Abstract
The aryl hydrocarbon receptor (AHR) is a cytoplasmic transcription factor, which plays an essential role in the xenobiotic metabolism in a wide variety of cells. The AHR gene is evolutionarily conserved and it has a central role not only in the differentiation and maturation of many tissues, but also in the toxicological metabolism of the cell by the activation of metabolizing enzymes. Several lines of evidence support that both AHR agonists and antagonists have profound immunological effects; and recently, the AHR has been implicated in antibacterial host defense. According to recent studies, the AHR is essential for the differentiation and activation of T helper 17 (Th17) cells. It is well known that Th17 cells have a central role in the development of inflammation, which is crucial in the defense against pathogens. In addition, Th17 cells play a major role in the pathogenesis of several autoimmune diseases such as rheumatoid arthritis. Therefore, the AHR may provide connection between the environmental chemicals, the immune regulation, and autoimmunity. In the present review, we summarize the role of the AHR in the Th17 cell functions.
Keywords: Aryl hydrocarbon receptor, Th17 cell, Polycyclic aromatic hydrocarbons, RAR-related orphan nuclear receptor gamma t
Overview of the domain structure, cytosolic complex and signal transduction of AHR
Although many recent investigations have implicated an immunological role of the aryl hydrocarbon receptor (AHR) [1, 2], this basic helix–loop–helix/Per–Arnt–Sim (bHLH/PAS) transcription factor was originally identified as a mediator of adaptive response to xenobiotics, including the dioxins and planar polychlorinated biphenyls (PCBs) [3, 4]. In addition to detoxification, this receptor has a role in cell proliferation, circadian rhythm, and neurogenesis as well [5]. In accordance with its diverse effects, AHR has many target genes, including: cytochrome (CYP1A1), cytochrome 1B1 (CYP1B1), UDP glucuronosyltransferase 1A1 (UGT1A1), multidrug resistance protein 1(MDR1), cytokines [interleukin 10 (IL-10), interleukin 21 (IL-21)], or receptors and transcription factors [estrogen receptor α (ERα), AHR repressor (AHRR)] and epiregulin [4–6].
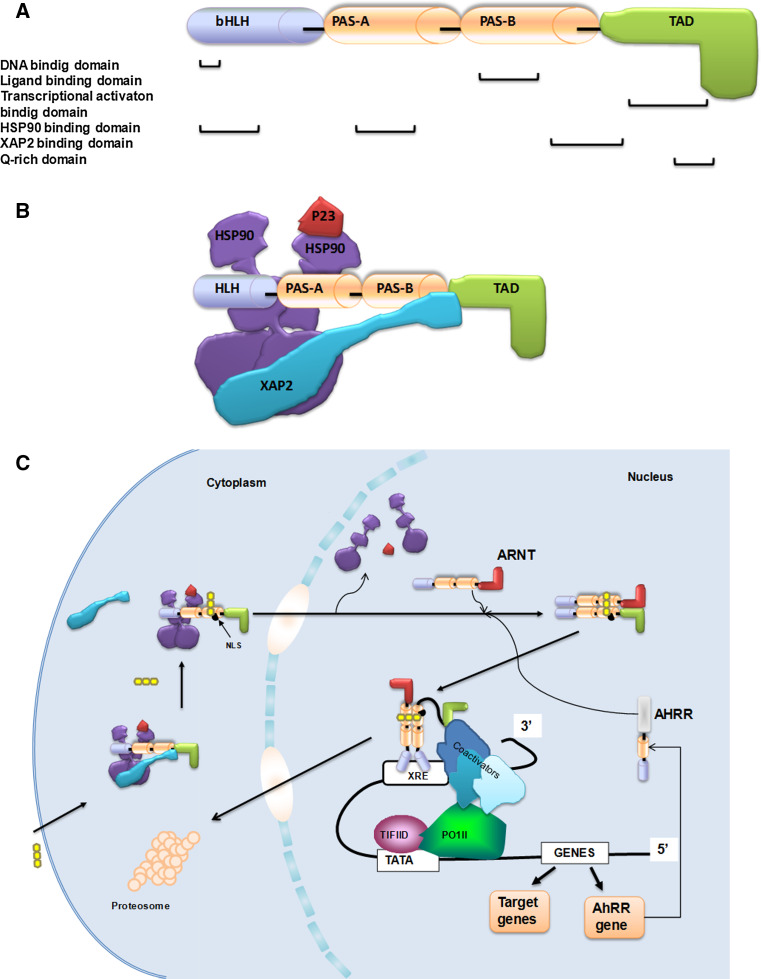
The human AHR gene is about 50 kilobases long, spans through 11 exons, and is located on chromosome 7 [7] (Fig. 1a). The gene encodes a 96 kDa protein, which has several functional domains. The bHLH domain mediates the ability of the ligand-activated AHR to bind to the DNA at the xenobiotic responsive elements (XREs) region [8, 9] and facilitate protein–protein interactions, such as the interaction with heat shock protein 90 (HSP90). The Per ArntSim (PAS) domain contains two highly conserved subdomains, namely the PAS-A and PAS-B. This region is required for the dimerization with AHR nuclear translocator (ARNT) and for the interaction with the ligand, or in inactive state, for the association with the two HSP90 proteins and with the hepatitis B virus X-associated protein 2 (XAP2, also known as ARA9 or AIP) [9, 10].
Fig. 1.
a The functional domains of human AHR gene [7–10]. b The structure of unliganded AHR complex [14, 15]. c The signaling pathway of AHR upon ligand binding [16–20]
The transactivation domain (TAD) of the AHR can facilitate the binding of the transcription factors, the TATA-box-binding protein (TBP) and nuclear factor 1 (NF-1) to the promoter of the target gene [11, 12]. Finally, a Q-rich domain is located in the C-terminal region of the protein and is involved in a co-activator recruitment and transactivation [13]. The unliganded AHR complex is localized in the cytosol (Fig. 1b). The AHR interacts directly with XAP2 and two HSP90 molecules through its central region (bHLH and PAS-A for HSP90 and PAS-B region for XAP2). XAP2 also binds to HSP90 molecules through their C-terminus. The HSP90-interacting bHLH domain of AHR regulates the ligand-dependent nuclear import of the receptor, while XAP2 plays a role in the protection of ligand-free AHR against degradation, by the inhibition of receptor ubiquitination and in the regulation of cytoplasmic retention of the receptor. There are other proteins participating in this interaction, e.g., the HSP90 accessory protein prostaglandin E synthase 3 (p23), which binds to the HSP90 and stabilizes the complex and modulates the ligand responsiveness during the AHR activation process [14, 15]. Upon the ligand binding to the AHR, XAP2 is released, promoting the movement of the AHR ligand complex to the nucleus [16]. After the nuclear translocation, the HSP90 dissociates, exposing the two PAS domains which allows the binding of ARNT [17]. The binding of the AHR/ARNT complex to the XREs stimulates the transcription of AHR target genes (e.g., in CYP1A1 the consensus XRE sequence is 5′-TNGCGTG-3′ and there are multiple copies of that upstream to the promoter) [18–20] (Fig. 1c).
The AHR/ARNT signaling pathway is negatively regulated by the nuclear AHRR proteins [21, 22]. The expression of AHRR is induced by the AHR/ARNT heterodimer through binding to XREs located in the 5′-flanking region of the AHRR gene. The AHRR protein was shown to repress AHR signaling through competition with AHR for ARNT and selective interference of AHRR/ARNT with AHR/ARNT in binding to the XRE sequences [23, 24]. Therefore, AHRR and AHR constitute a regulatory loop in which the heterodimer AHR/ARNT activates the expression of the AHRR gene, while the expressed AHRR inhibits the function of the AHR. Following the activation of the target genes, the ligand-bound AHR is removed rapidly from the nucleus by its nuclear export signal (NES) and is degraded in the cytosol in an ubiquitin-proteasome-dependent way [24, 25].
It was also described that AHR may bind to other dimerization partners than ARNT, such as V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog (c-MAF) or nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB). In type 1 regulatory T (Tr1) cells, the AHR/c-MAF complex induced the transactivation of the IL-10 and IL-21 promoters, which resulted in the generation of more Tr1 cells and the amelioration of the experimental autoimmune encephalomyelitis (EAE) [6, 26]. Chen and co-workers showed that AHR associates with NFκB protein in lung cancer cells. The AHR/NFκB complex then binds to the IL-6 gene and increases its expression [27].
AHR and ER
ERs have prominent effects on immune functions [28]. Recent studies show that in mice the modulation of ERα-dependent pathways decreases the sensitivity to autoimmune diseases such as lupus or arthritis and result prolonged survival [29, 30]. There are extensive data showing an intricate relationship between the AHR and ER pathways, which suggest a therapeutic potential of the AHR agonists in estrogen-related diseases [31–33]. Inhibitory crosstalk between the AHR and ER signaling was first suggested by the results of Kociba and co-workers, whose results suggest that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment decreases both mammary and uterine tumors in female Sprague–Dawley rats [34]. Other AHR agonists, such as 3-methylcholanthrene (3-MC), have the opposite effect compared to the TCDD, as they could directly activate ERα, and may therefore represent a new class of mixed AhR/ER agonists [35].
Recent data show that TCDD-activated AHR and ARNT may interfere with the ER machinery in several ways. They compete for promoter binding, which leads to the inhibition of the transcription of ERα. In addition, they also compete for the same transcriptional partners such as NF-1 and other co-activators, or co-repressors [13, 36]. Moreover, it has been shown that TCDD activation of AHR increases the proteasomal degradation of the ERα [37, 38]. Ligand-bound AHR/ARNT complex regulates the levels of circulating 17β-estradiol (E2) by controlling the gene expression of cytochrome P450 enzymes such as CYP19 (aromatase), CYP1A1 and CYP1B1 [39]. It has been demonstrated, that upon activation of AHR/ARNT, the heterodimer associates directly with ERα in the absence of estrogen through the N-terminal A/B region of the receptor. This fully competent transcriptional unit recruits CREB-binding protein/E1A binding protein p300 protein (CBP/P300) and binds to either XRE or estrogen-response element (ERE) through the attachment functions of AHR or ERα, respectively [38, 40]. Therefore, genes that are normally controlled by estrogens might become modulated by AHR agonists and vice versa.
AHR and other transcription factors
The orphan nuclear receptor liver X receptor (LXR) has two isoforms; liver X receptor alpha (LXRα) and liver X receptor beta (LXRβ) [41, 42]. In addition to lipid metabolism [43], LXRs also play an important role in the immune response. Both isoforms of the LXRs are expressed by human CD4+ T cells. Both the production of Th1 cytokines (such as interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα) and IL-2) and Th17 cell polarization are inhibited by LXR activation [44, 45]. Importantly, AHR plays a role in the LXR-mediated inhibition of Th17 differentiation as an interacting partner of LXR. LXR-induced sterol regulatory element-binding protein 1 (Srebp-1) binds to the IL-17 promoter (to the same site as AHR), leading to the inhibition of AHR-dependent IL-17 expression [44].
