Abstract
In our study, ghrelin was investigated with respect to its capacity on proliferative effects and molecular correlations on oral tumor cells. The presence of all molecular components of the ghrelin system, i.e., ghrelin and its receptors, was analyzed and could be detected using real-time PCR and immunohistochemistry. To examine cellular effects caused by ghrelin and to clarify downstream-regulatory mechanisms, two different oral tumor cell lines (BHY and HN) were used in cell culture experiments. Stimulation of either cell line with ghrelin led to a significantly increased proliferation. Signal transduction occurred through phosphorylation of GSK-3β and nuclear translocation of β-catenin. This effect could be inhibited by blocking protein kinase A. Glucose transporter1 (GLUT1), as an important factor for delivering sufficient amounts of glucose to tumor cells having high requirements for this carbohydrate (Warburg effect) was up-regulated by exogenous and endogenous ghrelin. Silencing intracellular ghrelin concentrations using siRNA led to a significant decreased expression of GLUT1 and proliferation. In conclusion, our study describes the role for the appetite-stimulating peptide hormone ghrelin in oral cancer proliferation under the particular aspect of glucose uptake: (1) tumor cells are a source of ghrelin. (2) Ghrelin affects tumor cell proliferation through autocrine and/or paracrine activity. (3) Ghrelin modulates GLUT1 expression and thus indirectly enhances tumor cell proliferation. These findings are of major relevance, because glucose uptake is assumed to be a promising target for cancer treatment.
Keywords: Glucose uptake, Orexigenic peptide, Oral squamous cell carcinoma cells
Introduction
Ghrelin is an appetite-stimulating and also GH-releasing peptide hormone which was first detected in the stomach [1, 2]. It has been isolated and identified as an endogenous ligand for the growth hormone secretagogue receptor (GHS-R) [1]. Ghrelin consists of 28 amino acids with an n-octanoyl moiety linked to the hydroxyl group of a conserved serine residue-3 leading to a molecular weight of 3.4 kDa and is synthesized as a 117 amino acid containing precursor protein with an amino-terminal signal peptide [1, 3]. The peptide is liberated by furin-like proteases and subsequently post-translationally n-octanoylated by the membrane-bound ghrelin O-acyl transferase (GOAT) to form the mature, biologically active hormone [1, 4–7]. Ghrelin has been shown to act as a growth factor and thus, stimulates the proliferation of various tumor cell lines, including of human hepatoma, leukemia, colorectum, pancreas, prostate, and breast [8, 9]. A number of studies indicate concentration depending controversial effects of ghrelin on the proliferation rate of tumor cell lines [8, 10–13].
The ghrelin receptor, also designated as growth hormone secretagogue receptor GHS-R, has been identified as a G protein-coupled receptor with typical seven transmembrane domains [1, 14–16]. Two subtypes of GHS-Rs have been isolated: GHS-R1a, full-length and biologically active, and GHS-R1b, a non-functional carboxy-terminal truncated isoform [1, 14]. GHS-R1a has first been described to be expressed in the hypothalamus and pituitary [14]. However, expression analysis of ghrelin receptors have depicted an inconsistent picture: while GHS-R1 has been found in various peripheral tissues, i.e., stomach, intestine, pancreas, kidney, liver, heart, lung and also in a variety of cancer entities (pituitary, prostate, ovarian), in contrast, some tumors and tumor cell lines are characterized by the absence of GHS-R1a expression, i.e., colorectal and non-small lung cancer, leukemia and breast cancer cell lines [8].
Cancer cells gain metabolic energy preferentially by inefficient glucose fermentation rather than by oxidative degradation, which is known as “Warburg effect” [17]. Thus, tumor cells have high requirements for glucose to provide sufficient amounts of energy equivalents for cell metabolism and growth. Hence, glucose uptake is significantly elevated in cancer compared to normal cells [17]. This process of glucose uptake across cellular membranes is mediated by glucose transporters (GLUTs). The most widespread and important member of the GLUT family is GLUT1 which is expressed in numerous normal and cancerous tissues and has been shown to be regulated through the GSK-3 pathway [18–21]. Various studies suggest a contribution of GLUT to cancer development by different glucosylation states of regulatory proteins, like p53 [22, 23]. The state of protein glucosylation is controlled through O-GlcNAc-cycling enzymes, O-linked β-N-acetylglucosamine transferase (OGT) and O-GlcNAcase (OGA) [24]. Recently, it has been observed that ghrelin affects cellular glucose uptake by modifying GLUT1 activity and gene expression [25, 26]. Thus, ghrelin might indirectly be involved in these glucose-based regulatory processes via its impact on GLUT1.
In summary, ghrelin plays an important role in food intake and intracellular glucose transport. We hypothesize that ghrelin, as an appetite-regulating hormone, displays a molecular link between the energy balance of the body and cellular energy uptake, and thus has a direct influence on cells with high energy requirements like tumor cells. Therefore, we investigated biopsies of oral lesions and cell lines of oral tumor origin for the presence of elements of ghrelin signaling and for their functioning.
Materials and methods
Tissue sampling
Tissue sampling was performed as described before [27]. Tissue biopsies of healthy gingiva (n = 15), irritation fibromas (n = 15), leukoplakias (n = 15), and head and neck tumors (n = 15) were obtained during routine surgical procedures. Procedures involving human tissue sampling followed a protocol approved by the ethical board of the University of Bonn. All patients had been informed about the study and had signed a letter of informed consent.
Isolation and cultivation of human oral epithelial cells
Human oral epithelial cells were isolated as described [28]. Before experiments started, all cell lines had been tested negative for mycoplasm contamination by PCR and DAPI test.
Culture of human cell lines
The following human cell lines were used in the experiments: BHY (bone invasive oral squamous cell carcinoma), HN (oral squamous cell carcinoma), and HaCaT (spontaneous immortal dermal keratinocytes). HaCaT was purchased from Cell Line Service (Eppelheim, Germany), BHY and HN from DSMZ (Braunschweig, Germany). The cells were cultured in DMEM/10 % FCS/1 % antibiotics in a humidified atmosphere at 37 °C under 5 % CO2. Before experiments started, all cell lines had been tested negative for mycoplasm contamination by PCR and DAPI test.
Cell stimulation
Cells were grown to 90 % confluency. Cells were stimulated with chemically synthesized human n-octanoylated ghrelin (>99 % purity; Peptanova, Sandhausen, Germany) in 10/100/1000 nM and the protein kinase A (PKA) inhibitor H-89 (N-[2-[[3-(4-Bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide; >98 % purity; Sigma-Aldrich, Taufkirchen, Germany) with 10 µM in serum-free medium. The GLUT1 inhibitors Fasentin (N-[4-Chloro-3-(trifluoromethyl)phenyl]-3-oxobutanamide; >95 % purity; Sigma-Aldrich), STF-31 (4-[[[[4-(1,1-Dimethylethyl)phenyl]sulfonyl]amino]methyl]-N-3-pyridinylbenzamide; >99 % purity), and WZB117 (3-Hydroxy-benzoic acid 1,1′-(3-fluoro-1,2-phenylene) ester; >98 % purity) (both Merckmillipore, Schwalbach, Germany) were applied in concentrations of 0.1/1/5/10/50/100 µM. Cells were adapted to serum-free conditions 16 h prior to stimulation. Optimal time periods and concentrations for cell stimulation were examined by prior pilot experiments.
