Abstract
Cholesterol plays a central role in numerous nervous system functions. Cholesterol is the major constituent of myelin sheaths, is essential for synapse and dendrite formation, axon guidance as well as neurotransmission. Among regulators of cholesterol homeostasis, liver X receptors (LXRs), two members of the nuclear receptor superfamily, play a determinant role. LXRs act as cholesterol sensors and respond to high intracellular cholesterol concentration by decreasing plasmatic and intracellular cholesterol content. Beyond their cholesterol-lowering role, LXRs have been proposed as regulators of immunity and anti-inflammatory factors. Dysregulation of cholesterol metabolism combined to neuroinflammatory context have been described in neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). ALS is characterized by the progressive loss of motoneurons in the brain and spinal cord, leading to severe paralytic condition and death of patients in a median time of 3 years. Motoneuron degeneration is accompanied by chronic neuroinflammatory response, involving microglial and astrocytic activation, infiltration of blood-derived immune cells and release of pro-inflammatory factors. We propose to discuss here the role of LXRs as a molecular link between the central nervous system cholesterol metabolism, neuroinflammation, motoneuron survival and their potential as promising therapeutic candidates for ALS therapy.
Keywords: Amyotrophic lateral sclerosis, Neuroinflammation, Astrocytes, Microglia, Liver X receptors, Cholesterol
Structure and mechanism of action of LXRs
Liver X receptor alpha (LXRα) and beta (LXRβ) are inducible transcription factors belonging to the nuclear receptor superfamily. Oxysterols, oxidized forms of cholesterol, are natural ligands activating LXRs. The initial discovery of LXRα [1] and LXRβ [2] was the result of a screening strategy based on the high sequence identity among nuclear receptors DNA-binding domains. This reverse endocrinology strategy [3], led to the identification of various nuclear receptors before the identification of their endogenous ligands, leading to the concepts of “orphan” (i.e., with no known endogenous ligand) and “adopted” (i.e., whose endogenous ligands were discovered after their receptor) receptors [4]. Based on this classification, LXRs can be considered adopted receptors.
LXRα and LXRβ are encoded by two distinct genes: NR1H3, located on chromosome 11 and NR1H2, located on chromosome 19 in human, respectively. They are composed of 447 and 460 amino acids [5] and encompass the classical domains of nuclear receptors: an N-terminal regulatory domain, a DNA-binding domain, a hinge domain and a ligand-binding domain. They share high sequence identity within their DNA- and ligand-binding domains (75 and 78 %, respectively) [6].
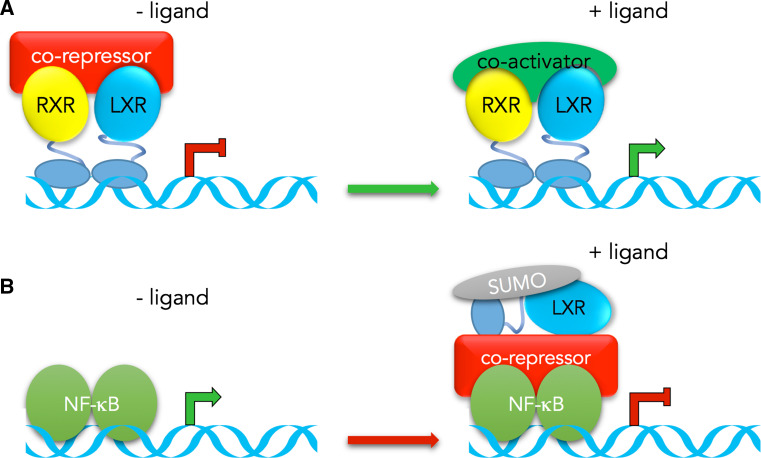
In their canonical mode of action, LXRs form obligate heterodimer with the nuclear receptors for 9-cis retinoic acid (RXRs). The RXR-LXR heterodimers are constitutively bound to their response element the LXR responsive elements (LXRE), within the promoter of their target genes [7]. The unliganded receptors inhibit target genes expression by contacting co-repressors (e.g., histone deacetylase, HDAC). The lipophilic nature of 9-cis retinoic acid and oxysterol allows them to pass through the cellular membranes and bind to the RXR–LXR heterodimer. Binding of ligand to either RXR and/or LXR induces conformational changes and the release of co-repressors and recruitment of coactivators (Fig. 1a). More recently, a direct inhibitory mechanism on several proinflammatory genes, including inducible nitric oxide synthase (iNOS), interleukin (IL)-1β, monocyte chemoattractant protein 1 (MCP-1) and tumor necrosis factor alpha (TNFα) has been described. This mechanism referred as transrepression implicates SUMOylation of LXRs upon ligand binding and subsequent stabilisation of the LXR-co-repressor complex on target gene promoter (Fig. 1b) [8]. These two distinct mechanisms allow LXRs to act either as activators or repressors, depending on the target gene.
