Abstract
The morphogenic factor sonic hedgehog (Shh) actively orchestrates many aspects of cerebellar development and maturation. During embryogenesis, Shh signaling is active in the ventricular germinal zone (VZ) and represents an essential signal for proliferation of VZ-derived progenitors. Later, Shh secreted by Purkinje cells sustains the amplification of postnatal neurogenic niches: the external granular layer and the prospective white matter, where excitatory granule cells and inhibitory interneurons are produced, respectively. Moreover, Shh signaling affects Bergmann glial differentiation and promotes cerebellar foliation during development. Here we review the most relevant functions of Shh during cerebellar ontogenesis, underlying its role in physiological and pathological conditions.
Keywords: Shh, Mitogen, Differentiation, Cerebellum
Introduction
Sonic hedgehog (Shh) signaling has been implicated in the regulation of key events during mammalian developmental processes [1]. The first gene of the Hedgehog family (Hh) was cloned in Drosophila in the early 1990s [2] and its role in controlling proper segmental identity during fruit fly embryonic development was identified [3, 4]. Shortly after, different Hh genes in vertebrates were described [5, 6] and their evolution was explained as the result of genome duplication. They were classified as desert hedgehog (Dhh), Indian hedgehog (Ihh), and Shh [7]. In mammals, Shh was found to be expressed from early embryogenesis and was established as one of the key molecules responsible for the regulation of central nervous system (CNS) patterning [8].
During CNS organogenesis, Shh plays key roles as a morphogen, mitogen, and guidance molecule [9, 10]. Shh is one of the master players in cerebellar patterning and maturation from early phases to adulthood (discussed in the following sections; [11]).
Given its prominent role during development, alterations of its physiological functions are implicated in many human cerebellar pathologies, such as ataxias [12], Joubert syndrome, and medulloblastoma (MB) [11, 13–18].
In this review, we discuss the broad range of actions of Shh in cerebellar genesis. Shh is produced by separate extracerebellar and cerebellar sources at distinct developmental stages. It sequentially targets different cell populations, thereby orchestrating the production of adequate numbers of diverse cell phenotypes and contributing to the shaping of cerebellar structure. We also discuss the broad range of actions of Shh in cerebellar genesis as a most remarkable example of the pleiotropic roles of this morphogen in CNS development.
Insight into the hedgehog pathway
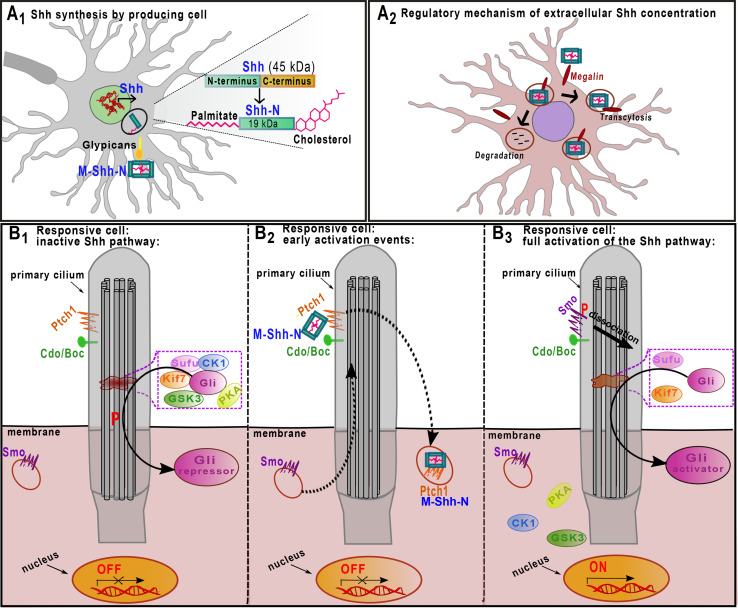
Shh is a protein capable of signaling both in an autocrine and a paracrine fashion. It is translated as a 45-kDa precursor and then auto-proteolytically cleaved by its own C-terminal domain into two secreted peptides: a 19-kDa amino terminus (Shh-N), with a signaling domain, and a 26-kDa carboxy terminus (Shh-C), devoid of any known signal transduction activity [19, 20] (Fig. 1).
Fig. 1.
Shh signaling pathway in vertebrates. A 1 Shh is translated as a 45-kDa precursor protein (Shh) and becomes an active signaling protein (Shh-N) after the addition of a cholesterol and a palmitate lipophilic moiety at its N-terminus. Shh is then trafficked to the cell surface and released from cells as a multimer (M-Shh-N). A 2 Interactions with both megalin and glypicans regulate long-range Shh signaling. Binding to the transmembrane protein megalin promotes Shh internalization, resulting in either degradation or subsequent exocytosis. The Shh affinity for megalin is increased by glypicans. B 1 Primary cilia are key organelles at which Shh signaling takes place. On their membranes, both receptors (Ptch1) and co-receptors (Cdo/Boc) are exposed. These structures also contain regulatory microtubule-associated complexes composed by Sufu, Kif7, PKA, GSK3, CK1, and Gli. In the absence of Shh, Ptch1 prevents membrane localization and activation of Smo, retaining it on intracellular vesicles. In this context, Gli2 and 3 (simply referred as Gli) proteins are held in the microtubule-associated complex, which induces their phosphorylation (red P). Upon phosphorylation, Gli leaves the complex and reaches the cytoplasm as a transcriptional repressor form (Gli repressor). B 2 When Shh interacts with Ptch1 and Cdo/Boc, Smo is shuttled from an endocytic vesicle to the cilium, while the hedgehog–patched complex is internalized and degraded by lysosomes. B 3 The de-repression of Smo, together with its phosphorylation, induces the dissociation of the Sufu–Gli-kinases complex, promoting the formation of the Gli activator form (Gli activator), which, after nuclear translocation, activates transcription of downstream targets
Next, lipophilic moieties are added to the Shh-N, which are essential first for its membrane insertion, followed by its multimerization and detachment [19, 21–23]. This leads to the release of soluble Shh-N, which diffuses away from the site of synthesis [24–27] (Fig. 1A1).
Extracellular levels and diffusion of Shh have been proposed to be regulated by multiple molecules and mechanisms, for example, those involving megalin and the heparin sulphate proteoglygan glypicans. Cell surface glypicans [28, 29] can sequester Shh and increase its affinity with the endocytic receptor megalin, which belongs to the low-density lipoprotein receptor family. Megalin mediates endocytosis of Shh into the cell and directs it to either lysosomal degradation, thereby contributing to the regulation of extracellular Shh concentration, or transcytosis and further diffusion [30, 31] (Fig. 1A2).
The cell surface machinery responsive to Shh comprises a complex interaction network [32]. Shh binds to its specific receptor patched 1 (Ptch1), a 12-pass transmembrane protein, and this binding is facilitated by other transmembrane proteins (e.g., Cdo, Boc, and Growth Arrest Specific 1 (Gas1), (Fig. 1B1–3; [33, 34]). In the absence of the ligand, the receptor Ptch1 represses the action of Shh signal transducer Smoothened (Smo). Smo is a seven-transmembrane-span receptor-like protein that is confined to intracellular endocytic vesicles in the absence of Shh [35]. After the interaction of Shh with Ptch1, Smo is phosphorylated by Casein kinase 1 α (CK1α) and G-protein coupled receptor kinase 2 (GRK2), resulting in the release of its inhibition [36] (Fig. 1B2). Phosphorylated Smo activates intracellular signals regulating several protein kinases, which in turn activate a class of transcription factors known as glioblastoma (Gli) proteins [37].