AHR expression may be affected by the ligand of peroxisome proliferator-activated receptors (PPARs) [5, 46]. PPARs are mediators of both the carcinogenic effects of AHR ligands (see below) in rodents and the lipid metabolism-controlled energy homeostasis in humans [47]. Peroxisome proliferator-activated receptor alpha (PPARα), a member of this ligand-activated transcription factor nuclear receptor superfamily, binds to the PPAR response element (PPRE) sites of genes [47]. Two PPRE sites were identified within the AHR-induced target gene CYP1A1 promoter region [48]. The PPARα agonist WY-14643 (WY) was shown to induce AHR expression in high doses, and it has an additive effect with the AHR ligand 3-MC on the induction of CYP1A1 expression in Caco-2 cell line [46]. PPARγ expression is also regulated by the AHR ligand TCDD in 3T3 cells during adipogenesis [49].
TCDD inhibits the testosterone-dependent transcriptional activity (for example the prostate-specific antigen expression) and cell growth of the androgen receptor (AR)-positive LNCaP prostate cancer cell line, while it does not change the expressions of AHR, ARNT or AR [50]. AHR is constitutively active in the castration-resistant C4-2 cell line and neither the AHR nor the AR is degraded by TCDD exposure. By contrast, in the androgen-sensitive LNCaP cell line both of them are degraded. Both the phosphorylation of AR and the expression of AR-inducing genes are enhanced by TCDD in LNCaP cells [51].
Activated AHR can also interact with p65 (RelA), which is a subunit of the NFκB heterodimer, and this way it is able to inhibit NFκB activity. It is an important connection point of the effects of cytokines and AHR signalization pathway [52]. This co-operation between RelA and AHR may induce the transactivation of c-Myc in Hs578T breast cancer cells via the binding of NFκB elements, and mediating malignant transformation by environmental carcinogens [53].
Th17 cells in autoimmunity
An IL-23 dependent T cell population was observed in mice by Langrish and his colleagues, which induced the autoimmune inflammation. These T cells were generated by the effect of IL-23 [54]. The Th17 cells were called as the third determinant population of the CD4+ effector T cells, in addition to the Th1 and Th2 subpopulations [55]. These cells produce proinflammatory cytokines, such as IL-17, IL-21 and IL-22. According to recent studies, the AHR plays an essential role in the T helper 17 (Th17) cell development.
The proinflammatory effects of the Th17 cells
The IL-17 cytokine family is evolutionary conserved and can be found in mammalian and non-mammalian vertebrates as well [56]. The IL-17 cytokine family consists of IL-17A, B, C, D, E and F cytokines, the IL-17A and F are co-produced by the Th17 cells. IL-17 induces the production of other proinflammatory cytokines (e.g., IL-6, TNFα) and interleukin-1 β (IL-1β), chemokines, and matrix metalloproteinases (MMP) along with colony-stimulating factors [granulocyte–monocyte colony-stimulating factor (GM-CSF)], granulocyte colony-stimulating factor [G-CSM]). IL-17 also recruits and activates neutrophils and promotes the production of nitric oxide (NO). The elevated levels of TNFα, IL-6 and IL-17 are predictors of joint destruction in rheumatoid arthritis (RA) [57–59]. IL-22 may contribute to the pathogenesis of psoriasis by promoting the abnormal differentiation and proliferation of keratinocytes [60]. IL-22 is upregulated in the mononuclear cells, isolated from the synovial fluid of RA patients; it induces the proliferation of synovial fibroblasts and promotes osteoclastogenesis [61]. IL-21 contributes to maintain the Th17 cell phenotype via proliferation induction and promotes the natural killer (NK) cell expansion, B cell differentiation, and immunoglobulin class switch [62, 63].
The Th17 cells play a major role in the pathogenesis of several autoimmune diseases including psoriasis, RA, multiple sclerosis (MS), inflammatory bowel disease (IBD), systemic lupus erythematosus (SLE) and asthma. The IL-23 and IL-1β induced IL-17 production of the microglial cells contribute to the inflammation in the central nervous system (CNS) in multiple sclerosis (MS) [64]. Both IL-17 and IL-22 receptors (IL-17R and IL-22R) are expressed in the blood–brain barrier endothelial cells (BBB-ECs). The Th17 cells may transmigrate through to the BBB-ECs and promote inflammation in the CNS [65]. The increased expression of Th17 pathway associated genes, such as growth factor beta 1 (TGFB1) signal transducer and activator of transcription 3 (STAT3), IL-6, IL-23R, TNF, IL17A, IL17F, and IL21 have been observed in IBD [66, 67]. The plasma concentration of IL-17A correlated with the disease activity in SLE [68]. The elevated number of circulating Th17 cells and the increased production of Th17 cell-related cytokines (IL-17, IL-22) were also described in allergic asthma [69].
Cytokine regulation of mouse Th17 differentiation
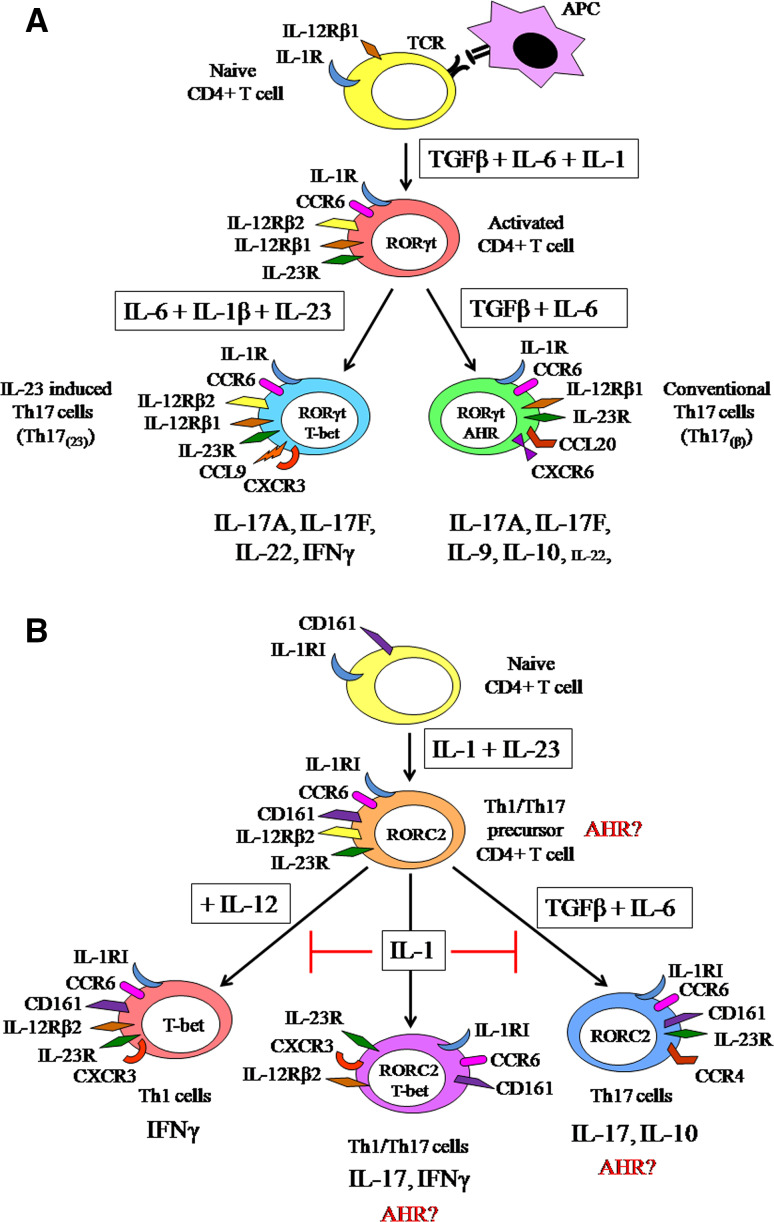
The Th17 differentiation in mice originates from CD4+ naive T cells which constitutively express IL-12Rβ1, induced by TGFβ, IL-6 and IL-1β; furthermore, maintained and amplified by IL-23, IL-21 and TNFα (Fig. 2a) [70–73]. The TGFβ-induced Forkhead box P3 (FoxP3) expression and Treg differentiation are inhibited by IL-6; the Th17 development is supported by the combination of TGFβ and IL-6. IL-23R [70–72, 74, 75] and CCR6 [76] expression are also induced by TGFβ and IL-6. IL-23 increased the ratio of IL-17 producing T cells, but Th17 cell differentiation from naive cells was not induced by IL-23 treatment or by the combination of IL-23 and TGFβ or IL-6 [70]. The T cell receptor signaling induced IL-1R expression is upregulated by IL-6 [73]. IL-21 and IL-23R expression are also induced by IL-6 in activated CD4+ T cells. IL-21 cooperates with TGFβ to induce Th17 cell differentiation, and IL-17 and IL-22 expression. In addition, IL-23R expression is upregulated by IL-21 as well, which also induces its own expression [70, 77]. Despite the Th17-induced milieu, Th17 cells may remain sensitive to IL-12, owing to their IL-12Rβ2 expression [78].
Fig. 2.
a The differentiation and phenotypical diversity of the Th17 cells in mice [70–74, 76–80]. b The differentiation and phenotypical plasticity of the Th17 cells in human. The role of AHR in Th17 cell subpopulations was not examined in the current studies, however, like in mouse AHR has expected role in the human Th17 differentiation as well [81–91]
In 2010, two functionally distinct Th17 cell populations were described. These populations need different cytokine milieu for their differentiation (Fig. 2a). The conventional Th17 cells [Th17(β)] are differentiated upon TGFβ and IL-6 treatment, express CCL20 and CXCR6, and produce IL-9 and IL-10 in addition to IL-17A and IL-17F. The IL-23 induced Th17 cells [Th17(23)] differentiate in the presence of IL-6, IL-23 and IL-1β. These Th17(23) cells produce similar amount of IL-17F, but more IL-22 than Th17(β) cells and express CCL9 and CXCR3. Remarkably, both population express RAR-related orphan nuclear receptor gamma t (RORγt), but the Th17(23) cells express T-box protein 21 (T-bet) as well [79]. The Th17(23) cells have more important roles in autoimmunity because they become IL-17/IFNγ double producers by the presence of IL-23 [79, 80].
