Abstract
Recent studies utilizing chemical-induced colitis-associated and sporadic colon cancer in mouse models indicated a protective role for absent in melanoma 2 (Aim2) in colon epithelial cells. Accordingly, mutations in the human AIM2 gene have been found in colorectal cancer (CRC), and reduced expression of AIM2 in CRC is associated with its progression. Furthermore, the overexpression of AIM2 protein in human cancer cell lines inhibits cell proliferation. Interferon-inducible Aim2 and AIM2 are members of the PYHIN (PYRIN and HIN domain-containing) protein family and share ~57 % amino acid identity. The family also includes murine p202, human PYRIN-only protein 3, and IFI16, which negatively regulate Aim2/AIM2 functions. Because the CRC incidence and mortality rates are higher among men compared with women and the expression of Aim2/AIM2 proteins and their regulators is dependent upon age, gender, and sex hormones, we discuss the potential roles of Aim2/AIM2 in the development of cancer. An improved understanding of the biological functions of the AIM2 in the development of CRC will likely identify new therapeutic approaches to treat patients.
Keywords: Colitis, Colorectal cancer, Inflammation, Inflammasome, PYHIN-family proteins
Introduction
Human and murine absent in melanoma 2 proteins (AIM2 and Aim2) are members of the interferon (IFN)-inducible PYHIN (PYRIN and HIN domain-containing) family proteins (also referred to as p200-family proteins) [1–5]. The family also includes the human (e.g., IFI16, IFIX, and POP3) and murine (e.g., p202 and p204) proteins [1, 2, 5–7]. These proteins share either one or two partially conserved repeats of 200-amino acid residues (the HIN domain) towards the C-terminus [2]. The HIN domain in the proteins allows them to bind double-stranded DNA (DNA) in sequence independent manner [3–6]. Most p200-family proteins (except p202) also contain a PYRIN domain (PYD) in the N-terminus. The PYD is a homotypic protein–protein interaction domain [3, 4]. The PYRIN-only protein 3 (POP3) is a recently identified member of the family [5]. Notably, AIM2 protein shares about 57 % amino acid identity with Aim2 protein [2]. Both Aim2 and AIM2 proteins sense cytosolic DNA (a “danger” signal) in myeloid and non-myeloid cells to activate the Aim2 and AIM2 inflammasome [3–6]. An inflammasome is a cytosolic protein complex that contains a “danger” sensing receptor (e.g., Aim2 and AIM2), an adaptor protein ASC, and pro-caspase-1 [3, 6, 8]. Upon sensing the cytosolic DNA, Aim2/AIM2 activate caspase-1 that then drives the maturation and secretion of proinflammatory cytokines (e.g., IL-1β and IL-18). Proliferation of epithelial cells in highly inflammatory microenvironment is thought to give rise to mutations in cells and their neoplastic transformation [9, 10]. Accordingly, dysregulation of inflammasomes in intestine is thought to contribute to inflammatory diseases and intestinal cancer [11–13].
Colorectal cancer (CRC) incidence and mortality rates are higher among men compared with women [14, 15]. Because the expression of Aim2/AIM2 proteins and their regulators in immune cells is dependent upon the age, gender and sex hormones, in this review, we discuss the roles of Aim2/AIM2 in the development of cancer. An improved understanding of the biological functions of the AIM2 in the development of cancer will likely identify new therapeutic approaches to treat patients.
AIM2/Aim2 expression
Cell-type and gender-dependent expression of AIM2
The AIM2 gene (located in the 1q21 region) that encodes for AIM2 protein was originally identified as a gene associated with growth suppression in human melanoma cell line UACC903 [16]. Furthermore, constitutive expression of the AIM2 mRNA was detected in spleen, small intestine, and peripheral blood leukocytes. Notably, the treatment of HL60 cell line with IFN-γ increased levels of AIM2 mRNA [16]. Studies indicate that constitutive and IFN-induced expression of AIM2 is detectable in myeloid and non-myeloid (e.g., keratinocytes, fibroblasts, epithelial, and vascular cells) cells [17–21]. Accordingly, constitutive levels of the AIM2 mRNA and protein were low in human normal prostate epithelial cells (PrECs), and IFN (α, β, or γ) treatment of cells robustly increased the levels [20]. Similarly, the treatment of human prostate cancer cell lines (e.g., RWPE-1, RWPE-2, DU-145, and PC-3) with IFN-γ robustly induced the expression of AIM2 protein. However, knockdown of the IFN-α/β receptor subunit-α (IFN-α/βRα) in human normal WI-38 lung fibroblasts, which decreased the constitutive levels of IFI16 protein, did not decrease the constitutive levels of AIM2 [19]. Furthermore, the treatment of WI-38 fibroblasts with bleomycin, which activated the ATM-p53 axis in cells and induced the IFI16 protein levels, did not change AIM2 protein levels [19]. However, the treatment-reduced steady-state levels of AIM2 mRNA. Interestingly, the treatment of WI-38 fibroblasts with IL-1β increased levels of AIM2 mRNA. In most cell types, two isoforms (~41 and ~39 kDa) of the AIM2 protein are detected in total cell lysates by a polyclonal antibody that was raised against the C-terminus peptide of the human AIM2 [19]. Of interest, macrophages from male SLE patients had higher levels of AIM2 mRNA than female patients [22]. These observations are consistent with gender-dependent regulation of AIM2 expression in certain cell types. Currently, it is not known how constitutive levels of AIM2 mRNA and protein are regulated in colon epithelial cells.
AIM2 expression during cellular and human aging
Increased levels of AIM2 protein in senescent (vs. young or old) WI-38 human diploid fibroblasts (HDFs) were associated with an increased production of IL-1β by cells [19]. Furthermore, increased constitutive levels of AIM2 protein in ataxia telangiectasia mutated (ATM) HDFs were associated with increased cellular levels of IL-1β [19]. Notably, knockdown of AIM2 expression in HDFs activated the IFN-signaling and increased the levels of IFI16 protein [19]. These observations suggested a role for the AIM2 in suppression of Type I IFN response and expression of IFI16, a potential negative regulator of AIM2 functions (see below). In addition, these observations raised the possibility of a role for AIM2 in senescence-associated secretory phenotype that is associated with the development of inflammation-associated cancers, such as colitis-associated cancer (CAC) [11, 12].
Interestingly, the stimulation of the whole blood cells from old donors (age 60–84 year) vs. young donors (age 20–39 year) with the AIM2 ligand poly(dA:dT) revealed that cells from the elderly donors produced significantly lower levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) [23]. Furthermore, the reduced production of these cytokines by the whole blood cells from elderly donors was associated with lower steady-state levels of AIM2 protein [23]. Thus, these observations suggested cellular aging-dependent regulation of the AIM2 expression in certain cell types. In addition, these observations raised the possibility that reduced sensing of the cytosolic DNA by AIM2 in cells with aging may contribute to an increased susceptibility to develop certain aging-associated cancers.
Genetic background, gender, cell-type, and sex hormone-dependent expression of Aim2
Constitutive levels of Aim2 mRNA and protein are lower in immune cells from the New Zealand black (NZB) strain (and NZB-derived B6.Nba2 congenic strain of mice) of female mice as compared with age and sex-matched C57BL/6 (B6) strain of mice [24, 25]. Steady-state levels of Aim2 mRNA and protein were detectable in bone marrow-derived macrophages (BMDMs) from the B6 female mice and treatment of cells with IFN-α appreciably increased the levels in time-dependent manner [26]. Expectedly, constitutive levels of the Aim2 mRNA were lower in Stat1-deficient splenic cells than the age and sex-matched wild-type mice. Notably, the treatment of purified splenic T or B cells from the B6 female mice with IFN-α did not increase levels of Aim2 mRNA [26]. Furthermore, the stimulation of purified splenic T cells (with α-CD3 + α-CD28) or B cells (with α-IgM) appreciably reduced levels of the Aim2 mRNA as compared with controls. Of interest, the levels of Aim2 mRNA and protein in immune cells (total splenic, B and T cells) were dependent upon the sex of mice (higher in males than the age-matched females) [26]. Furthermore, the treatment of cells that expressed the androgen receptor (AR) with the male sex hormone dihydro-testosterone (DHT) significantly increased the steady-state levels of Aim2 mRNA and protein [26]. These observations are consistent with the gender and male sex hormone-dependent regulation of Aim2 expression in certain immune cell types. Currently, it is not known how constitutive levels of Aim2 mRNA and protein are regulated in colon epithelial cells.
