Abstract
l-Dopa continues to be the gold drug in Parkinson’s disease (PD) treatment from 1967. The failure to translate successful results from preclinical to clinical studies can be explained by the use of preclinical models which do not reflect what happens in the disease since these induce a rapid and extensive degeneration; for example, MPTP induces a severe Parkinsonism in only 3 days in humans contrasting with the slow degeneration and progression of PD. This study presents a new anatomy and develops preclinical model based on aminochrome which induces a slow and progressive dysfunction of dopaminergic neurons. The unilateral injection of aminochrome into rat striatum resulted in (1) contralateral rotation when the animals are stimulated with apomorphine; (2) absence of significant loss of tyrosine hydroxylase-positive neuronal elements both in substantia nigra and striatum; (3) cell shrinkage; (4) significant reduction of dopamine release; (5) significant increase in GABA release; (6) significant decrease in the number of monoaminergic presynaptic vesicles; (7) significant increase of dopamine concentration inside of monoaminergic vesicles; (8) significant increase of damaged mitochondria; (9) significant decrease of ATP level in the striatum (10) significant decrease in basal and maximal mitochondrial respiration. These results suggest that aminochrome induces dysfunction of dopaminergic neurons where the contralateral behavior can be explained by aminochrome-induced ATP decrease required both for anterograde transport of synaptic vesicles and dopamine release. Aminochrome could be implemented as a new model neurotoxin to study Parkinson’s disease.
Keywords: Preclinical model; Dopamine, neurodegeneration; Drugs; Mitochondria; Presynaptic vesicles
Introduction
l-Dopa is an old drug which was introduced to Parkinson’s disease treatment for five decades ago. However, l-dopa continues to be the most important and successful drug despite the severe side effects observed after 4–6 years of use [1]. The failure to translate successful preclinical result to clinical studies and new therapies [2, 3] is probably due to lack of preclinical models that reflect what happens in the degenerative process in Parkinson’s disease where dopaminergic neurons containing neuromelanin are lost. Therefore, successful preclinical studies cannot be translated to new PD treatments [2, 3]. Actually, the most common preclinical models used for Parkinson’s disease use exogenous neurotoxins such as 6-hydroxydopamine, MPTP and rotenone [4]. The question is whether these exogenous neurotoxin are able to mimic what happens in the degenerative processes of the nigrostriatal system in Parkinson’s disease. All these neurotoxins induce a rapid and extensive loss of dopaminergic neurons contrasting with the very slow degenerative process in the disease that take years to degenerate the 60 % of these neurons to develop motor symptoms. For example, MPTP was found to induce a severe Parkinsonism in only 3 days after drug addicts injected themselves daily the pethidine drug containing MPTP [4], suggesting that the neurotoxin involved in the degenerative process of nigrostriatal system in Parkinson’s disease cannot be of exogenous origin.
Although, the neurotoxin identity and the mechanisms responsible for the loss of dopaminergic neurons containing neuromelanin in the nigrostriatal system are still unknown, there is a general agreement in the scientific community that the loss of dopaminergic neurons containing neuromelanin is related to mitochondria dysfunction, aggregation of alpha synuclein to neurotoxic oligomers, protein degradation dysfunction, oxidative stress, neuroinflammation and endoplasmic reticulum stress [5–9]. Interestingly, the endogenous neurotoxin, aminochrome, formed in dopaminergic neurons containing neuromelanin during dopamine oxidation has been reported to be directly involved in five of these six mechanisms related to the degenerative process in Parkinson’s disease with the exception of neuroinflammation [10–15]. In our opinion, it is time to introduce another preclinical model for Parkinson disease based on the endogenous neurotoxin aminochrome to decipher the molecular mechanism of the disease. We cannot discard the possibility that other endogenous neurotoxin can be responsible for the loss of dopaminergic neurons containing neuromelanin but aminochrome seems to be a good candidate due to its relationship with the formation of neuromelanin. Therefore, the aim of this study was to validate aminochrome as a new preclinical model for Parkinson’s disease that does not induce massive loss of dopaminergic neurons but only a dysfunction of dopaminergic neurons.
Materials and methods
Chemicals
CM-Sephadex C50-100, DEAE Sephadex 50-120, Sephadex G-25, dopamine, tyrosinase (EC 1.14.18.1) from mushroom were from Sigma-Aldrich (St. Louis, MO, USA); apomorphine (Apo-Go, Genes Pharmaceutical). Antibodies for tyrosine hydroxylase were from US Biological (TH, Rabbit polyclonal antibody, T9237-13).
Synthesis and purification of aminochrome
For aminochrome synthesis, dopamine 5 mM and 10 ng of tyrosinase were incubated in 1 ml of 2-(N-morpholino) ethanesulfonic acid hemisodium salt, 4-morpholineethanesulfonic acid hemisodium salt (MES) buffer 25 mM pH 6.0 for 10 min at room temperature to purify formed aminochrome, and the incubation solution was loaded into a column of 3 × 0.4 cm resin CM-Sephadex C-50-120 which was eluted with 3 ml of 25 mM MES buffer pH 6.0 [24]. We used the pH 6.0 to prevent aminochrome polymerization to neuromelanin. The concentration of aminochrome was determined using the molar extinction coefficient 3058 M−1cm−1 [16].
Animals
Adult Sprague–Dawley rats, weighing 220–250 g at the time of striatal stereotaxical injection and 250–350 g at the time of behavioral experiments, were housed in groups of six in a well-ventilated, temperature (22 ± 1 °C)-controlled environment, under a 12:12 light–dark cycle (lights on from 08.00 to 20.00 h) with free access to food and water. The minimum number of animals and duration of observation required to obtain consistent data were employed. All experiments were conducted in accordance with international standards of animal welfare recommended by the Society for Neuroscience (Handbook for the Use of Animals in Neuroscience Research, 1997). The local Animal Care and Use Committee of Universidad de Chile approved the experimental protocols.
Stereotaxic injection
According to a previous protocol described [17], for the left striatal injection the rats were anesthetized with sodium pentobarbital (30 mg/kg) and placed in a David Kopf stereotaxic frame. Stereotaxic coordinates relative to bregma for left striatum used were AP −0.48 mm, L −2.5 mm, V −4.5 mm according to Paxinos and Watson. A total of 1.6 nmol aminochrome in 4 μl solution was injected into the left side of striatum with a rate of 4 µl/min using a 5-µl Hamilton microsyringe. To minimize the possibility of back flow, the needle remained in position for an additional minute after the injection. After surgery the animals were allowed to recover for 7 days before conducting the rotational behavior test induced by 0.5 mg/kg sc apomorphine. As positive control (C+) we injected 32 nmol 6-hydroxydopamine dissolved in physiological saline containing 0.1 % ascorbic acid. For control group we injected the buffer used for aminochrome synthesis (25 mM MES pH 6.0) at the same rate of injection and we added a third control group, the sham-operated group; this third control group underwent the same surgical procedure but with an empty microsyringe.
Rotational behavior
The rotational model is based on a unilateral lesion of the nigrostriatal dopamine system with the neurotoxin 6-hydroxydopamine. Rats with this lesion of the nigrostriatal dopamine pathway show a postural deviation, which can be expressed as a contralateral rotation behavior when they are stimulated with apomorphine (0.5 mg/kg, sc). In our work, the rotational behavior was measured at 7, 14, 21 and 28 days after the intrastriatal injection of 1.6 mmol of aminochrome, for this measure each rat was placed in an LE 902 Rotometer (Panlab, Barcelona, Spain) connected to an LE 3806 multicounter (Panlab, Barcelona, Spain), to evaluate the presence of motor asymmetry. For rotational behavior 1 ml/kg body weight apomorphine was injected. Rotational behavior testing was carried out allowing free movement of the rat during 60 min. The sensor of rotations measured and provided a separate output for continuous movement in the clockwise or counterclockwise direction. Results were expressed as the mean total number of complete 360° turns in either direction during the entire period of observation.
