Abstract
In the central nervous system, most excitatory post-synapses are small subcellular structures called dendritic spines. Their structure and morphological remodeling are tightly coupled to changes in synaptic transmission. The F-actin cytoskeleton is the main driving force of dendritic spine remodeling and sustains synaptic plasticity. It is therefore essential to understand how changes in synaptic transmission can regulate the organization and dynamics of actin binding proteins (ABPs). In this review, we will provide a detailed description of the organization and dynamics of F-actin and ABPs in dendritic spines and will discuss the current models explaining how the actin cytoskeleton sustains both structural and functional synaptic plasticity.
Keywords: Dendritic spines, Actin cytoskeleton, Actin binding proteins, PSD, CAMKII, Synaptic plasticity, Super-resolution microscopy
Introduction
In 1888, Ramón y Cajal described for the first time that the surface of a neuron “appears bristling with thorns or short spines” [1, 2]. In subsequent work, he speculated that dendritic spines received axonal contacts and that their morphological changes were associated with neuronal function and learning processes [3–5]. Cajal’s hypothesis that spines connect axons and dendrites was confirmed in 1959 by the first visualization of pre- and post-synaptic contacts by electron microscopy (EM) [6–8]. Although it was widely believed that long-lasting changes in synaptic function are the cellular basis of learning and memory, [9–11] it was only recently that direct links between synaptic activity, dendritic spine morphology and the formation of a memory trace in vivo were demonstrated [12, 13].
Dendritic spines are generally composed of a spine head (200 nm to 1 µm in diameter) that is connected to dendrites by a thin spine neck (100–200 nm thick) [14–17]. Spine heads contain the post-synaptic density (PSD), a macro-molecular structure essential for synaptic transmission which mediates adhesion to the pre-synapse and anchoring of post-synaptic glutamate receptors. Despite their architectural role, dendritic spines are highly dynamic structures that undergo distinct morphological changes on timescales of seconds to minutes, hours and years, both in vitro and in vivo [18–22]. The morphological remodeling of spines is correlated with changes in synaptic transmission. Synaptic plasticity-induced stimuli such as long-term potentiation (LTP) or long-term depression (LTD) cause not only the formation or removal of new spines [23–27] but also the enlargement or shrinkage of pre-existing spines [27–31]. However, the in vivo longitudinal measurement of a single spine experiencing morphological remodeling during LTP and LTD has not yet been observed [32–34]. In this review, this type of spine morphological remodeling will be referred to as structural plasticity, while functional plasticity is defined as increased or decreased synaptic transmission.
While the function of dendritic spines had been the major scope of pioneer studies, current studies are thoroughly focusing on their molecular composition. Biochemical and proteomic studies have shown that dendritic spines are composed of a plethora of proteins covering a wide range of functions including membrane receptors and channels, scaffolding proteins, adhesion proteins, molecular motors, GTPases, kinases/phosphatases and cytoskeleton proteins [35]. Actin and actin binding proteins (ABPs) are particularly enriched in PSD and accumulate in dendritic spine heads [35–39], with F-actin dynamics being the driving force of spine morphological remodeling [19, 40, 41]. Indeed, the actin cytoskeleton sustains the formation of dendritic spines during neuron development and their enlargement and shrinkage upon increase and decrease synaptic activity, respectively [28, 29, 42–44].
Filamentous actin (F-actin) consists of two-stranded helical polymers arising from the polymerization of globular actin (G-actin) (Fig. 1a) [45, 46]. Actin assembles in a head-to-tail manner giving rise to the structural polarity of F-actin. Therefore, polymerization and depolymerization occur preferentially at the barbed end and the pointed end, respectively. This creates a net flow of actin monomers within the filament called actin treadmilling. Fluorescence microscopy revealed that the nature of ABPs but also the spatiotemporal coordination of their activity determine the organization and dynamics of F-actin in motile structures such as the lamellipodium and filopodium (Fig. 1a) [45, 47]. In the past decade, seminal studies have established the central role of F-actin dynamics and ABPs in spine morphogenesis and in synaptic plasticity-induced morphological remodeling [43, 44, 48, 49]. The spatial resolution of conventional fluorescence microscopy is, however, limited by the diffraction of light (around 250 nm in the lateral direction), whereas many sub-synaptic structures are typically below this diffraction limit (synaptic cleft: 20 nm; PSD thickness: 25–50 nm; spine neck width: 100–200 nm). To date, EM provided most of our knowledge about synapse ultrastructure, including the synaptic cleft size, the PSD and F-actin organization in dendritic spines [15, 39, 50, 51]. In 2014, Eric Betzig, William Moerner and Stefan Hell were awarded the Nobel Prize in Chemistry for the recent development of single-molecule and super-resolution light microscopy. These techniques can circumvent the diffraction limit of light and have provided new insights into protein organization and dynamics in different cellular systems including neurons [52, 53]. We are therefore just starting to reach a deep understanding of the sequence of molecular events leading to dendritic spines functional and structural plasticity, such as neurotransmitter receptor dynamics and organization, PSD remodeling, and spine neck morphology [54–56]. In this review, we will focus on the organization and dynamics of the F-actin cytoskeleton in dendritic spines, the signaling cascades driving F-actin remodeling upon changes in synaptic activity, and the interplay between F-actin remodeling and spine structural and functional plasticity. We will also highlight how super-resolution microscopy and single-molecule tracking approaches provided new understanding on the architecture and the dynamics of actin and actin regulatory proteins in dendritic spines.
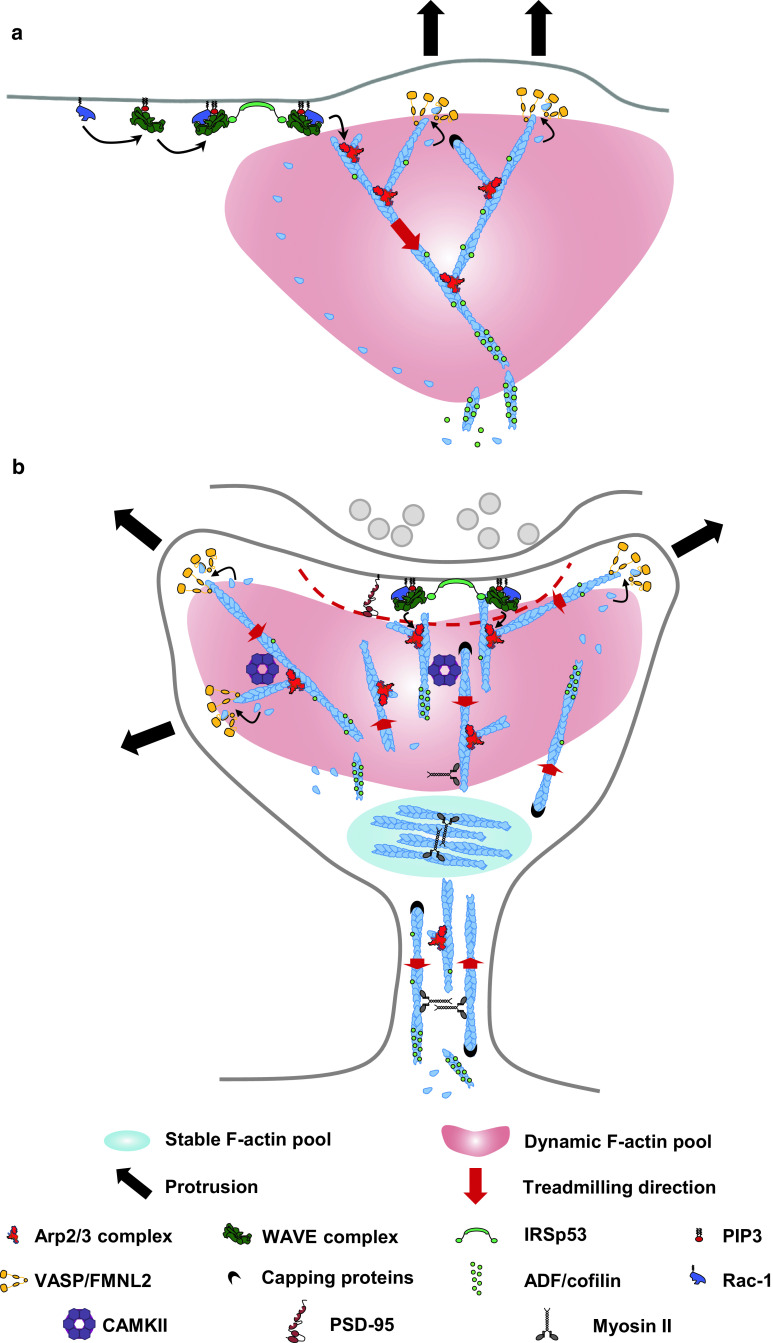
Fig. 1.
Comparison of F-actin and ABPs organization in the lamellipodium and in dendritic spines. a In lamellipodia of motile cells, nucleation and elongation of F-actin are co-localized at the tip of membrane protrusion triggering a fast and concerted rearward flow of the branched F-actin network. Activation of the Arp2/3 complex is mediated by the synchronized convergence of prenylated Rac-GTP, PIP3, IRSp53 and the WAVE complex, while elongation of F-actin filaments are powered by VASP and FMNL2, all these proteins being localized at the lamellipodium tip. Capping proteins are also enriched at the lamellipodium tip, while cortactin associates with the entire F-actin network. ADF/cofilin associates with the entire F-actin network and induces F-actin severing. b Dendritic spine heads are characterized by slow and non-polarized motions of the branched F-actin pool. This distinct F-actin dynamics in spines could be sustained by spatial segregation between stationary nucleation zones and delocalizing elongation zones. Indeed, since the PSD is a persistent confinement zone for the WAVE complex and IRSp53, branched F-actin nucleation occurs with a higher probability at the PSD vicinity, while elongation of F-actin filaments by VASP and FMNL2 occur at the tip of finger-like protrusions. Dendritic spines are also composed of a stable F-actin pool localizing at the base of the spine head and stabilized by the crosslinking activity of myosin II. Dendritic spine necks are composed of both branched and non-branched long F-actin filaments that exhibit slow and non-polarized motions
Actin dynamics in dendritic spines
Actin dynamics in dendritic filopodia
During neuronal development, highly motile structures called dendritic filopodia will emerge from dendrites [18]. Enlargement of dendritic filopodia occurs soon after their contact with axons leading to the formation of a dendritic spine [39, 57–60]. As opposed to the tight linear F-actin bundles present in filopodia from motile cells, dendritic filopodia are composed of unbundled F-actin of mixed orientations [39, 61]. However, dendritic filopodia display a fast retrograde actin flow powered by continuous polymerization of actin against the tip membrane protrusion, as observed for the lamellipodium and filopodium of motile cells and neuronal growth cones [62–67]. The molecular motor myosin II, accumulates at the base of dendritic filopodia and contributes to the retrograde F-actin flow [39, 66]. The initial contact between dendritic filopodia and the axon is driven by N-cadherin adhesion proteins and drastically reduces the retrograde F-actin flow [60]. This interplay between cell adhesion and actin dynamics is characteristic of a model used to explain cell migration called the ‘molecular clutch’ [68–72]. The molecular clutch consists of an assembly of ABPs that transmit forces generated by the F-actin flow to adhesive structures allowing cells to exert grip on the surrounding substrate or cells and to generate membrane protrusions [70]. According to this model, dendritic filopodia that are not contacting axons are in the slipping mode and display fast rearward F-actin motion driven by F-actin polymerization and myosin II activity. Upon filopodia/axon contact, clutch engagement between N-cadherin and F-actin, possibly mediated by α-catenin, will immobilize actin filaments counteracting myosin II activity and leading to a reduction of the F-actin flow, which causes F-actin growth to push against the plasma membrane. The resulting F-actin accumulation, decreased motility and dendritic filopodia enlargement might therefore be required for the initiation of dendritic spines [60].
