Abstract
To establish a functional bipolar mitotic spindle, the centrosome expands and matures, acquiring enhanced activities for microtubule (MT) nucleation and assembly at the onset of mitosis. However, the regulatory mechanisms of centrosome maturation and MT assembly from the matured centrosome are largely unknown. In this study, we showed that heat shock protein (HSP) 70 considerably accumulates at the mitotic centrosome during prometaphase to metaphase and is required for bipolar spindle assembly. Inhibition or depletion of HSP70 impaired the function of mitotic centrosome and disrupted MT nucleation and polymerization from the spindle pole, and may thus result in formation of abnormal mitotic spindles. In addition, HSP70 may associate with NEDD1 and γ-tubulin, two pericentriolar material (PCM) components essential for centrosome maturation and MT nucleation. Loss of HSP70 function disrupted the interaction between NEDD1 and γ-tubulin, and reduced their accumulation at the mitotic centrosome. Our results thus demonstrate a role for HSP70 in regulating centrosome integrity during mitosis, and indicate that HSP70 is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle.
Keywords: HSP70, Microtubule nucleation, Microtubule polymerization, Mitotic spindle, Spindle pole
Introduction
The centrosome is a remarkably complex structure with diverse functions. It comprises a pair of centrioles and the surrounding pericentriolar material (PCM). The centrosome is the major site for microtubule (MT) nucleation, resulting in MT emanation and elongation; in addition, the centrosome coordinates all MT-related functions, including vesicle transportation, cell polarity, cell shape, motility, adhesion, and cell division [1]. Similar to DNA replication, the centrosome duplicates once per cell cycle in a semi-conservative manner; as such, at mitosis a single interphase centrosome yields two mature centrosomes, which participate in the organization of bipolar spindles, faithful segregation of chromosomes, and correct division of a cell into two daughter cells with an exact set of chromosomes [2]. Thus, centrosome function is under differential regulation at each stage of the cell cycle, and the MTs undergo regulated nucleation and growth, shrinkage, or stabilization from the centrosome in response to intracellular signals, allowing cells to rapidly meet their physiological needs [3, 4]. Of a special note, the centrosome expands and matures upon entry into mitosis—at this time it acquires additional MT nucleation activities through increasing the recruitment of essential proteins, to prepare for the assembly of mitotic spindles [5]; this suggests the existence of distinctive regulatory processes that control mitotic centrosome functions. Inadequate recruitment of additional PCM components results in the emergence of disorganized spindles, which may trigger mitotic arrest, cell death, and/or aneuploidy [1, 6, 7]. Thus, loss of the integrity of the mitotic centrosome hampers the establishment of bipolar spindles and induces chromosome missegregation.
It is known that the recruitment of γ-tubulin to the centrosome is essential and correlates with increased MT nucleation as cells enter mitosis [8], suggesting that γ-tubulin recruitment must be increased for organization of a functional spindle pole. Gamma-tubulin and γ-tubulin complex proteins (GCPs) are the major components of the γ-tubulin ring complex (γTuRC), and these, along with NEDD1 and other factors regulating the localization and activation of γ-tubulin, are sequentially recruited to the centrosomes, where they enable assembly of the γTuRC to promote the polymerization of centrosomal MTs in the mitotic spindle during mitosis [9–11]. However, it is not completely understood how the interphase centrosomes form a mature mitotic centrosome. In addition, centrosome defects, such as loss of γ-tubulin or other centrosomal proteins, compromise MT nucleation/organization, leading to spindle defects [6]. Depletion or inhibition of NEDD1 results in loss of γTuRC from the centrosome, thereby abolishing centrosomal MT nucleation [9]. Overexpression or excessive accumulation of γ-tubulin or other centrosomal proteins induces defects in mitotic spindles and is characteristic of tumorigenesis and human malignancies in multiple tissues [12–16]. Thus, maintaining a homeostatic level of these proteins at the spindle pole is critical for a well-functioning mitotic centrosome.
Molecular chaperones have different but cooperative roles in the formation and function of the eukaryotic cell cytoskeleton [17]. It has been demonstrated that heat shock proteins (HSPs), the molecular chaperones essential for protein quality control and intracellular proteostasis [18, 19], associate with tubulin or MTs [20, 21] or accumulate at the centrioles [22] under physiological or stressed conditions. In addition, members of the HSP70 family from S. cerevisiae interact with different components of the spindle pole, kinetochore, and MT motors [20]. HSPB1 mutations that result in higher chaperone activity increase its binding to tubulin or MTs, and thereby stabilize the MT network [23]. Morgana/CHP-1, a HSP90 binding co-chaperone, prevents centrosome amplification and tumorigenesis by interfering with nucleophosmin-mediated ROCK activation [24]. In addition, HSP70 protects mitotic cells against heat-induced centrosome damage and division abnormalities [25]. HSPA2, like HSP70 protein, protects the integrity of nucleoli and centrosomes in cancer cells subjected to heat shock [26]. These studies indicate that HSPs may interact with components of the spindle pole, and be involved in the regulation and maintenance of centrosome function.
We have previously demonstrated that the inducible form of HSP70 (encoded by genes HSPA1A and HSPA1B) may modulate spindle dynamics and prevent mitotic cell apoptosis induced by arsenite [27], a HSP70 inducer. In this study, we further investigated whether HSP70 regulates the mitotic centrosome and controls the assembly of mitotic spindles. We demonstrate that HSP70 accumulates at the spindle pole and is required for spindle pole accumulation of NEDD1 and γ-tubulin and MT nucleation, and thus may support the assembly of a functional bipolar spindle and the completion of cell division.
Materials and methods
Cell culture
CGL2 cells (a HeLa cell/normal human fibroblast hybrid [28]) were kindly provided by Dr. E. J. Stanbridge (University of California, Irvine) and were cultured as previously described [29]. HeLa cells stably expressing EGFP-EB1 were established by transfecting cells with the expression vector pEGFP-N1-EB1 (a gift from Dr. Tim Mitchison, Addgene plasmid # 12345), and selecting under G418. Cell cycle synchronization at the mitotic stage was induced by double-thymidine block [29]. CGL2 cells overexpressing the FLAG-tagged wild type HSP70 (wt) or HSP70 mutant (d5) lacking the C-terminal CHIP binding domain were established by transducing cells with virions containing wt or d5-expressing constructs, respectively, and then selecting under puromycin [30]. Analyses of cell viability and cell cycle progression were carried out as previously described [29, 30].
Depletion of HSP70
The shRNAs specifically targeting HSPA1A (TRCN8757 and TRCN342860) and HSPA1B (TRCN8760 and TRCN8763), the two genes encoding HSP70, were obtained from the National RNAi core Facility Platform (Genomic Research Center, Academia Sinica). The shRNA-containing virions were collected, and depletion of HSP70 and establishment of cells transiently or stably depleted of HSP70 were carried out as previously described [29, 30]. For rescue experiments, cellular HSP70 was first stably depleted with shRNAs targeting at the 3′-untranslated regions (TRCN342860 and TRCN8759). Then the FLAG-tagged wild type or mutant HSP70 cDNA constructed under the control of a CMV promoter (to prevent the interference by the above-mentioned shRNAs) was delivered into cells to overexpress the ectopic HSP70.
