Abstract
The Helicase-like Transcription Factor (HLTF) belongs to the SWI/SNF family of proteins involved in chromatin remodeling. In addition to its role in gene transcription, HLTF has been implicated in DNA repair, which suggests that this protein acts as a tumor suppressor. Accumulating evidence indicates that HLTF expression is altered in various cancers via two mechanisms: gene silencing through promoter hypermethylation or alternative mRNA splicing, which leads to the expression of truncated proteins that lack DNA repair domains. In either case, the alteration of HLTF expression in cancer has a poor prognosis. In this review, we gathered published clinical and molecular data on HLTF. Our purposes are (a) to address whether HLTF alterations could be considered as cancer drivers or passengers and (b) to determine whether its different functions (transcription or DNA repair) could be diverted in clonal selection during cancer progression.
Keywords: HLTF, Tumor suppressor gene, Oncogene, Cancer, Post-replication DNA repair, Alternative mRNA splicing
Introduction
Tumors comprise different cell types that harbor characteristic phenotypic properties, which are referred to as the hallmarks of cancer [1]. The underlying mechanisms include (a) the high genomic instability of cancer cells and (b) the continuous Darwinian selection of tumor cells harboring optimal genomic configurations, which facilitates the clonal expansion of these cells [2]. This clonal progression is driven by mutations, epigenetic changes and chromosome alterations in cancer-related genes (oncogenes and tumor suppressor genes), called “drivers”, which are beneficial to cancer development. Drivers, however, are concomitant with other alterations, called “passengers”, which are randomly dispersed throughout the genome and have no direct beneficial effect, although these alterations modulate cell fate during neoplastic transformation. In this context, a collection of cell populations, or “subclones”, with various genotypes coexist within a tumor and undergo selective pressure from the tumor microenvironment. Thus, specific clones that acquire the fittest genotype at a given time will survive and build the tumor mass. During carcinogenesis, changes in tumor environment (cancer progression, treatment) and genomic instability constantly participate in the evolution of the intra-tumor heterogeneity [3].
The Helicase-like transcription factor (HLTF) is a member of the yeast mating SWItch/Sucrose Non-Fermenting (SWI/SNF) family of proteins which are involved in chromatin remodeling. Several studies have demonstrated the function of HLTF in gene transcription, DNA repair and genome stability maintenance, supporting a role for tumor suppression. In cancer, two different alterations in HLTF expression have been reported: epigenetic silencing through promoter hypermethylation or alternative mRNA splicing leading to the production of truncated proteins lacking DNA repair domains. In this review, we discuss the reported data on the functions of HLTF as a tumor suppressor gene (TSG), describe how this function is altered in cancer, and determine whether these alterations could be considered drivers or passengers.
The HLTF gene, transcripts and proteins
HLTF: the gene
Human HLTF is a 56.4 kb gene that is located on chromosome 3q25.1-q26.1 (chr3:148,747,904-148,804,341; UCSC) and comprises 26 exons. HLTF has been described using different names (Table 1) because several groups have independently discovered this gene through screening cDNA expression libraries to identify proteins binding to a specific DNA target sequence (cis-element). The aliases of HLTF are HIP116 [4], SNF2L3 [5], RUSH (rabbit homolog; [6]), P113 (mouse homolog; [7]), Zbu1 [8], and SMARCA3 (in OMIM), but HLTF [9] has recently been considered the official gene name (HUGO Gene Nomenclature Committee, HGNC, www.genenames.org).
Table 1.
DNA targets of HLTF
| Cis-elements | Processes | Protein partners | Organism | Sequence (5′–3′) | Assay | Groups |
|---|---|---|---|---|---|---|
| HIV-1 LTR | Undefined | – | H. sapiens, S. Pombe | TCGCATATAAGCAGCTGCTTTTTGCCTGTACTGGGT | EMSA | Sheridan et al. [4], Brys et al. [29] |
| SV-40 enhancer | Undefined | – | H. sapiens, S. Pombe |
SPH-I motif: TGCATGCTTT SPH-II motif: GCATACTTC |
EMSA | Sheridan et al. [4], Brys et al. [29] |
| Myosin Light Chain 1/3 enhancer | Muscle differentiation | – | H. sapiens | TCGACAAATTACCATGTGTG | EMSA | Gong et al. [8] and Ceccarelli et al. [36] |
| TNF response element | Undefined | – | H. sapiens | TGGCAGCCATGGCAGCCA | EMSA | Zhang et al. [7] |
| HEFT1 loci | Undefined | – | H. sapiens | TGAAGGGGTGTGGAGCCTGCCTG | EMSA | Ding et al. [30] |
| Beta-globin promoter | Control of Beta-globin transcription | – | H. sapiens | GCCAAGGACAGGTACGGCTGTCATCACTT |
EMSA, Luciferase Assay |
Mahajan and Weissman [31] |
| Beta-globin enhancer HS2 (LCR) | – | H. sapiens | TCCAAGCACAGCAATGCTGAGTCATGATGA |
EMSA, Luciferase Assay |
Mahajan and Weissman [31] | |
| SERPINE1 (PAI-1) promoter (B-box) | SERPINE1 transcription (Fibrinolysis, cell motility) | Sp1 and Sp3 | H. sapiens, S. Pombe, M. musculus |
CCAGTGAGTGGGTGGGGCTGGAACATG (A/G)G(T/C)(G/T)G (2 sites) |
EMSA, Luciferase Assay, Target definition assay |
Ding et al. [32] |
| – | Gong et al. [8], Zhang et al. [7] and Brys et al. [29] | |||||
| Uteroglobin (Scgb1a1) promoter | Uterogloblin transcription (Endometrium maturation) | – | O. cuniculus | (C/A)C(T/A)TN(T/G) | EMSA, ChIP, CASTing | Hayward-Lester et al. [6], Hewetson et al. [33] |
| Enhancer of OCA2 (in intron 86 of HERC2) | Control of OCA2 transcription [Melanocyte maturation (eye)] | LEF1 and MITF | H. sapiens | TGACA(T/C)TTAAT | MatInspector (Genomatix), ChIP | Visser et al. [21], Sturm et al. [34, 35] |
|
Prl promoter (E box) |
Prolactin transcription (circadian cycle) | NONO and SPQF | R. norvegicus | AATAC CAT TTGATGTTT | MatInspector (Genomatix), ChIP | Guillaumond et al. [25] |
| Gata4 promoter | Gata4 transcription (Angiogenesis, apoptosis, cell cycle) | – | M. musculus | (A/C)CTT(T/A)G (2 sites) | ChIP | Helmer et al. [27] |
| Hif-1a promoter | Hif-1 alpha transcription | – | M. musculus | CCATTG (2 sites) | ChIP | Helmer et al. [27] |
| Myh7b/miR499 promoter |
Myosin expression (cardiac myofiber) miR-499 expression (Gata4 regulation) |
– | M. musculus | (C/A)C(T/A)T(T/A)(G/T) (3 sites) | ChIP | Helmer et al. [27] |
| Scgb3a1 promoter | Secretoglobin transcription | – | M. musculus | CCATAG | ChIP | Helmer et al. [23] |
| P-element in chr. 7 | Olfactory and taste receptors (sperm cells) | – | M. musculus | ACTTTT | ChIP | Helmer et al. [23] |
Known DNA targets and protein partners of HLTF involved in transcription are summarized in this table. Cellular processes in which HLTF is involved are indicated. Sequences bound by HLTF are shown in the 5′–3′ orientation, and the consensus sequences recognized by HLTF are indicated in bold, when available. For each study, the assays used to confirm HLTF binding to DNA and the organisms in which these interactions occur are indicated. HIV-1 LTR, Human Immunodeficiency Virus 1 Long Terminal Repeat; SV-40, Simian Virus 40; TNF, Tumor Necrosis Factor; PAI-1, Plasminogen-Activator Inhibitor 1; SCGB, Secretoglobin; OCA2, OculoCutaneous Albinism 2; HERC2, HECT And RLD Domain Containing E3 Ubiquitin Protein Ligase 2; Prl, Prolactin; Gata4, GATA binding protein 4; Hif-1, Hypoxia Induced Factor 1; Myh7b, Myosin Heavy Chain 7Beta Cardiac Muscle; Sp1/Sp3, Sp1/Sp3 transcription factor; LEF1, Lymphoid enhancer-binding factor 1; MITF, Microphthalmia-associated transcription factor; NONO, Non-POU domain containing-octamer-binding; SPQF, Splicing factor proline/glutamine-rich; EMSA, Electrophoretic Mobility Shift Assay; ChIP, Chromatin Immunoprecipitation assay; HLTF cannot activate gene transcription alone and requires different protein partners according to the cell type and target gene; HLTF activates the PAI-1/SERPINE1 gene transcription by binding to a double “(A/G)G(T/C)(G/T)G” cis-element overlapping a GT box (Sp1/Sp3 recognition site) in HeLa cells [32], mouse preadipocytes (30A5 cells, [8]) and yeast (S; pombe, [29]); Studies have shown that HLTF activates transcription in synergy with Sp1/Sp3, the HLTF 80 carboxyl-terminal residues specifically interacted with the Sp1 zinc finger domain [32]. HLTF mediates cyclic Prl expression in rat lactotropic pituitary cells (GH4C1 cells) through direct interactions with its cis-element “(C/A)C(T/A)TN(T/G)”, Non-POU domain containing-octamer-binding (NONO) and Splicing factor proline/glutamine-rich (SPQF) proteins, the latter two proteins are expressed according to the circadian cycle [25]. HLTF (RUSH) alone activated Uteroglobin gene expression in rabbit endometrial cells through binding to the “(C/A)C(T/A)TN(T/G)” sequence in its promoter [33]. HLTF, in concert with Lymphoid enhancer-binding factor 1 (LEF1) and Microphthalmia-associated transcription factor (MITF), activates OCA2 transcription in melanocytes by binding to the cis-element in an enhancer (intron 86 of HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2) gene) and controlling OCA2 expression [21]
HLTF transcripts
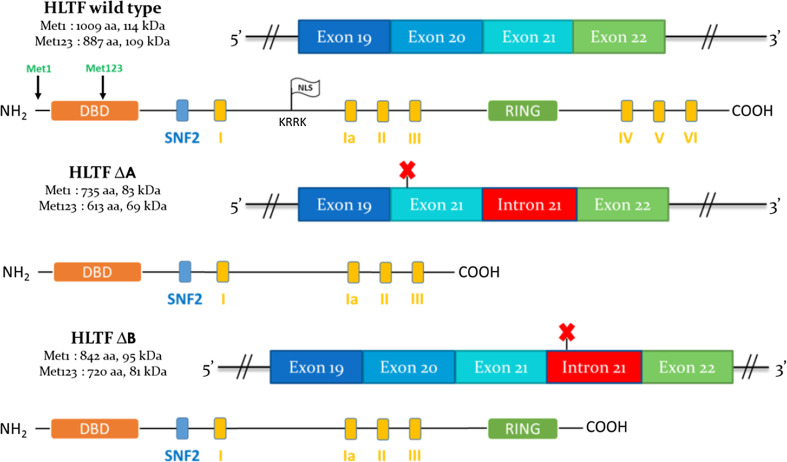
Two HLTF mRNAs are expressed in most human tissues and cell lines (www.genecards.org), and these 5.5- and 4.5-kb mRNAs [4, 8, 9] differ based on alternative splicing in the 3′ untranslated region (UTR). Both mRNAs harbor two translation start sites in the same reading frame (Met1 and Met123 codons) leading to the synthesis of two protein forms with distinct amino-termini (Fig. 1; [9]). In addition, Hayward-Lester et al. [6] described that the mRNA transcript of RUSH, the rabbit HLTF homolog, undergoes an estrogen-driven alternative splicing in the uterus, resulting in the retention of intron 21, which confers an early stop codon. A similar event was later described in HeLa cells (cervix adenocarcinoma) [10] without any evidence of hormone influence: the retention of intron 21 occurs alternatively with the retention or the loss of exon 20, which leads to frameshifts and early stop codons (Fig. 1). Accordingly, two protein forms are produced that possess shorter carboxyl-termini and lack functional domains (RING and DNA helicase, see below).
