Abstract
Autophagy is a lysosome-dependent mechanism of intracellular degradation. The cellular and molecular mechanisms underlying this process are highly complex and involve multiple proteins, including the kinases ULK1 and Vps34. The main function of autophagy is the maintenance of cell survival when modifications occur in the cellular environment. During the past decade, extensive studies have greatly improved our knowledge and autophagy has exploded as a research field. This process is now widely implicated in pathophysiological processes such as cancer, metabolic, and neurodegenerative disorders, making it an attractive target for drug discovery. In this review, we will summarize the different types of inhibitors that affect the autophagy machinery and provide some potential therapeutic perspectives.
Keywords: Autophagy, Autophagosomes, PI3K, PIK3C3/Vps34, ULK, Lysosomotropic agent, Hydroxychloroquine
Introduction
The maintenance of cellular homeostasis is continuously challenged by abrupt or long-lasting changes in the environment. In order to adapt, cells have to modify their anabolic and catabolic processes. Autophagy and the proteasome constitute the two universal intracellular catabolic pathways. The term autophagy, derived from the Greek word for “self-eating”, was coined by Christian de Duve to account for observations of vesicles containing components of the cytoplasm in various degrees of disintegration [1]. This was after his discovery of the lysosome in 1955, which won him the Nobel Prize in 1974 [2]. In contrast to the proteasome which allows the direct degradation of intracytoplasmic proteins, the process of autophagy enables cells to degrade proteins, protein complexes and organelles via a lysosome-dependent mechanism. Present in all eukaryotic cells, three different types of autophagy can be distinguished: macroautophagy, microautophagy and chaperone-mediated autophagy, that differ in their mechanisms and functions [3]. Macroautophagy (hereafter referred to as autophagy) is the most characterized form and will be the subject of this review. Readers interested by microautophagy and chaperone-mediated autophagy should consult other reviews [4, 5].
Autophagy machinery
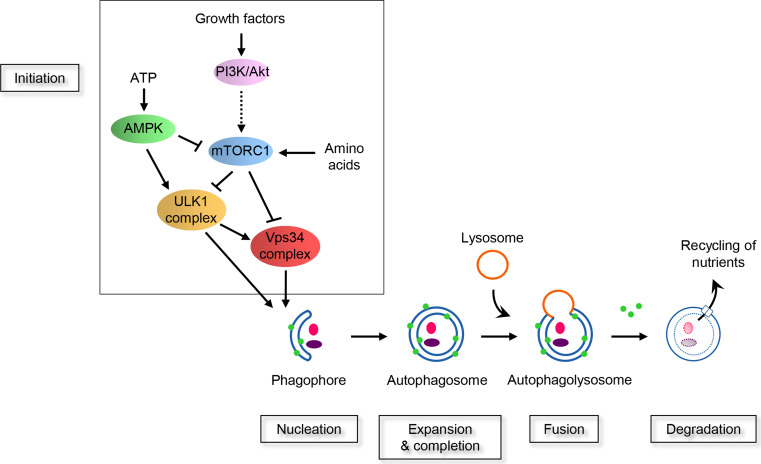
Autophagy is characterized by the formation of double-membrane vesicles called autophagosomes within the cytoplasm, which engulf bulk cytosol or organelles. Autophagosomes then fuse with the lysosomal system converting them into autolysosomes. The content of the autophagosomes is therefore degraded by lysosomal hydrolases and the metabolites generated as a result of autophagy are reused either as sources of energy or building blocks for the synthesis of new macromolecules (Fig. 1). The origin of the autophagosomal membrane that forms the phagophore is still not well understood, but the endoplasmic reticulum seems to be one of the main components of nascent autophagosomes [6, 7].
Fig. 1.
Schematic representation of the signalling pathways that control autophagy machinery. Autophagy is regulated by various growth and nutrient signalling. Growth factors activate the class I PI3K/Akt signalling pathway and mTOR complex 1 (mTORC1). In this condition, mTORC1 negatively regulated the two key kinase complexes, Vps34 and ULK1, leading to a prevention of autophagy. Under growth factors starvation, the activity of mTORC1 is suppressed, releasing the inhibition of Vps34 and ULK1 complexes. mTORC1 can also sense the cellular concentration of amino acids. High amino acids concentration positively affects mTORC1. When a decrease in amino acids occurs, mTORC1 is inhibited. This leads to Vps34 and ULK1 complexes activation. The level of ATP is detected by the kinase AMPK that negatively regulate mTORC1 and positively affect ULK1. In case of a decrease in ATP, AMPK is activated and this results in mTORC1 inhibition and activation of ULK1 complex allowing the induction of the autophagy machinery. The first step of the autophagy machinery is the formation of a double membrane named phagophore. This membrane then expands and forms the autophagosome. This vesicle sequesters complex proteins and organelles and is characterized by the presence of LC3 (green circles) attached at their membranes. Autophagosomes finally fuse with lysosomes and their content is degraded by the lysosomal hydrolases. The resulting metabolites are transported into the cytoplasm and used for the synthesis of new macromolecules or as a source of energy
The machinery of autophagy is tightly regulated by complex signaling pathways and core autophagy proteins. The identification of the autophagy-related (ATG) genes, using genetic screens for autophagy-defective mutants in yeast, was historically a major achievement for the field [8]. More than 36 ATG genes have been identified in yeast, and most of them are evolutionarily conserved. The hierarchy and the recruitment of ATG proteins have been reviewed in mammals [9, 10]. Proteins involved in the autophagy machinery, including ATG proteins, can be categorized into six functional groups that cooperate to allow the formation of autophagosomes: (1) UNC-51-like kinase 1 (ULK1) kinase complex; (2) PIK3C3, the class III phosphatidylinositol 3-kinase (PtdIns3K), also known and hereafter named vacuolar protein sorting 34 (Vps34), kinase complex; (3) FYVE or PX domain-containing proteins that bind to PtdIns 3-phosphate (PtdIns3P); (4) the ATG5-12 ubiquitin-like conjugation system; (5) the microtubule-associated protein 1-light chain 3 (LC3) phosphatidylethanolamine conjugation system; and (6) ATG9a, the only transmembrane protein among the ATG proteins [7, 11, 12].
Autophagy signaling pathways
The signaling pathways upstream of the autophagy machinery are complex and involve multiple mediators. The best characterized regulator of autophagy is the mammalian target of rapamycin (mTOR), also known to control cellular homeostasis. Indeed, as a serine/threonine kinase, mTOR can sense multiple stimuli including the presence of growth factors, nutrient and energy levels and stress in order to maintain cellular metabolism and growth. At the molecular level, mTOR integrates the signaling of many upstream pathways such as for example the phosphoinositide 3-kinase (PI3K)-Akt and AMP-activated kinase (AMPK) pathways [13]. Of note, the complex 1 of mTOR (mTORC1) acts as a major checkpoint in signaling pathways regulating autophagy (Fig. 1), whereas mTORC2 is not a direct autophagy regulator [14]. Activated mTORC1 downregulates autophagy by preventing the activity of ULK1 and Vps34 complexes [15–18]. During cell starvation, i.e. when the amino acids or growth factors concentration in the cell environment drops, mTORC1 is inhibited. This releases its break on the two downstream ULK1 and Vps34 complexes and as a consequence allows the nucleation of autophagosomes (Fig. 1).
ULK1 is a serine/threonine protein kinase that exists within the cells as protein complexes with Atg13, FIP200 (FAK family kinase-interacting protein of 200 kDa) and Atg101. This complex is regulated by the energy sensor AMPK (Fig. 1). One of the substrates of the ULK1 complex is the Vps34 complex [19–24].
Vps34 is a lipid kinase that converts phosphatidylinositol (PtdIns) to phosphatidylinositol 3-phosphate (PtdIns3P) [25–27]. Of note the pool of PtdIns3P is tightly regulated by phosphatases such as the two myotubularins, MTMR3 and MTMR14 (also called Jumpy) and the dual-domain protein tyrosine phosphatase σ (PTPσ) [28, 29]. The formation of PtdIns3P by the Vps34 complex recruits downstream proteins containing either a FYVE (Fab1, YOTB, Vac1 and EEA1) zinc-finger domain or a PX (Phox homology) domain such as double FYVE-containing protein 1 (DFCP1) and WD-repeat domain phosphoinositide-interacting protein (WIPI). Subsequently, an expansion phase of the phagophore occurs with the recruitment of the two ubiquitin-like conjugation system, the ATG12-ATG5 complex and the LC3 complex, which ends with the formation of an autophagosome [7, 11].
Autophagy function in physiology
Autophagy occurs under many physiological conditions. Notably it has been demonstrated that autophagy is critical during mammalian development and also during the period of nutrient deprivation immediately after birth [30, 31]. At the cellular level, autophagy is crucial in removing unfolded proteins and damaged or superfluous organelles such as mitochondria, peroxisomes, ribosomes, endoplasmic reticulum, or endosomes, and therefore acts as a “quality control” process [32]. Under starvation and stress, autophagy is dramatically induced to recycle cellular components and to supply the building blocks so that cellular homeostasis is maintained. Autophagy is thus viewed as a cell survival process in response to stress.
Autophagy and diseases
Dysregulated autophagy is implicated in numerous pathophysiological processes such as neurodegenerative diseases, infectious or metabolic diseases and cancer [33–37]. In the case of cancer, most of the compounds in the clinic cause cellular stress and activate autophagy after drug treatment in vitro [38]. Even if it was originally proposed that autophagic cell death is part of the mechanism of anticancer drugs, the emerging picture is that the increased autophagy seen during anticancer drug treatment could be a survival response of the dying cells, rather than a cause of cell death. One illustration of this concept was highlighted using a chemical screening study with 59 compounds that induced both autophagy and cell death. It was found that one of the ATG proteins, ATG7, when knockdown prevented autophagy, but not cell death. This study led the authors to conclude that “cell death is rarely, if ever, executed by autophagy in human cells” [39, 40].
