Abstract
Rheumatoid arthritis (RA) is a chronic, autoimmune, inflammatory disease destroying articular cartilage and bone. The female preponderance and the influence of reproductive states in RA have long linked this disease to sexually dimorphic, reproductive hormones such as prolactin (PRL). PRL has immune-enhancing properties and increases in the circulation of some patients with RA. However, PRL also suppresses the immune system, stimulates the formation and survival of joint tissues, acquires antiangiogenic properties upon its cleavage to vasoinhibins, and protects against joint destruction and inflammation in the adjuvant-induced model of RA. This review addresses risk factors for RA linked to PRL, the effects of PRL and vasoinhibins on joint tissues, blood vessels, and immune cells, and the clinical and experimental data associating PRL with RA. This information provides important insights into the pathophysiology of RA and highlights protective actions of the PRL/vasoinhibin axis that could lead to therapeutic benefits.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-016-2187-0) contains supplementary material, which is available to authorized users.
Keywords: Gender, Stress, Reproduction, Angiogenesis, Joint tissues, Immune cells, Cartilage, Bone, Blood vessels
Introduction
Rheumatoid arthritis (RA) is a disabling disease affecting nearly 1 % of the adult population worldwide with a female:male ratio of 3:1. The causes of RA remain unknown but may involve a combination of genetic, environmental, and host factors that initiate the response to an unidentified trigger and result in the recruitment of immune cells into the joints. Production of autoantibodies, inflammatory interactions between activated immune cells and synoviocytes, and synovial angiogenesis sustain and amplify the inflammatory reaction. Chronic inflammation, invasion, cell death, and proteolytic matrix degradation destroy the adjacent cartilage and bone and lead to systemic complications and increased comorbidities.
Among host factors able to affect the pathophysiology of RA is the sexually dimorphic hormone, prolactin (PRL). PRL is a stress-related [1, 2] and reproductive hormone, essential for lactation, that regulates a wide diversity of processes [3–5] including events in joint tissues and immune cells. PRL regulates cartilage survival [6, 7], bone formation and resorption [6, 8], the growth of blood vessels [9], the proliferation, survival, and function of immune cells [10–12], and the progression of rheumatic diseases [6, 10, 11]. Some of these actions are affected by the proteolytic conversion of PRL to vasoinhibins, a family of PRL fragments with effects opposite to those of PRL on inflammation and angiogenesis [13]. The generation, secretion, and action of PRL and vasoinhibins are integrated at the hypothalamus, the pituitary, and the target tissue levels and this organization has been recently defined as the PRL/vasoinhibin axis [14]. In this review, we briefly summarize current knowledge of risk factors for RA that can be linked to PRL, the effects of PRL and vasoinhibins on joint tissues, blood vessels, and immune cells, and the experimental and clinical evidences associating PRL with RA. We propose that the ability of the PRL/vasoinhibin axis to exert opposing effects on inflammatory reactions and angiogenesis contributes to the controversial role of PRL on the pathophysiology of RA and offers opportunities for the development of new treatments.
RA essentials
RA is the most common form of inflammatory arthritis; it occurs at any age but its incidence is higher among those aged 40–70 years. Chronic synovitis underlies the primary manifestations of this disease, including pain, stiffness, and swelling, which are eventually followed by cartilage destruction, bone erosion, subsequent joint deformities, and systemic complications.
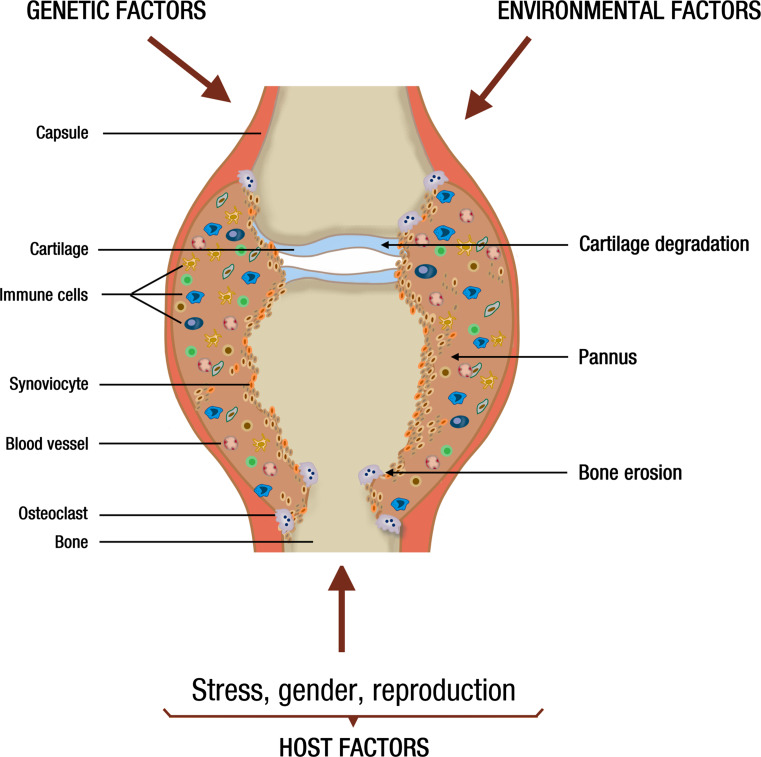
The detailed essentials of RA are beyond the scope of this article and can be found in several reviews [15–18]. A major challenge is that RA manifests in distinct disease subsets with different phenotype, fluctuating course, and uncertain progression. While some patients show a mild development that can be self-limiting, others acquire severe, erosive disease with systemic manifestations. Nonetheless, it is increasingly clear that the outcome of RA depends on the complex interaction of genetic, environmental, and host-related factors, which suggests the existence of parameters that can be evaluated to facilitate early diagnosis and prevention (Fig. 1).
Fig. 1.
Schematic view of a joint affected by RA indicating that a combination of genetic, environmental, and host factors (including stress, gender, and reproduction) influence the recruitment of immune cells and the proliferation of synovial cells, which together with the local formation of blood vessels (angiogenesis), sustain the proliferation of an inflamed, invasive pannus causing cartilage degradation and bone erosion
Studies in monozygotic twins have estimated the relative contribution of genetic factors to be about 50 % for RA predisposition, leaving the remaining part to environmental and host issues [19]. The association between RA and major histocompatibility complex class II polymorphisms, specifically those having a conserved sequence of amino acids in a number of human leukocyte antigen DRBl alleles, called the RA-shared epitope, closely correlates with the presence of rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA), or both [16]. This is consistent with T cell selection and antigen presentation playing a role in the induction of the autoimmune response in RA. However, the shared epitope has not been found in forms of RA negative for RF or ACPA, which may account for up to 20–30 % of patients [16, 20]. Other gene polymorphisms that differ among RA subsets include PTPN22 (coding a tyrosine phosphatase that has a role in T-cell and B-cell signaling) [21] and genes implicated in nuclear factor κB (NF- κβ)-dependent signaling [Tumor necrosis factor receptor-associated factor 1(TRAF1-C5)] [22], interferon regulatory factors [interferon regulatory factor 5] [23], and T-cell stimulation, activation, or differentiation [signal transduction and activator of transcription 4 (STAT4)] [24].
In general, individual gene polymorphisms are of minor value for predicting disease risk unless they combine with environmental or host RA risk factors. For example, smoking is the best-defined environmental risk factor for RA, and the risk for RA increases 21-fold in smokers that are ACPA positive and homozygous for the RA shared epitope, compared to non-smokers without the shared epitope gene [25]. Likewise, stress, gender, and reproduction are host factors influencing the susceptibility for RA in genetically restricted individuals (Fig. 1).
Stress
The long-held perception that psychological stress triggers and worsens RA has been the focus of much basic and clinical research [26–28]. Different forms of stress act in the brain (hypothalamus and brain stem) to activate efferent neuronal (i.e., norepinephrine) and hormonal (i.e., glucocorticoids, epinephrine, and PRL) pathways modifying the level of inflammation and pain [28–31]. The high prevalence of stressful events preceding the onset of RA supports the pathogenic role of stress [32], although this conclusion remains controversial [27]. Moreover, the influence of stress on RA appears to vary depending on the duration, intensity, and type of stressor, with short-lived, minor stress increasing [27, 28, 33, 34] and chronic, stronger stress ameliorating [27, 28, 35] disease activity. Similarly, exposure to different psychological or physical stressors can promote or reduce the onset and activity of experimental arthritis in rodents [36–38].
In general, stress has been considered as immunosuppressive. Exposure to stress mediators (glucocorticoids and catecholamines) counter-regulates increases in inflammatory activity by suppressing T helper 1 (Th1) cell responses and proinflammatory cytokine [interleukin-12 (IL-12), interferon γ (IFNγ), and tumor necrosis factor α (TNFα)] production and by boosting T helper 2 (Th2) responses and production of anti-inflammatory cytokines (IL-10, IL-4) [39–41]. However, glucocorticoids and catecholamines can also worsen inflammation by eliciting the expression of pro-inflammatory mediators (IL-12, IFN-γ, TNFα, IL-6, IL-23) and the down-regulation of IL-10 [28, 39, 42–44]. These opposing actions may relate to the concentration of stress hormones. There is evidence that lower cortisol, catecholamine, and PRL concentrations are immunostimulatory, whereas higher levels are immunosuppressive [26, 45–47]. Along this line, stressors can increase PRL levels in the hypothalamus by downregulating its proteolytic conversion to vasoinhibins [48], which have anxiogenic [48] and proinflammatory effects [49]. Of note, stress axes are hyporesponsive in patients with RA [50–52] and in experimental models of RA [53], and it has been proposed that the inadequate secretion of stress hormones constitutes the basis for stress-induced aggravation of the disease [26, 28]. In this regard, elevating the levels of endogenous glucocorticoids, which appear insufficient to reduce inflammation, is the basis for their successful long-term therapeutic use in RA patients [54].
Gender and reproduction
Women are more susceptible to RA than men. Increased incidence is established before menopause, implying that hormonal and reproductive exposures are involved [55]. The sex hormones estrogens, androgens, and PRL influence susceptibility to RA. However, the metabolism of androgens to estrogens, hormone levels, age, reproductive state, and the fact that each hormone is able to exert opposing effects on immune responses have complicated the study of their role in gender- and reproduction-related RA susceptibility.
In general, androgens are anti-inflammatory and suppress both humoral and cellular immune responses, whereas estrogens reduce cellular and enhance humoral immunity [56]. Ovariectomy increases and estrogens reduce the severity of collagen-induced arthritis in female mice [57–60]. In castrated male and female arthritic rats, administration of androgens that can (testosterone) and cannot (dihydrotestosterone) be converted to estrogens alleviates the disease [61]. Because lower androgen levels occur in men [62] and in women [63] with RA, androgen deficiency could explain gender differences in RA [55, 64].
The protective role of ovarian steroids is further suggested by studies showing that contraceptive treatment decreases RA risk [65, 66] and ameliorates its severity [67], although a lack of effect has also been reported [68]. More importantly, RA often shows remission in pregnancy and exacerbation during the postpartum period in women [69] and in rodents subjected to the experimental disease [70–72]. In the context of RA being characterized by a Th1-driven immunity, the shift to a dominant Th2 phenotype occurring during pregnancy is beneficial [73], and the high gestational levels of estrogens and progesterone promote the Th2-mediated responses [74]. Therefore, protection against RA after delivery would be withdrawn by the decrease in steroid hormones and the re-establishment of a Th1 immune response [75]. Indeed, estradiol treatment immediately after parturition protects mice from a postpartum flare of the disease. Estradiol administration also increases systemic PRL, and lactating mice show a reduced postpartum exacerbation [70]. Nonetheless, breastfeeding, a stimulus that elevates circulating PRL levels, enhances the severity [76] but reduces the risk [77] of RA.
While it is evident that the influence of stress, gender, and reproduction on RA results from complex interactions between neuroendocrine and immunologic systems among persons genetically prone to RA, optimal management of such interactions could help to prevent and control the disease. More in-depth studies are needed to address the role of hormonal mediators upregulated during conditions affecting the course of RA. One such influence is the activation of the PRL/vasoinhibin axis.
PRL and vasoinhibins
PRL is a multifaceted anterior pituitary hormone discovered by its stimulatory effect on milk production; it is known to regulate a strikingly diverse array of physiological functions, which include events in reproduction, osmoregulation, growth, brain function, metabolism, immune response, and angiogenesis [3, 5, 9, 10, 13, 78]. PRL synthesis has been demonstrated in numerous extra-pituitary tissues [79, 80] which, together with the ubiquitous expression of its receptors [3, 79], has led to the concept of PRL acting both as a circulating hormone and as a local regulator or cytokine. The tenet of PRL as a classical cytokine is further established by the fact that PRL is a member of the hematopoietic family of cytokines. Like other members of this family, PRL has a three-dimensional structure consisting of four long α-helices arranged in antiparallel fashion and linked by flexible loops [81]. PRL receptors also share structural and functional characteristics with the hematopoietin receptor superfamily [81], and they signal by activating various kinases including its canonical Janus kinase 2 (JAK2)-signal transducer and activator of transcription (STAT) pathway, the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and the mitogen-activated protein kinase (MAPK) pathway [82].
Adding complexity to PRL actions is the structural polymorphism of the hormone [83]. PRL is proteolytically cleaved to vasoinhibins, a family of PRL fragments with molecular masses ranging from 11 to 18 kDa, that signal through receptor complexes distinct from the PRL receptor [84, 85] to exert effects opposite to those of the full-length hormone (23 kDa) on blood vessels. PRL stimulates blood vessel growth (angiogenesis), whereas vasoinhibins inhibit angiogenesis, vasodilation, and vasopermeability [9, 13]. Beside vascular actions, vasoinhibins have other effects opposite to PRL. Vasoinhibins promote anxiety [48] whereas PRL is anxiolytic [86]; and vasoinhibins [49] and PRL [87] stimulate and inhibit inflammatory reactions in lung tissues, respectively. The structural diversity of vasoinhibins derives from the fact that different proteases, including cathepsin D, matrix metalloproteases (MMP) and bone morphogenetic protein-1 (BMP-1), generate the various fragments by cleaving near or within various sites of the large disulfide loop linking α-helixes 3 and 4 of the PRL molecule (for reviews [13, 14, 88]). Of relevance to RA, joint tissues are targets of the PRL/vasoinhibin axis. PRL is present in synovial fluid [89], and both PRL and vasoinhibins are produced in joint tissues, including cartilage [90], vascular endothelium [91], synoviocytes [92], fibroblasts [49], and immune cells [10, 79, 92], where they can act in local- and hormonal-related fashion.