Cytokine regulation of human Th17 differentiation
The IL-17 producing cells originate from CD161+/CD4+ cells. CD161 is a human homolog of murine natural killer (NK) cell-associated marker (NK1.1) (Fig. 2b).The Th1/Th17 precursor cells constitutively expresses retinoic acid-related orphan receptor variant 2 (RORC2),IL-23R, IL-1RI, and CCR6 [81–83]. The human Th17 polarization from CD4+ CD45RA+ naive T cells is initiated by IL-1β and IL-6 and is inhibited by TGFβ and IL-12 [84]. The Th17 differentiation from CD4+ naive T cells was not induced after the treatment by TGFβ and IL-6 [85]. The combination of IL-23 and IL-1β stimulated the Th17 differentiation from CD4+ naive T cells, while the IL-23 induced IL-17 and IL-22 production were reduced by the effect of TGFβ and IL-6 [86]. TGFβ is not able to induce human Th17 development [84–86]. Interestingly, TGFβ may serve as an indirect inducer of Th17 development via the inhibition of IL-1β and IL-23 induced T-bet expression, IFNγ production, and Th1 cell differentiation of CD161+ precursors [87]. According to other observations, the TGFβ and IL-21 are necessary for the development of Th17 cells from naive cells [88].
The CD161+ precursor cells differentiate to mature Th17 cells after IL-1β and IL-23 stimulus. These cells express T-bet, IL-12Rβ2, CCR6, CD161 and RORC2 [81, 89, 90]. Two functionally different IL-17 producing memory T cell populations were also observed (Fig. 2b). CCR6 and CCR4 are expressed on a homologue population of IL-17 producing cells; the IL-17/IFNγ double producers like Th1 cells express CXCR3 [89, 91], CCR6 and T-bet [89, 90]. The Candida albicans specific memory Th17 cells were shown to be CCR6+ CCR4+; however, the CCR6+ CXCR3+ cells were specific for Mycobacterium tuberculosis [89, 90]. Zielinski and her colleagues described that Candida albicans and Staphylococcus aureus-primed Th17 cells produced different cytokines. C. albicans-primed cells produced IL-17 and IFN-γ, while S. aureus-specific Th17 cells produced IL-17 and IL-10 [92]. The role of AHR in the functionally different IL-17 producing human T cells needs to be investigated.
Transcriptional regulation of mouse Th17 differentiation
The transcription factors regulating Th17 cell differentiation are: the RORγt/RORC2, the AHR (see below), and the non-specific positive regulator STAT3. The differentiation of IL-17 producing T cells in mice is controlled by RORγt, which is encoded inside the RoRc locus and expressed by immature lymphocytes [93–95]. RORγt has an essential role in the lymphoid organogenesis; furthermore, it controls the thymopoiesis and the apoptosis of the thymocytes. In RORγt-deficient mice, the absence of lymph nodes and Peyer’s patches and also the infiltration of Th17 cells were observed [96]. In TGFβ and IL-6 primed murine cells, the RORγt expression is regulated by STAT3, which is activated by IL-6, IL-21, and IL-23. The TGFβ, IL-6, and IL-23-stimulated IL-17 secreting phenotype is also regulated by STAT3 [97–99]. RORγt alone is sufficient for IL-17 production, and STAT3 preferably contributes to the adequate Th17 phenotype. High number of IL-17+ cells and high IL-17 expression were observed in RORγt+ cells upon the activation of STAT3 [77, 98, 99]. Th17 cell differentiation, including RORγt expression, IL-21 production, and IL-23R expression are induced by low TGFβ concentration in the presence of IL-6 [75, 100]. STAT3 induces several important Th17-related genes including IL17A, IL17F, IL21, IL21R, IL23R and regulates the expression of other Th17 related transcription factors [e.g., RORγt, interferon regulatory factor 4(IRF4), and basic leucine zipper transcription factor (BATF)] [79, 101].
Transcriptional regulation of human Th17 differentiation
Human Th17 cells express (RORC2), which is an orthologue of murine RORγt [84, 86, 90, 102, 103]. The overexpression of RORC in naive T cells promotes the Th17 phenotype via induction of IL-17A, IL-17F, IL-26 production, and CCR6 expression, but downregulates IFNγ secretion. The overexpression of RORC2 was not associated with the increased expression of IL-22, CCR4, and CCR2 [102]. In addition, the overexpression of RORC2 is sufficient not only for the Th17-related cytokine production (IL-17, IL-22, IL-6, and TNF-α), but also for the chemokine receptor expression (CCR6 and CCR4 but not CXCR3) [104]. The IL-17 producing cells differentiate from CD161+ precursors, interestingly the expression of CD161 is upregulated by RORC2.
Th17/Th1 cells are phenotypically halfway towards Th1 cells, but they have many “Th17-like” properties such as RORC2 expression, IL-17 production, and cell surface markers [87]. The CD161+ cells are common precursors of both Th17 and Th1 differentiation (Fig. 2b) and both pathways can be induced by IL-1β and IL-23 [84, 87, 105]. IL-12 promotes the Th1 pathway via reduction of IL-17 and induction of IFNγ producing cells while the number of double producers is not altered. In this IL-17+ IFNγ+ population, the IL-22 and/or IL-17F producing cells are decreased by IL-12 [105]. In addition, TGFβ inhibits Th1 development via inhibition of T-bet expression; thus, indirectly promotes the Th17 differentiation [87].
Mouse Th17 differentiation and AHR
In mice, AHR shows ubiquitous expression in the immune system, including high expression in several immunologically important tissues and cells [106]. In murine Th17 cells, the receptor is highly expressed, there is a slight expression in regulatory T (Treg) cells while it is not detectable in naïve Th1 and Th2 cells [107–109]. Marc Veldhoven and his colleagues described that natural agonists of AHR are required for the optimal Th17 cell differentiation [110].The IL-17 production depends on the RORγt and RORα expression, while the IL-22 production appears to be less ROR dependent [111]. The ligand-dependent AHR signalization plays a major role in the regulation of IL-22 production of CD4+ T cells [109, 112]. The STAT proteins are important factors in the differentiation of helper T cell subpopulations [97]. STAT signalization is regulated by AHR signalization; thus, indirectly controls Th17 cell differentiation [107]. The IFNγ and IL-27 induced STAT1 activation is selectively regulated by AHR, which can suppress Th17 cell differentiation under Th17 cell polarizing conditions (in the presence of TGFβ+ IL-6 or TGFβ+ IL-21). AHR is able to bind STAT1 and STAT5, but not STAT3 or STAT6, and negatively regulates the Th17 differentiation [107]. The presence of TGFβ is required for AHR expression, by contrast, the RORγt expression is also induced without TGFβ signaling (Fig. 2a). The AHR expression was observed only in the non-pathogenic conventional Th17 cells but not in the IL-23 induced Th17 cells [79]. Interestingly, both Th17 and Treg cells express AHR in mice, but the receptor has a ligand-specific effect on the cells. An increased Th17 cell number was observed after the treatment of endogen ligands such as the 6-formylindolo[3,2-b]carbazole (FICZ), while Treg induction was described in the presence of exogen ligands like TCDD [108]. The effect of AHR also depends on the expressing cell and the origin of the activating ligand. This regulation may represent a way to avoid the potential harmful consequences of the AHR ligands. Endogen ligands promote Th17 development to eliminate pathogens via inflammation, while exogen ligands support Tregs, protecting from the autoimmune processes, elicited by the overstimulated Th17 cells [113]. The mortality of the AHR-deficient mice generated by Pedro M. Fernandez-Salguero and his colleagues was 50 %. A decreased accumulation of lymphocytes was observed in the spleen and lymph nodes but not in the thymus in these animals. Although the AHR-deficient mice had reduced liver size (by 50 %) and were resistant to dioxin exposure, high doses of dioxin caused limited vasculitis and scattered single cell necrosis in the livers and lungs of these animals [114, 115].
Human Th17 cell differentiation and AHR
AHR is highly expressed in several human tissues such as lung, heart, kidney, liver, esophagus, pancreas, placenta, testicle, thymus gland, and retina [116, 117]. AHR is also highly expressed in synovial tissues of RA patients which are regulated by TNFα. The AHR agonist TCDD treatment increases the expression of IL-1β and IL-6 of the synovial cells [84, 88, 118]. The IL-17 and IL-22 production of CD4+ T cells are also modulated by AHR [119]. The increased AHR expression was correlated with the inflammatory responses in allergic rhinitis [120]. A non-toxic AHR ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) upregulated AHR in healthy controls and inhibited the Th17 differentiation and the production of IL-17 and IL-22 both in allergic rhinitis patients and healthy donors [120]. TCDD also regulates the IL-22 production of IL-22 single positive cells via AHR binding. Furthermore, TCDD decreases the IL-17 production, RORC2 expression and IL-23R expression, and increases IL-22 production in an IL-23 and IL-1β-independent way [121].
The role of AHR in RORγt+ innate lymphoid cells
AHR is expressed by the RORγt+ innate lymphoid cells (ILCs), and contributes to the formation of intestinal lymphoid follicules and to the expansion of RORγt+ ILCs in mice. The expression of Kit is stabilized by AHR-mediated transcription, which also correlates with the postnatal expansion of RORγt+ ILC cell pool [122]. The number of ILC22 cells is significantly decreased in the AHR KO mice, leading to decreased production of IL-22. These animals lack postnatal but not embryonic ‘imprinted’ cryptopatches and isolated lymphoid follicles (ILFs), indicating that AHR is crucial for the development of postnatal intestinal lymphoid tissues [123]. AHR ligands, especially cruciferous vegetables from Brassicaceae family, are essential to maintain the population of intraepithelial lymphocytes (IELs) via AHR signalization in the skin and intestine in mice. IELs highly express AHR and they represent a first line of defense of epithelial barriers and wound repair. The reduced AHR activity in mice causes reduced antimicrobial capacity (against Bacteroidetes species) [124, 125]. AHR is also expressed by other innate IL-17 producing cells such as lymphoid tissue inducer-like cells (LTi-like cells), gamma delta T cells (γδ T) cells, and NK cells. These innate sentinel cells represent a first line of the defense against pathogens, and they have a primary role in the proper formation of lymphoid tissues. In addition, the expression of AHR enables these cells to respond to environmental effects [123, 126–128].
Aryl hydrocarbon receptor ligands and Th17 cells
Several AHR agonists and antagonists regulate the Th17 lymphocyte differentiation and activation and may contribute to the pathogenesis of numerous inflammatory diseases (Tables 1, 2).
Table 1.