Actin dynamics in mature dendritic spines
Motility of dendritic spines is characterized by the formation and retraction of membrane protrusions [22, 73, 74]. However, the precise role of those spine protrusions is still unknown but might be needed to continuously adjust the position of the post-synaptic structure to the pre-synaptic one. Dendritic spines are F-actin-rich protrusions with a dynamic equilibrium between G-actin and F-actin [75]. As for dendritic filopodia, F-actin dynamics was demonstrated to be the driving force of dendritic spine motility. Indeed, pharmacological treatments preventing F-actin polymerization (cytochalasin-D) or promoting F-actin depolymerization (latrunculin-A) stop spine dynamics [19, 41]. Fluorescence recovery after photobleaching (FRAP) and photoactivation experiments provided more insights into actin dynamics in dendritic spines [76, 77]. These studies revealed that F-actin was composed of a large dynamic pool (80–95 %; 40 s turnover time constant) and a small stable pool (5–20 %; 17 min turnover time constant) (Fig. 1b); the size of the stable pool being positively correlated with spine volume. While the stable pool remains stationary at the base of the spine heads, the dynamic pool exhibits a slow retrograde flow (5–20 nm/s) from the tip to the base of the spine head [77, 78]. The recent development of single-particle tracking photoactivation localization microscopy (sptPALM) [79, 80] has enabled F-actin tracking in dendritic spines [67, 78, 81]. Those studies did not reveal a concerted flow of F-actin from the tip to the base of the spine head but rather an overall non-polarized motion. A large fraction of actin molecules had no detectable motion, while the remaining ones displayed retrograde and anterograde motions with heterogeneous speeds (8–40 nm/s). These dynamic properties are different from those found in other protrusive structures such as the lamellipodium of motile cells, axonal growth cones or dendritic filopodia where F-actin movements are faster, highly polarized rearward and driven by F-actin growth against the tip of membrane protrusions [66, 67, 82, 83]. Therefore, slow actin movements found in dendritic spines might result from a complex pattern of force generation, involving polymerization of new filaments pushing older filaments, F-actin severing and recycling, but also protrusion ruffling [22, 77, 81, 84–86]. Interestingly, F-actin motions in dendritic spine necks are also slow and non-polarized [39, 66]. This complex F-actin dynamics could also arise from the distinct spatial distribution and dynamics characterizing F-actin pools and from a specific spatiotemporal organization of ABPs in spines.
Actin organization in dendritic spines
Formation and organization of the branched F-actin network in dendritic spines
Essential F-actin regulators are located in spines, and have been found to critically control spinogenesis, spine morphology and synaptic plasticity [44]. Despite having different F-actin dynamics as compared to the lamellipodium, dendritic spine heads are also composed of a dense branched F-actin network, while the spine neck is composed of both branched and non-branched long F-actin filaments [39, 61, 87, 88]. The actin-related protein 2/3 (Arp2/3) complex is the only known actin nucleator forming side branches on a “mother” actin filament [89–92]. Knockout of a single Arp2/3 subunit decreases F-actin turnover and spine head size, induces a progressive spine loss, alters spine structural plasticity and leads to behavioral abnormalities [93]. However, the Arp2/3 complex possesses low intrinsic activity and requires nucleation promoting factors (NPFs) for an efficient activation [92]. NPFs such as the WAVE complex and N-WASP can activate Arp2/3 to induce branched actin nucleation; while cortactin stabilizes Arp2/3 during and after nucleation [92, 94, 95]. The Arp2/3 complex can also be inhibited by PICK1 [96]. Importantly, these proteins are all present in dendritic spines, interact directly or indirectly with PSD components and are involved in the regulation of spine density, morphology and plasticity (WAVE [97–101]; cortactin [102, 103]; N-WASP [104, 105]; PICK1 [96, 106]).
Filament length is also an important factor determining F-actin network dynamics [107]. Profilin binds with a one-to-one ratio to G-actin favoring F-actin polymerization at the barbed end [108, 109]. Profilin-II is enriched in spines upon increased synaptic activity and preventing its binding to G-actin destabilizes dendritic spines [110]. Vasodilator-stimulated phosphoprotein (VASP) and formins promote barbed end F-actin polymerization while capping proteins (CP or CapZ) and EGFR substrate pathway #8 (Eps8) cap the barbed end to prevent F-actin polymerization [111–115]. Interestingly, knockout of Eps8 and knockdown of CP lead to the formation of thin long spines [116, 117], whereas decreasing the activity of the formin mouse diaphanous 2 (mDia2) increases the proportion of large spines [61]. Actin filaments decoration by sub-stoichiometric densities of actin depolymerization factor/cofilin (ADF/cofilin), in coordination with coronin and actin interacting protein 1 (AIP1) recruitment, will induce severing and stochastic disassembly of F-actin [118–121]. Numerous studies have shown that ADF/cofilin and its upstream regulators are tightly regulating dendritic spine shape [29, 61, 122, 123].
Electron microscopy provided the first insights into F-actin organization in dendritic spines [36, 39, 51]. By performing single-molecule localization microscopy (SMLM) and single-particle tracking, our recent study has now revealed the specific nanoscale organization of ABPs in dendritic spines [67] (Fig. 1b). Incorporation of Arp2/3 complexes into the F-actin network occurred in close vicinity to the PSD, and the WAVE complex was found to form a single stable domain overlapping with PSD-95 [67]. The insulin receptor substrate p53 (IRSp53), an I-BAR protein binding and regulating the WAVE complex, was also shown to form a stable domain overlapping with PSD-95 [67, 124, 125]. PSD-95, IRSp53 and the WAVE complex share similar diffusion properties suggesting that branched F-actin nucleation occurs at the membrane apposed to the PSD [67]. Consistently, IRSp53 and subunits of the WAVE and Arp2/3 complexes can directly and/or indirectly bind to components of the PSD, including PSD-95, Shank1, Shank3 and CaMKII [101, 126–129]. In addition, the post-synaptic cell adhesion protein neuroligin-1 bears an interacting sequence binding directly to the WAVE complex [130]. Thus, sequestration of the WAVE complex and IRSp53 at the PSD might provide an efficient mechanism for spatiotemporal control of the branched F-actin network during changes in synaptic transmission. SMLM has also shown that VASP and the formin-like proteins2 (FMNL2), but not the WAVE complex, accumulate at the tip of finger-like protrusions growing away from the PSD [67]. Thus, in contrast to the lamellipodium, zones of branched F-actin nucleation and elongation do not colocalize at protrusion tips [47, 82, 83, 131] (Fig. 1). This demonstrates that it is the respective nanoscale organization of F-actin regulators and not their nature that determines the shape and dynamics of protrusive structures. Those finger-like protrusions might arise from the branched F-actin network but are unlikely to be formed by elongation of tight F-actin bundles, as demonstrated for filopodia emerging from the lamellipodium [39, 132]. The densely packed PSD could represent a physical barrier forcing F-actin barbed ends to grow away and generate finger-like protrusions in dendritic spines. Consistent with this hypothesis, in an in vitro reconstituted system, branched F-actin networks can elongate their barbed ends away for a nucleating surface [84]. Because of the inverse relationship between the rate of F-actin flow and membrane protrusion, the nanoscale segregation between stationary nucleation zones and the delocalizing elongation zones could also explain the slow and non-polarized motion of branched F-actin networks in dendritic spines [67, 133] (Fig. 1b). Despite these recent findings, the current models cannot yet assign a specific F-actin nanoscale organization to the previously described stable and dynamic F-actin pools.
Heterogeneity of F-actin crosslinker organization in dendritic spines
F-actin organization is also controlled by crosslinking proteins. Dendritic spines are for instance enriched in F-actin crosslinkers including α-actinin, neurabins and the neuron-specific F-actin parallel bundler drebrin (α-actinin [134–136]; neurabins [137–141]; drebrin [142–145]). Fascin, however, which stabilizes parallel F-actin bundles in conventional filopodia, appears absent from dendritic filopodia and spines [39, 146, 147]. While α-actinin knockdown delays spine maturation [136] and neurabin knockdown decreases surface glutamate receptor surface expression [148], drebrin knockdown was suggested to promote spine maturation [149]. This discrepancy might arise from specific binding capabilities to different fractions of the F-actin networks. On the one hand, α-actinin was shown to be enriched at the PSD where it could bind subunits of the GluN receptor (NMDA receptor), and therefore link and stabilize GluN receptors with the branched F-actin network [150]. On the other hand, drebrin is located at the spine center where it can associate with the stable F-actin pool and prevent the F-actin remodeling required for spine maturation [51, 151, 152].
The Ca2+/calmodulin-dependent protein kinase II (CaMKII), an essential protein involved in post-synaptic plasticity [153–155], can directly bundle and stabilize F-actin in its inactive state via its CaMKIIβ subunit [156, 157]. Under basal conditions, the binding of CaMKII to F-actin prevents other ABPs, such as the Arp2/3 complex and ADF/cofilin, to interact with F-actin, but upon GluN receptor activation, autophosphorylation of CaMKII prompts its release from F-actin allowing these ABPs to bind and regulate F-actin organization and dynamics [158]. Accordingly, recent sptPALM experiments showed that CaMKIIβ shares similar dynamics to F-actin and that depolymerization of the F-actin network (latrunculin-A) or dissociation of the CaMKIIβ subunit from F-actin increase CaMKII diffusion [158, 159]. The formation of F-actin bundles by CaMKIIβ could suggest an association with the stable F-actin pool [156]. However, CaMKIIβ displays inward and forward movements in dendritic spines similar to actin and Arp2/3, rather suggesting an association with the dynamic F-actin pool [158]. In the following sections, we will see how the activation status of CaMKII during synaptic transmission can act as a gating mechanism to trigger the nanoscale reorganization of the F-actin network [49, 128, 158].