Reverse transcription-PCR
Total RNA isolation and reverse transcription-PCR were performed according to [27]. The gene expression levels of ghrelin, its receptors (GHS-R1a, GHS-R1b), c-myc, cyclin D1, GLUT1, and Ki-67 were detected by real-time PCR using the 7300 Real-Time PCR System (Applied Biosystems®, Darmstadt, Germany), SYBR® Green (Bio-Rad Laboratories), and specific primers. All primers were verified by computational analysis for specification (BLAST) and synthesized of high quality (Metabion, Martinsried, Germany). Primer sequences, annealing temperatures (T) and efficiencies (E) are as follows: β-actin: sense 5′-CATGGATGATGATATCGCCGCG-3′, antisense 5′-ACATGATCTGGGTCATCTTCTCG-3′(T = 69 °C; E = 1.84); GAPDH: sense 5′-TGGTATCGTGGAAGGACTCA-3′, antisense 5′-CCAGTAGAGGCAGGGATGAT-3′ (T = 67 °C; E = 1.93); Ghrelin: sense 5′-GAGGATGAACTGGAAGTCCG-3′, antisense 5′-CATTTATTCGCCTCCTGAGC-3′ (T = 60 °C; E = 1.92); GHS-R1a: sense 5′-CCTCGCTCAGGGACCAGAACC-3′, antisense 5′-GTTGATGGCAGCACTGAGGTAGAA-3′ (T = 60 °C; E = 2.03); GHS-R1b: sense 5′-GGTCCTCTACAGTCTCATCGG-3′, antisense 5′-CAGAGAGAAGGGAGAAGGCACA-3′ (T = 61 °C; E = 1.97); c-myc: sense 5′-CCGAGGAGAATGTCAAGAGGCG-3′, antisense 5′-GCACAAGAGTTCCGTAGCTGTTC-3′ (T = 66 °C; E = 2.01); Cyclin D1: sense 5′-AGCTCCTGTGCTGCGAAGTGGAA-3′, antisense 5′-AGTGTTCAATGAAATCGTGCGGGG-3′ (T = 69 °C; E = 2.07); GLUT1: sense 5′-GCATCCTCATCGCCCAGGTG-3′, antisense 5′-CGCAGCTTCTTTAGCACACTCTTGG-3′ (T = 69 °C; E = 1.94); Ki-67: sense 5′-AAATTCAGACTCCATGTGCCTGAG-3′, antisense 5′-TCAAATACTTCACTGTCCCTATGAC-3′ (T = 66 °C; E = 1.91). PCR conditions were defined as follows: a 5-min preceding denaturation step at 95 °C was succeeded by 50 cycles of 15 s at 95 °C, 30 s at annealing temperatures specific for the primers, and 30 s at 72 °C for elongation. Relative differential gene expression was calculated using the method described by Pfaffl [29] with β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as reference genes. Real-time PCR experiments were carried out according to “the MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments” [30].
Proliferation assay
BHY and HN cells were seeded in 96-well plates (Greiner Bio-One, Frickenhausen, Germany) at a density of 5000 cells per well and cultured overnight. Cell proliferation was determined by using the PromoKine XTT Cell Proliferation Kit (Promocell) by photometric measurement at a wavelength of 490 nm with correction wavelength at 670 nm.
Immunohistochemistry
Immunohistochemistry was performed as described in [27] with different primary antibodies as follows: rabbit IgG anti-ghrelin (dilution of 1:50; sc-50297), goat IgG anti-GHS-R1a (dilution of 1:25; sc-10359) (all from Santa Cruz Biotechnology, Heidelberg, Germany), and rabbit IgG anti-GHS-R1b (dilution of 1:500; H-001-61) (Phoenix Pharmaceuticals, Karlsruhe, Germany) in a humid chamber at 4 °C overnight. Antigen–antibody binding was visualized using the EnVision Detection System Peroxidase/DAB (Dako, Hamburg, Germany). Images were captured by transmitted light microscopy (Axioskop 2, Carl-Zeiss Jena GmbH, Jena, Germany).
Western blot
Total protein and nuclear protein extracts of BHY and HN cells were isolated from 60-mm cell culture dishes (Greiner Bio-One). After the culture medium was aspirated, cells were washed with 1-ml ice-cold PBS and drained. For total protein extraction, cells were lysed with 0.5 ml RIPA (Radio-Immunoprecipitation Assay) buffer (50 mM Tris/Cl− pH 8.0; 150 mM NaCl; 1 % Igepal CA-630; 0.5 % sodium deoxycholate; 0.1 % SDS) containing protease inhibitors (0.5 mM PMSF; Roche cOmplete Mini ULTRA mix) and phosphatase inhibitors (10 mM sodium fluoride; 1 mM sodium orthovanadate; 10 mM 2-glycerophosphate) for 20 min on ice. The extract was spun down in a microcentrifuge (5.000 rpm, 4 °C, 5 min) and the supernatant, referred as total cellular protein, was stored at −80 °C. Nuclear proteins were isolated according to the method described elsewhere [31]. Total cellular protein concentration was determined using the PierceTM BCA Protein Assay Kit [32], and nuclear protein was quantified according to the Bio-Rad Protein Assay [33].
Proteins were separated by SDS-PAGE [34] and subsequently electrophoretically transferred onto a PVDF membrane (0.45 µm) (Millipore, Darmstadt, Germany) [35]. 30 µg of cytosolic proteins or 10 µg of nuclear proteins, respectively, were loaded per lane on a 10 % polyacrylamide gel. β-actin served as internal standard in the cytoplasmic and lamin B1 in the nuclear fraction. The following primary antibodies were used: rabbit IgG anti-GSK-3β (dilution of 1:200; sc-9166), goat IgG anti-phospho(Ser9)-GSK-3β (dilution of 1:400; sc-11757), rabbit IgG anti-β-actin (dilution of 1:200; sc-130656), mouse IgG anti-β-catenin (dilution of 1:200; sc-59737), and mouse IgG anti-lamin B1 (dilution of 1:200; sc-377000). All antibodies were from Santa Cruz Biotechnology, except for mouse IgG anti-glucose transporter 1 (dilution of 1:500; #07-1401; Merckmillipore). Horse radish peroxidase (HRP)-conjugated secondary antibodies were used from Dianova (Hamburg, Germany): rabbit anti-goat IgG (dilution of 1:10.000; #305-035-003), goat anti-mouse IgG (dilution of 1:10.000; #115-035-003), and goat anti-rabbit IgG (dilution of 1:10.000; #111-035-003). Detection was carried out using the “SuperSignal® West Femto Maximum Sensitivity Substrate” or “Pierce® ECL Plus Western Blotting Substrate” (Thermo Scientific, Bonn, Germany). Corresponding protein bands were visualized using the ChemiDoc XRS system and analyzed with the “Quantity One®” software (Bio-Rad Laboratories). Relative protein band intensities were standardized to the loading controls. Non-stimulated cells were used as controls and set to 1.0.
Small interfering RNA (siRNA) experiments
Experiments with siRNA were carried out using FlexiTube Hs GHRL1 siRNA for ghrelin and AllStars Negative control siRNA from Qiagen. Cell transfection was performed with Qiagen´s HiPerFect Reagent according to the manufacturer´s protocol. RNA silencing effects were verified by real-time RT-PCR.