The chemical structure of the AHR ligands in relation to Th17 cell development (↑ enhance; ↓ inhibit)
| Receptor ligand | Chemical name | Action | Effect on Th17 development | 2D structure |
|---|---|---|---|---|
| TCDD [108] | 2,3,7,8-tetrachlorodibenzo-p-dioxin | Exogen agonist | ↓ |
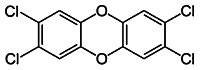
|
| CH-223191 [116] | 1-methyl-N-{2-methyl-4-[2-(2-methylphenyl)diazen-1-yl]phenyl}-1H-pyrazole-5-carboxamide | Synthetic antagonist | ↓ |
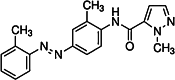
|
| B[a]P [109] | Benzo[alpha]pyrene | Exogen agonist | ↑ |
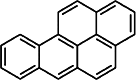
|
| 3-MC [109] | 3-Methylcholanthrene | Exogen agonist | ↑ |
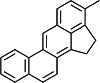
|
| Leflunomide [118] | 5-methyl-N-[4-(trifluoromethyl) phenyl]-isoxazole-4-carboxamide | Exogen agonist | ↓ |
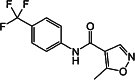
|
| ITE [120] | 1′H-indole-3′-carbonyl-thiazole-4-carboxylic acid methyl ester | Endogen agonist | ↓ |
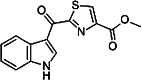
|
| FICZ [126] | 6-Formylindolo(3,2-b)carbazole | Endogen agonist | ↑ |
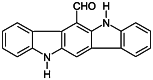
|
| L-Kynurenine (KYN) [167] | (S)-2-Amino-4-(2-aminophenyl)- 4-oxo-butanoic acid | Endogen agonist | ↓ |
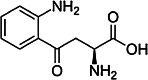
|
| Kynurenic acid (KYNA) [167] | (4-hydroxyquinoline-2-carboxylic acid) | Endogen agonist | ↑ |
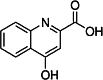
|
| Indoxyl 3-sulfate (I3S) [133] | 1H-Indol-3-yl hydrogen sulfate | Endogen agonist | ↑ |
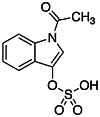
|
| Indole-3-carbinol (I3C) [168] | 1H-Indol-3-ylmethanol | Natural agonist | ↓ |
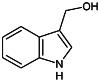
|
| 3,3’-Diindolylmethane (DIM) [168] | 3,3′-methanediylbis (1H-indole) | Natural agonist | ↓ |
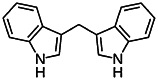
|
| Curcumin [169] | (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione | Natural agonist/ antagonist | ↓ (presumably indirectly) |
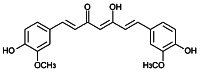
|
| trans Resveratrol (RES) [148] | 3,5,4’-trihydroxy-trans-stilbene | Natural agonist/ antagonist |
↓ (high concentration) ↑ (low concentration) |
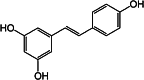
|
| Polydatin (POLY) [148] | resveratrol-3-O-β-mono-d-glucoside | Modified natural antagonist | ↑ (high concentration) |
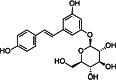
|
| Baicalin [158] | (2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-chromen-7-yl)oxy-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic acid | Natural agonist | ↓ |
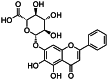
|
| alpha-naphthoflavone (α-NF) [170] | 2-phenylbenzo[h]chromen-4-one | Exogen antagonist | ↓ |
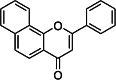
|
Table 2.
AHR ligands linkage with human autoimmune diseases and animal models related with Th17 development
| AHR ligand | Ligand type | Sources of ligand | Cell type/animal model used | Observed effect | Relevant diseases | Biological relevance in the pathology of disease |
|---|---|---|---|---|---|---|
| TCDD | Agonist | Combustion products, forest fire, cigarette smoke | Fibroblast-like synoviocyte cell line (MH7A) | Enhanced IL-1 mRNA | Rheumatoid arthritis | Aggravate [112] |
| RA patient-derived synoviocites | Upregulated IL-1β, IL-6 and IL-8 production | Rheumatoid arthritis and osteoarthritis | Aggravate [105] | |||
| 2,4,6-trinitrobenzenesulfonic acid (TNBS) murine model of colitis | Decreased IL-6 and induced FoxP3+ Tregs | Inflammatory bowel disease | Suppress [164] | |||
| C57BL/6 mice | Induced expansion of CD4+ CD25+ Foxp3+ Treg population | Experimental autoimmune encephalomyelitis | Suppress [95] | |||
| B[a]P | Agonist | Wood burning, exhaust fumes (especially diesel engine), charbroiled food, cigarette smoke | Fibroblast-like synoviocyte cell line (MH7A) | Enhanced IL-1 expression | Rheumatoid arthritis | Aggravate [112] |
| Human monocyte-derived DCs | Reduced IL-6 production | Rheumatoid arthritis | Contribute to pathogenesis [165] | |||
| 3-MC | Agonist | Combustion products | Fibroblast-like synoviocyte cell line (mh7a) | Enhanced IL-1 expression | Rheumatoid arthritis | Aggravate [112] |
| ITE | Agonist | Originally isolated from porcine lung | Murine T cells from thymus and mesenteric lymph nodes | Induced FoxP3+ Treg cells | Collagen-induced arthritis | Suppress [121] |
| PBMCs from AR patients | Inhibited Th17 differentiation and production of IL-17 and IL-22 | Allergic rhinitis | Suppress [107] | |||
| DCs and CD4+ T cells from AR patients | Inhibited IL-1β and IL-6, induced IL-10 secretion by DCs, inhibited IL-17 and induced IL-10 expression and by CD4+ T cells and Th17 cell expansion in vitro | Allergic rhinitis | Suppress [123] | |||
| monocyte-derived DCs and CD4+ T cells from BD patients | Inhibited production of IL-1β, IL-6, IL-23, TNFα and induced IL-10 by DCs and inhibited Th17 response | Behcet’s disease | Suppress [122] | |||
| FICZ | Agonist | Tryptophan photoproduct | CD4+ T cells from B6 mice and human | Increased expression of Il17a, Il17f and Il22 | Experimental autoimmune encephalomyelitis | Aggravate [94] |
| Lamina propria mononuclear cell from IBD patients and TNBS murine model of colitis | Upregulated production of IL-22 | Inflammatory bowel disease | Suppress [167] | |||
| KYNA | Agonist | Generated from tryptophan by enzymatic process via kynurenine pathway | CD4+ T cells from BALB/c mice | Increased production of IL-17 | Autoimmune gastritis | Aggravate [132] |
| I3S | Agonist | Originate from a tryptophan metabolite indole by intestinal bacteria | T cells from BALB/c mice | Increased phosphorylation of STAT3 and stimulated expression of RORγt, and in vitro Th17 differentiation | Collagen-induced arthritis | Aggravate [136] |
| I3C | Agonists | Generated from tryptophan from cruciferous vegetables (broccoli, brussel sprouts, cabbage, cauliflower, collard greens, sprouts, kale) | T cells from C57BL/6 mice | Increased number of Tregs and reduced number of Th17 cells | Experimental autoimmune encephalomyelitis | Suppress [138] |
| DIM | ||||||
| RES | Antagonist/agonist | Polyphenolic phytoalexin from red grape, red wine, cacao, peanut, mulberries, and pines | Lymphocytes from DBA1 mice | Inhibited production of IL-6, IL-17 and Th17 cell differentiation | Collagen induced arthritis | Suppress [166] |
| Splenic and brain derived mononuclear cells and macrophages from SJL/J mice | Induced number of IL-17+ IL-10+ T cells and increased IL-12/23 p19 and p35 production by macrophages, decreased expression of IL-6 and IL-12/23p40 expression by macrophages | Experimental autoimmune encephalomyelitis | Suppress [151] | |||
| C57BL/6 mice and mouse T lymphoma cell line (EL4) | Increased expression of FoxP3 | Experimental allergic encephalomyelitis | Suppress [150] | |||
| Baicalin | Agonist | Flavone type flavonoid in the genus Scutellaria (e.g., Blue skullcap) | CD4+ T cells from C57BL/6 (B6) and lupus-prone MRL/lpr mice | Inhibited in vitro and in vivo differentiation of Th17, inhibited expression of CCR6, IL-6 receptor and RORγt, upregulated expression of Foxp3 and down-regulated RORγt-mediated IL-17 | Lupus nephritis | Suppress [158] |
| α-NF | Antagonist | Flavone type flavonoid | Fibroblast-like synoviocyte cell line (MH7A) | Suppressed IL-1 expression via inhibition of B[a]P and 3-MC | Rheumatoid arthritis | Suppress [112] |
Exogen ligands and xenobiotics
The typically carcinogenic and teratogenic exogenic ligands and xenobiotics are originated from the environment due to anthropogenic influence [129].
Halogenated dioxins (HAHs) and their congeners
HAHs are mainly derived from industrial activity and halogenation makes them to be metabolically and environmentally stabile. They are generally AHR agonists with different binding affinity, especially if the lateral rings are halogenated. TCDD, one of the best known AHR ligands, belongs to this group (Tables 1, 2) [130]. As it was previously described by Ramirez and co-workers, in vitro TCDD treatment of human PBMC increased the number of human IL-22 producing cells; however, decreased the IL-17 production and RORC2 expression [121]. In vivo TCDD treatment promoted the Treg differentiation and inhibited the Th17 differentiation in mice [108, 131]. The IL-1β mRNA level was enhanced in several different human cell lines, including fibroblast-like synoviocytes (MH7A) [132], keratinocytes [133] and endometrial stromal cells [134] after TCDD exposure.
A synthetic ligand, 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191), was identified from a chemical library as a potent AHR antagonist (Tables 1, 2). In vitro treatment of CH-223191 inhibited TCDD-induced transcription of AHR [135]. CH-223191, unlike most dietary compounds, is a pure antagonist, including flavones, which act both as agonists or antagonists on Th17 development, in a concentration-dependent way [110]. Interestingly, CH-223191 is a ligand selective AHR antagonist which only inhibits the binding of HAHs to AHR, and does not alter the binding of other AHR ligands [136].
Polycyclic aromatic hydrocarbons (PAHs)
PAHs represent a group of AHR agonists (less effective inducers of the AHR signalization than TCDD), which originate mainly from soot, grilled, or smoked food, smoke exhaust, and cigarette smoke. They are composed of four or more conjugate benzene rings [130]. The carcinogenic effect of benzo[alpha]pyrene (B[a]P) (Tables 1, 2), similarly to TCDD, is associated with the AHR activation. AHR knockout mice was resistant to the topical or subcutaneous B[a]P injection-induced tumor induction [137]. The IL-6, IL-1β mRNA level is upregulated in human MH7A cells by B[a]P and 3-MC, which also belongs to this group (Table 1) [132].
Leflunomide
Leflunomide, a widely used disease modifying anti-rheumatic drug (DMARD) in RA, is a potent AHR agonist (Table 1). Unlike its metabolite teriflunomide (A771726), leflunomide induced the translocation of the AHR to the nucleus and the induction of the AHR target genes in both human HepG2 and mouse WT Hepa1 cells [138]. The number of FoxP3+ Tregs and the IL-10-producing cells were increased, while the number of IL-17 and IL-23 producing, presumably Th17 cells were reduced in both murine peripheral blood and kidney cells following in vivo leflunomide treatment [139].
