production of secondary messengers, activation of intracellular signaling cascades and consequent chemotaxis of immune cells (for example, dendritic cells, monocytes and neutrophils) towards chemokine gradient. PARPs respond to DNA damage by infectious agents originating poly(ADP-rybosyl)ation of targeted proteins. This may have a role in chromatin stabilization and epigenetic modifications. Furthermore, PARP activation results in NAD+ depletion and cell apoptosis due to decreased energy availability. The seven isoforms of sirtuins are spread in the nucleus (SIRT1, SIRT6 and SIRT7), the cytosol (SIRT2) and the mitochondria (SIRT3, SIRT4 and SIRT5), although SIRT1 may shuttle between the nuclear and cytosolic compartment and SIRT2 was already shown to be able to translocate to the nucleus. Upon sirtuin modulation by the presence of an infectious agent, NAD+ is degraded and ultimately SIRT1 activity originates mitochondrial biogenesis and increased lipid oxidation. These processes lead to an increase of intracellular ATP levels, in an attempt to restore energy homeostasis. Sirtuins and PARPs are also able to cause epigenetic modifications in cellular DNA, which may also contribute to metabolism modulation. ATP adenosine triphosphate, ADP adenosine diphosphate, cADP cyclic adenosine diphosphate, NAADP nicotinic acid adenine dinucleotide phosphate, NAD + nicotinamide adenine dinucleotide, SIRT sirtuin, PARP poly-(ADP-ribose) polymerase
cADP-ribose synthases
These ectoenzymes, known as lymphocyte antigens CD38 and CD157, are multifunctional proteins involved in the generation of second messengers in intracellular signaling. cADP-synthases are the major NAD+-regulating proteins: for each cADP-ribose molecule produced, around 100 NAD+ molecules are broken [17, 21]. Hence, CD38 is a main cellular NADase in mammalian tissues being a critical regulator of NAD+ levels by modulating its bioavailability. Under homeostatic conditions, very little NAD+ is found free in the serum of normal mice [42]. Previous studies have shown that NAD+ is consistently released or actively transported to the extracellular medium and rapidly catabolized by CD38 to maintain its levels to a minimum [84]. Nevertheless, upon damage or infection, local levels of extracellular NAD+ can rise quite dramatically, leading to an increased activity of CD38. The generated cADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) contribute to Ca2+ mobilization [47] and will enhance the ability of monocytes, neutrophils and dendritic cells to migrate to sites where danger was felt and secondary lymphoid tissues in response to chemokines [69]. Studies performed with CD38−/− mice demonstrated its crucial role in the regulation of both innate and adaptive immune responses against infections [69]. As illustrative examples, CD38−/− mice are more susceptible to infection by S. pneumoniae [68] and M. avium [98], while presenting a decrease hepatic elimination of Entamoeba histolytica [24] due to reduced neutrophil recruitment and limited inflammatory response. A similar failure to induce an appropriate inflammatory response was observed in Naegleria fowleri-induced primary amoebic meningoencephalitis [12]. Therefore, understanding how to alter NAD+ extracellular levels or CD38 enzyme activity is an exciting prospect in the modulation of inflammatory responses during infections.
PARPs
NAD+ is also used as a substrate for mono- or poly(ADP-ribosyl)ation (PARylation) reactions mediated by ADP-ribose transferases (ARTs) or poly(ADP-ribose) polymerases, respectively. ARTs catalyze the formation of mono(ADP-ribosyl)ation, but generally the attached ADP-riboses are built as polymers by PARPs, which are the most common ADP-ribosyltransferases [8]. PARP-1, one of five confirmed PARPs, is the most abundant and highly expressed nuclear enzymes widely involved in DNA-damage response, apoptosis, chromatin stabilization and epigenetic modifications in mammalians [82]. Overactivity of PARP-1 driven by DNA strand breaks or metabolic insults leads to NAD+ exhaustion and bioenergetic failure [89] inducing caspase-independent apoptosis [106]. Indeed, this phenomenon was estimated to contribute for a 75 % depletion of NAD+ [31]. Some pathogens were shown to take advantage of the loss of PARP-1 function to its own advantage. As example, Chlamydia trachomatis release a protease-like activity factor (CPAF) leading to the cleavage of PARP-1, assuring a reduced inflammatory response to membrane-damaged cells [104]. In opposition, PARP-1 activation was detected in the brains of Vietnamese patients with fatal Plasmodium falciparum malaria [52]. Interestingly, the use of PARP-1 inhibitor, 3-aminobenzamide, was protective against meningitis-associated central nervous system complications resulting from Streptococcus pneumoniae infection [43]. However, the role of PARP-1 in the integration of retroviral DNA and consequent steps of retroviral infections, as HIV and Moloney murine leukemia virus, remains controversial [2, 88]. Whereas some groups found that PARP-1 by decreasing the intracellular levels of NAD+ in infected host cells contribute for the maintenance of infection [30], others report a viral transcriptional repression through epigenetic mechanisms [7].
