Abstract
Neurotrophins and their receptors act as important proliferative and pro-survival factors in a variety of cell types. Neurotrophins are produced by multiple cell types in both pro- and mature forms, and can act in an autocrine or paracrine fashion. The p75NTR and Trk receptors can elicit signalling in response to the presence or absence of their corresponding neurotrophin ligands. This signalling, along with neurotrophin and receptor expression, varies between different cell types. Neurotrophins and their receptors have been shown to be expressed by and elicit signalling in B lymphocytes. In general, most neurotrophins are expressed by activated B-cells and memory B-cells. Likewise, the TrkB95 receptor is seen on activated B-cells, while TrkA and p75NTR are expressed by both resting and active B-cells as well as memory B-cells. Nerve growth factor stimulates B-cell proliferation, memory B-cell survival, antibody production and CD40 expression. Brain-derived neurotrophic factor is involved in B-cell maturation in the bone marrow through TrkB95. Overall neurotrophins and their receptors have been shown to be involved in B-cell proliferation, development, differentiation, antibody secretion and survival. As well as expression and activity in healthy B-cells, the neurotrophins and their receptors can contribute to B-cell malignancies including acute lymphoblastic leukaemia, diffuse large B-cell lymphoma, Burkitt’s lymphoma and multiple myeloma. They are involved in B-cell malignancy survival and potentially in drug resistance.
Keywords: Neurotrophins, Nerve growth factor (NGF), Tropomyosin-related kinase (Trk) receptors, p75NTR, B lymphocytes, B-cell malignancies
Introduction
B lymphocytes are one of the key types of immune cells. Their main role as antibody-producing and memory cells is vital for the proper functioning of the adaptive immune response. Apart from antibody-dependent B-cell receptor signalling to stimulate antibody production, B-cells are also responsive to a variety of cytokines and other growth factors. Neurotrophins are a small family of secreted proteins which are vital for the development, survival and functioning of neurons. Although they were originally discovered for these functions, they have since been shown to affect many other organs and tissues in both health and disease. In particular, neurotrophins are thought to contribute to the survival, growth and metastasis of several types of cancer. There is a growing body of evidence showing that neurotrophins play a role in the normal growth and development of B lymphocytes and that they may also contribute to the development of B lymphoid malignancies and subsequent drug resistance. Here, we review the literature on the role of neurotrophins in B lymphocytes including what is known concerning their role in B-cell malignancies.
B lymphocytes
B lymphocytes are a key component of the immune system and arise from common pluripotent stem cells in the bone marrow. They are distinguished from other lymphocytes by cell surface expression of an antigen-binding protein receptor called the B-cell receptor (BCR). The principal functions of B-cells are to make antibodies in response to antigens, to perform the role of antigen-presenting cells (APCs) and to develop into memory B-cells following activation by antigen interaction. B-cells also release cytokines, which are used for signalling immune regulatory functions [1].
The first steps in B-cell maturation are the assembly of immunoglobulin heavy (IGH) and light chain (IGL) loci, respectively, via V(D)J recombination [2]. Various stages of B-cell maturation are recognised according to how advanced this process is and include Pro-B cells, Pre-B cells, immature and mature B cells. This distinction can be important in correct classification of certain B-cell leukaemias and lymphomas. For example, Pre-B cells express pan B-cell antigens and the immunoglobulin heavy chain is rearranged, but the immunoglobulin light chain is not yet rearranged [3]. Cells with a fully functional BCR are positively selected to migrate into the peripheral lymphoid organs as mature, naïve B cells, whereas cells lacking a functional BCR undergo apoptosis within the bone marrow [4]. For most B-cells, subsequent maturation occurs within the germinal centre of the lymphoid organs following exposure to a foreign antigen, which cross links the BCR, followed by a second co-stimulatory signal from CD4-positive T (follicular helper T cells or Th2 cells) and antigen-presenting cells [5]. Binding of an antigen by the BCR leads to its internalisation, and fragments of the antigen are presented to the T cells by Class II major histocompatibility complex (MHC) [6].
Following exposure to a T-cell-dependent antigen, B-cells become proliferating centroblasts in the germinal centre of lymphoid tissues and eventually mature into centrocytes [7]. While in the germinal centre the processes of somatic hypermutation (SHM) and class switch recombination (CSR) are activated. Centroblasts use SHM to modify the variable regions of their immunoglobulin variable genes producing BCR’s higher affinity for antigen [8]. Following differentiation into centrocytes, B-cells are re-challenged with antigen via interaction with CD4 positive T cells and follicular dendritic cells. Only those B-cells with the highest affinity for antigen are selected to exit the germinal centre and further differentiate into memory B-cells or antibody secreting plasma cells, with the remainder being eliminated by apoptosis [9, 10]. Survival of B-cells with high affinity BCRs results from a variety of microenvironmental signals such as BCR engagement and activation of the CD40 receptor following engagement with CD40 ligand, expressed on CD4 positive cells [11]. These signals induce activation of the NF kappa B pathway which downregulates BCL6, thus turning off proliferation, allowing differentiation with expression of the transcription factor BLIMP1 leading to differentiation [9]. Genetic lesions within these survival pathways are frequently seen in B-cell neoplasms. Centrocytes subsequently undergo CSR, which results in the formation of the different isotypes of antibodies, IgG, IgA, etc. Both SHM and CSR are dependent on the activity of the activation-induced cytidine deaminase (AID) enzyme, and result in B-cell specific genomic modifications via the generation of single and double strand breaks with subsequent repair [12]. Mistakes in these processes are frequent oncogenic events in B-cell neoplasms, such as lymphoma. Importantly, B-cells are tested for self-reactivity before leaving the bone marrow, and undergo apoptosis, receptor editing or anergy if they bind to host antigens [13]. Defects in this latter process can lead to autoimmunity.
Memory B-cells remain in circulation to enable a rapid immune response if the same antigen is encountered again. CD20 is a marker of mature B-cells, present on naïve B-cells and memory B-cells, but not on plasma cells [14]. B-cells interact with T cells, macrophages and dendritic cells, as well as their soluble factors, to patrol the circulatory system for invading pathogens and danger signals [15]. Circulating plasma cells require colony-stimulating factors for survival. On effective removal of a pathogen, these factors are no longer secreted and the clonally expanded population of circulating plasma cells undergo apoptosis [1].
B-cell malignancies
B-cell malignancies are usually classified based on cell surface expression of various clusters of differentiation molecules, which in turn is usually determined by the stage of maturation/differentiation of the cell of origin. The two main types of B-cell leukaemia are as follows: acute lymphoblastic leukaemia (ALL) and chronic lymphocytic leukaemia (CLL). These diseases differ in terms of the speed of disease progression (acute vs. chronic), though both originate in the lymphoid cells in the bone marrow [16]. Other B-cell leukaemias include prolymphocytic leukaemia (PLL, which also originates in the bone marrow), pre-B lymphoblastic leukaemia and hairy cell leukaemia (originating in post-germinal centre B-cells). Other types of B-cell cancers include marginal-zone lymphoma, follicular lymphoma, mantle-cell lymphoma (originating in the lymph node primary follicle), diffuse large B-cell lymphoma (DLBCL; originating in the germinal centre), multiple myeloma (MM; originating in post-germinal centre B-cells) and Burkitt’s lymphoma (originating in the germinal centre) [17, 18].
B-cell cancers are largely heterogeneous, making it difficult to find common molecular targets and widely suitable therapies [18]. Treatments and prognoses for patients with B-cell cancers differ vastly, not only based on the type of B-cell cancer and speed of diagnosis, but also on subtype within each cancer [16, 19]. Classical therapy relies on chemotherapeutic agents such as fludarabine and cyclophosphamide, which interfere with DNA replication [20, 21]. The most effective targeted therapy to date is rituximab, a chimeric monoclonal antibody which binds to CD20. Binding of this antibody leads to apoptosis induction, BCR downregulation [22], activation of complement and enhanced B-cell death by natural killer cells [23]. Although rituximab targets all CD20+ B-cells, the absence of normal B-cells during treatment does not appear to cause any long-term effects [24]. Overcoming rituximab resistance is one of the major goals of upcoming therapies. Resistance can emerge through reduced CD20 expression, altered BCR signalling, reduced activation of complement or natural killer cells, or resistance to apoptosis [25]. For example, CLL is known for expression of low levels of CD20 and high levels of the anti-apoptotic protein Bcl-2, and these patients are generally resistant to rituximab as a single agent [24]. In light of this resistance, research into new therapies has focused on the downstream mediators of BCR signalling. BCR signalling leads to the activation of Bruton’s tyrosine kinase (Btk) and PI3K, among others [26]. Ibrutinib is a Btk inhibitor recently approved for the treatment of CLL [27]. By inhibiting Btk, there is reduced ERK and NF-κB signalling, in turn leading to reduced proliferation and migration [28]. Idelalisib is a PI3Kδ inhibitor, also recently approved for the treatment of CLL. By inhibiting PI3K, there is reduced Akt and mammalian target of rapamycin (mTOR) activation, leading to reduced migration, proliferation and survival [29]. Another promising approach is to target Bcl-2 directly. In this context, ABT-199, a so-called BH3 mimetic, is a potent and selective inhibitor for Bcl-2 with anti-tumour activity in several B-cell malignancies [30]. A further line of research is the use of cytotoxic T cells genetically engineered to express chimeric antigen receptors on their surface [31]. These receptors combine single-chain variable fragments (scFv) for antigen recognition with transmembrane and intracytoplasmic domains (e.g. CD3-zeta), which induce T cell activation and cytotoxicity on binding to their corresponding antigen (e.g. CD19 on B-cells) [32]. Future research will also focus on the importance of the microenvironment for B-cell malignancies. Despite these advances, relapse and resistance to therapy is an ongoing issue, and new druggable targets are always worthy of investigation.
