Abstract
Next to classical diffusion-based models, filopodia-like cellular protrusions have been proposed to mediate long range signaling events and morphogen gradient formation during communication between distant cells. An increasing wealth of data indicates that in spite of variable characteristics of signaling filopodia in different biological contexts, they represent a paradigm of intercellular crosstalk which is presently being unraveled in a growing literature. Here, we summarize recent advances in investigating the morphology, cellular basis and function of signaling filopodia, with focus on their role during embryonic development in vertebrates.
Keywords: Cell–cell communication, Actin cytoskeleton, Microtubules
Introduction
The development of a vertebrate embryo is a process of immense complexity. To establish the body plan from a single cell, the zygote, very large numbers of cells need to coordinately divide, specify, differentiate and spatially rearrange during embryogenesis. In many instances, namely during induction of developmental processes by neighboring cell populations, extensive cell–cell communication is required to exchange instructive signaling molecules between cells. This can occur between closely adjacent cells, but also between cells separated by larger gaps, which can be filled by interstitial fluid, extracellular matrix or other cells.
In the last years, long range signaling mechanisms have been intensely investigated. In the classical morphogen model of long range cellular interaction, diffusible signaling molecules are secreted by donor cells, diffuse to form a concentration gradient in the interstitial space, and are sensed by recipient cells in a concentration-dependent manner. Next to this model, recent research has established that cells are able to communicate over large distances by long, thin cellular protrusions which bridge the space between them, and which enable distant cells to exchange signaling molecules even if they are not diffusible as postulated for classical morphogens. In this review, we will provide an overview on the current knowledge on cellular protrusions arising during vertebrate development. Regarding their proper designation, there is not yet a consensus between researchers, and terms vary between, e.g., cytonemes, specialized filopodia, filopodia-like protrusions and others [1]. Here, we will therefore simply refer to “signaling filopodia” for signaling cellular protrusions, unless in certain cases special expressions have been already established, like cytonemes in the Drosophila imaginal disc.
Principles of intercellular communication
Morphogen gradients by diffusion
The classical diffusion-based morphogen model is based on the concept that cells close to the secreting cell are exposed to high concentrations of morphogen, whereas cells at further distances receive less. Given threshold-dependent specific reactions of the recipient cells to the morphogen, this model can explain that cells behave differently along the morphogen gradient, thus establishing for instance positional information along body axes like in the vertebrate limb [2]. A problem of this model is its apparent lack of precision in the complex environment of embryonic tissues. By further elaborating the morphogen concept, it has been shown that morphogen uptake or degradation by the recipient cells as well as extracellular matrix properties can shape morphogen gradients [3, 4]. In vertebrates, morphogen gradients have been suggested for BMP, Sonic hedgehog (Shh) or Wnt signaling [5, 6]. Even though wingless (Wg) gradients have been demonstrated in Drosophila [3], it remains controversial how gradients of the vertebrate homologues, Wnts, can be established. Wnt proteins have highly hydrophobic residues, which are essential for their secretion and function but due to which they remain largely attached to the secreting cells [7–9]. Yet they are known to act at considerable distances from their sources, e.g., in the developing chick embryo, where Wnt6 is secreted by the surface ectoderm and controls dermomyotomal cell fates [10, 11], even though both cell sheets are separated by the subectodermal space, which measures about 20 µm and contains a mesh of extracellular matrix.
Morphogen distribution by migrating cells
A different principle of intercellular communication has been suggested for the communication between the dorsal neural tube and the medial lip of the dermomyotome, which is achieved by migrating neural crest cells. Wnt1 and Wnt3a, expressed in the dorsal neural tube, induce medial fate and Wnt11 expression in the dermomyotome, which requires Wnts to bridge a distance of about 100 µm [12]. Wnt proteins have been shown to attach to migrating neural crest cells and to be transported, hitchhiking as it were, to the recipient cells in the dermomyotome [13]. This trackway could be mediated by the extracellular matrix and by cellular extensions from adjacent cells [14]. Whether dermomyotomal filopodia, which are formed by the dorsomedial lip cells of the dermomyotome [13] are involved in this process remains to be shown. However, it becomes increasingly clear that extracellular matrix proteins actively participate in this intercellular communication [13, 15].
Morphogen transportation by filopodia
Morphogen transportation by filopodia-like extensions has first been shown in Drosophila imaginal discs, where these extensions have been named cytonemes. Cytonemes have a dual role. On one hand, they are essential for the establishment of a morphogen gradient [16]; on the other hand, they serve as signaling filopodia bridging the space between distantly located cells to deliver either morphogens such as Wnt and Shh proteins to recipient cells or to extend receptors such as the Wnt-receptor frizzled or Shh co-receptors to the morphogen source [17–20]. In the following, we will focus on this mode of filopodia-based intercellular communication.
Filopodia-mediated cell–cell signaling
Signaling filopodia in Drosophila
Filopodia as cellular signaling extensions were first extensively characterized in Drosophila and addressed as cytonemes [21]. These cytonemes are cytoplasmic threads that extend towards morphogen-producing regions to bring the respective receptors in close vicinity to the ligand producing cells [22]. In Drosophila, cytonemes are described in different tissues such as the eye, wing disc, and tracheal cells. The cytonemal transport of various receptors to the morphogen source has been reported in a number of signaling pathways: Branchless (Bnl, the Drosophila homolog of FGF) induces the extension of cytoneme-like, Btl (FGF) receptor-bearing filopodia to the Bnl/FGF-expressing cells [23], Notch-delta ligands, where the extensions are induced by Delta to promote Notch signaling in distantly located cells [24], Spitz/EGF that induces formation of filopodia transporting EGF receptor in form of motile puncta [22], and the Dpp receptor thickveins (Tkv) on cytonemes that orient toward the Dpp producing disc cells [25]. Recently, Hedgehog-coated cytonemes were characterized that regulate BMP signaling in Drosophila female germline stem cells to prevent their differentiation [26]. Thus, Drosophila cytonemes transport receptors or ligands and emanate either from the receptor-bearing target cell or extend from the morphogen-secreting cell [27].