The molecular motor myosin II can bind and generate forces on F-actin, leading to F-actin networks’ contraction and/or disassembly. Myosin II is therefore a crucial regulator of F-actin dynamics and network architecture in the lamellipodium and neuronal growth cones [64, 65, 133, 160–164]. EM and SMLM studies have shown that myosin II is found in both dendritic spine heads and necks [39, 66] and that knockdown or pharmacological inhibition of myosin II induces filopodium-like morphologies [165–168]. Myosin II function in dendritic spines might be more complex than just enhancing the retrograde F-actin flow [60, 169, 170]. A recent study suggests that myosin IIb can stabilize the stable F-actin pool through actin crosslinking, while its contractile activity increases the turnover rate of the dynamic F-actin pool [170]. This dual function might arise from differences in the structural organization of actin filaments in the stable versus dynamic pool. A very elegant in vitro study has shown that myosin II, as well as myosin VI, can disassemble antiparallel but not parallel F-actin bundles leading to a selective actomyosin contractility and disassembly [163]. Taken together with the localization of the parallel bundler drebrin, this suggests that the stable F-actin pool is mainly composed of parallel F-actin bundles stabilized by drebrin and myosin II.
Other ABPs, such as actin binding protein 1 (ABP1), synaptopodin and MAP1B, can be enriched in dendritic spine and regulate spine morphology [171–176]. However, the mechanism of F-actin regulation by those ABPs remains largely unclear, and precludes any speculation about their role in F-actin network organization and dynamics.
Revealing the nanoscale organization and dynamics of a subset of ABPs led to a deeper understanding of the mechanisms linking F-actin generated forces to dendritic spine motility during basal synaptic transmission. In the following sections, we will focus on how changes in synaptic activity can lead to transient and long-lasting reorganization of the F-actin network.
The interplay between the actin cytoskeleton and synaptic plasticity
The strength of synaptic transmission can be either increased or decreased on a timescale of seconds to months. These processes, called long-term potentiation (LTP) and long-term depression (LTD), correlate with the enlargement and shrinkage of dendritic spines, respectively [27–29, 31, 43]. Therefore, the organization and dynamics of the F-actin network requires to be adjusted accordingly to the strength of synaptic transmission [177]. In this section, we will describe the signaling pathways that can regulate F-actin networks during synaptic plasticity, the sequence of events leading to the morphological remodeling of dendritic spines and how the actin cytoskeleton sustains both structural and functional synaptic plasticity.
Signaling pathways that control activity-induced reorganization of the actin cytoskeleton
Neuron depolarization leads to the pre-synaptic release of glutamate and to the activation of post-synaptic receptors. During synaptic plasticity, the opening of post-synaptic GluN receptors (NMDA receptors) and voltage-gated calcium channels induces Ca2+ influx into dendritic spines and activation of calmodulin (CaM) sensitive proteins [153, 178]. CaM binding to CaMKII releases its auto-inhibition allowing autophosphorylation and leading to transient kinase activity [153, 155] (Fig. 2a). As mentioned previously, CaMKII autophosphorylation induces its dissociation from F-actin. Once activated, CaMKII can in turn phosphorylate several synaptic proteins, including regulators of small Rho-familly GTPases. RhoGTPases can switch from an active GTP-bound state to an inactive GDP-bound state by intrinsic GTPase activity. Guanine nucleotide exchange factors (GEFs) activate RhoGTPases by stimulating the release of bound GDP and allowing the binding of GTP. Conversely, GTP-activating proteins (GAPs) can inactivate RhoGTPases by increasing GTPase activity. Rac1, Cdc42 and RhoA are among the best characterized small RhoGTPases and have been extensively described as F-actin regulators in non-neuronal cells [179–182]. Rac1 is responsible for lamellipodium formation, while Cdc42 is more involved in the formation of conventional filopodia, and RhoA plays a role in the assembly of stress fibers and adhesion sites. CaMKII can directly phosphorylate and activate Rac1 GEFs such as Tiam1 and Kalirin7 but can also directly phosphorylate and inactivate Rac1 GAP, p250GAP, both signaling cascades leading to enhanced Rac1 activity [183–186]. CaMKII phosphorylation of neurabin-II leads to the recruitment of a RhoA GEF, Lfc (“Lbc’s first cousin”) [187, 188]. CaMKII activity is also partially involved in Cdc42 activation, although the signaling pathway has not been described in dendritic spines [189]. An increase in intracellular Ca2+ can activate other CaM sensitive proteins involved in the regulation of F-actin networks. CaMKK and CaMKI form a multiprotein complex with another Rac1 GEF, βPIX (“Pak-interacting exchange factor”), resulting in Rac1 activation [190, 191].
Fig. 2.
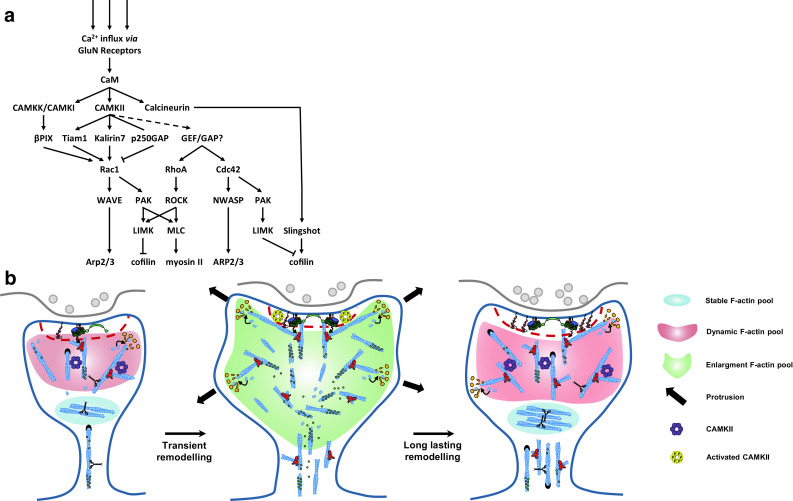
Activation of ABPs and reorganization of the F-actin cytoskeleton during LTP. a Selected signaling cascades driving F-actin remodeling upon Ca2+ influx via GluN Receptors. CAMKII is a key triggering protein both involved in functional and structural LTP. CAMKII can activate small RhoGTPases (Rac1, Cdc42, RhoA) by phosphorylating GEFs (Tiam1, Karilin7) and GAPs (p250GAAP). This will control the spatiotemporal activation of several ABPs (Arp2/3, myosin II, cofilin). Arrows indicate positive regulation. T-shaped bars indicate negative regulation. GluN NMDA receptor, CaM calmodulin, CaMK calcium-calmodulin dependent protein kinase, GEF guanine nucleotide exchange factor, GAP GTPase activating protein, βPIX β PAK interacting exchange factor, Tiam1 T cell lymphoma invasion and metastasis-inducing protein 1, Rac1 Ras-related C3 botulinum toxin substrate 1, Cdc42 cell division cycle 42, RhoA Ras homologous member A, ROCK Rho-associated, coiled-coil containing protein kinase, PAK p21-activated kinase, LIMK LIM-kinase, N-WASP neuronal Wiskott–Aldrich syndrome protein, WAVE WASP-family verprolin homologous protein, MLC myosin light chain, Arp2/3 actin-related-protein 2/3. b A model for F-actin reorganization during LTP. LTP induces a transient and long-lasting increase of the spine head size, a shortening and widening of spine neck and a concomitant ABPs and CaMKII spine recruitment. Activated CAMKII will dissociate from F-actin and phosphorylate multiple proteins leading to a fast F-actin reorganization and a transient spine head enlargement. This transient reorganization is characterized by the formation of an enlargement F-actin pool, an increase in the F-actin/G-actin ratio and increased concentration of cofilin and Arp2/3. Note that inactivation of cofilin is detected only 2 min after LTP induction, suggesting that cofilin might be only transiently active before it gets inactivated to prevent further depolymerization. This might be important to only transiently sever F-actin filaments in order to generate new F-actin barbed ends, and to increase F-actin polymerization and branching. During the long-lasting spine head enlargement, most ABPs return to their basal concentration, suggesting the formation of a larger dynamic and stable F-actin pool. Those larger dendritic spines most likely provides a “tag” for the capture of newly synthetized synaptic proteins in order to sustain late LTP
Many targets of RhoGTPases are present in dendritic spines and are critical regulators of the actin cytoskeleton (Fig. 2a). The WAVE complex and N-WASP, NPFs of the Arp2/3 complex, are two main downstream targets of Rac1 and Cdc42, respectively [192–194]. p21-activated kinase (PAK) is a downstream target of Rac1 and Cdc42, while Rho-associated, coiled-coil-containing protein kinase (ROCK), is a downstream target of RhoA [180, 195–197]. Both PAK and ROCK can activate LIM-kinase (LIMK) that can in turn phosphorylate ADF/cofilin preventing its binding and severing activities. This inhibition of ADF/cofilin can be counterbalanced by other signaling pathways. CaM activates protein phosphatase 2B (PP2B or calcineurin) triggering slingshot activation, which dephosphorylates and activates ADF/cofilin leading to enhanced F-actin severing [198, 199]. PAK and ROCK can also phosphorylate the myosin light chain (MLC) leading to increased myosin II activity [177, 195]. Finally, the Arp2/3 complex inhibitor PICK1 can also be regulated by synaptic activity since it is a low-affinity Ca2+ sensor, which binds to Rac1 and Cdc42 and is regulated by the GTPase ADP-ribosylation factor 1 (Arf1) [200–202]. Although many studies revealed the complex signaling pathways leading to F-actin network reorganization, their spatiotemporal integration during synaptic plasticity is far from being understood [181, 189, 203–205].
Morphological remodeling of dendritic spines and F-actin reorganization during LTP and LTD
Early EM studies already reported that LTP induction would increase dendritic spine head and PSD size [23, 206–208], increase the number of dendritic spine protrusions [25] and induce wider and shorter spine necks [207]. However, EM studies cannot provide longitudinal measurements for single spines during LTP induction. Kasai and co-workers performed two-photon glutamate uncaging to release glutamate at single dendritic spines [28, 209]. This stimulation induced spine head enlargement, GluA1 recruitment and an increase in GluA-mediated current [28, 210]. More recently, time-lapse STED microscopy revealed that shortening and widening of the spine neck occurred in a concerted fashion with spine head enlargement [31]. These changes in spine head volume display two distinct phases, a transient large volume increase (<5 min; Δvolume 200–400 % increase) followed by long-lasting smaller volume increase for small dendritic spines (60 min; Δvolume 50–150 % increase), whereas spine neck changes seem to display only a long-lasting change (Δ with 30 % increase; Δlength 30 % decrease; [31]). Consistent with previous studies demonstrating that F-actin dynamics supports spine motility, pharmacological treatments that increased F-actin depolymerization (latrunculin-A) prevented transient and long-lasting changes of dendritic spine head [28].
In vivo, LTP induction was shown to correlate with increased F-actin content in dendritic spines visualized by EM [211]. Fluorescence resonance energy transfer (FRET) also revealed an increased F-actin/G-actin ratio after pre-synaptic fiber tetanic stimulation, suggesting that LTP promotes F-actin polymerization [75]. Subsequently, a very elegant study explored F-actin reorganization by probing photoactivable actin dynamics after two-photon glutamate uncaging [77]. Single spine activation, which triggered spine enlargement, induced the formation of a transient enlargement F-actin pool distributed throughout the spine head, displaying a distinct turnover compared to the stable and dynamic F-actin pools (Fig. 2b). The localization and dynamics of this enlargement pool often synchronized with spine membrane protrusions, as if F-actin polymerization drove the enlargement. However, increased volumes triggered by LTP last longer than the life-time of the enlargement pool, suggesting that the long-lasting spine enlargement might be sustained by transfer of F-actin from the enlargement into the stable pool.
