Abstract
As the primary protective barrier for neurons in the brain, the blood–brain barrier (BBB) exists between the blood microcirculation system and the brain parenchyma. The normal BBB integrity is essential in protecting the brain from systemic toxins and maintaining the necessary level of nutrients and ions for neuronal function. This integrity is mediated by structural BBB components, such as tight junction proteins, integrins, annexins, and agrin, of a multicellular system including endothelial cells, astrocytes, pericytes, etc. BBB dysfunction is a significant contributor to the pathogeneses of a variety of brain disorders. Many signaling factors have been identified to be able to control BBB permeability through regulating the structural components. Among those signaling factors are inflammatory mediators, free radicals, vascular endothelial growth factor, matrix metalloproteinases, microRNAs, etc. In this review, we provide a summary of recent progress regarding these structural components and signaling factors, relating to their roles in various brain disorders. Attention is also devoted to recent research regarding impact of pharmacological agents such as isoflurane on BBB permeability and how iron ion passes across BBB. Hopefully, a better understanding of the factors controlling BBB permeability helps develop novel pharmacological interventions of BBB hyperpermeability under pathological conditions.
Keywords: Inflammatory mediators, Integrins, Annexins, Agrin, MicroRNAs, Anesthetics
Introduction
The blood–brain barrier (BBB) refers to an interface between the brain parenchyma and the systemic circulation. It regulates the entry of nutrients, vitamins, ions, and other molecules into the brain, protects the brain from harm caused by toxins and pathogens, and is critical to normal brain functions [1]. BBB consists of a non-fenestrated layer of brain microvascular endothelial cells (BMECs). The BMECs are primary components of BBB and play a critical role in regulating BBB permeability by a complex tight junction (TJ) structure [2, 3]. A more recent and complete view of BBB is that it refers to a neurovascular unit of BMECs interacting with other brain cells, such as astrocytes, pericytes, neurons, and microglial cells [3, 4]. It was clearly demonstrated as early as in 1987 that astrocytes contribute to BMEC-mediated regulation of BBB permeability and maintain cerebrovascular integrity [5]. It has been shown that astrocytes release sonic hedgehog that up-regulates TJ proteins in BMECs [5, 6]. However, activated astrocytes seem to disrupt endothelial barrier integrity by releasing biologically active molecules that activate ubiquitin proteasome and degrade TJ proteins [7]. In addition, microglia, pericytes and neurons maintain BBB integrity and function via contributing to cerebral vessel stability and modulating signaling mediators [8–11]. Disruption of BBB integrity and functions is involved in a growing list of brain disorders (Table 1). Particularly, diminished BBB function is an early event of multiple sclerosis (MS) and amyotrophic lateral sclerosis [12–16], chronic inflammatory disorders of the central nervous system (CNS), and a hallmark of stroke [17–20]. In this review, factors controlling BBB permeability are discussed in three chapters, junctional proteins at the BBB, proteins of the basement membranes of the BBB, and signaling mediators, in line with their contributions to neurodegenerative diseases and neurological disorders (Table 1). In addition, the impact of pharmacological agents such as the volatile anesthetic isoflurane on BBB permeability is reviewed. Recently, how iron passes across the BBB has drawn much attention; progress regarding this issue is also briefly reviewed. Understanding the molecular mechanisms by which these factors induce disruption of BBB integrity and function may lead to develop safe and effective therapeutic approaches to protect or restore the BBB integrity in many brain disorders.
Table 1.
Mechanisms underlying BBB disruption in brain disorders
| Disorders | Mechanisms |
|---|---|
| Alzheimer’s |
↓ ZO-1 by MMPs [146, 154, 225], cytokines [172], and ROS [226, 227] |
| Parkinson’s |
↓ Occludin and ZO-1 by MMP-3 [141] |
| Huntington’s | Degradation of endothelial basal lamina and ↓ TJ proteins (claudin, occludin and ZOs) by MMP-2/9 [142] |
| ALS | ↓ TJ proteins expression (ZO-1, occludin, and claudin) by MMP-2/9 [15, 16] |
| Migraine | ↓ TJ proteins expression (ZO-1, occludin, and claudin) by MMP-2 [143] and MMP-9 [144, 145] |
| Traumatic brain injury | ↓ TJ proteins expression (occludin, claudin-5, and ZO-1) by iNOS [164] and MMP-9 [100, 129, 149, 153] |
| Stroke |
↓ TJ proteins expression (occludin, claudin-5, and ZO-1) by MMP-2 [20], MMP-9 [73, 100, 129, 130, 148, 151, 156, 158, 163], ROS [19, 23, 130, 156, 158, 228], VEGF [181, 182], pericyte-derived VEGF [11], and astrocytes-derived VEGF [54–56, 183] ↓ Loss of α6β4 and β1 integrins [54] ↓ Agrin [110] ↓ AA1 [73] ↑ miRNA-21 → ERK-mediated upregulation of MMP-9 [196] ↑ miRNA-15a [197] |
| Intracerebral hemorrhage |
↑ miRNA-130a → ↑ MMP-2/9 expression [159] ↓ TJ proteins expression by MMPs induced by NF-κB [161] |
| Multiple sclerosis |
↑ MMPs and NO [175] ↓ AA2 and claudin-1 by miRNA-155 [28] |
| Japanese encephalitis | ↓ ZO-1 and claudin-5 by VEGF, IL-6, and MMP2/9 [7] |
| Autoimmune encephalomyelitis | ↓ Claudin-5 by VEGF [180] |
ALS amyotrophic lateral sclerosis, ↓ decrease in protein expression or activity, ↑ increase in protein expression or activity
Junctional proteins at the BBB
TJ proteins
Brain microvascular endothelial cells have a highly specialized phenotype characterized by the presence of intercellular TJs with high P-face association [21], which restrict molecules from moving between the blood and the brain and are responsible for the severe restrictions on diffusion. Transmembrane TJs consist of three major integral proteins—claudins, occludin, and junction adhesion molecules. ZOs are cytoplasmic membrane-associated accessory proteins that connect the cytoplasmic tails of claudins and occludin to the actin cytoskeleton to maintain the TJ structure. Altering TJ-associated proteins changes BBB permeability [22]. Occludin, claudin-5, and ZO-1 are considered sensitive indicators of normal and disturbed functional states of the BBB. As described in the latter paragraphs, many signaling mediators and pharmacological agents result in BBB dysfunction through modulating the expression of TJ proteins or TJ related proteins, particularly occludin, claudin-5, and/or ZO-1 [23, 24]. For example, claudin-5-deficiency induced size-selective loosening of the BBB [25], and we have observed that hyperglycemia caused endothelial hyperpermeability by altering the expression and distribution of ZO-1 in cultured BMECs [26] and in brain microvessels from diabetic mice (unpublished data). It is noteworthy that the role of claudin-1 in BBB permeability has recently been pursued by several groups. Although the presence or absence of claudin-1 in BMECs is still controversial [27, 28], ectopic expression of claudin-1 in Tie-2 tTA//TRE-claudin-1 double transgenic C57BL/6 mice was able to prevent BBB leakage in chronic experimental autoimmune encephalomyelitis [27], indicating the sealing function of claudins. Loss of claudin-1 at the BBB has been associated with barrier dysfunction in human glioblastoma multiforme [29] and hepatitis C infection [30]. In addition, the crosstalk between adherens junctions and TJs in maintaining barrier integrity is an important and emerging topic (see recent review [31] by Tietz and Engelhardt for details).
Integrins
Integrins are cell adhesion proteins that either link to the extracellular matrix (ECM) or mediate dynamic contacts to other cells via binding to cell adhesion molecules [32]. They widely exist and vary among species. Integrins are heterodimers and consist of α and β subunits that bind to each other non-covalently. There are over 18 α and 8 β subunits, which assemble over 24 distinct integrin proteins. Integrins have various cellular functions and can activate many intracellular signaling pathways [33]. Integrins express at the BBB at a high level and play important roles in the interactions between astrocytes and the endothelium. Integrin receptors, working with dystroglycan, contribute to endothelial cell–matrix adhesion and maintain adjacency between abluminal endothelial cell face to astrocyte end-feet [34, 35]. Specifically, αvβ8 integrin has been demonstrated to play an essential role in formation of the adhesive interaction, which was expressed by astrocytes and induced transforming growth factor-β (TGF-β) to stabilize the endothelium [36]. In a primary culture, αvβ5 and αvβ8 induced astrocyte adhesion to vitronectin and migration [37]. Alphavβ8 derived from neuroepithelial cells was able to activate TGF-β1 and TGFBR1-ALK5-Smad3 signaling in endothelial cells to maintain normal vascular patterning and suppress endothelial sprouting [38, 39]. Alpha6β4 expressed by astrocytes was reported to strengthen the attachment between end-feet and basal lamina in order to maintain apposition to endothelium of microvessels [35]. Alphavβ3 integrin promoted endothelial cell proliferation and survival through mediating capillary endothelial cell adhesion to fibronectin, which was introduced in the progress of angiogenesis [40]. It is clear that β1 integrins (the αβ heterodimers with β1 subunit) play a key role in forming the matrix adhesion between cerebral endothelial cells as a part of the BBB. In order to stabilize the TJ integrity, β1 integrins mediate the attachment of ECM to endothelial cells [41]. Expressed by endothelial cells along with other receptors, β1 integrins have an important function regulating the permeability of BBB [35]. In addition, in the process of CNS development, an obvious increased β1 integrin expression is a sign of cerebral blood vessel maturation [42].
β2 Integrins are a group of integrins that is essential for immune cell trafficking. Under inflammatory conditions, it has been found that β2 integrins are involved in neutrophil extravasation across inflamed BBB. There are many isoforms of β2 integrins such as lymphocyte function-associated antigen (LFA-1) and macrophage-1 antigen (Mac-1) [43, 44]. Many studies have investigated the roles of β2 integrins and their ligands, such as intercellular adhesion molecule 1 (ICAM-1), ICAM-2, and junctional adhesion molecule-A, in immune cell extravasation across the BBB under inflammatory conditions [45]. The adhesive interaction between immune cells and BMECs during inflammation requires β2 integrin activation to engage with their endothelial ICAMs. Both LFA-1 and Mac-1 with their endothelial ligands (ICAM-1 and ICAM-2) mediate neutrophil crawling on the BBB during inflammation [43, 45]. It seems that LFA-1 mediates shear-resistant arrest of neutrophils by ICAM-1 on the flamed BBB while neutrophil polarization is subsequently mediated by Mac-1 [45]. It was suggested that G protein-coupled receptors (GPCR) mediated β2 integrin activation [46] because GPCR inhibition resulted in reduction of neutrophil arrest and polarization. In the complete absence of either β2 integrins or their ligands, crawling of neutrophils on the inflamed BBB was abrogated [45].