Immunoblotting and immunoprecipitation
Cell lysis, immunoblotting, and immunoprecipitation were carried out as previously described [27, 30]. For immunoprecipitation, cells were lysed with RIPA buffer for 30 min on ice. HSP70, NEDD1, or γ-tubulin was immunoprecipitated with specific antibodies. Immunoprecipitated complexes were then subjected to immunoblot analysis. Specific proteins were immunoprecipitated or detected using antibodies against HSP70 (GeneTex, Hsinchu, Taiwan) and FLAG, NEDD1, α-tubulin, and γ-tubulin (Sigma, St. Louis, MO, USA). Beta-actin or GAPDH was detected with anti-β-actin (Chemicon, Temecula, CA) or anti-GAPDH (Genetex), respectively, for use as loading controls.
Immunofluorescence staining and confocal microscopy
Cells were seeded onto coverslips, incubated for 20 h prior to drug treatments, and then fixed in PTEMF buffer (20 mM PIPES, 4 % para-formaldehyde, 0.2 % Triton-X, 10 mM EGTA and 1 mM MgCl2) for 15 min. For depletion of HSP70, cells were transduced with virion containing HSP70 shRNAs for 48 h, and were then seeded onto coverslips for another 20 h before fixation. Immunostaining was carried out as previously described [27]. Anti-HSP70 (GeneTex or StressMarq, Victoria, Canada), anti-NEDD1 (Sigma), anti-α-tubulin (Sigma), anti-γ-tubulin (Sigma), and anti-pericentrin (Covance, Princeton, NJ, USA) were used for immunostaining. Alexa Fluor 488-, 568-, or 633-conjugated goat anti-mouse and rabbit IgG were obtained from Invitrogen. The stained cells were observed and images were obtained by z projection with an upright confocal microscope (Leica TCS-SP5). Exposure time was set and kept constant throughout each independent experiment. The region of interest (ROI) was defined by drawing a circle enclosing the centrosome. The intensities of HSP70, NEDD1, α-tubulin, or γ-tubulin within ROI were determined by measuring average pixel intensity with MetaMorph COMPLETE software (Molecular Device, Sunnyvale, CA). Background fluorescence (based on a circle of corresponding size in an adjacent region) was subtracted from each measurement.
Analysis of MT polymerization
The effect of HSP70 on spindle MT polymerization was assessed cold treatment or nocodazole washout assay. CGL2 cells seeded on coverslips in six-well plates were either treated with PES for 16 h or transduced with virion containing empty vector (pLKO.1) or shRNA targeting HSPA1A and HSPA1B (shHSPA1A/B) for 48 h before being subjected to cold or nocodazole treatment. For cold treatment, six-well plates were immersed in ice-cold water for 0, 10, or 30 min, and then the cells were immediately fixed in PTEMF buffer, stained with antibodies against α-tubulin and γ-tubulin, and imaged using a confocal microscope (Leica TCS-SP5). Cells with different levels of MT disassembly (as shown in Fig. 3E) were identified and counted.
Fig. 3.
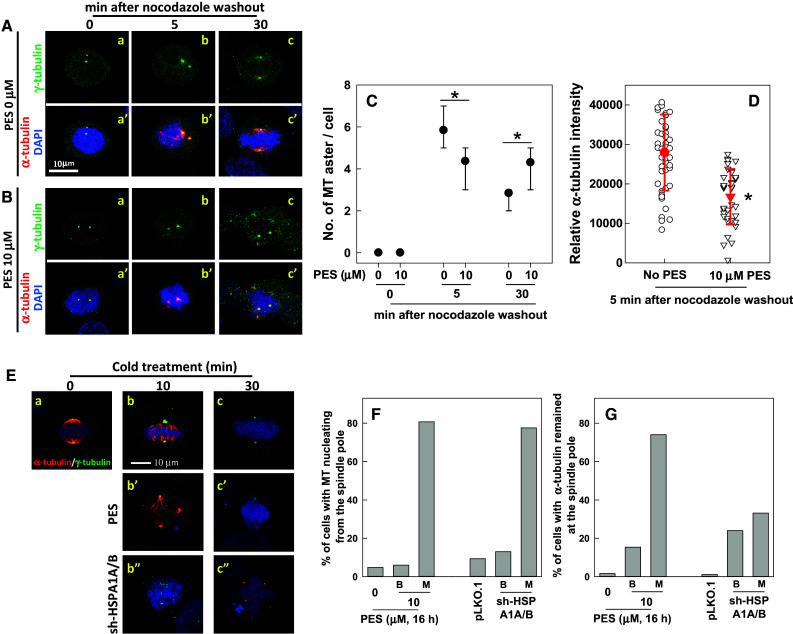
Inhibition or depletion of HSP70 induces stabilization of spindle MTs and impedes spindle MT polymerization. Representative images of the mitotic spindle after nocodazole treatment and washout. CGL2 cells were untreated (A) or treated with HSP70 10 μM PES for 16 h (B). At the last 3 h of PES treatment, nocodazole was added into culture medium to 3 μM. Nocodazole was then completely washed out and the cells were incubated in drug-free medium for the indicated time before being immediately fixed and immunostained for α-tubulin (red) and γ-tubulin (green). The chromosomes were counterstained with DAPI (blue). C The number of MT asters in cells. Results are the median ± 25 percentiles of at least 250 mitotic cells for each condition, as determined from three experiments. *p < 0.05 by Mann–Whitney rank sum test as compared with the PES-untreated control. D The fluorescence intensity of centrosomal α-tubulin. Results are the mean ± SD of at least 50 mitotic centrosomes from three independent experiments. *p < 0.05 by Student’s t test as compared with the PES-untreated control. E Representative images of the mitotic spindle after cold treatment. CGL2 cells were untreated (a, b, c) or treated with 10 μM PES (b′, c′) for 16 h. The cells were then subjected to cold treatment for the indicated time before being stained for mitotic spindles as described in A. For depletion of HSP70 (b″, c″), the cells were transduced with virion containing the empty vector (pLKO.1) or HSP70-specific shRNAs (sh-HSPA1A/B). After 48 h, the cells were subjected to cold treatment and then immunofluorescence staining. F The percentages of mitotic cells that still exhibited MTs nucleating from the spindle pole after cold treatment for 5 min. G The percentages of mitotic cells with α-tubulin signals remaining at the spindle pole after cold treatment for 30 min. Results are the average of at least 250 cells for each treatment, determined from two experiments. B, bipolar spindle; M, multipolar spindle
For nocodazole washout assay, cells after inhibition or depletion of HSP70 were incubated in medium containing 3 μM nocodazole for 3 h to completely depolymerize MTs. Nocodazole was then washed out and replaced with warmed media, followed by incubation at 37 °C for the indicated time. The cells were fixed in PTEMF buffer and immunostained with antibodies against α- and γ-tubulin after the indicated period of re-growth. The number of MT asters was counted under a fluorescent microscope (Zeiss Axioplan 2 Imaging MOT).