Alterations in AIM2 expression in cancer
AIM2 expression is reduced or lost in colorectal and prostate cancer
Consistent with a role for AIM2 gene in growth and tumor suppression, the gene contains a microsatellite instability site that results in the inactivation of the gene in ~47 % of colorectal tumors with high microsatellite instability [27, 28]. Mutations in the AIM2 gene have also been associated with the development of colon, gastric, and endometrial cancers [27]. Furthermore, AIM2 gene is frequently silenced by DNA-hypermethylation [28]. Therefore, it is likely that the loss of AIM2 function in cells provides growth advantage to the cancer cells (Table 1).
Table 1.
Alterations in the expression of AIM2 in human cancers
| Cancer type | Alteration | Phenotype/clinical outcome |
|---|---|---|
| Cervical cancer (CC) | Overexpression due to hypomethylation of AIM2 | Not known [23] |
| HNPCC-associated small bowl cancer (SBC) | Loss of expression (frame-shift mutation) | Poor prognosis [27] |
| Colorectal cancer | Loss of expression (microsatellite instability) | Poor prognosis [29, 30] |
| Nasopharyngeal carcinoma (NPC) | Overexpression | Correlated with survival [31] |
| Oral squamous cell carcinoma (OSCC) | Overexpression | p53-dependent inhibition [32] |
| Non-small cell lung cancer (NSCLC) | Overexpression | Poor prognosis [33] |
| Oral cancer | Overexpression | Not known [34] |
| Cervical cancer | Overexpression (amplification of 1q22–23 region) | Not known [75] |
Accordingly, a tissue microarray analysis (TMA) of matched tissue pairs (colorectal tumor and adjacent normal colon epithelium) indicated that ~67 % tumor tissue exhibited reduced AIM2 protein levels as compared with matched normal tissue [29]. Furthermore, ~9 % tumors had completely lost the AIM2 expression. Notably, after adjustment for sex, age, cancer stage, tumor site, tumor grade, and chemotherapy, complete lack of AIM2 expression in tumors was associated with up to an threefold increase in the overall mortality (HR 2.40; 95 % CI 1.44–3.99) and disease specific mortality (HR 3.14; 95 % CI 1.75–5.65) in comparison with AIM2-positive tumor samples [29]. Similarly, the expression profile of AIM2 in human colorectal cancer correlated with cancer stages [30]. Thus, suggesting that the lack of AIM2 expression in colon cancer is closely associated with a poor outcome in colorectal cancer patients.
In prostate cancer, the levels of AIM2 mRNA were significantly lower in the prostate adenocarcinoma (n = 40; Gleason scores 3–5 + 3–5 = 6–9/10) [20]. Accordingly, constitutive levels of the AIM2 mRNA and protein were lower in a variety of prostate cancer (PCa) cell lines as compared to benign prostate hyperplasia and normal prostate. However, IFN (IFN-α, β, or γ) treatment of normal prostate epithelial cells and certain prostate cancer cell lines induced AIM2 expression [20]. Together, these studies indicate a role for AIM2 in the suppression of CRC and PCa. However, the molecular mechanisms through which the AIM2 functions as a tumor suppressor remain to be elucidated.
Increased expression of AIM2 in certain virus-associated and other cancers
Increased levels of AIM2 protein in Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma (NPC) are correlated with patient survival [31]. In tumor cells, AIM2 expression was required for IL-1β induction by EBV genomic DNA and irradiation of cells further activated the AIM2 inflammasome activity. In mice, NPC tumor-derived IL-1β inhibited the tumor growth and enhanced the survival through host responses, which was depended upon infiltrated immunostimulatory neutrophils [31]. Accordingly, increased infiltration of NPC tumor with neutrophils was associated with increased survival of patients (Table 1). These observations suggested that the activation of AIM2 inflammasome in NPC tumors plays a protective role through altering the tumor microenvironment.
The development of oral squamous cell carcinoma (OSCC) is associated with the amplification of the 1q23 region, and genes within this region, such as AIM2, are overexpressed [32]. Intriguingly, knockdown of AIM2 expression in OSCC cell lines resulted in decreased activation of NF-κB and suppression of cell growth and apoptosis [32]. Furthermore, the restoration of AIM2 expression in p53-deficient OSCC cells increased cell proliferation and activated NF-κB activity. In contrast, the restoration of AIM2 expression in OSCC cells that expressed the functional p53 inhibited cell growth [32]. Importantly, these observations raised an interesting possibility that AIM2-mediated inhibition of cell growth in OSCC cells and possibly other cell types depends on the p53 functions.
AIM2 inflammasome was overexpressed in non-small cell lung cancer (NSCLC) as compared with lung adenocarcinoma (ADC) and small cell lung cancer (SCLC) [33]. The high-metastatic or cisplatin-sensitive NSCLC cells expressed more inflammasome components and products than their counterpart low-metastatic or cisplatin-resistant NSCLC cells, respectively. Furthermore, in lung cancer tissues, high-grade ADC expressed more inflammasome components and products than low-grade ADC [33]. Similarly, in chewing-tobacco-associated oral cancer tissues, AIM2 mRNA levels were higher than clinically normal oral buccal mucosa [34].
In human papillomavirus (HPV)-associated cervical cancer (CC), the AIM2 expression was up-regulated due to demethylation of the promoter region [35]. Interestingly, the HPV16 DNA triggered IL-1β and IL-18 release through AIM2 inflammasome activation in normal human keratinocytes [36]. Notably, HPV DNA did not induce the production of IFN-β in keratinocytes. However, the IFN-β secretion was observed when AIM2 function was blocked in cells [36]. Furthermore, blocking of the IFI16 function in keratinocytes increased the HPV16 DNA-induced IL-1β, but not IL-18, secretion [36], thus suggesting a functional crosstalk between the IFI16 and AIM2 proteins in human keratinocytes.
Currently, it remains unknown whether HPV or EBV virus-mediated inactivation of certain cell growth-inhibitory pathways (such as the p53 and pRb-E2F) up-regulates the expression of AIM2 in cancer cells. Because AIM2-mediated cell growth inhibition in OSCC was dependent upon the p53 status [32], it is conceivable that p53 regulates the expression of AIM2. Therefore, further work is needed to identify the functional interactions between AIM2 and p53 during the development of cancer.
Cell-type-dependent sub-cellular localization of Aim2/AIM2
The amino acid sequence of Aim2 or AIM2 protein lacks a classical nuclear localization signal (NLS) [2, 37]. Therefore, it is intriguing that both Aim2 and AIM2 are detected in the nucleus to a varying extent in certain normal and cancer cell types examined (Table 2). Furthermore, it is likely that both Aim2 and AIM2 are transported to the nucleus in a cell-type-dependent manner by their interactions with other proteins through a “piggyback” transport.
Table 2.