Effects of PRL and vasoinhibins on joint tissues
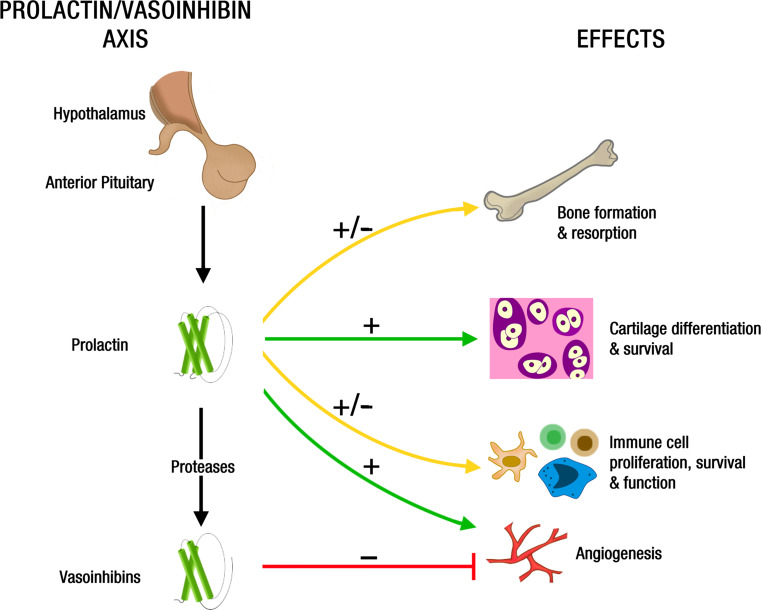
A summary of PRL and vasoinhibin effects on joint tissues is illustrated in Fig. 2.
Fig. 2.
Schematic representation of PRL and vasoinhibin effects on joint tissues. The anterior pituitary gland secretes PRL and vasoinhibins into the systemic circulation. Also, PRL produced locally by joint tissues can be proteolytically converted to Vi. By virtue of mechanisms poorly understood, PRL stimulates and inhibits (±) bone formation and resorption, promotes (+) cartilage differentiation and survival, and stimulates and inhibits (±) immune cell proliferation, survival, and function. In addition, PRL is proangiogenic (+) and, upon proteolytic cleavage to vasoinhibins, acquires antiangiogenic (−) properties. The effects of Vi on bone, cartilage, and immune cells are unknown
Bone and cartilage
Bone loss occurs in association with hyperprolactinemia during lactation, in patients with prolactinomas, and in patients treated with antipsychotic drugs [93–96]. Although increased bone loss involves PRL-induced hypogonadism [93, 97] and the interaction of PRL with other hormones (parathyroid hormone related peptide [98]), clinical [96] and experimental evidence support direct effects of PRL on bone metabolism. PRL receptor null mice are osteopenic and display a reduced rate of bone formation [8], indicating that PRL promotes the maintenance of bone mass. However, PRL has opposite effects on bone depending on the age and physiological state of the animals. Prolonged treatment with PRL increases bone formation and bone calcium content in growing rats [99], whereas it stimulates bone resorption and calcium turnover in sexually mature rats [100] and in pregnant and lactating rats [101]. In the latter, bromocriptine-induced inhibition of PRL secretion results in higher bone volume, lower mineral apposition rates, and reduced bone resorption [102]. It has been proposed that PRL stimulates maternal bone turnover during pregnancy and lactation as a means to satisfy the demands of calcium and phosphate needed for fetal growth and milk production [102].
Of note, PRL can act directly on bone. PRL receptors have been found in bone tissues of humans, mice, and rats [103, 104] and in osteoblasts from neonatal rodents [8, 105] and human fetuses [106]. Also, high levels of PRL stimulate bone resorption over bone formation in cultured osteoblasts derived from adult tissues. In these cells, PRL reduces the expression of Runx2 and osteocalcin, lowers alkaline phosphatase activity, and increases the ratio of receptor activator of nuclear factor κB ligand (RANKL) to osteoprotegerin [106, 107]. In contrast to these findings and consistent with the net bone gain effect of PRL observed in young animals, studies on PRL-exposed osteoblasts from fetal bone showed enhanced Runx2 and osteocalcin expression and a decreased RANKL/osteoprotegerin ratio, suggesting stimulation of bone formation and suppression of bone resorption, respectively [106, 108].
Bone tissues affected by PRL include cartilage. Chondrocytes in both reserve and proliferating regions of the developing long bone express PRL receptors [105], but cartilage formation and growth do not seem to be adversely affected in the PRL receptor null mice [8] or after elevating PRL levels during early life [105]. Nonetheless, PRL may contribute to the chondrogenic process by regulating cartilage formation and maintenance in the adult. Human adult bone marrow pluripotential mesenchymal stem cells express PRL receptors and PRL stimulates their proliferation and differentiation into chondrocytes (type II collagen and proteoglycan synthesis) in culture [89]. Also, PRL activates their receptors in articular chondrocytes from rat postpubescent and adult cartilage to inhibit apoptosis [7]. Because cartilage lacks blood vessels, the sparse distribution of chondrocytes encased within the extracellular matrix suggests that autocrine/paracrine anti-apoptotic factors are an efficient mechanism for maintaining cartilage survival. Along this line, PRL is a component of synovial fluid [89] and is generated by articular chondrocytes [90] and bone-marrow mesenchymal cells undergoing chondrogenic differentiation [89]. Therefore, PRL may help preserve the generation and functional integrity of cartilage. Moreover, chondrocytes produce several MMP that generate vasoinhibins from circulating and locally produced PRL and release vasoinhibins into their conditioned medium [90], suggesting that the PRL/vasoinhibin axis contributes to the local maintenance of cartilage avascularity.
Blood vessels
The regulation of blood vessel growth and function underlies the physiology of joint tissues. Fenestrated capillaries close to the synovial surface are a main source of synovial fluid, a plasma ultra-filtrate transporting lubricants, nutrients, waste products, hormones, growth factors, and cytokines within the joint, particularly to the avascular cartilage. Avascularity is essential for the biomechanical properties of cartilage and, although the subchondral vasculature contributes to the metabolic support of normal articular cartilage [109], vascular invasion from bone into cartilage leads to chondrocyte apoptosis and is a mechanism mediating cartilage substitution by bone during growth [110]. While blood vessels are known targets of PRL and vasoinhibins, and chondrocytes produce these peptides [90], no study has addressed the actions of the PRL/vasoinhibin axis on the joint vasculature.
The effects and signaling mechanisms of PRL and vasoinhibins on blood vessels have been well documented and reviewed [9, 13, 88]. The following summarizes some of the relevant findings. PRL promotes in vivo angiogenesis in the corpus luteum [111], the testis [112], the heart [113], the pancreas [114], and the liver [115]. This action involves a direct effect on endothelial cells [116–118], through both long and short isoforms of the PRL receptor [119–121], but also through the PRL receptor on other cell types (epithelial, immune, and stromal cells) where PRL induces the expression of proangiogenic factors, such as vascular endothelial growth factor (VEGF) [122, 123] and fibroblast growth factor-2 (FGF-2) [124, 125]. PRL signals to promote endothelial cell proliferation, migration, and tube formation through the JAK2/MAPK/early growth response gene-1 pathway [122], the heme oxygenase-1 (HO-1) [116], and the JAK2/STAT5 pathways [118]. However, controversial findings (for a review see [9, 13, 126]) have shown that PRL is unable to stimulate angiogenesis in the cornea and the in vitro proliferation of some endothelial cell types. Also, lack of PRL expression increases angiogenesis in the retina [127] and associates with highly vascularized pituitary tumors [128]. Moreover, PRL has opposing effects on vascular resistance, blood flow, and vasopermeability that depend on experimental conditions (for a review see [126]). These inconsistencies may result from a variable rate of proteolytic conversion of PRL to vasoinhibins.
Vasoinhibins inhibit vasoproliferation, vasodilation, and vasopermeability and promote vascular regression in the cornea [129], retina [127, 130], heart [113], and xenografted tumors [85]. They act directly on endothelial cells to inhibit the action of several vasoactive substances, including VEGF, FGF-2, interleukin 1β, bradykinin, and acetylcholine [130–135]. Binding sites for vasoinhibins were reported in endothelial cell membranes, but their chemical nature was not resolved [84]. Recently, vasoinhibins were shown to bind to a multicomponent complex conformed by plasminogen activator inhibitor-1 (PAI-1), urokinase plasminogen activator (uPA), and the urokinase plasminogen activator receptor (uPAR) on endothelial cells and that such binding was required for some antiangiogenic properties of vasoinhibins [85]. Vasoinhibins signal by blocking the activation of the Ras–Raf–MAPK pathway [136, 137], the Ras–Tiam1–Rac1–Pak1 pathway [133], and the Ca2+/calmodulin-mediated activation of endothelial nitric oxide synthase (eNOS) [134]. They also promote protein phosphatase 2A-induced dephosphorylation and inactivation of eNOS [130], downregulate transient receptor potential channel 5 expression [138], stimulate the activation of proapototic proteins of the Bcl-2 family, and the NFκB-mediated activation of caspases [139] and upregulation of microRNA-146a expression [140].
Therefore, PRL and vasoinhibins act through the activation of distinct receptors and target cells to stimulate and inhibit blood vessels, respectively. Vasoinhibins bind to a multi-component complex involving PAI-1, uPA, and uPAR on endothelial cells, while PRL signals through the PRL receptor not only on endothelial cells, but also on other cells in the capillary microenvironment, including immune cells (Fig. 2).
Immune cells
The action of PRL on immunocytes has been known for more than three decades, when PRL effects [141] and PRL receptors [142] on immune cells were first discovered. PRL operates not only by systemic but also by paracrine/autocrine mechanisms to regulate immune responses [10–12, 29, 80, 143–145]. PRL and the PRL receptor (PRLR) are expressed on T cells, B cells, natural killer (NK) cells, macrophages, neutrophils, and antigen-presenting dendritic cells; and PRL regulates various immune cell responses, including T-cell proliferation and survival, B-cell antibody production, NK-cell proliferation and mediated cytotoxicity, and macrophage effector functions. Notably, PRL exhibits both inmunoenhancing and immunosuppressive properties depending on its concentration (with lower levels augmenting and higher levels inhibiting immune responses) [12, 143, 145].
The first observation linking high PRL levels with immunosuppression was made more than 40 years ago, when lactation [146], as well as PRL treatment [147], was shown to increase susceptibility to worm-infection resulting from impaired differentiation of lymphocytes to mature effector cells. The opposite actions of different PRL concentrations was initially suggested by showing that a lower dose of PRL (100 μg/rat) stimulated antibody production and T cell proliferation, whereas a higher dose (400 μg/rat) was inhibitory [148]. Also, the number and function of circulating NK cells and mature T cells were reduced in patients with hyperprolactinemia (32–394 ng/ml) [149–151], and in vitro studies showed that higher (100–200 ng/ml) and lower (12–25 ng/ml) PRL levels inhibit and stimulate, respectively, the proliferation of blood NK cells and T cells [47]. Likewise, lower concentrations of PRL (<20 ng/ml) were more effective than higher PRL levels (100 ng/ml) in stimulating antibody production by circulating lymphocytes from patients with systemic lupus erythematosus [152]; while high doses of PRL enhanced the in vitro release of proinflammatory mediators by peritoneal macrophages and peripheral blood mononuclear cells, a much higher dose induced the release of anti-inflammatory IL-10 [153, 154].
Nevertheless, it has been widely assumed that PRL functions as an immuno-enhancing hormone. This notion is largely based on the use of experimental models of PRL deficit, including dwarf mice [155], hypophysectomized rats [144], and treatment with bromocriptine (a dopamine D2 receptor agonist that inhibits pituitary PRL release) in rodents and humans [11, 144]. These experimental approaches result in deficient B- and T-cell-mediated immune responses that can be restored by PRL treatment. However, it should be noted that multiple endocrine pathways are being altered in these experimental models, PRL is also produced by extrapituitary sources [79, 80], and bromocriptine can affect immune function independently of PRL [156, 157]. A more direct evaluation of loss of PRL function on the immune system has been done using PRL [158] and PRLR [159] null mice. Both mice exhibit normal numbers of lymphocytes and myeloid cells and mount effective innate and adaptive immune responses when subjected to immune challenges, thus implying that PRL is not essential for normal immune system development and function. Nonetheless, compensatory actions by other cytokines have not been examined, and experiments using these mice have not ruled out a contribution of PRL to immune-system homeostasis under conditions characterized by the up-regulation of PRL levels, such as under stress [29], reproductive adaptations, and pathologic hyperprolactinemia [160].
Indeed, renewed interest has focused on the possibility that elevated circulating PRL levels following stress [1, 2] represent an adaptation to help adjust the frequently observed stress-related immunosuppression [29]. PRL improves macrophage and splenocyte functions following trauma-hemorrhage and infections [161, 162], stimulates lymph node cellularity and antigen-specific proliferative responses under stressful housing conditions [155, 163], and it can help counteract glucocorticoid-induced lymphocyte apoptosis and signaling [164, 165]. However, PRL can also inhibit immune responses during stress. Bone marrow myelopoiesis and splenic lymphocyte proliferation induced by a burn injury are enhanced in PRL null mice [166], PRLR knockout mice show increased mortality and elevated IL-6 after partial hepatectomy [115], and PRL administration associates with decreased survival and an inhibition of cellular immune functions in septic mice [167]. The reason for the opposite effects of PRL on immune function during stress is unclear, but may relate to differences in PRL levels and in immune processes occurring in response to specific stressors. A possible explanation also involves the generation of vasoinhibins from PRL. Exposure to physical restraint increased PRL levels in the hypothalamus partly by reducing its rate of cleavage to vasoinhibins [48]. Because vasoinhibins are anxiogenic, this change would favor the anxiolytic effect of PRL, thereby reducing the emotional response to stress [48]. Similarly, such reciprocal interplay during stress could favor PRL-induced suppression of inflammatory responses. Vasoinhibins act as potent proinflammatory cytokines, via the activation of NFκB signaling pathways, to stimulate inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) production in fibroblasts and type II epithelial cells of the lung [49, 168], which are important cells for inflammatory reactions in the airways. Conversely, PRL has anti-inflammatory effects on the same cells [87].
Pregnancy and lactation constitute other important contexts for understanding the role of PRL in the immune system. Elevated PRL levels in pregnancy and early stages of lactation may help suppress immune responses required for successful maternal-fetal and maternal-nursing young interactions. Excessive production of IL-6 in the decidua can trigger an inflammatory response that leads to the termination of pregnancy, and estradiol and PRL inhibit IL-6 expression and signaling by decidual cells [169]. Moreover, PRL produced by the decidua [170] and PRL-like proteins produced by the placenta [171] interact with maternal immune cells (NK cells and megakaryocytes) to ensure successful fetal development [172, 173], and PRL regulation of humoral immunity can influence the levels of immunoglobulins in milk [174–176].