Fig. 1.
Alternative splicing and encoded proteins of HLTF. For each HLTF protein form, the name, number of amino acids and the theoretical molecular weight (kDa) are indicated (left). Met1 is the first methionine, corresponding to the first translation start site, and Met123 is the methionine 123, corresponding to the alternative translation start site (exon 3). The protein domains are indicated as colored boxes. DBD (orange): DNA-binding domain. SNF2 (blue): SNF2 domain. Yellow boxes from I to VI: Helicase/ATPase domains. RING (green): RING domain. The white flag “NLS” represents the nuclear localization signal (amino acids KRRK at position 380 encoded by exons 10–11; [23]). Above each protein, the mRNA area of exons 19-22 is represented. This area is subjected to alternative splicing, resulting in protein forms truncated at the carboxyl-terminus. Exons and introns are represented as colored rectangles. The red crosses indicate the early stop codons, resulting from reading frameshifts. Adapted from [10]
HLTF transcripts are expressed in most normal human tissues [8, 9], and HLTF expression is 20-fold higher in a variety of transformed cell lines compared with primary human fibroblasts or normal tissues. Gong et al. [8] showed that in late passages of cultured fibroblasts from Li–Fraumeni patients (germline mutation in TP53), HLTF transcript levels were higher than in normal fibroblasts, but no putative role for HLTF in carcinogenic transformation was investigated. The same group described the evolution of HLTF expression during mouse ontogeny. HLTF mRNAs accumulate at late embryonic stages (17–18 days post-coitum), whereas HLTF protein accumulates asynchronously (in contrast with the transcript) in tissues. After birth, HLTF protein persists in several differentiated organs (skeletal muscle, brain and testes). However, Zhang et al. [7] showed a different HLTF expression profile in rats, with high levels of HLTF expression observed in brain, spleen, kidney, testes and heart and undetectable levels found in skeletal muscle. In human tissues, HLTF transcript profile was studied using northern blot [9], microarray (www.biogps.org) and RNAseq (Illumina Human Bodymap) analysis, and the results were similar to those obtained in mice, with the highest expression occurring in testes.
Regulation of HLTF gene transcription
Hewetson and Chilton [16] described the HLTF (RUSH) promoter in rabbit and identified highly conserved elements between rabbits and humans, which suggested that HLTF regulation was similar in both species. HLTF promoter lacks a TATAA box near the transcription start site. However, a unique Initiator/Downstream Promoter Element (Inr/DPE) controls HLTF transcription, which is negatively regulated at two Sp1 binding sites through HLTF/RUSH itself. Promoters frequently occur in CpG islands. These regions of several hundred base pairs contain many CpG dinucleotides that are recognized by DNA methyltransferases (DNMTs), which in turn methylate (5-methylcytosines), hemimethylate (DNMT1, 3a and 3b) or unmethylate (DNMT3a and 3b) cytosines on DNA. This epigenetic mark facilitates the transcriptional repression of most repeated elements and specific genes via two mechanisms: (i) DNA methylation sterically blocks access to some transcription factors, and (ii) methylcytosines recruit Methyl-CpG binding proteins (MBPs) that interact with Histone deacetylases (HDAC), thereby compacting chromatin in promoter regions. In normal cells, CpG islands are typically unmethylated, but in cancer, epigenetic deregulations occur, which lead to the promoter hypermethylation of tumor suppressor genes, which are subsequently repressed (reviewed in [17]). The HLTF promoter contains 59 CpGs in a 653-bp CpG island (http://genome-euro.ucsc.edu), and silencing of HLTF through promoter hypermethylation was initially reported in colon [13], then in gastric, cervix, bladder, hepatocellular and lung cancers (summarized in Table 3).
Table 3.
HLTF methylation frequency in cancer
| Group | Ethnicity | Assay | Sample | Methylation frequency (n, %) | ||
|---|---|---|---|---|---|---|
| Normal | Adenoma/dysplasia | Cancer | ||||
| Colon carcinoma | ||||||
| Moinova et al. [13] | USA | MSP | Tumor | 9/78 (11.5 %) | 7/28 (25 %) | 27/63 (42.8 %) |
| Kim et al. [56] | USA | MSP | Tumor | 0/10 (0.0 %) | 11/42 (26.1 %) | 27/77 (35 %) |
| Brandes et al. [57] | USA | MSP | Tumor | NA | NA | 28/58 (48.2 %) |
| Total USA | 9/88 (10.2 %) | 18/70 (25.7 %) | 82/198 (41.4 %) | |||
| Bai et al. [58] | Asia | MSP | Tumor | 2/16 (12.5 %) | 24/35 (68.5 %) | 27/47 (57.4 %) |
| Hibi et al. [59] | Asia | MSP | Tumor | 0/20 (0.0 %) | NA | 25/76 (32.8 %) |
| Hibi et al. [60] | Asia | MSP | Tumor | NA | NA | 20/61 (32.7 %) |
| Hibi et al. [61] | Asia | MSP | Tumor | NA | NA | 18/58 (31.0 %) |
| Kang et al. [55] | Asia | MSP | Tumor | NA | NA | 74/219 (33.7 %) |
| Lee et al. [62] | Asia | MSP | Tumor | 3/148 (2.0 %) | NA | 78/243 (32.0 %) |
| Total Asia | 5/164 (3.0 %) | 24/35 (68.5 %) | 242/704 (34.3 %) | |||
| General total | 14/252 (5.5 %) | 42/105 (40.0 %) | 324/902 (35.9 %) | |||
| Wallner et al. [63] | Europe | MSP | Serum | 0/20 (0 %) | NA | 31/103 (30.0 %) |
| Herbst et al. [64] | Europe | MSP | Serum | NA | NA | 13/106 (12.2 %) |
| Herbst et al., 2011 [65] | Europe | MSP | Serum | 1/32 (3.1 %) | NA | 19/110 (17.2 %) |
| Philipp et al., 2012 [66] | Europe | MSP | Serum | NA | NA | 48/311 (15.4 %) |
| Philipp et al. [67] | Europe | MSP | Serum | NA | NA | 41/259 (15.8 %) |
| Total Europe | 1/52 (1.9 %) | NA | 152/889 (17.0 %) | |||
| Leung et al. [68] | Asia | MSP | Serum | 3/41 (7.3 %) | NA | 14/49 (28.6 %) |
| Lee et al. [62] | Asia | MSP | Serum | 3/148 (2.0 %) | NA | 78/243 (32.0 %) |
| Total Asia | 6/189 (3.1 %) | NA | 92/292 (31.5 %) | |||
| General total | 7/241 (2.9 %) | NA | 244/1181 (20.6 %) | |||
| Itzkowitz et al. [69] | USA | MSP | Feces | 9/122 (7.3 %) | NA | 15/40 (37.5 %) |
| Leung et al. [70] | Asia | MSP | Feces | 1/30 (3.3 %) | 6/30 (20.0 %) | 4/20 (20.0 %) |
| General total | 10/152 (6.6 %) | 6/30 (20 %) | 19/60 (31.6 %) | |||
| Gastric carcinoma | ||||||
| Hamai et al. [71] | Asia | MSP | Tumor | 0/10 (0.0 %) | NA | 25/50 (50 %) |
| Leung et al., 2003 [72] | Asia | MSP | Tumor | 1/10 (10 %) | NA | 13/46 (28.3 %) |
| Hibi et al. [59] | Asia | MSP | Tumor | 0/20 (0.0 %) | NA | 11/65 (16.9 %) |
| Oue et al. [73] | Asia | MSP | Tumor | 0/10 (0.0 %) | NA | 40/75 (53.3 %) |
| Kim et al. [74] | Asia | MSP | Tumor | NA | NA | 98/256 (38.2 %) |
| Guo et al. [75] | Asia | MSP | Tumor | 8/96 (8.3 %) | 21/102 (20.5 %) | 44/96 (45.8 %) |
| Plasma | NA | 1/25 (4.0 %) | 20/96 (20.8 %) | |||
| Total | 9/146 (6.1 %) | 21/102 (20.5 %) | 231/588 (39.2 %) | |||
| Esophageal Squamous Cell Carcinoma (ESCC) | ||||||
| Hibi et al. [59] | Asia | MSP | Tumor | 0/10 (0.0 %) | NA | 1/40 (2.5 %) |
| Fukuoka et al. [76] | Asia | MSP | Tumor | NA | NA | 1/35 (2.8 %) |
| Total | 0/10 (0.0 %) | NA | 2/75 (2.6 %) | |||
| Uterine carcinoma: Cervical Squamous Cell Carcinoma (CSCC), Cervical Adenocarcinoma (CA) and Endometrial Adenocarcinoma (EA) | ||||||
| Kang et al. [77] | Asia | MSP | Tumor | NA | NA |
CSCC: 2/62 (3.2 %) CA: 13/30 (43.3 %) EA: 10/21 (47.6 %) |
| Jo et al. [78] | Asia | MSP | Tumor | NA | NA | 4/82 (5 %) |
| Total | NA | NA |
CSCC + CA: 19/174 (10.9 %) EA: 10/21 (47.6 %) |
|||
| Lung Carcinoma: Squamous Cell Carcinoma (SCC) and Adenocarcinoma (ADC) | ||||||
| Castro et al. [79] | Europe | MS-MLPA | Tumor | NA | NA |
SCC: 9/20 (45 %) ADC: 12/33 (37.5 %) |
| Hepatocellular Carcinoma | ||||||
| Zhang et al. [80] | Asia | MSP | Tumor | 0/48 (0 %) | NA | 3/48 (6.3 %) |
| Bladder Carcinoma | ||||||
| Garcia-Baquero et al. [81, 82] | Europe | MS-MLPA | Urine | 3/23 (13 %) | NA | 16/100 (16 %) |
| Tumor | NA | NA | 2/63 (3 %) | |||
The number of patients (n) and the frequency (%) are indicated for each study. MSP methylation-specific PCR, MS-MLPA methylation-specific multiplexes ligation probe amplification
HLTF proteins
HLTF proteins contain the following functional domains (Fig. 1): (a) a HIP116 Rad5p N-terminal (HIRAN) domain embedded in a larger DNA-binding domain, (b) a Sucrose Non-Fermenting 2 (SNF2) amino-terminal dimerization domain, (c) seven conserved DNA helicase/ATPase domains, characteristic of SWI/SNF2 family members and (d) a Really Interesting New Gene (RING) finger domain. These domains are highly conserved across species [11]. In section II, we discuss the involvement of these domains in the intracellular roles of HLTF.