Analysis of centrosome volume
Centrosome volume was determined by fluorescent staining intensity of pericentrin, a critical PCM component essential for centrosome maturation; recruitment of pericentrin is significantly augmented at the centrosome at the onset of mitosis [31, 32]. Immunofluorescence staining of pericentrin at the centrosomes of cells was carried out as described above. A sample thickness of 10 μm (in Z-steps of 0.5 μm) was imaged with a Leica confocal microscope (TCS-SP5) and analyzed by Imaris. To obtain pericentrin intensity volume, a “surface” was created within a manually determined ROI, which covered the fluorescence signal with a surface area detail level at 0.241, a threshold of absolute intensity at 35, and the number of voxels at 10.
Analysis of MT nucleation
MT nucleation was assessed based on the intensity of centrosomal MT arrays, which were stained with immunofluorescent antibodies against α-tubulin. Images were obtained by z projection with a Leica confocal microscope (TCS-SP5), and α-tubulin intensity at the mitotic centrosome was determined by MetaMorph as described above.
MT nucleation was also visualized by live imaging of EGFP-EB1 [33]. HeLa cells stably expressing EGFP-EB1 were seeded and treated in chambered coverslips (μ-slide 4 well, ibidi GmbH, Germany). Alternatively, cells were first depleted of HSP70 and then seeded in chambered coverslips. The chambered coverslips were then placed on the objective stage set up in a humidified chamber with 10 % CO2 in air at 37 °C, and imaged under an inverted confocal microscope (Leica TCS-SP5) at a fixed focal plane with a sample thickness of 1 μm. EGFP-EB1 images were recorded every 1.25 s for a total of 3 min. Time lapse images were then compiled and analyzed using MetaMorph software. The EB1 comet emanating from the spindle pole and visible for three to five consecutive frames was manually identified and counted.
Results
HSP70 accumulates at the mitotic centrosome and is required for the assembly of bipolar mitotic spindles
Whether or not HSP70 plays a role in regulating the assembly of mitotic spindles was determined by examining the cellular localization of HSP70 and its effects on the assembly of mitotic spindles. Immunofluorescence staining was performed to show that HSP70 is ubiquitously expressed in interphase cells, with relatively higher expression in the nucleus. HSP70 staining at the spindle pole became evident at prophase, with staining intensity being most prominent at the spindle poles and the distal ends of spindle MTs from prometaphase to metaphase; this was followed by slight fading from metaphase to telophase, and HSP70 then translocated to the cleavage furrow during cytokinesis (Fig. 1a). In addition, HSP70 colocalized with γ-tubulin (Fig. 1b) and pericentrin (Fig. 1c). The accumulation of HSP70 at the mitotic centrosome was also observed in other cultured cells, such as human diploid fibroblasts and the bronchial epithelial cell line BEAS2B, as well as cancer cell lines A549 and HeLa (data not shown). We then inhibited cellular HSP70 with PES, an inhibitor that disrupts the binding of HSP70 to its substrates [34]. We observed that PES treatment resulted in dose-dependent induction of disorganized mitotic spindle formation (Fig. 2a, b), accumulation of mitotic cells (Fig. 2c), and cell death (Fig. 2d). We proceeded to deplete cellular HSP70 by transduction of virions containing HSP70-specific shRNAs (targeting HSPA1A and HSPA1B). Figure 2e shows that the expression of basal and arsenic trioxide (ATO)- or PES-induced HSP70 were efficiently reduced after transduction of the specific shRNAs. Depletion of HSP70 also significantly reduced HSP70 accumulation at the mitotic centrosome (Fig. 2f, g). In addition to inducing multipolar spindles, Fig. 2h shows that depletion of HSP70 also induced the formation of disorganized mitotic spindles with un-aligned or missegregated chromosomes in the cells. As shown in Fig. 2i, depletion of HSP70 significantly induced the accumulation of mitotic cells containing multipolar or disorganized mitotic spindles. The formation of abnormal spindles in mitotic cells was considerably reduced by overexpression of a CMV promoter-controlled wild type HSP70 (HSP70-wt) but not a deletion mutant (HSP70-d5) lacking the C-terminal co-chaperone binding domain (Fig. 2i). These results indicate that HSP70 may be required at the mitotic centrosome for spindle assembly, and hence absence of this protein hampers mitotic progression and induces cell death.
Fig. 1.
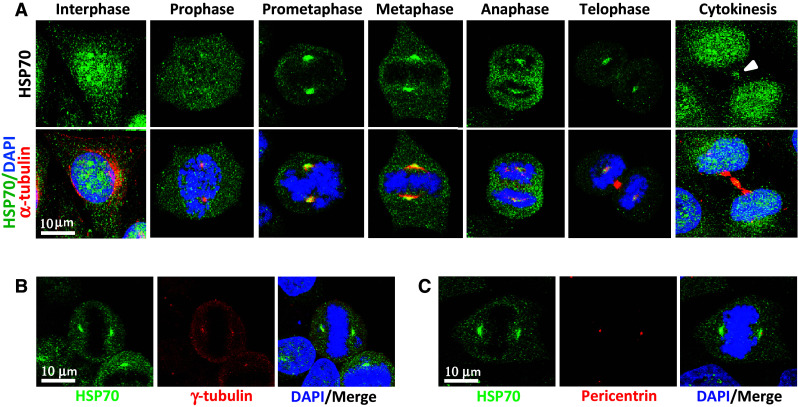
HSP70 accumulates at the mitotic centrosome. a Representative images of cellular distribution of HSP70 in CGL2 cells. Logarithmically growing cells were fixed and stained for HSP70 (green) and α-tubulin (red). The nuclei or chromosomes were counterstained with DAPI (blue). Colocalization of HSP70 with γ-tubulin (b) and pericentrin (c). Logarithmically growing CGL2 cells were fixed and stained for HSP70 (green) and γ-tubulin or pericentrin (red)
Fig. 2.
