Abstract
Ectodomain shedding of integral membrane receptors results in the release of soluble molecules and modification of the transmembrane portions to mediate or modulate extracellular and intracellular signalling. Ectodomain shedding is stimulated by a variety of mechanisms, including the activation of P2 receptors by extracellular nucleotides. This review describes in detail the roles of extracellular nucleotides and P2 receptors in the shedding of various cell surface molecules, including amyloid precursor protein, CD23, CD62L, and members of the epidermal growth factor, immunoglobulin and tumour necrosis factor families. This review discusses the mechanisms involved in P2 receptor-mediated shedding, demonstrating central roles for the P2 receptors, P2X7 and P2Y2, and the sheddases, ADAM10 and ADAM17, in this process in a number of cell types.
Keywords: Purinergic signalling, P2X receptors, P2Y receptors, Extracellular ATP, Extracellular UTP, Metalloprotease
Introduction
Ectodomain shedding is a post-translational modification of many cell surface molecules that results in their release as soluble molecules, which can subsequently induce cellular responses in an autocrine or paracrine manner, and in the modulation of the intracellular signalling properties of the remaining transmembrane portions [1]. Ectodomain shedding is exhibited by a diverse range of molecules, including cell adhesion molecules, cytokine members and epidermal growth factors, and can be stimulated by a variety of mechanisms [1], including the activation of P2 receptors by extracellular nucleotides (Fig. 1).
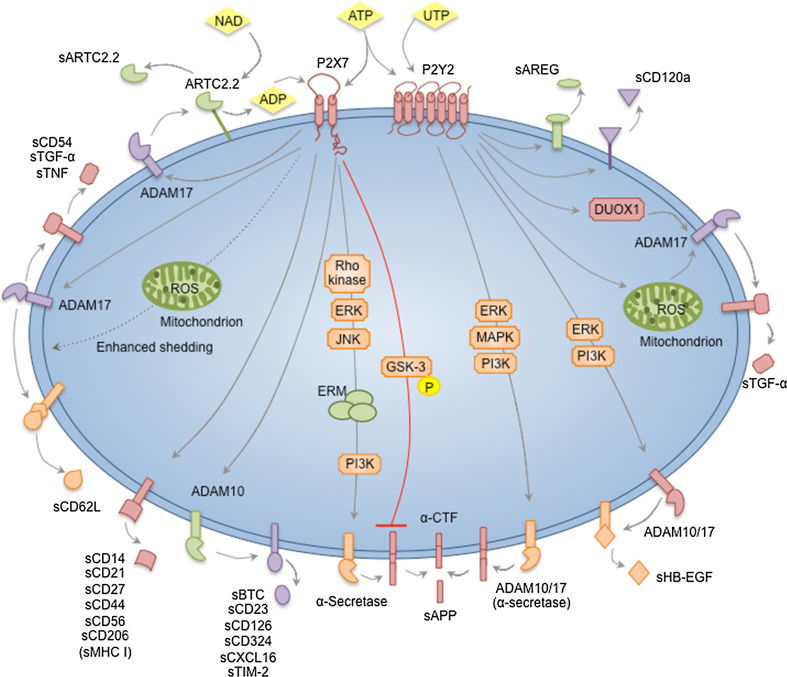
Fig. 1.
Nucleotide-induced ectodomain shedding. ATP activates P2X7 receptors. NAD can also activate P2X7 via the ADP-ribosyltransferase, ARTC2.2. P2X7 activation can stimulate ADAM10 to induce the shedding of BTC, CD23, CD126, CD324, CXCL16 and TIM-2. P2X7 activation can stimulate ADAM17 to induce the shedding of ARTC2.2, CD54, CD62L, TGF-α and TNF. P2X7-induced shedding of CD62L can be enhanced by mitochondrial ROS formation. P2X7 activation can stimulate α-secretase to induce APP shedding via an intracellular signalling cascade involving Rho-kinase, ERK, JNK, ERM and PI3K. An opposing role for P2X7 in APP processing is also suggested, whereby APP processing is inhibited by GSK-3 downstream of P2X7 activation. Finally, P2X7 activation can induce the shedding of CD14, CD21, CD27, CD44, CD56, CD206, and possibly MHC class I molecules, but the sheddases involved remain unknown. ATP and UTP can stimulate P2Y2 receptors. P2Y2 activation can stimulate ADAM17, via DUOX1 and mitochondrial ROS generating pathways, to induce the shedding of TGF-α. P2Y2 activation can stimulate ADAM10 and ADAM17 to induce HB-EGF shedding via an intracellular signalling cascade involving ERK and PI3K. P2Y2 activation can also stimulate ADAM10 and ADAM17, which serve as α-secretases, to induce APP shedding via an intracellular signalling cascade involving ERK, MAPK and PI3K. Finally, P2Y2 activation (or possibly activation of other P2Y receptors) can induce the shedding of AREG and CD120a, but the sheddases involved remain unknown. ADAM a disintegrin and metalloprotease, ADP adenosine 5′-diphosphate, APP amyloid precursor protein, AREG amphiregulin, ATP adenosine 5′-triphosphate, BTC betacellulin, CD21 complement receptor 2, CD23 IgE receptor, CD27 tumour necrosis factor receptor, CD44 hyaluronic acid receptor, CD54 intercellular adhesion molecule-1, CD56 neural cell adhesion molecule, CD62L l-selectin, CD120a tumour necrosis factor receptor 1, CD126 interleukin-6 receptor, CD324 E-cadherin, CTF carboxyl-terminal fragment, DUOX1 NADPH oxidase homolog dual oxidase 1, ERK extracellular signal-regulated kinase, ERM ezrin radixin moesin, GSK glycogen synthase kinase, HB-EGF heparin-binding-epidermal growth factor, JNK c-Jun N-terminal kinase, MHC major histocompatibility complex, NAD nicotinamide adenine nucleotide, PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase, ROS reactive oxygen species, TIM T-cell immunoglobulin and mucin domain, TGF transforming growth factor, TNF tumour necrosis factor, UTP uridine 5′-triphosphate
P2 receptors are classified as either P2X or P2Y receptors [2]. P2X receptors are trimeric ligand-gated cation channels that are activated by adenosine 5′-triphosphate (ATP) to mediate the rapid flux of Na+, K+ and Ca2+, and in some instances organic ions [3]. In humans and rodents, seven P2X receptor subunits exist (P2X1–P2X7), which combine to form homomeric or heteromeric receptors [4]. P2Y receptors are G protein-coupled receptors and modulate various signalling events, including adenylyl cyclase, phospholipase C and ion channel activation [5]. Eight P2Y receptors have been identified in humans and rodents (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11–P2Y14) [5]. P2Y receptors are predominately activated by ATP or adenosine 5′-diphosphate (ADP), but some are preferentially activated by other nucleotides, such as P2Y2 by uridine 5′-triphosphate (UTP) [5]. In addition, synthetic analogues of ATP and other nucleotides, including 2′,3′-O-(4-benzoylbenzoyl) ATP (BzATP) and adenosine 5′-(γ-thio)triphosphate (ATPγS), can activate a number of P2X and P2Y receptors [6]. P2X and P2Y activation stimulates various downstream signalling pathways that affect many cellular processes. Although these pathways remain to be fully elucidated, it is widely accepted that purinergic signalling is involved in physiological and pathophysiological responses, including neurotransmission, coagulation, inflammation, tissue regeneration, and cell proliferation, differentiation and death [7].
As discussed below, P2X7 and P2Y2 are the main P2 receptors implicated in nucleotide-induced ectodomain shedding. P2X7 is present on immune, bone, neural, epithelial and other cell types [8]. P2X7 is activated by BzATP and ATP, and to a lesser extent ATPγS [9]. Nicotinamide adenine dinucleotide (NAD) can also activate murine P2X7 following ADP-ribosylation by the ADP-ribosyltransferase ARTC2.2 [10]. P2X7 can be inhibited by broad-spectrum antagonists, including pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), Reactive Blue 2 and suramin, and by more specific antagonists, including Brilliant Blue G (BBG), KN-62, oxidised ATP and A 438079 [9]. P2X7 activity varies between human individuals and mouse strains due to single nucleotide polymorphisms, resulting in loss or gain of function variants, which can be used to establish the role of this receptor in nucleotide-induced responses [11]. P2Y2 is present on neural, bone, epithelial, endothelial, immune and renal cell types [12]. P2Y2 is activated by UTP and ATP, and to a lesser extent ATPγS [13]. Furthermore, there are synthetic P2Y2 agonists, including PSB1114, MRS2698 and INS37217 [13], and synthetic P2Y2/P2Y4 agonists, such as Diquafosol (INS365), which is used clinically to treat dry eye [6]. P2Y2 can be inhibited by broad-spectrum antagonists, including Reactive Blue 2 and suramin [13], and by specific antagonists, such as AR-C 118925XX [6]. This review aims to describe in detail the roles of extracellular nucleotides and P2 receptors in the shedding of various cell surface molecules and the mechanisms involved in this process.