The exacerbated activation of PARP-1 was shown to be associated with NAD+ depletion, followed by the opening of mitochondrial permeability transition (MPT) pore [1]. In parallel, the disturbance of mitochondria homeostasis and the rupture of membrane potential cause mitochondrial and cellular NAD+ depletion, culminating in cell death. Recently, the role of NAD+ in different types of cell death has been vastly addressed [25]. The modulation of mitochondria damage by pathogens has also been demonstrated in several studies [55, 79]. Because mitochondria are also the primary site for reactive oxygen species (ROS) production, which constitute essential microbicidal molecules, it seems likely that NAD+ modulation at the mitochondrial level may have an impact on microbe infections, but further studies are required.
Sirtuins
The mammalian sirtuin family comprises seven members, named SIRT1-7 [53], with distinct subcellular localizations reverting acetyl modifications of lysine residues or acting as ADP-rybosiltransferases in histones and other proteins. Sirtuins are activated in situations of energy deficit and prompt the utilization of non-carbohydrate energy sources, such as fatty acids [36]. The induced metabolic shift allows the organism to increase the efficiency of energy production.
SIRT1 was shown to be upregulated in hepatitis B virus (HBV) infected-liver cells. Its pharmacological inhibition by sirtinol was associated with a suppression of viral DNA replication, suggesting that SIRT1 inhibitors might be used as novel therapies to treat HBV infection [76]. The overexpression of hepatitis C virus (HCV) core proteins in HepG2 cells leads to an alteration in the cellular redox state, with decreased NAD+/NADH ratio. This imbalance was suggested to derive from a decreased signaling in the SIRT1-AMPK pathway, contributing to the hepatic metabolic disorder and influencing disease progression and anti-viral therapy efficacy [105]. Along the same line, Moreira and colleagues demonstrated the role of energy sensors AMPK and SIRT1 in Leishmania parasites survival and proliferation [56]. NAD+/NADH fluctuations during the course of infection reflected a correlation between SIRT1 activity and host metabolism driving pathogen persistence. Conversely, SIRT1 knockdown or inhibition by NAM and sirtinol in Kaposi’s Sarcoma-Associated Herpesvirus (KSHV)-infected cells resulted in increased concentration of infectious virions, which could reactivate the virus from the latent stage [32, 48]. Finally, the infection of human biliary epithelial cells with Cryptosporidium parvum, a coccidian parasite, resulted in higher SIRT1 expression, in a let-71-dependent manner that ultimately regulates NF-κB-driven innate immune response [101].
Interestingly, Listeria monocytogenes infection was impaired through H3K18 deacetylation-dependent fashion in SIRT2 knockout mice or blocking SIRT2 activity [23]. The role played by SIRT2 during infection appears to be pathogen-specific since the modulation of SIRT2 activity in vivo did not affect chronic infection with M. tuberculosis [11]. Therefore, it is critical to further explore the implications of sirtuin modulation during infections, especially since several sirtuin activators/inhibitors are in the biopharmaceutical pipeline to tackle metabolic, cardiovascular, neurodegenerative and neoplastic diseases.
The importance of NADP(H) in host–pathogen interactions
In contrast to NAD+/NADH, the NADP+/NADPH ratio must be maintained at very low levels. NADPH, the reduced form of NADP+, is known to provide reducing equivalents for anabolic reactions, such as fatty acid biosynthesis. It is also vital for protecting cells against reactive oxygen species (ROS), produced notably by the mitochondrial metabolism, through its role as cofactor for NADPH-dependent glutathione reductases, which ultimately ensures the regeneration of reduced glutathione (GSH). During host–pathogens interactions, NADPH is further used as an electron donor for NADPH oxidases activation that play both effector and signalling roles in the course of infection, through generation of microbicidal ROS [34, 66, 96]. Seven NADPH oxidases isoforms have been characterized in humans; the five NOX enzymes produce superoxide anion, while the two Dual Oxidase enzymes (DUOX1-2) generate hydrogen peroxide in a Ca2+-dependent manner (reviewed in [75]). NADPH oxidase NOX2, found in the membranes of neutrophils and macrophages phagosomes, has been extensively studied. During pathogen phagocytosis, this membrane-linked complex (composed of gp91phox; p22 phox; p47 phox; p40 phox; p67 phox; and small GTPase Rac) associates, resulting in the oxidation of NADPH to NADP+ with the concomitant production of superoxide (O·−2) from oxygen and the remaining downstream ROS to eliminate invading pathogens [67]. DUOX 1 and 2 are expressed in the epithelial surfaces of salivary glands, airways and along the gastrointestinal tract. The initial evidences of the in vivo role of DUOX enzymes in the crosstalk gut-microbiota were first provided in a Drosophila gut infection model system. The knockdown of DUOX on flies was shown to severely increase the susceptibility to gut infections (reviewed in [41]). Accordingly, Ha et al. [29] demonstrated that DUOX activity is essential for the maintenance of homeostasis in the fly gut in an infectious context through the development of an oxidative burst that is capable of limiting microbial proliferation. The tight control of DUOX enzymes allows the host gut–microbe homeostasis by efficiently controlling infection while tolerating commensal microbes [28]. In this context, the differential sensing of gut microbiota by innate immune sensors (Nod and Toll-like receptors) as well as the activation of distinct signalling pathways (Myd88, TRIF or NF-κB) has been shown to play a critical role by controlling the expression and activity of DUOX enzymes [33, 90]. Similarly, DUOX-derived ROS has been demonstrated to be essential for the innate immune response against bacterial or viral infections in airway mucosa [37, 39, 49, 92]. Thus, DUOX enzymes work in close collaboration with innate immune recognition receptors to develop an efficient innate immune response to viral or bacterial pathogens [37, 39]. However, its role in controlling parasite infections has never been addressed.
