Abstract
Insulin, insulin-like growth factors (IGFs) and insulin-like peptides (ILPs) are important regulators of metabolism, growth, reproduction and lifespan, and mechanisms of insulin/IGF signaling (IIS) have been well conserved over evolution. In insects, between one and 38 ILPs have been identified in each species. Relatively few insect species have been investigated in depth with respect to ILP functions, and therefore we focus mainly on the well-studied fruitfly Drosophila melanogaster. In Drosophila eight ILPs (DILP1-8), but only two receptors (dInR and Lgr3) are known. DILP2, 3 and 5 are produced by a set of neurosecretory cells (IPCs) in the brain and their biosynthesis and release are controlled by a number of mechanisms differing between larvae and adults. Adult IPCs display cell-autonomous sensing of circulating glucose, coupled to evolutionarily conserved mechanisms for DILP release. The glucose-mediated DILP secretion is modulated by neurotransmitters and neuropeptides, as well as by factors released from the intestine and adipocytes. Larval IPCs, however, are indirectly regulated by glucose-sensing endocrine cells producing adipokinetic hormone, or by circulating factors from the intestine and fat body. Furthermore, IIS is situated within a complex physiological regulatory network that also encompasses the lipophilic hormones, 20-hydroxyecdysone and juvenile hormone. After release from IPCs, the ILP action can be modulated by circulating proteins that act either as protective carriers (binding proteins), or competitive inhibitors. Some of these proteins appear to have additional functions that are independent of ILPs. Taken together, the signaling with multiple ILPs is under complex control, ensuring tightly regulated IIS in the organism.
Keywords: Insulin, Insulin-like growth factors, Neuropeptide release, Nutrient sensing, Metabolism
Introduction
Insulin is one of the most extensively investigated peptide hormones due to its critical role in carbohydrate metabolism and thus importance in diabetes and obesity. Since its discovery in 1922 [1], insulin and insulin-like peptides have been identified in a large number of animals from invertebrates, such as nematode worms, mollusks, and insects to chordates [2–5]. In humans, there is one insulin, two insulin-like growth factors (IGFs), one relaxin and a number of human insulin-like peptides (INSL3-7) that are members of the insulin superfamily of peptides [6–10]. These peptides display a variety of functions in different tissues both during development and in the mature organism. In insects, varying numbers of insulin-like peptides (ILPs) have been identified in different species, ranging from one in the locusts, Locusta migratoria and Schistocerca gregaria, to 38 in the silkmoth Bombyx mori [11–15]. The classification of insect ILPs as insulin-like is based on similarities in the amino acid sequence of the mature peptides to those of mammalian insulins, especially the number and positions of cysteine residues [4, 12, 16]. Another conserved feature is the arrangement of the precursor (pre-proinsulin) protein with B, C and A-chains that can be processed into dimeric peptides with an A and a B-chain linked by disulphide bridges. An exception to this structure is seen for the insulin-like growth factors (IGFs), where a short C-peptide is retained and the extended peptide is a single chain with internal cysteine bridges. In insects, ILPs of both insulin and IGF type have been identified (see [3, 13, 16]).
In Drosophila eight ILPs (DILP1-8), encoded on separate genes, have been detected [16–21]. One of these, DILP6, is IGF-like structurally and functionally [18, 22], and DILP7 and DILP8 have been proposed to be relaxin-like [19, 20, 23, 24]. Only one of the eight Drosophila DILPs seems to have conserved orthologs in insects outside the Drosophilids and that is DILP7 [4, 15]. The different DILPs are produced in a distinct spatio-temporal pattern that suggests separate functions of the peptides, although some redundancy has been detected [4, 16, 20]. One conserved feature among insects is that certain ILPs are produced in a set of median neurosecretory cells in the pars intercerebralis of the brain (see [3, 11, 13, 16]). In Drosophila, these cells, designated as insulin-producing cells (IPCs) express DILP2, 3 and 5, which seem to be independently regulated transcriptionally (see [4, 25, 26]).
Whereas there are several receptors for the different members of the insulin family in mammals [6, 27, 28], only one receptor has as yet been identified in most invertebrate species. In mammals, the receptors are of the tyrosine kinase type (insulins and IGFs) and G-protein coupled receptors (GPCRs; relaxin and INSLs), while in invertebrates only receptor tyrosine kinases have been clearly identified as cognate ILP receptors (see [3, 16]). However, two relaxin receptor-like leucine-rich repeats containing GPCRs (LGR type C) were recently discovered in Drosophila [29]. For one of these, Lgr3 (CG31096) it was established that the relaxin-like DILP8 is the ligand [30]. Also, there are two reports of additional receptor tyrosine kinases with ILP ligands in a single arthropod species, two in the hemipteran planthopper Nilaparvata lugens [31] and four putative receptors in the water flea Daphnia pulex [32]. The signaling pathway downstream of the insulin receptor (InR) is also well conserved across phyla, and among invertebrates it has been extensively studied in Caenorhabditis elegans and D. melanogaster (see [2, 3, 5, 33–36]). In the planthopper, the two insulin receptors (InR1 and InR2) couple differently to downstream signaling: InR1 stimulates phosphatidylinositol-3-kinase (PI3K)—protein kinase B (Akt) signaling, whereas InR2 inhibits the PI3K-Akt pathway in regulation of wing polyphenism [31].
In the last 15 years, the interest in insulin/IGF signaling (IIS) in invertebrates has increased dramatically. This was probably triggered by the discovery that defects in IIS increased lifespan in the worm C. elegans and in Drosophila [37–43]. However, it was soon found that IIS also plays multiple roles in cell and organism growth, cell cycle regulation, including stem cell activation, and in stress responses, fecundity and not least in metabolic homeostasis (see [3, 16, 34, 36, 44–49]). Furthermore, it seems that IIS plays an important role in regulating the dormancy state known as diapause in insects [50–52] and as dauer stage in C. elegans [53], and may modulate certain behaviors [54–57]. A common feature of the above aspects of IIS is that they integrate nutrient and energy storage information with cellular and physiological activities that regulate the anabolic branches of metabolism, in association with processes such as growth and reproduction.
Over the years, several reviews on various aspects of IIS have been published. Thus, we try to focus this review on what happens before and after release of ILPs in insects: cell-autonomous activation of IPCs, neuronal and hormonal regulation of IPC activity and thereby production and release of ILPs, circulating factors interacting with secreted ILPs, as well as peripheral modulators of IIS and feedback mechanisms. Among the latter are insulin-binding proteins that recently have emerged as important regulators of growth and organ wasting [17, 58–63]. We also discuss the complex interactions between IIS and the lipophilic hormones 20-hydroxyecdysone (20E) and juvenile hormone (JH). Since much of the available data derive from studies of D. melanogaster, this review will be somewhat biased towards this species, but we make an effort to discuss other insects. References to Drosophila in the text refer to D. melanogaster, other species will be given with full name.
A brief overview of insulin/IGF-like peptides in insects
Different ILPs are produced in different cell types and tissues at different developmental stages. However, in all insects studied at least one of the ILPs is produced by neurosecretory cells in the brain. Thus, a set of median neurosecretory cells (MNCs) in the pars intercerebralis, referred to as IPCs (Fig. 1), is an important source of ILPs in postembryonic insects [11, 16, 26, 64, 65]. In some insects such as the mosquitos Aedes aegypti and Anopheles gambiae, lateral neurosecretory cells of the brain also express ILPs [64, 66, 67], and in the hemipteran bug Rhodnius prolixus additional brain neuroendocrine cells stain with antisera to ILPs [68]. The ILPs of the brain IPCs can be released into the circulation via axon terminations in neurohemal areas of the corpora cardiaca and corpora allata, anterior aorta and anterior intestine (Fig. 1). Although conclusive data are lacking, there are indications that ILPs can be released within the Drosophila brain from IPCs, or at least ILPs can enter into the brain after release into the circulation. One line of evidence for modulatory action of ILPs within the brain is sequestering of DILP2 by specific sets of neuroendocrine cells in the Drosophila brain and activation of Akt1 (protein kinase B) in these neurons [58].
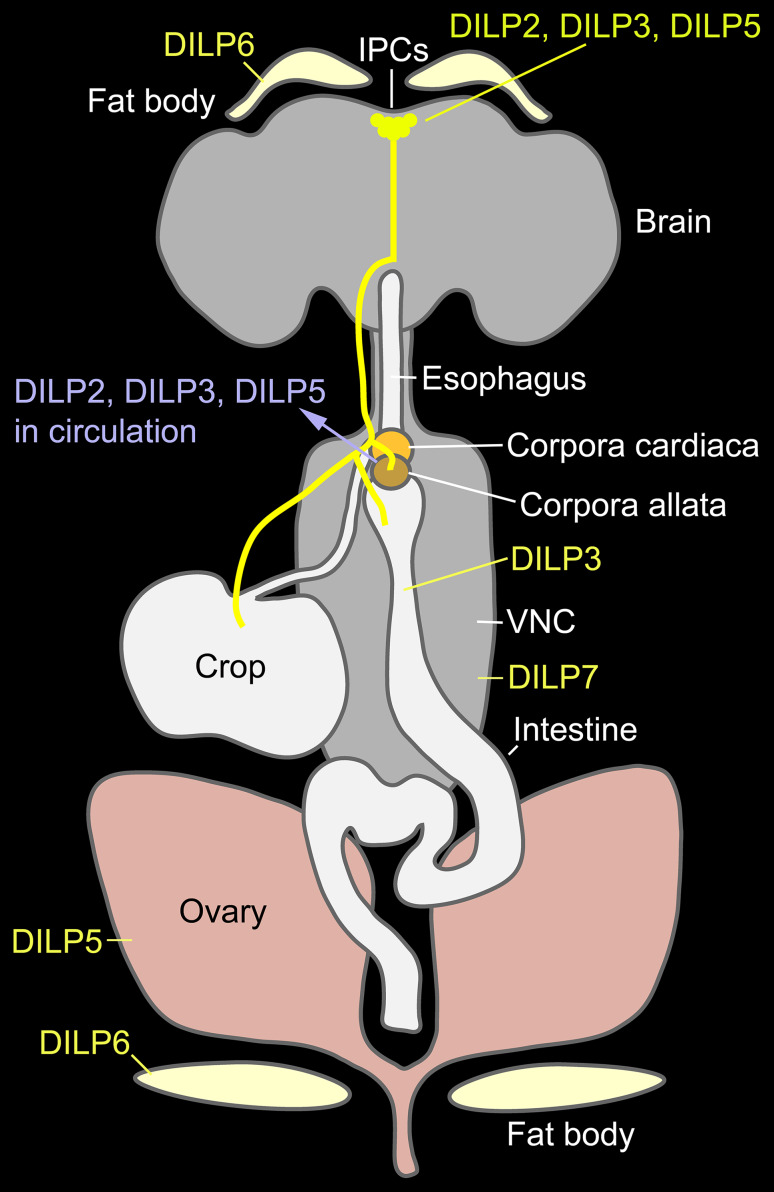
Fig. 1.
Overview of sites of production and release of DILPs in the CNS and other organs of adult Drosophila. Three DILPs are produced in the insulin-producing cells (IPCs) of the brain, shown in yellow: DILP2, 3 and 5. These DILPs are released from axon terminations in the corpora cardiaca (CC), corpora allata (CA), crop and anterior intestine. DILP3 is additionally produced by the intestinal muscle and DILP5 in the ovaries and Malpighian tubules (not shown). DILP6 is mainly produced by adipocytes of the fat body in the head and body of the fly. Finally, DILP7 is produced by about 20 neurons in the abdominal neuromeres of the ventral nerve cord (VNC) and may be released onto the posterior intestine and oviduct, as well as inside the CNS. This figure is redrawn and altered from [76], which was based on an illustration by Toivonen and Partridge [192]
The IGF-like ILPs in Drosophila (DILP6) and moths are produced in the adipose cells of the fat body [18, 22, 69, 70]. In adult Drosophila, the cellular expression of the eight DILPs has been analyzed in some detail: DILP2, 3 and 5 are expressed in the brain IPCs (also in larvae), DILP3 in the intestine, DILP5 in ovaries and Malpighian tubules, and DILP7 in neurons of abdominal ganglia [16, 23, 24, 26, 71, 72] (Fig. 1). A developmental expression of some of the Drosophila peptides has been recorded: DILP1 in IPCs mainly during pupal stages, DILP2-5 in the embryo (mesoderm), and DILP8 primarily in imaginal discs of the larva [19, 20, 25]. In the following, we will focus on ILPs produced by brain IPCs and the fat body.
