Abstract
Animal models of vitamin A (retinol) deficiency have highlighted its crucial role in reproduction and placentation, whereas an excess of retinoids (structurally or functionally related entities) can cause toxic and teratogenic effects in the embryo and foetus, especially in the first trimester of human pregnancy. Knock-out experimental strategies-targeting retinoid nuclear receptors RARs and RXRs have confirmed that the effects of vitamin A are mediated by retinoic acid (especially all-trans retinoic acid) and that this vitamin is essential for the developmental process. All these data show that the vitamin A pathway and metabolism are as important for the well-being of the foetus, as they are for that of the adult. Accordingly, during this last decade, extensive research on retinoid metabolism has yielded detailed knowledge on all the actors in this pathway, spurring the development of antagonists and agonists for therapeutic and research applications. Natural and synthetic retinoids are currently used in clinical practice, most often on the skin for the treatment of acne, and as anti-oncogenic agents in acute promyelocytic leukaemia. However, because of the toxicity and teratogenicity of retinoids during pregnancy, their pharmacological use needs a sound knowledge of their metabolism, molecular aspects, placental transfer, and action.
Keywords: Vitamin A, Placentation, Retinoid receptors, Agonists, Antagonists
Introduction
Since the demonstration by Hale in 1933 that vitamin A deficiency (VAD) leads to congenital ocular malformations [1], the absolute requirement of vitamin A, and its active derivatives, the retinoic acids (RAs) in a broad range of developmental and physiological processes has largely been documented. For example, vertebrate placentation, morphogenesis, cellular proliferation, differentiation, and apoptosis have been shown to come under the influence of this canonical signalling pathway. This pathway acts through the activation of ligand-dependent nuclear receptors called retinoic acid receptors (RARs) α, β, and γ and retinoid X receptors (RXRs) α, β, and γ. In this review, mammalian placentation and general vitamin A mechanisms of action will be briefly described. The importance of the pathway during mammalian pregnancy and, in particular, human pregnancy, will then be extensively reviewed with a particular emphasis on the pharmacological/pathological aspects of this signalling cascade during life in utero.
Placentation among species
The placenta is a transient but essential organ for the successful maintenance of gestation in mammals [2, 3]. As an autonomous organ, it permits the harmonious development of the embryo/foetus till the term birth. According to the species, the establishment and type of placentation are different, being principally defined by morphological and histological characteristics. Morphologically, the placenta can be diffuse (pig and horse), cotyledonic (ruminants), or disk-shaped (humans, rodents). Histologically, different types of placenta are classified according to the nature and number of tissue layers separating maternal and foetal blood. There are four different types: epitheliochorial (equine), synepitheliochorial (ruminant), endotheliochorial (carnivore), and haemochorial (human and rodent), the most invasive type of placenta [4–6]. As the placental functions are the same across species, many different animal models are currently being studied to gain a fuller understanding of the importance of this organ. For example, the sheep, rabbit, and, especially, the mouse are widely used, because they share some developmental phenomena [7]. The first essential function of the placenta is to connect the foetus physically to the uterine wall and allow the formation of an exchange surface between mother and foetus. Beside this anatomical function, the placenta ensures foetal growth especially by providing the nutrients and metabolites required for foetal development [8]. To this end, maternal blood, rich in nutrients and oxygen, enters the intervillous space through the spiral endometrial arteries, passes into the foetal circulation through the capillary system of the villi and reaches the foetus via the umbilical vein. Metabolic waste products then return to the intervillous space via two umbilical arteries [9]. Thus, these nutrient exchanges are well controlled within the foeto-maternal interface, which becomes a “selective filter”. This interface is also an imperfect barrier against many pathogens, drugs, and toxins principally by the syncytial actin cytoskeleton as described in humans and mice [10, 11]. Among other functions, the placenta also exerts an endocrine action that is essential for the maintenance of pregnancy, foetal maturation, and parturition. For example, it secretes several important gestational hormones, such as progesterone and prostaglandins, into both the maternal and foetal circulation [12].
Through all these functions, the placenta should normally be able to ensure the healthy growth and development of the foetus, but if we consider only human reproduction, this vital process is unfortunately inefficient, as 50–60 % of human embryos die before birth [13, 14]. Placental abnormalities can induce obstetric pathologies, such as spontaneous abortion, intra-uterine growth retardation, or preeclampsia. To gain a better understanding of how the placenta could be involved in these many pregnancy losses and problems, the most widely used strategy is gene inactivation in the mouse model, owing to the similarity of human and mouse placentation and potential extrapolation. Such mutations could result in embryonic and foetal mortality in utero principally by affecting implantation, choriovitelline, or chorioallantoic placentation, in correlation with faulty timing during gestation [15]. Among all the essential genes for placentation demonstrated by KO strategies, those codings for nuclear receptors are absolutely essential.
Nuclear receptors and placentation
General considerations
Nuclear receptors belong to the vast family of transcription factors, and are activated by their cognate ligands. The first complete coding sequences to be isolated were the glucocorticoid [16] and oestrogen [17] receptors. Comparison of these newfound sequences and their crystallographic study [18] showed conserved domains, disclosing the nuclear receptor superfamily.
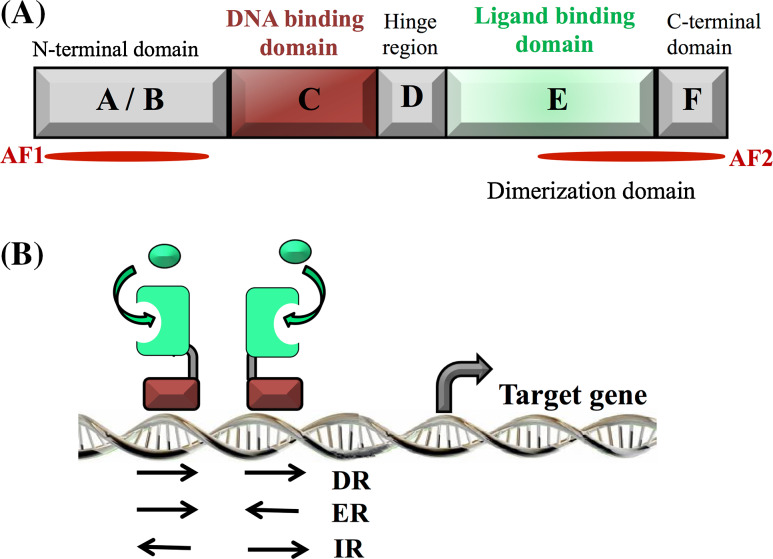
Briefly, nuclear receptors share a common structure composed of six conserved domains named A–F (for supplementary information, see [19]) (Fig. 1). The most important region, designated A/B, contains a ligand-independent transactivation function (AF-1), and constitutes the only variable domain of the nuclear receptors, giving them their own identity and specificity. This domain also often undergoes post-translational modifications. The DBD (or C-region) is highly conserved and confers on the nuclear receptor its specific DNA recognition property and its ability to regulate the transcription of target genes. In detail, this domain is composed of two zinc finger motifs with a spatial structure composed of two α-helices and a COOH-terminal extension (CTE) [20]. Nuclear receptors fall into three subgroups depending on their fixation to DNA and the consensus DNA sequence involved. For steroid receptors, e.g., glucocorticoid, oestrogen, progesterone, and androgen receptors, they act in a homodimer form and bind onto a response element configured as a palindrome (inverted repeat of two hexameric sequences separated by three nucleotide spacers) [21]. Non-steroid receptors, e.g., retinoic acid receptors (RARs), vitamin D receptors (VDRs), liver X receptors (LXRs), thyroid receptors (TRs), and peroxisome proliferator-activated receptors (PPARs) are characterized by their ability to heterodimerise with the retinoid X receptors (RXRs), and unlike the steroid receptor, their response elements are direct repeats separated by a ranging number of bases depending on each nuclear receptor considered [22–24]. Finally, orphan receptors, e.g., steroidogenic factor 1 (SF1), and the steroid hormone receptor ERR2 function as monomers and bind to a consensus sequence of six base pairs, as core motif preceded by three nucleotides, e.g., oestrogen-related receptor alpha (ERRα) recognizes TCAAGGTCA) [25]. The DNA-binding domain (DBD) is connected to the ligand-binding domain (LBD) by the hinge region (noted D-region). The LBD, noted E-region, mainly contains a “ligand pocket”, composed of hydrophobic residues responsible for the specific and unique ligand recognition of each receptor [26]. This domain also contains the dimerization domain and the ligand-dependent transactivation function (AF-2). Both AF-1, described earlier, and AF-2 constitute real interaction interfaces able to recruit many co-regulator partners (repressors and activators) to mediate the transcriptional response [27].