Cell-type-dependent sub-cellular localization of AIM2/Aim2
| Localization | Approach | Cell line(s)/tissue | Protein detected |
|---|---|---|---|
| Nuclear | Cell fractionation | WI-38 HDF | Endogenous AIM2 [19] |
| Cytoplasm | Cell fractionation | WI-38 HDF | IFN-induced AIM2 [19] |
| Cytoplasm | Cell fractionation | PC-3 | IFN-γ-induced AIM2 [20] |
| Nuclear and cytoplasmic | Cell fractionation | RAW264.7 | Endogenous Aim2 [26] |
| Cytoplasm | Cell fractionation | RAW264.7 | IFN-α induced Aim2 [26] |
| Nuclear > cytoplasm | Cell fractionation | J774.A1 | IFN-α induced Aim2 [26] |
| Cytoplasm | Indirect immunofluorescence | AKR-2B (MEF) | Transiently overexpressed AIM2 [36] |
| Nuclear | Indirect immunofluorescence | UACC903 | Constitutively overexpressed AIM2 [37] |
| Cytoplasm | Cell fractionation | THP-1 | Endogenous AIM2 [38] |
| Cytoplasm | Cell fractionation | THP-1 | PMA-induced AIM2 [38] |
| Cytoplasm > nucleus | Cell fractionation | THP-1 | IFN-γ induced AIM2 [38] |
| Nuclear > cytoplasm | Indirect immunofluorescence | MCF-7 | Inducibly overexpressed AIM2 [39] |
CF CELL fractionation, IIF indirect immunofluorescence, MEF mouse embryonic fibroblast, HDF human diploid fibroblast
Endogenous constitutive levels of Aim2 protein in murine RAW264.7 macrophage cell line were detected in the nuclear as well as in the cytoplasmic fractions [26]. Furthermore, IFN-α treatment of RAW264.7 cells that moderately induced Aim2 protein levels in cells increased the accumulation of the protein in the cytoplasm. In contrast, the endogenous constitutive levels of Aim2 protein were not detectable in J774.A1 murine macrophage cell line, and IFN-α treatment of cells robustly induced the Aim2 protein levels. Notably, the bulk of the induced Aim2 protein was detected in the nuclear fraction of the cells as compared with the cytoplasm [26].
Endogenous levels of AIM2 protein were not detectable in human prostate cancer cell line PC-3; IFN-γ treatment of cells robustly induced the levels of the AIM2 protein, and the induced protein was detected in the cytoplasm [20]. Similarly, endogenous constitutive levels of AIM2 protein in human THP-1 monocytic cancer cells were detected in the cytoplasm and treatment of cells with PMA that resulted in differentiation of cells increased the levels of AIM2 protein and the protein was detected in the cytoplasm [38]. Notably, IFN-γ treatment of THP-1 or PMA-treated THP-1 cells robustly increased AIM2 protein levels in the cytoplasm and very little protein was detected in the nucleus. Furthermore, constitutively overexpressed human AIM2 protein in the murine AKR-2B fibroblasts (spontaneously immortalized) was primarily detected in the cytoplasm [37]. However, when constitutively overexpressed in human melanoma cell line UACC903, the AIM2 protein was detected within the nucleus of transfected or interferon-treated cells [39]. Furthermore, when AIM2 was inducibly expressed in human breast cancer cell line MCF-7, the bulk of the AIM2 protein was detected in the nucleus, whereas less was detected in the cytoplasmic fraction [40].
In contrast to immortalized or cancer cells, the endogenous levels of AIM2 protein in human young (population doubling ~30) proliferating normal WI-38 lung fibroblasts were primarily detected in the nucleus and IFN-β treatment of cells, which induced AIM2 protein levels; the protein was primarily detected in the cytoplasm [19]. Together, these observations suggest that sub-cellular localization of the endogenous and overexpressed Aim2 and AIM2 proteins may depend upon several factors, such as normal vs. cancer phenotype, cell differentiation status, and cancer-associated cell signaling alterations in cells. Therefore, additional studies are needed to identify the molecular mechanisms that regulate the sub-cellular localization of the endogenous Aim2 and AIM2 protein in normal and cancer cells.
Homo and heterodimerization by Aim2/AIM2
The presence of PYD in the N-terminus of most PYHIN-family proteins allows them to interact with other proteins that contain a PYD [3, 4, 6]. Accordingly, AIM2 protein binds to apoptotic speck protein containing a caspase recruitment domain (CARD) (ASC), an adaptor protein, and POP3 though PYD [3–5]. Similarly, Aim2 also binds to ASC. Furthermore, studies involving overexpression of AIM2 in transfected cells, followed by immunoprecipitation-Western assays, indicated that the AIM2 protein binds with IFI16 protein [38] and can homodimerize [39]. Interestingly, studies indicated that the HIN domain in certain PYHIN-family proteins also allows proteins to form homo and heterodimers [41–46]. For example, the HIN2 domain in the p202 protein formed homodimers and bound to the HIN domain of Aim2 protein [42]. These observations make it likely that Aim2/AIM2 proteins physically interact with other proteins through the PYD as well as HIN domains. Therefore, further work is needed to determine whether protein–protein interactions involving PYD and/or HIN domain in the Aim2/AIM2 proteins with the nuclear PYHIN-family proteins, such as p202 and IFI16 (or other proteins), contribute to their nuclear localization in certain cell types.
AIM2 in cell growth suppression
Ectopic overexpression of AIM2 in murine AKR-2B fibroblasts retarded cell proliferation and, under reduced serum conditions, increased the propensity of cells to undergo cell death [37]. Increased expression of AIM2 in human breast cancer cell lines suppressed cell proliferation and tumorigenicity [40]. Furthermore, increased expression of the AIM2 protein inhibited mammary tumor growth in an orthotopic tumor model, and increased levels of AIM2 protein in breast cancer cells increased apoptosis [40, 47]. Interestingly, the susceptibility to apoptosis in cells was associated with inhibition of the transcriptional activity of NF-κB and desensitization of the NF-κB activation after TNF-α treatment [40]. Similarly, the restoration of AIM2 expression in HCT116 (a colon cancer cell line) cells suppressed cell proliferation and viability [48] and also down-regulated the core 1 mucin-type O-glycosylation [49]. Furthermore, cell cycle analyses of colon cancer cells revealed that AIM2-mediated inhibition of cell proliferation of HCT 116 cells was associated with an accumulation of cells at the late S-phase, resulting in the G2/M cell cycle arrest. The latter correlated well with an up-regulation of cyclin D3 and p21WAF1/CIP1 as well as with inhibition of Cdc2 activity through Tyr-15 phosphorylation [48]. Although, these observations suggested that overexpression of the AIM2 in colon cancer cell line that does not express AIM2 protein inhibits cell proliferation in part through inhibiting the cell cycle progression; intriguingly, overexpression of the AIM2 did not significantly affect the growth or survival of human melanoma cells UACC903 or an another human melanoma cell line [39].
Consistent with the above observations, constitutive expression of AIM2 in HCT 116 cell line, which does not express detectable levels of endogenous AIM2, significantly up-regulated levels of 111 transcripts, whereas down-regulated 80 transcripts as compared with control cells [50]. The up-regulated genes included the interferon-stimulated genes (ISGs) and genes that are involved in intercellular adhesion and matrix remodeling. Of note, the up-regulation was independent of stimulation of the cells with IFN-γ [50]. Accordingly, knockdown of AIM2 expression in cells reduced the expression of HLA-DRA, HLA-DRB, and CIITA in IFN-γ-treated HT-29 cells. These observations raised the possibility that AIM2 in colorectal cancer cell lines mediated both IFN-γ-dependent and independent induction of certain ISGs, including the genes that encode for the major histocompatibility complex (MHC) class II antigens HLA-DR-α and β [50].
In summary, a number of studies involving constitutive or inducible overexpression of AIM2 in a variety of cancer cell lines, including a colon cancer cell lines (HCT 116), have indicated a growth-suppressive role for the AIM2. However, the molecular mechanisms through which AIM2 inhibits cell growth may depend on the cell type and the functional status of other growth suppressors, such as p53 [32]. As noted above, it is likely that AIM2-mediated inhibition of cell growth in transfected cells depends upon its expression levels, its sub-cellular localization, and its interactions with other PYHIN-family proteins through the PYD and/or HIN domain. Therefore, further studies involving human normal cells and cancer cells are needed to identify the molecular mechanisms through which AIM2 inhibits cell proliferation.
