Abstract
As motile organelles and sensors, cilia play pivotal roles in cell physiology, development and organ homeostasis. Ciliary defects are associated with a class of cilia-related diseases or developmental disorders, termed ciliopathies. Even though the presence of cilia is required for diverse functions, cilia can be removed through ciliary shortening or resorption that necessitates disassembly of the cilium, which occurs normally during cell cycle progression, cell differentiation and in response to cellular stress. The functional significance of ciliary resorption is highlighted in controlling the G1-S transition during cell cycle progression. Internal or external cues that trigger ciliary resorption initiate signaling cascades that regulate several downstream events including depolymerization of axonemal microtubules, dynamic changes in actin and the ciliary membrane, regulation of intraflagellar transport and posttranslational modifications of ciliary proteins. To ensure ciliary resorption, both the active disassembly of the cilium and the simultaneous inhibition of ciliary assembly must be coordinately regulated.
Keywords: Chlamydomonas, IFT, Aurora-A, Plk1, Protein phosphorylation, Wnt signaling, Ciliary length control
Introduction
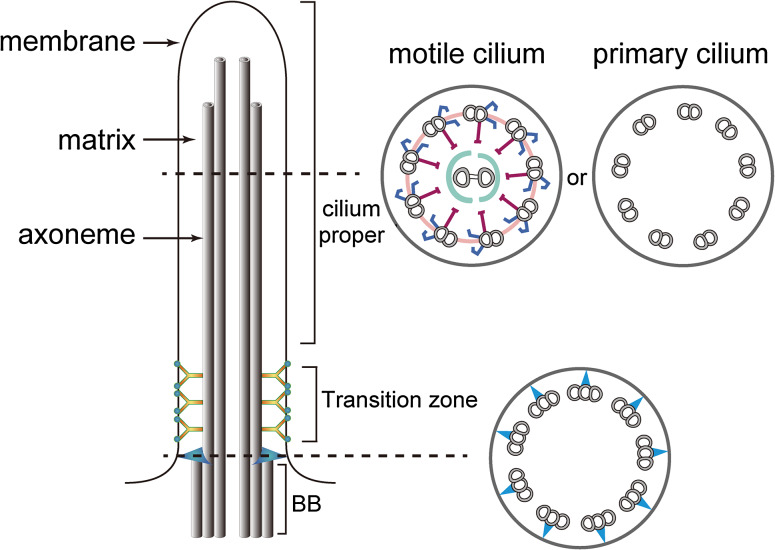
Cilia, microtubule (MT)-based cellular organelles, are evolutionally conserved and present in divergent organisms ranging from protists to human. As eukaryotic flagella are essentially identical organelles as cilia, the terms cilia and flagella are often used interchangeably. Cilia are composed of the axoneme (MTs and their associated structures), the ciliary membrane that is continuous with the cell membrane and the matrix between the axoneme and the ciliary membrane (Fig. 1). The axoneme of a motile cilium usually contains one pair of singlet MTs surrounded by nine outer doublet MTs organized in a circle. The axonemal MTs and their associated accessory substructures including central pair sheath, dynein arms, radial spokes, etc. coordinate to control the motility of cilia. In contrast, primary cilia usually lack the central pair MTs and the substructures associated with the outer doublet MTs that are required for motility, and thus are immotile. The ciliary membrane is continuous with the plasma membrane. However, it has a distinct composition of proteins and lipids due to the presence of a diffusion barrier at the ciliary base [1, 2]. The enrichment of various receptors and ion channels in the ciliary membrane makes the cilium a unique sensory organelle, involved in the control of cell differentiation, cell division and physiology in response to various stimuli [3–5]. Defects in ciliogenesis, integrity of ciliary structures or cilia-related signaling are associated with a spectrum of developmental disorders and/or diseases, collectively called ciliopathies (see recent review [6]).
Fig. 1.
The structure of a cilium. A motile cilium usually contains one central pair of MTs surrounded by nine outer MT doublets whereas a non-motile cilium lacks the central pair MTs. Both types of cilia are formed from a modified centriole, called a basal body (BB). Transitional fibers (blue) link the distal basal body to the base of the ciliary membrane. The transition zone that connects the basal body and the cilium proper contains Y-links (yellow) and the ciliary necklace (cyan). For simplicity, the depiction of the cilium in the left panel only shows two outer doublet MTs (grey tube) in the cilium proper and transition zone and two triplet MTs (grey tubes) in the basal body
At the base of the cilium is the basal body, which contains a cylindrical array of nine triplet MTs derived from the mother centriole and is anchored to the ciliary membrane through transition fibers. A short region that is contiguous with the basal body and distal to it is called the transition zone, which contains nine outer doublet MTs, Y-shaped structures extending from the MTs to the ciliary membrane, and membrane-associated protein complexes called the ciliary necklace (Fig. 1). This region is proposed to serve as a diffusion barrier for non ciliary proteins [2, 7–10]. After initial formation of the basal body and transition zone, the cilium grows by elongation of the axonemal MTs at the distal tip, formation of ciliary substructures, and extension of the ciliary membrane. The delivery of ciliary precursors to the assembly site requires a bidirectional transport of protein complexes called intraflagellar transport (IFT) [8, 11].
Cilia are dynamic structures. In a fully assembled cilium, assembly activity is balanced by disassembly activity, and alteration of this balance may lead to lengthening or shortening of the cilium [12]. As ciliary length is determines optimal ciliary signaling capacity and motility, the maintenance of cilia at the proper length is crucial for cells to perform their physiological functions. However, cilia may be lost during cell state transitions (e.g. during cell cycle progression or cell differentiation) or in response to environmental stress. Though the significance of ciliary loss during these processes is largely unknown, emerging evidences suggest that ciliary loss during cell cycle progression is required for the G1-S transition [13, 14].