Amyloid precursor protein
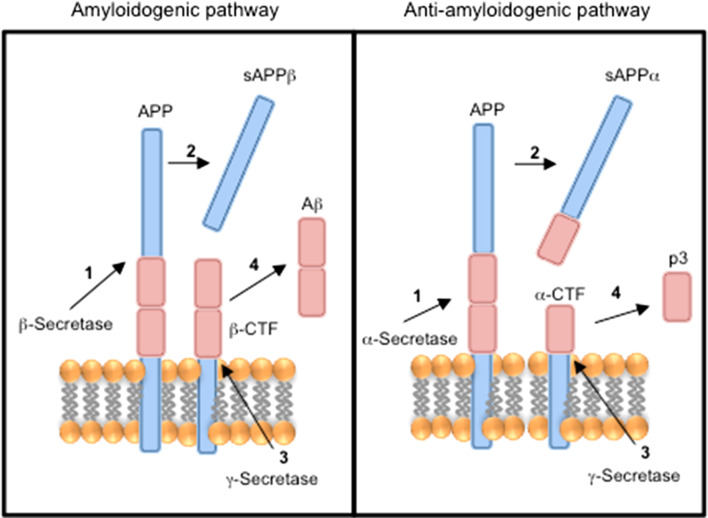
The amyloid precursor protein is a single transmembrane receptor with an extracellular N terminus and intracellular C terminus (type I integral membrane protein), which can be processed to amyloid- related peptides by either the amyloidogenic or the anti-amyloidogenic pathways [14] (Fig. 2). Amyloid-β peptide (Aβ) accumulation is a major pathological hallmark of Alzheimer’s disease [14]. In the amyloidogenic pathway, the ectodomain of amyloid precursor protein is cleaved by β-secretase to yield soluble amyloid precursor protein β (sAPPβ) and the remaining transmembrane protein, β-carboxyl-terminal fragment (β-CTF), which is subsequently cleaved by γ-secretase to release Aβ [14]. In the anti-amyloidogenic pathway, the ectodomain of β-amyloid precursor protein is cleaved by α-secretase to yield soluble amyloid precursor protein α (sAPPα) and the remaining transmembrane protein, α-carboxyl-terminal fragment (α-CTF), which is subsequently cleaved by γ-secretase to generate a truncated, non-amyloidogenic Aβ fragment, p3 [14]. Several metalloproteases, including ADAM9, ADAM10, ADAM17 and ADAM19, can function as α-secretases [14], while both P2Y2 and P2X7 activation are involved in the release of sAPPα from cells [15, 16].
Fig. 2.
APP processing pathways. The amyloidogenic pathway (left) is (1) initiated with the cleavage of APP by β-secretase (2) to generate sAPPβ and β-CTF. (3) β-CTF is subsequently cleaved by γ-secretase (4) to generate Aβ. The anti-amyloidogenic pathway (right) is (1) initiated with the cleavage of APP by α-secretase (2) to generate sAPPα and α-CTF. (3) α-CTF is subsequently cleaved by γ-secretase (4) to generate truncated Aβ peptide termed p3. Aβ amyloid-β peptide, APP amyloid precursor protein, sAPPβ soluble amyloid precursor protein β, β-CTF β-carboxyl-terminal fragment, sAPPα soluble amyloid precursor protein α, α-CTF α-carboxyl-terminal fragment (after [14])
P2Y2 was first implicated in the shedding of sAPPα using P2Y2-transfected human 1321N1 astrocytoma cells. UTP induced rapid shedding of sAPPα from 1321N1 cells in a concentration-dependent manner [17]. This process was dependent on extracellular Ca2+ and partly on extracellular signal-regulated protein kinase (ERK) phosphorylation, but independent of protein kinase C (PKC), Src and epidermal growth factor receptor (EGFR) activation [17]. Moreover, the metalloprotease inhibitors, phenanthroline and TAPI-2, and the proprotein convertase inhibitor, decanoyl-RVKR-CMK ketone (see Table 1 for sheddase inhibitors used in purinergic signalling studies), impaired UTP-induced sAPPα shedding [17]. Furthermore, short interfering RNA (siRNA) silencing of ADAM10 and ADAM17 reduced UTP-induced sAPPα shedding, and simultaneous silencing of both ADAMs near-completely suppressed this shedding [17]. This indicated that both ADAM10 and ADAM17 are involved in P2Y2-induced sAPPα shedding. Similarly, UTP induced sAPPα shedding via ADAM10 and ADAM17 in rat primary cortical neurons treated with interleukin (IL)-1β (to up-regulate P2Y2) or following transfection with P2Y2 [18]. This study also demonstrated a role for phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) in this pathway. UTP, and to a lesser extent ATP and BzATP, can also induce slow (48 h) sAPPα shedding from primary rat cortical astrocytes [19]. The use of P2 antagonists supported a role for both P2Y2 and P2Y4, but not P2Y6, in this process [19]. Both ATP-induced sAPPα shedding and UTP-induced sAPPα shedding from primary rat cortical astrocytes were dependent on ERK and p38 mitogen-activated protein kinase (MAPK) activity [19].
Table 1.
Sheddase inhibitors used in studies of nucleotide- and P2 receptor-induced ectodomain shedding
| Inhibitor | Target | References |
|---|---|---|
| A10-(23-213)a | ADAMb10 | [43] |
| BB-2516 (marimastat) | Metalloproteases (broad-spectrum) | [104] |
| BB-3103 | Metalloproteases (broad-spectrum) | [105] |
| BB-94 (batimastat) | Metalloproteases (broad-spectrum) | [106] |
| Decanoyl-RVKR-CMK | Convertase (non-selective) | [107] |
| GI 254023X | ADAM10 | [108] |
| GM 6001 | Metalloproteases (broad-spectrum) | [109] |
| GW 280264X | ADAM10 and ADAM17 | [108] |
| Phenanthroline | Metalloproteases (broad-spectrum) | [110] |
| Ro 31-9790 | Metalloproteases (broad-spectrum) | [111] |
| TAPI-1 | Metalloproteases (broad-spectrum) | [112] |
| TAPI-2 | Metalloproteases (broad-spectrum) | [113] |
aProdomain construct, b ADAM, a disintegrin and metalloprotease
P2X7 activation can also induce the rapid shedding of sAPPα from neural cell types. Both ATP and BzATP induced sAPPα shedding from human APP-transfected murine Neuro2a neuroblastoma cells, processes that were impaired by the P2X7 antagonists, BBG and A 438079, as well as by siRNA silencing of P2X7 [20]. Similarly, BzATP also induced sAPPα shedding from human SK-N-BE neuroblastoma cells, a process that was impaired by the P2X7 antagonists, oxidised ATP and A 438079 [20]. Furthermore, BzATP induced sAPPα shedding from primary astrocytes and neural progenitor cells from wild-type, but not Pfizer P2X7 knockout, mice [20]. The broad-spectrum metalloprotease inhibitors, TAPI-2 and GM 6001, inhibited BzATP-induced sAPPα shedding from Neuro2a cells, but siRNA silencing of ADAM9, ADAM10 and ADAM17 alone or in combination did not inhibit P2X7-induced shedding of sAPPα from these cells, suggesting the involvement of an alternate α-secretase in P2X7-induced shedding [20]. Additional pharmacological and siRNA evidence from this study [20] and a subsequent study [21] indicated that P2X7-induced shedding of sAPPα from neural cells depends on Rho and MAPK kinase modulation of ERK1/2 and c-Jun N-terminal kinase (JNK) upstream of the intracellular signalling complex, ezrin radixin and moesin, and downstream of PI3K.
In contrast to the above studies, BzATP decreased α-secretase activity (as assessed by expression of α-CTF) in murine Neuro2a neuroblastoma cells, while BBG or A 438079 increased basal α-secretase activity in these cells [22, 23], as well as in P2X7-transfected human embryonic kidney (HEK) 293 cells [22]. This increased activity in the presence of P2X7 inhibition was mediated through inhibition of glycogen synthase kinase-3 (GSK-3) [22]. In vivo blockade of P2X7 by BBG in a murine model of early onset familial Alzheimer’s disease decreased the number of hippocampal plaques, which correlated with decreased GSK-3 activity but increased α-secretase activity [22]. Differences between these and the above studies on P2X7 and amyloid precursor protein processing may possibly be explained by use of different BzATP concentrations, with lower concentrations (<100 µM) decreasing α-secretase activity through P2X7 activation and higher concentrations (>100 µM) increasing α-secretase activity through P2Y2 activation [22, 23].