Alpha 4 integrins are also involved in mediating the migration of immune cells across the BBB during neuroinflammation [47, 48]. Alpha4 integrin family consists of several isoforms such as α4β1 [very late antigen-4 (VLA-4)] and α4β7 [lymphocyte Peyer’s patch adhesion molecules (LPAM-1)]. It has been well known that the interaction between α4 integrins and vascular cell adhesion protein 1 (VCAM-1) contributes to T cell recruitment across BMECs. Lacking α4 integrins or VCAM-1 activity reduced the adhesion of encephalitogenic T cell blast to the BBB [49, 50]. It has been reported that VLA-4 predominantly regulated leukocyte extravasation to the inflamed brain while LPAM-1 had a role in immune cell trafficking to other inflamed systems such as the gut [50, 51]. The integrin α4 expression was up-regulated during MS brain lesions [52]. Moreover, recombinant VCAM-1 has been shown to bind to α4 integrins and to compromise BBB function in vitro through a mechanism that is mediated by activation of intracellular signaling pathways including MAP kinase. Pharmacological inhibitors (e.g. natalizumab) of the α4 integrin-VCAM-1 interaction protected the BBB during inflammatory conditions via blocking leukocyte adhesion to BMECs [51, 52]. Furthermore, preventing the interaction between α4 integrins and VCAM-1 by anti-α4 integrin monoclonal antibodies reduced inflammatory cell recruitment across the BBB during experimental autoimmune encephalomyelitis (EAE) in vivo [47]. Early treatment of EAE mice with a synthetic VLA-4 antagonist significantly reduced the clinical severity of the EAE condition [53].
Decreased expression of integrins has been found in neurological disorders and is considered to contribute to abnormal BBB permeability in such conditions. For example, it was shown that α6β4 was lost rapidly from astrocyte end-feet after focal ischemia [54]. Endothelial expression of β1 integrin, accompanied with the expression of subunits α1, α3, and α6, was significantly decreased within ischemic core 2 h after middle cerebral artery occlusion [55, 56]. In addition, Rice et al. and von Andrian et al. demonstrated that the counter-receptor on the vascular endothelium interacting with the α4β1 integrin on leukocytes had a significant effect on MS [57, 58]. Taken together, the above-mentioned findings suggest that normalizing integrin expression would be a potential approach to secure BBB integrity in these brain disorders.
Annexins
Annexins are Ca2+ and phospholipid-binding proteins that regulate cellular Ca2+ concentrations and membrane organization and traffic. Among many annexins, annexin A1 (AA1) is the most investigated. AA1 is a protein of 37 kDa and has been shown to function in intercellular trafficking due to its relation to membrane components and cytoskeletal proteins [59]. It was reported that AA1 possessed anti-inflammatory effects by regulating macrophage phagocytosis and neutrophil migration [60]. It might also play a significant role in differentiation [61], plasma membrane repair [62], proliferation [63], and apoptosis [64]. In the brain, AA1 is expressed in pericytes [65] and BMECs [66], where it may regulate prostaglandin (PG) E2 production [67]. AA1 is also strongly expressed in the ependymal cells of rats [68] and humans [69]. Glial expressions of AA1 are mainly seen in microglia [65], but not in oligodendrocytes [70, 71]. In CNS, the protective role of AA1 has been observed in neurodegeneration [72] and ischemia [73].
There is compelling evidence supporting AA1 as a critical physiological regulator of BBB integrity. First, endogenous AA1 regulates cytoskeleton by binding to actin. It can directly bind to β-actin, especially the cytosolic form rather than the fibrillar one. With predominant binding to soluble actin, AA1 may have a potential function in stabilizing actin oligomers before forming cytoskeleton [74]. It also works as a linkage between cytoskeleton and plasma membrane and helps to facilitate TJ functions. Second, AA1 signaling inhibits GTPase RhoA [75] that is able to lead to a downstream reaction, destabilizing actin cytoskeleton and enhancing paracellular permeability [76]. Third, AA1 knockout mice have a significant decrease in occludin and vascular endothelial (VE)-cadherin and an increase in BBB permeability although levels of claudin-5 and ZO-1 remain the same [75]. Furthermore, neutrophil extravasation in an animal model of stroke can be blocked by AA1 active fragments [73], corroborating AA1’s protective effect on BBB. In addition, AA2 has been shown to regulate endothelial cell morphology and junctional integrity via its association with VE-cadherin because silencing AA2 expression increased phosphorylation of VE-cadherin and BBB permeability in vitro [28, 77].
Vascular endothelial cell-specific phosphotyrosine phosphatase
Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) is a member of the receptor-type protein tyrosine phosphatase family that is exclusively expressed in endothelial cells. VE-PTP plays a critical role in embryonic development and angiogenesis. Mice lacking VE-PTP exhibited severe vascular malformation and died during early embryonic period [78, 79]. Furthermore, VE-PTP, working with its adhesive VE-cadherin partner, regulates the endothelial barrier function by an increased VE-PTP/VE-cadherin interaction [80]. Dissociation of VE-PTP from VE-cadherin is mediated by many inflammatory factors such as VEGF and then results in destabilization of endothelial cell integrity. Indeed, down-regulation of VE-PTP expression increased trans-endothelial permeability due to reduction of VE-cadherin adhesiveness [81, 82]. Moreover, cytokines (e.g. IL-1) that inhibit PTP activity increased BBB permeability [83]. Collectively, these findings suggest that modulating PTP activity could be an approach to regulate BBB permeability properties.
Platelet endothelial cell adhesion molecule-1
Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a transmembrane glycoprotein that is expressed in the intercellular junctions of the endothelial cells. PECAM-1 mediates angiogenesis in BBB development as well as migration and interaction of leukocytes to the endothelial cells. These effects indicate that PECAM-1 is a crucial molecule in neuroinflammation [84, 85]. Mice lacking PECAM-1 had deficiency in their inflammatory responses and exhibited an increased ability of inflammatory cells to cross the BBB due to prolonged changes in the BBB permeability [86, 87]. In addition, PECAM-1 seems to have an indirect role in modulating leukocyte extravasation behavior via increased β1 and β2 integrin affinity. This increase in integrin affinity mediates adhesion of leukocytes to vascular endothelial cells and enhances their trans-endothelial migration during neuroinflammation [87–89].
Proteins of the basement membranes of the BBB
The BBB is composed of both cellular components and basement membranes (BMs). Two BMs exist between blood and brain, endothelial BM and parenchymal BM. Endothelial BM lining the capillaries consists of collagen IV, fibronectin, laminins, etc. [90]. Each of these membrane components contributes to BBB integrity [91]. Some cells such as pericytes are embedded within the endothelial membrane. Parenchymal BM is primarily formed by the end-foot processes of astrocytes and neurons, ECM, and laminins. The BMs line the perivascular space and act as a barrier for leukocyte migration into the CNS during inflammatory perivascular cuff conditions [92].
Laminins
Laminins belong to a family of high-molecular weight (~400 kDa) glycoproteins of the ECM. There are four major isoforms of laminins at the BBB, including laminins 1, 2, 8, and 10. Laminins 8 and 10 are secreted in the endothelial BM while laminins 1 and 2 are mainly secreted by astrocytes in the parenchymal BM [93–95]. It has been known that pericytes regulate the secretion of these laminins [96]. Results from studies performed on mice lacking the major γ1 subunit of laminins support the notion that laminins play a crucial role in BM assembly. This targeted mutation resulted in early embryonic lethality due to defects in BM formation [97]. Yao et al. recently demonstrated that in conditional knockout mice and an acute adenovirus-mediated knockdown model, lack of astrocytic laminins induced BBB breakdown, possibly by increasing pericyte differentiation from the BBB-stabilizing resting stage to the BBB-disrupting contractile stage and decreasing TJ protein expression [98]. Moreover, it has been shown that decreased levels of laminins contribute to BBB damage caused by ischemia. Hamann et al. reported that ischemia significantly reduced the content of diverse basal lamina antigens including laminins [99]. Mostly recently, Cai et al. revealed that degradation of collagen IV and laminins was accompanied by increased BBB leakage after recombinant tissue plasminogen activator treatments, indicating that the BM works as an important barrier for preventing BBB disruption and hemorrhagic transformation after thrombolysis in stroke [100].
Collagen IV and fibronectin
All three major cells at BBB, endothelial cells, astrocytes, and pericytes, secrete collagen IV and fibronectin. Collagen IV chains form a covalently stabilized polygonal framework. It is responsible for the mechanical resistance of the basal lamina and mediates BM stabilization and integrity through retaining laminins [101]. Fibronectin exists in two forms, insoluble glycoprotein dimer and soluble disulphide linked dimer. The insoluble fibronectin serves as a linker in the ECM and is an important component of the ECM. Fibronectin binds other ECM components such as collagen, fibrin, and heparan sulfate proteoglycans. Collagen IV- or fibronectin-mutated mice exhibited embryonic death due to defects in vascular development and loss of BM stability, respectively [101, 102]. Similar to laminins, collagen IV and fibronectin are susceptible to ischemic damage and contribute to BBB hyperpermeability in ischemic stroke [99]. Collagen IV has been reported to decrease significantly at 3 and 24 h after focal ischemic brain injury [103, 104]. The loss of basal lamina components including laminins, collagen IV and fibronectin during ischemic brain injury seems to be a result of protein degradation caused by plasmin, MMPs and cathepsins (see review for detail [105]).
Agrin
Agrin is an extracellular heparan sulfate proteoglycan and accumulates extensively in the BM of the brain microvasculature [106]. It may play a role in BBB development due to its presence around brain microvessels during embryonic development [107]. Many lines of evidence have revealed that agrin is involved in maintaining BBB integrity and function. It was shown that loss of agrin was correlated with the loss of TJ protein expression in glioblastoma [108], Alzheimer’s disease (AD) [109], and cerebral ischemia [110]. Recently, the role of agrin in AD has attracted more attention due to its contribution to BBB dysfunction. Donahue et al. reported that a large fraction of the agrin in AD brains is insoluble in 1 % SDS while all the agrin in a normal brain is soluble [111]. The authors proposed that the insolubility was due to agrin’s tight association with β-amyloid and that altered agrin expression in the microvasculature and the brain parenchyma contributed to the pathogenesis of AD. Rauch et al. stated that in mice lacking endothelial cell expression of agrin, the level of aquaporin 4, a BBB-associated component, was reduced, and overexpression of agrin decreased Aβ deposition, indicating that agrin is important for maintaining BBB composition and influences Aβ homeostasis in mouse models of AD [112]. Most recently, Steiner et al. reported that agrin contributed to barrier characteristics of brain endothelial cells by stabilizing junctional localization of the adherent junction proteins VE-cadherin, β-catenin, and ZO-1 [113]. Their results demonstrated that agrin significantly enhanced the barrier characteristics of bEnd5 monolayers, accompanied by enhanced localization of VE-cadherin, β-catenin, and ZO-1, but not of claudin-5 and occludin. They further argued that agrin might stabilize these proteins at cell-to-cell junctions rather than inducing their enhanced-expression because protein levels of VE-cadherin, β-catenin, and ZO-1 did not change. Their argument was strengthened by results from an agrin deficiency mouse model that expressed mini-agrin, in which the vascular BMs had decreased junctional staining for VE-cadherin.
Signaling mediators that regulate BBB permeability
Cytokines
Cytokines are a group of polypeptides involved in inflammatory responses. These polypeptides include many families, such as interleukins (ILs) and tumor necrosis factors (TNFs), which are predominantly present in the brain during inflammation [114]. The normal activity of neuroinflammation is mainly to restore the homeostasis in the brain [115]. However, upon prolonged CNS inflammation, inflammatory responses may influence the BBB integrity and further result in a wide variety of CNS pathologies. In fact, many studies have recently demonstrated that neuroinflammation is a risk factor mediating BBB dysfunction in various brain disorders [116]. For example, early inflammation of the BBB was observed together with changes in permeability in experimental models of neurodegeneration [117]. Emerging evidence suggests that the chronic release of pro-inflammatory mediators from neurovascular cells is a major player in the disruption of BBB integrity in MS [118]. Although the mechanisms behind inflammation-induced BBB disruption and hyperpermeability are not fully understood, there is strong evidence that integrity of the BBB components can be threatened in response to pro-inflammatory mediators.