It is important to note that the current view is that the three DILPs produced by the IPCs of the Drosophila brain have distinct, but overlapping, functional roles. This is based on data showing a differential regulation of DILP2, 3 and 5 at the transcript and protein levels (see [25, 73–76]). A recent report suggests that release of these individual DILPs from the IPCs may occur independently from distinct storage vesicle populations [77]. Another important aspect of Drosophila IPCs is that most of these cells also produce the peptide drosulfakinin (DSK), a peptide that induces satiety [78].
The regulation of ILP production and release
The mechanisms behind secretion of insulin from mammalian pancreatic beta cells have been intensely studied (see [79–81]). Insulin secretion is triggered by cell-autonomous sensing of increased circulating glucose by the beta cells, which induces membrane depolarization and subsequent membrane fusion of insulin containing vesicles and thereby release. The glucose sensing is mediated by a glucose transporter (GLUT1), that allows entry of glucose into the glycolysis pathway. Subsequent production of ATP in mitochondria leads to inactivation of ATP-sensitive potassium channels (KATP) in the cell membrane and subsequent depolarization of the beta cell. This depolarization activates voltage-sensitive calcium channels resulting in secretion of insulin by exocytosis in a fashion similar to regular neurotransmitter release, and requires similar synapse and channel proteins (see [80, 81]). Insulin secretion from pancreatic beta cells also depends on fuel stimuli additional to glucose, such as free fatty acids and amino acids. After uptake into the beta cells, the fatty acids and amino acids enter appropriate metabolic pathways, similar to glucose, and key signaling molecules are produced that influence insulin secretion, as described in detail by Prentki et al. [81].
The calcium-dependent exocytosis can be modulated by neurotransmitters and circulating hormones via action on membrane receptors that change ion channel activity or recruit second messenger systems [79]. Thus, in addition to glucose-induced depolarization of the beta cells, various secreted factors regulate the exact amount of secreted insulin. Several stimulatory (incretins) and inhibitory (decretins) modulators of glucose-induced insulin secretion from beta cells are known in mammals [80, 81]. These are both neurotransmitters produced locally in the islets of the pancreas and hormones released from other sources. Somatostatin released from pancreatic delta cells and adrenalin from circulation hyperpolarize beta cells and inhibit insulin secretion via somatostatin and α2-adrenergic receptors, respectively. Another inhibitor is the gut peptide galanin. Among the stimulators of insulin secretion are acetylcholine acting on M3 muscarinic receptors, GABA acting on ionotropic GABAA receptors, as well as glucagon and glucagon-like peptide-1 (GLP-1) acting on their GPCRs. Another stimulator is ATP, which is co-released with insulin and acts in an autocrine loop on P2X3 receptors. The GABA signaling to beta cells is complex. GABA is produced in beta cells and after release it acts both on GABAA and metabotropic GABAB receptors on the same cells in an autocrine fashion [80]. Whereas GABAA activation stimulates insulin secretion, GABAB activation decreases it by inhibiting vesicle exocytosis, thus GABA signaling seems to be part of a local fine-tuning of insulin release.
Recent reviews have summarized some mechanisms that regulate the activity of brain IPCs in Drosophila and the production and release of DILPs [76, 82, 83]. However, quite a few studies on this topic have been published recently and will be reviewed here. It is evident that the functional roles of DILPs differ between larvae that feed constantly, grow, develop and undergo molting, and adult flies that feed intermittently, reproduce and age. In line with this, it has been shown in Drosophila that sensing of circulating nutrients by IPCs and other cells, as well as regulation of DILP production and release, differs between larvae and adults. Thus, we will describe IPC regulation in these developmental stages separately.
Mechanisms in adult Drosophila
It was shown relatively recently that the brain IPCs have cell-autonomous glucose-sensing capacity and that a sugar meal triggers DILP release by direct activation of these cells [84]. The mechanisms for glucose-induced DILP release are similar to those in mammalian pancreatic beta cells. Thus, the IPCs express an ATP-sensitive potassium channel (KATP), a glucose transporter (GluT1) and voltage-sensitive calcium channels [84, 85]. Until now the only peptide that was clearly shown to be released from IPCs by any mechanism is DILP2; and its release is glucose dependent [84]. Since it is known that the different DILPs are individually regulated at the transcriptional level in the IPCs [25, 73–75] and also suggested to be released separately by different triggers [77], we cannot generalize mechanisms for regulation of production and release of these peptides. Several neurotransmitters, neuropeptides and peptide hormones have been implicated in acting on IPCs to alter expression of DILPs (or their transcripts) and affect readouts of systemic insulin signaling. However, we will first discuss some factors released from the nutrient sensing fat body. In Fig. 2, the nutrient-derived signals to IPCs are shown, and in Fig. 3 and Table 1 the receptors (mostly GPCRs) expressed by IPCs.
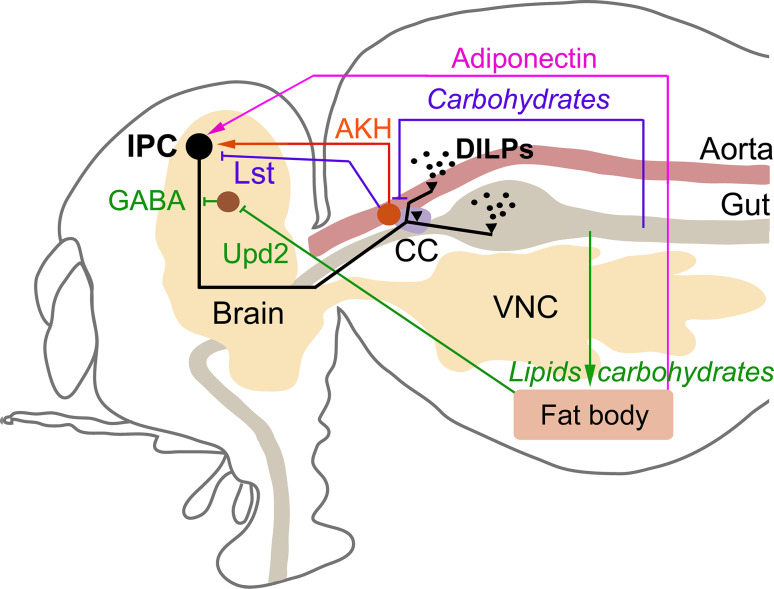
Fig. 2.
Scheme showing factors regulating IPCs in Drosophila. The IPCs (black cells) are regulated by multiple factors released from the intestine, fat body, endocrine cells (APCs) of the corpora cardiaca (CC) and brain. Green pathway shows lipid and carbohydrate-mediated Upd2 release from fat body that inhibits GABAeric neurons and thereby lifts tonic inhibition of IPCs. Red pathway shows glucose-mediated AKH release from CC that stimulates IPCs. Blue pathway is carbohydrate-mediated inhibition of AKH-producing cells that triggers release of limostatin (Lst) which inhibits IPCs. Finally, the magenta pathway shows adiponectin release from fat body that stimulates IPCs
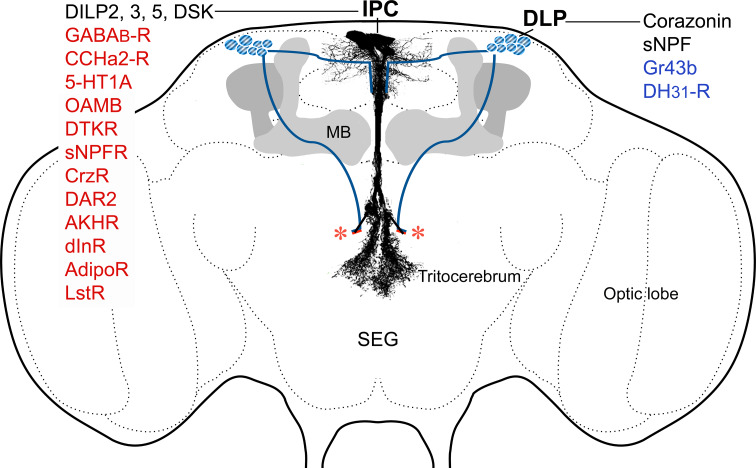
Fig. 3.
Local and remote regulation of IPCs in the brain. The IPCs are regulated by sNPF and corazonin from the DLP (dorsal lateral peptidergic) neurons that send processes to the IPC dendrites in the pars intercerebralis. These DLPs express sNPF and corazonin, the fructose receptor Gr43b and a GPCR for diuretic hormone 31 (DH31), a peptide that could be released from the intestine. The IPCs express DILP2, 3 and 5, drosulfakinin (DSK) and a number of GPCRs (in red text), as well as the insulin receptor (dInR). The GPCRs are discussed and acronyms explained in the text. The DILPs are released from the peripheral axon terminations (not shown) of the IPCs; asterisks where these axons originate in the brain and project to the corpora cardiaca, aorta and anterior gut structures. MB mushroom bodies (shown for orientation)
Table 1.
Neurotransmitters, neuropeptides and peptide hormones acting on IPCs in Drosophila melanogaster
| Substance | Production sitea | Receptor on IPCs | Stage | References |
|---|---|---|---|---|
| GABA | Brain neurons | GABAB | Adult | [86, 87] |
| Serotonin | Brain neurons | 5-HT1A | Adult | [108] |
| Octopamine | Brain neurons | OAMB | Adult | [57] |
| sNPF | Brain neuronsb | sNPFR1 | Adult | [91] |
| Corazonin | Brain neuronsb | CrzR (GRHRII)c | Adult | [91] |
| Tachykinin | Brain neurons, ECd | DTKR (TkR99D) | Adult | [94] |
| Allatostatin A | Brain neurons, ECd | DAR2 (AstA-R2) | Adult | [96] |
| CCHamide2 | EC (fat body?) | CCHa2-R | Adulte/larva | [100, 101] |
| Limostatin | Corpora cardiacaf | LstR (PK1-R)g | Adult | [103] |
| DILP6 | Fat body | dInR (InR) | Adult | [90] |
| Adiponectin | Fat body | AdipoR | Adult/larva | [89] |
| AKH | Corpora cardiaca | AkhR (GRHR) | Adult/larva | [77, 96] |
aIn most cases, tentative site of production is shown and actual cells releasing onto IPCs have rarely been identified
bThe DLP neurons of pars lateralis
cIn brackets synonyms are given
d EC enteroendocrine cells. Like suggested for CCHamide2 [100], these peptides may enter the circulation and act on IPCs
eThe adult action on IPCs is less well documented
fMore specifically, the endocrine cells that also produce AKH
gAlso known as PK1-R, a pyrokinin 1 receptor (CG9918, a neuromedin U-receptor-like GPCR)
Unpaired 2 and brain GABA
The glucose-induced DILP release seems to be modulated by alteration of the membrane potential of the IPCs. It was shown that IPCs express metabotropic GABAB receptors, GABAB-R [86] and that these cells are tonically hyperpolarized by GABA [87]. This GABAergic inhibition can be disengaged by a signal from the fat body, the cytokine peptide Unpaired 2 (Upd2), considered to be leptin-like [87]. Upd2 release from the fat body is triggered by elevated levels of lipid or carbohydrate in the hemolymph and circulating Upd2 acts on the cytokine receptor domeless (Dome) that activates JAK/STAT signaling in the GABAergic neurons and thereby blocks GABA release and diminishes IPC hyperpolarization (Fig. 2). It is, however, not known how Upd2 passes through the blood–brain barrier to act on the GABAergic neurons. These findings suggest involvement of GABA signaling in modulation of insulin release both in mammals and Drosophila, but with different mechanisms.