Autophagy inhibitors
Given its potential roles in many diseases, various preclinical studies have been undertaken to develop therapeutic agents targeting autophagy. However, the field had to face major challenges because autophagy is a dynamic process that is difficult to measure and quantify. For example, the accumulation of autophagosomes does not necessarily demonstrate an increase in autophagy by itself, but could simply reflect that autophagy is blocked at a late stage [41]. The recommendations on relevant assays for investigating autophagy are published elsewhere [42]. One of the widely used readout for measuring autophagy is LC3. Most researchers use a chimeric protein of LC3 conjugated to a fluorescent protein such as Green Fluorescent Protein (GFP). This probe, when transfected into cell lines, is a useful tool to measure autophagy and to perform cell-based screens.
Different compounds are described in the literature as potential inhibitors of the autophagy process. However, most of them are poorly selective, limiting their application. This review will be divided into two parts and will focus on the best characterized autophagy inhibitors (Table 1). The first part will cover the proximal inhibitors, targeting druggable proteins or pathways involved in the initial steps of the core autophagy machinery. The second part will present the late stage inhibitors of autophagy which target the functions of lysosomes.
Table 1.
List of the compounds discussed in the article
| Target | Inhibitor | References |
|---|---|---|
| PI3K | 3-methyladenine | [45–48] |
| Wortmannin | [48, 52–54] | |
| LY294002 | [47, 54, 58] | |
| PT210 | [60] | |
| GSK-2126458 | [63] | |
| Vps34 | Spautin-1 | [79] |
| SAR405 | [82] | |
| Compound 31 | [84] | |
| VPS34-IN1 | [85] | |
| PIK-III | [86] | |
| ULK | Compound 6 | [98] |
| MRT68921 | [100] | |
| SBI-0206965 | [24] | |
| Proteases | Pepstatin A | [101, 103] |
| E64d | [101, 103] | |
| V-ATPase | Bafilomycin A1 | [105–107] |
| Lysosomes | Clomipramine | [108] |
| Lucanthone | [109] | |
| Chloroquine | [110–112, 118–124] | |
| Hydroxychlorquine | [110–112, 118–124] | |
| Lys05 | [127] | |
| ARN5187 | [128] | |
| Compound 30 | [129] |
Pan-PI3K inhibitors
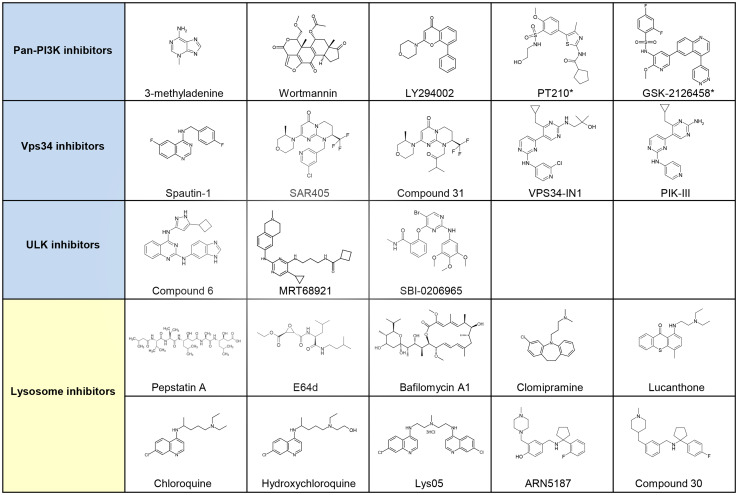
Phosphoinositide 3-kinases (PI3Ks) are divided into class I, class II and class III, and encompass eight isoforms. The class III isoform, corresponding to Vps34, activates the autophagy machinery, while the class I PI3K triggers mTOR signaling pathway and inhibits autophagy. The contribution of class II PI3K activity on autophagy is unclear [25, 27, 43, 44]. A group of pan-PI3K inhibitors, including 3-methyladenine (3-MA), Wortmannin and LY294002 have been used as autophagy inhibitors. The following section will present the knowledge on these three compounds as well as two additional novel pan-PI3K inhibitors, PT210 and GSK-2126458 (Fig. 2).
Fig. 2.
Selected small molecule inhibitors of autophagy. Autophagy inhibitors can be classified as early stage inhibitors (in blue) and late stage inhibitors (in yellow) of the autophagy machinery. The early stage inhibitors encompass the Pan-PI3K, Vps34 and ULK inhibitors. The second category corresponds to compounds that affect the function of lysosomes. All of these compounds are at the preclinical phase except for GSK-2126458, which is being evaluated in clinical trials. Clomipramine, chloroquine and hydroxychloroquine have been FDA-approved. Compounds with a asterisk correspond to putative autophagy inhibitors, as no report clearly demonstrate their inhibitory effect on autophagy
3-Methyladenine
3-MA was first discovered via the screening of purine-related substances using isolated hepatocytes from starved rats [45], and is the most widely used autophagy inhibitor [46]. One of the targets for 3-MA is Vps34 [47], but this compound also inhibits the activity of class I PI3K, and, as a consequence, exerts a dual effect on autophagy. It was described that 3-MA not only suppresses starvation-induced autophagy, but it can also promote autophagic flux when administrated under nutrient-rich conditions with a prolonged period of treatment [48]. This pan-PI3K inhibitor has a low potency, requiring its use at ~10 mM concentrations to prevent autophagy in vitro on cellular assays. At such high concentrations, it is known that 3-MA can also affect other kinases, including class I PI3K as mentioned earlier, p38MAPK or c-Jun kinase. With such off-target activities, it is recognized that this compound affects many cellular processes such as glycogen metabolism, lysosomal acidification, endocytosis, and mitochondrial permeability transition [49]. Another caveat of this compound is its poor solubility. To improve the solubility and also the potency of 3-MA, a library of 29 derivatives was synthetized [50]. While the authors described three novel 3-MA derivatives with improved solubility, the potency of these compounds on autophagy readout remains limited with IC50s between 18 and 670 µM [50].
Wortmannin
Wortmannin, a mold metabolite initially described as having anti-inflammatory activities [51], is a highly potent pan-PI3K inhibitor with IC50s from 10 to 50 nM for PI3K classes I, II and III [52, 53]. Wortmannin seems to exert persistent effects on Vps34 while it inhibits transiently the class I PI3K regardless of the nutrient conditions [48, 54]. It is known that Wortmannin achieves its inhibitory effect via covalent irreversible binding [52, 55]. However, it also has inhibitory effects on phylogenetically related kinases such as mTOR, DNA-dependent protein kinase (DNA-PK) or ataxia telangiectasia mutated (ATM) protein kinase [56, 57].
LY294002
LY294002 is the first synthetic inhibitor of PI3K and it inhibits autophagy on cellular assays [47, 54, 58]. This compound has limited potency (IC50s at the µM level) for the class I PI3K, Vps34, and affects mTOR and DNA-PK [59].
Although the three inhibitors described above block autophagy, they have limited potency (excepted for Wortmannin) and have off-target effects on different related lipid and protein kinases (Fig. 3). Moreover, these compounds are not fully characterized for their pharmacological properties such as solubility, stability, ADME or pharmacokinetic (PK) parameters. Therefore great caution needs to be exercised when analyzing data with these inhibitors in preclinical studies and on in vivo experiments.
Fig. 3.
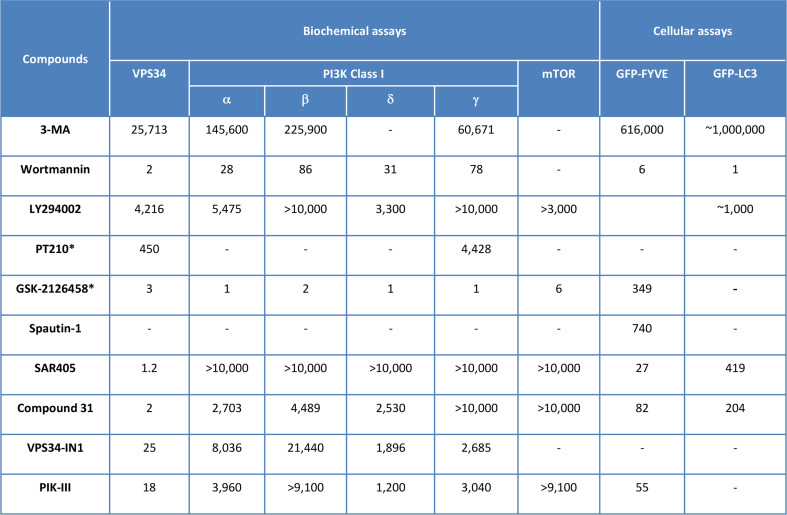
In vitro profiling of pan-PI3K and Vps34 inhibitors. The activity of the compounds in biochemical assays and recombinant enzymes are indicated. Results of the compounds on the inhibition of Vps34 target dependent cellular assay (GFP-FYVE transfected cells) and of autophagy cellular assay (GFP-LC3 transfected cells) are also mentioned in this table. The data correspond to IC50s (nM) determination and are from [55, 60, 79, 82, 84–86]. Compounds with a asterisk correspond to putative autophagy inhibitors, as no report clearly demonstrate their inhibitory effect on autophagy
Novel pan-PI3K inhibitors
PT210
Using the structure of Drosophila Vps34 in complex with pan-PI3K inhibitors, the group of RL. Williams performed structure-based design to identify compounds selective for Vps34 versus the isoform γ of the class I PI3K [60]. In particular, starting from PIK-93, another pan-PI3K inhibitor [61], the authors were able to select PT210 which shows a tenfold in vitro selectivity ratio between Vps34 (IC50 = 450 nM) and the isoform γ of the class I PI3K (IC50 = 4.4 µM) (Fig. 3). The effect of PT210 on autophagy is yet to be demonstrated.