It has been revealed that the BBB disruption during neuroinflammation is largely associated with the release of numerous cytokines, such as IL-1, IL-6, TNFα, and eicosanoids [119–121]. Inflammation initiates cytokine production mainly through activation of leukocytes, astrocytes, and microglial cells in the brain [122, 123]. Increase in alteration of TJ function and BBB permeability is strongly linked with elevation of the cytokines (IL-1, IL-6, and TNFα) in the brain. For example, Quagliarello et al. reported that an increase in the level of IL-1 induced meningitis and BBB breakdown in the rat [124]. Wang et al. demonstrated that IL-1β increased BBB permeability via suppression of astrocytic sonic hedgehog production, leading to down-regulating TJ proteins such as claudin and occludin [125]. In addition, IL-6 and TNFα were able to elevate paracellular permeability in BMECs through a down-regulation of TJ proteins and an increase in reactive oxygen species (ROS) generation [126]. Furthermore, it was found that TNFα mediated BBB permeability via induction of cyclooxygenase-2 (COX2) and PG release in BMECs [127]. Results reported by Nishioku et al. showed that lipopolysaccharide (LPS)-activated microglia could release TNFα and result in BBB dysfunction [128]. HPI201 and minocycline, which prevented the up-regulation of TNF-α, IL-1β, and IL-6, improved TJ protein expression and BBB integrity [129, 130]. In addition, TNFα up-regulated expression of chemokines and cell adhesion molecules (e.g. ICAM-1) in BMECs; and these molecules played a critical role in adhesion and migration of leukocytes across BMECs [131]. Most recently, Rosenberg et al. proposed that inflammatory cytokines that increase BBB permeability could be used as biomarkers for vascular cognitive impairment [132]. Taken together, it is clear that certain cytokines have the ability to increase BBB permeability through down-regulating TJ proteins as well as inducing other pro-inflammatory mediators such as PGs.
Eicosanoids
Eicosanoids are derived from arachidonic acid and include PGs and leukotrienes (LTs). They are released by COX1 and COX2 in response to inflammation [133]. In addition to the cytokines, these inflammatory mediators are predominant in CNS inflammation. Many studies have shown that PGE2 causes BBB damage and dysfunction. For instance, it was reported in 1998 that PGE2 contributed to BBB disruption during experimental rat meningitis [134]. A recent study showed that increased PG production facilitated the permeability of an in vitro model of BBB with human BMECs [135]. Moreover, studies have demonstrated that PGs enhanced BBB permeability in LPS- and TNFα-administered mice. Furthermore, Candelario-Jalil et al. demonstrated that COX inhibitors limited BBB disruption during neuroinflammation [136]. Besides PGs, LTs are also known to contribute to BBB hyperpermeability. They are inflammatory mediators derived from the arachidonic acid/lipoxygenase pathway. Injecting LTs (e.g. LTB4, LTC4, and LTE4) directly into the brain parenchyma increased BBB permeability. This effect could be limited by pretreatment with lipoxygenase inhibitors [137]. Although the specific mechanisms underlying arachidonic acid-induced BBB disruption have not been fully elucidated, some studies demonstrated that arachidonic acid could increase BBB permeability via ROS, which can be generated from COX and lipoxygenase signaling pathways [138, 139]. In addition, up-regulation of MMPs during neuroinflammation could be a link between PG production and BBB dysfunction because increased MMPs can destruct the BMs [136].
MMPs
MMPs belong to a family of at least 25 zinc-dependent endopeptidases and are extracellular enzymes that play a pivotal role in CNS physiology and physiopathology. Numerous studies have demonstrated that dysregulation of MMPs is a major cause of increased BBB leakage, cerebral edema, hemorrhage, leukocyte infiltration, progressive inflammatory reactions, and cerebral injury in many neurological conditions, such as Parkinson’s disease [140, 141], Huntington’s disease [142], migraine [143–145], and stroke [20]. MMP-2 and -9 are two prominent proteins that cause BBB disruption in many conditions. For example, Aβ1-42 significantly up-regulated the level of MMP-2 and -9 and induced alterations in TJ scaffold and BBB leakage through a mechanism that involved receptor for advanced glycation end-products [146]. MMP-2 and -9 were reported to be responsible for BBB damage in HIV encephalopathy [147]. Early appearance of MMP-9 triggered BBB dysfunction after focal cerebral ischemia in mice [148]. Increased MMP-9 expression was observed in pericontusional brain; and MMP-9 inhibitors reduced brain swelling and final lesion volume in focal injury models [149]. MMP-2 seemed not to play a role in the vasogenic edema [149]. In addition, it was reported that MMP-1 was highly expressed in brain metastatic cells and was capable of degrading claudin and occludin but not ZO-1 [150]. The expression of MMP-1 seemed to be induced by COX2-mediated PGs. MMP-3 was also able to induce BBB abnormality in Parkinson’s disease [141]. Mechanistically, MMP-induced endothelial barrier disruption was accompanied by MMP-mediated proteolytic degradation of claudin-5 in Japanese encephalitis-associated BBB breakdown [7]. Suppressing MMP-9-mediated degradation of the claudin-5 and occludin reduced BBB permeability in ischemic stroke [151].
Recent research has focused on pericytes as an important source of MMPs and inflammatory cytokines. Takata et al. reported that MMP-9 derived from pericytes induced migration of the cells from the endothelium, leading to loss of pericytes and ensuing BBB damage [152]. Machida et al. recently demonstrated that pericytes played a pivotal role, as a highly thrombin-sensitive and MMP-9-producing cell, at the BBB in brain damage [153]. Halliday’s research confirmed that pericytes maintained the integrity of the BBB and degenerated in AD. It was also revealed that apolipoprotein E4 (APOE4), a major genetic risk factor for late-onset AD, led to accelerated pericyte loss and enhanced activation of the low-density lipoprotein receptor-related protein-1-dependent cyclophilin A-MMP-9 pathway that caused BBB opening via degradation of endothelial TJ proteins and BMs in AD models [154, 155].
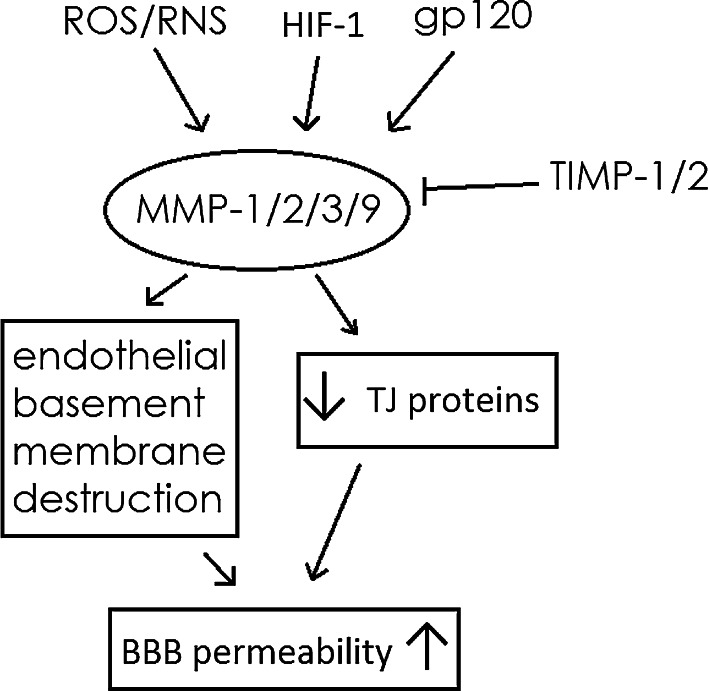
Another focus of research in the field is to identify potential pharmacological approaches to inhibit MMPs, at least in animal models. Takeuchi’s data demonstrated that hydrogen was able to attenuate BBB disruption via suppressing MMP-9 activity in the hippocampus and improved neurological function outcome in a hypertensive stroke model [156]. Hydrogen sulfide inhalation was found to be able to decrease early BBB permeability and brain edema induced by cardiac arrest and resuscitation, possibly by decreasing MMP-9 and VEGF activity [157]. Allahtavakoli et al. revealed that ascorbic acid might be a useful candidate to reduce the side effects of delayed application of recombinant tissue plasminogen activator in stroke therapy by suppressing overexpression of MMP-9 [158]. Yang et al. reported that minocycline reduced reperfusion injury by inhibiting MMPs and microglial activity and enhancing TJ protein expression in ischemic brains [130]. Neurotensin receptor agonist HPI201 and TGF-β1 significantly decreased MMP-2 and -9 levels in stroke and traumatic brain injury (TBI) models of adult rodents [100, 129]. Shi et al. argued that ethyl pyruvate conferred long-term neuroprotection against TBI, possibly through breaking the vicious cycle among MMP-9-mediated BBB disruption, neuroinflammation, and long-lasting brain damage [153]. Wang et al. observed that miRNA-130a inhibitors reduced brain edema, BBB permeability, and increased neurological deficit scores, possibly due to decreased MMP-2/9 expression in an acute intracerebral hemorrhage (ICH) model [159]. Wu et al. found that angiotensin 1–7 could effectively restore claudin-5 and ZO-1 expression in rats by down-regulating hypoxia-induced MMP-9 [160]. In addition, BBB disruption and brain edema formation play important roles in the secondary neuronal death and neurological dysfunction induced by ICH. Poloxamer 188, a multiblock copolymer surfactant, was found to protect against ICH, and the protective effect was associated with preventing BBB disruption through NF-κB-MMP-mediated TJ protein degradation [161]. Furthermore, tissue destruction by MMPs is regulated by their endogenous tissue inhibitors (TIMPs). TIMPs prevent excessive MMP-related degradation of ECM components. Promoting secretion of TIMPs has been explored as an effect way to inhibit MMP activities. Reuter et al. showed that simvastatin decreased MMP expression in human BMECs and experimental stroke mainly by means of increasing expression and secretion of TIMP-1 and TIMP-2 [162]. Resveratrol was also reported to attenuate the cerebral ischemia by maintaining the integrity of BBB via regulation of MMP-9 and TIMP-1 [163] (Fig. 1).
Fig. 1.
A brief summary of MMPs as a factor controlling BBB permeability
Free radicals
Human body normally produces free radicals at low concentrations as a defense mechanism. Overproduction of free radicals is implicated in the pathogeneses of many neurological diseases [140, 164, 165]. Reactive oxygen and nitrogen species are important in the early and delayed BBB disruption as the neuroinflammatory responses progress (see Rosenberg’s reviews for details [166, 167]). For example, studies have shown that LPS-induced BBB disruption is mediated by ROS and reactive nitrogen species (RNS) production [168, 169]. LPS-induced microglial activation can increase NADPH oxidase, one of the major enzymes that generate ROS. Many reports have clearly demonstrated that ROS play a central role in BBB dysfunction during ischemia–reperfusion [166, 167]. For instance, Kamada et al. reported that hyperglycemia increased ROS levels and MMP-9 activity, exacerbating BBB dysfunction after ischemia and reperfusion [23]. Other studies showed that ROS induced BM degradation, enhanced tyrosine phosphorylation of TJ proteins by activating MMP-1/2/9 and decreasing TIMP-1 and -2, and led to increased permeability and monocyte infiltration [24]. In addition, HIV-1 envelope protein gp120 up-regulated MMP-2/9 expression, probably by gp120-mediated ROS [147].
