Abstract
Single-nucleotide polymorphism studies have linked the chromosome 17q12-q21 region, where the human orosomucoid-like (ORMDL)3 gene is localized, to the risk of asthma and several other inflammatory diseases. Although mast cells are involved in the development of these diseases, the contribution of ORMDL3 to the mast cell physiology is unknown. In this study, we examined the role of ORMDL3 in antigen-induced activation of murine mast cells with reduced or enhanced ORMDL3 expression. Our data show that in antigen-activated mast cells, reduced expression of the ORMDL3 protein had no effect on degranulation and calcium response, but significantly enhanced phosphorylation of AKT kinase at Ser 473 followed by enhanced phosphorylation and degradation of IκBα and translocation of the NF-κB p65 subunit into the nucleus. These events were associated with an increased expression of proinflammatory cytokines (TNF-α, IL-6, and IL-13), chemokines (CCL3 and CCL4), and cyclooxygenase-2 dependent synthesis of prostaglandin D2. Antigen-mediated chemotaxis was also enhanced in ORMDL3-deficient cells, whereas spreading on fibronectin was decreased. On the other hand, increased expression of ORMDL3 had no significant effect on the studied signaling events, except for reduced antigen-mediated chemotaxis. These data were corroborated by increased IgE-antigen-dependent passive cutaneous anaphylaxis in mice with locally silenced ORMDL3 using short interfering RNAs. Our data also show that antigen triggers suppression of ORMDL3 expression in the mast cells. In summary, we provide evidence that downregulation of ORMDL3 expression in mast cells enhances AKT and NF-κB-directed signaling pathways and chemotaxis and contributes to the development of mast cell-mediated local inflammation in vivo.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-015-2047-3) contains supplementary material, which is available to authorized users.
Keywords: Mast cell, RNA interference, ORMDL3 knockdown, Prostaglandin D2, Degranulation, Chemotaxis, Proinflammatory cytokines
Introduction
Orosomucoid-like (ORMDL)3 protein has attracted increased attention since the discovery of single-nucleotide polymorphisms (SNPs) in the chromosome 17q12-q21 region that were associated with onset of asthma in childhood [1]. Further studies confirmed these data in ethnically diverse populations [2, 3]. Interestingly, SNPs associated with 17q12-q21 were also linked to chronic obstructive pulmonary disease [4], ulcerative colitis [5], primary biliary cirrhosis [6], type 1 diabetes [6], Crohn disease [6, 7] and rheumatoid arthritis [8]. It has been suggested that the risk alleles for asthma mapped in the 17q12-q21 region are linked to increased levels of the ORMDL3 gene transcript [1, 6], but the risk alleles implicated in the predisposition to primary biliary cirrhosis, type 1 diabetes and Crohn disease are associated with decreased ORMDL3 mRNA levels [6]. Since ORMDL3 is only one of several genes in the chromosome 17q12-q21 region, the observed data underline the complexity of inflammatory diseases [6, 9].
Human and mouse ORMDL3 genes encode 153 aa proteins belonging to the conserved ORMDL family consisting of three members. All three members of this family (ORMDL1–3) are embedded in the endoplasmic reticulum membrane [10]. Pivotal studies of ORMDL3 yeast homologs, orosomucoid (ORM)1 and ORM2, showed that ORM proteins are negative regulators of sphingolipid synthesis [11, 12] and that knockdown of all three ORMDL isoforms resulted in increased ceramide production in distinct mammalian cell lines [11, 13, 14]. Further experiments showed that disruption of sphingolipid homeostasis in the mouse model increased bronchial reactivity in the absence of inflammatory stimulus [15]. ORMDL3 has also been found to play a role as a regulator of Ca2+ homeostasis in the endoplasmic reticulum [16, 17] and as a trigger of unfolded protein response [16, 18, 19].
Several studies attempted to explain the physiological role of ORMDL3 in cells and tissues relevant to asthma. Mice challenged with allergen exhibited enhanced expression of ORMDL3 in macrophages, eosinophils and lung epithelial cells [18]. Furthermore, a lung epithelial cell line transfected with ORMDL3 cDNA showed increased transcription of genes encoding metalloproteases, chemokines and oligoadenylate synthetases [18]. In addition, bone marrow-derived eosinophils transduced with ORMDL3 cDNA showed enhanced rolling and nuclear localization of the phosphorylated NF-κB p65 subunit [20]. Transgenic mice overexpressing human ORMDL3 exhibited increased levels of serum IgE and showed spontaneous development of enhanced airway responsiveness. This correlated with increased numbers of CD4+ cells, macrophages, eosinophils and neutrophils, and enhanced Th2 cytokine levels in the lungs of transgenic mice [19]. Although mast cells are crucial effector cells in IgE-dependent allergic disorders [21, 22] and are critical for promoting the severity of collagen-induced arthritis, which is an autoimmune inflammatory disease [23], the involvement of ORMDL3 in FcεRI-dependent activation and mast cell-mediated inflammation has not yet been determined.
In this study, we decided to test the hypothesis that changes in the expression levels of ORMDL3 could modulate mast cell proinflammatory responses. Bone marrow-derived mast cells (BMMCs) with enhanced or reduced expression of ORMDL3 were produced and examined. We focused on the NF-κB signaling axis, which is an important player in the transcription of proinflammatory cytokines and immunoregulatory proteins [24] and is involved in the promotion of inflammation [25–27]. We found that ORMDL3-deficient cells exhibited significantly increased translocation of the NF-κB p65 subunit into the nucleus. These events were accompanied by enhanced expression of genes encoding proinflammatory cytokines, chemokines, and cyclooxygenase (COX)-2, an inducible enzyme involved in prostaglandin (PG)D2 synthesis [28]. In an attempt to identify the signaling pathway that precedes activation of NF-κB, we found that AKT kinase exhibited increased phosphorylation. We also studied the roles of ORMDL3 in the migration of mast cells toward antigen (Ag) and changes in ORMDL3 expression after high-affinity IgE receptor (FcεRI) triggering. Altogether, our data provide evidence that in mast cells, ORMDL3 functions predominantly as a negative regulator of FcεRI-mediated signaling events leading to the expression of proinflammatory mediators and chemotaxis. These data were corroborated by the finding of enhanced passive cutaneous anaphylaxis (PCA) in mice with locally silenced ORMDL3.
Materials and methods
Cells and lentiviral infection
BMMCs were derived from femurs and tibias of 8- to 10-week-old BALB/c mice bred, maintained, and used in accordance with the Institute of Molecular Genetics guidelines (Permit number 12135/2010-17210) and national guidelines (2048/2004-1020). The cells were cultured in RPMI-1640 medium supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 71 µM 2-mercaptoethanol, MEM non-essential amino acids, 0.7 mM sodium pyruvate, 2.5 mM l-glutamine, 12 mM d-glucose, recombinant mouse stem cell factor (15 ng/ml, PeproTech EC), mouse recombinant IL-3 (20 ng/ml, PeproTech EC) and 10 % FCS. Lentiviral transductions were done as described previously using HEK 293 T/17 packaging cells for virus preparation [29]. A set of murine ORMDL3 short hairpin (sh)RNAs cloned into the pLKO.1 vector (TRCN0000126200, TRCN0000126201, TRCN0000126202, TRCN0000126203; denoted in further text as indicated by numbers in bold) were purchased from Open Biosystems. To generate the myc-ORMDL3 vector, mouse ORMDL3 cDNA (Open Biosystems) was first amplified using forward primer 5′-AAAGAATTCGGAATGTGGGCACAGCACAC-3′ and reverse complementary primer 5′-TTTGCGGCCGCTCAGTACTTATTGATTCCAAA-3′, introducing EcoRI and NotI restriction endonuclease sites (underlined), respectively. Amplified ORMDL3 was then inserted into a pCMV-myc eukaryotic expression vector (Clontech). Myc-ORMDL3 was recloned by PCR into the vector pCDH-CMV-MCS-EF1-Puro (Systembio; pCDH) using forward primer 5′-AAATCTAGAGCCGCCACCATGGAGCAGAAGCTGATCTCA-3′ and reverse complementary primer 5′-TTTGCGGCCGCTCAGTACTTATTGATTCCAAA-3′, introducing XbaI and NotI restriction endonuclease sites (underlined), respectively. The construct was verified by sequencing and denoted myc-O3. The pCDH vector Myc-ORMDL3 was also used to clone murine ORMDL1 and ORMDL2 cDNAs (OpenBiosystems). Specific primers for ORMDL1 (forward primer 5′-AAAGAATTCGGAATGTTGGAGTTGCCCAC-3′ and reverse complementary primer 5′-TTTGCGGCCGCTCAATACTTATTAATTCCAAA-3′) and ORMDL2 (forward primer 5′-AAAGAATTCGGAATGTGGGGGTGGCACAC-3′ and reverse complementary primer 5′-TTTGCGGCCGTTAGTATTTGTTGATTCCAAA-3′) were designed to be cloned into pCDH using EcoRI and NotI restriction endonuclease sites (underlined). To prepare an expression vector for ORMDL3 with C-terminal myc-tag, ORMDL3 cDNA was amplified using forward primer 5′-AAAGAATTCGCCGCCACCATGAATGTGGGCACAGCACAC-3′ and reverse complementary primer 5′-TTTGGATCCGTACTTATTGATTCCAAA-3′, introducing EcoRI and BamHI restriction endonuclease sites (underlined), respectively. Amplified ORMDL3 was then cloned into pCDH-myc. The construct, denoted O3-myc, was verified by sequencing. Both constructs were used to produce lentiviruses as described [29]. BMMCs were transduced with the lentiviruses and stable transfectants were obtained by culturing the transduced cells for 2 weeks in the presence of puromycin (5 μg/ml). Cells were analyzed for FcεRI and c-Kit expression using flow cytometry, while ORMDL3 expression was examined by qPCR and immunoblotting as described below. In all experiments, more than 80 % of cells were alive and the selected populations were maintained for less than 3 weeks in culture. In all experiments, empty pLKO.1 or empty pCDH vectors were used as controls. In most of the functional studies, comparison between nontransduced cells and cells transduced with pLKO.1 was performed. In some experiments related to AKT/IκB signaling, we also used as a control pLKO.1 vector containing nontarget (NT) shRNA (Sigma-Aldrich) as a control.
Antibodies and reagents
Mouse IgE mAb specific for 2,4,6-trinitrophenol (TNP), clone IGEL b4 1 [30], SYK-specific mAb [31], and Lyn-specific mAb [32] were produced in our laboratory. Anti-mouse KIT (CD117)-allophycocyanin conjugate [catalog number (#) 17-1171] and anti-mouse FcεRI α-FITC conjugate (#11-5898) were obtained from eBioscience. Rabbit anti-COX-2 (#12282), rabbit anti-pSYK (Tyr525/526; #2710), rabbit anti-p65 subunit of NF-κB (#8242), rabbit phospho-AKT (Thr 308; #2965) and mouse anti-pIκBα (Ser32/36; #9246) were obtained from Cell Signaling. Anti-HPRT mAb (#sc-376938), rabbit anti-actin polyclonal Ab (#sc-8432), rabbit anti-p65 subunit of NF-κB (#sc-372), rabbit phospho-AKT1/2/3 (#sc-7985), rabbit ERK1 (#sc-93), rabbit phospho-ERK (Tyr204; #sc-7976), goat anti-AKT1 (#sc-1618), rabbit anti-IκBα (#sc-371), mouse anti-Myc (#sc-40), rabbit anti-GRB2 (#sc-255), HRP-conjugated goat anti-mouse IgG (#sc-2005), HRP-goat anti-rabbit IgG (#sc-2004), and HRP-donkey anti-goat IgG (#sc-2056) were obtained from Santa Cruz Biotechnology, Inc. Donkey anti-rabbit-IgG Alexa Fluor 488 conjugate (#A21206) and goat anti-rabbit-IgG Alexa Fluor 568 (#A11036) were from Molecular Probes. Antibodies specific for mouse TNF-α (#500-P64), IL-13 (#500-P178), the corresponding recombinant proteins, and recombinant IL-6 were obtained from PeproTech. Murine anti-IL-6 (#553414) was purchased from Becton–Dickinson. Antibodies used for immunoblotting of ORMDL proteins were obtained after immunization of rabbits with a peptide corresponding to the middle part of ORMDL3 (TPFETPDQGKARLLTHWEQMDC) conjugated to keyhole limpet hemocyanin via an extra cysteine residue (underlined). This antibody is designated TPF. For immunofluorescence studies, rabbit polyclonal serum raised against the N-terminal ORMDL3 peptide (CNVGTAHSEVNPNTR) was used. This antibody was prepared as described above and is designated NVG. The peptides used for immunization share high similarities with ORMDL1 and ORMDL2 proteins and both polyclonal sera recognize all three members of the ORMDL family. Therefore, we refer to the 17 kDa band on immunoblots as ORMDL. The sera were affinity purified using a SulfoLink™ Immobilization Kit for Peptides (Pierce) according to the manufacturer’s instructions. Fura-2, AM was obtained from Life Technologies. All other reagents were obtained from Sigma-Aldrich.