PRL and RA
The association of RA with stress, gender, and reproduction prompted the investigation of PRL as a biological explanation, but the significance of PRL to RA pathogenesis remains unclear. Several reviews have addressed this topic under the general assumption that PRL is a negative (proinflammatory) influence aggravating the disease [10, 177–186]. This conclusion contrasts with the lack of agreement between circulating PRL levels and disease severity, the inconsistent action of dopamine agonists and antagonists, the protective and worsening influence of physiological hyperprolactinemia (pregnancy and lactation), and the ability of PRL to exert both stimulatory and inhibitory effects on immune cells and joint tissues. Our work reviews this controversial field with the idea that its solution requires understanding the mechanisms mediating the dichotomous actions of PRL. Because PRL and vasoinhibins have opposite effects on immune reactions and angiogenesis, we propose that explanatory mechanisms lie within the PRL/vasoinhibin axis, an integrative framework influencing not only the levels of systemic and local PRL, but also the proteolytic conversion of PRL to vasoinhibins.
Clinical data
Several [182, 187–189], albeit not all [189–191], epidemiological studies have claimed that nulliparity and, paradoxically, the postpartum period increase the risk for developing RA. Nulliparity could be linked to hyperprolactinemia-induced infertility [97] and, of course, breastfeeding in the postpartum period elicits hyperprolactinemia. Originally, the connection between breastfeeding and RA generated controversial findings. Exposure to breastfeeding associated with increased risk [192, 193], had no effect [189] or was protective [194] against RA development. However, large cohort studies have now shown that breastfeeding for more than 12 months is inversely related to the development of RA and suggested that the protective effect of parity could be attributed to confounding breastfeeding in previous lactations among parous women [77, 191, 195].
The associations above encouraged an investigation of PRL levels in the circulation of patients with RA (Table 1). Several reports found that PRL levels were higher, albeit usually within the normal range (≤20 ng/ml), in patients with RA compared to healthy or osteoarthritic subjects [196–204]. Nonetheless, no differences [51, 52, 205–209], as well as lower than normal levels of the systemic hormone [210], have also been reported in RA. Because PRL levels vary according to the time of day and stress exposure [1, 2, 211], studies attempting to control for these variations showed that the RA group had significantly higher PRL levels at the diurnal hormonal peak, before and after stress (surgery), and following thyrotrophin releasing hormone (TRH) stimulation [197, 202]. However, no difference in PRL levels after TRH or even a decreased PRL release in response to other forms of stress (hypoglycemia), were also found [51, 205, 209]. Moreover, lowering circulating levels of PRL with dopamine D2 receptor agonists (bromocriptine, quinagolide, cabergoline) proved to be effective [177, 212–214], or ineffective [215–217] for reducing RA. In one report, RA improved after treatment with cabergoline in a hyperprolactinemic patient (76.5 ng/ml) with a microprolactinoma [214]. However, cases of PRL excess due to prolactinomas appear to be extremely rare in autoimmune diseases and are not consistent with their severity [218]. Also, PRL levels above normal have been detected in only 1 or 6 % of patients with RA and have either no correlation with disease activity [219] or a low disease activity score [220]. Along this line, human pathological hyperprolactinemia has been associated with immunosuppression (decreased number and function of NK cells and T cells) [149–151].
Table 1.
Circulating PRL levels in RA
| PRL levels | RA (n) | Control (n) | Serum PRL (ng/ml) | p value | Special features | References | |
|---|---|---|---|---|---|---|---|
| RA | Control | ||||||
| Higher | 99 | 68 (OA) | 11.7 ± 7.6a | 8.9 ± 4.0 | <0.01 | PRL levels correlate with RA duration and severity | [196] |
| 10 | 10 (OA) | 21.2−26.9a | 4.7−20.3 | <0.005 | Higher PRL diurnal levels and after surgery in RA | [197] | |
| 39 | 22 | 16.0 ± 11.2a | 10.4 ± 6.9 | <0.05 | PRL levels correlate with chemokine MIP-1α serum levels | [198] | |
| 29 | 30 (OA) | 13.1 ± 1.7b | 7.5 ± 0.5 | <0.01 | PRL levels correlate with RA duration | [199] | |
| 10 | 10 | 20.6 ± 5.1 | 12.1 ± 4.3 | <0.005 | Higher PRL levels in RA only at 0200 h | [200] | |
| 29 | 26 (OA) | 14.1 ± 1.3a | 10.9 ± 0.8 | <0.04 | PRL levels correlate with RA severity | [201] | |
| 23 | 8 | 27.3 ± 3.8b | 8.8 ± 2.2 | <0.01 | Higher PRL levels in response to TRH | [202] | |
| 60 | 31 (OA) | 10.6 ± 4.9a | 8.3 ± 3.2 | <0.04 | Both total and *free PRL were measured | [203] | |
| 20 | 20 | 40.2 ± 5.6a | 16.2 ± 3.1 | <0.001 | PRL levels correlate with ESR and C-reactive protein | [204] | |
| Not different | 38 | 23 | 10 | 8 | n.s. | Higher and lower PRL levels in response to hypoglycemia and TRH, respectively | [205] |
| 27 | 12 (OA) | 6.05 ± 1.1b | 8.49 ± 2.4 | n.s. | PRL levels in plasma and synovial fluid correlate positively | [206] | |
| 20 | 28 | 11.5 ± 7.4a | 12.5 ± 6.5 | n.s. | No association with thyroid autoantibodies in RA | [207] | |
| 10 | 10 | 10.1 ± 1.4b | 13.7 ± 2.4 | 0.32 | Similar PRL levels in response to TRH between RA and control | [208] | |
| 10 | 9 | 10 | 12.5 | n.s. | Similar PRL levels in response to TRH between RA and control | [209] | |
| 20 | 20 | 13.0 ± 7.5a | 15.0 ± 8.0 | 0.2 | Reduced PRL levels in response to hypoglycemia in RA | [51] | |
| 7 | 10 | 10.6 | 10.3 | n.s. | Lower increase in PRL levels in response to exercise in RA vs control | [52] | |
| Lower | 48 | 23 | 7.9 ± 0.3b | 9.5 ± 0.5 | <0.05 | Association between RA and PRL deficiency | [210] |
In some studies PRL data were converted from units to mass (ng/mL × 21.2 = mIU/L). OA indicates osteoarthritic patients as control group
MIP-1α macrophage inflammatory protein-1α, TRH thyrotropin releasing hormone, ESR erythrocyte sedimentation rate, ns non-significant
PRL values are a means ± SD, b mean ± SEM, or means
* Free PRL refers to monomeric, unbound PRL
The major endpoint of these studies is the concentration of systemic PRL. However, it is now clear that local PRL produced and metabolized in the tissues can be influential. In RA, synovium infiltrating T lymphocytes and fibroblast-like synovial cells produce PRL able to promote inflammation at the local level [92]. PRL stimulates proliferation and the production of proinflammatory cytokines and MMP, and it inhibits the synthesis of tissue inhibitor of MMP (TIMP-I) by RA synovial cells in culture [92]. Moreover, bromocriptine inhibits the expression of PRL mRNA by primary cultures of RA synovial cells [92] and can suppress their activity in a PRL-dependent and PRL-independent manner [92, 156, 157]. Also, the presence of a PRL-1149 T polymorphism in the promoter region controlling the production of extrapituitary PRL associates with reduced PRL production by lymphocytes [221], but not with the systemic levels of the hormone, and correlates with a decreased risk of developing RA [222, 223]. Therefore, local PRL levels may correlate better than systemic PRL levels with the ongoing inflammation.
An additional complexity is added by the paradox that activation of dopamine D2 receptors may also protect against RA. Haloperidol, a D2-receptor antagonist leading to hyperprolactinemia [224], alleviates inflammation in RA [225] by mechanisms that may include PRL-induced immunosuppression [6] and the direct blockage of D2 receptors on immune cells able to reduce proinflammatory cytokine release and action [226, 227].
The fact that both dopamine agonists and antagonists ameliorate RA may be due to the negative and positive effects of PRL on immune cells and joint tissues. To understand these opposite actions, it is important to study how PRL levels in the circulation and within the tissue microenvironment are being regulated to affect the function of the various targets. Experimental studies exploring the role of PRL in inflammatory arthritis mirror these conflicting observations but have also contributed to their clarification.
Experimental data
The causative link between PRL and RA is supported by studies using complete Freund’s adjuvant (CFA)-induced arthritis in rats, a well-documented model for the induction of inflammation within joint tissues and for having cartilage and bone destruction similar to that in RA [228, 229]. Early studies claimed that the development of adjuvant arthritis was at least partially dependent on PRL. Hypophysectomized rats did not develop CFA arthritis unless treated with PRL, and the CFA-induced joint swelling in intact rats was reduced by treatment with bromocriptine [230]. Moreover, hypophysectomized rats made hyperprolactinemic by placing anterior pituitary glands (AP) under the kidney capsule developed a more severe arthritis than sham-operated controls [230, 231]. It was reasoned that adrenocortical deficiency due to hypophysectomy favored the manifestation of PRL proinflammatory effects [231]. However, hypophysectomy could be a confounding factor. AP grafts and high PRL levels in the intact organism stimulate glucocorticoid release [232], and APs grafted into sham-operated rats result in a delay in the onset and a reduction in the severity of CFA-induced arthritis and higher corticosterone circulating levels [231]. Therefore, adrenocortical dysfunction due to hypophysectomy could also be counterbalancing the beneficial effects of PRL.
Recent findings support the protective and regenerative effects of hyperprolactinemia in CFA-induced arthritis [6]. Increasing prolactinemia, either by PRL infusion or treatment with haloperidol, before or after inducing arthritis with CFA, ameliorated inflammation and joint destruction as revealed by lower proinflammatory cytokine (TNFα, IL-1β, IFNγ, IL-6) and iNOS expression in joint tissues, and reduced chondrocyte apoptosis, pannus formation, bone erosion, joint swelling, and pain. Proinflammatory cytokines are crucial for initiating the inflammatory process leading to joint destruction in RA [233, 234]. The concentration and expression of proinflammatory cytokines are significantly elevated in serum [228] and joint tissues [6] of CFA-injected rats, respectively, and IL-1 antagonists and TNFα-neutralizing antibodies reduce the severity of arthritis in these animals [229, 235]. Accordingly, PRL could be protecting against CFA-induced arthritis not only by reducing the levels of proinflammatory cytokines, but also by counteracting their proapoptotic and inflammatory effects. PRL inhibited the apoptosis of cultured chondrocytes in response to a combination of TNFα, IL-1β, and IFNγ by blocking the induction of p53 and decreasing the BAX/BCL-2 ratio through a NO-independent, JAK2/STAT3-dependent pathway [6]. Local treatment with PRL or increasing PRL circulating levels also prevented chondrocyte apoptosis evoked by injecting the cytokines into the knee joints of rats, whereas the proapoptotic effect of cytokines was enhanced in PRL receptor null mice [6]. Similar findings were also reported in pulmonary fibroblasts, where the induction of iNOS expression and NO production in response to TNFα, IL-1β, and IFNγ was reduced by PRL [87].
However, other studies have argued against the anti-inflammatory effect of PRL in arthritis. PRL treatment enhances disease progression of collagen-induced arthritis only when delivered during the immunization phase [236], although the same report revealed that treatment with bromocriptine caused exacerbation at a later stage of the disease. In addition, PRL enhances the proliferation and release of IL-6, IL-8, and MMP-3 by RA synovial cells in culture and lowers their production of TIMP-1 [92]. While variations in experimental conditions may contribute to these discrepancies, it cannot be disregarded that PRL can have both protective and exacerbating effects on arthritis and that addressing this paradox is challenging.
Opposing effects may relate to PRL levels since, as previously described, low doses can be proinflammatory and higher doses anti-inflammatory [47, 148, 152]. PRL levels are affected by exposure to a variety of active substances and hormones in the inflammatory milieu. There are reports showing that CFA-induced arthritis lowers [237, 238], enhances [239], or has no effect [6, 240] on PRL circulating levels. Proinflammatory cytokines, such as TNFα and IL-6, stimulate the release of PRL by AP cells [241, 242], and IL-1β, IL-2, and IL-4 downregulate PRL expression in immune cells [243].
Importantly, the inflammatory milieu could influence the conversion of PRL to vasoinhibins. MMP are upregulated in the joints of patients with RA [244] and pro-inflammatory cytokines [233] and PRL [92] stimulate MMP production by RA synovial cells. MMP cleave PRL to vasoinhibins [90] which, by being antiangiogenic, are able to suppress the neovascularization required for pannus formation [245]. Angiogenesis occurs from the early stage of RA and supports the invasive pannus by enabling the continued accumulation of immune cells, the proliferation of the inflamed tissue, and the swelling of the joints [246, 247]. The upregulation of MMP in the arthritic joint (in response to cytokines including PRL) and the fact that hyperprolactinemia reduces pannus formation [6] raise the possibility that, under a sustained administration of PRL, there is an enhanced generation of vasoinhibins which, by virtue of their antiangiogenic properties, contribute to the protective and regenerative effects of PRL.
The above proposal is challenged by the fact that increased collagenase activity leads to joint destruction and is the basis for the use of MMP inhibitors in RA clinical trials [248]. There is also evidence that vasoinhibins act as potent proinflammatory cytokines in lung tissues [49, 168], where PRL is anti-inflammatory [87, 249]. Much work is needed to characterize the proinflammatory effects of vasoinhibins, and nothing is known regarding the actions of these peptides in joint tissues. However, the notion that full-length PRL has anti-inflammatory and proangiogenic effects and acquires pro-inflammatory and antiangiogenic properties after undergoing proteolytic cleavage to vasoinhibins provides an efficient mechanism for balancing these two processes and a new avenue that may clarify the controversial role of this hormone in pathologies such as RA that are characterized by inflammation and exacerbated angiogenesis.
Enhanced RA risk and severity correlate with pregnancy complications affecting the functionality of the PRL/vasoinhibin axis, which may help illustrate the considerations above. Such adverse pregnancy outcomes include preterm and small-for-gestational-age delivery [250], delivery of infants with very low and extremely low birth weight [251], low birth weight in general [252], and preeclampsia [253]. The levels of vasoinhibins are elevated in the serum, urine, and amniotic fluid of patients with preeclampsia, and vasoinhibin values in amniotic fluid are inversely correlated with birth weight [254–256]. Excessive inflammation and dysregulation of angiogenesis inhibitors are key factors in the etiology of preeclampsia [257] that may be influenced by an imbalance of the PRL/vasoinhibin axis [254, 258] and lead to the increased risk and severity of RA.
Evolutionary implications
RA belongs to a large family of acute and chronic arthritic diseases defined as inflammatory arthropathies that, in humans, also include spondyloarthritis (comprising ankylosing spondylitis, reactive arthritis, psoriatic arthritis, arthritis conditions associated with inflammatory bowel disease), septic arthritis, gout, and osteoarthritis. These are ancient diseases with cases identified in fossil records from millions of years ago in Triassic dinosaurs and Pleistocene mammals, and from thousands of years ago in Neolithic man (reviewed by [259]). In spite of evolutionary pressures, inflammatory arthropathies stand as prevalent disorders across contemporary terrestrial vertebrates. To explain such prevalence, it is proposed that the painful and limiting characteristics of arthritis could have represented an adaptation selected to restrain animals from physically demanding activities and energy expenditure when facing adverse conditions such as starvation, stress, pregnancy, lactation, and aging [260]. The strong association between inflammatory arthropathies and age, gender, and reproduction suggests that factors upregulated under these conditions helped to maintain these diseases throughout evolution. One such factor is PRL, a stress-related, sexually dimorphic, reproductive hormone with an ancient origin. The reader is directed to a recent overview addressing this idea [259].