ARTC2.2
ARTC2.2 is a glycosylphosphatidylinositol-anchored enzyme expressed on the cell surface of murine T-cells, which can catalyse the ADP-ribosylation of P2X7 resulting in its activation [10]. NAD induced the rapid shedding of ARTC2.2 from murine YAC-1 lymphoma cells, a process that was abrogated by either a blocking antibody or a blocking nanobody [24]. Moreover, NAD induced ARTC2.2 shedding from splenic CD4+ and CD8+ T-cells of wild-type, but not Pfizer P2X7 knockout, mice [24]. The metalloprotease inhibitor, BB-2516 (marimastat), and the ADAM10/ADAM17 inhibitor, GW 280264X, but not the ADAM10 inhibitor, GI 254023X, blocked this process in YAC-1 cells and T-cells [24], indicating that ADAM17 is the major sheddase involved in P2X7-induced ARTC2.2 shedding. Notably, shed ARTC2.2 retains enzymatic activity, but with substrate specificity favouring secreted proteins over membrane proteins [24]. To the best of our knowledge, this remains the only example of nucleotide-induced shedding of an ectodomain from a cell surface enzyme.
CD14 (lipopolysaccharide receptor)
CD14 is a glycosylphosphatidylinositol-anchored receptor (type V integral membrane protein) expressed on the surface of monocytes and macrophages, and to a lesser extent endothelial and epithelial cells [25]. CD14 functions as a receptor for the lipopolysaccharide (LPS)/LPS binding complex [25]. ATP induced the rapid shedding of CD14 from resting, M1 and M2 murine macrophages, a process that was impaired by A 438079, or in macrophages from Pfizer P2X7 knockout mice [26]. GM 6001 also impaired ATP-induced CD14 shedding from murine macrophages [26], but the specific metalloprotease mediating this effect is yet to be identified. P2X7-induced CD14 shedding may also occur in humans, as ATP incubation caused a loss in CD14+, but not CD33+, monocytes, in whole blood [27]; however, evidence for P2X7 in ATP-induced CD14 shedding in this setting remains to be determined.
CD21 (C3d receptor)
CD21 (C3d receptor) and CD35 (C3d/C4b receptor) comprise the two main complement receptors [28]. In humans, different genes code these receptors, whilst in mice, they are alternative splice products of the same gene [28]. CD21 is a type I integral membrane glycoprotein predominantly expressed on follicular dendritic cells and B-cells [28]. BzATP induced rapid CD21 shedding from human B-cells, which could be inhibited by the P2X7 antagonists, KN-62 and oxidised ATP [29] confirming a role for P2X7 in this process. The mechanism by which this process occurs remains unknown. CD21 shedding is a redox-regulated process involving oxidation of a tyrosine kinase pathway and reduction of metalloproteases [30]. Thus, the possibility remains that ATP-induced reactive oxygen species (ROS) formation may be involved in P2X7-induced CD21 shedding.
CD23 (low affinity IgE receptor)
CD23 is a single transmembrane receptor with an extracellular C terminus and intracellular N terminus (type II integral membrane protein), and is predominately present on B-cells, and some dendritic and epithelial cells [31] CD23 is principally involved in the regulation of immunoglobulin (Ig)E, but can also function as a cell adhesion molecule [32]. Following CD23 shedding, the soluble form exerts cytokine-like properties [33]. ATP and BzATP were first shown to induce the rapid shedding of CD23 from human chronic lymphocytic leukaemic (CLL) lymphocytes [34]. KN-62 and oxidised ATP impaired this nucleotide-induced CD23 shedding [34], confirming a role for P2X7 in this process. Since then, P2X7-induced CD23 shedding has been described in human monocyte-derived dendritic cells [35], human monocyte-derived Langerhans cells [36], human RPMI 8226 multiple myeloma B-cells [37], human B-cells [38] and murine B-cells [38, 39]. Monocyte-derived dendritic and Langerhans cells, from subjects homozygous for the loss-of-function polymorphism E496A, exhibited attenuated ATP-induced CD23 shedding [35, 36] further supporting a role for P2X7 in this process. Finally, BzATP induced CD23 shedding from CD23-transfected Chinese hamster ovary (CHO) cells [40]. However, a direct role for P2X7 in this process was not established in this study, especially given that these cells express both endogenous P2X7 [41] and P2Y receptors [42].
P2X7-induced CD23 shedding is primarily mediated by ADAM10. Comparisons of phorbol ester- and P2X7-induced shedding of CD23 and CD62L from CLL cells revealed that this process was mediated by different metalloproteases [34]. First, either phorbol ester treatment or P2X7 activation induced CD62L shedding, but only P2X7 activation induced CD23 shedding. Second, the hydroxamic acid-based protease inhibitor of Zn2+-dependent metalloprotease, Ro 31-9790, inhibited P2X7-induced CD23 shedding more potently than P2X7-induced CD62L shedding. A role for ADAM10 in ATP- or BzATP-induced CD23 shedding was first shown in human histiocytic lymphoma U937 cells using an inhibitory prodomain construct of ADAM10, A10-(23-213) [43], and subsequently in CD23-transfected CHO cells and murine B-cells using GI 254023X [39, 40]. However, a direct role for P2X7 in CD23 shedding was not established in any of these studies. Subsequently, the broad-spectrum metalloprotease inhibitors, BB-94 (batimastat) and GM 6001, were shown to impair P2X7-induced CD23 shedding from RPMI 8226 cells [37], supporting a role for a metalloprotease in this process. Finally, using GI 254023X, direct evidence for ADAM10 in mediating P2X7-induced CD23 shedding was established in RPMI 8226 cells [44], and in human and murine B-cells [38].
P2X7-induced CD23 shedding appears to occur independently of ion channel activity. Studies of P2X7-induced CD23 shedding in RPMI 8226 cells indicated that neither K+ efflux, Na+ influx, Ca2+ influx nor increases in intracellular Ca2+ were essential for this process [45]. In fact, extracellular Ca2+ partly impaired P2X7-induced CD23 shedding from CLL cells [34]. Similarly, Mn2+ and Mg2+ partly impaired P2X7-induced CD23 shedding from these cells [34]. However, the mechanism by which these divalent cations impair P2X7-induced CD23 shedding remains unknown. Finally, attempts using small molecule inhibitors in RPMI 8226 cells have failed to establish a role of many enzymes, commonly downstream of P2X7 activation, in P2X7-induced CD23 shedding, including PKC, JNK, Rho kinase, PI3K, GSK-3, MAPK, acid sphingomyelinase and phospholipases [45].
CD44 (phagocyte glycoprotein-1)
CD44 (phagocyte glycoprotein-1) is a type I integral membrane glycoprotein expressed on leukocytes, fibroblasts, and epithelial and endothelial cells [46]. CD44 plays roles in cell adhesion, lymphocyte signalling, inflammation, angiogenesis and tumour metastasis [46]. ATP, but not ADP, induced the rapid shedding of CD44 from murine P388D1 lymphoid tumour cells, which was impaired by KN-62 and short hairpin RNA silencing of P2X7 [47], indicating a role for P2X7 in the process. In contrast, ATP was unable to alter the expression of cell surface CD44 on CLL cells [34]. Thus, P2X7-induced CD44 shedding may be cell or species specific. Nevertheless, the mechanism involved in P2X7-induced CD44 shedding from P388D1 cells remains undefined. ADAM10 [48], ADAM17 [49], MMP (matrix metalloproteinase) 9 [50] and MMP14 [51] are involved in constitutive CD44 shedding, and thus, one or more of these sheddases may play a role in P2X7-induced CD44 shedding. Notably, the glycosaminoglycan chains of soluble CD44 have been reported to associate with P2X7 to function as a positive allosteric modulator of P2X7 activation [52]. Thus, it was proposed that P2X7 activation results in CD44 shedding, with the resulting soluble CD44 forming part of a regulatory positive feedback loop facilitating ATP-induced cell signalling via P2X7 [52, 53]. Whether other glycosaminoglycan chain-containing ectodomains shed following P2X7 activation can also modulate P2X7 remains unknown.