Signaling filopodia in vertebrates
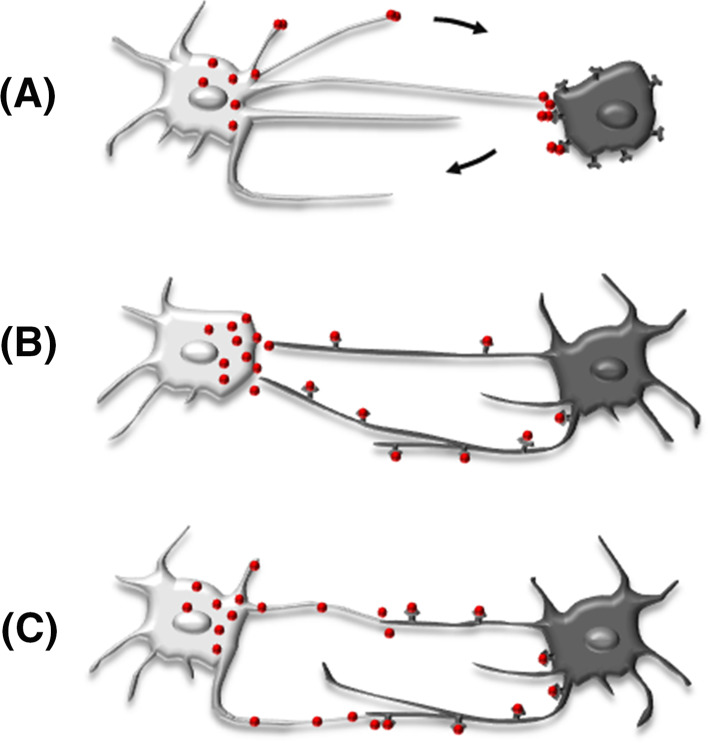
Increasing experimental evidence demonstrates that intercellular communication via signaling filopodia also exists in vertebrates. As they have been independently detected in various cell types, researchers have differently named them as, e.g., tunneling nanotubes in different cell lines [28], cytoplasmic bridges in zebrafish epiblast cells [29], filopodia-like protrusions (FiLiPs) in chick dermomyotomal cells [20], or specialized filopodia in chick limb buds [19]. As in Drosophila, vertebrate signaling filopodia transport receptors to the morphogen-producing cell. Likewise, ligands can be transported via protrusions extending from the signaling cell to contact the target cell either at the cell body or at receptor-presenting protrusions. In spite of species and cell type specific differences, signaling filopodia seem to represent a general concept of cell–cell communication (see Fig. 1).
Fig. 1.
Three variants of long-distance intercellular communication via signaling filopodia. Stable connections are formed between signaling cell (light gray cells on the left) and target cell (dark gray cells on the right). a The ligand secreting cell locates the ligand directly at the target cell and retracts the filopodium after ligand delivery. b The receptor-bearing target cell extends long signaling filopodia to the ligand secreting target cell. c Both, the ligand secreting and the receptor-bearing target cell extend signaling protrusions to contact
Except for mammalian blastocyst traversing filopodia that are formed early and keep intercellular contact when the cells are separated over long distances [30], most signaling filopodia are highly dynamic and show probing movements, but can form stable contacts with transport activity as soon as they contact their proper target cells [19, 20, 30–32]. Such stable contacts were also described as tunneling nanotubes (TNTs), which connect cells over long distances and transfer various cellular components from cell to cell [29, 33]. TNTs are used for the intercellular exchange of cellular cargos such as eGFP-actin, organelles/vesicles of endocytic origin, plasma membrane molecules, MHCI molecules in case of immune cells, and even mitochondria, prions and human immunodeficiency virus (HIV), but also for electrical coupling and mechanotransduction [34]. Hallmarks of signaling filopodia in vertebrates are listed in Table 1.
Table 1.
Features of signaling filopodia described and characterized in vertebrate species
| Species | Location and function of filopodia | Length and diameter (µm) Dynamics |
Characteristics of dynamic and stable filopodia | References |
|---|---|---|---|---|
| Zebrafish embryo |
Epiblast cells in blastula Function Delta-notch signaling |
Average length 215 µm (range 60–380 µm) Diameter: 1 µm |
Stable filopodia: “intercellular bridges” Delta-positive filopodia Actin positive Tubulin only at the bases Cargo transport: v = 55 nm/s |
[29] |
| Zebrafish embryo | A subset of migrating NCCs |
CXCR4-GFP localizes to fscn1a-dependent filopodia Actin positive |
[50] | |
| Zebrafish embryo | Epithelial cells in the presumptive neuroectoderm |
Wnt8a-venus positive “puncta” at filopodial tip is released as the filopodium retracts Formation of Wnt8a/Fzd9b clusters |
[37] | |
| Zebrafish embryo |
Transfected epiblast cells Function Neural plate patterning |
Average length: 16.6 µm (up to 50 µm) Diameter max 1.5 µm |
Wnt8a-eGFP-positive filopodial tips give rise to extracellular puncta, which are attached to target cells On stable filopodia formation of “signalosomes”: clusters of Wnt8a and Lrp6-GFP, Dvl-mCherry (or Axin1) on the target cell |
[17] |
| Zebrafish embryo | HEK293 cells, fish fibroblasts PAC2, mouse 3T3 |
Length in PAC2 cells: up to 70 µm Elongation: average v = 110 nm/s Max. v = 240 nm/s |
Wnt8a-eGFP-positive, stable filopodia Actin positive Tubulin at the base Myosin10 positive Cdc42 determines the length of Wnt-positive filopodia |
[17] |
| Xenopus |
Blastocyst Function Connecting blastomeres |
Length Short dynamic filopodia 10 µm Long blastocoel traversing filopodia up to 250 µm Diameter: 0.2–0.7 µm |
Dynamic filopodia PFA stable Stable filopodia spanning the blastocoel cavity Exchange of cytoplasm and membrane Caveolar endocytosis at proximal base Actin positive Tubulin negative (α-tubulin staining) Retro- and anterograde vesicle transport (v = 1–3 µm/s) PFA stable |
[31] |
| Xenopus fibroblasts |
Stable filopodia Actin positive Tubulin-positive Fluorophore spots: (v = 763 nm/s) colocalize only with mCherry tagged tubulin Active Wnt2a-eGFP is transported along microtubules to the plasma membrane, located at the very tips of filopodia and handed over to a Wnt receiving cell where it is rapidly internalized |
[18] | ||
| Chick embryo |
Limb bud, mesenchymal cells Function Limb patterning |
Average length 34.27 ± 9.6 µm Max. length 150 µm Diameter: 0.2–0.8 µm Elongation v = 150 nm/s |
ShhN-eGFP-positive filopodia Shh-eGFP particles: anterograde movement with a maximal net v = 120 nm/s Subpopulation positive for Shh co-receptors Cdo and Boc contact formation between Cdo/Boc- and Shh-positive filopodia PFA sensitive Cdc42 independent Actin positive Tubulin (Tau, EB3) only at the proximal base of a subset of mesenchymal filopodia myosin10 positive |
[19] |
| Chick embryo |
Between dermomyotome and ectoderm, spanning the subectodermal space, penetrate through the basal lamina Function Wnt-signaling (?) |
Length limited by the overlying ectoderm about 20 µm Diameter: 0.2–0.5 µm v (extending) 53.8 nm/s v (retracting) 68.8 nm/s |