Adiponectin
In mammals, adiponectin (a 244 amino acid polypeptide adipokine) acts on two GPCRs, AdipoR1 and AdipoR2 in muscle and liver to regulate lipid and carbohydrate homeostasis [88]. One adiponectin receptor ortholog (dAdipoR; CG5315) was identified in Drosophila and shown to be expressed by the IPCs and some other brain neurons in larvae and adult flies [89]. Knockdown of the dAdipoR in IPCs leads to increased circulating trehalose and glucose levels and stored lipids, as well as increased sensitivity to high lipid diet. Furthermore, the dilp3 mRNA was slightly decreased in these flies and DILP2 release from IPCs in starved and re-fed flies was inhibited [89]. As a consequence of decreased DILP release in dAdipoR knockdown flies, these authors noted effects on FOXO and 4E-BP suggesting reduced IIS in the fat body. The Drosophila adiponectin itself was not yet identified, but human adiponectin acts on IPCs (in larvae) to decrease DILP2 immunolabeling [89]. It was suggested that the Drosophila adiponectin is produced by the fat body and constitutes yet another adipokine connecting the nutrient sensing fat body and the IPCs [89] (Fig. 2).
DILP6
The IGF-like DILP6 is produced in the adult fat body, is under control by FOXO and regulates carbohydrate and lipid storage as well as oxidative stress resistance [90]. Overexpression of dilp6 in the fat body decreases the expression of dilp2 and dilp5 mRNA in the brain and reduces release of DILP2 and thereby increases longevity [90]. Thus, DILP6 may serve as another adipokine signal from fat body to IPCs.
Short neuropeptide F and corazonin
Several neuropeptides, peptide hormones and neurotransmitters have been proposed as modulators of IPC activity. However, only for a few of these ligands has it been established which cells or neurons that are likely to deliver them to the IPCs, and what triggers their action on these target cells. Two neuropeptides, short neuropeptide F (sNPF) and corazonin, are produced in a bilateral set of neurons, the so-called dorsal lateral peptidergic neurons (DLPs; Fig. 3), located in the pars lateralis [91]. These neurons impinge on the median processes of the IPCs in the pars intercerebralis. The IPCs express the sNPF receptor (sNPFR1) and indirect evidence suggests that this is also the case for the corazonin receptor (CrzR) [91]. Targeted knockdown of these receptors in IPCs produces a phenotype suggesting that systemic insulin signaling is down regulated [91]. Knockdown of sNPF in the DLPs also results in diminished dilp2 and dilp5 expression, whereas diminishing corazonin in these neurons has no effect on dilp transcription. Thus, the two peptides of the DLPs seem to have different actions on the IPCs. Interestingly, a subset of the DLPs also express a gustatory receptor, Gr43a (see Fig. 3), that responds to circulating fructose, a carbohydrate that is transiently increased in the circulation after a sugar meal [92]. Thus, the DLPs may be nutrient sensors that stimulate production and/or release of DILPs from IPCs. The DLPs are also known to express receptors for diuretic hormones 31 and 44 (DH31 and DH44) [93]. Therefore, these peptides may also modulate DLP activity, either via brain neurons, or in the case of DH31 via circulating peptide released from endocrine cells of the intestine (see [71]).
Drosophila tachykinin
Another neuropeptide, Drosophila tachykinin (DTK; actually five peptides, DTK1-5, encoded on the same precursor gene), has been proposed to regulate IPCs. The DTK receptor, DTKR, is expressed by IPCs and targeted knockdown of DTKR induces up regulation of dilp2 and 3 in fed flies and up regulation of dilp2 and down regulation of dilp3 in starved flies [94]. Although there are processes of DTK-expressing brain neurons superimposing the presumed IPC dendrites, the individual neurons of origin could not be identified. Another possible source of DTK is enteroendocrine cells of the midgut [95] signaling via the circulation, but this remains to be tested experimentally.
Allatostatin A
Recently, it was shown that the IPCs express a galanin receptor-like GPCR (DAR2) for the neuropeptide allatostatin A (AstA) and that several AstA producing neurons have branches superimposing the IPC branches in the pars intercerebralis and the tritocerebrum [96]. Another possible source of AstA is the numerous AstA producing enteroendocrine cells of the midgut [71, 97]. Interestingly, also the AKH-producing cells (APCs) in the corpora cardiaca express the DAR2 receptor. Thus, it was shown that both IIS and AKH signaling is stimulated by AstA via DAR2. More specifically, it was shown that genetic inactivation of AstA producing cells, or knockdown of DAR2 in IPCs or APCs, induced changes in the expression of several genes indicative of reduced IIS or AKH signaling. Targeted stimulation of AstA neurons with a depolarizing channel (NaChBac) increases expression of dilp3 and target of brain insulin (tobi), an α-glucosidase known to be activated by both AKH and DILPs [98], while an AstA loss-of-function mutant displays the opposite phenotype, suggesting that AstA stimulates AKH and DILP signaling [96].
To separately analyze the role of AstA signaling on APCs and IPCs, targeted dar2-RNAi was employed using Akh-Gal4 and dilp2-Gal4 lines. Both APC and IPC knockdown of DAR2 lead to increased starvation resistance (suggestive of decreased systemic IIS). However, when reducing DAR2 only in APCs, specific features of AKH signaling were affected that suggest that AstA regulates AKH release: increased expression of the AkhR and two metabolic genes brummer lipase (Bmm) and phosphoenolpyruvate carboxykinase (PEPCK), and decreased tobi [96]. Knockdown of DAR2 in IPCs decreases dilp2 and tobi, and increases the dFOXO target 4E-BP indicating that AstA normally stimulates IIS via the IPCs [96]. Thus, both AKH and DILP signaling is stimulated by AstA suggesting a complex interaction between these signal systems. As suggested previously [98, 99], APCs and IPCs may interact directly via contacts in the corpora cardiaca and intestine. This interaction could be seen after dar2-RNAi in APCs of female flies where dilp2 and dilp3 transcripts were up and down regulated, respectively, and 4E-BP was reduced; in males only dilp2 was affected and 4E-BP was up regulated [96]. These findings indicate that APCs regulate activity in IPCs (see Fig. 2) and that there is a sexual dimorphism in the interaction.
Another interesting finding was that AstA and Dar2 mRNA expression is dependent on diet. Flies kept on restricted diet and then transferred to either protein- or carbohydrate-rich diet displayed different transcript levels. Whereas both AstA and Dar2 are down regulated after nutrient restriction, both are strongly up regulated after re-feeding on high sugar diet, but only a weak up regulation of AstA is seen after high yeast diet [96]. Furthermore, flies kept on standard food prefer sucrose-rich food, whereas flies with AstA neurons activated by expression of NaChBac displayed a preference for protein-rich food, especially in females [96]. In contrast, the AstA mutant flies consumed more sucrose than control flies. In summary, the AstA signaling may be part of a nutrient-sensing mechanism and act on APCs and IPCs to guide feeding decisions to uphold a balance between energy requirements and feeding, and the sex-specific differences may be related to specific nutrient requirements during egg development [96]. As mentioned earlier, the mammalian galanin receptor is known to regulate pancreatic beta cells, suggesting another conserved signal pathway.
CCHamide2
The bombesin-like peptide CCHamide2 has been more extensively studied in larval Drosophila as a modulator of IPCs and DILP signaling [100, 101] and will be discussed in more detail below. This peptide may also act on adult IPCs, but more conclusive experiments are required. In adult flies, CCHa2 is produced by enteroendocrine cells of the midgut [102], as well as by brain neuroendocrine cells [100]. What is known in adults is that CCHa2 mutant flies feed less and display strongly reduced locomotor activity during the day.
Limostatin
A gene encoding a polypeptide, named limostatin (Lst) after Limos a Greek goddess of starvation, was recently identified as a suppressor of insulin production and release [103]. From the limostatin precursor, it was proposed that a peptide consisting of 15 amino acids with the sequence AIVFRPLFVYKQQEI (Lst-15) is liberated. The Lst gene is expressed in the fat body and together with AKH in the APCs of the corpora cardiaca and is produced during fasting [103]. Lst mutant flies were found hypoglycemic, with reduced lifespan and they display increased levels of Dilp2, 3 and 5 mRNA and circulating DILP2. Knockdown of Lst in the APCs produced the same phenotype as the Lst mutation suggesting that these cells are a sufficient source of secreted peptide (see Fig. 2). Furthermore, the authors showed that Lst expression is regulated by carbohydrate, but not protein, diet after a period of starvation, and that synthetic Lst-15 attenuated Ca2+ in IPCs and thereby depressed DILP release [103]. A GPCR (CG9918), related to neuromedin-U receptors in mammals, was detected in IPCs and its knockdown produced a phenotype similar to Lst mutant flies or Lst knockdown in AKH cells [103]. The CG9918 receptor has previously been identified as a pyrokinin-1 (Capability-PK), receptor (PK1-R) [104] and therefore it is possible that IPCs are modulated by both Lst peptide from APCs and PK-1 released from neurons in the subesophageal ganglion (see [105, 106]). In summary, Lst produced by APCs is induced by carbohydrate restriction and after release it suppresses DILP production and release [103] (Fig. 2).
Serotonin and octopamine
The monoamines serotonin and octopamine have also been implicated in regulation of IPCs [55, 57, 107, 108]. The IPCs express the serotonin receptor 5-HT1A [108] and octopamine receptor OAMB [55, 57, 109] and branches of non-identified serotonin and octopamine producing neurons impinge on the IPCs in the pars intercerebralis. Knockdown of the 5-HT1A receptor in IPCs increases expression of dilp2 and dilp5 mRNA, reduces starvation resistance and food intake and increases circulating glucose as well as stored trehalose and glycogen in fed flies [57, 108]. Furthermore, the diminished serotonin signaling to IPCs increases sensitivity to heat and cold, but increases resistance to oxidative stress [57, 108]. Diminishing OAMB in IPCs leads to elevated dilp3 transcript levels, increased starvation resistance and food ingestion, but has no effect on carbohydrate levels in fed flies [57]. At present, it is not clear what the modulatory roles of octopamine and serotonin are in the IPCs of adult Drosophila or what neuronal circuits underlie this signaling.
Olfactory inputs
Another signal that affects IPCs and dilp transcription is food odors, such as vinegar [110]. Even a brief (15–30 min) exposure to vinegar odor triggers a transient increase in Akh, Dilp2, Dilp3 and Dilp5 mRNA in the starved fly. This exposure is accompanied by increases in dilp6, Upd2 and tobi transcripts and, taken together, food odor appears to induce an anticipatory endocrine response in the hungry fly to get it ready for food ingestion and digestion [110]. The circuits connecting the odor inputs to the IPCs and APCs are not known.
Mechanisms in larval Drosophila
In Drosophila, the larval IPCs do not appear to sense nutrient levels cell-autonomously, in contrast to the adult ones and to mammalian pancreatic beta cells. Instead, nutrient sensing occurs in the adjacent cells of the ring gland that produce AKH, the APCs (see Fig. 4) and by adipocytes in the fat body [74, 99] or maybe even gut endocrine cells [100]. The APC sensors respond to glucose and trehalose and induce release of AKH that in turn triggers secretion of DILP3 from the IPCs [99], whereas the sensors in the fat body, such as the slimfast-TOR pathway, are activated by dietary amino acids and trigger release of an unidentified factor from the fat body that activates the IPCs to secrete DILP2 [74]. A recently proposed adipokine signaling via an adiponectin receptor in the IPCs that controls DILP2 release suggests another pathway from the nutrient sensing fat body [89]. As mentioned in the previous section, this adiponectin signaling seems to be present also in adults. Interestingly, blocking this adiponectin pathway does not affect growth of larvae, but alters carbohydrate and lipid homeostasis in both developmental stages [89].
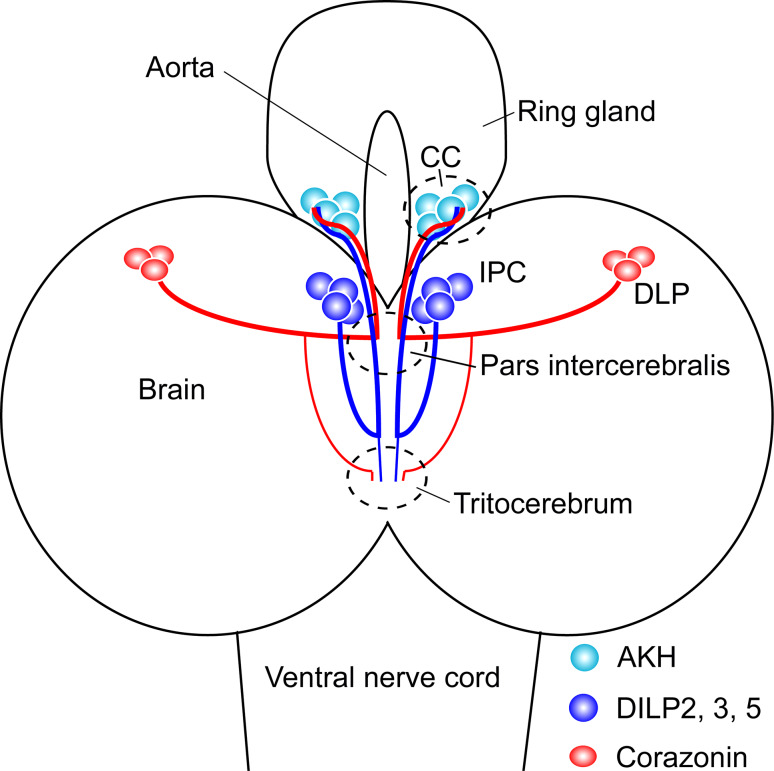
Fig. 4.