Negative regulation of AIM2/Aim2 functions
POP3, a negative regulator of AIM2
POP3, a newly identified member of the PYHIN family of proteins, shares ~61 % amino acid identity with AIM2 [5]. Interestingly, constitutive levels of POP3 mRNA in human macrophages were low, and IFN-β treatment of cells induced the levels 4–6-fold within 2 h. Furthermore, the levels of the POP3 mRNA decreased in cells after 4 h of IFN-β treatment. Similar to POP3 mRNA levels, constitutive levels of AIM2 mRNA in macrophages were low, and IFN-β treatment of cells induced the levels ~300-fold within 2 h. Of note, AIM2 mRNA levels remained high during the IFN-β treatment up to 48 h. After 48 h of IFN treatment, the levels of POP3 mRNA increased again. Thus, the IFN-mediated induction of POP3 expression in macrophages was biphasic. Notably, the induced levels of POP3 protein competed with ASC adaptor protein for recruitment to AIM2, thus inhibiting activation of the AIM2 inflammasome in macrophages [5] (Fig. 1). These observations suggested that AIM2 inflammasome activity is negatively regulated by increased expression of POP3 in human myeloid cells. Accordingly, IFNs-mediated induction of POP3 expression in human PrECs cells also inhibited activation of the AIM2 inflammasome (unpublished data). Therefore, it is likely that the activation of AIM2 inflammasome in non-myeloid cells is negatively regulated by increased expression of POP3. Currently, it is not known whether POP3 expression is dysregulated in human cancers.
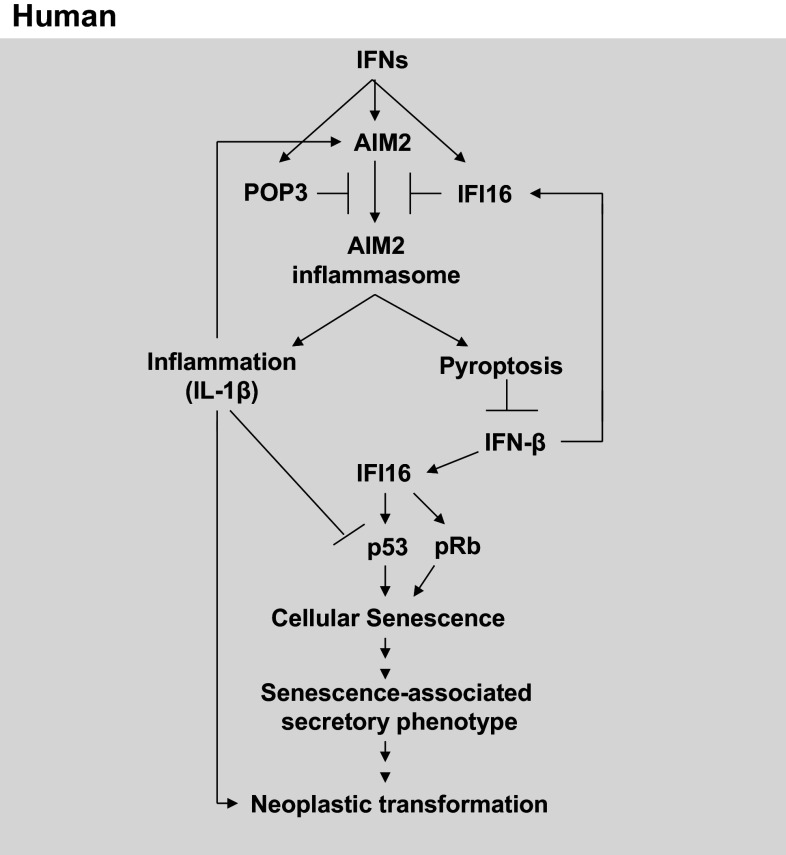
Fig. 1.
Regulation and role of AIM2 in cancer development. AIM2 expression is induced in a variety of cell types by Type I IFNs or IL-1β-induced signaling. Increased levels of POP3 or IFI16 inhibit activation of the AIM2 inflammasome. Activation of AIM2 inflammasome in macrophages and dendritic cells leads to inflammatory cell death by pyroptosis. However, pyroptosis suppresses the expression of IFN-β and Type I IFN response. IFN-induced IFI16 levels potentiate cellular senescence-associated permanent cell cycle arrest in cells through activation of the p53 and pRb pathway. Increased production of IL-1β inhibits p53-mediated functions. Accumulation of senescent cells leads to senescent-associated secretory phenotype and inflammation. Proliferation of epithelial cells in inflammatory microenvironment results in accumulation of mutations and neoplastic transformation
IFI16, a negative regulator of AIM2
Constitutive and IFN-induced levels of the IFI16 PYHIN-family protein are detected in both cytoplasm and nucleus, and the relative distribution between these two sub-cellular compartments depends upon the cell type [reviewed in 51]. Furthermore, the IFI16 also senses pathogen and self-derived DNA mainly in the nucleus to assemble an inflammasome [6, 7]. Notably, increased levels of IFI16 protein in a variety of cell types are associated with senescence-associated permanent cell cycle arrest through the activation of the p53 and pRb pathway [reviewed in 52]. Accordingly, reduced levels of IFI16 protein in certain cell types result in immortalization of cells, the first step in cell transformation. Because senescent cells eventually acquire a proinflammatory secretory phenotype that is associated with activation of the inflammasome [52], the accumulation of senescent cells in tissues and organs with aging or in response to the activation of DNA-damage response gives rise to an inflammatory environment in the tissue/organ. Proliferation of epithelial cells in such inflammatory tissue microenvironment contributes to neoplastic transformation [9, 10] (Fig. 1).
Treatment of human monocytic cell line THP-1 with Type-I IFN (IFN-α or β) or Type-II IFN (IFN-γ) induced levels of IFI16 and AIM2 mRNAs and corresponding proteins [38]. The induced levels of both IFI16 and AIM2 proteins were detected primarily in the cytoplasm. Notably, the IFI16 protein bound with AIM2 in immunoprecipitation-Western blotting assays and relatively more IFI16 protein bound with the AIM2 in the cytoplasmic fraction [38]. Expectedly, overexpression of IFI16 protein in transfected HEK-293 cells inhibited activation of caspase-1 by the AIM2-ASC inflammasome. Furthermore, the constitutive stable knockdown of IFI16 expression in THP-1 cells increased the spontaneous and poly(dA:dT)-induced activation of caspase-1 by the AIM2 inflammasome in PMA-treated THP-1 cells [38]. These observations suggested that increased levels of IFI16 protein in THP-1 cells inhibit activation of AIM2 inflammasome by binding to the AIM2 protein (Fig. 1). Currently, it is not known whether increased levels of the IFI16 protein also inhibit activation of the AIM2 inflammasome in epithelial cells.
p202, a negative regulator of Aim2
Constitutive levels of p202 are detectable in splenic B cells isolated from B6 female mice and treatment of cells with IFN-α induced the levels [53]. Notably, the induced levels of the p202 were primarily detected in the cytoplasm. In addition, IL-6 [54] and the female sex hormone estrogen [55] also induce the expression of p202 in immune cells. Accordingly, the constitutive levels of p202 mRNA and protein are higher in cells from female mice than the age-matched males [55]. However, the p202 expression is suppressed by activated p53 [56], growth factors [57], and E2F1 [58]. p202 lacks the PYD in the N-terminus [2]. Of note, constitutive levels of the p202 are detected in the cytoplasm as well as in the nucleus, and the activation of the IFN signaling in cells potentiates the nuclear localization [59]. The nuclear localization of p202 may be important for its ability to inhibit the transcriptional activity of p53 [46]. Based on the structural studies, p202 binds the cytosolic DNA with higher affinity than Aim2 [42] and increased levels of the p202 in macrophages from the New Zealand Black (NZB) mice inhibited activation of the Aim2 inflammasome [60]. Furthermore, Aim2 protein bound with p202 and increased levels of p202 in cells inhibited activation of the Aim2 inflammasome [42, 60] (Fig. 2).