In the meantime, hedgehog–patched complex is internalized and degraded by lysosomes [37] (Fig. 1B2). In this context, Ptch1 plays an important role in correct signal transduction, since the lack of its function induces abnormal activation of the Shh pathway leading to MB (see “Shh and cerebellar pathology: evidence from medulloblastoma” section).
Notably, in vertebrates, Shh receptor and co-receptors are concentrated in the primary cilium, a microtubule-based membrane protrusion present in nearly all nucleated cells [38]. Several studies suggest a crucial role of primary cilia during development of the cerebellum and, specifically, in Shh-induced proliferation of granule cell progenitors (GCPs) [18, 39, 40]. The inhibition of ciliogenesis in GCPs does not affect their initial specification but only their proliferation, with a consequent hypoplasia of the cerebellar vermis typical of human ciliopathies, such as Joubert and Meckel syndromes [16, 17, 39].
The transcriptional effectors of the Hedgehog signaling are the Gli proteins. In the absence of Shh, their nuclear localization is prevented by the binding of Gli2 and Gli3 to the Suppressor of Fused (Sufu) in the cytoplasm (Fig. 1B1; [41–44]). Sufu is essential for correct cerebellar development (see “Cerebellar territory and germinal zones” section) and its altered function is implicated in the onset of MB (see “Shh and cerebellar pathology: evidence from medulloblastoma” section). The Gli–Sufu complex, which also includes several scaffolding proteins and kinases (e.g., the scaffold kinesin family member 7 (Kif7), protein kinase A (PKA), glycogen synthase kinase 3 (GSK3) and CK1) contains phosphorylated Gli proteins. Consequently, Gli2 is rapidly degraded, whereas Gli3 is cleaved and then acts as a repressor, blocking transcription of downstream targets [25, 45–48]. The relevance of this regulatory complex in cerebellar morphogenesis is indicated by studies showing that deregulation of PKA-mediated inhibition of Gli activity leads to the uncontrolled proliferation of GCPs [49, 50].
Upon activation, Smo induces the inhibition of Gli2 and Gli3 phosphorylation by dissociating the Sufu–Gli-kinase complex and regulating PKA cytoplasmic availability. This results in the stabilization and nuclear accumulation of these two Gli family members, which in turn activate Gli1 transcription (Fig. 1B3; [48, 50–52]). The Gli1 transcriptional level is a popular biomarker for activated Shh signaling. Its experimental manipulation, together with that of Gli2, is widely used to alter physiological Hedgehog signaling in the cerebellum (see “Shh and GABAergic interneurons”, “Shh and cerebellar glia”, “Shh orchestrates normal cerebellar foliation” sections).
The target genes of Shh signaling include genes involved in cell growth and division, various transcription factors and a number of components of the Shh pathway itself presumably for positive and negative feedback (e.g., Gli1 and Ptch1). This list continues to grow as research progresses [15, 53–57].
Distinct functions of Shh during cerebellar development
During neurulation, Shh is produced by the ventral midline mesoderm as well as by the ventral neural tube. Its activity is required for the determination of ventral characteristics along the anterior–posterior neuraxis [58]. At successive stages of development, Shh signaling sustains the proper formation of several CNS regions, including the cerebellum, where it critically influences the initial phases of territorial determination and regulates cerebellar progenitor maturation in both primary and secondary germinal zones.
Cerebellar territory and germinal zones
The cerebellum arises from a specialized area at the midbrain/hindbrain boundary [59–61]. Here, at embryonic day 8.5 (E8.5), the interaction between homeobox genes Otx2 and Gbx2 defines the isthmic organizer region (IO; [62, 63]). The IO orchestrates the development of cerebellar and mesencephalic structures through the morphogenic activity of the secreted factors Fgf8 and Wnt1 [64–67]. At this early stage, Shh expressed in the ventral midline of the midbrain also promotes FgF8 expression by the IO (from E8.5 to E12; [9, 68]). In line with an early action of Shh at the onset of cerebellar morphogenesis, Hoxb.7-Cre-driven conditional knock-out of the Shh inhibitor SuFu leads to abnormal midbrain–hindbrain morphology and FgF8 misexpression, followed by delayed differentiation and atypical migration of different cerebellar cellular populations [69]. Similarly, as a confirmation of this early role of Shh, retrovirus-mediated misexpression of Shh in the early chick neural tube disrupts midbrain–hindbrain boundary formation, leading to later defects in the cerebellar primordia [58].
After territorial specification, cerebellar histogenesis starts at E9 in the mouse. At this age, the cerebellar anlage comprises two separate protuberances that during the following days grow and fuse together. This gives rise to the unitary cerebellar plate, consisting of the vermis and the two hemispheres [70].
During the initial phases of cerebellar histogenesis, Shh is secreted by the choroid plexus [71]. Later, from E17.5, it is released by PCs [72–74]. Whichever the source, Shh is a key regulator of the cerebellar progenitors residing in the two cerebellar germinative compartments: the rhombic lip (RL), located at the outer aspect of the cerebellar plate, adjacent to the roof-plate, and the ventricular zone (VZ), lining the fourth ventricle. These germinative zones are defined by the region-specific expression of two basic helix-loop-helix transcription factors: pancreas transcription factor 1a (Ptf1a), expressed in the VZ [75] and the mouse homolog of Drosophila atonal (Atoh1), present in the RL [76]. This spatially restricted expression pattern determines the neurochemical compartmentalization of cerebellar precursors. All GABAergic neurons [PCs, nucleo-olivary projection neurons of the cerebellar nuclei (CN), and all inhibitory interneurons—basket, stellate, Golgi, and Lugaro cells] originate from Ptf1a+ precursors [75, 77, 78], while the glutamatergic lineages (large projection neurons of the CN, unipolar brush cells (UBCs), and granule cells) derive from Atoh1+ progenitors [78–84].
The two primary germinal epithelia disappear at birth. Dividing VZ precursors migrate into the cerebellar prospective white matter (PWM), whereas those of the RL move along the cerebellar pial surface and form the external granular layer (EGL). Neurogenesis is active in secondary epithelia (PWM and EGL) up to the third postnatal week to generate appropriate numbers of GABAergic and glutamatergic interneurons, respectively [67, 70, 85].
It is well established that Shh actively regulates the amplification of cerebellar progenitors in both embryonic and postnatal germinal zones [71, 72, 86]. This morphogen controls the production of appropriate numbers of excitatory and inhibitory interneurons (see “Shh and granule cells”, “Shh and GABAergic interneurons” sections; Fig. 2). Moreover, it modulates the correct generation and development of glial progenitors (see “Shh and cerebellar glia” section) and has specific functions in different phases of granule cell development, both in normal and pathological conditions, such as MB (see “Shh and cerebellar pathology: evidence from medulloblastoma” section). Shh also actively orchestrates the major dynamics of cerebellar foliation, sustaining normal processes of cerebellar growth and maturation (see “Shh orchestrates normal cerebellar foliation” section).