Two major mechanisms are involved in ciliary loss: deflagellation (deciliation) and ciliary shortening (resorption or disassembly) [15]. Deflagellation involves severing of the axoneme and its associated membrane distal to the transition zone, resulting in detachment of the cilium from the cell body [16–18]. In contrast, ciliary shortening or resorption entails in situ disassembly of ciliary components, which are returned to the cell body for recycling or degradation. The shortening is initiated at the ciliary tip rather at the base. This was shown by labeling of flagella with HA-tubulin followed by fluorescence microscopy in a dikaryon assay in Chlamydomonas [12]. To support this notion, electron microscopy studies in Chlamydomonas showed that the basal body and the transition zone remain intact during flagellar shortening [19].
In this review, we will focus on the cellular processes and molecular mechanisms currently known to underly ciliary disassembly during shortening. Several excellent reviews have also covered other related aspects of ciliary disassembly [20–26].
Ciliary disassembly is induced during various biological processes
Ciliary disassembly is tightly associated with three cellular events: cellular stress responses, cell differentiation, and cell cycle progression; these will be discussed below in detail.
Ciliary disassembly in response to cellular stress
Cilia can be induced to disassemble upon changes in environmental conditions both in mammalian cells and in protists. However, the physiological significance has not been examined. Ciliary resorption may simply be a passive consequence of stress. On the other hand, it may play an active role in sensing environmental changes. Loss of cilia could abolish cilia mediated signaling to bring about changes in the cell body to face environmental challenges.
In mammalian cells, two different types of stress such as heat shock and mechanical strain have been reported to induce ciliary disassembly. Heat shock treatment of NIH3T3 cells for 30 min induces complete ciliary disassembly in 50 % of ciliated cells [27]. Several studies have examined mechanical stress. Cilia protrude outside the cell surface and thus may experience constant mechanical load from contact with the surrounding fluid, matrix or adjacent cells. The primary cilia of chondrocytes of articular cartilage have been proposed to act as mechanosensory organelles. Application of compressive strain on 3D cultures of bovine chondrocytes reduces ciliary incidence and length [28]. Exposing cultured human umbilical vein endothelial cells to laminar shear stress induces ciliary disassembly [29]. The trabecular meshwork in the eye regulates intraocular pressure. Primary cilia of cells in the meshwork shorten in response to fluid flow and elevated hydrostatic pressure [30]. Interestingly, gene knockout of transient receptor potential vanilloid 4 in mice, a ciliary mechanosensory channel, results in ciliary shortening accompanied by increased intraocular pressure [30]. These data implicate a link between ciliary disassembly and mechanical loads sensed by primary cilia. It is likely that cells respond to at least some mechanical challenges by modulation of ciliary signaling through ciliary shortening.
The impact of cellular stress on ciliary disassembly has been well studied in Chlamydomonas, a unicellular green alga with two flagella, each 12 μm long. Decreasing osmolarity by transferring cells into water induces flagellar elongation while increasing osmolarity by adding sucrose or mannitol to the medium results in flagellar shortening [31, 32]. Flagellar shortening induced by sucrose treatment is transient. For example, at 0.2 M sucrose, flagella shorten to approximately half length around 1 h after treatment and then gradually elongate back to normal length. Low concentrations of sodium chloride, e.g. 0.125 M, induces almost linear shortening within the first 2 h to around one-third of normal length; the flagella barely shorten further, even after 6 h of treatment [31]. Flagellar shortening can also be induced by a number of chemicals including 5 mM ATP or GTP, 20 mM sodium pyrophosphate (NaPPi), and 25 mM citrate [33]. In addition, treatments of cells with low concentrations of various drugs including amiprophos-methyl, caffeine, isobutyl methylxanthine, and halothane result in similar effects though the flagella are not completely resorbed even after several hours of treatment [24].
Inducing flagellar shortening by chemical treatments can be used as a superb experimental system to address the mechanism of ciliary disassembly. Among all the chemicals or drugs that have been tested so far, NaPPi emerges as an excellent reagent. First, NaPPi treatment induces complete flagellar resorption with almost linear kinetics within 3 h. Second, NaPPi is not toxic as treated cells could proliferate for several days in the presence of NaPPi [33].
Cellular stress that impacts an individual cilium on a multiciliated cell may induce ciliary shortening of the remaining cilia of the same cell. In Chlamydomonas, mechanical shearing stress normally causes deflagellation of both flagella followed by flagellar regrowth. Occasionally, only one flagellum is lost. When the new flagellum starts to grow, the remaining flagellum shorten simultaneously until it reaches the same length as the new regenerating flagellum [34]. A similar phenomenon is also observed in Tetrahymena in that the remaining cilia are resorbed when some of the cilia are amputated, [35].
Ciliary disassembly during cell differentiation
Ciliary resorption has been observed during cell differentiation in uni-cellular organisms and mammalian cells, but its functional significance has not been thoroughly studied. The kinocilum in cochlear hair cells is implicated in organizing the sterocilia (actually microvilli) which are important for transducing sound waves into neural signals. When sterocilia are formed and properly organized, the kinocilium is disassembled [36, 37]. Myofibroblasts can be differentiated from mesenchymal fibroblasts or epithelial cells. During this transition, primary cilia are eventually lost through ciliary resorption instead of deciliation, resulting in impaired PDGF and hedgehog signaling [38–40]. Ciliary disassembly also occurs during differentiation of osteoblasts and adipocytes [41–43]. Interestingly, during apidocyte differentiation primary cilia initially elongate followed by gradual shortening and they are fully resorbed [42].
Cell differentiation in the life cycle of algae, fungi and protozoa involves ciliary loss. In most cases, the loss of cilia or flagella during formation of a zoospore or zygote is not mediated by ciliary resorption but rather by retraction of the entire axoneme into the cell body [15]. However, ciliary disassembly does occur in a few organisms during cell differentiation. In Chlamydomonas, the flagella of newly formed zygotes gradually shorten during zygote maturation until they are fully resorbed. Complete resorption has been reported to take about 30 min [19, 44]. However, live cell imaging of individual zygotes shows that it takes around 1 h [45]. In Leishmania mexicana, the long, flagellated promastigote differentiates into the amastigote with only a rudimentary flagellum, indicating that the flagellum undergoes disassembly during the differentiation process [46].