Caspase-3 activity
Cells were cultured in 12-well plates to 50–60 % confluency (200.000 cells/well) prior to 24 h stimulation. Subsequently, medium was removed, cells were washed once with PBS and lysed with 125 µl lysis buffer (50 mM HEPES, 5 mM CHAPS, 5 mM DTT, pH 7.4) for 20 min on ice. The lysate was isolated by scraping and centrifuged for 10 min at 10,000g. The supernatant was used for measuring caspase-3 activity (modified after a protocol from Sigma-Aldrich): 20 µl of cell lysate was added to 80 µl assay buffer (20 mM HEPES, 1.6 mM CHAPS, 5 mM DTT, 2 mM EDTA, 0.2 mM Ac-DEVD-pNA, pH 7.4) and incubated for 90 min. at 37 °C. Enzyme activity was measured by detecting p-Nitroaniline at a wavelength of 405 nm. Various concentrations of p-Nitroaniline were used for generating a standard curve. Cell lysate samples were subjected to protein quantification using the Pierce™ BCA Protein Assay Kit [32] with BSA serving as standard to determine specific enzyme activities. All chemicals were from Sigma-Aldrich. 5 µM Staurosporine (>98 % purity; Biomol, Hamburg, Germany) served as positive control in cell stimulation experiments.
Statistics
Standard error means (SEM) of hexatuplicates (n = 6) were calculated. One-way ANOVA and the post hoc Tukey’s multiple comparison test were applied using a statistical software program (GraphPad Prism version 6 Software, San Diego, CA, USA). P values < 0.05 were considered to be statistically significant.
Results
Detection of members of the ghrelin system in oral tissues and tumor cells
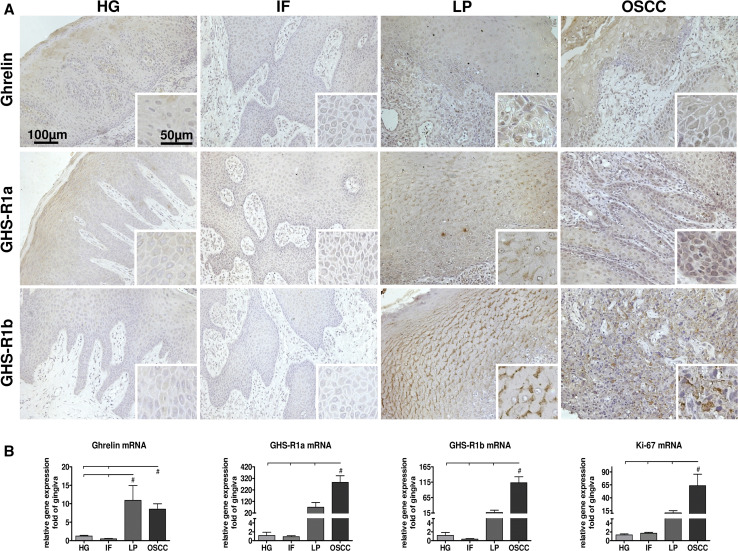
We first analyzed the occurrence of ghrelin and its receptors in oral tissues (Fig. 1a). We have investigated three different types of oral lesions in comparison to healthy gingiva: oral irritation fibromas served as a benign, leukoplakias as a precancerogenic and oral squamous cell carcinomas (OSCC) as a malignant entity. Gene expression of ghrelin and its receptors could be detected in all tissues being studied. Oral leukoplakias (OLP) and oral squamous cell carcinoma exhibited significantly higher transcript levels for ghrelin and both receptors, GHS-R1a and GHS-R1b (Fig. 1b), than the other tissues: ghrelin mRNA level was elevated tenfold in leukoplakias and 8-fold in OSCCs, GHS-R1a 70-fold increased in OLPs and 15-fold for GHS-R1b both not reaching the level of significance. OSCCs exhibited an even higher transcript level of 270-fold for GHS-R1a and 110-fold for GHS-R1b. These observations could also be verified on protein level by immunohistochemical investigations (Fig. 1a). Furthermore, the marker gene for cell proliferation, Ki-67, was analyzed. The relative expression level corresponds to the malignancy of the neoplasm: no differences of expression could be detected for healthy gingiva and benign irritation fibromas while precancerogenic leukoplakias showed a 15-fold increase and OSCCs of even 60-fold (Fig. 1b). In addition, gene expression of ghrelin and its receptors was analyzed in two cell lines derived from oral squamous cell carcinomas, BHY and HN, and oral epithelial cells (OEC) which served as general reference control: both tumor cell lines exhibited elevated transcript levels of these three genes compared to normal keratinocyte cells (Table 1). An increased expression level could be detected for ghrelin (4-fold), GHS-R1a (5-fold), GHR-S1b (5-fold) in BHY cells and for ghrelin (15-fold), GHR-S1a (4-fold) and GHR-S1b (9-fold) in HN cells (Table 1). Non-malignant OECs did not differ significantly for any of the three examined mRNAs compared to dermal keratinocyte HaCat cells (Table 1).
Fig. 1.
Occurrence and gene expression of ghrelin and its receptors in oral tissues. a Immunohistochemical staining of different oral biopsies with anti-ghrelin and anti-growth hormone secretagogue receptor (GHS-R)1a, and 1b antibodies. Primary magnification was 20× (40× for insets). b Relative gene expression of ghrelin, GHS-R1a, GHS-R1b, and Ki-67 in different oral biopsies (n = 15). Statistically significant differences (p < 0.05) between groups are marked with (#). HG healthy gingiva, IF irritation fibroma, LP leukoplakia, OSCC oral squamous cell carcinoma
Table 1.
Relative gene expression of ghrelin, its receptors GHS-R1a and GHS-R1b in various cell lines and healthy primary oral epithelial cells (OEC) analyzed by quantitative real-time PCR
| Ghrelin | GHS-R1a | GHS-R1b | |
|---|---|---|---|
| BHY | 3.34 ± 0.14# | 10.91 ± 0.79# | 4.50 ± 0.50# |
| HN | 14.98 ± 0.66# | 7.79 ± 1.38# | 8.71 ± 0.74# |
| OEC | 1.15 ± 0.34 | 2.91 ± 1.02 | 1.20 ± 0.31 |
| HaCat | 1.00 ± 0.11 | 1.00 ± 0.4 | 1.00 ± 0.07 |
GAPDH and β-actin served as reference genes. Relative gene expression was standardized to the mean values for both reference genes. Data are shown with standard error means. Significant differences compared to HaCat cells (set arbitrarily to “1”) are marked with # for p < 0.05
Ghrelin is capable of increasing the proliferation rate of oral tumor cells via GSK-3β/β-catenin pathway through up-regulation of cyclin D1 and c-myc
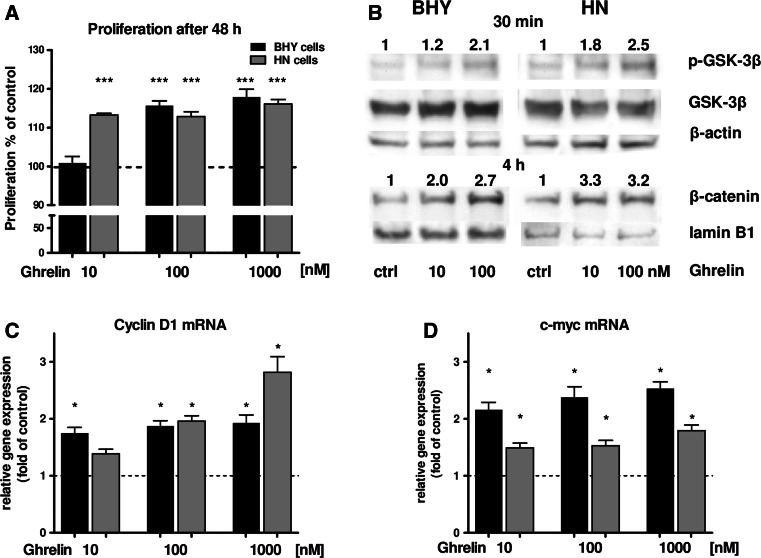
In the next step, the potential of ghrelin to modulate cancer cell proliferation was investigated: BHY and HN cells were stimulated with three ghrelin concentrations. It turned out that the proliferation rate of BHY cells could be enhanced by 100 nM and 1 µM ghrelin (Fig. 2a). HN cells were even more sensitive to ghrelin: already 10 nM of this peptide led to a significant increase in proliferation (Fig. 2a). To determine putative signaling pathways which could be activated by ghrelin to promote cell proliferation, phosphorylation of GSK-3β and nuclear translocation of β-catenin was examined. An increase of phospho-GSK-3β could be detected by Western Blot analysis for BHY and HN cells after 30 min of ghrelin stimulation (Fig. 2b). Also, nuclear β-catenin was demonstrated to be enhanced in BHY and HN cells after four hours of stimulation (Fig. 2b). Periods of incubation were determined in pilot experiments before. Next, gene expression analyses of β-catenin downstream target genes involved in proliferative processes were carried out. For this purpose, differential gene expression of cyclin D1 and c-myc was investigated: both genes were up-regulated by ghrelin stimulation in BHY and also HN cells (Fig. 2c, d).