Dynamic filopodia Actin positive Tubulin positive PFA stable Stable filopodia 82 % stable protrusions Actin positive Tubulin positive PFA stable |
[20] |
| Mouse embryo |
Between cells of the inner cell mass and the mural trophoectoderm (mTE) Function Signal transduction to induce cell proliferation of TE cells |
Short filopodia Length 3 – 8 µm Diameter: 0.2–1 µm Long, traversing filopodia Length up to 34.6 µm Diameter at narrowest point: 0.2–0.4 µm |
Short filopodia Actin positive Microtubule negative PFA stable TE-derived filopodia: FGFR2 positive/ErbB3 negative ICM-derived filopodia: FGFR2 negative/ErbB3 negative Long, traversing filopodia Actin positive Microtubule negative (as deduced due to their colchicine resistance) Retrograde vesicle transport PFA stable TE-derived filopodia: FGFR2 positive/ErbB3 positive ICM-derived filopodia: FGFR2 negative Junctional filopodia (see also Fleming et al. [73]) Actin positive Tubulin positive PFA stable |
[30] |
| Mouse embryo | Between cells of the inner cell mass and the mural trophoectoderm (mTE) |
Junctional filopodia Length: 0.1–3 µm |
Junctional filopodia Microtubule positive (as visualized electron microscopically) PFA stable |
[73] |
| Mouse embryo |
Between cells of the late morula stage Function Providing tension, stabilizing mechanical force necessary for compaction of preimplantation embryo |
Length 10.9 ± 0.8 µm |
Dynamic filopodia Actin positive Microtubule negative Stable filopodia Filopodia to neighboring cells Dependent on E-cadherin, a- and b-catenin F-actin positive myosin10 positive |
[74] |
| Mouse mesenchymal cells C3H/10T1/2 |
Length <10 µm Length <20 µm Long: length >60 µm |
Dynamic, short filopodia Cdc42-induced filopodia Actin positive Tubulin positive Fascin positive RhoD-induced filopodia Short filopodia Actin positive Tubulin negative Fascin positive and negative PFA stable Stable filopodia Actin positive Tubulin negative PFA sensitive Net retrograde transport of fluorescent nodules and of FGF/FGFRs Coexpression of Rab5 (early endosome-like vesicle) |
[41] | |
|
Human cell lines A431 HeLa MCF7 |
ErbB1 receptor positive Actin positive Tubulin negative Retrograde transport of EGF/ErbB1 complex (v = 22 nm/s) Activation of the receptor tyrosine kinase essential for retrograde transport Endocytosis of the EGF/ErbB1 complex at the filopodial base |
[42] | ||
|
Human cell lines HEK cells |
Mammalian stem cells Lgr4- and Lgr5-induced filopodia |
Length 80 µm Diameter 0.53 µm |
Dynamic filopodia Actin positive myosin10 positive PFA stable Stable filopodia Mature Lgr5-induced cytonemes |
[32] |
Signaling filopodia in Wnt signaling
Next to Shh signaling in the limb bud mesenchyme [19, 35, 36], Wnt signaling has been a focus in recent research on filopodia-mediated cell–cell signaling in vertebrate embryos. In zebrafish and Xenopus, and presumably also in chick, the transfer of Wnt protein from the secreting to the responding cell is mediated by signaling filopodia (see below).
In zebrafish, recent work has shown that specialized filopodia mediate the paracrine transport of canonical Wnt8a during neural plate patterning [17]. Wnt8a is presented at the filopodial tips of the Wnt8a producing cells and colocalizes with Evi/Wls, a specialized cargo receptor, which binds to Wnts through palmitate moieties. Approximately, 10 min after contact formation, the extensions are shortened, and Wnt8a-positive tips form extracellular punctate, which are attached to neighboring cells [17, 37] (Fig. 1a). In the recipient cell Wnt8a co-localizes with the Wnt-receptor Frizzled (Fzd) and its co-receptor Lrp6 [17]. The clustering of ligand, receptor and co-receptor initiates the recruitment of intracellular Wnt transducers, such as Dishevelled2 (Dvl2) and Axin1, to assemble the so-called “signalosome” [17, 38, 39]. This mechanism is similar to the synaptic contact of neurons, where the transmitter is transported along the entire axon to be released from the presynaptic membrane and taken up postsynaptically by the responding neuron.
In Xenopus fibroblasts, EGFP-labeled Xwnt2a has been shown to move along microtubules in protrusions of Wnt-producing cells and is transferred to filopodia of the recipient cells (Fig. 1b). Subsequently, Xwnt2b–EGFP becomes endocytosed by the Wnt receiving cell and moves retrogradely together with the retracting protrusions [18].
In chicken embryos, Wnt signals from the surface ectoderm regulate development of the somite epithelium across the subectodermal space, which contains dense extracellular matrix [10, 11, 40]. Recently, filopodia have been documented in chick dermomyotomal cells that reach out through the subectodermal space to the overlying ectoderm (Fig. 1c). These filopodia contain motile transmembranous Fzd7 receptors, which are extended to close vicinity to the Wnt-producing cells and are likely to mediate the observed ectodermal–dermomyotomal crosstalk [20] (Fig. 2).
Fig. 2.
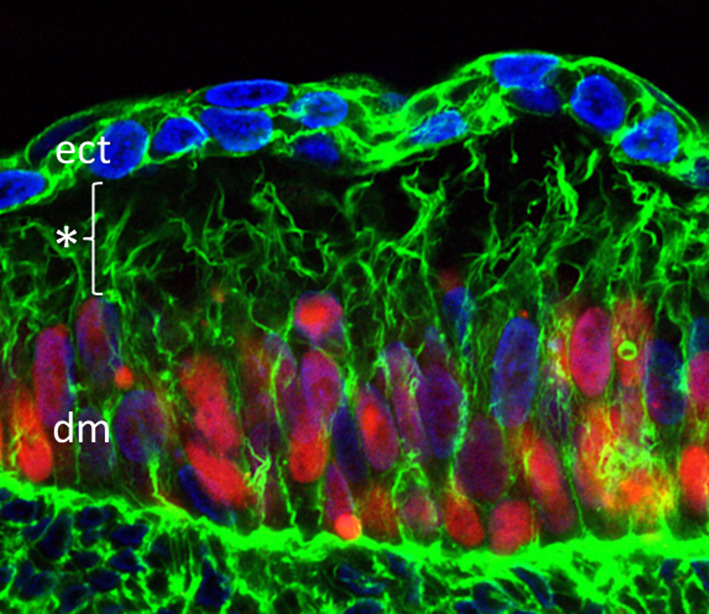
Signaling filopodia spanning the subectodermal space in the chicken embryo. Somitic cells (red) were labeled by overexpression of pCAGGS-H2B-mCherry-pA, nuclei (blue) are stained with DAPI, actin (green) was stained using Alexa Fluor 488 phalloidin. Filopodia are spanning the distance of about 20 µm (*) between the dermomyotome (dm) and the ectoderm (ect)
In mammalian embryos, filopodia have so far not been linked to Wnt signaling. However, a similar concept has emerged for FGFR2 and ErbB3-positive protrusions that span over the murine blastocoelic cavity to connect the mural trophectoderm with the cells of the inner cell mass [30].