Interactions between AKH- and insulin-producing cells in the larva. Schematic representation of the relations between insulin-producing cells (IPC), AKH-producing cells in corpora cardiaca (CC) and DLP neurons that produce corazonin. These three cell types may interact in the CC and the AKH-mediated regulation of IPCs is likely to occur here. IPCs and DLPs may also interact in the pars intercerebralis and tritocerebrum. All three cell types are likely to release their peptide hormones into the aorta via the CC part of the ring gland. Note that the AKH cells have neurite-like extensions along the aorta that are not shown here, and that for simplicity the full number of AKH cells and IPCs are not drawn. This figure is slightly altered from [105]
The cell-autonomous sensing of glucose by the APCs relies on the expression of ATP-sensitive potassium channels (KATP) formed by a sulphonylurea receptor and inward-rectifying potassium channel (Kir) subunits [99]. Thus, these channels are cellular sensors of the ADP/ATP ratio that, depending on glucose uptake and ensuing glycolysis, depolarize the cell membrane and thus increase calcium currents that control hormone release. AKH is often referred to as a functional analog of mammalian glucagon and, thus, its action is primarily antagonistic to that of ILPs; AKH acts on a GPCR to increase lipolysis, glycogenolysis and production of trehalose in the fat body [111–113]. Of interest here is that it was shown that carbohydrate-dependent release of DILP3 from IPCs depends on activation of AKH signaling in these cells [77]. These authors showed that trehalose triggers AKH release from the corpora cardiaca and that this hormone acts on AKH receptors on the IPCs to induce secretion of DILP3 (see Fig. 4 for cell interrelations), which in turn activates TOR signaling in the fat body of the larva. This mechanism is presumed to operate during nutritional stress where sugar homeostasis is promoted at the same time as insulin signaling is kept high to ensure cell growth during development of the larva [77]. Apparently simultaneous release of DILPs and AKH occurs under specific conditions both in adult flies and larvae to keep a balance between carbohydrate homeostasis and other IIS-mediated functions [77, 96]. It is interesting to note that interference with AKH signaling to the IPCs did not affect dilp3 mRNA levels, suggesting that release, but not production of DILP3 is controlled by AKH [77]. The role of the carbohydrate/AKH-regulated DILP3 signaling in larvae seems to be to link dietary sugar to growth and metabolism.
The fat body sensing of nutrients may recruit three different signals that enter the circulation, the aforementioned unidentified factor [74], an adiponectin-like adipokine [89], and a recently suggested peptide hormone, CCHamide-2 [101]. This peptide hormone is expressed in the fat body and intestine of Drosophila and expression of the CCHamide-2 receptor (CCHa2-R) was detected in the IPCs of larval Drosophila [101]. Knockdown of the peptide or its receptor diminished release of DILP2 and 5, and decreased the mRNA of Dilp5, and furthermore lead to reduced growth and a developmental delay. The CCHa2 transcript is increased after feeding glucose or yeast and is dependent on active TOR signaling in the fat body [101]. Another study found that CCHa2 mutant larvae display reduced feeding, delayed development and down regulated dilp2 and dilp3 mRNA levels [100]. These authors also showed that the ccha2 mRNA is produced mainly in the intestine and to a lesser extent by the brain, whereas the fat body displays very low expression [100]. In summary, the CCHa2 signaling links glucose levels, to DILP production and release and growth, which is in contrast to the previously described fat body sensing that relies on dietary amino acids [74], but reminiscent of the previously described Upd2 signaling in adults that is glucose and lipid sensitive [87]. It is not clear whether the glucose sensing resides in CCHa2-expressing enteroendocrine cells or in fat body, but it seems that there are several nutrient-sensing pathways regulating IIS both in larvae and adults. This may ensure that energy balance and growth can be tightly regulated even under unbalanced nutritional conditions.
Genes in IPCs that affect DILP production and release
In addition to the GluT1 [84] and specific membrane channels [84, 114], a number of genes and microRNAs expressed in the IPCs have been shown to affect expression of dilp mRNA, or influence transport or release of DILPs under certain conditions (see Table 2). These genes encode protein kinases, transcription factors and other proteins and due to their action in IPCs they play roles in regulating IIS. Moreover, microRNAs (miRNAs), small endogenous RNAs that regulate the stability and/or the translation of a number of target mRNAs, also constitute an important level of control that can be situated both upstream and downstream of hormonal signaling pathways such as IIS. Different miRNAs have indeed been found to directly or indirectly control the production of ILPs by controlling targets in the Drosophila IPCs (miR-14 [115]; miR-9a [116]) and/or in the fat body (miR-278 [117]; miR-8 [118]). Deregulation of these miRNAs can lead to IIS-associated defects in hormone signaling, metabolic homeostasis and body growth. The complex interactions between miRNAs and IIS in Drosophila have recently been reviewed by Luhur et al. [119].
Table 2.
Genes in IPCs of Drosophila melanogaster with roles in DILP production, transport or release
| Gene | Proposed function | Role in IPCs | Stage | References |
|---|---|---|---|---|
| Apsa (Nudt3-like) | Regulation of signaling nucleotides | dilp3 increase | Adult | [193] |
| IDEb | Insulin-degrading enzyme | Not determined affects IISb | Adult | [146] |
| JNKc (basket) | MAP kinase, stress tolerance | dilp2 repression | Adult | [194] |
| Lmdd (Glis3-like) | Transcription factor | dilp2 increase | Adult | [84] |
| Mioe | Carbohydrate response element binding protein | dilp3 repression | Adult | [195] |
| miR-9a | MicroRNA | dilp2, dilp3, dilp5 repression | Larvaf/adult | [116] |
| miR-14 | MicroRNA | dilp3, dilp5 increase | Adult | [115] |
| Ogtg | O-GlcNAc transferase | dilp2, dilp3, dilp5 increase | Larva | [196] |
| Ogag | O-GlcNAcase | dilp2, dilp3, dilp5 repression | Larva | [196] |
| Pax6 (eyeless) | Transcription factor | dilp5 increase | Larva | [197] |
| Rab1h | Small GTPase | DILP transport | Larva | [198] |
| Taillessi | Transcriptional repressor | Affects peptide release | Adult | [199] |
| Atrophini | Tailless co-repressor | Affects peptide release | Adult | [199] |
| Unc-104j | Kinesin 3 family gene | DILP transport | Larva | [198] |
aAps (CG6391) encodes a Drosophila ortholog of a nudix hydrolase family protein, nucleoside diphosphate-linked moiety X motif 3 (NUDT3). Aps knockdown also lowers Akh and dilp6 mRNA
bIDE (CG5517), a metalloprotease, known to degrade insulin. IDE knockdown in IPCs reduces hemolymph carbohydrate and increases fecundity as well as body weight and decreases lifespan, all suggesting upregulated DILP signaling
c JNK Jun-N-terminal Kinase (stress-responsive mitogen-activated protein kinase, MAP kinase). Basket (CG5680) is a Drosophila JNK
d Lmd lame duck (CG4677) is an ortholog of Glis3, a mammalian transcription factor required for insulin expression in pancreatic beta cells
eMio (missing oocyte; CG7074) functions as a transcription factor and regulates dilp3 and feeding
fIn larvae, only dilp2 and dilp3 decrease
gOgt (supersex combs; CG10392) and Oga (CG5871) serve in the final step of a nutrient-driven hexosamine-signaling pathway and play a role in insulin signaling in mammals
hRab1 regulates membrane trafficking, e.g., in ER-Golgi transition and affects DILP transport in IPCs
iTailless (CG1378) and athrophin (grunge; CG6964) form a transcriptional control module that regulates DILP release from IPCs
jUnc-104 (C. elegans) ortholog (CG8566)
Functional interactions between lipophilic hormones and ILP signaling in insect reproduction
In many insect species, belonging to different orders, it is well documented that IIS also plays a prominent role in the control of reproduction, as recently reviewed by Badisco et al. [120]. In adult female insects, vitellogenesis and oocyte growth, processes that depend on the availability and/or reallocation of nutrients and energy, are controlled by nutrient-sensing and hormonal pathways. In these processes, the role of IIS is situated within a complex physiological regulatory network that also encompasses the lipophilic insect hormones, 20-hydroxyecdysone (20E) and juvenile hormone (JH), which are produced in adult insect gonads and corpora allata (CA), respectively. While the nutritional status is likely to be a crucial determinant of the regulatory network’s output, the exact regulatory hierarchy of hormones and ILPs, which can influence each other, seems to be species dependent. Moreover, sensitivity and responses to lipophilic hormones and ILPs may also be cell-type and stage-dependent [120].
The relationship between IIS and ovarian ecdysteroid synthesis has been most profoundly investigated in dipteran species, such as the mosquito A. aegypti. In adult female mosquitoes, the amino acids required for yolk protein production are derived from an ingested protein-rich blood meal, which initiates a complex endocrine regulation [121]. After this blood meal, neurohormones are released which induce ecdysteroidogenesis in the ovaries, and this will result in increased levels of circulating ecdysteroids that will further induce the process of vitellogenesis [122, 123]. Interestingly, this ecdysteroidogenic response could also be induced by injection of vertebrate insulin [124, 125] and more recently an endogenous ILP (ILP3) was indeed shown to bind the mosquito insulin receptor and to stimulate ovarian ecdysteroidogenesis [126, 127]. In addition to ILPs, another mosquito ecdysteroidogenic neurohormone was also shown to function after the ingestion of a blood meal, the “ovary ecdysteroidogenic hormone” (OEH) [128], which displays sequence similarity to neuroparsins (see sections below).
In many insects, JH appears to be the lipophilic hormone that acts, in concert with the insulin and TOR signaling pathways, as a major regulator of nutrient and energy allocation during vitellogenesis. In some species, IIS was suggested to elicit stimulatory influences on JH biosynthesis in the CA [129–134]. However, in others, JH signaling was shown to exert its effects by inducing IIS. For instance, the vitellogenic effect of JH in adult female Tribolium castaneum beetles may be mediated via a stimulatory effect of JH on ILP gene expression in the fat body [135]. RNAi-mediated silencing of IIS and TOR signaling components in the red flour beetle mimicked the effects of starvation and resulted in reduced vitellogenin transcript levels [136], whereas knockdown of JH biosynthesis and signaling components did not only reduce ILP gene expression, but also resulted in a FOXO-mediated inhibition of vitellogenin gene expression [135]. In this beetle species, it was also suggested that JH controls trehalose homeostasis and starvation resistance by regulating the synthesis of ILP2 [137]. The extended life span under reduced ILP signaling conditions could be normalized by injection of bovine insulin, but not by treatment with JH [137]. In adult female desert locusts, RNAi-mediated knockdown of the ILP precursor transcripts also resulted in significantly reduced vitellogenin transcript levels, while silencing of neuroparsin mRNA led to the opposite effects [138]. Since the locust ILP precursor gene is highly expressed in both brain and fat body, it remains to be proven whether this peptide acts directly or indirectly on the fat body to stimulate vitellogenin synthesis [11].
All this information illustrates the complexity and diversity of functional interactions between these intersecting signaling pathways in insects. In addition to reproduction, functional interactions between ecdysteroids, JH and IIS have also been reported in the control of postembryonic growth and development, further demonstrating the complex relationships between these pathways [139–144]. Moreover, recent studies have also implicated the role of multiple miRNAs at the crossroads between these pathways [119].