β-Glucuronidase release and Ca2+ response
BMMCs were sensitized with TNP-specific IgE (IGEL b4 1 mAb, 1 μg/ml) in stem cell factor- and IL-3-free culture medium for 16 h, unless stated otherwise. Then the cells were washed in buffered saline solution (BSS; 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 5.6 mM glucose, 20 mM HEPES, pH 7.4) supplemented with 0.1 % BSA and activated with Ag (TNP–BSA conjugate, 15–25 mol TNP/mol BSA). The extent of degranulation was evaluated by determining β-glucuronidase concentrations as described previously [33] except that an Infinite 200 M (TECAN) plate reader was used at 355 nm excitation and 460 nm emission wavelengths. Calcium mobilization was determined using Fura-2 acetoxymethyl ester (Fura-2 AM; Life Technologies) as a reporter. Cells were incubated with Fura-2 AM (1 ng/ml) and probenecid (2.5 mM) in BSS supplemented with 0.1 % BSA and incubated in the shaker for 30 min at 37 °C. Fura-2 AM-loaded cells were washed twice with 2.5 mM probenecid in BSS-0.1 % BSA and then transferred to BSS-0.1 % BSA supplemented with 2.5 mM probenecid and incubated in Thermomixer (Eppendorf; 15 min, 37 °C, 500 rpm). Cells were pelleted by centrifugation at 500×g for 3 min, resuspended in BSS-0.1 % BSA and transferred to white polysorp 96 well plate (NUNC, Thermo Scientific). After 1 min, BSS-0.1 % BSA supplemented with Ag (final concentration 100 ng/ml) was added using the TECAN injector system. Measurement continued up to 200 s. Levels of Ca2+ were determined by spectrofluorometry using the Infinite 200 M plate reader with excitation wavelengths at 340 and 380 nm and with a constant emission at 510 nm. To determine basal concentrations of free intracellular Ca2+ ([Ca2+]i) in various cell types, Triton X-100 (0.1 % final concentration) was added to the Fura-2-loaded cells and Fura-2 fluorescence was determined. Then, EGTA (16 mM final concentration) was added and Fura-2 fluorescence was again determined. Calcium concentrations were then calculated using the formula: [Ca2+]i = K d × S f2/S b2 × (R − R min)/(R max − R), where K d represents the dissociation constant for Fura-2 (224 nM), S f2 the average fluorescence obtained at 380 nm after EGTA addition, S b2 the average fluorescence obtained at 380 nm after addition of Triton X-100, R min the mean ratio calculated from the data acquired after EGTA addition, R max the mean of ratio calculated from data acquired after addition of Triton X-100, and R the ratio of fluorescence obtained at 340/380.
Detection of ORMDL, cytokines, and chemokines at the mRNA level
IgE-sensitized BMMCs were activated with different concentrations of Ag. One hour later, mRNA was extracted using a TurboCapture 96 mRNA kit or RNeasy miniKit (Qiagen). Single-stranded cDNA was synthesized with M-MLV reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Real-time PCR amplifications of cDNAs were performed in 10 μl reaction volumes of qPCR mix containing 1 M 1,2-propanediol, 0.2 M trehalose and SYBR green 1 [34] in 384-well plates sealed with LightCycler 480 sealing foil and analyzed by LightCycler 480 (Roche Diagnostics). The following cycling conditions were used: 3 min at 95 °C, followed by 50 cycles of 10 s at 95 °C, 20 s at 60 °C and 20 s at 72 °C. Threshold cycle (Ct) values were determined by automated threshold analysis of the cycler. Specificity of the PCR was evaluated by examining the melting curves. For data presented in Fig. 2a, actin, GAPDH, ubiquitin, TBP, SDHA, and HPRT were used as reference genes and the expression levels of ORMDL3 mRNA were normalized to the geometric mean of the reference genes in nonactivated control cells. In all other experiments, actin, GAPDH, and ubiquitin were used as the reference genes. The relative changes in the mRNA expression levels were normalized to the ones of the corresponding controls. The following primer sets were used for amplification of different cDNA fragments (sense/antisense; numbers in square brackets are sizes of the fragments in bps): actin, 5′-GATCTGGCACCACACCTTCT-3′/5′-GGGGTGTTGAAGGTCTCAAA-3′ [138]; GAPDH, 5′-AACTTTGGCATTGTGGAAGG-3′/5′-ATCCACAGTCTTCTGGGTGG-3′ [69]; ubiquitin, 5′-ATGTGAAGGCCAAGATCCAG-3′/5′-TAATAGCCACCCCTCAGACG-3′ [160]; HPRT, 5′-CTGGTGAAAAGGACCTCTCGAA-3′/5′-CTGAAGTACTCATTATAGTCAAGGGCAT-3′ [109]; TBP, 5′-GAAGAACAATCCAGACTAGCAGCA-3′/5′-CCTTATAGGGAACTTCACATCACAG-3′ [128]; SDHA, 5′-AAGGCAAATGCTGGAGAAGA-3′/5′-TGGTTCTGCATCGACTTCTG-3′ [112]; ORMDL3, 5′- CCAACCTTATCCACAACCTGG-3′/5′-GACCCCGTAGTCCATCTGC-3′ [124]; ORMDL2, 5′-CACAGCGAAGTAAACCCCAAC-3′/5′-AGGGTCCAGACAACAGGAATG-3′ [134]; ORMDL1, 5′-ACAGTGAGGTAAACCCCAATACT-3′/5′-GCAAAAACACATACATCCCCAGA-3′ [174]; TNF-α, 5′-CCCTCACACTCAGATCATCTTCT-3′/5′-GCTACGACGTGGGCTACAG-3′ [61]; IL-6, 5′-GAGGATACCACTCCCAACAGACC-3′/5′-AAGTGCATCATCGTTGTTCATACA-3′ [141]; IL-13, 5′-AGACCAGACTCCCCTGTGCA-3′/5′-TGGGTCCTGTAGATGGATTG-3′ [123]; CCL3, 5′-CATCGTTGACTATTTTGAAACCAG-3′/5′-GCCGGTTTCTCTTAGTCAGGAA-3′ [72]; CCL4, 5′-CTTGGAGTTGAACTGAGCAGC-3′/5′-AGAGGGGCAGGAAATCTGAA-3′ [126]; SERCA2b, 5′-GAGAACGCTCACACAAAGACC-3′/5′-CAATTCGTTGGAGCCCCAT-3′ [120]; COX-2, 5′-TGAGCAACTATTCCAAACCAGC-3′/5′-GCACGTAGTCTTCGATCACTATC-3′ [74].
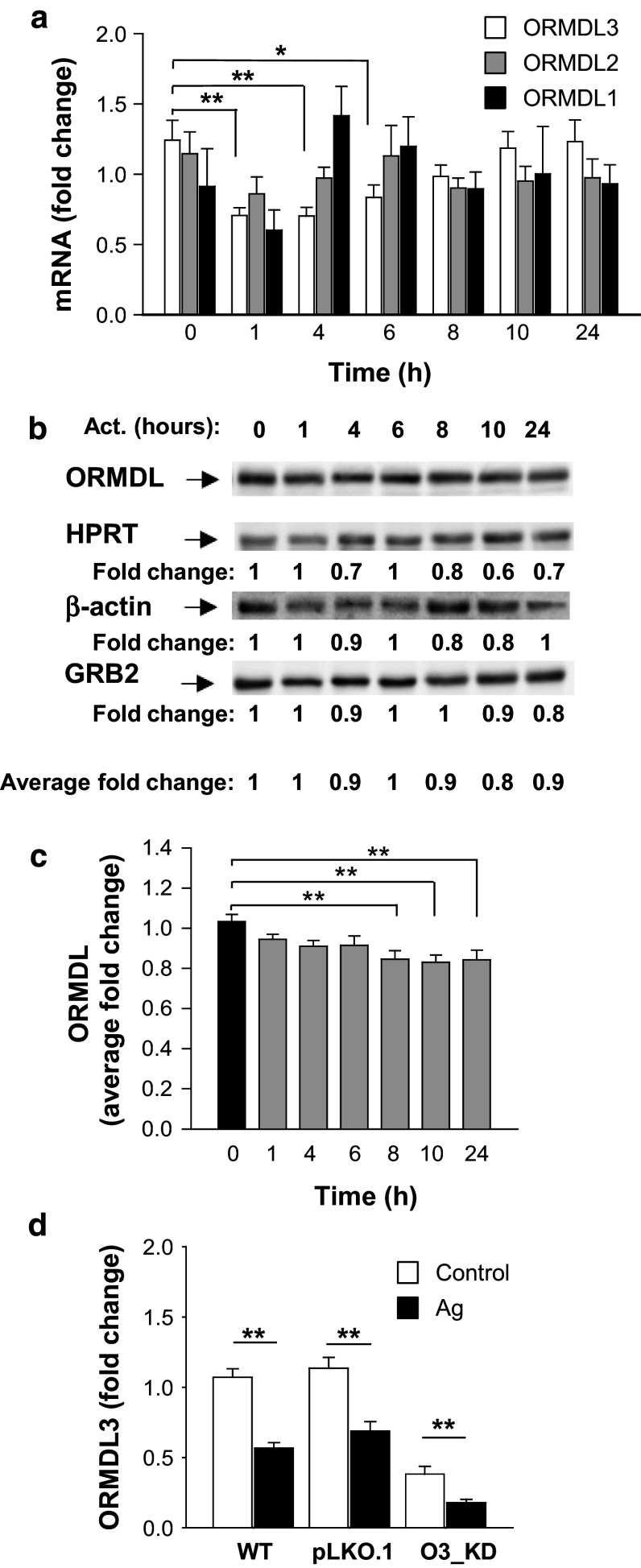
Fig. 2.
Changes in ORMDL3 expression in Ag-activated BMMCs. a BMMCs were sensitized for 16 h with IgE and then activated with Ag (100 ng/ml) or not (0 h) for the indicated time intervals. ORMDL3, ORMDL2, and ORMDL1 mRNAs were quantified by RT-qPCR and normalized as described in “Materials and methods”. The means and SEMs were calculated from three independent experiments performed in duplicate. b Immunoblot quantification of ORMDL proteins at various time intervals after activation of IgE-sensitized BMMCs with Ag (100 ng/ml). Numbers under the immunoblots indicate the relative amounts of ORMDL3 normalized to its amount in nonactivated cells and the amounts of HPRT, β-actin, and GRB2 were used as loading controls (Average fold change). A representative immunoblot from seven independent experiments with similar results is shown. c Statistical evaluation of ORMDL proteins expression is shown. ORMDL3 was normalized to its amount in nonactivated cells and the amount of the average fold change of three loading controls mentioned above. d RT-qPCR quantification of ORMDL3 in nonactivated (control) or Ag-activated (100 ng/ml; 1 h) BMMCs with the empty vector (pLKO.1) or O3_KD. Data are normalized as in a. The means and SEMs were calculated from six independent experiments performed in duplicate
For detection of cytokine secretion, the nano-iPCR method was used as described [35]. Briefly, anti-TNF-α, anti-IL-13 (each at 1 µg/ml), or anti-IL-6 (2 µg/ml) in 100 mM borate buffer (pH 9.5) was dispensed in 50 µl aliquots into wells of a real-time 96-well plate (Eppendorf). After overnight incubation at 4 °C, each well was washed four times with 200 µl of TBST (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 0.05 % Tween 20) and the remaining binding sites were blocked by 2 h incubation at 37 °C with TBST supplemented with 2 % BSA. After washing, 50 µl of serial dilutions (0.1–100 ng/ml) of recombinant TNF-α, IL-13 or IL-6, or the tested samples diluted in PBS-1 % BSA were added. The samples were incubated 1 h at 37 °C and after washing with TBST, 50 µl of gold nanoparticles armed with thiolated DNA oligonucleotide template were added and further processed as described [35]. For calculation of the cytokine concentrations, the obtained Ct values were compared with those from the corresponding calibration curves.
