Abstract
Introduction:
Due to its biological and antibacterial qualities, many plants, including curcumin, are used as phytomedicines in dentistry. They are primarily used as intracanal medication in endodontics to prevent probable chemical side effects and also to address antimicrobial resistance. Curcumin nanoformulations have improved antibacterial activity and improved dispersion, making them the superior form of curcumin. The purpose of this study was to assess curcumin and nanocurcumin’s antibacterial properties. As a gutta-percha coating, they are to be tested against Escherichia coli.
Materials and Methods:
The study employs the standard strain of E. coli, ATCC 25922. The antibacterial activity of gutta-percha cones against E. coli is assessed after coating them with suspensions of curcumin and nanocurcumin. Scanning electron microscopy is utilized to evaluate the coatings’ continuity.
Results:
The gutta-percha cones that are untreated, coated with curcumin, and coated with nanocurcumin exhibit significantly different levels of antibacterial activity. There is statistically significant variation in their antibacterial activity.
Conclusion:
(1) Compared to curcumin-coated and untreated gutta-percha cones, those coated with nanocurcumin exhibit a stronger antibacterial activity. (2) Compared to uncoated gutta-percha cones, gutta-percha cones coated with curcumin exhibit more antibacterial action.
Keywords: Curcumin, gutta-percha, nanocurcumin, scanning electron microscopy
INTRODUCTION
Endodontic treatment points to dispense with diseases from the internal root canal framework and avoid reinfection by obturation.[1] The complexity of the root canal can be attributed to the presence of isthmuses, accessory canals, and dental tubules. Root canals’ conventional cleaning procedures do not prevent microbes from surviving due to this complex anatomy.[2] In addition to helping to prevent and treat apical periodontitis, the removal of bacteria from the root canal system is essential in lowering the number of re-treatment cases and, consequently, treatment failure.[3]
There are many different types of bacteria present at the primary endodontic level of infection, with Gram-negative bacteria, primarily anaerobes, predominate. In contrast, the diversity of bacterial flora at the secondary level of infection is minimal and distinct. Because of their virulence factors and pathogenicity markers, these bacteria are able to reenter the roots even after treatment. Secondary or persistent root canal infections usually contain facultative bacteria that were formerly hard to isolate from primary infections.[4,5,6]
The primary virulence characteristics of these Gram-negative bacteria that are common in root canals include their ability to build biofilms, their resistance to intracanal medications, their ability to produce endotoxin lipopolysaccharide (LPS), and their capacity to survive on their own in an environment deficient in nutrients.
Antibiotic resistance has resulted in a shift toward the use of phytochemicals and a search for other methods of treating these infections.[7,8,9] Direct broad-spectrum antibacterial activity of curcumin against both Gram-positive and Gram-negative bacteria has been demonstrated.[10,11,12] The anti-inflammatory, anti-arthritic, anti-asthmatic, anti-oxidant, anti-microbial, cardiac protein, and immune-modulatory properties of curcumin have been demonstrated.[13] Curcumin’s broad-spectrum pharmacological characteristics notwithstanding, its low intestinal absorption, hydrophobic nature, and fast metabolism provide a significant obstacle to the drug’s desired therapeutic applications. After oral administration, it has extremely little systemic bioavailability.[14]
A number of methods have been developed recently to boost curcumin’s effectiveness.[15] Using curcumin nanoformulations is the most popular and efficient way to improve the stability and solubility of curcumin.[16] The purpose of this study is to evaluate the antibacterial activity of uncoated, curcumin-coated, and nanocurcumin-coated gutta-percha against Escherichia coli.
MATERIALS AND METHODS
Nanocurcumin synthesis
Ionic gelation method was used to prepare curcumin-loaded chitosan nanoparticles, the methodology procedure was described by Nair et al.[17] To prepare this, to 1% (v/v) acetic acid solution, 0.2% w/v chitosan were added and it was homogenized by overnight stirring at 500 rpm on magnetic stirrer. 4M NaOH was used to adjust the pH of the solution to 5. Then, the curcumin (6%) dissolved in tween 80 was gently mixed with the chitosan solution. To achieve mass ratios of chitosan and tripolyphosphate (TPP) of between 3:1 ratio, an anionic cross-linker TPP (0.1% w/v) was added dropwise to this solution. The suspension obtained was further stirred using magnetic stirrer for 10–45 min at room temperature.
Bacterial strain
Reference strain E. coli ATCC 25922 was the bacterial strain employed in this investigation. To obtain the isolated colonies, the freeze-dried strain preparation was reconstituted using brain heart infusion (BHI) broth, subcultured on BHI agar plates, and then incubated at 37°C for 18–24 h. Following that, the bacterial solution was diluted until the final concentration met the McFarland criterion of 0.5.