The modulation of the ILP signal after release
A number of polypeptides have been identified in mammals, as well as in insects, that interact with IGFs and ILPs after their release. Some function as carrier proteins in the circulation, others have actions antagonistic to ILPs, and several seem to have functions additional to interactions with ILPs. One factor that deserves further study is the insulin-degrading enzyme (IDE) [145]. In Drosophila, IDE knockdown in IPCs reduces hemolymph carbohydrate and increases fecundity and lifespan, suggesting elevated circulating DILP levels and increased systemic IIS [146]. It is not known how IDE acts to degrade DILPs within or outside the IPCs.
Binding proteins for vertebrate insulin-like peptides
In vertebrates, IGFs are expressed in a wide variety of tissues and play a prominent role as regulators of cellular metabolism, proliferation and survival [147, 148]. Their action is modulated by the presence of a set of IGF binding proteins (IGFBP) that function as IGF carriers within the circulatory system [149–151]. When released in the circulation, IGFs are mainly occurring in ternary protein complexes of IGF:IGFBP with the acid-labile subunit (ALS), while binary IGF:IGFBP complexes are also encountered. When circulating within these complexes, the half-life of IGF increases. The smaller binary complexes are capable of crossing the vascular epithelial barrier and can thus deliver the IGFs to their target tissues. Both circulating and locally produced IGFBPs can modulate the availability of bioactive IGFs to their receptor sites at target cells. Release of bioactive IGF from protein complexes can be mediated by extracellular matrix interactions and/or proteolytic cleavage, which may reduce the affinity of the IGFBP to the IGF [152]. However, it should be mentioned that IGFBPs are also known to play many functional roles that are IGF-independent. Indeed, apart from IGFs, IGFBPs can also interact with many other molecules and binding partners [153–158]. Some IGFBPs were even shown to enter cells and migrate into the nucleus, where they may interact with particular transcription factors and nuclear receptors [149, 152, 159–161].
In mammals, six different IGFBPs (IGFBP1-6) have been identified which differ in their structural and functional properties [149, 152, 162]. Nevertheless, they share an overall structural organization consisting of two well-conserved protein domains, separated by a more variable region. The N-terminal IGFBP domain is highly Cys-rich and the conserved pattern with 10–12 Cys-residues can also be found in several other proteins that display lower affinities to IGFs. The C-terminal domain contains three pairs of Cys-residues and adopts a thyroglobulin-type onefold. Isolated N-terminal IGFBP domains have affinities, which are up to 100× lower than the intact IGFBPs, while the isolated C-terminal domains have even lower affinities to the IGFs. Therefore, each of the separate domains is capable of interacting with IGF, but the combination of both seems to be required for high affinity binding [152]. In addition to mammals, IGFBP sequences have also been identified in a wide selection of other vertebrates, as well as more primitive chordates, such as lancelets and tunicates [162]. The IGFBP of Amphioxus was also shown to enter the nucleus of cultured cells and to possess transcriptional activation activity, suggesting that the IGF-independent role(s) of these proteins may refer to ancient functions [161]. However, the evolutionary origins of this protein family become more vague when looking for clear homologs in invertebrate phyla, as seen in the following sections.
ILP-binding proteins in Drosophila
Imaginal morphogenesis protein-late 2 (IMP-L2)
Imp-L2 was originally identified as a 20-hydroxyecdysone (20E) inducible late gene transcript in mass cultures of Drosophila imaginal discs associated with membrane-bound polysomes [163, 164]. In vivo, this transcript was found to be abundant at the onset of metamorphosis, reaching maximal levels in pupae ca. 14 h after puparium formation [163]. Further studies indicated that the Imp-L2 gene is already expressed in the cellular blastoderm stage and that it is essential during development. Its transcript can be observed in several cell types throughout development [165]. In addition, IMP-L2 was immunolocalized in specific neuronal structures late in embryogenesis, suggesting a role of this immunoglobulin (Ig) type domains containing factor in the development of the nervous system.
Interestingly, Sloth Andersen et al. [61] described an insulin-related peptide binding protein that was secreted by cultured cells derived from the lepidopteran insect, Spodoptera frugiperda. The protein was also shown to be capable of inhibiting human insulin action at its receptor. The secreted lepidopteran protein contains two Ig-like domains and displays clear sequence similarity with Drosophila IMP-L2. The second domain also shows some similarity with the C-terminal Ig-like domain of the mammalian tumor suppressor protein Mac25, which possesses an additional N-terminal IGFBP-like domain (Mac25 was previously also designated as “IGFBP7”, but it is not considered as a genuine IGFBP). In Drosophila, IMP-L2 was shown to counteract DILP signaling and to be essential for the tolerance to starvation stress [59]. IMP-L2 was also shown to be capable of interacting with some of the DILPs. Therefore, IMP-L2 may act as a secreted antagonist of insulin-like signaling in this fly species. In addition, the elimination of germ cells in the fruit fly resulted in increased lifespan, while IMP-L2 expression was up regulated [166]. Indeed, overexpression of IMP-L2 was shown to extend lifespan in Drosophila [167]. More locally restricted effects of IMP-L2 have also been described recently. Some neurons that are directly contacted by DILP producing cells in the larval brain appear to express IMP-L2, and this was demonstrated to be necessary for local neuronal uptake of DILP2 resulting in induced insulin signaling activity in the target neurons [58]. In this case, DILP2 and IMP-L2 seem to act together to enhance the insulin signaling activity within this distinct subset of neurons. An intact insulin receptor InR is also required for this process. Furthermore, Sarraf-Zadeh et al. [60] discovered that some IMP-L2 expressing neurons directly contact the prothoracic gland (PG) portion of the larval ring gland and that these neurons are responsible for sensing the nutritional status of the animals and for coordinating ecdysone production to adjust the developmental timing under conditions of starvation. Increased IMP-L2 production in these neurons severely delayed pupariation, while its loss-of-function resulted in a reduction of the developmental delay caused by starvation as well as in increased levels of ecdysone production [60].
Two recent studies in the fruit fly pointed at an important regulatory role for the ILP antagonist Imp-L2 in the organ wasting process that can occur in conditions of extreme nutritional starvation and resembles the human wasting disorder designated as cancer-associated cachexia. In one study, a fruit fly model with overproliferating gut tissue was generated and shown to produce high levels of IMP-L2, resulting in a dramatic systemic reduction of IIS and in wasting of fat body, muscle and ovary [62]. The reduced IIS mimicks the starved state in these organs, while upregulation of IIS components and glycolytic enzymes probably overrules this effect in the overproliferating gut. Interestingly, the wasting phenotype could be rescued by a loss-of-function of Imp-L2. In the other study, transplantation of malignant tumors also induced wasting of adipose, muscular and gonadal fly tissues [63]. Again, IMP-L2 was shown to be secreted from these tumors and responsible for the organ wasting phenotype, suggesting that its upregulation in the tumors resulted in insulin resistance of the wasting organs.
Drosophila acid-labile subunit (dALS)
Arquier et al. [168] identified dALS, a Drosophila homolog of the acid-labile subunit (ALS). In vertebrates, together with IGF and IGFBP, ALS is a component of the ternary IGF-containing complex that can be found in the circulation. The fruit fly homolog consists of a series of Leu-rich repeats (LRRs), which also form the core of vertebrate ALS. In larvae, dALS appears to be expressed in the DILP producing cells of the brain and in the fat body. Its expression level in the fat body depends on the nutritional state of the animals. Similar to its mammalian homolog, dALS has been proposed to be part of a circulating heterotrimeric complex, together with an insulin-like peptide and IMP-L2. Moreover, it was shown to functionally antagonize the role of DILPs in controlling body growth as well as the metabolism of carbohydrates and lipids [168].
Secreted decoy of insulin receptor (SDR)
Recently, Okamoto et al. [17] discovered a protein (SDR, CG3837) that displays structural similarities to the extracellular domain of the Drosophila insulin receptor (InR). This protein, designated Secreted Decoy of InR (SDR), was demonstrated to be capable of interacting with several DILPs, independently of IMP-L2. Its expression remains relatively constant under changing nutritional conditions, which also contrasts with the situation described for IMP-L2. Glia-derived SDR was shown to have an inhibitory effect on larval body growth and on the outgrowth of peripheral structures, such as the wings, while the RNAi-based knockdown conditions resulted in the opposite effects. Therefore, SDR was proposed to function as a negative regulator of DILP signaling in Drosophila [17].
Insulin-like peptide binding proteins in other species
Genuine IGFBPs, containing both the N-terminal (IGFBP-type) and C-terminal (thyroglobulin-type) domains, have only been identified in deuterostomes [162]. Nevertheless, the IGFBP-type domain appears to have a more ancient origin and it can be found in several protostomian proteins, for which the evolutionary relationships remain difficult to resolve. Some of these protostomian proteins have even been shown to be capable of interacting with IGFs and/or insulin-like peptides [11, 169, 170]. In the mollusk, Haliotis laevigata, perlustrin, a nacre protein was discovered that contains an IGFBP-like domain [169]. This protein displayed binding affinities to IGFs that were in a similar range as those of the N-terminal domains of the mammalian IGFBPs, while perlustrin also showed affinity to bovine insulin. In addition, Claeys et al. [171] discovered that neuroparsins (NPs), a family of small cysteine-rich proteins present in several insect and crustacean species, also displayed clear sequence similarities with perlustrin, as well as with the N-terminal hormone-binding module of IGFBPs. A few years later, they indeed showed that a recombinant locust NP was capable of interacting in vitro with the purified locust insulin-related peptide [11]. When searching the available sequence databases, the same authors also found that several other arthropod proteins, designated as neuroparsin-like peptides (NPLPs), displayed sequence similarity with the N-terminal IGFBP module [172]. More recently, a multidomain protein of the crayfish, Cherax quadricarinatus, was also shown to contain this N-terminal module (followed by a kazal-type serine protease inhibitor domain and an Ig-type C2 domain) [170]. Interestingly, this protein (Cq-IGFBP) was demonstrated to specifically interact with the insulin-like androgenic gland hormone (Cq-IAG) of this crayfish species. From the information above, it can be concluded that at least three types of IGFBP-like domain containing proteins occur in arthropods, i.e., NPs, NPLPs (also designated as single IGFBP-like domain containing proteins or SIBD) and IGFBP-like multidomain factors. Interestingly, all three types were found to be present in the mud crab, Scylla paramamosain; their gene expression appeared to be under control of IIS and a regulatory role in reproduction was suggested [173]. Based on data obtained in these different reports, the hypothesis can be suggested that some of these invertebrate IGFBP-like proteins may act in vivo by controlling ILP availability, in a similar way as IGFBP in mammals. However, as in the case of mammalian IGFBP, the existence of ILP-independent effects cannot be ruled out.
Other roles of neuroparsins and the ovary ecdysteroidogenic hormone
Some NPs indeed appear to have functions independent of ILPs. The first member of the NP family was initially isolated from the pars intercerebralis—corpora cardiaca (CC) neurohemal complex of the migratory locust, Locusta migratoria [174, 175]. This peptide was functionally characterized as an anti-gonadotrophic factor that showed various effects opposite to those elicited by JH, without directly affecting JH biosynthesis. Later on, it was shown that locusts possess several other NP variants, which probably result from alternative splicing and are expressed in a tissue-, stage-, and locust phase-dependent manner [171, 172, 176, 177]. Searches in sequence databases indicate that neuroparsins and related peptides are present in several insect and crustacean species. However, their sequences appear to be more divergent in higher insect orders, such as Diptera, and even seem to be absent in D. melanogaster, as well as in a number of related species in the melanogaster subgroup within the Drosophila genus [178]. In the mosquito, A. aegypti, a NP-like neurohormonal factor, the ovary ecdysteroidogenic hormone (OEH), plays an important role in stimulating ovarian ecdysteroid synthesis in response to a protein-rich blood meal [128]. Recent studies revealed that the mode of action of OEH is exerted independently from the mosquito insulin receptor (MIR), although it also activates Akt/PKB, a well-known downstream target of the IIS pathway [179]. Very recently, a previously orphan receptor tyrosine kinase (RTK) has been shown to mediate the effects of OEH on egg formation in A. aegypti [180]. This receptor is distantly related to the insulin receptor and belongs to an evolutionary distinct group of RTKs characterized by the presence of a Venus flytrap module, typical of amino acid receptors. Although the exact evolutionary origin of this OEH receptor type remains unclear, close relatives can be found in several other Diptera, including a number of Drosophila species in which a NP sequence has been described previously [178]. Interestingly, the Venus kinase receptor, which has been identified in the platyhelminth Schistosoma mansoni, can be bound and activated by amino acids and also seems to play a role in reproduction, in addition to larval growth and development [181]. Therefore, it is possible that a flatworm peptide activating this receptor still has to be discovered. Alternatively, at some point in insect/arthropod evolution, a NP-like ligand may have been “hijacked” by a member of this RTK subfamily for small amino acid ligands (or vice versa).