GSK-2126458
The PI3K/Akt signaling pathway has been extensively investigated during the last 20 years, and oncogene activation or tumor suppressor gene inactivation has been described in this pathway. As a consequence, numerous novel pan-PI3K inhibitors have been developed by pharmaceutical industries as targeted therapies for cancer. These molecules demonstrate improved drug-like properties that led to many clinical trials [62]. Among the pan-PI3K compounds in clinical studies, GSK-2126458 [63], developed as an inhibitor for the class I PI3K and mTOR, is also highly active on Vps34 using biochemical assays and recombinant proteins (Fig. 3). To the best of our knowledge, no report was available on the regulation of autophagy by GSK-2126458. With such a pan-PI3K/mTOR/Vps34 profile, GSK-2126458 could, in theory, both activate and inhibit autophagy. While it will be challenging to demonstrate the inhibition of autophagy after treatment with GSK-2126458, the results from clinical trials could confirm the therapeutic advantage of this inhibitor.
Vps34 inhibitors
Overview of Vps34
Vps34, the most ancient paralog of the PI3Ks, was originally identified in S. cerevisiae as a protein involved in endosomal sorting of proteins towards the vacuole, the yeast equivalent of the mammalian lysosome [64]. It is now recognized that Vps34 is involved in a broad range of vesicular trafficking pathways [26, 27]. This includes not only autophagy [65, 66], but also the endosomal trafficking of receptors, for example the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR) or Transferrin receptor [67, 68]. The molecular explanation behind these multiple pathways comes from the fact that Vps34 is active within complexes with several accessory proteins [25, 69]. At least two distinct complexes are described, both encompass Vps34, Vps15, Beclin-1 plus either Atg14L/Barkor or UVRAG. Atg14L-containing Vps34 complex is localized in autophagosomal structures and participates in the generation of autophagosomes, while the UVRAG-containing Vps34 complex is found at the endosomes [25, 69]. Another dimension for the functions associated to Vps34 come from the multitude of PtdIns3P-binding proteins, including phosphatases, kinases and adaptor proteins that are present within the cells [26].
Target credentialing of Vps34 in the autophagy process
During the last 50 years, different in vitro studies have validated Vps34 as a key target for autophagy using antisense oligonucleotides or siRNA [47, 70, 71]. However, the recent results from Vps34 knockout mice have generated controversy about the role of Vps34 on the activation of autophagy in vivo. While two initial studies have shown that conditional knockout of Vps34 in sensory neurons and in T lymphocytes does not elicit an autophagy defect [72, 73], more recent results indicated that the deletion of Vps34 prevents the formation of autophagosomes [68, 74, 75]. In fact when looking at the constructs of the Vps34 knockout mice models, it appears that two different strategies were used, with the deletion of exon 17/18 and the targeting of exon 4, respectively. Whether this difference can explain the discrepancy observed on autophagy remains to be demonstrated.
Considering the key role of Vps34 in the autophagy machinery, many groups were interested in developing compounds targeting this lipid kinase. The following section will first present the characterization of an indirect Vps34 inhibitor, Spautin-1 and will then discuss the two recently described catalytic inhibitors of Vps34 (Fig. 2).
Spautin-1
4-[[3,4-(methylenedioxy)benzyl]amino]-6-chloroquinazoline (MBCQ) was originally characterized as a potent and selective inhibitor of phosphodiesterase type 5 (PDE5) [76, 77]. Using a cell-based screen with the GFP-LC3 cell line, this compound was able to inhibit autophagy [78, 79]. Derivatives of MBCQ were synthetized to improve the effect on autophagy and to decrease the off-target effect on PDE5 leading to the identification of the quinazoline derivative Spautin-1 (specific and potent inhibitor of autophagy 1) [79]. This compound does not affect the catalytic activity of Vps34, but promotes the degradation of Vps34 complexes. The exact mechanism of action of Spautin-1 was identified and it was demonstrated that it inhibits the ubiquitin-specific peptidases, USP10 and USP13 [79]. These two enzymes deubiquitinate the protein Beclin-1. Under glucose-free conditions, treatment with Spautin-1 indirectly promotes the ubiquitination of Beclin-1 and leads to its proteasomal degradation. The consequence of this is the destabilization and degradation of Vps34 complexes and an inhibition of autophagy. The IC50 of Spautin-1 in GFP-LC3 cellular assay is 740 nM. Moreover, this compound promotes the death of cancer cells under nutrient deprivation, when autophagy is activated, but not in nutrient-rich conditions. Spautin-1 also appears to be essentially non cytotoxic to normal cells. It could be interesting to explore whether Spautin-1 could also affect the endosomal trafficking of receptors. Preclinical studies using Spautin-1 showed a synergy with Imatinib, indicating a potential therapeutic application of this compound in chronic myeloid leukemia [80].
Catalytic inhibitors of Vps34
A major breakthrough in the field has been made with the very recent characterization of two series, the pyrimidinone and the bisaminopyrimidine, originated from compound libraries at Sanofi and Novartis, respectively.
Pyrimidinones
With the aim to screen for autophagy inhibitors in a physiologically relevant environment, HeLa cells stably expressing GFP-LC3 were employed in a cell image-based assay with a subset of our proprietary compound collection, consisting of ~250,000 compounds. The screen identified compounds with as yet unidentified targets, and chemical series active against autophagy-relevant biological enzymes, ULK and Vps34 in biochemical assays using recombinant enzymes [81]. The initial hit compounds displayed an activity on Vps34 in the nM range, but they showed a lack of selectivity regarding the class I PI3K. A chemical optimization program was initiated and allowed the identification of SAR405, a highly potent and selective inhibitor of Vps34. SAR405 has a binding equilibrium constant K D of 1.5 nM. In order to evaluate its activity on a dedicated Vps34 cellular assay, HeLa cells were transfected with a fluorescent probe, GFP-FYVE, that binds to PtdIns3P, the reaction product of Vps34. SAR405 completely inhibited the signal and an IC50 activity was measured at 27 nM. As compared to the pan-PI3K inhibitors, SAR405 presented an exquisitely selective profile with regards to class I, class II PI3K, mTOR and other lipid kinases (Fig. 3). Moreover, no activity was found when profiled on a panel of more than 200 protein kinases present in a cellular extract at 1 µM [82, 83]. Using SAR405 we showed that the catalytic activity of Vps34 is necessary for maintaining the size of late endosomes/lysosomes and the function of lysosomes during vesicle trafficking, thus confirming previous data with knockdown of Vps34 [67, 68]. We then demonstrated that SAR405 is able to inhibit autophagy induced by two different stimuli, starvation and treatment with a catalytic mTOR inhibitor AZD8055 [82, 83]. These results suggest that SAR405 might prevent the catalytic activity of the two Vps34 complexes, Atg14L- and UVRAG-containing Vps34 complexes. Finally we showed that adding the Vps34 inhibitor in combination with the FDA-approved mTOR inhibitor everolimus synergized to trigger antiproliferative activity in renal tumor cell lines, indicating a potential therapeutic application in renal cell carcinoma.
As SAR405 demonstrated an excellent Vps34-specific inhibitory profile, we went on to find compounds that were chemically optimized for in vivo applications. Compound 31 was then characterized as a Vps34 inhibitor for oral route with excellent in vitro ADME and PK properties in rats and mice. When tested in vivo, compound 31 induced a sustained target inhibition after a single administration at 100 mg/kg. The in vivo effective plasma concentration to achieve 50 % inhibition was estimated at 0.37 µM [84]. To our knowledge, this is the first report of an optimized Vps34 inhibitor that can be used to investigate the biology of this target, including autophagy.
Bisaminopyrimidines
The bisaminopyrimidine family is exemplified by VPS34-IN1 and PIK-III, with an activity of 25 and 18 nM, respectively, using biochemical assays and Vps34 recombinant enzyme [85, 86]. These two compounds display a highly selectivity profile when tested on a panel of protein kinases and lipid kinases at 1 µM or using dose–response experiments (Fig. 3). The group of D. Alessi demonstrated that Vps34 regulates the activity of serum- and glucocorticoid-regulated kinase-3 (SGK3), a PX-bearing domain protein, indicating that SGK3 phosphorylation can be used as a biomarker of Vps34 activity in vitro [85]. Unfortunately, the drug-like properties of VPS34-IN1 and PIK-III, including their in vitro ADME properties and in vivo target engagement, were not disclosed.
While no data concerning autophagy was provided with VPS34-IN1 [85], the effect of the PIK-III compound was well described using LC3 fluorescent readout and western blot [86]. It was shown that PIK-III inhibits the degradation of autophagy substrate receptors such as p62, NBR1 and NDP52 when autophagy is induced by the catalytic mTOR inhibitor AZD8055. In addition PIK-III can inhibit mitophagy, a subtype of autophagy that triggers the selective degradation of mitochondria. Using SILAC proteomics, the group of L. Murphy identified a novel molecule namely NCOA4 (nuclear receptor co-activator 4) that is increased following treatment with PIK-III. NCOA4 interacts with ferritin and is required for its degradation into autophagolysosomes after starvation. This study and the discovery of PIK-III allowed them to identify a new function for autophagy, the regulation of iron levels [86].
Collectively, the three catalytic inhibitors mentioned above illustrate the dual role of Vps34 in autophagy and in endosomal pathway. Rather than being considered as autophagy-specific inhibitors, these agents are powerful tools to dissect the role of Vps34 in these two processes.
ULK inhibitors
ULK1, present in higher eukaryotes, corresponds to Atg1 kinase in yeast. This kinase has four orthologues in humans: ULK2, ULK3, ULK4 and STK36 [87]. ULK1 function as the most upstream protein kinase in the autophagy machinery [9, 16].
Target credentialing of ULK in the autophagy process
Multiple evidences have validated ULK as a target for autophagy modulation [88–92]. In particular, in a siRNA screen with a library that targeted 753 human kinases, ULK1 was found as a key protein involved in autophagy [93]. Of note, a possible redundancy between ULK1 and ULK2 was described in mouse embryonic fibroblasts (MEFs) [94]. The ULK knockout mouse indicated that this kinase plays a role in mitophagy during erythroid differentiation [95], but also in axon growth, endocytosis and in lung development [96, 97].
Catalytic inhibitors of ULK
Because of its role in autophagy and its druggable properties, ULK has attracted attention for drug discovery. Three groups very recently disclosed the structures of potential ULK inhibitors (Fig. 2). Of note our group also identified hit compounds on ULK2 after the phenotypic screen on GFP-LC3 HeLa cells [81].