Neurotrophins and neurotrophin receptors
Neurotrophins are a class of growth factor proteins. Although originally described for their role in the development, survival and functioning of neurons, they have since been shown to have roles in many other systems. They can regulate sodium channels in skeletal muscle cells and are important for ovulation [33, 34]. In particular, there is interplay between neurotrophins and the immune system.
The prototypical member of the neurotrophins is nerve growth factor (NGF), discovered by Levi-Montalcini in the 1950s [35, 36]. Other members include brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3 and NT-4/5 [37–41]. A common characteristic of the neurotrophins is that they are produced in a pro-form, requiring cleavage to the mature form [42]. This occurs either intracellularly (e.g. by furin) or extracellularly (e.g. by matrix metalloproteinases) [43]. They may be secreted from cells in either form [44–46]. Neurotrophins all share a similar structure, with a cysteine knot between beta sheets which are linked by disulphide bridges [47]. Neurotrophins naturally form homo-dimers and have been shown to form hetero-dimers experimentally, though the functionality of hetero-dimers is somewhat in question [48, 49].
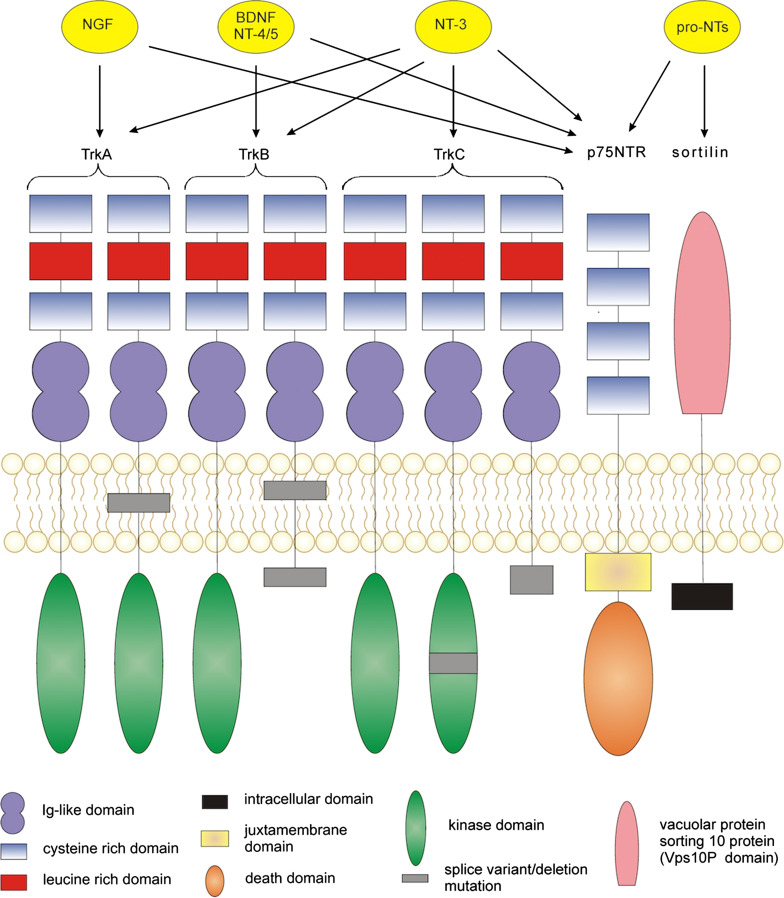
Mature neurotrophins bind to two main types of receptor—the high affinity tropomyosin-related kinases (Trk receptors) and the so-called low affinity p75NTR receptor [50]. A third type of receptor, known as sortilin, is also to known to bind to pro-neurotrophins in association with p75NTR [51, 52]. All of the mature and pro-neurotrophins can bind to the p75NTR receptor. Each neurotrophin binds specifically to a different Trk receptor—NGF to TrkA, BDNF and NT4/5 to TrkB, and NT-3 to TrkC [53]. In the absence of p75NTR, NT-3 can also bind to TrkA and TrkB, albeit with lower affinity [54]. Low affinity binding of pro-neurotrophins to the Trk receptors has also been described [55]. Normally proNGF binding requires processing to mature NGF to activate Trk signalling [56]. However, proNGF may mediate signalling through TrkA, albeit at lower levels of activity than mature NGF, while also inhibiting NGF-TrkA binding [57]. In the case of breast cancer, proNGF has been shown to directly stimulate cancer cell proliferation/invasion through a sortilin-TrkA heterodimer, although direct proNGF-TrkA binding was not shown [58]. To date, this pathway has not been demonstrated in other malignancies and in melanoma for example, proNGF mediates cell proliferation and migration through p75NTR and sortilin [59]. ProBDNF has also been shown to be able to bind TrkB and elicit signalling [60]. The neurotrophins and the receptors they bind to are depicted in Fig. 1.
Fig. 1.
Structure of the neurotrophin receptors found in humans and their ligand-receptor affinities. Tropomyosin-related kinase (Trk) receptors are typical tyrosine kinase receptors. The three Trk receptors bind neurotrophins with different affinities. NGF binds to TrkA, BDNF and NT-4/5 to TrkB, with NT-3 able to bind to all of the Trk receptors (though preferentially to TrkC). Alternative splicing can lead to truncated Trk receptors, which may still retain some signalling ability. p75NTR is a member of the TNF receptor superfamily. It contains an intracellular death domain which can recruit adaptor molecules to propagate signalling. All of the neurotrophins can bind to p75NTR, as well as the pro-neurotrophins. p75NTR can also signal in the absence of a ligand. Sortilin is a vesicular sorting protein which acts as a co-receptor for p75NTR-pro-neurotrophin binding. It lacks a catalytic domain only allowing signalling through p75NTR
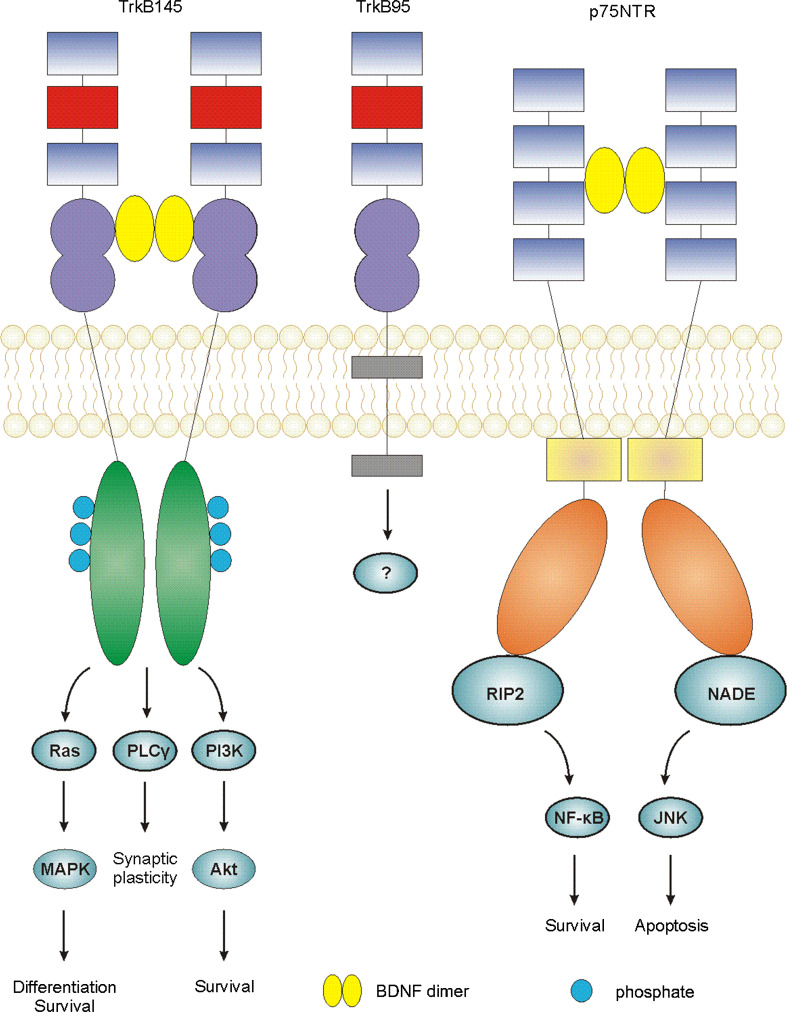
The Trk receptors are typical receptor tyrosine kinases which consist of highly conserved extracellular domains, with varying cytoplasmic domains [61]. Full-length Trk receptors homodimerize and trans-autophosphorylate upon ligand binding. This leads to the induction of three signalling pathways—the Ras/mitogen-activated protein kinase pathway (MAPK), the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the phospholipase Cγ (PLCγ) pathway [62, 63], outlined in Fig. 2. Trk signalling is important for neuronal cell growth, survival and differentiation [64]. It is also linked to survival, proliferation and invasion in several types of cancer including breast, neuroblastoma, lung, pancreatic, and colorectal cancers [65–71]. Truncated Trk receptors can also be produced, by alternative splicing. These receptors lack the intracellular kinase domain and so cannot signal in the same way as the full-length receptors [53]. Truncated Trk receptors have been shown to bind to neurotrophins, particularly truncated TrkB (TrkB95) to BDNF. TrkB has two truncated splice variants, TrkB95 (also known as TrkB.T1) and TrkB.T2. It was initially thought that these receptors functioned purely as dominant negative receptors, through hetero-dimerisation with full-length TrkB in neurons [72, 73]. However, TrkB.T2 does not appear to have signalling ability and has not been found in humans [74]. TrkB95, though lacking the catalytic kinase domain, still retains some signalling ability of its own [75]. For example, BDNF has been shown to induce inositol 1,4,5-trisphosphate (IP3)-dependent calcium release in glia cells through TrkB95 [73]. In addition, BDNF can induce the release of Rho GDP-dissociation inhibitor (GDI)1 from TrkB95 in glial cells, leading to a decrease in signalling by extracellular signal-regulated kinase (ERK). This signalling system has been shown to alter cell morphology [76]. A novel 61 kDa protein, labelled ‘Truncated TrkB Interacting Protein’ (TTIP), has also been shown to bind the intracellular region of TrkB95 in a ligand-independent manner in neuroblastoma cells [77]. However, TrkB95-TTIP signalling has so far not been elucidated.