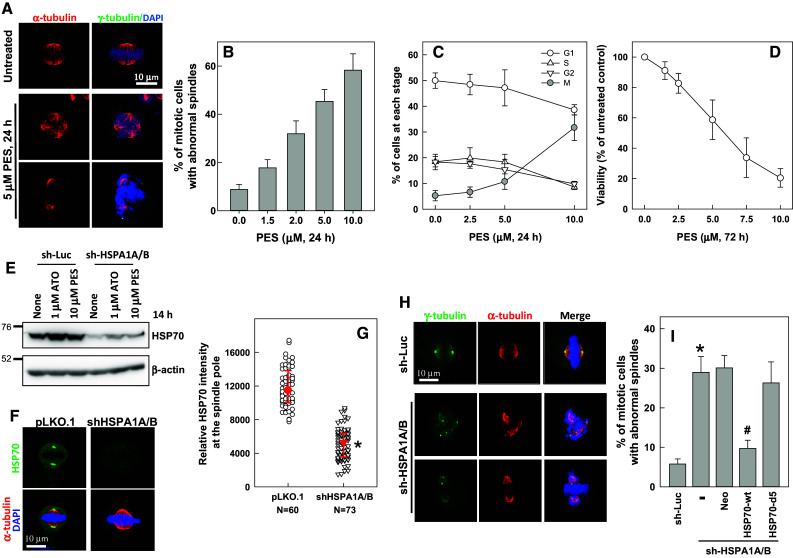
HSP70 is required for assembly of a bipolar mitotic spindle. a, b Inhibition of HSP70 induces spindle defects. CGL2 cells were treated with PES at the indicated concentrations for 24 h and were then fixed and stained for mitotic spindles with antibodies against α-tubulin (red) and γ-tubulin (green). The chromosomes were counterstained with DAPI (blue). The percentage of mitotic cells containing abnormal mitotic spindles was determined using at least 500 mitotic cells from three independent experiments. c PES induces mitotic arrest. Cells were treated as in A and then fixed for analysis of cell cycle distribution. The results (mean ± SD) are from four independent experiments. d PES induces cell death. Cells were treated with PES at the indicated concentrations for 72 h, and then subjected to viability assays. The results (mean ± SD) are from three independent experiments. e Expression level of HSP70 in cells stably depleted of HSP70 (sh-HSPA1A/B). Control (sh-Luc) and sh-HSPA1A/B cells were untreated or treated with arsenic trioxide (ATO) or PES for 14 h; the expression of HSP70 was then examined by immunoblotting. f, g Decreased accumulation of HSP70 at the spindle pole in cells stably depleted of HSP70 (sh-HSPA1A/B). Logarithmically growing control (pLKO.1) and sh-HSPA1A/B cells were fixed and immunostained for HSP70 (green) and α-tubulin (red). Relative HSP70 intensity at the spindle pole was determined (median ± 25 percentiles) from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the control transduced cells (pLKO.1). h, i Depletion of HSP70 induces spindle defects. Control cells (sh-Luc) and cells depleted of HSP70 (sh-HSPA1A/B) were fixed and stained for mitotic spindles as in A. The percentage of mitotic cells containing abnormal mitotic spindles was determined using at least 500 mitotic cells from three independent experiments. Cells stably depleted of HSP70 (sh-HSPA1A/B) were further transduced with a CMV promoter-driven wild type HSP70 (HSP70-wt) or a deletion mutant (HSP70-d5). pFB-Neo is an empty vector and serves as a control. *p < 0.05 by Student’s t test compared to the control depleted cells (pLKO.1). # p < 0.05 by Student’s t test compared to the no ectopic HSP70 control
Inhibition or depletion of HSP70 impedes spindle MT polymerization
The effect of HSP70 on spindle MT polymerization was examined using nocodazole washout assay. As shown in Fig. 3A-a and a′, nocodazole treatment resulted in complete disassembly of the spindle MTs, leaving two γ-tubulin spots without α-tubulin fibers in the mitotic cells. Up to six MT asters (two of which were centrosomal as indicated by the staining of γ-tubulin, while the others were non-centrosomal, Fig. 3A-b, b′, C) reformed within 5 min in mitotic cells recovering from nocodazole-induced MT disassembly. The non-centrosomal asters gradually merged into centrosomal asters, and a bipolar spindle reassembled within 30 min after release from nocodazole (Fig. 3A-c, c′, C). Two centrosomal MT asters also appeared in mitotic cells from PES-treated culture, but in contrast to the control, the intensities of α-tubulin fibers nucleated from the mitotic centrosome were significantly reduced compared to those in untreated cells after release from nocodazole for 5 min (Fig. 3B-b, b′, D), indicating that centrosomal MT nucleation and/or polymerization was impaired. In addition, fewer additional asters emerged after release from nocodazole for 5 min (Fig. 3B-b, b′, C). After release from nocodazole-induced spindle disassembly for 30 min, many of the PES-treated cells exhibited more than two MT asters (Fig. 3B-c, c′, C), indicating that reformation of the bipolar spindle was also delayed. These results indicate that inhibition of HSP70 may impair centrosomal MT aster formation, and hence delay reassembly of a bipolar spindle.
The dynamic instability of spindle MTs is essential for a functional mitotic spindle; therefore, whether loss of HSP70 function alters spindle MT dynamics was determined based on the sensitivity of spindle MTs to cold-induced disassembly. Cells treated with PES or depleted of HSP70 were subjected to ice treatment to destabilize the MTs. Cold treatment for 10 min resulted in significant disassembly of centrosomal MTs [35], leaving the kinetochore-MTs bundled together in mitotic cells released from a double-thymidine block (Fig. 3E-b). After 30 min of cold treatment, all of the spindle MTs had disassembled, leaving two γ-tubulin spots without any α-tubulin staining (Fig. 3E-c). The kinetics of disassembly of spindle MT after cold treatment in the control-depleted cells (pLKO.1) is similar to that in untreated CGL2 cells. In the mitotic cells from PES-treated (Fig. 3E-b′, c′) or HSP70-depleted cultures (Fig. 3E-b″, c″), the disassembly of centrosomal MTs after cold treatment was considerably delayed compared to that in the respective control cells. The absence of HSP70 significantly elevated the percentage of mitotic cells that still exhibited MTs nucleating from the spindle pole after cold treatment for 10 min (Fig. 3E-b′, b″, F) and the percentage of cells with α-tubulin signals remaining at the spindle pole after cold treatment for 30 min (Fig. 3E-c′, c″, G). These results indicate that centrosomal MTs in mitotic cells without HSP70 function are more stable and more resistant to cold treatment than those from control cells, suggesting that the absence of functional HSP70 results in less dynamic spindle MTs.
HSP70 is required for centrosome maturation and MT nucleation from the spindle pole
Since centrosome maturation is prerequisite to the enhanced MT nucleation activity required for assembling a functional mitotic spindle, we examined whether HSP70 depletion alters centrosome maturation. Pericentrin is a critical PCM component essential for centrosome maturation, and its recruitment is significantly augmented at the centrosome at the onset of mitosis [31, 32]. Therefore, we examined centrosome maturation based on the fluorescent staining intensity of pericentrin at the mitotic centrosomes. Figure 4a, b shows that the volume of pericentrin intensity at the mitotic centrosome significantly decreased in HSP70-depleted mitotic cells, indicating that HSP70 accumulation at the mitotic centrosome is required for pericentrin expansion during centrosome maturation.
Fig. 4.