The beneficial versus detrimental role of NADPH oxidase activation in infectious contexts has been recently addressed. While some pathogenic bacteria, viruses and parasites have developed different means to limit NADPH oxidase activation and escape oxidative burst [93, 96], others seem to use NADPH oxidase activation and ROS production for their own benefit [34, 66]. Moreover, this is not limited to phagocytic cells. NOX/DUOX enzymes in the lung epithelium has been shown to participate in the host defense against respiratory viruses [27]. In opposition, hepatic NOX proteins during chronic hepatitis C virus were associated with exacerbated oxidative stress that leads to hepatocellular carcinoma [15], while ROS-generating NOX5 are essential for HTLV-I virus mediated T cell transformation phenotype [86].
For pathogens, the maintenance of high levels of NADPH is considered fundamental for survival. If in one hand, the pathogens have to adapt to the oxidative burst naturally present in the phagosomes, on the other this hostile environment may be aggravated by antiparasitic drugs act that act through generation of oxidative stress. Similarly to what happens in host cells, the majority of the antioxidant cofactor NADPH produced by parasites and bacteria derives from the concomitant action of G6PDH and 6PGD in the pentose phosphate pathway (PPP), which makes this metabolism an attractive target for weakening of pathogen’s defenses. G6PDH deficiency is one of the most common enzymopathy found in humans, affecting over 400 million people. This genetic disorder is more frequent in Africa and it mostly affects red blood cells (RBC) that are unable to produce sufficient NADPH levels and become therefore highly susceptible to oxidative stress. Paradoxically, G6PDH-deficient persons are more resistant to Plasmodium infections in Africa. The possible explanations are that chronic oxidative stress generated in G6PDH-deficient infected RBCs limit infection by Plasmodium or that these RBCs cannot sustain a normal infection and will be rapidly eliminated by macrophages [10, 59]. All these examples underline a complex role of NADPH in host/pathogen interactions, with both a role in the generation and the resistance to produced ROS during infection.
Concluding remarks
The biosynthetic pathways that culminate with NAD+ production are currently being used to fight non-infectious diseases, demonstrating its importance in novel drug design [3, 9, 57, 80]. It is possible to draw an analogy of this principle for infectious diseases. The remaining outstanding questions are: (1) Would it be more efficient to target host and/or pathogens NAD+ metabolism? (2) Upon blockage of a biosynthetic enzyme and consequent NAD+ depletion, are the pathogens able to evade elimination by upregulating other enzymes or by retrieving NAD+ from other sources? (3) Will the targeting of such an important molecule affect not only the host infected cells, but also bystander or non-infected cells? A rapid and considerable drop in NAD+ levels may cause massive cell death. More importantly, do the pathogen’s enzymes differ significantly, in terms of homology, from host’s ones, allowing its specific target? Or should the therapeutic approaches focus only in pathogen-specific and unique enzymes, as the nicotinamidases? It is also important to acknowledge the importance of maintaining NAD+ levels for the activation of metabolic sensors, as the sirtuins, and downstream signaling pathways. The correct functioning of host cells depends on the intricate connection between processes that drive cell survival, proliferation and host defense. The modulation of NAD+ levels is predicted to affect effector functions of immune cells and, consequently, the clearance or persistence of infections. Therefore, the study of NAD+ biology may be a promising approach for the discovery of new targets against infectious diseases. Table 1 synthetizes the major mechanisms used by pathogens to modulate host NAD+ levels. Remarkably, the first trials targeting NAD+ biology in infectious diseases go back to 1945, where nicotinamide was explored as an anti-M. tuberculosis agent, and later on during the 1990’s as an anti-HIV drug [61]. Although all of this information had fallen into obscurity, the past decade has seen the renaissance of targeting NAD+ biology to tackle infectious diseases, which has been accompanied by the arrival of new structure-based chemical modulators [6, 65, 74, 80, 94]. Nonetheless, the investigation of NAD+ metabolome is taking its first steps and it is expected to convey important updates regarding the interface between metabolism and immunity.
Table 1.