CD62L (l-selectin)
CD62L (l-selectin) is a type I integral membrane glycoprotein present predominately on leukocytes [54]. CD62L is involved in constitutive trafficking of lymphocytes through lymphoid organs, and rolling of leukocytes on inflamed vascular endothelium [54]. Seminal studies by Wiley et al. demonstrated that P2X7 activation could induce the rapid shedding of CD62L, revealing for the first time the potential importance of extracellular nucleotides and P2 receptors in ectodomain shedding. ATP was first shown to induce rapid CD62L shedding from the surface of CLL cells [55]. Adenosine, ADP and UTP did not cause CD62L shedding, while oxidised ATP inhibited both ATP- and BzATP-induced CD62L shedding from these cells [55]. Furthermore, KN-62 inhibited ATP-induced CD62L shedding from CLL lymphocytes [34, 56], while BzATP-induced CD62L shedding was impaired in CLL cells expressing non-functional P2X7 [57]. Collectively, confirming a role for P2X7 in this process.
Following the studies above, it was shown that P2X7 activation can induce CD62L shedding from human B-cells and T-cells [29], and subsets of these cells from humans or mice, including CD27− and CD27+ B-cells [29], and CD4+ and CD8+ T-cells [29, 58–60]. In murine T-cells, NAD was also able to induce P2X7-induced CD62L shedding [61, 62]. ATP-induced CD62L shedding was impaired in lymphocytes, as well as monocytes, from subjects coding the P2X7 E496A loss-of-function polymorphism [60, 63] and from Pfizer P2X7 knockout mice [64]. In contrast, BzATP-induced CD62L shedding was found to be more rapid in T-cells from GlaxoSmithKline P2X7 knockout mice [65]; presumably due to the presence of the highly functional escape variant P2X7k in T-cells from these mice [66, 67].
A physiological role for P2X7-induced CD62L shedding was implicated in T-cell transendothelial migration to the heart in a murine model of Duchenne muscular dystrophy [68]. This study showed that treatment of mdx/mdx mice with the P2X7 antagonist, BBG, allowed the transendothelial migration of T-cells to the heart by allowing sustained CD62L expression [68]. This suggested that impaired P2X7-induced CD62L shedding might contribute to the pathology of Duchenne muscular dystrophy. In contrast, oxidised ATP did not affect the loss of CD62L during CLL lymphocyte transmigration [69], suggesting that P2X7 may not play a role in this process. This difference may be due to different experimental conditions, including cell type and species.
ADAM17 is thought to be the principal sheddase involved in P2X7-induced CD62L shedding. A metalloproteases was first implicated in this process when Ro 31-9790 was shown to inhibit P2X7-induced CD62L shedding in CLL cells [34]. Subsequently, a role for ADAM17 in this process was shown in murine B-cells and T-cells [39, 40]. In the absence of ADAM17, ADAM10 is able to mediate BzATP-induced CD62L shedding [39, 40]. However, BzATP-stimulated CD62L shedding mediated by ADAM17 was significantly more rapid than shedding mediated by ADAM10 [39], corroborating a role for ADAM17 as the principal sheddase. Such studies highlight the complexity in ectodomain shedding, often revealing dominant but redundant roles for many sheddases.
Other studies have identified other molecules important in P2X7-induced CD62L shedding. An inhibitor of phosphatidylserine exposure, 4,4′-diisothiocyanatosilbene-2,2′-disulphonic acid, prevented P2X7-induced CD62L shedding from murine effector/memory CD4+ T-cells [70], indicating a role for rapid, non-apoptotic phosphatidylserine exposure in this process, although the precise mechanism involved remains undefined. P2X7-induced CD62L shedding does not involve conventional PKC isoforms [55], PI3K, ERK or the NADPH oxidase [71]. In contrast, P2X7-induced loss of CD62L from human CD4+ T-cells was enhanced by diphenyleneiodonium, rotenone and antimycin A, which uncouple complexes of the mitochondrial respiratory chain to cause ROS formation [71]. Moreover, rottlerin, an inhibitor of novel PKC isoforms that can also promote ROS formation, potentiated P2X7-induced CD62L loss from human CD4+ T-cells [71]. Although this study did not directly study ADAM17, ROS are able to activate ADAM17 via oxidation of cysteine motifs, which are critical for CD62L cleavage [72]. Therefore, ROS may play a role in P2X7-induced activation of ADAM17 and subsequent shedding of CD62L.
CD206 (macrophage mannose receptor 1)
CD206 (macrophage mannose receptor 1) is a type I integral membrane glycoprotein mainly expressed on the surface of macrophages and dendritic cells, where it functions as an antigen receptor promoting phagocytosis of pathogens [73]. As for CD14, ATP induced the rapid shedding of CD206 from resting, M1 and M2 murine macrophages, a process that was impaired by A 438079 and GM 6001, as well as in macrophages from Pfizer P2X7 knockout mice [26]. The specific metalloprotease mediating P2X7-induced CD206 shedding is yet to be determined.
CD324 (E-cadherin)
CD324 (E-cadherin) is a type I integral membrane glycoprotein that mediates cell-to-cell adhesion in epithelial tissues, while loss of this molecule promotes cell migration, including epithelial tumour metastasis [74]. In contrast to most studies that use exogenous ATP to induce ectodomain shedding, one study showed that melittin, the major component of bee venom, induced the rapid shedding of CD324 from human HaCaT keratinocytes via a purinergic pathway [75]. Although evidence for the specific P2 receptor involved in this process is limited, melittin caused ATP release from HaCaT keratinocytes, and the ATP-degrading enzyme, apyrase, and broad-spectrum P2 antagonists, PPADS, suramin and Evans Blue, impaired ERK1/2 phosphorylation, which was associated with melittin-induced CD324 shedding. Furthermore, P2X7-transfected, but not mock transfected, HEK 293 cells showed increased melittin-induced phosphorylation of ERK, which could also be abrogated by apyrase [75]. Collectively, this suggested a role for an ATP-P2X7 axis in melittin-induced CD324 shedding. Finally, BB-2516, GI 254023X and GW 280264X inhibited melittin-induced CD324 shedding [75], thus supporting a role for ADAM10 in this pathway.
CXCL16 (CXC-chemokine ligand 16)
CXCL16 (CXC-chemokine ligand 16) is a type I integral membrane glycoprotein, which predominantly functions as an adhesion receptor for cells expressing the CXC-chemokine receptor CXCR6, but can also serve as a scavenger receptor capable of binding cells displaying exposed phosphatidylserine or oxidised low-density lipoprotein [76]. Following shedding, soluble CXCL16 can induce lymphocyte chemotaxis, promote angiogenesis and prevent excitotoxicity of neurons [76]. P2X7 activation can induce CXCL16 shedding, and this process is mediated by ADAM10. ATP and BzATP, but neither ADP nor UTP, induced the rapid shedding of CXCL16 from RPMI 8226 cells, a process that was impaired by the P2X7 antagonists, KN-62 and AZ 10606120 [44]. Moreover, BB-94 and GM 6001, as well as GI 254023X, impaired ATP-induced CXCL16 shedding [44].
Epidermal growth factor members
Activation of EGFR involves the binding of members of the epidermal growth factor (EGF) family, including amphiregulin, betacellulin, EGF, heparin-binding EGF-like growth factor (HB-EGF) and transforming growth factor-α (TGF-α) [77]. EGF family members are type I integral membrane proteins and ectodomain shedding of these proteins generates soluble ligands for EGFR [77]. EGF members are involved in the modulation of cell proliferation, apoptosis and migration, and play important roles in processes, such as bone formation, wound healing and tumourigenesis [77].
Amphiregulin
ATP and ATPγS induced slow (24 h) shedding of amphiregulin from LPS-stimulated human monocyte-derived and murine bone marrow-derived dendritic cells [78]. UTP also induced amphiregulin shedding from LPS-stimulated murine bone marrow-derived, but not from human monocyte-derived, dendritic cells [78]. Moreover, suramin inhibited ATPγS-induced amphiregulin shedding from human monocyte-derived dendritic cells [78], but the specific P2 receptors involved were not identified. Notably, amphiregulin released from murine bone marrow-derived dendritic cells stimulated tumour growth in vivo [78] indicating that nucleotides can confer tumourigenic properties to dendritic cells by inducing amphiregulin release. This contrasts the anti-tumourigenic properties of dendritic cells following P2X7 activation and subsequent NLRP3 inflammasome stimulation to promote anti-tumour immunity [79].
Betacellulin
BzATP induced the rapid shedding of betacellulin from betacellulin-transfected CHO cells, and from P2X7 and betacellulin co-transfected murine embryonic fibroblasts (mEFs) [39, 40]. BzATP-induced betacellulin shedding was impaired by BB-2516 and GI 254023X, as well as by transfection of dominant-negative ADAM10 plasmid DNA [40]. GI 254023X impaired BzATP-induced betacellulin shedding in ADAM17 knockout mEFs (which expressed ADAM10). Conversely, BzATP did not induce betacellulin shedding in ADAM10 knockout mEFs (which expressed ADAM17) [40]. Collectively, this indicated that P2X7 stimulates ADAM10 to induce betacellulin shedding.