Compound 6
The initial aim of the group of KM. Shokat was to solve the structure of human ULK1. As they met some issues with the apo protein, they decided to look for a compound that could bind to ULK1 and increase its stability in order to facilitate its crystallization. A screening of 764 molecules against ULK1 was done using a 32P-ATP radioactive assay with myelin basic protein (MBP) as a generic substrate [98]. Using the enzymatic assay, they identified compound 6 as their best hit. This inhibitor displayed a high affinity with an IC50 on ULK1 of 8 nM. While the effect of this compound on autophagy was not depicted, the authors showed that it is not specific toward ULK1 when profiled in a small panel of protein kinases. Indeed compound 6 inhibited the activity of 37 kinases by more than 50 % at a concentration of 250 nM and is therefore not yet optimized for in vitro testing on cellular assays.
MRT68921
Starting from the TANK-binding kinase 1 (TBK1) inhibitor, MRT67307 (IC50 = 19 nM) [99], the group of IG. Ganley investigated its effect on kinases with similar catalytic domain [100]. They found that MRT67307 potently inhibits ULK1 and ULK2 (IC50 of 45 and 38 nM, respectively) using a similar biochemical assay as the one used by KM. Shokat. When looking at a closely related series of analogues generated during the original TBK1 screen, the authors selected MRT68921 as a more potent inhibitor for ULK. This compound displays a tenfold increased affinity as compared to MRT67307 on ULK1 and ULK2 (IC50 of 2.9 and 1.1 nM, respectively) [100]. However, when they investigated its potential off-target effect on a panel of 80 protein kinases, they found that 44 kinases were inhibited (<50 % activity remaining) by treatment of MRT68921 at 1 µM. Among them, TBK1 and AMPK, two kinases known to play a role in the autophagy process, were found. Using knockout MEF cells, it was demonstrated that MRT68921 was still capable of inhibiting autophagy in the absence of TBK1 and AMPK pathways. To rule out the fact that MRT68921 could inhibit autophagy through other kinases, the authors generated a drug-resistant ULK1 mutant. Using biochemical studies they selected the mutation of the gatekeeper residue of ULK1 methionine 92 to threonine (M92T) that retains kinase activity in the presence of MRT68921. It was shown that ULK1/2 double knockout MEF cells reconstituted with M92T ULK1 protein restored autophagy in the presence of MRT68921, while this compound was still able to inhibit autophagy in MEF cells reconstituted with wild type ULK1. This result demonstrates that MRT68921 inhibits autophagy specifically through ULK1.
SBI-0206965
The group of RJ. Shaw performed a small screen with analogues of a focal adhesion kinase (FAK) inhibitor [24]. SBI-0206965 was selected as the best ULK inhibitor (IC50 = 108 and 711 nM for ULK1 and ULK2, respectively). Having identified that the phosphorylation of Vps34 Ser249 is one of the reaction catalyzed by ULK1, they confirmed the activity of the compound on ULK1 using this biomarker in overexpressing Vps34 HEK293T cells. When profiled in a panel of 456 protein kinases at a concentration of 1 µM, this compound inhibited the activity of 33 kinases by more than 50 % [24]. Finally they investigated the effect of SBI-0206965 on the GFP-LC3 cell line. It was shown that this compound could prevent autophagy induced by the ATP-competitive mTOR kinase inhibitor AZD8055 [24]. Similar results were obtained with ULK1 siRNA suggesting that this target is essential under these experimental conditions [24]. One caveat of this recent study is that SBI-0206965 was used at 5 or 10 µM concentration on cellular assays. At such high concentrations and given the weak specificity of the compound, caution needs to be taken when interpreting the in vitro results with SBI-0206965.
As a conclusion, the three catalytic inhibitors of ULK1 mentioned above correspond to the very early stages of drug discovery. Although they demonstrate potency in biochemical assays (IC50s from 3 to 100 nM), their poor selectivity limits their use as chemical tools in vitro. Chemical optimization is needed for further deciphering the role of ULK in cellular processes, including autophagy, and also in human pathophysiologies using this class of inhibitors.
Late inhibitors
Another way to tackle autophagy is to act on the later stages of the autophagy process, the degradation of autophagosome content by the lysosomes (Fig. 1). In the following section we will briefly present the different category of drugs that were described as late inhibitor of autophagy in vitro. A main focus will be done on the lysosomotropic agents and their applications in cancer (Figs. 2, 4).
Fig. 4.
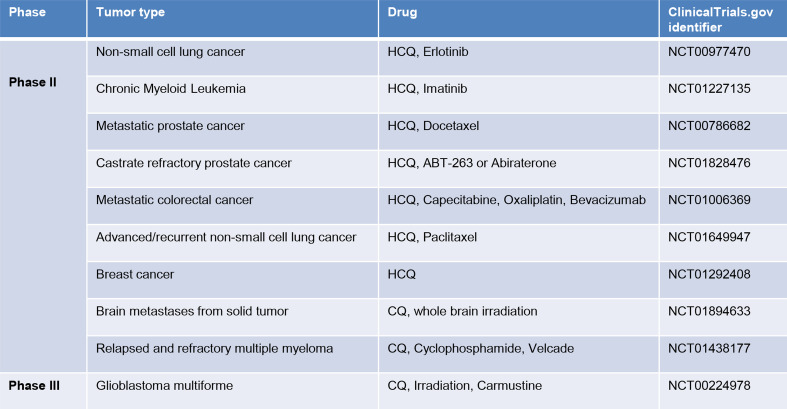
Selected phase II and III clinical trials of chloroquine (CQ) and hydroxychloroquine (HCQ) in cancer treatment. These information’s were queried from ClinicalTrial.gov website (http://clinicaltrials.gov/)
Acid protease inhibitors
Lysosomal proteases are involved in the degradation of autophagolysosomes and were reviewed earlier [101]. Among the lysosomal hydrolases and proteases, cathepsins have a major role. Pepstatin A and E64d inhibit cathepsins D, E and B, H, L respectively [102]. These two drugs by inhibiting lysosomal proteases can effectively suppress autophagy [103] and are commonly used to monitor the autophagy flux [104].
Vacuolar-type H+-ATPase inhibitors
Vacuolar-type H+-ATPases (V-ATPases) are found within the membranes of many organelles including lysosomes, endosomes, and secretory vesicles. V-ATPases couple proton transport with ATP hydrolysis and maintain the acidic pH microenvironment of the vacuole [105]. Bafilomycin A1 blocks lysosomal proton transport and lead to the inhibition of lysosomal hydrolases, which are only activated at low pH. As a consequence, Bafilomycin A1 inhibits autophagy flux [105–107].
Other inhibitors of autophagic degradation
Clomipramine is a FDA-approved drug used for the treatment of psychiatric disorders. Its active metabolite, desmethylclomipramine have been shown to interfere with autophagic flux by blocking autophagosome-lysosome fusion [108]. Lucanthone, an anti-schistosome agent, inhibits autophagy by disrupting lysosomal membrane permeabilization leading to markedly increased cathepsin D cytosolic levels [109]. Importantly, lucanthone was found more effective at reducing the viability on a panel of 7 breast cancer cells than chloroquine.
Lysosomotropic agents
Chloroquine (CQ) and Hydroxychloroquine (HCQ)
CQ and HCQ function primarily by inhibiting lysosomal acidification. At neutral pH, both drugs remain uncharged and freely diffuse across the plasma membrane. In acidic environments, CQ and HCQ become protonated and trapped in lysosomes, causing an increase in the lysosomal pH and therefore inhibiting the activity of the degradative enzymes [110–112]. Consequently, cells treated with such agents are unable to undergo lysosomal digestion and exhibit vesicular organelle accumulation in the cytoplasm consistent with blocked autophagy.
For the past 60 years, CQ and HCQ have been prescribed for malaria [110–112] and autoimmune disorders such as rheumatoid arthritis [113] and lupus erythematosus [114]. These compounds were also evaluated for HIV infection [115]. CQ and HCQ are oral drugs well tolerated in human. The only major side effect of CQ is retinal toxicity [116]. HCQ was later developed in response to this problem. Structurally, HCQ differs from CQ by the addition of a single hydroxyl group to the beta carbon of a tertiary amino ethyl located at the terminus of the side chain attached to the quinolone base. This addition limits the ability of the drug to cross the blood-retinal barrier and decreases the potential for retinal toxicity [117].
Many preclinical studies demonstrated that effective autophagy inhibition can be achieved with CQ and HCQ, and that they can have therapeutic application in cancer [118–121]. The potency of CQ as an anticancer agent seems to be very different among tumor cell lines [123]. It is still not clear if the cytotoxic effects of these agents in cancer cells are specifically from autophagy inhibition and not from another effect on lysosomal. However, cancer cells exhibiting increased autophagy flux are sensitive to, and could die, in response to treatment with lysosomotropic agents [124].
Because these drugs are well tolerated, numerous clinical trials for cancer patients were undertaken with the primary aim to measure efficacy and safety. Most of the trials are investigating HCQ in various tumors in combination with standard of care treatments, such as allosteric mTOR inhibitors (Temsirolimus, Everolimus), DNA damaging agents (Cyclophosphanmide, Oxaliplatin, Capecitabine), Tyrosine kinase inhibitors (Erlotinib, Imatinib), proteasome inhibitors (Velcade), microtubule inhibitors (Docetaxel, Paclitaxel) and radiation (Fig. 4). In patients suffering from glioblastoma multiforme, a phase III clinical trial combining conventional treatment, radiation and carmustine, with chloroquine demonstrated a median survival of 24 months in the chloroquine arm compared to 11 months in the placebo arm. However these results were not statistically significant due to the limited number of patients enrolled and in addition autophagy was not examined in this study [124].
Few of the current clinical trials for cancer patients included endpoints on autophagy biomarkers. HCQ dose-dependent autophagy inhibition was observed using electron microscopy assay, as a pharmacodynamic readout, on blood mononuclear cells [125, 126]. However, it is unclear today whether safely tolerated doses of HCQ or CQ can effectively and consistently inhibit autophagy in human tumors.