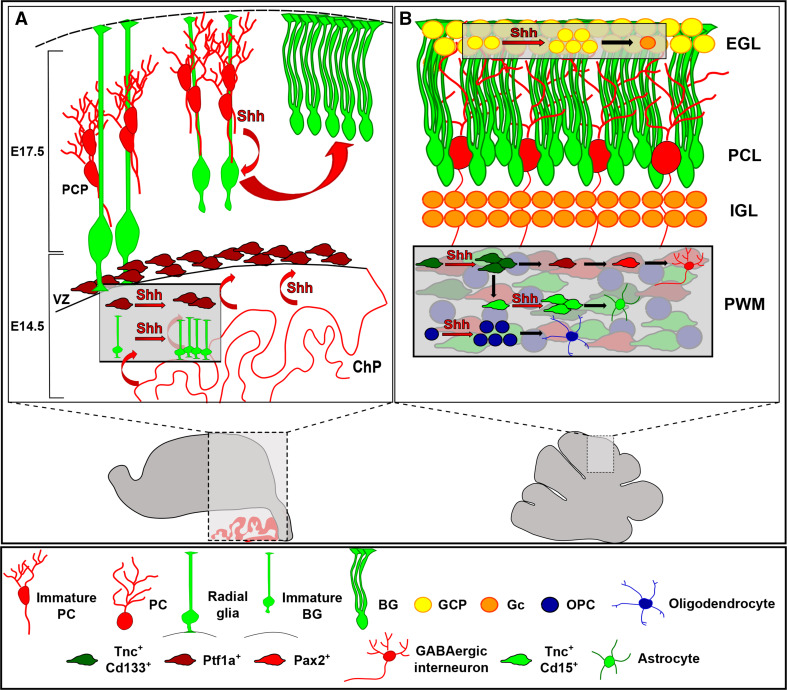
Fig. 2.
Shh functions during cerebellar development. a During embryonic development, Shh is first secreted by the choroid plexus (ChP) and is essential to radial glial cell proliferation and the expansion of Ptf1a+ progenitors of GABAergic neurons. Purkinje cells start Shh secretion by E17.5, modulating the correct differentiation of glial progenitors into mature BG. b Postnatally, Shh acts as a mitogen on both granule and oligodendrocyte precursor cells (GCPs and OPCs), in the EGL and PWM, respectively. In the PWM, Shh also exerts a proliferative function on the neural stem cell-like progenitors (Tnc+CD133+) that generate both intermediate astrocyte precursors (Tnc+CD15+) and GABAergic transient amplifying cells (Ptf1a+). PCP Purkinje cell progenitor, ChP choroid plexus, PWM prospective white matter, GL granular layer, PCL Purkinje cell layer, EGL external granular layer
Shh and granule cells
Granule cells (GCs) represent the most abundant neuron type in the brain (there are about 108 granule cells in the adult murine cerebellum [87, 88]). GCs derive from Atoh1+ progenitors that migrate from the RL to the EGL. The entire process of GC production lasts from E12.5 to P14 and is fundamental for the acquisition of normal cerebellar size and foliation [70]. It has been observed that reduction in GC number leads to the formation of smaller cerebella [89–91] and abnormal foliation, such as the persistence of just the five cerebellar cardinal lobules at P14 in rats ([92, 93], see “Shh orchestrates normal cerebellar foliation” section). In addition, abnormal proliferation of GCPs is at the basis of pathological conditions such as MB, the most common form of malignant brain tumor in children (for review see [94, 95]; see “Shh and cerebellar pathology: evidence from medulloblastoma” section).
Numerous studies have analyzed the mechanisms underlying the initial phases of GCP proliferation and migration from the mitogenic niche of the EGL [74, 96, 97]. It has been demonstrated that these stages are actively controlled by mitogenic factors secreted by PCs, in particular Shh. Indeed, the relative number of granule cells is reduced in animal models characterized by primary PC degeneration [98–100], as a consequence of both the loss of mitogenic Shh and the degeneration of their targets (the PCs). Conversely, if loss of PCs occurs later in the postnatal period (as in the pcd mutant mouse) the Shh mitogenic effect is preserved and consequently the granule cell layer appears nearly normal [100, 101].
Treatment of GCPs with Shh prevents differentiation and induces a long-lasting proliferative response, while inhibition of Shh signaling dramatically reduces their mitotic activity [72, 74, 102–104]. The pathway activated involves the upregulation of the target genes Patched, Gli1, and Gli2 [53, 93, 102]. For example, the activation of a Gli2-mediated pathway is important to ensure the correct expansion of GCPs in the EGL and the consequent normal patterning of cortical folia ([93], see “Shh orchestrates normal cerebellar foliation” section). Other important mediators of Shh-induced proliferation are N-myc, cyclin D1, and cyclin D2, which directly promote the entry of precursors into the cell-cycle and DNA replication [53, 105, 106]. Overexpression of these molecules is sufficient to boost GCP proliferation, but the amplitude of the elicited response is strictly dependent upon the particular molecule involved [53].
Notably, a negative regulatory action of GCPs on the production of Shh by PCs has been proposed based on the evidence that PCs produce increased quantities of Shh in the Kif3a mutant mouse, characterized by defective GCP cilia and reduced GCP numbers [39].
The decreased expression of Gli1 in the innermost part of the EGL indicates that the response to the Shh signal is progressively switched off in GCs [93, 96]. How exactly do GCPs modify their response to Shh and become insensitive to its mitogenic effect? Both intrinsic and extrinsic factors may contribute to this process. In parallel with the loss of Gli1 expression, the Shh effector cyclin-D1 is not detected in GCPs of the deepest part of EGL. The same is observed in GCs of the granular layer (GL), indicating their status as non-proliferating cells [107–109]. In addition, the GCPs located in the deeper EGL start the expression of cyclin-dependent kinase inhibitors such as p27. This molecule arrests proliferation and induces the differentiation programme both in vitro and in vivo [110, 111]. However, in p27 knockout mice GCPs are still able to leave the cell-cycle and differentiate into mature granule cells, indicating that p27 is not the sole factor responsible for this effect [97]. This switch in GCP sensitivity and response to Shh has been suggested to depend on extracellular matrix (ECM) glycoproteins. Both laminin and vitronectin can modulate GPC responses to Shh [112]. GCPs actively proliferate in the presence of Shh when cultured on laminin, which is present in vivo in the outer EGL, but not on vitronectin, which is normally contacted by granule cells in the deepest EGL and GL [73, 112]. Therefore, the same molecular elements may regulate proliferation of GCPs, depending on the pattern of ECM molecules and receptors expressed in different parts of the EGL.
Overall, these data highlight the reciprocal interactions between GCPs and PCs mediated by Shh and the dynamic changes in the pathway due to different molecules in the local environment.
Shh and GABAergic interneurons
GABAergic interneurons comprise multiple subsets of morphologically and neurochemically distinct phenotypes integrated in the cerebellar cortex and CN. These cells are produced from late embryonic life to the second postnatal week; the peak is around P5 and the production of 75 % of all inhibitory interneurons occurs prior to P7 [113]. Maricich and Herrup [114] identified the progenitors of inhibitory interneurons as a population of Pax-2+ cells that arise in the VZ around E12 and later migrate into the cerebellar parenchyma. Inhibitory interneuron precursors continue to proliferate during their migration in the PWM [113, 115–117] and generate the various interneuron phenotypes according to an inside-out progression. CN interneurons are the first to be born during embryonic and early postnatal life, followed by GL interneurons (Golgi and Lugaro cells) and lastly the interneurons residing in the molecular layer (ML, basket and stellate cells; [113, 114, 116, 118]). Interestingly, transplantation experiments have demonstrated that all these different interneuron subsets derive from a single population of Pax-2+ immature interneurons that acquire mature phenotypic traits under the influence of local instructive cues provided by the PWM microenvironment [67, 116, 117].
During embryonic development Shh is secreted from the choroid plexus and transventricularly delivered by the embryonic cerebrospinal fluid to the target niches. Here it induces the expansion of the early generated subset of GABAergic interneurons, as shown both in vivo [71] and after exogenous administration of the recombinant amino-terminal active fragment Shh-N to embryonic explants [119]. Interestingly, the observation of double-immunopositive Gli1/Pax-2 cells in the VZ/SVZ regions or scattered in the cerebellar parenchyma shows that during embryonic development, the Shh pathway is already active in young Pax-2+ interneurons located in germinal and migratory sites [119].