Immunohistochemistry
When the behavioral study was complete, rats were killed with an overdose of sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9 % saline followed by 4 % buffered formaldehyde solution at 4 °C. The brains were removed, post-fixed in a 4 % buffered formaldehyde solution for 24 h at 4 °C and cryopreserved in 30 % sucrose. All samples were cut into 30-μm-thick frozen sections in a cryostat (Leica CM1800, Leica Microsystem Inc., NY, USA). Tissue was stored in brain antifreeze solution (phosphate buffer pH 7.4 containing 30 % glycerol and 30 % ethylene glycol) and maintained at 4 °C until further use. Coronal sections were processed for tyrosine hydroxylase (TH) immunostaining at the level of the striatum or the substantia nigra. For the immunohistochemistry, free-floating sections from striatum and substantia nigra were washed in a solution of PBS 0.1 M + Triton X-100 0.15 % and then incubated for 30 min with 0.3 % H2O2 at room temperature. After incubation, samples were blocked in 5 % BSA in 0.1 M PBS containing 0.15 % Triton X-100, rinsed and incubated overnight at room temperature with 1:500 dilution of rabbit anti-TH antibody (US Biological) anti-Rabbit polyclonal antibody, T9237-13, in 0.15 % Triton X-100 in 0.1 M PBS. After washing, the sections were incubated with 1:200 dilution of biotinylated goat anti-rabbit secondary antibody (Vector laboratories, Burlingame, CA, USA) in 0.15 % Triton X-100 in 0.1 M PBS during 1 h followed by an incubation with avidin–biotin–peroxidase complex diluted 1/125 during 1 h (Vectastain, ABC kit, Vector laboratories). Finally, the substrate H2O2 30 % was added in the presence of DAB (0.5 mg/ml) to visualize the antigen–antibody complex.
Quantification
The degree of dopaminergic nigrostriatal lesion in striatum was expressed as the percentage of reduction in tyrosine hydroxylase (TH)-positive fiber density in the injected side as compared to the contralateral non-injected side. To estimate the specific TH staining density, the optical density readings were corrected for nonspecific background density, as measured from the completely denervated parts of the striatum in the animals. The degree of dopaminergic nigrostriatal lesion in substantia nigra was expressed as the reduction in the number TH-positive cells.
In vivo microdialysis
Fourteen days after intrastriatal injection into the left striatum of 1.6 nmol aminochrome, microdialysis experiments were performed using a protocol previously described [18]. Rats were deeply anesthetized with choral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus (Stoelting, Wood Dale, MA, USA). Corporal temperature of the rats was maintained at 37 °C with an electrical blanket controlled by a thermostat. A quarter of the initial dose of choral hydrate was given every hour to maintain the rat anesthetized during the course of the experiments. Concentric brain microdialysis probes (2 mm membrane; CMA 11 model, CMA Carniege Medicine) were implanted in the animals left striatum. The following coordinates, according to the atlas of Paxinos and Watson were used: AP −0.48 mm, L −2.5 mm, V −4.5 mm. The microdialysis probe was perfused with Krebs–Ringer’s phosphate buffer (KRP, pH 7.4) at a flow rate of 2 μl/min, using a Harvard infusion pump (Model 22; Dover, MA, USA). After stabilization period of 90 min, eight perfusion samples of 10 min each were collected in 3 μl of perchloric acid (0.2 N). After the third basal fraction, the KRP solution was changed for 10 min to 70 mM K+KRP. Between 40 and 80 min, the KRP solution was again perfused through the microdialysis probe. All perfusion samples were maintained on ice during the experiment and stored at −80 °C until analysis. Rats were killed at the end of the experiment.
Dopamine determination
HPLC-electrochemical determination of dopamine was performed as described previously [18]. Briefly, 10 μl of dialysates were injected into an HPLC system with the following configuration: an isocratic pump (model PU-2080 Plus, Jasco Co. Ltd., Tokyo, Japan), a UniJet microbore column (MF-8949, BAS, West Lafayette, IN, USA) and an amperometric detector (set at 650 mV, 0.5 nA; model LC-4C). The mobile phase, containing 0.1 M NaH2PO4, 1.2 mM 1-octanesulfonic acid, 1 mM EDTA and 4.0 % (v/v), CH3CN (pH adjusted to 2.8) was pumped at a flow rate of 80 μl/min. The retention time for DA was 7 min and the detection limit was 0.1 fmol/μl.
GABA and glutamate determination
HPLC-fluorometric determination of GABA and glutamate were performed as described previously (50). Briefly, 10 μl of dialysis perfusate were mixed with 10 μl of 3× distillated H2O, 4 μl of borate buffer (pH 10.8), and then the mixture was derivatized by adding 4 μl of fluorogenic reagent (20 mg of orthophthaldehyde and 10 μl of β-mercaptoethanol in 5 ml of ethanol). At 90 s after derivatization, samples were injected into an HPLC system with the following configuration: quaternary gradient pump (Jasco Co. Ltd., Tokyo, Japan), a C-18 reverse phase column (Kromasil®; Eka Chemicals, Bohus, Sweden), and a fluorescence detector (Jasco Co. Ltd.). A mobile phase containing 0.1 M NaH2PO4 and 26 % CH3CN (pH 5.7) was pumped for 4 min. Thereafter, a continuous gradient of CH3CN (14.5–50.0 %) was used as mobile phase for 16 min. From 16 to 18 min, mobile phase was maintained at a concentration of 50 % CH3CN to remove all fluorescent products of the column. Finally, from 18 to 22 min mobile phase was returned to a final concentration of 14.5 % CH3CN (pH 5.7). The flow rate of the mobile phase was set at 1.2 ml/min and the retention time for GABA was 15.7 min. Dialysate samples were analyzed by comparing the peak area and elution time with reference standards. The fluorometer filters were configured for an excitation wavelength of 340–380 nm and an emission wavelength of 418–700 nm. Under these experimental conditions, the retention time for glutamate was 2 and 11 min for GABA.
Transmission electron microscopy
Seven days after intrastriatal injection into the left striatum of 1.6 nmol aminochrome and rotational behavior the brains of three aminochrome-treated and three control animals were used for transmission electron microscopical studies. The tissue was dissected and small pieces were fixed in 2 % glutaraldehyde + 1 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at room temperature for 30 min and stored at 4 °C. Specimens were rinsed in 0.1 M phosphate buffer, pH 7.4 and post-fixed in 2 % osmium tetroxide 0.1 M phosphate buffer, pH 7.4 at 4 °C for 2 h, dehydrated in ethanol followed by acetone and embedded in LX-112 (Ladd, Burlington, Vermont, USA). Semi-thin sections (approximately 0.5 µm) were cut and stained with toluidine blue O and used for light microscopic analysis. Ultrathin sections (approximately 50–60 nm) were cut at a Leica EM UC 6 (Leica, Wien, Austria) and contrasted with uranyl acetate followed by lead citrate and examined in a Tecnai 12 Spirit Bio TWIN transmission electron microscope (FEI company, Eindhoven, The Netherlands) at 100 kV. Digital images were taken by using a Veleta camera (Olympus Soft Imaging Solutions, GmbH, Münster, Germany). The quantification of presynaptic vesicles was performed by counting the number of presynaptic vesicles in ten randomly selected images in the striatum of aminochrome-treated and control animals. The quantification of damaged mitochondria (with loss of outer membrane integrity or cristae) was performed by counting damaged mitochondria per nm2 in the substantia nigra or per µm2 in the striatum of aminochrome-treated and control animals in eight randomly selected images.
Synaptic vesicles isolation and dopamine determination
The isolation of synaptic vesicle was performed using the synaptic vesicles isolation kit (Sigma-Aldrich #SV0100). The isolation procedure involves rat striatum homogenization, hypo-osmotic lysis of the synaptosomes, and differential centrifugation followed by an OptiPrep density gradient. Antibodies against synaptophysin, a marker of synaptic vesicles, were used to follow the enrichment procedure. The isolated vesicles were quantified by the method of Lowry. The synaptic vesicles were lysed using 100 µl buffer MES 25 mM pH 6.0 and Triton X-100 0.01 % to measure dopamine concentration inside the synaptic vesicles. The released dopamine was oxidized to aminochrome by adding 0.3 mg/ml tyrosinase and incubating 1 h at room temperature. The absorbance was measured at 475 nm and the concentration of aminochrome was determined using the molar extinction coefficient 3058 M−1 cm−1 [16].
Determination of oxygen consumption rate (OCR)
For the functional mitochondrial analysis [19], acute section of rat intrastriatal injected with aminochrome (14 days) was obtained and processed. Briefly, animals were anesthetized with sodium pentobarbital (100 mg/kg) and thereafter decapitated. Brains were immediately dissected to obtain only striatum and substantia nigra and with a tissue punch of 1 mm we obtained the desired section diameter for analysis in XFe96 Analyzer plates (Seahorse Bioscience). Before running the assay the sections dissected were placed into a 50-ml beaker at 37 °C with CSF (120 mM NaCl, 3.5 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2 hexahydrate, 0.4 mM KH2PO4 and 5 mM HEPES with 10 mM added on day of experiment, pH to 7.4 using 10 N NaOH). Substantia nigra and striatal tissue were incubated at 37 °C throughout the entire experimental period of sectioning. The incubation mixture was replaced with Seahorse assay medium supplemented with 1 mM sodium pyruvate and 1 mM GlutaMAX™ and the pH was adjusted to 7.4. The following compounds were sequentially added to the incubation mixture: 10 mM glucose, 1 µM oligomycin, which was injected to differentiate the ATP-linked respiration (oligomycin-sensitive fraction) from the proton leak; 1 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), membrane protonophore and shows maximal OCR; 1 μM rotenone, which blocks complex I and shows NADH-driven OCR together with 1 µM antimycin A which a block complex III. There were 3–4 wells per treatment group. We measured OCR at three time points during basal respiration and after each injection using a Seahorse XFe96 Flux Analyzer (Seahorse Biosciences).