Lys05
Even if HCQ displays a very large tissue distribution resulting in high intratumoral concentration, it is anticipated that CQ or HCQ might not be sufficiently potent to demonstrate clinical efficacy in cancer at tolerable doses. To increase the activity of CQ, derivatives are being developed. A bivalent aminoquinoline analog of HCQ, Lys05, was identified [127]. This compound is water soluble and accumulates more readily within the lysosome. Lys05 causes robust lysosomal pH elevation, blocks autophagy and induces cytotoxicity in cancer cell lines with a ten-fold greater potency than HCQ. When tested in vivo, this compound induced an increase of autophagic vesicles, using electron microscopy and LC3 biomarker, in human tumor xenografts after 2 days of dosing at 76 mg/kg (138 nmol/g) i.p, as compared to equimolar dose of HCQ (60 mg/kg) or PBS. Administrated as a single agent, Lys05 showed a significant antitumor activity on two different tumor models without causing toxicity at effective doses in mice [127]. Of note, antitumor activity was not observed with equimolar dose of HCQ. While no extensive in vivo PK data was provided it this report, the authors measured a better accumulation of Lys05 within tumors at effective dose (138 nmol/g, i.p.) as compared to equimolar dose of HCQ, using mass spectrometry. These preclinical data with Lys05 are promising and investigations on alternative route of administration, such as intravenous or oral, would be of great interest for a clinical perspective.
ARN5187
Starting from an in silico screen of lysosomotropic small molecule interacting with the circadian nuclear receptor REV-ERBβ, ARN5187 was recently identified [128]. This lysosomotropic compound blocked autophagolysosome final maturation and inhibited autophagy. Although ARN5187 displayed a similar potency to equimolar concentrations of CQ on inhibiting autophagy readout, it was more cytotoxic in cancer cell lines. This was recently explained by structure–activity relationship (SAR) of the chemical series of ARN5187 and also using knockdown of REV-ERBβ in CQ-treated cancer cell lines [129]. The improved cytotoxic effect of ARN5187 as compared to CQ, is due to its dual inhibitory activity on REV-ERB and autophagy. The group of B. Grimaldi finally selected a lead compound, the compound 30, with 5–50 superior in vitro cytotoxic activity on 6 tumor cell lines, as compared to equimolar concentrations of CQ. Testing this dual inhibitor in vivo for PK and efficacy studies will demonstrate the benefit of this novel pharmacological approach.
Conclusions
The housekeeping function of autophagy has attracted major attention over the last decade as it has a broad variety of roles in health and diseases. While the learnings in the autophagy machinery markedly improved, the researchers still had to deal with poorly selective tools to manipulate it. During the last 2 years, significant progress was made with the discovery of catalytic inhibitors of the two main kinases involved in the autophagy process, Vps34 and ULK1. We must keep in mind that these enzymes do not exclusively belong to autophagy pathway, and chemical optimization is needed in the case of ULK inhibitors. Nonetheless, we anticipate that these new compounds would advantageously replace the pan-PI3K inhibitors, such as 3-methyladenine, to interrogate the fundamental questions in the field of autophagy.
Whether inhibiting autophagy could have some benefits for patients is still an open question. Targeting this process could be of great interest for the treatment of cancer, as autophagy has been associated with tumor initiation, progression and resistance to treatment [37]. New information could come from the current clinical trials with chloroquine analogs, that affect lysosomal functions. However, many challenges remain to be addressed because autophagy is a dynamic process and a clear and suitable pharmacodynamic readout is not yet available for patients. The identification of the responder patient population and the best combination will also be critical to increase the probability of success of such therapy. Given the “addiction to autophagy” of tumor associated K-RAS mutation, such as pancreas cancer or non-small cell lung cancer [123, 130, 131], and the possibility of combining with mTOR inhibitors in renal cell carcinoma [82, 132], the development of new autophagy inhibitors is an attractive field of investigations.
Acknowledgments
I thank Baptiste Ronan, Magali Mathieu, Florence Fassy, Ellen Ross and Michael Shaw for their comments and suggestions during the preparation of this manuscript.
Abbreviations
- ADME
Absorption, distribution, metabolism, excretion
- AMPK
AMP-activated protein kinase
- ATG
Autophagy-related genes or proteins
- Beclin-1
Coiled-coil, myosin-like BCL2-ineracting protein
- CQ
Chloroquine
- DNA-PK
DNA-dependent protein kinase
- FYVE
Fab1, YOTB, Vac1 and EEA1
- GFP
Green fluorescent protein
- HCQ
Hydroxychloroquine
- LC3
Microtubule-associated protein light chain 3
- mTOR
Mammalian target of rapamycine
- mTORC1
Mammalian target of rapamycine complex 1
- MEF
Mouse embryonic fibroblasts
- PK
Pharmacokinetic
- PI3K
Phosphoinositide 3-kinase
- PIK3C3
Phosphatidylinositol 3-kinase, catalytic subunit type 3
- PtdIns3K
Phosphatidylinositol 3-kinase
- PtdIns3P
Phosphatidylinositol 3-phosphate
- ULK
Unc-51-like kinase
- ULK1
Unc-51-like kinase 1
- Vps34
Vacuolar protein sorting 34
- 3-MA
3-methyladenine
Compliance with ethical standards
Conflict of interest
BP is a Sanofi employee and also a shareholder of Sanofi stock.
References
- 1.de Duve C. The lysosome concept. In: De Reuck A, Cameron MP, editors. Ciba foundation symposium on lysosomes. Boston: Little Brown; 1963. [Google Scholar]
- 2.de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fight disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li WW, Li J, Bao JK. Microautophagy: lesser-known self eating. Cell Mol Life Sci. 2012;69(7):1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in diseases and aging. Cell Res. 2014;24(1):92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinsztein DC, Shpika T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22(1):R29–R34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Shibutani ST, Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res. 2014;24:58–68. doi: 10.1038/cr.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9:1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- 11.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 12.Murrow L, Malhotra R, Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17(3):300–310. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15(7):741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341(6144):1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy. 2013;9:1983–1995. doi: 10.4161/auto.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22(2):132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirht M, Joachim J, Tooze SA. Autophagosome formation-the role of ULK1 and beclin1-PI3 KC3 complexes in setting the stage. Semin Cancer Biol. 2013;23(5):301–309. doi: 10.1016/j.semcancer.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noda NN, Fujioka Y. Atg1 family kinases in autophagy initiation. Cell Mol Life Sci. 2015;72(16):3083–3096. doi: 10.1007/s00018-015-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND, Shaw RJ. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 2015;59(2):285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signaling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 26.Raiborg C, Schink KO, Stenmark H. Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic. FEBS J. 2013;280(12):2730–2742. doi: 10.1111/febs.12116. [DOI] [PubMed] [Google Scholar]
- 27.O’Farrell F, Rusten TE, Stenmark H. Phosphoinositide 3-kinases as accelerators and brakes of autophagy. FEBS J. 2013;280(24):6322–6337. doi: 10.1111/febs.12486. [DOI] [PubMed] [Google Scholar]
- 28.Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T, Laporte J, Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin KR, Xu Y, Looyenga BD, Davis RJ, Wu CL, Tremblay ML, Xu E, MacKeigan JP. Identification of PTPσ as an autophagic phosphatase. J Cell Sci. 2010;124:812–819. doi: 10.1242/jcs.080341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321(5885):117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 31.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 32.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. New Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 34.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang P, Mizushima N. Autophagy in human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, Maiuri MC, Martin SJ, Penninger J, Piacentini M, Rubinsztein DC, Simon HU, Simonsen A, Thorburn AM, Velasco G, Ryan KM, Kroemer G. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34(7):856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White E. The role for autophagy in cancer. J Clin Invest. 2015;1:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nature Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 39.Shen S, Kepp O, Michaud M, Martins I, Minoux H, Métivier D, Maiuri MC, Kroemer RT, Kroemer G. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30(45):4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- 40.Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19(1):87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–324. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Bánhegyi G, Bartholomew CR, Bassham DC, Bast RC, Jr, Batoko H, Bay BH, Beau I, Béchet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahová M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen X, Chen X, Chen YG, Chen Y, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronjé MJ, Cuervo AM, Cullen JJ, Czaja MJ, D’Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De Felici M, de Groot JF, de Haan CA, De Martino L, De Milito A, De Tata V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di Gioacchino M, Di Paolo G, Di Pietro C, Díaz-Araya G, Díaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC, Dosenko V, Dowling JJ, Doxsey S, Dreux M, Drew ME, Duan Q, Duchosal MA, Duff K, Dugail I, Durbeej M, Duszenko M, Edelstein CL, Edinger AL, Egea G, Eichinger L, Eissa NT, Ekmekcioglu S, El-Deiry WS, Elazar Z, Elgendy M, Ellerby LM, Eng KE, Engelbrecht AM, Engelender S, Erenpreisa J, Escalante R, Esclatine A, Eskelinen EL, Espert L, Espina V, Fan H, Fan J, Fan QW, Fan Z, Fang S, Fang Y, Fanto M, Fanzani A, Farkas T, Farré JC, Faure M, Fechheimer M, Feng CG, Feng J, Feng Q, Feng Y, Fésüs L, Feuer R, Figueiredo-Pereira ME, Fimia GM, Fingar DC, Finkbeiner S, Finkel T, Finley KD, Fiorito F, Fisher EA, Fisher PB, Flajolet M, Florez-McClure ML, Florio S, Fon EA, Fornai F, Fortunato F, Fotedar R, Fowler DH, Fox HS, Franco R, Frankel LB, Fransen M, Fuentes JM, Fueyo J, Fujii J, Fujisaki K, Fujita E, Fukuda M, Furukawa RH, Gaestel M, Gailly P, Gajewska M, Galliot B, Galy V, Ganesh S, Ganetzky B, Ganley IG, Gao FB, Gao GF, Gao J, Garcia L, Garcia-Manero G, Garcia-Marcos M, Garmyn M, Gartel AL, Gatti E, Gautel M, Gawriluk TR, Gegg ME, Geng J, Germain M, Gestwicki JE, Gewirtz DA, Ghavami S, Ghosh P, Giammarioli AM, Giatromanolaki AN, Gibson SB, Gilkerson RW, Ginger ML, Ginsberg HN, Golab J, Goligorsky MS, Golstein P, Gomez-Manzano C, Goncu E, Gongora C, Gonzalez CD, Gonzalez R, González-Estévez C, González-Polo RA, Gonzalez-Rey E, Gorbunov NV, Gorski S, Goruppi S, Gottlieb RA, Gozuacik D, Granato GE, Grant GD, Green KN, Gregorc A, Gros F, Grose C, Grunt TW, Gual P, Guan JL, Guan KL, Guichard SM, Gukovskaya AS, Gukovsky I, Gunst J, Gustafsson AB, Halayko AJ, Hale AN, Halonen SK, Hamasaki M, Han F, Han T, Hancock MK, Hansen M, Harada H, Harada M, Hardt SE, Harper JW, Harris AL, Harris J, Harris SD, Hashimoto M, Haspel JA, Hayashi S, Hazelhurst LA, He C, He YW, Hébert MJ, Heidenreich KA, Helfrich MH, Helgason GV, Henske EP, Herman B, Herman PK, Hetz C, Hilfiker S, Hill JA, Hocking LJ, Hofman P, Hofmann TG, Höhfeld J, Holyoake TL, Hong MH, Hood DA, Hotamisligil GS, Houwerzijl EJ, Høyer-Hansen M, Hu B, Hu CA, Hu HM, Hua Y, Huang C, Huang J, Huang S, Huang WP, Huber TB, Huh WK, Hung TH, Hupp TR, Hur GM, Hurley JB, Hussain SN, Hussey PJ, Hwang JJ, Hwang S, Ichihara A, Ilkhanizadeh S, Inoki K, Into T, Iovane V, Iovanna JL, Ip NY, Isaka Y, Ishida H, Isidoro C, Isobe K, Iwasaki A, Izquierdo M, Izumi Y, Jaakkola PM, Jäättelä M, Jackson GR, Jackson WT, Janji B, Jendrach M, Jeon JH, Jeung EB, Jiang H, Jiang H, Jiang JX, Jiang M, Jiang Q, Jiang X, Jiang X, Jiménez A, Jin M, Jin S, Joe CO, Johansen T, Johnson DE, Johnson GV, Jones NL, Joseph B, Joseph SK, Joubert AM, Juhász G, Juillerat-Jeanneret L, Jung CH, Jung YK, Kaarniranta K, Kaasik A, Kabuta T, Kadowaki M, Kagedal K, Kamada Y, Kaminskyy VO, Kampinga HH, Kanamori H, Kang C, Kang KB, Kang KI, Kang R, Kang YA, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kanthasamy A, Karantza V, Kaushal GP, Kaushik S, Kawazoe Y, Ke PY, Kehrl JH, Kelekar A, Kerkhoff C, Kessel DH, Khalil H, Kiel JA, Kiger AA, Kihara A, Kim DR, Kim DH, Kim DH, Kim EK, Kim HR, Kim JS, Kim JH, Kim JC, Kim JK, Kim PK, Kim SW, Kim YS, Kim Y, Kimchi A, Kimmelman AC, King JS, Kinsella TJ, Kirkin V, Kirshenbaum LA, Kitamoto K, Kitazato K, Klein L, Klimecki WT, Klucken J, Knecht E, Ko BC, Koch JC, Koga H, Koh JY, Koh YH, Koike M, Komatsu M, Kominami E, Kong HJ, Kong WJ, Korolchuk VI, Kotake Y, Koukourakis MI, Kouri Flores JB, Kovács AL, Kraft C, Krainc D, Krämer H, Kretz-Remy C, Krichevsky AM, Kroemer G, Krüger R, Krut O, Ktistakis NT, Kuan CY, Kucharczyk R, Kumar A, Kumar R, Kumar S, Kundu M, Kung HJ, Kurz T, Kwon HJ, La Spada AR, Lafont F, Lamark T, Landry J, Lane JD, Lapaquette P, Laporte JF, László L, Lavandero S, Lavoie JN, Layfield R, Lazo PA, Le W, Le Cam L, Ledbetter DJ, Lee AJ, Lee BW, Lee GM, Lee J, Lee JH, Lee M, Lee MS, Lee SH, Leeuwenburgh C, Legembre P, Legouis R, Lehmann M, Lei HY, Lei QY, Leib DA, Leiro J, Lemasters JJ, Lemoine A, Lesniak MS, Lev D, Levenson VV, Levine B, Levy E, Li F, Li JL, Li L, Li S, Li W, Li XJ, Li YB, Li YP, Liang C, Liang Q, Liao YF, Liberski PP, Lieberman A, Lim HJ, Lim KL, Lim K, Lin CF, Lin FC, Lin J, Lin JD, Lin K, Lin WW, Lin WC, Lin YL, Linden R, Lingor P, Lippincott-Schwartz J, Lisanti MP, Liton PB, Liu B, Liu CF, Liu K, Liu L, Liu QA, Liu W, Liu YC, Liu Y, Lockshin RA, Lok CN, Lonial S, Loos B, Lopez-Berestein G, López-Otín C, Lossi L, Lotze MT, Lőw P, Lu B, Lu B, Lu B, Lu Z, Luciano F, Lukacs NW, Lund AH, Lynch-Day MA, Ma Y, Macian F, MacKeigan JP, Macleod KF, Madeo F, Maiuri L, Maiuri MC, Malagoli D, Malicdan MC, Malorni W, Man N, Mandelkow EM, Manon S, Manov I, Mao K, Mao X, Mao Z, Marambaud P, Marazziti D, Marcel YL, Marchbank K, Marchetti P, Marciniak SJ, Marcondes M, Mardi M, Marfe G, Mariño G, Markaki M, Marten MR, Martin SJ, Martinand-Mari C, Martinet W, Martinez-Vicente M, Masini M, Matarrese P, Matsuo S, Matteoni R, Mayer A, Mazure NM, McConkey DJ, McConnell MJ, McDermott C, McDonald C, McInerney GM, McKenna SL, McLaughlin B, McLean PJ, McMaster CR, McQuibban GA, Meijer AJ, Meisler MH, Meléndez A, Melia TJ, Melino G, Mena MA, Menendez JA, Menna-Barreto RF, Menon MB, Menzies FM, Mercer CA, Merighi A, Merry DE, Meschini S, Meyer CG, Meyer TF, Miao CY, Miao JY, Michels PA, Michiels C, Mijaljica D, Milojkovic A, Minucci S, Miracco C, Miranti CK, Mitroulis I, Miyazawa K, Mizushima N, Mograbi B, Mohseni S, Molero X, Mollereau B, Mollinedo F, Momoi T, Monastyrska I, Monick MM, Monteiro MJ, Moore MN, Mora R, Moreau K, Moreira PI, Moriyasu Y, Moscat J, Mostowy S, Mottram JC, Motyl T, Moussa CE, Müller S, Muller S, Münger K, Münz C, Murphy LO, Murphy ME, Musarò A, Mysorekar I, Nagata E, Nagata K, Nahimana A, Nair U, Nakagawa T, Nakahira K, Nakano H, Nakatogawa H, Nanjundan M, Naqvi NI, Narendra DP, Narita M, Navarro M, Nawrocki ST, Nazarko TY, Nemchenko A, Netea MG, Neufeld TP, Ney PA, Nezis IP, Nguyen HP, Nie D, Nishino I, Nislow C, Nixon RA, Noda T, Noegel AA, Nogalska A, Noguchi S, Notterpek L, Novak I, Nozaki T, Nukina N, Nürnberger T, Nyfeler B, Obara K, Oberley TD, Oddo S, Ogawa M, Ohashi T, Okamoto K, Oleinick NL, Oliver FJ, Olsen LJ, Olsson S, Opota O, Osborne TF, Ostrander GK, Otsu K, Ou JH, Ouimet M, Overholtzer M, Ozpolat B, Paganetti P, Pagnini U, Pallet N, Palmer GE, Palumbo C, Pan T, Panaretakis T, Pandey UB, Papackova Z, Papassideri I, Paris I, Park J, Park OK, Parys JB, Parzych KR, Patschan S, Patterson C, Pattingre S, Pawelek JM, Peng J, Perlmutter DH, Perrotta I, Perry G, Pervaiz S, Peter M, Peters GJ, Petersen M, Petrovski G, Phang JM, Piacentini M, Pierre P, Pierrefite-Carle V, Pierron G, Pinkas-Kramarski R, Piras A, Piri N, Platanias LC, Pöggeler S, Poirot M, Poletti A, Poüs C, Pozuelo-Rubio M, Prætorius-Ibba M, Prasad A, Prescott M, Priault M, Produit-Zengaffinen N, Progulske-Fox A, Proikas-Cezanne T, Przedborski S, Przyklenk K, Puertollano R, Puyal J, Qian SB, Qin L, Qin ZH, Quaggin SE, Raben N, Rabinowich H, Rabkin SW, Rahman I, Rami A, Ramm G, Randall G, Randow F, Rao VA, Rathmell JC, Ravikumar B, Ray SK, Reed BH, Reed JC, Reggiori F, Régnier-Vigouroux A, Reichert AS, Reiners JJ, Jr, Reiter RJ, Ren J, Revuelta JL, Rhodes CJ, Ritis K, Rizzo E, Robbins J, Roberge M, Roca H, Roccheri MC, Rocchi S, Rodemann HP, Rodríguez de Córdoba S, Rohrer B, Roninson IB, Rosen K, Rost-Roszkowska MM, Rouis M, Rouschop KM, Rovetta F, Rubin BP, Rubinsztein DC, Ruckdeschel K, Rucker EB, 3rd, Rudich A, Rudolf E, Ruiz-Opazo N, Russo R, Rusten TE, Ryan KM, Ryter SW, Sabatini DM, Sadoshima J, Saha T, Saitoh T, Sakagami H, Sakai Y, Salekdeh GH, Salomoni P, Salvaterra PM, Salvesen G, Salvioli R, Sanchez AM, Sánchez-Alcázar JA, Sánchez-Prieto R, Sandri M, Sankar U, Sansanwal P, Santambrogio L, Saran S, Sarkar S, Sarwal M, Sasakawa C, Sasnauskiene A, Sass M, Sato K, Sato M, Schapira AH, Scharl M, Schätzl HM, Scheper W, Schiaffino S, Schneider C, Schneider ME, Schneider-Stock R, Schoenlein PV, Schorderet DF, Schüller C, Schwartz GK, Scorrano L, Sealy L, Seglen PO, Segura-Aguilar J, Seiliez I, Seleverstov O, Sell C, Seo JB, Separovic D, Setaluri V, Setoguchi T, Settembre C, Shacka JJ, Shanmugam M, Shapiro IM, Shaulian E, Shaw RJ, Shelhamer JH, Shen HM, Shen WC, Sheng ZH, Shi Y, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shintani T, Shirihai OS, Shore GC, Sibirny AA, Sidhu SB, Sikorska B, Silva-Zacarin EC, Simmons A, Simon AK, Simon HU, Simone C, Simonsen A, Sinclair DA, Singh R, Sinha D, Sinicrope FA, Sirko A, Siu PM, Sivridis E, Skop V, Skulachev VP, Slack RS, Smaili SS, Smith DR, Soengas MS, Soldati T, Song X, Sood AK, Soong TW, Sotgia F, Spector SA, Spies CD, Springer W, Srinivasula SM, Stefanis L, Steffan JS, Stendel R, Stenmark H, Stephanou A, Stern ST, Sternberg C, Stork B, Strålfors P, Subauste CS, Sui X, Sulzer D, Sun J, Sun SY, Sun ZJ, Sung JJ, Suzuki K, Suzuki T, Swanson MS, Swanton C, Sweeney ST, Sy LK, Szabadkai G, Tabas I, Taegtmeyer H, Tafani M, Takács-Vellai K, Takano Y, Takegawa K, Takemura G, Takeshita F, Talbot NJ, Tan KS, Tanaka