Pathogen-specific and host enzymes involved in host intracellular NAD+ modulation during infectious diseases
| Pathogen-specific enzymes | Pathogen | Observations | References |
|---|---|---|---|
| Nicotinamidase | Leishmania spp. | Maintenance of parasite homeostasis and infectivity | [26] |
| Brucella abortus | Contribution for intracellular replication and infectivity | [40] | |
| Borrelia burgdorferi | [40] | ||
| NAD+ glycohydrolase | Group A streptococci | Depletes intracellular NAD+ and ATP | [3, 13, 54, 64, 95] |
| NadN | Haemophilus influenzae | NAD+ synthesis from NMR and NR by NadN | [38, 44, 87] |
| NadA | Shigella spp. | NAD+ synthesis from quinolinate and aspartate by NadA and NadB | [72] |
| NadB | [73] | ||
| Pathogen’s homolog enzymes | |||
| PfNMNAT | Plasmodium spp. | Inhibition of PfNMNAT: parasite growth arrest | [63] |
| NAD+ synthase | Mycobacterium tuberculosis | De novo and salvage synthesis using NAD+ synthase | [99] |
| Host enzyme modulation | |||
| IDO/TDO | Staphylococcus aureus | Induction of IDO by IFNy: inhibition of replication | [83] |
| Mycobacterium tuberculosis | Starving of tryptophan: bacteria elimination | [108] | |
| Toxoplasma gondii | Blockage of IDO: attenuation of T. gondii replication | [60] | |
| Clostridium difficile | IDO KO mice: increased pathology | [22] | |
| LP-BM5 murine leukemia virus | IDO inhibition: suppression of virus replication via type I IFN | [35] | |
| HIV | Induction of IDO: dysfunction of CD8 T cells; shift Th22 to Tregs | [4, 16] | |
| Hepatitis B virus | Infection up-regulates IDO expression | [81] | |
| Hepatitis C virus | [81] | ||
| Influenza virus | [81] | ||
| NAMPT | HIV | NAMPT induction: prevents proviral DNA integration | [14, 97] |
| Plasmodium spp. | NAMPT induction: increased NAD+ levels in infected erythrocytes | [107] | |
| Staphylococcus aureus | Inhibition of NAMPT: decreased inflammation | [78] | |
| CD38 | Streptococcus pneumoniae | CD38 KO mice: more susceptible to infection | [68] |
| Mycobacterium avium | [98] | ||
| Entamoeba histolytica | CD38 KO mice: decreased protozoa elimination | [24] | |
| Naegleria fowleri | CD38 KO mice: loss of ability to induce inflammatory response | [12] | |
| PARP | Chlamydia trachomatis | Cleavage of PARP-1 by secreted protease-like activity factor | [104] |
| Plasmodium falciparum | PARP-1 activation in patients with fatal malaria | [52] | |
| Streptococcus pneumoniae | PARP-1 inhibitor: protective against meningitis | [43] | |
| HIV | Controversial role for PARP-1 | [2, 7, 30, 88] | |
| Sirtuins | Hepatitis B virus | SIRT1: upregulated in infection; sirtinol: protective | [76] |
| Hepatitis C virus | Imbalanced SIRT1/AMPK axis: hepatic disorder | [105] | |
| Leishmania infantum | Activation of the SIRT1/AMPK axis contributes | [56] | |
| Kaposi’s Sarcoma-Associated Herpesvirus | SIRT1 knockdown or inhibition: increase in infectious virions | [32, 48] | |
| Cryptosporidium parvum | Increased expression of SIRT1 | [101] | |
| Listeria monocytogenes | SIRT2 knockdown or inhibition: impairment of infection | [23] | |
| Mycobacterium tuberculosis | SIRT2 knockdown: no impairment in chronic infection | [11] | |
Acknowledgments
JG was supported by PD/BD/106053/2015. BV was supported by IRD (Institut de Recherche pour le Développement) institutional funding. JE was supported by a European Community’s Seventh Framework Program under grant agreement No. 602773 (Project KINDRED), an ANR grant (LEISH-APO, France) and a Partenariat Hubert Curien (PHC) (program Volubilis, MA/11/262). JE also thanks the Canada Research Chair program for his support. RS thank FCT—Foundation for Science and Technology—for their Investigator FCT Grant (IF/00021/2014)
Compliance with ethical standards
Conflict of interest
The authors have declared that no competing interests exist.
Contributor Information
Jérôme Estaquier, Phone: +418 525 44 44, Email: estaquier@yahoo.fr.
Ricardo Silvestre, Phone: +351 253 604 811, Email: ricardosilvestre@ecsaude.uminho.pt.