Fig. 2.
Ligand-induced signalling through neurotrophin receptors. Mature neurotrophins bind the receptors in dimeric form, inducing receptor dimerization. Ligand binding to Trk receptors induces autophosphorylation and three downstream pathways: the Ras/MAPK pathway resulting in differentiation/survival; the PLCγ pathway inducing synaptic plasticity in neurons and the PI3K/Akt pathway resulting in cell survival. Signalling downstream of the truncated TrkB (TrkB95) receptor is not well characterised and requires further investigation. Ligand binding to the p75NTR receptor induces receptor dimerization and recruitment of adaptor molecules such as RIP2 and NADE to the death domain which mediate pro-survival NF-κB signalling or pro-apoptotic JNK signalling, respectively
p75NTR is a member of the tumour necrosis factor (TNF) receptor superfamily, and is also known as TNFRSF16 or CD271 [78]. Its structure, as determined independently by three groups, contains a cysteine-rich cytoplasmic domain, a single-pass transmembrane domain and an intracellular death domain [79–81]. It can signal in either monomeric or dimeric form upon ligand binding [82]. Unlike TrkA, p75NTR requires the recruitment of intracellular adaptor proteins to induce signalling [78]. It has been shown to recruit a number of proteins, such as TRAF6, to initiate signal transduction [83]. p75NTR can elicit a variety of responses based on the binding of pro- or mature neurotrophins, co-receptor expression and availability of adaptors [84]. Even upon binding of mature neurotrophins, p75NTR can stimulate nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) activation, promoting cell survival, or c-Jun N-terminal kinase (JNK) activation, promoting apoptosis, depending on cell type [85]. Pro-neurotrophins can bind p75NTR in combination with the co-receptor sortilin, inducing apoptosis in neural cells [51]. In cancer, p75NTR can act as either an oncogene or a tumour suppressor, depending on cell type. For example, increased p75NTR expression is seen in triple-negative breast cancer [86], while reduced p75NTR expression is seen in prostate cancer [87].
Interestingly, the neurotrophin receptors, p75NTR, TrkA and TrkC, have all been proposed as ‘dependence receptors’. The dependence receptor concept asserts that cells rely on signalling induced by ligand binding to the receptor in order to survive. In the absence of ligand binding, the receptor does not cease signalling, but actively induces pro-apoptotic signalling [88]. p75NTR was described as the first ‘dependence receptor’ [88]. This p75NTR pro-apoptotic signalling is thought to be different from the pro-apoptotic signalling induced by ligand binding in some cell types. It utilizes the ‘addiction/dependence domain’ described by Dale Bredesen [89], and later identified as the ‘Chopper’ domain [90], rather than the death domain (DD) which binds to adaptors on ligand binding. Furthermore, p75NTR is known to enhance ligand binding to the Trk receptors, increasing their affinity for neurotrophins by tenfold [91]. TrkA has also recently been proposed as a dependence receptor, causing neuronal death during NGF depletion [92]. Both p75NTR and TrkA are atypical dependence receptors as they are not cleaved by caspases to induce pro-apoptotic signalling [93]. However, they may still be cleaved by other proteases leading to a similar pro-apoptotic signalling initiation mechanism [94]. TrkC is known to have caspase cleavage sites in the intracellular domain, making it a more typical dependence receptor [95].
Sortilin is a member of the vesicular sorting proteins (Vsps) and is involved in protein secretion. It was later discovered to also play a role in neurotrophin signalling [96]. Sortilin cannot induce intracellular signalling alone as it lacks a cytoplasmic catalytic domain. It therefore acts as a co-receptor, in combination with p75NTR. It is known to promote apoptosis upon binding of pro-neurotrophins [51], but is not known to bind to mature neurotrophins.
Neurotrophins in B lymphocytes
Neurotrophins are known to affect many cells of the immune system [97]. The interactions between the immune and nervous systems have undergone much study, including investigation into the expression and secretion of neurotrophins by cells of the immune system (comprising both cells of the lymphoid organs and circulating immunocompetent cells). NGF is known to enhance survival, activation and differentiation of B and T lymphocytes, monocytes, eosinophils, neutrophils, basophils and mast cells [98]. Dendritic cells have been shown to express both TrkA and p75NTR [99], while T lymphocytes produce NGF and express p75NTR [100]. Monocytes express TrkA, which is lost upon differentiation into macrophages [101]. Macrophages do however secrete NGF, BDNF and NT-4/5 [102–104]. As for B lymphocytes, neurotrophins have been shown to be involved in proliferation, development, differentiation, antibody secretion and survival. An overview of neurotrophin expression, receptor expression and signalling activities in B lymphocytes is shown in Table 1.
Table 1.
Expression and activity of neurotrophins and their receptors in healthy B-cells
| Gene/protein | Expression | Activity |
|---|---|---|
| NGF |
Resting and activated human B-cells [105] Higher in memory B-cells [105] and memory-like B-cell line [106] |
Proliferation of memory-like B-cell line (CESS) and B lymphocytes in vitro [106, 118] Survival of memory B lymphocytes in vitro and memory-like B-cell line (CESS) [105, 106] Differentiation/IgM production of B lymphocytes in vitro [105, 119] IgG and IgA production from resting and activated B lymphocytes in vitro [119] IL-2R expression by activated B lymphocytes in vitro [120] CD40 expression by B lymphocytes in vitro [120] IgE inhibition in B lymphocytes in vitro [122] |
| BDNF |
Activated human B-cells [105] B-cell lines [107] |
Maturation of B-cells in vivo from Pre-BII stage in murine bone marrow [109] Survival of B lymphocytes in vitro with serum deprivation [107] Apoptosis induction (by proBDNF) in a mature B-cell line (BL2) in vitro [107] |
| NT-3 | Activated human B-cells [105] | No data |
| NT-4/5 | No data | No data |
| TrkA | Resting and activated human B-cells [105] | No data |
| TrkB145 | B-cell lines [111] | No data |
| TrkB95 | Murine spleen and bone marrow [109] activated B-cell lines [105] | Maturation of B-cells in vivo from Pre-BII stage in murine bone marrow [109] |
| TrkC | No data | No data |
| p75NTR | Murine spleen and bone marrow [109] resting and activated human B-cells [105] memory-like B-cell line [106] | Apoptosis induction (by proBDNF) in a mature B-cell line (BL2) in vitro [107] |
| Sortilin | B-cell lines [107] | Apoptosis induction of a mature B-cell line (BL20) [107] |
Expression of neurotrophins and their receptors in B lymphocytes
Examination of the expression of neurotrophins in B-cells has revealed basal NGF expression in both resting and activated human B-cells [105]. Memory B-cells have been shown to produce eight times more NGF than virgin B lymphocytes, while levels of the neurotrophin receptors were similar [105]. High levels of basal NGF expression and production are also seen in the memory B-cell-like cell line CESS, and it has been suggested that NGF production is restricted to long-lived B-cells [106]. BDNF has been shown to be secreted from activated human B-cells [103]. NT-3 mRNA expression has been reported from activated human B-cells [103, 107]. Expression of NT-4/5 has not been examined in B lymphocytes or cell lines.