Fig. 1.
Schematic representation of LXRs mechanism of action. a. In their canonical mode of action, the RXR-LXR heterodimer is constitutively bound to its response elements (LXRE) in the target gene promoter. Binding of RXR and/or LXR ligand induces conformational changes resulting in the release of co-repressors and recruitment of co-activators and subsequent activation of target genes. b. Transrepression mechanism has been described for pro-inflammatory factors encoding genes. Binding of LXR ligand induces its SUMOylation, thus stabilizing co-repressors on NF-κB, resulting in a down-regulation of the target gene
The first attempt to identify putative ligands for LXRs led to the discovery of some oxysterols as activators of these nuclear receptors [9]. Oxysterols, which are oxidized forms of cholesterol, can be either synthesized enzymatically through an endogenous pathway from cholesterol or they can originate directly from the alimentation [10]. The nature of oxysterols in the organism may depend on the site of synthesis, for instance 22(R)-hydroxycholesterol in steroidogenic tissues, 24(S),25-epoxycholesterol in liver or 27-hydroxycholesterol in plasma and macrophages [11]. It is interesting to notice the presence of high levels of 24(S)-hydroxycholesterol, also named cerebrosterol, which act as LXRs ligand, in the brain. The definite proof that oxysterol are endogenous ligands for LXRs came in 2007. On one hand, Wong et al. [12] showed a correlation between some oxysterols concentrations and cholesterol concentrations. On the other hand, Chen et al. [13] generated mice with targeted deletions in three oxysterol biosynthetic enzyme genes, cholesterol 24-hydroxylase, cholesterol 25-hydroxylase and sterol 27-hydroxylase, unable to synthesize 24(S)-hydroxycholesterol, 25-hydroxycholesterol and 27-hydroxycholesterol. These mice totally lost their ability to induce several LXRs target genes in response to dietary cholesterol. Finally, beyond their well-known activation by oxysterols, the LXRs are today considered as endogenous cholesterol sensors. Over the last years intensive efforts have been made to produce synthetic ligands for LXRs. Among these ligands, T0901317 and GW3965 are widely used in cell culture and animal studies (for a review, see [11]). To date, only one agonist, LXR-623, has been published in clinical trial, but the trial had to be stopped due to neurologic- or psychiatric-related adverse events [14]. This trial indirectly supported the putative role of LXRs in the human central nervous system (CNS). The aim of many pharmaceutic companies is now directed towards the development of synthetic agonists of these nuclear receptors, referred as selective liver X receptors modulators (SLIMS) [11], that could be specific of an organ, a cell type or an isoform of LXRs. One of these new modulators has recently entered new clinical trials and could represent the future for new LXR ligands development [15].
Role of LXRs in cholesterol metabolism
The discovery of oxysterols as ligands for LXRs implied that they could control cholesterol metabolism. Indeed, a functional LXRE has initially been discovered in cyp7a1 gene promoter [16]. This gene encodes a key enzyme in the hepatic conversion of cholesterol into bile acid and its subsequent elimination from the body. The generation of Lxrα-deficient mice then confirmed the link in vivo between LXRα and CYP7a1. When fed with a high cholesterol diet, these mice developed hepatic steatosis due to the lack of bile acids into cholesterol conversion by CYP7a1. Beside their role on bile acid synthesis [17, 18], LXRs also control cholesterol de novo synthesis [19–21] and reverse cholesterol transport to the liver [5, 22]. All taken together, the LXRs, indirectly activated by dietary cholesterol, work to lower intracellular and plasmatic concentrations and can be considered today as real endogenous cholesterol sensors.
LXRs and inflammation
The involvement of LXRs in immunity process has been initially suggested as their activation reduces the expression of pro-inflammatory factors including cyclooxygenase-2 (COX-2), iNOS, IL-1β and IL-6 [23]. A wealth of studies further demonstrated that LXRs attenuate inflammatory process through inhibition of iNOS, COX-2, osteopontin, MCP-1 or interferon-gamma-dependent pathways (for review [24]). As the atherosclerotic lesion is highly immunogenic and a high inflammatory status is a risk factor for atherosclerosis [25, 26], the anti-inflammatory role of LXRs makes them promising candidates for the treatment of this disease. Indeed, their activation attenuates atherosclerosis in animal models for this pathology. The study of these nuclear receptors in other diseases involving inflammation aspects has today become a milestone in the research on inflammatory diseases [27].