Endogen ligands
ITE
The AHR agonist 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) was first isolated from porcine lung (Table 1, 2) [140]. Treatment with ITE promoted the generation of FoxP3+ Treg cells in vivo and suppressed EAE in the mouse model. ITE treatment also induced the generation of tolerogenic dendritic cells that produce low levels of proinflammatory cytokines (IL-1β, IL-6, IL-12, IL-23), osteopontin, high levels of TGF-β1 and IL-10, and support the generation of FoxP3+ Tregs in vitro, via AHR activation [141]. ITE treatment of DCs, isolated from healthy donors and from patients with Bechet’s disease (BD), inhibited the Th17 cell differentiation and activation. ITE profoundly altered the cytokine production of DCs from active BD patients; the production of IL-1β, IL-6, IL-23, and TNFα were significantly inhibited while the IL-10 production was increased upon ITE treatment [142]. According to recently published data, the expression of AHR was increased and correlated with the clinical parameters in allergic rhinitis. ITE inhibited the expansion of Th17 cells and decreased the production of IL-17 in the samples of patients with allergic rhinitis [120, 143].
Dietary compounds
The anti-inflammatory properties of several dietary compounds including flavonoids and their derivatives (herbs, vegetables and fruits) might be explained by their effects on the AHR [144].
Tryptophan metabolites and derivatives
The essential amino acid tryptophan plays a central role in the Th17 cell development. The high affinity AHR agonist 6-formylindolo(3,2-b)carbazole (FICZ) is generated from tryptophan through photo-oxidation (Tables 1, 2) [145]. As it was mentioned earlier, natural AHR agonists were shown to be required for the optimal Th17 cell differentiation [110]. AHR is expressed both in mouse and human Th17 cells, and the activation of AHR with FICZ treatment promotes the Th17 cell expansion and IL-17 production in vitro [109]. Importantly, normal laboratory lightning may also induce the generation of FICZ, potentially leading to AHR activation in the cultured cells [146].
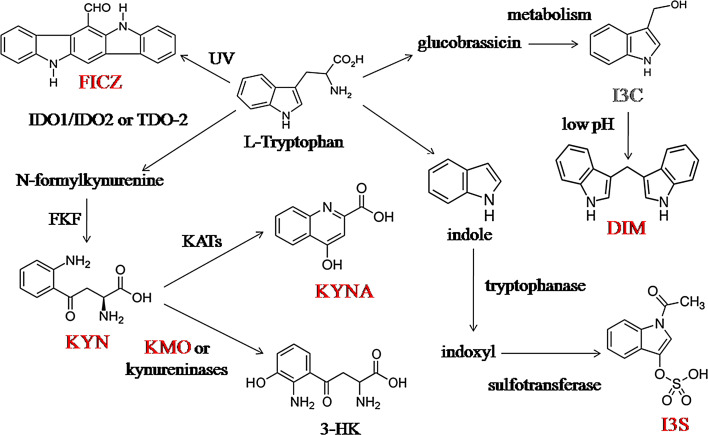
Tryptophan is also metabolized via the kynurenine (KYN) pathway. In mammals, l-tryptophan is oxidized into N-formylkynurenine first, either by the indoleamine 2,3-dioxygenase 1 (IDO1), or by the IDO2 or by the tryptophan 2,3-dioxygenase (TDO-2) enzymes. N-formylkynurenine is further degraded into L-kynurenine (KYN) by formylkynurenine formamidase (FKF), then L-kynurenine might be irreversibly transaminated into kynurenic acid (KYNA) by kynurenine aminotransferases (KATs) (Fig. 3) [147, 148].
Fig. 3.
The tryptophan originated AHR ligands were indicated red bold letters. 3-HK: 3-hydroxykynurenine, DIM: 3,3′-diindolylmethane, FICZ: 6-formylindolo(3,2-b)carbazole, FKF: formylkynurenine formamidase, I3C: indole-3-carbinol, I3S: indoxyl 3-sulfate, IDO1: 2,3-dioxygenase 1, IDO2: 2,3-dioxygenase 2, KATs: kynurenine aminotransferases, KMO: kynurenine 3-monooxygenase, KYN: kynurenine, KYNA: kynurenic acid, TDO: tryptophan 2,3-dioxygenase [147, 148, 151, 154, 155, 158, 159]
Both KYN and KYNA are endogen agonists of the AHR (Table 1 and 2). KYN is a potent long-acting AHR ligand which directly activates the receptor. In mice, the differentiation of FoxP3+ Tregs, but not the Th17 cells, was promoted by KYN via AHR-dependent pathway. This effect of KYN was further potentiated by TGFβ [149]. KYNA induces IL-6 production in synergism with IL-1β in breast cancer cell line MCF-7 [150]. Kynurenine might be metabolized into 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO) and by kynureninase enzymes (Fig. 3) [151]. KMO enzymes are preferentially expressed by murine Th17 cells and contribute to the differentiation of Th17 cells in vitro. If the KMO enzyme is inhibited, the KYNA is highly formed from KYN in the Th17 cells by KATs. The Th17-driven inflammation is enhanced by this process in an AHR-dependent way [152].
Indoxyl 3-sulfate (I3S), another potent AHR agonist (Tables 1, 2; Fig. 3), is an uremic toxin, which is not completely cleared from the blood of hemodialyzed patients, due to its albumin binding capacity. I3S is synthetized in the liver from the intestinal bacterial metabolite indole [153, 154]. Indole is metabolized in the intestinal tract from dietary tryptophan by the tryptophanase enzymes of the intestinal bacteria [155]. In mice, I3S promotes Th17 cell differentiation via the increased RORγt expression and STAT3 phosphorylation in an AHR-dependent way. Furthermore, the treatment of I3S aggravated EAE by increasing the number of Th17 cells in vivo [153, 156]. The kynurenine/tryptophan ratio was found to be increased in the sera of patients with RA [157]. These data suggest that tryptophan metabolites regulate the development of autoimmunity, through mediating the AHR activation.
Indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM) (Tables 1, 2; Fig. 3) are also potent AHR agonists, which are originated in dietary tryptophan in cruciferous vegetables, such as broccoli, brussels sprouts, cabbage, cauliflower, collard greens, sprouts, and kale) [158]. Glucobrassicins are degraded to I3C from cruciferous vegetables via metabolism which is further condensated to DIM under acidic conditions [159]. The Th17 differentiation was inhibited, while the generation of Tregs was promoted in vivo and in vitro in mice by both I3C and DIM via an AHR-dependent pathway. The previously mentioned AHR antagonist CH223191 was capable to reverse the Th17 suppressing effects of I3C and DIM in vivo; however, Th17 differentiation was also inhibited by this antagonist in vitro [158] (Fig. 4).
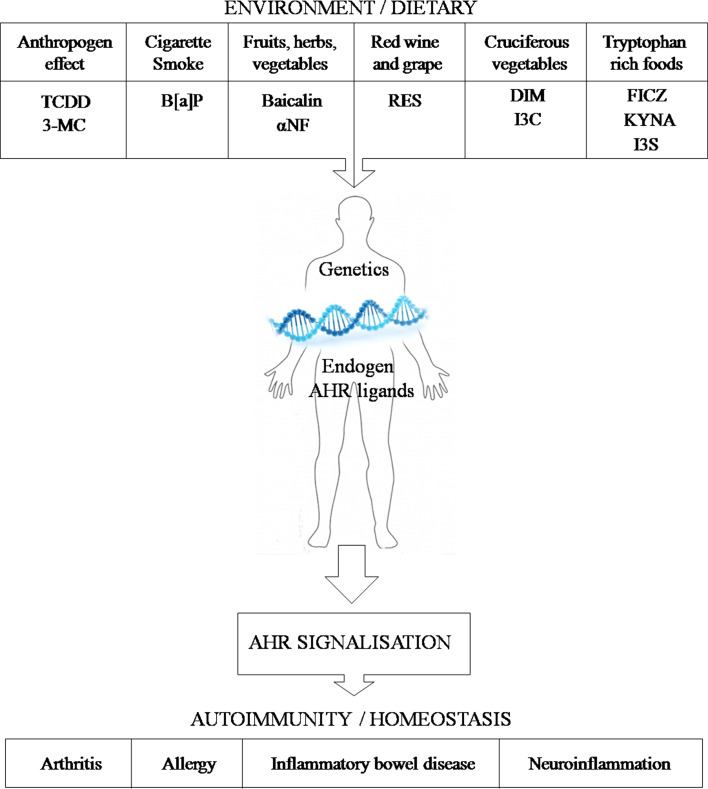
Fig. 4.
The aryl hydrocarbon receptor as a potential regulator of inflammation via synchronize the environmental effects and the dietary compounds which cooperates with genetics
Curcumin
Curcumin a lipophilic polyphenol is well known as a traditional (Ayurvedic) medicine derived from the rhizome of the plant Curcuma longa (turmeric) (Table 1) [160]. AHR shows both agonistic and antagonistic effect in different cells, but acts as antagonist in the presence of HAHs and PAHs in both human breast carcinoma cell line MCF-7 and mouse hepatoma (Hepa-1c1c7) cells [161, 162]. Curcumin has anti-cancer, antioxidant, and anti-inflammatory effects and it may protect the skin, kidneys, brain, heart, liver, and lungs from oxidative effects [163].
Although the direct role of curcumin on the AHR was not studied yet, it inhibited the expression of proinflammatory cytokines (TNFα, IL-1β, IL-6, IL-12, and IFNγ) [164] and the IL-6-induced activation, translocation, and phosphorylation of STAT3 in a dose-dependent manner in head and neck squamous cell carcinoma (HNSCC) cell line [165]. Curcumin stimulated the TCDD-induced AHR translocation to the nucleus and facilitated the heterodimerization with ARNT but it inhibited the binding to DRE in the nucleus in mouse Hepa-1c1c7 cells. These data suggest that curcumin suppresses the transformation of AHR by inhibiting its phosphorylation [161].