PRL is thought to have evolved 400 millions years ago [261] to regulate osmoregulation and dispersion of skin pigments in fish and amphibians, ancestral activities that continue to be present in the PRLs of higher vertebrates [262]. Likewise, providing nutrients to the young and stimulating parental behavior, the best-known effects of PRL, also emerged in fish and have been retained in fish, birds, and mammals [259, 262].
The high-energy demands associated with feeding and tending the young make wild organisms particularly susceptible to adverse stressful conditions. Stress downregulates PRL levels in birds, thereby interfering with parental behavior [263]. In mammals, there is an attenuation of physiological and behavioral stress responses during pregnancy and lactation [264] that is counteracted by inhibiting the expression of PRL receptors in the brain [86]. Reduced reaction to stress not only ensures maternal care under aversive conditions, but can also be detrimental for parent survival. PRL inhibits anxiety [86] but acquires anxiogenic properties upon conversion to vasoinhibins [48]; thus, the PRL/vasoinhibin axis [14] may represent an efficient mechanism for adjusting stress responses and maximizing reproduction and survival under challenging conditions such as arthritis.
While ample evidence exists in rodents and humans, there have been remarkably few or no studies in non-mammalian vertebrates regarding effects of PRL on joint tissues and immune cells. However, the facts that reproductive effects of PRL and PRL stress-related interactions are phylogenetically conserved and that PRL receptors are present in immune, bone, and muscle cells of fish [265, 266] and birds [267, 268] prompt the speculation that the PRL functions in non-mammalian vertebrates, similar to its role in mammals, as an epigenetic, adaptive regulator of arthritis development, influencing its prevalence throughout evolution.
As for the PRL/vasoinhibin axis, a PRL sequence comparison between species of several taxons indicates the possibility that vasoinhibins first emerged in tetrapods as no vasoinhibin-generating cleavage site was identified in Teleost fish (Zebrafish), Ray-finned-fish (Spotted gar), and Lobe-finned fish (Coelacanth). Amphibians, reptiles and birds, however, possess cleavage sites required for the generation of vasoinhibins, indicating the possible existence of a PRL/vasoinhibin axis in these vertebrates [14]. The emergence of the PRL/vasoinhibin axis in tetrapods correlates well with the effects of this axis on joint physiology and disease, as arthritis essentially occurs in terrestrial vertebrates [259].
Concluding remarks
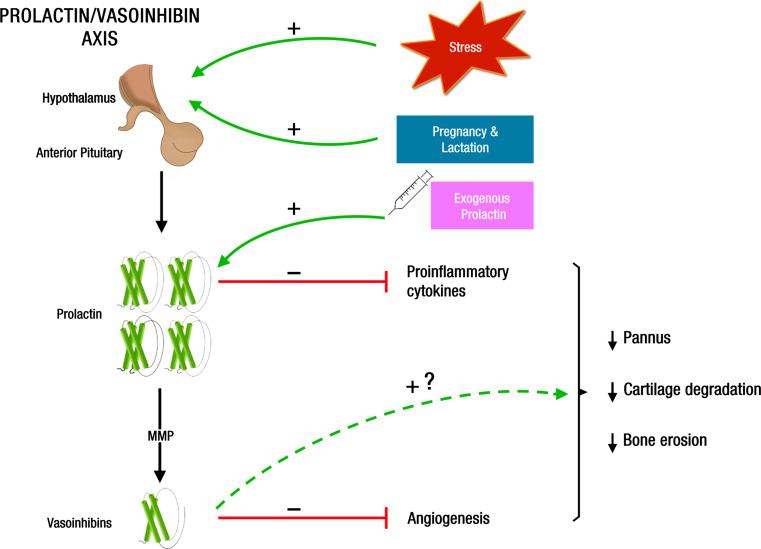
The influence of stress, gender, and reproduction on the pathogenesis of RA points to a possible contribution of PRL, a stress-related, sexually dimorphic, reproductive hormone with effects on joint tissues and immune cells. The female preponderance of RA, the increased levels of PRL in the circulation of patients with RA, and the ability of PRL to promote inflammation have been the basis for the long-held notion that this hormone is a negative factor aggravating RA. However, the risk and severity of RA are downregulated in reproductive states characterized by hyperprolactinemia (pregnancy and lactation); the circulating levels of PRL only increase in a small proportion of RA patients, and this hormone is also immunosuppressive. The paradox of PRL having both protective and exacerbating effects in RA is better explained when considering the PRL/vasoinhibin axis. This organizational principle takes into account that PRL actions are regulated at the hypothalamus, pituitary gland, and target-tissue levels not only by altering the synthesis and release of systemic and local PRL, but also by regulating the proteolytic conversion of PRL to vasoinhibins as PRL and vasoinhibins activate distinct receptor complexes and cell types to exert their opposite effects on blood vessels and inflammatory reactions. The PRL/vasoinhibin axis is exposed to a variety of active substances that vary according to stress-related and reproductive events preceding and modifying the progression of RA. Some of these agents (catecholamines, glucocorticoids, sex steroids, proinflammatory cytokines, anti-inflammatory cytokines) alter the systemic and local concentrations of PRL and vasoinhibins. Variations in the levels of PRL and vasoinhibins influence the direction of their action in RA. Lower and higher levels of PRL are immunostimulatory and immunosuppressive, respectively, and vasoinhibins counteract the anti-inflammatory and proangiogenic effects of PRL. Of much interest are observations in which hyperprolactinemic states (stress, pregnancy, and lactation) and PRL treatment protect against inflammation and joint destruction in arthritis by mechanisms that may include reduced proinflammatory cytokine expression and action and the upregulation of cleaving proteases (MMP) leading to vasoinhibin-mediated antiangiogenesis, actions that lead to reduced pannus formation, cartilage degradation, and bone erosion (Fig. 3). A therapy based on elevating PRL serum levels may be comparable to the well-established use of glucocorticoids in patients with RA, whose levels of the endogenous hormones appear insufficient to control the disease [269].
Fig. 3.
Mechanisms that contribute to PRL-mediated protection against RA. Hyperprolactinemia induced by stress, reproduction (pregnancy and lactation), and PRL administration acts on joint tissues to reduce proinflammatory cytokine expression and action and to upregulate PRL cleaving proteases (MMP) leading to vasoinhibin-mediated antiangiogenesis; these effects, in turn, lead to reduced pannus formation, cartilage degradation, and bone erosion. Vasoinhibins may act as proinflammatory factors able to counteract PRL anti-inflammatory effects in arthritis, a possibility that needs to be investigated
There is much to learn about the mechanisms mediating the opposite effects of PRL on joint tissues and immune cells and about how such mechanisms interact with those activated by vasoinhibins to result in specific outcomes in RA. Unraveling interactions at the level of the PRL/vasoinhibin axis will very likely help to understand the pathogenesis of RA and offer new treatment approaches to control this complex disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Fernando Lopez-Barrera, Gabriel Nava, and Francisco Javier Valles Valenzuela for their technical assistance, Guadalupe Calderon for artistic contribution, and Dorothy D. Pless for critically editing the manuscript. N. Adan, M.G. Ledesma-Colunga, and M. Solis-Gutierrez were supported by fellowships from the Council of Science and Technology of Mexico (CONACYT) and the Ph.D. Program in Biomedical Sciences of the National University of Mexico (UNAM). The study was supported by UNAM Grant IN201315 to C. Clapp.
Abbreviations
- RA
Rheumatoid arthritis
- PRL
Prolactin
- ACPA
Anti-citrullinated peptide antibodies
- RF
Rheumatoid factor
- TRAF1-C5
Tumor necrosis factor receptor-associated factor 1
- STAT
Signal transducer and activator of transcription
- NF-κβ
Nuclear factor κβ
- Th1
T helper 1
- Th2
T helper 2
- IL
Interleukin
- IFNγ
Interferon γ
- TNF α
Tumor necrosis factor α
- JAK
Janus kinase
- PI3k
Phosphatidylinositol 3-kinase
- MAPK
Mitogen-activated protein kinase
- BMP-1
Bone morphogenetic protein-1
- RANKL
Receptor activator of NF-κβ ligand
- VEGF
Vascular endothelial growth factor
- FGF-2
Fibroblast growth factor-2
- HO-1
Heme oxigenase-1
- BK
Bradykinin
- ACh
Acetylcholine
- PAI-1
Plasminogen activator inhibitor-1
- uPA
Urokinase plasminogen activator
- uPAR
Urokinase plasminogen activator receptor
- eNOS
Endothelial nitric oxide synthase
- PP2A
Protein phosphatase 2A
- TRPC5
Transient receptor potential channel 5
- PRLR
Prolactin receptor
- NK
Natural killer
- iNOS
Inducible nitric oxide synthase
- NO
Nitric oxide
- TRH
Thyrotropin-releasing hormone
- TIMP-1
Tissue inhibitor of MMP
- CFA
Complete Freund´s adjuvant
- AP
Anterior pituitary gland
References
- 1.Gala RR. The physiology and mechanisms of the stress-induced changes in prolactin secretion in the rat. Life Sci. 1990;46:1407–1420. doi: 10.1016/0024-3205(90)90456-2. [DOI] [PubMed] [Google Scholar]
- 2.Reichlin S. Prolactin and growth hormone secretion in stress. Adv Exp Med Biol. 1988;245:353–376. doi: 10.1007/978-1-4899-2064-5_28. [DOI] [PubMed] [Google Scholar]
- 3.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 4.Goffin V, Bouchard B, Ormandy CJ, Weimann E, Ferrag F, Touraine P, Bole-Feysot C, Maaskant RA, Clement-Lacroix P, Edery M, Binart N, Kelly PA. Prolactin: a hormone at the crossroads of neuroimmunoendocrinology. Ann N Y Acad Sci. 1998;840:498–509. doi: 10.1111/j.1749-6632.1998.tb09588.x. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adan N, Guzman-Morales J, Ledesma-Colunga MG, Perales-Canales SI, Quintanar-Stephano A, Lopez-Barrera F, Mendez I, Moreno-Carranza B, Triebel J, Binart N, Martinez de la Escalera G, Thebault S, Clapp C. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Invest. 2013;123:3902–3913. doi: 10.1172/JCI69485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zermeno C, Guzman-Morales J, Macotela Y, Nava G, Lopez-Barrera F, Kouri JB, Lavalle C, de la Escalera GM, Clapp C. Prolactin inhibits the apoptosis of chondrocytes induced by serum starvation. J Endocrinol. 2006;189:R1–R8. doi: 10.1677/joe.1.06766. [DOI] [PubMed] [Google Scholar]
- 8.Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96–105. doi: 10.1210/endo.140.1.6436. [DOI] [PubMed] [Google Scholar]
- 9.Clapp C, Thebault S, Macotela Y, Moreno-Carranza B, Triebel J, Martinez de la Escalera G. Regulation of blood vessels by prolactin and vasoinhibins. Adv Exp Med Biol. 2015;846:83–95. doi: 10.1007/978-3-319-12114-7_4. [DOI] [PubMed] [Google Scholar]
- 10.Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmun Rev. 2015;14:223–230. doi: 10.1016/j.autrev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Yu-Lee LY. Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res. 2002;57:435–455. doi: 10.1210/rp.57.1.435. [DOI] [PubMed] [Google Scholar]
- 12.Suarez AL, Lopez-Rincon G, Martinez Neri PA, Estrada-Chavez C. Prolactin in inflammatory response. Adv Exp Med Biol. 2015;846:243–264. doi: 10.1007/978-3-319-12114-7_11. [DOI] [PubMed] [Google Scholar]
- 13.Clapp C, Thebault S, Jeziorski MC, Martinez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–1215. doi: 10.1152/physrev.00024.2009. [DOI] [PubMed] [Google Scholar]
- 14.Triebel J, Bertsch T, Bollheimer C, Rios-Barrera D, Pearce CF, Hufner M, Martinez de la Escalera G, Clapp C. Principles of the prolactin/vasoinhibin axis. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1193–R1203. doi: 10.1152/ajpregu.00256.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 16.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 17.Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac’h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–228. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 18.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 19.Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–735. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 20.Huizinga TW, Amos CI, van der Helm-van Mil AH, Chen W, van Gaalen FA, Jawaheer D, Schreuder GM, Wener M, Breedveld FC, Ahmad N, Lum RF, de Vries RR, Gregersen PK, Toes RE, Criswell LA. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum. 2005;52:3433–3438. doi: 10.1002/art.21385. [DOI] [PubMed] [Google Scholar]
- 21.Lee AT, Li W, Liew A, Bombardier C, Weisman M, Massarotti EM, Kent J, Wolfe F, Begovich AB, Gregersen PK. The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose-dependent manner but not with HLA-SE status. Genes Immun. 2005;6:129–133. doi: 10.1038/sj.gene.6364159. [DOI] [PubMed] [Google Scholar]
- 22.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, Liew A, Khalili H, Chandrasekaran A, Davies LR, Li W, Tan AK, Bonnard C, Ong RT, Thalamuthu A, Pettersson S, Liu C, Tian C, Chen WV, Carulli JP, Beckman EM, Altshuler D, Alfredsson L, Criswell LA, Amos CI, Seldin MF, Kastner DL, Klareskog L, Gregersen PK. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med. 2007;357:1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigurdsson S, Padyukov L, Kurreeman FA, Liljedahl U, Wiman AC, Alfredsson L, Toes R, Ronnelid J, Klareskog L, Huizinga TW, Alm G, Syvanen AC, Ronnblom L. Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 2007;56:2202–2210. doi: 10.1002/art.22704. [DOI] [PubMed] [Google Scholar]
- 24.Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, de Bakker PI, Le JM, Lee HS, Batliwalla F, Li W, Masters SL, Booty MG, Carulli JP, Padyukov L, Alfredsson L, Klareskog L, Chen WV, Amos CI, Criswell LA, Seldin MF, Kastner DL, Gregersen PK. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 26.Straub RH, Dhabhar FS, Bijlsma JW, Cutolo M. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum. 2005;52:16–26. doi: 10.1002/art.20747. [DOI] [PubMed] [Google Scholar]
- 27.McCray CJ, Agarwal SK. Stress and autoimmunity. Immunol Allergy Clin North Am. 2011;31:1–18. doi: 10.1016/j.iac.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Cutolo M, Straub RH. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation. 2006;13:277–282. doi: 10.1159/000104855. [DOI] [PubMed] [Google Scholar]
