Abstract
Vaccination is a successful strategy to proactively develop immunity to a certain pathogen, but most vaccines fail to trigger a specific immune response at the mucosal surfaces, which are the first port of entry for infectious agents. At the mucosal surfaces, the predominant immunoglobulin is secretory IgA (SIgA) that specifically neutralizes viruses and prevents bacterial colonization. Mucosal passive immunization, i.e. the application of pathogen-specific SIgAs at the mucosae, can be an effective alternative to achieve mucosal protection. However, this approach is not straightforward, mainly because SIgAs are difficult to obtain from convalescent sources, while recombinant SIgA production is challenging due to its complex structure. This review provides an overview of manufacturing difficulties presented by the unique structural diversity of SIgAs, and the innovative solutions being explored for SIgA production in mammalian and plant expression systems.
Keywords: Mucosal infections, Plant-based production, IgA purification, Topical application, Immune prophylaxis, Molecular farming
Introduction
Each of the immunoglobulin (Ig) isotypes—IgG, IgA, IgM, IgE or IgD—has its own peculiarities, but the one that stands out is IgA, being highly distinct, with high heterogeneity, occurring in formats with different valances, each existing in different subclasses and allotypes. The uniqueness is also reflected in its functionality and spatial distribution in the body. Unlike most Igs, which are circulatory, IgAs are predominantly secreted at mucosal surfaces and as such, they form the hallmark of mucosal immunity, maintaining a barrier function [1].
Most pathogenic invasions start at the mucosae, which are extensively vast surfaces lining the external orifice of the body, such as the gastro-intestinal, the urogenital and the respiratory tracts [2, 3]. In human beings, the mucosal surfaces measure about 400 m2, at which about 3 g of IgAs are secreted daily [3] to provide protection against colonization, entry and invasion of infectious pathogens [4]. More and more, mucosal secretory IgAs (SIgAs) are suggested to have a protective role against viral and bacterial infections like HIV, rotavirus, Clostridium, Helicobacter pylori, tuberculosis, Vibrio cholera, etc. [5–7].
Though the presence of SIgAs is desirable at the mucosae, most vaccines fail to elicit this protective mucosal immune response [5, 8–10]. Successful proof-of-concept experiments of mucosal vaccines in animal models often do not translate into a similarly protective efficacy in humans [6]. In contrast to the better-studied systemic immunity, our understanding of the human mucosal immune system has only recently increased, showing that it operates very distinctly, and standard principles may not always apply. For example, the mucosal immune system has a degree of compartmentalization, due to which vaccination at one site does not lead to a systemic mucosal immune response. Oral vaccination leads to an immune response restricted to the gastro-intestinal mucosa, while nasal vaccination leads to an immune response at the respiratory tract, but may also lead to a vaginal SIgA response [5, 11, 12]. Furthermore, the principle of ‘prime and boost’ in context of mucosal memory is yet to be ascertained in clinical trials. Evidences thus far from mice experiments suggest that the predominant SIgA response is always swayed toward the most predominant microbe, thus having a ‘threshold effect’ [3]. Also, the unavailability of safe mucosal adjuvants is a major reason for the difficulty and delay in mucosal vaccine development [5]. As the field develops and the challenges are overcome, more promising mucosal vaccines would start arriving in the clinics, but until then, a ‘short-cut’ solution to attain protection at the mucosal surfaces would be passive immunization. Mucosal passive immunization involves the topical application of pathogen-specific IgA antibodies at the mucosal surfaces [13]. Using disease-specific SIgAs, the defense borders can be strengthened and infection of the specific pathogen can be prevented [6], thus achieving ‘instant protection’ and circumventing the need for priming of the immune system [14, 15].