GluN receptor-dependent Ca2+ influx during LTP, which leads to activation of the intracellular signaling pathways, could increase F-actin polymerization and reorganization [153, 212]. CaMKII is necessary and sufficient for LTP induction; it is therefore not surprising that pharmacological inhibition of CaMKII or expression of a CaMKIIα kinase dead mutant in knock-in mouse disrupt the long-lasting volume increase of dendritic spines [28, 205, 213]. Combining FRET with two-photon glutamate uncaging, Yasuda and co-workers demonstrated that CaMKII activity was only transiently increased (~2 min) during spine head enlargement [205]. As mentioned in “Actin organization in dendritic spines” and “The interplay between the actin cytoskeleton and synaptic plasticity”, active CaMKIIβ will dissociate from F-actin and phosphorylate multiple proteins. Recently, Okamoto and colleagues showed that CaMKIIβ dissociation from F-actin is required for structural and functional plasticity of spines, and that this dissociation is not sufficient to induce structural plasticity but permits ADF/cofilin binding to F-actin [158]. The small RhoGTPases Cdc42 and RhoA showed increases in transient (~2 min) but also long-lasting (~40 min) activity [189]. These increased activities are dependent on CaMKII and GluN receptor. Although this latter study did not study Rac1 activity pattern during LTP, a previous study reported that kalirin-7 phosphorylation by CaMKII and subsequent Rac1 activation would lead to dendritic spine enlargement [186]. Overall, those studies suggest that the sustained activities of Rac1, Cdc42 and RhoA might be the main mechanism to relay the transient CaMKII activity for efficient F-actin remodeling.
The main downstream targets of those proteins will regulate F-actin branched nucleation by Arp2/3, ADF/cofilin severing activity and myosin II function (Fig. 2a). Studying the spatiotemporal coordination of various proteins, Hayashi and co-workers have provided new insights into F-actin cytoskeleton reorganization during two-photon glutamate uncaging-induced LTP [49] (Fig. 2b). Concomitantly with spine enlargement, ABPs and CaMKII were translocated into spines. However, while the concentration of cofilin, AIP1 and Arp2/3 in spines increased during the transient spine enlargement phase, the concentration of the crosslinking proteins CaMKIIβ, α-actinin and drebrin A decreased (relative to the spine volume) [49]. This qualitative switch in ABPs concentration in spines suggests that the F-actin network undergoes a fast reorganization that might be important for the formation of the F-actin enlargement pool. Phosphorylation and inactivation of cofilin is detected only 2 min after LTP induction [214], suggesting that cofilin might be only transiently active before it gets inactivated by LIMK to prevent further F-actin severing [49]. Activation of Rac1 downstream pathways might be the key for this short time window activation of ADF/cofilin. Indeed, activation of Rac1 will lead to increased F-actin branching via activation of WAVE and Arp2/3 complexes but also decreased F-actin severing via phosphorylation of ADF/cofilin by PAK/LIMK pathways. A delayed activation of the PAK/LIMK pathway as compared to the fast increase of AIP1 and ADF/cofilin concentrations might provide this 2-min time window for efficient F-actin severing [120]. Interestingly, this transient activation fits with the CaMKII time window of activity and its dissociation from the F-actin network [158, 189]. This model is in agreement with the role that ADF/cofilin might play to trigger membrane protrusions [83, 215]. In that model, transient F-actin severing generates new F-actin barbeds ends, increasing F-actin branching and polymerization without totally depolymerizing the F-actin network. Thus, during these 2-min time windows, the actin cytoskeleton in spines could experience drastic remodeling. Our recent findings provided some insights into the links between spine enlargement and the nanoscale reorganization of ABPs [67]. SMLM experiments demonstrated that Rac1 activation correlates with its immobilization in spines, spine enlargement and delocalization of the WAVE complex from the PSD. In addition, we found that Shank3 overexpression, which causes spine enlargement and neuropsychiatric disorders [129, 216], also induces delocalization of the WAVE complex from the PSD [67]. Thus, alteration of spine morphology could rely on the long-lasting or transient nanoscale relocalization of ABPs, e.g., the WAVE complex, leading to remodeling of the entire dendritic spine structure.
During the long-lasting spine volume increase, most ABPs returned to their basal concentration, whereas cofilin showed a persistent accumulation at the base of the spine and increased F-actin binding [49]. Actin filaments fully decorated by ADF/cofilin are stable, promoting F-actin bundle stabilization in the absence of AIP1 [120, 121, 217]. The long-lasting ADF/cofilin pool might therefore be associated with the stable F-actin pool. Accordingly, drebrin-A is also slightly enriched at potentiated spines during the long-lasting spine head enlargement [49, 218]. Maintaining high-concentration levels of ADF/cofilin during a precise time window could be an interesting mechanism to efficiently depotentiate newly potentiated synapses [49, 219].
Although GluN receptor antagonist and F-actin depolymerization (latrunculin-A) totally disrupt transient and long-lasting spine remodeling, inhibition of CaMKII, Cdc42, ADF/cofilin or Arp2/3 blocks only the long-lasting volume changes [28, 49, 93, 189, 205]. Thus, Ca2+ influx might remodel the F-actin network via distinct signaling pathways such as activation of CaMKI or release of internal calcium stores [174, 220]. Activation of GluN receptor can also recruit and stabilize β-catenin in dendritic spines [221]. According to the molecular clutch hypothesis, connection of N-cadherin/β-catenin to the acto-myosin network could transmit forces that will lead to spine enlargement [60, 70, 222, 223]. Supporting the requirement of a clutch mechanism for dendritic spine enlargement during LTP, inhibition of RhoA and ROCK, needed for myosin II activity, also disrupted transient and long-lasting volume changes [60, 189]. All these studies highlight the need for a spatiotemporal coordination of F-actin polymerization, branching, severing and bundling for transient and long-lasting remodeling of the different F-actin pools in order to sustain structural LTP.
Induction of synaptic LTD by electrical or chemical stimulation can lead to dendritic spine shrinkage [29, 30, 75, 224, 225]. Adapting the two-photon glutamate uncaging to induce LTP, Zito and co-workers established a low frequency uncaging (LFU) protocol to induce LTD at individual spines [30]. Although LFU does not allow the probing of structural remodeling at early time scales, the authors were able to induce a long-lasting and saturable volume shrinkage of small and large dendritic spines (Δvolume 25 % decrease). Combining two-photon uncaging of glutamate and GABA to induce a distinct form of LTD, Kasai and coworkers could induce larger volume changes (ΔVolume 40 % decrease) and even spine elimination [226]. Spine shrinkage was first shown to correlate with a decreased F-actin/G-actin ratio after pre-synaptic fiber tetanic stimulation, suggesting F-actin depolymerization [75], which, during GluN receptor-dependent LTD, would be powered by activation of ADF/cofilin by calcineurin via slingshot [29, 123, 198, 224]. Inhibition of GluN receptors and calcineurin and also a phospho-cofilin peptide can therefore abolish spine shrinkage [224, 226]. However, shrinkage of large spines requires additional signaling from mGluR and internal calcium stores [30]. Dendritic spine shrinkage is also blocked after knockdown of the Arp2/3 inhibitor PICK1 or expression of a PICK1 mutant unable to bind Arp2/3 [106]. Therefore, as for LTP, regulation of F-actin organization and dynamics might be critical mechanisms for structural LTD.
How does the actin cytoskeleton sustain functional LTP and LTD?
Dendritic spine structural plasticity and functional plasticity share similar triggering molecules, such as CaMKII for LTP and calcineurin for LTD. However, structural plasticity and functional plasticity can be dissociated as they do not strictly rely on the same intracellular signaling pathways [43, 199, 224, 227–230]. Although regulation of F-actin network assembly and disassembly is not sufficient to induce synaptic plasticity, it is required for its formation in vitro and in vivo [211, 231–233]. Likewise, knocking out proteins involved in F-actin network organization and dynamics, such as Rac1, IRSp53, subunits of WAVE or Arp2/3 complexes, PAK1, PAK3, LIMK, ADF/cofilin and Eps8 displayed impaired LTP and/or LTD and also spatial and working memory deficits [93, 98, 100, 117, 122, 234–239]. Neurological disorders associated with abnormal spine morphologies such as autism spectrum disorders (ASD) and schizophrenia can also be triggered by genetic deregulation of proteins such as IRSp53, Shank3 and FMRP (fragile X mental retardation protein) that directly interact with subunits of the WAVE and Arp2/3 complexes [129, 216, 239–242]. What could therefore be the mechanisms behind F-actin cytoskeleton sustainment of synaptic plasticity?
GluN and GluA receptors play a major role in the induction and maintenance of synaptic plasticity, respectively [199, 243, 244]. Trafficking of GluN and GluA in and out of dendritic spines, but also their anchorage and stabilization via interaction with PSD scaffold proteins, are crucial mechanisms for LTP and LTD [245–248]). PSD dynamic architecture and receptor trafficking are supported and regulated by the F-actin cytoskeleton [43, 86, 249]. F-actin can regulate in many ways the dynamics of key PSD scaffold proteins such as PSD-95, GKAP, Shank and Homer [35]. First, actin filaments are in close proximity with the PSD [36]. Second, the Arp2/3 and WAVE complexes and cortactin can directly bind Shank3 [101, 129]. Third, proteins involved in the activation of the WAVE complex, such as IRSp53 and the Rac1 GEF βPIX, can interact with Shank family proteins and PSD95 [126, 127, 250–252]. Acute pharmacological treatment inducing F-actin depolymerization (latrunculin-A) disrupts PSD-95 nanoscale organization and dynamics but only slightly affects its content in dendritic spines, probably because of its direct association with the plasma membrane [253–255]. A similar treatment decreases GKAP, Homer1C and Shank2 content in immature but not mature spines [253, 254]. Thus, PSD scaffold matrix remodeling relies on F-actin dynamics, but the intermolecular assembly between PSD proteins might be strengthened during dendritic spine maturation to become independent from the F-actin network [256]. Interestingly, as for PSD95 proteins, the WAVE complex and IRSp53 are still present in dendritic spines after F-actin depolymerization, highlighting the tight functional coupling between the PSD and F-actin regulators [67]. Pharmacological treatments which induce F-actin depolymerization also disrupt GluA dynamic architecture, increasing their surface diffusion, decreasing their spine content and preventing their spine insertion during LTP [230, 238, 254, 257]. Furthermore, inhibiting F-actin dynamics without disrupting the F-actin network (low concentrations of latrunculin-A) does not decrease GluA content but still prevents insertion of new GluA receptors upon synaptic stimulation [228, 230, 232]. Altogether, these findings suggest, first, that PSD reorganization by F-actin controls the anchorage of a synaptic pool of GluA receptors; second that PSD remodeling provides constant accessibility of new binding sites for GluA; and finally that maintenance and insertion of GluA receptors relies on the F-actin cytoskeleton [230, 238, 254, 258–260].