Fig. 1.
a Structural organization of nuclear receptors. The N-terminal domain contains the transactivation ligand-independent domain AF1. The DNA-binding domain (DBD) recognizes a specific sequence on the DNA called hormone responsive element (HRE). The ligand-binding domain, allowing the binding of the ligand(s), also contains the activation function 2 (AF2) and contributes to the dimerization interface. b Binding of nuclear receptors to the hormones responsive elements (HRE). Nuclear receptors bind as homodimer or heterodimer on specific sequences present on the DNA. HRE are composed of a repeat of one core motif that can be direct (DR), everted (ER), or inverted (IR), depending on the nuclear receptor subtype
Nuclear receptors and placentation
It is already well known that this superfamily of nuclear receptors is required for the regulation and homeostasis of several physiological mechanisms, such as metabolism, inflammation, placentation, development, and parturition [28–30]. Concerning placentation and development, the gene inactivation strategy in mice demonstrates that the inactivation of the orphan receptor ERRβ is required for early placentation. Embryo mutants exhibit placental malformations (abnormal chorion and trophoblast) and have been observed to die at 10.5 days postcoitum (dpc) [31]. It has also been demonstrated that the peroxisome proliferator-activated receptor (PPAR), through their pleiotropic functions in the placenta [32], is essential for placental development and functions [33, 34]. In particular, the involvement of PPARδ in implantation and decidualisation has been established in mouse model [35] and in vitro studies have already demonstrated the crucial role of PPARγ in the regulation of human trophoblasts invasion [36, 37]. More importantly, PPARγ null embryos were found to die only due to placental defects, including vascularisation and trophoblastic differentiation abnormalities. Indeed, embryo death in PPARγ−/− was due to defective trophoblast differentiation, since PPARγ−/− mice are viable when the trophectoderm express PPARγ (SOX2cre/PPARL2/L2 mice) [38–40]. All these results were completed in humans by direct relations between the expression level of this nuclear receptor and the appearance of well-known pregnancy pathologies. Nevertheless, these studies remain controversial and need to be deepened. For example, PPARα and γ are down-regulated in the placenta of women with gestational diabetes, whereas these receptors are found up-regulated in the placenta of women with preeclampsia conjugated with intra-uterine growth restriction [41]. At the opposite, it has been demonstrated that the activation of PPARα and γ is weaker among women having a severe preeclamptic pregnancy compare with normal pregnant women [42]. Moreover, another study on PPARγ expression showed that no change was observed between healthy women and women having preeclampsia or intra-uterine growth restriction [43], likewise for LXR (liver X receptor), which seems important for the development of the placenta. At transcriptional and protein levels, LXRα and LXRβ were significantly decreased in placental tissues from mothers with preeclampsia [44]. Moreover, the potential role of LXRβ in the inhibition of human trophoblasts invasion has been described [45]. A partner of both the above nuclear receptors (PPAR and LXR), RXR is also the heterodimer partner of RAR, two receptor families stimulated by active derivatives of vitamin A. The importance of RXRs in mammalian placentation and development has been demonstrated, and a harmonious pregnancy found to require a well-controlled supply and transformation of vitamin A.
Retinoids and reproduction
RAR/RXR and their partners
Two different research groups discovered the first retinoid acid receptor, called RARα, in 1987 [46, 47]. Some years later, other isoforms, RARβ and RARγ, were also identified [48–51]. Finally, retinoid X receptor alpha, the non-exclusive partner of RARs, was also characterized [52], soon joined by two other isoforms logically named RXRβ and RXRγ [53]. RARs and RXRs are highly conserved among mammals, and have specific patterns of expression, especially during embryogenesis, highlighting their important specific functions during mammalian development [54]. Briefly, RARα, RXRα, and RXRβ are ubiquitously expressed in embryonic and adult tissues, whereas RARβ, RARγ, and RXRγ expression is more restricted, and depends on the tissue specificity [55]. It was demonstrated that all isoforms of RXRs and RARs (to a lesser extent) could bind the 9-cis retinoic acid (9cisRA) form ligand, whereas the all-trans retinoic acid (atRA) form could enter the ligand pocket only of the RARs isoforms. Unlike RARs, RXRs are not specific to the retinoic pathway, and can be involved in other signalling by binding either orphan nuclear receptors, e.g., LXR and farnesyl X receptor (FXR), or other ligand-dependent nuclear receptors, such as TRs, VDR or PPARs. As above, among these nuclear receptors, the PPAR family is of particularly interest, because it is first involved in the RA signalling pathway, developing a heterodimer with RXR, binding the 9cisRA ligand. In addition, the subtype PPARβ/δ has been described as binding RA and reveals new RA functions in the regulation of energy homeostasis and insulin response [56].
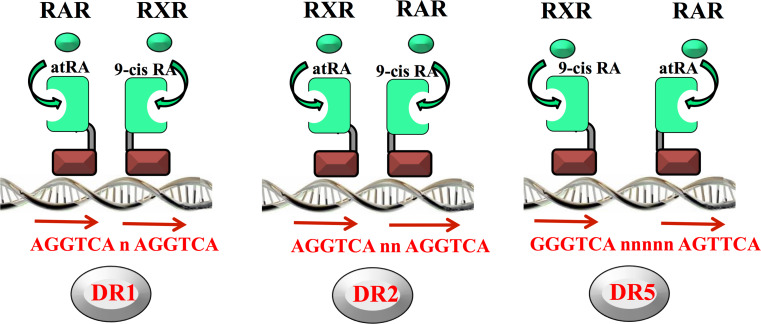
RXR can act as either a homodimer or heterodimer (with RARs) [57]. In the second case, regulation of gene transcription is achieved by the binding of the heterodimer RAR/RXR to a specific promoter sequence called RAREs (retinoid acid response elements) composed classically of two direct repeats of a hexameric motif [58]. The most classical RARE motif is commonly called DR5, where the direct repeats are spaced by five base pairs. However, it can also be a DR1 or DR2, where the direct repeats are separated by only one or two bps, respectively (Fig. 2). These RAREs have now been experimentally verified or identified, using bio-informatics, in promoters of a large and ever-growing number of retinoid-target genes involved in many different physiological functions, in particular, the DR5 type [59].
Fig. 2.
Binding of the heterodimers RAR/RXR to the retinoic acid responsive elements (RARE). RAR and RXR heterodimerize and recognize the direct repeat of the motif 5′-Pu G (G/T)TCA spaced by 1 (DR1), 2 (DR2), or 5 (DR5) nucleotides located in target gene promoters
Vitamin A and development
Retinol (vitamin A) and its active derivatives can underpin many physiological functions (morphogenesis, growth, reproduction, immunity, and vision) by the transduction of their signal using retinoic acid receptors [60]. Although the identification of vitamin A by McCollum and Davis [61] dates back to 1913, the vitamin A signalling pathway and actions are very complex and remain to be more thoroughly studied. The retinoid pathway is critical for harmonious mammalian gestation (normal embryonic, foetal, and neonatal development) as demonstrated in many nutritional studies in animal models [62]. Furthermore, the embryo is unable to synthesize retinol, and is strongly dependent on the maternal delivery of retinol itself or its precursors (retinyl esters and carotenoids) through transplacental transfer. As the foetus grows quickly in the third trimester, it needs vitamin A throughout pregnancy [63]. Consequently, both deficiency and excess of vitamin A cause serious damage during prenatal and postnatal development and during adult life. Nutritional and clinical studies on animals and human models have shown that a vitamin A deficiency leads to VAD syndrome characterized by a number of developmental defects, including ocular defects, infectious diseases, growth delay, respiratory and urogenital system and heart defects, and iron deficiency anemia [64, 65]. Moreover, it is suggested that the high incidence of heart malformations in developing countries could be mostly explained by a low availability of retinol due to vitamin A deficiency in the diet [66]. This has also been shown in the rat model, where mating can induce a failure of reproduction, foetal resorption, and prolonged gestation. Interestingly, an administration of retinoic acid can reverse the detrimental effects of VAD diet. Conversely, an excess of vitamin A in the diet leads to toxicity of many organs like the liver, central nervous system, internal organs, and skin. A high level of retinol during pregnancy is teratogenic for the foetus, especially during the first trimester [67–70]. All these instances of vitamin A deregulation show that this signalling pathway has to be closely regulated, particularly for the bioavailability of active forms, to perfectly ensure these pleiotropic roles during development.
Vitamin A regulation pathway
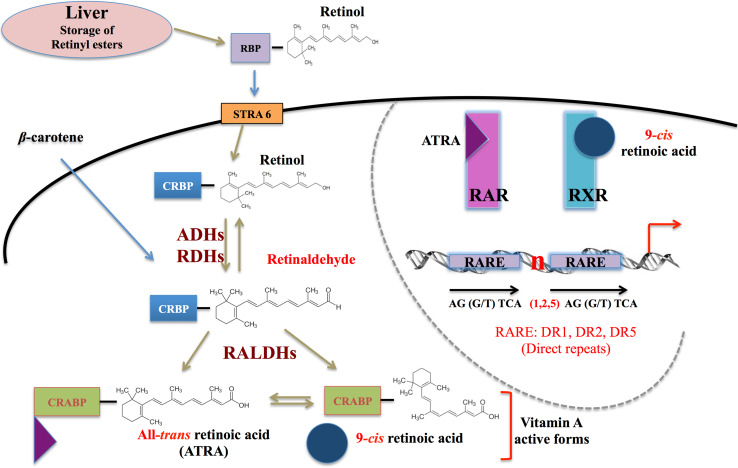
Retinol homeostasis is dependent on several levels of regulation from dietary uptake, via storage, considering the distribution to each tissue, including at the end the production, and degradation of active forms. These different regulation steps involve many proteins and enzymes (a simplified representation is shown in Fig. 3). First of all, vitamin A cannot be synthesized de novo by mammals, and comes exclusively from the diet as β-carotene (plant sources) or retinyl esters (animal sources). Conversions by key enzymes are required to synthesize vitamin A active forms [71]. Retinol is stored in the liver as retinyl esters, or distributed in the blood to target tissues. Because retinol is highly hydrophobic, a carrier protein called retinol-binding protein (RBP) is required for its circulation and recognition by a receptor called STRA6 [72]. This latter is present at the cell surface and allows the retinol to enter the cells, where it is carried mostly by cytoplasmic cellular retinol-binding protein 1 (CRBP1). To be biologically active, retinol must first be oxidized to retinaldehyde, and then to retinoic acid. The first reversible oxidation is catalysed by cytosolic alcohol dehydrogenases (ADHs) and microsomal retinol dehydrogenase (RDH), and the retinaldehyde is then irreversibly oxidized to retinoic acid by several retinal dehydrogenases (RALDHs) [73, 74]. As described above, the pleiotropic effects of retinoids are mediated by the two main endogenous active forms of vitamin A: atRA and 9cisRA, specific ligands of the RAR/RXR heterodimer. In this pathway, catabolism feedback and regulation feedback of atRA and 9cisRA are important mechanisms for controlling endogenous RA levels. To achieve this, catabolism of retinoic acid is under the control of the cytochrome p450 superfamily, and especially CYP26 proteins (CYP26A1, B1, and C1 for mammalian species). This subfamily allows the transformation of atRA into polar metabolites [75, 76]. Furthermore, some of the components of the RA biosynthesis are controlled by atRA via negative feedback. atRA induces its own catabolism by inducing the CYP26 enzyme gene transcription, allowing the degradation of excess atRA. Conversely, atRA can also regulate its biosynthesis by positive feedback, up-regulating the expression of some RA biosynthesis components, such as RBP4, CRBP1, and STRA6 [77, 78].