Compared with monoclonal IgGs, which are frequently administered to patients, either as prophylaxis or as therapy, the use of monoclonal IgAs has yet to catch up. The complex structural diversity of IgA has presented a challenge in its recombinant production [1, 16, 17], requiring the development of a wide range of innovative technological solutions. This article discusses these challenges and solutions developed, also highlighting issues such as improving assembly and purification, that need to be addressed to realize IgA-based mucosal passive immunization.
Structural formats of IgA
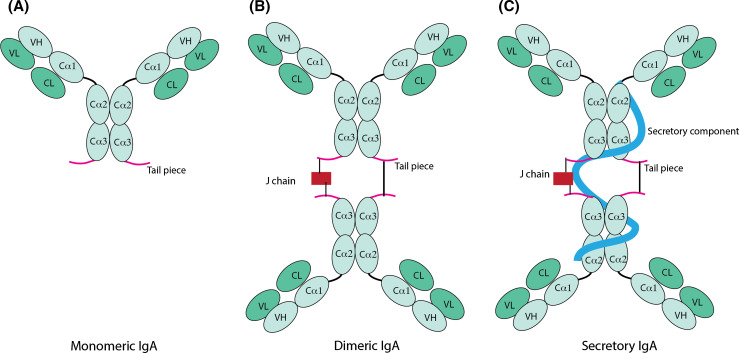
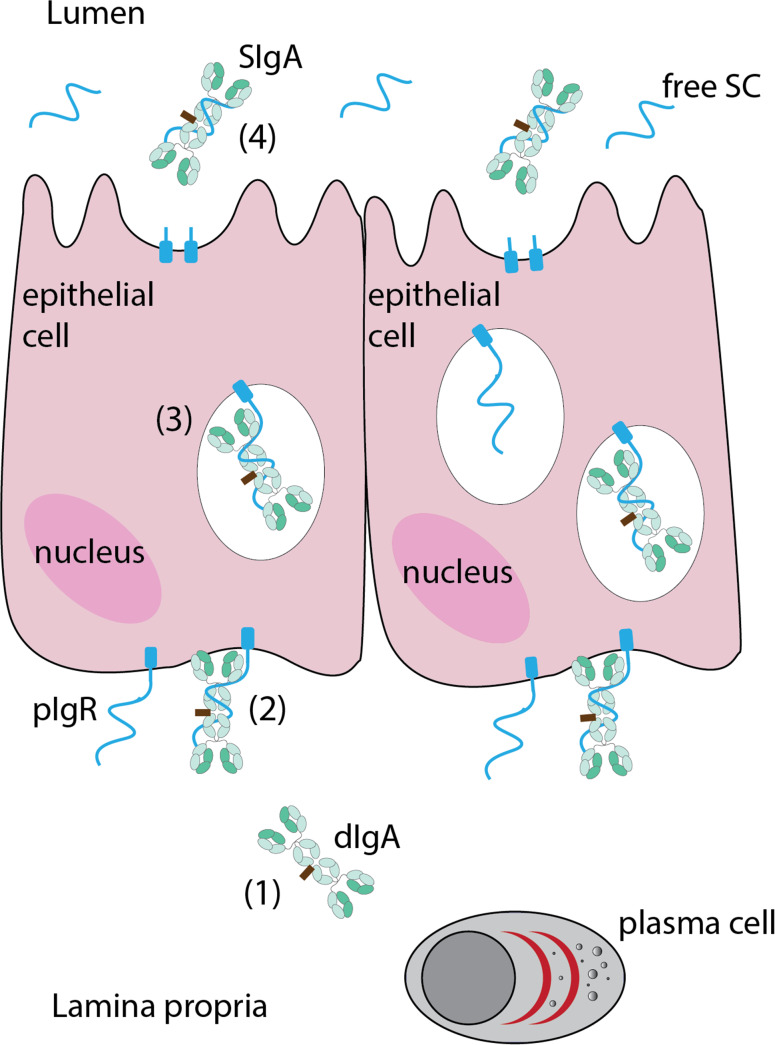
The serum of mammals contains IgA mainly in the monomeric format (mIgA) and only a fraction (1–5 %) in the polymeric format (pIgA), the predominant proportion of which occurs as dimeric IgA (dIgA). At the mucosal surfaces, IgA occurs as SIgA. Monomeric IgAs can be regarded as the basic IgA unit, and are composed of the quintessential paired heavy and light chains (Fig. 1a), having two antigen binding domains (bivalent, like IgG). Dimeric and polymeric IgAs are composed of two (dIgA) or four (pIgA) such mIgA units, which become covalently linked via disulfide bridges between the 18-amino-acid-long heavy chain C-terminal ends, called tailpiece. A 15-kDa joining chain (J chain) further facilitates the IgA polymerization by connecting one of the two heavy chains of each monomeric unit, while the other heavy chains form a direct disulfide linkage with each other (Fig. 1b). For the biosynthesis and mucosal secretion of SIgAs (Fig. 2), dIgAs produced by the plasma cells in the lamina propria bind to the polymeric immunoglobulin receptor (pIgR) at the basal surface of the epithelial cell via the J chain, and are subsequently transcytosed to the apical surface. During vesicular transit, the pIgR undergoes proteolysis and the association of the 80-kDa extracellular ligand-binding portion, called secretory component (SC) with the dIgA molecule is stabilized by disulfide bonds. The resulting SC-bound dIgA (Fig. 1c) is secreted into the lumen as SIgA (Fig. 2). Thus, the biosynthesis of SIgAs needs the activity of two different cell types, i.e. the plasma cells for producing the dIgAs and the columnar epithelial cells for transcytosis and providing the SC (Fig. 2).
Fig. 1.
Schematic representation of IgA formats, a monomeric IgA, b dimeric IgA, c secretory IgA. The monomeric IgA (mIgA) is formed by the paired heavy and light chains. The respective domains have been labeled, namely variable domains of the heavy (VH) and light (VL) chains, and constant domains of the heavy (Cα1, Cα2 or Cα3) and light (CL) chains. The joining chain (J chain) facilitates the dimerization of two mIgAs into a dimeric IgA (dIgA); the association of the dIgA with the secretory component results in a secretory IgA (SIgA)
Fig. 2.
Secretory IgA biosynthesis. The plasma cells in the lamina propria produce dimeric IgA (dIgA) (1), which binds to the polymeric immunoglobulin receptor (pIgR) on the basal surface of the columnar epithelial cells (2), after which the complex is internalized and the vesicle traffics toward the apical, luminal surface (3), during which the dIgA-binding extracellular domain of the pIgR cleaves off as the secretory component (SC); the SC-dIgA complex secreted at the apical surface is called secretory IgA (SIgA) (4). Secretory component free of IgAs is also found in the lumen, resulting from the transcytosis and proteolysis of unbound pIgR
Diversity in IgA subclasses
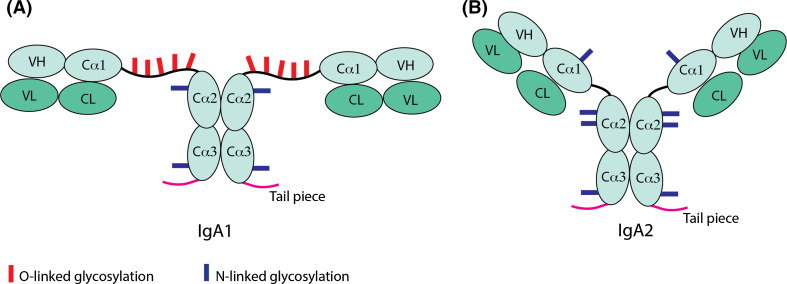
The most frequently used animal models to study the antibody response are mice and rabbits, which have one or 13 Cα genes, respectively, encoding the constant domain of the IgA heavy chains. However, humans and hominids, like gorilla and chimpanzee, have two Cα genes coding for two different subclasses of IgAs—IgA1 and IgA2, differing in hinge length and other structural features, including the extent of and difference in glycosylation (Fig. 3) [4, 7]. Also, the distribution of these two subclasses depends on the mucosal tissue systems in which they are expressed, e.g. IgA2 is more predominant in endocrine secretions, suggesting a difference in functional specialization. However, the specific roles of IgA1 and IgA2 with respect to different mucosal infections is yet to be unraveled [7]; most clinical publications studying IgA responses to infections do not distinguish between IgA1 and IgA2. This distinction could conveniently be made with IgA1- or IgA2-specific monoclonal antibodies [7] or by Jacalin, a ligand from jackfruit, which selectively binds IgA1 [18].
Fig. 3.