Preparation of coated gutta-percha cones
In this study, curcumin and nanocurcumin coatings were applied to gutta-percha. Size 35 taper 4% gutta-percha cones certified from the International Organization for Standardization were autoclaved to sterilize them. The sterilized gutta-percha cones were then placed in Eppendorf tubes containing the tested coatings of nanocurcumin and curcumin for a duration of 24 h. Cones were taken out and let to air dry for an additional day. Together with the untreated gutta-percha cone samples, these coated gutta-percha cones were also submitted for scanning electron microscopy (SEM) to check the uniformity of the coatings on the surface of the gutta-percha cones. Furthermore, tests were conducted on the antibacterial activity of the three different varieties of gutta-percha.
Antimicrobial activity of coated gutta-percha and uncoated gutta-percha cones
The agar diffusion method was utilized to ascertain the antibacterial activity.[18] The nanoparticle-coated gutta-percha cones and the curcumin-coated cones were compared for their antibacterial activity along with that of ordinary uncoated gutta-percha. For this, a reference strain of E. coli was used to seed the BHI plates. The plates were split into two sections. On one half of the plate, normal gutta-percha and coated gutta-percha were inserted on the other half. The plates were incubated for 18–24 h at 37°C. The results were compared and read as a zone of inhibition. Three assays were run, and the mean value was taken into account for statistical analysis.
Scanning electron microscopy study
SEM was used to assess the surface topography of the coated and uncoated gutta-percha cones. Cones coated with curcumin and nanocurcumin and uncoated gutta-percha were critical point dried and then gold coated using an ion sputter JFC 1100. After the cones were cut horizontally, pictures were made by scanning the cross sections of the cones.[19] A JSM-6100 scanning electron microscope with a slub was used to investigate the coated gutta-percha cones’ outer surface at Punjab University Chandigarh’s Central Instrument Laboratory.
Statistical analysis
The comparison between the results of the antibacterial activity of three groups (uncoated gutta-percha, curcumin-coated gutta-percha, and nanocurcumin-coated gutta-percha) was done using a one-way ANOVA test.
RESULTS
Antimicrobial activity of coated gutta-percha versus uncoated gutta-percha cones
The agar diffusion method was utilized to assess antimicrobial activity. The results clearly indicate that coated gutta-percha exhibits a well-defined distinct zone of inhibition against E. coli, while the assay findings demonstrate no antimicrobial activity of conventional gutta-percha against E. coli. When we compared the zones of inhibition of the three groups, we found out that nanocurcumin had wider zones as compared to the other two groups. The test outcomes are shown in Figures 1a and b.
Figure 1.
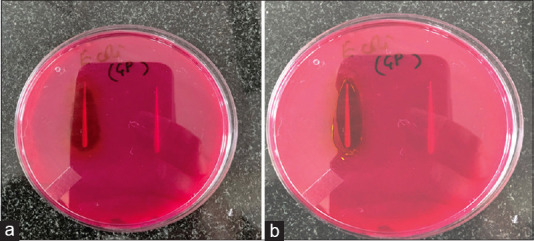
(a) Antibacterial activity of nanocurcumin-coated gutta-percha versus uncoated gutta-percha, (b) Antibacterial activity of curcumin-coated gutta-percha versus uncoated gutta-percha
Scanning electron microscopy study
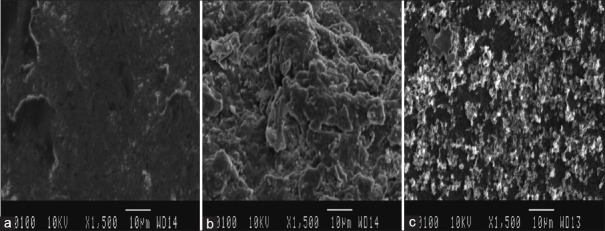
The gutta-percha cones’ surface coatings have dramatically altered the topography of the object. Comparing the uncoated gutta-percha surface to coated cones, unevenness and irregularities have been observed. While curcumin coatings and nanocurcumin coatings were closely adherent to the surface, but latter showed greater uniformity and evenness; for further details, see Figure 2a-c.
Figure 2.
(a) Scanning electron microscopy (SEM) image of uncoated gutta percha at ×1500, (b) SEM image of curcumin-coated gutta-percha at ×1500, (c) SEM image of nanocurcumin-coated gutta-percha at ×1500
Statistical analysis result
As clear from Table 1 which shows test results of one-way ANOVA that there is significant difference between the antibacterial activity of nanocurcumin-coated gutta-percha as compared to curcumin-coated and uncoated gutta-percha. The F-ratio value is 6056. The P < 0.00001. The result is significant at P < 0.05, indicating that nanocurcumin-coated gutta-percha has the highest antibacterial activity and uncoated gutta-percha has the least antibacterial activity.