Disrupting F-actin dynamics could also lead to impaired exocytosis and endocytosis therefore preventing insertion and removal of GluA receptors during LTP and LTD, respectively [249, 261–265]. The plasma membrane t-SNARE syntaxin 4 binds to actin and is involved in exocytosis in dendritic spines and LTP [261, 266]. However, F-actin depolymerization does not change the frequency and proportion of exocytotic events in the somatodendritic compartment [267]. Spine-localized endocytosis of GluA receptors is, on the other hand, well established and relies on the actin cytoskeleton [249]. The Arp2/3 inhibitor PICK1 binds to GluA2/3 subunits and plays a critical role in their surface expression. Preventing PICK1 binding to Arp2/3 disrupts GluA internalization during LTD induction therefore occluding LTD [96, 106]. Interestingly, PICK1 is a Ca2+ sensor, and the PICK1-mediated inhibition of Arp2/3 is enhanced by GluA2-PICK1 interaction thus providing spatiotemporal control of GluA endocytosis during LTD [96, 200]. Since PICK1 knockdown also prevents LTP, branched F-actin nucleation and its regulation by PICK1 could be more generally involved in the recycling endosomal pathway and in the constant supply of GluA receptors [249, 268, 269].
Intracellular transport of recycling endosomes (RE) into and out of dendritic spines relies on myosin motors moving on actin filaments: myosin V and myosin VI walking towards the barbed- and pointed-end, respectively. Spine necks are composed of both branched and non-branched long F-actin filaments of mixed orientation, thus providing tracks for myosin V and VI to enter and exit dendritic spines [39, 66]. As mentioned previously, LTP induction induces shortening and widening of the spine neck and could therefore facilitate in and out trafficking mediated by myosin motors [31]. Myosin VI was first shown to be involved in stimulation-induced GluA1 endocytosis [270]. Myosin Vb was also shown to be recruited to RE upon Ca2+ influx and LTP induction, triggering translocation of RE into spines and GluA1 surface insertion [271]. Furthermore, acute blockade of Myosin Vb ATP activity, needed for its movements along F-actin, attenuates LTP maintenance [271].
While the mechanisms described above were shown to be essential to sustain the early phase of LTP and LTD (1–4 h), protein synthesis is required to sustain the later phases (≥4 h) [49, 199, 272–277]. Late LTP (L-LTP) requires, for instance, the supply of newly synthetized PSD proteins to increase the number of available binding sites for GluA receptors in order to prevent synaptic saturation [278]. Interestingly, inhibiting CaMKII activity only during LTP induction or inhibiting F-actin dynamics during LTP induction up to 30 min after induction are sufficient to occlude L-LTP [233, 279]. Therefore, activation of the CaMKII signaling cascade, reorganization of the F-actin cytoskeleton and dendritic spine morphological remodeling could all represent a synaptic “tag” for efficient delivery and capture of newly synthetized proteins in order to sustain long-term synaptic plasticity and memory storage [49, 280].
Conclusion/outlook
In this review, we have highlighted that, as for many other motile sub-cellular compartments, dendritic spine motility and remodeling are sustained by F-actin cytoskeleton dynamics. The unique F-actin dynamics of dendritic spines results from the nanoscale organization of various ABPs within different sub-spine compartments [67]. However, to completely understand how the nanoscale organization of ABPs can lead to the coexistence of several F-actin pools with distinct dynamic properties will require further studies. Correlative live cell and super-resolution microscopy could, for instance, enable correlation between F-actin dynamics and ABP nanoscale organization [281]. Because of the heterogeneity of dendritic spine morphologies, multiplex SMLM could represent a powerful tool to visualize membrane, ABPs, PSD proteins and post-synaptic receptors within an individual spine. Multiplex SMLM can be achieved by linking DNA-PAINT (DNA-Point Accumulation for Imaging in Nanoscale Topography) docking strands to antibodies [282, 283]; by using virtual grids and sequential immunostaining and imaging [284]; or more recently by spectrally resolving single molecules from different dyes [285]. This last approach might, however, be challenging regarding the density of proteins in dendritic spines.
Regulation of the spatiotemporal activity of ABPs is a fundamental mechanism to induce spine structural plasticity during LTP and LTD. Recent work has provided a detailed picture of this spatiotemporal coordination [49, 189, 286]. However, those studies could not provide the nanoscale organization of ABPs at sub-spine resolution. So far, SMLM has reached the highest resolution for fluorescence microscopy on both live and fixed biological samples in 2D and 3D [53, 80, 287, 288]. Deep tissue and in vivo imaging of dendritic spines is, however, still a challenge [289, 290]. Super-resolution microscopy techniques, such as simulated emission depletion microscopy (STED), can perform live recordings of dendritic spines in brain slices and in vivo [22, 31, 291]. However, high-power lasers used for STED induce high photobleaching and requires high labeling density or replenishment of a cytosolic fluorophore. Reversible saturable optical fluorescence transitions microscopy can overcome those drawbacks by exploiting reversibly photoswitchable fluorophores [292, 293]. Structured illumination microscopy is also well suited for three-dimensional multi-color live imaging, and recent improvements of its spatial resolution in combination with light sheet microscopy could represent a valuable tool to study the spatiotemporal reorganization of synaptic proteins in brain slices and in vivo during synaptic plasticity [294–299].
Spine structural plasticity and the underlying reorganization of F-actin networks could represent a synaptic “tag” required for the maintenance of functional plasticity [75, 153, 233, 280]. Recently, Kasai and co-workers have demonstrated that motor task learning could be disrupted by optical shrinkage of the potentiated spines, therefore establishing a direct link between dendritic spine morphology and the formation of a memory trace in vivo [13]. The exact mechanism by which the F-actin cytoskeleton sustains functional plasticity is, however, still unclear. Most likely, it involves efficient delivery and capture of newly synthetized proteins as well as the regulation of PSD proteins and post-synaptic receptors dynamics and stability. The coordinated structural remodeling of pre- and post-synaptic structures could also be a key mechanism for maintenance of functional plasticity and might require tight coordination between the F-actin cytoskeleton and trans-synaptic adhesion proteins [286, 300]. Further development in optogenetics will allow the manipulation of synaptic protein interactions in vitro and in vivo and could tackle those hypotheses to refine the model behind the sustainment of functional synaptic plasticity by F-actin cytoskeleton reorganization [301, 302]. However, how this knowledge, acquired at a single spine and neuron level, can be implemented in neural networks models that can generate behavior, cognition and mental disease remains a main challenge in neuroscience [303, 304].
Acknowledgments
We would like to thank Dr. Harold D. McGillavry and Dr Laura F. Gumy for helpful comments on the manuscript. We acknowledge financial support from the French Ministry of Research and CNRS, ANR grant Nanomotility, LabEx BRAIN, Conseil Régional Aquitaine, Fondation pour la Recherche Médicale, and from Marie Skłodowska-Curie fellowship to Anaël Chazeau.
Abbreviations
- ABP
Actin binding proteins
- ASD
Autism spectrum disorders
- EM
Electron microscopy
- FRAP
Fluorescence recovery after photobleaching
- FRET
Fluorescence resonance energy transfer
- LTD
Long-term depression
- LTP
Long-term potentiation
- NPFs
Nucleation promoting factors
- PSD
Post-synaptic density
- SMLM
Single molecule localization microscopy
- sptPALM
Single particle tracking photoactivation localization microscopy
- STED
Simulated emission depletion microscopy
References
- 1.Ramón y Cajal S S. Estructura de los centros nerviosos de las aves. Rev Trim Histol Norm Pat. 1888;1:1–10. [Google Scholar]
- 2.Yuste R. The discovery of dendritic spines by Cajal. Front Neuroanat. 2015;9:1–6. doi: 10.3389/fnana.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramón y Cajal S. Significación fisiológica de las expansiones protoplásmicas y nerviosas de la sustancia gris. Rev Cienc Med Barcelona. 1891;22:23. [Google Scholar]
- 4.Ramón y Cajal S (1893) Neue darstellung vom histologischen bau des centralnervensystem. Arch Anat Entwick 319–428
- 5.Ramón y Cajal S. La fine structure des centres nerveux. The croonian lecture. Proc R Soc Lond B. 1894;55:443–468. [Google Scholar]
- 6.Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- 7.Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 8.Guillery RW. Early electron microscopic observations of synaptic structures in the cerebral cortex: a view of the contributions made by George Gray (1924–1999) Trends Neurosci. 2000;23:594–598. doi: 10.1016/S0166-2236(00)01635-0. [DOI] [PubMed] [Google Scholar]
- 9.Hebb DO. The organization of behaviour: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- 10.Kandel ER. The biology of memory: a forty-year perspective. J Neurosci. 2009;29:12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Squire LR. Memory and brain systems: 1969–2009. J Neurosci. 2009;29:12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nägerl UV, Willig KI, Hein B, Hell SW, Bonhoeffer T. Live-cell imaging of dendritic spines by STED microscopy. Proc Natl Acad Sci USA. 2008;105:18982–18987. doi: 10.1073/pnas.0810028105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izeddin I, Specht CG, Lelek M, Darzacq X, Triller A, Zimmer C, Dahan M. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE. 2011;6:e15611. doi: 10.1371/journal.pone.0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dailey E, Smith S. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/S0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 20.Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 21.Grutzendler J, Kasthuri N, Gan W-B. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 22.Berning S, Willig KI, Steffens H, Dibaj P, Hell SW. Nanoscopy in a living mouse brain. Science. 2012;335:551. doi: 10.1126/science.1215369. [DOI] [PubMed] [Google Scholar]
- 23.Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc Natl Acad Sci USA. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 25.Toni N, Buchs P, Nikonenko I, Bron C, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 26.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama J, Yasuda R. Biochemical computation for spine structural plasticity. Neuron. 2015;87:63–75. doi: 10.1016/j.neuron.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Oh WC, Hill TC, Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc Natl Acad Sci USA. 2013;110:E305–E312. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tønnesen J, Katona G, Rózsa B, Nägerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci. 2014;17:678–685. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GCR, Kitamura K, Kano M, Matsuzaki M, Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loewenstein Y, Kuras A, Rumpel S. Multiplicative dynamics underlie the emergence of the log-normal distribution of spine sizes in the neocortex in vivo. J Neurosci. 2011;31:9481–9488. doi: 10.1523/JNEUROSCI.6130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Cudmore RH, Lin D-T, Linden DJ, Huganir RL. Visualization of NMDA receptor-dependent AMPA receptor synaptic plasticity in vivo. Nat Neurosci. 2015;18:402–407. doi: 10.1038/nn.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 36.Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng D, Hoogenraad C, Rush J, Schlager M, Duong D, Xu P, Wijayawardana S, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korkotian E, Segal M. Regulation of dendritic spine motility in cultured hippocampal neurons. J Neurosci. 2001;21:6115–6124. doi: 10.1523/JNEUROSCI.21-16-06115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 44.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlier M-F, Pernier J, Montaville P, Shekhar S, Kühn S. Control of polarized assembly of actin filaments in cell motility. Cell Mol Life Sci. 2015;72:3051–3067. doi: 10.1007/s00018-015-1914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilo Boyl P, Witke W. Small, smaller. dendritic spine. EMBO J. 2014;33:2737–2739. doi: 10.15252/embj.201490137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin W-H, Webb DJ. Actin and actin-binding proteins: masters of dendritic spine formation, morphology, and function. Open Neurosci J. 2009;3:54–66. doi: 10.2174/1874082000903020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci USA. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rácz B, Weinberg RJ. Microdomains in forebrain spines: an ultrastructural perspective. Mol Neurobiol. 2013;47:77–89. doi: 10.1007/s12035-012-8345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang B, Bates M, Zhuang X. Super-resolution fluorescence microscopy. Annu Rev Biochem. 2009;78:993–1016. doi: 10.1146/annurev.biochem.77.061906.092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maglione M, Sigrist SJ. Seeing the forest tree by tree: super-resolution light microscopy meets the neurosciences. Nat Neurosci. 2013;16:790–797. doi: 10.1038/nn.3403. [DOI] [PubMed] [Google Scholar]