LXRs in the CNS
The brain has the highest content of cholesterol in the body as well as the highest metabolic rate with 95 % of brain cholesterol content being synthetized de novo [28, 29]. Cholesterol is essential for the formation of myelin sheath (where it represents approximately 70 % of brain content) and is also a main actor promoting synaptogenesis, axonal plasticity and neuroprotection [30]. Cholesterol has also been suggested to be involved in neuronal regeneration after injury [31]. LXRs, which are both expressed in the brain, are the main regulators of cholesterol metabolism in the CNS. One of their first described roles was the control of brain cholesterol efflux [32]. Indeed, LXRs control exchange of cholesterol between neurons and astrocytes, the latter being the main source of cholesterol for neurons [29]. The supply of cholesterol from astrocytes to neurons is mediated by apolipoprotein-E (APOE), ATP binding cassette A1 (ABCA1) and ABCG1 in a LXR-dependent manner [33]. The neuronal content of cholesterol is also regulated by LXRs that (1) regulate cholesterol uptake via an indirect mechanism involving degradation of LDR receptor (LDLR) by inducible degrader of LDLR (IDOL) and (2) induce expression of APOE, ABCA1 and ABCG1, promoting its efflux and avoiding therefore its accumulation [34]. In the myelin sheath, the cholesterol is provided by oligodendrocytes and Schwann cells, where LXRs regulate cholesterol homeostasis, myelination and remyelination [35, 36]. Another intriguing role of LXRs can be depicted in microglia, where beside their role in regulating cholesterol concentrations, they also control neuroinflammation [37].
The role of LXRs in neuroinflammation
In the CNS, immune and inflammatory responses are defense mechanisms that can be triggered by accumulation of misfolded proteins, release of cell membrane components resulting from neuronal damage, blood components that result from blood–brain barrier disruption, or oxidative stress following hypoxia [38]. If improperly regulated, neuroinflammation can be one of the pathophysiologic mechanisms underlying many neurodegenerative diseases. The activation of microglial cells, considered as the resident immune cells of the CNS [39], is a hallmark of many neurodegenerative diseases, such as Alzheimer [40] or Parkinson diseases [41], multiple sclerosis [42] as well as amyotrophic lateral sclerosis (ALS) [43]. The first evidence that LXRs play a role in microglia came in 2006 with the finding that LXRs agonists were able to inhibit iNOS and COX-2 expression in LPS-activated microglia in vitro [44]. Zhang-Gandhi and Drew further showed that treatment of microglial cells with a synthetic LXR agonist inhibited nitric oxide (NO) production as well as LPS-induced release of IL-1β and IL-6 cytokines and MCP-1 chemokine. The same results were obtained in cultured astrocytes [45]. The authors also showed that the agonist impaired the LPS-induced IκB-NF-κB pathway, an important modulator of many cytokines and chemokines. Inhibitory action of LXRs on iNOS expression, and the subsequent NO production, and IL-1β production at both mRNA and protein levels was recently confirmed on LPS-stimulated microglial cells [46]. Interestingly, intraperitoneal injections of LXR agonist in an experimental allergic encephalomyelitis mouse model delayed the onset of symptoms [46]. A recent study highlighted the neuroprotective and anti-inflammatory role of LXRs in experimental intracerebral hemorrhage (ICH) mouse model [47]. The authors showed that both LXR isoforms were expressed in neurons and microglia in the peri-ICH region. Interestingly, administration of T0901317, a synthetic agonist of LXRs conferred protective effects on behavioral and motor recovery following ICH. T0901317 reduced hemorrhagic injury volume, brain edema and blood–brain barrier permeability. It also decreased neutrophil infiltration, microglial activation, and macrophage infiltration. The ligand finally reduced LPS- and thrombin-induced pro-inflammatory responses in cultured microglial cells, including IL-1β, IL-6 and p38 and JNK phosphorylation.
Taken together, these studies suggest a pivotal role for LXRs in the regulation of microglial activation. Therefore, the development of LXR ligands that could selectively target microglia-dependent mechanisms (i.e. SLiMS) is an appealing objective to modulate neuroinflammation in several neurodegenerative diseases.
The contribution of LXRs in ALS pathogenesis
ALS is an adult-onset neurological disorder characterized by the selective degeneration of both upper and lower motoneurons. ALS leads to progressive paralysis and eventual death of patients within 3–5 years after diagnosis [48]. The incidence and prevalence of ALS are variable among studies and are approximately 2/100,000 and 7/100,000, respectively [49]. The typical modes of presentation are schematically limb-onset (about 70 %), bulbar-onset, mainly presenting speech or swallowing difficulties as firsts symptoms (about 25 %) or trunk or respiratory onset (about 5 %) [50]. Depending on the site of onset and the type of degenerated motoneuron (i.e., upper or lower motoneuron), the clinical features may vary, including spasticity, weakness, tendinous reflexes anomalies, fasciculations, wasting, dysarthria and dysphagia. Independently on the onset site, the symptoms spread from the initial onset site and progressive weakening of respiratory muscles participates to the causes of death.