PGD2 measurements
IgE-sensitized mast cells were seeded at 2 × 105 cells per well in 100 μl of BSS–BSA buffer in a 96-well culture plate. Cells were stimulated by adding 100 µl of Ag to a final concentration of 100 ng/ml for 5 h. Cell-free supernatants were collected and assessed for PGD2 using competitive enzyme immunoassay based on measurement of PGD2-methoxylamine hydrochloride derivate according to the manufacturer’s conditions (Cayman Chemicals). For detection, the Infinite 200 M plate reader was used. Supernatants of nonactivated and activated cells were diluted 1/10 and 1/40, respectively, to be read within the range of the respective standard curve.
Gel electrophoresis and immunoblotting
Whole-cell extracts were prepared by washing the cells in cold PBS, solubilizing them in hot SDS-sample buffer [36], sonicating, and boiling for 5 min. Proteins were size fractionated on 10 or 12.5 % SDS-PAGE gels, electrophoretically transferred onto nitrocellulose membrane, and analyzed by immunoblotting with protein- or phosphoprotein-specific Abs. Bound primary Abs were detected with HRP-conjugated secondary Abs. The HRP signal was detected with chemiluminescence reagent [37] and quantified by a Luminescent Image Analyzer LAS 3000 (Fuji Photo Film Co.). Aida software (Raytest GmbH) was used for signal quantification. Protein levels were normalized to the corresponding controls.
Flow cytometry analysis
To quantify the surface expression of FcεRI and KIT, BMMCs (3 × 105/ml) were exposed simultaneously to anti-mouse FcεRI-FITC and anti-mouse KIT-allophycocyanin for 30 min on ice. Then, the cells were washed with ice-cold PBS and evaluated using an Accuri C6 flow cytometer (BD Biosciences).
Cell adhesion and spreading
IgE-sensitized BMMCs were loaded with calcein-AM and transferred into a 96-well plate (Thermo Scientific) coated with fibronectin (10 ng/ml) diluted in PBS [38]. Cells activated for 30 min with 100 ng/ml of Ag were examined for adhesion assay using the Infinite 200 M plate reader with excitation and emission filters at 485 nm and 538 nm, respectively. For cell spreading, wells in 96-well glass-bottom plates (InVitroSci) were coated with fibronectin (50 µl; 50 µg/ml) diluted in PBS. Wells were then washed with PBS, and 30 × 103 cells in BSS-0.1 % BSA were added per well. Cells were allowed to attach for 30 min at 37 °C, washed, and activated or not with Ag. After 30 min, cells were fixed with 3 % paraformaldehyde in PBS for 30 min at room temperature. For filamentous (F)-actin staining, cells were exposed to Alexa Fluor 488-phalloidin conjugate (Invitrogen, #A12379), diluted 1:100 in PBS supplemented with L-α-lysophosphatidylcholine (80 μg/ml). After 1 h, cells were washed and kept in PBS supplemented with Hoechst 33258 stain (1 µg/ml) until measurement. Cells were then examined with the ScanR system (Olympus).
Chemotactic response
Ag-Mediated chemotactic responses were evaluated using 24-well Transwell chambers (Corning) with 8 μm polycarbonate filters as described previously [38]. Cells migrating into lower compartments within the 8-h incubation period were counted using an Accuri C6 Flow Cytometer (Becton–Dickinson).
F-actin assay
The total amount of F-actin in nonactivated and Ag-activated cells was determined by flow cytometry. BMMCs in a 96-well plate (5 × 104 cells per well) were exposed to various stimuli at 37 °C, fixed with 3 % paraformaldehyde in phosphate-buffered saline, and then permeabilized and stained in a single step by a mixture of lysophosphatidylcholine (200 mg/ml) and 1000× diluted Alexa Fluor 488-phalloidin (Molecular Probes) in phosphate-buffered saline. The fluorescence intensity was measured using LSRII flow cytometer (Becton–Dickinson). The acquired data were analyzed using FlowJo software (Tree Star Inc).
Confocal microscopy
IgE-sensitized BMMCs (3 × 105) were attached (60 min at 37 °C) to a fibronectin-coated multitest slide (MP Biomedicals) and then activated by Ag. After 30 min, the cells were fixed with 4 % paraformaldehyde for 15 min at room temperature and permeabilized with 0.3 % Triton X-100 for 20 min. Free binding sites were blocked with 5 % donkey serum (Jackson ImmunoResearch Laboratories) in PBS and the cells were stained with a mixture of rabbit Abs against p65 subunit of NF-κB diluted 1:400 (#8242) and 1:200 (#sc-372), followed by labeling with secondary antibody, donkey anti-rabbit-Alexa Fluor 488 conjugate, and diluted 1:200 in PBS containing 1 % BSA. ORMDL was labeled with rabbit polyclonal serum (described in Antibodies and reagents) followed by labeling with donkey anti-rabbit-Alexa Fluor 568 (1:100). After 60 min incubation, the cells were washed and mounted in Mowiol 4-88, supplemented with 1 µg/ml Hoechst 33258 to label nuclei. Samples were examined with a confocal laser scanning microscope Leica TCS SP5 equipped with an X63/1.4.N.A. oil-immersion objective. STIM1-YFP (kind gift of Dr. T. Meyer, Stanford University Medical School) was used as an endoplasmic reticulum marker. Golgi marker (YFP-GT) was from Clontech Laboratories. ORMDL3-YFP was cloned into the N1-YFP plasmid (Clontech) using forward primer 5′-AAACTCGAGGCCGCCACCATGAATGTG GGCACAGCAC-3′ and reverse complementary primer 5′-AAAGAATTCGGTACTTATTGATTCCAAAG-3′, introducing XhoI and EcoRI restriction endonuclease sites (underlined), respectively. Amaxa Nucleofector II (Lonza Cologne AG, Cologne, Germany) was used to nucleofect mast cells using a Mouse Macrophage Kit and program Y-001.
Image analysis
Image processing and analysis were performed using CellProfiler software (Broad Institute, Boston, MA) [39]. For cell spreading, areas corresponding to individual cells were identified by F-actin staining with Alexa Fluor 488-phalloidin and the mean values were calculated from 200 to 400 cells in each sample. The fraction of NF-κB p65 subunit in the nucleus was determined as the difference between NF-κB mean fluorescence in the nucleus (stained with Hoechst 332578) and NF-κB mean fluorescence in the cytoplasm as described [40]. Fifty to hundred cells were evaluated per sample and the values obtained for each cell were plotted in GraphPad Prism (San Diego, CA, USA). Other charts were done in SigmaPlot 8.0 (San Jose, CA, USA).
Short interfering RNA (siRNA)-mediated inhibition of ORMDL3 expression and measurements of PCA
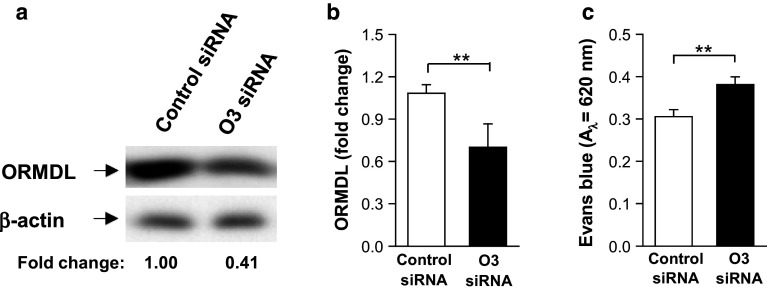
To silence ORMDL3 in the mouse ear, we followed the experimental procedure described by Kanada et al. [41] with some modifications. The specificity of ORMDL3 siRNAs was first confirmed by immunoblotting analysis of lysates from BMMCs nucleofected by Amaxa Nucleofector II 48 h earlier with 1 µg of ORMDL3 siRNA pool (Accell Mouse Ormdl3 siRNA; E-049023-00-0005; Dharmacon) or 1 μg control siRNAs ORMDL3 (O3_siRNA; Accell Non-targeting Pool; D-001910-10-05; Dharmacon) using a Mouse Macrophage Kit and program Y-001 as recommended by the manufacturer (Lonza). For PCA, 20 μL of PBS containing 4 μg/ml of anti-TNP-specific IgE and 2.5 μg of ORMDL3 siRNAs pool was injected into the right ear. As a control, 20 μL of anti-TNP-specific IgE (4 μg/ml) and 2.5 μg of control siRNA was injected intradermally into the left ear. After 48 h, the mice were challenged with an intravenously injected PBS (200 µl) containing Ag (100 µg TNP–BSA) and 1 mg of Evans blue. Two hours later, the mice were killed and the ears removed for measurement of the amount of extravasated dye. Formamide (0.75 ml) was then added to each ear, which was then homogenized with the T-25 ULTRA-TURRAX Digital High-Speed Homogenizer Systems (IKA) and incubated at 80OC for 2 h. The samples were centrifuged at 14,000×g for 15 min and supernatants were used for measurement of absorbance at 620 nm.
Statistical analyses
Unless stated otherwise, the significance of intergroup differences was evaluated by Student’s t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Microsoft Excel 2010 or GraphPad Prism was used for statistical analysis evaluation.
Results
Properties of BMMCs with reduced or enhanced expression of ORMDL3
Although ORMDL3 expression was described in various cells of the immune system [1, 18], its presence in mast cells has not yet been determined. Here, we used RT-PCR to show the expression of ORMDL3 in BMMCs. We found that ORMDL3 as well as ORMDL1 and ORMDL2 are expressed in BMMCs (Fig. 1a). We did not observe any variations in the expression of ORMDL3 during BMMC cultivation in medium supplemented with IL-3 and SCF (data not shown). Using BMMCs transduced with markers of endoplasmic reticulum or Golgi apparatus, we confirmed previous findings [16, 18] that endogenous ORMDL proteins detected by the NVG antibody recognizing all three ORMDLs (Fig. S1a, c, d) are associated with the endoplasmic reticulum and perinuclear membrane, but not with the Golgi apparatus (Fig. 1b). Next, we examined whether the changes in ORMDL3 expression influence mast cell effector functions. To induce ORMDL3 knockdown (O3_KD), BMMCs were transduced using the pLKO.1 lentiviral vector with ORMDL3 shRNAs (200, 201, 202, and 203, Fig. 1c). As controls, BMMCs were transduced with the empty pLKO.1 vector. Quantification of ORMDL3 mRNA expression by RT-qPCR showed its 30–80 % decrease after transduction of ORMDL3 shRNAs (Fig. 1c). To find out whether silencing of ORMDL3 is accompanied by changes in expression of other ORMDL family members, we assessed the expression of all members of the ORMDL family in BMMCs with O3_KD. Data in Fig. 1d indicate that silencing with the O3_KD vector (shRNA 200) led to a significant reduction of ORMDL3 mRNA without any effect on the expression of ORMDL1 and ORMDL2 mRNA. For production of cells with enhanced expression of ORMDL3, we used pCDH vectors containing ORMDL3 cDNA tagged with myc at the N terminus (myc-O3) or C terminus (O3-myc). Cells transduced with ORMDL3 cDNAs exhibited an 8- to 12-fold increase in ORMDL3 mRNA when compared with cells transduced with the empty pCDH vector, but expression of ORMDL1 and ORMDL2 mRNAs was not significantly changed (Fig. 1e). It should be noted that orientation of the tag attached to ORMDL3 influenced the mobility of the proteins in the gel ([11]; Fig. 1f). However, because in functional assays we did not see any significant differences between cells transduced with the two constructs, the data obtained with both of them were pooled and are presented as cells with ORMDL3 overexpressors (O3_OE). The observed downregulation or upregulation of ORMDL3 mRNA in cells with O3_KD or O3_OE, respectively, resulted in the expected changes in the intensity of the band corresponding to the ORMDL family proteins as detected by immunoblotting with polyclonal serum. The amount of the band corresponding to the ORMDL proteins was reduced by 60–80 % depending on the shRNA used (Fig. 1f). The characteristics of the rabbit serum used for immunoblotting (TPF) are shown in Fig. S1b, c. Both sera (NVG and TPF) recognize other members of the ORMDL family, as determined by their reactivity with cells expressing cDNAs encoding ORMDL1 and ORMDL2 (Fig. S1c). Therefore, we refer to the corresponding band of 17 kDa as ORMDL. In BMMCs transfected with myc-O3 or O3-myc vectors, ORMDL3 protein was enhanced approximately three to fourfold (Fig. 1f), although the increase of ORMDL3 expression at the mRNA level was more prominent (Fig. 1e). BMMCs with both O3_KD (all four shRNAs) and O3_OE (myc-O3 or O3-myc vectors) had preserved expression of FcεRI and KIT receptors on the plasma membrane (Fig. S2), but statistical evaluation revealed that shRNAs 201 and 202 exhibited significant changes in the expression of c-Kit (P < 0.05) and shRNA 201 as well as in FcεRI expression (Fig. 1g; P < 0.05). Based on these results, we excluded shRNAs 201 and 202 from further work. All experiments with O3_KD are based on shRNA 200. Cells transduced with shRNA 203 exhibited similar properties to those with shRNA 200. Based on these findings, experimental data were pooled and all data are presented as O3_KD. Alcian blue staining of cytospin slides showed that the morphology of O3_KD and O3_OE mast cells was not different from that of cells transduced with appropriate controls (see examples in Fig. 1h).