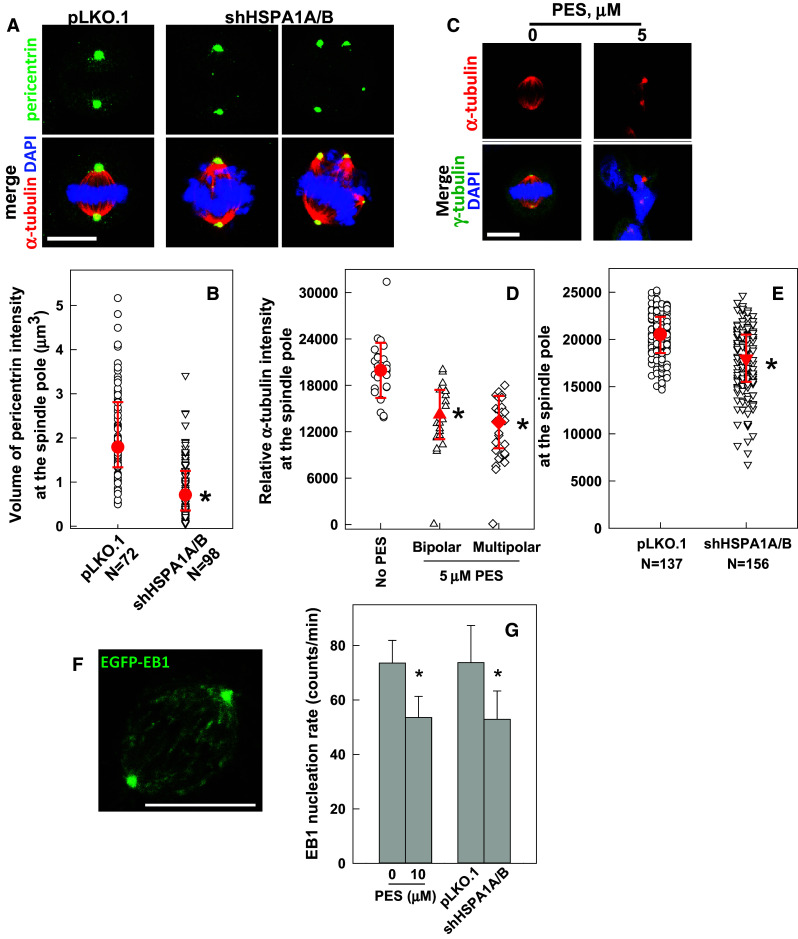
Inhibition or depletion of HSP70 reduces MT nucleation from the spindle pole. a Representative images of cells immunostained for pericentrin (green) and α-tubulin (red). The CGL2 cells were depleted of HSP70 as described in Fig. 2e and then fixed and stained for pericentrin. Scale bar, 10 μm. b Pericentrin volumes at mitotic centrosomes. Pericentrin volume was determined as described in the “Materials and methods”. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the control transduced cells (pLKO.1). c Representative images of PES-treated cells immunostained for α-tubulin (red) and γ-tubulin (green). The cells were treated with 5 μM PES for 16 h and fixed and immunostained as described in Fig. 2a. Scale bar, 10 μm. d, e Relative α-tubulin intensity at the spindle pole in cells lacking HSP70 function. Cells were treated with PES or depleted of HSP70 as described in Fig. 2e. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the untreated or control transduced cells (pLKO.1), as appropriate. f A representative image frame of a time-lapse sequence from a cell stably expressing EGFP-EB1 (HeLa-EB1-GFP). Scale bar 10 μm. g Inhibition or depletion of HSP70 reduces the nucleation of EGFP-EB1 comets. HeLa-EGFP-EB1 cells were treated with PES or depleted of HSP70 as described in Fig. 2e, and then subjected to time-lapse imaging under a confocal microscope as described in the “Materials and methods”. Typically, each comet was visible for three to five frames. Results are the mean ± SD of EB1 comets nucleated from 30 to 50 spindle poles in three independent experiments. *p < 0.05 by Student’s t test compared to the untreated or control transduced cells (pLKO.1), as appropriate
We subsequently examined the role of HSP70 on MT nucleation from the spindle pole. Immunofluorescence staining of α-tubulin revealed that the intensities of α-tubulin arrays emanating from the poles of either bipolar or multipolar spindles in PES-arrested mitotic cells were considerably reduced compared to those in untreated mitotic cells (Fig. 4c, d). Depletion of HSP70 also decreased the intensity of α-tubulin nucleating from the spindle pole (Fig. 4e). The effect of HSP70 on MT nucleation was also examined by live-imaging of EGFP-tagged EB1, a microtubule tip-binding protein, and subsequent calculation of the rate of EB1-positive comet emanation from the mitotic centrosome (Fig. 4f). As shown in Fig. 4g, the rates of EGFP-EB1 comet nucleation from the spindle pole in mitotic cells of the PES-treated or HSP70-depleted cultures were also significantly decreased compared to those of untreated or undepleted mitotic cells. These results indicate that HSP70 is required for MT nucleation from the spindle pole.
HSP70 is required for NEDD1 and γ-tubulin recruitment to the mitotic centrosome
Establishment of a well-functioning bipolar spindle requires that the mitotic centrosome acquire elevated nucleation activities through increased recruitment of essential proteins to the centrosome. To understand how HSP70 regulates MT nucleation, we examined HSP70 effects on centrosomal accumulation of NEDD1, a γ-tubulin-targeting factor required for centrosomal MT nucleation [9]. We observed that inhibition (Fig. 5a, b) or depletion (Fig. 5c, d) of HSP70 significantly decreased the fluorescence intensities of NEDD1 (left-side panels in Fig. 5a–d) and γ-tubulin (right-side panels of Fig. 5a–d) at the spindle pole. These results indicate that HSP70 accumulation at the spindle pole is required for the localization of sufficient amounts of NEDD1 and γ-tubulin at the spindle pole.
Fig. 5.
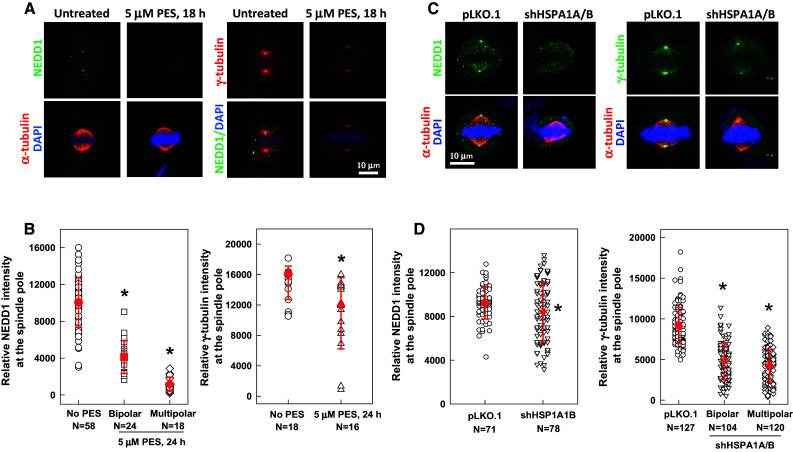
Inhibition or depletion of HSP70 reduces the accumulation of NEDD1 and γ-tubulin at the spindle pole. Representative images of PES-treated (a) or HSP70-depleted (c) CGL2 cells immunostained for NEDD1 (left-side panels) or γ-tubulin (right-side panels). The cells were treated with PES or depleted of HSP70 as described in Fig. 2e, and then fixed and stained for NEDD1 and γ-tubulin. b and d Relative intensities of NEDD1 (left) or γ-tubulin (right) at the spindle pole. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the untreated or control transduced cells (pLKO.1), as appropriate
We next examined whether HSP70 associates with NEDD1 to facilitate its spindle pole accumulation. We found that NEDD1 is present in the immunocomplex pulled down by HSP70 antibodies from synchronized untreated mitotic cells (Fig. 6a), indicating that HSP70 is associated with NEDD1 during mitosis. However, this association was significantly reduced in mitotic cells from PES-treated cultures (Fig. 6a). HSP70 depletion reduced both the amount of NEDD1 observed in the immunocomplex pulled down by γ-tubulin antibodies (Fig. 6b) and the amount of γ-tubulin from the immunocomplex pulled down by NEDD1 antibodies (Fig. 6c), indicating that HSP70 is required for the interaction between NEDD1 and γ-tubulin. In addition, the interaction between NEDD1 and FLAG-tagged HSP70-d5, a deletion mutant lacking the C-terminal co-chaperone binding domain and not able to rescue the detrimental effect of HSP70 depletion on spindle assembly, was considerably reduced as compared to that with wildtype HSP70 (Fig. 6d). Thus, the results shown in Figs. 5 and 6 collectively imply that HSP70 associates with NEDD1 during mitosis, and disruption of this association may hamper the assembly of NEDD1 and γ-tubulin at the spindle pole, resulting in impairment of centrosome maturation and disruption of MT nucleation.