Schematic representation of the structural differences between human IgA1 and IgA2. The typical ‘T’ and ‘Y’ shape structure of IgA1 (a) and IgA2 (b), due to varying hinge length, are denoted in the figure, as are the light chain and heavy chain domains, namely, variable light (VL), constant light (CL), variable heavy (VH), the three heavy chain constant domains (Cα1, Cα2, Cα3) and the tail piece, which enables IgA polymerization. The difference in extent of and the positions of O-linked and N-linked glycosylation sites are shown with red and blue bars, respectively
The human IgA1 molecule, as compared with IgA2, is rather ‘T-shaped’ (Fig. 3a), due to its predominant difference in the hinge region. IgA1 has a 19-amino-acid-long hinge, with up to five serine and threonine residues with O-linked glycosylation. The site occupancy may however vary, giving rise to glycosylation variants of IgA1 [19, 20]. The long proline-rich hinge of IgA1 may present an extended antigen reach, but the length comes at the cost of being susceptible to bacterial proteases. A range of infectious bacteria, such as Neisseria meningitidis, N. gonorrhoea, Haemophilus influenza, Streptococcus pneumonia, and S. sanguis, have evolved to produce IgA1-proteases as an important virulence factor to successfully colonize mucosal surfaces [21]. The more proteolytically resistant IgA2 has a shorter hinge of six amino acids, lacks the O-linked glycosylation, and is ‘Y-shaped’ (Fig. 3b). Additional allotypes of IgA2, namely IgA2m(1), IgA2m(2), and IgA2m(3), have been defined. Differences between both IgA subclasses also exist in the extent of N-linked glycosylation. The IgA1 molecule has two, whereas IgA2 has four N-linked glycosylation sites on each heavy chain (Fig. 3) [22]. These variations in glycosylation affect the stability and the Fc-mediated biological functionality of the IgA [16].
In conclusion, the structural diversity of IgAs, and their specific modifications may influence their stability, assembly, and ultimately their biological function.
Recombinant IgA production
To study the biological functions of the different IgA classes and subclasses, and to apply them for mucosal passive immunization, a sound recombinant production platform capable of producing assembled hetero-multimeric glycoproteins is required [4]. The production of mIgA is analogous to the well-established production of IgG, involving only heavy and light chains. However, the recombinant production of multivalent dIgA and SIgA formats, which are more relevant for mucosal passive immunization, becomes tedious, because of its additional requirement of J chain- and SC-encoding sequences. The SIgAs are high-molecular weight molecules (>400 kDa), which need precise folding, glycosylation and disulfide bridge formation for stabilizing intra- and interchain assembly. Despite the complexity of the SIgA molecule, the benefits of SIgA in passive mucosal protection have attracted considerable attention for its recombinant production. Recently, Reinhart and Kunert have reviewed the “upstream and downstream processing of recombinant IgA”, listing SIgAs produced in mammalian cell cultures [23]. Here, we present a brief overview of different mammalian and plant-based technological innovations used for SIgA production (Fig. 4).
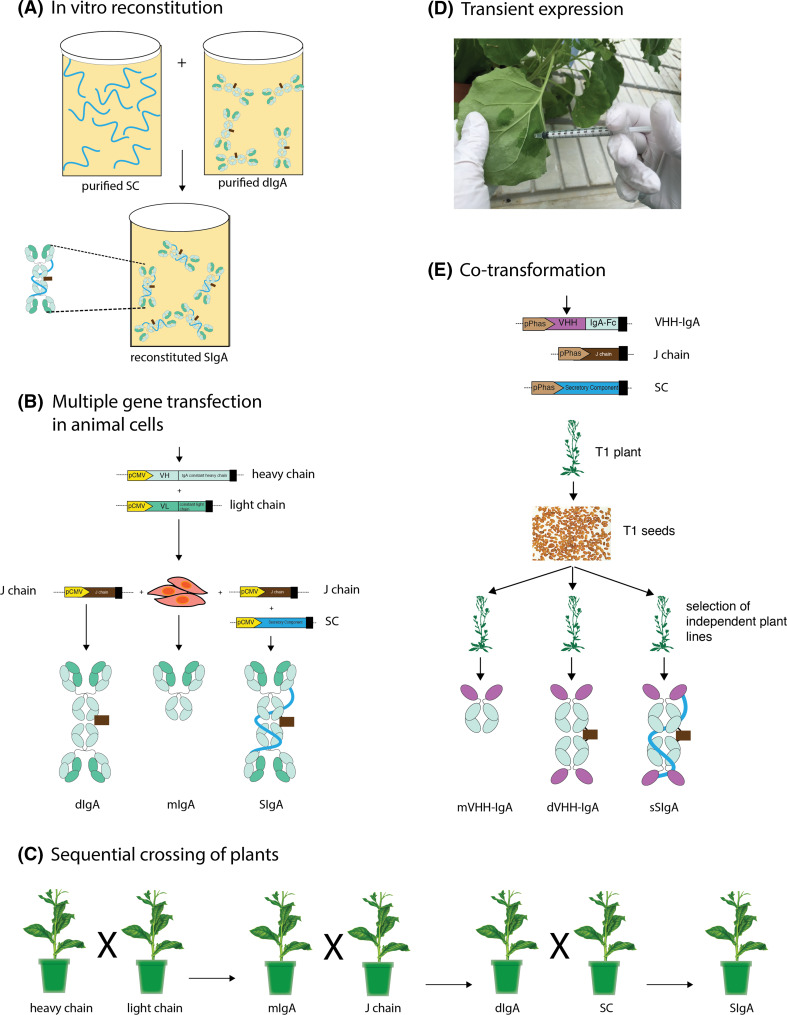
Fig. 4.
Representation of different technological innovations for recombinant secretory IgA production. a In vitro reconstitution, a process of auto-association of secretory component (SC) and dimeric IgA (dIgA) in solution; b production of monomeric (mIgA), dimeric (dIgA) and secretory (SIgA) IgA in CHO cells by transfection of respective constituting protein-chain encoding genes; c method for generating stable plant lines expressing SIgA by sequential crossing of plants expressing the constituting elements (indicated below the plants); d transient expression in Nicotiana benthamiana leaves via a needle-less syringe based infiltration of transformed Agrobacterium tumefaciens for rapid production of SIgAs; e Co-transformation of Arabidopsis with VHH-IgA, J chain and SC to produce lines expressing VHH-IgA based, monomeric (mVHH-IgA), dimeric (dVHH-IgA) and simplified secretory IgA (sSIgA). The protein domains and the gene elements coding for the heavy chain are indicated in light blue, the light chain in lime-green, the joining chain (J chain) in brown, the SC in cyan, and the VHH domain in magenta
An initial breakthrough was the method for production of SIgA in test tubes, by combining purified pIgA from hybridoma cell lines with recombinant SC (Fig. 4a) [24–26]. This process was later refined by stable transfection of the human SC in myeloma cells expressing mouse–human chimeric pIgAs [27]. Since then, the importance of using a single cell was reaffirmed by many groups, given its convenience in streamlining the cell culture conditions, downstream processing, and establishing cell lines for sustained manufacturing [4]. Starting from 1999, several groups successfully produced milligram amounts of SIgA in Chinese hamster ovary (CHO) cells using co-transfection techniques (Fig. 4b) [23, 28, 29]. Predominantly in this approach, all essential components were derived from human cDNA libraries with the exception of the variable regions, which were cloned from murine hybridomas. These are commendable developments in the SIgA production in mammalian expression system. However, to reach the current production bench mark of therapeutic IgGs, the production levels of SIgA in mammalian cell cultures would have to be increased by at least tenfold [23]. To boost the production levels, novel innovations may be needed, involving the engineering of cell lines, a better clone selection and the optimization of growth conditions, like incorporation of feed-batch or perfusion batch fermentation processes in addition to new medium formulations [23]. Low production levels have been by far the major hurdle in realizing the full potential of SIgA for clinical use [4], both in human and domesticated animals.