Ciliary disassembly during cell cycle progression
The primary cilium is nucleated by the mother centriole after mitosis, and is present on quiescent and differentiated cells of many cell types [47]. It has been noted that a reciprocal relationship exists between the presence of a cilium on a cell and cell division [48, 49]. The question then arises whether a primary cilium is present in any stages of the cell cycle (G1, S, G2 and M) in proliferating cells. Examination of chick limb cells by electron microscopy has showed that the majority of cells that are proliferating possess cilia, but cells actively in mitosis do not [50], indicating that cells in G1, S or G2 still have cilia. In cultures of exponentially growing mouse BALB/c3T3 cells and hamster kidney BHK21/C13 cells primary cilia are also found on interphase cells but not on cells in mitosis [51, 52]. The presence or length of cilia in proliferating cells may depend on the cell types and cell growth conditions.
The disappearance of primary cilia from cells entering mitosis is a general phenomenon, as stated by Wheatley and colleagues [52]. Complete ciliary disassembly occurs by G2 or early mitosis, this may be due to cell type and cell growth conditions. Primary cilia are no longer observed in human RPE-1 cells at late G2 [53, 54]. In BHK21/C13 cells, cilia were observed on cells with two sets of mature centrioles, i.e. after centriole replication, but not in cells at late prophase [52]. By contrast, complete loss of cilia occurs in prometaphase in Female Rat Kangaroo Kidney Epithelial Cells (PtK1 line) [55].
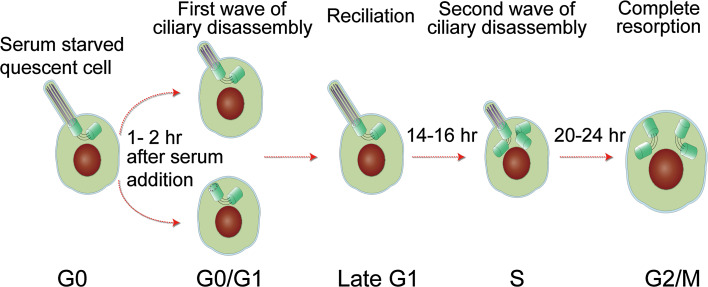
Ciliary assembly and disassembly during cell cycle progression has been systematically studied in mouse 3T3 and human RPE-1 cells and similar results have been reported [51, 53, 54]. Serum starved cells (in G0) can be stimulated to re-enter the cell cycle after addition of serum to the growth medium. Around 1–2 h after stimulation, the first wave of ciliary disassembly occurs followed by reciliation with an increase in the number of ciliated cells and an increase in average ciliary length. When cells enter S phase, around 14–16 h after serum addition, a second wave of ciliary disassembly occurs. When cells reach late G2 or early mitosis around 20–24 h, cilia are completely resorbed (Fig. 2).
Fig. 2.
Ciliary disassembly during cell cycle progression. This figure is based on results from studies in RPE-1 and 3T3 cells as discussed in the text. The first wave of ciliary disassembly is associated with the G0/G1 transition, followed by reciliation. The second wave is initiated at the G1/S transition. Complete ciliary resorption occurs in G2 or early M
The second wave of ciliary shortening noted above is coupled to DNA replication [51, 53]. Primary cilia persist in cells blocked in S phase by treatment with thymidine or hydroxyurea, and gradually resorb after washout of these chemicals. These data argue that ciliary disassembly is initiated after S phase entry. However, it was also shown that cilia are shorter in cells blocked in S phase compared to those cells in late G1 [53]. This suggests that cilia undergo some distal shortening prior to S phase entry and continue to disassemble during S phase progression.
Initiation of ciliary disassembly prior to S phase entry suggests that ciliary shortening may play a role in controlling the G1-S transition, an idea initially proposed by Tucker et al. [51, 56]. Evidence from recent studies supports this hypothesis. Sung and colleagues showed that phosphorylated Tctex-1, a dynein light chain, is recruited to the area of the transition zone. It promotes ciliary disassembly, and suppression of Tctex-1 delays cell cycle progression [57]. As Tctex-1 is dispensable for S-phase entry in non-ciliated cells, these data suggest that ciliary shortening is required for S-phase entry in ciliated cells. Second, depletion of the centrosomal protein Nde1, which is a negative regulator of ciliary length, induces longer cilia and also results in a delay in the G1-S phase [58]. Lastly, activation of the Aurora-A protein kinase induces ciliary shortening [54], and its activator, Trichoplein, promotes ciliary disassembly [59]. Knockdown of Aurora-A or Trichoplein prevents ciliary disassembly and cells arrest in G1. Collectively, these data suggest that ciliary disassembly serves as a checkpoint to control G1-S phase transition [13].
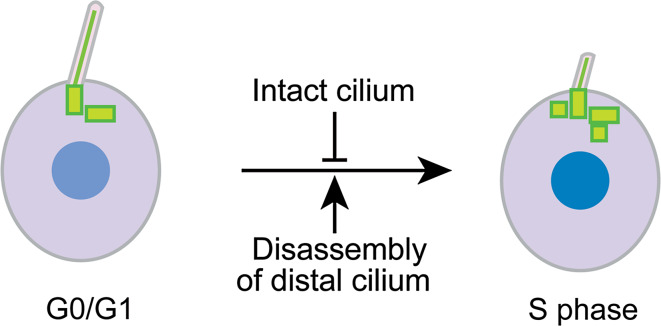
How ciliary shortening triggers the G1-S transition remains enigmatic. As cilia are still present during S phase, complete ciliary resorption is unlikely required. Because ciliary disassembly is initiated prior to S-phase, shortening of the distal portion of cilia might be required to reduce or abolish inhibitory signals that intact cilia might generate (Fig. 3). The evidence that shortening cilia are able to initiate a novel signaling cascade comes from studies in Chlamydomonas [60]. Chlamydomonas cells gradually resorb their flagella during cell cycle progression, with complete resorption in about 30 min to 1 h [19, 44, 60, 61]. By using a mutant defective in flagellar shortening, it was revealed that disassembly of the distal portion of the flagella initiates a phosphorylation cascade (see “CDK-like kinase FLS1” for further details) [60]. It remains to be demonstrated whether a similar cascade occurs during partial ciliary disassembly in mammalian cells.