Formation and retrograde transport of ligand/receptor complexes in signaling filopodia
In vitro studies indicate that in EGF and FGF signaling, after delivery of the ligand, the ligand–receptor complex travels to the cell body to induce the signaling cascade at the base of the filopodia. In mouse mesenchymal cells, FGF receptor-bearing protrusions grow in the direction of the FGF ligand and bind FGF and Rab5, which is localized to the early endosome membranes, to form a ternary complex. This complex associates with nodules that move retrogradely through the protrusions to the cell’s body [41]. The same has been shown for complexes containing EGF ligand and the activated ErbB1 receptor that are retrogradely transported to the cell body and endocytosed, a process that only occurs at the base of the filopodia [42]. In zebrafish, Wnt8, presented on cellular protrusions, clusters with Fzd9b and intracellular signal transducers on the membrane of the target cell [37]. For signal transduction this complex needs to be endocytosed [39]. In this case no filopodia are formed by the target cell so that the ligand/receptor complex is formed directly on the membrane of the recipient cell body and no retrograde transport is required prior to signaling.
Specificity of signaling filopodia
Very interestingly, in Drosophila tracheal cells, different subsets of cytonemes have been reported. One type of cytonemes is made in response to Bnl/FGF, to which the cells synthesize the respective Bnl receptor, and another type of cytonemes of the same cell harbors the Dpp receptor [22]. This means that receptor-harboring cytonemes are not restricted to a specific subset of cells but that one cell can send out different subsets of cytonemes with the selective presence of a particular receptor. A similar phenomenon has been reported for the filopodia-based transport of Shh and the co-receptors Boc and Cdo in chicken mesenchymal cells. Shh particles of appr. 200 nm in size travel only to specific subsets of filopodia of donor cells. Similarly, in recipient cells, Boc and Cdo localize to microdomains within only a subset of filopodia [19], revealing that the spatial distribution of ligands and receptors is tightly regulated. In chick, only a subset of dermomyotomal filopodia harbor the Wnt-receptor Fzd7 and seem to fetch their respective Wnt signaling protein from the overlying Wnt-producing ectoderm [20]. Whether these or the other Fzd7-negative protrusions harbor other receptors has not been investigated in this study.
Endogenous cellular ability to form signaling filopodia
The current data indicate that the formation of signaling filopodia is a cell autonomous process, which is endogenously regulated by the filopodia-forming cells. In mammalian cells, it has been shown that expression of the stem cell markers Lgr4 and Lgr5 induces and drives the formation of membrane protrusions [32]. Lgr4 and Lgr5 are G-protein-coupled receptors (GPCR) that identify a subpopulation of intestinal crypt stem cells and progenitor cells in the intestinal crypt, respectively [43]. In chicken and zebrafish embryos and also in cells cultured in vitro, filopodia formation occurs in vitro in the absence of target cells [17, 20, 32, 41, 42], a feature that clearly documents the cell autonomy of protrusion formation. However, these cellular protrusions can be very long compared to the in vivo situation, are highly dynamic and not polarized but extending in all directions [17, 20]. The length of filopodia seems not only to be controlled by contact formation with the target cell but also depend on the intactness of the downstream signaling. In Drosophila Hh signaling, it was shown that filopodia that reach their target are not only stabilized but respond by up to sixfold elongation in case of experimentally impaired signaling of the target cell [26].
Length and dynamics of signaling filopodia
Signaling filopodia have been described as stable and dynamic subtypes, which are likely different states of the same structures, meaning that dynamic filopodia are intermediate stages without contact to the target cell and presumably without signaling activity, and stable filopodia have established contact to the target cell and are active in signaling as shown by, e.g., Frz7 receptor trafficking [20]. The length of signaling filopodia is obviously dependent on the distance to the target cells and can be very variable within and between different embryonic structures.
In zebrafish, it has been shown that intercellular bridges are formed between Delta- and Notch-positive filopodia. These epiblast-derived filopodia in the zebrafish blastula have an average length of 215 µm (60–380 µm) [29]. Also in zebrafish, Wnt8a is transported in filopodia that extend from epithelial cells of the presumptive neuroectoderm to the target site. These Wnt8a containing filopodia retract and leave Wnt8a in the vicinity of target cells [37]. The average length of these signaling filopodia is 16.6 µm, reaching up to 50 µm in vivo and up to 70 µm in vitro. They are formed at an average speed of 110 nm/s as has been determined in cultured zebrafish fibroblasts [17].
Signaling filopodia in mouse and chick are known to carry the morphogen receptors FGFR2, ErbB3 or Fzd7, respectively [20, 30]. These filopodia are initially formed as short protrusions that extend in all directions in a probing manner until they reach their target and form stable connections. The length of the epithelial filopodia in the mouse blastocyst ranges up to 34.6 µm, depending on the degree of blastocyst extension [30]. Epithelial filopodia in the chicken dermomyotome extend up to 20 µm, which is the width of the subectodermal space between the dermomyotomal and ectodermal layer. In the absence of the ectoderm, filopodia can become considerably longer. The extension and retraction speed of the dynamic subtype is at an average of 53.76 and 68.80 nm/s, respectively [20]. In the chicken limb, the filopodia connecting distantly located mesenchymal cells have an average length of 34.27 µm but can reach up to a length of 150 µm, with a maximum speed of elongation velocity of 150 nm/s [19]. These filopodia extend from both sites, the signaling and the responding cells, being equipped with the morphogen Shh and the Shh co-receptors Cdo and Boc [19]. Both receptor- and ligand bearing filopodia, which are bound to their target cell, are stabilized and less dynamic than non-target bound filopodia, a process that has been described by Sanders et al. [19] as “dynamic probing of the extracellular environment”.