Expression of all three Trk receptors has been shown in human B-cells [108]. TrkA is expressed in both resting and activated B-cells [105] as well as in the CESS cell line [106]. Cells from murine spleen and bone marrow have been shown to express TrkB95, but full-length TrkB was not detected [109]. Expression of TrkB95 has also been reported in Epstein–Barr virus (EBV)-transformed lymphoblastoid B-cell lines, but only after cell activation [103]. Expression of full-length TrkB has also been observed in unstimulated EBV-transformed B-cells, but with very low levels at the cell surface [110]. It is worth noting that EBV can act as a mitogen for B lymphocytes [111], and may induce TrkB expression. It has been suggested that TrkB may be cytoplasmically sequestered in resting B-cells, and relocated to the cell surface upon B-cell stimulation [107]. Relatively little is known about TrkC expression in B lymphocytes, but mRNA expression has been seen in human B-cells [108]. In general, it is agreed that B-cells express at least the TrkB95 receptor, with TrkA also expressed by memory B-cells. This suggests that activated B-cells are responsive to BDNF and NT-3, with memory B-cells also responsive to NGF. The intracellular signalling pathways activated following neurotrophin binding to B-cells are proposed to be similar to those activated in other cell types. NGF binding has been shown to induce tyrosine phosphorylation and activation of MAPK and p90rsk in lymphoblastoid B-cell lines [112]. This was followed by confirmation of Ras activation by Trk signalling in B-cells [113]. It was later shown that NGF-mediated inhibition of apoptosis is through the activation of protein kinase C zeta (PKCζ) (via TrkA signalling activating the PI3K pathway) in an EBV-negative Burkitt’s lymphoma B-cell line, which expresses surface proteins characteristic of normal early B-cells [114]. In terms of TrkB95 signalling, the kinase domain is absent so activation of classical Trk signalling does not occur. However, it may be the case that TrkB95 induces calcium release or ERK signalling, as seen in glial cells [73, 76], or may interact with adaptor proteins, e.g. TTIP [77].
There are conflicting reports as to whether normal B-cells express the p75 NTR receptor. p75NTR has been shown to be expressed by both resting and activated human B lymphocytes, [105] and by the CESS cell line [106]. p75NTR mRNA expression was observed in human B-cells, but could not be detected at the protein level [108]. p75NTR expression has also been shown in B-cells derived from murine spleen and bone marrow [109]. Expression of the p75NTR co-receptor sortilin has been shown in B-cell lines representing several stages of B-cell maturation and in primary B-cells [107]. Together these findings indicate that B-cells have the potential to respond to both neurotrophins and pro-neurotrophins through p75NTR. p75NTR signalling in B-cells has not been well studied. However, signalling is likely to be similar to that seen in non-immune cells, i.e. by adaptor recruitment leading to a pro-survival or pro-death response through the NF-κB or JNK signalling pathways, respectively [78, 85].
Both expression of neurotrophins and their receptors, by both T and B lymphocytes, can be induced/enhanced by cell activation, usually by stress but theoretically also by danger-associated molecular pattern/pathogen-associated molecular pattern (DAMP/PAMP) recognition. This is an important consideration to be taken into account when studying the effect of neurotrophins on these immune cells.
Effect of neurotrophin signalling on B-cells
As neurotrophins are secreted by numerous immune cell types, they may act in an autocrine or paracrine fashion on B lymphocytes. Bone marrow stromal cells have been shown to produce neurotrophins [115] potentially leading to paracrine signalling towards developing and mature B-cells present in the bone marrow. Both BDNF and NGF have been shown to affect B-cell function; however, in contrast, the effects of NT-3 and NT-4/5 on B-cells have not been well characterised.
BDNF has been shown to be involved in B lymphocyte development, through the use of BDNF knockout mice. These mice showed reduced B-cell numbers and a block in B-cell maturation at the pre-B II stage, with no disruption seen in T cell number/development. BDNF from stromal cells promoted pre-B cell maturation through TrkB signalling by immature B-cells [109]. BDNF has been shown to be able to reduce the level of apoptosis in mature B-cell lines during serum starvation [108]. It has also been suggested that the BDNF-mediated anti-apoptotic signalling may be linked to the upregulation of FLICE-inhibitory protein (FLIP), as shown in neural cells [116]. In experimental allergic encephalomyelitis, used as a model for multiple sclerosis, BDNF has been shown to be upregulated to provide neuroprotection, though it is unclear whether this is due to increased local expression or expression by the infiltrating Breg cells [117].
NGF has been shown to increase the rate of proliferation of the CESS cell line and primary B lymphocytes, by binding to a high affinity receptor [106, 118], most likely TrkA. NGF can act as an autocrine pro-survival factor for memory B lymphocytes (which produce high levels of NGF), by maintaining high levels of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) [105]. Use of anti-NGF antibodies, or Trk inhibitors, has been shown to reduce Bcl-2 levels in CESS cells. In these studies, the absence of neurotrophin signalling caused activation of p38 MAPK, leading to phosphorylation of Bcl-2, caspase activation and subsequent apoptosis [106]. It has been suggested that early claims of NGF as a proliferative factor for B lymphocytes need to be re-examined, to consider that the relatively higher numbers of B lymphocytes seen in the presence of NGF may be due to a reduction in B lymphocyte death, leading to an accumulation of cells rather than mitogenic stimulation per se [105]. NGF can induce IgM production from B lymphocytes in vitro, indicating that NGF can induce differentiation into antibody-producing B-cells [118]. However, it has been shown that endogenous NGF is not involved in the antibody constant region class switch that converts virgin B-cells to antigen-targeting and memory B-cells [105]. NGF has been shown to promote IgG production by resting B lymphocytes, while enhancing production of IgG, IgM and IgA by activated B lymphocytes in vitro [119]. NGF and interleukin-2 (IL-2) have been shown to enhance B-cell progression synergistically, with NGF causing an increase in IL-2 receptor expression and IL-2 causing an increase in p75NTR expression in B lymphocytes [120, 121]. NGF has also been shown to increase expression of CD40 (a co-stimulatory protein that binds T cells) on B-cell lines [120]. However, p75NTR and CD40 have opposing effects on IgE production by B lymphocytes, with NGF inhibiting IL-4-mediated IgE production [122]. In allergic airway inflammation, NGF and NT-3 act as pro-survival factors for pulmonary plasma cells, through increased expression of the anti-apoptotic Bcl-2 protein. NGF and NT-3 also enhance IgE production from pulmonary plasma cells in allergic airway inflammation by causing increased expression of the transcription factors X-box binding protein 1 and NF-κB [123].
Neurotrophins and neurotrophin receptors in B-cell malignancies
Considerably more studies have been carried out regarding the levels of expression and activities of neurotrophins and their receptors in healthy B lymphocytes compared with B lymphocyte cancers. However, many of these ‘healthy’ B-cell studies use non-normal B-cell models, particularly cell lines derived from EBV-transformed Burkitt’s lymphoma or DLBCL cells. Therefore, it is reasonable to suggest that many of the functions of neurotrophins and their receptors as described above also apply to B-cell malignancies. An overview of neurotrophin expression, receptor expression and signalling activities in B lymphocyte cancers is provided in Table 2.
Table 2.