Fig. 1.
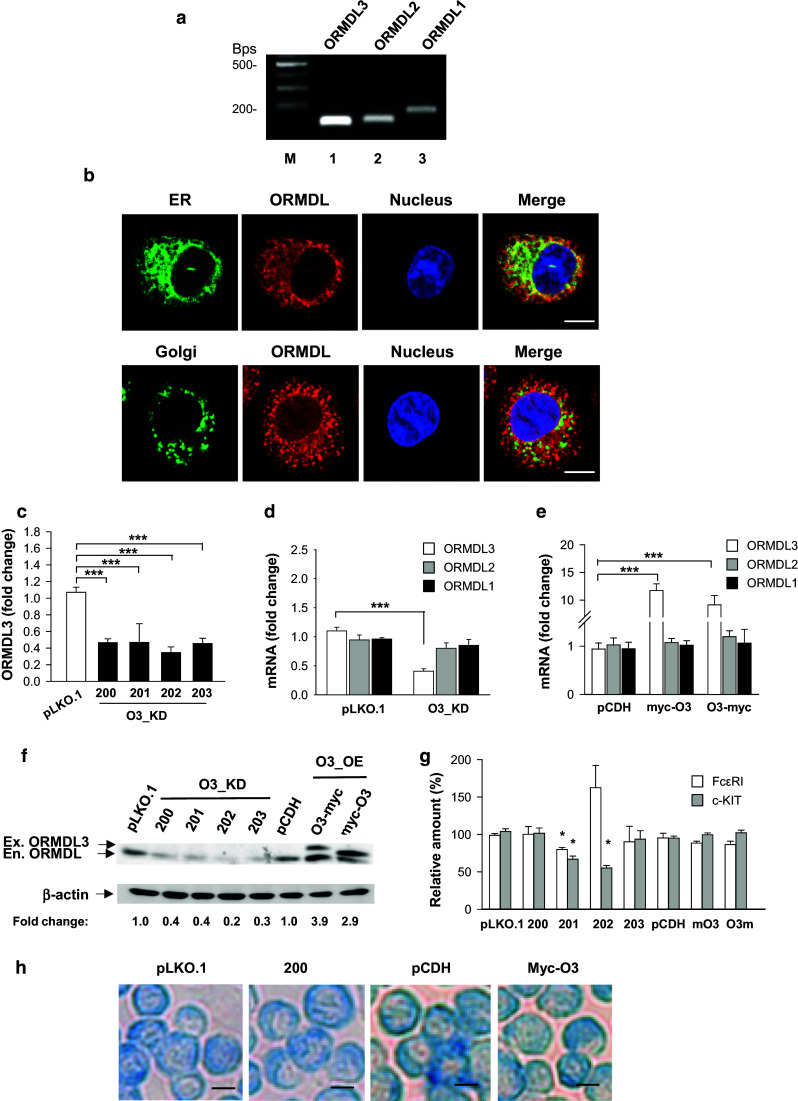
Properties of BMMCs with reduced or enhanced ORMDL3 expression. a Expression of ORMDL1-3 mRNAs in BMMCs as determined by RT-PCR followed by agarose gel electrophoresis and staining with ethidium bromide. DNA marker in bps is shown in line M. b Colocalization of endogenous ORMDL proteins (shown in red pseudocolor) with STIM1-YFP, a marker of endoplasmic reticulum (ER upper panel; green pseudocolor), but not with GT-YFP, a marker of Golgi (lower panel green pseudocolor) is shown. Nuclei were stained with Hoechst 33258 (shown in blue pseudocolor); bars 5 µm. c BMMCs with O3_KD were obtained after transduction with lentiviruses containing four shRNAs denoted 200, 201, 202, and 203. Control cells were transduced with empty pLKO.1 vector. ORMDL3 mRNAs were quantified by RT-qPCR. d RT-qPCR quantification of individual ORMDL family members in cells with O3_KD or in control cells transduced with empty pLKO.1 vector. e BMMCs with O3_OE were obtained after transduction with lentiviruses containing ORMDL3 cDNAs terminally tagged with myc at the N-(myc-O3) or C-(O3-myc) end. Control cells were transduced with empty pCDH vector. The levels of ORMDL1 and ORMDL2 mRNAs are shown. RT-qPCR data in c–e were normalized as described in “Materials and methods” and represent the means and SEMs calculated from three to seven independent experiments. f Quantification of ORMDL proteins (serum not specific for ORMDL3) by immunoblotting in whole-cell lysates after transduction of the cells as in c and e. Numbers under the immunoblots indicate the amounts of ORMDL proteins normalized to control cells transfected with pLKO.1 vector (for O3_KD) or pCDH vector (for O3_OE) and to the amount of β-actin used as a loading control (fold change). The positions of endogenous (En.) and exogenous (Ex.) ORMDL3 and β-actin are indicated by arrows. g Statistical evaluation of FcεRI and c-Kit expression in the transduced BMMCs. h Alcian blue staining of cytospin preparations of BMMCs transduced as above; bars 10 µm
Ag activation of BMMCs induces downregulation of ORMDL3
A previous study showed that challenge with allergen induced expression of ORMDL3 in mouse bronchial epithelial cells, lung macrophages, or BM-derived eosinophils, but not in peripheral blood neutrophils [18]. Moreover, increased ORMDL3 expression in bronchial airway epithelial cells was stimulated with IL-4 and IL-13 [18] and in eosinophils triggered with IL-3 and eotaxin-1 [20]. In further experiments, we therefore examined whether FcεRI activation of BMMCs would also lead to changes in ORMDL3 expression. Interestingly, in IgE-sensitized cells, activation with Ag resulted in a significant decrease of ORMDL3 mRNA expression 1, 4, and 6 h after FcεRI triggering, but ORMDL1 and ORMDL2 mRNA expression was not significantly affected (Fig. 2a). At the protein level, for detection we used polyclonal antibodies recognizing all members of the ORMDL family (Fig. S1a–c). Data presented in Fig. 2b and c indicate that there is a slight, but significant decrease in ORMDL family member expression in cells activated for 8–24 h with Ag. Attenuation of ORMDL3 transcription in activated cells was also observed in cells with O3_KD, in which the residual amount of ORMDL3 mRNA was further lowered (Fig. 2d). Thus, FcεRI-activated mast cells exhibit downregulation of ORMDL3 and in this regard differ from activated eosinophils, lung macrophages, and bronchial airway epithelial cells.
BMMCs with reduced or enhanced expression of ORMDL3 do not exhibit changes in SYK tyrosine phosphorylation, degranulation, and Ca2+ response after FcεRI triggering
FcεRI-mediated signaling events are initiated by tyrosine phosphorylation of the FcεRI β and γ subunits by Src family kinase LYN, followed by propagation of the signal through activity of the Src family kinases and SYK kinase [22]. To examine a possible role of ORMDL3 in FcεRI signaling, we first examined phosphorylation of SYK in Ag-activated BMMCs with reduced or enhanced ORMDL3 expression. Our data indicate that cells with O3_KD (Fig. 3a) or O3_OE (Fig. 3b) show similar SYK phosphorylation as the corresponding controls.
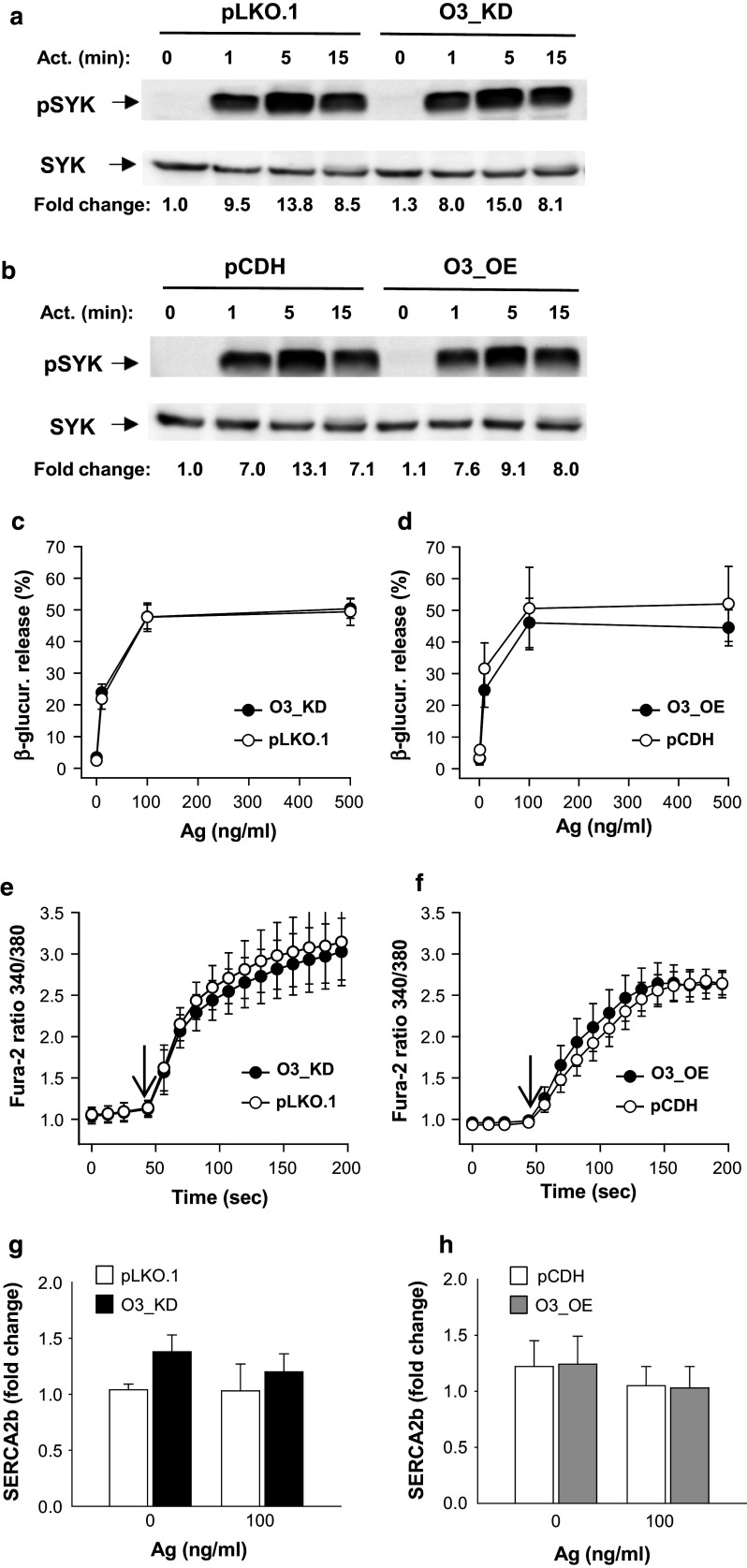
Fig. 3.