Several prognostic factors have been described for ALS, including older age at onset, bulbar onset and shorter time from first symptoms to diagnosis, weight loss or vital capacity [50, 51]. Although not distinguishable from a clinical point of view, familial forms (FALS) and sporadic forms (SALS) co-exist. The FALS rate may vary among the studies and is below 10 % [52]. Mutations in 4 major independent genes: C9ORF72, superoxide dismutase-1 (SOD1), TAR DNA-binding protein (TARDBP) and fused in sarcoma (FUS) occur in more than 70 % of FALS patients. The recent efforts and the emergence of next-generation sequencing technologies have led to the identification of dozens of new genes, although found mutated in only few percent of patients (reviewed in [53]). Genetic mutations are rarely found in SALS forms and mainly involve C9ORF72 gene. The majority of ALS cases can subsequently not be explained by genetic factors. It thus appears likely that ALS is a multifactorial disease with a tight interaction of genetic and environmental factors.
The pathophysiology of ALS is complex. Many cellular and molecular mechanisms underlying the disease have been described so far. They include nuclear transport dysfunction, protein misfolding and aggregation and altered RNA metabolism [54–56]. Interestingly, energetic metabolism, especially lipid metabolism seems to participate to the natural history of ALS. Hypermetabolism has been linked to ALS and resting energy expenditure has been proposed as a prognostic factor for survival in ALS patients [57]. Dyslipidemia is also often seen in ALS patients [58]. In a cohort of 369 patients, it has been shown that plasma levels of total cholesterol were twofold higher than in the 286 healthy controls and that elevated LDL/HDL ratio in ALS patients was associated with longer survival [59]. However, these results remain debated. On one hand, similar results in favor of a better ALS prognosis in patients with dyslipidemia were presented in another cohort of 498 patients. On the other hand, dyslipidemia was not a factor associated independently with survival [60, 61].
Neuroinflammation, typified by microglia and astrocyte activation as well as infiltration of immune cells in the CNS, is a hallmark of the disease [43, 62]. According to the role of LXRs in lipid metabolism and in inflammatory process, their implication in ALS pathogenesis represents a new avenue to explore. Indeed, LXRs have also been proposed to confer neuroprotection in neurological disorders such as Alzheimer’s diseases [63]. An involvement of these nuclear receptors in motoneuron function has first been suggested in transgenic mice lacking LXRβ. Mice with a targeted deletion of Lxrβ gene showed an impairment of motor performance compared to wild-type animals, starting from 7 months of age, that progresses to hind limb paralysis [64]. This phenotype was associated with a marked degeneration of large alpha-motoneurons in the spinal cord, a decrease in the mean diameter of axons in spinal ventral roots and astrocytic activation. Later, the same team provided a more detailed analysis of Lxrβ −/− mice and showed that motor defects appeared as early as 3 months of age and were associated with a dramatic loss of neuromuscular junctions [65]. In addition, Lxrβ-deficient mice showed signs of neuroinflammation as revealed by increased levels of IL-6, TNF, IL-1β and MCP-1 in spinal cord. Interestingly, lxrβ-deficient mice showed signs of microglial and astrocytic activation and an increase in spinal cord cholesterol content, associated with an up-regulation of ApoE expression. Moreover, analysis of spinal cord of Lxrβ knockout mice also revealed the presence of inclusions of ubiquitin and TDP-43 in the cytoplasm of motoneurons [66]. All the pathological features of mice with lxrβ deletion were interestingly reminiscent with those of ALS patients and mouse models.
A link between cholesterol metabolism and neurodegeneration has been made using Lxrβ-deficient mice [67]. Based on the finding that a β-sitosterol analog (β-sitosterol-β-d-glucoside) had neurotoxic effects that caused ALS-like symptoms in mice [68], the authors orally administrated β-sitosterol to both wild-type and Lxrβ −/− mice. This phytosterol, is a well-known ligand for LXRs that is directly excreted at the intestinal level by the sterol transporters ABCG5 and ABCG8 [66, 69]. Interestingly, the administration of ββ-sitosterol exacerbated motor signs of Lxrβ-deficient mice and was associated with an increased loss of spinal motoneurons, as well as activation of microglial cells. However, these defects were not observed in wild-type mice, suggesting that LXRβ is neuroprotective. The oral administration of ββ-sitosterol induced an accumulation of 24-hydroxycholesterol in brains of transgenic mice but not wild-type animals. 24-hydroxycholesterol is an oxysterol produced from the hydroxylation of cholesterol by the cholesterol 24-hydroxylase enzyme (encoded by CYP46A1) and can cross the blood–brain barrier. This conversion of cholesterol into 24-hydroxycholesterol represents the major cholesterol excretion route in the brain [29]. These findings suggested that the role of LXRβ in maintaining cholesterol homeostasis in CNS could participate to its neuroprotective effects. However, the precise molecular link made by LXRs between cholesterol metabolism and neuroprotection is still unclear. It was indeed shown that specific cholestenoic acids, intermediates in the conversion of cholesterol into bile acids, can activate LXRs, thus promoting motoneurons survival [70]. The endogenous ligands of LXRs and their exact target genes thus represent an unexplored field of investigation in the understanding of the molecular mechanisms involving LXRs in motoneuron survival.