Fig. 3.
A model for ciliary disassembly serving as a checkpoint for the G1-S transition. The inhibitory signal generated by the presence of an intact cilium is abolished when ciliary disassembly is initiated, resulting in activation of a signaling cascade leading to the G1-S transition. Note that S phase entry is not simply induced by ciliary disassembly unless cells already become committed for S phase entry. And complete ciliary resorption is not a prerequisite for S phase entry either
In addition to mammalian cells and Chlamydomonas, resorption of existing cilium prior to mitotic entry also occurs in other algae and ciliates or flagellates [15]. However, this does not occur in all ciliated cells. For example, during cell division of Trypanosome brucei, the old flagellum is retained [62]. During meiotic division of spermatocytes in some insects including fruit fly, cilia persist [63, 64].
Cellular responses and biochemical changes during ciliary disassembly
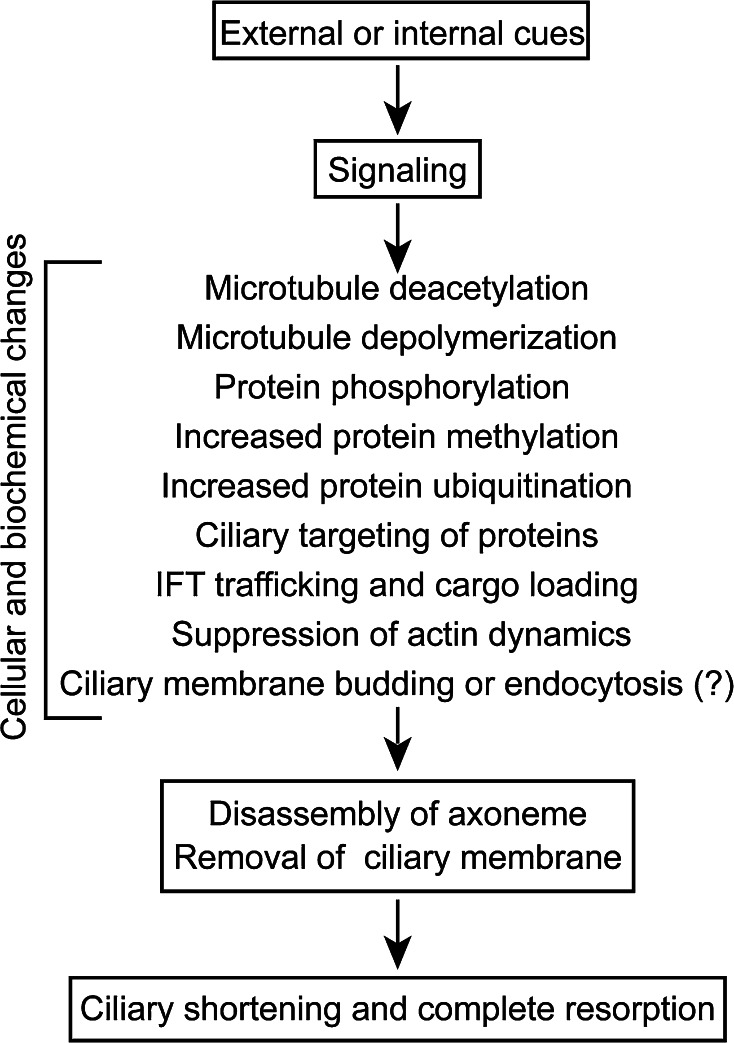
The cilium is a complex organelle consisting of several hundreds of proteins and forming distinct substructures [65] (Fig. 1): a unique ciliary membrane, axonemal MTs and their associated substructures or protein complexes. During ciliary shortening, the axonemal MTs need to be disassembled; the MT-associated complexes must be dissociated to form individual molecules or smaller protein complexes, which are then transported back to the cytoplasm via retrograde IFT. The area of the ciliary membrane has to be decreased in coordination with disassembly of the axoneme. Upon the stimulation of ciliary resorption, a signaling cascade is activated that brings about these changes, including posttranslational modifications of ciliary components, leading to ciliary disassembly (Fig. 4). Emerging data also suggest that the reactions involved in ciliary assembly also need to be suppressed simultaneously to ensure ciliary shortening.
Fig. 4.
Cellular process and biochemical changes during ciliary disassembly. See text for detail
Deacetylation of axonemal MTs
Microtubule acetylation was first demonstrated in the flagella of Chlamydomonas [66, 67] and subsequently found in diverse cilia and other stable MT arrays such as centrioles [68–71]. During flagellar assembly and disassembly in Chlamydomonas, axonemal microtubules are acetylated and deacetylated, respectively [66, 67]. The acetylation occurs after tubulin dimers have been incorporated into the axonemal MTs as MTs in nascent flagella are not acetylated [72, 73]. MT acetylation is mediated by the acetyltransferase MEC-17 or αTAT1 [73–75], whereas deacetylation is mediated by the MT deacetylase HDAC6 or SIRT2 [71].
To define the ciliary function of α-tubulin acetylation and deacetylation, the α-tubulin gene was mutated by changing Lys40 to Arg and transformed into Chlamydomonas [76]. Although in these experiments the nonacetylatable α-tubulins comprised 50–70 % of the total flagellar α-tubulin, no gross phenotypic effects on ciliary assembly, maintenance, or disassembly were observed. However, recent data in mammalian cells has shown that MT acetylation and deacetylation regulate ciliary assembly and disassembly, respectively, as depletion of the acetylase, αTAT1, delays ciliogenesis in RPE-1 cells [75]. Acetylation-mimicking mutants of α-tubulin or suppression of HDAC6, the deacetylase, by RNAi or chemical inhibitors prevents ciliary disassembly [54, 77].