Fig. 2.
a Proliferation rates of BHY and HN cells after stimulation with ghrelin. b Western Blot of GSK-3β and phospho-GSK-3β (p-GSK-3β) in BHY and HN cells after ghrelin treatment. β-actin served as cytosolic loading control for comparison. For densitometric analysis, band intensities of p-GSK-3β and GSK-3β were normalized to β-actin. The numbers indicate relative ratios of p-GSK-3β vs. GSK-3β compared to the control (set to 1). Nuclear detection of β-catenin in BHY and HN cells after ghrelin stimulation. Lamin B1 was used as nuclear loading control. Protein band intensities were normalized to lamin B1. The numbers indicate relative ratios of β-catenin compared to the control (set to 1). c Relative cyclin D1 and d c-myc expression of BHY and HN cells after ghrelin stimulation. Statistically significant differences vs. control are marked with asterisks [p < 0.05 (*); p < 0.001 (***)]
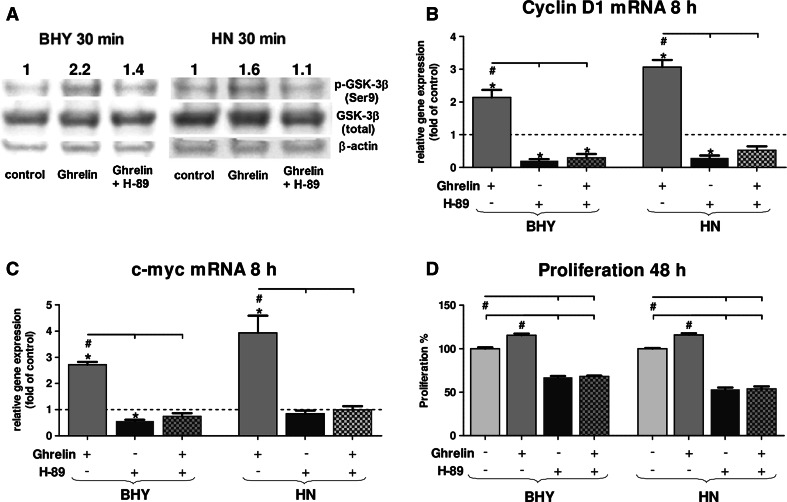
To verify whether GHS-R1 mediated signaling via the PKA pathway, BHY and HN cells were treated with ghrelin and the PKA inhibitor H-89. PKA activity was measured by detecting phosphorylation of its downstream target GSK-3β (Fig. 3a). Cells treated with ghrelin and H-89 simultaneously showed a decrease in the amount of phospho-GSK-3β (Fig. 3a), indicating the presence of PKA activity in ghrelin signaling. Furthermore, downstream genes of phospho-GSK-3β signaling, cyclin D1 and c-myc, were negatively affected by the PKA inhibitor (Fig. 3b, c). Consequently, the proliferation rate of either cell line markedly dropped to about 50 % independent of externally applied ghrelin when treated with H-89 (Fig. 3d). Taken together, incubation of BHY and HN cells with ghrelin induced up-regulation of the investigated metabolites of the ghrelin pathway resulting in stimulation of the proliferation rate. Both receptors for ghrelin were detected, also the receptor activity including downstream target signaling molecules (GSK-3β; β-catenin) and the β-catenin target genes cyclin D1 and c-myc leading to enhanced proliferation. In addition, PKA inhibition prevented up-regulation of downstream signaling steps.
Fig. 3.