As described above (HLTF transcripts), two wild-type HLTF proteins that differ at the amino terminus are encoded by the same transcripts, which contain two translation start sites (Met1 and Met123; Fig. 1) [9]. In addition, alternative pre-mRNA splicing in the exons 19-22 region generates two reading frameshifts, which result in truncated protein forms [14]. Considering both translation start sites and splicing events, six different protein forms could be produced from the HLTF gene (Fig. 1).
Regulation of HLTF protein levels
Kim et al. [18] showed that HLTF undergoes negative regulation through the E3 ubiquitin ligase Checkpoint with Forkhead and RING finger domains (CHFR) that promotes HLTF degradation mediated by the proteasome. Both proteins interact with each other via their RING domain (confirmed by immunoaffinity combined with mass spectrometry, co-immunoprecipitation and GST-pull down). An in vitro ubiquitination assay in the presence of CHFR and HLTF showed that HLTF is poly-ubiquitinated by CHFR on sites that were not identified [18]. Cell treatment by MG132, a proteasome inhibitor, prevented CHFR-mediated degradation of HLTF. In addition, a pulse-chase assay confirmed that HLTF half-life was shortened in the presence of CHFR. The consequence of HLTF degradation on downstream events was assessed on a direct target gene: PAI-1 (Table 1). In cells expressing CHFR, triggering HLTF degradation, PAI-1 expression was not detected and cell migration was reduced [18]. Further experiments on the effect of HLTF degradation on other downstream targets should be performed, as HLTF is an important actor in DNA damage repair (see "The HLTF gene, transcripts and proteins").
In the reverse process, the protein half-life is increased through the removal of the conjugated ubiquitin. Qing et al. [19] demonstrated that Ubiquitin Specific Peptidase 7 (USP7), a deubiquination enzyme (DUB), interacts with and stabilizes HLTF both in vitro and in vivo. In USP7-depleted cells, the levels of HLTF protein, but not mRNA, were decreased. In contrast, the HLTF protein levels were restored through proteasome inhibition, which suggested that USP7 protects HLTF from ubiquitin–proteasome-mediated degradation. Moreover, USP7 deubiquitinates CHFR-ubiquitinated HLTF. However, there was no binding competition between USP7 and CHFR to HLTF. Therefore, HLTF is negatively and positively regulated via CHFR and USP7, respectively.
Cellular functions of HLTF
HLTF is a transcription factor
HLTF was identified as a transcription factor based on its capacity to specifically bind DNA sequences (Table 1; Fig. 1). Studies were performed in different species (human, rabbit, mouse, rat and yeast; see Table 1) and suggest that most HLTF target genes are conserved in humans: (a) HLTF protein binds the same cis-element in different species (Table 1), (b) HLTF is highly conserved across species [11] and (c) the additional roles for HLTF are similar (DNA repair, chromatin remodeling) across species [12, 20, 21]. Several studies have highlighted the importance of HLTF in the induction of genes involved in a variety of processes, summarized in Table 1. Two different consensus sequences recognized by HLTF were proposed: (A/G)G(T/C)(G/T)G [9] and (C/A)C(T/A)TN(T/G) [6]. The latter sequence was successfully used in a Genomatix analysis (MatInspector; [22]) to develop an algorithm for identifying putative HLTF binding sites in genomic sequences ([23]; Table 1). The two HLTF binding consensus sequences were defined using a similar screening strategy from a collection of random sequence DNA oligonucleotides: a target definition assay [32] or CASTing [33]. The only difference was that the screening was performed using either the HLTF DNA-binding domain expressed in E. coli as a glutathione-S-transferase (GST) fusion [32] or HLTF in rabbit nuclear extracts [33]. HLTF binding to these sequences was confirmed through site-directed mutagenesis and luciferase assays [32] and through chromatin immunoprecipitation (ChIP; [21, 23, 25, 27, 33–36]).
We cannot exclude the fact that HLTF binds other DNA sequences. Indeed, HLTF recognizes replication fork-like DNA structures [12] that are associated with its ATPase activity. HLTF can activate gene transcription either alone or with different protein partners (Table 1).
HLTF-mediated regulation involves long-distance chromatin looping [21] for Prl and OCA2 expression [25] and its own gene downregulation mediated with Early Growth Response 1 (EGR1) and V-Rel Avian Reticuloendotheliosis Viral Oncogene Homolog (c-Rel) proteins [26].
Recently, splice variants of Hltf mRNA were quantified in control mice, which showed that the wt mRNA (exons 1-25) and the splice variant (exons 1-21 extended by intron 21) were present in a 5:1 ratio in the brain and at a 26:1 ratio in the heart. The protein forms (116 and 97 kDa, respectively) encoded by these alternatively spliced transcripts were both expressed in the brain, but only the full-length protein (116 kDa) was expressed in the heart [27]. These findings underscore a role for HLTF in development (particularly for brain and heart), consistent with the findings of Gong et al., showing that HLTF transcripts were expressed in these organs [8]. The brain and heart transcriptomes were studied after the specific inactivation of Hltf in mice [23, 27] and suggested additional roles for HLTF in different pathways (Table 2). HLTF is involved in the G2/M transition of the cell cycle via 4 mechanisms: sister chromatid holding (HLTF deletion reduces the mRNAs of genes involved in chromosome cohesion), chromosome condensation (compaction of sister chromatids), centrosome separation and aberrant chromosome segregation and cell cycle arrest (resulting from HLTF deletion). The deletion of Hltf severely impairs post-replication and double-strand DNA repair (see below) [28]. This disturbance of the Hltf function in the maintenance of genome stability triggers apoptosis. Hltf participates in cardiac development by coordinating the genes involved in (a) the genesis of the collagen fibrillar network (posttranslational modification of collagen, trimerization and association with elastin), (b) angiogenesis and (c) contractile function.
Table 2.
Main pathways/transcripts dysregulated in Hltf knockdown mice
| Pathways | Transcripts dysregulation |
|---|---|
| 1. Cell cycle: G2/M transition | |
| 1.1. Chromosome cohesion | ↓ Rad21/Scc1 |
| 1.2. Chromosome condensation | ↓ Smc2/Cap-E, ↓ Cap-G/G2 |
| 1.3. Centrosome separation and chromosome separation | ↓ Aurora B kinase, ↓Histone H3 |
| 1.4. Cell cycle arrest in G2/M | ↓ Rad52 |
| 2. DNA Repair |
↓ Brca1 and 12 members of BASC ↓ Fanconi Anemia members |
| 3. Apoptosis |
↓ Cflar (inhibitor of apoptosis) Loss of DNA repair role of Hltf ↓ Brca1 and 12 members of BASC |
| 4. Collagen biogenesis | ↓ Hif1a and collagen hydroxylases, trimerization PPIases and lysyl oxidase |
| 5. Angiogenesis | ↓ Gata4, Hif1a, Wt1 and Vegfa |
| 6. Contractile function | ↓ Myh7/miR499 |
HLTF and DNA damage tolerance pathways: post-replication DNA repair
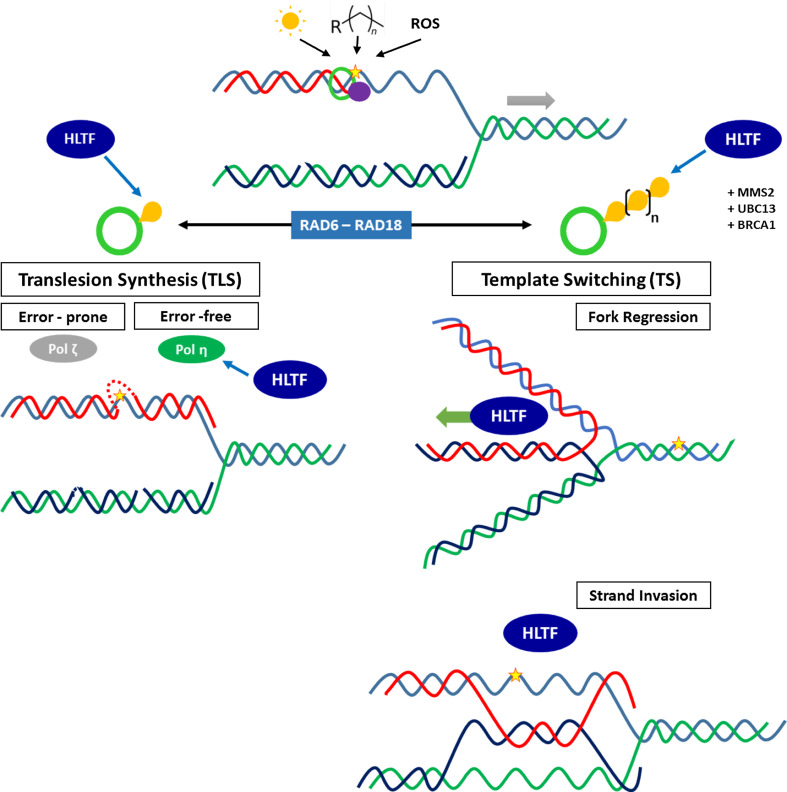
DNA constantly undergoes endogenous and exogenous events, which cause damage. DNA repair processes remove lesions to avoid aberrant DNA replication, a source of mutations, double-strand breaks and chromosome rearrangements associated with pathological disorders, such as cancer [37]. However, DNA damage can escape repair and interfere with DNA replication. A prolonged stalling of the replication fork induces replisome dislocation and fork collapse, which leads to cell death [12, 37]. To sustain replication progression, DNA damage tolerance pathways are activated: these mechanisms do not repair lesions but instead promote error-free (non-mutagenic) or error-prone (mutagenic) replication of damaged DNA [12]. These mechanisms, which are discussed below, were extensively studied in the budding yeast S. cerevisiae but are highly conserved within eukaryotes [37]. Here, we focus on HLTF (the ortholog of yeast Rad5) involvement in the RAD6-RAD18-dependent damage tolerance pathway, which is primarily activated in response to bulky DNA lesions (caused by UV irradiation, alkylating agents, reactive oxygen species) and facilitates DNA damage bypass. This pathway includes two mechanisms: (a) translesion synthesis and (b) template switching. They are activated through ubiquitination of Proliferating Cell Nuclear Antigen (PCNA), a DNA polymerase sliding clamp and key element of the replisome [12]. The different activities of HLTF in these mechanisms are discussed to show the important role of this protein in the maintenance of genome stability (Fig. 2).
Fig. 2.