Depolymerization of axonemal MTs
Axonemal MTs comprise the main structural elements of the central structures of the cilium, and they must be depolymerized during ciliary resorption. MT depolymerization can be mediated by several classes of MT-depolymerizing kinesins including members of the kinesin-13, kinesin-8, and kinesin-14A families [78]. Genetic data in mammals and protists have demonstrated that several microtubule depolymerizing kinesins regulate ciliary assembly, length or shortening [79, 80]. KIF19A of the kinesin-8 family in mammalian cells and members of the kinesin-13 family in Leishmania, Trypanosome and Giardia have been implicated in ciliary length control, although it is not yet clear whether these kinesin-13s participate in ciliary resorption [81–84].
CrKinesin13 of Chlamydomonas and KIF2A of mammalian cells, members of the kinesin-13 family, have been examined for their role in ciliary shortening. In Chlamydomonas, a single kinesin-13 is present. CrKinesin-13 is predominantly localized to the cell body and is barely detectable in the flagella of steady state cells. However, it is increased several fold in the flagella via transport by IFT upon induction of flagellar disassembly. Depletion of CrKinesin-13 suppresses flagellar shortening [85].
In mammalian cells, three kinesin-13 family members, KIF2A, 2B and 2C, were examined for their function in ciliary resorption and KIF2A was found to play a major role [86]. Upon stimulation of ciliary disassembly, phosphorylation of KIF2A at residue T554 is mediated by polo-like kinase 1 (Plk1) and occurs at the mother centriole. Overexpression of the phosphomimetic mutant T554E suppresses ciliogenesis whereas that of the phosphoresistant mutant T554A does not. Depletion of KIF2A impairs ciliary disassembly in serum-stimulated RPE-1 quiescent cells. As MT deacetylation is required for ciliary disassembly, it is reasonable to conclude that MT depolymerases act on deacetylated axonemal MTs to promote ciliary shortening.
Protein methylation
Protein methylation increases hydrophobicity and steric bulk, and thus affects protein–protein interactions. During ciliary resorption, cilia are disassembled into individual protein components or protein complexes, which are carried back to the cell body by IFT [87]. Thus, alteration of protein–protein interactions is expected to occur and protein methylation is likely involved. Sloboda’s group has shown that the amount of several methylated proteins increases during ciliary disassembly in Chlamydomonas [88–90]. Arginine methyltransferase 1 is enriched at the flagellar tip and likely phosphorylated during flagellar resorption [88]. Meanwhile, methionine synthase (MetE) also increases in the flagella [89]. MetE catalyzes the synthesis of methionine, which is converted to the methyl donor S-adenosyl methionine (SAM) by the action of SAM synthase. Thus, a highly regulated methylation pathway must be activated to mediate ciliary disassembly.
Protein ubiquitination
Protein ubiquitination is involved in diverse cellular processes including cell cycle progression, endocytosis, transcription and DNA repair through protein degradative and nondegradative functions [91, 92]. Huang et al. [93] showed that the ubiquitination system is involved in ciliary disassembly in Chlamydomonas. An in vitro ubiquitination assay using isolated flagella showed the presence of a functional ubiquitination system in cilia. Protein ubiquitinating activity increases during flagellar disassembly and the ubiquitinated proteins include membrane and axonemal proteins such as PKD2 and α-tubulin, respectively. Though the ciliary ubiquitination system was first discovered in Chlamydomonas, it likely functions in cilia of other organisms including mammal, as free ubiquitin and the enzymes E1 and E2 have also been identified in various ciliomes [94]. Indeed, in mammalian cells, APC, an E3 ligase, together with its co-activator cdc20 maintains ciliary length in quiescent cells, and also promotes serum induced ciliary disassembly [95].
The function of protein ubiquitination during ciliary disassembly is not clear. The proteasome machinery is not present in the flagella [93] and there is no evidence for the presence of the lysosome pathway in cilia. Thus, it is unlikely that any ciliary components tagged by ubiquitin are degraded in situ. Ubiquitin may be tagging disassembled proteins for transport into the cell body, as defects in anterograde or retrograde IFT motors lead to accumulation of ubiquitinated proteins in the flagella [93]. Protein ubiquitination may also alter the conformation of proteins, leading to dissociation of ciliary protein complexes. Lastly, as in other systems ubiquitination may affect the signaling pathway that regulates ciliary disassembly [96]. Many questions remain as to how the ubiquitination system is involved in ciliary disassembly. How is this system activated in response to cues that trigger ciliary disassembly? Because several E2 and E3 enzymes are present in the Chlamydomonas flagellar proteome [65], do they play redundant roles or have specific functions? The exact function of protein ubiquitination in cilia remains to be defined.
Protein phosphorylation
Initiation of ciliary disassembly is mediated at least partly by a protein phosphorylation mediated signaling cascade, as aurora-like kinase CALK in Chlamydomonas and Aurora-A in mammalian cells actively participate in ciliary disassembly [23, 54, 97]. Several key molecules that are involved in ciliary resorption are in turn regulated by phosphorylation, and these are discussed in more detail in “Signaling molecules and signaling pathways that mediate ciliary disassembly”.
To gain insight into the overall phosphorylation changes that occur during ciliary resorption, Pan and colleagues performed a comparative phosphoproteome analysis in Chlamydomonas [98]. Control and resorbing flagella were isolated and extracted to generate membrane/matrix fraction, which was analyzed by mass spectrometry. A total of 224 phosphoproteins have been identified, among which 89 are only detected in resorbing flagella. This result demonstrates that protein phosphorylation is intensively involved in ciliary disassembly. Newly appeared phosphoproteins and proteins with increased phosphorylation in resorbing flagella include protein kinases, small G proteins, IFT motors, and proteins involved in methylation and ubiquitination. Thus, phosphorylation participates in ciliary disassembly likely through regulating signal transduction, IFT, protein methylation and ubiquitination. Interestingly, the level of phosphorylated α-tubulin and β-tubulin is also increased in resorbing flagella. However, the significance of this phosphorylation in ciliary disassembly is unknown.