Determination of ATP level in striatum
To quantify ATP in rats injected with aminochrome, we selected a group of rats after 14 days of intrastriatal injection of aminochrome. We measured the ATP level in the striatum of animals injected with aminochrome. Freshly isolated striatum were homogenized with 1 ml of homogenization buffer (ice-cold phenol-TE; 10 mM Tris–HCl, pH 8.0, and 1 mM EDTA-saturated phenol). After tissue homogenization, we transferred the homogenized sample into 2.0-ml microtubes containing 200 μl of chloroform and 150 μl de-ionized water. The homogenized sample was shaken for 20 s and centrifuged at 10,000 g for 5 min at 4 °C. After the centrifugation, an aliquot of the supernatant was diluted 1000-fold and only 10 μl was used for Luciferin–Luciferase assay (Enliten® ATP assay System, Promega,Madison, WI, USA).
Data expression and statistical analysis
In all cases, values are presented as mean ± SEM. Statistically analysis was performed using the GraphPad Prims 5.0 (GraphPad Software, Inc., Chicago, IL, USA). For behavior analysis in the diverse groups of treatment, a one-way ANOVA was used followed by Bonferroni multiple comparison test to detect difference between groups and days of treatment. In the case of microdialysis studies, fentomoles of dopamine and picomoles of GABA and glutamate were measured and values are presented as percentages of basal levels. Data are presented as absolute values, percentage of basal level. Unpaired t test was used to determine significant differences in DA and GABA basal level and basal release between groups. Two-way ANOVA followed by Bonferroni multiple comparison test was used to analyze the data of time course in the microdialysis experiment. Values were expressed as mean ± SEM, and *P < 0.05 and ***P < 0.0001 were considered statistically significant.
Results
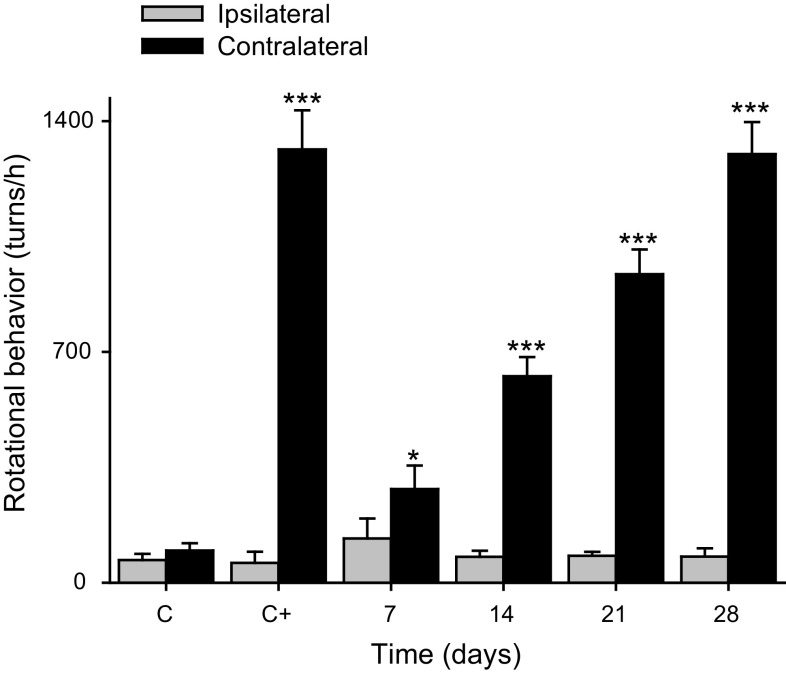
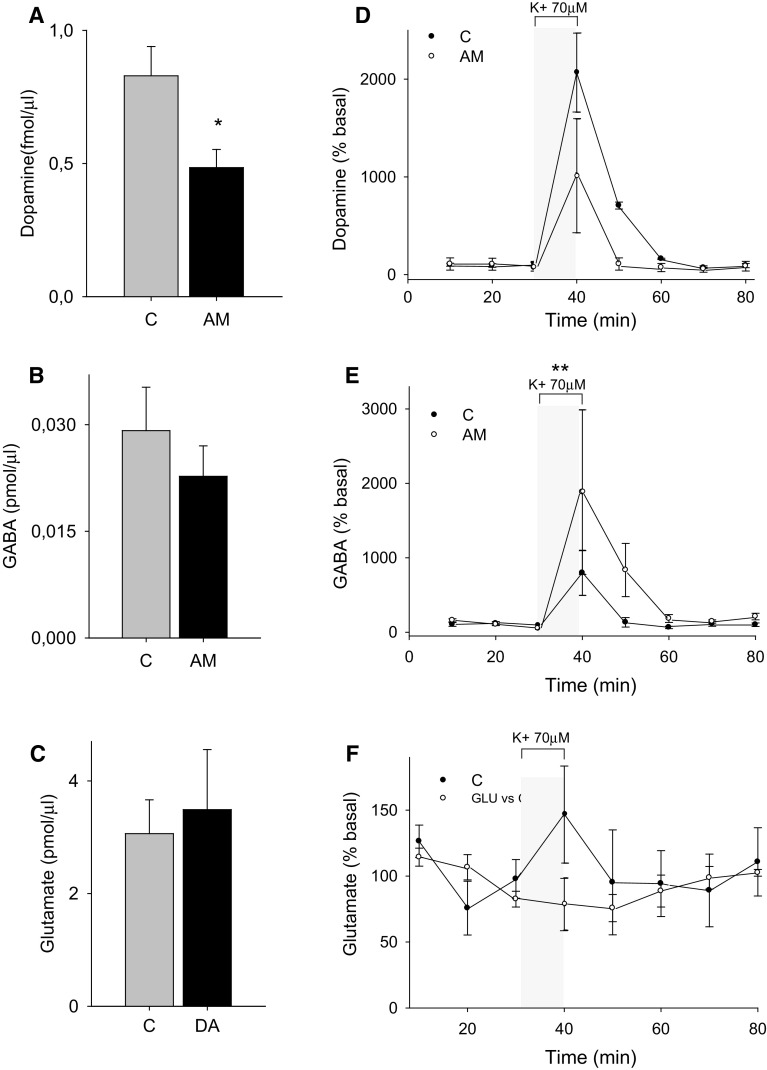
We have injected once 1.6 nmol aminochrome unilaterally into the left side of rat striatum and we determined rotational behavior after 7, 14, 21 and 28 days. The aminochrome injection induced a significant contralateral rotation after 7 days (P < 0.05) that increased with the time at 14, 21 and 28 days (P < 0.001) when the animals were injected subcutaneously with 0.5 mg/kg apomorphine (Fig. 1). Interestingly, aminochrome, which has been shown to be neurotoxic in cellular models, did not induce a significant loss of tyrosine hydroxylase-positive cells in the substantia nigra (Fig. 2) and tyrosine hydroxylase-positive fibers in the striatum (data not shown). The lack of degeneration in the substantia nigra and striatum combined with the positive contralateral rotation opened the question whether dopamine release is normal. Therefore, we measured dopamine release by using microdialysis after 14 days of the unilateral striatal injection of one dose of 1.6 nmol aminochrome (Fig. 3). The basal level of dopamine was also significantly decreased (0.48 ± 0.06 fmol/µl aminochrome animal versus 0.83 ± 0.1 fmol/µl for control animal; P < 0.05; Fig. 3a). No significant differences were observed in the basal level of GABA and glutamate (Fig. 3b, c, respectively). The addition of a depolarizing stimulus of 70 mM potassium resulted in a significant decrease of dopamine release in animals treated with aminochrome (0.40 ± 0.07 fmol/µl; P < 0.05) in comparison with animals injected with the buffer MES (1.79 ± 0.49 fmol/µl) used for the synthesis of aminochrome (Fig. 3d). Interestingly, the treatment of aminochrome resulted in a significant increase of GABA release with the depolarizing stimulus of 70 mM potassium from 0.022 ± 0.004 to 0.08 ± 0.02 pmol/µl after K+ 70 mM stimulation (P < 0.01; Fig. 3e). No significant differences in glutamate were observed after the depolarizing stimulus of 70 mM potassium (Fig. 3f).
Fig. 1.