Table 1.
Statistical details
| Summary of Data | ||||
|---|---|---|---|---|
|
| ||||
| Treatments | Total | |||
|
| ||||
| 1 | 2 | 3 | ||
| N | 5 | 5 | 5 | 15 |
| ∑X | 2.25 | 4.85 | 7.75 | 14.85 |
| Mean | 0.45 | 0.97 | 1.55 | 0.99 |
| ∑X2 | 1.0135 | 4.7055 | 12.0135 | 17.7325 |
| Std.Dev. | 0.0158 | 0.0158 | 0.0158 | 0.4653 |
|
| ||||
| Result Details | ||||
|
| ||||
| Source | SS | df | MS | F ratio |
|
| ||||
| Between-treatments | 3.028 | 2 | 1.514 | F=6056 |
| Within-treatments | 0.003 | 12 | 0.0003 | |
| Total | 3.031 | 14 | ||
SS: sum of squares; df: degree of freedom and MS: mean sum of squares
DISCUSSION
A number of microbial taxa, primarily facultative anaerobes, are important contributors to endodontic case failure.[20,21] These microorganisms either enter the canal through coronal or apical leaks following obturation, or they survive all endodontic cleaning methods and remain inside the canal and dentine tubule.[22,23] Microbes that cause endodontic failure and flare-ups include Gram-positive bacteria (Staphylococcus, Enterococcus), Gram-negative bacteria (E. coli), and yeasts (Candida albicans).[24,25] Bacterial prevalence differences in the literature may be due to differences in detection techniques, sample collection, and patients’ clinical conditions. Some microorganisms have a similar prevalence in both primary infections and secondary infections, suggesting that they may not be completely eliminated during treatment with endodontics.[26,27]
The aim of the study was to synthesize and test the antibacterial activity of a curcumin-loaded chitosan nanoparticle against E. coli, as well as to compare it with that of curcumin alone and also with that of uncoated gutta-percha. Curcumin has a wide range of therapeutic potential due to its antimicrobial properties, anti-inflammatory properties, anticancer properties, and anti-oxidation properties. However, its potential remains limited due to low bioavailability, large size, and stability problems.[14] However, because nanocurcumin is much smaller than curcumin, it penetrates cells more easily and is taken up by them more fully. Our study’s findings show that gutta-percha coated with nanocurcumin has higher antibacterial activity than gutta-percha coated with conventional curcumin, suggesting that nanocurcumin has more antimicrobial potential than curcumin. To match the zone of inhibition of curcumin to that of nanocurcumin, curcumin at higher concertation is to be used; since at the same concentration levels, they showed different zone diameters. In this investigation, a size 35 gutta-percha cone was selected since it is an intermediate size that works well with most canals and obturation methods.[28] When compared to the surface of uncoated and curcumin-coated gutta-percha cones, the nanocurcumin coating is more consistent and tightly adheres to the gutta-percha cones.
Our study’s findings concur with those of Adahoun et al., who also showed that curcumin nanoformulations have improved antibacterial activity and increased water solubility.[29] A further study by Mun et al. showed that curcumin and other antibiotics had a synergistic impact on methicillin-resistant Staphylococcus aureus and that using curcumin in combination with each of these drugs significantly improved the inhibition of bacterial growth.[30] Researchers Panwar et al. showed that gutta-percha coated with nanocurcumin had an antibacterial impact on Enterococcus faecalis,[31] whereas Sharma et al. observed an antimicrobial effect on S. aureus.[32] Our findings also support the findings of Tyagi et al., who demonstrated that curcumin had potent antibacterial activity against E. coli, S. aureus, and E. faecalis.[33] Our investigation revealed that conventional gutta-percha lacked antibacterial activity; these findings are consistent with those of Melker et al.,[20] who also showed that normal gutta-percha was unable to eradicate crucial endodontic pathogens.
CONCLUSION
Nanocurcumin represents a significant advance as an antimicrobial agent against E. coli and other pathogens prevalent in endodontic infections. Curcumin nanoparticles have good stability and solubility, therefore, difficulties inherent in curcumin administration can be circumventers. Gutta-percha cones coated with nanocurcumin have antimicrobial activity against various microbes and may be of great help in combating residual microbes in the root canals after obturation, thus limiting flare-ups and decreasing the number of failure cases in endodontic treatment.
Limitations of the study
Minimum inhibitory concentration of curcumin as well as nanocurcumin would add to the precision of the study
A study designed on large population sample is required to generalize the results
Carrier used for nanoparticle preparation may also alter the properties of nanoparticles. Detailed characterization of nanoparticles is required for precise results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors would like to express their sincere gratitude to Panjab University, Chandigarh for proving financial support in the form of publication grant to publish this article in Journal of Conservative Dentistry and Endodontics.