- 54.Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Adrian M, Kusters R, Wierenga CJ, Storm C, Hoogenraad CC, Kapitein LC. Barriers in the brain: resolving dendritic spine morphology and compartmentalization. Front Neuroanat. 2014;8:1–12. doi: 10.3389/fnana.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacGillavry HD, Hoogenraad CC. The internal architecture of dendritic spines revealed by super-resolution imaging: what did we learn so far? Exp Cell Res. 2015;335:180–186. doi: 10.1016/j.yexcr.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/S0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 58.Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 59.Zuo Y, Lin A, Chang P, Gan W-B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Chazeau A, Garcia M, Czondor K, Perrais D, Tessier B, Giannone G, Thoumine O. Mechanical coupling between transsynaptic N-cadherin adhesions and actin flow stabilizes dendritic spines. Mol Biol Cell. 2015;26:859–873. doi: 10.1091/mbc.E14-06-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotulainen P, Llano O, Smirnov S, Tanhuanpää K, Faix J, Rivera C, Lappalainen P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol. 2009;185:323–339. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 64.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 65.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner H-G, Freund Y, Borisy G, Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tatavarty V, Das S, Yu J. Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron. Mol Biol Cell. 2012;23:3167–3177. doi: 10.1091/mbc.E12-02-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chazeau A, Mehidi A, Nair D, Gautier JJ, Leduc C, Chamma I, Kage F, Kechkar A, Thoumine O, Rottner K, Choquet D, Gautreau A, Sibarita J-B, Giannone G. Nanoscale segregation of actin nucleation and elongation factors determines dendritic spine protrusion. EMBO J. 2014;33:2745–2764. doi: 10.15252/embj.201488837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 69.Suter DM, Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J Neurobiol. 2000;44:97–113. doi: 10.1002/1097-4695(200008)44:2<97::AID-NEU2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 70.Giannone G, Mège R-M, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Bard L, Boscher C, Lambert M, Mège R-M, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia M, Leduc C, Lagardère M, Argento A, Sibarita J-B, Thoumine O. Two-tiered coupling between flowing actin and immobilized N-cadherin/catenin complexes in neuronal growth cones. Proc Natl Acad Sci USA. 2015;112:201423455. doi: 10.1073/pnas.1423455112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao-Cheng J-H, Dosemeci A, Gallant PE, Miller S, Galbraith JA, Winters CA, Azzam R, Reese TS. Rapid turnover of spinules at synaptic terminals. Neuroscience. 2009;160:42–50. doi: 10.1016/j.neuroscience.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 76.Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 77.Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GCR, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 80.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 81.Tatavarty V, Kim E-J, Rodionov V, Yu J. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS ONE. 2009;4:e7724. doi: 10.1371/journal.pone.0007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pollard TD. The cytoskeleton, cellular motility and the reductionist agenda. Nature. 2003;422:741–745. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- 83.Lai FPL, Szczodrak M, Block J, Faix J, Breitsprecher D, Mannherz HG, Stradal TEB, Dunn GA, Small JV, Rottner K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008;27:982–992. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Achard V, Martiel J-L, Michelot A, Guérin C, Reymann A-C, Blanchoin L, Boujemaa-Paterski R. A “primer”-based mechanism underlies branched actin filament network formation and motility. Curr Biol. 2010;20:423–428. doi: 10.1016/j.cub.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 85.Sykes C, Plastino J. Cell biology: actin filaments up against a wall. Nature. 2010;464:365–366. doi: 10.1038/464365a. [DOI] [PubMed] [Google Scholar]
- 86.Frost NA, Kerr JM, Lu HE, Blanpied TA. A network of networks: cytoskeletal control of compartmentalized function within dendritic spines. Curr Opin Neurobiol. 2010;20:578–587. doi: 10.1016/j.conb.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rácz B, Weinberg RJ. Organization of the Arp2/3 complex in hippocampal spines. J Neurosci. 2008;28:5654–5659. doi: 10.1523/JNEUROSCI.0756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burette AC, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman MH, Weinberg RJ. Electron tomographic analysis of synaptic ultrastructure. J Comp Neurol. 2012;520:2697–2711. doi: 10.1002/cne.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanchoin L, Amann KJ, Higgs HN, Marchand J, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;171:1007–1011. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- 90.Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA. 2001;98:15009–15013. doi: 10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 92.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, Wetsel WC, Soderling SH. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J Neurosci. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Helgeson LA, Nolen BJ. Mechanism of synergistic activation of Arp2/3 complex by cortactin and N-WASP. Elife. 2013;2:e00884. doi: 10.7554/eLife.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Helgeson LA, Prendergast JG, Wagner AR, Rodnick-Smith M, Nolen BJ. Interactions with actin monomers, actin filaments, and Arp2/3 complex define the roles of WASP family proteins and cortactin in coordinately regulating branched actin networks. J Biol Chem. 2014;289:28856–28869. doi: 10.1074/jbc.M114.587527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim Y, Sung JY, Ceglia I, Lee K-W, Ahn J-H, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 98.Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pilpel Y, Segal M. Rapid WAVE dynamics in dendritic spines of cultured hippocampal neurons is mediated by actin polymerization. J Neurochem. 2005;95:1401–1410. doi: 10.1111/j.1471-4159.2005.03467.x. [DOI] [PubMed] [Google Scholar]
- 100.Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM. Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation. EMBO J. 2007;26:1397–1409. doi: 10.1038/sj.emboj.7601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Racz B, Weinberg RJ. The subcellular organization of cortactin in hippocampus. J Neurosci. 2004;24:10310–10317. doi: 10.1523/JNEUROSCI.2080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee S, Lee K, Hwang S, Kim SH, Song WK, Park ZY, Chang S. SPIN90/WISH interacts with PSD-95 and regulates dendritic spinogenesis via an N-WASP-independent mechanism. EMBO J. 2006;25:4983–4995. doi: 10.1038/sj.emboj.7601349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura Y, Wood CL, Patton AP, Jaafari N, Henley JM, Mellor JR, Hanley JG. PICK1 inhibition of the Arp2/3 complex controls dendritic spine size and synaptic plasticity. EMBO J. 2011;30:719–730. doi: 10.1038/emboj.2010.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 108.Goldschmidt-Clermont PJ, Machesky LM, Doberstein SK, Pollard TD. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991;113:1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology, 4th edn. Section 18.2: the dynamics of actin assembly. W. H. Freeman, New York
- 110.Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- 111.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 112.Breitsprecher D, Kiesewetter AK, Linkner J, Vinzenz M, Stradal TEB, Small JV, Curth U, Dickinson RB, Faix J. Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J. 2011;30:456–467. doi: 10.1038/emboj.2010.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 2012;336:1164–1168. doi: 10.1126/science.1218062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Disanza A, Carlier M-F, Stradal TEB, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- 116.Fan Y, Tang X, Vitriol E, Chen G, Zheng JQ. Actin capping protein is required for dendritic spine development and synapse formation. J Neurosci. 2011;31:10228–10233. doi: 10.1523/JNEUROSCI.0115-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menna E, Zambetti S, Morini R, Donzelli A, Disanza A, Calvigioni D, Braida D, Nicolini C, Orlando M, Fossati G, Cristina Regondi M, Pattini L, Frassoni C, Francolini M, Scita G, Sala M, Fahnestock M, Matteoli M. Eps8 controls dendritic spine density and synaptic plasticity through its actin-capping activity. EMBO J. 2013;32:1730–1744. doi: 10.1038/emboj.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suarez C, Roland J, Boujemaa-Paterski R, Kang H, McCullough BR, Reymann A-C, Guérin C, Martiel J-L, De la Cruz EM, Blanchoin L. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr Biol. 2011;21:862–868. doi: 10.1016/j.cub.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCullough BR, Grintsevich EE, Chen CK, Kang H, Hutchison AL, Henn A, Cao W, Suarez C, Martiel J-L, Blanchoin L, Reisler E, De La Cruz EM. Cofilin-linked changes in actin filament flexibility promote severing. Biophys J. 2011;101:151–159. doi: 10.1016/j.bpj.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jansen S, Collins A, Chin SM, Ydenberg CA, Gelles J, Goode BL. Single-molecule imaging of a three-component ordered actin disassembly mechanism. Nat Commun. 2015;6:7202. doi: 10.1038/ncomms8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gressin L, Guillotin A, Guérin C, Blanchoin L, Michelot A. Architecture dependence of actin filament network disassembly. Curr Biol. 2015;25:1437–1447. doi: 10.1016/j.cub.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 122.Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/S0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 123.Zhou L, Jones EV, Murai KK. EphA signaling promotes actin-based dendritic spine remodeling through slingshot phosphatase. J Biol Chem. 2012;287:9346–9359. doi: 10.1074/jbc.M111.302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scita G, Confalonieri S, Lappalainen P, Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18:52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Suetsugu S, Gautreau A. Synergistic BAR-NPF interactions in actin-driven membrane remodeling. Trends Cell Biol. 2012;22:141–150. doi: 10.1016/j.tcb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Bockmann J, Kreutz MR, Gundelfinger ED, Böckers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–1017. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 127.Choi J, Ko J, Racz B, Burette A, Lee J-R, Kim S, Na M, Lee HW, Kim K, Weinberg RJ, Kim E. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25:869–879. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park E, Chi S, Park D. Activity-dependent modulation of the interaction between CaMKIIα and Abi1 and its involvement in spine maturation. J Neurosci. 2012;32:13177–13188. doi: 10.1523/JNEUROSCI.2257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han K, Holder JL, Schaaf CP, Lu H, Chen H, Kang H, Tang J, Wu Z, Hao S, Cheung SW, Yu P, Sun H, Breman AM, Patel A, Lu H-C, Zoghbi HY. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature. 2013;503:72–77. doi: 10.1038/nature12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen B, Brinkmann K, Chen Z, Pak CW, Liao Y, Shi S, Henry L, Grishin NV, Bogdan S, Rosen MK. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell. 2014;156:195–207. doi: 10.1016/j.cell.