References
- 1.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariumi Y, Turelli P, Masutani M, Trono D. DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensable for human immunodeficiency virus type 1 integration. J Virol. 2005;79:2973–2978. doi: 10.1128/JVI.79.5.2973-2978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastiat-Sempe B, Love JF, Lomayesva N, Wessels MR. Streptolysin O and NAD-glycohydrolase prevent phagolysosome acidification and promote group a streptococcus survival in macrophages. mBio. 2014;5:e01690-01614. doi: 10.1128/mBio.01690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boasso A. Wounding the immune system with its own blade: HIV-induced tryptophan catabolism and pathogenesis. Curr Med Chem. 2011;18:2247–2256. doi: 10.2174/092986711795656126. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nat Rev Microbiol. 2008;6:657–666. doi: 10.1038/nrmicro1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruzzone S, Parenti MD, Grozio A, Ballestrero A, Bauer I, Del Rio A, Nencioni A. Rejuvenating sirtuins: the rise of a new family of cancer drug targets. Curr Pharm Des. 2013;19:614–623. doi: 10.2174/138161213804581954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bueno MT, Reyes D, Valdes L, Saheba A, Urias E, Mendoza C, et al. Poly(ADP-ribose) polymerase 1 promotes transcriptional repression of integrated retroviruses. J Virol. 2013;87:2496–2507. doi: 10.1128/JVI.01668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkle A. Poly(ADP-ribose). The most elaborate metabolite of NAD+ . FEBS J. 2005;272:4576–4589. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- 9.Cagnetta A, Soncini D, Caffa I, Acharya C, Acharya P, Adamia S, et al. Apo866 increases anti-tumor activity of cyclosporin-a by inducing mitochondrial and endoplasmic reticulum stress in leukemia cells. Clin Cancer Res. 2015;21(17):3934–3945. doi: 10.1158/1078-0432.CCR-14-3023. [DOI] [PubMed] [Google Scholar]
- 10.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso F, Castro F, Moreira-Teixeira L, Sousa J, Torrado E, Silvestre R, et al. Myeloid sirtuin 2 expression does not impact long-term Mycobacterium tuberculosis control. PLoS One. 2015;10:e0131904. doi: 10.1371/journal.pone.0131904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervantes-Sandoval I, Serrano-Luna Jde J, Garcia-Latorre E, Tsutsumi V, Shibayama M. Characterization of brain inflammation during primary amoebic meningoencephalitis. Parasitol Int. 2008;57:307–313. doi: 10.1016/j.parint.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekaran S, Caparon MG. The Streptococcus pyogenes NAD glycohydrolase modulates epithelial cell PARylation and HMGB1 release. Cell Microbiol. 2015;17(9):1376–1390. doi: 10.1111/cmi.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XY, Zhang HS, Wu TC, Sang WW, Ruan Z. Down-regulation of NAMPT expression by miR-182 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. Int J Biochem Cell Biol. 2013;45:292–298. doi: 10.1016/j.biocel.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Corder NL, Koduru B, Wang Y. Oxidative stress and hepatic Nox proteins in chronic hepatitis C and hepatocellular carcinoma. Free Radic Biol Med. 2014;72:267–284. doi: 10.1016/j.freeradbiomed.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, Estaquier J. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ. 2007;14:1747–1758. doi: 10.1038/sj.cdd.4402192. [DOI] [PubMed] [Google Scholar]
- 17.de Toledo FG, Cheng J, Liang M, Chini EN, Dousa TP. ADP-Ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res. 2000;86:1153–1159. doi: 10.1161/01.RES.86.11.1153. [DOI] [PubMed] [Google Scholar]
- 18.Di Stefano M, Conforti L. Diversification of NAD biological role: the importance of location. FEBS J. 2013;280:4711–4728. doi: 10.1111/febs.12433. [DOI] [PubMed] [Google Scholar]
- 19.Dolle C, Niere M, Lohndal E, Ziegler M. Visualization of subcellular NAD pools and intra-organellar protein localization by poly-ADP-ribose formation. Cell Mol Life Sci. 2010;67:433–443. doi: 10.1007/s00018-009-0190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domergue R, Castano I, De Las Penas A, Zupancic M, Lockatell V, Hebel JR, et al. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science. 2005;308:866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- 21.Dousa TP, Chini EN, Beers KW. Adenine nucleotide diphosphates: emerging second messengers acting via intracellular Ca2+ release. Am J Physiol. 1996;271:C1007–C1024. doi: 10.1152/ajpcell.1996.271.4.C1007. [DOI] [PubMed] [Google Scholar]
- 22.El-Zaatari M, Chang YM, Zhang M, Franz M, Shreiner A, McDermott AJ, et al. Tryptophan catabolism restricts IFN-gamma-expressing neutrophils and Clostridium difficile immunopathology. J Immunol. 2014;193:807–816. doi: 10.4049/jimmunol.1302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppee JY, Cossart P, Hamon MA. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science. 2013;341:1238858. doi: 10.1126/science.1238858. [DOI] [PubMed] [Google Scholar]
- 24.Estrada-Figueroa LA, Ramirez-Jimenez Y, Osorio-Trujillo C, Shibayama M, Navarro-Garcia F, Garcia-Tovar C, Talamas-Rohana P. Absence of CD38 delays arrival of neutrophils to the liver and innate immune response development during hepatic amoebiasis by Entamoeba histolytica . Parasite Immunol. 2011;33:661–668. doi: 10.1111/j.1365-3024.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- 25.Fouquerel E, Sobol RW. ARTD1 (PARP1) activation and NAD(+) in DNA repair and cell death. DNA Repair (Amst) 2014;23:27–32. doi: 10.1016/j.dnarep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazanion E, Garcia D, Silvestre R, Gerard C, Guichou JF, Labesse G, et al. The Leishmania nicotinamidase is essential for NAD+ production and parasite proliferation. Mol Microbiol. 2011;82:21–38. doi: 10.1111/j.1365-2958.2011.07799.x. [DOI] [PubMed] [Google Scholar]