Heparin-binding epidermal growth factor-like growth factor (HB-EGF)
The first indication of nucleotide-induced HB-EGF release was indirectly shown using a neutralising antibody against HB-EGF, which inhibited ATP-induced mitogenic effects in guinea pig Muller glial cells [80]. This study postulated that ATP-induced P2Y activation leads to the release of HB-EGF from cells, which mediated EGFR activation [80]. Later, direct evidence of nucleotide-induced HB-EGF shedding was shown using ATPγS in HB-EGF-transfected SV-40 immortalised human corneal epithelial cells [81, 82]. Wounding, which increased ATP in the culture medium, as well as ADP, also induced rapid HB-EGF shedding from these cells [81, 82]. Reactive Blue 2 inhibited ATPγS-induced HB-EGF shedding [81] indicating a role for a P2 receptor in this process. GM 6001, GW 280264X [81] and GI 254023X [82] inhibited HB-EGF shedding from these cells. Furthermore, the mitogen-activated protein kinase kinase (MEK) and ERK1/2 inhibitors, PD98059 and U0126, respectively, impaired ATPγS- and wound-induced HB-EGF shedding [81, 82]. Overall, these studies suggest that ADAM10, ADAM17, MEK and ERK1/2 are involved in nucleotide-induced HB-EGF shedding from corneal epithelial cells, and that this process may be relevant during wound healing. However, since Reactive Blue 2 and ATPγS can inhibit and activate a range of P2X and P2Y receptors, respectively [6], further studies are required to determine, which P2 receptor(s) is (are) involved in this process.
Transforming growth factor-α (TGF-α)
Several studies have shown that nucleotides induce TGF-α shedding from cells. BzATP induced the rapid shedding of TGF-α from TGF-α-transfected CHO cells [40], and from P2X7 and TGF-α co-transfected mEFs [39, 40]. TGF-α shedding from CHO cells was impaired by BB-2516, but not GI 254023X [40], excluding a role for ADAM10 in the process. P2X7 was thought to be involved in this process, but direct evidence for this receptor is required, especially since P2Y receptors have also been implicated in TGF-α shedding as discussed below.
Both ATP and UTP can induce rapid TGF-α shedding from TGF-α-transfected CHO cells, which express P2Y2 mRNA [42]. The broad-spectrum metalloprotease inhibitor, TAPI-2, inhibited ATP-induced TGF-α shedding from CHO cells, but ATP did not induce TGF-α shedding from CHO cells deficient in ADAM17 [42]. Moreover, ATP, ATPγS and UTP induced TGF-α shedding from EC-4 murine fibroblasts (which express ADAM17), but not from EC-2 murine fibroblasts (which are ADAM17 deficient) [42]. Collectively, these data indicate that P2Y2 activation stimulates ADAM17 to induce the rapid shedding of TGF-α, but an additional role for P2X7 in this process cannot be excluded. Further data from this study, using the Ca2+ chelators, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) and ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), indicate that this process is regulated by both intracellular and extracellular Ca2+ [42]. Furthermore, this process involved mitochondrial, but not NADPH oxidase, ROS formation, as the ROS scavenger N-acetylcysteine, and the mitochondrial complex inhibitors, rotenone and myxothiazol, but not the NADPH oxidase inhibitor, apocynin, impaired ATP-induced TGF-α shedding [42].
ATP also induced TGF-α shedding from human bronchial epithelial 1 cells over 2 h [83], while melittin (which causes ATP release) induced rapid TGF-α shedding from HaCaT keratinocytes [75]. Although a role for P2 receptor activation in this process was only assessed in the former study and then only using the broad-spectrum P2 antagonist, suramin, this further supports a role for P2 receptor activation in TGF-α shedding. Notably, both studies identified ADAM17 as the main sheddase involved in this process [75, 83]. Furthermore, through the use of siRNA knockdown and chemical inhibitors, ATP-induced ADAM17 stimulation in human bronchial epithelial 1 cells was mediated by a pathway involving activation of the NADPH oxidase homolog dual oxidase 1 and ERK1/2 [83].
Collectively, the above studies suggest a role for both P2X7 and P2Y2 receptor activation in nucleotide-induced TGF-α shedding, and that this process is primarily mediated by ADAM17. However, further evidence is required to establish the specific roles for each receptor in this process.
Immunoglobulin superfamily members
Extracellular nucleotides induce the shedding of several molecules of the Ig superfamily, including CD54, CD56, CD126, T-cell immunoglobulin and mucin domain (TIM)-2, and possibly major histocompatibility complex (MHC) class I molecules.
CD54 (intercellular adhesion molecule-1, ICAM-1)
CD54 (intercellular adhesion molecule-1, ICAM-1) is a type I integral membrane glycoprotein important in cell adhesion mediating immune cell trafficking, antigen presentation and cell signalling [84]. BzATP stimulated the rapid shedding of CD54 from P2X7 and CD54 co-transfected mEFs, and from CHO cells [40], but a direct role for P2X7 in this process in CHO cells was not directly established. GI 254023X inhibited BzATP-induced CD54 shedding in ADAM17 knockout mEF cells (which expressed ADAM10), but not in ADAM10 knockout mEF cells (which expressed ADAM17) [40]. Furthermore, BzATP failed to induce CD54 shedding in ADAM10/ADAM17 knockout mEF cells [40], but this process could be reversed following transfection with ADAM17 [39]. BzATP-induced CD54 shedding from CHO cells was impaired by BB-2516 but not by GI 254023X [40]. Collectively, this suggested that ADAM17 is the main sheddase involved in BzATP-induced CD54 shedding when both ADAM10 and ADAM17 are present.
CD56 (neural cell adhesion molecule, NCAM)
CD56 (neural cell adhesion molecule, NCAM) is an integral membrane glycoprotein and comprises three major forms, with molecular weights of 120, 140 and 180 kDa [85]. These molecules are involved in cell adhesion, migration, and survival, as well as axon guidance and synaptic targeting [85]. ATP induced the shedding of all three forms from embryonic rat hippocampal neurons and from CD56-transfected L929 murine fibroblasts over 1.5–6 h [86]. ATP, at concentrations of 2.5 mM, was required to induce CD56 shedding [86], indicating a possible role for P2X7 in this process. The broad-spectrum metalloprotease inhibitors, BB-3103 and GM 6001, impaired ATP-induced CD56 shedding from CD56-transfected L929 cells [86]. In contrast, ATP-induced CD56 shedding was independent of lysosomal, proteasomal and calpain proteolytic activity, as well as PI3K and PKC activation [86]. Finally, the extracellular ATP binding site of CD56 was not required for ATP-induced CD56 shedding [86], indirectly supporting a role for a P2 receptor in this process.
CD126 (interleukin-6 receptor, IL-6 receptor)
IL-6 is a cytokine involved in homeostasis, as well as immune responses, and is secreted by both immune and non-immune cells [87]. This cytokine induces the proliferation and differentiation of T-cells, macrophages and neutrophils, and can induce fever in the presence of tumour necrosis factor (TNF) and IL-1β [87]. The biological activities of IL-6 are mediated by binding CD126 (IL-6 receptor), a type I integral membrane glycoprotein, which subsequently recruits two integral membrane glycoproteins, CD130 (gp130), to form a signalling complex [87]. Alternatively, CD126 shed from cells can bind IL-6 to form a complex with two membrane CD130 molecules to mediate signalling in cells that do not express CD126 or have shed this receptor [87]. P2X7 can mediate the rapid shedding of CD126. BzATP induced the rapid shedding of CD126 from wild-type murine splenocytes, a process that was impaired by KN-62 or in splenocytes from Pfizer P2X7 knockout [88]. Notably, CD126 serum concentrations from these knockout mice are reduced compared to wild-type mice, suggesting a role for P2X7 in CD126 shedding in vivo [88]. GI 254023X and GW 280264X both inhibited BzATP-induced CD126 shedding in P2X7 and CD126 co-transfected NIH3T3 mouse embryonic fibroblasts and HEK 293 cells, while the ADAM10 prodomain, A10-(23-213), inhibited BzATP-induced CD126 shedding from wild-type murine T-cells and T-cells with minimal ADAM17 expression [88]. Thus, indicating that ADAM10, but not ADAM17, plays a predominant role in P2X7-induced CD126 shedding.