Inhibition of PKA signaling by H-89 in BHY and HN cells in the presence of externally applied ghrelin (100 nM). a Western Blot of GSK-3β and phospho-GSK-3β in BHY and HN cells after PKA inhibition. β-actin served as cytosolic loading control. For densitometric analysis, band intensities of p-GSK-3β and GSK-3β were normalized to β-actin. The numbers indicate relatives ratios of p-GSK-3β vs. GSK-3β compared to the unstimulated control (set to 1). b Relative gene expression of cyclin D1. c Relative gene expression of c-myc. d Corresponding proliferation rates of BHY and HN cells. Significant differences (p < 0.05) between groups are marked with (hash) and significant deviations from control with (asterisks)
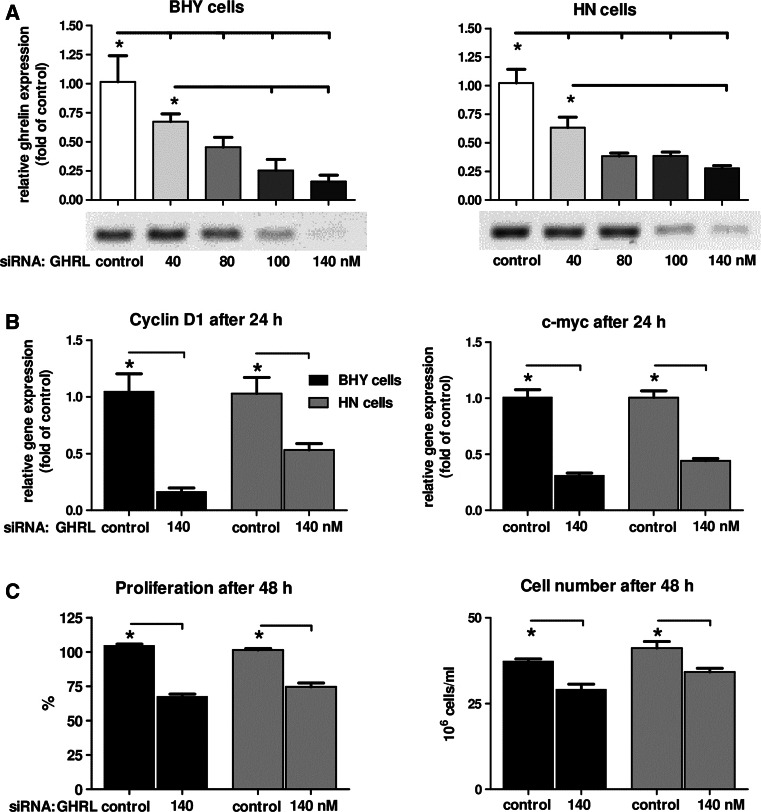
Down-regulation of endogenous ghrelin by siRNA led to a decreased proliferation rate in oral tumor cells
To examine the role of endogenous intracellular ghrelin on the proliferation rate, both oral tumor cell lines were subjected to siRNA experiments: ghrelin siRNA was administered in three concentrations (40/80/140 nM) to the cells. Subsequently, gene expression analyses were carried out to verify the optimal expression inhibiting concentrations. It turned out that 140 nM ghrelin siRNA was sufficient to dramatically decrease ghrelin transcript levels, in BHY as well as in HN cells (Fig. 4a). Furthermore, also lower expression levels of the two cell cycle involved genes, cyclin D1 and c-myc, were detectable (Fig. 4h). These data corresponded with negatively affected proliferation behavior for either cell line (Fig. 4h). Cell viability analyses were carried out to clarify whether the decrease in proliferation rates was due to reduced cell division (Fig. 4h) or to enhanced apoptosis. Cell viability was not influenced by 140 nM ghrelin siRNA (99 % for BHY; 98 % for HN), thus suggesting no apoptotic effects being involved in a lower proliferation rate. Caspase-3 activity was analyzed to further examine putative effects on apoptosis by intracellular ghrelin: both cell lines showed no change in caspase-3 activity with 140 nM ghrelin siRNA (0.95 for BHY; 1.06 for HN compared to 1.0 for unstimulated control). Staurosporine as a positive control was shown to lead in either cell line to strongly increased caspase-3 activities (5-fold for BHY; 10-fold for HN). Hence, apoptosis was assumed not to be the reason for the decrease in cell proliferation after down-regulating endogenous ghrelin mRNA using siRNA technology.
Fig. 4.
Effect of ghrelin mRNA silencing on the proliferation of BHY and HN cells. a Relative gene expression of ghrelin in BHY and HN cells after siRNA treatment. b Relative expression of cyclin D1 and c-myc. c Proliferation rates and cell numbers after treatment with 140 nM GHRL siRNA. Significant differences (p < 0.05) between groups are marked with (asterisks)
Exogenous and endogenous ghrelin affect GLUT1 expression
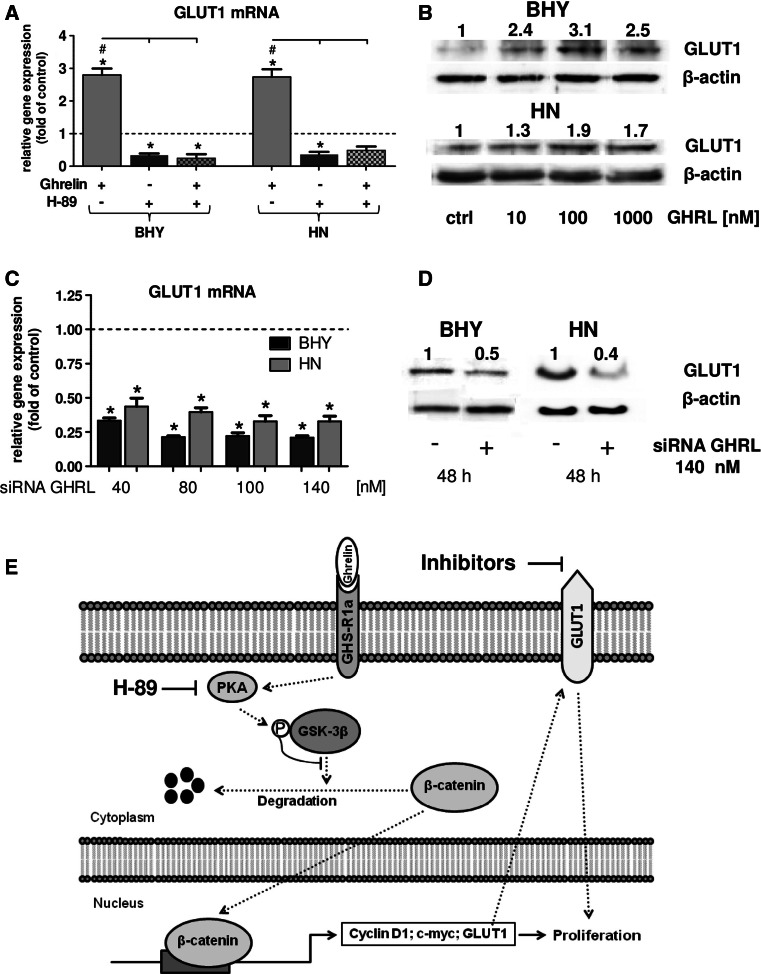
As described above, cancer cells have high requirements for glucose to conserve sufficient quantities of metabolic energy. In this respect, glucose transporters play an important role in cancer by providing tumor cells with adequate amounts of glucose. As demonstrated in our studies, ghrelin modulated oral tumor cell proliferation through the GSK-3 signal pathway. GLUT1 expression was observed to be also regulated by the same kinase [20]. Consequently, we investigated whether ghrelin had an impact on GLUT1-based effects. First, the effect of GLUT1 on the viability of the OSCC cells was investigated. For this purpose, BHY and HN cells were subjected to various concentrations of the GLUT1 inhibitors STF-31, WZB117, and Fasentin (Table 2): all three inhibitors negatively affected the viability of the cell lines, with STF-31 being the most effective. This compound reduced viability of BHY cells to 85 % at 0.1 µM. HN cells were even more susceptible with 75 % viability at the same concentration. All three inhibitors diminished viabilities of both cell lines to 50 % at concentration between 50 and 100 µM. Hence, GLUT1 plays a prominent role in cell viability. As ghrelin has been demonstrated to activate GSK-3β, it should be figured out whether exogenous ghrelin would also affect GLUT1 gene expression. Indeed, ghrelin led to a significant up-regulation of GLUT1 mRNA (Fig. 5 a) in both cell lines. The constitutive GLUT1 expression was down-regulated and ghrelin was not able to stimulate GLUT1 expression anymore when blocking protein kinase A with H-89 (Fig. 5 a). These data strongly suggest ghrelin to regulate GLUT1 gene expression via GSK-3β. To demonstrate this on protein level, ghrelin was applied to BHY and HN cells in concentrations of 10/100/1000 nM resulting in a concentration-dependent up-regulation of GLUT1 (Fig. 5 b).
Table 2.