Rotational behavior induced by apomorphine. The unilateral injection of 1.6 nmol aminochrome into the left side of rat striatum induces contralateral behavior after 7, 14, 21 and 28 days when the animals were injected with 0.5 mg/kg apomorphine. The rotational behavior increased with the time (mean ± SEM). As positive control 32 nmol 6-hydroxydopamine was injected (C+) and the control buffer group was also used in this experiment (C). The significance was determined using one-way ANOVA followed by Bonferroni multiple comparison test which was used to detect differences between the groups and days of treatment (***P < 0.0001). A statistical difference was observed between the contralateral rotation and ipsilateral rotation for each time evaluated
Fig. 2.
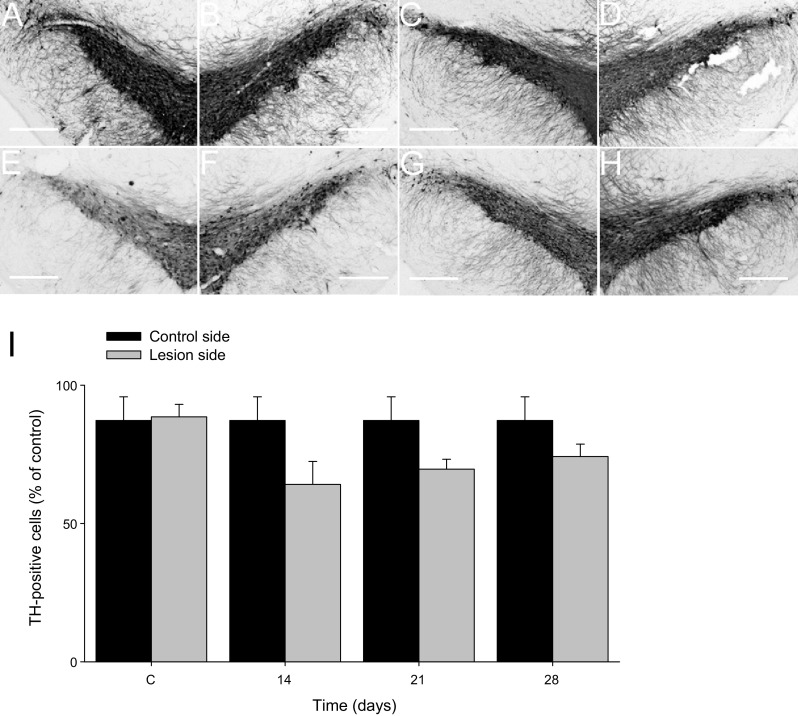
Effect of aminochrome on tyrosine hydroxylase immunoreactivity in substantia nigra pars compacta (SNc). Microphotographs showing no effect on tyrosine hydroxylase (TH) immunoreactivity in substantia nigra pars compacta of Sham control group (a, b) or aminochrome 1.6 nmol at 14 days (c, d); at 21 days (e, f) and at 28 days after aminochrome injection (g, h). The left panels are the injection side and the right panels are the contralateral side of each evaluated condition. In i the unilateral intrastriatal injection does not induce a decrease in the number of TH-immunoreactive cells in the SNc. Bars values are expressed as mean ± SEM of percent lost of TH-immunoreactive cell on the lesioned side compared to the non-lesioned SN (contralateral side)
Fig. 3.
a–c Intrastriatal aminochrome administration decreased basal dopamine extracellular levels but not GABA and glutamate levels. a Striatal dopamine extracellular levels, b GABA extracellular levels and c glutamate extracellular levels in animals treated with 1.6 nmol of aminochrome intrastriatum (black bars) and respective controls (gray bars). Dopamine basal levels are expressed in fmol/µl while GABA and glutamate are expressed in pmol/µl. Values are expressed as mean ± SEM; n = 4, unpaired t test was used to determine significant differences (*P < 0.05). d–f Intrastriatal aminochrome administration decreased depolarization-induced dopamine and increased GABA extracellular levels but not glutamate levels. d–f The time course of DA, GABA and glutamate extracellular levels in animals treated with 1.6 nmol of aminochrome intrastriatum (open diamonds) and respective controls (filled diamonds). Thirty minutes after beginning the collection of samples a KRP solution containing 70 mM K+ was perfused through the dialysis probe during 10 min (n = 4 per group) to induce the release of DA, GABA and Glutamate. Two-way ANOVA followed by Bonferroni post test for multiple comparison was used to compare the effect of K+ 70 mM depolarization in control versus aminochrome-treated animals (*P < 0.05 and **P < 0.01 K+ stimulus-aminochrome group vs. K+ stimulus-sham group). Values are expressed as % of basal levels (mean ± SEM); n = 4, independent experiments
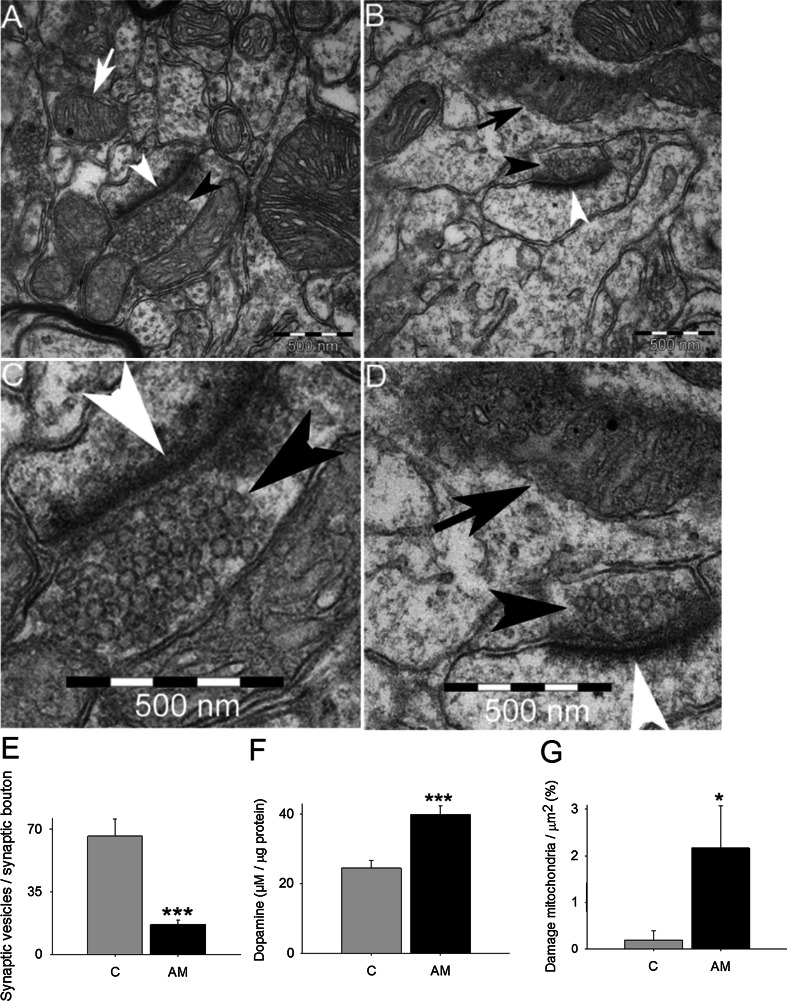
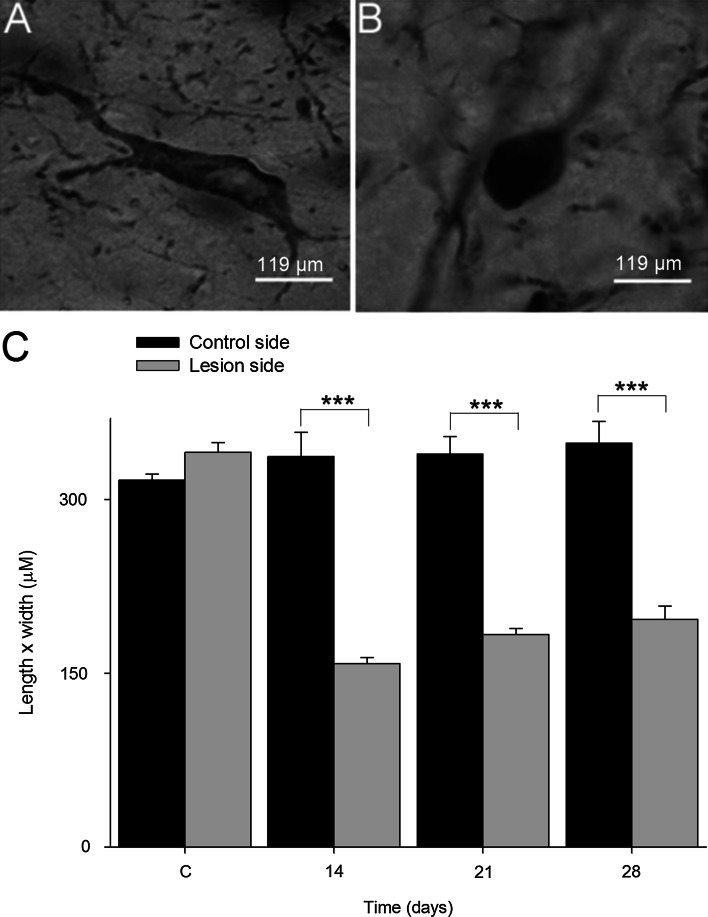
The decrease in dopamine release opens the question about possible differences in the number of monoaminergic vesicles that are able to release dopamine in the presynaptic terminals of dopaminergic neurons. We used transmission electron microscopy to analyze the number of vesicles in the terminals. We found that the amount of monoaminergic vesicles was significantly decreased (4.1-fold; P < 0.001) in the terminals of dopaminergic neurons treated with aminochrome in comparison to control animals (Fig. 4a–e). We isolated synaptic vesicles to measure dopamine level in these vesicles and found that the vesicles from animals treated with aminochrome have 1.6-fold more dopamine than in control animals (Fig. 4f). Microtubules play an important role in the axonal transport of proteins and organelles. Therefore, we analyzed the possible effect of aminochrome on dopaminergic neurons morphology and we found that aminochrome induced significant morphological changes in dopaminergic neurons characterized by a reduction of cell volume, a phenomenon also called as cell shrinkage (Fig. 5; P < 0.001).