Fig. 3.
Simplified version of the retinoid pathway. Retinol (combined with retinol-binding protein-RBP) passes through the cellular membrane using the stimulated by retinoic acid 6 receptor (STRA6), is carried by the cellular retinol-binding protein (CRBPs), and transformed into retinaldehyde (Ral) by alcohol dehydrogenase (ADH) or retinol dehydrogenase (RDH). β-Carotene after its entry into the cells is directly transformed into Ral, which is then transformed into retinoic acids (RAs) by retinaldehyde dehydrogenase. RAs are then bound to cellular retinoic acid-binding protein (CRABPs) and enter the nucleus
Vitamin A pathways in development and placentation
Studies of KO members of the vitamin A pathway, especially for RARs and RXRs in mutant mice, have demonstrated their crucial role in functions, such as development and reproduction mediated by vitamin A. Postnatal damage and foetal damage, equivalent to that found in VAD syndrome, were found in RAR or RXR single-mutant mice, but defects are less severe, suggesting a high degree of functional redundancy among these receptors [79, 80]. The absence of one receptor can be partly compensated by another nuclear receptor expressed in the same tissue. More specifically, RARα null mutants die before the age of two months due to severe malformations, and males are sterile through the degeneration of the seminiferous epithelium. RARβ mutant mice are growth-deficient, and present behavioural and eye defects, but are fertile and have a normal longevity. RARγ null mice exhibit foetal malformations due to skeletal and epithelial defects, leading to extensive postnatal lethality [81, 82]. RAR double mutants (RARα/β; RARα/γ and RARβ/γ null mutants) died in utero or shortly after birth, highlighting the essential role of RARs in vertebrate development [83, 84]. Such genetic studies have also been conducted on the other heterodimeric partner, RXR. The knock-out mouse models of RXRα and RXRβ are lethal during embryogenesis or shortly after birth. RXRα−/− mice exhibit eye defects and their mortality is due to severe heart damage (myocardium defect), suggesting a role of RXRα in myocardial growth and eye morphogenesis [85]. RXRβ males are sterile, because of an abnormal spermatogenesis, and present behavioural defects. Finally, RXRγ null mice survive and present not developmental defects, but fertility defects. Defects in double RXR mutants are similar to those in RAR double mutants. For example, the double knock-out RXRβ and RXRγ, and the triple mutant RXRα+/− RXRβ−/− and RXRγ−/− are viable, but have growth defects, and the males are sterile. A single copy of RXRα suffices to rescue most of the functions of RXRs.
Besides the many roles of retinoids described and extensively studied in vertebrate development [85], the molecular role of retinoid signalling during placentation remains imperfectly understood. Several studies conducted on the mouse model demonstrate an involvement of the retinoid pathway in placentation. First, retinoid-binding proteins (RBP) and retinoic acid receptors (RARs and RXRs) have been shown to be expressed in placental mice tissues in a specific spatio-temporal expression pattern [86, 87]. In particular, RARγ expression is increased after 15.5 dpc, whereas it is only weakly expressed during early placentation. A strong expression of RXRα has also been demonstrated at the late stages of placentation, and persists until birth. Expression patterns of these two receptors suggest their involvement in the formation and function of the definitive placenta. In addition to this specific expression pattern of retinoid pathway components, retinoic acid-inducible (STRA) genes have also been shown to exhibit a specific expression in mouse placenta, depending on tissue specificity and placentation stages [88]. Briefly, they are expressed in two major placental components depending on the placental stage: the regions of maternal–embryonic exchanges (yolk sac and labyrinthine zone) and the trophoblastic giant cells. For example, from mid-late gestation until birth, Stra6 was no longer expressed in the yolk sac membrane, but its expression was detected in the labyrinthine zone of the chorioallantoic placenta. Such spatio-temporal patterns of expression are observed for most of the genes studied during both yolk sac (primitive placenta) and chorioallantoic (definitive placenta) placentation. The way in which the expression of these retinoic acid-inducible genes (STRA) is controlled during placentation remains unknown, but the important role of the retinoid pathway for normal placentation has clearly been demonstrated in mice. VAD in rats [89], and PPARγ [38], RXRα, and β [90, 91] knock-out in mice led to chorioallantoic placentation defects resulting in embryo death. RXR being essential for heterodimerization with PPARγ, all these knock-out receptors contribute to the same defect. In more detail, they alter the formation of the labyrinth, a crucial structure for mice placentation, allowing gas, and nutrient exchange between the mother and the foetus in the placenta. In the heterozygous phenotype, the labyrinth was not totally affected, resulting in the reduction of transfers between mother and conceptus and intra-uterine growth retardation. When the placental defect is total in the homozygous situation, the transfers are completely abolished, causing in utero death.
In humans, the retinoid pathway has been described as present in the placenta and in foetal membranes. Several studies of the human placenta indicate its ability to isomerize and oxidize retinoids [92, 93]. Studies also strongly suggest the contribution of retinoids in the regulation of human placental lactogen (hPL) and other hormones or target genes fundamental for the maintenance of pregnancy [94, 95], e.g., chorionic gonadotropin hormone [36, 96], epidermal growth factor receptor [97], apolipoprotein A-II [98], the thyroid hormone receptor Mct8 [99], and leptin [100]. By the heterodimer PPAR/RXR, retinoids regulate some important placental phenomena, such as trophoblast invasion [36] and the processes of human term parturition [101]. As expected, their abnormal expression or activities are described in spontaneous abortion [102], in preterm labour, and in infection-complicated preterm deliveries [103]. In addition, an abnormal activation of RXR by the tributyltin (an environmental contaminant) was found to alter human placental functions [104]. In the foetal membranes, the functionality of the retinoid pathway [105] and its role in physiopathology have been demonstrated. Retinoids regulate the expression of genes involved in both amniotic fluid homeostasis [Prat et al., unpublished data] and extracellular matrix dynamics [106].