Expression and activity of neurotrophins and their receptors in B-cell cancers
| Protein | Acute lymphoblastic leukaemia | Diffuse large B-cell lymphoma | Burkitt’s lymphoma | Multiple myeloma | Follicular/small lymphocytic lymphomas |
|---|---|---|---|---|---|
| NGF |
ProNGF expressed intracellularly in SUDHL4, SUDH6, OCI-LY3 and OCI-LY19 cell lines [126, 127] No NGF secretion by SUDHL4, SUDH6, OCI-LY3 or OCI-LY19 cell lines [126, 127] SUDHL4 and SUDHL6 cell lines increased expression and secretion of NGF on anti-CD20 treatment [126] |
No secretion by Ramos, Raji or Daudi cell lines [127] |
No secretion by RPMI-8226 cell line [127] Prolongs patient sample survival in vitro, in the presence of stromal cells [129] |
mRNA expressed in patient samples [127] | |
| BDNF |
ProBDNF expressed intracellularly in SUDHL4, SUDHL6, OCI-LY3 and OCI-LY19 cell lines [126, 127] SUDHL4 and SUDHL6 cell lines secrete low levels of BDNF and proBDNF, downregulated on stress [126] OCI-LY3 and OCI-LY19 cell lines do not secrete BDNF [127] |
No secretion by Ramos, Raji or Daudi cell lines [127] Knockdown induces cell cycle arrest, apoptosis and sensitivity to 5-fluorouracil in Ramos and Raji cell lines [127] |
No secretion by RPMI-8226 cell line [126] BDNF secreted by several MM cell lines (including RPMI-8226) [129] BDNF expressed intracellularly in 12/25 patient samples [129] Protects U266 and RPMI-8226 cell lines from stress-induced apoptosis [108] Protects RPMI-8226 cell line from dexamethasone-induced apoptosis [129] Delays bortezomib-induced apoptosis in JJN3 cells [129] Prolongs patient sample survival in vitro, with and without stromal cells [129] proBDNF induces apoptosis in U266 and RPMI-8226 cell lines [108] |
mRNA expressed in 1/8 patients [126] | |
| NT-3 |
ProNT-3 expressed intracellularly in OCI-LY3 and OCI-LY19 cell lines [127] NT-3 secreted by OCI-LY3 and OCI-LY19 cell lines [127] |
mRNA expressed in all patient samples [127] | |||
| NT-4/5 | |||||
| TrkA | Expressed in OCI-LY19 cell line [127] | Expressed in patient samples [127] | |||
| TrkB145 | Not detected in SUDHL4, SUDHL6, OCI-LY3 or OCI-LY19 cell lines [126, 127] |
mRNA and protein expressed in KMS11, RPMI-8226 and LP1 cells [129] Low expression upregulated on dexamethasone treatment in OPM1, U266, JJN3, H929 and UTMC2 cell lines [129] Expressed in 24/25 patient samples [129] |
|||
| TrkB95 | Expressed in SUDHL4, SUDHL6, OCI-LY3 and OCI-LY19 cell lines [126, 127], membranous expression upregulated in SUDHL4 and SUDHL6 cell lines on stress [126] | ||||
| TrkC | Expressed in OCI-LY3 and OCI-LY19 cell lines [127] | ||||
| p75NTR | mRNA expressed in 22–38 % ALL patient samples [124] | Expressed in SUDHL4 and SUDHL6 cell lines, upregulated on stress [126] |
Not detected in any cell lines tested [129] Not detected at mRNA or protein level in patient samples [129] |
||
| Sortilin |
mRNA and protein expressed in RS4 and Nalm6 cell line [125, 130] Low membranous expression, increased on stress in Nalm6 cell line [125] |
Expressed in SUDHL4 and SUDHL6 cell lines [126] |
mRNA and protein expressed in BL2 and BL41 cell lines [108, 125], increased on stress [125] Low membranous expression, increased on stress in BL41 cell line [125] |
mRNA and protein expressed in U266 and RPMI-8226 cell lines [125, 130], increased on stress [125] Membranous expression increased on stress in RPMI-8226 cell line [125] Induces apoptosis in response to proBDNF in U266 and RPMI-8226 cell lines via [108] |
|
Acute leukaemias
A study in 2005 examined the expression of p75NTR in acute leukaemias using flow cytometry and qPCR, and in two different cohorts of patients, they found expression in 22 and 38 % of ALL samples [124]. They also found that p75NTR was preferentially expressed in ALL patients with the BCR/ABL translocation, suggesting that the BCR/ABL translocation and p75NTR expression are not interdependent, as p75NTR expression was not observed in chronic myelogenous leukaemia which also expresses the BCR/ABL mutation [124]. Sortilin expression has also been observed in the Nalm6 ALL cell line, at both the mRNA and protein levels. Levels of sortilin seen at the cell membrane were very low, but increased significantly on serum starvation, and was shown to inhibit apoptosis due to serum starvation in these cells [125]. This suggests that sortilin, in particular, may be involved in acute leukaemia resistance to chemotherapeutics aimed at apoptosis induction.
DLBCL
Neurotrophin and neurotrophin receptor expression has been examined further in DLBCL cell lines. SUDHL4 and SUDHL6 DLBCL cell lines have been shown to express proNGF and proBDNF intracellularly, while secreting only low levels of pro- and mature BDNF and not NGF in normal culture conditions [126]. OCI-LY3 (activated B-cell subtype) and OCI-LY19 (germinal centre origin) DLBCL cell lines express proNGF, proBDNF and proNT-3, but only secrete NT-3 at detectable levels [127]. These cells also express p75NTR, TrkB95 and sortilin (SUDHL4/6 cells; [126]; TrkA, TrkB95 and TrkC (OCI-LY19); or TrkB95 and TrkC (OCI-LY3; [127]). In none of these lines was full-length TrkB detected. This suggests that these cells may have some basal BDNF-TrkB95 signalling. Constitutive PI3K/Akt signalling was also observed in SUDHL cells [126]; however, the involvement of BDNF/TrkB in this would need to be confirmed by inhibiting BDNF signalling. TrkA was largely not expressed, consistent with a lack of NGF secretion [126].
Further to looking at basal expression of the neurotrophins and their receptors, Bellanger et al. also examined changes in expression on cellular stress (induced by serum deprivation). Upon stress, the cells upregulated expression of the receptors (p75NTR and TrkB95) while downregulating BDNF production. They also underwent constitutive processing of p75NTR, indicating activation of this receptor. A large increase in TrkB95 on the cell membrane suggests that cellular stress leads to translocation of this receptor. These data suggest that DLBCL cells respond to stress by altering the neurotrophin:receptor ratio. This may allow them to better survive the tumour microenvironment, such as the lymph nodes, or to increase resistance to therapy. In response to the therapeutically relevant anti-CD20 monoclonal antibody rituximab, the cell lines increased expression and secretion of NGF, presumably leading to NGF-p75NTR pro-survival signalling, as TrkA was not observed [126]. In contrast, no effect was seen on BDNF, TrkB95, p75NTR or sortilin expression.
Finally, this group also showed that inhibition of Trk receptor signalling, with the kinase inhibitor K252a, induced apoptosis in the SUDHL4 DLBCL cell line, suggesting an important role for Trk signalling in DLBCL survival. It is noted however that K252a may also be inhibiting other kinases at the concentration used [126]. Similar experiments were carried out by Sniderhan et al. in OCI-LY3 and OCI-LY19 DLBCL cell lines [127]. They found that K252a reduced proliferation of OCI-LY3 and OCI-LY19 cells, but could only induce apoptosis in the OCI-LY3 cell line (up to 70 % at 200 nM), suggesting a preferential effect on the activated B-cell subtype of DLBCL. They suggest that the OCI-LY19 cells are resistant to K252a-induced apoptosis due to high basal levels of X-linked inhibitor of apoptosis protein (XIAP). They also found that K252a treatment of OCI-LY3 cells resulted in inhibition of NF-κB transcriptional activity with a concomitant decrease in IL-6 production, which may act as an autocrine/paracrine pro-survival signal [127]. It is likely that K252a is targeting TrkC signalling in these cells as it is unable to fully inhibit TrkB95 [128]. This signifies a possible avenue of investigation into new targeted therapies for DLBCL.
Multiple myeloma
In contrast to DLBCL cells, several multiple myeloma (MM) cell lines (including RPMI-8226) were found to produce functional BDNF (as shown by PC12 neurite outgrowth; [129]). However, BDNF expression and secretion from these cells are controversial, as a more recent report did not find BDNF expression in the RPMI-8226 MM cell line. This group also found low-intermediate levels of Trk expression, though Trk type was not determined [127]. This confirmed earlier reports where full-length TrkB mRNA and protein were seen in RPMI-8226 [129]. Constitutive TrkB expression was seen in KMS11, RPMI-8226 and LP1 cells, with low TrkB levels increasing on dexamethasone exposure in OPM1, U266, JJN3, H929 and UTMC2 cell lines. BDNF-TrkB signalling was examined and shown to be functional, resulting in increased phosphorylation of Akt and MAPK p42/44. In contrast, p75NTR was not detected in any of the MM cell lines used. Following on from the increased TrkB expression after dexamethasone exposure, cell survival was examined, and BDNF was shown to protect RPMI-8226 cells from dexamethasone-induced apoptosis [129]. As expected, in the absence of its receptors, NGF had no effect on survival. BDNF could also delay bortezomib-induced apoptosis in JJN3 cells, but not prevent it. This is thought to be partly due to activation of PI3K signalling [129].
As well as cell lines, Pearse et al. also examined BDNF-TrkB expression and signalling in MM patient samples. They found that MM patients produced pro- and mature BNDF mRNA, and increased BDNF in one marrow sample from MM patients in comparison to control (though the cellular source of BDNF was undetermined; [129]). Full-length TrkB was found in 24/25 patients samples tested, with no p75NTR detected at either mRNA or protein levels. Consistent with their cell line findings, BDNF led to an increase in Akt phosphorylation in primary samples. Finally, they tested the effect of BDNF on primary MM cell survival, with and without stromal cell co-culture, and saw prolonged survival in culture conditions in both cases. NGF also aided MM cell survival in co-culture, presumably by acting on the stromal cells which then stimulate plasma cell survival [129].
These data contribute to the hypothesis that neurotrophins have a pro-survival role, in both an autocrine and paracrine manner, in MM. Sortilin expression has also been examined in MM cell lines, with mRNA and protein expression seen in all cases. This expression was mainly intracellular, increasing upon serum deprivation [125]. Apoptosis induction in myeloma-derived cell lines has been shown by proBDNF binding to sortilin (and presumably p75NTR), while mature BDNF reduced apoptosis, presumably through TrkB activation [107]. However, sortilin, as a component of the secretory pathway, has been shown to be required for BDNF secretion [107]. This identifies a potential indirect role for sortilin in pro-survival signalling, through promotion of mature BDNF secretion and subsequent BDNF-TrkB signalling, which has previously not been reported. Overall this may indicate a dual role for sortilin in both pro- and anti-death signalling in B-cell malignancies.