Although from E17.5 onward Shh is produced by a different source (i.e., PCs), it continues to control the generation of GABAergic interneurons by regulating the activity of PWM neural stem-cell-like primary progenitors. These generate both interneurons and parenchymal astrocytes and are characterized by the expression of prominin and low levels of tenascin [86]. This idea is supported by the observation that blockade of Shh signaling in these cells disrupts the proliferative activity of intermediate precursors, consequently decreasing the final numbers of both interneurons and parenchymal astrocytes [86]. This specific effect of Shh is independent of its classical role in regulating GCP proliferation [74], as direct perturbation of GCP expansion does not alter GABAergic progenitor production [86].
In line with these observations, the exogenous administration of Shh-N to cerebellar slices from P2 mice induces an amplification of Pax-2+ cells [119]. This effect is significant both at day 1 and day 2 in vitro, whereas in the presence of the Shh inhibitor cyclopamine, the effect is lost [119]. In addition, the mitogenic effect of Shh on newborn Pax-2+ cells disappears at later postnatal stages such as P7, by which time the bulk of GABAergic interneurons has been already produced [113, 120].
Gene expression analysis of sorted GFP+ cells from Pax-2-GFP transgenic mice revealed that the Shh pathway is active not only in stem cell-like progenitors and intermediate precursors but also in immature early postmitotic Pax-2+ interneurons, which express both the Shh receptor Ptch1 and the Shh target gene Gli1. These results were confirmed by in situ hybridization [119]. However, the role of Shh during interneuron maturation remains to be clarified.
Taken together, these recent studies highlight the fundamental roles of Shh in regulating the interneuron population by maintaining the embryonic and postnatal niches in which these cells are produced.
Shh and cerebellar glia
Cerebellar astrocytes and oligodendrocytes are morphologically distinct cell types located at different sites in the cerebellar cortex and white matter [121–123].
Experimental evidence indicates that astrocytes [comprising Bergman glia (BG) and parenchymal astrocytes] derive from the VZ [118, 121, 124–126], whereas oligodendrocyte precursors (OPCs) appear to originate mainly from exogenous sources and populate the cerebellar parenchyma at later embryonic stages [127–129].
A few studies have investigated the action of Shh signaling on both astrocytes [130–132] and oligodendrocytes [133–138] in the developing and mature brain. In the cerebellum, Shh has been reported to act directly on both BG and oligodendrocytes, regulating their differentiation and proliferation, respectively. Moreover, this ligand critically maintains the VZ, controlling the proliferation of radial glia cells via their cilia [39, 71], and the PWM niche, controlling the amplification of Tnc+CD15+ intermediate progenitors of parenchymal astrocytes ([71, 86]; see previous section). It is not clear whether Shh also has effects on mature parenchymal astrocytes.
Wallace [102] and Traiffort et al. [139] observed high expression levels of Ptch and Gli1 in small cells in the PC layer, presumably BG, suggesting that the Shh signaling pathway is active in these cells. Confirmation of a direct action of Shh came shortly after, when Dahmane and Ruiz-I-Altaba [72] first demonstrated the role of this ligand in BG differentiation but, intriguingly, not in BG progenitor proliferation [72]. Given the PC origin of Shh, these results confirmed previous data revealing a key role for PCs in the control of BG maturation [66, 140]. However, BG persist in both Gli2 mutant embryos [73] and Gli2-En1 conditional knock-out mutants, in which Gli2 deletion is restricted to cerebellar precursors [93], thus demonstrating that Shh signaling through Gli2 is not essential for BG specification. However, the latter mutant model did show an abnormal glial morphology, characterized by a disorganization and deformity of the glial fibers. This phenotype was explained as a secondary effect of the abnormal PC morphology [93]. Lewis et al. [74], using transgenic mice models specifically developed to prevent Shh production by PCs at different ages, observed alterations in BG only after P5, while BG morphology was normal at previous developmental stages. In this case, the results were interpreted as a secondary effect of PC disorganization and absence of parallel fibers and not as a direct consequence of Shh absence on BG differentiation. In contrast, Mecklenburg et al. [141] proposed a direct role for Shh in the regulation of BG maturation, suggesting that the altered glial morphology observed in conditional Shh mutants [73] was not subordinate to defective PCs. In this study, conditional ablation of Shh in the developing cerebellum led to a rapid reduction of Gdf10, which is a member of the transforming growth factor beta (TGF-β)-superfamily strongly expressed in BG cells from E15. The rapid down-regulation of this glial-specific gene in the absence of Shh suggested that it may have a direct role in BG specification, although the exact mechanisms still need to be clarified.
Shh has also been shown to affect the proliferation of cerebellar oligodendrocytes, the glia responsible for myelin synthesis. Bouslama-Oueghlani et al. [142] used cerebellar organotypic cultures to investigate the influence of PCs (the only type of cerebellar neuron to be myelinated) on the timing of oligodendrocyte differentiation. In particular, by using cerebellar slice cultures in which numbers of PCs were significantly different, these authors found that soluble factors produced by PCs were able to affect the OPC population. Among these factors, Shh was shown to be downregulated during PC postnatal maturation, whereas vitronectin was upregulated. Importantly, either Shh or vitronectin when administrated to postnatal organotypic slices, had opposite effects on OPCs, stimulating either their proliferation or differentiation [142]. These effects were reversed by Shh or vitronectin antagonists.
In summary, these results highlight that both neuronal and glial development is highly synchronized in the cerebellum [142], as it is in other CNS regions [143–145].
Shh orchestrates normal cerebellar foliation
A prominent feature of cerebellar morphology is its folded appearance, whereby fissures divide its anterior–posterior extent into lobules [67, 146]. By E18.5, four principal fissures are evident in midsagittal sections of the mouse cerebellar vermis, allowing the distinction of five cardinal lobes (the anterobasal, anterodorsal, central, posterior, and inferior lobes [146]). Subsequently, additional (non-principal) fissures further divide the cardinal lobes into lobules, resulting in a total of ten lobules identifiable in the adult murine cerebellum [146]. The process of fissure formation strictly depends on GCP proliferation [73, 93]. Therefore Shh, acting as a mitogen on GCPs, indirectly plays a role in cerebellar foliation.
In particular, it has been shown that Shh signaling spatially and temporally correlates with fissure formation. Gli2 is the principal activator of Shh-induced GCP proliferation, which in turn is the driving force for the initial establishment of the fissures [73]. Indeed, Gli2-null mutants show decreased foliation at birth and reduced numbers of GCPs, whereas Shh overexpression in wild-type cerebella leads to normal cerebellar foliation but also to an increased cerebellar size, a consequence of prolonged GCP proliferation [73]. Other experiments have clarified that the level of Shh signaling regulates the extent and complexity of cerebellar foliation, but not its typical pattern [93]. In the absence of Gli2, foliation proceeds but the process of lobulation is delayed and prematurely arrested and further reduction in foliation occurs in double Gli2 and Gli1 null mutant mice. Whereas, when the entire Shh signaling is removed, foliation is totally inhibited because of a rapid exhaustion of GCPs after E17.5 [93]. Collectively, these findings suggest that Shh is not necessary to initiate foliation, nor does it determine the position of the fissures, but is a regulator of the extent and complexity of foliation. This interpretation implies that at embryonic stages even in the absence of Shh, some GPCs should be able to start mitosis, allowing the first sulci to form. However, at both late prenatal and postnatal stages, Shh becomes necessary to sustain the expansion of the EGL [73].