Pharmaceutical aspects
Agonists/antagonists of the vitamin A pathway and examples of clinical applications
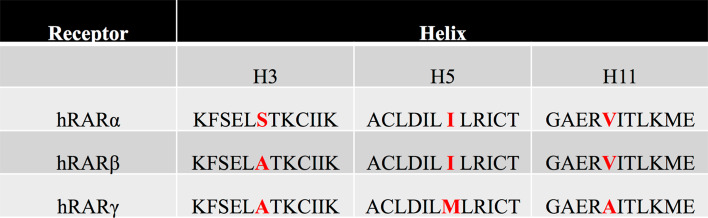
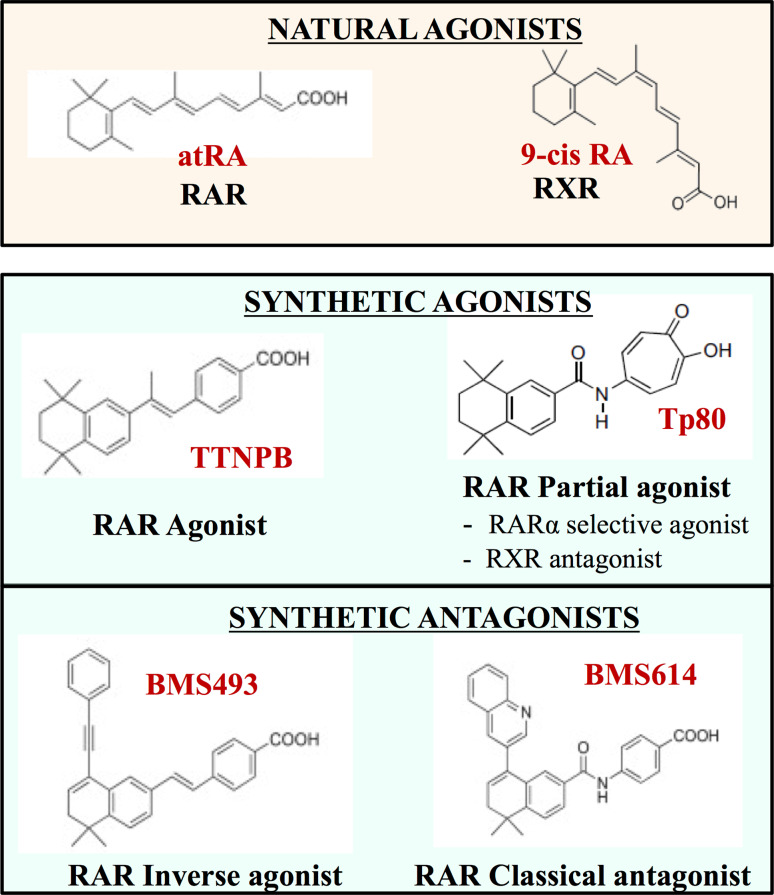
For a better understanding of these nuclear receptors (specific biological responses of retinoid receptor isotypes), and for therapeutic applications in several diseases, e.g., acne, psoriasis, cancer, acute promyelocytic leukaemia (APL), and photo-aging, retinoid receptors have become new targets for the development of several synthetic retinoid agonists (mimicking the action of endogenous ligands), and antagonists (blocking the action of endogenous ligands) [107–109]. A major problem in such a strategy is that it generates synthetic ligands specifically targeting one isotype of RARs or RXRs, and it is extremely difficult due to the closed LBD domain between the different subtypes α, β, and γ. As an illustration of this, only one residue in the ligand-binding pocket (LBP) differs between RARβ and RARα, and only two between RARβ and RARγ (Fig. 4). This major problem could be by-passed using crystallographic studies of the empty ligand-binding domain (apo-receptor) compared with the filled receptor (halo-receptor) and allowed a better understanding of the binding mechanism to develop synthetic agonists or antagonists of nuclear receptors [110, 111]. In a cellular environment, in the absence of endogenous ligands (natural agonists, such as atRA) or in the presence of certain antagonists, RAR-RXR heterodimers recruit corepressor (CoR) complexes, associated with histone deacetylases (HDACs), which cause transcriptional silencing through chromatin condensation [112]. Thus, the ligand binding leads to a conformational change in the nuclear receptor LBD domain [113]. This allosteric change alters the ability of the receptor surface to interact with CoRs, and allows the recruitment of coactivator (CoA) complexes on the LBD domain, which contains histone acetyltransferases (HATs) and so result in active transcription through chromatin decondensation. More precisely, the ligand binding really modulates the communication between the nuclear receptor and its intracellular environment (receptor-protein, receptor-DNA, receptor-chromatin interaction) [114]. Focusing on synthetic compounds, there are actually different classes of agonists and antagonists according to their mechanism of action (Fig. 5) [115]. Whereas stilbene-based retinoid TTNPB (4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl] benzoic acid) and CD3254 are examples of RAR and RXR agonists, respectively, “partial agonists” are considered as weaker agonists than the endogenous ligands, and can act as antagonists. For their classification, the “neutral” or “classical” ones prevent the recruitment of CoAs and maintain the receptor in an inactive state (such as for example BSM614), whereas “inverse agonists”, such as BMS493, exert their antagonist action by strengthening the interaction of receptors with CoRs [116].
Fig. 4.
Sequence alignment of LBD regions of RARα, β, and γ. These regions contain the three divergent amino acid residues responsible for the retinoic acid receptor isotype specificity. These residues belong to the helix 3, 5, and 11 within the ligand-binding pocket, which is composed of 12 α-helix units arranged in a 3D conformation and separated by β-sheets
Fig. 5.
Examples of agonists/antagonists of RARs and RXRs. AtRA and 9cisRA are the natural agonists of RAR and RXR, respectively: these endogenous ligands exert a physiological agonist action on the receptors. Synthetic agonists/antagonists are shown in blue; different classes of agonists and antagonists are presented and illustrated by one example
From the clinical and pharmaceutical points of view, generation of new synthetic ligands for RARs and RXRs is highly developed for “retinoid therapies” to optimize already demonstrated beneficial effects, and, in particular, to eliminate or minimize the side effects of these drugs. Retinoids used in therapies are powerful tools to improve cell differentiation, decrease cell proliferation, and promote cell death. However, retinoids can also exhibit teratogenicity (see below) and produce side effects in patients (e.g., mucocutaneous toxicity characterized by sore, dry skin, as particularly demonstrated for RARγ targets) [117]. All these negative clinical effects, added to biological ones (thrombopenia or blood lipid homeostasis abnormalities), constitute the “retinoic acid syndrome” potentially present after chronic retinoid administration. Several retinoid ligands have been developed and used to treat psoriasis: etretinate shows pro-differentiation and anti-inflammatory effects, and after a treatment of 8 months decreases the psoriatic plaque by 64 % on average [118]. Acitretin is the treatment of choice for pustular psoriasis, and can be used in combination with another treatment to improve efficacy for psoriatic plaque. It is well tolerated at low doses, and it is not immunosuppressive [119, 120]. Finally, clinical studies have also shown that TTNPB, a pan-RAR agonist, is a good candidate for the treatment of psoriasis, but has mucocutaneous side effects [121]. Retinoids are also major targets in cancer research, because of their antiproliferative and cancer-preventive properties: RARβ has already been demonstrated to be the “major actor” in the anticancer action of retinoids, as it presents some tumour-suppressive properties [122]. It has been proved that RARβ is reduced or absent during cancer progression (breast cancer, head and neck tumours, oral leukoplakia) [123, 124], and that the restoration of normal RARβ expression can inhibit, through retinoid action, cancer progression and thus constitute a new therapy. For example, the use of isotretinoin in the treatment of oral leukoplakia allows restoration of a normal RARβ expression [125]. Retinoic acids (all-trans and 13-cis forms) are also known for the therapeutic revolution they have brought to acute promyelocytic leukaemia. By destabilizing the haemopoietic cells presenting the chromosomal abnormalities “PML-RARalpha”, the retinoic acids induce the differentiation of cancer cells and cure this leukaemia, which survey results had previously shown to be a very pejorative disorder [126]. Finally, in dermatology, isotretinoin (13cisRA) was the first and is still the main drug used in the treatment of severe recalcitrant acne, and success rates are ≥60 % following an oral dose of isotretinoin for 3–4 months [127]. Because of common side effects (eye irritation, serum lipid alterations, and mucocutaneous reactions from ATRA syndrome), this drug should be reserved for patients with severe conditions who are unresponsive to the conventional therapy [128]. Adapalene® (differin), a naphthoic acid derivative with retinoid-like activity, is also used as an anti-acne drug. Several studies have shown that adapalene 0.1 % gel has the same efficacy as tretinoin (atRA) 0.025 % gel, but a better tolerability than isotretinoin (13-cisRA) [129].
Retinoid placental transfer and toxicity
In 1982, the first published scientific work established the dynamics of the transfer of retinol between foetuses and their mothers through the placenta in the rat [130] and sheep [131]. Some years later, the placental transfer of retinoic acid and retinyl acetate was also demonstrated in pregnant rats [132]. It is now well established that the uptake of dietary retinoids at the maternal–foetal barrier involves two pathways: the RBP-retinol complex through the STRA6 and the retinyl esters bound to lipoproteins modified by lipoprotein lipase [133]. The human placenta is also able to esterify retinol and convert it into retinoic acid [134]. A decrease in placental transfer of retinol was proposed to explain the intra-uterine growth restrictions [135]. In a toxic model of congenital diaphragmatic hernia (foetal lung abnormalities), the mobilization of retinol from placenta to foetal circulation was disturbed [136].
The use of RAR agonists/antagonists as therapeutic agents during pregnancy has widely been discussed and strongly depends on the stage of pregnancy. The toxicity of retinoids during the first trimester has abundantly been demonstrated [137], but is more controversial at later stages, i.e., second and third trimesters. In later pregnancy, several studies on animal models show that teratogenic consequences are strongly dependent on the chemical structure of the retinoid analogue. These differences are partly due to binding affinities for both cellular retinoic-acid-binding proteins (CRABPs) and later to the pathway for the nuclear receptors themselves. For example, placental transfer of 13-cisRA, which does not bind to CRABP, is less efficient than for atRA, which passes rapidly through the placenta and reaches the developing embryo, causing teratogenicity from a low concentration [138, 139]. However, 13-cisRA can be highly teratogen by its spontaneous isomerisation in atRA which passes through the placenta. This isomer can also exert his action by his fixation on membrane receptors and his ability to inhibit key enzymes of the retinoid pathway [140, 141]. Such pharmacokinetic differences between isomers are the first explanation for why one agonist may be less toxic than another (e.g. 13-cisRA compared with atRA). It seems that this explanation can be extended to all the cis isoforms of retinoids, as also 9-cis [142]. Nevertheless, continuous isomerisation of cis isoforms (for example 13-cis into all-trans RA) during placental transfer could explain why even if these isoforms are not themselves teratogenic, their conversion can generate some compounds that are toxic for the embryo [139]. Another explanation for the different negative effects of retinoids during organogenesis may be their frequency of administration. It has clearly been demonstrated that multiple administration of all-trans retinoic acid inducing its own elimination pathways (via Cyp26 degradation enzymes) results in substantially lowered blood levels in the embryo, and teratogenicity [143]. The synthetic agonist CD394, which is also unable to bind to CRABP, has pharmacokinetics close to 13-cisRA [144]. Ro 23-9223, a highly lipophilic aromatic retinoid, presents a reduced placental transfer and is established as four times less teratogenic than 13-cis RA for the same orally administered quantity [145]. By contrast, some agonists do not present any correlation between their toxic potency and their ability to bind the binding proteins and/or receptors. For example, the synthetic agonist TTNPB binds to RARα, RARβ, and CRABP with a lower affinity than atRA [146], and yet, its teratogenic potency is 700 times higher than atRA in mice [147]. This may be due to a lower metabolic degradation of some synthetic ligands compared with the natural retinoids [148]. Another point in terms of potential teratogenicity has to be considered, namely receptor-binding affinity. This was demonstrated using three RAR agonists: CD336, CD2019, and CD437 for alpha, beta, and gamma isoforms, respectively. The teratogenic potential of these three RAR agonists was clearly established as the resultant of the placental transfer properties added to the receptor-binding activities linked to the tissue regioselectivity of each entity [149].