Cytoskeleton of signaling filopodia
For building up morphogen gradients [16] and for delivering ligands to distantly located cells, ligands have to be transported with or along the filopodia in an anterograde direction. In the responding cell, receptors and ligand–receptor complexes need to be transported retrogradely towards the cell body to induce the respective signaling cascades. The formation of filopodia as well as antero- and retrograde transport are based on the cytoskeleton, the major constituents of which are actin and tubulin.
Actin
Similar to cytonemata in Drosophila [21], the cytoskeleton of all signaling filopodia in vertebrates is actin based. In chick dermomyotomal filopodia, which span the subectodermal space, phalloidin staining showed the entire length of filopodia to contain actin filaments [20]. Phalloidin also stained the entire length of mouse blastocoel spanning filopodia that connect the inner cell mass with the trophoectodermal cells [30] as well as the long Lgr5-induced filopodia in HEK cells [32]. Filopodia of different mammalian cells in culture as well as Xenopus fibroblasts stained positive for fluorophore labeled actin [18, 42]. Intercellular bridges of zebrafish epiblast cells expressed transgenic EGFP-actin along their entire length [29], and also in zebrafish epiblast cells and in PAC2 cells the entire length of the Wnt8a-positive filopodia stained with LifeAct [17]. Yet, in chick mesenchymal filopodia, the highly actin-specific LifeAct staining was limited to the proximal base while the high affinity F-actin probe utrophin calponin homology domain (UCHD)-EGFP and moesin-GFP labeled the entire length of the filopodia [19] indicating that, though LifeAct negative, the entire filopodia are actin positive. The failure of LifeAct to stain actin filaments in the shaft and tip of signaling filopodia is discussed to be due to either actin modifications or to decoration of the actin filaments with binding proteins that disturb actin recognition by LifeAct [19, 44]. Actin filaments are stabilized when highly decorated with ADP/cofilin [45, 46]. Whether this is the protein decoration that inhibits LifeAct staining is not known. Yet, it has been reported that these protein decorations or actin modifications very likely vary in time but also between species or filopodial types [46]. According to the data presently available all signaling filopodia have an actin-based cytoskeleton irrespective of LifeAct negative results.
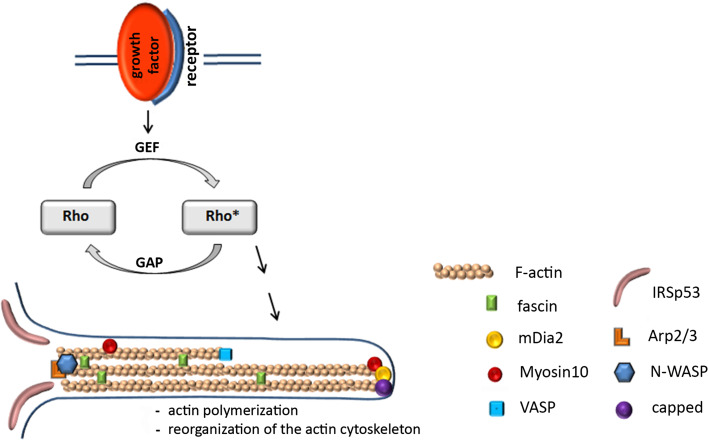
While the cytoskeleton of lamellipodia consists of branched actin filaments, the cytoskeleton of filopodia is based on straight, parallelly aligned actin filaments, the initiation, elongation and termination of which is a highly complex and strictly regulated process. Initiation of the actin filament formation is mediated by a nucleation complex, where different kinds of actin nucleators can work synergistically to promote actin polymerization [47]. N-WASP (neural Wiskott-Aldrich syndrome protein) functions as a nucleation promoting factor, which stimulates the ability of Arp2/3 to induce actin polymerization. Instead of N-WASP, members of the formin family, such as mDia2, can also mediate actin nucleation. In addition, mDia2 can promote continued elongation of uncapped actin filaments [41], a process that is also mediated by another formin, mDia3C, when complexed with the small GTPase RhoD [41]. Prerequisite for actin elongation is the uncapped stage of the growing, barbed end of actin filaments, which is achieved by VASP (vasodilator uncapping protein), which next to its uncapping function also delivers actin monomers to the growing tip of filopodia [48] (Fig. 3).
Fig. 3.
The Rho family of small GTPases regulates actin polymerization and the reorganization of the actin cytoskeleton by cycling between inactive GDP-bound and active GTP-bound forms (*). GTPase-stimulating protein (GAP); guanine nucleotide exchange factor (GEF). Members of the Rho family are RhoD, Rac1, cdc42. The formin mDia2 nucleates unbranched filaments, continues elongation of uncapped actin filaments and can also replace N-WASP in its function as nucleator protein. For details on the other actin-associated molecules shown, please refer to “Actin” section
Bundling of the straight actin filaments is mediated by fascin1 (FSCN1), which cross-links actin filaments to become stiff bundles in the shaft of filopodia [49, 50]. FSCN1 labels filopodial extensions [51] and can increase the efficiency of Ena/VASP-associated actin polymerization [52]. It is also essential for the localization of receptors in filopodial extensions as has been shown in neural crest cells, in which FSCN1a promotes the directional migration by localizing the receptor CXCR4a to filopodia, which allows efficient responses to local chemokine signals such as Cxcl12b [50]. In Lgr5-induced, in Cdc42-induced, and in chick dermomyotomal filopodia, fascin is homogenously distributed throughout the protrusions [20, 32, 41]. In contrast, in RhoD-induced filopodia fascin was either only intermittently distributed along the filopodia or they contained no fascin at all. This incomplete association with fascin is discussed to render the protrusions more flexible [41].
Filopodia formation is a dynamic process: in vivo imaging of cell migration showed that filopodia are rapidly generated in the direction of chemo-attractive cues but collapse when exposed to repulsive cues [53]. The dynamic remodeling of the actin cytoskeleton requires assembly and disassembly of actin filaments. Stabilization is supported by high ADP/cofilin concentrations [45] while disassembly is mediated by the combined action of ADP/cofilin and Aip1 [46, 54]. In accordance with previously described filopodia [55], chicken dermomyotomal as well as mesenchymal filopodia contain cofilin [19, 20]. Cofilin has been shown to localize to specific microdomains along chick mesenchymal filopodia [19]. It accumulates rapidly at the tips of limb mesenchymal filopodia and its subsequent retraction back to the cell soma prefigures the rapid and dynamic retraction of filopodial extensions [19].