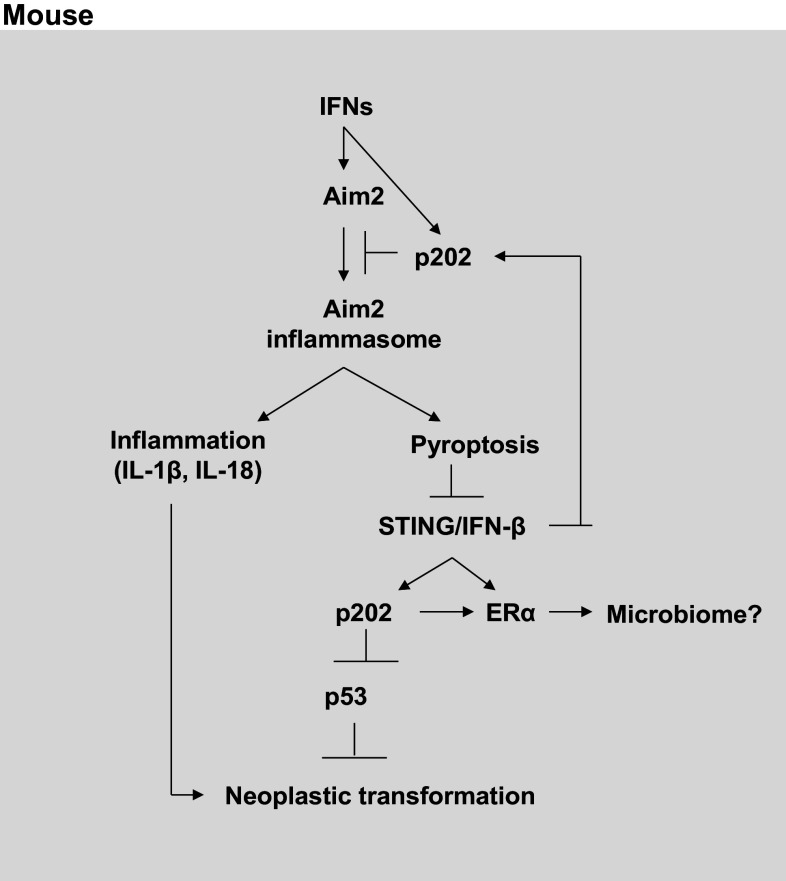
Fig. 2.
Regulation and role of Aim2 in cancer development. Aim2 expression is induced in bone marrow-derived macrophages (BMDMs) from C57BL/6 male and female mice by Type I IFNs. Increased levels of p202 inhibit activation of the Aim2 inflammasome. Activation of Aim2 inflammasome in macrophages leads to cell death by pyroptosis. Furthermore, pyroptosis suppresses the production of the IFN-β and activation of the Type I IFN response. The IFN-induced p202 levels inhibit p53-mediated transcriptional activation of pro-apoptotic genes and increase cell survival. Increased production of IL-1β also inhibits the p53-mediated functions. Proliferation of epithelial cells in inflammatory microenvironment and in the absence of p53 function results in accumulation of mutations and neoplastic transformation. p202 also potentiate the estrogen receptor-α (ERα), thus altering the ERα signaling in estrogen-responsive cells
BMDMs from Aim2-deficient mice (mice on the C57BL/6 genetic background), when transfected with DNA, produced higher levels of IFN-β as compared with wild-type mice [61]. Similarly, the Aim2-deficient macrophages activated the STING pathway in response to cytosolic DNA and produced more IFN-β than the wild-type macrophages [62]. The above observations indicated that, such as AIM2 protein [19, 36], the Aim2 protein also suppresses the production of IFN-β and activation of Type I IFN-signaling. Accordingly, knockdown of Aim2 expression in the murine macrophage cell line J774.A1 activated a robust Type I IFN response as compared with control cells and induced p202 expression [24]. These observations revealed an important functional antagonism between Aim2 and p202 in immune cells (Fig. 2). However, it is not known whether a similar functional antagonism exits between these two proteins in colon epithelial cells.
Aim2 in colitis and colitis-associated cancer
Role of Aim2 in colitis
A study [63] utilizing Salmonella-induced colitis mouse model noted that Aim2-deficient mice on the mixed (129/Sv × C57BL/6) genetic background, as compared with wild-type mice, exhibited an increased susceptibility to mucosal infection by Salmonella typhimurium. The Aim2-deficient mice exhibited greater body weight loss, intestinal damage and associated inflammation, and disruption of basal and activated epithelial cell turnover. This increased susceptibility to infection in the Aim2-deficient mice was due to increased epithelial permeability due to reduced expression of tight-junction proteins that contribute to epithelial integrity [63]. Accordingly, Aim2 overexpression in cells increased the expression of tight-junction proteins through Akt activation. Therefore, the study concluded that the Aim2 expression in colon epithelial cells contributes to the maintenance of intestinal barrier integrity and provides protection against certain bacterial infections [63].
An another study [64] that utilized Aim2-deficient mice (presumably both males and females) on the mixed genetic background and induced colitis using DSS noted a higher colonic burden of commensal Escherichia coli as compared with wild-type mice. Of interest, colonization of germ-free mice with microbiota from Aim2-deficient mice resulted in higher colitis susceptibility. The study noted that Aim2-deficiency in mice resulted in suppression of caspase-1 activation and IL-1β and IL-18 production. Furthermore, IL-18 infusion in mice reduced E. coli burden as well as colitis. Importantly, the study found that an altered microbiota in the Aim2-deficient mice correlated with reduced expression of certain antimicrobial peptides in intestinal epithelial cells [64]. These observations implicated a role for Aim2 in maintaining intestinal homeostasis.
Because Aim2 deficiency in mice with mixed genetic background exhibited activation of Type I IFN-signaling and also increased levels of the p202 in immune cells [24], it remains unknown whether the Aim2 deficiency in mice in the studies [63, 64] that are described above resulted in an increased expression of p202 in colon cells and, more importantly, whether the increased levels of the p202 in cells contributed to the maintenance of the intestinal homeostasis [64] and an increased susceptibility to mucosal infection by S. typhimurium [63]. Therefore, further work will be needed to assess the role of Aim2 in mouse models of colitis.
Multiple factors affect colitis-associated cancer development in the AOM/DSS mouse model
Among the chemically induced colitis-associated cancer (CAC) mouse models, a single injection with azoxymethane (AOM) is followed by cyclic treatment with DSS [65, 66]. In this model, besides the genetic background of the mice, a number of other factors contribute to the development and progression of the disease. These factors include the environment of the vivarium, which significantly influences the load and composition of gut microbiome, and the sex of mice [66]. Of note, alterations in colonization by microbes in gut are known to alter serum levels of sex hormones and the sex hormone levels in mice in turn influence the composition of the gut microbiome [67]. Accordingly, under germ-free conditions, colitis development is mild [66]. Furthermore, both male and female mice develop DSS-induced colitis robustly; however, the males rapidly develop more significant and aggressive disease than the age-matched females. Thus, the development of DSS-induced colitis in mouse models and its severity may depend on the genetic background of mice, their gut microbiome composition, sex, and sex hormone levels [65–67].
In the AOM/DSS-induced colitis-associated cancer (CAC) mouse model, a number of signaling pathways have been implicated in the development of cancer [68, 69]. These pathways include the Wnt/APC/β-catanin, TLR, JAK/STAT, and p53. Interestingly, a study [68] utilizing AOM/DSS-induced CAC in mice noted that levels of the Ifi202 mRNA were low in control mice and the mRNA levels induced appreciably following treatment of mice with AOM/DSS that resulted in inflamed colorectal mucosa (within 2 weeks), colorectal dysplasia (week 4), high-grade dysplasia (weeks 6–8), and colorectal adenocarcinoma (week 20). In contrast, levels of the Aim2 mRNA were high in the control mice and the treatment decreased the mRNA levels significantly in inflamed colorectal mucosa (within 2 weeks). However, levels of Aim2 mRNA increased again to the levels comparable to the levels detected prior to the AOM/DSS treatment of mice and the levels stayed higher during the development of colorectal dysplasia, high-grade dysplasia, and colorectal adenocarcinoma [68]. These observations are consistent with the possibility that changes in the ratio between p202 and Aim2 protein levels in colon epithelial cells in AOM/DSS-treated mice contribute to the development of CAC.