Cell viability of BHY and HN cells after treatment with GLUT inhibitors STF-31, WZB117, and Fasentin for 24 h
| BHY | HN | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | p values | Mean | SEM | p values | |
| STF-31 | STF-31 | |||||
| Control | 100.00 | 2.03 | 100.00 | 1.21 | ||
| 0.1 µM | 87.58* | 1.32 | <0.0001 | 71.70* | 0.75 | <0.0001 |
| 1 µM | 74.47* | 2.02 | <0.0001 | 61.89* | 1.69 | <0.0001 |
| 5 µM | 63.37* | 2.42 | <0.0001 | 60.06* | 1.42 | <0.0001 |
| 10 µM | 61.14* | 1.60 | <0.0001 | 60.59* | 1.88 | <0.0001 |
| 50 µM | 54.66* | 3.21 | <0.0001 | 56.40* | 1.82 | <0.0001 |
| 100 µM | 50.12* | 2.42 | <0.0001 | 50.30* | 1.32 | <0.0001 |
| WZB117 | WZB117 | |||||
|---|---|---|---|---|---|---|
| Control | 100.00 | 2.25 | 100.00 | 1.47 | ||
| 0.1 µM | 100.84 | 0.78 | 96.66 | 0.64 | ||
| 1 µM | 95.27 | 1.95 | 94.60* | 0.34 | 0.0336 | |
| 5 µM | 95.85 | 1.56 | 93.07* | 1.71 | 0.0026 | |
| 10 µM | 98.08 | 1.16 | 92.71* | 2.25 | 0.0014 | |
| 50 µM | 68.29* | 2.13 | <0.0001 | 62.50* | 0.88 | <0.0001 |
| 100 µM | 38.25* | 2.45 | <0.0001 | 45.39* | 0.98 | <0.0001 |
| Fasentin | Fasentin | |||||
|---|---|---|---|---|---|---|
| Control | 100.00 | 2.07 | 100.00 | 1.01 | ||
| 0.1 µM | 97.39 | 0.92 | 100.00 | 0.63 | ||
| 1 µM | 97.54 | 1.20 | 98.40 | 1.31 | ||
| 5 µM | 89.64* | 1.05 | 0.0006 | 97.82 | 0.53 | |
| 10 µM | 91.81* | 1.39 | 0.0103 | 98.40 | 1.20 | |
| 50 µM | 66.88* | 2.24 | <0.0001 | 68.99* | 1.21 | <0.0001 |
| 100 µM | 47.06* | 2.62 | <0.0001 | 21.71* | 3.28 | <0.0001 |
Non-treated cells were used as controls (set to 100 %)
Data are described in mean values with standard error means (SEM) and adjusted p values in (). Each set of experiments was carried out with n = 6. Significant differences vs. control are marked with asterisks [p < 0.05 (*)]
Fig. 5.
Influence of exogenous and endogenous ghrelin on GLUT1. a Relative gene expression of GLUT1 after PKA inhibition by H-89 in the presence of ghrelin (100 nM). Statistically significant differences (p < 0.05) between groups are marked with (hash), to controls with (asterisks). b Western Blot of GLUT1 after stimulation of BHY and HN cells with various concentrations of ghrelin. Protein band intensities were normalized to β-actin. The numbers indicate relative ratios of GLUT1 compared to the control (set to 1). c Relative gene expression of GLUT1 after ghrelin siRNA treatment. Significant differences (p < 0.05) between groups are marked with (asterisks). d Western Blot of GLUT1 after incubation with 140 nM ghrelin siRNA in BHY cells. The numbers indicate the ratio of GLUT1 vs. β-actin. e Schematic summary of ghrelin signaling in oral tumor cells. Activation of GHS-R1a by ghrelin leads to nuclear translocation of β-catenin regulated through PKA and GSK-3β. As a consequence, nuclear β-catenin up-regulates its target genes cyclin D1 and c-myc, thus promoting proliferation. In addition, GLUT1 expression is also positively influenced leading to enhanced proliferative effects
Data shown above demonstrated endogenous ghrelin expression to be up-regulated in OSCC cells compared to normal oral epithelial cells (Table 1). To investigate whether endogenous ghrelin would have an effect on GLUT1 transcript level, siRNA experiments were carried out: it could be shown that ghrelin siRNA significantly decreased the level of GLUT1 mRNA in BHY as well as in HN cells (Fig. 5c). These effects could also be observed for GLUT1 protein which was down-regulated by 140 nM of ghrelin siRNA (Fig. 5d). A schematic summary of ghrelin signaling in oral tumor cells is shown in Fig. 5e: activation of GHS-R1a by ghrelin leads to nuclear translocation of β-catenin regulated through PKA and GSK-3β. As a consequence, nuclear β-catenin up-regulates its target genes cyclin D1 and c-myc, thus promoting proliferation. In addition, GLUT1 expression is also positively influenced leading to enhanced proliferative effects.
Discussion
Although members of the ghrelin axis have been detected in various tumors, their role in carcinogenesis lacks detailed understanding [36]. One hypothesis which explains the role of ghrelin in the development of various neoplasms is based upon the evidence that ghrelin affects the GH/IGF-1 axis, whose inappropriate regulation is known to stimulate colon cancer carcinogenesis [37, 38]. In renal carcinoma cells, ghrelin has been shown to promote cell migration via Snail activation [39]. On the other hand, breast cancer might be affected by ghrelin-based inhibition of aromatase expression and activity in human adipose stromal cells independently of GHS-R. Hence, ghrelin may be used as therapeutical tool for estrogen-dependent breast cancer [40]. Little is known about ghrelin expression in oral tissues [41]. Nevertheless, ghrelin was detected in saliva, salivary gland, and oral squamous cell carcinoma [42, 43]. Cells of the oral cavity are permanently in contact with saliva and consequently with ghrelin because of being a salivary component. Hence, ghrelin might be chronically effective on oral tumor initiation and progression. For this reason, we have focused our study on the impact of the ghrelin signaling on oral tumor cells.
Four different oral tissue specimens have been analyzed for ghrelin and its receptors, GHS-R1a and GHS-R1b: expression of proteins of the ghrelin signaling was verified in all tissues examined with all three components being highly up-regulated in carcinomas compared to healthy tissue. A comparative review of expression patterns of ghrelin and its receptors in a number of various tumors exhibited a heterogeneous picture [36]: only in a minority of the investigated tumors and cell lines all three members were identified on mRNA and protein level. However, some tumor entities showed increased levels of ghrelin compared to healthy controls, i.e., breast, colorectal adenocarcinoma, and medullar and follicular thyroid cancer [36]. Other tumors were shown to have decreased levels: endometrial, renal, and salivary gland mucoepidermoid cancer [36]. The same picture can be drawn for both receptors [36]. In esophageal squamous cell carcinoma, ghrelin expression has been described to correlate with tumor depth and differentiation state [44]. In human pituitary adenomas, ghrelin mRNA expression levels differ significantly in invasive compared to noninvasive tumors with higher levels detected in invasive entities [45]. Only one study investigating ghrelin in oral squamous cell carcinoma has been published up to date [43]: ghrelin was shown to be decreased or even absent in carcinoma tissues compared to benign control biopsies and the transcript level correlated inversely to cancer invasiveness. This is in contrast to the results of the present study demonstrating that ghrelin and ist receptors are up-regulated in oral squamous cell carcinoma tissue and tumor cell lines derived from this tumor entity. In summary, the majority of cancer cells are a source of ghrelin, and thus may play a role in affecting tumor growth in a paracrine and/or autocrine mode, since silencing of endogenous ghrelin led to decreased ghrelin transcript levels with a subsequent attenuated proliferation rate.