Although the exact mechanism by which ROS induce BBB breakdown has not fully clarified, their harmful effects on BBB integrity could be mediated by many mechanisms such as MMP activation and TJ protein down-regulation [170–172]. Moreover, ROS can change the vascular tone and therefore influence cerebral blood flow. Their vascular effects also include increasing platelet aggregability and endothelial cell permeability, altering reactivity to vasodilators, and leading to the formation of focal lesions in endothelial cell membranes [14].
The brain has an essential defense system in response to free radical generation, and this antioxidant system contains small molecules such as glutathione and enzymes such as superoxide dismutase and GSH peroxidase that decrease levels of free radicals. It is believed that free radical scavengers could reduce oxidative stress and subsequently rescue BBB integrity [173]. Clinically, glucocorticoids have been used to treat neuroinflammatory conditions such as MS. Glucocorticoids seem to restore BBB integrity via a broad spectrum by down-regulating multiple inflammatory genes, such as cytokines [174], enzymes (e.g. inducible NO synthase and MMPs) [175], adhesion molecules [176]. Antioxidant gene delivery of Cu/Zn superoxide dismutase or GSH peroxidase blunted gp120-induced MMP production though down-regulating TIMP-1 and -2 [147]. Moreover, administration of a peroxynitrite decomposition catalyst, 5,10,15,20-tetrakis (4-sulfonatophenyl) porphyrinato iron (III), protected against hemoglobin-induced neurovascular injuries, which possibly in part by suppressing MMP-9 activation [177].
VEGF
VEGF is a specific mitogen; it binds to the vascular BM and causes proliferation and migration of endothelial cells [178]. Besides being the most prominent member of the angiogenic growth factor family, VEGF has been known since the 1980s to increase vascular permeability. VEGF has been characterized as an inducer of vascular leakage in response to hypoxia [17]. It has been reported that VEGF alters the expression and distribution of TJ proteins, leading to BBB hyperpermeability in hypoxia and autoimmune encephalomyelitis [179, 180]. VEGF exerted a major role in BBB disruption leading to subsequent edema during ischemic brain injury [181, 182]. It is noteworthy that VEGF seemed to have a dual role in both enhancing cerebral microvascular perfusion and increasing the BBB leakage in the ischemic brain. Zhang et al. reported that rhVEGF165 significantly enhanced cerebral microvascular plasma perfusion and improved functional neurological recovery when it was administered to ischemic rats at 48 h after a stroke while administration at 1 h after stroke exacerbated BBB leakage [182]. Pericytes and astrocytes seem to be the major sources of VEGF in pathological conditions such as stroke [11, 183].
The mechanism that contributes to VEGF-mediated BBB hyperpermeability is not fully understood. It was reported that VEGF bound to the receptor tyrosine kinase, VEGF receptor 2, to induce BBB permeability [184]. In vitro studies suggest that the receptors for VEGF are located on the abluminal side of microvessels [185, 186]. Although it is known that VEGF increases BBB permeability by down-regulating the expression of TJ proteins [180], the specific target proteins of VEGF may vary, depending on the pathological condition and other factors. For example, our study has shown that claudin-5 seemed not to play a role in hyperglycemia-induced BBB hyperpermeability [26]. In contrast, Argaw’s data suggest that down-regulation of claudin-5 by VEGF was central to disruption of the BBB in autoimmune encephalomyelitis [180]. Most recently, Japanese encephalitis virus-infected astrocytes were found to release VEGF that activated ubiquitin proteasome, degraded ZO-1, and disrupted endothelial barrier integrity in cultured BMECs [7]. In addition, several groups have reported that effect of exogenous VEGF on endothelial cells is partially attributed to NO generation from these cells through a NO synthase/cGMP-dependent pathway [182, 187]. VEGF may also induce microvascular hyperpermeability through activation of vesicular–vacuolar organelles [188] (Fig. 2).
Fig. 2.
A brief summary of VEGF as a factor controlling BBB permeability
Hypoxia inducible factor 1
The transcriptional factor hypoxia inducible factor 1 (HIF-1) is implicated in many cerebral vascular pathological disorders. HIF-1 is a heterodimeric complex consisting of a hypoxia inducible subunit HIF-1α and a constitutively expressed subunit HIF-1β. Under hypoxic conditions, HIF-1α is stabilized and translocated to the nucleus where it dimerizes with HIF-1β. The activated HIF-1 complex subsequently binds to hypoxic response elements in the regulatory regions of targeted genes. It induces transcription of more than a hundred genes with various functions [62]. VEGF is one of the most well-known HIF-1 target genes in vascular biology. Thus, one mechanism of HIF-1-mediated BBB disruption is VEGF up-regulation. Moreover, HIF-1 may cause BBB damage via the induction of MMP-2 and -9 [180]. It was reported that increased MMP activity during ischemia resulted in fragmentation/relocalization of occludin and claudin-5, leading to BBB opening [189]. Inhibition of HIF-1 ameliorated hypoxia-induced BBB disruption and the subsequent brain injury in animal ischemic models [190, 191]. Suppression of HIF-1α and its downstream VEGF and MMPs reduced hemorrhagic transformation induced by hyperglycemia in ischemic rat brains, which indicates a recovery of BBB function [192]. Yeh et al. showed that inhibition of HIF-1 by YC-1 decreased VEGF expression and suppressed the increase in BBB permeability [191]. We were the first to report that high glucose induced a time-dependent up-regulation of HIF-1α in mouse and human primary BMECs, in which it induced disarrangement of TJ proteins [26]. In addition, HIF-1 was responsible for MMP-9-mediated BBB disruption that contributed white matter injury in hypertensive rats with carotid artery occlusion and a Japanese permissive diet [193].
MicroRNAs
MicroRNAs (miRNAs) are a family of non-protein-coding small RNA molecules that negatively regulate protein expression. Many miRNAs have recently been found to regulate cellular signaling pathways and cell survival. Various miRNAs have been discovered in different cellular components of the BBB (Table 2). Several groups have reported that certain miRNAs control endothelial cell functions and BBB permeability. For example, Reijerkerk et al. demonstrated that a large number of miRNAs were down-regulated in BMECs with impaired BBB function and that strengthening BBB function was generally associated with increased miRNA expression [194]. Specifically, they reported that brain endothelial cells overexpressing miRNA-125a-5p formed thicker and more continuous junctional complexes of VE-cadherin and ZO-1 and increased BMEC barrier function. Conversely, specific knockdown of the miRNA reduced the levels of VE-cadherin and ZO-1 along the BBB. In contrast, others have demonstrated negative effects of miRNAs on BMECs and BBB function. Lopez-Ramirez et al. observed that miRNA-155 negatively affected BBB function during neuroinflammation [28]. MiRNA-155 was up-regulated by inflammatory cytokines and expressed at the neurovascular unit of MS patients and of mice with experimental autoimmune encephalomyelitis. The miRNA up-regulation mimicked cytokine-induced alterations in junctional organization and BBB permeability. Furthermore, they demonstrated that miRNA-155 modulated brain endothelial barrier function by targeting cell–cell complex molecules, such as AA2, claudin-1, and molecules that are critical in cell-to-ECM interactions including dedicator of cytokinesis 1 and syntenin-1. Kalani et al. reported that miRNA-29b caused BBB hyperpermeability by regulating DNA (cytosine-5-)-methyltransferase 3 beta and MMP-9 that can disrupt the membrane and junction proteins leading to leaky vasculature [195]. Furthermore, Deng et al. showed that miRNA-21, but not -224, involved in ERK-mediated upregulation of MMP-9 in rat hippocampus and BBB dysfunction following cerebral ischemia [196]. Yin’s results suggested that miRNA-15a was involved in ischemia-induced cerebral vascular endothelial injury [197]. Another group showed that miRNA-26b and -28 seemed to contribute to BBB dysfunction in lupus mouse brain [198]. Slava et al. demonstrated that miRNA-98 overexpression rescued the BBB during neuroinflammation through reduction of leukocyte adhesion and cytokine production. Their study also showed that inhibition of glycogen synthase kinase 3β increased miRNA-98 expression in inflamed brain [199]. Moreover, miRNA-181c has been reported to be induced in many malignancies such as breast cancer [50]. Despite the exact mechanism by which miRNA-181c mediates BBB breakdown is not yet fully understood, Tominaga et al. found an increased level of miRNA-181c in the blood circulation of breast cancer patients with brain metastasis, indicating that the miRNA plays a crucial role in brain metastasis [52]. In addition, a recent study showed that miRNA-181c triggered the Toll-like receptor 4 pathway, resulting in microglial activation and neuroinflammation [200]. These observations suggest the miRNAs are a new set of controllers of BBB permeability under stress and pathological conditions.
Table 2.
MiRNA expression in different cells at the BBB
| miRNAs | Cellular components of the BBB |
|---|---|
| miRNA-15a | Astrocytes, microglia [230] |
| miRNA-21 | BMECs, astrocytes, microglia [231] |
| miRNA-26b | BMECs [198] |
| miRNA-28 | BMECs [198] |
| miRNA-29 | Astrocytes [232] |
| miRNA-98 | BMECs [199] |
| miRNA-125a-5p | BMECs [194] |
| miRNA-155 | BMECs [28], astrocytes [233] |
| miRNA-181c | Microglia [200] |
Anesthetic agents
Besides endogenous mediators that control/regulate BBB function and permeability, many pharmacological agents have great impact on BBB permeability through regulating the signaling mediators and the structural components. One widely studied pharmacological agent is isoflurane, a popular volatile anesthetic routinely used in human patients. As early as in 1992, Chi et al. reported that isoflurane decreased the transport of small hydrophilic molecules across the BBB by measuring blood–brain transfer coefficient and capillary permeability-surface area [201]. Later on, Tetrault et al. demonstrated that high doses of isoflurane (3 %) opened the BBB in the cortex and thalamus while milder doses (1 %) only opened the BBB in the thalamus in cats by monitoring the extravasation of Evans blue [202]. Their results also showed that the opening of the BBB was associated with an evaluated increase in cerebral volume of 2–2.8 %, indicating brain edema [202].
The mechanism of isoflurane-mediated hyperpermeability of BBB seems to involve in its direct or indirect effects on the TJ proteins. Most recently, Cao et al. studied the effects of isoflurane on BBB in aged rats. Their results showed that 1.5 % isoflurane resulted in reversible time-dependent morphological damage of the BBB ultrastructure and significant decreases in expression of the TJ protein occludin in 24-month old rats. They concluded that occludin down-regulation was one of the mediators of isoflurane-induced BBB disruption, and might contribute to hippocampus-dependent cognitive impairment after isoflurane exposure in aged rats [203]. In a model of TBI, ZO-1 expression was more significantly disrupted in sevoflurane than isoflurane-exposed animals with controlled cortical impact, while claudin-5 was less affected in the pericontusional area [155]. Moreover, Zhao et al. reported in 2014 that isoflurane treatment induced a time- and concentration-dependent decrease in occludin mRNA and protein levels in human BMECs, which was partially abrogated by silencing the alpha subunit of HIF-1 [204]. Isoflurane-mediated HIF-1α expression up-regulated the level of VEGF, which decreased the expression of occludin and TGF-β3 that accelerated the endocytosis of occludin [204]. If HIF-1 activation is indeed responsible for isoflurane-induced BBB abnormality, it is still not clear how isoflurane up-regulates the transcriptional factor (Fig. 3).