HLTF involvement in DNA damage tolerance pathways (DDT) at stalled replication forks (RF). Parental DNA strands are indicated in blue and green; nascent strands are indicated in red (leading strand) and dark blue (lagging strand, Okazaki fragments). The RF direction is indicated as a gray arrow. DNA might be damaged through UV (sun), alkylating agents (Rn) or reactive oxygen species (ROS) that induce bulky lesions (yellow star). In this case, the RF might stall because the replisome is reduced to PCNA (green ring), and DNA polymerase δ (purple circle) stalls at the lesion site. PCNA ubiquitination (Ub are yellow bubbles), governed by RAD6-RAD18, directs the activation of either TLS (mono-Ub PCNA) or TS (poly-Ub PCNA). In TLS, the random recruitment of low-fidelity DNA polymerases occurs, facilitating the lesion bypass in an error-prone or an error-free way. HLTF promotes mono-Ub of PCNA as an additional error-free translesion polymerase recruitment. In TS (poly-Ub PCNA), HLTF is involved in the Fork Regression and Strand Invasion mechanisms. HLTF first promotes poly-Ub of PCNA, with other partners, such as BRCA1, MMS2-UBC13 and RAD6-RAD18 complexes. In Fork Regression, HLTF translocase activity facilitates the annealing of the stalled nascent strand (red) with the newly synthesized one of the sister duplex (dark blue), which leads to an error-free bypass of the lesion. In Strand Invasion, HLTF mediates D-loop formation in a homologous region of the lesion of the sister duplex in an ATP-independent or/and ATP-dependent fashion. The single-strand nascent strand (red) invades the D-loop and anneals with the donor strand (dark blue). Here, the error-free bypass of the lesion is also promoted through HLTF
HLTF as an E3-ubiquitin ligase
When high-fidelity polymerases (Polδ and Polε) encounter a bulky DNA lesion, the replisome is blocked and the replication fork stalls. PCNA ubiquitination at its K164 residue serves as a molecular switch for DNA damage tolerance pathways [38] and does not promote its proteasome-mediated degradation. PCNA can be either mono- or poly-ubiquitinated and activate translesion synthesis or template switching, respectively (Fig. 2).
In the case of translesion synthesis, a switch occurs from high- to low-fidelity DNA polymerases, which bypass the damage in an error-prone (Polζ) or error-free (Polη) manner [38, 39]. This switch is activated by PCNA mono-ubiquitination (K164) via E2-ubiquitin ligase RAD6 and RING type E3-ubiquitin ligases RAD18, HLTF and BRCA1 [38, 39, 41, 42]. The RING domain is a protein loop structure stabilized through the interactions of two zinc ions with eight residues (7 cysteines and 1 histidine; C3HC4 motif [40]), which confers E3 ubiquitin ligases with substrate specificity and the ability to interact with other RING-containing proteins. After UV-directed DNA damage, HLTF promotes PCNA mono-ubiquitination and the recruitment of Polη (Fig. 2) [28, 43]. BRCA1 recruits HLTF, RAD18 and Polη through its RING domain [28]. Hltf inactivation reduces the expression of Brca1 and Brca1-associated genome surveillance complex (Basc), which is also involved in double-strand DNA break and post-replication repair (Table 2) [23].
Activation of template switching is triggered via poly-ubiquitination of a previously mono-ubiquitinated PCNA (K63-linked polyubiquitin chain). This process is mediated by RAD6 (E2 ubiquitin ligase), MMS2-UBC13 (E2 variants), and RAD18, HLTF and BRCA1 (E3 ubiquitin ligases) [28, 37, 38].
HLTF promotes PCNA poly-ubiquitination by forming a thioester linked Ub chain (via Cys 760 in its RING domain) on UBC13 and subsequently transfers this chain to the RAD6-RAD18 complex. The chain is then transferred to PCNA by RAD18 (Fig. 2) [44].
In addition, another ortholog of yeast Rad5 is present in mammalian cells: SNF2 Histone-linker PHD-finger RING-finger Helicase (SHPRH). Yeast Rad5, human SHPRH, and HLTF share a unique structural feature and sequence identities, whereby the RING domain is embedded between the conserved helicase motifs IV and V of the SWI2/SNF2 domain (Fig. 1). Both HLTF and SHPRH proteins poly-ubiquitinate PCNA, but they are recruited through different DNA lesion types [43]. HLTF is exclusively recruited after UV damage and inhibits SHPRH via direct protein/protein interaction. In contrast DNA alkylation by methyl-methane-sulfonate (MMS) triggers HLTF degradation through auto-ubiquitination, whereas SHPRH is recruited to the damaged sites. HLTF-deficient cells exhibit higher UV and MMS sensitivity [38] and chromosome aberrations [39]. HLTF can also complement Rad5 deficient yeast for resistance to UV [38]. However, some studies [45, 46] showed that HLTF and PCNA ubiquitination had minimal importance in DNA damage tolerance pathways, which suggests that alternative pathways exist. The studies discussed above endorse the role of HLTF in preventing mutagenesis through both translesion synthesis and template switching. The latter occurs through two mechanisms: replication fork regression and strand invasion. Both mechanisms result in the annealing of the stalled nascent strand to the newly synthesized strand of the undamaged sister duplex [47]. The two following points summarize HLTF involvement in these processes, called post-replication DNA repair (PRR; Fig. 2).
HLTF as a branch-migrating enzyme (replication fork regression)
The seven conserved SWI/SNF helicase motifs confer HLTF a double-stranded DNA translocase activity to facilitate fork regression at a lesion site [12]. In vitro, HLTF mediates the concerted unwinding of leading and lagging strands and subsequently anneals the nascent and parental strands without exposing any single-stranded DNA (Fig. 2). HLTF translocase activity is ATP-dependent and has a 3′-5′ polarity [48]. Both RING and helicase domains are critical for this process [48]. Studies on HLTF HIRAN domain (Fig. 1) revealed how HLTF recognizes a stalled replication fork and restarts it by fork regression [49, 50]. Stalled replication forks contain a 3′-hydroxyl group (3′-OH) on the nascent leading strand, which mimics a site of two unpaired nucleotides («lesion»). HLTF specifically recognizes this «lesion» by its HIRAN domain pocket in which (a) the two unpaired nucleotides are stuck between two tyrosines (Y72 and Y93) and (b) the 3′-OH single DNA (ssDNA) end binds to an aspartate (D94) [51, 52].
A stalled replication fork is fragile because of (a) a delay between the leading and the lagging strand replication and (b) exposure of single-stranded DNA regions. This unstable structure is surrounded with DNA-binding proteins, such as RPA, PCNA or RFC. They are part of the paused replisome or hide single-strand DNA regions and inhibit access to damage bypass players. HLTF remodels DNA–protein complexes at stalled replication fork with its ATPase function: it catalyzes the clearance of both double- and single-strand DNA-binding proteins [47, 53] and thus provides access to the DNA bypass players. In addition, through its DNA translocase activity and its HIRAN domain, HLTF regresses the replication fork by annealing the stalled nascent strand to the undamaged newly synthesized strand [47, 48, 51]. These observations indicate that HLTF leads to an error-free bypass of DNA damage, thereby preventing mutations to spread to newly replicated DNA.
HLTF as a strand invasion promoter
A DNA lesion can be bypassed through homologous recombination as an alternative pathway to fork regression. This mechanism is mediated by several proteins, such as RAD51, RAD54 or BRCA2, and is characterized by the formation of a nucleoprotein filament, which facilitates the alignment of a single-strand DNA end to the homologous region through sister chromatid invasion. This process leads to the formation of a D-loop structure to access the homologous region [54]. HLTF facilitates DNA strand invasion and the formation of a D-loop structure with no requirement for ATP (Fig. 2). Burkovics et al. [54] suggested that HLTF forms a D-loop in two ways: (a) dimerization to bridge the invading and donor strands and (b) changes in the DNA conformation by inducing a topological stress that facilitates single-strand DNA invasion into the donor double-strand DNA. This latter mechanism is, however, ATP-dependent. Because these experiments were performed in vitro, it cannot be excluded that in vivo, both ATP-dependent and ATP-independent mechanisms have a functional relevance [54].
HLTF and cancer
HLTF expression is altered in cancer through either of two mechanisms: gene silencing by promoter hypermethylation or expression of truncated proteins that lack functional domains. Here, we summarize these data and emphasize the clinical relevance of HLTF expression disturbance.
HLTF silencing through promoter hypermethylation
Moinova et al. initially observed the hypermethylation of HLTF gene promoter [13] in colon cancer and associated it with chromatin compaction and transcription silencing. This group identified HLTF promoter sequences (GenBank accession no. NT_005616) in a CG rich area (CpG island, see above), presenting a consensus initiator element, two Sp1 binding sites and no TATA box. This structure is typical of TATA-less housekeeping gene promoters (see Regulation of HLTF gene transcription, [16]).
HLTF expression is inversely correlated with the methylation status of its promoter, as expected for this epigenetic marker. However, the mechanism by which HLTF promoter is methylated remains unknown. Kang et al. [55] compared the methylation of HLTF promoter with DNMT1 (DNA Methyltransferase 1) mRNA level, particularly in colorectal cancer, but no correlation was reported. It suggested that HLTF promoter might be hypermethylated via another mechanism. HLTF promoter hypermethylation was primarily examined in colon and gastric cancers, but it was also reported in esophageal, uterine, lung, hepatocellular and bladder cancers (Table 3).
In patients with colon cancer, HLTF promoter hypermethylation was detected in primary tumors and in cell-free DNA resulting from cancer cell necrosis in serum/plasma and feces. HLTF promoter methylation is rarely observed in normal colon tissue (3.0–10.2 %). However, it is increased in colon adenoma (25.7–68.5 %) [13, 56] and remains stable or slightly increased in invasive carcinomas (34.3–41.4 %), independently of tumor stage [13, 55–70] (Table 3). HLTF promoter hypermethylation was analyzed in association with the methylation of other genes. Colorectal cancers with a highly methylated panel of genes, including HLTF, are associated with the absence of lymph nodes metastasis and with a poorly differentiated histology [60, 61]. HLTF promoter hypermethylation alone was significantly less frequent in non-metastatic (Dukes’s stages B and C) than in metastatic (Duke’s stage D) primary cancers (Table 3).
Several groups detected HLTF promoter hypermethylation in serum/plasma of patients with colon cancer (Table 3) [62–68]. The purpose of analyzing gene methylation in cancer patients is for use as a biomarker to predict outcomes, including survival and disease recurrence. Currently, alongside the established TNM staging system, Carcino-Embryogenic Antigen (CEA) is the only recommended seric marker in colon cancer for post-operative and treatment monitoring [66]. HLTF promoter hypermethylation is detected in patient serum with colon cancer but at a lower frequency than in tumors (ranging between 17.0 and 31.5 % in serum compared with 34.3–41.4 % in tissue) (Table 3). HLTF promoter hypermethylation alone or with a panel of other genes is an independent pejorative prognostic factor in colorectal cancer, associated with a shorter survival and higher risk of disease recurrence [63–65, 68]. It also correlated with larger tumor size, higher stage and grade, and metastatic disease [63, 66, 68].
Two studies report the detection of HLTF promoter methylation in feces of patients with colon cancer (Table 3). The combined detection of HLTF and vimentin gene hypermethylation has a sensitivity of 87.5 % and a specificity of 82 % for predicting colorectal cancer [69]. The detection of aberrant methylation in a panel of 7 tumor-related genes including HLTF in patient feces was used as markers for predicting colon cancer. It was found with a 90 % sensitivity that 75 % of cancer and 68 % of colonic adenoma patients had methylated DNA detected in their stool samples [70].