As stated above, we have demonstrated for the first time that ghrelin enhanced the proliferation rate of OSCC cells via the PKA pathway leading to phosphorylation of GSK-3β and nuclear translocation of β-catenin with up-regulated cell cycle genes, cyclin D1 and c-myc. Recently, it has been described that ghrelin is able to induce gastric carcinoma cell proliferation, migration and invasion via the GHS-R/NF-κB signaling pathway [46]. In human renal adenocarcinoma cells, ghrelin promotes metastasis via GHS-R-based activation of PI3 K-Akt [39]. Obviously, ghrelin and GHS-R act through different cellular signaling pathways depending on tumor cell types. A variety of different cells of healthy and tumor origin have been examined with respect to proliferation, apoptosis and cell migration after ghrelin stimulation [36, 47]: pro-proliferative effects have been observed in cells derived from breast, pancreas, prostate, and colorectum cancer [36]. In contrast, various other cells did not respond to or were negatively affected by ghrelin: small cell lung, thyroid, and breast cancer cells [36, 47]. These contradictory data might be caused by different ghrelin concentrations used for stimulation. It seems that micromolar concentrations lead to less or even reduced proliferation rates [10, 36]. The role of ghrelin in cancer cell apoptosis is ambiguous as ghrelin stimulation induced both pro- and also anti-apoptotic effects in published experiments [36, 47]. However, in our experiments, no apoptotic effects have been observed.
It has been described that the most widespread glucose transporter GLUT1 is regulated via the GSK-3 pathway and transactivation of β-catenin [20, 21]. Our studies have revealed the same kinase being involved in ghrelin signaling. Hence, a putative correlation between ghrelin and GLUT1 was investigated. Indeed, ghrelin was shown to up-regulate GLUT1 gene expression through PKA and thus also via GSK-3β phosphorylation.
The role of ghrelin is further substantiated by the observation that ghrelin transcript levels are elevated in oral tumor cell lines compared to normal epithelial cells. Furthermore, decreasing the mRNA transcript level of ghrelin by siRNA technology led as a result to a down-regulation of GLUT1 gene expression. Hence, ghrelin might indirectly influence the intracellular glucose level of tumor cells. This observation might be of serious relevance since the contribution of glucose-based regulatory processes in cancer development has recently been described [22–24].
In summary, cancer cells being an endogenous source for ghrelin per se have the ability to arrange an improved environment for tumor growth.
There is another aspect of importance regarding ghrelin being used as therapeutical tool in cancer treatment. Cancer-associated dyspepsia syndrome (CADS) is known as a side effect of chemotherapy treatment with cisplatin [48]. Patients suffering from esophageal cancer develop a decreased ghrelin plasma concentration after cisplatin-based chemotherapy [49]. Cancer patients which were treated with this orexigenic peptide to overcome chemotherapeutic-based cachexia have consequently developed reduced symptoms of gastrointestinal disorders induced by this cytostatic agent [49]. In addition, it has been shown that ghrelin is able to improve weight stabilization of cancer patients [50]. Moreover, a recent study describes the ability of ghrelin to prevent tumor- and cisplatin-induced muscle atrophy [51]. Besides that, the orexigenic peptide might be useful in the treatment of estrogen-dependent breast cancer by its inhibitory capability regarding the aromatase system [40]. Although therapeutical application of ghrelin exhibits some advantageous effects on cancer patients, our studies strongly suggest to reconsider the use of ghrelin as a therapeutic supplement to minimize cancer-associated cachexia. The cellular effects of exogenous applied ghrelin might counteract cancer treatment. At the end, these disadvantageous effects might prevail the positive implications of ghrelin treatment in long-term usage.
In conclusion, our study describes an important role for the peptide hormone ghrelin in oral cancer proliferation particularly under the following aspects: (1) tumor cells are a source of ghrelin, thus might affect their own growth. (2) Ghrelin affects tumor cell proliferation through autocrine and/or paracrine activity. (3) Ghrelin modulates GLUT1 expression, thereby indirectly enhances tumor cell proliferation. The ghrelin axis is definitely of substantial relevance in this context: not only paracrine but also autocrine ghrelin is capable of modulating GLUT1 expression. Thus, this peptide hormone is of crucial significance for the energy metabolism of oral cancer cells. This makes the ghrelin axis to a potential target for cancer therapy.
Acknowledgments
D.K. was supported by a grant of the BONFOR program of the Medical Faculty of the University of Bonn. We are grateful to Imke Beier for her excellent technical assistance.
Abbreviations
- Ac-DEVD-pNA
N-Acetyl-Asp-Glu-Val-Asp p-nitroanilide
- CHAPS
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- Ghrl
Ghrelin
- GlcNAC
N-acetylglucosamine
- GSK-3β
Glycogen synthase kinase-3β
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists. DK, RP, and JW are founders of OnContra. Nevertheless, this company has not been involved nor has any interest in the scientific field related to the work this manuscript deals with. Furthermore, this does not alter the authors´ adherence to all the policies on sharing data and materials as stated for the journal.
Footnotes
R. Meyer and J. Winter have equally contributed to this work.
References
- 1.Kojima M, Kangawa K. Ghrelin: structure and Function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 2.Korbonits M, Grossman AB. Ghrelin: update on a novel hormonal system. Eur J Endocrinol. 2004;151(Suppl 1):S67–S70. doi: 10.1530/eje.0.151S067. [DOI] [PubMed] [Google Scholar]
- 3.Rauh M, Gröschl M, Rascher W. Simultaneous quantification of ghrelin and desacylghrelin by liquid chromatography-tandem mass spectrometry in plasma, serum, and cell supernatants. Clin Chem. 2007;53:902–910. doi: 10.1373/clinchem.2006.078956. [DOI] [PubMed] [Google Scholar]
- 4.Seim I, Herington AC, Chopin LK. New insights into the molecular complexity of the ghrelin gene locus. Cytokine Growth Factor Rev. 2009;20:297–304. doi: 10.1016/j.cytogfr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Seim I, Josh P, Cunningham P, Herington A, Chopin L. Ghrelin axis genes, peptides and receptors: recent findings and future challenges. Mol Cell Endocrinol. 2011;340:3–9. doi: 10.1016/j.mce.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Chopin L, Walpole C, Seim I, Cunningham P, Murray R, Whiteside E, Josh P, Herington A. Ghrelin and cancer. Mol Cell Endocrinol. 2011;340:65–69. doi: 10.1016/j.mce.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery PL, Herington AC, Chopin LK. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone dependent cancer. Cytokine Growth Factor Rev. 2003;14:113–122. doi: 10.1016/S1359-6101(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 10.Cassoni P, Ghé C, Marrocco T, Tarabra E, Allia E, Catapano F, Deghenghi R, Ghigo E, Papotti M, Muccioli G. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol. 2004;150:173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- 11.Díaz-Lezama N, Hernández-Elvira M, Sandoval A, Monroy A, Felix R, Monjaraz E. Ghrelin inhibits proliferation and increases T-type Ca2+ channel expression in PC-3 human prostate carcinoma cells. Biochem Biophys Res Commun. 2010;403:24–29. doi: 10.1016/j.bbrc.2010.10.100. [DOI] [PubMed] [Google Scholar]