Major histocompatibility complex (MHC) molecules
MHC class I molecules are cell surface receptors comprised of a single transmembrane protein paired with β2 microglobulin, and are highly expressed on nearly all cell types [89]. MHC class I molecules present antigenic peptides to CD8+ T-cells [89]. BzATP was shown to induce the loss of cell surface MHC class I molecules from murine CD8+ T-cells [58], but the identity of the P2 receptor involved, and whether this loss was due to shedding or internalisation was not determined. However, ATP also induced the rapid loss of MHC class I molecules from murine bone marrow-derived macrophages from wild-type, but not Pfizer P2X7 knockout, mice [90]. Furthermore, the P2X7 antagonists, A 438079 and A 740003, impaired ATP-induced MHC class I molecule loss from these cells from wild-type mice [90]. Collectively, confirming a role for P2X7 in this process. The use of GM 6001, or the Zn2+ chelator, N,N,N′,N′-tetra-2-picolylethylenediamine, failed to impair ATP-induced MHC class I molecule loss [90], suggesting that this process was not due to shedding. Moreover, the lysosome cysteine protease inhibitor, E64, or the proteasome inhibitor, MG132, failed to impair ATP-induced MHC class I loss [90], suggesting that this process was not due to a lysosome-mediated pathway. Thus, the authors proposed that ATP-induced MHC class I loss was the result of microvesicle release rather than shedding. Consistent with this, P2X7 activation induced the release of MHC class II molecule-containing microvesicles and exosomes from murine macrophages and dendritic cells [91, 92], but did not induce a loss of MHC class II molecules from the surface of CLL cells [34]. Finally, P2X7 activation inhibited the release of the non-classical MHC molecule, soluble human leukocyte antigen-G, from LPS-activated peripheral blood mononuclear cells [93]. Collectively, these studies demonstrate a complex role for P2X7 activation in the loss of MHC molecules from cells, and it remains to be determined if P2 receptor activation induces ectodomain shedding of MHC molecules.
T-cell immunoglobulin and mucin domain-2 (TIM-2)
T-cell immunoglobulin and mucin domain (TIM) molecules are type I integral membrane glycoproteins comprising three members in humans (TIM-1, TIM-3 and TIM-4) and eight members (TIM1-8) in mice [94]. TIM-2 is present in mice, but not humans, and functions as an H-ferritin receptor on T helper 2 cells and B-cells to modulate immune responses [94]. BzATP induced the rapid shedding of TIM-2 from murine splenic B-cells, as well as from P2X7 and TIM-2 co-transfected HEK 293 cells [95]. Both GI 254023X and GW 280264X inhibited BzATP-induced TIM-2 shedding from these cells [95], supporting a role for ADAM10 in this process. However, a direct role for P2X7 activation in BzATP-induced TIM-2 shedding from splenic B-cells remains to be established.
Tumour necrosis factor-related members
TNF and TNF receptors (TNFRs) belong to TNF and TNFR superfamilies, respectively, and have numerous roles in biology, including the immune, nervous and skeletal systems [96]. TNF is a proinflammatory cytokine that plays a critical role in inflammation and immunity by binding to TNFRs [97]. TNF functions as a transmembrane protein (to transmit signals as either a ligand or as a receptor) and as a soluble protein (ligand) following shedding by ADAM17 [97]. The type I integral membrane glycoprotein, CD27, is another member of the TNFR superfamily with important roles in immunity and tolerance [98]. CD27 binds CD70 and functions as both a membrane receptor and soluble molecule with biological activity [98].
CD27
ATP induced a rapid loss of surface CD27 from murine B- and T-cells [99]. In contrast to most other studies, which utilised enzyme-linked immunosorbent assays or immunoblotting to directly demonstrate P2 receptor-induced ectodomain shedding, surface plasmon resonance was used to show that supernatants from ATP-treated splenocytes displayed significant binding to immobilised anti-CD27 antibody [99] indicating that ATP-induced loss of CD27 is due to shedding. BzATP induced CD27 shedding more potently than ATP, whilst ATP-induced CD27 shedding was impaired by KN-62 [99] indicating a role for P2X7 activation in this process. GM 6001 also impaired P2X7-induced CD27 shedding [99], indicating a role for a metalloprotease in this process, but the identity of which remains unknown. Inhibitors of tyrosine kinases, PI3K, MEK and p38 MAPK did not impair P2X7-induced CD27 shedding [99]. Of note, NAD, released during tissue dissociation, promoted the P2X7-mediated loss of cell surface CD27 from murine splenic T regulatory cells and liver NK T-cells, a process that could be impaired with a blocking nanobody against ARTC2.2 [100, 101]. Thus, P2X7 activation can induce CD27 shedding from various lymphocyte subsets.
CD120a (tumour necrosis factor receptor 1, TNFR1)
The P2Y2 and P2Y4 agonist, UTP, induced the rapid shedding of CD120a (tumour necrosis factor receptor 1, TNFR1) from SV-40 immortalised human corneal epithelial cells, a process that was impaired by the broad-spectrum metalloprotease inhibitor, TAPI-1 [102]. Moreover, eye drops containing the P2Y2/P2Y4 agonist, Diquafosol, increased soluble CD120a in tears of patients with short break-up time dry eye [102]. Collectively, this suggests that P2Y2 or P2Y4 activation can induce CD120a shedding. Of note, P2Y2 is required for sustained TNF-induced Ca2+ oscillations in murine lung endothelial cells, a process that accompanies ADAM17-mediated CD120a shedding [103], providing indirect evidence that P2Y2 activation can induce CD120a shedding.
Tumour necrosis factor
BzATP induced the rapid shedding of TNF from P2X7 and TNF co-transfected mEFs [40], and from either ADAM17 knockout mEF cells (which expressed ADAM10) or ADAM10 knockout mEF cells (which expressed ADAM17) [40]. Furthermore, GI 254023X impaired BzATP-induced shedding of TNF in ADAM17 knockout mEF cells, but not in ADAM10 knockout mEF cells or normal CHO cells [40]. This indicates that ADAM10 contributes to BzATP-induced TNF shedding only in the absence of ADAM17, and that ADAM17, when present, is the main sheddase involved in this process. The contribution of P2X7 in this process remains to be established.
Summary
In conclusion, it is evident that extracellular nucleotides through the activation of P2 receptors can mediate the ectodomain shedding of numerous cell surface molecules. Both P2X7 and P2Y2 appear to be the major P2 receptors involved in this process, with well-established roles for various cell surface molecules, including amyloid precursor protein, CD23, CD27 and CD62L, although a role for P2X7 in amyloid precursor protein shedding remains to be clearly defined. Importantly, a number of studies have shown that P2 receptor-mediated ectodomain shedding for some molecules, such as ARTC2.2, CD62L and CD126, can occur in vivo, but such evidence for most molecules is still required. In addition, the biological significance of P2 receptor-mediated ectodomain shedding remains to be determined in nearly all cases. In most instances, it remains unknown if the soluble molecules or the remaining transmembrane regions, following P2 receptor-mediated ectodomain shedding, have any biological properties, although this is often inferred from ectodomain shedding studies initiated by pathways other than purinergic signalling. Furthermore, a large body of evidence supporting a role for P2 receptor-induced ectodomain shedding is based on immortalised cell lines and in many cases transfection of P2 receptors into such cell lines. Thus, further work is required to study this process in primary cells expressing endogenous P2 receptors, especially in human cell types. In this regard, the identity of the P2 receptors involved in nucleotide-induced ectodomain shedding remains to be determined in many cases. Finally, although the major sheddases, most notably ADAM10 and ADAM17, have been identified in many examples of P2 receptor-induced ectodomain shedding, it remains unclear in most instances how such metalloproteases are activated downstream of P2 receptor activation.
Acknowledgments
Monash University and the Biomedicine Discovery Institute currently support AP. The American Kennel Club Canine Health Foundation, the University of Wollongong, the Centre for Medical and Molecular Bioscience, and the Illawarra Health and Medical Research Institute currently support the laboratory of RS.