Reduced levels of p202 in a variety of cell types stimulate the p53-mediated transcription [46]. Accordingly, increased expression of the p202 in immune cells inhibits the p53-mediated transcription of pro-apoptotic genes [46]. Furthermore, p202 binds to p53 in the N-terminal region (aa 44–83) comprising the proline-rich region that is important for p53-mediated apoptosis. In p202, the HINB domain bound to p53 [46]. Considering that the p202 inhibits p53-mediated pro-apoptotic functions, and the likelihood that in AOM/DSS mouse model of CAC, an increase in the ratio between p202 and Aim2 protein levels functionally inactivated the p53 functions, additional studies will be required to assess the role of the p202–p53 axis in the development of colitis and CAC in mouse models.
Aim2 in the development of CAC in the AOM/DSS mouse model
Two recent studies [70, 71] that utilized AOM/DSS-induced CAC development in Aim2-deficient B6 mice, as compared with wild-type mice, concluded that Aim2 expression in mice protects against CAC. The study by Wilson et al. [70] used Aim2-deficient male mice along with sex-matched wild-type mice. The study noted that Aim2-deficient mice had greater tumor load burden than Asc-deficient mice. Furthermore, the tumor burden was also higher in the Aim2−/−/ApcMin/+ than in APCMin/+ mice. Notably, Aim2-mediated functions during the development of CAC did not involve its ability to assemble an active inflammasome and IL-1β production. Significantly, Aim2 expression in mice-reduced activation of Akt and tumor burden in colorectal cancer models [70]. However, this study did not analyze gut microbiome alterations between the control and the Aim-deficient male mice following the induction of colitis and CAC.
In contrast to the study by Wilson et al, [70], the study by Man et al. [71] presumably used female Aim2-deficient mice to investigate the role of Aim2 in AOM/DSS-induced CAC. Of interest, injection of wild-type mice with AOM significantly reduced the expression of Aim2 in colon tissues as compared with untreated wild-type mice after 14-days of injection and the levels of Aim2 mRNA remained low even after 80-days post-injection [71]. The study noted that Aim2-deficient mice, when housed in specific pathogen-free vivarium, were hyper-susceptible to colonic tumor development as compared with wild-type mice. Furthermore, the study noted alterations in the gut microbiome composition following induction of colitis and tumors. Of note, Aim2-deficient intestinal stem cells exhibited uncontrolled proliferation and an aberrant activation of Wnt-signaling [71].
As noted above and by others [13], the genetic background and sex-dependent alterations in colonization by microbes in inflamed gut of mice could alter serum levels of sex hormones and influence the composition of gut microbiome [65–67]. Because sex hormone levels are predicted to influence the ratio between p202 and Aim2 protein levels in colon epithelial and stem cells [26, 55, 72], additional studies will be needed to identify the molecular mechanisms through which Aim2 deficiency in the male and female mice renders them more susceptible to the development of CAC in the AOM/DSS mouse model.
Conclusions and perspectives
In this review, we have discussed the potential roles of the absent in melanoma 2 proteins (AIM2/Aim2) in cell growth suppression and the development of inflammation-associated cancers. Because the functions of these proteins are negatively regulated by the species-specific proteins (POP3, IFI16, and p202) [5, 19, 38, 60], we have highlighted the role of these negative regulators in the development of cancer (Fig. 3).
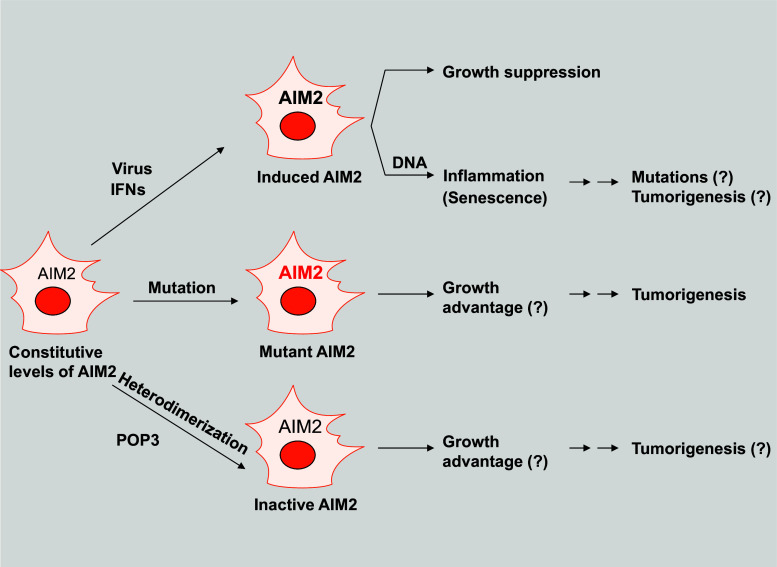
Fig. 3.
Proposed model for the regulation and potential roles of the AIM2 in the suppression of tumorigenesis. Most cell types express constitutive low levels of AIM2 mRNA or protein. However, treatment of cells with IFNs or certain viral infections induces the expression of AIM2. The increased levels of AIM2 protein in most cell types that have been tested inhibited cell growth. Furthermore, the cell growth inhibition was dependent upon the status of p53 in cells. In the presence of cytosolic DNA, keratinocytes, fibroblasts (both young and senescent), and epithelial cells assemble the AIM2 inflammasome, resulting in the production of proinflammatory cytokines, such as IL-1β and IL-18. Increased production of proinflammatory cytokines in tissue leads to inflammation. Notably, chronic inflammation is associated with mutations in cells and the development of certain cancers. Mutations in the AIM2 gene or its reduced expression in cells due to hypermethylation of the gene promoter is associated with the development of colorectal cancer, thus suggesting that the mutant AIM2 or the lack of its expression provides growth advantage to cancer cells. Furthermore, increased expression of POP3 in cancer cells and its heterodimerization with AIM2 could inactivate the AIM2 functions, thus leading to cell growth advantage and tumorigenesis
In vitro studies that involved overexpression of AIM2 in a variety of human cancer cell lines have indicated that increased levels of AIM2 in certain tumor cell lines suppress cell growth [37, 40, 47, 48], whereas no cell growth inhibition was noted in other cell lines [39]. A study that analyzed expression of AIM2 protein in colon cancer tissue microarray (TMA) [29] or studies that analyzed AIM2 mRNA levels in colorectal [29, 30] and prostate [20] cancer specimen indicated that reduced or loss of AIM2 expression in cells is associated with cancer development and its progression to an advanced stage. However, increased levels of the AIM2 mRNA and protein have been reported in a number of inflammation and virus-associated cancers [31–36]. Therefore, given that AIM2 expression is also increased in senescent cells [19], further work will be needed to determine whether cancer therapy-induced accumulation of senescent cells in tumors contributes to the observed increased levels of AIM2 mRNA or protein. Furthermore, malignant cells or tumor-infiltrating dendritic cells produce Type I IFNs (IFN-α/β) that could up-regulate the expression of AIM2 (and other ISGs) in tumors [73]. Therefore, to understand the role of AIM2 in cancer development and its progression, it is critical to identify the downstream effectors of the AIM2. Based on the accumulating data [32], it is likely that AIM2-mediated inhibition of cell growth, in part, depends upon the functional status of p53 in cancer cells.
As compared with AIM2, the role of Aim2 is less clear in cell growth suppression and the development of epithelial cancer. Therefore, recent studies [63, 64, 70, 71, 74] that utilized mouse models to investigate the role of Aim2 in colitis and AOM/DSS-induced CAC are informative. These studies suggested an inflammasome-dependent and independent roles for Aim2: in the maintenance of intestinal barrier integrity and protection against certain bacterial infections [63]; in the maintenance of intestinal homeostasis through the regulation of epithelial antimicrobial host defense [64]; and in providing protection against the development of CAC [70, 71]. Because gut microbiome appears to play a significant role in the development of colitis and multiple factors (such as the genetic background of mice, sex of mice, and sex hormone levels) that are likely to influence the development of CAC through altering the ratio between p202 and Aim2 protein levels [65–67], further studies in the future need to assess the relative contributions of the p202 and Aim2 in the disease development phenotype. This is important because studies in the future could provide important molecular insights with respect to the role of AIM2 in the development of inflammation-associated cancers.