Conclusions and perspectives
In insects, the production and release of multiple ILPs is under complex control, ensuring a tightly regulated systemic IIS in the organism. In adult Drosophila, the major sources of ILPs, the brain IPCs and adipose cells of the fat body, cell-autonomously monitor nutrient levels and energy stores by several mechanisms to allow for optimal ILP secretion into circulation. In addition, the IPCs are under modulatory control by neurotransmitters, neuropeptides and other secreted factors, derived from brain neurons, adipocytes and gut endocrine cells, some of which may be nutrient sensing. During growth and development, as well as in reproduction and diapause, IIS is interfaced signaling with the lipophilic hormones JH and 20E. Finally, several proteins, such as binding proteins and proteases regulate IIS availability and action after release of ILPs.
It is obvious from reviewing the literature on IIS in insects that there are many gaps in our knowledge. One major gap is at the biochemical level. In most insects, and certainly in Drosophila, it is not clear in what form the ILPs are released (or if they are indeed released). Only in a few cases have ILPs been chemically isolated and sequenced (e.g., from silkmoths and locusts) and shown to have structures reminiscent of insulin-like peptides with A and B chains, or an IGF-like structure in the case of Bombyx IGF-like peptide [11, 13, 70, 182, 183]. Thus, it would be of interest to unveil whether ILPs encoding genes expressed in different tissues give rise to tissue-specific forms of ILPs. Another question is whether the C-peptide, when cleaved off from the precursor, is released in equimolar concentration, as in mammals [184] where it seems to have a function [185]. Synthetic C-peptide from L. migratoria was found to depolarize neurons explanted from thoracic ganglia [186]. In this species, another peptide was isolated that was proposed to be cleaved off from the extended B-chain of the ILP precursor, and was shown to inhibit glycogen phosphorylase activity in the fat body [187]. In this context, it should also be noted that there are very few studies actually analyzing ILP ligand binding to and activation of the insulin receptor (see [127, 188, 189]). Thus, many conclusions on receptor activation are drawn from genetic interactions.
In most insects studied, only one ILP receptor has been identified in spite of multiple ILPs. Are there further receptors, especially for the ILPs that have been classified as relaxin-like, such as DILP7 and 8 in Drosophila [19, 20, 23, 24, 190]? Or is there a single receptor with different affinities for the ILPs and associated coupling to different downstream signaling pathways? Just after this paper was accepted, it was shown that DILP8 is acting via the GPCR Lgr3 in a pathway controlling PPTH release during late larval development and thus regulating ecdysone pulses to coordinate organ growth with developmental transition [30]. Alternatively, the ILPs might have different access to the receptor due to specific proximity (spatial or temporal), sensitivity to peptidase degradation, or affinity to circulating binding proteins. It might also be of interest to analyze cell type-, stage- or species-dependent differences in the interactome of the ILP receptor protein.
The functional interactions between ILPs and other hormones need to be further explored. Some studies, reviewed here, have investigated the relations between IIS, JH and 20E, but further work is required to understand feedback between the different ILPs (see [4, 90, 191]), as well as between ILPs and for instance circulating sulfakinins, AKH and corazonin and other peptides that regulate feeding, metabolism and stress responses.
Finally, it might also be of interest to determine the most ancient and conserved functions of ILP signaling, as compared to derived species-specific functions. Whereas the intracellular IIS pathways appear well conserved over evolution, it remains to be determined to what extent functions and physiological roles in intercellular signaling are conserved between different insect species. Another intriguing question is how locusts manage with one molecular form of ILPs, when fruitflies and silkmoths require at least 8 forms? Obviously ILP signaling needs to be further explored in more detail in a variety of insect species.
References
- 1.Banting FG, Best CH. The internal secretion of the pancreas. J Lab Clin Med. 1922;7:251–266. [PubMed] [Google Scholar]
- 2.Claeys I, Simonet G, Poels J, Van Loy T, Vercammen L, De Loof A, Vanden Broeck J. Insulin-related peptides and their conserved signal transduction pathway. Peptides. 2002;23(4):807–816. doi: 10.1016/S0196-9781(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 3.Antonova Y, Arik AJ, Moore W, Riehle MR, Brown MR. Insulin-like peptides: structure, signaling, and function. In: Gilbert LI, editor. Insect endocrinology. New York: Elsevier/Academic Press; 2012. pp. 63–92. [Google Scholar]
- 4.Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6(2):e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garofalo RS. Genetic analysis of insulin signaling in Drosophila . Trends Endocrinol Metab. 2002;13(4):156–162. doi: 10.1016/S1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M. Relaxin-3/INSL7 regulates the stress-response system in the rat hypothalamus. J Mol Neurosci. 2011;43(2):169–174. doi: 10.1007/s12031-010-9468-0. [DOI] [PubMed] [Google Scholar]
- 7.Lok S, Johnston DS, Conklin D, Lofton-Day CE, Adams RL, Jelmberg AC, Whitmore TE, Schrader S, Griswold MD, Jaspers SR. Identification of INSL6, a new member of the insulin family that is expressed in the testis of the human and rat. Biol Reprod. 2000;62(6):1593–1599. doi: 10.1095/biolreprod62.6.1593. [DOI] [PubMed] [Google Scholar]
- 8.Chassin D, Laurent A, Janneau JL, Berger R, Bellet D. Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics. 1995;29(2):465–470. doi: 10.1006/geno.1995.9980. [DOI] [PubMed] [Google Scholar]
- 9.Froesch ER, Zapf J. Insulin-like growth factors and insulin: comparative aspects. Diabetologia. 1985;28(8):485–493. doi: 10.1007/BF00281982. [DOI] [PubMed] [Google Scholar]
- 10.Hudson P, Haley J, John M, Cronk M, Crawford R, Haralambidis J, Tregear G, Shine J, Niall H. Structure of a genomic clone encoding biologically active human relaxin. Nature. 1983;301(5901):628–631. doi: 10.1038/301628a0. [DOI] [PubMed] [Google Scholar]
- 11.Badisco L, Claeys I, Van Hiel M, Clynen E, Huybrechts J, Vandersmissen T, Van Soest S, Vanden Bosch L, Simonet G, Vanden Broeck J. Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J Mol Endocrinol. 2008;40(3):137–150. doi: 10.1677/JME-07-0161. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida I, Moto K, Sakurai S, Iwami M. A novel member of the bombyxin gene family: structure and expression of bombyxin G1 gene, an insulin-related peptide gene of the silkmoth Bombyx mori . Dev Genes Evol. 1998;208(7):407–410. doi: 10.1007/s004270050197. [DOI] [PubMed] [Google Scholar]
- 13.Mizoguchi A, Okamoto N. Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front Physiol. 2013;4:217. doi: 10.3389/fphys.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagueux M, Lwoff L, Meister M, Goltzene F, Hoffmann JA. cDNAs from neurosecretory cells of brains of Locusta migratoria (Insecta, Orthoptera) encoding a novel member of the superfamily of insulins. Eur J Biochem. 1990;187(1):249–254. doi: 10.1111/j.1432-1033.1990.tb15302.x. [DOI] [PubMed] [Google Scholar]
- 15.Veenstra JA. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front Physiol. 2014;5:454. doi: 10.3389/fphys.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11(4):213–221. doi: 10.1016/S0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto N, Nakamori R, Murai T, Yamauchi Y, Masuda A, Nishimura T. A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes Dev. 2013;27(1):87–97. doi: 10.1101/gad.204479.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slaidina M, Delanoue R, Grönke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17(6):874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336(6081):582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 20.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336(6081):579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- 21.Vanden Broeck J. Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster . Peptides. 2001;22(2):241–254. doi: 10.1016/S0196-9781(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila . Dev Cell. 2009;17(6):885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miguel-Aliaga I, Thor S, Gould AP. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol. 2008;6(3):e58. doi: 10.1371/journal.pbio.0060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319(5870):1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila . Curr Biol. 2002;12(15):1293–1300. doi: 10.1016/S0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 26.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296(5570):1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 27.Ward CW, Lawrence MC. Landmarks in insulin research. Front Endocrinol (Lausanne) 2011;2:76. doi: 10.3389/fendo.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13(4):225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 29.Van Hiel MB, Vandersmissen HP, Proost P, Vanden Broeck J. Cloning, constitutive activity and expression profiling of two receptors related to relaxin receptors in Drosophila melanogaster . Peptides. 2015;68:83–90. doi: 10.1016/j.peptides.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Colombani J, Andersn DS, Boulan L, Boone E, Romero N, Virolle V, Tecxada M, Leopold P. Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Curr Biol. 2015 doi: 10.1016/j.cub.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Xu HJ, Xue J, Lu B, Zhang XC, Zhuo JC, He SF, Ma XF, Jiang YQ, Fan HW, Xu JY, Ye YX, Pan PL, Li Q, Bao YY, Nijhout HF, Zhang CX. Two insulin receptors determine alternative wing morphs in planthoppers. Nature. 2015;519(7544):464–467. doi: 10.1038/nature14286. [DOI] [PubMed] [Google Scholar]
- 32.Boucher P, Ditlecadet D, Dube C, Dufresne F. Unusual duplication of the insulin-like receptor in the crustacean Daphnia pulex . BMC Evol Biol. 2010;10:305. doi: 10.1186/1471-2148-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila . Biochem J. 2010;425(1):13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 34.Goberdhan DC, Wilson C. The functions of insulin signaling: size isn’t everything, even in Drosophila . Differentiation. 2003;71(7):375–397. doi: 10.1046/j.1432-0436.2003.7107001.x. [DOI] [PubMed] [Google Scholar]
- 35.Braeckman BP, Houthoofd K, Vanfleteren JR. Insulin-like signaling, metabolism, stress resistance and aging in Caenorhabditis elegans . Mech Ageing Dev. 2001;122(7):673–693. doi: 10.1016/S0047-6374(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 36.Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190(2):191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- 37.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 38.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 39.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 40.Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20(23):2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila . Nature. 2011;471(7339):508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padmanabha D, Baker KD. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metabol. 2014 doi: 10.1016/j.tem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Owusu-Ansah E, Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis Model Mech. 2014;7(3):343–350. doi: 10.1242/dmm.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Géminard G, Arquier N, Layalle S, Bourouis M, Slaidina M, Delanoue R, Bjordal M, Ohanna M, Ma M, Colombani J, Léopold P. Control of metabolism and growth through insulin-like peptides in Drosophila . Diabetes. 2006;55:S5–S8. doi: 10.2337/db06-S001. [DOI] [Google Scholar]
- 47.Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32(4):180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol. 2009;41(5):1006–1010. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol Metabol. 2014;25(10):509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens . Proc Natl Acad Sci USA. 2008;105(18):6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubrak OI, Kucerova L, Theopold U, Nassel DR. The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster . PLoS ONE. 2014;9(11):e113051. doi: 10.1371/journal.pone.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn DA, Denlinger DL. Energetics of insect diapause. Ann Rev Entomol. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- 53.McElwee JJ, Schuster E, Blanc E, Thornton J, Gems D. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans . Mech Ageing Dev. 2006;127(5):458–472. doi: 10.1016/j.mad.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145(1):133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65(5):670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corl AB, Rodan AR, Heberlein U. Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster . Nat Neurosci. 2005;8(1):18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- 57.Luo J, Lushchak OV, Goergen P, Williams MJ, Nassel DR. Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS ONE. 2014;9(6):e99732. doi: 10.1371/journal.pone.0099732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bader R, Sarraf-Zadeh L, Peters M, Moderau N, Stocker H, Kohler K, Pankratz MJ, Hafen E. The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J Cell Sci. 2013;126(Pt 12):2571–2576. doi: 10.1242/jcs.120261. [DOI] [PubMed] [Google Scholar]