Fig. 4.
The effect of aminochrome on presynaptic monoaminergic vesicles in the neuron terminals. Aminochrome induces a significant decrease in the number of monoaminergic presynaptic vesicles in the terminals of dopaminergic neurons (b and d, a magnification b) in comparison with control animals (a and c, a magnification of a), determined using electron transmission microscopy in the striatum. The quantification of monoaminergic synaptic vesicles per terminal was plotted in e and the values correspond to the mean ± standard error. A significant increase in the amount dopamine was observed in isolated presynaptic vesicles of animals treated with aminochrome in comparison with control animals (f). In g is shown that aminochrome induced a significant increase in the number of damaged mitochondria per µm2 in animal treated with 1.6 nmol aminochrome (AM) in comparison to control animals in presynaptic terminals (c). The black head arrow shows synaptic vesicles. White head arrows show the synapses. White arrow shows normal mitochondria and black arrow shows damaged mitochondria. The inset is a magnification of synapses and synaptic vesicles in animals treated with aminochrome. The significance was measured with unpaired Student’s t test (***P < 0.001; *P < 0.05)
Fig. 5.
The effect of aminochrome on the morphology of TH-immunoreactive neurons. Aminochrome (1.6 nmol) induces significant changes in TH-positive neurons in substantia nigra pars compact characterized by a cell shrinkage (b) in comparison with control animals (a). The cell shrinkage was quantified by determining the length and width (values expressed in µm ± SEM) in 12 neurons per side (n = 3 animals per timescale; c). The microphotographs were obtained using a ×40 objective. One-way ANOVA, followed by Bonferroni multiple comparison tests, was used to compare control side versus lesion side at each time (***P < 0.0001). The scale bar was of 119 µm
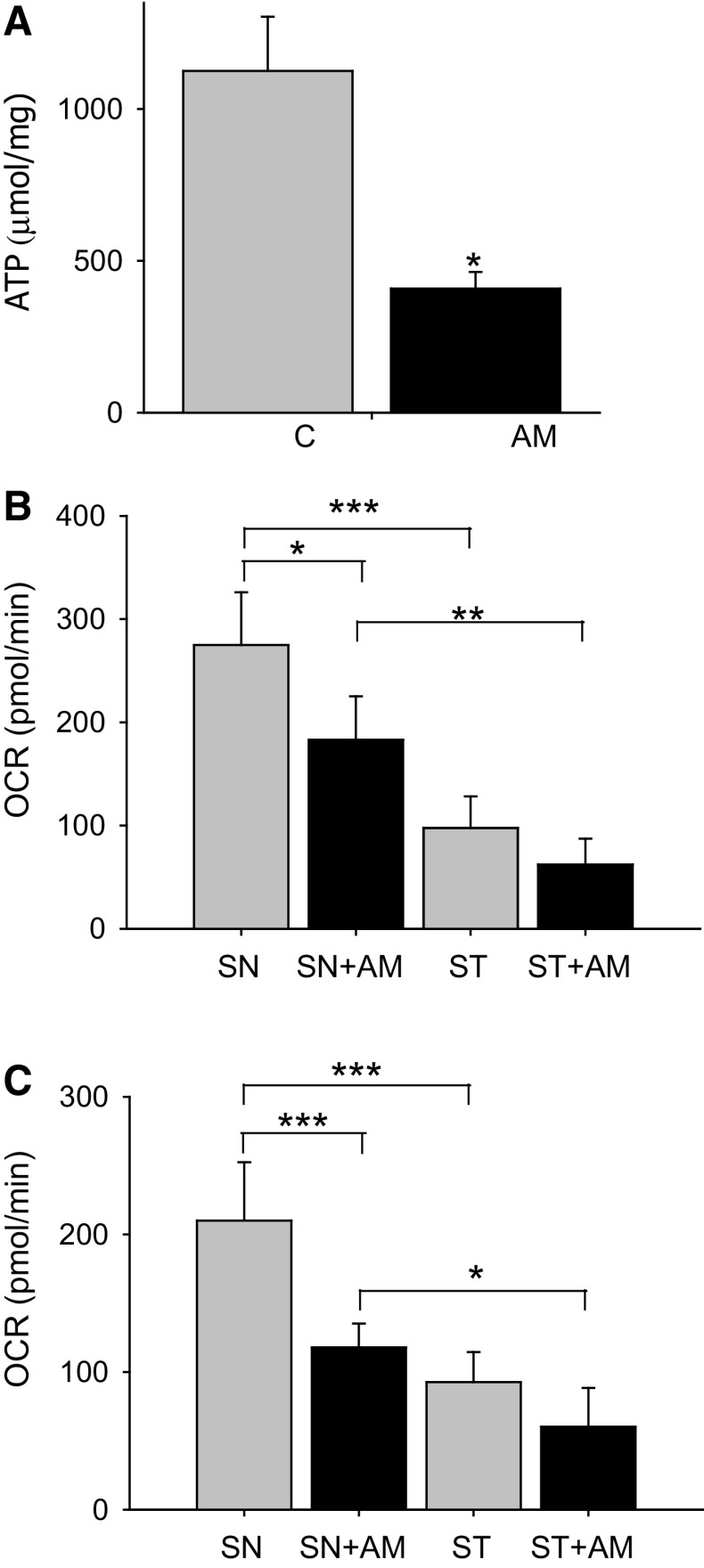
Ultrastructural analysis of mitochondria performed with transmission electron microscopy revealed a significant increase in the number of damaged mitochondria per µm2 in the striatum of animals treated with aminochrome (11-fold increase; P < 0.05) in comparison to control (Fig. 4b, d, g). In the substantia nigra we also found a significant increase in damaged mitochondria per nm2 in animals treated with aminochrome (Fig. 6). The increase of damaged mitochondria both in substantia nigra and striatum in animals treated with aminochrome suggested that aminochrome must induce mitochondria dysfunction followed with a decrease in ATP level. Therefore, we determined the total ATP level in striatum tissue and we observed a significant decrease in aminochrome-treated animals (36 % of control, P < 0.05) (Fig. 7a). In addition, we analyzed mitochondria respiratory function in animals injected with 1.6 nmol aminochrome and control animals injected with MES buffer by measuring the oxygen consumption rate (OCR) in isolated mitochondria from substantia nigra and striatum. Aminochrome induces a significant decrease in the basal respiration in substantia nigra in comparison with the control side (66 % of control; P < 0.05, Fig. 8b). No significant difference in the basal respiration was observed in the striatum in the presence of aminochrome. However, a significant decrease in the basal respiration in the striatum was observed in comparison with substantia nigra both in control animals and aminochrome-treated animals (P < 0.001 and P < 0.01, respectively; Fig. 7b). Aminochrome also induces a significant decrease in maximal respiratory rate both in substantia nigra a (56 % of control; P < 0.001; Fig. 7c). No significant difference in the maximal respiration was observed in the striatum in the presence of aminochrome. However, a significant decrease in the maximal respiration in the striatum was observed in comparison with substantia nigra both in control animals and aminochrome-treated animals (P < 0.001 and P < 0.05, respectively; Fig. 7c).
Fig. 6.