It is generally difficult to predict drug teratogenesis in humans from these animal studies, because of the many differences in drug pharmacokinetics between species that strongly depend on the placentation type [150, 151]. Teratogenesis studies have been conducted on a wide range of animal models, and so far, none of the test animals have proved to offer a reliable model to predict teratogenesis in humans. For example, rats, which historically have been the most extensively studied model, exhibit low teratogenicity for cortisone [152], thalidomide, and trimethadione [153], which are nonetheless highly teratogenic for humans. Among non-rodent species, rabbits have been routinely used for teratogenic studies, but in the same way, some important human teratogens, such as alcohol, exhibit a poor teratogenic response in the rabbit model [154]. Surprisingly, these differences from humans are also observed in primate models, e.g., methotrexate is weakly teratogenic in macaque and rhesus monkey models, whereas it has been demonstrated to be severely teratogenic at low concentrations in humans and rats [155]. Owing to these differences between species, identifying teratogens in humans remains difficult, and unfortunately, mostly happens after detrimental outcomes have been recorded in clinical reports and observations of individual cases. The most serious adverse effects have been described for the isotretinoin drug (13-cis retinoic acid or accutane). This substance, approved in 1982 for the treatment of severe recalcitrant nodular acne, was responsible within the first year of its commercialisation for 29 cases of adverse reproductive outcomes in pregnant women who took isotretinoin during the first trimester of gestation [156]. It was reported that this chemical-induced abortion and several foetal malformations, including cardiovascular, central nervous system, and craniofacial defects, known as retinoid acid embryopathy [157]. As a consequence of this finding, women were advised to take contraception in association with treatment by isotretinoin or to stop any such treatment if they were seeking to become pregnant. Such recommendations have also been made from results concerning the half-lives of isotretinoin (10–20 h) and its metabolite, 4-oxo-isotretinoin (17–50 h), which is quite short. Thus, it is recommended to wait five times the half-life (i.e. 1 month) for safe conception to return to the baseline of this drug and its metabolite [158, 159].
What possibilities of treatment during pregnancy?
As described above, beside the toxicity of RAR agonists during pregnancy, and especially during the first trimester, the use of such agonists remains possible in some clinical emergency cases during the second and the third trimesters [160]. This would be the case for women diagnosed for acute promyelocytic leukaemia (APL) during pregnancy. The standard treatment of APL for newly diagnosed non-pregnant patients consists in administering atRA combined with anthracycline-based chemotherapy. This therapeutic protocol exhibits high efficacy, with 90–95 % complete remission [161]. Considering that APL diagnosis is a medical emergency, its management becomes a real clinical challenge when it occurs during the first trimester of pregnancy. Given the high teratogenic potency of RAR agonists at this stage (demonstrated for 13-cisRA), the usual APL treatment with atRA administration could not be conducted without termination of pregnancy decided by the patient. However, if the diagnosis is made at later stages of pregnancy, the current therapy with atRA administration becomes possible and presents only a low teratogenic risk. To date, no malformations related to retinoid embryopathies have been reported for mothers treated with atRA at the second and third trimesters [162], arguing in this case for a possible use of RAR agonists during later stages of pregnancy. Given the important implications of the RXR-PPARs in the obstetric pathology preeclampsia, some questions arise about using RXR agonists to modulate the activation of this heterodimer. Owing to the banning of PPAR agonists during pregnancy, the use of RXR agonists might offer a useful opportunity. In fact, several in vivo and in vitro studies have already demonstrated that RXR agonist and antagonist can be useful in the treatment for cancer [163] Alzheimer’s disease [164], endocrine disorders, and metabolic syndrome [165]. Nevertheless, the use of RXRs as pharmacological targets remains difficult, because of its pleiotropic roles involving numerous heterodimeric partners. Indeed, many agonists induced side effects, such as blood triglyceride elevation or hypothyroidism [166, 167]. Nowadays, the only agonist used in clinical practice is LGD1069 (12) in the treatment of cutaneous T-cell lymphoma [168]. Studies of mouse model permitted to develop specific agonists targeting only one heterodimer of RXR and may further overcome the adverse effects cited above [169, 170]. The same questions arise with respect to some specific aspects of chorioamnionitis, where partners of RARs and RXRs are also described.
Conclusive remarks
The retinoids are deeply involved in the development of the mammalian embryo and foetus. The nuclear retinoid signalling pathway is very complex in its metabolic and molecular aspects, with the involvement of other nuclear receptors when RXRs are present. Pharmacological properties of natural and/or synthetic retinoids are difficult to model during gestation, due to complex links with placentation. Taken together, these points highlight the difficulties in using such natural compounds or synthetic ligands during pregnancy in the case of obstetric pathologies or treatment of maternal diseases already present before conception.
Acknowledgments
A. C. was supported by a THEA laboratories grant. M. R. was supported by a thesis grant from the French Ministry of Education and Research (MENRT).
Footnotes
A. Comptour and M. Rouzaire contributed equally to this work.
References
- 1.Hale F. Pigs born without eyeballs. J Heredity. 1933;24:105. [Google Scholar]
- 2.Deligdish L. Pathology of the placenta. In: Pasqualini JRT, Scholler R, editors. Hormone and fetal pathophysiology. New York: Marcel Dekker Inc.; 1992. pp. 203–249. [Google Scholar]
- 3.Rinkenberger JL, Cross JC, Werb Z. Molecular genetics of implantation in the mouse. Dev Genet. 1997;21:6–20. doi: 10.1002/(SICI)1520-6408(1997)21:1<6::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann P, Burton G. Anatomy and genesis of the placenta. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. New York: Raven Press Ltd; 1994. pp. 441–484. [Google Scholar]
- 5.Enders AC, Blankenship TN. Interstitial trophoblast cells: an enigmatic and variable component of the developing macaque placenta. Placenta. 2012;9:672–676. doi: 10.1016/j.placenta.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Enders AC, Carter AM. The evolving placenta: convergent evolution of variations in the endotheliochorial relationship. Placenta. 2012;5:319–326. doi: 10.1016/j.placenta.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Carter AM, Croy BA, Dantzer V, Enders AC, Hayakawa S, Mess A, Soma H (2007) Comparative aspects of placental evolution: a workshop report. Placenta, pp 129–132 [DOI] [PubMed]
- 8.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD. Fetal growth and placental function. Mol Cell Endocrinol. 1998;140:115–120. doi: 10.1016/S0303-7207(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 9.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Zeldovich VB, Clausen CH, Bradford E, Fletcher DA, Maltepe E, Robbins JR, Bakardjiev AI. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013;9:12. doi: 10.1371/journal.ppat.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caserta D, Graziano A, Monte GL, Bordi G, Moscarini M. Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci. 2013;17:2198–2206. [PubMed] [Google Scholar]
- 12.Evain-Brion D, Malassine A. Human placenta as an endocrine organ. Growth Horm IGF Res. 2003;13:34–37. doi: 10.1016/S1096-6374(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 13.Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. Early embryonic mortality in women. Fertil Steril. 1982;4:447–453. doi: 10.1016/S0015-0282(16)46579-9. [DOI] [PubMed] [Google Scholar]
- 14.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;3:503–509. doi: 10.1016/S0015-0282(16)58144-8. [DOI] [PubMed] [Google Scholar]
- 15.Sapin V, Blanchon L, Serre AF, Lémery D, Dastugue B, Ward SW. Use of transgenic mice model for understanding the placentation: towards clinical applications in human obstetrical pathologies? Transgenic Res. 2001;10:377–398. doi: 10.1023/A:1012085713898. [DOI] [PubMed] [Google Scholar]
- 16.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 18.Khan SH, Arnott JA, Kumar R. Naturally occurring osmolyte, trehalose induces functional conformation in an intrinsically disordered activation domain of glucocorticoid receptor. PLoS One. 2011;6:e19689. doi: 10.1371/journal.pone.0019689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Zechel C, Shen XQ, Chambon P, Gronemeyer H. Dimerization interfaces formed between the DNA binding domains determine the cooperative binding of RXR/RAR and RXR/TR heterodimers to DR5 and DR4 elements. EMBO J. 1994;13:1414–1424. doi: 10.1002/j.1460-2075.1994.tb06395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991;5:2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- 22.Rastinejad F, Perlmann T, Evans RM, Sigler PB. Structural determinants of nuclear receptor assembly on DNA direct repeats. Nature. 1995;375:203–211. doi: 10.1038/375203a0. [DOI] [PubMed] [Google Scholar]