Fig. 6.
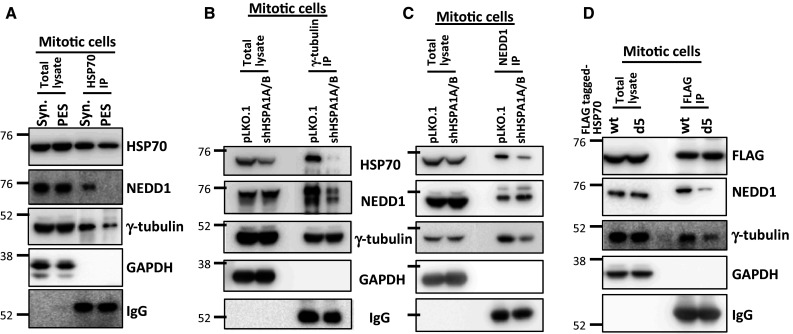
HSP70 associates with NEDD1, and inhibition or depletion of HSP70 disrupts the interaction between NEDD1 and γ-tubulin. a Inhibition of HSP70 by PES treatment reduces the interaction between HSP70 and NEDD1. Mitotic CGL2 cells from untreated cultures were obtained by synchronization with double-thymidine block as described in the “Materials and methods”. The PES-arrested mitotic cells were removed by shaking after treatment with 10 μM PES for 16 h. HSP70 was then immunoprecipitated (IP) using a specific antibody. The immunocomplex was analyzed by immunoblotting using anti-NEDD1 or anti-γ-tubulin. GAPDH and immunoglobin heavy chain (IgG) served as loading controls for total lysate and HSP70 IP, respectively. b and c Depletion of HSP70 impairs the interaction between NEDD1 and γ-tubulin. HSP70 was depleted as described in Fig. 2e. The mitotic cells were then collected as described in a. Gamma-tubulin (b) or NEDD1 (c) in mitotic cells were immunoprecipitated with specific antibodies. The immunocomplex was analyzed by immunoblotting as described in a. d A HSP70 mutant lacking the C-terminal co-chaperone binding domain exhibits a reduced interaction with NEDD1. Mitotic cells from cultures stably expressing the wild type (wt) or the mutant (d5) HSP70 were collected, and ectopic HSP70 was immunoprecipitated with anti-FLAG. The immunocomplex was then analyzed by immunoblotting as described in a
Discussion
Before entering mitosis, the centrosome matures, and the amount of PCM components recruited to the mitotic centrosome dramatically increases to prepare for MT nucleation and assembly. In this study, we provide evidence that HSP70 accumulates at the mitotic centrosome, where it contributes to the preservation of centrosome integrity by maintaining the level of PCM components, which facilitates elevated MT nucleation and polymerization, and thereby supports the establishment of mitotic spindles.
HSP70 is a molecular chaperone that participates in maintaining protein homeostasis through controlling the activity, stability, and/or oligomeric state of its substrate proteins [36]. It has been reported to be targeted to the lysosome [37] and nucleus [38] after heat shock, where it stabilizes and protects organelles under stress. HSP70 has also been reported to protect the mitotic centrosome from heat damage, and maintain cell survival [25, 39]. In yeast, HSP70 has been demonstrated to interact with several spindle pole proteins [20]. Recently, HSP70 was shown to accumulate at the mitotic centrosome during fever and prevent the degradation of critical centrosomal proteins [40]. These results indicate that HSP70 may interact with centrosomal proteins to regulate centrosome functions. Since profound structural and proteomic transitions occur during the G2–M transition and centrosome maturation [41], HSP70 may play a role in controlling the mobility and local interactions of centrosomal proteins to maintain the function of the mitotic centrosomes. In this study, we employed several methods to delineate the putative role of HSP70 at mitotic centrosomes. First, loss of HSP70 function was observed to hamper the ability of cells to reassemble a bipolar spindle after nocodazole washout. Second, the spindle MTs were found to be more resistant to cold treatment, and thus less dynamic, in mitotic cells lacking HSP70 function. In addition, HSP70 was found to be required for MT nucleation, as indicated by the decreased intensity of centrosomal α-tubulin and the reduced rate of EB1 comet emanation from the spindle pole. We also demonstrated that loss of HSP70 function considerably decreased the levels of pericentrin, NEDD1, and γ-tubulin at the mitotic centrosome. Since a decreased level of these centrosomal proteins and a decrease in MT nucleation from the spindle pole may indicate lost or compromised centrosome integrity [7], our results thus confirm that HSP70 acts to maintain the integrity of mitotic centrosomes, and is essential for the assembly of a functional mitotic centrosome.
Our results showed that inhibition or depletion of HSP70 induces the formation of disorganized mitotic spindles with unaligned or missegregated chromosomes in the cells. Among these mitotic cells, very few anaphases were observed. These results imply that inhibition or depletion of HSP70 may prevent anaphase onset due to the presence of dysfunctional metaphase spindles. Since our results demonstrate HSP70 is required for normal functioning of mitotic centrosomes, we propose that HSP70 may contribute to spindle assembly and chromosome segregation through maintaining the function of mitotic centrosome, although HSP70 may also play additional roles independent of the mitotic centrosome in the assembly of spindle MT.