Regulation of IFT and cargo loading onto IFT particles
IFT mediates the transport of ciliary precursors to the ciliary tip for ciliary assembly and maintenance, and returns the turnover products to the cell body for recycling [87]. Cilia are disassembled from the ciliary tip [12]. It has been shown that the length of flagella regenerated in the absence of protein synthesis varies with the amount of prior flagellar resorption [33, 99]. Thus, like ciliary precursors, disassembled ciliary components may be transported to the cell body by IFT and can be reutilized during flagellar regeneration. Indeed, the amount of IFT proteins in flagella increase 2–4 fold during flagellar shortening in Chlamydomonas [45]. The increased amount of IFT proteins is constant at different stages of flagelar shortening, suggesting that the injection rate of IFT is upregulated, which may be required to meet the increasing demand for retrieval of disassembled products. The molecular mechanism underlying regulation of IFT injection rate is largely unknown. Recently, a calcium dependent kinase, CDPK1, was found to phosphorylate the kinesin-II motor subunit FLA8 to control IFT entry during ciliary assembly [100]. It will be interesting to determine whether this regulation also operates during ciliary disassembly.
Another aspect of IFT regulation during ciliary disassembly is cargo loading onto IFT particles. It has been shown that the loading of anterograde cargo is inhibited whereas retrograde cargo loading is permitted during flagellar disassembly [45]. During flagellar assembly, 12S radial spoke complexes are transported into the flagella by IFT, where they assemble into mature 20S radial spoke complexes. During flagellar disassembly, the 20S complex is released from the axoneme and returns to the cell body [87]. Thus, 12S and 20S complexes of radial spoke can be used as markers for anterograde and retrograde cargos, respectively. During flagellar shortening, the amount of 20S complex in the flagella increases while the amount of the 12S complex decreases [45]. Because the amount of IFT particles increases in resorbing flagella, this observation suggests that anterograde cargo loading is inhibited to suppress ciliary assembly and the increased IFT particles would allow binding of disassembled components for retrograde transport. The manner in which IFT cargo loading and unloading during ciliary growth and resorption is regulated, however, remains elusive. Posttranslational modification of individual IFT polypeptides and/or ciliary proteins are very likely involved.
Ciliary targeting of proteins involved in ciliary disassembly
As discussed earlier, CrKinesin-13 is specifically targeted to cilia at the time when cells initiate ciliary disassembly [85]. These data raise several intriguing questions. Are there any other proteins that are targeted to the flagella and required for flagellar shortening? What are they and what are their functions? In mammalian cells, HDAC6 is phosphorylated and activated by Aurora-A [54]. However, Aurora-A is concentrated and activated at the basal body region. Thus, phosphorylated HDAC6 would be expected to be targeted to cilia for deacetylation of axonemal MTs, yet this is yet to be explicitly shown.
Disassembly or removal of the ciliary membrane
When the axoneme shortens during ciliary disassembly, the surface area of the ciliary membrane that encloses the axoneme must be simultaneously reduced. At present little is known about the disassembly or removal of the ciliary membranes. Theoretically, ciliary membrane budding at the ciliary tip or endocytosis at the ciliary base may be involved. Studies in various organisms have shown that the cilium has the capacity to release membrane vesicles extracellularly. In Chlamydomonas, gametic flagella release vesicles during flagellar agglutination and vegetative flagella release membrane vesicles during release of daughter cells from mother cell wall [101–103]. In addition, neuronal cilia in C. elegans, and neuroepithelial cilia and renal cilia in mice can also release membrane vesicles [104–106]. Evidence shows that the release of membrane vesicles is through the formation of ectosomes [102]. Thus, studies are needed to determine if this system is involved in the removal of ciliary membranes during ciliary resorption. Though endocytosis at the base of cilium is another attractive model for removal of the ciliary membrane, no evidence is currently available to support this notion.
Actin dynamics
Increasing evidence suggests that actin dynamics play an active role in cilia formation and shortening [107]. The current theme is that depolymerization of filamentous actin (F-actin) promotes ciliary assembly while inhibition of actin disassembly facilitates ciliary disassembly. First, inducing F-actin depolymerization with cytochalasin D, an F-actin depolymerizing compound, stimulates ciliogenesis and increases ciliary length [108–111]. Second, depletion of positive regulators of actin polymerization including ARP3, a subunit of ARP2/3 complex, LIM kinase 2 (LIMK2) and testicular protein kinase 1 (TESK1) [109, 111], or overexpression of the microRNA mir-129-3p that targets several regulators required for F-actin polymerization increases cilia formation [108]. Similarly, ablation of MIM, a monomeric globular actin binding protein, induces phosphorylation of Cortactin that promotes actin nucleation, coincident with increased cilia formation [110, 112]. In contrast, knockdown of Gelsolin, a F-actin depolymerizing protein, induces formation of shorter cilia [111].
Two mechanisms may explain the role of actin dynamics in affecting ciliogenesis. First, depolymerization of F-actin induces accumulation of ciliogenic vesicles to the basal body [108, 109, 111] though the detailed mechanism is unknown. Recently, it has been shown that F-actin depolymerization also results in cytoplasmic retention and inactivation of the transcription coactivators YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) [109]. YAP/TAZ regulates the expression of several proteins, including Aurora-A and Plk1, which promote ciliary shortening (see “Signaling molecules and signaling pathways that mediate ciliary disassembly”). Thus, F-actin depolymerization may promote cilia formation by suppressing ciliary disassembly.
Given that modulation of actin dynamics impacts ciliary formation and length, it is intriguing whether this cellular process directly participates in ciliary resorption. Treatment with cytochalasin D blocks serum induced ciliary shortening [57, 77]. During ciliary disassembly the cellular level of MIM decreases whereas phosphorylation of Cortactin increases, which is predicted to increase F-actin polymerization [110]. In addition, the acetylation of Cortactin abolishes its actin polymerization activity [113]. Mutants mimicking Cortactin acetylation prevent actin polymerization and inhibit ciliary shortening [77]. Tctex-1, a dynein light chain, has a dynein-independent role in the regulation of actin dynamics, and phosphorylation of Tctex-1 promotes ciliary disassembly [57, 114]. Thus, actin dynamics are actively involved in ciliary shortening in that actin polymerization blocks ciliary assembly and promotes disassembly simultaneously by upregulation of the genes encoding Aurora-A and Plk1.