Cargo transport along filopodia is closely linked to the presence of the unconventional Myosin10 (Myo10, MyoX) protein, a member of the filopodial tip complex [56]. It is an actin motor protein and has been shown to travel along actin-rich filopodia and deliver cargo (ligands as well as receptors) to the filopodial tips [57, 58]. In zebrafish embryos, Myo10-GFP move to the distal tips of Wnt8a-positive filopodia during the contact formation process where it accumulates [17]. In Lgr5-induced filopodia, Myo10 travels along the filopodia and, when co-expressed with the receptor β2-AR, translocates the adapter protein β-arrestin2 [32]. Translocation of β-arrestin2 is a hallmark of prototypical G-protein-coupled receptor signaling and receptor desensitization [59, 60]. Also in chick mesenchymal filopodia, Myo10-GFP has been shown to move to the distal tips, where it accumulates [19].
Myo10 plays an essential role not only in the formation and cargo transport of signaling filopodia but also in the delivery of integrins to the cell surface to increase cell adhesion [57, 58]. This Myo10-dependent transport of integrins to the filopodial tips is not primarily linked to the signaling properties of filopodia but is discussed to be essential for tumor invasiveness [61] and metastasis [62].
Signaling molecules
Key regulators of the actin cytoskeleton are members of the Rho family of small guanosine triphosphatases (Rho-GTPases) such as RhoD, Rac1, cdc42. These Rho-GTPases act as molecular switches by cycling from the active GTP-bound form to the inactive GDP-bound form, thereby activating downstream targets. The GTPase activity is stimulated by GAPs (GTPase activating proteins) while GEPs (guanine nucleotide exchange factor) recycle the inactive GDP-bound form into the active GTP-bound form (Fig. 3). Filopodia formation is mediated by either one of the above listed RhoGTPases or even by a concerted interaction between them. Accordingly, modulation of these small RhoGTPases affects filopodia formation [63].
In zebrafish, the formation of Wnt8a-positive filopodia depends on Cdc42 activity. Overexpression of Cdc42 results in filopodial branching in PAC2 cells while treatment with the Cdc42 GTPase inhibitor ML14139 resulted in a strong reduction in the number of protrusions formed [17]. Furthermore, Toca1 (transducer of Cdc42-dependent actin assembly1 [64] ), which is a core member of the Cdc42/N-WASP nucleation complex and one of the earliest localization markers of outgrowing filopodia, co-localizes with Wnt8a-mCherry at the plasma membrane before protrusion formation and remains at outgrowing filopodial tips [17]. Active Cdc42 and N-WASP are bound by IRSp53 (Rho family GTPase effector insulin tyrosine kinase substrate p53), the localization of which reveals a spotted pattern along the Zebrafish epiblast-derived filopodia [17]. Interestingly, conditional inactivation of Cdc42 in the chicken limb bud did not perturb mesenchymal filopodia formation [19], indicating that filopodial protrusion is also controlled by GTPases other than Cdc42. Activation of RhoD promotes, e.g., the formation of FGF-induced filopodia in murine mesenchymal cells. RhoD was shown to facilitate actin polymerization by complexing with mDia3c. Accordingly, knockdown of RhoD interfered with FGF-induced protrusion formation [41].
In murine mesenchymal cells, Cdc42-induced filopodia differ from RhoD-induced filopodia with respect to their length, thickness, straightness and microtubular composition (see below). This is discussed to be due to the formation of focal adhesions in Cdc42-induced filopodia whereas focal adhesions collapse in the presence of activated RhoD [41]. Cdc42 plays a pivotal role in cytoskeletal rearrangements [65] and induces de novo filopodia formation via activation of N-WASP as has been shown in cell culture [66] as well as during murine embryonic development, in which Cdc42 largely controls skin development and tubulogenesis in pancreas, kidney and intestine [63].
The RhoGTPase Rac1 has also the ability to coordinate the activation of signaling cascades that control the reorganization of the actin cytoskeleton and ultimately the formation of cellular protrusions. Genetic ablation of Rac1 in mouse embryonic limb bud ectoderm results in severe truncation of limbs. Molecular analyses revealed a disruption of the Wnt-dependent, Gαq/11/βγ-PI3K-Rac1-JNK2-mediated, canonical pathway [67]. Rac1-dependent pathways predominantly affect lamellipodia formation and as such cell movement [53, 68, 69], yet Rac1 has also been implicated in endocytotic processes [70–72]. Interestingly, overexpression of a dominant-negative form of Rac1 (dnRac1) resulted in abrogation of dermomyotomal signaling filopodia in the chick [20]. Dysfunctional signaling cascades, in general, seem to affect filopodial dynamics. In Drosophila disruption of the signaling pathway of the receptor-bearing recipient cells leads to considerable elongation of ligand transporting cytonemata [26] pointing towards the plasticity of the signaling filopodia. Likewise, abrogated filopodia formation in dnRac1 transfected dermomyotomal cells could be due to defective endocytosis and subsequent dysfunctional signaling, a postulated feedback mechanism that remains to be shown.
In summary, filopodia formation is largely controlled by individual members of the family of small Rho-GTPases, which rearrange actin filaments along which ligands and receptors can be transported.
Microtubules
Most of the signaling filopodia described so far are negative for microtubules, which is in accordance with those described in Drosophila [21]. However, in several organisms microtubules have been described in at least a subset of filopodial subtypes.
In zebrafish, microtubule-positive filopodial bases exist in epiblast cells with α-tubulin being present only in the proximal portion of the intercellular bridges [29]. Microtubule-positive proximal bases also exist in Wnt-positive extensions as shown by the localization of the microtubule-associated protein DCK-GFP in microinjected zebrafish epiblast cells while shafts and tips of the filopodia are tubulin negative [17]. For a subset of filopodia in the chicken limb bud the microtubular markers Tau and EB3 were described to be restricted to proximal bases of mesenchymal filopodia [19]. In contrast, the entire lengths of filopodia were actin and tubulin positive in Xenopus fibroblasts where Wnt protein is transported along these tubulin-positive filopodia [18]. Tubulin and actin-positive filopodia also exist in chicken dermomyotomal cells [20]. These actin- and tubulin-positive filopodia are resistant to PFA fixation [18, 20].
In mouse blastocysts, three types of filopodia have been described: long blastocoel traversing filopodia that connect the inner cell mass (ICM) with the trophectoderm (TE), short filopodia that underly the ICM and TE surface and cellular projections, named “junctional extensions” that originate in junctional TE cells and lie in the transition area between ICM and TE. The analysis of microtubular structures within these filopodia reveals a diverse pattern [30]. While short and traversing extensions were unaffected by colchicine treatment, which inhibits tubulin polymerization, colchicine treatment resulted in retraction of the junctional extensions in 72 % of the embryos. This suggests that only a subpopulation is tubulin positive [30, 73]. Different types of filopodial extensions were also characterized in mouse mesenchymal cells, in which formation of filopodia were induced by overexpression of constitutively active Cdc42 or by overexpression of constitutively active RhoD. The Cdc42-induced filopodia are shorter (<10 µm), thicker, straight and contain actin filament bundles and microtubules. Constitutively activated RhoD-induced short and long filopodia, both of which contained no microtubules [41].