- 12.Lanfranco F, Baldi M, Cassoni P, Bosco M, Ghé C, Muccioli G. Ghrelin and prostate cancer. Vitam Horm. 2008;7:301–324. doi: 10.1016/S0083-6729(06)77013-3. [DOI] [PubMed] [Google Scholar]
- 13.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin: a potential therapeutic target for cancer. Regul Pept. 2010;163:7–17. doi: 10.1016/j.regpep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 15.McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, Smith RG, Van der Ploeg LH, Howard AD. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997;11:415–423. doi: 10.1210/mend.11.4.9908. [DOI] [PubMed] [Google Scholar]
- 16.Smith RG, Feighner S, Prendergast K, Guan X, Howard A. A new orphan receptor involved in pulsatile growth hormone release. Trends Endocrinol Metab. 1999;10:128–135. doi: 10.1016/S1043-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- 17.Warburg O. On the origin of cancer cells. Science. 1956;123:209–214. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 18.Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustin R. The protein family of glucose transport facilitators: it´s not only about glucose after all. IUBMB Life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 20.Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC., 3rd A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol. 2008;295:C836–C843. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PMK2 promotes the Warburg effect. Nat Cell Biol. 2012;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X, Lin M, Yu H, Liu L, Levine AJ, Hu W, Feng Z. Tumor-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;2013(4):2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starska K, Forma E, Jóźwiak P, Bryś M, Lewy-Trenda I, Brzezińska- Błaszczyk E, Krześlak A. Gene and protein expression of glucose transporter 1 and glucose transporter 3 in human laryngeal cancer-the relationship with regulatory hypoxia-inducible factor-1α expression, tumor invasiveness, and patient prognosis. Tumour Biol. 2015;36(4):2309–2321. doi: 10.1007/s13277-014-2838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starska K, Forma E, Brzezińska-Błaszczyk E, Lewy-Trenda I, Bryś M, Jóźwiak P, Krześlak A (2014) Gene and protein expression of O-GlcNAc-cycling enzymes in human laryngeal cancer. Clin Exp Med. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 25.Lear PV, Iglesias MJ, Feijóo-Bandín S, Rodríguez-Penas D, Mosquera-Leal A, García-Rúa V, Gualillo O, Ghè C, Arnoletti E, Muccioli G, Diéguez C, González-Juanatey JR, Lago F. Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology. 2010;151:3286–3298. doi: 10.1210/en.2009-1205. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Lin P, Yu S. Effects of ghrelin on developmental competence and gene expression of in vitro fertilized ovine embryos. Theriogenology. 2013;79:695–701. doi: 10.1016/j.theriogenology.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Winter J, Pantelis A, Reich R, Jepsen S, Allam JP, Novak N, Wenghoefer M. Risk estimation for a malignant transformation of oral lesions by S100A7 and Doc-1 gene expression. Cancer Invest. 2011;29:478–484. doi: 10.3109/07357907.2010.543210. [DOI] [PubMed] [Google Scholar]
- 28.Kraus D, Winter J, Jepsen S, Jäger A, Meyer R, Deschner J. Interactions of adiponectin and lipopolysaccharide from porphyromonas gingivalis on human oral epithelial cells. PLoS One. 2012;7(2):e30716. doi: 10.1371/journal.pone.0030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 31.Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1β and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- 32.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1971;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology. 1992;24:145–149. [PubMed] [Google Scholar]
- 36.Chopin LK, Seim I, Walpole CM, Herington AC. The ghrelin axis-does it have an appetite for cancer progression? Endocr Rev. 2012;33:849–891. doi: 10.1210/er.2011-1007. [DOI] [PubMed] [Google Scholar]
- 37.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20(18):5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustin SA, Jenkins PJ. The growth hormone-insulin-like growth factor-I axis and colorectal cancer. Trends Mol Med. 2001;7(10):447–454. doi: 10.1016/S1471-4914(01)02104-9. [DOI] [PubMed] [Google Scholar]
- 39.Lin TC, Liu YP, Chan YC, Su CY, Lin YF, Hsu SL, Yang CS, Hsiao M. Ghrelin promotes renal cell carcinoma metastasis via Snail activation and is associated with poor prognosis. J Pathol. 2015;237(1):50–61. doi: 10.1002/path.4552. [DOI] [PubMed] [Google Scholar]
- 40.Docanto MM, Yang F, Callaghan B, Au CC, Ragavan R, Wang X, Furness JB, Andrews ZB, Brown KA. Ghrelin and des-acyl ghrelin inhibit aromatase expression and activity in human adipose stromal cells: suppression of cAMP as a possible mechanism. Breast Cancer Res Treat. 2014;147(1):193–201. doi: 10.1007/s10549-014-3060-1. [DOI] [PubMed] [Google Scholar]
- 41.Ohta K, Laborde NJ, Kajiya M, Shin J, Zhu T, Thondukolam AK, Min C, Kamata N, Karimbux NY, Stashenko P, Kawai T. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J Dent Res. 2011;90:1286–1292. doi: 10.1177/0022034511420431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gröschl M, Topf HG, Bohlender J, Zenk J, Klussmann S, Dötsch J, Rascher W, Rauh M. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin Chem. 2005;51:997–1006. doi: 10.1373/clinchem.2004.040667. [DOI] [PubMed] [Google Scholar]
- 43.Alnema MM, Aydin S, Ozkan Y, Dagli AF, Ozercan HI, Yildirim N, Sahin I, Karaoglu A, Kilic N, Yilmaz M, Ozercan MR, Donder E. Ghrelin and obestatin expression in oral squamous cell carcinoma: an immunohistochemical and biochemical study. Mol Cell Biochem. 2010;339:173–179. doi: 10.1007/s11010-009-0381-1. [DOI] [PubMed] [Google Scholar]
- 44.Omoto I, Matsumoto M, Uchikado Y, Kita Y, Sakurai T, Sasaki K, Setoyama T, Okumura H, Owaki T, Ishigami S, Natsugoe S. Immunohistochemical evidence of association between ghrelin expression and tumor growth in esophageal carcinoma. Anticancer Res. 2014;34(6):2727–2733. [PubMed] [Google Scholar]
- 45.Wang J, Guo S, Han L, Fang M, Wang L, Bartsch JW, Li J. Correlation of ghrelin and growth hormone secretagogue receptor expression with clinical features in human pituitary adenomas. Exp Ther Med. 2015;9(5):1909–1914. doi: 10.3892/etm.2015.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian C, Zhang L, Hu D, Ji J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol Cell Biochem. 2013;382(1–2):163–172. doi: 10.1007/s11010-013-1731-6. [DOI] [PubMed] [Google Scholar]
- 47.Papotti M, Duregon E, Volante M. Ghrelin and tumors. Endocr Dev. 2013;25:122–134. doi: 10.1159/000346061. [DOI] [PubMed] [Google Scholar]
- 48.Hiura Y, Takiguchi S, Yamamoto K, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Fujiwara Y, Mori M, Doki Y. Fall in plasma ghrelin concentrations after cisplatin-based chemotherapy in esophageal cancer patients. Int J Clin Oncol. 2012;17:316–323. doi: 10.1007/s10147-011-0289-0. [DOI] [PubMed] [Google Scholar]
- 49.Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Fujiwara Y, Mori M, Kangawa K, Doki Y. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer. 2012;118:4785–4794. doi: 10.1002/cncr.27430. [DOI] [PubMed] [Google Scholar]
- 50.Ali S, Chen JA, Garcia JM. Clinical development of ghrelin axis-derived molecules for cancer cachexia treatment. Curr Opin Support Palliat Care. 2013;7(4):368–375. doi: 10.1097/SPC.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle. 2015;6(2):132–143. doi: 10.1002/jcsm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]