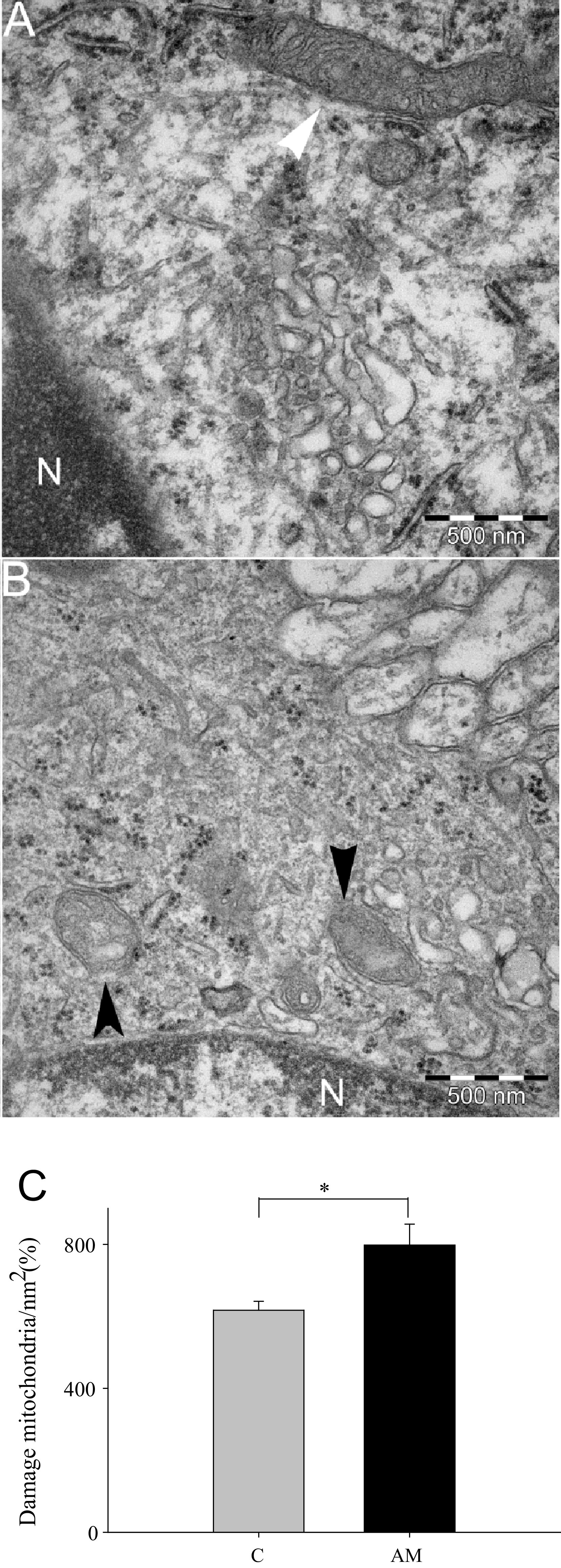
The effect of aminochrome on substantia nigral mitochondria. The ultrastructure of mitochondria determined using transmission electron microscopy revealed that aminochrome induces mitochondria damage (black arrow head; b) in comparison with control animals with normal mitochondria (white arrow head; a). A significant increase in the number of damaged mitochondria per nm2 was observed when we count the number of damaged mitochondria per normal mitochondria and plotted in c. Scale bar is 500 nm. The significance was measured with unpaired Student’s t test (*P < 0.05)
Fig. 7.
The effect of aminochrome on mitochondrial respiration and ATP levels. Aminochrome induces a significant decrease in ATP levels in the striatum compared to control animals (a). The OCR of mitochondria in the striatum and substantia nigra of animals treated with aminochrome (AM) or the buffer used for aminochrome synthesis (c) revealed that aminochrome induces a significant decrease in the basal respiration in the substantia nigra (SN) (b) and in the maximal respiration (c) but not in the striatum (ST). The values were expressed in pmol/min ± SEM. The significance was measured with unpaired Student’s t test (***P < 0.001)
Fig. 8.
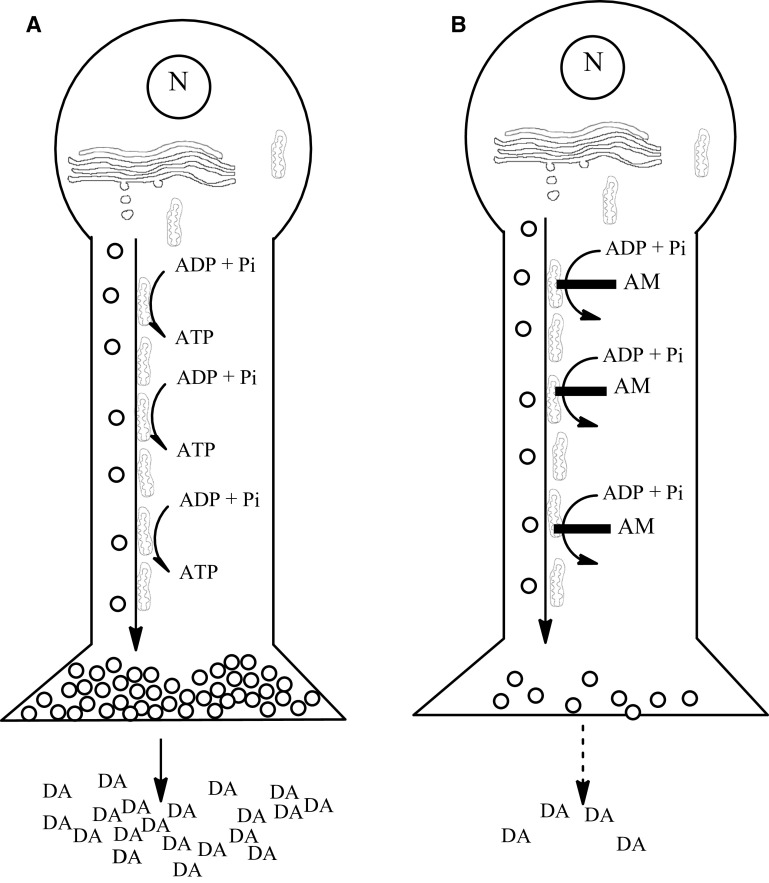
Possible mechanism for aminochrome-induced dopaminergic neuronal dysfunction. The unilateral injection of aminochrome into the striatum induces contralateral behavior without significant loss of dopaminergic neurons. The contralateral behavior can be a consequence of (1) the significant decrease of dopamine release as a consequence of the significant decrease in the number of monoaminergic vesicles in the presynaptic terminals of animals treated with aminochrome; and (2) significant decrease of ATP levels in the animals treated with aminochrome since ATP is necessary for both dopamine release from monoaminergic vesicles into intersynaptic space and also for axonal transport of monoaminergic vesicles from the neuron soma to the neuron presynaptic terminals. Aminochrome induces mitochondria dysfunction, explaining the significant decrease in cellular ATP level. Aminochrome has been reported to inhibit the formation of microtubules [25] that play a key role in axonal transport of vesicles and organelles. N nucleus, AM aminochrome, DA dopamine
Discussion
The contralateral behavior induced by apomorphine observed in animals with unilateral lesion treated with neurotoxins such as 6-hydroxydopamine is a consequence of the extensive degeneration of the nigrostriatal dopaminergic system induced by the neurotoxin [20]. Interestingly, aminochrome induces contralateral behavior without significant loss of dopaminergic neurons in substantia nigra and striatum probably as a consequence of the decrease of dopamine release. Reserpine is an inhibitor of vesicular monoamine transporter-2 (VMAT-2) and the treatment of animals with reserpine results in dopamine depletion in monoaminergic vesicles for neurotransmission. Reserpine has been used as preclinical model of Parkinson’s disease since it induces temporal akinesia in animals up to 24 h after injection. After 24 h of reserpine treatment, the akinesia begins to disappear with concomitant replenishment of striatal dopamine [21]. Interestingly, aminochrome-induced contralateral behavior does not disappear with the time as it was observed for reserpine. Aminochrome-induced contralateral behavior significantly increase with time despite the fact that aminochrome does not induce degeneration of dopaminergic neurons, contrasting with exogenous neurotoxins where the contralateral behavior is based on the loss of nigrostriatal neurons even at low doses of 6-hydroxydopamine, a partial loss is observed [22]. In Parkinson’s disease the loss of dopaminergic neurons containing neuromelanin impairs dopaminergic modulation of the flow of information within the extrapyramidal motor system resulting in an imbalance between the level of dopamine with respect to GABA and glutamate. The level of glutamate and GABA are higher than dopamine in Parkinson’s disease and the treatment of the disease is focused to obtain a balance between dopamine and glutamate and GABA with dopaminergic compounds [23]. The loss of dopamine also disrupts the balance between dopamine and acetylcholine that contributes to motor symptoms as a consequence of an increase in acetylcholine concentration, explaining the use of anti-cholinergic drugs in Parkinson’s disease treatment [1].