The only recombinant SIgA to successfully complete human clinical trials is the plant (tobacco)-made anti-dental caries SIgA antibody called CaroRx®, which is now approved for use as a medical device in the European Union [30]. This was produced by stable transformation of the heavy chain, light chain, J chain and SC in individual tobacco lines, followed by successive crossing of the selected final transformant lines (Fig. 4c). First, plants expressing the heavy chain and light chain were crossed, to obtain a line producing the mIgA [31]. Crossing of this mIgA daughter line with a J chain-expressing tobacco line led to the production of dIgA and further crossing of this dIgA line with a tobacco line expressing the SC resulted in an SIgA-producing plant [32]. The example of CaroRx® antibody demonstrates the capacity of plants to stably produce complex SIgAs, which are functional in preventing the disease, and can be easily scaled up for bulk production. However, obtaining plants producing SIgAs via sequential filial crossing is a lengthy process, requiring transformation of plants, identification of suitable lines for crossing and finally generation of a homozygous seed stock for subsequent upscaling of SIgA-producing plants. Simultaneous introduction of the sequences encoding the four elements, i.e. IgA heavy chain, light chain, J chain and SC, into the plants would help in shortening the time required for developing SIgA-expressing plants [33]. This can be achieved either via co-transformation of the respective elements, either cloned in individual plasmids (4 T-DNAs); or stacking of the elements in tandem on a single plasmid (1 T-DNA) [34].
Juarez et al. [34] used the single plasmid system for the production of human SIgAs against rotavirus in tobacco leaves via the transient leaf infiltration method. In this method, a suspension of Agrobacterium transformed with the plasmid bearing the coding sequences for the heavy, the light the J chains, and the SC, each under the control of a constitutive promoter, was infiltrated into leaves using a needle-less syringe (Fig. 4d). Speed of production is a unique advantage of this transient expression system, since, by the fifth day post infiltration, SIgAs could be extracted and purified from homogenized leaf tissue.
In contrast to the two above-described leaf-based expression systems (stable and transient), seeds can also be used for SIgA production [35]. Production of SIgAs in seeds has the advantage of stock piling at ambient temperatures, which could be a strategy of choice for periodic or recurrent seasonal infection outbreaks. Being cold-chain-free, seed-produced SIgAs are relevant for tropical diseases in remote access regions. Moreover, seeds can be incorporated into food and feed for oral delivery, avoiding the necessity for purification [35]. To achieve SIgA production in seeds, the specific β-phaseolin promoter that leads to high expression in dicotyledonous seeds has been used [36]. Furthermore, in case of an anti-bacterial SIgA produced in seeds, the recombinant SIgA molecule was engineered to simplify its structure, but maintaining all advantages of a classical SIgA. This was achieved essentially using the VHH-IgA fusion strategy to build the monomeric units of the SIgA [35]. More explicitly, instead of using the antigen-binding domains formed by the paired heavy and light chain (the Fab, ~55 kDa), the antigen-binding Nanobody® (~15 kDa), which is the variable domain of a heavy chain-only antibody of camelids (VHH) [37], was used. This substitution also eliminated the necessity for the light chain and the light chain-binding Cα1 domain of the heavy chain, thereby simplifying the >400-kDa complex SIgA molecule to a 230-kDa simplified SIgA (sSIgA) (Fig. 4e). Such an sSIgA may be convenient to produce in all platforms. The sSIgA-producing seeds were obtained via triple co-transformation of Arabidopsis thaliana (thale cress) with three Agrobacterium cultures, each bearing the VHH-IgA, the J chain or the SC construct, mixed in equal proportions (Fig. 4e). This method is faster than the sequential crossing method, and although it requires time to screen the pool of co-transformants, one can screen and select every interesting combination, e.g. plants expressing only VHH-IgA (mVHH-IgA, one T-DNA), VHH-IgA and the J chain (dVHH-IgA, two T-DNAs), or VHH-IgA, the J chain and the SC (sSIgA, three T-DNAs) (Fig. 4e). In this proof-of-concept study, the sSIgAs were made by grafting the VHHs against diarrhea-causing enterotoxigenic Escherichia coli (ETEC) onto the Fc fragment of porcine IgA, oral feed based delivery of these VHH-IgAs protected piglets in a challenge experiment [35]. Evaluating the efficacy of sSIgAs meant for human oral application is more pertinent in large monogastric animals like pigs than in mouse models. The sheer length of the pig alimentary canal enables a better assessment of VHH-IgA stability during gut transit. Essentially, the VHH-IgA fusion strategy can be used for producing sSIgAs for other species. We are currently investigating a murine and humanized version of VHH-based sSIgAs.
Another clever innovation has been the LEX system, a Lemna-based platform for SIgA production with modified glycosylation patterns [38]. Lemna or duckweed is an aquatic monocot that can be grown in transparent bags placed under a light source and yields SIgAs up to 15 % of the total soluble protein (Table 1). This gives the unique advantage of having contained, cost-effective and scalable SIgA production.
Table 1.