K, Tanaka K, Tang D, Tang D, Tanida I, Tannous BA, Tavernarakis N, Taylor GS, Taylor GA, Taylor JP, Terada LS, Terman A, Tettamanti G, Thevissen K, Thompson CB, Thorburn A, Thumm M, Tian F, Tian Y, Tocchini-Valentini G, Tolkovsky AM, Tomino Y, Tönges L, Tooze SA, Tournier C, Tower J, Towns R, Trajkovic V, Travassos LH, Tsai TF, Tschan MP, Tsubata T, Tsung A, Turk B, Turner LS, Tyagi SC, Uchiyama Y, Ueno T, Umekawa M, Umemiya-Shirafuji R, Unni VK, Vaccaro MI, Valente EM, Van den Berghe G, van der Klei IJ, van Doorn W, van Dyk LF, van Egmond M, van Grunsven LA, Vandenabeele P, Vandenberghe WP, Vanhorebeek I, Vaquero EC, Velasco G, Vellai T, Vicencio JM, Vierstra RD, Vila M, Vindis C, Viola G, Viscomi MT, Voitsekhovskaja OV, von Haefen C, Votruba M, Wada K, Wade-Martins R, Walker CL, Walsh CM, Walter J, Wan XB, Wang A, Wang C, Wang D, Wang F, Wang F, Wang G, Wang H, Wang HG, Wang HD, Wang J, Wang K, Wang M, Wang RC, Wang X, Wang X, Wang YJ, Wang Y, Wang Z, Wang ZC, Wang Z, Wansink DG, Ward DM, Watada H, Waters SL, Webster P, Wei L, Weihl CC, Weiss WA, Welford SM, Wen LP, Whitehouse CA, Whitton JL, Whitworth AJ, Wileman T, Wiley JW, Wilkinson S, Willbold D, Williams RL, Williamson PR, Wouters BG, Wu C, Wu DC, Wu WK, Wyttenbach A, Xavier RJ, Xi Z, Xia P, Xiao G, Xie Z, Xie Z, Xu DZ, Xu J, Xu L, Xu X, Yamamoto A, Yamamoto A, Yamashina S, Yamashita M, Yan X, Yanagida M, Yang DS, Yang E, Yang JM, Yang SY, Yang W, Yang WY, Yang Z, Yao MC, Yao TP, Yeganeh B, Yen WL, Yin JJ, Yin XM, Yoo OJ, Yoon G, Yoon SY, Yorimitsu T, Yoshikawa Y, Yoshimori T, Yoshimoto K, You HJ, Youle RJ, Younes A, Yu L, Yu L, Yu SW, Yu WH, Yuan ZM, Yue Z, Yun CH, Yuzaki M, Zabirnyk O, Silva-Zacarin E, Zacks D, Zacksenhaus E, Zaffaroni N, Zakeri Z, Zeh HJ, 3rd, Zeitlin SO, Zhang H, Zhang HL, Zhang J, Zhang JP, Zhang L, Zhang L, Zhang MY, Zhang XD, Zhao M, Zhao YF, Zhao Y, Zhao ZJ, Zheng X, Zhivotovsky B, Zhong Q, Zhou CZ, Zhu C, Zhu WG, Zhu XF, Zhu X, Zhu Y, Zoladek T, Zong WX, Zorzano A, Zschocke J, Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;4:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, Suzuki A. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res. 2009;48:307–343. doi: 10.1016/j.plipres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Devereaux K, Dall’Armi C, Alcazar-Roman A, Ogasawara Y, Zhou X, Wang F, Yamamoto A, De Camilli P, Di Paolo G. Regulation of mammalian autophagy by class II and III PI3-kinases through PI3P synthesis. PLoS ONE. 2013;8(10):e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seglen PO, Gordon PB. 3-methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79(6):1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinod V, Padmakrishnan CJ, Vijayan B, Gopala S. How can I halt thee? The puzzles involved in autophagic inhibition. Pharmacol Res. 2014;82:1–8. doi: 10.1016/j.phrs.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275(2):992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 48.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition of class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285(14):10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caro LH, Plomp PJ, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur J Biochem. 1988;175(2):325–329. doi: 10.1111/j.1432-1033.1988.tb14200.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Wang X, Guo H, Zhang B, Zhang XB, Shi ZJ, Yu L. Synthesis and screening of 3_MA derivatives for autophagy inhibitors. Autophagy. 2013;9(4):595–603. doi: 10.4161/auto.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiesinger D, Gubler HU, Haefliger W, Hauser D. Antiinflammatory activity of the new mould metabolite 11-desacetoxy-wortmannin and some of its derivatives. Experientia. 1974;30:135–136. doi: 10.1007/BF01927691. [DOI] [PubMed] [Google Scholar]
- 52.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, Vlahos CJ. Wortmannin, a potent and selective inhibitor of phosphatidylinositol 3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 53.Thelen M, Wymann MP, Langen H. Wortmannin binds specifically to 1-phosphatidylinositol 3-kinase while inhibiting guanine nucleotide-binding protein coupled receptor signaling in neutrophil leukocytes. Proc Natl Acad Sci USA. 1994;91:4960–4964. doi: 10.1073/pnas.91.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blommaart EFC, Krause U, Schellens JPM, Vreeling-Sindelarova Meijer AJ. The phosphatidylinositol 3-kinase inhbitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 55.Walker EH, Pacold ME, Perisic O, Stepehens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin and staurosporine. Mol Cell. 2000;6(4):909–919. doi: 10.1016/S1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 56.Brunn GJ, Hudson CC, Sekulić A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277(5322):99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 57.Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58(19):4375–4382. [PubMed] [Google Scholar]
- 58.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)8-phenyl64H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 59.Knight ZA, Shokat KM. Chemically targeting the PI3 K family. Biochem Soc Trans. 2007;35(Pt 2):245–249. doi: 10.1042/BST0350245. [DOI] [PubMed] [Google Scholar]
- 60.Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, Williams RL. Shapping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327:1638–1642. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125(4):733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH, Sarpong MA, Schmidt SJ, Van Aller GS, Carson JD, Diamond MA, Elkins PA, Gardiner CM, Garver E, Gilbert SA, Gontarek RR, Jackson JR, Kershner KL, Luo L, Raha K, Sherk CS, Sung CM, Sutton D, Tummino PJ, Wegrzyn RJ, Auger KR, Dhanak D. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herman PK, Emr SD. Characterization of Vps34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6742–6754. doi: 10.1128/MCB.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kihara A, Noda T, Ishihara N, Oshui Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase. J Cell Biol. 2001;152(3):519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obara K, Noda T, Niimi K, Oshumi Y. Transport of phosphatidylinositol 3-phosphate into vacuole via autophagic membranes in Saccharomyces cerevisiae. Genes Cells. 2008;13(6):537–547. doi: 10.1111/j.1365-2443.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 67.Johnson EE, Overmeyer JH, Gunning WT, Maltese WA. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J Cell Sci. 2006;119:1219–1232. doi: 10.1242/jcs.02833. [DOI] [PubMed] [Google Scholar]
- 68.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funderburk SF, Wang QJ, Yue Z. The beclin 1-Vps34 complex: at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgo J, Diaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphophate receptor. Cell Death Differ. 2007;14(5):1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 71.Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, Liu X, Eisenberg J, Liu J, Blenis J, Xavier RJ, Yuan J. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev Cell. 2010;18(6):1041–1052. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, He Y, Wang F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci USA. 2010;107(20):9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLeod IX, Zhou X, Li QJ, Wang F, He YW. The class III kinase Vps34 promote T lymphocyte survival through regulating IL-7Rα surface expression. J Immunol. 2011;187:5051–5061. doi: 10.4049/jimmunol.1100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parekh VV, Wu L, Boyd KL, Wiliams JA, Gaddy JA, Olivared-Villagomez D, Cover TL, Zong WX, Zhang J, Van Kaer L. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J Immunol. 2013;190:5086–5101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide 3-kinase Vps34 is required for naïve T cell homeostasis. Proc Natl Acad Sci USA. 2012;109(22):8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takase Y, Saeki T, Watanabe N, Adachi H, Souda S, Saito I. Cyclic-GMP phosphodiesterase inhibitors: II. Requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. J Med Chem. 1994;37:2106–2111. doi: 10.1021/jm00039a024. [DOI] [PubMed] [Google Scholar]
- 77.MacPherson JD, Gillespie TD, Dunkerley HA, Maurice DH, Bennett BM. Inhibition of phosphodiesterase 5 selectively reverses nitrate tolerance in the venous circulation. J Pharmacol Exp Ther. 2006;317:188–195. doi: 10.1124/jpet.105.094763. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Yu AD, Xie X, Ma D, Yuan J. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci USA. 2007;104(48):19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Vakifahmetoglu Norberg H, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Li Y, Hao Y, Choi A, Ke H, Ma D, Yuan J. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147(1):223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao S, Li S, Qin Y, Wang X, Yang Y, Bai H, Zhou L, Zhao C, Wang C. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol. 2014;44(5):1661–1668. doi: 10.3892/ijo.2014.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peppard JV, Rugg C, Smicker M, Dureuil C, Ronan B, Flamand O, Durand L, Pasquier B. Identifying small molecules which inhibit autophagy: a phenotypic screen using image-based high-content cell analysis. Curr Chem Genomics Transl Med. 2014;8:3–15. doi: 10.2174/2213988501408010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, Marquette J, El-Ahmad Y, Filoche-Romme B, Schio L, Garcia-Echeverria C, Goulaouic H, Pasquier B. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. 2014;10(12):1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- 83.Pasquier B. SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with mTOR inhibition in tumor cells. Autophagy. 2015;4:725–726. doi: 10.1080/15548627.2015.1033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasquier B, El-Ahmad Y, Filoche-Rommé B, Dureuil C, Fassy F, Abecassis PY, Mathieu M, Bertrand T, Benard T, Barrière C, El Batti S, Letallec JP, Sonnefraud V, Brollo M, Delbarre L, Loyau V, Pilorge F, Bertin L, Richepin P, Arigon J, Labrosse JR, Clément J, Durand F, Combet R, Perraut P, Leroy V, Gay F, Lefrançois D, Bretin F, Marquette JP, Michot N, Caron A, Castell C, Schio L, McCort G, Goulaouic H, Garcia-Echeverria C, Ronan B. Discovery of (2S)-8-[(3R)-3-methylmorpholin-4-yl]-1-(3-methyl-2-oxo-butyl)-2-(trifluoromethyl)-3,4-dihydro-2H-pyrimido[1,2-a]pyrimidin-6-one: a novel potent and selective inhibitor of Vps34 for the treatment of solid tumors. J Med Chem. 2015;58(1):376–400. doi: 10.1021/jm5013352. [DOI] [PubMed] [Google Scholar]