HSP70 has been recognized as a stress-induced chaperone. Its transcription is often elevated in response to stress due to the activation of heat shock transcription factor 1. It has been reported that HSP70 is also constitutively expressed under non-stressed conditions [42]. Our immunofluorescence staining results showed that HSP70 is expressed in interphase cells, with high levels in nuclei; it then translocates to the centrosome at the onset of mitosis. Similar results were also obtained using primary cell lines (human fibroblasts and bronchial epithelial cells (BEAS2B)) and cancer cell lines (A549 and HeLa), indicating that the expression and distribution of HSP70 is cell-cycle dependent. HSP70 was demonstrated to be delivered to the centrosome in a MT-independent manner after heat stress [40]. It has been demonstrated that phosphorylation of HSP70 by Nek6 targets HSP70 to the spindle pole and outer kinetochores and that HSP70 promotes stable kinetochore-fiber assembly through recruitment of the ch-TOG–TACC3 complex [43]. These results indicated that HSP70 is a critical target of Nek6 and may mediate Nek6 function in spindle assembly. Since Nek6 plays limited role in the mitotic centrosome [44], the study provides evidence demonstrating that HSP70 may regulate spindle assembly independent of centrosome. We have previously demonstrated that phosphorylation of HSP70 by PLK1 induces its spindle pole localization during mitosis [27]. Since PLK1 controls many key events for the assembly of mitotic centrosomes and mitosis onset [31], these results indicate that HSP70 may be a PLK1 downstream effector regulating centrosome maturation.
It has been reported that inhibition of proteasome activity impairs centrosome-dependent MT nucleation and organization due to excessive accumulation of several proteins (including γ-tubulin and NEDD1) at the PCM [45], suggesting that the turnover of centrosome proteins contributes greatly toward normal centrosome function. Here, we observed that loss of HSP70 function considerably reduces the levels of NEDD1 and γ-tubulin at the mitotic centrosome, thereby indicating that HSP70 may also regulate the turnover of centrosome proteins. HSP70, together with its co-chaperone (HOP or CHIP), regulates the stability of its substrate proteins [46, 47]. Our results show that deletion of the CHIP-binding domain of HSP70 significantly hampered its interaction with NEDD1, indicating that HSP70-CHIP may mediate NEDD1 retention at the mitotic centrosome. CHIP is an ubiquitin E3 ligase that targets ubiquitinated proteins for proteasome degradation [48]. Therefore, we hypothesize that HSP70–CHIP may mediate the degradation of a negative regulator of NEDD1 at the mitotic centrosome. Loss of HSP70 function or loss of HSP70–CHIP interaction would therefore lead to reduced recruitment of NEDD1 at the mitotic centrosomes. Future identification of HSP70 substrate proteins that regulate the recruitment of NEDD1 and γ-tubulin to the mitotic centrosome and subsequent MT nucleation will provide pivotal insights into the underlying mechanisms.
We have demonstrated that loss of HSP70 function induces defects in mitotic spindles and results in altered mitosis progression and subsequent cell death. HSP70 overexpression has been reported in many tumors, and is typically a marker for therapeutic resistance and poor prognosis [42]. In addition to the previously reported roles of HSP70 in preventing apoptosis and inducing drug resistance [49], our current results indicate that HSP70 is also required for the assembly of the mitotic centrosome and bipolar spindles. Our results provide a possible mechanistic explanation for the role of HSP70 in cancer cell proliferation and survival, and identify HSP70 as a putative drug target for improving the efficacy of mitosis-disrupting drugs in cancer therapy.
Acknowledgments
The authors thank the Core Facility of the Institute of Cellular and Organismic Biology, Academia Sinica, for their assistance with confocal microscopy and image analysis. This work was supported by grants from Academia Sinica and the Ministry of Science and Technology (NSC 102-2320-B-001-023-MY3), Taiwan.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
C.-T. Fang and H.-H. Kuo contribute equally.
References
- 1.Chavali PL, Putz M, Gergely F. Small organelle, big responsibility: the role of centrosomes in development and disease. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014 doi: 10.1098/rstb.2013.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigg EA, Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13(10):1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colello D, Reverte CG, Ward R, Jones CW, Magidson V, Khodjakov A, LaFlamme SE. Androgen and Src signaling regulate centrosome activity. J Cell Sci. 2010;123(Pt 12):2094–2102. doi: 10.1242/jcs.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colello D, Mathew S, Ward R, Pumiglia K, LaFlamme SE. Integrins regulate microtubule nucleating activity of centrosome through mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling. J Biol Chem. 2012;287(4):2520–2530. doi: 10.1074/jbc.M111.254128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mennella V, Agard DA, Huang B, Pelletier L. Amorphous no more: subdiffraction view of the pericentriolar material architecture. Trends Cell Biol. 2014;24(3):188–197. doi: 10.1016/j.tcb.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura M, Yoshioka T, Saio M, Banno Y, Nagaoka H, Okano Y. Mitotic catastrophe and cell death induced by depletion of centrosomal proteins. Cell Death Dis. 2013;4:e603. doi: 10.1038/cddis.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiato H, Logarinho E. Mitotic spindle multipolarity without centrosome amplification. Nat Cell Biol. 2014;16(5):386–394. doi: 10.1038/ncb2958. [DOI] [PubMed] [Google Scholar]
- 8.Teixido-Travesa N, Roig J, Luders J. The where, when and how of microtubule nucleation—one ring to rule them all. J Cell Sci. 2012;125(Pt 19):4445–4456. doi: 10.1242/jcs.106971. [DOI] [PubMed] [Google Scholar]
- 9.Luders J, Patel UK, Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat Cell Biol. 2006;8(2):137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- 10.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by gamma-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12(11):709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petry S, Vale RD. Microtubule nucleation at the centrosome and beyond. Nat Cell Biol. 2015;17(9):1089–1093. doi: 10.1038/ncb3220. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Ferreria MA, Rath U, Buster DW, Chanda SK, Caldwell JS, Rines DR, Sharp DJ. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr Biol CB. 2007;17(22):1960–1966. doi: 10.1016/j.cub.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Katsetos CD, Draberova E, Smejkalova B, Reddy G, Bertrand L, de Chadarevian JP, Legido A, Nissanov J, Baas PW, Draber P. Class III beta-tubulin and gamma-tubulin are co-expressed and form complexes in human glioblastoma cells. Neurochem Res. 2007;32(8):1387–1398. doi: 10.1007/s11064-007-9321-1. [DOI] [PubMed] [Google Scholar]
- 14.Katsetos CD, Draberova E, Legido A, Draber P. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme,II. gamma-Tubulin. J Cell Physiol. 2009;221(3):514–520. doi: 10.1002/jcp.21884. [DOI] [PubMed] [Google Scholar]