Secretory IgAs produced in plants
| Plant | Tissue/organ | Antibody targets | Antibody details | Method of transformation | T-DNA construct | Expression levels | Efficacy | References |
|---|---|---|---|---|---|---|---|---|
| Nicotiana benthamiana | Leaves | Anti-VP8, Rotavirus | Human IgAs, 16 variants | Transient | Tandem genes in a single T-DNA | 32.5 µg IgA/g fresh weight (33 % assembly) | Specifically binds antigen- VP8 in ELISA | [34] |
| Nicotiana benthamiana | Leaves | Anti-Eimeria acervulina, (Coccidiosis) | Chicken IgA | Transient | Quadruple infiltration (four distinct T-DNAs) | Variability in expression, of different idiotypes, highest ~1.6 % of soluble protein | Specifically binds antigen in ELISA | [61] |
| Nicotiana benthamiana | Whole plant (constitutive expression) | Anti-HIV | Human IgA (class switched IgG1 to IgA1) | Transient | Quadruple infiltration (four distinct T-DNAs) | 25 µg/g fresh weight (48 % assembly) | Binds GP120, neutralizes HIV strains and aggregates HIV virions | [41] |
| Nicotiana tabacum | Stable | Sequential crossing | 15.2 µg/g fresh weight (76 % assembly) | |||||
| Nicotiana tabacum | Whole plant (constitutive expression) | Anti-Streptococcus mutans | Human IgA (class switched) | Stable | Sequential crossing | 200–500 µg/g fresh weight (50 % assembly) | Prevents dental caries in humans, approved for use as a medical device in European Union | [32] |
| Arabidopsis thaliana | Seeds | Anti-Enterotoxigenic Escherichia coli (ETEC) | Anti-ETEC lama VHHs fused to Porcine IgA Fc | Stable | Triple co-transformation | 0.2 % of seed weight (~50 % assembly) | Protected piglets in a challenge model by preventing ETEC colonization | [35] |
| Lemna minor (Duckweed) | Whole plant (Constitutive expression) | Anti-inflammatory | Human IgA (class switched) | Stable | Tandem genes in a single T-DNA | 8.5–15.1 % of soluble protein | Binds interleukin-12 | [38] |
Issues with recombinant IgA production, improving expression and assembly levels
Overall, it appears that the plant cellular machinery may be well suited for expression and assembly of SIgAs [30, 39], but there is room for further improvement in the overall expression of the SIgA elements and their assembly (Table 1). Partial assembly seems to be one of the common issues affecting both mammalian cell- and plant-based SIgA production. Of the total recombinant IgA heavy and light chains expressed, only a fraction seems to assemble into SIgAs (Table 1) [23, 35, 40]. The strategy for fine-tuning the expression levels to obtain ideal molar ratios that may lead to higher SIgA-assembled fractions is resounded in many publications. Theoretically, this can be achieved using promoters of varying strength, or selecting differential copy numbers, but in practice, it remains more an empirical method. In plants, the assembly rate may also be influenced by the transformation method used. Transient expression of an Fc class-switched (IgG1 to IgA1) version of anti-HIV SIgA produced up to 25 µg/g fresh leaf weight, of which 48 % was assembled as SIgA, whereas the yield from leaves of transgenic tobacco (stable nuclear transformation) of the same antibody led to lower IgA expression levels of 15.2 µg/g fresh leaf weight, but these plants had a higher rate (76 %) of SIgA assembly [41]. Overexpression of chaperones, foldases, specific glycosyltransferases and protein disulfide isomerases may also help in achieving efficient expression and assembly [4, 42], and avoid wastage of unassembled chains, which also co-purify in affinity chromatographic steps and, hence, require additional processing to obtain pure recombinant SIgA.
Another strategy to achieve higher amounts of assembled SIgAs might be to perform in vitro re-association (reconstitution). Basically, when free SC is incubated with dIgA (bearing the J chain), it autonomously leads to the formation of covalently linked SIgA in vitro (Fig. 4a) [24, 43]. The recombination can be achieved in solution, or when the dIgA is immobilized to a membrane of nitrocellulose or polyvinylidene fluoride, or even on an IgA affinity column [23, 44–46]. However, the reconstitution efficiency reported by different groups seems to vary vastly (15–90 %) [23], perhaps due to the subtle post-translational differences between the recombinant molecules. These may include glycosylation differences and particular folds that defy access to cysteine residues. Addition of a thiol-disulfide redox buffer has been reported to render the dIgA into a structural conformation necessary for SC reconstitution [47], which may help in some cases for increasing the post-production in vitro reconstitution of plant- and CHO cell-made SIgAs.
Purification
The need, process and degree of purification depends on the required application of the recombinant IgA (e.g. orally delivered against enteric infections, or nasally for a respiratory infection, etc.) and the production system used (CHO cells, leaves or seeds). However, purification of IgA has been a lingering issue since its discovery. Unlike the robust scalable solutions that exist for IgG purification, like protein-A and protein-G resins, a comparable, high efficiency resin for IgA purification is yet to make its mark in the IgA research and manufacturing front. There are sets of commercially available resins, which in proverbial terms ‘get the job done’ but have certain drawbacks. Again, the high variation in formats with molecular weights ranging from ~300 (pIgA) to ~400 (SIgA) kDa, with different subclasses, and with variation in glycosylation, makes it challenging to identify and establish a generally applicable standard affinity-based method that is both specific (high purity levels) and shows omnipotent IgA-binding (for all formats of IgA).
The use of jacalin, an α-d-galactose-binding lectin derived from jackfruit, has been widely reported for purifying IgA from mucosal specimens [23, 48]. However, because the lectin binds via the O-linked glycosylation site, it is only effective for IgA1 purification and not for IgA2 [18, 49]. Using jacalin for clinical research specimens inevitably induces a bias in IgA1 enrichment, but also enables to focus on the role of subclasses in infection. Another issue is the co-elution of host proteins bearing O-linked glycosylation along with IgA1. In a purification analysis of IgAs from serum-free CHO supernatant, two different IgAs, each with the κ and λ light chain, showed high recovery efficiencies (97–98 %) and acceptable purities (80–90 %), but with contaminating proteins of diverse molecular weights [17]. In case of tobacco-produced SIgAs, be it of the IgA1 or IgA2 subtype, the jacalin-based method seems inefficient [34, 41], perhaps due to the lack of the precise O-linked glycosylation. Lastly, the elution step in jacalin-based chromatography requires competing galactose, the cost of which and difficulty in removing it from purified IgA1 also raises questions about scalability [23].