Burkitt’s lymphoma
Analysis of the expression of neurotrophins and receptors in some Burkitt’s lymphoma (Ramos, Raji, Daudi) cell lines revealed low-intermediate levels of Trk receptors (of undetermined type), with no NGF or BDNF expression [127]. In line with this, none of the cell lines exhibited an anti-proliferative or apoptotic response to K252a exposure [127]. In contrast to this, knockdown of BDNF in the Ramos/Raji cell lines has been reported to induce cell cycle arrest and apoptosis [130]. This was shown to occur through downregulation of Bcl-2, upregulation of Bax and activation of caspase −3 and −9 [130]. This suggests a constitutively active BDNF signalling pathway, as seen in DLBCL cell lines. BDNF knockdown could also sensitise the cells to 5-Fluorouracil (a chemotherapeutic pyrimidine analogue; [130]).
Sortilin expression has been shown in Burkitt’s lymphoma cell lines at both the mRNA and protein levels. Expression was mainly intracellular, with basal plasma membrane expression lower than that observed in MM cell lines. However, plasma membrane expression greatly increased in BL41 cells on serum deprivation [125]. This highlights sortilin translocation to the membrane as a response to stress in Burkitt’s lymphoma and potential mediator of cell survival through its dual role in pro- and anti-death signalling, as previously mentioned.
Other B-cell malignancies
Less is known about neurotrophins in other B-cell malignancies. As well as investigating neurotrophins and their receptors in DLBCL cell lines, Sniderhan et al. also investigated their role in cells derived from patients with non-Hodgkin’s lymphomas (i.e. follicular and small lymphocytic lymphomas). They found TrkA to be expressed in all of the patient samples, along with production of NGF and NT-3 mRNA. However, they did not examine TrkB95 expression and only found BDNF mRNA in 1 of 8 patients tested. Further to their cell line results with K252a, a reduced number of cells in S phase were seen in primary samples treated with K252a. Although they showed induction of low levels of apoptosis in normal resting B-cells exposed to K252a, and slightly higher levels in activated B-cells, they failed to examine K252a-induced apoptosis in their patient samples [127].
Significance of neurotrophin signalling in B-cell malignancies and future perspectives
Neurotrophin and neurotrophin receptor expression and signalling is now known to occur in many cell types beyond the nervous system. It constitutes part of the pro-survival mechanism of many cells, both transformed and not. This signalling has been shown to be involved in many cellular activities of normal B-cells, including, but not limited to, proliferation, survival and maturation. Further research has shown that neurotrophin signalling plays an equally important role in B-cell malignancies. In particular, expression of neurotrophins and their receptors is generally upregulated on stress indicating a pro-survival response. Knockdown of BDNF was shown to enhance apoptosis and chemosensitivity in Burkitt’s lymphoma cell lines [130]. Similarly, BDNF protected MM cell lines from apoptosis and chemotherapeutic agents [107, 129]. Trk inhibition induced apoptosis in an activated B-cell DLBCL cell line [127], the subtype known to have the worse prognosis. These studies indicate potential value for neurotrophin-targeting as either a mono- or an adjuvant therapy in these diseases. As well as the potential for inhibition of signalling by mature neurotrophins, enhanced pro-neurotrophin signalling through sortilin could be a potential new avenue of therapy. In combination with Bcl-2 inhibition, pro-neurotrophins could lead to significant levels of apoptosis in these malignancies. Neurotrophins are also known to be expressed and secreted by cells of the microenvironment, such as bone marrow stromal cells [131, 132]. Targeting neurotrophins could significantly decrease the proliferative and pro-survival effects of the microenvironment as well as affecting the B-cells directly.
Acknowledgments
This work was funded by Molecular Medicine Ireland as part of the Clinical and Translational Research Scholars Programme.
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack C, Hirama M, Lenhardschuller R, Tonegawa S. Complete immunoglobulin gene is created by somatic recombination. Cell. 1978;15:1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- 3.Gathings WE, Lawton AR, Cooper MD. Immunofluorescent studies of development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977;7:804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- 4.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/S0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 5.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–349. doi: 10.1016/S1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia, A, Bove, S (1985) Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring Institute Mitteilungen, pp 82–87 [PubMed]
- 7.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 8.Kincade PW, Lawton AR, Bockman DE, Cooper MD. Suppression of immunoglobulin G synthesis as a result of antibody-mediated suppression of immunoglobulin M synthesis in chickens. Proc Natl Acad Sci USA. 1970;67:1918–1925. doi: 10.1073/pnas.67.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 10.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 11.Kehry MR. CD40-mediated signaling in B cells—balancing cell survival, growth, and death. J Immunol. 1996;156:2345–2348. [PubMed] [Google Scholar]
- 12.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/S0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 13.Goodnow CC, Adelstein S, Basten A. The need for central and peripheral tolerance in the B cell repertoire. Science. 1990;248:1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- 14.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human lymphocyte B specific antigen. Journal of Immunology. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 15.Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer AL, III, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30(30):565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83–87. [PubMed] [Google Scholar]
- 19.Plawny L, Ries F. Emerging new anticancer biological therapies in 2013 (haematological malignancies) Curr Opin Oncol. 2014;26:363–370. doi: 10.1097/CCO.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 20.Plunkett W, Huang P, Gandhi V. Metabolism and action of fludarabine phosphate. Semin Oncol. 1990;17:3–17. [PubMed] [Google Scholar]
- 21.Rauen HM, Reisch A, Schriewer H. On the biochemical mechanism of action of cyclophosphamide. Arzneimittelforschung. 1964;14:176–178. [PubMed] [Google Scholar]
- 22.Shaw T, Quan J, Totoritis MC. B cell therapy for rheumatoid arthritis: the rituximab (anti-CD20) experience. Ann Rheum Dis. 2003;62(suppl 2):ii55–ii59. doi: 10.1136/ard.62.suppl_2.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudnicka D, Oszmiana A, Finch DK, Strickland I, Schofield DJ, Lowe DC, Sleeman MA, Davis DM. Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity. Blood. 2013;121:4694–4702. doi: 10.1182/blood-2013-02-482570. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin P, Grillo-Lopez AJ, Maloney DG, Link BK, Levy R, Czuczman MS, Cabanillas F, Dallaire BK, White CA. Efficacy controls and long-term follow-up of patients (pts) treated with rituximab for relapsed or refractory, low-grade or follicular (R-LG/F) NHL. Blood. 1998;92:414A–415A. [Google Scholar]
- 25.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 26.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 27.Burger JA. Targeting the microenvironment in chronic lymphocytic leukemia is changing the therapeutic landscape. Curr Opin Oncol. 2012;24:643–649. doi: 10.1097/CCO.0b013e3283589950. [DOI] [PubMed] [Google Scholar]
- 28.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O’Brien SM. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park C-M, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 31.Cheadle EJ, Sheard V, Hombach AA, Chmielewski M, Riet T, Berrevoets C, Schooten E, Lamers C, Abken H, Debets R, Gilham DE. Chimeric antigen receptors for T-cell based therapy. Methods Mol Biol (Clifton, N.J.) 2012;907:645–666. doi: 10.1007/978-1-61779-974-7_36. [DOI] [PubMed] [Google Scholar]
- 32.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65(65):333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodie C, Sampson SR. Nerve growth factor and fibroblast growth factor influence postfusion expression of Na channels in cultured rat skeletal muscle. J Cell Physiol. 1990;144:492–497. doi: 10.1002/jcp.1041440317. [DOI] [PubMed] [Google Scholar]
- 34.Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, Costa ME, Skinner MK, Ojeda SR. Involvement of nerve growth factor in the ovulatory cascade: trkA receptor activation inhibits gap junctional communication between thecal cells. Endocrinology. 1996;137:5662–5670. doi: 10.1210/endo.137.12.8940397. [DOI] [PubMed] [Google Scholar]