Early activation events in BMMCs with enhanced or reduced ORMDL3 expression. a Ag-induced tyrosine phosphorylation of SYK as determined by immunoblotting of whole-cell lysates from BMMCs with O3_KD or control cells transfected with empty pLKO.1. IgE-sensitized cells were activated for various time intervals with Ag (100 ng/ml). The positions of pSYK and SYK, used as loading controls, are indicated by arrows. b Phosphorylation of SYK was examined as in a, except that the lysates were from cells with O3_OE or control cells transfected with empty pCDH. Representative immunoblots from at least three performed in each group are shown in a and b. Numbers under the immunoblots indicate the relative amounts of pSYK normalized to its amounts in nonactivated cells transduced with empty vectors and total amounts of SYK in individual samples (fold change). c, d β-Glucuronidase release in IgE-sensitized BMMCs. The cells were activated with various concentrations of Ag and after 30 min β-glucuronidase released into the supernatant was determined. Data presented are means and SEMs for cells with O3_KD (c, n = 19), pLKO.1 controls (c, n = 19), O3_OE (d, n = 8), and pCDH control cells (d, n = 5). e, f Calcium response in IgE-sensitized BMMCs activated with Ag (100 ng/ml) for various time intervals. Arrows show the time points when Ag was added. Means and SEMs were calculated for cells with O3_KD (e, n = 16), pLKO.1 controls (e, n = 8), O3_OE (f, n = 3), and pCDH controls (f, n = 5). g, h RT-qPCR quantification of SERCA2b mRNA in resting and activated BMMCs with O3_KD (g, n = 4) pLKO.1 controls (g, n = 4), O3_OE (h, n = 4) and pCDH controls (h, n = 4). Data for SERCA2b mRNA were normalized as described in “Materials and methods”
Next, we examined Ag-induced degranulation estimated by the release of β-glucuronidase from preformed secretory lysosomes. We found that BMMCs with O3_KD (Fig. 3c) or O3_OE (Fig. 3d) exhibited similar degranulation as the control cells. We also compared cells transduced with pLKO.1 and nontransduced wild-type (WT) cells to show that mast cell degranulation is not changed by lentivirus transduction (Fig. S3a).
Previous studies have shown that ORMDL3 affects Ca2+ mobilization in lymphocytes and eosinophils [16, 17, 20] and regulates the expression of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA)2b [19]. Interestingly, BMMCs with O3_KD (Fig. 3e) or O3_OE (Fig. 3f) exhibited similar Ca2+ mobilization to cells transduced with empty pLKO.1 or pCDH vectors. We also compared cells transduced with pLKO.1 and WT cells to show that calcium mobilization in mast cells is not changed by lentivirus transduction (Fig. S3b). We also found that expression of mRNA for SERCA2b was not significantly changed in activated and nonactivated mast cells with O3_KD (Fig. 3g) or O3_OE (Fig. 3h) when compared with the corresponding controls. The mRNA levels of SERCA2b in cells transduced with pLKO.1 were comparable to those in WT cells (Fig. S3c). In line with the SERCA2b expression, the basal levels of [Ca2+]i were not changed in O3_KD (Fig. S4a) or O3_OE (Fig. S4b) when compared with the corresponding controls. We conclude that early FcεRI-induced activation events, including SYK tyrosine phosphorylation, degranulation, and calcium responses in mast cells, are not affected by enhanced or reduced expression of ORMDL3. Furthermore, we show that changes in ORMDL3 protein levels have no significant effect on SERCA2b expression.
Negative regulatory role of ORMDL3 in the expression of cytokines and chemokines
Mast cells are potent producers of various cytokines and chemokines involved in both asthma and autoimmune diseases [22, 23, 42, 43]. In further experiments, we therefore quantified the levels of mRNA encoding cytokines (TNF-α, IL-6, and IL-13; Fig. 4a) and chemokines (CCL3 and CCL4; Fig. 4b) in BMMCs with O3_KD. Using RT-qPCR, we found that all the studied transcripts for cytokines and chemokines were significantly increased in cells with O3_KD when compared with control cells transduced with empty pLKO.1 vector in both nonactivated and Ag-activated cells (Fig. 4a, b). The observed increase in cytokine mRNA levels corresponded to enhanced secretion of the cytokines TNF-α, IL-6 and IL-13 from Ag-activated BMMCs with O3_KD (Fig. 4c). In contrast, no significant changes in the transcription of TNF-α, IL-6, and IL-13 at the RNA (not shown) or protein (Fig. 4d) level were observed when BMMCs with O3_OE were compared with the corresponding controls. These data indicate that ORMDL3 in mast cells is a negative regulator of the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-13 and chemokines CCL3 and CCL4.
Fig. 4.
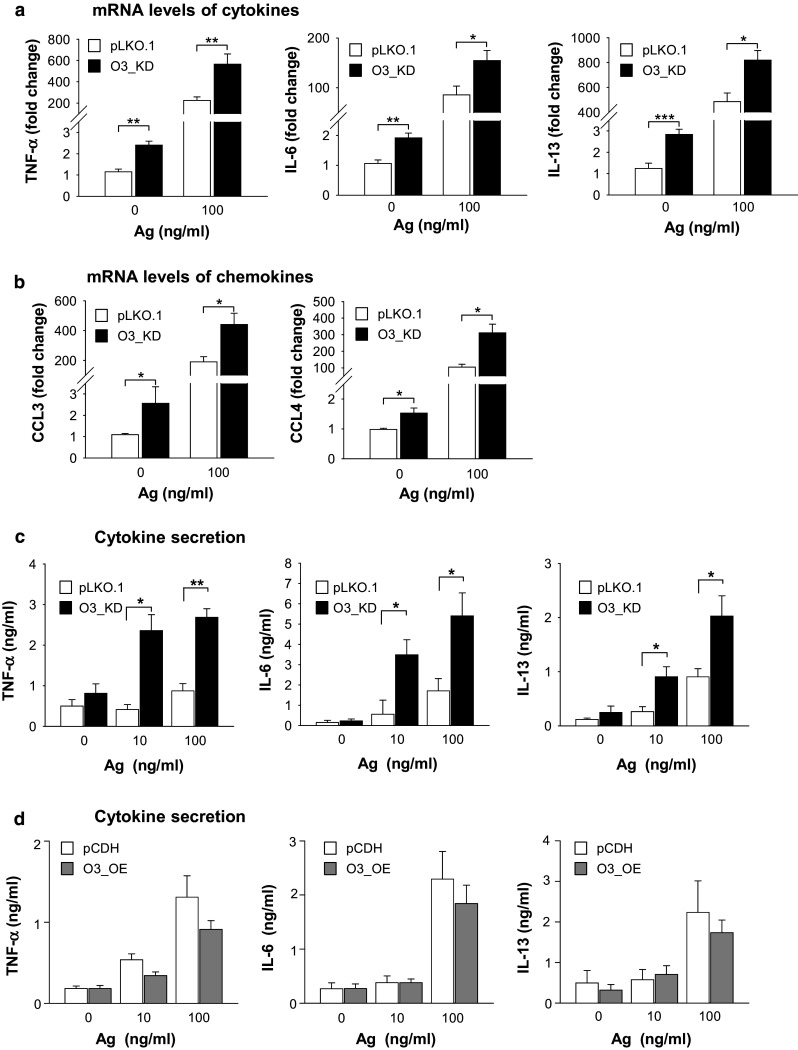
Expression of cytokine and chemokine genes and production of cytokines in BMMCs with O3_KD and O3_OE. a RT-qPCR quantification of mRNAs encoding TNF-α, IL-6, and IL-13 in nonactivated or Ag-activated (100 ng/ml; 1 h) BMMCs with O3_KD and the corresponding control cells, pLKO.1. Means and SEMs were calculated from 13 to 16 independent experiments. b RT-qPCR quantification of mRNA for CCL3 and CCL4 chemokines from cells as in a. The means and SEMs were calculated from six to eight experiments. Data in a and b were normalized as described in “Materials and methods”. c, d Quantification of cytokines TNF-α, IL-6, and IL-13 secreted into the supernatants of nonactivated or Ag-activated (100 ng/ml; 6 h) BMMCs with O3_KD or pLKO.1 control (c) and O3_OE or pCDH control (d) as determined by nano-iPCR. The means and SEMs were calculated from four to five independent experiments
ORMDL3 functions as a negative regulator of Ag-mediated chemotaxis
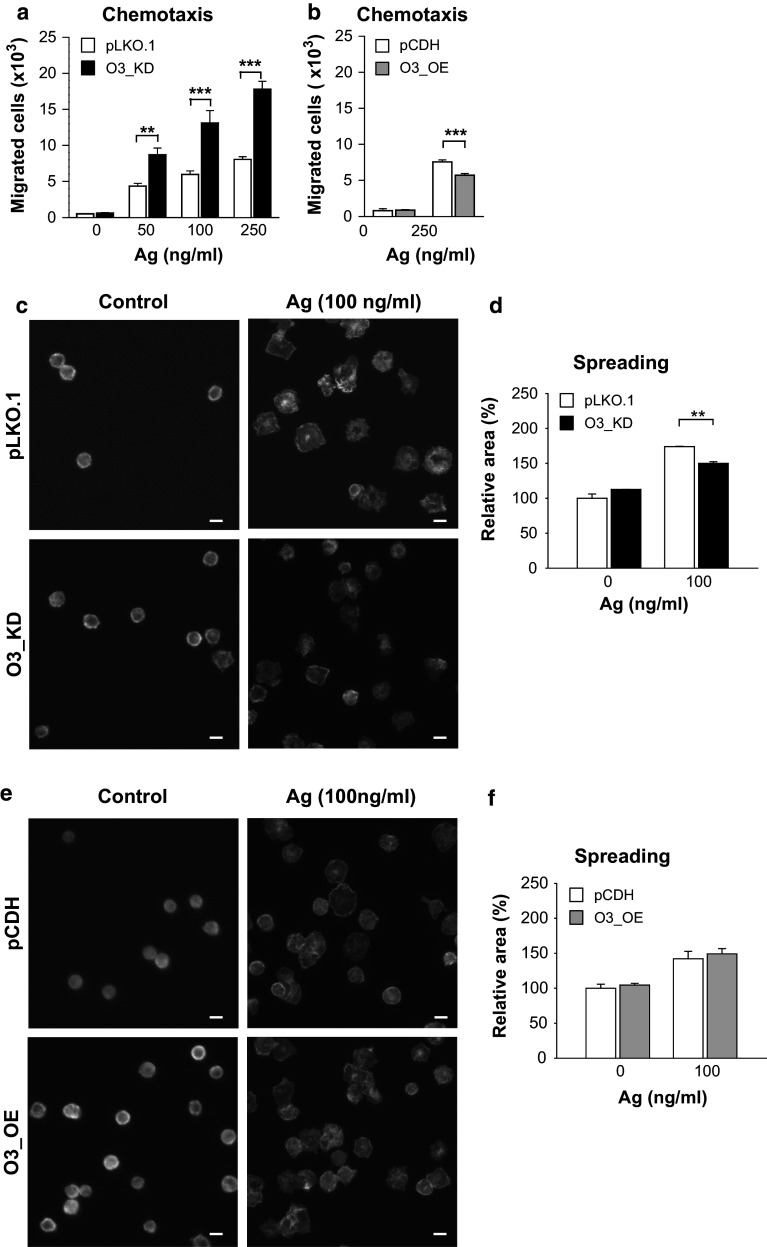
The observed changes in the expression of genes for cytokines and chemokines depending on the expression of ORMDL3 led us to examine the capability of IgE-sensitized cells to migrate toward Ag and to adhere and spread on fibronectin-coated substrates. BMMCs with O3_KD exhibited a significantly stronger chemotactic response to all tested concentrations of Ag when compared with control cells transfected with empty pLKO.1 vector (Fig. 5a). In contrast, BMMCs with O3_OE showed a less efficient chemotactic response than the corresponding control cells (Fig. 5b). We also compared cells transduced with pLKO.1 and WT cells to show that mast cell migration is not changed by lentivirus transduction (Fig. S3d). When adhesion to fibronectin was measured, cells with O3_KD (Fig. S5a) or O3_OE (Fig. S5b) exhibited adhesion similar to the corresponding control cells. In contrast, spreading on fibronectin-coated surfaces after exposure to Ag was reduced in cells with O3_KD (Fig. 5c, d). When compared to control cells, cells with O3_OE exhibited no significant difference in Ag-induced spreading (Fig. 5e, f). We also compared cells transduced with pLKO.1 and WT cells and showed that Ag-induced mast cells adhesion to and spreading on fibronectin was not changed by lentivirus transduction (Fig. S3e, f). Finally, we examined the depolymerization of F-actin in Ag-activated cells with O3_KD (Fig. S5c) or O3_OE (Fig. S5d) and corresponding controls and found that F-actin content was independent of ORMDL3 expression levels.
Fig. 5.