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen XJ, Squarr AJ, Stephan R, Chen B, Higgins TE, Barry DJ, Martin MC, Rosen MK, Bogdan S, Way M. Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev Cell. 2014;30:569–584. doi: 10.1016/j.devcel.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang C, Svitkina T. Filopodia initiation: focus on the Arp2/3 complex and formins. Cell Adh Migr. 2011;5(5):402–408. doi: 10.4161/cam.5.5.16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giannone G, Dubin-Thaler BJ, Döbereiner H-G, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/S0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 134.Ylänne J, Scheffzek K, Young P, Saraste M. Crystal structure of the alpha-actinin rod reveals an extensive torsional twist. Structure. 2001;9:597–604. doi: 10.1016/S0969-2126(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 135.Nakagawa T, Engler JA, Sheng M. The dynamic turnover and functional roles of α-actinin in dendritic spines. Neuropharmacology. 2004;47:734–745. doi: 10.1016/j.neuropharm.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 136.Hodges JL, Vilchez SM, Asmussen H, Whitmore LA, Horwitz AR. α-actinin-2 mediates spine morphology and assembly of the post-synaptic density in hippocampal neurons. PLoS ONE. 2014;9:e101770. doi: 10.1371/journal.pone.0101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J Cell Biol. 1997;139:951–961. doi: 10.1083/jcb.139.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Al E. Neurabin-II/spinophilin: an actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- 140.Zito K, Knott G, Shepherd GMG, Shenolikar S, Svoboda K. Induction of spine growth and synapse formation by regulation of the spine actin cytoskeleton. Neuron. 2004;44:321–334. doi: 10.1016/j.neuron.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 141.Sarrouilhe D, di Tommaso A, Métayé T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 142.Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483:383–402. doi: 10.1002/cne.20449. [DOI] [PubMed] [Google Scholar]
- 143.Mikati MA, Grintsevich EE, Reisler E. Drebrin-induced stabilization of actin filaments. J Biol Chem. 2013;288:19926–19938. doi: 10.1074/jbc.M113.472647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Worth DC, Daly CN, Geraldo S, Oozeer F, Gordon-Weeks PR. Drebrin contains a cryptic F-actin-bundling activity regulated by Cdk5 phosphorylation. J Cell Biol. 2013;202:793–806. doi: 10.1083/jcb.201303005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, Parsons M, Eickholt BJ. Phosphorylation of the actin binding protein drebrin at S647 is regulated by neuronal activity and PTEN. PLoS ONE. 2013;8:1–12. doi: 10.1371/journal.pone.0071957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jayo A, Parsons M. Fascin: a key regulator of cytoskeletal dynamics. Int J Biochem Cell Biol. 2010;42:1614–1617. doi: 10.1016/j.biocel.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 147.Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J Cell Sci. 2011;124:3305–3318. doi: 10.1242/jcs.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Terry-Lorenzo R, Roadcap D, Otsuka T, Blanpied T, Zamorano P, Garner C, Shenolikar S, Ehlers M. Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and maturation. Mol Biol Cell. 2005;16:2349–2362. doi: 10.1091/mbc.E04-12-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur J Neurosci. 2008;27:2847–2859. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- 150.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- 151.Shirao T, González-Billault C. Actin filaments and microtubules in dendritic spines. J Neurochem. 2013;126:155–164. doi: 10.1111/jnc.12313. [DOI] [PubMed] [Google Scholar]
- 152.Mizui T, Sekino Y, Yamazaki H, Ishizuka Y, Takahashi H, Kojima N, Kojima M, Shirao T. Myosin II ATPase activity mediates the long-term potentiation-induced exodus of stable F-actin bound by drebrin A from dendritic spines. PLoS ONE. 2014;9:e85367. doi: 10.1371/journal.pone.0085367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- 154.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Okamoto K-I, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin Y-C, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA. 2008;105:15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim K, Lakhanpal G, Lu HE, Khan M, Suzuki A, Kato Hayashi M, Narayanan R, Luyben TT, Matsuda T, Nagai T, Blanpied TA, Hayashi Y, Okamoto K. A temporary gating of actin remodeling during synaptic plasticity consists of the interplay between the kinase and structural functions of CaMKII. Neuron. 2015;87:813–826. doi: 10.1016/j.neuron.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu HE, MacGillavry HD, Frost NA, Blanpied TA. Multiple spatial and kinetic subpopulations of CaMKII in spines and dendrites as resolved by single-molecule tracking PALM. J Neurosci. 2014;34:7600–7610. doi: 10.1523/JNEUROSCI.4364-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, Lippincott-Schwartz J. A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol. 2011;13:371–381. doi: 10.1038/ncb2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Q, Zhang X-F, Pollard TD, Forscher P. Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J Cell Biol. 2012;197:939–956. doi: 10.1083/jcb.201111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reymann A-C, Boujemaa-Paterski R, Martiel J-L, Guérin C, Cao W, Chin HF, De La Cruz EM, Théry M, Blanchoin L. Actin network architecture can determine myosin motor activity. Science. 2012;336:1310–1314. doi: 10.1126/science.1221708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burnette DT, Shao L, Ott C, Pasapera AM, Fischer RS, Baird MA, Der Loughian C, Delanoe-Ayari H, Paszek MJ, Davidson MW, Betzig E, Lippincott-Schwartz J. A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J Cell Biol. 2014;205:83–96. doi: 10.1083/jcb.201311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ryu J, Liu L, Wong TP, Wu DC, Burette A, Weinberg R, Wang YT, Sheng M. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 167.Hodges JL, Newell-Litwa K, Asmussen H, Vicente-Manzanares M, Horwitz AR. Myosin IIb activity and phosphorylation status determines dendritic spine and post-synaptic density morphology. PLoS ONE. 2011;6:e24149. doi: 10.1371/journal.pone.0024149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rubio MDM, Johnson R, Miller CA, Huganir RL, Rumbaugh G. Regulation of synapse structure and function by distinct myosin II motors. J Neurosci. 2011;31:1448–1460. doi: 10.1523/JNEUROSCI.3294-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, Lynch G, Rumbaugh G. Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron. 2010;67:603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koskinen M, Bertling E, Hotulainen R, Tanhuanpää K, Hotulainen P. Myosin IIb controls actin dynamics underlying the dendritic spine maturation. Mol Cell Neurosci. 2014;61:56–64. doi: 10.1016/j.mcn.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 171.Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24:2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haeckel A, Ahuja R, Gundelfinger ED, Qualmann B, Kessels MM. The actin-binding protein Abp1 controls dendritic spine morphology and is important for spine head and synapse formation. J Neurosci. 2008;28:10031–10044. doi: 10.1523/JNEUROSCI.0336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J Neurosci. 2009;29:1017–1033. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Korkotian E, Frotscher M, Segal M. Synaptopodin regulates spine plasticity: mediation by calcium stores. J Neurosci. 2014;34:11641–11651. doi: 10.1523/JNEUROSCI.0381-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cueille N, Blanc CT, Popa-Nita S, Kasas S, Catsicas S, Dietler G, Riederer BM. Characterization of MAP1B heavy chain interaction with actin. Brain Res Bull. 2007;71:610–618. doi: 10.1016/j.brainresbull.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 176.Tortosa E, Montenegro-Venegas C, Benoist M, Hartel S, Gonzalez-Billault C, Esteban JA, Avila J. Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J Biol Chem. 2011;286:40638–40648. doi: 10.1074/jbc.M111.271320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saneyoshi T, Hayashi Y. The Ca(2+) and Rho GTPase signaling pathways underlying activity-dependent actin remodeling at dendritic spines. Cytoskeleton (Hoboken) 2012;69:545–554. doi: 10.1002/cm.21037. [DOI] [PubMed] [Google Scholar]
- 178.Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. doi: 10.1038/nrn2191. [DOI] [PubMed] [Google Scholar]
- 179.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 180.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 181.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fleming IN, Elliott CM, Buchanan FG, Downes CP, Exton JH. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem. 1999;274:12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- 184.Okabe T, Nakamura T, Nishimura YN, Kohu K, Ohwada S, Morishita Y, Akiyama T. RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-d-aspartate receptor signaling. J Biol Chem. 2003;278:9920–9927. doi: 10.1074/jbc.M208872200. [DOI] [PubMed] [Google Scholar]
- 185.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 186.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grossman SD, Futter M, Snyder GL, Allen PB, Nairn AC, Greengard P, Hsieh-Wilson LC. Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. J Neurochem. 2004;90:317–324. doi: 10.1111/j.1471-4159.2004.02491.x. [DOI] [PubMed] [Google Scholar]
- 188.Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu G-Y, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47:85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 189.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wayman GA, Lee Y-S, Tokumitsu H, Silva AJ, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 193.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 194.Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, Umetani J, Billadeau DD, Otwinowski Z, Rosen MK. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 196.Rane CK, Minden A. P21 activated kinases: structure, regulation, and functions. Small GTPases. 2014;5:37–41. doi: 10.4161/sgtp.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/S0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 199.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 200.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rocca DL, Amici M, Antoniou A, Blanco Suarez E, Halemani N, Murk K, McGarvey J, Jaafari N, Mellor JR, Collingridge GL, Hanley JG. The small GTPase Arf1 modulates Arp2/3-mediated actin polymerization via PICK1 to regulate synaptic plasticity. Neuron. 2013;79:293–307. doi: 10.1016/j.neuron.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rocca DL, Hanley JG. PICK1 links AMPA receptor stimulation to Cdc42. Neurosci Lett. 2015;585:155–159. doi: 10.1016/j.neulet.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee SR, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Van Harreveld A, Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exp Neurol. 1975;49:736–749. doi: 10.1016/0014-4886(75)90055-2. [DOI] [PubMed] [Google Scholar]
- 207.Fifková E, Anderson CL. Stimulation-induced changes in dimensions of stalks of dendritic spines in the dentate molecular layer. Exp Neurol. 1981;74:621–627. doi: 10.1016/0014-4886(81)90197-7. [DOI] [PubMed] [Google Scholar]
- 208.Desmond N, Levy W. Changes in the postsynaptic density with long-term potentiation in the dentate gyrus. J Comp Neurol. 1986;253:476–482. doi: 10.1002/cne.902530405. [DOI] [PubMed] [Google Scholar]
- 209.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/S0896-6273(03)00206-X. [DOI] [PubMed] [Google Scholar]
- 212.Rochefort NL, Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yamagata Y, Kobayashi S, Umeda T, Inoue A, Sakagami H, Fukaya M, Watanabe M, Hatanaka N, Totsuka M, Yagi T, Obata K, Imoto K, Yanagawa Y, Manabe T, Okabe S. Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning. J Neurosci. 2009;29:7607–7618. doi: 10.1523/JNEUROSCI.0707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 216.Durand CM, Perroy J, Loll F, Perrais D, Fagni L, Bourgeron T, Montcouquiol M, Sans N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol Psychiatry. 2012;17:71–84. doi: 10.1038/mp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 218.Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang Y, Wang X-B, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30:11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tai C-Y, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 222.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng L-H, Colman DR, Miki N. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang X, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.He K, Lee A, Song L, Kanold PO, Lee H-K. AMPA receptor subunit GluR1 (GluA1) serine-845 site is involved in synaptic depression but not in spine shrinkage associated with chemical long-term depression. J Neurophysiol. 2011;105:1897–1907. doi: 10.1152/jn.00913.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GCR, Matsuzaki M, Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat Neurosci. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sdrulla AD, Linden DJ. Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci. 2007;10:546–548. doi: 10.1038/nn1889. [DOI] [PubMed] [Google Scholar]