- 15.Caracciolo V, D’Agostino L, Draberova E, Sladkova V, Crozier-Fitzgerald C, Agamanolis DP, de Chadarevian JP, Legido A, Giordano A, Draber P, Katsetos CD. Differential expression and cellular distribution of gamma-tubulin and betaIII-tubulin in medulloblastomas and human medulloblastoma cell lines. J Cell Physiol. 2010;223(2):519–529. doi: 10.1002/jcp.22077. [DOI] [PubMed] [Google Scholar]
- 16.Maounis NF, Draberova E, Mahera E, Chorti M, Caracciolo V, Sulimenko T, Riga D, Trakas N, Emmanouilidou A, Giordano A, Draber P, Katsetos CD. Overexpression of gamma-tubulin in non-small cell lung cancer. Histol Histopathol. 2012;27(9):1183–1194. doi: 10.14670/HH-27.1183. [DOI] [PubMed] [Google Scholar]
- 17.Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- 18.Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI. A chaperone subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Reports. 2014;9(3):1135–1150. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treweek TM, Meehan S, Ecroyd H, Carver JA. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci CMLS. 2015;72(3):429–451. doi: 10.1007/s00018-014-1754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhnevych T, Wong P, Pogoutse O, Vizeacoumar FJ, Greenblatt JF, Emili A, Houry WA. Hsp110 is required for spindle length control. J Cell Biol. 2012;198(4):623–636. doi: 10.1083/jcb.201111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida-Souza L, Asselbergh B, De Winter V, Goethals S, Timmerman V, Janssens S. HSPB1 facilitates the formation of non-centrosomal microtubules. PLoS One. 2013;8(6):e66541. doi: 10.1371/journal.pone.0066541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalouei S, Chow AM, Brown IR. Stress-induced localization of HSPA6 (HSP70B’) and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells. Cell Stress Chaperones. 2014;19(3):321–327. doi: 10.1007/s12192-013-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida-Souza L, Asselbergh B, d’Ydewalle C, Moonens K, Goethals S, de Winter V, Azmi A, Irobi J, Timmermans JP, Gevaert K, Remaut H, Van Den Bosch L, Timmerman V, Janssens S. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci Off J Soc Neurosci. 2011;31(43):15320–15328. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferretti R, Palumbo V, Di Savino A, Velasco S, Sbroggio M, Sportoletti P, Micale L, Turco E, Silengo L, Palumbo G, Hirsch E, Teruya-Feldstein J, Bonaccorsi S, Pandolfi PP, Gatti M, Tarone G, Brancaccio M. Morgana/chp-1, a ROCK inhibitor involved in centrosome duplication and tumorigenesis. Dev Cell. 2010;18(3):486–495. doi: 10.1016/j.devcel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Hut HM, Kampinga HH, Sibon OC. Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities. Mol Biol Cell. 2005;16(8):3776–3785. doi: 10.1091/mbc.E05-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scieglinska D, Piglowski W, Mazurek A, Malusecka E, Zebracka J, Filipczak P, Krawczyk Z. The HspA2 protein localizes in nucleoli and centrosomes of heat shocked cancer cells. J Cell Biochem. 2008;104(6):2193–2206. doi: 10.1002/jcb.21778. [DOI] [PubMed] [Google Scholar]
- 27.Chen YJ, Lai KC, Kuo HH, Chow LP, Yih LH, Lee TC. HSP70 colocalizes with PLK1 at the centrosome and disturbs spindle dynamics in cells arrested in mitosis by arsenic trioxide. Arch Toxicol. 2014;88(9):1711–1723. doi: 10.1007/s00204-014-1222-x. [DOI] [PubMed] [Google Scholar]
- 28.Stanbridge EJ, Flandermeyer RR, Daniels DW, Nelson-Rees WA. Specific chromosome loss associated with the expression of tumorigenicity in human cell hybrids. Somatic cell Gene. 1981;7(6):699–712. doi: 10.1007/BF01538758. [DOI] [PubMed] [Google Scholar]
- 29.Yih LH, Wu YC, Hsu NC, Kuo HH. Arsenic trioxide induces abnormal mitotic spindles through a PIP4KIIgamma/Rho pathway. Toxicol Sci Off J Soc Toxicol. 2012;128(1):115–125. doi: 10.1093/toxsci/kfs129. [DOI] [PubMed] [Google Scholar]
- 30.Yih LH, Hsu NC, Kuo HH, Wu YC. Inhibition of the heat shock response by PI103 enhances the cytotoxicity of arsenic trioxide. Toxicol Sci Off J Soc Toxicol. 2012;128(1):126–136. doi: 10.1093/toxsci/kfs130. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Rhee K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J Cell Biol. 2011;195(7):1093–1101. doi: 10.1083/jcb.201106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luders J. The amorphous pericentriolar cloud takes shape. Nat Cell Biol. 2012;14(11):1126–1128. doi: 10.1038/ncb2617. [DOI] [PubMed] [Google Scholar]
- 33.Piehl M, Tulu US, Wadsworth P, Cassimeris L. Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc Natl Acad Sci USA. 2004;101(6):1584–1588. doi: 10.1073/pnas.0308205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84(1):145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- 36.Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38(10):507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, Zylicz A, Knudsen J, Sandhoff K, Arenz C, Kinnunen PK, Nylandsted J, Jaattela M. Hsp70 stabilizes lysosomes and reverts Niemann–Pick disease-associated lysosomal pathology. Nature. 2010;463(7280):549–553. doi: 10.1038/nature08710. [DOI] [PubMed] [Google Scholar]
- 38.Kose S, Furuta M, Imamoto N. Hikeshi, a nuclear import carrier for Hsp70s, protects cells from heat shock-induced nuclear damage. Cell. 2012;149(3):578–589. doi: 10.1016/j.cell.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 39.Brown CR, Hong-Brown LQ, Doxsey SJ, Welch WJ. Molecular chaperones and the centrosome. A role for HSP 73 in centrosomal repair following heat shock treatment. J Biol Chem. 1996;271(2):833–840. doi: 10.1074/jbc.271.2.833. [DOI] [PubMed] [Google Scholar]
- 40.Vertii A, Zimmerman W, Ivshina M, Doxsey S. Centrosome-intrinsic mechanisms modulate centrosome integrity during fever. Mol Biol Cell. 2015;26(19):3451–3463. doi: 10.1091/mbc.E15-03-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahen R, Venkitaraman AR. Pattern formation in centrosome assembly. Curr Opin Cell Biol. 2012;24(1):14–23. doi: 10.1016/j.ceb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34(6):1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Regan L, Sampson J, Richards MW, Knebel A, Roth D, Hood FE, Straube A, Royle SJ, Bayliss R, Fry AM. Hsp72 is targeted to the mitotic spindle by Nek6 to promote K-fiber assembly and mitotic progression. J Cell Biol. 2015;209(3):349–358. doi: 10.1083/jcb.201409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fry AM, O’Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125(Pt 19):4423–4433. doi: 10.1242/jcs.111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Didier C, Merdes A, Gairin JE, Jabrane-Ferrat N. Inhibition of proteasome activity impairs centrosome-dependent microtubule nucleation and organization. Mol Biol Cell. 2008;19(3):1220–1229. doi: 10.1091/mbc.E06-12-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32(25):3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Amick J, Chakravarti R, Santarriaga S, Schlanger S, McGlone C, Dare M, Nix JC, Scaglione KM, Stuehr DJ, Misra S, Page RC (2015) A bipartite interaction between Hsp70 and CHIP regulates ubiquitination of chaperoned client proteins. Structure (London, England : 1993) 23 (3):472–482. doi:10.1016/j.str.2015.01.003 [DOI] [PMC free article] [PubMed]
- 48.McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8(4):303–308. doi: 10.1379/1466-1268(2003)008<0303:CALBTC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, Dionigi G, Roukos DH. The role of heat shock proteins in cancer. Cancer Lett. 2015;360(2):114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]