- 27.Grandvaux N, Mariani M, Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin Sci (Lond) 2015;128:337–347. doi: 10.1042/CS20140321. [DOI] [PubMed] [Google Scholar]
- 28.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 29.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 30.Ha HC, Juluri K, Zhou Y, Leung S, Hermankova M, Snyder SH. Poly(ADP-ribose) polymerase-1 is required for efficient HIV-1 integration. Proc Natl Acad Sci USA. 2001;98:3364–3368. doi: 10.1073/pnas.051633498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: Where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M, Gao SJ. A novel role of SIRT1 in gammaherpesvirus latency and replication. Cell Cycle. 2014;13:3328–3330. doi: 10.4161/15384101.2014.968431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill T, 3rd, Xu C, Harper RW. IFNgamma mediates DUOX2 expression via a STAT-independent signaling pathway. Biochem Biophys Res Commun. 2010;395:270–274. doi: 10.1016/j.bbrc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan D, Wheeler RT. The complex roles of NADPH oxidases in fungal infection. Cell Microbiol. 2014;16:1156–1167. doi: 10.1111/cmi.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshi M, Saito K, Hara A, Taguchi A, Ohtaki H, Tanaka R, et al. The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. J Immunol. 2010;185:3305–3312. doi: 10.4049/jimmunol.0901150. [DOI] [PubMed] [Google Scholar]
- 36.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrn3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joo JH, Ryu JH, Kim CH, Kim HJ, Suh MS, Kim JO, et al. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxid Redox Signal. 2012;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- 38.Kemmer G, Reilly TJ, Schmidt-Brauns J, Zlotnik GW, Green BA, Fiske MJ, et al. NadN and e (P4) are essential for utilization of NAD and nicotinamide mononucleotide but not nicotinamide riboside in Haemophilus influenzae. J Bacteriol. 2001;183:3974–3981. doi: 10.1128/JB.183.13.3974-3981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim CH, Kim MJ, Ryu JH, Seong SY, Kim S, et al. The Induction of pattern-recognition receptor expression against influenza A virus through Duox2-derived reactive oxygen species in Nasal Mucosa. Am J Respir Cell Mol Biol. 2015;53:525–535. doi: 10.1165/rcmb.2014-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Kurokawa D, Watanabe K, Makino S, Shirahata T, Watarai M. Brucella abortus nicotinamidase (PncA) contributes to its intracellular replication and infectivity in mice. FEMS Microbiol Lett. 2004;234:289–295. doi: 10.1111/j.1574-6968.2004.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Lee WJ. Role of DUOX in gut inflammation: lessons from Drosophila model of gut–microbiota interactions. Front Cell Infect Microbiol. 2014;3:116. doi: 10.3389/fcimb.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim UH, Kim MK, Kim JS, Han MK, Park BH, Kim HR. Purification and characterization of NAD glycohydrolase from rabbit erythrocytes. Arch Biochem Biophys. 1993;305:147–152. doi: 10.1006/abbi.1993.1404. [DOI] [PubMed] [Google Scholar]
- 43.Koedel U, Winkler F, Angele B, Fontana A, Pfister HW. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 2002;22:39–49. doi: 10.1097/00004647-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kurnasov OV, Polanuyer BM, Ananta S, Sloutsky R, Tam A, Gerdes SY, Osterman AL. Ribosylnicotinamide kinase domain of NadR protein: identification and implications in NAD biosynthesis. J Bacteriol. 2002;184:6906–6917. doi: 10.1128/JB.184.24.6906-6917.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau C, Dolle C, Gossmann TI, Agledal L, Niere M, Ziegler M. Isoform-specific targeting and interaction domains in human nicotinamide mononucleotide adenylyltransferases. J Biol Chem. 2010;285:18868–18876. doi: 10.1074/jbc.M110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci (Landmark Ed) 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 47.Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317–323. doi: 10.1007/s00894-005-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q, He M, Zhou F, Ye F, Gao SJ. Activation of Kaposi’s sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle. J Virol. 2014;88:6355–6367. doi: 10.1128/JVI.00219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu H, Wu Q, Yang H. DUOX2 promotes the elimination of the Klebsiella pneumoniae strain K5 from T24 cells through the reactive oxygen species pathway. Int J Mol Med. 2015;36:551–558. doi: 10.3892/ijmm.2015.2234. [DOI] [PubMed] [Google Scholar]
- 50.Magni G, Orsomando G, Raffelli N, Ruggieri S. Enzymology of mammalian NAD metabolism in health and disease. Front Biosci. 2008;13:6135–6154. doi: 10.2741/3143. [DOI] [PubMed] [Google Scholar]
- 51.Mantis NJ, Sansonetti PJ. The nadB gene of Salmonella typhimurium complements the nicotinic acid auxotrophy of Shigella flexneri. Mol Gen Genet. 1996;252:626–629. doi: 10.1007/BF02172409. [DOI] [PubMed] [Google Scholar]