Fig. 3.
Proposed mechanism responsible for isoflurane-mediated BBB hyperpermeability
Furthermore, several recent reports suggest that under pathological conditions, isoflurane may exaggerate brain injury by further opening the BBB. Dittmar et al. investigated the effect of a 2-h isoflurane exposure on apoptosis of the cerebral endothelium following 24 h of hypoxia in an in vitro BBB model. They found that isoflurane treatments resulted in severe cellular morphological changes and a significant dose-dependent increase in DNA fragmentation in trans-differentiated human umbilical vein endothelial cells, possibly due to increases in pro-apoptotic Bax levels and decreases in the level of anti-apoptotic Bcl-2 [205]. In a rat stroke model of embolic occlusion of middle cerebral artery, Bezerra et al. observed that BBB permeability measured by blood-to-brain forward rate constant was elevated in the ipsilateral hemisphere in animals under either halothane or isoflurane anesthesia. However, isoflurane-anesthetized rats demonstrated less BBB damage than halothane-anesthetized group [155]. In addition, BBB disruption causes cerebral edema formation and is a major cause for high mortality after TBI. Results from Thal’s study demonstrated that 24 h after controlled cortical impact, brain water content of mice subjected to either sevoflurane or isoflurane increased significantly while in healthy mice, anesthesia did not influence brain water content [206].
In summary, isoflurane is able to affect BBB permeability at normal conditions, to induce brain edema, and to exaggerate BBB injuries under pathological conditions such as stroke and TBI. This effect may be attributed to its impact on TJ protein expression, possibly through regulating HIF-1 activity. In addition, other anesthetic agents have also been shown to affect BBB permeability. Similar to isoflurane, sevoflurane was able to induce structural changes in BMECs and increase BBB permeability [155, 206, 207]. An in vitro study showed that thiopental induced BBB opening at high concentrations (~100 µg/ml) while fentanyl and methohexital did not affect BBB permeability [208]. Future research is required to investigate the exact molecular mechanisms of anesthetic-mediated BBB opening and to mitigate the adverse effect on patients.
Factors that regulate transcellular transport of iron through BBB
The above discussion focuses on paracellular permeability of the BBB. Transcellular transport across BBB might be affected by the same or different signaling mediators as those discussed above. In this section, recent progress on transcellular transport of iron ion is briefly reviewed. Brain is one of the most abundant iron-containing organs. When there is iron deficiency or overload in the brain, the nervous system suffers from disturbed functions. Hence, it is essential to maintain brain iron homeostasis. The key to iron balance in the brain lays in the regulation of brain iron uptake. The regulation of iron transport across the BBB is of major importance to brain iron uptake [209]. Available data suggest that uptake of plasma transferrin binding iron (Tf-Fe) occurs at the luminal membrane of BMECs, mainly through the classic transferrin receptors (TfR)-mediated endocytosis [210]. In BMECs, due to the acidification of the microenvironment within endosomes, iron ions dissociate from Tf and are reduced from Fe3+ to Fe2+. Ferrous ion can translocate across the endosomal membrane via a proton driven transporter, divalent metal transport 1 [211]. Most of the Tf and TfR will return to the luminal membrane. Iron ion is proposed to be transported across the abluminal membrane of the BBB into the interstitial fluid of the brain [212]. This process likely involves iron exporter ferroportin1 (FPN1) on the abluminal membrane, but the exact mechanism remains to be explored. Available studies showed that both hephaestin and FPN1 have been identified in BMECs. In addition, iron efflux from BMECs by FPN1 may require the action of an exocytoplasmic ferroxidase, possibly ceruloplasmin produced by glial cells [213].
At the systematic level, brain iron homeostasis may be regulated by a peptide ‘hormone’ named hepcidin. Hepcidin binds to the extracellular loop of FPN1 and causes its internalization and degradation, and thereby reduces cellular iron efflux. Recent data indicate that brain hepcidin stimulates internalization of FPN1 to regulate iron efflux from the BMECs [214]. The observation indicates that hepcidin may play an important regulatory role in brain iron metabolism. The expression of hepcidin can be regulated by high iron levels, inflammation, erythropoiesis, or hypoxia [215]. When iron levels are high, hemojuvelin and TfR2 increase hepcidin expression. In addition, IL-6, a cytokine that regulates paracellular permeability, is able to stimulate hepcidin expression through molecular pathways that could include binding of STAT3 to the hepcidin promoter.
Perspectives
Blood–brain barrier permeability is tightly controlled by many component factors of BMECs, astrocytes, pericytes and microglial cells. The TJ structure is essential to the integrity of BBB. It establishes a linkage between endothelial cells, which is tight enough to form a barrier to separate brain cells from exposure to the systemic circulation. As discussed above, altered TJ protein expression and structural arrangement underlines the mechanism of BBB dysfunction in many brain disorders. Hence, normalizing TJ protein expression and arrangement is an important pharmacological goal to enhance the BBB integrity under pathological conditions. The regulation of the expression of TJ proteins including their interactions, stereochemistry, and their signaling mediators are directions of future studies. Meanwhile, many other proteins play important roles in maintaining BBB permeability and functions. As evident from many recent reports, integrins binding to ECM together with annexins and agrin play an essential role in BBB integrity and functions. Yet, the functions of integrins, annexin and agrin in BMEC are still not well revealed, especially their interactions with TJs. These are interesting areas to be explored in the future.
Inflammation plays a central role in the pathogeneses of neurodegenerative and neurological disorders. Evidence is being accumulated for the harmful effects of neuroinflammation on BBB integrity. Inflammatory mediators such as cytokines, eicosanoids, or free radicals have been reported to cause BBB damage. Results from recent studies are consistent in supporting that TJ protein alternation is the most predominant mechanism underlying neuroinflammation-induced BBB disruption. Although the mechanism has not fully been understood, activation of microglial cells may act as the first step in BBB disruption in CNS inflammation. Recent studies have established a link between microglial activation and BBB breakdown [216]. Interestingly, microglial cells seem to be a double-edge sword. On one hand, these cells regulate the integrity of the mature BBB. On the other hand, the microglial response may activate different signaling pathways through pro-inflammatory mediators. Inactivation of microglial cells is most likely not the suitable therapeutic approach because their activation can mediate many beneficial physiological functions such as phagocytosis of damaged neurons [217]. Thus, targeting the microglial signaling pathways that mediate inflammation-induced BBB breakdown would be a promising therapeutic strategy. Sonic hedgehog has been known to maintain BBB integrity via TJ protein up-regulation [218]. Overexpression of sonic hedgehog could be an effective experimental approach to restore BBB properties after inflammation. Furthermore, many studies have shown that anti-inflammatory and antioxidative agents could limit BBB disruption after neuroinflammation. Inhibition of the COX signaling pathway with indomethacin protected against TNF-α-induced BBB disruption in vivo. This COX inhibition was associated with a decrease in MMP-9 and -3 activities [136]. Recently, a report showed that ginsenosides, a group of anti-inflammatory and antioxidant bioactive compounds, reduced BBB disruption following cerebral ischemia [53] by suppressing the expression of pro-inflammatory mediators such as NO synthase, IL-1β, and MMP-9 [53, 200]. Moreover, caffeic acid phenethyl ester, an anti-inflammatory and antioxidative agent [219], limited BBB opening in a TBI rodent model through preserving TJ proteins such as claudin-5 [220].
Emerging evidence suggests that the brain is a target of diabetes, resulting in diabetic encephalopathy [221]. The pathophysiology of diabetic encephalopathy is still largely elusive. Cerebral microvascular disturbances could play an important role in diabetes-induced brain abnormality. In fact, BBB dysfunction (i.e. hyperpermeability) has been demonstrated in diabetic patients by magnetic resonance imaging [222] and in animal models of diabetes [223]. However, the mechanism responsible for BBB hyperpermeability in diabetes is not known. To understand the mechanism of diabetes-induced BBB dysfunction is crucial for the prevention and treatment of diabetes-induced neurological diseases. Future studies may focus on the following to reveal the mechanism, inflammatory mediators, MMPs, HIF-1, VEGF, ROS, and miRNAs that control the permeability of BBB. In fact, excessive generation of ROS has been recognized as an integral part to the pathophysiology of diabetes, particularly in diabetes-induced vascular diseases [224]. Expression and activation of HIF-1 in hyperglycemia-exposed brain endothelial cells are believed to be closely associated with the brain microvascular damage and to be induced by free radicals. We recently demonstrated that high glucose activated HIF-1 in mouse and human BMECs [26]. It will be useful to determine what specific ROS and inflammatory mediators are generated and responsible for the enhanced activities of HIF-1 and BBB dysfunction in diabetes. In addition, anesthetic agents such as isoflurane may change BBB permeability in diabetic patients. More research is needed to find its molecular mechanism to minimize the adverse effects on patients. Moreover, change in iron transport across BBB in diabetic patients is also an important and interesting topic to be addressed.
In summary, BBB is a very important component in the body regulating the brain homeostasis. Permeability of BBB is regulated by many factors such as junctional proteins, BM proteins, cytokines, VEGF, HIF-1, ROS, miRNAs, etc. Recent research has made great progress in revealing the mechanisms by which they regulate BBB permeability. Changes of BBB permeability are involved in the pathogeneses of many brain disorders. Further studies are needed to reveal a better understanding of BBB permeability regulation, particularly in neuroinflammatory and diabetic conditions, and to develop novel and specific therapeutic approach to mitigate the harmful effect of BBB dysfunction.
Acknowledgments
This work was supported in part by the University of Kansas Center for Research and National Natural Science Foundation of China (31520103908 and 31528013). Mohammed M. A. Almutairi was supported by a scholarship from King Saud University (Riyadh, Saudi Arabia).