Our experiments conducted with microdialysis revealed that in aminochrome-treated animals, the level of released dopamine was significant decreased after the depolarizing stimulus with potassium, contrasting with a significant increase of GABA. In addition, the basal extracellular level of dopamine after aminochrome was also lower than in control conditions. This imbalance between dopamine and GABA is similar to what happens in Parkinson disease but the level of glutamate does not have a significant increase. The decrease of dopamine release from dopaminergic neurons in animals treated with aminochrome is probably due to a dysfunction of anterograde axonal transport. Kinesin and microtubules play an essential role in the anterograde axonal transport of organelles and synaptic vesicles to the terminals in neurons. Aminochrome induces aggregation of α- and β-tubulin and inhibits tubulin polymerization and microtubules disassembly required for the formation of microtubules in dopaminergic neurons [24, 25]. Aminochrome-dependent impairment of microtubules formation should result in a dysfunction of anterograde transport of dopaminergic neurons, explaining the low amount of synaptic vesicles observed with transmission electron microscopy in the presynaptic terminals of dopaminergic neurons. Therefore, it seems to be plausible that (1) aminochrome impairs the anterograde axonal transport resulting in lower amount of vesicles in the terminal of dopaminergic neurons in the striatum; and (2) that aminochrome induces mitochondria dysfunction by forming adducts with mitochondrial complex I and inhibiting ATP formation [10]. ATP is essential both for anterograde axonal transport and for dopamine release from synaptic monoaminergic vesicles. Our results suggest that aminochrome induces mitochondria dysfunction in vivo since the levels of ATP were found to be significantly decreased in the striatum where ATP is required for dopamine release. In addition, aminochrome induces a significant decrease of basal respiration both in substantia nigra with respect to control and in the striatum when compared to the aminochrome-treated side of substantia nigra. The maximal respiration reflects how the system reacts to an increased ATP demand [26]. Aminochrome significantly decreased the maximal respiration both in the substantia nigra and in striatum when compared to the aminochrome-treated side of substantia nigra. This leads to an energetic crisis that can explain the lower amount of monoaminergic synaptic vesicles and the significant decrease in dopamine release (Fig. 8).
Several evidences support a possible link between dopamine oxidation and the loss of dopaminergic neurons containing neuromelanin in Parkinson’s disease. The formation of neuromelanin requires dopamine oxidation to o-quinones. Although, in the majority of healthy individuals, dopamine oxidation finally ends in the formation of neuromelanin [4, 5], the formation of aminochrome formed during dopamine oxidation under certain conditions can be neurotoxic by inducing (1) mitochondria dysfunction—aminochrome forms adducts with complex I inducing mitochondria dysfunction and inhibit the formation of ATP in SH-SY5Y cells [10, 13, 27, 28]; (ii) protein degradation dysfunction—aminochrome induces inactivation of the proteasomal system [11, 14]. Aminochrome prevents the fusion between autophagy vacuoles and lysosomes [12, 29]. In addition, aminochrome induces lysosome dysfunction [12]; (iii) alpha synuclein aggregation—aminochrome induces the formation of adducts with alpha synuclein generating neurotoxic oligomers [15]; (iv) oxidative stress—aminochrome can be one-electron reduced to leukoaminochrome radical which is extremely unstable in the presence of oxygen inducing the generation of hydroxyl radicals [13, 30]; and (5) aminochrome induces endoplasmic reticulum stress [14]. The reason why o-quinones generated during dopamine oxidation to generate neuromelanin are not neurotoxic in healthy individuals is because there are two enzymes that prevent the neurotoxic effects of these o-quinones: DT-diaphorase and glutathione transferase M2-2. DT-diaphorase is expressed both in dopaminergic neurons and astrocytes [31]. DT-diaphorase is a unique flavoenzyme that catalyzes the two-electron reduction of aminochrome to leukoaminochrome [16]. DT-diaphorase prevents (1) aminochrome-induced cell death [32]; (2) the formation of alpha synuclein neurotoxic oligomers [15]; (3) aminochrome-induced mitochondria dysfunction and inhibition of ATP production in cell cultures [10, 13, 27, 28]; (4) aminochrome-induced autophagy inhibition and proteasome inactivation [11]; (5) aminochrome-induced oxidative stress [13]; (6) α- and β-tubulin aggregation required for microtubules formation that play a key role in cell cytoskeleton structure, in the fusion between autophagy vacuoles and lysosomes and in the axonal transport [24, 25]. Glutathione transferase M2-2 is expressed in human astrocytes and catalyzes the GSH conjugation of both aminochrome to 4-S-glutathionyl-5,6-dihydroxyindoline which is resistant to biological oxidizing agents [33, 34] and dopamine o-quinone to 5-glutathionyl dopamine [35] that normally will be degraded to 5-cysteinyl dopamine that it has been found in the cerebrospinal fluid of Parkinson’s disease patients and neuromelanin [36, 37]. Glutathione transferase M2-2 prevents aminochrome-induced cell death, autophagy and lysosome dysfunction [12]. Astrocytes protect dopaminergic neurons in co-cultures by secreting glutathione transferase M2-2 into conditioned medium where dopaminergic neurons take up glutathione transferase M2-2 into the neuron to protect them against aminochrome neurotoxicity [38, 39].
Studies performed in a cell culture revealed that aminochrome induces the formation of adducts with α- and β-tubulin generating aggregates that disrupt the cytoskeleton architecture and cell morphology [24]. Interestingly, the unilateral injection of only one single dose of aminochrome into striatum also induces morphological changes in tyrosine hydroxylase-positive neurons, resulting in a significant reduction of neurons volume during the time. Interesting is that neurons with reduced volume or cell shrinkage do not die suggesting that the dopaminergic neurons survive but they have dopamine release dysfunction creating an imbalance between dopamine and GABA. However, we did not observe a significant change in glutamate. In Parkinson’s disease, the decrease in dopamine release generated by the loss of dopaminergic neurons generates an imbalance between dopamine, GABA and glutamate and an intensive research is being performed to balance the basal ganglia circuitry [40].
Recently, it has been reported that the unilateral injection of 10 nmol dopaminochrome into substantia nigra induced a slow and progressive degeneration of dopaminergic neurons of substantia nigra pars compacta after 10 days [41]. However, dopaminochrome is a dopamine oxidation product which is different from aminochrome since their absorption maximum are at 303 and 479 nm [42] while aminochrome absorption maximum are at 280 and 475 nm and its structure was confirmed by NMR analysis [24]. In our study, we do not observe significant degeneration of dopaminergic neurons in substantia nigra or dopaminergic fibers in the striatum using aminochrome (1.6 nmol) but aminochrome induces several signals of neuronal dysfunction such as dopamine release decrease, increased release of GABA, cell shrinkage, mitochondria dysfunction and disturbance of axonal transport of vesicles. Aminochrome induces a model for neuronal dysfunction of dopaminergic neurons that is more similar to the very slow degeneration of dopaminergic neurons containing neuromelanin in the substantia nigra in Parkinson’s disease.
The failure to translate successful results from preclinical studies, using MPTP, 6-hydroxydopamine or rotenone, to clinical studies and new therapies for PD [2, 3] suggests an enormous need to find new preclinical models that resemble to what happens in degeneration of the nigrostriatal system resulting in the loss of dopaminergic neurons containing neuromelanin [43]. The problem with these preclinical models is that all are exogenous neurotoxins and induce a massive and rapid degeneration of dopaminergic neurons contrasting with the very slow degeneration and progression of human Parkinson’s disease. The advantages of using aminochrome as a preclinical model to study the molecular mechanisms of Parkinson’s disease are (1) aminochrome is an endogenous compound formed inside the neurons that are lost under Parkinson disease, i.e., it is formed inside dopaminergic neurons containing neuromelanin; (2) aminochrome does not induce a rapid and extensive neurodegeneration. Instead, aminochrome induces a slow progressive dysfunction of dopaminergic neurons that increases with time as we have observed in the rotational behavior; (3) aminochrome induces an imbalance between the levels of dopamine and GABA; (4) aminochrome induces mitochondrial dysfunction. It seems to be plausible that the aminochrome-dependent dysfunction induced in dopaminergic neurons will evolve to degeneration of the nigrostriatal system at long term as a consequence of the imbalance between neurotransmitters in the basal ganglia induced by aminochrome. We have used rats as animal model in this study but animals such as mice or monkey can also be suitable to use aminochrome. More studies observing aminochrome effects during a longer period of time are needed to follow the fate of dopaminergic neurons with dopamine release dysfunction, the effect of aminochrome on other monoaminergic systems and the possible formation of Lewy bodies containing alpha synuclein aggregates.