In summary, studies suggest a cell growth-suppressive role for both Aim2/AIM2 proteins (Fig. 3). Since the relationship between inflammation and development of cancer is complex [9, 10], additional studies are needed to investigate the role of AIM2/Aim2 in the development of inflammation-associated cancers and their progression. These studies will require investigation of the role of the species-specific negative regulators of the AIM2/Aim2. An improved understanding of the biological functions of the AIM2 in epithelial and myeloid cells is likely identify new therapeutic approaches to effectively treat inflammation-associated cancers in patients.
Acknowledgments
Research work in author’s laboratory was supported by a Merit Award (I01BX001133) from the Veterans Administration (VA). The author apologizes to those colleagues, whose research work could not be cited directly due to space limitations.
Abbreviations
- Aim2
Absent in melanoma 2 (murine)
- AIM2
Absent in melanoma 2 (human)
- AR
Androgen receptor
- ASC
Apoptotic speck protein containing a caspase recruitment domain
- ATM
Ataxia telangiectasia
- BMDM
Bone marrow-derived macrophage
- CAC
Colitis-associated cancer
- CRC
Colorectal cancer
- DHT
Dihydrotestosterone
- ERα
Estrogen receptor-α
- IFN
Interferon
- ISGs
Interferon-stimulated genes
- POP3
PYRIN-only protein 3
- PrECs
Prostate epithelial cells
- PYD
PYRIN domain
- PYHIN
YRIN and HIN domain-containing
- TMA
Tissue microarray
References
- 1.Asefa B, Klarmann KD, Copeland NG, Gilbert DJ, Jenkins NA, Keller JR. The interferon-inducible p200 family of proteins: a perspective on their roles in cell cycle regulation and differentiation. Blood Cells Mol Dis. 2004;32:155–167. doi: 10.1016/j.bcmd.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Choubey D, Duan X, Dickerson E, Ponomareva L, Panchanathan R, Shen H, Srivastava R. Interferon-inducible p200-family proteins as novel sensors of cytoplasmic DNA: role in inflammation and autoimmunity. J Interferon Cytokine Res. 2010;30:371–380. doi: 10.1089/jir.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schattgen SA, Fitzgerald KA. The PYHIN protein family as mediators of host defenses. Immunol Rev. 2011;243:109–118. doi: 10.1111/j.1600-065X.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 4.Choubey D. DNA-responsive inflammasomes and their regulators in autoimmunity. Clin Immunol. 2012;142:223–231. doi: 10.1016/j.clim.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khare S, Ratsimandresy RA, de Almeida L, Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte E, Gottwein E, Perlman H, Reed JC, Greaves DR, Dorfleutner A, Stehlik C. The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol. 2014;15:343–353. doi: 10.1038/ni.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly DJ, Bowie AG. The emerging role of human PYHIN proteins in innate immunity: implications for health and disease. Biochem Pharmacol. 2014;92:405–414. doi: 10.1016/j.bcp.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269–280. doi: 10.1002/eji.201545839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 10.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 11.Chen GY, Núñez G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology. 2011;141:1986–1999. doi: 10.1053/j.gastro.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliani N, Palm NW, de Zoete MR, Flavell RA. Inflammasomes and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. Int Immunol. 2014;26:495–499. doi: 10.1093/intimm/dxu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man SM, Karki R, Kanneganti TD (2016) DNA-sensing inflammasomes: regulation of bacterial host defense and the gut microbiota. Pathog Dis 74(4). doi:10.1093/femspd/ftw028 [DOI] [PMC free article] [PubMed]
- 14.Nguyen SP, Bent S, Chen YH, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:676.e1-3–681.e1-3. doi: 10.1016/j.cgh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, Nennecke A, Eberle A, Brenner H, GEKID Cancer Survival Working Group Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One. 2013;8:e68077. doi: 10.1371/journal.pone.0068077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, Meltzer PS, Trent JM. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997;15:453–457. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- 17.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, Simanski M, Gläser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopfnagel V, Wittmann M, Werfel T. Human keratinocytes express AIM2 and respond to dsDNA with IL-1β secretion. Exp Dermatol. 2011;20:1027–1029. doi: 10.1111/j.1600-0625.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- 19.Duan X, Ponomareva L, Veeranki S, Panchanathan R, Dickerson E, Choubey D. Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol Cancer Res. 2011;9:589–602. doi: 10.1158/1541-7786.MCR-10-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11:1193–1202. doi: 10.1158/1541-7786.MCR-13-0145. [DOI] [PubMed] [Google Scholar]
- 21.Hakimi M, Peters A, Becker A, Böckler D, Dihlmann S. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59:794–803. doi: 10.1016/j.jvs.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Yang CA, Huang ST, Chiang BL. Sex-dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology (Oxford) 2015;54:324–331. doi: 10.1093/rheumatology/keu318. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Westra J, van der Geest KS, Moser J, Bijzet J, Kuiper T, Lorencetti PG, Joosten LA, Netea MG, Heeringa P, Brouwer E, Boots AM. Reduced levels of cytosolic DNA sensor AIM2 are associated with impaired cytokine responses in healthy elderly. Exp Gerontol. 2016;78:39–46. doi: 10.1016/j.exger.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Panchanathan R, Duan X, Shen H, Rathinam VA, Erickson LD, Fitzgerald KA, Choubey D. Aim2-deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;185:7385–7393. doi: 10.4049/jimmunol.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sester DP, Sagulenko V, Thygesen SJ, Cridland JA, Loi YS, Cridland SO, Masters SL, Genske U, Hornung V, Andoniou CE, Sweet MJ, Degli-Esposti MA, Schroder K, Stacey KJ. Deficient NLRP3 and AIM2 inflammasome function in autoimmune NZB Mice. J Immunol. 2015;195:1233–1241. doi: 10.4049/jimmunol.1402859. [DOI] [PubMed] [Google Scholar]
- 26.Panchanathan R, Duan X, Arumugam M, Shen H, Liu H, Choubey D. Cell type and gender-dependent differential regulation of the p202 and Aim2 proteins: implications for the regulation of innate immune responses in SLE. Mol Immunol. 2011;49:273–280. doi: 10.1016/j.molimm.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Krüger S, Vogel T, Knaebel HP, Rüschoff J, Hahn SA, Knebel-Doeberitz MV, Moeslein G, Meltzer SJ, Schackert HK, Tympner C, Mangold E, Schmiegel W, German HNPCC Consortium HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology. 2005;128:590–599. doi: 10.1053/j.gastro.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Woerner SM, Kloor M, Schwitalle Y, Youmans H, Mv Doeberitz, Gebert J, Dihlmann S. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer. 2007;46:1080–1089. doi: 10.1002/gcc.20493. [DOI] [PubMed] [Google Scholar]
- 29.Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135:2387–2396. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Truax AD, Chen L, Hu P, Li Z, Chen J, Song C, Chen L, Ting JP. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget. 2015;6:33456–33469. doi: 10.18632/oncotarget.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med. 2012;4:1276–1293. doi: 10.1002/emmm.201201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo Y, Nagai K, Nakahata S, Saito Y, Ichikawa T, Suekane A, Taki T, Iwakawa R, Enari M, Taniwaki M, Yokota J, Sakoda S, Morishita K. Overexpression of the DNA sensor proteins, absent in melanoma 2 and interferon-inducible 16, contributes to tumorigenesis of oral squamous cell carcinoma with p53 inactivation. Cancer Sci. 2012;103:782–790. doi: 10.1111/j.1349-7006.2012.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36:7501–7513. doi: 10.1007/s13277-015-3473-4. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti S, Multani S, Dabholkar J, Saranath D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: a pilot study. Med Oncol. 2015;32:60. doi: 10.1007/s12032-015-0483-4. [DOI] [PubMed] [Google Scholar]