References
- 1.Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 5.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 6.Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood–brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 7.Chang CY, Li JR, Chen WY, Ou YC, Lai CY, Hu YH, Wu CC, Chang CJ, Chen CJ. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-Infected astrocytes. Glia. 2015 doi: 10.1002/glia.22857. [DOI] [PubMed] [Google Scholar]
- 8.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spindler KR, Hsu TH. Viral disruption of the blood–brain barrier. Trends Microbiol. 2012;20(6):282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y, Zhu X, Chao J, Zhang Y, Qian C, Li P, Liu D, Han B, Zhao L, Zhang J, Buch S, Teng G, Hu G, Yao H. Pericytes contribute to the disruption of the cerebral endothelial barrier via increasing VEGF expression: implications for stroke. PLoS One. 2015;10(4):e0124362. doi: 10.1371/journal.pone.0124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea E, Urrutia A, Green AR, Colado MI. Current preclinical studies on neuroinflammation and changes in blood–brain barrier integrity by MDMA and methamphetamine. Neuropharmacology. 2014;87:125–134. doi: 10.1016/j.neuropharm.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Correale J, Villa A. The blood–brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40(2):148–160. doi: 10.1080/08916930601183522. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011;89(5):718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 16.Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MC, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval KE, Witt KA. Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kratzer I, Chip S, Vexler ZS. Barrier mechanisms in neonatal stroke. Front Neurosci. 2014;8:359. doi: 10.3389/fnins.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood–brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood–brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32(9):3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood–brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107(Pt 5):1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 23.Dobrogowska DH, Vorbrodt AW. Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood–brain barrier. J Mol Histol. 2004;35(5):529–539. doi: 10.1007/10.1007/s10735-004-1318-3. [DOI] [PubMed] [Google Scholar]
- 24.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24(19):8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Zhang Z, Shi H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci. 2012;69(1):115–128. doi: 10.1007/s00018-011-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122(5):601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. MicroRNA-155 negatively affects blood–brain barrier function during neuroinflammation. FASEB J. 2014;28(6):2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 29.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher NF, Wilson GK, Murray J, Hu K, Lewis A, Reynolds GM, Stamataki Z, Meredith LW, Rowe IA, Luo G, Lopez-Ramirez MA, Baumert TF, Weksler B, Couraud PO, Kim KS, Romero IA, Jopling C, Morgello S, Balfe P, McKeating JA. Hepatitis C virus infects the endothelial cells of the blood–brain barrier. Gastroenterology. 2012;142(3):634–643.e636. doi: 10.1053/j.gastro.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4):493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 33.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 34.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 2001;24(12):719–725. doi: 10.1016/S0166-2236(00)02004-X. [DOI] [PubMed] [Google Scholar]
- 35.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26(9):1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 36.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25(43):9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milner R, Huang X, Wu J, Nishimura S, Pytela R, Sheppard D, ffrench-Constant C Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J Cell Sci. 1999;112(Pt 23):4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HL, Lee YJ, Shin J, Lee E, Park SO, McCarty JH, Oh SP. TGF-beta signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab Invest. 2011;91(11):1554–1563. doi: 10.1038/labinvest.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama YS, Fuxe J, Akhurst R, Betsholtz C, Sheppard D, Reichardt LF. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development. 2014;141(23):4489–4499. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5beta1 and alphavbeta3 integrins via MAP kinase signalling. J Neurochem. 2006;96(1):148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- 41.Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab. 2011;31(10):1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milner R, Campbell IL. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20(4):616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- 43.Randi AM, Hogg N. I domain of beta 2 integrin lymphocyte function-associated antigen-1 contains a binding site for ligand intercellular adhesion molecule-1. J Biol Chem. 1994;269(17):12395–12398. [PubMed] [Google Scholar]
- 44.Hermand P, Huet M, Callebaut I, Gane P, Ihanus E, Gahmberg CG, Cartron JP, Bailly P. Binding sites of leukocyte beta 2 integrins (LFA-1, Mac-1) on the human ICAM-4/LW blood group protein. J Biol Chem. 2000;275(34):26002–26010. doi: 10.1074/jbc.M002823200. [DOI] [PubMed] [Google Scholar]
- 45.Gorina R, Lyck R, Vestweber D, Engelhardt B. beta2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood–brain barrier. J Immunol. 2014;192(1):324–337. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- 46.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 47.Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102(12):2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Laschinger M, Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102(1):32–43. doi: 10.1016/S0165-5728(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 50.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J Neural Transm. 2006;113(4):477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto E, Nakahashi S, Okamoto T, Imai H, Shimaoka M. Anti-integrin therapy for multiple sclerosis. Autoimmune Dis. 2012;2012:357101. doi: 10.1155/2012/357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haarmann A, Nowak E, Deiss A, van der Pol S, Monoranu CM, Kooij G, Muller N, van der Valk P, Stoll G, de Vries HE, Berberich-Siebelt F, Buttmann M. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin alpha-4-transduced outside-in signalling. Acta Neuropathol. 2015;129(5):639–652. doi: 10.1007/s00401-015-1417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W. Protective effect of ginsenoside Rb1 on integrity of blood–brain barrier following cerebral ischemia. Exp Brain Res. 2015 doi: 10.1007/s00221-015-4352-3. [DOI] [PubMed] [Google Scholar]
- 54.Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28(4):858–865. doi: 10.1161/01.STR.28.4.858. [DOI] [PubMed] [Google Scholar]
- 55.Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21(7):835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Haring HP, Akamine BS, Habermann R, Koziol JA, Del Zoppo GJ. Distribution of integrin-like immunoreactivity on primate brain microvasculature. J Neuropathol Exp Neurol. 1996;55(2):236–245. doi: 10.1097/00005072-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 58.von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348(1):68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 59.Schlaepfer DD, Haigler HT. Characterization of Ca2+-dependent phospholipid binding and phosphorylation of lipocortin I. J Biol Chem. 1987;262(14):6931–6937. [PubMed] [Google Scholar]
- 60.Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci USA. 1998;95(24):14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. Human annexin 1 is highly expressed during the differentiation of the epithelial cell line A 549: involvement of nuclear factor interleukin 6 in phorbol ester induction of annexin 1. Cell Growth Differ. 1998;9(4):327–336. [PubMed] [Google Scholar]
- 62.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281(46):35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 63.de Coupade C, Gillet R, Bennoun M, Briand P, Russo-Marie F, Solito E. Annexin 1 expression and phosphorylation are upregulated during liver regeneration and transformation in antithrombin III SV40 T large antigen transgenic mice. Hepatology. 2000;31(2):371–380. doi: 10.1002/hep.510310217. [DOI] [PubMed] [Google Scholar]
- 64.Solito E, de Coupade C, Canaider S, Goulding NJ, Perretti M. Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation. Br J Pharmacol. 2001;133(2):217–228. doi: 10.1038/sj.bjp.0704054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solito E, McArthur S, Christian H, Gavins F, Buckingham JC, Gillies GE. Annexin A1 in the brain—undiscovered roles? Trends Pharmacol Sci. 2008;29(3):135–142. doi: 10.1016/j.tips.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Eberhard DA, Brown MD, VandenBerg SR. Alterations of annexin expression in pathological neuronal and glial reactions. Immunohistochemical localization of annexins I, II (p36 and p11 subunits), IV, and VI in the human hippocampus. Am J Pathol. 1994;145(3):640–649. [PMC free article] [PubMed] [Google Scholar]
- 67.Herbert SP, Odell AF, Ponnambalam S, Walker JH. The confluence-dependent interaction of cytosolic phospholipase A2-alpha with annexin A1 regulates endothelial cell prostaglandin E2 generation. J Biol Chem. 2007;282(47):34468–34478. doi: 10.1074/jbc.M701541200. [DOI] [PubMed] [Google Scholar]
- 68.Smith T, Flower RJ, Buckingham JC. Lipocortins 1, 2 and 5 in the central nervous system and pituitary gland of the rat: selective induction by dexamethasone of lipocortin 1 in the anterior pituitary gland. Mol Neuropharmacol. 1993;3(1):45–55. [Google Scholar]
- 69.Johnson MD, Kamso-Pratt JM, Whetsell WO, Jr, Pepinsky RB. Lipocortin-1 immunoreactivity in the normal human central nervous system and lesions with astrocytosis. Am J Clin Pathol. 1989;92(4):424–429. doi: 10.1093/ajcp/92.4.424. [DOI] [PubMed] [Google Scholar]
- 70.McKanna JA, Zhang MZ. Immunohistochemical localization of lipocortin 1 in rat brain is sensitive to pH, freezing, and dehydration. J Histochem Cytochem. 1997;45(4):527–538. doi: 10.1177/002215549704500405. [DOI] [PubMed] [Google Scholar]
- 71.Dreier R, Schmid KW, Gerke V, Riehemann K. Differential expression of annexins I, II and IV in human tissues: an immunohistochemical study. Histochem Cell Biol. 1998;110(2):137–148. doi: 10.1007/s004180050275. [DOI] [PubMed] [Google Scholar]
- 72.Young KA, Hirst WD, Solito E, Wilkin GP. De novo expression of lipocortin-1 in reactive microglia and astrocytes in kainic acid lesioned rat cerebellum. Glia. 1999;26(4):333–343. doi: 10.1002/(SICI)1098-1136(199906)26:4<333::AID-GLIA7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 73.Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21(8):1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- 74.Dominguez R. Structural insights into de novo actin polymerization. Curr Opin Struct Biol. 2010;20(2):217–225. doi: 10.1016/j.sbi.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J, Christian HC, Weksler BB, Malaspina A, Solito E. Identification of an essential endogenous regulator of blood–brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci USA. 2013;110(3):832–841. doi: 10.1073/pnas.1209362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology. 2010;25(1):16–26. doi: 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 77.Su SC, Maxwell SA, Bayless KJ. Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J Biol Chem. 2010;285(52):40624–40634. doi: 10.1074/jbc.M110.157271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107(12):4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 79.Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, Noguera-Troise I, Murphy AJ, Valenzuela DM, Davis S, Thurston G, Yancopoulos GD, Gale NW. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci USA. 2007;104(9):3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21(18):4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205(12):2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med. 2011;208(12):2393–2401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gloor SM, Weber A, Adachi N, Frei K. Interleukin-1 modulates protein tyrosine phosphatase activity and permeability of brain endothelial cells. Biochem Biophys Res Commun. 1997;239(3):804–809. doi: 10.1006/bbrc.1997.7557. [DOI] [PubMed] [Google Scholar]
- 84.Iovino F, Molema G, Bijlsma JJ. Platelet endothelial cell adhesion molecule-1, a putative receptor for the adhesion of Streptococcus pneumoniae to the vascular endothelium of the blood–brain barrier. Infect Immun. 2014;82(9):3555–3566. doi: 10.1128/IAI.00046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong D, Prameya R, Dorovini-Zis K. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J Neuroimmunol. 2007;184(1–2):136–148. doi: 10.1016/j.jneuroim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 86.DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc Res. 2008;75(2):188–201. doi: 10.1016/j.mvr.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109(3):383–392. doi: 10.1172/JCI0213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winger RC, Koblinski JE, Kanda T, Ransohoff RM, Muller WA. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood–brain barrier. J Immunol. 2014;193(5):2427–2437. doi: 10.4049/jimmunol.1400700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaitanya GV, Cromer W, Wells S, Jennings M, Mathis JM, Minagar A, Alexander JS. Metabolic modulation of cytokine-induced brain endothelial adhesion molecule expression. Microcirculation. 2012;19(2):155–165. doi: 10.1111/j.1549-8719.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- 90.Tilling T, Korte D, Hoheisel D, Galla HJ. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J Neurochem. 1998;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 91.Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood–brain barrier and its role in the transport of amino acids. J Nutr. 2006;136(1 Suppl):218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 92.van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol. 2005;64(8):722–729. doi: 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- 93.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 94.Sorokin L, Girg W, Gopfert T, Hallmann R, Deutzmann R. Expression of novel 400-kDa laminin chains by mouse and bovine endothelial cells. Eur J Biochem. 1994;223(2):603–610. doi: 10.1111/j.1432-1033.1994.tb19031.x. [DOI] [PubMed] [Google Scholar]