REFERENCES
- 1.Pantera EA. Wiley-Blackwell, United States: American Dental Education Association; 2008. Essential Endodontology: Prevention and Treatment of Apical Periodontitis; pp. 1212–3. [Google Scholar]
- 2.Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36:322–8. doi: 10.1016/j.joen.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siqueira JF, Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291–301.e3. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–78. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siqueira JF, Jr, de Uzeda M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod. 1996;22:674–6. doi: 10.1016/S0099-2399(96)80062-8. [DOI] [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int Endod J. 1999;32:361–9. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 7.Khameneh B, Iranshahy M, Soheili V, Bazzaz BS. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8:1–28. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother Res. 2019;33:13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- 9.Shadifar H, Bahreini M, Khameneh B, Emami SA, Fatemi N, Sharifmoghadam MR. Antibacterial and synergistic effects of aqueous and methanol extracts of artemisia annua against multidrug-resistant isolates of acinetobacter. Anti InfectAgents. 2021;19:28–35. [Google Scholar]
- 10.Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. 2019;10:912. doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasbolaghi Sharahi J, Aliakbar Ahovan Z, Taghizadeh Maleki D, Riahi Rad Z, Riahi Rad Z, Goudarzi M, et al. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J Phytomed. 2020;10:3–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Sundaramoorthy NS, Sivasubramanian A, Nagarajan S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb Pathog. 2020;148:104445. doi: 10.1016/j.micpath.2020.104445. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piao ZZ, Choe JS, Oh KT, Rhee YS, Lee BJ. Formulation and in vivo human bioavailability of dissolving tablets containing a self-nanoemulsifying itraconazole solid dispersion without precipitation in simulated gastrointestinal fluid. Eur J Pharm Sci. 2014;51:67–74. doi: 10.1016/j.ejps.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–83. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 17.Nair RS, Morris A, Billa N, Leong CO. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20:69. doi: 10.1208/s12249-018-1279-6. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. CLSI Approved Standard M100-S15. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 19.Afanasyev SS, Kychkina TV, Savvinova LN. Scanning electron microscope (advantages and disadvantages). Research paper on medical technology. Colloq J. 2019;2:25–7. [Google Scholar]
- 20.Melker KB, Vertucci FJ, Rojas MF, Progulske Fox A, Bélanger M. Antimicrobial efficacy of medicated root canal filling materials. J Endod. 2006;32:148–51. doi: 10.1016/j.joen.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 22.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–84. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira JF., Jr Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 24.Fabricius L, Dahlén G, Holm SE, Möller AJ. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res. 1982;90:200–6. doi: 10.1111/j.1600-0722.1982.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 25.Endo MS, Ferraz CC, Zaia AA, Almeida JF, Gomes BP. Quantitative and qualitative analysis of microorganisms in root-filled teeth with persistent infection: Monitoring of the endodontic retreatment. Eur J Dent. 2013;7:302–9. doi: 10.4103/1305-7456.115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chugal N, Wang JK, Wang R, He X, Kang M, Li J, et al. Molecular characterization of the microbial flora residing at the apical portion of infected root canals of human teeth. J Endod. 2011;37:1359–64. doi: 10.1016/j.joen.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tennert C, Fuhrmann M, Wittmer A, Karygianni L, Altenburger MJ, Pelz K, et al. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J Endod. 2014;40:670–7. doi: 10.1016/j.joen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Marciano MA, Bramante CM, Duarte MA, Delgado RJ, Ordinola Zapata R, Garcia RB. Evaluation of single root canals filled using the lateral compaction, tagger's hybrid, microseal and guttaflow techniques. Braz Dent J. 2010;21:411–5. doi: 10.1590/s0103-64402010000500006. [DOI] [PubMed] [Google Scholar]
- 29.Adahoun MA, Al Akhras MH, Jaafar MS, Bououdina M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif Cells Nanomed Biotechnol. 2017;45:98–107. doi: 10.3109/21691401.2015.1129628. [DOI] [PubMed] [Google Scholar]
- 30.Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20:714–8. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Panwar D, Sidhu K, Bhushan J, Kakkar V, Mehta M, Sharma J. Evaluation of antimicrobial efficacy of nanocurcumin-coated gutta-percha against Enterococcus faecalis: An in vitro study. J Conserv Dent. 2023;26:160–4. doi: 10.4103/jcd.jcd_512_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma J, Panwar D, Bhushan J, Mehta M, Sidhu K, Jhamb S, et al. An in vitro evaluation of antibacterial effect of curcumin-loaded chitosan nanoparticle-coated gutta-percha against Staphylococcus aureus. J Conserv Dent Endod. 2023;26:560–3. doi: 10.4103/jcd.jcd_302_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10:e0121313. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]