In general, actin-positive/tubulin-negative filopodia, as described for filopodia in zebrafish, mouse, and chick embryos as well as for mammalian cells lines, are very fragile and cannot be fixed with conventional fixation techniques. These signaling filopodia are described to transport receptors as well as ligands [17, 19, 29, 42, 74].
Actin- and tubulin-positive filopodia, in contrast, seem to be less fragile and more resistant to PFA fixation. These filopodia contain actin and tubulin filaments along their entire lengths [18, 20, 30, 41]. Up to now, only receptors have been reported to be transported along these actin- and tubulin-positive filopodia [20].
Cargo transport along signaling filopodia
Antero- and retrograde cargo transport along the signaling filopodia seem to be largely linked to the actin cytoskeleton. In chick and zebrafish embryos ligand net anterograde transport is reported for Shh and Wnt8a proteins [17, 19]. Both filopodia were characterized by the existence of microtubules at the proximal base (see above) while filopodial shaft and tip were tubulin negative. Accordingly, Shh transport, with a maximum velocity of anterograde particle movement of 120 nm/s, is consistent with actin-based myosin motors [19, 57]. Wnt8a transport is also actin based and characterized in detail. Before the outgrowth of filopodial protrusions Wnta8a is localized at the plasma membrane together with Toca1, and transported to the filopodial tip via the actin-coupled motor protein Myo10 [17].
Microtubule-assisted transport has been reported in cultured Xenopus fibroblast cells, where Wnt2b is transported anterogradely along the microtubules at a velocity of 763 nm/s and this transport was impaired after nocodazole treatment [18]. The velocity of the transport as well as the fact that the transport is nocodazole sensitive are strong indicators that Wnt2b transport is mediated by the microtubular system.
The retrograde transport of receptors also seems basically to be actin associated. In chick mesenchymal cells with predominantly tubulin-negative filopodial structures, the Shh coreceptors Cdo and Boc are transported retrogradely [19]. Due to the availability of microtubules in chick dermomyotomal filopodia, the reported retrograde transport of Fzd7 receptor could be tubulin based. Interestingly, these filopodia contain the microtubule-associated motor proteins dynein and kinesin. Yet, with the measured average velocities ranging between 56 and 63 nm/s [20] Fzd7 trafficking is still well in the range of actin-associated retrograde cargo transport [75]. Also in cultured mammalian cells the retrograde transport of the ErbB1 receptor is linked, by its binding to a postulated adapter protein, to the actin cytoskeleton. This transport can be disrupted by cytochalasin D, an inhibitor of actin polymerization, but not by nocodazole, a disruptor of microtubule dynamics [42]. The measured velocities of the ErbB1 transport on the filopodia (mean velocity 22 nm/s) is within the range of values (up to 55 nm/s) that were reported for actin retrograde flow in vivo [75, 76]. The experimental differentiation between actin- and tubulin-based cargo is not trivial since chemical disruption of F-actin not only impairs actin-based cargo transport but also filopodia formation. Yet, the estimated microtubular-assisted transport is expected to be very fast. The maximum velocity of kinesin measured in cells is 1.5–2.4 µm/s [77].
Outlook
Next to classical diffusion models, filopodia-based long range signaling has emerged as a general concept of intercellular crosstalk, both in cell culture and during embryonic development. This concept will likely be discovered in many more biological contexts than the presently known model systems and will help to understand the signaling capacity over a distance even of low-diffusible morphogens like Wnts. As yet, a reliable tool to specifically disrupt filopodia formation is missing, which will be essential to study the importance of filopodial signaling in greater detail. With the rapidly growing research efforts on signaling filopodia in both cell and developmental biology, the avenue to establish their role as a general principle of biological signaling is open.
Acknowledgments
We would like to apologize to the authors whose work we could not mention in this review for reasons of space. We thank the Deutsche Forschungsgemeinschaft (DFG, SFB591 grant to M.S.), the Imhoff Stiftung, Cologne (to M.S.) and the Moritz-Stiftung, Cologne (to F.P.) for financial support.
References
- 1.Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development. 2014;141:729–736. doi: 10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/S0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 3.Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol (CB) 2000;10:293–300. doi: 10.1016/S0960-9822(00)00378-X. [DOI] [PubMed] [Google Scholar]
- 4.Muller P, Rogers KW, Yu SR, Brand M, Schier AF. Morphogen transport. Development. 2013;140:1621–1638. doi: 10.1242/dev.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen M, Page KM, Perez-Carrasco R, Barnes CP, Briscoe J. A theoretical framework for the regulation of Shh morphogen-controlled gene expression. Development. 2014;141:3868–3878. doi: 10.1242/dev.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bier E, De Robertis EM (2015) Embryo development. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 348:aaa5838 [DOI] [PubMed]
- 7.Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11:1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 8.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 10.Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132:3895–3905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K. Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Developmental biology. 2004;271:198–209. doi: 10.1016/j.ydbio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 13.Serralbo O, Marcelle C. Migrating cells mediate long-range WNT signaling. Development. 2014;141:2057–2063. doi: 10.1242/dev.107656. [DOI] [PubMed] [Google Scholar]
- 14.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 15.Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell. 2009;17:470–481. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff M, Gradilla AC, Seijo I, Andres G, Rodriguez-Navas C, Gonzalez-Mendez L, Guerrero I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, Schug A, Raz E, Scholpp S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- 18.Holzer T, Liffers K, Rahm K, Trageser B, Ozbek S, Gradl D. Live imaging of active fluorophore labelled Wnt proteins. FEBS Lett. 2012;586:1638–1644. doi: 10.1016/j.febslet.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagar, Prols F, Wiegreffe C, Scaal M. Communication between distant epithelial cells by filopodia-like protrusions during embryonic development. Development. 2015;142:665–671. doi: 10.1242/dev.115964. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/S0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/S1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 24.De Joussineau C, Soule J, Martin M, Anguille C, Montcourrier P, Alexandre D. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 2003;426:555–559. doi: 10.1038/nature02157. [DOI] [PubMed] [Google Scholar]