- 95.Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153(5):933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 97.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144(1):151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, DeGeorgia M, Krieger DW. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. 2004;35(3):764–769. doi: 10.1161/01.STR.0000116866.60794.21. [DOI] [PubMed] [Google Scholar]
- 100.Cai Y, Liu X, Chen W, Wang Z, Xu G, Zeng Y, Ma Y. TGF-beta1 prevents blood–brain barrier damage and hemorrhagic transformation after thrombolysis in rats. Exp Neurol. 2015;266:120–126. doi: 10.1016/j.expneurol.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 101.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 102.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 103.Hamann GF, Liebetrau M, Martens H, Burggraf D, Kloss CU, Bultemeier G, Wunderlich N, Jager G, Pfefferkorn T. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 2002;22(5):526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Trinkl A, Vosko MR, Wunderlich N, Dichgans M, Hamann GF. Pravastatin reduces microvascular basal lamina damage following focal cerebral ischemia and reperfusion. Eur J Neurosci. 2006;24(2):520–526. doi: 10.1111/j.1460-9568.2006.04920.x. [DOI] [PubMed] [Google Scholar]
- 105.Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007;83(3):140–148. doi: 10.1016/j.pneurobio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Kroger S, Mann S. Biochemical and functional characterization of basal lamina-bound agrin in the chick central nervous system. Eur J Neurosci. 1996;8(3):500–509. doi: 10.1111/j.1460-9568.1996.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 107.Barber AJ, Lieth E (1997) Agrin accumulates in the brain microvascular basal lamina during development of the blood–brain barrier. Dev Dyn 208(1):62–74. doi:10.1002/(SICI)1097-0177(199701)208:1<62:AID-AJA6>3.0.CO;2-# [DOI] [PubMed]
- 108.Rascher G, Fischmann A, Kroger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood–brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104(1):85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 109.Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging. 2000;21(2):349–355. doi: 10.1016/S0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 110.Baumann E, Preston E, Slinn J, Stanimirovic D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood–brain barrier disruption after global cerebral ischemia. Brain Res. 2009;1269:185–197. doi: 10.1016/j.brainres.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 111.Donahue JE, Berzin TM, Rafii MS, Glass DJ, Yancopoulos GD, Fallon JR, Stopa EG. Agrin in Alzheimer’s disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc Natl Acad Sci USA. 1999;96(11):6468–6472. doi: 10.1073/pnas.96.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rauch SM, Huen K, Miller MC, Chaudry H, Lau M, Sanes JR, Johanson CE, Stopa EG, Burgess RW. Changes in brain beta-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exp Neurol. 2011;70(12):1124–1137. doi: 10.1097/NEN.0b013e31823b0b12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steiner E, Enzmann GU, Lyck R, Lin S, Ruegg MA, Kroger S, Engelhardt B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014;358(2):465–479. doi: 10.1007/s00441-014-1969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood–brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49(2):143–155. [PubMed] [Google Scholar]
- 115.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15(1):43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 116.Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood–brain barrier. Neuroimmunomodulation. 2012;19(2):121–130. doi: 10.1159/000330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood–brain barrier permeability. Eur J Neurosci. 2005;22(5):1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- 118.Minagar A, Alexander JS. Blood–brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9(6):540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 119.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood–brain barrier in vitro. J Neuroimmunol. 1996;64(1):37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 120.Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58(3):245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 121.Wei EP, Ellison MD, Kontos HA, Povlishock JT. O2 radicals in arachidonate-induced increased blood–brain barrier permeability to proteins. Am J Physiol. 1986;251(4 Pt 2):H693–699. doi: 10.1152/ajpheart.1986.251.4.H693. [DOI] [PubMed] [Google Scholar]
- 122.Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, Pfizenmaier K, Male DK, Sharrack B, Romero IA. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol. 2012;189(6):3130–3139. doi: 10.4049/jimmunol.1103460. [DOI] [PubMed] [Google Scholar]
- 123.Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 124.Quagliarello VJ, Wispelwey B, Long WJ, Jr, Scheld WM. Recombinant human interleukin-1 induces meningitis and blood–brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991;87(4):1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1beta induces blood–brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9(10):e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rochfort KD, Collins LE, Murphy RP, Cummins PM. Downregulation of blood–brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One. 2014;9(7):e101815. doi: 10.1371/journal.pone.0101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 1999;64(21):1941–1953. doi: 10.1016/S0024-3205(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 128.Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y. Tumor necrosis factor-alpha mediates the blood–brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci. 2010;112(2):251–254. doi: 10.1254/jphs.09292SC. [DOI] [PubMed] [Google Scholar]
- 129.Gu X, Wei ZZ, Espinera A, Lee JH, Ji X, Wei L, Dix TA, Yu SP. Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats. Exp Neurol. 2015;267:135–142. doi: 10.1016/j.expneurol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood–brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflamm. 2015;12(1):26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett. 2011;585(23):3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 132.Rosenberg GA, Prestopnik J, Adair JC, Huisa BN, Knoefel J, Caprihan A, Gasparovic C, Thompson J, Erhardt EB, Schrader R. Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: approach to targeted treatment trials. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2014-309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jaworowicz DJ, Jr, Korytko PJ, Singh Lakhman S, Boje KM. Nitric oxide and prostaglandin E2 formation parallels blood–brain barrier disruption in an experimental rat model of bacterial meningitis. Brain Res Bull. 1998;46(6):541–546. doi: 10.1016/S0361-9230(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 135.Dalvi S, Nguyen HH, On N, Mitchell RW, Aukema HM, Miller DW, Hatch GM. Exogenous arachidonic acid mediates permeability of human brain microvessel endothelial cells through prostaglandin E activation of EP and EP receptors. J Neurochem. 2015 doi: 10.1111/jnc.13117. [DOI] [PubMed] [Google Scholar]
- 136.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase inhibition limits blood–brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007;323(2):488–498. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- 137.Black KL, Hoff JT. Leukotrienes increase blood–brain barrier permeability following intraparenchymal injections in rats. Ann Neurol. 1985;18(3):349–351. doi: 10.1002/ana.410180313. [DOI] [PubMed] [Google Scholar]
- 138.Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell Mol Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Easton AS, Fraser PA. Arachidonic acid increases cerebral microvascular permeability by free radicals in single pial microvessels of the anaesthetized rat. J Physiol. 1998;507(Pt 2):541–547. doi: 10.1111/j.1469-7793.1998.541bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee H, Pienaar IS. Disruption of the blood–brain barrier in Parkinson’s disease: curse or route to a cure? Front Biosci. 2014;19:272–280. doi: 10.2741/4206. [DOI] [PubMed] [Google Scholar]
- 141.Chung YC, Kim YS, Bok E, Yune TY, Maeng S, Jin BK. MMP-3 contributes to nigrostriatal dopaminergic neuronal loss, BBB damage, and neuroinflammation in an MPTP mouse model of Parkinson’s disease. Mediators Inflamm. 2013;2013:370526. doi: 10.1155/2013/370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duran-Vilaregut J, del Valle J, Manich G, Camins A, Pallas M, Vilaplana J, Pelegri C. Role of matrix metalloproteinase-9 (MMP-9) in striatal blood–brain barrier disruption in a 3-nitropropionic acid model of Huntington’s disease. Neuropathol Appl Neurobiol. 2011;37(5):525–537. doi: 10.1111/j.1365-2990.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 143.Goncalves FM, Martins-Oliveira A, Lacchini R, Belo VA, Speciali JG, Dach F, Tanus-Santos JE. Matrix metalloproteinase (MMP)-2 gene polymorphisms affect circulating MMP-2 levels in patients with migraine with aura. Gene. 2013;512(1):35–40. doi: 10.1016/j.gene.2012.09.109. [DOI] [PubMed] [Google Scholar]
- 144.Martins-Oliveira A, Speciali JG, Dach F, Marcaccini AM, Goncalves FM, Gerlach RF, Tanus-Santos JE. Different circulating metalloproteinases profiles in women with migraine with and without aura. Clin Chim Acta. 2009;408(1–2):60–64. doi: 10.1016/j.cca.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 145.Gupta VK. CSD, BBB and MMP-9 elevations: animal experiments versus clinical phenomena in migraine. Expert Rev Neurother. 2009;9(11):1595–1614. doi: 10.1586/ern.09.103. [DOI] [PubMed] [Google Scholar]
- 146.Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S. Abeta oligomer-induced leakage in an in vitro blood–brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem. 2015;134(2):382–393. doi: 10.1111/jnc.13122. [DOI] [PubMed] [Google Scholar]
- 147.Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele EJ, Strayer DS. HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur J Neurosci. 2011;34(12):2015–2023. doi: 10.1111/j.1460-9568.2011.07908.x. [DOI] [PubMed] [Google Scholar]
- 148.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood–brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19(9):1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 149.Guilfoyle MR, Carpenter KL, Helmy A, Pickard JD, Menon DK, Hutchinson PJ. Matrix metalloproteinase expression in contusional traumatic brain injury: a paired microdialysis study. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, Chan MD, Zhou X, Qasem SA, Pochampally R, Mo YY, Watabe K. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–9854. doi: 10.1074/jbc.M114.602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu L, Cao F, Xu F, He B, Dong Z. Bexarotene reduces blood–brain barrier permeability in cerebral ischemia-reperfusion injured rats. PLoS One. 2015;10(4):e0122744. doi: 10.1371/journal.pone.0122744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, Harada E, Miyaji H, Koga M, Nishioku T, Yamauchi A, Kataoka Y. Brain pericytes among cells constituting the blood–brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflamm. 2011;106(8):106. doi: 10.1186/1742-2094-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Machida T, Takata F, Matsumoto J, Takenoshita H, Kimura I, Yamauchi A, Dohgu S, Kataoka Y. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood–brain barrier in vitro. Neurosci Lett. 2015 doi: 10.1016/j.neulet.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 154.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bezerra FJ, Brown M, Alarcon W, Karki K, Knight RA, Keenan KA, Nagaraja TN. Rate and extent of leakage of a magnetic resonance contrast agent tend to be lower under isoflurane anesthesia in comparison to halothane in a rat model of embolic stroke. Neurol Res. 2014;36(9):847–850. doi: 10.1179/1743132814Y.0000000341. [DOI] [PubMed] [Google Scholar]
- 156.Takeuchi S, Nagatani K, Otani N, Nawashiro H, Sugawara T, Wada K, Mori K. Hydrogen improves neurological function through attenuation of blood–brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015;16(1):22. doi: 10.1186/s12868-015-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Geng Y, Li E, Mu Q, Zhang Y, Wei X, Li H, Cheng L, Zhang B. Hydrogen sulfide inhalation decreases early blood–brain barrier permeability and brain edema induced by cardiac arrest and resuscitation. J Cereb Blood Flow Metab. 2015;35(3):494–500. doi: 10.1038/jcbfm.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Allahtavakoli M, Amin F, Esmaeeli-Nadimi A, Shamsizadeh A, Kazemi-Arababadi M, Kennedy D. Ascorbic acid reduces the adverse effects of delayed administration of tissue plasminogen activator in a rat stroke model. Basic Clin Pharmacol Toxicol. 2015 doi: 10.1111/bcpt.12413. [DOI] [PubMed] [Google Scholar]