- 59.Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7(3):10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarraf-Zadeh L, Christen S, Sauer U, Cognigni P, Miguel-Aliaga I, Stocker H, Kohler K, Hafen E. Local requirement of the Drosophila insulin binding protein imp-L2 in coordinating developmental progression with nutritional conditions. Dev Biol. 2013;381(1):97–106. doi: 10.1016/j.ydbio.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem. 2000;275(22):16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- 62.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell. 2015;33(1):36–46. doi: 10.1016/j.devcel.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueroa-Clarevega A, Bilder D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell. 2015;33(1):47–55. doi: 10.1016/j.devcel.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001;304(2):317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- 65.Van de Velde S, Badisco L, Claeys I, Verleyen P, Chen X, Vanden Bosch L, Vanden Broeck J, Smagghe G. Insulin-like peptides in Spodoptera littoralis (Lepidoptera): detection, localization and identification. Gen Comp Endocrinol. 2007;153(1–3):72–79. doi: 10.1016/j.ygcen.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum . Gen Comp Endocrinol. 2011;173(2):303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27(11):2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Vafopoulou X, Steel CG. Insulin-like and testis ecdysiotropin neuropeptides are regulated by the circadian timing system in the brain during larval-adult development in the insect Rhodnius prolixus (Hemiptera) Gen Comp Endocrinol. 2012;179(2):277–288. doi: 10.1016/j.ygcen.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 69.Okamoto N, Yamanaka N, Endo Y, Kataoka H, Mizoguchi A. Spatiotemporal patterns of IGF-like peptide expression in the silkmoth Bombyx mori predict its pleiotropic actions. Gen Comp Endocrinol. 2011;173(1):171–182. doi: 10.1016/j.ygcen.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Okamoto N, Yamanaka N, Satake H, Saegusa H, Kataoka H, Mizoguchi A. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori . FEBS J. 2009;276(5):1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x. [DOI] [PubMed] [Google Scholar]
- 71.Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334(3):499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 72.Söderberg JA, Birse RT, Nässel DR. Insulin production and signaling in renal tubules of Drosophila is under control of tachykinin-related peptide and regulates stress resistance. PLoS ONE. 2011;6(5):e19866. doi: 10.1371/journal.pone.0019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9(3):336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila . Cell Metab. 2009;10(3):199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4(6):842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nässel DR, Liu Y, Luo J (2015) Insulin/IGF signaling and its regulation in Drosophila. Gen Comp Endocrinol. doi:10.1016/j.ygcen.2014.11.021(in press) [DOI] [PubMed]
- 77.Kim J, Neufeld TP. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat Commun. 2015;6:6846. doi: 10.1038/ncomms7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Söderberg JA, Carlsson MA, Nässel DR. Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, Drosulfakinin. Front Endocrinol. 2012;3:109. doi: 10.3389/fendo.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 80.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 81.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 82.Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV. Factors that regulate insulin producing cells and their output in Drosophila . Front Physiol. 2013;4:252. doi: 10.3389/fphys.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto N, Yamanaka N. Nutrition-dependent control of insect development by insulin-like peptides. Curr Opin Insect Sci. 2015;11:21–30. doi: 10.1016/j.cois.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park S, Alfa RW, Topper SM, Kim GE, Kockel L, Kim SK. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 2014;10(8):e1004555. doi: 10.1371/journal.pgen.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, Scantling D, Mulkey DK, Helfand SL. Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 2009;1(8):699–713. doi: 10.18632/aging.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enell LE, Kapan N, Soderberg JA, Kahsai L, Nässel DR. Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila . PLoS ONE. 2010;5(12):e15780. doi: 10.1371/journal.pone.0015780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 89.Kwak SJ, Hong SH, Bajracharya R, Yang SY, Lee KS, Yu K. Drosophila adiponectin receptor in insulin producing cells regulates glucose and lipid metabolism by controlling insulin secretion. PLoS ONE. 2013;8(7):e68641. doi: 10.1371/journal.pone.0068641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai H, Kang P, Tatar M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell. 2012;11(6):978–985. doi: 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapan N, Lushchak OV, Luo J, Nässel DR. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci. 2012;69:4051–4066. doi: 10.1007/s00018-012-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151(5):1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, Taghert PH. A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol. 2005;208(Pt 7):1239–1246. doi: 10.1242/jeb.01529. [DOI] [PubMed] [Google Scholar]
- 94.Birse RT, Söderberg JA, Luo J, Winther ÅM, Nässel DR. Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol. 2011;214:4201–4208. doi: 10.1242/jeb.062091. [DOI] [PubMed] [Google Scholar]
- 95.Siviter RJ, Coast GM, Winther ÅM, Nachman RJ, Taylor CA, Shirras AD, Coates D, Isaac RE, Nässel DR. Expression and functional characterization of a Drosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J Biol Chem. 2000;275(30):23273–23280. doi: 10.1074/jbc.M002875200. [DOI] [PubMed] [Google Scholar]
- 96.Hentze JL, Carlsson MA, Kondo S, Nässel DR, Rewitz KF. The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila . Sci Rep. 2015;5:11680. doi: 10.1038/srep11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoon JG, Stay B. Immunocytochemical localization of Diploptera punctata allatostatin-like peptide in Drosophila melanogaster . J Comp Neurol. 1995;363(3):475–488. doi: 10.1002/cne.903630310. [DOI] [PubMed] [Google Scholar]
- 98.Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7(4):321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 99.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431(7006):316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 100.Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, Didriksen AK, Schjott SR, Sembach FE, Li S, Sogaard KC, Sondergaard L, Grimmelikhuijzen CJ. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila . PLoS ONE. 2015;10(7):e0133017. doi: 10.1371/journal.pone.0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sano H, Nakamura A, Texada MJ, Truman JW, Ishimoto H, Kamikouchi A, Nibu Y, Kume K, Ida T, Kojima M. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster . PLoS Genet. 2015;11(5):e1005209. doi: 10.1371/journal.pgen.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Veenstra JA, Ida T. More Drosophila enteroendocrine peptides: orcokinin B and the CCHamides 1 and 2. Cell Tissue Res. 2014;357(3):607–621. doi: 10.1007/s00441-014-1880-2. [DOI] [PubMed] [Google Scholar]
- 103.Alfa RW, Park S, Skelly KR, Poffenberger G, Jain N, Gu X, Kockel L, Wang J, Liu Y, Powers AC, Kim SK. Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 2015;21(2):323–333. doi: 10.1016/j.cmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJ. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun. 2005;335(1):14–19. doi: 10.1016/j.bbrc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 105.Nässel DR, Winther ÅM. Drosophila neuropeptides in regulation of physiology and behavior. Progr Neurobiol. 2010;92(1):42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 106.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JA. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster . Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1297–R1307. doi: 10.1152/ajpregu.00584.2001. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP. A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev. 2008;22(14):1877–1893. doi: 10.1101/gad.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo J, Becnel J, Nichols CD, Nässel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT(1A) receptor. Cell Mol Life Sci. 2012;69(3):471–484. doi: 10.1007/s00018-011-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Kholy S, Stephano F, Li Y, Bhandari A, Fink C, Roeder T. Expression analysis of octopamine and tyramine receptors in Drosophila . Cell Tissue Res. 2015;361(3):669–684. doi: 10.1007/s00441-015-2137-4. [DOI] [PubMed] [Google Scholar]
- 110.Lushchak OV, Carlsson MA, Nässel DR. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci. 2015;72(16):3143–3155. doi: 10.1007/s00018-015-1884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bednarova A, Kodrik D, Krishnan N. Unique roles of glucagon and glucagon-like peptides: parallels in understanding the functions of adipokinetic hormones in stress responses in insects. Comp Biochem Physiol A: Mol Integr Physiol. 2013;164(1):91–100. doi: 10.1016/j.cbpa.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 112.Baumbach J, Xu Y, Hehlert P, Kuhnlein RP. Galphaq, Ggamma1 and Plc21C control Drosophila body fat storage. J Genet Genomics. 2014;41(5):283–292. doi: 10.1016/j.jgg.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Van der Horst DJ, Van Marrewijk WJ, Diederen JH. Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol. 2001;211:179–240. doi: 10.1016/S0074-7696(01)11019-3. [DOI] [PubMed] [Google Scholar]
- 114.Sheldon AL, Zhang J, Fei H, Levitan IB. SLOB, a SLOWPOKE channel binding protein, regulates insulin pathway signaling and metabolism in Drosophila. PLoS ONE. 2011;6(8):e23343. doi: 10.1371/journal.pone.0023343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Varghese J, Lim SF, Cohen SM. Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev. 2010;24(24):2748–2753. doi: 10.1101/gad.1995910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suh YS, Bhat S, Hong SH, Shin M, Bahk S, Cho KS, Kim SW, Lee KS, Kim YJ, Jones WD, Yu K. Genome-wide microRNA screening reveals that the evolutionary conserved miR-9a regulates body growth by targeting sNPFR1/NPYR. Nat Commun. 2015;6:7693. doi: 10.1038/ncomms8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev. 2006;20(4):417–422. doi: 10.1101/gad.374406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee GJ, Jun JW, Hyun S. MicroRNA miR-8 regulates multiple growth factor hormones produced from Drosophila fat cells. Insect Mol Biol. 2015;24(3):311–318. doi: 10.1111/imb.12156. [DOI] [PubMed] [Google Scholar]
- 119.Luhur A, Chawla G, Sokol NS. MicroRNAs as components of systemic signaling pathways in Drosophila melanogaster . Curr Top Dev Biol. 2013;105:97–123. doi: 10.1016/B978-0-12-396968-2.00004-X. [DOI] [PubMed] [Google Scholar]
- 120.Badisco L, Van Wielendaele P, Vanden Broeck J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front Physiol. 2013;4:202. doi: 10.3389/fphys.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fallon AM, Hagedorn HH, Wyatt GR, Laufer H. Activation of vitellogenin synthesis in the mosquito Aedes aegypti by ecdysone. J Insect Physiol. 1974;20(9):1815–1823. doi: 10.1016/0022-1910(74)90211-X. [DOI] [PubMed] [Google Scholar]
- 123.Martin D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol Cell Endocrinol. 2001;173(1–2):75–86. doi: 10.1016/S0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 124.Riehle MA, Brown MR. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti . Insect Biochem Mol Biol. 1999;29(10):855–860. doi: 10.1016/S0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 125.Riehle MA, Brown MR. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti . Cell Tissue Res. 2002;308(3):409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- 126.Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti . Proc Natl Acad Sci USA. 2008;105(15):5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol. 2010;328(1–2):47–55. doi: 10.1016/j.mce.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem. 1998;273(7):3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- 129.Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster . Gen Comp Endocrinol. 2005;142(3):347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 130.Mutti NS, Dolezal AG, Wolschin F, Mutti JS, Gill KS, Amdam GV. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J Exp Biol. 2011;214(Pt 23):3977–3984. doi: 10.1242/jeb.061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suren-Castillo S, Abrisqueta M, Maestro JL. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol. 2012;42(7):491–498. doi: 10.1016/j.ibmb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 132.Perez-Hedo M, Rivera-Perez C, Noriega FG. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti . Insect Biochem Mol Biol. 2013;43(6):495–500. doi: 10.1016/j.ibmb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sim C, Denlinger DL. Insulin signaling and the regulation of insect diapause. Front Physiol. 2013;4:189. doi: 10.3389/fphys.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abrisqueta M, Suren-Castillo S, Maestro JL. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol. 2014;49:14–23. doi: 10.1016/j.ibmb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Sheng Z, Xu J, Bai H, Zhu F, Palli SR. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum . J Biol Chem. 2011;286(49):41924–41936. doi: 10.1074/jbc.M111.269845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parthasarathy R, Palli SR. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum . Insect Biochem Mol Biol. 2011;41(5):294–305. doi: 10.1016/j.ibmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J, Sheng Z, Palli SR. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum . PLoS genetics. 2013;9(6):e1003535. doi: 10.1371/journal.pgen.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badisco L, Marchal E, Van Wielendaele P, Verlinden H, Vleugels R, Vanden Broeck J. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria . Peptides. 2011;32(3):573–580. doi: 10.1016/j.peptides.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 139.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science. 2012;337(6096):860–864. doi: 10.1126/science.1224286. [DOI] [PubMed] [Google Scholar]