- 23.Koenig RJ, Brent GA, Warne RL, Larsen PR, Moore DD. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc Natl Acad Sci. 1987;84:5670–5674. doi: 10.1073/pnas.84.16.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sladek R, Bader JA, Giguère V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. doi: 10.1128/MCB.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaholz BP, Renaud JP, Mitschler A, Zusi C, Chambon P, Gronemeyer H, Moras D. Conformational adaptation of agonists to the human nuclear receptor RAR gamma. Nat Struct Biol. 1998;5:199–202. doi: 10.1038/nsb0398-199. [DOI] [PubMed] [Google Scholar]
- 27.Lavery DN, McEwan IJ. Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J. 2005;391:449–464. doi: 10.1042/BJ20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoeven G, Willems A, Denolet E, Swinnen JV, DeGendt K. Androgens and spermatogenesis: lessons from transgenic mouse models. Philos Trans R Soc Lond B Biol Sci. 2010;365:1537–1556. doi: 10.1098/rstb.2009.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borel V, Gallot D, Marceau G, Sapin V, Blanchon L. Placental implications of peroxisome proliferator-activated receptors in gestation and parturition. PPAR Res. 2008;2008:758562. doi: 10.1155/2008/758562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serpente P, Tümpel S, Ghyselinck NB, Niederreither K, Wiedemann LM, Dollé P, Chambon P, Krumlauf R, Gould AP. Direct crossregulation between retinoic acid receptor beta and Hox genes during hindbrain segmentation. Development. 2005;132:503–513. doi: 10.1242/dev.01593. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 32.Schaiff WT, Barak Y, Sadovsky Y. The pleiotropic function of PPAR gamma in the placenta. Mol Cell Endocrinol. 2006;249(1–2):10–15. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Barak Y, Sadovsky Y, Shalom-Barak T. PPAR signaling in placental development and function. PPAR Res. 2008;2008:142082. doi: 10.1155/2008/142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthi P, Kalionis B, Cocquebert M, Rajaraman G, Chui A, Keogh RJ, Evain-Brion D, Fournier T. Homeobox genes and down-stream transcription factor PPARγ in normal and pathological human placental development. Placenta. 2013;34(4):299–309. doi: 10.1016/j.placenta.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Ding NZ, Teng CB, Ma H, Ni H, Ma XH, Xu LB, Yang ZM. Peroxisome proliferator-activated receptor delta expression and regulation in mouse uterus during embryo implantation and decidualization. Mol Reprod Dev. 2003;66:218–224. doi: 10.1002/mrd.10348. [DOI] [PubMed] [Google Scholar]
- 36.Tarrade A, Schoonjans K, Pavan L, Auwerx J, Rochette-Egly C, Evain-Brion D, Fournier T. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab. 2001;86:5017–5024. doi: 10.1210/jcem.86.10.7924. [DOI] [PubMed] [Google Scholar]
- 37.Fournier T, Handschuh K, Tsatsaris V, Evain-Brion D. Involvement of PPARgamma in human trophoblast invasion. Placenta. 2007;28 Suppl A:S76–S81. doi: 10.1016/j.placenta.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/S1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 39.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4(4):597–609. doi: 10.1016/S1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 40.Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, Desvergne B. PPARgamma in placental angiogenesis. Endocrinology. 2010;151(10):4969–4981. doi: 10.1210/en.2010-0131. [DOI] [PubMed] [Google Scholar]
- 41.Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M, Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–229. doi: 10.1016/j.placenta.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Waite LL, Louie RE, Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. J Clin Endocrinol Metab. 2005;90(2):620–626. doi: 10.1210/jc.2004-0849. [DOI] [PubMed] [Google Scholar]
- 43.Rodie VA, Young A, Jordan F, Sattar N, Greer IA, Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig. 2005;12(5):320–329. doi: 10.1016/j.jsgi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Weedon-Fekjær MS, Johnsen GM, Anthonisen EH, Sugulle M, Nebb HI, Duttaroy AK, Staff AC. Expression of liver X receptors in pregnancies complicated by preeclampsia. Placenta. 2010;31:818–824. doi: 10.1016/j.placenta.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Pavan L, Hermouet A, Tsatsaris V, Thérond P, Sawamura T, Evain-Brion D, Fournier T. Lipids from oxidized low-density lipoprotein modulate human trophoblast invasion: involvement of nuclear liver X receptors. Endocrinology. 2004;145(10):4583–4591. doi: 10.1210/en.2003-1747. [DOI] [PubMed] [Google Scholar]
- 46.Petkovich M, Brand NJ, Krust A, Chambon P. Human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 47.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 48.Brand N, Petkovich M, Krust A, Chambon P, de Thé H, Marchio A, Tiollais P, Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 49.Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- 50.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 51.Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci. 1989;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 53.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–954. [PubMed] [Google Scholar]
- 54.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub M-P, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dollé P. Developmental expression of retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor/and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 58.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17(10):427–433. doi: 10.1016/0968-0004(92)90014-Z. [DOI] [PubMed] [Google Scholar]
- 59.Lalevee S, Anno YN, Chatagnon A, Samarut E, Poch O, Laudet V, Benoit G, Lecompte O, Rochette-Egly C. Genome-wide in silico identification of new conserved and functional retinoic acid receptor response elements (direct repeats separated by 5 bp) J Biol Chem. 2011;286:33322–33334. doi: 10.1074/jbc.M111.263681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 61.McCollum EV, Davis M. The necessity of certain lipids in the diet during growth. J Biol Chem. 1913;15:167–175. [Google Scholar]
- 62.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 63.Venkatachalam PS, Belavady B, Gopalan C. Studies on vitamin A nutritional status of mothers and infants in poor communities of India. J Pediatr. 1962;61:262–268. doi: 10.1016/S0022-3476(62)80261-3. [DOI] [PubMed] [Google Scholar]
- 64.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 65.Mason KE. Fetal death, prolonged gestation, and difficult parturition in the rat as a result of vitamin A-deficiency. Am J Anat. 1935;57:303–349. doi: 10.1002/aja.1000570204. [DOI] [Google Scholar]
- 66.Sommer A, Tarwotjo I, Djunaedi E, West KP, Jr, Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169–1173. doi: 10.1016/S0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- 67.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 68.Cohlan SQ. Excessive intake of vitamin A as a cause of congenital anomalies in the rat. Science. 1953;117:535–536. doi: 10.1126/science.117.3046.535. [DOI] [PubMed] [Google Scholar]
- 69.Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, Nieuwkoop PD. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 70.Avantaggiato V, Acampora D, Tuorto F, Simeone A. Retinoic acid induces stage-specific repatterning of the rostral central nervous system. Dev Biol. 1996;175(2):347–357. doi: 10.1006/dbio.1996.0120. [DOI] [PubMed] [Google Scholar]
- 71.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 73.Parés X, Farrés J, Kedishvili N, Duester G. Medium- and short-chain dehydrogenase/reductase gene and protein families: medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci. 2008;65:3936–3949. doi: 10.1007/s00018-008-8591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 75.Chithalen JV, Luu L, Petkovich M, Jones G. HPLC-MS/MS analysis of the products generated from all-trans-retinoic acid using recombinant human CYP26A. J Lipid Res. 2002;43:1133–1142. doi: 10.1194/jlr.M100343-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Reijntjes S, Blentic A, Gale E, Maden M. The control of morphogen signalling: regulation of the synthesis and catabolism of retinoic acid in the developing embryo. Dev Biol. 2005;285:224–237. doi: 10.1016/j.ydbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 77.Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res. 2010;51:378–387. doi: 10.1194/jlr.M001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Décimo D, Dollé P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/S0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 79.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 80.Mark M, Chambon P. Functions of RARs and RXRs in vivo: genetic dissection of the retinoid signaling pathway. Pure Appl Chem. 2003;75:1709–1732. doi: 10.1351/pac200375111709. [DOI] [Google Scholar]
- 81.Mark M, Ghyselinck N, Chambon P. Function of retinoic acid receptors during embryonic development. J Nucl Recept Signal Atlas. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 83.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 84.Luo J, Sucov HM, Bader JA, Evans RM, Giguère V. Compound mutants for retinoic acid receptor (RAR) beta and RAR alpha 1 reveal developmental functions for multiple RAR beta isoforms. Mech Dev. 1996;55:33–44. doi: 10.1016/0925-4773(95)00488-2. [DOI] [PubMed] [Google Scholar]