Staphyloccocal superantigen like-7 (SSL7) is another popular alternative commercially available for IgA purification. SSL7 is one of the molecular arsenals that Staphylococcus aureus has evolved to evade the host immunity by specifically binding IgA and preventing its FcαR-mediated functions [50]. The specificity of SSL7 binding to only IgA and no other Igs makes it particularly attractive as IgA-purifying reagent for a wide range of sources like milk, serum, tissue washes, etc. SSL7 binds IgA at the Cα2–Cα3 junction of both human IgA1 and IgA2. However, it does not bind murine IgA, due to an N-linked glycosylation in the middle of the Cα2–Cα3 SSL7-binding site [51]. Thus, SSL7 remains useful for human, primates, rats and most other mammals, but not for mice, which has been developed as a model to study several infectious diseases. SSL7 is also efficient for purification of plant-made human IgAs [34, 41]. In case of seed-produced porcine sSIgAs, which cannot be purified with jacalin or light chain-based affinity chromatography, SSL7 remained one of the limited options for purification [35]. Though the purity of the eluted sSIgA was high (~90 %) [35], the rate and capacity of capture was low, making it feasible for analytical purposes only. Paul and colleagues also used SSL7 for small-scale purification of anti-HIV 2G12 SIgA and used protein L affinity resin for large batches of tobacco leaves (up to 0.5 kg) [41].
Protein L is a bacterial surface protein from Peptococcus magnus that binds certain κ light chains from different Ig classes, i.e. IgG, IgM, IgA, IgD and IgE [52]. Given its broad spectrum, the application of protein L in the context of IgA passive immunization can only be envisioned for recombinant IgA and not for IgA derived from milk or convalescent serum. Even for recombinant IgAs, it is effective only for the κ light chain of IgA1. However, the protein L binding framework can be introduced into the non-binding κ light chains to enable protein L-based purification [53]. This was successfully demonstrated for IgA transiently expressed in tobacco leaves without bearing detrimental effects for the IgA antigen-binding capacity or expression level [54].
Protein M is another major bacterial virulence factor from S. pyrogenes that binds the IgA-Fc and enables the bacteria to resist phagocytosis [55]. An engineered version of this protein, with a cysteine residue incorporated in the C-terminal end called the peptide M, enables purification of human IgA1 and IgA2 with high specificity, and with a recovery of 99 % from serum and 45 % from saliva samples in a single-step purification [56]. But again, the peptide M does not bind murine IgA.
A recent study compared the use of different commercially available IgA affinity resins, namely SSL7, peptide M and jacalin, for the characterization of mucosal IgA from macaque in a simian immunodeficiency virus infection study [48]. Apparently, the SSL7 and peptide M showed much better results with a 96.7 and 83.7 % recovery, respectively; whereas jacalin had a poor recovery of 0.2 %. Apart from the resin binding capacity, the quality of purified IgA is an equally important parameter to consider. The authors observed that there was a single heavy chain moiety in the jacalin fraction, whereas elution from the other resins also contained additional heavy chain degradation fragments. Analysis on Western blots from native gels showed a higher IgA homogeneity in the jacalin-purified material, whereas multiple formats of IgA (ranging from ~300 to ~720 kDa) were found in the SSL7-purified material. Further, the SSL7-purified IgA had the poorest neutralization and phagocytosis capacity. In the end, the authors argued the merit of peptide M-purified IgA, which performed relatively well across all analyses [48]. This example shows that not only the binding capacity, but also the binding specificity of a particular functional IgA format are important considerations when purifying IgAs for passive immunization.
Conclusion
Specific SIgAs play an important role in evading, neutralizing and even ‘cleaning up’ infectious pathogens from the mucosal surface. Recombinant IgA application can be an immediate procedure to impart protective capacity [35]. Immediate protection is a unique advantage of passive immunization, which is needed when there is a small window for intervention, like post-weaning infections, emergency in bio-terrorism and epidemics. SIgA is an alternative to the antibiotic use, and may help in contributing to lower the indiscriminate use of antibiotics, which is inevitably tied to the risk of introducing bacterial resistance [57]. Protective SIgAs may be applied as prophylaxis prior to the contingency of being infected, e.g. oral application against traveler’s diarrhea prior to traveling or intranasal application in case of seasonal respiratory infections such as influenza, human respiratory syncytial virus [58], or at the urogenital tract against sexually transmitted diseases like HIV [41, 59] and chlamydia [60]. Importantly for the end users, mucosal passive immunization with SIgA means: ‘non-invasive, immediate protection’. A formulation of SIgA may eventually be applied by susceptible individuals themselves with or without medical supervision, and thus has a wider coverage, especially important in resource-poor areas or in case of epidemics.
But before these SIgA-based prophylactic approaches can be realized, improvements are needed to obtain cost-effective, high amounts of assembled monoclonal SIgAs. CHO cells have come far from the initial proof of concept to the current production levels [23]. Given the well-established tools and platform for CHO-based recombinant protein production, it is likely that the SIgA production levels in these cells will also increase in the future. Maintaining low production and purification costs for SIgA-based prophylaxis is also essential for universal accessibility. Seed- and leaf-based production offers a cost effectiveness, as well as delivery solution. Because plants are free from mammalian viruses and pathogens, oral administration of minimally processed or crude extracts as ‘SIgA juice’ can be envisioned for enteric infections. Simultaneously, we need to better understand the role of SIgA in various diseases and study the influence of applying recombinant SIgAs in preventing those specific infections at the mucosal surfaces. In the future, the SIgA production platform would possibly need to be flexible enough to produce different variants of SIgA with specific glycan-modification. Thus, for the SIgA-based passive immunization to become a reality, we require outstanding solutions for the manufacturing of this outstanding immunoglobulin.
Acknowledgments
We would like to thank T. De Meyer and A. Bleys for their advise and proof reading the manuscript. This work was supported by Research Foundation Flanders (FWO project G0C9714N), the European Commission (H2020-MSCA-IF-2014 Proposal 658701-ImmunoFarm) the VIB and the Agency for Innovation by Science and Technology (IWT-innovation mandate 140851).