References
- 1.Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 2010;293:925–937. doi: 10.1002/ar.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 3.Wei L, Caseley E, Li D, Jiang LH. ATP-induced P2X receptor-dependent large pore formation: how much do we know? Front Pharmacol. 2016;7:5. doi: 10.3389/fphar.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 8.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 10.Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. ADP-ribosylation of P2X7: a matter of life and death for regulatory T cells and natural killer T cells. Curr Top Microbiol Immunol. 2015;384:107–126. doi: 10.1007/82_2014_420. [DOI] [PubMed] [Google Scholar]
- 11.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq. 2011;5:41–54. doi: 10.2174/187221511794839219. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 13.von Kugelgen I, Hoffmann K. Pharmacology and structure of P2Y receptors. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb L, Cao C, Ajit D, Weisman GA. P2Y receptors in Alzheimer’s disease. Biol Cell. 2015;107:1–21. doi: 10.1111/boc.201400043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miras-Portugal MT, Diaz-Hernandez JI, Gomez-Villafuertes R, Diaz-Hernandez M, Artalejo AR, Gualix J. Role of P2X7 and P2Y2 receptors on alpha-secretase-dependent APP processing: control of amyloid plaques formation “in vivo” by P2X7 receptor. Comput Struct Biotechnol J. 2015;13:176–181. doi: 10.1016/j.csbj.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- 18.Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY, Weisman GA. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109:1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran MD. P2 receptor stimulation induces amyloid precursor protein production and secretion in rat cortical astrocytes. Neurosci Lett. 2011;492:155–159. doi: 10.1016/j.neulet.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 20.Delarasse C, Auger R, Gonnord P, Fontaine B, Kanellopoulos JM. The purinergic receptor P2X7 triggers α-secretase-dependent processing of the amyloid precursor protein. J Biol Chem. 2011;286:2596–2606. doi: 10.1074/jbc.M110.200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darmellah A, Rayah A, Auger R, Cuif MH, Prigent M, Arpin M, Alcover A, Delarasse C, Kanellopoulos JM. Ezrin/radixin/moesin are required for the purinergic P2X7 receptor (P2X7R)-dependent processing of the amyloid precursor protein. J Biol Chem. 2012;287:34583–34595. doi: 10.1074/jbc.M112.400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Hernandez JI, Gomez-Villafuertes R, Leon-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, Diaz-Hernandez M. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol Aging. 2012;33:1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Leon-Otegui M, Gomez-Villafuertes R, Diaz-Hernandez JI, Diaz-Hernandez M, Miras-Portugal MT, Gualix J. Opposite effects of P2X7 and P2Y2 nucleotide receptors on α-secretase-dependent APP processing in Neuro-2a cells. FEBS Lett. 2011;585:2255–2262. doi: 10.1016/j.febslet.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Menzel S, Rissiek B, Bannas P, Jakoby T, Miksiewicz M, Schwarz N, Nissen M, Haag F, Tholey A, Koch-Nolte F. Nucleotide-induced membrane-proximal proteolysis controls the substrate specificity of T cell ecto-ADP-ribosyltransferase ARTC2.2. J Immunol. 2015;195:2057–2066. doi: 10.4049/jimmunol.1401677. [DOI] [PubMed] [Google Scholar]
- 25.Jersmann HP. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol Cell Biol. 2005;83:462–467. doi: 10.1111/j.1440-1711.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 26.de Torre-Minguela C, Barbera-Cremades M, Gomez AI, Martin-Sanchez F, Pelegrin P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep. 2016;6:22586. doi: 10.1038/srep22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1β and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Koralov SB, Kelsoe G. Regulation of humoral immune responses by CD21/CD35. Immunol Rev. 2000;176:194–204. doi: 10.1034/j.1600-065X.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengstake S, Boneberg EM, Illges H. CD21 and CD62L shedding are both inducible via P2X7Rs. Int Immunol. 2006;18:1171–1178. doi: 10.1093/intimm/dxl051. [DOI] [PubMed] [Google Scholar]
- 30.Aichem A, Masilamani M, Illges H. Redox regulation of CD21 shedding involves signaling via PKC and indicates the formation of a juxtamembrane stalk. J Cell Sci. 2006;119:2892–2902. doi: 10.1242/jcs.02984. [DOI] [PubMed] [Google Scholar]
- 31.Rosenwasser LJ, Meng J. Anti-CD23. Clin Rev Allergy Immunol. 2005;29:61–72. doi: 10.1385/CRIAI:29:1:061. [DOI] [PubMed] [Google Scholar]
- 32.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 33.Acharya M, Borland G, Edkins AL, Maclellan LM, Matheson J, Ozanne BW, Cushley W. CD23/FcεRII: molecular multi-tasking. Clin Exp Immunol. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and l-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–951. [PubMed] [Google Scholar]
- 35.Sluyter R, Wiley JS. Extracellular adenosine 5′-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors. Int Immunol. 2002;14:1415–1421. doi: 10.1093/intimm/dxf111. [DOI] [PubMed] [Google Scholar]
- 36.Georgiou JG, Skarratt KK, Fuller SJ, Martin CJ, Christopherson RI, Wiley JS, Sluyter R. Human epidermal and monocyte-derived Langerhans cells express functional P2X receptors. J Invest Dermatol. 2005;125:482–490. doi: 10.1111/j.0022-202X.2005.23835.x. [DOI] [PubMed] [Google Scholar]
- 37.Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, Sluyter R. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophys Acta. 2010;1800:1173–1182. doi: 10.1016/j.bbagen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Pupovac A, Geraghty NJ, Watson D, Sluyter R. Activation of the P2X7 receptor induces the rapid shedding of CD23 from human and murine B cells. Immunol Cell Biol. 2015;93:77–85. doi: 10.1038/icb.2014.69. [DOI] [PubMed] [Google Scholar]
- 39.Le Gall SM, Maretzky T, Issuree PD, Niu XD, Reiss K, Saftig P, Khokha R, Lundell D, Blobel CP. ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J Cell Sci. 2010;123:3913–3922. doi: 10.1242/jcs.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Gall SM, Bobe P, Reiss K, Horiuchi K, Niu XD, Lundell D, Gibb DR, Conrad D, Saftig P, Blobel CP. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor α, l-selectin, and tumor necrosis factor α. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel AD, Chessell IP, Hibell AD, Simon J, Humphrey PP. Identification and characterization of an endogenous P2X7 (P2Z) receptor in CHO-K1 cells. Br J Pharmacol. 1998;125:1194–1201. doi: 10.1038/sj.bjp.0702205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, Sunnarborg SW. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-α shedding. Mol Biol Cell. 2009;20:5236–5249. doi: 10.1091/mbc.E08-12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, Petrovich R, Lipsky PE, Moss ML, Werb Z. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem. 2007;282:14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pupovac A, Foster CM, Sluyter R. Human P2X7 receptor activation induces the rapid shedding of CXCL16. Biochem Biophys Res Commun. 2013;432:626–631. doi: 10.1016/j.bbrc.2013.01.134. [DOI] [PubMed] [Google Scholar]
- 45.Pupovac A, Stokes L, Sluyter R. CAY10593 inhibits the human P2X7 receptor independently of phospholipase D1 stimulation. Purinergic Signal. 2013;9:609–619. doi: 10.1007/s11302-013-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin C, Ren S, Zhang L, Jin H, Sun J, Zuo Y. Extracellular ATP induces CD44 shedding from macrophage-like P388D1 cells via the P2X7 receptor. Hematol Oncol. 2012;30:70–75. doi: 10.1002/hon.1008. [DOI] [PubMed] [Google Scholar]
- 48.Anderegg U, Eichenberg T, Parthaune T, Haiduk C, Saalbach A, Milkova L, Ludwig A, Grosche J, Averbeck M, Gebhardt C, Voelcker V, Sleeman JP, Simon JC. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J Invest Dermatol. 2009;129:1471–1482. doi: 10.1038/jid.2008.323. [DOI] [PubMed] [Google Scholar]
- 49.Takamune Y, Ikebe T, Nagano O, Nakayama H, Ota K, Obayashi T, Saya H, Shinohara M. ADAM-17 associated with CD44 cleavage and metastasis in oral squamous cell carcinoma. Virchows Arch. 2007;450:169–177. doi: 10.1007/s00428-006-0350-y. [DOI] [PubMed] [Google Scholar]
- 50.Chetty C, Vanamala SK, Gondi CS, Dinh DH, Gujrati M, Rao JS. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal. 2012;24:549–559. doi: 10.1016/j.cellsig.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moura GE, Lucena SV, Lima MA, Nascimento FD, Gesteira TF, Nader HB, Paredes-Gamero EJ, Tersariol IL. P2X7 receptor activity regulation: the role of CD44 proteoglycan GAG chains. Cell Death Dis. 2015;6:e1997. doi: 10.1038/cddis.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moura G, Lucena SV, Lima MA, Nascimento FD, Gesteira TF, Nader HB, Paredes-Gamero EJ, Tersariol ILS. Post-translational allosteric activation of the P2X7 receptor through glycosaminoglycan chains of CD44 proteoglycans. Cell Death Dis. 2015;6:e1997. doi: 10.1038/cddis.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smalley DM, Ley K. l-Selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of l-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS. Expression of P2X7 purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X7 receptors. Am J Physiol Cell Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- 58.Elliott JI, Higgins CF. Major histocompatibility complex class I shedding and programmed cell death stimulated through the proinflammatory P2X7 receptor: a candidate susceptibility gene for NOD diabetes. Diabetes. 2004;53:2012–2017. doi: 10.2337/diabetes.53.8.2012. [DOI] [PubMed] [Google Scholar]