Although depletion of LXRβ leads to motoneuron loss in mice, the contribution of LXR pathway in ALS remains to be explored. Recently, a cell type-specific translational profiling study based on high throughput sequencing of ribosome-bound RNA predicted LXRs as major transcription coactivators in mutant SOD1-expressing astrocytes [71]. Additional studies will be important to determine the contribution of LXR pathway in disease process.
Single nucleotide polymorphisms (SNPs) within LXRα and LXRβ-encoding genes, NR1H3 and NR1H2, respectively, have been associated with several metabolic conditions in humans, including serum total, LDL and HDL cholesterol concentrations [72–75] as well as obesity [76]. Smith et al. [75] showed that an intronic SNP in LXRα-encoding gene was strongly associated with HDL-C levels. Moreover, SNPs in these genes have also been linked to other pathologies associated with deregulation of the immune system, such as preeclampsia [77]. Implication of both LXRs in nervous system has also been suggested as SNPs within both LXRs-encoding genes might contribute to Alzheimer’s disease risk and its progression [78–81]. One LXRα SNP has also been associated with soluble Aβ42 in the temporal cortex of Alzheimer’s disease patients. Such an involvement of LXRs in Alzheimer’s disease is not surprising as APOE encoding gene, whose implication in the pathology is well known, is a target gene of these nuclear receptors (see [82] for a review). Altogether, these studies could suggest an implication of both LXRs in the natural history of neurologic diseases in which neuroinflammation takes place.
Although no LXR genes mutation has been identified in ALS patients so far, a recent work showed for the first time that a nonsense mutation in LXRα gene could be responsible for primary progressive multiple sclerosis (PPMS) [83]. This study is the first report of a mutation in LXR genes in a neurologic disease. This mutation (p.Arg415Gln) has been shown to disrupt RXR-LXR heterodimerisation and to counteract the transcriptional activity of the heterodimer. The authors also showed that one SNP within LXRα was associated with PPMS, suggesting that the identified mutation was not isolated and that additional LXRα mutation could be identified in PPMS patients in the future. This disease is a progressive form of multiple sclerosis associated with neuronal loss. This study is the first to directly link one LXR-encoding gene to neuronal degeneration and opens new perspectives in the search for such mutation in ALS patients.
Conclusion
Over the last years, lipids, and especially cholesterol, have been demonstrated to exert deleterious effects on the CNS when imbalanced. The LXRs were initially identified as cholesterol “safety valves”, detecting cholesterol excess (through its conversion to oxysterols) and thus lowering cholesterol cellular content. With the goal to understand the role of these intriguing nuclear receptors, they are now recognized as central inflammatory regulators, especially in the CNS. LXRs act as neuroprotective factors, limiting neuroinflammation as well as astrocytic and microglial activation (Fig. 2). It is highly tempting to argue that LXRs are central modulators of neuroinflammation by regulating CNS cholesterol content, but this molecular link has still yet to be explored. Besides, oxysterols have been shown to cross the blood–brain barrier and to modulate several actors of lipid metabolism as well as brain functions [84], but the participation of LXRs to these mechanisms is still unclear. The findings of motor impairment and ALS-like symptoms in Lxr deficient animal models and the recent discovery of a LXRα mutation causative of a disease in which a neuronal degeneration occurs have opened a new field of investigation to further understand the natural history of neurodegenerative diseases, such as ALS. The use of LXRs as pharmacologic targets in the management of neurodegenerative diseases might not be a utopic project anymore, and will probably arise with the development of synthetic selective LXR modulators, specific of CNS. Studying LXRs is still not over!
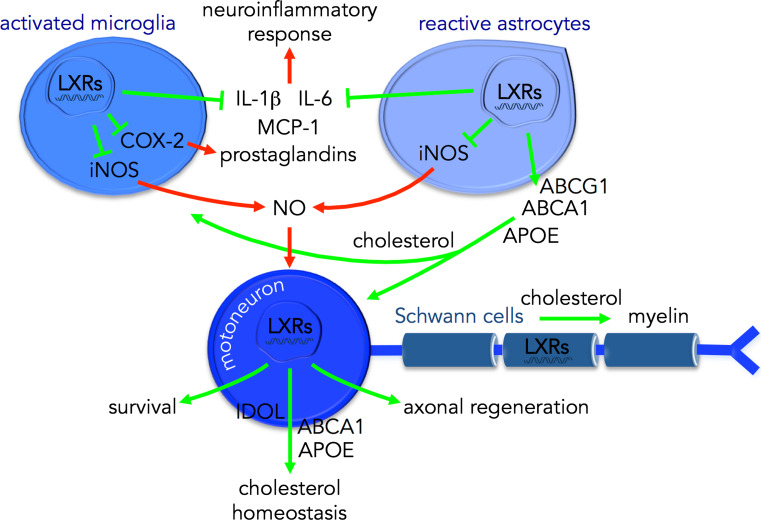
Fig. 2.