The role of actin dynamics in ciliary assembly and disassembly has also been examined in Chlamydomonas. Interestingly, inducing actin filament depolymerization by treatment with cytochalasin D or inhibiting polymerization by latrunculin B results in flagellar shortening, which is contrary to the observations in mammalian cells described above [115, 116]. More perplexing is that a null mutant of actin has flagella of normal length instead of short flagella. [117, 118]. The finding that actin dynamics regulates IFT recruitment to the basal body may provide some insight [115]. Disruption of actin dynamics inhibits IFT recruitment, which may tilt the assembly/disassembly balance toward disassembly, leading to flagellar shortening.
Inhibition of activities for ciliary assembly during ciliary shortening
Several lines of evidences suggest that assembly activities are inhibited at the time of ciliary shortening. F-actin polymerization, which inhibits ciliary assembly, occurs during ciliary shortening [110], and inhibition of anterograde cargo loading during flagellar shortening in Chlamydomonas has been predicted to suppress flagellar assembly [45]. In addition, a NIMA-related kinase, Nek2, phosphorylates at S/G2 Kif24, a microtubule depolymerizing kinesin, and prevents ciliary growth while depletion of Nek2 or Kif24 induces aberrant cilia formation [119]. Thus, during ciliary shortening, assembly related activities are inhibited to coordinate with active disassembly to ensure ciliary disassembly.
Signaling molecules and signaling pathways that mediate ciliary disassembly
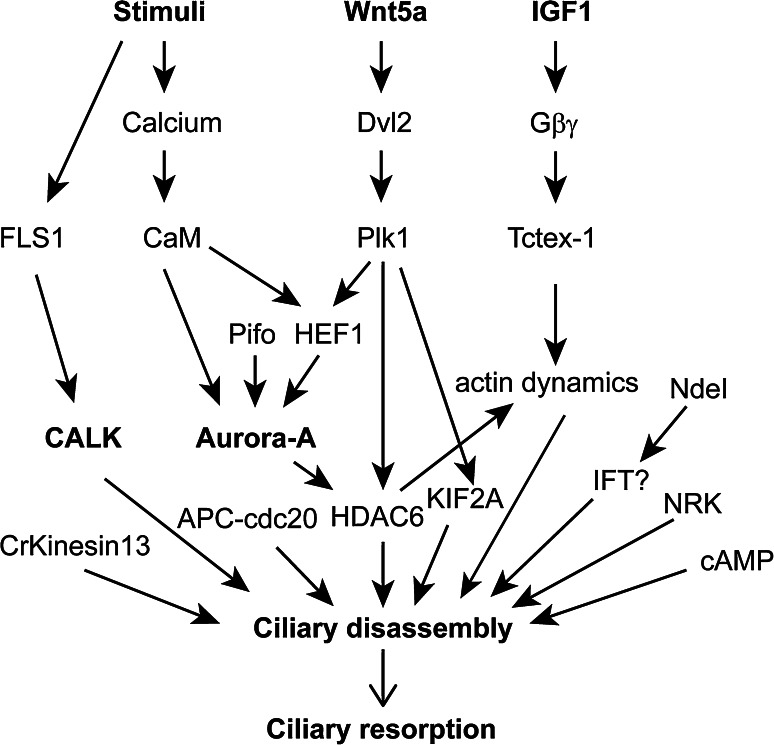
Ciliary disassembly has been found to be associated with cell cycle progression, cell differentiation, and cellular stress in different cell types or organisms. The internal or external cues that trigger ciliary disassembly in different situations vary, and the perception of these cues is expected to be distinct and cell-type specific. However, the downstream cellular events should be identical or similar as cilia are conserved organelles. For example, the activation of aurora kinases during ciliary disassembly is conserved in Chlamydomonas and mammalian cells [54, 97]. In this section, we will discuss different signaling molecules that have been shown to be directly involved in ciliary disassembly; these are summarized in Fig. 5. Though signaling molecules that are involved in ciliary length control may also function in ciliary disassembly activities, they will not be focused on here unless they are implicated directly in ciliary disassembly during ciliary shortening or resorption.
Fig. 5.
Signaling and important regulators for ciliary disassembly. See text for detail
Ciliary disassembly is a signaling driven, active process
The Chlamydomonas temperature sensitive mutants fla10 and fla8, which are defective in the IFT motor kinesin-II subunits, KIF3A and KIF3B, respectively, fail to maintain their flagella at the restrictive temperature and the flagella gradually resorb [120–123]. As cilia are maintained by a balance between IFT-mediated assembly and continuous protein turnover [12], ciliary disassembly is induced by constitutive protein turnover when anterograde IFT is blocked. This seems to suggest that ciliary disassembly may be a passive process mediated by an imbalance in ciliary inherent protein turnover. However, the discovery of several signaling molecules, including aurora kinases and various posttranslational modifications that are tightly associated with ciliary disassembly, suggests that ciliary disassembly is an active process and is highly regulated.
Calcium
Calcium is essential for ciliogenesis, however, alteration of calcium homeostasis may also induce ciliary disassembly. In Chlamydomonas, depletion of extracellular calcium blocks flagellar regeneration after deflagellation [124–126]. Increasing the salt concentration from 3 to 12.5 mM does not affect flagellar length. However, if extracellular calcium is removed at the same time, the flagella undergo shortening, which is reversed when calcium is added back [33, 127]. In addition, when cell cultures are treated with calcium chelators such as 5 mM ATP or GTP, 20 mM NaPPi, or 25 mM citrate, the flagella also shorten [33]. The depletion of extracellular calcium likely results in an internal calcium increase mediated by the calcium homeostasis system of the cell.