Resveratrol (RES)
Resveratrol is a polyphenolic phytoalexin, which can be found in red grape, red wine, cacao, peanut, mulberries, and pines. It has cis and trans isoforms. The trans isomer is more stable and more bioactive. RES may act both as agonist and antagonist of the AHR receptor (Tables 1 and 2). Resveratrol binds to the AHR and promotes its translocation to the nucleus, but does not induce subsequent transactivation as a competitive antagonist in human carcinoma cell line [166]. The anti-inflammatory effects of resveratrol are associated with its effect on the Th17 development [167–169]. The IL-17 production of human peripheral blood mononuclear cells (PBMCs) was stimulated by low concentrations of RES (0,01 µg/ml), while higher concentrations (20 µg/ml) had inhibitory effect. Trans-resveratrol is easily converted into the less stable cis form by the effect of UV radiation unlike polydatin (resveratrol-3-O-β-mono-d-glucoside, POLY) (Tables 1, 2). POLY is a major glucoside derivative of RES which more effectively inhibits the IL-17 production of human PBMCs than RES [167, 168]. STAT3 activity was shown to be modulated by RES in human leukemia cell lines (Jurkat, SUP-B15, and Kasumi-1) [169]. Resveratrol induced the AHR expression in activated T cells in vitro and suppressed the inflammation in mice with induced experimental allergic encephalomyelitis [170]. In these animals, increased IL-17+ IL-10+ cell number, decreased IL-6 expression by macrophage and induced FoxP3 and IL10 expression were observed after oral exposition of RES [170, 171]. Thus, AHR-dependent inhibition of IL-17 production may have important role in the protective effect of moderate alcohol consumption in RA [172].
Flavonoids
Flavonoids are naturally occurring plant metabolites [173]. Their agonistic or antagonistic effect on the AHR largely depends on their actual concentration and on the affected cell type [174]. Many flavonoids have antioxidant, anti-inflammatory, and anti-cancer activity which make them suitable for use in natural therapy [175]. Baicalin is a flavone type flavonoid which originates from the roots of Scutellariae Radix (Tables 1 , 2). Baicalin inhibited the dioxin-induced activation of AHR, by contrast it may serve as a receptor antagonist as well [176, 177]. The Th17 development was inhibited by baicalin both in vivo and in vitro in mice. The IL-17 production and RORγt expression were inhibited, while FoxP3 expression was upregulated by baicalin in vitro. The expression of CCR6 and IL-6 receptor were also suppressed by baicalin in vivo, which may contribute to the decreased infiltration of Th17 cells into the inflamed kidney tissue in lupus-prone MRL/lpr mice [178]. The AHR antagonist alpha-naphthoflavone (α-NF) inhibits the TCDD-induced AHR signalization by forming an inactive complex with AHR (Table 1, 2) [179]. The B[a]Pand 3-MC induced IL-1β mRNA expression of human MH7A cells was also inhibited by α-NF [132].
AHR ligands linkage with autoimmune diseases
Several endogenous and exogenous AHR ligands regulate the differentiation and activation of Th17 cells (Table 2), thereafter the activation of the AHR may shape the immune response in infections, tumors, and autoimmune diseases. AHR appears to represent a link between environmental effects (smoking, dietary compounds, alcohol consumption, air pollutants, etc.) and the immune responses. According to the latest results, AHR has a prominent role in the antimicrobial defense against both acute and chronic bacterial infections. AHR is capable of sensing the bacterial pigments as pathogen-associated molecular patterns and regulates inflammation in the infected organ [180].
Cigarette smoke
Cigarette smoke, a major risk factor for RA [181], contains significant amounts of several AHR ligands including HAHs such as TCDD and PAHs like B[a]P and 3-MC. Cigarette smoking appears to be a risk factor for RA, especially in patients, who carry the shared epitope (SE), representing a well-established gene–environmental interaction [182]. AHR may provide a link between smoking and autoimmunity in RA [118, 183]. The TNF-α induced AHR expression in RA synovial tissue is referring to the potential pathogenic role of AHR activation in RA. TCDD upregulates the expression of proinflammatory cytokines (IL-1β, IL-6, IL-17, IL-22) via AHR activation, suggesting that TCDD exposure contributes to the pathophysiology of RA. On the other hand, the development of Tregs was upregulated by TCDD in murine models of other inflammatory diseases such as inflammatory bowel disease (IBD) or EAE [108, 184]. The expression of IL-1 was also induced by PAHs: B[a]P and 3-MC [132]. Decreased IL-17A gene expression was measured in the synovial samples of smoker RA patients compared to the non-smokers. AHR is mainly expressed in the monocyte-derived early differentiating DCs. B[a]P exposure decreased the toll-like receptor agonist polyinosinic: polycytidylic acid (Polyl:C) induced IL-6 expression in DCs [185].
Norture
AHR appears to have a central role in regulating the inflammatory processes and it has numerous ligands in both food and drink. Tryptophan from nutriment is metabolized via either photo-oxidation or enzymatic pathways, which leads to the generation of different AHR ligands. The IL-17 and IL-22 expressions are induced in mice by a tryptophan photoproduct FICZ, which aggravates the development of EAE [109]. Other tryptophan metabolites such as I3C and its derivative DIM support the generation of Tregs, which suppress the inflammation in EAE [158]. EAE was also suppressed by resveratrol [171]. Interestingly, the IL-17 production of human PBMCs was inhibited by higher resveratrol concentrations [167]. However, the number of IL-17+ IL-10+ producing cells was not decreased in mice during EAE, in addition, FoxP3 expression was upregulated in experimental allergic encephalomyelitis by resveratrol [170, 171]. Collagen induced arthritis in mice is also suppressed by resveratrol (via the inhibition of IL-6 and IL-17 production) [186], by contrast it was aggravated through the inhibition of RORγt expression and STAT3 phosphorylation by I3S [156]. The 3-HK pathway, one of the KYN metabolization pathways, is catalyzed by a Th17-specific enzyme KMO, which is upregulated in Th17 cells. The inhibition of KMO enzyme leads to the aggravation of the autoimmune responses and promotes Th17 differentiation in an AHR-dependent way. The KMO enzyme activation may represent a self-limiting mechanism in the regulation of inflammation [152]. Several flavonoids, such as baicalin, are also AHR ligands and have anti-inflammatory roles through the inhibition of Th17 development and the promotion of Tregs [178].
Conclusion
Numerous endogen and exogen AHR ligands regulate the differentiation and activation of Th17 and Treg cells; thereafter, the activation of the AHR may shape the immune response in infections, tumors, and autoimmune diseases. AHR appears to represent a link between environmental effects (smoking, dietary compounds, alcohol consumption, air pollutants, etc.) and the immune response. Several AHR ligands contribute to the inflammation via the promotion of the Th17 differentiation such as cigarette smoke components (PAHs and HAHs), or tryptophan derivatives (I3S, KYNA). By contrast, other AHR ligands suppress inflammation and may support the Treg development. AHR ligands can be found in the environment as pollutants or in different foods such as flavonoids, fruits, vegetables, and herbs. Some of these directly inhibit the Th17 development like baicalin, resveratrol, I3C and DIM, while others indirectly block the Th17 induced inflammation such as curcumin and αNF. A better understanding of the immunomodulatory effects of the AHR ligands may contribute to the development of new medications of immune-mediated diseases.
Acknowledgments
The study was supported by the Hungarian Scientific Research Fund (Grant: OTKA-PD 108297, OTKA-NN 111023 and NK84043and K111958, MEDINPROT, FP7 COST ME HAD BM1202 and FP7 Marie Curie ITN “DYNANO”).
Abbreviations
- 3-HK
3-Hydroxykynurenine
- 3-MC
3-Methylcholanthrene
- AHR
Aryl hydrocarbon receptor
- AHRR
Nuclear AHR repressor
- AR
Androgen receptor
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- B[a]P
Benzo[alpha]pyrene
- BBB-ECs
Blood–brain barrier endothelial cells
- BD
Bechet’s disease
- bHLH/PAS
Basic helix–loop–helix/Per–Arnt–Sim
- CBP/P300
CREB-binding protein/E1A binding protein p300 protein
- CCL20
Chemokine (C–C motif) ligand 20
- CCR6
Chemokine receptor 6
- CH-223191
2-Methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide
- c-MAF
V-Maf avian musculoaponeuroticfibrosarcoma oncogene homolog
- CNS
Central nervous system
- CXCL1
Chemokine (C-X-C motif) ligand 1
- CYP1A1
Cytochrome P450, family 1, subfamily A, polypeptide 1
- DCs
Dendritic cells
- DIM
3,3′-Diindolylmethane
- E2
17β-Estradiol
- EAE
Experimental autoimmune encephalomyelitis
- eNOS
Endothelial NO synthase
- ER
Estrogen receptor
- ERE
Estrogen response element
- ERα
Estrogen receptor alpha
- FICZ
6-Formylindolo(3,2-b)carbazole
- FoxP3
Forkhead box P3
- FKF
Formylkynurenineformamidase
- G-CSM
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte–monocyte colony-stimulating factor
- HAHs
Halogenated-dioxins and their congeners
- HSP90
Heat shock protein 90
- I3C
Indole-3-carbinol
- I3S
Indoxyl 3-sulfate
- IBD
Inflammatory bowel disease
- IDO1
2,3-Dioxygenase 1
- IDO2
2,3-Dioxygenase 2
- IFNγ
Interferon gamma
- ILC
Innate lymphoid cell
- ILFs
Isolated lymphoid follicles
- IELs
Intraepithelial lymphocytes
- LTi
Lymphoid tissue inducer
- IL-10
Interleukin 10
- IL-1β
Interleukin-1 beta
- ITE
2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester
- KATs
Kynurenine aminotransferases
- KLRB1
Killer cell lectin-like receptor B1
- KMO
Kynurenine 3-monooxygenase
- KYN
Kynurenine
- KYNA
Kynurenic acid
- LXR
Liver X receptor
- LXRα
Liver X receptor alpha
- LXRβ
Liver X receptor beta
- MMP
Matrix metalloproteinase
- MDR1
Multidrug resistance protein 1
- MS
Multiple sclerosis
- NES
Nuclear export signal
- NF-1
Nuclear factor 1
- NFκB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural killer
- NKT
Natural killer T
- NO
Nitric oxide
- p23
Prostaglandin E synthase 3
- PAHs
Polycyclic aromatic hydrocarbons
- PAS
PerArntSim
- PBMCs
Peripherial blood mononuclear cells
- PCBs
Planar polychlorinated biphenyls
- POLY
Polydatin
- PolylC
Toll-like receptor agonist polyinosinic:polycytidylic acid
- PPARs
Peroxisome proliferator-activated receptors
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPRE
PPAR response element
- RA
Rheumatoid arthritis
- RES
Resveratrol
- RORC2
Retinoic acid-related orphan receptor variant 2
- RORγt
RAR-related orphan nuclear receptor gamma t
- SE
Shared epitope
- SLE
Systemic lupus erythematosus
- SOCS3
Suppressor of cytokine signaling 3
- Srebp-1
Sterol regulatory element-binding protein 1
- TAD
Transactivation domain
- T-bet
T-box expressed in T cells
- TBP
TATA-box-binding protein
- TDO
Tryptophan 2,3-dioxygenase
- TGFB1
Tumor growth factor beta 1
- Th17
T helper 17
- Th17(23)
IL-23 induced Th17 cells
- Th17(β)
Conventional Th17 cells
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- TNFα
Tumor necrosis factor alpha
- Tr1
Type 1 regulatory T
- Treg
Regulatory T cell
- UGT1A1
UDP glucuronosyltransferase 1 family, polypeptide A1
- XAP2
Hepatitis B virus X-associated protein
- XREs
Xenobiotic responsive elements
- WY
WY-14643
- α-NF
Alpha-napthoflavone
- γδ T
Gamma delta T cells
References
- 1.Pot C. Aryl hydrocarbon receptor controls regulatory CD4+ T cell function. Swiss Med Wkly. 2012;142:w13592. doi: 10.4414/smw.2012.13592. [DOI] [PubMed] [Google Scholar]
- 2.Allan S. T cells: tuning T cells through the aryl hydrocarbon receptor. Nat Rev Immunol. 2008;8(5):326. doi: 10.1038/nri2319. [DOI] [Google Scholar]
- 3.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 4.Conney AH, Miller EC, Miller JA. Substrate-induced synthesis and other properties of benzpyrene hydroxylase in rat liver. J Biol Chem. 1957;228(2):753–766. [PubMed] [Google Scholar]
- 5.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–250. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett P, Ramsden DB, Williams AC. Complete structural characterisation of the human aryl hydrocarbon receptor gene. Clin Mol Pathol. 1996;49(1):M12–M16. doi: 10.1136/mp.49.1.M12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong L, Ma Q, Whitlock JP., Jr DNA binding by the heterodimeric Ah receptor. Relationship to dioxin-induced CYP1A1 transcription in vivo. J Biol Chem. 1996;271(14):7942–7948. doi: 10.1074/jbc.271.14.7942. [DOI] [PubMed] [Google Scholar]