- 29.Dorshkind K, Horseman ND. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays. 2001;23:288–294. doi: 10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Tian R, Hou G, Li D, Yuan TF. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci World J. 2014;2014:780616. doi: 10.1155/2014/780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 32.Marcenaro M, Prete C, Badini A, Sulli A, Magi E, Cutolo M. Rheumatoid arthritis, personality, stress response style, and coping with illness. A preliminary survey. Ann N Y Acad Sci. 1999;876:419–425. doi: 10.1111/j.1749-6632.1999.tb07666.x. [DOI] [PubMed] [Google Scholar]
- 33.Feigenbaum SL, Masi AT, Kaplan SB. Prognosis in rheumatoid arthritis. A longitudinal study of newly diagnosed younger adult patients. Am J Med. 1979;66:377–384. doi: 10.1016/0002-9343(79)91055-6. [DOI] [PubMed] [Google Scholar]
- 34.Zautra AJ, Hoffman JM, Matt KS, Yocum D, Potter PT, Castro WL, Roth S. An examination of individual differences in the relationship between interpersonal stress and disease activity among women with rheumatoid arthritis. Arthritis Care Res. 1998;11:271–279. doi: 10.1002/art.1790110408. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann M, Scholmerich J, Straub RH. Stress and rheumatic diseases. Rheum Dis Clin North Am. 2000;26:737–763. doi: 10.1016/S0889-857X(05)70167-8. [DOI] [PubMed] [Google Scholar]
- 36.Rogers MP, Trentham DE, Dynesius-Trentham R, Daffner K, Reich P. Exacerbation of collagen arthritis by noise stress. J Rheumatol. 1983;10:651–654. [PubMed] [Google Scholar]
- 37.Jurcovicova J, Stancikova M, Svik K, Ondrejickova Krsova D, Seres J, Rokyta R. Stress of chronic food restriction attenuates the development of adjuvant arthritis in male Long Evans rats. Clin Exp Rheumatol. 2001;19:371–376. [PubMed] [Google Scholar]
- 38.Chover-Gonzalez AJ, Jessop DS, Tejedor-Real P, Gibert-Rahola J, Harbuz MS. Onset and severity of inflammation in rats exposed to the learned helplessness paradigm. Rheumatology (Oxford) 2000;39:764–771. doi: 10.1093/rheumatology/39.7.764. [DOI] [PubMed] [Google Scholar]
- 39.Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, Pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/S1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 40.Curtin NM, Boyle NT, Mills KH, Connor TJ. Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23:535–547. doi: 10.1016/j.bbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Curtin NM, Mills KH, Connor TJ. Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23:371–379. doi: 10.1016/j.bbi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 43.Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013;8:e58488. doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kittner JM, Jacobs R, Pawlak CR, Heijnen CJ, Schedlowski M, Schmidt RE. Adrenaline-induced immunological changes are altered in patients with rheumatoid arthritis. Rheumatology (Oxford) 2002;41:1031–1039. doi: 10.1093/rheumatology/41.9.1031. [DOI] [PubMed] [Google Scholar]
- 45.Lappin D, Whaley K. Adrenergic receptors on monocytes modulate complement component synthesis. Clin Exp Immunol. 1982;47:606–612. [PMC free article] [PubMed] [Google Scholar]
- 46.Broug-Holub E, Kraal G. Dose- and time-dependent activation of rat alveolar macrophages by glucocorticoids. Clin Exp Immunol. 1996;104:332–336. doi: 10.1046/j.1365-2249.1996.29733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matera L, Cesano A, Bellone G, Oberholtzer E. Modulatory effect of prolactin on the resting and mitogen-induced activity of T, B, and NK lymphocytes. Brain Behav Immun. 1992;6:409–417. doi: 10.1016/0889-1591(92)90039-Q. [DOI] [PubMed] [Google Scholar]
- 48.Zamorano M, Ledesma-Colunga MG, Adan N, Vera-Massieu C, Lemini M, Mendez I, Moreno-Carranza B, Neumann ID, Thebault S, Martinez de la Escalera G, Torner L, Clapp C. Prolactin-derived vasoinhibins increase anxiety- and depression-related behaviors. Psychoneuroendocrinology. 2014;44:123–132. doi: 10.1016/j.psyneuen.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Corbacho AM, Nava G, Eiserich JP, Noris G, Macotela Y, Struman I, Martinez De La Escalera G, Freeman BA, Clapp C. Proteolytic cleavage confers nitric oxide synthase inducing activity upon prolactin. J Biol Chem. 2000;275:13183–13186. doi: 10.1074/jbc.275.18.13183. [DOI] [PubMed] [Google Scholar]
- 50.Eijsbouts AM, van den Hoogen FH, Laan RF, Hermus AR, Sweep CG, van de Putte LB. Hypothalamic-pituitary-adrenal axis activity in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:658–664. [PubMed] [Google Scholar]
- 51.Eijsbouts AM, van den Hoogen FH, Laan RF, Sweep CG, Hermus AR, van de Putte LB. Decreased prolactin response to hypoglycaemia in patients with rheumatoid arthritis: correlation with disease activity. Ann Rheum Dis. 2005;64:433–437. doi: 10.1136/ard.2002.001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pool AJ, Whipp BJ, Skasick AJ, Alavi A, Bland JM, Axford JS. Serum cortisol reduction and abnormal prolactin and CD4+/CD8+ T-cell response as a result of controlled exercise in patients with rheumatoid arthritis and systemic lupus erythematosus despite unaltered muscle energetics. Rheumatology (Oxford) 2004;43:43–48. doi: 10.1093/rheumatology/keg425. [DOI] [PubMed] [Google Scholar]
- 53.Harbuz MS, Richards LJ, Chover-Gonzalez AJ, Marti-Sistac O, Jessop DS. Stress in autoimmune disease models. Ann N Y Acad Sci. 2006;1069:51–61. doi: 10.1196/annals.1351.005. [DOI] [PubMed] [Google Scholar]
- 54.van Everdingen AA, Jacobs JW, Siewertsz Van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002;136:1–12. doi: 10.7326/0003-4819-136-1-200201010-00006. [DOI] [PubMed] [Google Scholar]
- 55.Brennan P, Silman A. Why the gender difference in susceptibility to rheumatoid arthritis? Ann Rheum Dis. 1995;54:694–695. doi: 10.1136/ard.54.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 57.Holmdahl R, Jansson L, Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986;29:1501–1509. doi: 10.1002/art.1780291212. [DOI] [PubMed] [Google Scholar]
- 58.Holmdahl R, Jansson L, Meyerson B, Klareskog L. Oestrogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987;70:372–378. [PMC free article] [PubMed] [Google Scholar]
- 59.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol. 1994;53:203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 60.Latham KA, Zamora A, Drought H, Subramanian S, Matejuk A, Offner H, Rosloniec EF. Estradiol treatment redirects the isotype of the autoantibody response and prevents the development of autoimmune arthritis. J Immunol. 2003;171:5820–5827. doi: 10.4049/jimmunol.171.11.5820. [DOI] [PubMed] [Google Scholar]
- 61.Ganesan K, Selvam R, Abhirami R, Raju KV, Manohar BM, Puvanakrishnan R. Gender differences and protective effects of testosterone in collagen induced arthritis in rats. Rheumatol Int. 2008;28:345–353. doi: 10.1007/s00296-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 62.Spector TD, Ollier W, Perry LA, Silman AJ, Thompson PW, Edwards A. Free and serum testosterone levels in 276 males: a comparative study of rheumatoid arthritis, ankylosing spondylitis and healthy controls. Clin Rheumatol. 1989;8:37–41. doi: 10.1007/BF02031066. [DOI] [PubMed] [Google Scholar]
- 63.Masi AT, Feigenbaum SL, Chatterton RT. Hormonal and pregnancy relationships to rheumatoid arthritis: convergent effects with immunologic and microvascular systems. Semin Arthritis Rheum. 1995;25:1–27. doi: 10.1016/S0049-0172(95)80014-X. [DOI] [PubMed] [Google Scholar]
- 64.Cutolo M, Villaggio B, Craviotto C, Pizzorni C, Seriolo B, Sulli A. Sex hormones and rheumatoid arthritis. Autoimmun Rev. 2002;1:284–289. doi: 10.1016/S1568-9972(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 65.Hazes JM, Dijkmans BC, Vandenbroucke JP, de Vries RR, Cats A. Reduction of the risk of rheumatoid arthritis among women who take oral contraceptives. Arthritis Rheum. 1990;33:173–179. doi: 10.1002/art.1780330204. [DOI] [PubMed] [Google Scholar]
- 66.Doran MF, Crowson CS, O’Fallon WM, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol. 2004;31:207–213. [PubMed] [Google Scholar]
- 67.Vandenbroucke JP, Valkenburg HA, Boersma JW, Cats A, Festen JJ, Huber-Bruning O, Rasker JJ. Oral contraceptives and rheumatoid arthritis: further evidence for a preventive effect. Lancet. 1982;2:839–842. doi: 10.1016/S0140-6736(82)90809-1. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez-Avila M, Liang MH, Willett WC, Stampfer MJ, Colditz GA, Rosner B, Chang RW, Hennekens CH, Speizer FE. Oral contraceptives, replacement oestrogens and the risk of rheumatoid arthritis. Br J Rheumatol. 1989;28(Suppl 1):31. doi: 10.1093/rheumatology/XXVIII.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 69.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- 70.Mattsson R, Mattsson A, Holmdahl R, Whyte A, Rook GA. Maintained pregnancy levels of oestrogen afford complete protection from post-partum exacerbation of collagen-induced arthritis. Clin Exp Immunol. 1991;85:41–47. doi: 10.1111/j.1365-2249.1991.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whyte A, Williams RO. Bromocriptine suppresses postpartum exacerbation of collagen-induced arthritis. Arthritis Rheum. 1988;31:927–928. doi: 10.1002/art.1780310717. [DOI] [PubMed] [Google Scholar]
- 72.Waites GT, Whyte A. Effect of pregnancy on collagen-induced arthritis in mice. Clin Exp Immunol. 1987;67:467–476. [PMC free article] [PubMed] [Google Scholar]
- 73.Straub RH, Buttgereit F, Cutolo M. Benefit of pregnancy in inflammatory arthritis. Ann Rheum Dis. 2005;64:801–803. doi: 10.1136/ard.2005.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113:224–230. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29:185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 76.Barrett JH, Brennan P, Fiddler M, Silman A. Breast-feeding and postpartum relapse in women with rheumatoid and inflammatory arthritis. Arthritis Rheum. 2000;43:1010–1015. doi: 10.1002/1529-0131(200005)43:5<1010::AID-ANR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 77.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50:3458–3467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006;17:110–116. doi: 10.1016/j.tem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Marano RJ, Ben-Jonathan N. Minireview: extrapituitary prolactin: an update on the distribution, regulation, and functions. Mol Endocrinol. 2014;28:622–633. doi: 10.1210/me.2013-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 81.Horseman ND, Yu-Lee LY. Transcriptional regulation by the helix bundle peptide hormones: growth hormone, prolactin, and hematopoietic cytokines. Endocr Rev. 1994;15:627–649. doi: 10.1210/edrv-15-5-627. [DOI] [PubMed] [Google Scholar]
- 82.Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11:265–275. doi: 10.1038/nrendo.2015.36. [DOI] [PubMed] [Google Scholar]
- 83.Sinha YN. Structural variants of prolactin: occurrence and physiological significance. Endocr Rev. 1995;16:354–369. doi: 10.1210/edrv-16-3-354. [DOI] [PubMed] [Google Scholar]
- 84.Clapp C, Weiner RI. A specific, high-affinity, saturable binding-site for the 16-KDa fragment of prolactin on capillary endothelial-cells. Endocrinology. 1992;130:1380–1386. doi: 10.1210/endo.130.3.1311239. [DOI] [PubMed] [Google Scholar]
- 85.Bajou K, Herkenne S, Thijssen VL, D’Amico S, Nguyen NQ, Bouche A, Tabruyn S, Srahna M, Carabin JY, Nivelles O, Paques C, Cornelissen I, Lion M, Noel A, Gils A, Vinckier S, Declerck PJ, Griffioen AW, Dewerchin M, Martial JA, Carmeliet P, Struman I. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat Med. 2014;20:741–747. doi: 10.1038/nm.3552. [DOI] [PubMed] [Google Scholar]
- 86.Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corbacho AM, Macotela Y, Nava G, Eiserich JP, Cross CE, Martinez de la Escalera G, Clapp C. Cytokine induction of prolactin receptors mediates prolactin inhibition of nitric oxide synthesis in pulmonary fibroblasts. FEBS Lett. 2003;544:171–175. doi: 10.1016/S0014-5793(03)00499-X. [DOI] [PubMed] [Google Scholar]
- 88.Clapp C, Aranda J, Gonzalez C, Jeziorski MC, Martinez de la Escalera G. Vasoinhibins: endogenous regulators of angiogenesis and vascular function. Trends Endocrinol Metab. 2006;17:301–307. doi: 10.1016/j.tem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Ogueta S, Munoz J, Obregon E, Delgado-Baeza E, Garcia-Ruiz JP. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol Cell Endocrinol. 2002;190:51–63. doi: 10.1016/S0303-7207(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 90.Macotela Y, Aguilar MB, Guzman-Morales J, Rivera JC, Zermeno C, Lopez-Barrera F, Nava G, Lavalle C, Martinez de la Escalera G, Clapp C. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci. 2006;119:1790–1800. doi: 10.1242/jcs.02887. [DOI] [PubMed] [Google Scholar]
- 91.Clapp C, Lopez-Gomez FJ, Nava G, Corbacho A, Torner L, Macotela Y, Duenas Z, Ochoa A, Noris G, Acosta E, Garay E, Martinez de la Escalera G. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol. 1998;158:137–144. doi: 10.1677/joe.0.1580137. [DOI] [PubMed] [Google Scholar]
- 92.Nagafuchi H, Suzuki N, Kaneko A, Asai T, Sakane T. Prolactin locally produced by synovium infiltrating T lymphocytes induces excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol. 1999;26:1890–1900. [PubMed] [Google Scholar]
- 93.Shibli-Rahhal A, Schlechte J. The effects of hyperprolactinemia on bone and fat. Pituitary. 2009;12:96–104. doi: 10.1007/s11102-008-0097-3. [DOI] [PubMed] [Google Scholar]
- 94.López JM, González G, Reyes V, Campino C, Díaz S. Bone turnover and density in healthy women during breastfeeding and after weaning. Osteoporos Int. 1996;6:153–159. doi: 10.1007/BF01623940. [DOI] [PubMed] [Google Scholar]