Shh and cerebellar pathology: evidence from medulloblastoma
MB is the most common form of neuroectodermal tumor of childhood, with an estimated lethality of 30 % and high clinical heterogeneity. It is widely accepted that MB originates from GCPs and four different subtypes of MBs have been identified and classified according to their transcriptional profiles: (1) WNT MBs; (2) Shh MBs; (3) Group C, frequently associated with TGF1 beta pathway abnormalities; (4) Group D, often related to tandem duplication of α-synuclein-interacting protein (SNCAIP) [67, 147–151].
Shh-MBs represent one-third of the total number of MB cases occurring both in childhood and adulthood [152, 153]. It has been demonstrated that infant and adult MBs exhibit different transcriptional and genetic profiles. Northcott et al. [149] identified a number of homeobox family members as the genes most strongly up-regulated in adult forms of Shh-MBs. Alternatively, infant MBs express high levels of transcriptional regulators participating in brain development, such as ZIC2 and ZIC5. These dissimilarities lead to large variability in clinical and prognostic aspects of the disease at different ages—one reason why the targeting of signaling molecules might be a fundamental step in developing new therapeutic approaches.
It has been demonstrated that Shh-MBs are caused by aberrations in various components of Shh pathway, such as Ptch1 [103, 154], Sufu [155, 156], Gli transcription factors [157] and Smo [158]. Studies in mice lacking Ptch function demonstrated that abnormal activation of the Shh pathway through repression of its inhibitors leads to the formation of MBs [159, 160]. Similar mutations have also been described in patients with nevoid basal cell carcinoma syndrome—also known as Gorin syndrome—often associated with childhood MB [161]. According to Kim et al. [162], Shh pathway alteration via Ptch in heterozygous mice induces a subset of GCPs to maintain their proliferative activity with consequent deregulation of developmental gene expression, rather than a global increase of GCPs proliferation during postnatal development or an interruption in programmed cell death. However, given the complexity of the Shh pathway, it is not possible to attribute MB formation to abnormalities of just one element of the network, as demonstrated by the fact that only a fraction of Ptch mutant mice develop MB. Taylor et al. [155] identified Sufu as a tumor-suppressor gene in a subset of desmoplastic MBs. They created a model in which several mutations in Sufu encoded truncated proteins that are unable to export the Gli transcription factor from nucleus to cytoplasm, with the subsequent activation of Shh signaling. More recently, it has been also demonstrated that nevoid basal cell carcinoma syndrome, traditionally associated with Ptch mutations, could be caused by heterozygous loss-of-function germline mutations in Sufu as well. These studies contributed to the redefinition of the risk of MB in Gorlin syndrome on the basis of the related gene: in Ptch-related forms, the risk of MB has been reduced from 5 to 2 % with a probability approximately 20 times higher in Sufu-related forms [163, 164].
Other studies specifically focused on the role of Gli1 expression in MB. Yoon et al. [165] identified the subset of Gli1 target genes responsible for cell transformation and specifically expressed in MBs. Moreover, the expression of the Shh signaling components was investigated in relation to prognosis, suggesting that Gli1 or Gli2 expression in pediatric MB might confer a worse outcome [166]. However, recently, new intriguing findings partly modified this classical view of Shh-dependent proliferation of GCPs. Li et al. [167] discovered a new population of progenitors cells in the EGL identified by the expression of the neural stem cells marker Nestin. Surprisingly, Nestin+ cells do not express Atoh1 and are not responsive to Shh in vivo, even if they express the signaling-associated machinery. Although they account for only 3–5 % of EGL cells, they display enhanced tumorigenic potential and chromosomal aberrations following loss of the Shh receptor Ptch1 compared to Atoh1+ GCPs [167], thus questioning the cellular origin of MB [168].
Concluding remarks
The role of Shh in the development of the CNS has been extensively investigated, leading to an expanded understanding of the extrinsic and intrinsic molecular machineries of its signaling pathway. Beyond its role in patterning, hedgehog signaling is now known to have multiple roles throughout development, favoring the processes of fate specification, oligodendrogenesis, stem cell maintenance, and axon path finding. In the cerebellum, the Shh pathway has been principally studied for many years in the context of GCP proliferation. However (as described above), the Shh ligand exerts additional roles on distinct cell populations throughout cerebellar development. In particular, Shh crucially sustains the expansion of neuronal and glial precursors within embryonic and postnatal niches, by means of different mechanisms that involve both cerebellar and extracerebellar strategies (Fig. 2). Shh also induces BG maturation and oligodendrocyte amplification. As described above, Shh signaling is a complex network involving distinct players that modulate Shh function at different levels and in a cell-context-dependent manner. Intriguingly, the sole source of Shh during the late embryonic and postnatal development is PCs. These strategically orchestrate postnatal cerebellar morphogenesis through the modulated secretion of Shh and vitronectin. Despite extensive data in these areas, a deeper knowledge of the processes regulating the timing and balance of Shh/vitronectin production by PCs will certainly shed further light on the mechanisms of cerebellar development.
Acknowledgments
We dedicate this work to the memory of Prof. F. Rossi for his continuous support and encouragement. This work was supported by the University of Turin and Research Fund for the Promotion of Basic Research Grant RBFR10A01S (K.L.). We thank Professor Richard Hawkes, Dr. Daniela Carulli, and Dr. Ishira Nanavaty for critical reading of this manuscript and helpful suggestions.
Compliance with ethical standards
Conflict of interest
The author(s) declare that they have no competing interests.
Footnotes
Annarita De Luca and Valentina Cerrato contributed equally to this work.