- 85.Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR α developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 86.Conlon RA. Retinoic acid and pattern formation in vertebrates. Trends Genet. 1995;11:314–319. doi: 10.1016/S0168-9525(00)89089-7. [DOI] [PubMed] [Google Scholar]
- 87.Sapin V, Ward SJ, Bronner S, Chambon P, Dollé P. Differential expression of transcripts encoding retinoid binding proteins and retinoic acid receptors during placentation of the mouse. Dev Dyn. 1997;208:199–210. doi: 10.1002/(SICI)1097-0177(199702)208:2<199::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 88.Sapin V, Bouillet P, Oulad-Abdelghani M, Dastugue B, Chambon P, Dollé P. Differential expression of retinoic acid-inducible (Stra) genes during mouse placentation. Mech Dev. 2000;92:295–299. doi: 10.1016/S0925-4773(00)00241-0. [DOI] [PubMed] [Google Scholar]
- 89.McC Howell, Thompson N, Pitt G. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. J Reprod Fertil. 1964;7:251–258. doi: 10.1530/jrf.0.0070251. [DOI] [PubMed] [Google Scholar]
- 90.Wendling O, Chambon P, Mark M. RXRs are essential for early mouse development and placentogenesis. Proc Natl Acad Sci. 1999;96:547–551. doi: 10.1073/pnas.96.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sapin V, Dollé P, Hindelang C, Kastner P, Chambon P. Defects of the chorioallantoic placenta in mouse RXRalpha null fetuses. Dev Biol. 1997;191:29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- 92.Datta K, Kulkarni AP. Co-oxidation of all-trans retinol acetate by human term placental lipoxygenase and soybean lipoxygenase. Reprod Toxicol. 1996;10:105–112. doi: 10.1016/0890-6238(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 93.Sapin V, Chaïb S, Blanchon L, Alexandre-Gouabau MC, Lémery D, Charbonne F, Gallot D, Jacquetin B, Dastugue B, Azais-Braesco V. Esterification of vitamin A by the human placenta involves villous mesenchymal fibroblasts. Pediatr Res. 2000;48(4):565–572. doi: 10.1203/00006450-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 94.Stephanou A, Handwerger S. Retinoic acid and thyroid hormone regulate placental lactogen expression in human trophoblast cells. Endocrinology. 1995;136(3):933–938. doi: 10.1210/endo.136.3.7867602. [DOI] [PubMed] [Google Scholar]
- 95.Stephanou A, Handwerger S. Identification of a composite steroid hormone response element on the human placental lactogen promoter. Mol Cell Endocrinol. 1995;112(1):123–129. doi: 10.1016/0303-7207(95)03598-2. [DOI] [PubMed] [Google Scholar]
- 96.Guibourdenche J, Alsat E, Soncin F, Rochette-Egly C, Evain-Brion D. Retinoid receptors expression in human term placenta: involvement of RXR alpha in retinoid induced-hCG secretion. J Clin Endocrinol Metab. 1998;4:1384–1387. doi: 10.1210/jcem.83.4.4860. [DOI] [PubMed] [Google Scholar]
- 97.Roulier S, Rochette-Egly C, Rebut-Bonneton C, Porquet D, Evain-Brion D. Nuclear retinoic acid receptor characterization in cultured human trophoblast cells: effect of retinoic acid on epidermal growth factor receptor expression. Mol Cell Endocrinol. 1994;2:165–173. doi: 10.1016/0303-7207(94)90166-X. [DOI] [PubMed] [Google Scholar]
- 98.Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Heyman RA, Staels B, Auwerx J. Retinoids increase human apolipoprotein A-11 expression through activation of the retinoid X receptor but not the retinoic acid receptor. Mol Cell Biol. 1996;7:3350–3360. doi: 10.1128/MCB.16.7.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kogai T, Liu YY, Richter LL, Mody K, Kagechika H, Brent GA. Retinoic acid induces expression of the thyroid hormone transporter, monocarboxylate transporter 8 (Mct8) J Biol Chem. 2010;35:27279–27288. doi: 10.1074/jbc.M110.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hollung K, Rise CP, Drevon CA, Reseland JE. Tissue-specific regulation of leptin expression and secretion by all-trans retinoic acid. J Cell Biochem. 2004;92(2):307–315. doi: 10.1002/jcb.20047. [DOI] [PubMed] [Google Scholar]
- 101.Holdsworth-Carson SJ, Permezel M, Riley C, Rice GE, Lappas M. Peroxisome proliferator-activated receptors and retinoid X receptor-alpha in term human gestational tissues: tissue specific and labour-associated changes. Placenta. 2009;30(2):176–186. doi: 10.1016/j.placenta.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Lin Q, Wang X, Lu P, Sheng Z, Ding C, Hong Y, Li W. Expression of lethal gene mRNA on placenta villi in patients with spontaneous abortion. Zhonghua Fu Chan Ke Za Zhi. 2002;37(9):542–545. [PubMed] [Google Scholar]
- 103.Holdsworth-Carson SJ, Permezel M, Rice GE, Lappas M. Preterm and infection-driven preterm labor: the role of peroxisome proliferator-activated receptors and retinoid X receptor. Reproduction. 2009;137(6):1007–1015. doi: 10.1530/REP-08-0496. [DOI] [PubMed] [Google Scholar]
- 104.Nakanishi T, Nishikawa J, Hiromori Y, Yokoyama H, Koyanagi M, Takasuga S, Ishizaki J, Watanabe M, Isa S, Utoguchi N, Itoh N, Kohno Y, Nishihara T, Tanaka K. Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol Endocrinol. 2005;19(10):2502–2516. doi: 10.1210/me.2004-0397. [DOI] [PubMed] [Google Scholar]
- 105.Marceau G, Gallot D, Borel V, Lémery D, Dastugue B, Dechelotte P, Sapin V. Molecular and metabolic retinoid pathways in human amniotic membranes. Biochem Biophys Res Commun. 2006;346(4):1207–1216. doi: 10.1016/j.bbrc.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 106.Borel V, Marceau G, Gallot D, Blanchon L, Sapin V. Retinoids regulate human amniotic tissue-type plasminogen activator gene by a two-step mechanism. J Cell Mol Med. 2010;6B:1793–1805. doi: 10.1111/j.1582-4934.2009.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Reese D. A screen for disruptors of the retinol (vitamin A) signaling pathway. Birth Defects Res B. 2013;98:276–282. doi: 10.1002/bdrb.21062. [DOI] [PubMed] [Google Scholar]
- 108.Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids. 2012;1821:21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thacher SM, Vasudevan J, Chandraratna RA. Therapeutic applications for ligands of retinoid receptors. Curr Pharm Des. 2000;6(1):25–58. doi: 10.2174/1381612003401415. [DOI] [PubMed] [Google Scholar]
- 110.Germain P, Kammerer S, Pérez E, Peluso-Iltis C, Tortolani D, Zusi FC, Starrett J, Lapointe P, Daris JP, Marinier A. Rational design of RAR-selective ligands revealed by RARβ crystal stucture. EMBO Rep. 2004;5:877–882. doi: 10.1038/sj.embor.7400235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bourguet W, Germain P, Gronemeyer H. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci. 2000;21:381–388. doi: 10.1016/S0165-6147(00)01548-0. [DOI] [PubMed] [Google Scholar]
- 112.Dilworth FJ, Chambon P. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene. 2001;20:3047–3054. doi: 10.1038/sj.onc.1204329. [DOI] [PubMed] [Google Scholar]
- 113.Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378(6558):681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 114.de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov. 2007;6(10):811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 115.Maire A, Alvarez S, Shankaranarayanan P, Lera AR, Bourguet W, Gronemeyer H. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr Top Med Chem. 2012;12(6):505–527. doi: 10.2174/156802612799436687. [DOI] [PubMed] [Google Scholar]
- 116.Germain P, Gaudon C, Pogenberg V, Sanglier S, Van Dorsselaer A, Royer CA, Lazar MA, Bourguet W, Gronemeyer H. Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists. Chem Biol. 2009;16(5):479–489. doi: 10.1016/j.chembiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Elias PM. Retinoid effects on the epidermis. Dermatologica. 1987;1:28–36. doi: 10.1159/000248851. [DOI] [PubMed] [Google Scholar]
- 118.Gottlieb S, Hayes E, Gilleaudeau P, Cardinale I, Gottlieb AB, Krueger JG. Cellular actions of etretinate in psoriasis enhanced epidermal differentiation and reduced cell -mediated inflammation are unexpected outcomes. J Cutan Pathol. 1996;23(5):404–418. doi: 10.1111/j.1600-0560.1996.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 119.Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158(6):1345–1349. doi: 10.1111/j.1365-2133.2008.08564.x. [DOI] [PubMed] [Google Scholar]
- 120.Lee CS, Li K. A review of acitretin for the treatment of psoriasis. Expert Opin Drug Saf. 2009;8(6):769–779. doi: 10.1517/14740330903393732. [DOI] [PubMed] [Google Scholar]
- 121.Mérot Y, Camenzind M, Geiger JM, Saurat JH. Arotinoid ethyl ester (Ro 13-6298): a long term pilot study in various dermatoses. Acta Derm Venereol. 1987;67(3):237–242. [PubMed] [Google Scholar]
- 122.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 123.Widschwendter M, Berger J, Müller HM, Zeimet AG, Marth C. Epigenetic downregulation of the retinoic acid receptor-beta2 gene in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6(2):193–201. doi: 10.1023/A:1011360724350. [DOI] [PubMed] [Google Scholar]
- 124.Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54(13):3580–3587. [PubMed] [Google Scholar]
- 125.Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332(21):1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 126.Ablain J, de Thé H. Retinoic acid signaling in cancer: the parable of acute promyelocytic leukemia. Int J Cancer. 2014;135(10):2262–2272. doi: 10.1002/ijc.29081. [DOI] [PubMed] [Google Scholar]