The role of HLTF loss of function in colon cancer was investigated in two murine models. Borinstein et al. [83] treated mice with azoxymethane (AOM) to induce aberrant methylation in tumor suppressor genes involved in colon carcinogenesis. However, in this model, no Hltf promoter hypermethylation was reported, suggesting a different pathway for Hltf silencing in mice or no association with AOM treatment. Sandhu et al. [84] inactivated Hltf in mice by replacing exons 1–5 with a LacZ cDNA fused in frame with the endogenous Hltf coding sequence. These mice showed a normal development. However, introducing the Hltf null mutation into Adenomatous polyposis coli (Apc gene)+/− mice promoted the malignant transformation of colonic adenomas to carcinomas through genomic instability. Conversely [13], restoration of HLTF expression in cells harboring HLTF promoter hypermethylation decreased the colony formation ability by 75 % in each of the colon cell lines treated.
In gastric cancer, HLTF promoter hypermethylation was detected in dysplasias (20.5 %), and the prevalence increased in invasive carcinomas (39.2 %) (Table 3). High methylation of tumor-related genes was associated with later stages of gastric cancer, but HLTF methylation status was not associated with the tumor stage in most series [59, 70–76]. HLTF promoter hypermethylation appears at an early stage in gastric carcinogenesis in patients with a family history of gastric cancers and might constitute a risk marker for gastric cancer in these families. The prevalence of HLTF promoter hypermethylation in patients with family history was 60 % (27/45 cases) compared with patients without family history (33 %, 17/51; [75]). With respect to colon cancer, HLTF promoter hypermethylation was detected in plasma [75] of patients with gastric adenocarcinomas in 20.8 % (20/96) of cases, which is twofold less than the prevalence observed in primary tumors (Table 3).
HLTF promoter hypermethylation was reported in esophageal, hepatocellular and bladder carcinomas, though with a low prevalence (Table 3), which suggests that HLTF is not a common methylation target in such cancers.
In uterine cancer, HLTF promoter hypermethylation was more frequent in adenocarcinomas (43.3 %) than in squamous cell carcinomas (3.2 %) (Table 3) [77]. In lung cancer, it was detected in 45 % of squamous cell carcinomas and 37.5 % of adenocarcinomas (Table 3) [79].
HLTF expression in cancer
Despite evidence in favor of a tumor suppressor function (see above), HLTF was overexpressed in many transformed cell lines [8] and as an early event in an experimental model of estrogen-induced renal carcinogenesis in rodent [85]. Studies on HLTF expression in head and neck or cervix cancers (Table 4) demonstrated the alternative splicing of HLTF mRNA (see HLTF transcripts) leading to an increased expression of truncated protein forms [10]. These shorter protein forms lack domains involved in DNA repair (RING and helicases domains, see Fig. 1) and progressively replace wild-type HLTF during the head and neck or cervix oncogenic processes (Table 4).
Table 4.
HLTF expression in cervix, head and neck and thyroid cancers
| Group | Ethnicity | Assay | Normal (20) | CIN I (32) | CIN II–III (35) | SCC (10) | ADC (12) | AIS (10) |
|---|---|---|---|---|---|---|---|---|
| HLTF expression in cervix cancer ( n ) | ||||||||
| Capouillez et al [15] | Europe | IHC | ± | + | ++ | +++ | +++ | +++ |
| WB | Wt form | / | Wt form | Truncated forms | / | / | ||
| Group | Ethnicity | Assay | Disease-free (16) | Recurrence (11) | ||||
|---|---|---|---|---|---|---|---|---|
| HLTF expression in cervix cancer after curative radiotherapy ( n ) | ||||||||
| Cho et al. [86] | Asia | IHC | + | ++ | +++ | + | ++ | +++ |
| 2 | 10 | 4 | 0 | 2 | 9 | |||
| Group | Ethnicity | Assay | HSCC | |||
|---|---|---|---|---|---|---|
| Tumor-free (27) | Dyspl. (48) | HSCC IV (100) | ||||
| HLTF expression in head and neck cancer ( n ): Hypopharyngeal Squamous Cell Carcinoma (HSCC) and Laryngeal Squamous Cell Carcinoma (LSCC) | ||||||
| Capouillez et al. [10, 14] | Europe | IHC | + | ++ | +++ | |
| Nucleocytopl. | Nucleocytopl. | Cytopl. shift | ||||
| WB | Truncated form (2) | / | Wt + truncated forms (3) | |||
| Assay | LSCC | |||||
| Tumor-free (33) | Dyspl. (44) | LSCC I-II (40) | LSCC IV (16) | |||
| IHC | +++ | ++ | ++ | ++ | ||
| Cytopl. | Nucleocytopl. | Nucleocytopl. | Nucleocytopl. | |||
| WB | / | / | Wt + truncated forms (3) | |||
| Group | Ethnicity | Assay | Cohort I (80) | |||
|---|---|---|---|---|---|---|
| Adenoma (20) | Papillary carcinoma (40) | Follicular carcinoma (12) | Anaplastic carcinoma (8) | |||
| HLTF expression in thyroid cancer ( n ) | ||||||
| Arcolia et al. [88] | Europe | IHC | +++ | ++ | ± | ± |
| Nucleus | Cytopl. | Nucleocytopl. | Nucleocytopl. | |||
| Cohort II (69) | ||||||
| Benign lesions (40) | Malignant lesions (29) | |||||
| +++ | + to ± | |||||
| Nucleus | Nucleocytopl. | |||||
The number of patients is indicated under the brackets. CIN, cervical intraepithelial neoplasia; SCC, squamous cell carcinoma; ADC, adenocarcinoma; AIS, adenocarcinoma in situ; IHC, immunohistochemistry; WB, western blotting; Wt, wild-type; for the staining intensity: ±, weak; +, low; ++, moderate; +++, high
In head and neck cancers, Capouillez et al. [10, 14] focused on hypopharyngeal squamous cell carcinoma (HSCC) and laryngeal SCC (LSCC). In both cases, HLTF expression was assessed using immunohistochemistry (IHC) followed by microscopy computer-assisted quantification (percentage of immunopositive cells and staining intensity) and immunodetection on western blots (WB); subsequently, HLTF expression was correlated with the patient clinical outcome. In HSCC, HLTF staining increased from tumor-free tissue to carcinoma (Table 4), associated with a protein shift from the nucleus to the cytoplasm between dyplasias and carcinomas. Truncated protein forms were detected in carcinomas. The quantitative determination of HLTF in HSCC was an independent prognostic marker of disease recurrence. In LSCC, HLTF expression decreased from tumor-free tissue to carcinomas, associated with a nucleo-cytoplasmic translocation in dyplasias and carcinomas (Table 4). Truncated protein forms were also detected in carcinomas. No significant association was reported in LSCC with clinical outcome, but a trend in carcinomas expressing low HLTF levels for a higher risk of recurrence was observed.
Similar to HSCC, HLTF immunodetection increased during the oncogenesis in cervical cancer [15]: cervix carcinomas (SCC, AD and AD in situ, Table 4) exhibited the highest HLTF immunostaining. Truncated protein forms were detected in SCC (no data about AD and AD in situ). No correlation with clinical data was presented in this study. In addition, Cho et al. [86] showed HLTF overexpression in recurrent cervical carcinoma following radiation treatment compared with patients with former cervical cancer without recurrence. These authors suggested that HLTF might confer radiation resistance in cervical cancer, but the HLTF protein form expressed in these cases was not determined. Ye et al. [87] also showed that HLTF mRNA was a target of miR-145, which is decreased in cervical cancers and is associated with radiosensitivity. Indeed, miR-145 and HLTF expression levels were inversely correlated in radio-resistant cervical cancers.
The relationship of HLTF expression with neoplastic progression was also examined using IHC followed by microscopy computer-assisted quantification in thyroid carcinoma ([88]; Table 4). HLTF expression was assessed in two independent cohorts. In the first cohort (Table 4), HLTF expression was compared among adenoma, papillary carcinoma, follicular carcinoma and anaplastic carcinoma: adenomas presented strong nuclear HLTF immunostaining, whereas carcinomas exhibited HLTF only in the cytoplasm. In the second cohort (Table 4), benign thyroid lesions (including 10 colloid nodules, 16 follicular adenomas, 7 Hashimoto’s thyroiditis, and 7 Grave’s disease) and malignant lesions (including 17 papillary carcinomas and 12 follicular variant of papillary carcinomas) were compared. HLTF staining was strong and primarily located in nuclei in benign lesions, and it was weaker and shifted to the cytoplasm in malignant lesions. Interestingly, thyroid immune diseases (Hashimoto and Graves’s diseases) harbored lower HLTF expression within the benign lesion group. The expression of HLTF truncated proteins was assessed in 3 cell lines derived from thyroid carcinoma. HLTF was proposed as a biomarker in the differential diagnosis of benign and malignant thyroid lesions.
Discussion
Approximately two decades ago, several groups independently cloned HLTF cDNA fragments by screening cDNA expression libraries with different double-stranded oligonucleotides representing cis-elements to which HLTF bound with a high affinity. Here, we reviewed the accumulated data clarifying the multiple roles of HLTF and the putative roles for this protein in cancer.
HLTF was first identified as a transcription factor by identification of its target genes, its DNA-binding sequences and its protein partners [8, 21, 25, 29, 32] in different species and by analysis of heart and brain transcriptomes from a Hltf null mouse [23, 27] (Tables 1, 2).
These data were collected from studies focusing on specific organ development in mouse or cell models. Because the transcriptomes of only two mouse organs with Hltf inactivation were studied, it would be interesting to extend the same differential expression analysis to additional organs and cancer types to better understand HLTF functions. In the context of cancer progression, HLTF could be considered an oncoprotein based on its transcriptional activity. Indeed, HLTF activates PAI-1/SERPINE1 expression, which is involved in tumor progression, invasion, metastasis and angiogenesis [89, 90]. HLTF activates HIF-1α, which controls genes that are involved in angiogenesis (VEGF) and in glucose metabolism (glucose transporters GLUT1 and 3, glycolytic enzymes, such as aldolase A and C, enolase 1, hexokinases 1 and 3, lactate dehydrogenase A), thereby facilitating tumor cells to adapt to a hypoxic environment [91]. Because HIF-1α expression in tumor cells is dependent on the distance from a blood vessel, one cannot exclude that HLTF expression could follow a similar profile, leading to a variety of cell clones that express different HLTF levels. HLTF also favors cell proliferation by activating genes (Table 2) that are involved in cell cycle and the anti-apoptotic genes CASP8 and FADD-like apoptosis regulator (CFLAR). These features are considered hallmarks of cancer [1], and the activation of HLTF expression could thus favor cancer development.