Schematic diagram illustrating the proposed neuroprotective role of LXRs in ALS. LXRs are key regulators of cholesterol homeostasis between CNS cell types. LXRs promote the supply of cholesterol from astrocytes to motoneurons and microglia. In motoneurons, LXRs also participate to cholesterol homeostasis (through IDOL, ABCA1 and APOE) and can promote survival and axonal regeneration. In Schwann cells, LXRs contribute to myelin sheath production and maintenance. In glial cells, LXRs can decrease the production of pro-inflammatory factors including IL-1β, IL-6, MCP-1, prostaglandins (through COX-2); expression of iNOS and therefore the subsequent production of NO, which can trigger a motoneuron-specific death pathway [85]
Acknowledgments
We are grateful to all technicians of the Department of Biochemistry and Molecular Biology at Nîmes University Hospital and team members for helpful discussion. Our work is supported by grants from the institut national de la santé et de la recherche médicale (Inserm), the Groupement de Coopération Sanitaire Montpellier-Nîmes (GCS-MERRI) and Association Française pour la Recherche sur la SLA (ARSLA).
References
- 1.Willy PJ, Umesono K, Ong ES, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 2.Shinar DM, Endo N, Rutledge SJ, et al. NER, a new member of the gene family encoding the human steroid hormone nuclear receptor. Gene. 1994;147:273–276. doi: 10.1016/0378-1119(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 3.Kliewer SA, Lehmann JM, Willson TM. Orphan nuclear receptors: shifting endocrinology into reverse. Science. 1999;284:757–760. doi: 10.1126/science.284.5415.757. [DOI] [PubMed] [Google Scholar]
- 4.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 5.Maqdasy S, Trousson A, Tauveron I, et al. Once and for all, LXRα and LXRβ are gatekeepers of the endocrine system. Mol Aspects Med. 2016;49:31–46. doi: 10.1016/j.mam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Mouzat K, Baron S, Marceau G, et al (2012) Emerging roles for LXRs and LRH-1 in female reproduction. Mol Cell Endocrinol [DOI] [PubMed]
- 7.Gabbi C, Warner M, Gustafsson J-Å. Action mechanisms of Liver X Receptors. Biochem Biophys Res Commun. 2014;446:647–650. doi: 10.1016/j.bbrc.2013.11.077. [DOI] [PubMed] [Google Scholar]
- 8.Ghisletti S, Huang W, Ogawa S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janowski BA, Willy PJ, Devi TR, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 10.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 11.Viennois E, Mouzat K, Dufour J, et al. Selective liver X receptor modulators (SLiMs): what use in human health? Mol Cell Endocrinol. 2012;351:129–141. doi: 10.1016/j.mce.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Wong J, Quinn CM, Brown AJ. Synthesis of the oxysterol, 24(S),25-epoxycholesterol, parallels cholesterol production and may protect against cellular accumulation of newly-synthesized cholesterol. Lipids Health Dis. 2007;6:10. doi: 10.1186/1476-511X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Chen G, Head DL, et al. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz A, Udata C, Ott E, et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–649. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Zhuang L, Fan KY, et al. Discovery of a Novel, Orally Efficacious Liver X Receptor (LXR) β Agonist. J Med Chem. 2016;59:3264–3271. doi: 10.1021/acs.jmedchem.5b02029. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann JM, Kliewer SA, Moore LB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Cooper AD, Levy-Wilson B. Hepatocyte nuclear factor 1 binds to and transactivates the human but not the rat CYP7A1 promoter. Biochem Biophys Res Commun. 1999;260:829–834. doi: 10.1006/bbrc.1999.0980. [DOI] [PubMed] [Google Scholar]
- 18.Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/S1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Alberti S, Schuster G, Parini P, et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Investig. 2001;107:565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 21.Schultz JR, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 23.Joseph SB, Castrillo A, Laffitte BA, et al. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 24.Michael DR, Ashlin TG, Buckley ML, Ramji DP. Liver X receptors, atherosclerosis and inflammation. Curr Atheroscler Rep. 2012;14:284–293. doi: 10.1007/s11883-012-0239-y. [DOI] [PubMed] [Google Scholar]
- 25.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 26.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffensen KR, Jakobsson T, Gustafsson J-Å. Targeting liver X receptors in inflammation. Expert Opin Ther Targets. 2013;17:977–990. doi: 10.1517/14728222.2013.806490. [DOI] [PubMed] [Google Scholar]
- 28.Björkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauch DH, Nägler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 31.Tassew NG, Mothe AJ, Shabanzadeh AP, et al. Modifying lipid rafts promotes regeneration and functional recovery. Cell Rep. 2014;8:1146–1159. doi: 10.1016/j.celrep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Whitney KD, Watson MA, Collins JL, et al. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol Endocrinol Baltim Md. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 33.Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, et al. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem. 2006;281:12799–12808. doi: 10.1074/jbc.M601019200. [DOI] [PubMed] [Google Scholar]