- 230.Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ramachandran B, Frey JU. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. J Neurosci. 2009;29:12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J Neurosci. 2005;25:6641–6650. doi: 10.1523/JNEUROSCI.0028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Asrar S, Meng Y, Zhou Z, Todorovski Z, Huang WW, Jia Z. Regulation of hippocampal long-term potentiation by p21-activated protein kinase 1 (PAK1) Neuropharmacology. 2009;56:73–80. doi: 10.1016/j.neuropharm.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 237.Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM, McConnell SK, Palmer TD. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol Cell Neurosci. 2009;41:409–419. doi: 10.1016/j.mcn.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Görlich A, Sassoè-Pognetto M, Al Banchaabouchi M, Giustetto M, Triller A, Choquet D, Witke W. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kang J, Park H, Kim E. IRSp53/BAIAP2 in dendritic spine development, NMDA receptor regulation, and psychiatric disorders. Neuropharmacology. 2016;100:27–39. doi: 10.1016/j.neuropharm.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 240.Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–898. doi: 10.1016/S0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 241.Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.De Rubeis S, Pasciuto E, Li KW, Fernández E, Di Marino D, Buzzi A, Ostroff LE, Klann E, Zwartkruis FJT, Komiyama NH, Grant SGN, Poujol C, Choquet D, Achsel T, Posthuma D, Smit AB, Bagni C. CYFIP1 coordinates mRNA translation and cytoskeleton remodeling to ensure proper dendritic spine formation. Neuron. 2013;79:1169–1182. doi: 10.1016/j.neuron.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 244.Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MVL, Zukin RS. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans. 2009;37:1369–1374. doi: 10.1042/BST0371369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 246.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol. 2012;22:453–460. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 248.Ladépêche L, Dupuis JP, Groc L. Surface trafficking of NMDA receptors: gathering from a partner to another. Semin Cell Dev Biol. 2014;27:3–13. doi: 10.1016/j.semcdb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 249.Hanley JG. Actin-dependent mechanisms in AMPA receptor trafficking. Front Cell Neurosci. 2014;8:1–8. doi: 10.3389/fncel.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Soltau M, Richter D, Kreienkamp H. The insulin receptor substrate IRSp53 links postsynaptic shank1 to the small G-protein cdc42. Mol Cell Neurosci. 2002;21:575–583. doi: 10.1006/mcne.2002.1201. [DOI] [PubMed] [Google Scholar]
- 251.Park E, Na M, Choi J, Kim S, Lee J-R, Yoon J, Park D, Sheng M, Kim E. The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the beta PIX guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem. 2003;278:19220–19229. doi: 10.1074/jbc.M301052200. [DOI] [PubMed] [Google Scholar]
- 252.Soltau M, Berhörster K, Kindler S, Buck F, Richter D, Kreienkamp H-J. Insulin receptor substrate of 53 kDa links postsynaptic shank to PSD-95. J Neurochem. 2004;90:659–665. doi: 10.1111/j.1471-4159.2004.02523.x. [DOI] [PubMed] [Google Scholar]
- 253.Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J Neurosci. 2006;26:7693–7706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kerr JM, Blanpied TA. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J Neurosci. 2012;32:658–673. doi: 10.1523/JNEUROSCI.2927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Renner M, Choquet D, Triller A. Control of the postsynaptic membrane viscosity. J Neurosci. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Czondor K, Mondin M, Garcia M, Heine M, Frischknecht R, Choquet D, Sibarita J-B, Thoumine OR. Unified quantitative model of AMPA receptor trafficking at synapses. Proc Natl Acad Sci USA. 2012;109:3522–3527. doi: 10.1073/pnas.1109818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kusters R, Kapitein LC, Hoogenraad CC, Storm C. Shape-induced asymmetric diffusion in dendritic spines allows efficient synaptic AMPA receptor trapping. Biophys J. 2013;105:2743–2750. doi: 10.1016/j.bpj.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J Biol Chem. 2008;283:10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang Y, Wang X-B, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci. 2013;70:2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 266.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jullie D, Choquet D, Perrais D. Recycling endosomes undergo rapid closure of a fusion pore on exocytosis in neuronal dendrites. J Neurosci. 2014;34:11106–11118. doi: 10.1523/JNEUROSCI.0799-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JTR. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kelly EE, Horgan CP, McCaffrey MW, Young P. The role of endosomal-recycling in long-term potentiation. Cell Mol Life Sci. 2011;68:185–194. doi: 10.1007/s00018-010-0516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang Z, Edwards JG, Riley N, Provance DW, Karcher R, Li X-D, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Huber K, Kayser M, Bear M. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 273.Nosyreva E, Huber K. Developmental Switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tanaka J-I, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GCR, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Holbro N, Grunditz Å, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci USA. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Govindarajan A, Israely I, Huang S-Y, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ramiro-Cortés Y, Israely I. Long lasting protein synthesis- and activity-dependent spine shrinkage and elimination after synaptic depression. PLoS ONE. 2013;8:e71155. doi: 10.1371/journal.pone.0071155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lynch G, Kramár EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. doi: 10.1016/j.neuropharm.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Redondo RL, Okuno H, Spooner PA, Frenguelli BG, Bito H, Morris RGM. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30:4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 281.Tam J, Cordier GA, Bálint Š, Sandoval Álvarez Á, Borbely JS, Lakadamyali M. A microfluidic platform for correlative live-cell and super-resolution microscopy. PLoS ONE. 2014;9:e115512. doi: 10.1371/journal.pone.0115512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jungmann R, Steinhauer C, Scheible M, Kuzyk A, Tinnefeld P, Simmel FC. Single-molecule kinetics and super-resolution microscopy by fluorescence imaging of transient binding on DNA origami. Nano Lett. 2010;10:4756–4761. doi: 10.1021/nl103427w. [DOI] [PubMed] [Google Scholar]
- 283.Jungmann R, Avendaño MS, Woehrstein JB, Dai M, Shih WM, Yin P. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat Methods. 2014;11:313–318. doi: 10.1038/nmeth.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tam J, Cordier GA, Borbely JS, Sandoval Álvarez Á, Lakadamyali M. Cross-talk-free multi-color STORM imaging using a single fluorophore. PLoS ONE. 2014;9:e101772. doi: 10.1371/journal.pone.0101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhang Z, Kenny SJ, Hauser M, Li W, Xu K. Ultrahigh-throughput resolved super-resolution microscopy. Nat Methods. 2015 doi: 10.1038/nmeth.3528. [DOI] [PubMed] [Google Scholar]
- 286.Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 287.Xu K, Babcock HP, Zhuang X. Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat Methods. 2012;9:185–188. doi: 10.1038/nmeth.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Galland R, Grenci G, Aravind A, Viasnoff V, Studer V, Sibarita J-B. 3D high- and super-resolution imaging using single-objective SPIM. Nat Methods. 2015;12:641–644. doi: 10.1038/nmeth.3402. [DOI] [PubMed] [Google Scholar]
- 289.Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Vangindertael J, Beets I, Rocha S, Dedecker P, Schoofs L, Vanhoorelbeeke K, Hofkens J, Mizuno H. Super-resolution mapping of glutamate receptors in C. elegans by confocal correlated PALM. Sci Rep. 2015;5:13532. doi: 10.1038/srep13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Urban NT, Willig KI, Hell SW, Nägerl UV. STED nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys J. 2011;101:1277–1284. doi: 10.1016/j.bpj.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hofmann M, Eggeling C, Jakobs S, Hell SW. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc Natl Acad Sci USA. 2005;102:17565–17569. doi: 10.1073/pnas.0506010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Testa I, Urban NT, Jakobs S, Eggeling C, Willig KI, Hell SW. Nanoscopy of living brain slices with low light levels. Neuron. 2012;75:992–1000. doi: 10.1016/j.neuron.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 294.Kner P, Chhun BB, Griffis ER, Winoto L, Gustafsson MGL. Super-resolution video microscopy of live cells by structured illumination. Nat Methods. 2009;6:339–342. doi: 10.1038/nmeth.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shao L, Kner P, Rego EH, Gustafsson MGL. Super-resolution 3D microscopy of live whole cells using structured illumination. Nat Methods. 2011;8:1044–1046. doi: 10.1038/nmeth.1734. [DOI] [PubMed] [Google Scholar]
- 296.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10:413–420. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- 297.Chen B-C, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA, 3rd, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Reymann A-C, Bembenek JN, Bohme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E, Reymann A-C, Bohme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science. 2014;346(6208):1257998. doi: 10.1126/science.1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Li D, Shao L, Chen B, Zhang X, Zhang M, Moses B, Milkie DE, Beach JR, Hammer J, Pasham M, Kirchhausen T, Baird MA, Davidson MW, Xu P, Betzig E. ADVANCED IMAGING. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science. 2015;349(6251):aab3500. doi: 10.1126/science.aab3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Keller PJ, Ahrens MB. Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy. Neuron. 2015;85:462–483. doi: 10.1016/j.neuron.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 300.Benson DL, Huntley GW. Synapse adhesion: a dynamic equilibrium conferring stability and flexibility. Curr Opin Neurobiol. 2012;22:397–404. doi: 10.1016/j.conb.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.van Bergeijk P, Adrian M, Hoogenraad CC, Kapitein LC. Optogenetic control of organelle transport and positioning. Nature. 2015;518:111–114. doi: 10.1038/nature14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, Tucker CL. An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun. 2014;5:4925. doi: 10.1038/ncomms5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 304.Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, Kahou GAA, Berger TK, Bilgili A, Buncic N, Chalimourda A, Chindemi G, Courcol J-D, Delalondre F, Delattre V, Druckmann S, Dumusc R, Dynes J, Eilemann S, Gal E, Gevaert ME, Ghobril J-P, Gidon A, Graham JW, Gupta A, Haenel V, Hay E, Heinis T, Hernando JB, Hines M, Kanari L, Keller D, Kenyon J, Khazen G, Kim Y, King JG, Kisvarday Z, Kumbhar P, Lasserre S, Le Bé J-V, Magalhães BRC, Merchán-Pérez A, Meystre J, Morrice BR, Muller J, Muñoz-Céspedes A, Muralidhar S, Muthurasa K, Nachbaur D, Newton TH, Nolte M, Ovcharenko A, Palacios J, Pastor L, Perin R, Ranjan R, Riachi I, Rodríguez J-R, Riquelme JL, Rössert C, Sfyrakis K, Shi Y, Shillcock JC, Silberberg G, Silva R, Tauheed F, Telefont M, Toledo-Rodriguez M, Tränkler T, Van Geit W, Díaz JV, Walker R, Wang Y, Zaninetta SM, DeFelipe J, Hill SL, Segev I, Schürmann F. Reconstruction and simulation of neocortical microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]