Abbreviation
- sSIgA
Simplified secretory IgA, an IgA format devoid of light chain, in which the variable domains, either a single chain variable fragment (scFv) or a variable domain of camelid heavy chain only (VHH/nanobody), are grafted onto the IgA Fc chain
References
- 1.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 2.Strugnell RA, Wijburg OLC. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8(9):656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 3.Macpherson AJ, Geuking MB, McCoy KD. Homeland Security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33(4):160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Corthésy B. Recombinant immunoglobulin A: powerful tools for fundamental and applied research. Trends Biotechnol. 2002;20(2):65–71. doi: 10.1016/S0167-7799(01)01874-1. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Holmgren J. Topical immunization strategies. Mucosal Immunol. 2010;3(6):545–555. doi: 10.1038/mi.2010.55. [DOI] [PubMed] [Google Scholar]
- 6.Corthésy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010;5(5):817–829. doi: 10.2217/fmb.10.39. [DOI] [PubMed] [Google Scholar]
- 7.Zhou M, Ruprecht RM. Are anti-HIV IgAs good guys or bad guys? Retrovirology. 2014;11(1):109. doi: 10.1186/s12977-014-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corthésy B. Recombinant secretory immunoglobulin A in passive immunotherapy: Linking Immunology and biotechnology. Curr Pharm Biotechno. 2003;4(1):51–67. doi: 10.2174/1389201033378020. [DOI] [PubMed] [Google Scholar]
- 9.De Magistnis MT. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv Drug Deliv Rev. 2006;58(1):52–67. doi: 10.1016/j.addr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 11.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5(12):905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 12.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29(2):158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virdi V, Depicker A. Role of plant expression systems in antibody production for passive immunization. Int J Dev Biol. 2013;57(6–8):587–593. doi: 10.1387/ijdb.130266ad. [DOI] [PubMed] [Google Scholar]
- 14.Naz RK, Rajesh C. Passive immunization for immunocontraception: lessons learned from infectious diseases. Front Mol Biosci. 2004;9:2457–2465. doi: 10.2741/1407. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin. 2010;31(9):1198–1207. doi: 10.1038/aps.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo EM, Morrison SL. IgA: an immune glycoprotein. Clin Immunol. 2005;116(1):3–10. doi: 10.1016/j.clim.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Reinhart D, Weik R, Kunert R. Recombinant IgA production: single step affinity purification using camelid ligands and product characterization. J Immunol Methods. 2012;378(1–2):95–101. doi: 10.1016/j.jim.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Kondoh H, Kobayashi K, Hagiwara K. A simple procedure for the isolation of human secretory IgA of IgA1 and IgA2 subclass by a jackfruit lectin, jacalin, affinity chromatography. Mol Immunol. 1987;24(11):1219–1222. doi: 10.1016/0161-5890(87)90169-6. [DOI] [PubMed] [Google Scholar]
- 19.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273(4):2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 20.Russel MW, Kilian M, Mantis NJ, Corthésy B. Biological activity of IgA. In: Mestecky J, Strober W, Russell MW, Cheroutre H, Lambrecht BN, Kelsall BL, editors. Mucosal immunology. 4. USA: Academic Press; 2015. pp. 429–454. [Google Scholar]
- 21.Mistry D, Stockley RA. IgA1 protease. Int J Biochem Cell Biol. 2006;38(8):1244–1248. doi: 10.1016/j.biocel.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2000;191(12):2171–2182. doi: 10.1084/jem.191.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhart D, Kunert R. Upstream and downstream processing of recombinant IgA. Biotechnol Lett. 2015;37(2):241–251. doi: 10.1007/s10529-014-1686-z. [DOI] [PubMed] [Google Scholar]
- 24.Rindisbacher L, Cottet S, Wittek R, Kraehenbuhl J-P, Corthésy B. Production of human secretory component with dimeric IgA binding-capacity using viral expression systems. J Biol Chem. 1995;270(23):14220–14228. doi: 10.1074/jbc.270.23.14220. [DOI] [PubMed] [Google Scholar]
- 25.Lüllau E, Heyse S, Vogel H, Marison I, von Stockar U, Kraehenbuhl J-P, Corthésy B. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J Biol Chem. 1996;271(27):16300–16309. doi: 10.1074/jbc.271.27.16300. [DOI] [PubMed] [Google Scholar]
- 26.Crottet P, Cottet S, Corthésy B. Expression, purification and biochemical characterization of recombinant murine secretary component: a novel tool in mucosal immunology. Biochem J. 1999;341(Pt 2):299–306. doi: 10.1042/bj3410299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chintalacharuvu KR, Morrison SL. Production of secretory immunoglobulin A by a single mammalian cell. Proc Natl Acad Sci USA. 1997;94(12):6364–6368. doi: 10.1073/pnas.94.12.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berdoz J, Blanc CT, Reinhardt M, Kraehenbuhl J-P, Corthésy B. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc Natl Acad Sci USA. 1999;96(6):3029–3034. doi: 10.1073/pnas.96.6.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen F-E, Norderhaug IN, Røe M, Sandlie I, Brandtzaeg P. Recombinant expression of polymeric IgA: incorporation of J chain and secretory component of human origin. Eur J Immunol. 1999;29(5):1701–1708. doi: 10.1002/(SICI)1521-4141(199905)29:05<1701::AID-IMMU1701>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Paul M, Ma JK-C. Plant-made pharmaceuticals: leading products and production platforms. Biotechnol Appl Biochem. 2011;58(1):58–67. doi: 10.1002/bab.6. [DOI] [PubMed] [Google Scholar]
- 31.Ma JK-C, Lehner T, Stabila P, Fux CI, Hiatt A. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur J Immunol. 1994;24(1):131–138. doi: 10.1002/eji.1830240120. [DOI] [PubMed] [Google Scholar]
- 32.Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, van Dolleweerd C, Mostov K, Lehner T. Generation and assembly of secretory antibodies in plants. Science. 1995;268(5211):716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- 33.Wycoff KL. Secretory IgA antibodies from plants. Curr Pharm Design. 2005;11(19):2429–2437. doi: 10.2174/1381612054367508. [DOI] [PubMed] [Google Scholar]