The other major role of HTLF is its ability to maintain genome stability, which suggests this protein is a tumor suppressor. Several studies detailed HLTF mechanisms of action in post-replication DNA repair (cell cycle G2/M transition). A stalled replication fork, due to a DNA lesion, is one of the main causes of genome instability. HLTF is recruited at such structures and helps damages to be bypassed by the error-free DNA polymerase Polη [42]. This HLTF function in DNA repair correlates with the transcriptome alterations observed upon Hltf gene inactivation in the mouse [24, 27]. Moreover, such a link between HLTF function and genome integrity was highlighted in cancers such as (a) in colon carcinogenesis, where an increased genomic instability was observed in another Hltf null mouse model [84] and (b) in cervical cancers, where radioresistance was associated with increased HLTF and decreased miR-145 expression: this microRNA targets the 3′ UTR of HLTF mRNA [86, 87]. Interestingly, CHFR, the E3 ubiquitin ligase that targets HLTF for degradation at the proteasome also functions as a mitotic checkpoint. As HLTF is a key player in post-replication DNA repair, the impact of HLTF degradation on such mechanisms should be studied.
The suppression of wt HLTF expression in many tumors would favor the dynamic occurrence of alterations in the genome. It underlies the high adaptability of cancer cells and suggests that HLTF inactivation is a “driver”, rather than a “passenger”, in carcinogenesis. Intriguingly, in all of the studies published to date, this suppression did not result from a genetic but rather epigenetic alteration: the hypermethylation of the promoter, which leads to HLTF silencing. Several groups reported this phenomenon, though primarily in colon and gastric cancers and at a lower level in uterine, lung or bladder cancers (Table 3). The combination of molecular and clinical data showed that HLTF methylation, when present, started at early stages of cancer and was clearly more frequent in advanced adenoma/dysplasia. In the majority of the cases, HLTF silencing correlated with a poor prognosis for patients, in relation to survival, disease recurrence and tumor aggressiveness.
The other alteration of HLTF expression discovered in cancer (head and neck, cervix and thyroid cancers; Table 4) is the alternative splicing of its transcripts, which leads to the expression of truncated protein forms that lack domains involved in DNA repair. The existence of such transcripts was shown at low levels in non-pathological conditions in rabbit uterus and in mouse brain and heart [16, 23, 27]. Interestingly, HLTF mRNA alternative splicing was estrogen-dependent in rabbit uterus (endometrium) [16]. Although the functions of the resulting truncated protein forms are still unknown, the fact that these proteins were generated by estrogens that play a major role in endometrium cell proliferation suggests that these proteins could activate the cell cycle. The expression of these proteins might be an early event: in an experimental model of estrogen-induced renal carcinogenesis in a hamster, HLTF expression was detected via IHC in each cell of the tumor buds, but it only appeared in scattered cells in larger tumors [85]. Although the nature of the immunodetected proteins was not investigated in that study, we suggest that truncated forms rather than wt HLTF were expressed in the positive tumor buds because estrogens favor HLTF mRNA splicing, which leads to the synthesis of the truncated proteins [6]. These shorter forms progressively replace wt HLTF during carcinogenesis in human cervical and hypopharyngeal cancers [10, 14, 15]. This switch from wt to truncated HTLF forms is also linked to a poor patient prognosis. These observations suggest that the HLTF tumor suppressor gene could express proteins contributing to cancer development, and such a phenomenon has previously been described for other tumor suppressors and named “oncomorphism”. For instance, some mutant p53 proteins can drive breast cancer [88], and the alternative splicing of cyclin D1 mRNA leads to distinct protein forms with opposite activities on cell cycling (G1-S progression inhibition, S-G2/M progression acceleration and apoptosis enhancement or delay) [92, 93, 94]. In addition, alternative RNA processing has emerged as a new pathway in genome maintenance that affects the expression of protein forms involved in both RNA splicing and genome surveillance [95]. The functions of HLTF truncated forms might act as oncogenic proteins and/or interfere with the normal activities of wt HLTF as dominant negative oncomorphic forms of a tumor suppressor. These shorter proteins lack domains involved in DNA repair and ubiquitin ligation, thus affecting the core tumor suppressor activity. However, they keep the DNA-binding domain essential for HLTF transcriptional ability, and could thus thereby act as oncoproteins. Recent data suggest HLTF proteins might harbor novel functions. Indeed HLTF interacts with messenger ribonucleoprotein (mRNP) granules such as HNRNPK and HNRNPU, and with nuclear paraspeckle-associated proteins such as NONO and SPQF which can also associate with mRNPs in the cytoplasm [25]. Further investigations should address a putative role for HLTF in nuclear paraspeckle formation with long non-coding RNAs [96], in RNA post-transcriptional processing, mRNA intracellular transport and translation regulation. As shorter HLTF forms are also located in the cytoplasm in cancer cells [10, 14, 15, 88], they might be associated with mRNP granules and thus favor cell growth.
HLTF molecular activities and alterations affect genome stability and might participate in cancer progression via Darwinian selection of best proliferation-fit cells. During carcinogenesis, wt HLTF is first expressed in early steps and progressively silenced or supplanted by its oncogenic protein forms. Clones expressing wt HLTF undergo proliferation via HLTF-driven pathways; however, the generation of a large mutation diversity required for cancer progression is impaired in the presence of HLTF genome maintenance activities. As a result, after HLTF allowed for adaptation to metabolism and growth under hypoxic conditions, cells showing silenced HLTF expression become free of its tumor suppressor activity, ultimately reaching higher proliferation rates. Other clones that express truncated HLTF forms and maintain oncogenic activity, block wt HLTF and loose genome maintenance control. In every case, wt HLTF activities are diverted and only tumor clones harboring HLTF alterations survive.
In conclusion, whatever the alteration of HLTF expression in cancer, the issue for patients is a shorter survival and a higher risk of disease recurrence. Intriguingly, in a large proportion of different cancer types where HLTF silencing through promoter hypermethylation was described, a number of tumors do express HLTF. It is currently not known which form of HLTF is expressed in these tumors and how this protein affects patient survival. Although the majority of analyzed tumors presented no mutation in HLTF gene per se, silencing of this gene through promoter hypermethylation and mRNA splicing activation could play an early role in carcinogenesis. Indeed, epigenetic alterations of cancer-associated genes were linked with most cancer initiation events.
In this review, we gathered and discussed evidence on the complex function of HLTF, a tumor suppressor involved in genome stability maintenance and the mechanisms commonly disturbed in cancer to divert the functions of this protein. These considerations suggest that HLTF alterations should be considered “drivers” in cancer progression.
Acknowledgments
L. D. is a F. R. S.-FNRS Research Fellow (Belgian National Fund for Scientific Research). C. M. was a Télévie Research Associate (Belgian National Fund for Scientific Research). This work was financially supported through funding from F. R. S.-FNRS, Télévie and FRMH (Fonds pour la Recherche Médicale dans le Hainaut, Belgium).
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Aguilera A, García-Muse T. Causes of genome instability. Annu Rev Genet. 2013;47:1–32. doi: 10.1146/annurev-genet-111212-133232. [DOI] [PubMed] [Google Scholar]
- 3.Gatenby RA, Cunningham JJ, Brown JS. Evolutionary triage governs fitness in driver and passenger mutations and suggests targeting never mutations. Nat Commun. 2014;5:5499. doi: 10.1038/ncomms6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheridan PL, Schorpp M, Voz ML, Jones KA. Cloning of an SNF2/SWI2-related protein that binds specifically to the SPH motifs of the SV40 enhancer and to the HIV-1 promoter. J Biol Chem. 1995;270:4575–4587. doi: 10.1074/jbc.270.9.4575. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Sheridan PL, Jones KA, Evans GA. The HIP116 SNF2/SW12-related transcription factor gene (SNF2L3) is located on human chromosome 3q25.1-q26.1. Genomics. 1995;27:381–382. doi: 10.1006/geno.1995.1064. [DOI] [PubMed] [Google Scholar]
- 6.Hayward-Lester A, Hewetson A, Beale EG, Oefner PJ, Doris PA, Chilton BS. Cloning, characterization, and steroid-dependent posttranscriptional processing of RUSH-1 alpha and beta, two uteroglobin promoter-binding proteins. Mol Endocrinol Baltim Md. 1996;10:1335–1349. doi: 10.1210/mend.10.11.8923460. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Ekhterae D, Kim KH. Molecular cloning and characterization of P113, a mouse SNF2/SWI2-related transcription factor. Gene. 1997;202:31–37. doi: 10.1016/S0378-1119(97)00446-0. [DOI] [PubMed] [Google Scholar]
- 8.Gong X, Kaushal S, Ceccarelli E, Bogdanova N, Neville C, Nguyen T, Clark H, Khatib ZA, Valentine M, Look AT, et al. Developmental regulation of Zbu1, a DNA-binding member of the SWI2/SNF2 family. Dev Biol. 1997;183:166–182. doi: 10.1006/dbio.1996.8486. [DOI] [PubMed] [Google Scholar]
- 9.Ding H, Descheemaeker K, Marynen P, Nelles L, Carvalho T, Carmo-Fonseca M, Collen D, Belayew A. Characterization of a helicase-like transcription factor involved in the expression of the human plasminogen activator inhibitor-1 gene. DNA Cell Biol. 1996;15:429–442. doi: 10.1089/dna.1996.15.429. [DOI] [PubMed] [Google Scholar]
- 10.Capouillez A, Decaestecker C, Filleul O, Chevalier D, Coppée F, Leroy X, Belayew A, Saussez S. Helicase-like transcription factor exhibits increased expression and altered intracellular distribution during tumor progression in hypopharyngeal and laryngeal squamous cell carcinomas. Virchows Arch Int J Pathol. 2008;453:491–499. doi: 10.1007/s00428-008-0675-9. [DOI] [PubMed] [Google Scholar]
- 11.Aguilera A, García-Muse T. Causes of genome instability. Annu Rev Genet. 2013;47:1–32. doi: 10.1146/annurev-genet-111212-133232. [DOI] [PubMed] [Google Scholar]