- 140.Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179(3):477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 141.Koyama T, Mendes CC, Mirth CK. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front Physiol. 2013;4:263. doi: 10.3389/fphys.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lozano J, Belles X. Role of Methoprene-tolerant (Met) in adult morphogenesis and in adult ecdysis of Blattella germanica . PLoS ONE. 2014;9(7):e103614. doi: 10.1371/journal.pone.0103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mirth CK, Tang HY, Makohon-Moore SC, Salhadar S, Gokhale RH, Warner RD, Koyama T, Riddiford LM, Shingleton AW. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila . Proc Natl Acad Sci USA. 2014;111(19):7018–7023. doi: 10.1073/pnas.1313058111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith WA, Lamattina A, Collins M. Insulin signaling pathways in lepidopteran ecdysone secretion. Front Physiol. 2014;5:19. doi: 10.3389/fphys.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsuda M, Kobayashi T, Matsuo T, Aigaki T. Insulin-degrading enzyme antagonizes insulin-dependent tissue growth and Abeta-induced neurotoxicity in Drosophila . FEBS Lett. 2010;584(13):2916–2920. doi: 10.1016/j.febslet.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 146.Hyun J, Hashimoto C. Physiological effects of manipulating the level of insulin-degrading enzyme in insulin-producing cells of Drosophila. Fly (Austin) 2011;5(1):53–57. doi: 10.4161/fly.5.1.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 148.Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26(7):916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 149.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14(5):329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 150.Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16(4–5):421–439. doi: 10.1016/j.cytogfr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 151.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 152.Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol (Lausanne) 2012;3:38. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beattie J, Kreiner M, Allan GJ, Flint DJ, Domingues D, van der Walle CF. IGFBP-3 and IGFBP-5 associate with the cell binding domain (CBD) of fibronectin. Biochem Biophys Res Commun. 2009;381(4):572–576. doi: 10.1016/j.bbrc.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 154.Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PH, Seth A. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5(255):ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- 155.Hwang JR, Huh JH, Lee Y, Lee SI, Rho SB, Lee JH. Insulin-like growth factor-binding protein-5 (IGFBP-5) inhibits TNF-α-induced NF-κB activity by binding to TNFR1. Biochem Biophys Res Commun. 2011;405(4):545–551. doi: 10.1016/j.bbrc.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 156.Kawai M, Breggia AC, DeMambro VE, Shen X, Canalis E, Bouxsein ML, Beamer WG, Clemmons DR, Rosen CJ. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286(16):14670–14680. doi: 10.1074/jbc.M110.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miljus G, Malenkovic V, Dukanovic B, Kolundzic N, Nedic O. IGFBP-3/transferrin/transferrin receptor 1 complexes as principal mediators of IGFBP-3 delivery to colon cells in non-cancer and cancer tissues. Exp Mol Pathol. 2015;98(3):431–438. doi: 10.1016/j.yexmp.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 158.Zhong Y, Lu L, Zhou J, Li Y, Liu Y, Clemmons DR, Duan C. IGF binding protein 3 exerts its ligand-independent action by antagonizing BMP in zebrafish embryos. J Cell Sci. 2011;124(Pt 11):1925–1935. doi: 10.1242/jcs.082644. [DOI] [PubMed] [Google Scholar]
- 159.Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33(5):578–588. doi: 10.1038/onc.2012.630. [DOI] [PubMed] [Google Scholar]
- 160.Dai W, Kamei H, Zhao Y, Ding J, Du Z, Duan C. Duplicated zebrafish insulin-like growth factor binding protein-5 genes with split functional domains: evidence for evolutionarily conserved IGF binding, nuclear localization, and transactivation activity. Faseb J. 2010;24(6):2020–2029. doi: 10.1096/fj.09-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou J, Xiang J, Zhang S, Duan C. Structural and functional analysis of the amphioxus IGFBP gene uncovers ancient origin of IGF-independent functions. Endocrinology. 2013;154(10):3753–3763. doi: 10.1210/en.2013-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152(6):2278–2289. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- 163.Osterbur DL, Fristrom DK, Natzle JE, Tojo SJ, Fristrom JW. Genes expressed during imaginal discs morphogenesis: IMP-L2, a gene expressed during imaginal disc and imaginal histoblast morphogenesis. Dev Biol. 1988;129(2):439–448. doi: 10.1016/0012-1606(88)90391-0. [DOI] [PubMed] [Google Scholar]
- 164.Zapf J, Schoenle E, Froesch ER. In vivo effects of the insulin-like growth factors (IGFs) in the hypophysectomized rat: comparison with human growth hormone and the possible role of the specific IGF carrier proteins. Ciba Found Symp. 1985;116:169–187. doi: 10.1002/9780470720974.ch11. [DOI] [PubMed] [Google Scholar]
- 165.Garbe JC, Yang E, Fristrom JW. IMP-L2: an essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development. 1993;119(4):1237–1250. doi: 10.1242/dev.119.4.1237. [DOI] [PubMed] [Google Scholar]
- 166.Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA. 2008;105(17):6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;10(1):137–147. doi: 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7(4):333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 169.Weiss IM, Gohring W, Fritz M, Mann K. Perlustrin, a Haliotis laevigata (abalone) nacre protein, is homologous to the insulin-like growth factor binding protein N-terminal module of vertebrates. Biochem Biophys Res Commun. 2001;285(2):244–249. doi: 10.1006/bbrc.2001.5170. [DOI] [PubMed] [Google Scholar]
- 170.Rosen O, Weil S, Manor R, Roth Z, Khalaila I, Sagi A. A crayfish insulin-like-binding protein: another piece in the androgenic gland insulin-like hormone puzzle is revealed. J Biol Chem. 2013;288(31):22289–22298. doi: 10.1074/jbc.M113.484279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Claeys I, Simonet G, Van Loy T, De Loof A, Vanden Broeck J. cDNA cloning and transcript distribution of two novel members of the neuroparsin family in the desert locust, Schistocerca gregaria . Insect Mol Biol. 2003;12(5):473–481. doi: 10.1046/j.1365-2583.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- 172.Badisco L, Claeys I, Van Loy T, Van Hiel M, Franssens V, Simonet G, Vanden Broeck J. Neuroparsins, a family of conserved arthropod neuropeptides. Gen Comp Endocrinol. 2007;153(1–3):64–71. doi: 10.1016/j.ygcen.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Huang X, Ye H, Feng B, Huang H. Insights into insulin-like peptide system in invertebrates from studies on IGF binding domain-containing proteins in the female Mud Crab, Scylla paramamosain . Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 174.Girardie J, Girardie A, Huet JC, Pernollet JC. Amino acid sequence of locust neuroparsins. FEBS Lett. 1989;245(1–2):4–8. doi: 10.1016/0014-5793(89)80179-6. [DOI] [PubMed] [Google Scholar]
- 175.Girardie J, Bourême D, Couillaud F, Tamarelle M, Girardie A. Anti-juvenile effect of neuroparsin-A, A neuroprotein isolated from locust corpora cardiaca. Insect Biochem. 1987;17:977–983. doi: 10.1016/0020-1790(87)90106-5. [DOI] [Google Scholar]
- 176.Janssen T, Claeys I, Simonet G, De Loof A, Girardie J, Vanden Broeck J. cDNA cloning and transcript distribution of two different neuroparsin precursors in the desert locust, Schistocerca gregaria . Insect Mol Biol. 2001;10(2):183–189. doi: 10.1046/j.1365-2583.2001.00257.x. [DOI] [PubMed] [Google Scholar]
- 177.Veenstra JA. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front Physiol. 2014;5:454. doi: 10.3389/fphys.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Veenstra JA. What the loss of the hormone neuroparsin in the melanogaster subgroup of Drosophila can tell us about its function. Insect Biochem Mol Biol. 2010;40(4):354–361. doi: 10.1016/j.ibmb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 179.Dhara A, Eum JH, Robertson A, Gulia-Nuss M, Vogel KJ, Clark KD, Graf R, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti . Insect Biochem Mol Biol. 2013;43(12):1100–1108. doi: 10.1016/j.ibmb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti . Proc Natl Acad Sci USA. 2015;112(16):5057–5062. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dissous C. Venus kinase receptors at the crossroads of insulin signaling: their role in reproduction for helminths and insects. Front Endocrinol (Lausanne) 2015;6:118. doi: 10.3389/fendo.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Mizoguchi A, Fujiwara Y, Takahashi SY, Ishizaki H. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori . Proc Natl Acad Sci USA. 1986;83(16):5840–5843. doi: 10.1073/pnas.83.16.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hetru C, Li KW, Bulet P, Lagueux M, Hoffmann JA. Isolation and structural characterization of an insulin-related molecule, a predominant neuropeptide from Locusta migratoria . Eur J Biochem. 1991;201(2):495–499. doi: 10.1111/j.1432-1033.1991.tb16308.x. [DOI] [PubMed] [Google Scholar]
- 184.Cobelli C, Pacini G. Insulin secretion and hepatic extraction in humans by minimal modeling of C-peptide and insulin kinetics. Diabetes. 1988;37(2):223–231. doi: 10.2337/diab.37.2.223. [DOI] [PubMed] [Google Scholar]
- 185.Steiner DF. The proinsulin C-peptide–a multirole model. Exp Diabesity Res. 2004;5(1):7–14. doi: 10.1080/15438600490424389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bermudez I, Beadle DJ, Trifilieff E, Luu B, Hietter H. Electrophysiological activity of the C-peptide of the Locusta insulin-related peptide. Effect on the membrane conductance of Locusta neurones in vitro. FEBS Lett. 1991;293(1–2):137–141. doi: 10.1016/0014-5793(91)81170-D. [DOI] [PubMed] [Google Scholar]
- 187.Clynen E, Huybrechts J, Baggerman G, Van Doorn J, Van Der Horst D, De Loof A, Schoofs L. Identification of a glycogenolysis-inhibiting peptide from the corpora cardiaca of locusts. Endocrinology. 2003;144(8):3441–3448. doi: 10.1210/en.2002-0107. [DOI] [PubMed] [Google Scholar]
- 188.Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 1995;14(14):3373–3384. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sajid W, Kulahin N, Schluckebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Norgaard P, Wahlund PO, Brandt J, Kohanski RA, Andersen AS, De Meyts P. Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem. 2011;286(1):661–673. doi: 10.1074/jbc.M110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Veenstra JA. The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii . Gen Comp Endocrinol. 2015 doi: 10.1016/j.ygcen.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 191.Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3(11):e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toivonen JM, Partridge L. Endocrine regulation of aging and reproduction in Drosophila . Mol Cell Endocrinol. 2009;299(1):39–50. doi: 10.1016/j.mce.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 193.Williams MJ, Eriksson A, Shaik M, Voisin S, Yamskova O, Paulsson J, Thombare K, Fredriksson R, Schioth HB. The obesity-linked gene Nudt3 Drosophila homolog Aps is associated with insulin signalling. Mol Endocrinol. 2015 doi: 10.1210/ME.2015-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karpac J, Hull-Thompson J, Falleur M, Jasper H. JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila . Aging Cell. 2009;8(3):288–295. doi: 10.1111/j.1474-9726.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Docherty JE, Manno JE, McDermott JE, DiAngelo JR. Mio acts in the Drosophila brain to control nutrient storage and feeding. Gene. 2015;568(2):190–195. doi: 10.1016/j.gene.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sekine O, Love DC, Rubenstein DS, Hanover JA. Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. J Biol Chem. 2010;285(49):38684–38691. doi: 10.1074/jbc.M110.155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Clements J, Hens K, Francis C, Schellens A, Callaerts P. Conserved role for the Drosophila Pax6 homolog eyeless in differentiation and function of insulin-producing neurons. Proc Natl Acad Sci USA. 2008;105(42):16183–16188. doi: 10.1073/pnas.0708330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cao J, Ni J, Ma W, Shiu V, Milla LA, Park S, Spletter ML, Tang S, Zhang J, Wei X, Kim SK, Scott MP. Insight into insulin secretion from transcriptome and genetic analysis of insulin-producing cells of Drosophila . Genetics. 2014;197(1):175–192. doi: 10.1534/genetics.113.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Davis SM, Thomas AL, Nomie KJ, Huang L, Dierick HA. Tailless and Atrophin control Drosophila aggression by regulating neuropeptide signalling in the pars intercerebralis. Nat Commun. 2014;5:3177. doi: 10.1038/ncomms4177. [DOI] [PubMed] [Google Scholar]