References
- 1.Simpson F, Kerr MC, Wicking C. Trafficking, development and hedgehog. Mech Dev. 2009;126(5–6):279–288. doi: 10.1016/j.mod.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Tashiro S, Michiue T, Higashijima S, Zenno S, Ishimaru S, Takahashi F, Orihara M, Kojima T, Saigo K. Structure and expression of hedgehog, a Drosophila segment-polarity gene required for cell–cell communication. Gene. 1993;124(2):183–189. doi: 10.1016/0378-1119(93)90392-G. [DOI] [PubMed] [Google Scholar]
- 3.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila . Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Arias A, Lawrence PA. Parasegments and compartments in the Drosophila embryo. Nature. 1985;313(6004):639–642. doi: 10.1038/313639a0. [DOI] [PubMed] [Google Scholar]
- 5.Wada H, Makabe K. Genome duplications of early vertebrates as a possible chronicle of the evolutionary history of the neural crest. Int J Biol Sci. 2006;2(3):133–141. doi: 10.7150/ijbs.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DT, Lopez A, von Kessler DP, Chiang C, Simandl BK, Zhao R, Seldin MF, Fallon JF, Beachy PA. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120(11):3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- 7.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 8.Machold R, Fishell G. Hedgehog patterns midbrain architecture. Trends Neurosci. 2002;25(1):10–11. doi: 10.1016/S0166-2236(00)01982-2. [DOI] [PubMed] [Google Scholar]
- 9.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signaling in vertebrate neural development. Nat Rev Neurosci. 2006;7(10):772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 10.Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23(6):965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Vaillant C, Monard D. SHH pathway and cerebellar development. Cerebellum. 2009;8(3):291–301. doi: 10.1007/s12311-009-0094-8. [DOI] [PubMed] [Google Scholar]
- 12.Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabási AL, Vidal M, Zoghbi HY. A protein–protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125(4):801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Bale AE. Hedgehog signaling and human disease. Annu Rev Genomics Hum Genet. 2002;3:47–65. doi: 10.1146/annurev.genom.3.022502.103031. [DOI] [PubMed] [Google Scholar]
- 14.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 1805;2:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 16.Aguilar A, Meunier A, Strehl L, Martinovic J, Bonniere M, Attie-Bitach T, Encha-Razavi F, Spassky N. Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome. Proc Natl Acad Sci USA. 2012;109(42):16951–16956. doi: 10.1073/pnas.1201408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millen KL, Gleeson JG. Cerebellar development and disease. Curr Opin Neurobiol. 2008;18(1):12–19. doi: 10.1016/j.conb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barakat MT, Humke EW, Scott MP. Kif3a is necessary for initiation and maintenance of medulloblastoma. Carcinogenesis. 2013;34(6):1382–1392. doi: 10.1093/carcin/bgt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MM Jr (2004) The hedgehog signaling network. Am J Med Genet A 123A(1):5–28. Erratum in: Am J Med Genet (2004) 124A(4):439–440 [DOI] [PubMed]
- 21.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293(5537):2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science. 1994;266(5190):1528–1537. doi: 10.1126/science.7985023. [DOI] [PubMed] [Google Scholar]
- 23.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, et al. Identification of a palmitic acid-modified form of human sonic hedgehog. J Biol Chem. 1998;273(22):14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 24.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105(5):599–612. doi: 10.1016/S0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19(2):159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Goetz A, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of sonic hedgehog mediates long-range signaling. Nature. 2001;411(6838):716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 27.Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18(6):641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fico A, Maina F, Dono R. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci. 2011;68(6):923–929. doi: 10.1007/s00018-007-7471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236(2):364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277(28):25660–25667. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- 31.Litingtung Y, Chiang C. Control of Shh activity and signaling in the neural tube. Dev Dyn. 2000;219(2):143–154. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1050>3.3.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Izzi L, Lévesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20(6):788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the hedgehog receptor. Nature. 1996;384(6605):176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 34.Kang JS, Zhang W, Krauss RS (2007) Hedgehog signaling: cooking with Gas1. Sci STKE 403:pe50 [DOI] [PubMed]
- 35.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J. Sonic hedgehog dependent phosphorylation by CK1α and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9(6):e1001083. doi: 10.1371/journal.pbio.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen MM., Jr Hedgehog signaling update. Am J Med Genet A. 2010;152A(8):1875–1914. doi: 10.1002/ajmg.a.32909. [DOI] [PubMed] [Google Scholar]
- 38.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spassky N, Han YG, Agular A, Strehl L, Besse L, Laclef C, Ros MR, Garcia Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317(1):246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27(36):9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol. 2004;24(19):8627–8641. doi: 10.1128/MCB.24.19.8627-8641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svärd J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergström A, Ericson J, Toftgård R, Teglund S. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian hedgehog signaling pathway. Dev Cell. 2006;10(2):187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25(16):7042–7053. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merchant M, Evangelista M, Luoh SM, Frantz GD, Chalasani S, Carano RA, van Hoy M, Raminez J, Ogasawara AK, McFarland LM, et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25(16):7054–7068. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100(4):423–434. doi: 10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 46.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26(9):3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell. 2006;10(6):719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, Wang B. A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. J Biol Chem. 2007;282(15):10846–10852. doi: 10.1074/jbc.M608599200. [DOI] [PubMed] [Google Scholar]
- 49.Lelievre V, Seksenyan A, Nobuta H, Yong WH, Chhith S, Niewiadomski P, Cohen JR, Dong H, Flores A, Liau LM, Kornblum HI, Scott MP, Wascheck JA. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Dev Biol. 2008;313(1):359–370. doi: 10.1016/j.ydbio.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cell Signal. 2013;25(11):2222–2230. doi: 10.1016/j.cellsig.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 52.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate hedgehog signaling: recruitment to cilia and dissociation of SuFu–Gli protein complexes. J Cell Biol. 2010;191(2):415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci USA. 2003;100(12):7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97(7):903–915. doi: 10.1016/S0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 55.Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP., Jr Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002;16(1):114–126. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4(8):761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, Longabaugh WJ, Davidson EH, Wong WH, McMahon AP. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134(10):1977–1989. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- 58.Zhang XM, Lin E, Yang XJ. Sonic hedgehog-mediated ventralization disrupts formation of the midbrain–hindbrain junction in the chick embryo. Dev Neurosci. 2000;22(3):207–216. doi: 10.1159/000017443. [DOI] [PubMed] [Google Scholar]
- 59.Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- 60.Hallonet ME, Le Douarin NM. Tracing neuroepithelial cells of the mesencephalic and metencephalic alar plates during cerebellar ontogeny in quail-chick chimaeras. Eur J Neurosci. 1993;5(9):1145–1155. doi: 10.1111/j.1460-9568.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 61.Hallonet ME, Alvarado-Mallart RM. The chick/quail chimeric system: a model for early cerebellar development. Perspect Dev Neurobiol. 1997;5(1):17–31. [PubMed] [Google Scholar]
- 62.Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401(6749):164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- 63.Li JY, Lao Z, Joyner AL. New regulatory interactions and cellular responses in the isthmic organizer region revealed by altering Gbx2 expression. Development. 2005;132(8):1971–1981. doi: 10.1242/dev.01727. [DOI] [PubMed] [Google Scholar]
- 64.Martinez S, Wassef M, Alvarado-Mallart RM. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene engrailed. Neuron. 1991;6:971–981. doi: 10.1016/0896-6273(91)90237-T. [DOI] [PubMed] [Google Scholar]