- 34.Eckert GP, Vardanian L, Rebeck GW, Burns MP. Regulation of central nervous system cholesterol homeostasis by the liver X receptor agonist TO-901317. Neurosci Lett. 2007;423:47–52. doi: 10.1016/j.neulet.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 35.Meffre D, Shackleford G, Hichor M, et al. Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc Natl Acad Sci USA. 2015;112:7587–7592. doi: 10.1073/pnas.1424951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makoukji J, Shackleford G, Meffre D, et al. Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci Off J Soc Neurosci. 2011;31:9620–9629. doi: 10.1523/JNEUROSCI.0761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courtney R, Landreth GE. LXR regulation of brain cholesterol: from development to disease. Trends Endocrinol Metab TEM. 2016;27:404–414. doi: 10.1016/j.tem.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo JH, Lee JH, Kim H, et al. Control of inflammatory responses: a new paradigm for the treatment of chronic neuronal diseases. Exp Neurobiol. 2015;24:95–102. doi: 10.5607/en.2015.24.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almolda B, González B, Castellano B. Are microglial cells the regulators of lymphocyte responses in the CNS? Front Cell Neurosci. 2015;9:440. doi: 10.3389/fncel.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. The relation between α-synuclein and microglia in Parkinson’s disease: recent developments. Neuroscience. 2015;302:47–58. doi: 10.1016/j.neuroscience.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Grigoriadis N, van Pesch V, ParadigMS Group A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;22(Suppl 2):3–13. doi: 10.1111/ene.12798. [DOI] [PubMed] [Google Scholar]
- 43.Bowerman M, Vincent T, Scamps F, et al. Neuroimmunity dynamics and the development of therapeutic strategies for amyotrophic lateral sclerosis. Front Cell Neurosci. 2013;7:214. doi: 10.3389/fncel.2013.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim OS, Lee CS, Joe E, Jou I. Oxidized low density lipoprotein suppresses lipopolysaccharide-induced inflammatory responses in microglia: oxidative stress acts through control of inflammation. Biochem Biophys Res Commun. 2006;342:9–18. doi: 10.1016/j.bbrc.2006.01.107. [DOI] [PubMed] [Google Scholar]
- 45.Zhang-Gandhi CX, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2007;183:50–59. doi: 10.1016/j.jneuroim.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Secor McVoy JR, Oughli HA, Oh U. Liver X receptor-dependent inhibition of microglial nitric oxide synthase 2. J Neuroinflammation. 2015;12:27. doi: 10.1186/s12974-015-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C-H, Chen C-C, Lai C-Y, et al. Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J Neuroinflammation. 2016;13:62. doi: 10.1186/s12974-016-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 49.Talbot K. Motor neuron disease: the bare essentials. Pract Neurol. 2009;9:303–309. doi: 10.1136/jnnp.2009.188151. [DOI] [PubMed] [Google Scholar]
- 50.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 51.Chiò A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler Off Publ World Fed Neurol Res Group Mot Neuron Dis. 2009;10:310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:623–627. doi: 10.1136/jnnp.2010.224501. [DOI] [PubMed] [Google Scholar]
- 53.Leblond CS, Kaneb HM, Dion PA, Rouleau GA. Dissection of genetic factors associated with amyotrophic lateral sclerosis. Exp Neurol. 2014;262 Pt B:91–101. doi: 10.1016/j.expneurol.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Jovičić A, Paul JW, Gitler AD. Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J Neurochem. 2016 doi: 10.1111/jnc.13642. [DOI] [PubMed] [Google Scholar]
- 55.Parakh S, Atkin JD. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. 2016 doi: 10.1016/j.brainres.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Walsh MJ, Cooper-Knock J, Dodd JE, et al. Invited review: decoding the pathophysiological mechanisms that underlie RNA dysregulation in neurodegenerative disorders: a review of the current state of the art. Neuropathol Appl Neurobiol. 2015;41:109–134. doi: 10.1111/nan.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desport J-C, Torny F, Lacoste M, et al. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. 2005;2:202–207. doi: 10.1159/000089626. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt F, Hussain G, Dupuis L, et al. A plural role for lipids in motor neuron diseases: energy, signaling and structure. Front Cell Neurosci. 2014;8:25. doi: 10.3389/fncel.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 60.Dorst J, Kühnlein P, Hendrich C, et al. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. 2011;258:613–617. doi: 10.1007/s00415-010-5805-z. [DOI] [PubMed] [Google Scholar]