- 34.Juarez P, Huet-Trujillo E, Sarrion-Perdigones A, Falconi EE, Granell A, Orzaez D. Combinatorial analysis of secretory immunoglobulin A (sIgA) expression in plants. Int J Mol Sci. 2013;14(3):6205–6222. doi: 10.3390/ijms14036205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virdi V, Coddens A, De Buck S, Millet S, Goddeeris BM, Cox E, De Greve H, Depicker A. Orally fed seeds producing designer IgAs protect weaned piglets against enterotoxigenic Escherichia coli infection. Proc Natl Acad Sci USA. 2013;110(29):11809–11814. doi: 10.1073/pnas.1301975110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jaeger G, Scheffer S, Jacobs A, Zambre M, Zobell O, Goossens A, Depicker A, Angenon G. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat Biotechnol. 2002;20(12):1265–1268. doi: 10.1038/nbt755. [DOI] [PubMed] [Google Scholar]
- 37.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 38.Ariaans GJA, De Roo G, Declan Nolan T, Regan J (2012) Expression of secretory IgA antibodies in duckweed (WO Patent App. PCT/EP2012/075,673). WO Patent App. PCT/EP2012/075,673
- 39.Stoger E, Fischer R, Moloney M, Ma JK-C. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol. 2014;65:743–768. doi: 10.1146/annurev-arplant-050213-035850. [DOI] [PubMed] [Google Scholar]
- 40.Ma JK-C, Hein MB. Immunotherapeutic potential of antibodies produced in plants. Trends Biotechnol. 1995;13(12):522–527. doi: 10.1016/S0167-7799(00)89016-2. [DOI] [PubMed] [Google Scholar]
- 41.Paul M, Reljic R, Klein K, Drake PM, van Dolleweerd C, Pabst M, Windwarder M, Arcalis E, Stoger E, Altmann F, Cosgrove C, Bartolf A, Baden S, Ma JK-C. Characterization of a plant-produced recombinant human secretory IgA with broad neutralizing activity against HIV. mAbs. 2014;6(6):1585–1597. doi: 10.4161/mabs.36336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pybus LP, Dean G, West NR, Smith A, Daramola O, Field R, Wilkinson SJ, James DC. Model-directed engineering of “difficult-to-express” monoclonal antibody production by Chinese hamster ovary cells. Biotechnol Bioeng. 2014;111(2):372–385. doi: 10.1002/bit.25116. [DOI] [PubMed] [Google Scholar]
- 43.Longet S, Vonarburg C, Lötscher M, Miescher S, Zuercher A, Corthésy B. Reconstituted human polyclonal plasma-derived secretory-like IgM and IgA maintain barrier function of epithelial cells infected with an enteropathogen. J Biol Chem. 2014;289:21617–21626. doi: 10.1074/jbc.M114.549139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longet S, Miled S, Lötscher M, Miescher SM, Zuercher AW, Corthésy B. Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J Biol Chem. 2013;288(6):4085–4094. doi: 10.1074/jbc.M112.410811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crottet P, Corthésy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: a possible implication for mucosal defense. J Immunol. 1998;161(10):5445–5453. [PubMed] [Google Scholar]
- 46.Moldt B, Saye-Francisco K, Schultz N, Burton DR, Hessell AJ. Simplifying the synthesis of SIgA: combination of dIgA and rhSC using affinity chromatography. Methods. 2014;65(1):127–132. doi: 10.1016/j.ymeth.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones RML, Schweikart F, Frutiger S, Jaton J-C, Hughes GJ. Thiol-disulfide redox buffers maintain a structure of immunoglobulin A that is essential for optimal in vitro binding to secretory component. Biochim Biophys Acta Protein Struct Mol Enzymol. 1998;1429(1):265–274. doi: 10.1016/S0167-4838(98)00239-8. [DOI] [PubMed] [Google Scholar]
- 48.Musich T, Demberg T, Morgan IL, Estes JD, Franchini G, Robert-Guroff M. Purification and functional characterization of mucosal IgA from vaccinated and SIV-infected rhesus macaques. Clin Immunol. 2015;158(2):127–139. doi: 10.1016/j.clim.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondoh H, Kobayashi K, Hagiwara K, Kajii T. Jacalin, a jackfruit lectin, precipitates IgA1 but not IgA2 subclass on gel diffusion reaction. J Immunol Methods. 1986;88(2):171–173. doi: 10.1016/0022-1759(86)90003-7. [DOI] [PubMed] [Google Scholar]
- 50.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-FcαRI binding and serum killing of bacteria. J Immunol. 2005;174(5):2926–2933. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- 51.Wines BD, Ramsland PA, Trist HM, Gardam S, Brink R, Fraser JD, Hogarth PM. Interaction of human, rat, and mouse immunoglobulin A (IgA) with Staphylococcal superantigen-like 7 (SSL7) decoy protein and leukocyte IgA receptor. J Biol Chem. 2011;286(38):33118–33124. doi: 10.1074/jbc.M111.272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björck L. Protein L. A novel bacterial cell wall protein with affinity for Ig L chains. J Immunol. 1988;140(4):1194–1197. [PubMed] [Google Scholar]
- 53.Nilson BHK, Lögdberg L, Kastern W, Björck L, Åkerström B. Purification of antibodies using protein L-binding framework structures in the light chain variable domain. J Immunol Methods. 1993;164(1):33–40. doi: 10.1016/0022-1759(93)90273-A. [DOI] [PubMed] [Google Scholar]
- 54.Boes A, Spiegel H, Delbrück H, Fischer R, Schillberg S, Sack M. Affinity purification of a framework 1 engineered mouse/human chimeric IgA2 antibody from tobacco. Biotechnol Bioeng. 2011;108(12):2804–2814. doi: 10.1002/bit.23262. [DOI] [PubMed] [Google Scholar]
- 55.Carlsson F, Berggård K, Stålhammar-Carlemalm M, Lindahl G. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J Exp Med. 2003;198(7):1057–1068. doi: 10.1084/jem.20030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandin C, Linse S, Areschoug T, Woof JM, Reinholdt J, Lindahl G. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J Immunol. 2002;169(3):1357–1364. doi: 10.4049/jimmunol.169.3.1357. [DOI] [PubMed] [Google Scholar]
- 57.Berghman LR, Abi-Ghanem D, Waghela SD, Ricke SC. Antibodies: an alternative for antibiotics? Poult Sci. 2005;84(4):660–666. doi: 10.1093/ps/84.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weltzin R, Traina-Dorge V, Soike K, Zhang J-Y, Mack P, Soman G, Drabik G, Monath TP. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis. 1996;174(2):256–261. doi: 10.1093/infdis/174.2.256. [DOI] [PubMed] [Google Scholar]
- 59.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau Q, Villinger F, Corti D, Ruprecht RM. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS. 2013;27(9):F13–F20. doi: 10.1097/QAD.0b013e328360eac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armitage C, O’Meara C, Harvie M, Timms P, Wijburg O, Beagley K. Evaluation of intra-and extra-epithelial secretory IgA in chlamydial infections. Immunology. 2014;143(4):520–530. doi: 10.1111/imm.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wieland WH, Lammers A, Schots A, Orzaez DV. Plant expression of chicken secretory antibodies derived from combinatorial libraries. J Biotechnol. 2006;122(3):382–391. doi: 10.1016/j.jbiotec.2005.12.020. [DOI] [PubMed] [Google Scholar]