- 159.Wang MD, Wang Y, Xia YP, Dai JW, Gao L, Wang SQ, Wang HJ, Mao L, Li M, Yu SM, Tu Y, He QW, Zhang GP, Wang L, Xu GZ, Xu HB, Zhu LQ, Hu B. High serum MiR-130a levels are associated with severe perihematomal edema and predict adverse outcome in acute ICH. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9099-0. [DOI] [PubMed] [Google Scholar]
- 160.Wu J, Zhao D, Wu S, Wang D. Ang-(1–7) exerts protective role in blood–brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol. 2015;748:30–36. doi: 10.1016/j.ejphar.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 161.Wang T, Chen X, Wang Z, Zhang M, Meng H, Gao Y, Luo B, Tao L, Chen Y. Poloxamer-188 can attenuate blood–brain barrier damage to exert neuroprotective effect in mice intracerebral hemorrhage model. J Mol Neurosci. 2015;55(1):240–250. doi: 10.1007/s12031-014-0313-8. [DOI] [PubMed] [Google Scholar]
- 162.Reuter B, Rodemer C, Grudzenski S, Meairs S, Bugert P, Hennerici MG, Fatar M. Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl Stroke Res. 2015;6(2):156–159. doi: 10.1007/s12975-014-0381-7. [DOI] [PubMed] [Google Scholar]
- 163.Wei H, Wang S, Zhen L, Yang Q, Wu Z, Lei X, Lv J, Xiong L, Xue R. Resveratrol attenuates the blood–brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia reperfusion in rats. J Mol Neurosci. 2015;55(4):872–879. doi: 10.1007/s12031-014-0441-1. [DOI] [PubMed] [Google Scholar]
- 164.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Floyd RA. Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic Biol Med. 1999;26(9–10):1346–1355. doi: 10.1016/S0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- 166.Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39(1):71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 167.Rosenberg GA. Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao Z, Hu J, Gao X, Liang H, Liu Z. Activation of AMPK attenuates lipopolysaccharide-impaired integrity and function of blood–brain barrier in human brain microvascular endothelial cells. Exp Mol Pathol. 2014;97(3):386–392. doi: 10.1016/j.yexmp.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 169.Minami T, Okazaki J, Kawabata A, Kawaki H, Okazaki Y, Tohno Y. Roles of nitric oxide and prostaglandins in the increased permeability of the blood–brain barrier caused by lipopolysaccharide. Environ Toxicol Pharmacol. 1998;5(1):35–41. doi: 10.1016/S1382-6689(97)10004-7. [DOI] [PubMed] [Google Scholar]
- 170.Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21(13):3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 171.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J Neurochem. 2007;101(2):566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 172.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15(5):1285–1303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 173.Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, Vosler PS, Chen J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28(10):2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gelati M, Corsini E, Dufour A, Massa G, Giombini S, Solero CL, Salmaggi A. High-dose methylprednisolone reduces cytokine-induced adhesion molecules on human brain endothelium. Can J Neurol Sci. 2000;27(3):241–244. doi: 10.1017/S0317167100000883. [DOI] [PubMed] [Google Scholar]
- 175.Schweingruber N, Reichardt SD, Luhder F, Reichardt HM. Mechanisms of glucocorticoids in the control of neuroinflammation. J Neuroendocrinol. 2012;24(1):174–182. doi: 10.1111/j.1365-2826.2011.02161.x. [DOI] [PubMed] [Google Scholar]
- 176.Dietrich JB. Endothelial cells of the blood–brain barrier: a target for glucocorticoids and estrogens? Front Biosci. 2004;9:684–693. doi: 10.2741/1272. [DOI] [PubMed] [Google Scholar]
- 177.Ding R, Feng L, He L, Chen Y, Wen P, Fu Z, Lin C, Yang S, Deng X, Zeng J, Sun G. Peroxynitrite decomposition catalyst prevents matrix metalloproteinase-9 activation and neurovascular injury after hemoglobin injection into the caudate nucleus of rats. Neuroscience. 2015;297:182–193. doi: 10.1016/j.neuroscience.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 178.Rosenstein JM, Mani N, Silverman WF, Krum JM. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95(12):7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125(Pt 11):2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 180.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood–brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171(5):1210–1230. doi: 10.1111/bph.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li YN, Pan R, Qin XJ, Yang WL, Qi Z, Liu W, Liu KJ. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J Neurochem. 2014;129(1):120–129. doi: 10.1111/jnc.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao LN, Yang ZH, Liu YH, Ying HQ, Zhang H, Xue YX. Vascular endothelial growth factor increases permeability of the blood–tumor barrier via caveolae-mediated transcellular pathway. J Mol Neurosci. 2011;44(2):122–129. doi: 10.1007/s12031-010-9487-x. [DOI] [PubMed] [Google Scholar]
- 185.Wang W, Merrill MJ, Borchardt RT. Vascular endothelial growth factor affects permeability of brain microvessel endothelial cells in vitro. Am J Physiol. 1996;271(6 Pt 1):C1973–1980. doi: 10.1152/ajpcell.1996.271.6.C1973. [DOI] [PubMed] [Google Scholar]
- 186.Zhao L, Zhang MM, Ng KY. Effects of vascular permeability factor on the permeability of cultured endothelial cells from brain capillaries. J Cardiovasc Pharmacol. 1998;32(1):1–4. doi: 10.1097/00005344-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 187.Mayhan WG. VEGF increases permeability of the blood–brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am J Physiol. 1999;276(5 Pt 1):C1148–1153. doi: 10.1152/ajpcell.1999.276.5.C1148. [DOI] [PubMed] [Google Scholar]
- 188.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 189.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 190.Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis. 2008;31(3):433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood–brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol. 2007;72(2):440–449. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- 192.Chen C, Ostrowski RP, Zhou C, Tang J, Zhang JH. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 2010;88(9):2046–2055. doi: 10.1002/jnr.22361. [DOI] [PubMed] [Google Scholar]
- 193.Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TC, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci. 2013;33(16):6857–6863. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, Tyagi SC, Tyagi N. Role of microRNA29b in blood–brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. J Cereb Blood Flow Metab. 2014;34(7):1212–1222. doi: 10.1038/jcbfm.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Deng X, Zhong Y, Gu L, Shen W, Guo J. MiR-21 involve in ERK-mediated upregulation of MMP9 in the rat hippocampus following cerebral ischemia. Brain Res Bull. 2013;94:56–62. doi: 10.1016/j.brainresbull.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 197.Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30(18):6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Eadon MT, Jacob A, Cunningham PN, Quigg RJ, Garcia JG, Alexander JJ. Transcriptional profiling reveals that C5a alters microRNA in brain endothelial cells. Immunology. 2014;143(3):363–373. doi: 10.1111/imm.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rom S, Dykstra H, Zuluaga-Ramirez V, Reichenbach NL, Persidsky Y. miR-98 and let-7g* protect the blood–brain barrier under neuroinflammatory conditions. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 201.Chi OZ, Anwar M, Sinha AK, Wei HM, Klein SL, Weiss HR. Effects of isoflurane on transport across the blood–brain barrier. Anesthesiology. 1992;76(3):426–431. doi: 10.1097/00000542-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 202.Tetrault S, Chever O, Sik A, Amzica F. Opening of the blood–brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28(7):1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- 203.Cao Y, Ni C, Li Z, Li L, Liu Y, Wang C, Zhong Y, Cui D, Guo X. Isoflurane anesthesia results in reversible ultrastructure and occludin tight junction protein expression changes in hippocampal blood–brain barrier in aged rats. Neurosci Lett. 2015;587:51–56. doi: 10.1016/j.neulet.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 204.Zhao J, Hao J, Fei X, Wang X, Hou Y, Deng C. Isoflurane inhibits occludin expression via up-regulation of hypoxia-inducible factor 1alpha. Brain Res. 2014;1562:1–10. doi: 10.1016/j.brainres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 205.Dittmar MS, Petermichl W, Schlachetzki F, Graf BM, Gruber M. Isoflurane induces endothelial apoptosis of the post-hypoxic blood–brain barrier in a transdifferentiated human umbilical vein endothelial cell model. PLoS One. 2012;7(6):e38260. doi: 10.1371/journal.pone.0038260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thal SC, Luh C, Schaible EV, Timaru-Kast R, Hedrich J, Luhmann HJ, Engelhard K, Zehendner CM. Volatile anesthetics influence blood–brain barrier integrity by modulation of tight junction protein expression in traumatic brain injury. PLoS One. 2012;7(12):e50752. doi: 10.1371/journal.pone.0050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Acharya NK, Goldwaser EL, Forsberg MM, Godsey GA, Johnson CA, Sarkar A, DeMarshall C, Kosciuk MC, Dash JM, Hale CP, Leonard DM, Appelt DM, Nagele RG. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood–brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015 doi: 10.1016/j.brainres.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 208.Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of fentanyl, methohexital, and thiopental on brain endothelial permeability. Anesthesiology. 1995;82(2):451–458. doi: 10.1097/00000542-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 209.Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83(3):149–173. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 210.Bradbury MW. Transport of iron in the blood–brain-cerebrospinal fluid system. J Neurochem. 1997;69(2):443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- 211.Qian ZM, Tang PL, Wang Q. Iron crosses the endosomal membrane by a carrier-mediated process. Prog Biophys Mol Biol. 1997;67(1):1–15. doi: 10.1016/S0079-6107(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 212.Moos T, Rosengren Nielsen T, Skjorringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. 2007;103(5):1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- 213.De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26(12):2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McCarthy RC, Kosman DJ. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One. 2014;9(2):e89003. doi: 10.1371/journal.pone.0089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI0215686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR. The impact of microglial activation on blood–brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Baby N, Patnala R, Ling EA, Dheen ST. Nanomedicine and its application in treatment of microglia-mediated neuroinflammation. Curr Med Chem. 2014;21(37):4215–4226. doi: 10.2174/0929867321666140716101258. [DOI] [PubMed] [Google Scholar]
- 218.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 219.Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz M, Oztas E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic Biol Med. 2004;37(3):386–394. doi: 10.1016/j.freeradbiomed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 220.Zhao J, Pati S, Redell JB, Zhang M, Moore AN, Dash PK. Caffeic Acid phenethyl ester protects blood–brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J Neurotrauma. 2012;29(6):1209–1218. doi: 10.1089/neu.2011.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ (2006) Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55(4):1106–1113. pii: 55/4/1106 [DOI] [PubMed]
- 222.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74(1):70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS (2005) Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 54(10):2977–2982. pii: 54/10/2977 [DOI] [PubMed]
- 224.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):E14–22. doi: 10.1161/01.RES.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 225.Kook SY, Seok Hong H, Moon M, Mook-Jung I. Disruption of blood–brain barrier in Alzheimer disease pathogenesis. TissueBarriers. 2013;1(2):e23993. doi: 10.4161/tisb.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wan W, Chen H, Li Y. The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood–brain barrier in Alzheimer’s disease. Int J Neurosci. 2014;124(2):75–81. doi: 10.3109/00207454.2013.825258. [DOI] [PubMed] [Google Scholar]
- 227.Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE. Amyloid Beta induces oxidative stress-mediated blood–brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15(5):1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 228.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69(14):2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356(25):2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 230.Wang WX, Danaher RJ, Miller CS, Berger JR, Nubia VG, Wilfred BS, Neltner JH, Norris CM, Nelson PT. Expression of miR-15/107 family microRNAs in human tissues and cultured rat brain cells. Genomics Proteomics Bioinform. 2014;12(1):19–30. doi: 10.1016/j.gpb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J. MiR-21 alleviates secondary blood–brain barrier damage after traumatic brain injury in rats. Brain Res. 2015;1603:150–157. doi: 10.1016/j.brainres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 232.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21(6):1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 233.Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132(Pt 12):3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]