- 12.Unk I, Hajdú I, Blastyák A, Haracska L. Role of yeast Rad5 and its human orthologs, HLTF and SHPRH in DNA damage tolerance. DNA Repair. 2010;9:257–267. doi: 10.1016/j.dnarep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Moinova HR, Chen W-D, Shen L, Smiraglia D, Olechnowicz J, Ravi L, Kasturi L, Myeroff L, Plass C, Parsons R, et al. HLTF gene silencing in human colon cancer. Proc Natl Acad Sci USA. 2002;99:4562–4567. doi: 10.1073/pnas.062459899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capouillez A, Debauve G, Decaestecker C, Filleul O, Chevalier D, Mortuaire G, Coppée F, Leroy X, Belayew A, Saussez S. The helicase-like transcription factor is a strong predictor of recurrence in hypopharyngeal but not in laryngeal squamous cell carcinomas. Histopathology. 2009;55:77–90. doi: 10.1111/j.1365-2559.2009.03330.x. [DOI] [PubMed] [Google Scholar]
- 15.Capouillez A, Noël J-C, Arafa M, Arcolia V, Mouallif M, Guenin S, Delvenne P, Belayew A, Saussez S. Expression of the helicase-like transcription factor and its variants during carcinogenesis of the uterine cervix: implications for tumour progression. Histopathology. 2011;58:984–988. doi: 10.1111/j.1365-2559.2011.03843.x. [DOI] [PubMed] [Google Scholar]
- 16.Hewetson A, Chilton BS. An Sp1-NF-Y/progesterone receptor DNA binding-dependent mechanism regulates progesterone-induced transcriptional activation of the rabbit RUSH/SMARCA3 gene. J Biol Chem. 2003;278:40177–40185. doi: 10.1074/jbc.M303921200. [DOI] [PubMed] [Google Scholar]
- 17.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 18.Kim JM, Cho EN, Kwon YE, Bae SJ, Kim M, Seol JH. CHFR functions as a ubiquitin ligase for HLTF to regulate its stability and functions. Biochem Biophys Res Commun. 2010;395:515–520. doi: 10.1016/j.bbrc.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Qing P, Han L, Bin L, Yan L, Ping WX. USP7 regulates the stability and function of HLTF through deubiquitination. J Cell Biochem. 2011;112:3856–3862. doi: 10.1002/jcb.23317. [DOI] [PubMed] [Google Scholar]
- 20.Hewetson A, Chilton BS. Progesterone-dependent deoxyribonucleic acid looping between RUSH/SMARCA3 and Egr-1 mediates repression by c-Rel. Mol Endocrinol Baltim Md. 2008;22:813–822. doi: 10.1210/me.2007-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser M, Kayser M, Palstra R-J. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22:446–455. doi: 10.1101/gr.128652.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinforma Oxf Engl. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 23.Helmer RA, Foreman O, Dertien JS, Panchoo M, Bhakta SM, Chilton BS. Role of helicase-like transcription factor (hltf) in the G2/m transition and apoptosis in brain. PLoS ONE. 2013;8:e66799. doi: 10.1371/journal.pone.0066799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmer RA, Dertien JS, Chilton BS. Prolactin induces Jak2 phosphorylation of RUSHY195. Mol Cell Endocrinol. 2011;338:79–83. doi: 10.1016/j.mce.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Guillaumond F, Boyer B, Becquet D, Guillen S, Kuhn L, Garin J, Belghazi M, Bosler O, Franc J-L, François-Bellan A-M. Chromatin remodeling as a mechanism for circadian prolactin transcription: rhythmic NONO and SFPQ recruitment to HLTF. FASEB. J Off Publ Fed Am Soc Exp Biol. 2011;25:2740–2756. doi: 10.1096/fj.10-178616. [DOI] [PubMed] [Google Scholar]
- 26.Hewetson A, Chilton BS. Progesterone-dependent deoxyribonucleic acid looping between RUSH/SMARCA3 and Egr-1 mediates repression by c-Rel. Mol Endocrinol Baltim Md. 2008;22:813–822. doi: 10.1210/me.2007-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmer RA, Martínez-Zaguilán R, Dertien JS, Fulford C, Foreman O, Peiris V, Chilton BS. Helicase-like transcription factor (hltf) regulates g2/m transition, wt1/gata4/hif-1a cardiac transcription networks, and collagen biogenesis. PLoS ONE. 2013;8:e80461. doi: 10.1371/journal.pone.0080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian F, Sharma S, Zou J, Lin S-Y, Wang B, Rezvani K, Wang H, Parvin JD, Ludwig T, Canman CE, et al. BRCA1 promotes the ubiquitination of PCNA and recruitment of translesion polymerases in response to replication blockade. Proc Natl Acad Sci USA. 2013;110:13558–13563. doi: 10.1073/pnas.1306534110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brys R, Nelles L, van der Schueren E, Silvestre N, Huylebroeck D, Remacle JE. Identical cis-acting elements and related trans-acting factors control activity of nonviral promoter in Schizosaccharomyces pombe and mammalian cells. DNA Cell Biol. 1998;17:349–358. doi: 10.1089/dna.1998.17.349. [DOI] [PubMed] [Google Scholar]
- 30.Ding H, Beckers MC, Plaisance S, Marynen P, Collen D, Belayew A. Characterization of a double homeodomain protein (DUX1) encoded by a cDNA homologous to 3.3 kb dispersed repeated elements. Hum Mol Genet. 1998;7:1681–1694. doi: 10.1093/hmg/7.11.1681. [DOI] [PubMed] [Google Scholar]
- 31.Mahajan MC, Weissman SM. DNA-dependent adenosine triphosphatase (helicaselike transcription factor) activates beta-globin transcription in K562 cells. Blood. 2002;99:348–356. doi: 10.1182/blood.V99.1.348. [DOI] [PubMed] [Google Scholar]
- 32.Ding H, Benotmane AM, Suske G, Collen D, Belayew A. Functional interactions between Sp1 or Sp3 and the helicase-like transcription factor mediate basal expression from the human plasminogen activator inhibitor-1 gene. J Biol Chem. 1999;274:19573–19580. doi: 10.1074/jbc.274.28.19573. [DOI] [PubMed] [Google Scholar]
- 33.Hewetson A, Hendrix EC, Mansharamani M, Lee VH, Chilton BS. Identification of the RUSH consensus-binding site by cyclic amplification and selection of targets: demonstration that RUSH mediates the ability of prolactin to augment progesterone-dependent gene expression. Mol Endocrinol Baltim Md. 2002;16:2101–2112. doi: 10.1210/me.2002-0064. [DOI] [PubMed] [Google Scholar]
- 34.Sturm RA, Duffy DL, Zhao ZZ, Leite FPN, Stark MS, Hayward NK, Martin NG, Montgomery GW. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–431. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturm RA, Larsson M. Genetics of human iris colour and patterns. Pigment Cell Melanoma Res. 2009;22:544–562. doi: 10.1111/j.1755-148X.2009.00606.x. [DOI] [PubMed] [Google Scholar]
- 36.Ceccarelli E, McGrew MJ, Nguyen T, Grieshammer U, Horgan D, Hughes SH, Rosenthal N. An E box comprises a positional sensor for regional differences in skeletal muscle gene expression and methylation. Dev Biol. 1999;213:217–229. doi: 10.1006/dbio.1999.9345. [DOI] [PubMed] [Google Scholar]
- 37.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 38.Unk I, Hajdú I, Fátyol K, Hurwitz J, Yoon J-H, Prakash L, Prakash S, Haracska L. Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination. Proc Natl Acad Sci USA. 2008;105:3768–3773. doi: 10.1073/pnas.0800563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motegi A, Liaw H-J, Lee K-Y, Roest HP, Maas A, Wu X, Moinova H, Markowitz SD, Ding H, Hoeijmakers JHJ, et al. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc Natl Acad Sci USA. 2008;105:12411–12416. doi: 10.1073/pnas.0805685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 41.Ding L, Forsburg SL (2014) Essential domains of Schizosaccharomyces pombe Rad8 required for DNA damage response. G3 Bethesda Md 4:1373–1384 [DOI] [PMC free article] [PubMed]
- 42.Ortiz-Bazán MÁ, Gallo-Fernández M, Saugar I, Jiménez-Martín A, Vázquez MV, Tercero JA. Rad5 plays a major role in the cellular response to DNA damage during chromosome replication. Cell Rep. 2014;9:460–468. doi: 10.1016/j.celrep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Lin J-R, Zeman MK, Chen J-Y, Yee M-C, Cimprich KA. SHPRH and HLTF act in a damage-specific manner to coordinate different forms of postreplication repair and prevent mutagenesis. Mol Cell. 2011;42:237–249. doi: 10.1016/j.molcel.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masuda Y, Suzuki M, Kawai H, Hishiki A, Hashimoto H, Masutani C, Hishida T, Suzuki F, Kamiya K (2012) En bloc transfer of polyubiquitin chains to PCNA in vitro is mediated by two different human E2–E3 pairs. Nucleic Acids Res [DOI] [PMC free article] [PubMed]
- 45.Krijger PHL, Lee K-Y, Wit N, van den Berk PCM, Wu X, Roest HP, Maas A, Ding H, Hoeijmakers JHJ, Myung K, et al. HLTF and SHPRH are not essential for PCNA polyubiquitination, survival and somatic hypermutation: existence of an alternative E3 ligase. DNA Repair. 2011;10:438–444. doi: 10.1016/j.dnarep.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendel A, Krijger PHL, Diamant N, Goren Z, Langerak P, Kim J, Reissner T, Lee K, Geacintov NE, Carell T, et al. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 2011;7:e1002262. doi: 10.1371/journal.pgen.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achar YJ, Balogh D, Haracska L. Coordinated protein and DNA remodeling by human HLTF on stalled replication fork. Proc Natl Acad Sci USA. 2011;108:14073–14078. doi: 10.1073/pnas.1101951108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blastyák A, Hajdú I, Unk I, Haracska L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol Cell Biol. 2010;30:684–693. doi: 10.1128/MCB.00863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hishiki A, Hara K, Ikegaya Y, Yokoyama H, Shimizu T, Sato M, Hashimoto H. Structure of a novel DNA-binding domain of Helicase-like Transcription Factor (HLTF) and its functional implication in DNA damage tolerance. J Biol Chem. 2015;290:13215–13223. doi: 10.1074/jbc.M115.643643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikegaya Y, Hara K, Hishiki A, Yokoyama H, Hashimoto H. Crystallographic study of a novel DNA-binding domain of human HLTF involved in the template-switching pathway to avoid the replication arrest caused by DNA damage. Acta Crystallogr. Sect F Struct Biol Commun. 2015;71:668–670. doi: 10.1107/S2053230X15005907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kile AC, Chavez DA, Bacal J, Eldirany S, Korzhnev DM, Bezsonova I, Eichman BF, Cimprich KA (2015) HLTF’s ancient HIRAN domain binds 3′ DNA ends to drive replication fork reversal. Mol Cell [DOI] [PMC free article] [PubMed]
- 52.Tsutakawa SE, Tainer JA. Bending forks and wagging dogs-It’s about the DNA 3′ tail. Mol Cell. 2015;58:972–973. doi: 10.1016/j.molcel.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longerich S, Sung P. Clearance of roadblocks in replication fork restart. Proc Natl Acad Sci USA. 2011;108:13881–13882. doi: 10.1073/pnas.1110698108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burkovics P, Sebesta M, Balogh D, Haracska L, Krejci L. Strand invasion by HLTF as a mechanism for template switch in fork rescue. Nucleic Acids Res. 2014;42:1711–1720. doi: 10.1093/nar/gkt1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang MY, Lee BB, Kim Y-H, Chang DK, Kyu Park S, Chun H-K, Song SY, Park J, Kim D-H. Association of the SUV39H1 histone methyltransferase with the DNA methyltransferase 1 at mRNA expression level in primary colorectal cancer. Int J Cancer J Int Cancer. 2007;121:2192–2197. doi: 10.1002/ijc.22953. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y-H, Petko Z, Dzieciatkowski S, Lin L, Ghiassi M, Stain S, Chapman WC, Washington MK, Willis J, Markowitz SD, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006;45:781–789. doi: 10.1002/gcc.20341. [DOI] [PubMed] [Google Scholar]