- 35.Milutin Gašperov N, Farkas SA, Nilsson TK, Grce M. Epigenetic activation of immune genes in cervical cancer. Immunol Lett. 2014;162(2 Pt B):256–257. doi: 10.1016/j.imlet.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T, Schauber J. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 2013;305:723–732. doi: 10.1007/s00403-013-1375-0. [DOI] [PubMed] [Google Scholar]
- 37.Choubey D, Walter S, Geng Y, Xin H. Cytoplasmic localization of the interferon- inducible protein that is encoded by the AIM2 (absent in melanoma) gene from the 200-gene family. FEBS Lett. 2000;474:38–42. doi: 10.1016/S0014-5793(00)01571-4. [DOI] [PubMed] [Google Scholar]
- 38.Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One. 2011;6:e27040. doi: 10.1371/journal.pone.0027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cresswell KS, Clarke CJ, Jackson JT, Darcy PK, Trapani JA, Johnstone RW. Biochemical and growth regulatory activities of the HIN-200 family member and putative tumor suppressor protein, AIM2. Biochem Biophys Res Commun. 2005;326:417–424. doi: 10.1016/j.bbrc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 40.Chen IF, Ou-Yang F, Hung JY, Liu JC, Wang H, Wang SC, Hou MF, Hortobagyi GN, Hung MC. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5:1–7. doi: 10.1158/1535-7163.MCT-05-0310. [DOI] [PubMed] [Google Scholar]
- 41.Liao JC, Lam R, Brazda V, Duan S, Ravichandran M, Ma J, Xiao T, Tempel W, Zuo X, Wang YX, Chirgadze NY. Arrowsmith CH (2011) Interferon-inducible protein 16: insight into the interaction with tumor suppressor p53. Structure. 2010;19:418–429. doi: 10.1016/j.str.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin Q, Sester DP, Tian Y, Hsiao YS, Lu A, Cridland JA, Sagulenko V, Thygesen SJ, Choubey D, Hornung V, Walz T, Stacey KJ, Wu H. Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell Rep. 2013;4:327–339. doi: 10.1016/j.celrep.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 auto-inhibition and inflammasome assembly. J Biol Chem. 2013;288:13225–13235. doi: 10.1074/jbc.M113.468033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Wang ZX, Wu JW. Comparative purification and characterization of two HIN domains, hematopoietic interferon-inducible nuclear antigens with a 200-amino-acid repeat, in murine AIM2-like receptors. Biosci Biotechnol Biochem. 2013;77:2283–2287. doi: 10.1271/bbb.130544. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Wang J, Wang J, Cao LS, Wang ZX, Wu JW. Structural mechanism of DNA recognition by the p202 HINa domain: insights into the inhibition of Aim2-mediated inflammatory signalling. Acta Crystallogr F Struct Biol Commun. 2014;70:21–29. doi: 10.1107/S2053230X1303135X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin H, D’Souza S, Jørgensen TN, Vaughan AT, Lengyel P, Kotzin BL, Choubey D. Increased expression of Ifi202, an IFN-activatable gene, in B6.Nba2 lupus susceptible mice inhibits p53-mediated apoptosis. J Immunol. 2006;176:5863–5870. doi: 10.4049/jimmunol.176.10.5863. [DOI] [PubMed] [Google Scholar]
- 47.Liu ZY, Yi J, Liu FE. The molecular mechanism of breast cancer cell apoptosis induction by absent in melanoma (AIM2) Int J Clin Exp Med. 2015;8:14750–14758. [PMC free article] [PubMed] [Google Scholar]
- 48.Patsos G, Germann A, Gebert J, Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer. 2010;126:1838–1849. doi: 10.1002/ijc.24905. [DOI] [PubMed] [Google Scholar]
- 49.Patsos G, André S, Roeckel N, Gromes R, Gebert J, Kopitz J, Gabius HJ. Compensation of loss of protein function in microsatellite-unstable colon cancer cells (HCT116): a gene-dependent effect on the cell surface glycan profile. Glycobiology. 2009;19:726–734. doi: 10.1093/glycob/cwp040. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Li L, Gretz N, Gebert J, Dihlmann S. Absent in Melanoma 2 (AIM2) is an important mediator of interferon-dependent and -independent HLA-DRA and HLA-DRB gene expression in colorectal cancers. Oncogene. 2012;31:1242–1253. doi: 10.1038/onc.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veeranki S, Choubey D. Interferon-inducible p200-family protein IFI16, an innate immune sensor for cytosolic and nuclear double-stranded DNA: regulation of subcellular localization. Mol Immunol. 2012;49:567–571. doi: 10.1016/j.molimm.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choubey D, Panchanathan R. IFI16, an amplifier of DNA-damage response: role in cellular senescence and aging-associated inflammatory diseases. Ageing Res Rev. 2016;28:27–36. doi: 10.1016/j.arr.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Choubey D, Panchanathan R, Shen H, Duan X. Comment on “Development of murine lupus involves the combined genetic contribution of the SLAM and Fc gamma R intervals within the Nba2 autoimmune susceptibility locus”. J Immunol. 2010;184:4051–4052. doi: 10.4049/jimmunol.1090015. [DOI] [PubMed] [Google Scholar]
- 54.Pramanik R, Jørgensen TN, Xin H, Kotzin BL, Choubey D. Interleukin-6 induces expression of Ifi202, an interferon-inducible candidate gene for lupus susceptibility. J Biol Chem. 2004;279:16121–16127. doi: 10.1074/jbc.M313140200. [DOI] [PubMed] [Google Scholar]
- 55.Panchanathan R, Shen H, Bupp MG, Gould KA, Choubey D. Female and male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J Immunol. 2009;183:7031–7038. doi: 10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Souza S, Xin H, Walter S, Choubey D. The gene encoding p202, an interferon-inducible negative regulator of the p53 tumor suppressor, is a target of p53-mediated transcriptional repression. J Biol Chem. 2001;276:298–305. doi: 10.1074/jbc.M007155200. [DOI] [PubMed] [Google Scholar]
- 57.Geng Y, D’Souza S, Xin H, Walter S, Choubey D. p202 levels are negatively regulated by serum growth factors. Cell Growth Differ. 2000;11:475–483. [PubMed] [Google Scholar]
- 58.Panchanathan R, Xin H, Choubey D. Disruption of mutually negative regulatory feedback loop between interferon-inducible p202 protein and the E2F family of transcription factors in lupus-prone mice. J Immunol. 2008;180:5927–5934. doi: 10.4049/jimmunol.180.9.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choubey D, Pramanik R, Xin H. Sub-cellular localization and mechanisms of nucleo-cytoplasmic distribution of p202, an interferon-inducible candidate for lupus susceptibility. FEBS Lett. 2003;553:245–249. doi: 10.1016/S0014-5793(03)01006-8. [DOI] [PubMed] [Google Scholar]
- 60.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 61.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis . Proc Natl Acad Sci USA. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW, Jr, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J Immunol. 2016;196:3191–3198. doi: 10.4049/jimmunol.1502538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu GQ, Song PX, Li N, Chen W, Lei QQ, Yu SX, Zhang XJ, Du CT, Deng XM, Han WY, Yang YJ. AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immunol. 2016 doi: 10.1038/mi.2015.142. [DOI] [PubMed] [Google Scholar]
- 64.Hu S, Peng L, Kwak YT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH. The DNA sensor AIM2 maintains intestinal homeostasis via regulation of epithelial antimicrobial host defense. Cell Rep. 2015;13:1922–1936. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: from pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 68.Tang A, Li N, Li X, Yang H, Wang W, Zhang L, Li G, Xiong W, Ma J, Shen S. Dynamic activation of the key pathways: linking colitis to colorectal cancer in a mouse model. Carcinogenesis. 2012;33:1375–1383. doi: 10.1093/carcin/bgs183. [DOI] [PubMed] [Google Scholar]
- 69.Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, Ono Y, Imura J, Yamaoka S, Sakamoto C, Ueda Y, Chiba T. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Mühlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906–913. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- 73.Minn AJ, Wherry EJ. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell. 2016;165:272–275. doi: 10.1016/j.cell.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 74.Rommereim LM, Subramanian N. AIMing 2 curtail cancer. Cell. 2015;162:18–20. doi: 10.1016/j.cell.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 75.Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, Gissmann L, Dürst M, Schneider A, Pothuri B, Mansukhani M, Basso K, Chaganti RS, Murty VV. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]