- 95.Kinon BJ, Liu-Seifert H, Stauffer VL, Jacob J. Bone loss associated with hyperprolactinemia in patients with schizophrenia. Clin Schizophr Relat Psychoses. 2013;7:115–123. doi: 10.3371/CSRP.KISE.020113. [DOI] [PubMed] [Google Scholar]
- 96.Mazziotti G, Porcelli T, Mormando M, De Menis E, Bianchi A, Mejia C, Mancini T, De Marinis L, Giustina A. Vertebral fractures in males with prolactinoma. Endocrine. 2011;39:288–293. doi: 10.1007/s12020-011-9462-5. [DOI] [PubMed] [Google Scholar]
- 97.Sonigo C, Bouilly J, Carre N, Tolle V, Caraty A, Tello J, Simony-Conesa FJ, Millar R, Young J, Binart N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122:3791–3795. doi: 10.1172/JCI63937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA. 1996;276:549–554. doi: 10.1001/jama.1996.03540070045029. [DOI] [PubMed] [Google Scholar]
- 99.Krishnamra N, Cheeveewattana V. Studies of acute effect of prolactin on distribution of absorbed calcium and long-term effect on calcium balance in weaned, young, and sexually mature rats. Can J Physiol Pharmacol. 1994;72:1521–1527. doi: 10.1139/y94-218. [DOI] [PubMed] [Google Scholar]
- 100.Krishnamra N, Seemoung J. Effects of acute and long-term administration of prolactin on bone Ca-45 uptake, calcium deposit, and calcium resorption in weaned, young, and mature rats. Can J Physiol Pharm. 1996;74:1157–1165. doi: 10.1139/y96-123. [DOI] [PubMed] [Google Scholar]
- 101.Lotinun S, Limlomwongse L, Krishnamra N. The study of a physiological significance of prolactin in the regulation of calcium metabolism during pregnancy and lactation in rats. Can J Physiol Pharmacol. 1998;76:218–228. doi: 10.1139/y98-016. [DOI] [PubMed] [Google Scholar]
- 102.Lotinun S, Limlomwongse LC, Sirikulchayanonta V, Krishnamra N. Bone calcium turnover, formation, and resorption in bromocriptine- and prolactin-treated lactating rats. Endocrine. 2003;20:163–170. doi: 10.1385/ENDO:20:1-2:163. [DOI] [PubMed] [Google Scholar]
- 103.Tzeng SJ, Linzer DI. Prolactin receptor expression in the developing mouse embryo. Mol Reprod Dev. 1997;48:45–52. doi: 10.1002/(SICI)1098-2795(199709)48:1<45::AID-MRD6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 104.Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest. 1997;99:1107–1117. doi: 10.1172/JCI119239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am J Physiol Endocrinol Metab. 2000;279:E1216–E1225. doi: 10.1152/ajpendo.2000.279.6.E1216. [DOI] [PubMed] [Google Scholar]
- 106.Seriwatanachai D, Charoenphandhu N, Suthiphongchai T, Krishnamra N. Prolactin decreases the expression ratio of receptor activator of nuclear factor kappaB ligand/osteoprotegerin in human fetal osteoblast cells. Cell Biol Int. 2008;32:1126–1135. doi: 10.1016/j.cellbi.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 107.Charoenphandhu N, Teerapornpuntakit J, Methawasin M, Wongdee K, Thongchote K, Krishnamra N. Prolactin decreases expression of Runx2, osteoprotegerin, and RANKL in primary osteoblasts derived from tibiae of adult female rats. Can J Physiol Pharmacol. 2008;86:240–248. doi: 10.1139/Y08-037. [DOI] [PubMed] [Google Scholar]
- 108.Seriwatanachai D, Krishnamra N, van Leeuwen JP. Evidence for direct effects of prolactin on human osteoblasts: inhibition of cell growth and mineralization. J Cell Biochem. 2009;107:677–685. doi: 10.1002/jcb.22161. [DOI] [PubMed] [Google Scholar]
- 109.Graf J, Neusel E, Freese U, Simank HG, Niethard FU. Subchondral vascularisation and osteoarthritis. Int Orthop. 1992;16:113–117. doi: 10.1007/BF00180198. [DOI] [PubMed] [Google Scholar]
- 110.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–228. doi: 10.1016/S1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 111.Gaytan F, Morales C, Bellido C, Aguilar E, Sanchez-Criado JE. Role of prolactin in the regulation of macrophages and in the proliferative activity of vascular cells in newly formed and regressing rat corpora lutea. Biol Reprod. 1997;57:478–486. doi: 10.1095/biolreprod57.2.478. [DOI] [PubMed] [Google Scholar]
- 112.Ko JY, Ahn YL, Cho BN. Angiogenesis and white blood cell proliferation induced in mice by injection of a prolactin-expressing plasmid into muscle. Mol Cells. 2003;15:262–270. [PubMed] [Google Scholar]
- 113.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 114.Johansson M, Olerud J, Jansson L, Carlsson PO. Prolactin treatment improves engraftment and function of transplanted pancreatic islets. Endocrinology. 2009;150:1646–1653. doi: 10.1210/en.2008-1318. [DOI] [PubMed] [Google Scholar]
- 115.Moreno-Carranza B, Goya-Arce M, Vega C, Adan N, Triebel J, Lopez-Barrera F, Quintanar-Stephano A, Binart N, Martinez de la Escalera G, Clapp C. Prolactin promotes normal liver growth, survival, and regeneration in rodents: effects on hepatic IL-6, suppressor of cytokine signaling-3, and angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;305:R720–R726. doi: 10.1152/ajpregu.00282.2013. [DOI] [PubMed] [Google Scholar]
- 116.Malaguarnera L, Pilastro MR, Quan S, Ghattas MH, Yang L, Mezentsev AV, Kushida T, Abraham NG, Kappas A. Significance of heme oxygenase in prolactin-mediated cell proliferation and angiogenesis in human endothelial cells. Int J Mol Med. 2002;10:433–440. [PubMed] [Google Scholar]
- 117.Reuwer AQ, Nowak-Sliwinska P, Mans LA, van der Loos CM, von der Thusen JH, Twickler MT, Spek CA, Goffin V, Griffioen AW, Borensztajn KS. Functional consequences of prolactin signalling in endothelial cells: a potential link with angiogenesis in pathophysiology? J Cell Mol Med. 2012;16:2035–2048. doi: 10.1111/j.1582-4934.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X, Meyer K, Friedl A. STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis. J Biol Chem. 2013;288:21184–21196. doi: 10.1074/jbc.M113.481119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merkle CJ, Schuler LA, Schaeffer RC, Jr, Gribbon JM, Montgomery DW. Structural and functional effects of high prolactin levels on injured endothelial cells: evidence for an endothelial prolactin receptor. Endocrine. 2000;13:37–46. doi: 10.1385/ENDO:13:1:37. [DOI] [PubMed] [Google Scholar]
- 120.Ricken AM, Traenkner A, Merkwitz C, Hummitzsch K, Grosche J, Spanel-Borowski K. The short prolactin receptor predominates in endothelial cells of micro- and macrovascular origin. J Vasc Res. 2007;44:19–30. doi: 10.1159/000097892. [DOI] [PubMed] [Google Scholar]
- 121.Castilla A, Garcia C, Cruz-Soto M, Martinez de la Escalera G, Thebault S, Clapp C. Prolactin in ovarian follicular fluid stimulates endothelial cell proliferation. J Vasc Res. 2010;47:45–53. doi: 10.1159/000231720. [DOI] [PubMed] [Google Scholar]
- 122.Goldhar AS, Vonderhaar BK, Trott JF, Hovey RC. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol Cell Endocrinol. 2005;232:9–19. doi: 10.1016/j.mce.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Malaguarnera L, Imbesi RM, Scuto A, D’Amico F, Licata F, Messina A, Sanfilippo S. Prolactin increases HO-1 expression and induces VEGF production in human macrophages. J Cell Biochem. 2004;93:197–206. doi: 10.1002/jcb.20167. [DOI] [PubMed] [Google Scholar]
- 124.Srivastava RK, Gu Y, Ayloo S, Zilberstein M, Gibori G. Developmental expression and regulation of basic fibroblast growth factor and vascular endothelial growth factor in rat decidua and in a decidual cell line. J Mol Endocrinol. 1998;21:355–362. doi: 10.1677/jme.0.0210355. [DOI] [PubMed] [Google Scholar]
- 125.Too CK, Knee R, Pinette AL, Li AW, Murphy PR. Prolactin induces expression of FGF-2 and a novel FGF-responsive NonO/p54nrb-related mRNA in rat lymphoma cells. Mol Cell Endocrinol. 1998;137:187–195. doi: 10.1016/S0303-7207(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 126.Clapp C, Thebault S, Martinez de la Escalera G. Role of prolactin and vasoinhibins in the regulation of vascular function in mammary gland. J Mammary Gland Biol Neoplasia. 2008;13:55–67. doi: 10.1007/s10911-008-9067-7. [DOI] [PubMed] [Google Scholar]
- 127.Aranda J, Rivera JC, Jeziorski MC, Riesgo-Escovar J, Nava G, Lopez-Barrera F, Quiroz-Mercado H, Berger P, Martinez de la Escalera G, Clapp C. Prolactins are natural inhibitors of angiogenesis in the retina. Invest Ophthalmol Vis Sci. 2005;46:2947–2953. doi: 10.1167/iovs.05-0173. [DOI] [PubMed] [Google Scholar]
- 128.Cruz-Soto ME, Scheiber MD, Gregerson KA, Boivin GP, Horseman ND. Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology. 2002;143:4429–4436. doi: 10.1210/en.2002-220173. [DOI] [PubMed] [Google Scholar]
- 129.Duenas Z, Torner L, Corbacho AM, Ochoa A, Gutierrez-Ospina G, Lopez-Barrera F, Barrios FA, Berger P, Martinez de la Escalera G, Clapp C. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest Ophthalmol Vis Sci. 1999;40:2498–2505. [PubMed] [Google Scholar]
- 130.Garcia C, Aranda J, Arnold E, Thebault S, Macotela Y, Lopez-Casillas F, Mendoza V, Quiroz-Mercado H, Hernandez-Montiel HL, Lin SH, de la Escalera GM, Clapp C. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J Clin Invest. 2008;118:2291–2300. doi: 10.1172/JCI34508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI. The 16-KDa N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology. 1993;133:1292–1299. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 132.Lee H, Struman I, Clapp C, Martial J, Weiner RI. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: activation of plasminogen activator inhibitor 1 expression. Endocrinology. 1998;139:3696–3703. doi: 10.1210/endo.139.9.6194. [DOI] [PubMed] [Google Scholar]
- 133.Lee SH, Kunz J, Lin SH, Yu-Lee LY. 16-kDa prolactin inhibits endothelial cell migration by down-regulating the Ras–Tiam1–Rac1–Pak1 signaling pathway. Cancer Res. 2007;67:11045–11053. doi: 10.1158/0008-5472.CAN-07-0986. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez C, Corbacho AM, Eiserich JP, Garcia C, Lopez-Barrera F, Morales-Tlalpan V, Barajas-Espinosa A, Diaz-Munoz M, Rubio R, Lin SH, Martinez de la Escalera G, Clapp C. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology. 2004;145:5714–5722. doi: 10.1210/en.2004-0647. [DOI] [PubMed] [Google Scholar]
- 135.Arredondo Zamarripa D, Diaz-Lezama N, Melendez Garcia R, Chavez Balderas J, Adan N, Ledesma-Colunga MG, Arnold E, Clapp C, Thebault S. Vasoinhibins regulate the inner and outer blood-retinal barrier and limit retinal oxidative stress. Front Cell Neurosci. 2014;8:333. doi: 10.3389/fncel.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.D’Angelo G, Martini JF, Iiri T, Fantl WJ, Martial J, Weiner RI. 16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells. Mol Endocrinol. 1999;13:692–704. doi: 10.1210/mend.13.5.0280. [DOI] [PubMed] [Google Scholar]
- 137.Tabruyn SP, Nguyen NQ, Cornet AM, Martial JA, Struman I. The antiangiogenic factor, 16-kDa human prolactin, induces endothelial cell cycle arrest by acting at both the G0–G1 and the G2–M phases. Mol Endocrinol. 2005;19:1932–1942. doi: 10.1210/me.2004-0515. [DOI] [PubMed] [Google Scholar]
- 138.Thebault S, González C, García C, Zamarripa DA, Nava G, Vaca L, López-Casillas F, Martínez de la Escalera G, Clapp C. Vasoinhibins prevent bradykinin-stimulated endothelial cell proliferation by inactivating eNOS via reduction of both intracellular Ca2 + Levels and eNOS phosphorylation at Ser1179. Pharmaceuticals. 2011;4:1052–1069. doi: 10.3390/ph4071052. [DOI] [Google Scholar]
- 139.Tabruyn SP, Sorlet CM, Rentier-Delrue F, Bours V, Weiner RI, Martial JA, Struman I. The antiangiogenic factor 16K human prolactin induces caspase-dependent apoptosis by a mechanism that requires activation of nuclear factor-kappaB. Mol Endocrinol. 2003;17:1815–1823. doi: 10.1210/me.2003-0132. [DOI] [PubMed] [Google Scholar]
- 140.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gout PW, Beer CT, Noble RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40:2433–2436. [PubMed] [Google Scholar]
- 142.Russell DH, Kibler R, Matrisian L, Larson DF, Poulos B, Magun BE. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985;134:3027–3031. [PubMed] [Google Scholar]
- 143.Matera L, Mori M, Geuna M, Buttiglieri S, Palestro G. Prolactin in autoimmunity and antitumor defence. J Neuroimmunol. 2000;109:47–55. doi: 10.1016/S0165-5728(00)00302-7. [DOI] [PubMed] [Google Scholar]
- 144.Chikanza IC. Prolactin and neuroimmunomodulation: in vitro and in vivo observations. Ann N Y Acad Sci. 1999;876:119–130. doi: 10.1111/j.1749-6632.1999.tb07629.x. [DOI] [PubMed] [Google Scholar]
- 145.Draca S. Prolactin as an immunoreactive agent. Immunol Cell Biol. 1995;73:481–483. doi: 10.1038/icb.1995.77. [DOI] [PubMed] [Google Scholar]
- 146.Dineen JK, Kelly JD. The suppression of rejection of Nippostrongylus brasiliensis in lactating rats: the nature of the immunological defect. Immunology. 1972;22:1–12. [PMC free article] [PubMed] [Google Scholar]
- 147.Kelly JD, Dineen JK. The suppression of rejection of Nippostrongylus brasiliensis in Lewis strain rats treated with ovine prolactin. The site of the immunological defect. Immunology. 1973;24:551–558. [PMC free article] [PubMed] [Google Scholar]
- 148.Spangelo BL, Hall NR, Ross PC, Goldstein AL. Stimulation of in vivo antibody production and concanavalin-A-induced mouse spleen cell mitogenesis by prolactin. Immunopharmacology. 1987;14:11–20. doi: 10.1016/0162-3109(87)90004-X. [DOI] [PubMed] [Google Scholar]
- 149.Gerli R, Rambotti P, Nicoletti I, Orlandi S, Migliorati G, Riccardi C. Reduced number of natural killer cells in patients with pathological hyperprolactinemia. Clin Exp Immunol. 1986;64:399–406. [PMC free article] [PubMed] [Google Scholar]