Negative regulatory role of ORMDL3 in Ag-mediated chemotaxis, but not cell spreading on fibronectin. a, b Migration of BMMCs toward various concentrations of Ag was determined in IgE-sensitized BMMCs with O3_KD (a) or O3_OE (b) and the corresponding controls. The means and SEMs were calculated from four to eight independent experiments performed in duplicate. c–f Spreading of BMMCs with O3_KD (c, d), O3_OE (e, f), and the corresponding controls. The cells were attached to fibronectin-coated slides and activated with Ag (100 ng/ml) for 30 min. Representative fluorescent images are shown in c and e; bars 10 µm. Quantification and statistical evaluation of data obtained as in c and e are shown in d and f, respectively. The means and SEMs were calculated from five independent experiments
Upregulation of NF-κB signaling axis in mast cells with O3_KD
Previous studies with eosinophils showed that increased expression of ORMDL3 enhanced translocation of NF-κB into the nucleus and its activation [20]. Based on our findings that reduced expression of ORMDL3 upregulated the expression of cytokine and chemokine genes (Fig. 4a–d) and that these mediators were directed by transcription factor NF-κB [44–48], we decided to analyze the NF-κB signaling axis in BMMCs with enhanced or decreased ORMDL3 protein levels. First, we examined the phosphorylation of IκBα, a negative regulator of NF-κB signaling. We found that 5 min after Ag activation, BMMCs with O3_KD exhibited higher phosphorylation of IκBα than control pLKO.1 cells. Interestingly, we found that IκBα in activated BMMCs with O3_KD is more degraded than in control cells (Fig. 6a). This is in accord with the previously described observation that phosphorylation of IκBα triggers its degradation [49]. Densitometry evaluation of the data and normalization of pIκBα to LYN kinase, used as a loading control, showed significantly higher IκBα phosphorylation in O3_KD cells than control pLKO.1 cells (Fig. 6b). BMMCs with O3_OE showed a small, but nonsignificant decrease of IκBα phosphorylation (not shown). Based on these data, we next examined the consequences of reduced ORMDL3 levels on translocation of the NF-κB p65 subunit into the nucleus in BMMCs. We found that Ag-induced translocation of the p65 subunit into the nucleus was higher in BMMCs with O3_KD than in control cells (Fig. 6c). The difference was statistically significant (Fig. 6d). Interestingly, resting BMMCs with O3_KD also exhibited increased p65 nuclear localization when compared with resting control pLKO.1 cells. These data are consistent with the findings that basal levels of mRNAs for cytokines (Fig. 4a) and chemokines (Fig. 4b) were higher in cells with O3_KD than in control cells. We also compared cells transduced with pLKO.1 and WT cells and found that p65 nuclear localization after activation was not changed by lentivirus transduction (Fig. S3g).
Fig. 6.
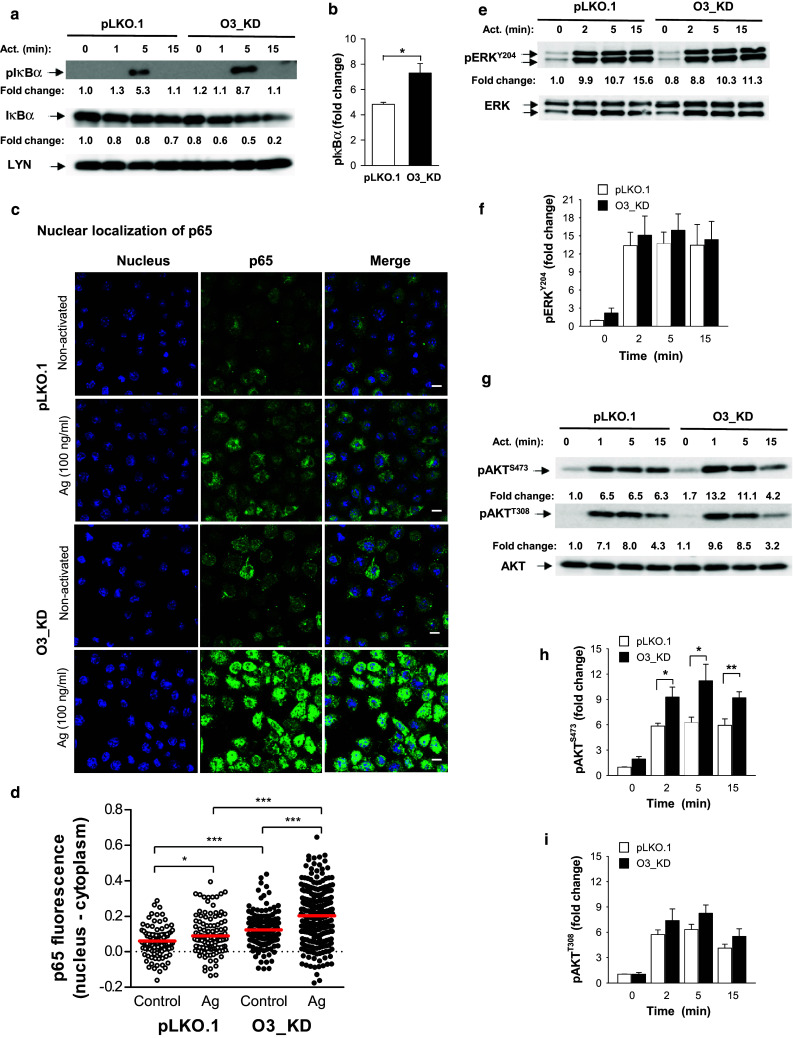
Negative regulatory role of ORMDL3 in AKT and NF-κB signaling axis in Ag-activated BMMCs. a Phosphorylation of IκBα and its amount was determined by immunoblotting of whole-cell lysates from BMMCs with O3_KD and control cells (pLKO.1) with the corresponding antibodies. Representative immunoblots from four independent experiments are shown. Numbers under the immunoblots indicate the relative amounts of pIκBα and IκBα normalized to their amounts in nonactivated pLKO.1 control cells and the amount of Lyn used as a loading control (Fold change). b Quantification and statistical evaluation of pIκBα in pLKO.1 control cells and O3_KD cells activated with Ag for 5 min as in a. Means and SEMs were calculated from four independent experiments. c Representative confocal microscopy pictures of NF-κB p65 subunit (p65) localization in the nuclei (stained with Hoechst 33258) and cytoplasm in nonactivated or Ag-activated (100 ng/ml; 30 min) BMMCs with O3_KD or pLKO.1 control; bars 10 µm. d Quantification of NF-κB p65 subunit localization in the nucleus of nonactivated or Ag-activated (100 ng/ml, 30 min) O3_KD and pLKO.1 control cells. Values from two independent experiments as in c (50–100 cells per experiment) were plotted; each symbol represents one cell, red horizontal lines indicate means. e–i Phosphorylation of ERK at Tyr 204 (e, f), AKT at Ser 473 (g, h), and AKT at Thr 308 (g, i) was assessed by immunoblotting with the corresponding antibodies of the whole-cell lysates from BMMCs with O3_KD or pLKO.1 controls. Representative immunoblots from at least three in each group are shown in e and g. Numbers under the immunoblots indicate the relative amounts of pERK (e) or pAKT (g) normalized to their amounts in nonactivated pLKO.1 control cells and the amount of ERK or AKT used as loading controls (fold changes). Quantification and statistical analyses of differences in fold changes between cells with O3_KD and pLKO.1 controls in pERKY204 (f), pAKTS473 (h), and pAKTT308 (i) are also shown. Means and SEMs were calculated from three experiments in each group
To determine which signaling pathways are involved in enhanced IκBα phosphorylation we examined phosphorylation of ERK kinase, which is involved in IκBα phosphorylation [50]. We found that mast cells with decreased expression of ORMDL3 exhibited similar ERK phosphorylation as pLKO.1 control cells (Fig. 6e, f). Next, we therefore tested phosphorylation of AKT kinase, which is a positive regulator of NF-κB signaling [51]. Interestingly, BMMCs with O3_KD exhibited significantly enhanced phosphorylation of AKT at Ser 473 at all examined time points, but phosphorylation of AKT at Thr 308 was not significantly affected (Fig. 6g–i). To exclude the possibility that the observed immunological responses are not specifically activated by siRNA [52], we compared the phosphorylation of IκBα and AKT (at Ser 473) in WT cells, BMMCs transduced with empty pLKO.1, or cells transduced with NT shRNA (Fig. S6a). We found that cells with O3_KD showed enhanced responses when compared with all controls. We also used the same controls in experiments with cytokines to demonstrate that lentiviral transduction had no impact on cytokine production and that cytokines were specifically increased by O3_KD (Fig. S6b–d). Collectively, these data demonstrate that decreased expression of ORMDL3 is accompanied in Ag-activated cells by enhanced phosphorylation of AKT, increased translocation of the p65 subunit into the nucleus, and enhanced production of cytokines.
ORMDL3 is a negative regulator of COX-2 expression and PGD2 synthesis
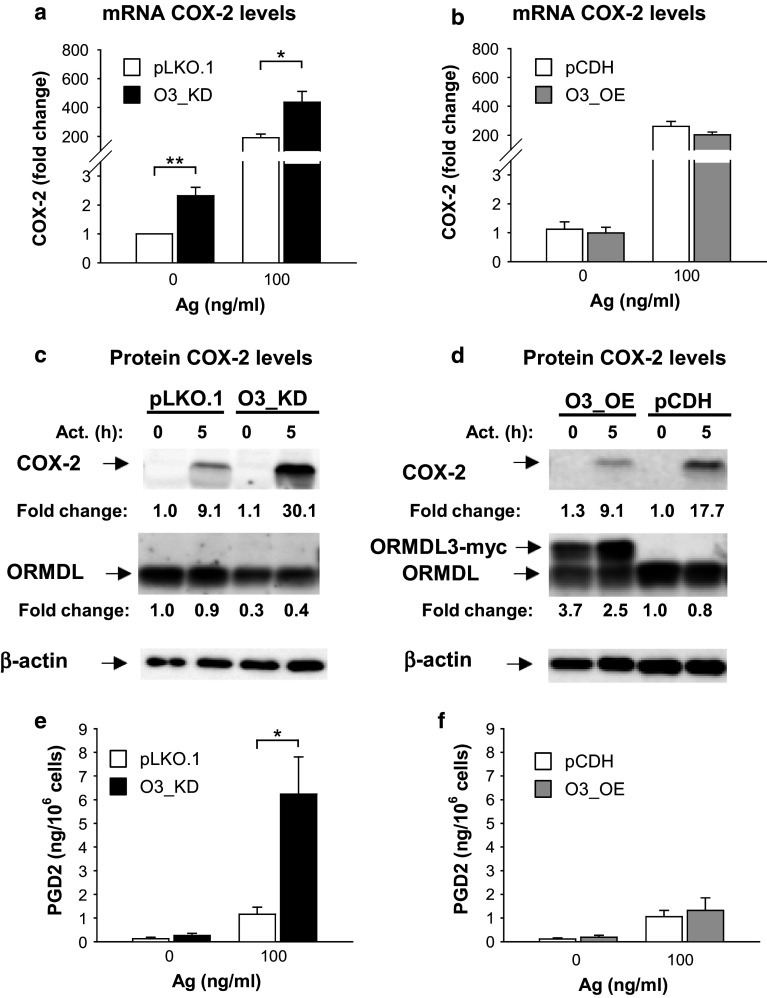
We also examined the expression of an inducible enzyme, COX-2, which is regulated by NF-κB [53, 54]. COX-2 converts arachidonic acid to PGH2, a precursor of PGD2 and PGE2 [28]. When compared with control cells (pLKO.1), BMMCs with O3_KD exhibited significantly higher levels of COX-2 mRNA than both resting and Ag-activated control cells (Fig. 7a), while cells with O3_OE showed decreased COX-2 mRNA expression when compared with the control cells (pCDH); however, the difference was not significant (Fig. 7b). These data were corroborated by studies of the COX-2 protein in Ag-activated BMMCs. After 5 h stimulation with Ag, we observed a higher increase of COX-2 levels in cells with O3_KD than in control cells (Fig. 7c); this difference was significant (P < 0.05; n = 3). On the other hand, Ag-activated cells with O3_OE showed lower COX-2 levels than the corresponding control cells (Fig. 7d); however, this difference was not significant (P > 0.05; n = 3). The stronger band of exogenous ORMDL3-myc after 5 h of activation in Fig. 7d was reproducibly observed. Ag-induced activation apparently induces enhanced transcription from the CMV promoter. We found that this enhancement was not ORMDL3 specific, rather it depended on the CMV promoter, as several other ORMDL family nonrelated proteins cloned into the same pCDH vector exhibited a similar increase (data not shown).
Fig. 7.