- 35.Levimontalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 36.Levimontalcini R. Nerve growth factor. Ann N Y Acad Sci. 1964;118:149–170. doi: 10.1111/j.1749-6632.1964.tb33978.x. [DOI] [PubMed] [Google Scholar]
- 37.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 39.Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallbook F, Ibanez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- 41.Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5—a novel neurotrophic factor that activates Trk and TrkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-A. [DOI] [PubMed] [Google Scholar]
- 42.Acklin C, Stoney K, Rosenfeld RA, Miller JA, Rohde MF, Haniu M. Recombinant human brain-derived neurotrophic factor (rHuBDNF)—disulfide structure and characterization of BDNF expressed in CHO cells. Int J Pept Protein Res. 1993;41:548–552. doi: 10.1111/j.1399-3011.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 43.Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 44.Hibbert AP, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-4, alone or heterodimerized with brain-derived neurotrophic factor, is sorted to the constitutive secretory pathway. J Biol Chem. 2003;278:48129–48136. doi: 10.1074/jbc.M300961200. [DOI] [PubMed] [Google Scholar]
- 45.Young M, Oger J, Blanchard MH, Asdourian H, Amos H, Arnason BGW. Secretion of a nerve growth factor by primary chick fibroblast cultures. Science. 1975;187:361–362. doi: 10.1126/science.1167427. [DOI] [PubMed] [Google Scholar]
- 46.Hasan W, Pedchenko T, Krizsan-Agbas D, Baum L, Smith PG. Sympathetic neurons synthesize and secrete pro-nerve growth factor protein. J Neurobiol. 2003;57:38–53. doi: 10.1002/neu.10250. [DOI] [PubMed] [Google Scholar]
- 47.McDonald NQ, Lapatto R, Murrayrust J, Gunning J, Wlodawer A, Blundell TL. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991;354:411–414. doi: 10.1038/354411a0. [DOI] [PubMed] [Google Scholar]
- 48.Jungbluth S, Bailey K, Barde YA. Purification and characterization of a brain-derived neurotrophic factor neurotrophin-3 (BDNF/NT-3) heterodimer. Eur J Biochem. 1994;221:677–685. doi: 10.1111/j.1432-1033.1994.tb18780.x. [DOI] [PubMed] [Google Scholar]
- 49.Heymach JV, Shooter EM. The biosynthesis of neurotrophin heterodimers by transfected mammalian cells. J Biol Chem. 1995;270:12297–12304. doi: 10.1074/jbc.270.20.12297. [DOI] [PubMed] [Google Scholar]
- 50.Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-C. [DOI] [PubMed] [Google Scholar]
- 51.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 52.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75(NTR) and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 55.Clewes O, Fahey MS, Tyler SJ, Watson JJ, Seok H, Catania C, Cho K, Dawbarn D, Allen SJ. Human ProNGF: biological effects and binding profiles at TrkA, P75NTR and sortilin. J Neurochem. 2008;107:1124–1135. doi: 10.1111/j.1471-4159.2008.05698.x. [DOI] [PubMed] [Google Scholar]
- 56.Boutilier J, Ceni C, Pagdala PC, Forgie A, Neet KE, Barker PA. Proneurotrophins require endocytosis and intracellular proteolysis to induce TrkA activation. J Biol Chem. 2008;283:12709–12716. doi: 10.1074/jbc.M710018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobottka B, Reinhardt D, Brockhaus M, Jacobsen H, Metzger F. ProNGF inhibits NGF-mediated TrkA activation in PC12 cells. J Neurochem. 2008;107:1294–1303. doi: 10.1111/j.1471-4159.2008.05690.x. [DOI] [PubMed] [Google Scholar]
- 58.Demont Y, Corbet C, Page A, Ataman-Onal Y, Choquet-Kastylevsky G, Fliniaux I, Le Bourhis X, Toillon RA, Bradshaw RA, Hondermarck H. Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J Biol Chem. 2012;287:1923–1931. doi: 10.1074/jbc.M110.211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Investig Dermatol. 2008;128:2031–2040. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- 60.Fayard B, Loeffler S, Weis J, Vogelin E, Kruttgen A. The secreted brain-derived neurotrophic factor precursor pro-BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J Neurosci Res. 2005;80:18–28. doi: 10.1002/jnr.20432. [DOI] [PubMed] [Google Scholar]
- 61.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 62.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 63.Klein R, Jing SQ, Nanduri V, Orourke E, Barbacid M. The Trk protooncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-Y. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan DR, Stephens RM. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 65.Descamps S, Toillon R, Adriaenssens E, Pawlowski V, Cool S, Nurcombe V, Le Bourhis X, Boilly B, Peyrat J, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–17870. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- 66.Lagadec C, Meignan S, Adriaenssens E, Foveau B, Vanhecke E, Romon R, Toillon RA, Oxombre B, Hondermarck H, Le Bourhis X. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28:1960–1970. doi: 10.1038/onc.2009.61. [DOI] [PubMed] [Google Scholar]
- 67.Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu XG, Azar CG, Lee CP, Evans AE. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997;31:49–55. doi: 10.1023/A:1005729329526. [DOI] [PubMed] [Google Scholar]
- 68.Perez-Pinera P, Hernandez T, Garcia-Suarez O, de Carlos F, Germana A, del Valle M, Astudillo A, Vega JA. The Trk tyrosine kinase inhibitor K252a regulates growth of lung adenocarcinomas. Mol Cell Biochem. 2007;295:19–26. doi: 10.1007/s11010-006-9267-7. [DOI] [PubMed] [Google Scholar]
- 69.Miknyoczki SJ, Lang D, Huang LY, Klein-Szanto AJP, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(SICI)1097-0215(19990505)81:3<417::AID-IJC16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Martin TA, Jiang WG. Biological influence of brain-derived neurotrophic factor (BDNF) on colon cancer cells. Exp Ther Med. 2013;6:1475–1481. doi: 10.3892/etm.2013.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S, Guo D, Luo W, Zhang Q, Zhang Y, Li C, Lu Y, Cui Z, Qiu X. TrkB is highly expressed in NSCLC and mediates BDNF-induced the activation of Pyk2 signaling and the invasion of A549 cells. BMC Cancer. 2010;10:43. doi: 10.1186/1471-2407-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eide FF, Vining ER, Eide BL, Zang KL, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 74.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human Trks—molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein R, Conway D, Parada LF, Barbacid M. The TrkB tyrosine protein kinase gene codes for a 2nd neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-U. [DOI] [PubMed] [Google Scholar]
- 76.Ohira K, Homma KJ, Hirai H, Nakamura S, Hayashi M. TrkB-T1 regulates the RhoA signaling and actin cytoskeleton in glioma cells. Biochem Biophys Res Commun. 2006;342:867–874. doi: 10.1016/j.bbrc.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 77.Kryl D, Barker PA. TTIP is a novel protein that interacts with the truncated T1 TrkB neurotrophin receptor. Biochem Biophys Res Commun. 2000;279:925–930. doi: 10.1006/bbrc.2000.4058. [DOI] [PubMed] [Google Scholar]
- 78.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/S0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 79.Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong Y, Cao P, Yu H-j, Jiang T. Crystal structure of the neurotrophin-3 and p75(NTR) symmetrical complex. Nature. 2008;454:789–793. doi: 10.1038/nature07089. [DOI] [PubMed] [Google Scholar]
- 81.Welcher AA, Bitler CM, Radeke MJ, Shooter EM. Nerve growth factor binding domain of the nerve growth factor receptor. Proc Natl Acad Sci USA. 1991;88:159–163. doi: 10.1073/pnas.88.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vilar M, Charalampopoulos I, Kenchappa RS, Simi A, Karaca E, Reversi A, Choi S, Bothwell M, Mingarro I, Friedman WJ, Schiavo G, Bastiaens PIH, Verveer PJ, Carter BD, Ibanez CF. Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron. 2009;62:72–83. doi: 10.1016/j.neuron.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- 84.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 85.Allen J, Khwaja F, Byers S, Djakiew D. The p75(NTR) mediates a bifurcated signal transduction cascade through the NF kappa B and JNK pathways to inhibit cell survival. Exp Cell Res. 2005;304:69–80. doi: 10.1016/j.yexcr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Tsang JYS, Wong KHY, Lai MWH, Lacambra MD, Ko C-W, Chan SK, Lam CCF, Yu AMC, Tan P-H, Tse GM. Nerve growth factor receptor (NGFR): a potential marker for specific molecular subtypes of breast cancer. J Clin Pathol. 2012;66:291–296. doi: 10.1136/jclinpath-2012-201027. [DOI] [PubMed] [Google Scholar]
- 87.Krygier S, Djakiew D. Molecular characterization of the loss of p75(NTR) expression in human prostate tumor cells. Mol Carcinog. 2001;31:46–55. doi: 10.1002/mc.1038. [DOI] [PubMed] [Google Scholar]
- 88.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 89.Bredesen DE, Ye X, Tasinato A, Sperandio S, Wang JJL, Assa-Munt N, Rabizadeh S. p75(NTR) and the concept of cellular dependence: seeing how the other half die. Cell Death Differ. 1998;5:365–371. doi: 10.1038/sj.cdd.4400378. [DOI] [PubMed] [Google Scholar]