- 150.Gerli R, Riccardi C, Nicoletti I, Orlandi S, Cernetti C, Spinozzi F, Rambotti P. Phenotypic and functional abnormalities of T lymphocytes in pathological hyperprolactinemia. J Clin Immunol. 1987;7:463–470. doi: 10.1007/BF00915056. [DOI] [PubMed] [Google Scholar]
- 151.Vidaller A, Guadarrama F, Llorente L, Mendez JB, Larrea F, Villa AR, Alarcon-Segovia D. Hyperprolactinemia inhibits natural killer (NK) cell function in vivo and its bromocriptine treatment not only corrects it but makes it more efficient. J Clin Immunol. 1992;12:210–215. doi: 10.1007/BF00918091. [DOI] [PubMed] [Google Scholar]
- 152.Jacobi AM, Rohde W, Volk HD, Dorner T, Burmester GR, Hiepe F. Prolactin enhances the in vitro production of IgG in peripheral blood mononuclear cells from patients with systemic lupus erythematosus but not from healthy controls. Ann Rheum Dis. 2001;60:242–247. doi: 10.1136/ard.60.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sodhi A, Tripathi A. Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-kappaB. Cytokine. 2008;41:162–173. doi: 10.1016/j.cyto.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw. 2004;15:99–104. [PubMed] [Google Scholar]
- 155.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- 156.Morikawa K, Oseko F, Morikawa S. Immunosuppressive activity of bromocriptine on human T lymphocyte function in vitro. Clin Exp Immunol. 1994;95:514–518. doi: 10.1111/j.1365-2249.1994.tb07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morkawa K, Oseko F, Morikawa S. Immunosuppressive property of bromocriptine on human B lymphocyte function in vitro. Clin Exp Immunol. 1993;93:200–205. doi: 10.1111/j.1365-2249.1993.tb07966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bouchard B, Ormandy CJ, Di Santo JP, Kelly PA. Immune system development and function in prolactin receptor-deficient mice. J Immunol. 1999;163:576–582. [PubMed] [Google Scholar]
- 160.Molitch ME. Pathologic hyperprolactinemia. Endocrinol Metab Clin North Am. 1992;21:877–901. [PubMed] [Google Scholar]
- 161.Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol. 1996;157:5748–5754. [PubMed] [Google Scholar]
- 162.Knoferl MW, Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Insight into the mechanism by which metoclopramide improves immune functions after trauma-hemorrhage. Am J Physiol Cell Physiol. 2000;279:C72–C80. doi: 10.1152/ajpcell.2000.279.1.C72. [DOI] [PubMed] [Google Scholar]
- 163.Murphy WJ, Durum SK, Longo DL. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993;178:231–236. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.LaVoie HA, Witorsch RJ. Investigation of intracellular signals mediating the anti-apoptotic action of prolactin in Nb2 lymphoma cells. Proc Soc Exp Biol Med. 1995;209:257–269. doi: 10.3181/00379727-209-43901. [DOI] [PubMed] [Google Scholar]
- 165.Krumenacker JS, Buckley DJ, Leff MA, McCormack JT, de Jong G, Gout PW, Reed JC, Miyashita T, Magnuson NS, Buckley AR. Prolactin-regulated apoptosis of Nb2 lymphoma cells: pim-1, bcl-2, and bax expression. Endocrine. 1998;9:163–170. doi: 10.1385/ENDO:9:2:163. [DOI] [PubMed] [Google Scholar]
- 166.Dugan AL, Thellin O, Buckley DJ, Buckley AR, Ogle CK, Horseman ND. Effects of prolactin deficiency on myelopoiesis and splenic T lymphocyte proliferation in thermally injured mice. Endocrinology. 2002;143:4147–4151. doi: 10.1210/en.2002-220515. [DOI] [PubMed] [Google Scholar]
- 167.Oberbeck R, Schmitz D, Wilsenack K, Schuler M, Biskup C, Schedlowski M, Nast-Kolb D, Exton MS. Prolactin modulates survival and cellular immune functions in septic mice. J Surg Res. 2003;113:248–256. doi: 10.1016/S0022-4804(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 168.Macotela Y, Mendoza C, Corbacho AM, Cosio G, Eiserich JP, Zentella A, Martinez de la Escalera G, Clapp C. 16K prolactin induces NF-kappaB activation in pulmonary fibroblasts. J Endocrinol. 2002;175:R13–R18. doi: 10.1677/joe.0.175R013. [DOI] [PubMed] [Google Scholar]
- 169.Deb S, Tessier C, Prigent-Tessier A, Barkai U, Ferguson-Gottschall S, Srivastava RK, Faliszek J, Gibori G. The expression of interleukin-6 (IL-6), IL-6 receptor, and gp130-KDa glycoprotein in the rat decidua and a decidual cell line: regulation by 17beta-estradiol and prolactin. Endocrinology. 1999;140:4442–4450. doi: 10.1210/endo.140.10.7063. [DOI] [PubMed] [Google Scholar]
- 170.Gu Y, Srivastava RK, Clarke DL, Linzer DI, Gibori G. The decidual prolactin receptor and its regulation by decidua-derived factors. Endocrinology. 1996;137:4878–4885. doi: 10.1210/endo.137.11.8895360. [DOI] [PubMed] [Google Scholar]
- 171.Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the prolactin family of hormones: coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991;12:402–423. doi: 10.1210/edrv-12-4-402. [DOI] [PubMed] [Google Scholar]
- 172.Lin J, Linzer DI. A novel megakaryocyte differentiation factor from mouse placenta. Trends Cardiovasc Med. 1999;9:167–171. doi: 10.1016/S1050-1738(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 173.Muller H, Liu B, Croy BA, Head JR, Hunt JS, Dai G, Soares MJ. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology. 1999;140:2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- 174.Guy MA, McFadden TB, Cockrell DC, Besser TE. Effects of unilateral prepartum milking on concentrations of immunoglobulin G1 and prolactin in colostrum. J Dairy Sci. 1994;77:3584–3591. doi: 10.3168/jds.S0022-0302(94)77302-1. [DOI] [PubMed] [Google Scholar]
- 175.Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991;53:32–39. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- 176.Zimmer JP, Garza C, Butte NF, Goldman AS. Maternal blood B-cell (CD19+) percentages and serum immunoglobulin concentrations correlate with breast-feeding behavior and serum prolactin concentration. Am J Reprod Immunol. 1998;40:57–62. doi: 10.1111/j.1600-0897.1998.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 177.McMurray RW. Bromocriptine in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 2001;31:21–32. doi: 10.1053/sarh.2001.25482. [DOI] [PubMed] [Google Scholar]
- 178.Chikanza IC. The neuroendocrine immunology of rheumatoid arthritis. Baillieres Clin Rheumatol. 1996;10:273–293. doi: 10.1016/S0950-3579(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 179.De Bellis A, Bizzarro A, Pivonello R, Lombardi G, Bellastella A. Prolactin and autoimmunity. Pituitary. 2005;8:25–30. doi: 10.1007/s11102-005-5082-5. [DOI] [PubMed] [Google Scholar]
- 180.Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev. 2007;6:537–542. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 181.Jara LJ, Medina G, Saavedra MA, Vera-Lastra O, Navarro C. Prolactin and autoimmunity. Clin Rev Allergy Immunol. 2011;40:50–59. doi: 10.1007/s12016-009-8185-3. [DOI] [PubMed] [Google Scholar]
- 182.Brennan P, Ollier B, Worthington J, Hajeer A, Silman A. Are both genetic and reproductive associations with rheumatoid arthritis linked to prolactin? Lancet. 1996;348:106–109. doi: 10.1016/S0140-6736(96)02037-5. [DOI] [PubMed] [Google Scholar]
- 183.Neidhart M, Gay RE, Gay S. Prolactin and prolactin-like polypeptides in rheumatoid arthritis. Biomed Pharmacother. 1999;53:218–222. doi: 10.1016/S0753-3322(99)80091-2. [DOI] [PubMed] [Google Scholar]
- 184.Yoshikawa H, Nara K, Suzuki N. Recent advances in neuro-endocrine-immune interactions in the pathophysiology of rheumatoid arthritis. Curr Rheumatol Rev. 2006;2:191–205. doi: 10.2174/157339706776876116. [DOI] [Google Scholar]
- 185.Chuang E, Molitch ME. Prolactin and autoimmune diseases in humans. Acta Biomed. 2007;78(Suppl 1):255–261. [PubMed] [Google Scholar]
- 186.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11:A465–A470. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 187.Spector TD, Hochberg MC. The protective effect of the oral contraceptive pill on rheumatoid arthritis: an overview of the analytical epidemiological studies using meta-analysis. Br J Rheumatol. 1989;28(Suppl 1):11–12. doi: 10.1093/rheumatology/XXVIII.suppl_1.11. [DOI] [PubMed] [Google Scholar]
- 188.Silman AJ. Parity status and the development of rheumatoid arthritis. Am J Reprod Immunol. 1992;28:228–230. doi: 10.1111/j.1600-0897.1992.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 189.Jorgensen C, Picot MC, Bologna C, Sany J. Oral contraception, parity, breast feeding, and severity of rheumatoid arthritis. Ann Rheum Dis. 1996;55:94–98. doi: 10.1136/ard.55.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pope JE, Bellamy N, Stevens A. The lack of associations between rheumatoid arthritis and both nulliparity and infertility. Semin Arthritis Rheum. 1999;28:342–350. doi: 10.1016/S0049-0172(99)80019-5. [DOI] [PubMed] [Google Scholar]
- 191.Pikwer M, Bergstrom U, Nilsson JA, Jacobsson L, Berglund G, Turesson C. Breast feeding, but not use of oral contraceptives, is associated with a reduced risk of rheumatoid arthritis. Ann Rheum Dis. 2009;68:526–530. doi: 10.1136/ard.2007.084707. [DOI] [PubMed] [Google Scholar]
- 192.Brennan P, Silman A. Breast-feeding and the onset of rheumatoid arthritis. Arthritis Rheum. 1994;37:808–813. doi: 10.1002/art.1780370605. [DOI] [PubMed] [Google Scholar]
- 193.Hampl JS, Papa DJ. Breastfeeding-related onset, flare, and relapse of rheumatoid arthritis. Nutr Rev. 2001;59:264–268. doi: 10.1111/j.1753-4887.2001.tb05511.x. [DOI] [PubMed] [Google Scholar]
- 194.Brun JG, Nilssen S, Kvale G. Breast feeding, other reproductive factors and rheumatoid arthritis. A prospective study. Br J Rheumatol. 1995;34:542–546. doi: 10.1093/rheumatology/34.6.542. [DOI] [PubMed] [Google Scholar]
- 195.Lahiri M, Morgan C, Symmons DP, Bruce IN. Modifiable risk factors for RA: prevention, better than cure? Rheumatology (Oxford) 2012;51:499–512. doi: 10.1093/rheumatology/ker299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet D. High serum prolactin levels in men with rheumatoid arthritis. J Rheumatol. 1998;25:2077–2082. [PubMed] [Google Scholar]
- 197.Chikanza IC, Petrou P, Chrousos G, Kingsley G, Panayi GS. Excessive and dysregulated secretion of prolactin in rheumatoid arthritis: immunopathogenetic and therapeutic implications. Br J Rheumatol. 1993;32:445–448. doi: 10.1093/rheumatology/32.6.445. [DOI] [PubMed] [Google Scholar]
- 198.Kullich WC, Klein G. High levels of macrophage inflammatory protein-1alpha correlate with prolactin in female patients with active rheumatoid arthritis. Clin Rheumatol. 1998;17:263–264. doi: 10.1007/BF01451064. [DOI] [PubMed] [Google Scholar]
- 199.Seriolo B, Ferretti V, Sulli A, Fasciolo D, Cutolo M. Serum prolactin concentrations in male patients with rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:258–262. doi: 10.1111/j.1749-6632.2002.tb04224.x. [DOI] [PubMed] [Google Scholar]
- 200.Zoli A, Lizzio MM, Ferlisi EM, Massafra V, Mirone L, Barini A, Scuderi F, Bartolozzi F, Magaro M. ACTH, cortisol and prolactin in active rheumatoid arthritis. Clin Rheumatol. 2002;21:289–293. doi: 10.1007/s100670200076. [DOI] [PubMed] [Google Scholar]
- 201.Fojtikova M, Tomasova Studynkova J, Filkova M, Lacinova Z, Gatterova J, Pavelka K, Vencovsky J, Senolt L. Elevated prolactin levels in patients with rheumatoid arthritis: association with disease activity and structural damage. Clin Exp Rheumatol. 2010;28:849–854. [PubMed] [Google Scholar]
- 202.Jorgensen C, Maziad H, Bologna C, Sany J. Kinetics of prolactin release in rheumatoid arthritis. Clin Exp Rheumatol. 1995;13:705–709. [PubMed] [Google Scholar]
- 203.Ram S, Blumberg D, Newton P, Anderson NR, Gama R. Raised serum prolactin in rheumatoid arthritis: genuine or laboratory artefact? Rheumatology (Oxford) 2004;43:1272–1274. doi: 10.1093/rheumatology/keh307. [DOI] [PubMed] [Google Scholar]
- 204.Ghule S, Dhotre A, Gupta M, Dharme P, Vaidya SM. Serum prolactin levels in women with rheumatoid arthritis. Biomed Res. 2009;20:115–118. [Google Scholar]
- 205.Rovensky J, Bakosova J, Koska J, Ksinantova L, Jezova D, Vigas M. Somatotropic, lactotropic and adrenocortical responses to insulin-induced hypoglycemia in patients with rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:263–270. doi: 10.1111/j.1749-6632.2002.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 206.Rovensky J, Imrich R, Radikova Z, Simorova E, Greguska O, Vigas M, Macho L. Peptide hormones and histamine in plasma and synovial fluid of patients with rheumatoid arthritis and osteoarthrosis. Endocr Regul. 2005;39:1–6. [PubMed] [Google Scholar]
- 207.Kramer CK, Tourinho TF, de Castro WP, da Costa Oliveira M. Association between systemic lupus erythematosus, rheumatoid arthritis, hyperprolactinemia and thyroid autoantibodies. Arch Med Res. 2005;36:54–58. doi: 10.1016/j.arcmed.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 208.Gutierrez MA, Garcia ME, Rodriguez JA, Mardonez G, Jacobelli S, Rivero S. Hypothalamic-pituitary-adrenal axis function in patients with active rheumatoid arthritis: a controlled study using insulin hypoglycemia stress test and prolactin stimulation. J Rheumatol. 1999;26:277–281. [PubMed] [Google Scholar]
- 209.Templ E, Koeller M, Riedl M, Wagner O, Graninger W, Luger A. Anterior pituitary function in patients with newly diagnosed rheumatoid arthritis. Br J Rheumatol. 1996;35:350–356. doi: 10.1093/rheumatology/35.4.350. [DOI] [PubMed] [Google Scholar]
- 210.Nagy E, Chalmers IM, Baragar FD, Friesen HG, Berczi I. Prolactin deficiency in rheumatoid arthritis. J Rheumatol. 1991;18:1662–1668. [PubMed] [Google Scholar]