- 9.Reisz-Porszasz S, Probst MR, Fukunaga BN, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT) Mol Cell Biol. 1994;14(9):6075–6086. doi: 10.1128/MCB.14.9.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270(49):29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433(2):379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Ko HP, Okino ST, Ma Q, Whitlock JP., Jr Transactivation domains facilitate promoter occupancy for the dioxin-inducible CYP1A1 gene in vivo. Mol Cell Biol. 1997;17(7):3497–3507. doi: 10.1128/MCB.17.7.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar MB, Tarpey RW, Perdew GH. Differential recruitment of coactivator RIP140 by Ah and estrogen receptors. Absence of a role for LXXLL motifs. J Biol Chem. 1999;274(32):22155–22164. doi: 10.1074/jbc.274.32.22155. [DOI] [PubMed] [Google Scholar]
- 14.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem. 1999;274(19):13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 15.Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141(1–2):25–40. doi: 10.1016/S0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 16.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273(5):2895–2904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 17.White TE, Gasiewicz TA. The human estrogen receptor structural gene contains a DNA sequence that binds activated mouse and human Ah receptors: a possible mechanism of estrogen receptor regulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochemical and biophysical research communications. 1993;193(3):956–962. doi: 10.1006/bbrc.1993.1718. [DOI] [PubMed] [Google Scholar]
- 18.Swanson HI, Chan WK, Bradfield CA. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270(44):26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- 19.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39(1):103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Shen ES, Whitlock JP., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992;267(10):6815–6819. [PubMed] [Google Scholar]
- 21.Haarmann-Stemmann T, Abel J. The arylhydrocarbon receptor repressor (AhRR): structure, expression, and function. Biol Chem. 2006;387(9):1195–1199. doi: 10.1515/BC.2006.147. [DOI] [PubMed] [Google Scholar]
- 22.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. Structure and expression of the Ah receptor repressor gene. J Biol Chem. 2001;276(35):33101–33110. doi: 10.1074/jbc.M011497200. [DOI] [PubMed] [Google Scholar]
- 23.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper PA, Riddick DS, Okey AB. Regulating the regulator: factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol. 2006;72(3):267–279. doi: 10.1016/j.bcp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochemical and biophysical research communications. 2005;338(1):311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- 26.Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, Weiner HL. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 2011;6(8):e23618. doi: 10.1371/journal.pone.0023618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen PH, Chang H, Chang JT, Lin P. Aryl hydrocarbon receptor in association with RelA modulates IL-6 expression in non-smoking lung cancer. Oncogene. 2012;31(20):2555–2565. doi: 10.1038/onc.2011.438. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 29.Apelgren LD, Bailey DL, Fouts RL, Short L, Bryan N, Evans GF, Sandusky GE, Zuckerman SH, Glasebrook A, Bumol TF. The effect of a selective estrogen receptor modulator on the progression of spontaneous autoimmune disease in MRL lpr/lpr mice. Cell Immunol. 1996;173(1):55–63. doi: 10.1006/cimm.1996.0251. [DOI] [PubMed] [Google Scholar]
- 30.Svenson JL, EuDaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin Immunol. 2008;128(2):259–268. doi: 10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268(3):132–138. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Matthews J, Gustafsson JA. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16(7):807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 34.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46(2):279–303. doi: 10.1016/0041-008X(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 35.Abdelrahim M, Ariazi E, Kim K, Khan S, Barhoumi R, Burghardt R, Liu S, Hill D, Finnell R, Wlodarczyk B, Jordan VC, Safe S. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res. 2006;66(4):2459–2467. doi: 10.1158/0008-5472.CAN-05-3132. [DOI] [PubMed] [Google Scholar]
- 36.Ricci MS, Toscano DG, Mattingly CJ, Toscano WA., Jr Estrogen receptor reduces CYP1A1 induction in cultured human endometrial cells. J Biol Chem. 1999;274(6):3430–3438. doi: 10.1074/jbc.274.6.3430. [DOI] [PubMed] [Google Scholar]
- 37.Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol Cell Biol. 2003;23(6):1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohtake F, Fujii-Kuriyama Y, Kawajiri K, Kato S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J Steroid Biochem Mol Biol. 2011;127(1–2):102–107. doi: 10.1016/j.jsbmb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144(8):3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 40.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 41.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 42.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol. 1994;14(10):7025–7035. doi: 10.1128/MCB.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272(6):3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 44.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Investig. 2011;121(2):658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, Hombach V, Marx N. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006;26(5):1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- 46.Fallone F, Villard PH, Decome L, Seree E, Meo M, Chacon C, Durand A, Barra Y, Lacarelle B. PPAR alpha activation potentiates AhR-induced CYP1A1 expression. Toxicology. 2005;216(2–3):122–128. doi: 10.1016/j.tox.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. Evidence that peroxisome proliferator-activated receptor alpha is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem. 2003;278(7):4467–4473. doi: 10.1074/jbc.M211261200. [DOI] [PubMed] [Google Scholar]
- 48.Seree E, Villard PH, Pascussi JM, Pineau T, Maurel P, Nguyen QB, Fallone F, Martin PM, Champion S, Lacarelle B, Savouret JF, Barra Y. Evidence for a new human CYP1A1 regulation pathway involving PPAR-alpha and 2 PPRE sites. Gastroenterology. 2004;127(5):1436–1445. doi: 10.1053/j.gastro.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Chen CL, Brodie AE, Hu CY. CCAAT/enhancer-binding protein beta is not affected by tetrachlorodibenzo-p-dioxin (TCDD) inhibition of 3T3-L1 preadipocyte differentiation. Obes Res. 1997;5(2):146–152. doi: 10.1002/j.1550-8528.1997.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 50.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochemical and biophysical research communications. 1999;256(3):462–468. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 51.Ghotbaddini M, Powell JB. The AhR Ligand, TCDD, Regulates Androgen Receptor Activity Differently in Androgen-Sensitive versus Castration-Resistant Human Prostate Cancer Cells. Int J Environ Res Public Health. 2015;12(7):7506–7518. doi: 10.3390/ijerph120707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274(1):510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 53.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19(48):5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 54.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19(6):652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunimaladevi I, Savan R, Sakai M. Identification, cloning and characterization of interleukin-17 and its family from zebrafish. Fish Shellfish Immunol. 2006;21(4):393–403. doi: 10.1016/j.fsi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 58.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Attur MG, Patel RN, Abramson SB, Amin AR. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 1997;40(6):1050–1053. doi: 10.1002/art.1780400609. [DOI] [PubMed] [Google Scholar]
- 60.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol (Baltimore, Md: 1950) 178(4):2229–2240 [DOI] [PubMed]
- 61.Kim K-W, Kim H-R, Park J-Y, Park J-S, Oh H-J, Woo Y-J, Park M-K, Cho M-L, Lee S-H. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64(4):1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 62.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 63.Pelletier M, Bouchard A, Girard D (2004) In vivo and in vitro roles of IL-21 in inflammation. J Immunol (Baltimore, Md: 1950) 173(12):7521–7530 [DOI] [PubMed]
- 64.Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A. Production and functions of IL-17 in microglia. J Neuroimmunol. 2008;194(1–2):54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bogaert S, Laukens D, Peeters H, Melis L, Olievier K, Boon N, Verbruggen G, Vandesompele J, Elewaut D, De Vos M. Differential mucosal expression of Th17-related genes between the inflamed colon and ileum of patients with inflammatory bowel disease. BMC Immunology. 2010;11(1):1–11. doi: 10.1186/1471-2172-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science (New York, NY) 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW, Yang M. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J Clin Immunol. 2010;30(2):221–225. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Yang J, Gao Y, Guo W. Th17 Immunity in Patients with Allergic Asthma. Int Arch Allergy Immunol. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 70.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 71.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT (2006) Transforming growth factor-[beta] induces development of the TH17 lineage. Nature 441(7090):231–234. doi:http://www.nature.com/nature/journal/v441/n7090/suppinfo/nature04754_S1.html [DOI] [PubMed]
- 72.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 75.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR (2007) IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8 (9):967–974. doi:http://www.nature.com/ni/journal/v8/n9/suppinfo/ni1488_S1.html [DOI] [PubMed]
- 78.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanier LL, Chang C, Phillips JH (1994) Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol (Baltimore, Md: 1950) 153(6):2417–2428 [PubMed]
- 83.Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, Querci V, Fambrini M, Liotta F, Levings MK, Maggi E, Cosmi L, Romagnani S, Annunziato F. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40(8):2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 84.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1 beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 85.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56(9):2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 87.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39(1):207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 88.Li Y, David EA, Clare B-A, William DH, Estelle B, Mohamed O, Vijay KK, David AH. IL-21 and TGF-beta are required for differentiation of human TH17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 90.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th17 cells: are they different from murine Th17 cells? Eur J Immunol. 2009;39(3):637–640. doi: 10.1002/eji.200839050. [DOI] [PubMed] [Google Scholar]
- 92.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 93.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science (New York, NY) 2004;305(5681):248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 94.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science (New York, NY) 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 95.Medvedev A, Chistokhina A, Hirose T, Jetten AM. Genomic structure and chromosomal mapping of the nuclear orphan receptor ROR gamma (RORC) gene. Genomics. 1997;46(1):93–102. doi: 10.1006/geno.1997.4980. [DOI] [PubMed] [Google Scholar]
- 96.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97(18):10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH (2007) Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol (Baltimore, Md: 1950) 178(8):4901–4907 [DOI] [PubMed]
- 98.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 99.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19(6):400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirahara K, Ghoreschi K, Laurence A, Yang XP, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21(6):425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Unutmaz D. RORC2: the master of human Th17 cell programming. Eur J Immunol. 2009;39(6):1452–1455. doi: 10.1002/eji.200939540. [DOI] [PubMed] [Google Scholar]