Increased COX-2 expression and PGD2 synthesis in nonactivated or Ag-activated BMMCs with O3_KD. a, b RT-qPCR quantification of COX-2 mRNAs in nonactivated or Ag-activated (100 ng/ml; 1 h) BMMCs with O3_KD (a), O3_OE (b), and the corresponding controls, pLKO.1 (a) and pCDH (b). The means and SEMs were calculated from five independent experiments. c, d Amount of COX-2 protein in nonactivated or Ag-activated (100 ng/ml; 5 h) BMMCs with O3_KD (c), O3_OE (d), and the corresponding controls, pLKO.1 (c) and pCDH (d). Numbers under the COX-2 immunoblots indicate the relative amount of COX-2 normalized to its levels in nonactivated control cells and to the amount of β-actin, used as a loading control, in individual samples (fold change). Levels of ORMDL3 normalized as above are also shown. Representative immunoblots from three independent experiments are shown. e, f Levels of PGD2 released into supernatants of nonactivated or Ag-activated (100 ng/ml; 5 h) BMMCs with O3_KD (e), O3_OE (f), and the corresponding controls, pLKO.1 (e) and pCDH (f). The means and SEMs were calculated from three independent experiments
Supernatants collected from the experiments as depicted in Fig. 7 c, d were assessed for PGD2 levels. We found that Ag-activated mast cells with O3_KD released significantly higher amounts of PGD2 than appropriate controls (Fig. 7e). Furthermore, PGD2 expression was not significantly changed in mast cells with O3_OE (Fig. 7f). To show that COX-2 expression is independent of immunological responses toward dsRNAs, we compared COX-2 production in cells with O3_KD and controls (NT and WT). We found that in O3_KD cells activated with Ag, the levels of COX2 were more elevated than in control cells (Fig. S6e). These data support the negative regulatory role of ORMDL3 in the activation of the NF-κB signaling axis.
Silencing of ORMDL3 enhances mast cell-dependent PCA
To evaluate whether ORMDL3 has a function in mast cells in vivo, we silenced ORMDL3 locally by intradermal injection of the ORMDL3 siRNA pool. First, we examined the efficiency of ORMDL3 siRNA in vitro. When compared with BMMCs exposed to nontargeting control siRNA pool, the ORMDL3 siRNA pool reduced ORMDL expression by approximately 60 % as determined by immunoblotting (Fig. 8a, b; serum specific for all members of murine ORMDL family). For FcεRI-mediated PCA reactions, mice were intradermally injected with ORMDL3 siRNAs together with TNP-specific IgE into the right ears and control siRNAs together with TNP-specific IgE into the left ears. After 48 h, mice were challenged intravenously with Ag (TNP–BSA) and Evans blue dye and 2 h later Evans blue in both ears was quantified. Data presented in Fig. 8c show that ears injected with ORMDL3 siRNA displayed significantly higher amounts of Evans blue than ears injected with control siRNA. These data corroborate previous results obtained with BMMCs in vitro. Based on these results, we conclude that silencing of ORMDL3 in vivo leads to enhanced local inflammation triggered by mast cells.
Fig. 8.
Enhanced PCA in mice pretreated with ORMDL3 siRNA (O3 siRNA). a Specificity of the O3 siRNA. BMMCs were exposed to O3 siRNA pool or non-targeting control siRNA. 48 h later, the cells were lysed and analyzed for ORMDL3 and β-actin by immunoblotting. The position of ORMDL3 and β-actin, used as a loading control, is indicated by an arrow. Numbers under immunoblots indicate the relative amount of ORMDL3 normalized to its level in cells nucleofected with control siRNA and the amount of β-actin. b Quantification of ORMDL3 from data as in a. Means ± SEMs are shown (n = 5). c Role of ORMDL3 in PCA. TNP-specific IgE and O3 siRNA were injected intradermally into the right ears and TNP-specific IgE and control siRNA into the left ears. After 48 h, TNP–BSA was administered intravenously together with Evans blue dye and mice were killed 2 h later. Extravasation of Evans blue in both ears was determined. Means ± SEMs are shown (n = 7)
Discussion
In this study, we examined the role of ORMDL3 protein in Ag-mediated mast cell signaling. Although enhanced or reduced expression of ORMDL3 had no effect on Ag-induced degranulation and calcium responses, several lines of evidence presented in this study indicate that ORMDL3 is a negative regulator of proinflammatory activities of mast cells (Table 1). Increased expression of cytokines, chemokines, and COX-2 in O3_KD cells could be explained by our observation that BMMCs with O3_KD exhibited enhanced phosphorylation of IκBα, which is a regulator of transcription factor NF-κB [24]. Indeed, localization of the NF-κB p65 subunit was increased in the nuclei of Ag-activated ORMDL3-deficient mast cells. It is known that enhanced nuclear localization of the NF-κB p65 subunit leads to increased transcription of NF-κB-regulated genes encoding various cytokines, including TNF-α, IL-6 and IL-13, chemokines, CCL3 and CCL4, and COX-2 enzyme [44–48, 53–55]. Moreover, improperly regulated NF-κB causes a broad variety of inflammatory diseases [25–27]. Our findings regarding the regulatory roles of ORMDL3 are different from those obtained with other cell types, demonstrating a positive regulatory role of ORMDL3 in cell signaling. For example, eosinophils overexpressing ORMDL3 exhibited enhanced localization of the phosphorylated NF-κB p65 subunit in the nucleus [20]. Furthermore, transfection of ORMDL3 into lung epithelial cell line A549 increased the expression of several genes, including those for metalloproteases, ADAM-8 and MMP-9, chemokines CXCL-10, CXCL-11, IL-8, CCL-20, and oligoadenylate synthetases [18]. It has also been shown that the expression of several genes, including TGF-β1, ADAM8, MMP9, and CXCL-10, is enhanced in the bronchial epithelium of transgenic mice overexpressing ORMDL3 [19]. Recent studies showed that in eosinophils with O3_OE, ERK kinase played a role in regulating NF-κB signaling [20]. However, mast cells with O3_KD or O3_OE (not shown) exhibited similar ERKY204 phosphorylation when compared with control cells. Although we did not exclude the possibility that phosphorylation of other targets in ERK could be affected by changing ORMDL3 levels, we focused on another potent activator of the NF-κB pathway, AKT kinase. We found that AKT kinase is more phosphorylated at the Ser 473 (but not at the Thr 308) in BMMCs with O3_KD than control cells. These data suggested a regulatory role of AKT in NF-κB signaling in mast cells with O3_KD. However, the exact molecular mechanism by which ORMDL3 activates AKT kinase remains unclear. To exclude the possibility that immunological responses toward dsRNAs could nonspecifically activate IκBα/AKT phosphorylation, COX-2 expression, and cytokine production, we included cells transduced with pLKO.1 containing NT shRNA as a control. It should be mentioned that BMMCs poorly expressed toll-like receptors recognizing dsRNA, and their activation by specific ligands had little impact on TNF-α, IL-6, and IL-13 expression [56].
Table 1.
Summary of Ag-induced responses of BMMCs with O3_KD or O3_OE when compared with control cells
| Parameter | BMMCs with | |
|---|---|---|
| O3_KD | O3_OE | |
| Degranulation | – | – |
| Ca2+ response | – | – |
| SERCA2b (mRNA) | – | – |
| Chemotaxis toward Ag | ↑ | ↓ |
| Adhesion to fibronectin | – | – |
| Spreading on fibronectin | ↓ | – |
| F-actin content | – | – |
| Phosphorylation of IκBα (Ser 32/36) | ↑ | – |
| Phosphorylation of AKT (Ser 473) | ↑ | – |
| Phosphorylation of AKT (Thr 308) | – | – |
| Phosphorylation of ERK (Tyr 204) | – | – |
| Nuclear p65 subunit of NF-κB | ↑ | – |
| Production of cytokines (mRNA and protein) | ||
| TNF-α | ↑ | – |
| IL-6 | ↑ | – |
| IL-13 | ↑ | – |
| Production of chemokines (mRNA) | ||
| CCL3 | ↑ | – |
| CCL4 | ↑ | – |
| Expression of COX-2 (mRNA and protein) | ↑ | – |
| PGD2 synthesis | ↑ | – |
BMMCs with O3_KD or O3_OE exhibited unchanged (–), decreased (↓) or increased (↑) responses when compared to control cells transduced with the corresponding empty vectors (pLKO.1 or pCDH)
It has been shown that silencing of all three members of the ORMDL family potentiates de novo synthesis of sphingolipids [11]. In our study, we used shRNA to decrease the expression of ORMDL3 and found that expression of the other two members, ORMDL1 and ORMDL2, remained unchanged. Thus, we propose that in mast cells, a decrease in ORMDL3 expression alone is capable of promoting inflammation without the coordinate silencing of the ORMDL family genes. It should be noted that the NF-κB transcription factor is activated by multiple stimuli and that the multiprotein NF-kB signaling complex is subjected to extensive regulation [24]. TNF-α is known to be involved in the phosphorylation of IκBα [57, 58], and autocrine activation of NF-κB by TNF-α was observed in human mast cells [59]. Collectively, our data suggest a unique role of ORMDL3 in mast cells in the negative regulation of proinflammatory mediator synthesis. Interestingly, mast cells with O3_OE exhibited no significant inhibition of IκBα/AKT signaling and no suppression of proinflammatory mediator production. This could be explained at least in part by a recently published study, which showed that the expression levels of ORMDL3 were crucial for regulation of cell signaling in lipopolysaccharide-mediated activation of RAW264.7 macrophage cell line and A549 lung epithelial cells [60]. Thus, the relatively low overexpression of ORMDL3 in BMMCs could be under the threshold to regulate signaling pathways leading to the changes in cytokine expression. Our attempt to increase the exogenous expression of ORMDL3 in BMMCs failed because of enhanced mortality of BMMCs.
As already mentioned, no significant changes in calcium mobilization were found in Ag-activated BMMCs with O3_KD or O3_OE. These findings are different from those obtained with ORMDL3-deficient BM-derived eosinophils which, when compared with wild-type cells, showed reduced Ca2+ response after activation with eotaxin-1 [20]. On the other hand, Jurkat T cells with an siRNA-mediated O3_KD exhibited elevated mobilization of Ca2+ when activated via CD3, while a decreased Ca2+ response was observed in Jurkat cells with O3_OE [17]. Recent data showed that transgenic mice with elevated expression of ORMDL3 exhibited enhanced lung expression of SERCA2b, a known regulator of cytosolic calcium concentrations [19]. In contrast, our data with BMMCs showed that the expression of SERCA2b was not affected by changes in the ORMDL3 levels. These data are in line with our finding that BMMCs with decreased or enhanced expression of ORMDL3 exhibited similar levels of basal [Ca2+]i, and Ag-induced calcium mobilization when compared with corresponding control cells.
ORMDL3 has been described as an inducible gene upon Alternaria allergen challenge of mouse lung epithelial cells, lung eosinophils, and lung macrophages, but not lung neutrophils [18]. Moreover, IL-4 and IL-13 were capable of inducing ORMDL3 expression in lung epithelial cells [18]. These data were extended to mouse BM-derived eosinophils, which exhibited IL-3- and eotaxin-1-dependent induction of ORMDL3 expression [20]. In contrast to these data, our studies with mouse mast cells showed that expression of the ORMDL3 gene was downregulated after FcεRI triggering. Interestingly, a similar decrease was observed in our previous study in which gene expression was analyzed in nonactivated and Ag-activated BMMCs (Gene Expression Omnibus repository dataset GSE40731 [61]). The data show that 2 h after FcεRI triggering, ORMDL3 was decreased to 0.6 in WT and to 0.43 in pLKO.1-transduced cells (n = 3 for each group, adj. P value <0.006). There were no significant changes between ORMDL1 and ORMDL2. The amounts of all ORMDL family members were not significantly changed between WT and pLKO.1 BMMCs. These data suggest a distinct regulation of ORMDL3 gene expression in Ag-activated mast cells when compared with activated eosinophils, macrophages, and lung epithelial cells [18, 20]. Recently, it has been shown that lipopolysaccharide activation of macrophage cell line RAW267.4 induces coordinated transient suppression of expression of genes encoding the ORMDL family and in this way regulates sphingolipids homeostasis [14]. Chemotaxis is another example of distinct ORMDL3 regulatory roles in mast cells and eosinophils. ORMDL3-deficient eosinophils exhibited decreased chemotaxis toward eotaxin-1 [20], while we showed that BMMCs with O3_KD increasingly migrate toward Ag. Moreover, O3_KD exhibited decreased Ag-induced spreading on fibronectin. These data are consistent with previous observations of indirect relationship between mast cell migration and cell spreading [62]. The observed difference between mast cells and eosinophils could be caused by distinct chemoattractants and different methods used and/or different involvement of ORMDL3 in chemotaxis of BMMCs and bone marrow-derived eosinophils.