- 65.Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development. 1999;126(6):1189–1200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- 66.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, et al. Consensus paper: cerebellar development. Cerebellum. 2015 doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Epstein DJ, McMahon AP, Joyner AL. Regionalization of sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126(2):281–292. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- 69.Kim JJ, Gill PS, Rotin L, van Eede M, Henkelman RM, Hui CC, Rosenblum ND. Suppressor of fused controls mid–hindbrain patterning and cerebellar morphogenesis via GLI3 repressor. J Neurosci. 2011;31(5):1825–1836. doi: 10.1523/JNEUROSCI.2166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. New York: CRC Press; 1997. [Google Scholar]
- 71.Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C. Transventricular delivery of sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci USA. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 73.Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development. 2004;131:5581–5590. doi: 10.1242/dev.01438. [DOI] [PubMed] [Google Scholar]
- 74.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- 77.Seto Y, Nakatani T, Masuyama N, Taya S, Kumai M, Minaki Y, Hamaguchi A, Inoue YU, Inoue T, Miyashita S, et al. Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum. Nat Commun. 2014;5:3337. doi: 10.1038/ncomms4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada M, Seto Y, Taya S, Owa T, Inoue YU, Inoue T, Kawaguchi Y, Nabeshima Y, Hoshino M. Specification of spatial identities of cerebellar neuronal progenitors by Ptf1a and Atoh1 for proper production of GABAergic and glutamatergic neurons. J Neurosci. 2014;34:4786–4800. doi: 10.1523/JNEUROSCI.2722-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/S0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 80.Wingate RJT. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/S0959-4388(00)00177-X. [DOI] [PubMed] [Google Scholar]
- 81.Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 82.Wang VY, Rose MF, Zoghbi H. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 83.Fink AJ, Englund C, Daza RA, Pham D, Lau C, Nivison M, Kowalczyk T, Hevner RF. Development of the deep cerebellar nuclei: transcription factors and cell migration from the rhombic lip. J Neurosci. 2006;26:3066–3076. doi: 10.1523/JNEUROSCI.5203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Englund C, Kowalczyk T, Daza RA, Dagan A, Lau C, Rose MF, Hevner RF. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J Neurosci. 2006;26:9184–9195. doi: 10.1523/JNEUROSCI.1610-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carletti B, Rossi F. Neurogenesis in the cerebellum. The Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- 86.Fleming JT, He W, Hao C, Ketova T, Pan FC, Wright CV, Litingtung Y, Chiang C. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev Cell. 2013;27:278–292. doi: 10.1016/j.devcel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 88.Fujita S, Simada M, Nakanuna T. 3H-thymidine autoradiographic studies on the cell proliferation and differentiation in the external and internal granular layers of the mouse cerebellum. J Comp Neurol. 1966;128:191–209. doi: 10.1002/cne.901280206. [DOI] [PubMed] [Google Scholar]
- 89.Sidman RL, Lane PW, Dickie MM. Staggerer, a new mutation in the mouse affecting the cerebellum. Science. 1962;137(3530):610–612. doi: 10.1126/science.137.3530.610. [DOI] [PubMed] [Google Scholar]
- 90.Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973;152(2):133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 91.Herrup K, Mullen RJ. Staggerer chimeras: intrinsic nature of Purkinje cell defects and implications for normal cerebellar development. Brain Res. 1979;178(2–3):443–457. doi: 10.1016/0006-8993(79)90705-4. [DOI] [PubMed] [Google Scholar]
- 92.Doughty ML, Delhaye-Bouchaud N, Mariani J. Quantitative analysis of cerebellar lobulation in normal and agranular rats. J Comp Neurol. 1998;399:306–320. doi: 10.1002/(SICI)1096-9861(19980928)399:3<306::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 93.Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133:1811–1821. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- 94.Behesti H, Marino S. Cerebellar granule cells: insights into proliferation, differentiation, and role in medulloblastoma pathogenesis. Int J Biochem Cell Biol. 2009;41(3):435–445. doi: 10.1016/j.biocel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr Top Dev Biol. 2011;94:235–282. doi: 10.1016/B978-0-12-380916-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi Y, Borghesani PR, Chan JA, Segal RA. Migration from a mitogenic niche promotes cell-cycle exit. J Neurosci. 2005;25(45):10437–10445. doi: 10.1523/JNEUROSCI.1559-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishizaki Y. Control of proliferation and differentiation of neural precursor cells: focusing on the developing cerebellum. J Pharmacol Sci. 2006;101(3):183–188. doi: 10.1254/jphs.CPJ06011X. [DOI] [PubMed] [Google Scholar]
- 98.Sonmez E, Herrup K. Role of staggerer gene in determining cell number in cerebellar cortex. II. Granule cell death and persistence of the external granule cell layer in young mouse chimeras. Brain Res. 1984;314(2):271–283. doi: 10.1016/0165-3806(84)90049-X. [DOI] [PubMed] [Google Scholar]
- 99.Vogel MW, Sunter K, Herrup K. Numerical matching between granule and Purkinje cells in lurcher chimeric mice: a hypothesis for the trophic rescue of granule cells from target-related cell death. J Neurosci. 1989;9(10):3454–3462. doi: 10.1523/JNEUROSCI.09-10-03454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smeyne RJ, Chu T, Lewin A, Bian F, S.-Crisman S, Kunsch C, Lira SA, Oberdick J. Local control of granule cell generation by cerebellar Purkinje cells. Mol Cell Neurosci. 1995;6:230–251. doi: 10.1006/mcne.1995.1019. [DOI] [PubMed] [Google Scholar]
- 101.Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci USA. 1976;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wallace VA. Purkinje-cell-derived sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/S0960-9822(99)80195-X. [DOI] [PubMed] [Google Scholar]
- 103.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/S0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 104.Nicot A, Lelièvre V, Tam J, Waschek JA, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. J Neurosci. 2002;22(21):9244–9254. doi: 10.1523/JNEUROSCI.22-21-09244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 107.Shambaugh GE, 3rd, Lee RJ, Watanabe G, Erfurth F, Karnezis AN, Koch AE, Haines GK, 3rd, Halloran M, Brody BA, Pestell RG. Reduced cyclin D1 expression in the cerebella of nutritionally deprived rats correlates with developmental delay and decreased cellular DNA synthesis. J Neuropathol Exp Neurol. 1996;55(9):1009–1020. doi: 10.1097/00005072-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe G, Pena P, Shambaugh GE, 3rd, Haines GK, 3rd, Pestell RG. Regulation of cyclin dependent kinase inhibitor proteins during neonatal cerebella development. Brain Res Dev Brain Res. 1998;108(1–2):77–87. doi: 10.1016/S0165-3806(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 109.Parmigiani E, Leto K, Rolando C, Figueres-Onãte M, López-Mascaraque L, Buffo A, Rossi F. Heterogeneity and bipotency of astroglial-like cerebellar progenitors along the interneuron and glial lineages. J Neurosci. 2015;35(19):7388–7402. doi: 10.1523/JNEUROSCI.5255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durand B, Fero ML, Roberts JM, Raff MC. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol. 1998;8(8):431–440. doi: 10.1016/S0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 111.Miyazawa K, Himi T, Garcia V, Yamagishi H, Sato S, Ishizaki Y. A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation. J Neurosci. 2000;20(15):5756–5763. doi: 10.1523/JNEUROSCI.20-15-05756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pons S, Trejo JL, Martinez-Morales JR, Marti E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development. 2001;128(9):1481–1492. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- 113.Weisheit G, Gliem M, Endl E, Pfeffer PL, Busslinger M, Schilling K. Postnatal development of the murine cerebellar cortex: formation and early dispersal of basket, stellate and Golgi neurons. Eur J Neurosci. 2006;24:466–478. doi: 10.1111/j.1460-9568.2006.04915.x. [DOI] [PubMed] [Google Scholar]
- 114.Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(SICI)1097-4695(19991105)41:2<281::AID-NEU10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16(1):47–54. doi: 10.1016/S0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 116.Leto K, Carletti B, Williams IM, Magrassi L, Rossi F. Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. J Neurosci. 2006;26:11682–11694. doi: 10.1523/JNEUROSCI.3656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leto K, Bartolini A, Yanagawa Y, Obata K, Magrassi L, Schilling K, Rossi F. Laminar fate and phenotype specification of cerebellar GABAergic interneurons. J Neurosci. 2009;29:7079–7091. doi: 10.1523/JNEUROSCI.0957-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sudarov A, Turnbull RK, Kim EJ, Lebel-Potter M, Guillemot F, Joyner AL. Ascl1 genetics reveals insights into cerebellum local circuit assembly. J Neurosci. 2011;31:11055–11069. doi: 10.1523/JNEUROSCI.0479-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Luca A, Parmigiani E, Tosatto G, Martire S, Hoshino M, Buffo A, Leto K, Rossi F. Exogenous sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development. Cerebellum. 2015;14:72–85. doi: 10.1007/s12311-014-0596-x. [DOI] [PubMed] [Google Scholar]