- 90.Coulson EJ, Reid K, Baca M, Shipham KA, Hulett SM, Kilpatrick TJ, Bartlett PF. Chopper, a new death domain of the p75 neurotrophin receptor that mediates rapid neuronal cell death. J Biol Chem. 2000;275:30537–30545. doi: 10.1074/jbc.M005214200. [DOI] [PubMed] [Google Scholar]
- 91.Chao MV. Neurotrophin receptors—a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- 92.Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, Bibel M, Barde Y-A. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467:59–63. doi: 10.1038/nature09336. [DOI] [PubMed] [Google Scholar]
- 93.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 94.Zupan AA, Johnson EM. Evidence for endocytosis-dependent proteolysis in the generation of soluble truncated nerve growth factor receptors by A875 human melanoma cells. J Biol Chem. 1991;266:15384–15390. [PubMed] [Google Scholar]
- 95.Tauszig-Delamasure S, Yu L-Y, Cabrera JR, Bouzas-Rodriguez J, Mermet-Bouvier C, Guix C, Bordeaux M-C, Arumae U, Mehlen P. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci USA. 2007;104:13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 97.Vega JA, Garcia-Suarez O, Hannestad J, Perez-Perez M, Germana A. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–137. doi: 10.1016/S1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 99.Labouyrie E, Parrens M, deMascarel A, Bloch B, Merlio JP. Distribution of NGF receptors in normal and pathologic human lymphoid tissues. J Neuroimmunol. 1997;77:161–173. doi: 10.1016/S0165-5728(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 100.Lambiase A, BracciLaudiero L, Bonini S, Starace G, Delios MM, DeCarli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–414. doi: 10.1016/S0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- 101.Ehrhard PB, Ganter U, Bauer J, Otten U. Expression of functional Trk protooncogene in human monocytes. Proc Natl Acad Sci USA. 1993;90:5423–5427. doi: 10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boven LA, Middel J, Portegies P, Verhoef J, Jansen GH, Nottet H. Overexpression of nerve growth factor and basic fibroblast growth factor in AIDS dementia complex. J Neuroimmunol. 1999;97:154–162. doi: 10.1016/S0165-5728(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 103.Besser M, Wank R. Cutting edge: clonally restricted production of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J Immunol. 1999;162:6303–6306. [PubMed] [Google Scholar]
- 104.Schober A, Huber K, Fey J, Unsicker K. Distinct populations of macrophages in the adult rat adrenal gland: a subpopulation with neurotrophin-4-like immunoreactivity. Cell Tissue Res. 1998;291:365–373. doi: 10.1007/s004410051006. [DOI] [PubMed] [Google Scholar]
- 105.Torcia M, BracciLaudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/S0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 106.Rosini P, De Chiara G, Lucibello M, Garaci E, Cozzolino F, Torcia M. NGF withdrawal induces apoptosis in CESS B cell line through p38 MAPK activation and Bcl-2 phosphorylation. Biochem Biophys Res Commun. 2000;278:753–759. doi: 10.1006/bbrc.2000.3871. [DOI] [PubMed] [Google Scholar]
- 107.Fauchais A-L, Lalloue F, Lise M-C, Boumediene A, Preud’homme J-L, Vidal E, Jauberteau M-O. Role of endogenous brain-derived neurotrophic factor and sortilin in B cell survival. J Immunol. 2008;181:3027–3038. doi: 10.4049/jimmunol.181.5.3027. [DOI] [PubMed] [Google Scholar]
- 108.D’Onofrio M, de Grazia U, Morrone S, Cuomo L, Spinsanti P, Frati L, Gulino A, Ragona G. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur Cytokine Netw. 2000;11:283–291. [PubMed] [Google Scholar]
- 109.Schuhmann B, Dietrich A, Sel S, Hahn C, Klingenspor M, Lommatzsch M, Gudermann T, Braun A, Renz H, Nockher WA. A role for brain-derived neurotrophic factor in B cell development. J Neuroimmunol. 2005;163:15–23. doi: 10.1016/j.jneuroim.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 110.Schenone A, Gill JS, Zacharias DA, Windebank AJ. Expression of high- and low-affinity neurotrophin receptors on human transformed B lymphocytes. J Neuroimmunol. 1996;64:141–149. doi: 10.1016/0165-5728(95)00162-X. [DOI] [PubMed] [Google Scholar]
- 111.Klein G. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 1994;77:791–793. doi: 10.1016/0092-8674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 112.Franklin RA, Brodie C, Melamed I, Terada N, Lucas JJ, Gelfand EW. Nerve growth factor induces activation of MAP kinase and p90 (RSK) in human B lymphocytes. J Immunol. 1995;154:4965–4972. [PubMed] [Google Scholar]
- 113.Melamed I, Patel H, Gelfand EW. Trk tyrosine kinase dependent activation of Vav/Ras in human B cells by nerve growth factor. Journal of Allergy and Clinical Immunology. 1997;99:1181. [Google Scholar]
- 114.Kronfeld I, Kazimirsky G, Gelfand EW, Brodie C. NGF rescues human B lymphocytes from anti-IgM induced apoptosis by activation of PKC zeta. Eur J Immunol. 2002;32:136–143. doi: 10.1002/1521-4141(200201)32:1<136::AID-IMMU136>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 115.Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, Reiffers J, de Mascarel A, Merlio JP. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154:405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1061. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Begum-Haque S, Christy M, Ochoa-Reparaz J, Nowak EC, Mielcarz D, Hague A, Kasper LH. Augmentation of regulatory B cell activity in experimental allergic encephalomyelitis by glatiramer acetate. J Neuroimmunol. 2011;232:136–144. doi: 10.1016/j.jneuroim.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA. 1989;86:10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimata H, Yoshida A, Ishioka C, Kusunoki T, Hosoi S, Mikawa H. Nerve growth factor specifically induces human IgG4 production. Eur J Immunol. 1991;21:137–141. doi: 10.1002/eji.1830210121. [DOI] [PubMed] [Google Scholar]
- 120.Brodie C, Gelfand EW. Functional nerve growth factor receptors on human B lymphocytes—interaction with IL-2. J Immunol. 1992;148:3492–3497. [PubMed] [Google Scholar]
- 121.Thorpe LW, Werrbachperez K, Perezpolo JR. Effects of nerve growth factor on the expression of interleukin 2 receptors on cultured human lymphocytes. Ann N Y Acad Sci. 1987;496:310–311. doi: 10.1111/j.1749-6632.1987.tb35781.x. [DOI] [PubMed] [Google Scholar]
- 122.Brodie C, Oshiba A, Renz H, Bradley K, Gelfand EW. Nerve growth-factor and anti-CD40 provide opposite signals for the production of IgE in interleukin-4-treated lymphocytes. Eur J Immunol. 1996;26:171–178. doi: 10.1002/eji.1830260127. [DOI] [PubMed] [Google Scholar]
- 123.Abram M, Wegmann M, Fokuhl V, Sonar S, Luger EO, Kerzel S, Radbruch A, Renz H, Zemlin M. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009;182:4705–4712. doi: 10.4049/jimmunol.0802814. [DOI] [PubMed] [Google Scholar]
- 124.Beutel G, Meyer J, Ma LP, Yin SM, Eder M, von Neuhoff N, Wilkens L, Wei J, Hertenstein B, Heil G, Schlegelberger B, Ganser A, Li ZX, Baum C. Expression of the p75 neurotrophin receptor in acute leukaemia. Br J Haematol. 2005;131:67–70. doi: 10.1111/j.1365-2141.2005.05717.x. [DOI] [PubMed] [Google Scholar]
- 125.Saada S, Marget P, Fauchais AL, Lise MC, Chemin G, Sindou P, Martel C, Delpy L, Vidal E, Jaccard A, Troutaud D, Lalloue F, Jauberteau MO. Differential expression of neurotensin and specific receptors, NTSR1 and NTSR2, in normal and malignant human B lymphocytes. J Immunol. 2012;189:5293–5303. doi: 10.4049/jimmunol.1102937. [DOI] [PubMed] [Google Scholar]
- 126.Bellanger C, Dubanet L, Lise M-C, Fauchais A-L, Bordessoule D, Jauberteau M-O, Troutaud D. Endogenous neurotrophins and Trk signaling in diffuse large B cell lymphoma cell lines are involved in sensitivity to rituximab-induced apoptosis. PLoS One. 2011;6:27213. doi: 10.1371/journal.pone.0027213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sniderhan LF, Garcia-Bates TM, Burgart M, Bernstein SH, Phipps RR, Maggirwar SB. Neurotrophin signaling through tropomyosin receptor kinases contributes to survival and proliferation of non-Hodgkin lymphoma. Exp Hematol. 2009;37:1295–1309. doi: 10.1016/j.exphem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, MedinaSelby A, Coit D, Valenzuela P, Feinstein SC. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429–4436. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- 130.Xia D, Li W, Zhang L, Qian H, Yao S, Qi X. RNA interference-mediated knockdown of brain-derived neurotrophic factor (BDNF) promotes cell cycle arrest and apoptosis in B-cell lymphoma cells. Neoplasma. 2014;61:523–532. doi: 10.4149/neo_2014_064. [DOI] [PubMed] [Google Scholar]
- 131.Auffray I, Chevalier S, Froger J, Izac B, Vainchenker W, Gascan H, Coulombel L. Nerve growth factor is involved in the supportive effect by bone marrow-derived stromal cells of the factor-dependent human cell line UT-7. Blood. 1996;88:1608–1618. [PubMed] [Google Scholar]
- 132.Laurenzi MA, Beccari T, Stenke L, Sjolinder M, Stinchi S, Lindgren JA. Expression of mRNA encoding neurotrophins and neurotrophin receptors in human granulocytes and bone marrow cells—enhanced neurotrophin-4 expression induced by LTB4. J Leukoc Biol. 1998;64:228–234. doi: 10.1002/jlb.64.2.228. [DOI] [PubMed] [Google Scholar]