- 61.Paganoni S, Deng J, Jaffa M, et al. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang J, Rivest S. Lipid metabolism and neuroinflammation in Alzheimer’s disease: a role for liver X receptors. Endocr Rev. 2012;33:715–746. doi: 10.1210/er.2011-1049. [DOI] [PubMed] [Google Scholar]
- 64.Andersson S, Gustafsson N, Warner M, Gustafsson JA. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102:3857–3862. doi: 10.1073/pnas.0500634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bigini P, Steffensen KR, Ferrario A, et al. Neuropathologic and biochemical changes during disease progression in liver X receptor beta−/− mice, a model of adult neuron disease. J Neuropathol Exp Neurol. 2010;69:593–605. doi: 10.1097/NEN.0b013e3181df20e1. [DOI] [PubMed] [Google Scholar]
- 66.Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 67.Kim HJ, Fan X, Gabbi C, et al. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson’s dementia. Proc Natl Acad Sci USA. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson JMB, Shaw CA. Late appearance of glutamate transporter defects in a murine model of ALS-parkinsonism dementia complex. Neurochem Int. 2007;50:1067–1077. doi: 10.1016/j.neuint.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 69.Plat J, Nichols JA, Mensink RP. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J Lipid Res. 2005;46:2468–2476. doi: 10.1194/jlr.M500272-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Theofilopoulos S, Griffiths WJ, Crick PJ, et al. Cholestenoic acids regulate motor neuron survival via liver X receptors. J Clin Investig. 2014;124:4829–4842. doi: 10.1172/JCI68506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun S, Sun Y, Ling S-C, et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc Natl Acad Sci USA. 2015;112:E6993–E7002. doi: 10.1073/pnas.1520639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Legry V, Cottel D, Ferrieres J, et al. Association between liver X receptor alpha gene polymorphisms and risk of metabolic syndrome in French populations. Int J Obes. 2008;32:421–428. doi: 10.1038/sj.ijo.0803705. [DOI] [PubMed] [Google Scholar]
- 73.Robitaille J, Houde A, Lemieux S, et al. The lipoprotein/lipid profile is modulated by a gene–diet interaction effect between polymorphisms in the liver X receptor-alpha and dietary cholesterol intake in French-Canadians. Br J Nutr. 2007;97:11–18. doi: 10.1017/S0007114507201722. [DOI] [PubMed] [Google Scholar]
- 74.Sabatti C, Service SK, Hartikainen AL, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith AJP, Howard P, Shah S, et al. Use of allele-specific FAIRE to determine functional regulatory polymorphism using large-scale genotyping arrays. PLoS Genet. 2012;8:e1002908. doi: 10.1371/journal.pgen.1002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dahlman I, Nilsson M, Jiao H, et al. Liver X receptor gene polymorphisms and adipose tissue expression levels in obesity. Pharmacogenet Genomics. 2006;16:881–889. doi: 10.1097/01.fpc.0000236334.49422.48. [DOI] [PubMed] [Google Scholar]
- 77.Mouzat K, Mercier E, Polge A, et al. A common polymorphism in NR1H2 (LXRbeta) is associated with preeclampsia. BMC Med Genet. 2011;12:145. doi: 10.1186/1471-2350-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adighibe O, Arepalli S, Duckworth J, et al. Genetic variability at the LXR gene (NR1H2) may contribute to the risk of Alzheimer’s disease. Neurobiol Aging. 2006;27:1431–1434. doi: 10.1016/j.neurobiolaging.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Infante J, Rodríguez-Rodríguez E, Mateo I, et al. Gene–gene interaction between heme oxygenase-1 and liver X receptor-beta and Alzheimer’s disease risk. Neurobiol Aging. 2010;31:710–714. doi: 10.1016/j.neurobiolaging.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 80.Natunen T, Martiskainen H, Sarajärvi T, et al. Effects of NR1H3 genetic variation on the expression of liver X receptor α and the progression of Alzheimer’s disease. PLoS One. 2013;8:e80700. doi: 10.1371/journal.pone.0080700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodríguez-Rodríguez E, Sánchez-Juan P, Mateo I, et al. Interaction between CD14 and LXRbeta genes modulates Alzheimer’s disease risk. J Neurol Sci. 2008;264:97–99. doi: 10.1016/j.jns.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Sodhi RK, Singh N. Liver X receptors: emerging therapeutic targets for Alzheimer’s disease. Pharmacol Res. 2013;72:45–51. doi: 10.1016/j.phrs.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Sadovnick AD, Traboulsee AL, et al. Nuclear receptor NR1H3 in familial multiple sclerosis. Neuron. 2016;90:948–954. doi: 10.1016/j.neuron.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gosselet F, Saint-Pol J, Fenart L. Effects of oxysterols on the blood-brain barrier: implications for Alzheimer’s disease. Biochem Biophys Res Commun. 2014;446:687–691. doi: 10.1016/j.bbrc.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 85.Raoul C, Estévez AG, Nishimune H, et al. Motoneuron death triggered by a specific pathway downstream of Fas. Potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/S0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]