- 57.Brandes JC, van Engeland M, Wouters KAD, Weijenberg MP, Herman JG. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis. 2005;26:1152–1156. doi: 10.1093/carcin/bgi058. [DOI] [PubMed] [Google Scholar]
- 58.Bai AHC, Tong JHM, To K-F, Chan MWY, Man EPS, Lo K-W, Lee JFY, Sung JJY, Leung WK. Promoter hypermethylation of tumor-related genes in the progression of colorectal neoplasia. Int J Cancer J Int Cancer. 2004;112:846–853. doi: 10.1002/ijc.20485. [DOI] [PubMed] [Google Scholar]
- 59.Hibi K, Nakayama H, Kanyama Y, Kodera Y, Ito K, Akiyama S, Nakao A. Methylation pattern of HLTF gene in digestive tract cancers. Int J Cancer J Int Cancer. 2003;104:433–436. doi: 10.1002/ijc.10985. [DOI] [PubMed] [Google Scholar]
- 60.Hibi K, Kodera Y, Ito K, Akiyama S, Nakao A. Aberrant methylation of HLTF, SOCS-1, and CDH13 genes is shown in colorectal cancers without lymph node metastasis. Dis Colon Rectum. 2005;48:1282–1286. doi: 10.1007/s10350-004-0947-7. [DOI] [PubMed] [Google Scholar]
- 61.Hibi K, Nakao A. Highly-methylated colorectal cancers show poorly-differentiated phenotype. Anticancer Res. 2006;26:4263–4266. [PubMed] [Google Scholar]
- 62.Lee BB, Lee EJ, Jung EH, Chun H-K, Chang DK, Song SY, Park J, Kim D-H. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin. Cancer Res. Off J Am Assoc Cancer Res. 2009;15:6185–6191. doi: 10.1158/1078-0432.CCR-09-0111. [DOI] [PubMed] [Google Scholar]
- 63.Wallner M, Herbst A, Behrens A, Crispin A, Stieber P, Göke B, Lamerz R, Kolligs FT. Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:7347–7352. doi: 10.1158/1078-0432.CCR-06-1264. [DOI] [PubMed] [Google Scholar]
- 64.Herbst A, Wallner M, Rahmig K, Stieber P, Crispin A, Lamerz R, Kolligs FT. Methylation of helicase-like transcription factor in serum of patients with colorectal cancer is an independent predictor of disease recurrence. Eur J Gastroenterol Hepatol. 2009;21:565–569. doi: 10.1097/MEG.0b013e328318ecf2. [DOI] [PubMed] [Google Scholar]
- 65.Herbst A, Rahmig K, Stieber P, Philipp A, Jung A, Ofner A, Crispin A, Neumann J, Lamerz R, Kolligs FT. Methylation of NEUROG1 in serum is a sensitive marker for the detection of early colorectal cancer. Am J Gastroenterol. 2011;106:1110–1118. doi: 10.1038/ajg.2011.6. [DOI] [PubMed] [Google Scholar]
- 66.Philipp AB, Stieber P, Nagel D, Neumann J, Spelsberg F, Jung A, Lamerz R, Herbst A, Kolligs FT. Prognostic role of methylated free circulating DNA in colorectal cancer. Int J Cancer J Int Cancer. 2012;131:2308–2319. doi: 10.1002/ijc.27505. [DOI] [PubMed] [Google Scholar]
- 67.Philipp AB, Nagel D, Stieber P, Lamerz R, Thalhammer I, Herbst A, Kolligs FT. Circulating cell-free methylated DNA and lactate dehydrogenase release in colorectal cancer. BMC Cancer. 2014;14:245. doi: 10.1186/1471-2407-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung WK, To K-F, Man EPS, Chan MWY, Bai AHC, Hui AJ, Chan FKL, Sung JJY. Quantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancer. Am J Gastroenterol. 2005;100:2274–2279. doi: 10.1111/j.1572-0241.2005.50412.x. [DOI] [PubMed] [Google Scholar]
- 69.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC, 3rd, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Leung WK, To K-F, Man EPS, Chan MWY, Hui AJ, Ng SSM, Lau JYW, Sung JJY. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol. 2007;102:1070–1076. doi: 10.1111/j.1572-0241.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 71.Hamai Y, Oue N, Mitani Y, Nakayama H, Ito R, Matsusaki K, Yoshida K, Toge T, Yasui W. DNA hypermethylation and histone hypoacetylation of the HLTF gene are associated with reduced expression in gastric carcinoma. Cancer Sci. 2003;94:692–698. doi: 10.1111/j.1349-7006.2003.tb01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung WK, Yu J, Bai AHC, Chan MWY, Chan K-K, To K-F, Chan FKL, Ng EKW, Chung SCS, Sung JJY. Inactivation of helicase-like transcription factor by promoter hypermethylation in human gastric cancer. Mol Carcinog. 2003;37:91–97. doi: 10.1002/mc.10124. [DOI] [PubMed] [Google Scholar]
- 73.Oue N, Mitani Y, Motoshita J, Matsumura S, Yoshida K, Kuniyasu H, Nakayama H, Yasui W. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006;106:1250–1259. doi: 10.1002/cncr.21754. [DOI] [PubMed] [Google Scholar]
- 74.Kim JJ, Chung SW, Kim JH, Kim JW, Oh JS, Kim S, Song SY, Park J, Kim D-H. Promoter methylation of helicase-like transcription factor is associated with the early stages of gastric cancer with family history. Ann. Oncol. Off J Eur Soc Med Oncol ESMO. 2006;17:657–662. doi: 10.1093/annonc/mdl018. [DOI] [PubMed] [Google Scholar]
- 75.Guo W, Dong Z, Guo Y, Chen Z, Kuang G, Yang Z. Aberrant methylation of the CpG island of HLTF gene in gastric cardia adenocarcinoma and dysplasia. Clin Biochem. 2011;44:784–788. doi: 10.1016/j.clinbiochem.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Fukuoka T, Hibi K, Nakao A. Aberrant methylation is frequently observed in advanced esophageal squamous cell carcinoma. Anticancer Res. 2006;26:3333–3335. [PubMed] [Google Scholar]
- 77.Kang S, Kim JW, Kang GH, Lee S, Park NH, Song YS, Park SY, Kang SB, Lee HP. Comparison of DNA hypermethylation patterns in different types of uterine cancer: cervical squamous cell carcinoma, cervical adenocarcinoma and endometrial adenocarcinoma. Int J Cancer J Int Cancer. 2006;118:2168–2171. doi: 10.1002/ijc.21609. [DOI] [PubMed] [Google Scholar]
- 78.Jo H, Kang S, Kim JW, Kang GH, Park NH, Song YS, Park SY, Kang SB, Lee HP. Hypermethylation of the COX-2 gene is a potential prognostic marker for cervical cancer. J Obstet Gynaecol Res. 2007;33:236–241. doi: 10.1111/j.1447-0756.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 79.Castro M, Grau L, Puerta P, Gimenez L, Venditti J, Quadrelli S, Sánchez-Carbayo M. Multiplexed methylation profiles of tumor suppressor genes and clinical outcome in lung cancer. J Trans Med. 2010;8:86. doi: 10.1186/1479-5876-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Li HM, Liu Z, Zhou G, Zhang Q, Zhang T, Zhang J, Zhang C. Loss of heterozygosity and methylation of multiple tumor suppressor genes on chromosome 3 in hepatocellular carcinoma. J Gastroenterol. 2013;48:132–143. doi: 10.1007/s00535-012-0621-0. [DOI] [PubMed] [Google Scholar]
- 81.García-Baquero R, Puerta P, Beltran M, Alvarez M, Sacristan R, Alvarez-Ossorio JL, Sánchez-Carbayo M. Methylation of a novel panel of tumor suppressor genes in urine moves forward noninvasive diagnosis and prognosis of bladder cancer: a 2-center prospective study. J Urol. 2013;190:723–730. doi: 10.1016/j.juro.2013.01.105. [DOI] [PubMed] [Google Scholar]
- 82.García-Baquero R, Puerta P, Beltran M, Alvarez-Mújica M, Alvarez-Ossorio JL, Sánchez-Carbayo M (2014) Methylation of tumor suppressor genes in a novel panel predicts clinical outcome in paraffin-embedded bladder tumors. Tumour Biol J Int Soc Oncodevelopmental Biol Med [DOI] [PubMed]
- 83.Borinstein SC, Conerly M, Dzieciatkowski S, Biswas S, Washington MK, Trobridge P, Henikoff S, Grady WM. Aberrant DNA methylation occurs in colon neoplasms arising in the azoxymethane colon cancer model. Mol Carcinog. 2010;49:94–103. doi: 10.1002/mc.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sandhu S, Wu X, Nabi Z, Rastegar M, Kung S, Mai S, Ding H. Loss of HLTF function promotes intestinal carcinogenesis. Mol Cancer. 2012;11:18. doi: 10.1186/1476-4598-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Debauve G, Nonclercq D, Ribaucour F, Wiedig M, Gerbaux C, Leo O, Laurent G, Journé F, Belayew A, Toubeau G. Early expression of the Helicase-Like Transcription Factor (HLTF/SMARCA3) in an experimental model of estrogen-induced renal carcinogenesis. Mol Cancer. 2006;5:23. doi: 10.1186/1476-4598-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho S, Cinghu S, Yu J-R, Park W-Y. Helicase-like transcription factor confers radiation resistance in cervical cancer through enhancing the DNA damage repair capacity. J Cancer Res Clin Oncol. 2011;137:629–637. doi: 10.1007/s00432-010-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye C, Sun N-X, Ma Y, Zhao Q, Zhang Q, Xu C, Wang S-B, Sun S-H, Wang F, Li W. MicroRNA-145 contributes to enhancing radiosensitivity of cervical cancer cells. FEBS Lett. 2015;589:702–709. doi: 10.1016/j.febslet.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 88.Arcolia V, Paci P, Dhont L, Chantrain G, Sirtaine N, Decaestecker C, Remmelink M, Belayew A, Saussez S. Helicase-like transcription factor: a new marker of well-differentiated thyroid cancers. BMC Cancer. 2014;14:492. doi: 10.1186/1471-2407-14-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Debauve G, Capouillez A, Belayew A, Saussez S. The helicase-like transcription factor and its implication in cancer progression. Cell. Mol. Life Sci. CMLS. 2008;65:591–604. doi: 10.1007/s00018-007-7392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masuda T, Hattori N, Senoo T, Akita S, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Kohno N. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol Cancer Ther. 2013;12:2378–2388. doi: 10.1158/1535-7163.MCT-13-0041. [DOI] [PubMed] [Google Scholar]
- 91.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. doi: 10.1016/S1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 92.Walerych D, Napoli M, Collavin L, Del Sal G. The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis. 2012;33:2007–2017. doi: 10.1093/carcin/bgs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Alternate splicing produces a novel cyclin D1 transcript. Oncogene. 1995;11:1005–1011. [PubMed] [Google Scholar]
- 94.Sun Y, Lou X, Yang M, Yuan C, Ma L, Xie B-K, Wu J-M, Yang W, Shen SX, Xu N, et al. Cyclin-dependent kinase 4 may be expressed as multiple proteins and have functions that are independent of binding to CCND and RB and occur at the S and G 2/M phases of the cell cycle. Cell Cycle Georget. Tex. 2013;12:3512–3525. doi: 10.4161/cc.26510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lenzken SC, Loffreda A, Barabino SML. RNA Splicing: a New Player in the DNA Damage Response. Int J Cell Biol. 2013;2013:153634. doi: 10.1155/2013/153634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bond CS, Fox AH. Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol. 2009;186:637–644. doi: 10.1083/jcb.200906113. [DOI] [PMC free article] [PubMed] [Google Scholar]