- 59.Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sluyter R, Wiley JS. P2X7 receptor activation induces CD62L shedding from human CD4+ and CD8+ T cells. Inflamm Cell Signal. 2014;1:e92. [Google Scholar]
- 61.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/S1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 62.Krebs C, Adriouch S, Braasch F, Koestner W, Leiter EH, Seman M, Lund FE, Oppenheimer N, Haag F, Koch-Nolte F. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J Immunol. 2005;174:3298–3305. doi: 10.4049/jimmunol.174.6.3298. [DOI] [PubMed] [Google Scholar]
- 63.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. J Immunol. 2004;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 64.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 65.Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, Gorecki DC, Murrell-Lagnado RD, Soto F. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem. 2009;284:25813–25822. doi: 10.1074/jbc.M109.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarz N, Drouot L, Nicke A, Fliegert R, Boyer O, Guse AH, Haag F, Adriouch S, Koch-Nolte F. Alternative splicing of the N-terminal cytosolic and transmembrane domains of P2X7 controls gating of the ion channel by ADP-ribosylation. PLoS One. 2012;7:e41269. doi: 10.1371/journal.pone.0041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cascabulho CM, Bani Correa C, Cotta-de-Almeida V, Henriques-Pons A. Defective T-lymphocyte migration to muscles in dystrophin-deficient mice. Am J Pathol. 2012;181:593–604. doi: 10.1016/j.ajpath.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 69.Chen JR, Gu BJ, Dao LP, Bradley CJ, Mulligan SP, Wiley JS. Transendothelial migration of lymphocytes in chronic lymphocytic leukaemia is impaired and involved down-regulation of both l-selectin and CD23. Br J Haematol. 1999;105:181–189. doi: 10.1111/j.1365-2141.1999.01278.x. [DOI] [PubMed] [Google Scholar]
- 70.Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C, Linton K, Alexander DR, Higgins CF. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 71.Foster JG, Carter E, Kilty I, MacKenzie AB, Ward SG. Mitochondrial superoxide generation enhances P2X7R-mediated loss of cell surface CD62L on naive human CD4+ T lymphocytes. J Immunol. 2013;190:1551–1559. doi: 10.4049/jimmunol.1201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for l-selectin shedding. J Immunol. 2009;182:2449–2457. doi: 10.4049/jimmunol.0802770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–192. doi: 10.1615/CritRevImmunol.v24.i3.20. [DOI] [PubMed] [Google Scholar]
- 74.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 75.Sommer A, Fries A, Cornelsen I, Speck N, Koch-Nolte F, Gimpl G, Andra J, Bhakdi S, Reiss K. Melittin modulates keratinocyte function through P2 receptor-dependent ADAM activation. J Biol Chem. 2012;287:23678–23689. doi: 10.1074/jbc.M112.362756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng L, Chen N, Li Y, Zheng H, Lei Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim Biophys Acta. 2010;1806:42–49. doi: 10.1016/j.bbcan.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Schneider MR, Wolf E. The epidermal growth factor receptor and its ligands in female reproduction: insights from rodent models. Cytokine Growth Factor Rev. 2008;19:173–181. doi: 10.1016/j.cytogfr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Bles N, Di Pietrantonio L, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116:3219–3226. doi: 10.1182/blood-2010-01-265611. [DOI] [PubMed] [Google Scholar]
- 79.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 80.Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci. 2003;44:1211–1220. doi: 10.1167/iovs.02-0260. [DOI] [PubMed] [Google Scholar]
- 81.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin J, Yu FS. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Invest Ophthalmol Vis Sci. 2009;50:132–139. doi: 10.1167/iovs.08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009;284:17858–17867. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hua S. Targeting sites of inflammation: intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front Pharmacol. 2013;4:127. doi: 10.3389/fphar.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dallerac G, Rampon C, Doyere V. NCAM function in the adult brain: lessons from mimetic peptides and therapeutic potential. Neurochem Res. 2013;38:1163–1173. doi: 10.1007/s11064-013-1007-2. [DOI] [PubMed] [Google Scholar]
- 86.Hubschmann MV, Skladchikova G, Bock E, Berezin V. Neural cell adhesion molecule function is regulated by metalloproteinase-mediated ectodomain release. J Neurosci Res. 2005;80:826–837. doi: 10.1002/jnr.20530. [DOI] [PubMed] [Google Scholar]
- 87.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90:484–494. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, Koch-Nolte F, Rose-John S, Scheller J. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011;286:14804–14811. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 90.Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. P2X7 receptor activation impairs exogenous MHC class I oligopeptides presentation in antigen presenting cells. PLoS One. 2013;8:e70577. doi: 10.1371/journal.pone.0070577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 92.Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rizzo R, Ferrari D, Melchiorri L, Stignani M, Gulinelli S, Baricordi OR, Di Virgilio F. Extracellular ATP acting at the P2X7 receptor inhibits secretion of soluble HLA-G from human monocytes. J Immunol. 2009;183:4302–4311. doi: 10.4049/jimmunol.0804265. [DOI] [PubMed] [Google Scholar]
- 94.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewitz C, Moller-Hackbarth K, Schweigert O, Reiss K, Chalaris A, Scheller J, Rose-John S. T-cell immunoglobulin and mucin domain 2 (TIM-2) is a target of ADAM10-mediated ectodomain shedding. FEBS J. 2014;281:157–174. doi: 10.1111/febs.12583. [DOI] [PubMed] [Google Scholar]
- 96.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49:1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 99.Moon H, Na HY, Chong KH, Kim TJ. P2X7 receptor-dependent ATP-induced shedding of CD27 in mouse lymphocytes. Immunol Lett. 2006;102:98–105. doi: 10.1016/j.imlet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Rissiek B, Danquah W, Haag F, Koch-Nolte F. Technical advance: a new cell preparation strategy that greatly improves the yield of vital and functional Tregs and NKT cells. J Leukoc Biol. 2014;95:543–549. doi: 10.1189/jlb.0713407. [DOI] [PubMed] [Google Scholar]
- 101.Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on mouse T cells: one channel, many functions. Front Immunol. 2015;6:204. doi: 10.3389/fimmu.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakimoto T, Ohnishi T, Ishimori A. Significance of ectodomain shedding of TNF receptor 1 in ocular surface. Invest Ophthalmol Vis Sci. 2014;55:2419–2423. doi: 10.1167/iovs.13-13265. [DOI] [PubMed] [Google Scholar]
- 103.Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest. 2011;121:1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wada N, Otani Y, Kubota T, Kimata M, Minagawa A, Yoshimizu N, Kameyama K, Saikawa Y, Yoshida M, Furukawa T, Fujii M, Kumai K, Okada Y, Kitajima M. Reduced angiogenesis in peritoneal dissemination of gastric cancer through gelatinase inhibition. Clin Exp Metastasis. 2003;20:431–435. doi: 10.1023/A:1025453500148. [DOI] [PubMed] [Google Scholar]
- 105.Mirastschijski U, Impola U, Karsdal MA, Saarialho-Kere U, Agren MS. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol. 2002;118:55–64. doi: 10.1046/j.0022-202x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 106.Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993;53:2087–2091. [PubMed] [Google Scholar]
- 107.Denault JB, D’Orleans-Juste P, Masaki T, Leduc R. Inhibition of convertase-related processing of proendothelin-1. J Cardiovasc Pharmacol. 1995;26:S47–S50. doi: 10.1097/00005344-199506263-00015. [DOI] [PubMed] [Google Scholar]
- 108.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 109.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 110.Sellers A, Woessner JF., Jr The extraction of a neutral metalloproteinase from the involuting rat uterus, and its action on cartilage proteoglycan. Biochem J. 1980;189:521–531. doi: 10.1042/bj1890521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinmann-Niggli K, Lukes M, Marti HP. Rat mesangial cells and matrix metalloproteinase inhibitor: inhibition of 72-kD type IV collagenase (MMP-2) and of cell proliferation. J Am Soc Nephrol. 1997;8:395–405. doi: 10.1681/ASN.V83395. [DOI] [PubMed] [Google Scholar]
- 112.Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995;155:5198–5205. [PubMed] [Google Scholar]
- 113.Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massague J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]