- 120.Schilling K, Oberdick J, Rossi F, Baader SL. Besides Purkinje cells and granule neurons: an appraisal of the cell biology of the interneurons of the cerebellar cortex. Histochem Cell Biol. 2008;130:601–615. doi: 10.1007/s00418-008-0483-y. [DOI] [PubMed] [Google Scholar]
- 121.Ramón y Cajal S. Histologie du système nerveux de l’homme et des vertébrés. Paris: Maloine; 1911. [Google Scholar]
- 122.Palay SL, Chan-Palay V. Cerebellar cortex. Berlin: Springer; 1974. [Google Scholar]
- 123.Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol. 2013;109:42–63. doi: 10.1016/j.pneurobio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 124.Yuasa S. Bergmann glial development in the mouse cerebellum as revealed by tenascin expression. Anat Embryol. 1996;194:223–234. doi: 10.1007/BF00187133. [DOI] [PubMed] [Google Scholar]
- 125.Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77:94–108. doi: 10.1046/j.0022-7722.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 126.Mori T, Tanaka K, Buffo A, Wurst W, Kuehn R, Goetz M. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 127.Sotelo C, Alvarado-Mallart RM. The reconstruction of cerebellar circuits. Trends Neurosci. 1991;14(8):350–355. doi: 10.1016/0166-2236(91)90161-M. [DOI] [PubMed] [Google Scholar]
- 128.Rossi F, Borsello T, Strata P. Embryonic Purkinje cells grafted on the surface of the cerebellar cortex integrate in the adult unlesioned cerebellum. Eur J Neurosci. 1992;4(6):589–593. doi: 10.1111/j.1460-9568.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 129.Grimaldi P, Parras C, Guillemot F, Rossi F, Wassef M. Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev Biol. 2009;328:422–433. doi: 10.1016/j.ydbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 130.Wallace VA, Raff MC. A role for sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126(13):2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- 131.Sehgal R, Sheibani N, Rhodes SJ, Belecky Adams TL. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332(2):429–443. doi: 10.1016/j.ydbio.2009.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30(41):13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poncet C, Soula C, Trousse F, Kan P, Hirsinger E, Pourquié O, Duprat AM, Cochard A. Induction of oligodendrocyte progenitors in the trunk neural tube by ventralizing signals: effects of notochord and floor plate grafts, and of sonic hedgehog. Mech Dev. 1996;60(1):13–32. doi: 10.1016/S0925-4773(96)00595-3. [DOI] [PubMed] [Google Scholar]
- 134.Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/S0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 135.Lu Q, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/S0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 136.Alberta JA, Park SK, Mora J, Yuk D, Pawlitzky I, Iannarelli P, Vartanian T, Stiles CD, Rowitch DH. Sonic hedgehog is required during an early phase of oligodendrocyte development in mammalian brain. Mol Cell Neurosci. 2001;18(4):434–441. doi: 10.1006/mcne.2001.1026. [DOI] [PubMed] [Google Scholar]
- 137.Merchán P, Bribián A, Sánchez-Camacho C, Lezameta M, Bovolenta P, de Castro F. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci. 2007;36(3):355–368. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 138.Ortega MC, Cases O, Merchán P, Kozyraki R, Clemente D, de Castro F. Megalin mediates the influence of sonic hedgehog on oligodendrocyte precursor cell migration and proliferation during development. Glia. 2012;60(6):851–866. doi: 10.1002/glia.22316. [DOI] [PubMed] [Google Scholar]
- 139.Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of sonic hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem. 1998;70(3):1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- 140.Fisher M, Trimmer P, Ruthel G. Bergmann glia require continuous association with Purkinje cells for normal phenotype expression. Glia. 1993;8(3):172–182. doi: 10.1002/glia.440080305. [DOI] [PubMed] [Google Scholar]
- 141.Mecklenburg N, Martinez-Lopez JE, Moreno-Bravo JA, Perez-Balaguer A, Puelles E, Martinez S. Growth and differentiation factor 10 (Gdf10) is involved in Bergmann glial cell development under Shh regulation. Glia. 2014;62(10):1713–1723. doi: 10.1002/glia.22710. [DOI] [PubMed] [Google Scholar]
- 142.Bouslama-Oueghlani L, Wehrlé R, Doulazmi M, Chen XR, Jaudon F, Lemaigre-Dubreuil Y, Rivals I, Sotelo C, Dusart I. Purkinje cell maturation participates in the control of oligodendrocyte differentiation: role of sonic hedgehog and vitronectin. PLoS One. 2012;7(11):e49015. doi: 10.1371/journal.pone.0049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361(6409):258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 144.Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16(6):2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137(2):459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2007;3:26. doi: 10.1186/1749-8104-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Northcott PA, Hielscher T, Dubuc A, Mack S, Shih D, Remke M, Al-Halabi H, Albrecht S, Jabado N, Eberhart CG, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropath. 2011;122:231–240. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stütz AM, Korshunov A, Reimand J, Schumacher SE, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aref D, Moffatt CJ, Agnihotri S, Ramaswamy V, Dubuc AM, Northcott PA, Taylor MD, Perry A, Olson JM, Eberhart CG, et al. Canonical TGF-beta pathway activity is a predictor of SHH-driven medulloblastoma survival and delineates putative precursors in cerebellar development. Brain Pathol. 2012;23(2):178–191. doi: 10.1111/j.1750-3639.2012.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TT, Lin SM, Wechsler-Reya RJ. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 153.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 154.Raffel C. Medulloblastoma: molecular genetics and animal models. Neoplasia. 2004;6:310–322. doi: 10.1593/neo.03454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiapa S, Gao L, Lowrance A, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 156.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eberhart CG. Medulloblastoma in Mice Lacking p53 and PARP. All roads lead to Gli. Am J Pathol. 2003;162(1):7–10. doi: 10.1016/S0002-9440(10)63792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, Hansen S, Knoblaugh SE, Lee D, Eberhart CG, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90 % incidence and leptomeningeal spread. Cancer Res. 2008;68(6):1768–1776. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 159.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 160.Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/S0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- 161.Gorlin RJ. Nevoid basal cell carcinoma syndrome. Dermatol Clin. 1995;13:113–125. [PubMed] [Google Scholar]
- 162.Kim J, Nelson AL, Algon SA, Graves O, Sturla LM, Goumnerova LC, Rowitch DH, Segal RA, Pomeroy SL. Medulloblastoma tumorigenesis diverges from cerebellar granule cell differentiation in patched heterozygous mice. Dev Biol. 2003;263:50–66. doi: 10.1016/S0012-1606(03)00434-2. [DOI] [PubMed] [Google Scholar]
- 163.Brugières L, Remenieras A, Pierron G, Varlet P, Forget S, Byrde V, Bombled J, Puget S, Caron O, Dufour C, et al. High frequency of germline SUFU mutations in children with desmoplastic/nodular medulloblastoma younger than 3 years of age. J Clin Oncol. 2012;30:2087–2093. doi: 10.1200/JCO.2011.38.7258. [DOI] [PubMed] [Google Scholar]
- 164.Smith MJ, Beetz C, Williams SG, Bhaskar SS, O’Sullivan J, Anderson B, Daly SB, Urquhart JE, Bholah Z, Oudit D, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–4161. doi: 10.1200/JCO.2014.58.2569. [DOI] [PubMed] [Google Scholar]
- 165.Yoon JW, Gilbertson R, Iannaccone S, Iannaccone P, Walterhouse D. Defining a role for sonic hedgehog pathway activation in desmoplastic medulloblastoma by identifying GLI1 target genes. Int J Cancer. 2008;124:109–119. doi: 10.1002/ijc.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Buczkowicz P, Ma J, Hawkins C. GLI2 is a potential therapeutic target in pediatric medulloblastoma. J Neuropathol Exp Neurol. 2011;70(6):430–437. doi: 10.1097/NEN.0b013e31821b94db. [DOI] [PubMed] [Google Scholar]
- 167.Li P, Du F, Yuelling LW, Lin T, Muradimova RE, Tricarico R, Wang J, Enikolopov G, Bellacosa A, Whechsler-Reya RJ, et al. A population of Nestin-expressing progenitors in the cerebellum exhibits increased tumorigenicity. Nat Neurosci. 2013;16(12):1737–1744. doi: 10.1038/nn.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Park M, Park HJ, Eom HS, Kwon YJ, Park JA, Lim YJ, Yoon JH, Kong SY, Ghim TT, Lee HW, et al. Safety and effects of prophylactic defibrotide for sinusoidal obstruction syndrome in hematopoietic stem cell transplantation. Ann Transplant. 2013;18:36–42. doi: 10.12659/AOT.883811. [DOI] [PubMed] [Google Scholar]