- 211.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 212.Figueroa FE, Carrion F, Martinez ME, Rivero S, Mamani I. Bromocriptine induces immunological changes related to disease parameters in rheumatoid arthritis. Br J Rheumatol. 1997;36:1022–1023. doi: 10.1093/rheumatology/36.9.1022. [DOI] [PubMed] [Google Scholar]
- 213.Mobini M, Kashi Z, Mohammad Pour AR, Adibi E. The effect of cabergoline on clinical and laboratory findings in active rheumatoid arthritis. Iran Red Crescent Med J. 2011;13:749–750. [PMC free article] [PubMed] [Google Scholar]
- 214.Erb N, Pace AV, Delamere JP, Kitas GD. Control of unremitting rheumatoid arthritis by the prolactin antagonist cabergoline. Rheumatology (Oxford) 2001;40:237–239. doi: 10.1093/rheumatology/40.2.237. [DOI] [PubMed] [Google Scholar]
- 215.Dougados M, Duchesne L, Amor B. Bromocriptine and cyclosporin A combination therapy in rheumatoid arthritis. Arthritis Rheum. 1988;31:1333–1334. doi: 10.1002/art.1780311022. [DOI] [PubMed] [Google Scholar]
- 216.Eijsbouts A, van den Hoogen F, Laan RF, Hermus RM, Sweep FC, van de Putte L. Treatment of rheumatoid arthritis with the dopamine agonist quinagolide. J Rheumatol. 1999;26:2284–2285. [PubMed] [Google Scholar]
- 217.Salesi M, Sadeghihaddadzavareh S, Nasri P, Namdarigharaghani N, Farajzadegan Z, Hajalikhani M. The role of bromocriptine in the treatment of patients with active rheumatoid arthritis. Int J Rheum Dis. 2013;16:662–666. doi: 10.1111/1756-185x.12015. [DOI] [PubMed] [Google Scholar]
- 218.Li M, Keiser HD, Peeva E. Prolactinoma and systemic lupus erythematosus: do serum prolactin levels matter? Clin Rheumatol. 2006;25:602–605. doi: 10.1007/s10067-005-0117-x. [DOI] [PubMed] [Google Scholar]
- 219.Orbach H, Zandman-Goddard G, Amital H, Barak V, Szekanecz Z, Szucs G, Danko K, Nagy E, Csepany T, Carvalho JF, Doria A, Shoenfeld Y. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci. 2007;1109:385–400. doi: 10.1196/annals.1398.044. [DOI] [PubMed] [Google Scholar]
- 220.Haga H, Martin Moreno A, Terp Anderson D, Peen E. Serum prolactin and the association to disease activity in patients with rheumatoid arthritis. Res Endocrinol. 2014;2014:h1–h6. doi: 10.5171/2014.396352. [DOI] [Google Scholar]
- 221.Stevens A, Ray D, Alansari A, Hajeer A, Thomson W, Donn R, Ollier WE, Worthington J, Davis JR. Characterization of a prolactin gene polymorphism and its associations with systemic lupus erythematosus. Arthritis Rheum. 2001;44:2358–2366. doi: 10.1002/1529-0131(200110)44:10<2358::AID-ART399>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 222.Lee YC, Raychaudhuri S, Cui J, De Vivo I, Ding B, Alfredsson L, Padyukov L, Costenbader KH, Seielstad M, Graham RR, Klareskog L, Gregersen PK, Plenge RM, Karlson EW. The PRL -1149 G/T polymorphism and rheumatoid arthritis susceptibility. Arthritis Rheum. 2009;60:1250–1254. doi: 10.1002/art.24468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Reyes-Castillo Z, Pereira-Suarez AL, Palafox-Sanchez CA, Rangel-Villalobos H, Estrada-Chavez C, Oregon-Romero E, Angel-Chavez LI, Munoz-Barrios S, Bueno-Topete MR, Munoz-Valle JF. The extrapituitary prolactin promoter polymorphism is associated with rheumatoid arthritis and anti-CCP antibodies in Mexican population. Gene. 2013;525:130–135. doi: 10.1016/j.gene.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 224.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 225.Grimaldi MG. Long-term low dose haloperidol treatment in rheumatoid patients: effects on serum sulphydryl levels, technetium index, ESR, and clinical response. Br J Clin Pharmacol. 1981;12:579–581. doi: 10.1111/j.1365-2125.1981.tb01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Moots RJ, Al-Saffar Z, Hutchinson D, Golding SP, Young SP, Bacon PA, McLaughlin PJ. Old drug, new tricks: haloperidol inhibits secretion of proinflammatory cytokines. Ann Rheum Dis. 1999;58:585–587. doi: 10.1136/ard.58.9.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Song C, Lin A, Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42:157–164. doi: 10.1016/S0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- 228.Cai X, Wong YF, Zhou H, Xie Y, Liu ZQ, Jiang ZH, Bian ZX, Xu HX, Liu L. The comparative study of Sprague-Dawley and Lewis rats in adjuvant-induced arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:140–147. doi: 10.1007/s00210-006-0062-5. [DOI] [PubMed] [Google Scholar]
- 229.Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001;1:377–385. [PubMed] [Google Scholar]
- 230.Berczi I, Nagy E. A possible role of prolactin in adjuvant arthritis. Arthritis Rheum. 1982;25:591–594. doi: 10.1002/art.1780250517. [DOI] [PubMed] [Google Scholar]
- 231.Neidhart M, Fluckiger EW. Hyperprolactinaemia in hypophysectomized or intact male rats and the development of adjuvant arthritis. Immunology. 1992;77:449–455. [PMC free article] [PubMed] [Google Scholar]
- 232.Adler RA. The anterior pituitary-grafted rat: a valid model of chronic hyperprolactinemia. Endocr Rev. 1986;7:302–313. doi: 10.1210/edrv-7-3-302. [DOI] [PubMed] [Google Scholar]
- 233.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 234.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 5):v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 235.Spears R, Oakes R, Bellinger LL, Hutchins B. Tumour necrosis factor-alpha and apoptosis in the rat temporomandibular joint. Arch Oral Biol. 2003;48:825–834. doi: 10.1016/S0003-9969(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 236.Mattsson R, Mattsson A, Hansson I, Holmdahl R, Rook GA, Whyte A. Increased levels of prolactin during, but not after, the immunisation with rat collagen II enhances the course of arthritis in DBA/1 mice. Autoimmunity. 1992;11:163–170. doi: 10.3109/08916939209035151. [DOI] [PubMed] [Google Scholar]
- 237.Parara S, Seres J, Rokyta R, Stancikova M, Jurcovicova J. Differences in hormonal and inflammatory parameters in male Lewis and Long Evans rats with adjuvant arthritis. Int J Tissue React. 2003;25:99–104. [PubMed] [Google Scholar]
- 238.Roman O, Seres J, Herichova I, Zeman M, Jurcovicova J. Daily profiles of plasma prolactin (PRL), growth hormone (GH), insulin-like growth factor-1 (IGF-1), luteinizing hormone (LH), testosterone, and melatonin, and of pituitary PRL mRNA and GH mRNA in male Long Evans rats in acute phase of adjuvant arthritis. Chronobiol Int. 2003;20:823–836. doi: 10.1081/CBI-120021085. [DOI] [PubMed] [Google Scholar]
- 239.Castrillon PO, Cardinali DP, Pazo D, Cutrera RA, Esquifino AI. Effect of superior cervical ganglionectomy on 24-h variations in hormone secretion from the anterior hypophysis and in hypothalamic monoamine turnover during the preclinical phase of Freund’s adjuvant arthritis in rats. J Neuroendocrinol. 2001;13:288–295. doi: 10.1046/j.1365-2826.2001.00627.x. [DOI] [PubMed] [Google Scholar]
- 240.Elhassan AM, Adem A, Suliman IA, Mustafa A, Lindgren JU. Prolactin, growth hormone, and IGF-1 in ankles and plasma of adjuvant arthritic rats. Scand J Rheumatol. 1999;28:368–373. doi: 10.1080/03009749950155364. [DOI] [PubMed] [Google Scholar]
- 241.Lemini M, Ruiz-Herrera X, Ledesma-Colunga MG, Diaz-Lezama N, De Los Rios EA, Lopez-Barrera F, Mendez I, Martinez de la Escalera G, Macotela Y, Clapp C. Prolactin anterior pituitary expression and circulating levels are reduced in obese and diabetic rats: role of TGF-beta and TNF-alpha. Am J Physiol Regul Integr Comp Physiol. 2015;308:R792–R799. doi: 10.1152/ajpregu.00327.2014. [DOI] [PubMed] [Google Scholar]
- 242.Arzt E, Pereda MP, Castro CP, Pagotto U, Renner U, Stalla GK. Pathophysiological role of the cytokine network in the anterior pituitary gland. Front Neuroendocrinol. 1999;20:71–95. doi: 10.1006/frne.1998.0176. [DOI] [PubMed] [Google Scholar]
- 243.Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R. Modulation of prolactin expression in human T lymphocytes by cytokines. J Neuroimmunol. 2005;162:190–193. doi: 10.1016/j.jneuroim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 244.Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- 245.Roccaro AM, Russo F, Cirulli T, Di Pietro G, Vacca A, Dammacco F. Antiangiogenesis for rheumatoid arthritis. Curr Drug Targets Inflamm Allergy. 2005;4:27–30. doi: 10.2174/1568010053622911. [DOI] [PubMed] [Google Scholar]
- 246.Bainbridge J, Sivakumar B, Paleolog E. Angiogenesis as a therapeutic target in arthritis: lessons from oncology. Curr Pharm Des. 2006;12:2631–2644. doi: 10.2174/138161206777698747. [DOI] [PubMed] [Google Scholar]
- 247.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 248.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 249.Ochoa-Amaya JE, Hamasato EK, Tobaruela CN, Queiroz-Hazarbassanov N, Anselmo Franci JA, Palermo-Neto J, Greiffo FR, de Britto AA, Vieira RP, Ligeiro de Oliveira AP, Massoco Salles-Gomes CO, Felicio LF. Short-term hyperprolactinemia decreases allergic inflammatory response of the lungs. Life Sci. 2015;142:66–75. doi: 10.1016/j.lfs.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 250.Bharti B, Lee SJ, Lindsay SP, Wingard DL, Jones KL, Lemus H, Chambers CD. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the organization of teratology information specialists autoimmune diseases in pregnancy project. J Rheumatol. 2015;42:1376–1382. doi: 10.3899/jrheum.140583. [DOI] [PubMed] [Google Scholar]
- 251.Ma KK, Nelson JL, Guthrie KA, Dugowson CE, Gammill HS. Adverse pregnancy outcomes and risk of subsequent rheumatoid arthritis. Arthritis Rheumatol. 2014;66:508–512. doi: 10.1002/art.38247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, Dolhain RJ. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60:3196–3206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- 253.Jorgensen KT, Harpsoe MC, Jacobsen S, Jess T, Frisch M. Increased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth Cohort. Rheumatology (Oxford) 2014;53:1513–1519. doi: 10.1093/rheumatology/keu150. [DOI] [PubMed] [Google Scholar]
- 254.Gonzalez C, Parra A, Ramirez-Peredo J, Garcia C, Rivera JC, Macotela Y, Aranda J, Lemini M, Arias J, Ibarguengoitia F, de la Escalera GM, Clapp C. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest. 2007;87:1009–1017. doi: 10.1038/labinvest.3700662. [DOI] [PubMed] [Google Scholar]
- 255.Leanos-Miranda A, Campos-Galicia I, Ramirez-Valenzuela KL, Chinolla-Arellano ZL, Isordia-Salas I. Circulating angiogenic factors and urinary prolactin as predictors of adverse outcomes in women with preeclampsia. Hypertension. 2013;61:1118–1125. doi: 10.1161/HYPERTENSIONAHA.111.00754. [DOI] [PubMed] [Google Scholar]
- 256.Leanos-Miranda A, Marquez-Acosta J, Cardenas-Mondragon GM, Chinolla-Arellano ZL, Rivera-Leanos R, Bermejo-Huerta S, Romero-Arauz JF, Alvarez-Jimenez G, Ramos-Leon JC, Ulloa-Aguirre A. Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab. 2008;93:2492–2499. doi: 10.1210/jc.2008-0305. [DOI] [PubMed] [Google Scholar]
- 257.Ramma W, Ahmed A. Is inflammation the cause of pre-eclampsia? Biochem Soc Trans. 2011;39:1619–1627. doi: 10.1042/BST20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nakajima R, Ishida M, Kamiya CA, Yoshimatsu J, Suzuki M, Hirota A, Ikeda T, Harigaya T. Elevated vasoinhibin derived from prolactin and cathepsin D activities in sera of patients with preeclampsia. Hypertens Res. 2015;38:899–901. doi: 10.1038/hr.2015.99. [DOI] [PubMed] [Google Scholar]
- 259.Adan N, Ledesma-Colunga MG, Reyes-Lopez AL, Martinez de la Escalera G, Clapp C. Arthritis and prolactin: a phylogenetic viewpoint. Gen Comp Endocrinol. 2014;203:132–136. doi: 10.1016/j.ygcen.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 260.Reser JE, Reser WW. Does rheumatoid arthritis represent an adaptive, thrifty condition? Med Hypotheses. 2010;74:189–194. doi: 10.1016/j.mehy.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 261.Cooke NE, Coit D, Shine J, Baxter JD, Martial JA. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981;256:4007–4016. [PubMed] [Google Scholar]
- 262.Nicoll CS. Physiological actions of prolactin. In: Knobil E, Sawyer WH, editors. Handbook of Physiology. Section 7: Endocrinology. Washington: American Physiological Society; 1974. pp. 253–292. [Google Scholar]
- 263.Angelier F, Chastel O. Stress, prolactin and parental investment in birds: a review. Gen Comp Endocrinol. 2009;163:142–148. doi: 10.1016/j.ygcen.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 264.Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 265.Manzon LA. The role of prolactin in fish osmoregulation: a review. Gen Comp Endocrinol. 2002;125:291–310. doi: 10.1006/gcen.2001.7746. [DOI] [PubMed] [Google Scholar]
- 266.Whittington CM, Wilson AB. The role of prolactin in fish reproduction. Gen Comp Endocrinol. 2013;191:123–136. doi: 10.1016/j.ygcen.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 267.Bu G, Ying Wang C, Cai G, Leung FC, Xu M, Wang H, Huang G, Li J, Wang Y. Molecular characterization of prolactin receptor (cPRLR) gene in chickens: gene structure, tissue expression, promoter analysis, and its interaction with chicken prolactin (cPRL) and prolactin-like protein (cPRL-L) Mol Cell Endocrinol. 2013;370:149–162. doi: 10.1016/j.mce.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 268.Zhou JF, Zadworny D, Guemene D, Kuhnlein U. Molecular cloning, tissue distribution, and expression of the prolactin receptor during various reproductive states in Meleagris gallopavo. Biol Reprod. 1996;55:1081–1090. doi: 10.1095/biolreprod55.5.1081. [DOI] [PubMed] [Google Scholar]
- 269.Hardy RS, Raza K, Cooper MS. Endogenous glucocorticoids in inflammation: contributions of systemic and local responses. Swiss Med Wkly. 2012;142:w13650. doi: 10.4414/smw.2012.13650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.