- 127.Shalita AR, Armstrong RB, Leyden JJ, Pochi PE, Strauss JS. Isotretinoin revisited. Cutis. 1988;42(6A):1–19. [PubMed] [Google Scholar]
- 128.Perry MD, McEvoy GK. Isotretinoin: new therapy for severe acne. Clin Pharmacol. 1983;2(1):12–19. [PubMed] [Google Scholar]
- 129.Brogden RN, Goa KE. Adapalene: a review of its pharmacological properties and clinical potential in the management of mild to moderate acne. Drugs. 1997;53(3):511–519. doi: 10.2165/00003495-199753030-00010. [DOI] [PubMed] [Google Scholar]
- 130.Ismadi SD, Olson JA. Dynamics of the fetal distribution and transfer of vitamin A between rat fetuses and their mother. Int J Vitam Nutr Res. 1982;52(2):112–119. [PubMed] [Google Scholar]
- 131.Donoghue S, Richardson DW, Sklan D, Kronfeld DS. Placental transport of retinol in sheep. J Nutr. 1982;112(11):2197–2203. doi: 10.1093/jn/112.11.2197. [DOI] [PubMed] [Google Scholar]
- 132.Shukla RR, Kumar V, Banerjee R, Misra UK. Placental transfer and fetal distribution of 3H-retinoic acid in rats. Int J Vitam Nutr Res. 1986;56(1):29–33. [PubMed] [Google Scholar]
- 133.Wassef L, Quadro L. Uptake of dietary retinoids at the maternal–fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem. 2011;286(37):32198–32207. doi: 10.1074/jbc.M111.253070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marceau G, Gallot D, Lemery D, Sapin V. Metabolism of retinol during mammalian placental and embryonic development. Vitam Horm. 2007;75:97–115. doi: 10.1016/S0083-6729(06)75004-X. [DOI] [PubMed] [Google Scholar]
- 135.Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E. Enhanced circulating retinol and non-esterified fatty acids in pregnancies complicated with intrauterine growth restriction. Clin Sci (Lond) 2009;118(5):351–358. doi: 10.1042/CS20090292. [DOI] [PubMed] [Google Scholar]
- 136.Kutasy B, Pes L, Friedmacher F, Paradisi F, Puri P. Nitrofen increases total retinol levels in placenta during lung morphogenesis in the nitrofen model of congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30(10):1017–1022. doi: 10.1007/s00383-014-3525-7. [DOI] [PubMed] [Google Scholar]
- 137.Sulik KK, Dehart DB, Rogers JM, Chernoff N. Teratogenicity of low doses of all-trans retinoic acid in presomite mouse embryos. Teratology. 1995;51(6):398–403. doi: 10.1002/tera.1420510605. [DOI] [PubMed] [Google Scholar]
- 138.Creech Kraft J, Kochhar DM, Scott WJ, Jr, Nau H. Low Teratogenicity of 13-cis-retinoic acid (isotretinoin) in the mouse corresponds to low embryo concentrations during organogenesis: comparison to the all-trans isomer. Toxicol Appl Pharmacol. 1987;87:474–482. doi: 10.1016/0041-008X(87)90253-5. [DOI] [PubMed] [Google Scholar]
- 139.Nau H. Teratogenicity of isotretinoin revisited: species variation and the role of all-trans-retinoic acid. J Am Acad Dermatol. 2001;45(5):S183–S187. doi: 10.1067/mjd.2001.113720. [DOI] [PubMed] [Google Scholar]
- 140.Tsukada M, Schröder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, Orfanos CE, Zouboulis CC. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115(2):321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 141.Blaner WS. Cellular metabolism and actions of 13-cis-retinoic acid. J Am Acad Dermatol. 2001;45(5):S129–S135. doi: 10.1067/mjd.2001.113714. [DOI] [PubMed] [Google Scholar]
- 142.Kochhar DM, Jiang H, Penner JD, Heyman RA. Placental transfer and developmental effects of 9-cis retinoic acid in mice. Teratology. 1995;51(4):257–265. doi: 10.1002/tera.1420510411. [DOI] [PubMed] [Google Scholar]
- 143.Collins MD, Tzimas G, Bürgin H, Hummler H, Nau H. Single versus multiple dose administration of all-trans-retinoic acid during organogenesis: differential metabolism and transplacental kinetics in rat and rabbit. Toxicol Appl Pharmacol. 1995;130(1):9–18. doi: 10.1006/taap.1995.1002. [DOI] [PubMed] [Google Scholar]
- 144.Sass JO, Hartmann J, Chahoud I, Shroot B, Nau H. Transplacental pharmacokinetics of a synthetic retinoid which is not bound by mouse embryonic cellular retinoic acid-binding protein. Toxicol Lett. 1995;75(1–3):159–168. doi: 10.1016/0378-4274(94)03175-7. [DOI] [PubMed] [Google Scholar]
- 145.Willhite CC, Lovey A, Eckhoff C. Distribution, teratogenicity, and embryonic delivered dose of retinoid Ro 23-9223. Toxicol Appl Pharmacol. 2000;164(2):171–175. doi: 10.1006/taap.1999.8866. [DOI] [PubMed] [Google Scholar]
- 146.Crettaz M, Baron A, Siegenthaler G, Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kochhar DM, Penner JD. Analysis of high dysmorphogenic activity of Ro 13-6307, a tetramethylated tetralin analog of retinoic acid. Teratology. 1992;45(6):637–645. doi: 10.1002/tera.1420450608. [DOI] [PubMed] [Google Scholar]
- 148.Loeliger P, Bollag W, Mayer H. Arotinoids, a new class of highly active retinoids. Eur J Med Chem. 1980;15:9–15. [Google Scholar]
- 149.Arafa HM, Elmazar MM, Hamada FM, Reichert U, Shroot B, Nau H. Selective agonists of retinoic acid receptors: comparative toxicokinetics and embryonic exposure. Arch Toxicol. 2000;73(10–11):547–556. doi: 10.1007/s002040050007. [DOI] [PubMed] [Google Scholar]
- 150.Brown NA, Fabro S. The value of animal teratogenicity testing for predicting human risk. Clin Obstet Gynecol. 1983;26(2):467–477. doi: 10.1097/00003081-198306000-00028. [DOI] [PubMed] [Google Scholar]
- 151.Schardein JL, Schwetz BA, Kenel MF. Species sensitivities and prediction of teratogenic potential. Environ Health Perspect. 1985;61:55–67. doi: 10.1289/ehp.856155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buresh JJ, Urban TJ. Palatal abnormalities induced by cortisone and corticosterone in the rat. J Acad Gen Dent. 1970;18(2):34–37. [PubMed] [Google Scholar]
- 153.Buttar HS, Dupuis I, Khera KS. Fetotoxicity of trimethadione and paramethadione in rats. Toxicol Appl Pharmacol. 1976;37:126. [Google Scholar]
- 154.Schwetz BA, Smith FA, Staples RE. Teratogenic potential of ethanol in mice, rats and rabbits. Teratology. 1978;18:385–392. doi: 10.1002/tera.1420180313. [DOI] [PubMed] [Google Scholar]
- 155.Neubert D, Merker HJ, Nau H and Langman J (1978) Role of pharmacokinetics in prenatal and perinatal toxicology: third symposium on prenatal development, Berlin, pp 499–506
- 156.Centers for Disease Control Isotretinoin—a newly recognized human teratogen. Morb Mortal Wkly Rep. 1984;33:171–173. [PubMed] [Google Scholar]
- 157.Loureiro KD, Kao KK, Jones KL, Alvarado S, Chavez C, Dick L, Felix R, Johnson D, Chambers CD. Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am J Med Genet A. 2005;136(2):117–121. doi: 10.1002/ajmg.a.30744. [DOI] [PubMed] [Google Scholar]
- 158.Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant woman. Dermatol Ther. 2013;26(4):302–311. doi: 10.1111/dth.12077. [DOI] [PubMed] [Google Scholar]
- 159.Nulman I, Berkovitch M, Klein J, Pastuszak A, Lester RS, Shear N, Koren G. Steady-state pharmacokinetics of isotretinoin and its 4-oxo metabolite: implications for fetal safety. J Clin Pharmacol. 1998;38(10):926–930. doi: 10.1002/j.1552-4604.1998.tb04388.x. [DOI] [PubMed] [Google Scholar]
- 160.Yang D, Hladnik L. Treatment of acute promyelocytic leukemia during pregnancy. Pharmacotherapy. 2009;29(6):709–724. doi: 10.1592/phco.29.6.709. [DOI] [PubMed] [Google Scholar]
- 161.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H, Burnett AK, Lo-Coco F. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 162.Culligan DJ, Merriman L, Kell J, Parker J, Jovanovic JV, Smith N, Grimwade D. The management of acute promyelocytic leucemia presenting during pregnancy. Clin Leukemia. 2007;1:183–191. doi: 10.3816/CLK.2007.n.006. [DOI] [Google Scholar]
- 163.Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6(5):R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shulman AI, Mangelsdorf DJ. Retinoid X receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 166.Lowe MN, Plosker GL. Bexarotene. Am J Clin Dermatol. 2000;1:245–250. doi: 10.2165/00128071-200001040-00006. [DOI] [PubMed] [Google Scholar]
- 167.Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, Duvic M. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340(14):1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]
- 168.Zhang C, Duvic M. Retinoids: therapeutic applications and mechanisms of action in cutaneous T-cell lymphoma. Dermatol Ther. 2003;16:322–330. doi: 10.1111/j.1396-0296.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- 169.Michellys PY, Ardecky RJ, Chen JH, Crombie DL, Etgen GJ, Faul MM, Faulkner AL, Grese TA, Heyman RA, Karanewsky DS, Klausing K, Leibowitz MD, Liu S, Mais DA, Mapes CM, Marschke KB, Reifel-Miller A, Ogilvie KM, Rungta D, Thompson AW, Tyhonas JS, Boehm MF. Novel (2E,4E,6Z)-7-(2-alkoxy-3,5-dialkylbenzene)-3-methylocta-2,4,6-trienoic acid retinoid X receptor modulators are active in models of type 2 diabetes. J Med Chem. 2003;46(13):2683–2696. doi: 10.1021/jm020340q. [DOI] [PubMed] [Google Scholar]
- 170.Michellys PY, Ardecky RJ, Chen JH, D’Arrigo J, Grese TA, Karanewsky DS, Leibowitz MD, Liu S, Mais DA, Mapes CM, Montrose-Rafizadeh C, Ogilvie KM, Reifel-Miller A, Rungta D, Thompson AW, Tyhonas JS, Boehm MF. Design, synthesis, and structure-activity relationship studies of novel 6,7-locked-[7-(2-alkoxy-3,5-dialkylbenzene)-3-methylocta]-2,4,6-trienoic acids. J Med Chem. 2003;46(19):4087–4103. doi: 10.1021/jm020401k. [DOI] [PubMed] [Google Scholar]