- 85.Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, Shipiro N, Ward R, Cross D, Ganley IG, Alessi DR. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J . 2014;463:413–427. doi: 10.1042/BJ20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D, Powers AF, Helliwell SB, D’Aquin S, Bastien J, Wang H, Wiederschain D, Kuerth J, Bergman P, Schwalb D, Thomas J, Ugwonali S, Harbinski F, Tallarico J, Wilson CJ, Myer VE, Porter JA, Bussiere DE, Finan PM, Labow MA, Mao X, Hamann LG, Manning BD, Valdez RA, Nicholson T, Schirle M, Knapp MS, Keaney EP, Murphy LO. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16(11):1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 87.Chan EY, Tooze SA. Evolution of Atg1 function and regulation. Autophagy. 2009;5:758–765. doi: 10.4161/auto.8709. [DOI] [PubMed] [Google Scholar]
- 88.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150(6):1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan EYW, Longatti A, McKnight NC, Tooze SA. Kinase inactivated ULK proteins inhibit autophagy via their conserved C-terminal domain using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Young ARJ, Chan EYW, Hu XW, Köchl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 92.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci USA. 2011;108(27):11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan EYW, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282:25464–25474. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 94.McAlpine F, Williamson LE, Tooze SA, Chan EY. Regulation of nutrient-sensitive autophagy by uncoordinated 51-like kinases 1 and 2. Autophagy. 2013;9:361–373. doi: 10.4161/auto.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112:1493–1502. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xhou X, Babu JR, da Silva S, Shu Q, Graef IA, Oliver T, Tomoda T, Tani T, Wooten MW, Wang F. Unc-51-like kinase 1/2-mediated endocytic processes regulate filipodia extension and branching of sensory axons. Proc Natl Acad Sci USA. 2007;104(14):5842–5847. doi: 10.1073/pnas.0701402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheong H, Wu J, Gonzales LK, Guttentag SH, Thompson CB, Lindsten T. Analysis of a lung defect in autophagy-deficient mouse strains. Autophagy. 2014;10(1):45–56. doi: 10.4161/auto.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lazarus MB, Novotny CJ, Shokat KM. Structure of the human autophagy initiating kinase ULK1 in complex with potent inhibitors. ACS Chem Biol. 2015;10(1):257–261. doi: 10.1021/cb500835z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clark K, Peggie M, Plater L, Sorcek RJ, Young ERR, Madwed JB, Hough J, McIver EG, Cohen P. Novel cross-talk within the IKK family controls innate immunity. Biochem J . 2011;434:93–104. doi: 10.1042/BJ20101701. [DOI] [PubMed] [Google Scholar]
- 100.Petherick KJ, Conway OJL, Mpamhanga C, Osborne SA, Kamal A, Saxty B, Ganley IG. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycine (mTOR)-dependent autophagy. J Biol Chem. 2015;290(18):11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Müller S, Dennemärker J, Reinheckel T. Specific functions of lysosomal proteases in endocytic and autophagic pathways. Biochim Biophys Acta. 2012;1824(1):34–43. doi: 10.1016/j.bbapap.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jung M, Lee J, Seo HY, Lim JS, Kim EK. Cathepsin inhibition-induced lysosomal dysfunction enhances pancreatic beta-cell apoptosis in high glucose. PLoS ONE. 2015;10(1):e0116972. doi: 10.1371/journal.pone.0116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy. 2011;7:188–204. doi: 10.4161/auto.7.2.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1(2):84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 106.Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC, Li HT, Sung JJ, Cho CH. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun. 2009;382:451–456. doi: 10.1016/j.bbrc.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 107.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes. Autophagy. 2008;4:849–950. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- 108.Rossi M, Munarriz ER, Bartesaghi S, Milanese M, Dinsdale D, Guerra-Martin MA, Bampton ET, Glynn P, Bonanno G, Knight RA, Nicotera P, Melino G. Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. J Cell Sci. 2009;122(Pt 18):3330–3339. doi: 10.1242/jcs.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carew JS, Espitia CM, Esquivel JA, 2nd, Mahalingam D, Kelly KR, Reddy G, Giles FJ, Nawrocki ST. Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J Biol Chem. 2011;286(8):6602–6613. doi: 10.1074/jbc.M110.151324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235:50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- 111.Slater AF. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum . Pharmacol Ther. 1993;57:203–235. doi: 10.1016/0163-7258(93)90056-J. [DOI] [PubMed] [Google Scholar]
- 112.O’Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines-past, present, and future: a chemical perspective. Pharmacol Ther. 1998;77:29–58. doi: 10.1016/S0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 113.Kremer JM. Rational use of new and existing disease-modifying agents in rheumatoid arthritis. Ann Intern Med. 2001;134:695–706. doi: 10.7326/0003-4819-134-8-200104170-00013. [DOI] [PubMed] [Google Scholar]
- 114.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 115.Romanelli F, Smith KM, Hoven AD. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr Pharm Des. 2004;10:2643–2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 116.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997;40(8):1482–1486. doi: 10.1002/art.1780400817. [DOI] [PubMed] [Google Scholar]
- 118.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, Van Etten RA, Donato N, Hunter A, Dinsdale D, Tirrò E, Vigneri P, Nicotera P, Dyer MJ, Holyoake T, Salomoni P, Calabretta B. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119(5):1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, Liao WS, Bast RC., Jr The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118(12):3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA, Debnath J, Shokat KM, Weiss WA. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3(147):81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harhaji-Trajkovic L, Arsikin K, Kravic-Stevovic T, Petricevic S, Tovilovic G, Pantovic A, Zogovic N, Ristic B, Janjetovic K, Bumbasirevic V, Trajkovic V. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm Res. 2012;29(8):2249–2263. doi: 10.1007/s11095-012-0753-1. [DOI] [PubMed] [Google Scholar]
- 123.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sotelo J, Briceño E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(5):337–344. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 125.Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, Mikkelson T, Wang D, Chang YC, Hu J, McAfee Q, Fisher J, Troxel AB, Piao S, Heitjan DF, Tan KS, Pontiggia L, O’Dwyer PJ, Davis LE, Amaravadi RK. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8):1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O’Dwyer PJ, Amaravadi RK. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8):1391–1402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, Winkler JD, Amaravadi RK. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci USA. 2012;109(21):8253–8258. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Mei C, Ercolani L, Parodi C, Veronesi M, Lo Vecchio C, Bottegoni G, Torrente E, Scarpelli R, Marotta R, Ruffili R, Mattioli M, Reggiani A, Wade M, Grimaldi B. Dual inhibition of REV-ERBβ and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene. 2015;34(20):2597–2608. doi: 10.1038/onc.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torrente E, Parodi C, Ercolani L, De Mei C, Ferrari A, Scarpelli R, Grimaldi B. Synthesis and in vitro anticancer activity of the first class of dual inhibitors of REV-ERBβ and autophagy. J Med Chem. 2015;58(15):5900–5915. doi: 10.1021/acs.jmedchem.5b00511. [DOI] [PubMed] [Google Scholar]
- 130.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4(8):914–927. doi: 10.1158/2159-8290.CD-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bray K, Mathew R, Lau A, Kamphorst JJ, Fan J, Chen J, Chen HY, Ghavami A, Stein M, DiPaola RS, Zhang D, Rabinowitz JD, White E. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLoS ONE. 2012;7(7):e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]