- 52.Medana IM, Mai NT, Day NP, Hien TT, Bethell D, Phu NH, et al. Cellular stress and injury responses in the brains of adult Vietnamese patients with fatal Plasmodium falciparum malaria. Neuropathol Appl Neurobiol. 2001;27:421–433. doi: 10.1046/j.0305-1846.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 53.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J . 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michos A, Gryllos I, Hakansson A, Srivastava A, Kokkotou E, Wessels MR. Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J Biol Chem. 2006;281:8216–8223. doi: 10.1074/jbc.M511674200. [DOI] [PubMed] [Google Scholar]
- 55.Mocarski ES, Guo H, Kaiser WJ. Necroptosis: the Trojan horse in cell autonomous antiviral host defense. Virology. 2015;479–480:160–166. doi: 10.1016/j.virol.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreira D, Rodrigues V, Abengozar M, Rivas L, Rial E, Laforge M, et al. Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog. 2015;11:e1004684. doi: 10.1371/journal.ppat.1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno-Vinasco L, Quijada H, Sammani S, Siegler J, Letsiou E, Deaton R, et al. Nicotinamide phosphoribosyltransferase inhibitor is a novel therapeutic candidate in murine models of inflammatory lung injury. Am J Respir Cell Mol Biol. 2014;51:223–228. doi: 10.1165/rcmb.2012-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori V, Amici A, Mazzola F, Di Stefano M, Conforti L, Magni G, et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS One. 2014;9:e113939. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol Microbiol. 2004;53:1291–1305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- 60.Murakami Y, Hoshi M, Hara A, Takemura M, Arioka Y, Yamamoto Y, et al. Inhibition of increased indoleamine 2,3-dioxygenase activity attenuates Toxoplasma gondii replication in the lung during acute infection. Cytokine. 2012;59:245–251. doi: 10.1016/j.cyto.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Murray MF. Nicotinamide: an oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin Infect Dis. 2003;36:453–460. doi: 10.1086/367544. [DOI] [PubMed] [Google Scholar]
- 62.Murray MF, Nghiem M, Srinivasan A. HIV infection decreases intracellular nicotinamide adenine dinucleotide [NAD] Biochem Biophys Res Commun. 1995;212:126–131. doi: 10.1006/bbrc.1995.1945. [DOI] [PubMed] [Google Scholar]
- 63.O’Hara JK, Kerwin LJ, Cobbold SA, Tai J, Bedell TA, Reider PJ, Llinas M. Targeting NAD+ metabolism in the human malaria parasite Plasmodium falciparum. PLoS One. 2014;9:e94061. doi: 10.1371/journal.pone.0094061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Seaghdha M, Wessels MR. Streptolysin O and its co-toxin NAD-glycohydrolase protect group A Streptococcus from Xenophagic killing. PLoS Pathog. 2013;9:e1003394. doi: 10.1371/journal.ppat.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olesen UH, Thougaard AV, Jensen PB, Sehested M. A preclinical study on the rescue of normal tissue by nicotinic acid in high-dose treatment with APO866, a specific nicotinamide phosphoribosyltransferase inhibitor. Mol Cancer Ther. 2010;9:1609–1617. doi: 10.1158/1535-7163.MCT-09-1130. [DOI] [PubMed] [Google Scholar]
- 66.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 69.Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–183. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- 70.Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, et al. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem. 2010;285:34106–34114. doi: 10.1074/jbc.M110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prunier AL, Schuch R, Fernandez RE, Maurelli AT. Genetic structure of the nadA and nadB antivirulence loci in Shigella spp. J Bacteriol. 2007;189:6482–6486. doi: 10.1128/JB.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prunier AL, Schuch R, Fernandez RE, Mumy KL, Kohler H, McCormick BA, Maurelli AT. nadA and nadB of Shigella flexneri 5a are antivirulence loci responsible for the synthesis of quinolinate, a small molecule inhibitor of Shigella pathogenicity. Microbiology. 2007;153:2363–2372. doi: 10.1099/mic.0.2007/006916-0. [DOI] [PubMed] [Google Scholar]
- 74.Pulla VK, Sriram DS, Soni V, Viswanadha S, Sriram D, Yogeeswari P. Targeting NAMPT for therapeutic intervention in cancer and inflammation: structure-based drug design and biological screening. Chem Biol Drug Des. 2015;86(4):881–894. doi: 10.1111/cbdd.12562. [DOI] [PubMed] [Google Scholar]
- 75.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren JH, Tao Y, Zhang ZZ, Chen WX, Cai XF, Chen K, et al. Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol. 2014;88:2442–2451. doi: 10.1128/JVI.02861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts KJ, Cross A, Vasieva O, Moots RJ, Edwards SW. Inhibition of pre-B cell colony-enhancing factor (PBEF/NAMPT/visfatin) decreases the ability of human neutrophils to generate reactive oxidants but does not impair bacterial killing. J Leukoc Biol. 2013;94:481–492. doi: 10.1189/jlb.1012527. [DOI] [PubMed] [Google Scholar]
- 79.Rodrigues V, Cordeiro-da-Silva A, Laforge M, Ouaissi A, Silvestre R, Estaquier J. Modulation of mammalian apoptotic pathways by intracellular protozoan parasites. Cell Microbiol. 2012;14:325–333. doi: 10.1111/j.1462-5822.2011.01737.x. [DOI] [PubMed] [Google Scholar]
- 80.Sampath D, Zabka TS, Misner DL, O’Brien T, Dragovich PS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther. 2015;151:16–31. doi: 10.1016/j.pharmthera.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt SV, Schultze JL. New insights into IDO biology in bacterial and viral infections. Front Immunol. 2014;5:384. doi: 10.3389/fimmu.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 83.Schroten H, Spors B, Hucke C, Stins M, Kim KS, Adam R, Daubener W. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics. 2001;32:206–210. doi: 10.1055/s-2001-17375. [DOI] [PubMed] [Google Scholar]