- 104.Crome SQ, Wang AY, Kang CY, Levings MK. The role of retinoic acid-related orphan receptor variant 2 and IL-17 in the development and function of human CD4+ T cells. Eur J Immunol. 2009;39(6):1480–1493. doi: 10.1002/eji.200838908. [DOI] [PubMed] [Google Scholar]
- 105.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R (2010) Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol (Baltimore, Md: 1950) 185(1):679–687. doi:10.4049/jimmunol.1000366 [DOI] [PubMed]
- 106.Frericks M, Meissner M, Esser C. Microarray analysis of the AHR system: tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol. 2007;220(3):320–332. doi: 10.1016/j.taap.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 107.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 109.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191):106–109. doi:http://www.nature.com/nature/journal/v453/n7191/suppinfo/nature06881_S1.html [DOI] [PubMed]
- 110.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23(3):159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 112.Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2010;107(13):5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ho PP, Steinman L. The aryl hydrocarbon receptor: a regulator of Th17 and Treg cell development in disease. Cell Res. 2008;18(6):605–608. doi: 10.1038/cr.2008.63. [DOI] [PubMed] [Google Scholar]
- 114.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science (New York, NY) 1995;268(5211):722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 116.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993;44(5):911–917. [PubMed] [Google Scholar]
- 117.Jiang YZ, Wang K, Fang R, Zheng J. Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem. 2010;58(8):679–685. doi: 10.1369/jhc.2010.955955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kobayashi S, Okamoto H, Iwamoto T, Toyama Y, Tomatsu T, Yamanaka H, Momohara S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford) 2008;47(9):1317–1322. doi: 10.1093/rheumatology/ken259. [DOI] [PubMed] [Google Scholar]
- 119.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 120.Wei P, Hu GH, Kang HY, Yao HB, Kou W, Liu H, Hong SL. Increased aryl hydrocarbon receptor expression in patients with allergic rhinitis. QJM. 2014;107(2):107–113. doi: 10.1093/qjmed/hct188. [DOI] [PubMed] [Google Scholar]
- 121.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, Saurat JH, Roosnek E, Chizzolini C. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40(9):2450–2459. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 122.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science (New York, NY) 2011;334(6062):1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 123.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 124.Hooper LV. You AhR what you eat: linking diet and immunity. Cell. 2011;147(3):489–491. doi: 10.1016/j.cell.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 125.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 126.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 127.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+ IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207(2):281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the deichmann lecture. International Congress of Toxicology-XI. Toxicol Sci. 2007;98(1):5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI (2005) Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol (Baltimore, Md: 1950) 175(7):4184–4188 [DOI] [PubMed]
- 132.Tamaki A, Hayashi H, Nakajima H, Takii T, Katagiri D, Miyazawa K, Hirose K, Onozaki K. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol Pharm Bull. 2004;27(3):407–410. doi: 10.1248/bpb.27.407. [DOI] [PubMed] [Google Scholar]
- 133.Sutter TR, Guzman K, Dold KM, Greenlee WF (1991) Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science (New York, NY) 254(5030):415–418 [DOI] [PubMed]
- 134.Yang JH. Expression of dioxin-responsive genes in human endometrial cells in culture. Biochem Biophys Res Commun. 1999;257(2):259–263. doi: 10.1006/bbrc.1999.0451. [DOI] [PubMed] [Google Scholar]
- 135.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69(6):1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 136.Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci. 2010;117(2):393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97(2):779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, Mathew LK, Sengupta S, Kerkvliet NI, Tanguay RL, Kolluri SK (2010) The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PLoS One 5(10). doi:10.1371/journal.pone.0013128 [DOI] [PMC free article] [PubMed]
- 139.Baban B, Liu JY, Mozaffari MS. Aryl hydrocarbon receptor agonist, leflunomide, protects the ischemic-reperfused kidney: role of Tregs and stem cells. Am J Physiol Regul Integr Comp Physiol. 2012;303(11):R1136–R1146. doi: 10.1152/ajpregu.00315.2012. [DOI] [PubMed] [Google Scholar]
- 140.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99(23):14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107(48):20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang C, Ye Z, Kijlstra A, Zhou Y, Yang P. Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells. Clin Exp Immunol. 2014;177(2):521–530. doi: 10.1111/cei.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei P, Hu GH, Kang HY, Yao HB, Kou W, Liu H, Zhang C, Hong SL. An aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress the Th17 response in allergic rhinitis patients. Lab Invest. 2014;94(5):528–535. doi: 10.1038/labinvest.2014.8. [DOI] [PubMed] [Google Scholar]
- 144.Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278(1):88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 145.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262(32):15422–15427. [PubMed] [Google Scholar]
- 146.Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85(2):935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 147.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJD, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 148.Kincses ZT, Toldi J, Vecsei L. Kynurenines, neurodegeneration and Alzheimer’s disease. J Cell Mol Med. 2010;14(8):2045–2054. doi: 10.1111/j.1582-4934.2010.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol (Baltimore, Md: 1950) 185(6):3190–3198. doi:10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed]
- 150.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alberati-Giani D, Cesura AM, Broger C, Warren WD, Rover S, Malherbe P. Cloning and functional expression of human kynurenine 3-monooxygenase. FEBS Lett. 1997;410(2–3):407–412. doi: 10.1016/S0014-5793(97)00627-3. [DOI] [PubMed] [Google Scholar]
- 152.Stephens GL, Wang Q, Swerdlow B, Bhat G, Kolbeck R, Fung M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur J Immunol. 2013;43(7):1727–1734. doi: 10.1002/eji.201242779. [DOI] [PubMed] [Google Scholar]
- 153.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci. 2009 doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49(2):393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Banoglu E, Jha GG, King RS. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur J Drug Metab Pharmacokinet. 2001;26(4):235–240. doi: 10.1007/BF03226377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hwang S-J, Hwang Y-J, Yun M-O, Kim J-H, Oh G-S, Park J-H. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol Lett. 2013;220(2):109–117. doi: 10.1016/j.toxlet.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 157.Schroecksnadel K, Kaser S, Ledochowski M, Neurauter G, Mur E, Herold M, Fuchs D. Increased degradation of tryptophan in blood of patients with rheumatoid arthritis. J Rheumatol. 2003;30(9):1935–1939. [PubMed] [Google Scholar]
- 158.Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol. 2013;169(6):1305–1321. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aggarwal BB, Ichikawa H (2005) Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell cycle (Georgetown, Tex) 4(9):1201–1215 [DOI] [PubMed]
- 160.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 161.Nishiumi S, K-i Yoshida, Ashida H. Curcumin suppresses the transformation of an aryl hydrocarbon receptor through its phosphorylation. Arch Biochem Biophys. 2007;466(2):267–273. doi: 10.1016/j.abb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 162.Ciolino HP, Daschner PJ, Wang TTY, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56(2):197–206. doi: 10.1016/S0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 163.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39(1):41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 165.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006;119(6):1268–1275. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 166.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56(4):784–790. [PubMed] [Google Scholar]
- 167.Lanzilli G, Cottarelli A, Nicotera G, Guida S, Ravagnan G, Fuggetta MP. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation. 2012;35(1):240–248. doi: 10.1007/s10753-011-9310-z. [DOI] [PubMed] [Google Scholar]
- 168.Fabris S, Momo F, Ravagnan G, Stevanato R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys Chem. 2008;135(1–3):76–83. doi: 10.1016/j.bpc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 169.Li T, Wang W, Chen H, Li T, Ye L. Evaluation of anti-leukemia effect of resveratrol by modulating SATA3 signaling. Int Immunopharmacol. 2010;10(1):18–25. doi: 10.1016/j.intimp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 170.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72(6):1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Imler TJ, Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+ IL-10+ T cells, CD4(−) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9(1):134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 172.Lu B, Solomon DH, Costenbader KH, Keenan BT, Chibnik LB, Karlson EW. Alcohol consumption and markers of inflammation in women with preclinical rheumatoid arthritis. Arthritis Rheum. 2010;62(12):3554–3559. doi: 10.1002/art.27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hollman PC, Katan MB (1997) Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharm 51(8):305–310 [DOI] [PubMed]
- 174.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111(16):1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, Pizzolatti MG, Silva FR. Flavonoids: prospective drug candidates. Mini Rev Med Chem. 2008;8(13):1429–1440. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 176.Kasai A, Hiramatsu N, Hayakawa K, Yao J, Kitamura M. Blockade of the dioxin pathway by herbal medicine Formula Bupleuri Minor: identification of active entities for suppression of AhR activation. Biol Pharm Bull. 2008;31(5):838–846. doi: 10.1248/bpb.31.838. [DOI] [PubMed] [Google Scholar]
- 177.Ashida H (2000) Suppressive effects of flavonoids on dioxin toxicity. BioFactors (Oxford, England) 12(1–4):201–206 [DOI] [PubMed]
- 178.Yang J, Yang X, Chu Y, Li M. Identification of Baicalin as an immunoregulatory compound by controlling T(H)17 cell differentiation. PLoS One. 2011;6(2):e17164. doi: 10.1371/journal.pone.0017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gasiewicz TA, Rucci G. Alpha-naphthoflavone acts as an antagonist of 2,3,7, 8-tetrachlorodibenzo-p-dioxin by forming an inactive complex with the Ah receptor. Mol Pharmacol. 1991;40(5):607–612. [PubMed] [Google Scholar]
- 180.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, Skrahina T, Guhlich-Bornhof U, Klemm M, Koehler AB, Bandermann S, Goosmann C, Mollenkopf HJ, Hurwitz R, Brinkmann V, Fillatreau S, Daffe M, Tummler B, Kolbe M, Oschkinat H, Krause G, Kaufmann SH. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512(7515):387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 181.Baka Z, Buzas E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther. 2009;11(4):238. doi: 10.1186/ar2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene–environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50(10):3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 183.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 184.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 2011;120(1):68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kazantseva MG, Highton J, Stamp LK, Hessian PA. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res Ther. 2012;14(5):R208. doi: 10.1186/ar4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71(1):129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]