Dysregulation of ORMDL3 expression is linked to various inflammatory diseases [1, 4–7]. It has been shown that upregulation of ORMDL3 stimulates the release of proinflammatory mediators [18, 20] and that ORMDL3 transgenic mice exhibit the proinflammatory phenotype [19]. On the other hand, decreased expression of all members of the ORMDL family led to activation of sphingolipid synthesis [11]. In this study, we showed for the first time that O3_KD alone is able to enhance the inflammatory response of mast cells. In BMMCs with O3_KD, we described a complex phenotype based on the enhanced AKT–NF-κB signaling axis, followed by increased secretion of TNF-α, IL-6, and IL-13 and enhanced expression of COX-2 associated with increased PGD2 synthesis. In addition, the chemotactic response of IgE-sensitized BMMCs to Ag was increased in cells with O3_KD and decreased in O3_OE. Moreover, we have found that ORMDL3 mRNA levels are decreased after challenge of IgE-sensitized BMMCs with Ag. These data were corroborated by an in vivo study, which showed that decreased ORMDL3 expression in mast cells was capable of inducing Ag-dependent inflammation. The opposite results obtained in mast cells versus other cell types described in the literature could be explained by formation of different functional complexes of ORMDL3 with other molecules in various cell types. Clarification of this issue and ORMDL3 properties in human mast cells requires further studies.
Collectively, our data show that ORMDL3 is predominantly a negative regulator of proinflammatory responses in Ag-activated murine mast cells, both in vitro and in vivo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by projects P305-14-00703S, P302/12/G101, and P302-14-09807S from the Grant Agency of the Czech Republic and Institutional project RVO 68378050 from the Academy of Sciences of the Czech Republic. L.P., H.D., M.B., and T.P. were supported in part by the Faculty of Science, Charles University, Prague. We thank J. Eitler for preparation of ORMDL3-myc plasmid, H. Mrazova, and R. Budovicova for technical assistance, and Pavel Draber, Peter Draber, and S. Takacova for critical reading of the manuscript.
Abbreviations
- Ag
Antigen
- BMMC
Bone marrow-derived mast cell
- BSS
Buffered saline solution
- Bp
Base pairs
- [Ca2+]i
Concentrations of free intracellular Ca2+
- COX
Cyclooxygenase
- Ct
Threshold cycle
- NT
Nontarget
- O3_KD
ORMDL3 knockdown
- O3_OE
ORMDL3 overexpression
- ORM
Orosomucoid
- ORMDL
Orosomucoid-like
- PCA
Passive cutaneous anaphylaxis
- pCDH
pCDH-CMV-MCS-EF1-Puro
- PG
Prostaglandin
- SERCA
Sarco/endoplasmic reticulum Ca2+ ATPase
- sh
Short hairpin
- siRNA
Short interfering RNA
- SNP
Single-nucleotide polymorphisms
- TNP
2,4,6-Trinitrophenol
- WT
Wild type
References
- 1.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 2.Galanter J, Choudhry S, Eng C, Nazario S, Rodriguez-Santana JR, Casal J, Torres-Palacios A, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med. 2008;177:1194–1200. doi: 10.1164/rccm.200711-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota T, Harada M, Sakashita M, Doi S, Miyatake A, Fujita K, Enomoto T et al (2008) Genetic polymorphism regulating ORM1-like 3 (Saccharomyces cerevisiae) expression is associated with childhood atopic asthma in a Japanese population. J Allergy Clin Immunol 121:769–770 [DOI] [PubMed]
- 4.Balantic M, Rijavec M, Flezar M, Camlek T, Hudoklin I, Kosnik M, Korosec P, Suskovic S. A polymorphism in ORMDL3 is associated not only with asthma without rhinitis but also with chronic obstructive pulmonary disease. J Investig Allergol Clin Immunol. 2013;23:256–261. [PubMed] [Google Scholar]
- 5.McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, Neale BM, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verlaan DJ, Berlivet S, Hunninghake GM, Madore AM, Lariviere M, Moussette S, Grundberg E, et al. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet. 2009;85:377–393. doi: 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurreeman FA, Stahl EA, Okada Y, Liao K, Diogo D, Raychaudhuri S, Freudenberg J, et al. Use of a multiethnic approach to identify rheumatoid- arthritis-susceptibility loci, 1p36 and 17q12. Am J Hum Genet. 2012;90:524–532. doi: 10.1016/j.ajhg.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Ampleford EJ, Howard TD, Moore WC, Torgerson DG, Li H, Busse WW, et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol. 2012;130:861–868. doi: 10.1016/j.jaci.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelmqvist L, Tuson M, Marfany G, Herrero E, Balcells S, Gonzalez-Duarte R (2002) ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol 3:RESEARCH0027-1-16 [DOI] [PMC free article] [PubMed]
- 11.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2011;108:19222–19227. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siow DL, Wattenberg BW. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J Biol Chem. 2012;287:40198–40204. doi: 10.1074/jbc.C112.404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiefer K, Carreras-Sureda A, Garcia-Lopez R, Rubio-Moscardo F, Casas J, Fabrias G, Vicente R. Coordinated regulation of the orosomucoid-like gene family expression controls de novo ceramide synthesis in mammalian cells. J Biol Chem. 2015;290:2822–2830. doi: 10.1074/jbc.M114.595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, Silver RB, Jiang XC, Worgall S (2013) Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med 5:186ra67 [DOI] [PubMed]
- 16.Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- 17.Carreras-Sureda A, Cantero-Recasens G, Rubio-Moscardo F, Kiefer K, Peinelt C, Niemeyer BA, Valverde MA, Vicente R. ORMDL3 modulates store-operated calcium entry and lymphocyte activation. Hum Mol Genet. 2013;22:519–530. doi: 10.1093/hmg/dds450. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, Rosenthal P, et al. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci USA. 2012;109:16648–16653. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Rosenthal P, Beppu A, Mueller JL, Hoffman HM, Tam AB, Doherty TA, et al. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014;192:3475–3487. doi: 10.4049/jimmunol.1303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha SG, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP, Sriramarao P. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 2013;4:2479. doi: 10.1038/ncomms3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert N, Dudeck J, Liu P, Karutz A, Speier S, Maurer M, Tuckermann J, Dudeck A. Mast cell promotion of T cell-driven antigen-induced arthritis despite being dispensable for antibody-induced arthritis in which T cells are bypassed. Arthritis Rheumatol. 2015;67:903–913. doi: 10.1002/art.38996. [DOI] [PubMed] [Google Scholar]
- 24.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollrath J, Greten FR. IKK/NF-κB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheller JR, Polosukhin VV, Mitchell D, Cheng DS, Peebles RS, Blackwell TS. Nuclear factor κB induction in airway epithelium increases lung inflammation in allergen-challenged mice. Exp Lung Res. 2009;35:883–895. doi: 10.3109/01902140903019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hájková Z, Bugajev V, Dráberová E, Vinopal S, Dráberov L, Janáček J, Dráber P, Dráber P. STIM1-directed reorganization of microtubules in activated mast cells. J Immunol. 2011;186:913–923. doi: 10.4049/jimmunol.1002074. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph AK, Burrows PD, Wabl MR. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981;11:527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- 31.Tolar P, Dráberová L, Dráber P. Protein tyrosine kinase Syk is involved in Thy-1 signaling in rat basophilic leukemia cells. Eur J Immunol. 1997;27:3389–3397. doi: 10.1002/eji.1830271238. [DOI] [PubMed] [Google Scholar]
- 32.Dráberová L, Amoui M, Dráber P. Thy-1-mediated activation of rat mast cells: the role of Thy-1 membrane microdomains. Immunology. 1996;87:141–148. [PMC free article] [PubMed] [Google Scholar]
- 33.Draberova L, Bugajev V, Potuckova L, Halova I, Bambouskova M, Polakovicova I, Xavier RJ, Seed B, Draber P. Transmembrane adaptor protein PAG/CBP is involved in both positive and negative regulation of mast cell signaling. Mol Cell Biol. 2014;34:4285–4300. doi: 10.1128/MCB.00983-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horáková H, Polakovičová I, Shaik GM, Eitler J, Bugajev V, Dráberová L, Dráber P. 1,2-propanediol-trehalose mixture as a potent quantitative real-time PCR enhancer. BMC Biotechnol. 2011;11:41. doi: 10.1186/1472-6750-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potůčková L, Franko F, Bambousková M, Dráber P. Rapid and sensitive detection of cytokines using functionalized gold nanoparticle-based immuno-PCR, comparison with immuno-PCR and ELISA. J Immunol Methods. 2011;371:38–47. doi: 10.1016/j.jim.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Haan C, Behrmann I. A cost effective non-commercial ECL-solution for Western blot detections yielding strong signals and low background. J Immunol Methods. 2007;318:11–19. doi: 10.1016/j.jim.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Hálová I, Dráberová L, Bambousková M, Machyna M, Stegurová L, Smrž D, Dráber P. Crosstalk between tetraspanin CD9 and transmembrane adaptor protein non-T cell activation linker (NTAL) in mast cell activation and chemotaxis. J Biol Chem. 2013;288:9801–9814. doi: 10.1074/jbc.M112.449231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, et al. Cell Profiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK. Characterization and quantitation of NF-κB nuclear translocation induced by interleukin-1 and tumor necrosis factor-α. Development and use of a high capacity fluorescence cytometric system. J Biol Chem. 1998;273:28897–28905. doi: 10.1074/jbc.273.44.28897. [DOI] [PubMed] [Google Scholar]
- 41.Kanada S, Nishiyama C, Nakano N, Suzuki R, Maeda K, Hara M, Kitamura N, Ogawa H, Okumura K. Critical role of transcription factor PU.1 in the expression of CD80 and CD86 on dendritic cells. Blood. 2011;117:2211–2222. doi: 10.1182/blood-2010-06-291898. [DOI] [PubMed] [Google Scholar]
- 42.Bradding P. Mast cell regulation of airway smooth muscle function in asthma. Eur Respir J. 2007;29:827–830. doi: 10.1183/09031936.00017707. [DOI] [PubMed] [Google Scholar]
- 43.Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822:57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S, Dorken B, Zenke M, Stein H, Scheidereit C. Nuclear factor κB-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196:605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klemm S, Gutermuth J, Hultner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. The Bcl10-Malt1 complex segregates FcεRI-mediated nuclear factor κB activation and cytokine production from mast cell degranulation. J Exp Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-κB activity. J Allergy Clin Immunol. 2000;105:500–505. doi: 10.1067/mai.2000.104942. [DOI] [PubMed] [Google Scholar]
- 47.Peng Y, Power MR, Li B, Lin TJ. Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: a role for the IKK-IκB-NF-κB pathway in IgE-dependent mast cell activation. J Leukoc Biol. 2005;77:975–983. doi: 10.1189/jlb.0204115. [DOI] [PubMed] [Google Scholar]
- 48.Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 α, and MIP-1 β, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993;150:4996–5012. [PubMed] [Google Scholar]
- 49.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 50.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/S0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 51.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 53.Kaltschmidt B, Linker RA, Deng J, Kaltschmidt C. Cyclooxygenase-2 is a neuronal target gene of NF-κB. BMC Mol Biol. 2002;3:16. doi: 10.1186/1471-2199-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortaz E, Redegeld FA, Nijkamp FP, Engels F. Dual effects of acetylsalicylic acid on mast cell degranulation, expression of cyclooxygenase-2 and release of pro-inflammatory cytokines. Biochem Pharmacol. 2005;69:1049–1057. doi: 10.1016/j.bcp.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Kurumbail RG, Kiefer JR, Marnett LJ. Cyclooxygenase enzymes: catalysis and inhibition. Curr Opin Struct Biol. 2001;11:752–760. doi: 10.1016/S0959-440X(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 56.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 57.Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor α signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-κB and TNF-α: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–5293. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- 60.Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, Newton J et al (2015) Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J Allergy Clin Immunol. doi:10.1016/j.jaci.2015.02.031 [DOI] [PMC free article] [PubMed]
- 61.Polakovicova I, Draberova L, Simicek M, Draber P. Multiple Regulatory Roles of the Mouse Transmembrane Adaptor Protein NTAL in Gene Transcription and Mast Cell Physiology. PLoS ONE. 2014;9:e105539. doi: 10.1371/journal.pone.0105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tůmová M, Koffer A, Šimíček M, Dráberová L, Dráber P. The transmembrane adaptor protein NTAL signals to mast cell cytoskeleton via the small GTPase Rho. Eur J Immunol. 2010;40:3235–3245. doi: 10.1002/eji.201040403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.