- 84.Seman M, Adriouch S, Haag F, Koch-Nolte F. Ecto-ADP-ribosyltransferases (ARTs): emerging actors in cell communication and signaling. Curr Med Chem. 2004;11:857–872. doi: 10.2174/0929867043455611. [DOI] [PubMed] [Google Scholar]
- 85.Sereno D, Alegre AM, Silvestre R, Vergnes B, Ouaissi A. In vitro antileishmanial activity of nicotinamide. Antimicrob Agents Chemother. 2005;49:808–812. doi: 10.1128/AAC.49.2.808-812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shigemura T, Shiohara M, Kato M, Furuta S, Kaneda K, Morishita K, et al. Superoxide-generating Nox5alpha is functionally required for the human T-cell leukemia virus Type 1-induced cell transformation phenotype. J Virol. 2015;89:9080–9089. doi: 10.1128/JVI.00983-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh SK, Kurnasov OV, Chen B, Robinson H, Grishin NV, Osterman AL, Zhang H. Crystal structure of Haemophilus influenzae NadR protein. A bifunctional enzyme endowed with NMN adenyltransferase and ribosylnicotinimide kinase activities. J Biol Chem. 2002;277:33291–33299. doi: 10.1074/jbc.M204368200. [DOI] [PubMed] [Google Scholar]
- 88.Siva AC, Bushman F. Poly(ADP-ribose) polymerase 1 is not strictly required for infection of murine cells by retroviruses. J Virol. 2002;76:11904–11910. doi: 10.1128/JVI.76.23.11904-11910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sodhi RK, Singh N, Jaggi AS. Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol. 2010;53:77–87. doi: 10.1016/j.vph.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Sommer F, Backhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8:372–379. doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 91.Sorci L, Kurnasov O, Rodionov D, Osterman A. Genomics and enzymology of NAD biosynthesis. In: Mander L, Liu H-W, editors. Comprehensive natural products II. Oxford: Elsevier; 2010. pp. 213–257. [Google Scholar]
- 92.Strengert M, Jennings R, Davanture S, Hayes P, Gabriel G, Knaus UG. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid Redox Signal. 2014;20:2695–2709. doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- 93.Sun K, Metzger DW. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol. 2014;192:3301–3307. doi: 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takeuchi M, Niimi T, Masumoto M, Orita M, Yokota H, Yamamoto T. Discovery of a novel nicotinamide phosphoribosyl transferase (NAMPT) inhibitor via in silico screening. Biol Pharm Bull. 2014;37:31–36. doi: 10.1248/bpb.b13-00495. [DOI] [PubMed] [Google Scholar]
- 95.Tatsuno I, Isaka M, Minami M, Hasegawa T. NADase as a target molecule of in vivo suppression of the toxicity in the invasive M-1 group A Streptococcal isolates. BMC Microbiol. 2010;10:144. doi: 10.1186/1471-2180-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 97.Van den Bergh R, Florence E, Vlieghe E, Boonefaes T, Grooten J, Houthuys E, et al. Transcriptome analysis of monocyte-HIV interactions. Retrovirology. 2010;7:53. doi: 10.1186/1742-4690-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Viegas MS, do Carmo A, Silva T, Seco F, Serra V, Lacerda M, Martins TC. CD38 plays a role in effective containment of mycobacteria within granulomata and polarization of Th1 immune responses against Mycobacterium avium . Microbes Infect. 2007;9:847–854. doi: 10.1016/j.micinf.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Vilcheze C, Weinrick B, Wong KW, Chen B, Jacobs WR., Jr NAD+ auxotrophy is bacteriocidal for the tubercle bacilli. Mol Microbiol. 2010;76:365–377. doi: 10.1111/j.1365-2958.2010.07099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J . 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie H, Lei N, Gong AY, Chen XM, Hu G. Cryptosporidium parvum induces SIRT1 expression in host epithelial cells through downregulating let-7i. Hum Immunol. 2014;75:760–765. doi: 10.1016/j.humimm.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamahira A, Narita M, Iwabuchi M, Uchiyama T, Iwaya S, Ohiwa R, et al. Activation of the leukemia plasmacytoid dendritic cell line PMDC05 by Toho-1, a novel IDO inhibitor. Anticancer Res. 2014;34:4021–4028. [PubMed] [Google Scholar]
- 103.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu H, Schwarzer K, Forster M, Kniemeyer O, Forsbach-Birk V, Straube E, Rodel J. Role of high-mobility group box 1 protein and poly(ADP-ribose) polymerase 1 degradation in Chlamydia trachomatis-induced cytopathicity. Infect Immun. 2010;78:3288–3297. doi: 10.1128/IAI.01404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu JW, Sun LJ, Liu W, Zhao YH, Kang P, Yan BZ. Hepatitis C virus core protein induces hepatic metabolism disorders through down-regulation of the SIRT1-AMPK signaling pathway. Int J Infect Dis. 2013;17:e539–e545. doi: 10.1016/j.ijid.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 106.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 107.Zerez CR, Roth EF, Jr, Schulman S, Tanaka KR. Increased nicotinamide adenine dinucleotide content and synthesis in Plasmodium falciparum-infected human erythrocytes. Blood. 1990;75:1705–1710. [PubMed] [Google Scholar]
- 108.Zhang YJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell. 2013;155:1296–1308. doi: 10.1016/j.cell.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]