- 25.Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 2014;343:1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gradilla AC, Guerrero I. Cytoneme-mediated cell-to-cell signaling during development. Cell Tissue Res. 2013;352:59–66. doi: 10.1007/s00441-013-1578-x. [DOI] [PubMed] [Google Scholar]
- 28.Austefjord MW, Gerdes HH, Wang X. Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol. 2014;7:e27934. doi: 10.4161/cib.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caneparo L, Pantazis P, Dempsey W, Fraser SE. Intercellular bridges in vertebrate gastrulation. PLoS One. 2011;6:e20230. doi: 10.1371/journal.pone.0020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salas-Vidal E, Lomeli H. Imaging filopodia dynamics in the mouse blastocyst. Dev Biol. 2004;265:75–89. doi: 10.1016/j.ydbio.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Danilchik M, Williams M, Brown E. Blastocoel-spanning filopodia in cleavage-stage Xenopus laevis: potential roles in morphogen distribution and detection. Dev Biol. 2013;382:70–81. doi: 10.1016/j.ydbio.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Snyder JC, Rochelle LK, Marion S, Lyerly HK, Barak LS, Caron MG. Lgr4 and Lgr5 drive the formation of long actin-rich cytoneme-like membrane protrusions. J Cell Sci. 2015;128:1230–1240. doi: 10.1242/jcs.166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 34.Gerdes HH, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013;130:381–387. doi: 10.1016/j.mod.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 35.McMahon AP, Hasso SM. Filopodia: the cellular quills of hedgehog signaling? Dev Cell. 2013;25:328–330. doi: 10.1016/j.devcel.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Fairchild CL, Barna M. Specialized filopodia: at the ‘tip’ of morphogen transport and vertebrate tissue patterning. Curr Opin Genet Dev. 2014;27:67–73. doi: 10.1016/j.gde.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luz M, Spannl-Muller S, Ozhan G, Kagermeier-Schenk B, Rhinn M, Weidinger G, Brand M. Dynamic association with donor cell filopodia and lipid-modification are essential features of Wnt8a during patterning of the zebrafish neuroectoderm. PLoS One. 2014;9:e84922. doi: 10.1371/journal.pone.0084922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 39.Hagemann AI, Kurz J, Kauffeld S, Chen Q, Reeves PM, Weber S, Schindler S, Davidson G, Kirchhausen T, Scholpp S. In vivo analysis of formation and endocytosis of the Wnt/beta-catenin signaling complex in zebrafish embryos. J Cell Sci. 2014;127:3970–3982. doi: 10.1242/jcs.148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rifes P, Thorsteinsdottir S. Extracellular matrix assembly and 3D organization during paraxial mesoderm development in the chick embryo. Dev Biol. 2012;368:370–381. doi: 10.1016/j.ydbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Koizumi K, Takano K, Kaneyasu A, Watanabe-Takano H, Tokuda E, Abe T, Watanabe N, Takenawa T, Endo T. RhoD activated by fibroblast growth factor induces cytoneme-like cellular protrusions through mDia3C. Mol Biol Cell. 2012;23:4647–4661. doi: 10.1091/mbc.E12-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 44.Munsie LN, Caron N, Desmond CR, Truant R. Lifeact cannot visualize some forms of stress-induced twisted F-actin. Nat Methods. 2009;6:317. doi: 10.1038/nmeth0509-317. [DOI] [PubMed] [Google Scholar]
- 45.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Winterhoff M, Faix J. Actin-filament disassembly: it takes two to shrink them fast. Curr Biol (CB) 2015;25:R450–R452. doi: 10.1016/j.cub.2015.04.050. [DOI] [PubMed] [Google Scholar]
- 47.Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224:289–300. doi: 10.1002/path.2894. [DOI] [PubMed] [Google Scholar]
- 50.Boer EF, Howell ED, Schilling TF, Jette CA, Stewart RA. Fascin1-dependent Filopodia are required for directional migration of a subset of neural crest cells. PLoS Genet. 2015;11:e1004946. doi: 10.1371/journal.pgen.1004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J Cell Sci. 2011;124:3305–3318. doi: 10.1242/jcs.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkelman JD, Bilancia CG, Peifer M, Kovar DR. Ena/VASP enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc Natl Acad Sci USA. 2014;111:4121–4126. doi: 10.1073/pnas.1322093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clay MR, Halloran MC. Control of neural crest cell behavior and migration: insights from live imaging. Cell Adhes Migr. 2010;4:586–594. doi: 10.4161/cam.4.4.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gressin L, Guillotin A, Guerin C, Blanchoin L, Michelot A. Architecture dependence of actin filament network disassembly. Curr Biol (CB) 2015;25:1437–1447. doi: 10.1016/j.cub.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci USA. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 59.Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 60.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arjonen A, Kaukonen R, Mattila E, Rouhi P, Hognas G, Sihto H, Miller BW, Morton JP, Bucher E, Taimen P, Virtakoivu R, Cao Y, Sansom OJ, Joensuu H, Ivaska J. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J Clin Investig. 2014;124:1069–1082. doi: 10.1172/JCI67280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao R, Chen J, Zhang X, Zhai Y, Qing X, Xing W, Zhang L, Malik YS, Yu H, Zhu X. Elevated expression of myosin X in tumours contributes to breast cancer aggressiveness and metastasis. Br J Cancer. 2014;111:539–550. doi: 10.1038/bjc.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duquette PM, Lamarche-Vane N. Rho GTPases in embryonic development. Small GTPases. 2014;5:8. doi: 10.4161/sgtp.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee K, Gallop JL, Rambani K, Kirschner MW. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 2010;329:1341–1345. doi: 10.1126/science.1191710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/S0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 66.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 67.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Theveneau E, Mayor R. Integrating chemotaxis and contact-inhibition during collective cell migration: small GTPases at work. Small GTPases. 2010;1:113–117. doi: 10.4161/sgtp.1.2.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 70.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 71.Nobes C, Marsh M. Dendritic cells: new roles for Cdc42 and Rac in antigen uptake? Curr Biol (CB) 2000;10:R739–R741. doi: 10.1016/S0960-9822(00)00736-3. [DOI] [PubMed] [Google Scholar]
- 72.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol life Sci (CMLS) 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fleming TP, Warren PD, Chisholm JC, Johnson MH. Trophectodermal processes regulate the expression of totipotency within the inner cell mass of the mouse expanding blastocyst. J Embryol Exp Morphol. 1984;84:63–90. [PubMed] [Google Scholar]
- 74.Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–1433. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 75.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 77.Yoo J, Kambara T, Gonda K, Higuchi H. Intracellular imaging of targeted proteins labeled with quantum dots. Exp Cell Res. 2008;314:3563–3569. doi: 10.1016/j.yexcr.2008.09.014. [DOI] [PubMed] [Google Scholar]