Acknowledgments
This work was supported by FONDECYT # 1100165 (JSA), University of Chile ENL014/14 (JSA); ECOS-CONICYT # C10S02 (JSA, RR-V). FONDECYT # 1120443, FONDAP # 15150012 (CC) and FONDECYT postdoctoral fellowship # 3140458 (FJ)
References
- 1.Segura-Aguilar J, Muñoz P, Paris I. Aminochrome as new preclinical model to find new pharmacological treatment that stop the development of Parkinson’s disease. Curr Med Chem. 2016;23:346–359. doi: 10.2174/0929867323666151223094103. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm D, Mäkelä J, Di Liberto V, Mudò G, Belluardo N, Eriksson O, Saarma M. Current disease modifying approaches to treat Parkinson’s disease. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olanow Bartus RT, Volpicelli-Daley LA, Kordower JH. Trophic factors for Parkinson’s disease: to live or let die. Mov Disord. 2015;30:1715–1724. doi: 10.1002/mds.26426. [DOI] [PubMed] [Google Scholar]
- 4.Segura-Aguilar J, Kostrzewa RM. Neurotoxin mechanisms and processes relevant to Parkinson’s disease: an update. Neurotox Res. 2015;27:328–354. doi: 10.1007/s12640-015-9519-y. [DOI] [PubMed] [Google Scholar]
- 5.Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- 6.Mullin S, Schapira A. α-Synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol Neurobiol. 2013;47:587–597. doi: 10.1007/s12035-013-8394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol. 2012;124:153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercado G, Valdés P, Hetz C. An ERcentric view of Parkinson’s disease. Trends Mol Med. 2013;19:165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J, Núñez MT. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals. 2012;25:795–803. doi: 10.1007/s10534-012-9525-y. [DOI] [PubMed] [Google Scholar]
- 11.Zafar KS, Siegel D, Ross D. A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol. 2006;70:1079–1086. doi: 10.1124/mol.106.024703. [DOI] [PubMed] [Google Scholar]
- 12.Huenchuguala S, Muñoz P, Zavala P, Villa M, Cuevas C, Ahumada U, Graumann R, Nore BF, Couve E, Mannervik B, Paris I, Segura-Aguilar J. Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy. 2014;10:618–630. doi: 10.4161/auto.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arriagada C, Paris I, Sanchez de las Matas MJ, Martinez-Alvarado P, Cardenas S, Castañeda P, Graumann R, Perez-Pastene C, Olea-Azar C, Couve E, Herrero MT, Caviedes P, Segura-Aguilar J. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol Dis. 2004;16:468–477. doi: 10.1016/j.nbd.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Xiong R, Siegel D, Ross D. Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol Appl Pharmacol. 2014;280:285–295. doi: 10.1016/j.taap.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz P, Cardenas S, Huenchuguala S, Briceño A, Couve E, Paris I, Segura-Aguilar J. DT Diaphorase prevents aminochrome-induced alpha-synuclein oligomer formation and neurotoxicity. Toxicol Sci. 2015;145:37–47. doi: 10.1093/toxsci/kfv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segura-Aguilar J, Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine: prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact. 1989;72:309–324. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 17.Segura-Aguilar J, Diaz-Veliz G, Mora S, Herrera-Marschitz M. Inhibition of DT-diaphorase is a requirement for Mn(III) to produce a 6-OH-dopamine like rotational behaviour. Neurotox Res. 2002;4:127–131. doi: 10.1080/10298420290015926. [DOI] [PubMed] [Google Scholar]
- 18.Sotomayor R, Forray MI, Gysling K. Acute morphine administration increases extracellular DA levels in the rat lateral septum by decreasing the GABAergic inhibitory tone in the ventral tegmental area. J Neurosci Res. 2005;81:132–139. doi: 10.1002/jnr.20537. [DOI] [PubMed] [Google Scholar]
- 19.Fried N, Moffat C, Seifert E, Oshinsky M. Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol. 2014;307:C1017–C1030. doi: 10.1152/ajpcell.00332.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera-Marschitz M, Arbuthnott G, Ungerstedt U. The rotational model and microdialysis: significance for dopamine signalling, clinical studies, and beyond. Prog Neurobiol. 2010;90:176–189. doi: 10.1016/j.pneurobio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penttinen AM, Suleymanova I, Albert K, Anttila J, Voutilainen MH, Airavaara M. Characterization of a new low-dose 6-hydroxydopamine model of Parkinson’s disease in rat. J Neurosci Res. 2016;94:318–328. doi: 10.1002/jnr.23708. [DOI] [PubMed] [Google Scholar]
- 23.di Michele F, Luchetti S, Bernardi G, Romeo E, Longone P. Neurosteroid and neurotransmitter alterations in Parkinson’s disease. Front Neuroendocrinol. 2013;34:132–142. doi: 10.1016/j.yfrne.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- 25.Briceño A, Muñoz P, Brito P, Huenchuguala S, Segura-Aguilar J, Paris IB. Aminochrome toxicity is mediated by inhibition of microtubules polymerization through the formation of adducts with tubulin. Neorotox Res. 2015 doi: 10.1007/s12640-015-9560-x. [DOI] [PubMed] [Google Scholar]
- 26.Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol. 2014;547:309–354. doi: 10.1016/B978-0-12-801415-8.00016-3. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz P, Paris I, Sanders LH, Greenamyre JT, Segura-Aguilar J. Overexpression of VMAT-2 and DT-diaphorase protects substantia nigra-derived cells against aminochrome neurotoxicity. Biochim Biophys Acta. 2012;1822:1125–1136. doi: 10.1016/j.bbadis.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paris I, Muñoz P, Huenchuguala S, Couve E, Sanders LH, Greenamyre JT, Caviedes P, Segura-Aguilar J. Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol Sci. 2011;121:376–388. doi: 10.1093/toxsci/kfr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinsons Dis. 2012;2012:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segura-Aguila J, Metodiewa D, Welch CJ. Metabolic activation of dopamine o-quinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim Biophys Acta. 1998;1381:1–6. doi: 10.1016/S0304-4165(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 31.Schultzberg M, Segura-Aguilar J, Lind C. Distribution of DT diaphorase in the rat brain: biochemical and immunohistochemical studies. Neuroscience. 1988;27:763–776. doi: 10.1016/0306-4522(88)90181-9. [DOI] [PubMed] [Google Scholar]
- 32.Lozano J, Muñoz P, Nore BF, Ledoux S, Segura-Aguilar J. Stable expression of short interfering RNA for DT-diaphorase induces neurotoxicity. Chem Res Toxicol. 2010;23:1492–1496. doi: 10.1021/tx100182a. [DOI] [PubMed] [Google Scholar]
- 33.Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem. 1997;272:5727–5731. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- 34.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun. 2000;274:32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 36.Carstam R, Brinck C, Hindemith-Augustsson A, Rorsman H, Rosengren E. The neuromelanin of the human substantia nigra. Biochim Biophys Acta. 1991;1097:152–160. doi: 10.1016/0925-4439(91)90100-N. [DOI] [PubMed] [Google Scholar]
- 37.Rosengren E, Linder-Eliasson E, Carlsson A. Detection of 5-S-cysteinyldopamine in human brain. J Neural Transm. 1985;63:247–253. doi: 10.1007/BF01252029. [DOI] [PubMed] [Google Scholar]
- 38.Cuevas C, Huenchuguala S, Muñoz P, Villa M, Paris I, Mannervik B, Segura-Aguilar J. Glutathione transferase-M2-2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox Res. 2015;27(217–228):429. doi: 10.1007/s12640-014-9500-1. [DOI] [PubMed] [Google Scholar]
- 39.Segura-Aguilar J. A new mechanism for protection of dopaminergic neurons mediated by astrocytes. Neural Regen Res. 2015;10:1225–1227. doi: 10.4103/1673-5374.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cazorla M, Kang UJ, Kellendonk C. Balancing the basal ganglia circuitry: a possible new role for dopamine D2 receptors in health and disease. Mov Disord. 2015;30:895–903. doi: 10.1002/mds.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touchette JC, Breckenridge JM, Wilken GH, Macarthur H. Direct intranigral injection of dopaminochrome causes degeneration of dopamine neurons. Neurosci Lett. 2015;612:178–184. doi: 10.1016/j.neulet.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochs SD, Westfall TC, Macarthur H. The separation and quantification of aminochromes using high-pressure liquid chromatography with electrochemical detection. J Neurosci Methods. 2005;142:201–208. doi: 10.1016/j.jneumeth.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Segura-Aguilar J, Paris I, Muñoz P. The need of a new and more physiological preclinical model for Parkinson’s disease. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]