In mammalian cells, application of the calcium ionphore (A23187) to BALB/C 3T3 or RPE-1 cells induces transient ciliary shortening [128, 129]. Various compounds that interfere with calcium homeostasis also induce changes of ciliary length in IMCD cells [130]. For example, blocking either (a) internal calcium release with dantrolene, an inhibitor of ryanodine receptor or (b) calcium entry with gadolinium, a calcium channel inhibitor, increases ciliary length. By contrast, elevation of the cellular level of calcium by inhibition of the endoplasmic reticulum calcium ATPase with thapsigargin results in shorter cilia. The critical inducer of ciliary disassembly, Aurora A kinase, can be activated by calcium and this activation induces ciliary disassembly [129, 131]. The results obtained in Chlamydomonas and mammalian cells are consistent in that elevation of internal calcium levels induces ciliary disassembly. One of the targets of calcium is aurora-A [129]. However, it remains to be demonstrated that calcium levels are actually increased during ciliary shortening.
Cyclic AMP (cAMP)
There are conflicting data on the role of cAMP in ciliary disassembly. Treatment of vegetative Chlamydomonas cells with IBMX, a phosphodiesterase inhibitor, in the presence or absence of cAMP induces flagellar shortening [132, 133]. However, it has no effect on flagellar length in Chlamydomonas gametes [134]. Similarly, in mammalian cells, inhibition of adenylyl cyclase induces formation of long cilia, also suggesting that cAMP promotes ciliary disassembly [135]. However, opposite results have been reported in another study [130, 136]. Collectively, these data imply that the function of cAMP in regulation of ciliary shortening depends on the cellular context or the cell type.
Chlamydomonas aurora-like kinase CALK
Chlamydomonas aurora-like kinase, CALK, is the first protein kinase identified that exhibits phosphorylation changes during ciliary resorption. CALK has 679 aa with a protein kinase domain at the N-terminus [137]. CALK is closely related to aurora kinases including Aurora A, B and C in mammalian cells, which are smaller, have no more than 400 aa. Reciprocal Blast analysis of aurora kinases identifies a single Chlamydomonas aurora kinase orthologue, ALK2, with 292 aa (Cre04.g220700). This suggests that CALK is an aurora-like kinase rather an orthologue of aurora kinases.
When flagellar shortening is induced with NaPPi treatment or during normal flagellar shortening in zygotes, CALK is phosphorylated and undergoes a mobility shift on SDS-PAGE [97]. Depletion of CALK by RNAi inhibits flagellar shortening. Subsequent studies show that CALK is phosphorylated at T193 in the activation loop, which renders it active; a second phosphorylation in the C-terminus accounts for the mobility shift [138]. CALK is predominately localized to the basal body region and activated upon induction of flagellar shortening. However, the cellular level of CALK phosphorylated at T193 gradually decreases along with flagellar shortening while that of CALK phosphorylated at the C-terminus persists through the whole process of flagellar resorption. These data suggests that transient but not constitutive activation of CALK is involved in ciliary disassembly, and phosphorylation of the C-terminal tail also plays a role.
How CALK regulates ciliary disassembly is unknown. In mammalian cells, Aurora A phosphorylates and activates HDAC6 to facilitate disassembly of the axonemal MTs during ciliary resorption [54]. This has yet to be shown for CALK. CALK may also have additional roles in regulating flagellar disassembly. The involvement of CALK in flagellar length control may provide some insights. CALK T193 phosphorylation is tightly linked with flagellar length [138]. After deflagellation, the cellular level of CALK phosphorylated at T193 is greatly diminished. During flagellar assembly, phosphorylation of T193 gradually increases and reaches the steady state level when full-length flagella are formed. This suggests that CALK T193 phosphorylation might inhibit flagellar assembly to control final flagellar length. As IFT cargo loading decreases along with flagellar assembly [139, 140], it is likely that CALK T193 phosphorylation inhibits IFT cargo loading. Consistent with this idea, anterograde IFT cargo loading is inhibited during flagellar shortening while CALK is transiently activated [45]. Thus, it is attractive to posit that CALK may inhibit flagellar assembly by blocking anterograde IFT cargo loading to ensure ciliary disassembly during flagellar shortening.
CDK-like kinase FLS1
CALK is immediately phosphorylated in response to cues that trigger flagellar shortening. Recent work shows that a CDK-like protein kinase regulates CALK phosphorylation [60]. A genetic screen identified a flagellar shortening mutant flagellar shortening 1 (fls1), which is defective in a gene encoding a CDK-like kinase. By examination of flagellar shortening prior to cell division as well as that induced by NaPPi treatment, it was revealed that fls1 exhibits slower shortening of the distal half of the flagella whereas the rate of shortening of the proximal half is not affected. FLS1 is immediately phosphorylated after NaPPi treatment and its kinase activity is required for its function, suggesting that FLS1 acts earlier in the signaling cascade that triggers flagellar disassembly.
After induction of flagellar shortening by treatment with NaPPi in fls1 cells, initial phosphorylation of CALK is blocked, indicating FLS1 acts upstream of CALK [60]. As flagella can undergo slower shortening in the absence of FLS1 and CALK phosphorylation, additional shortening mechanisms independent of CALK phosphorylation and FLS1 may exist. Surprisingly, at around 90 min after NaPPi treatment of fls1 cells, CALK phosphorylation appears, which is independent of FLS1. It is likely that flagellar shortening itself may generate a signaling to control CALK phosphorylation at this stage.
In addition to the regulation of CALK, FLS1 also controls CrKinesin13 phosphorylation. CrKinesin13 is targeted to the flagella at the time when flagellar shortening is triggered and becomes phosphorylated around 1 h after the induction of shortening [85]. The fls1 mutation does not affect flagellar targeting of CrKinesin13 [60]. However, fls1 mutation induces early onset of CrKinesin13 phosphorylation, which has been shown to suppress MT depolymerization in vitro [141]. Thus, the early onset of CrKinesin13 phosphorylation is consistent with the slower flagellar shortening phenotype of fls1.
Aurora A and its regulation
The discovery that the aurora-like kinase CALK is involved in ciliary disassembly in Chlamydomonas raises an interesting question as to whether a similar mechanism operates in mammalian cells. Golemis and colleagues demonstrated that the aurora kinase, Aurora-A, plays a pivotal role in regulating ciliary disassembly [54]. Serum starvation of RPE1 cells promotes ciliary assembly with more than 80 % of cells forming 3–4 μm long cilia. After serum stimulation, cells exhibit two waves of ciliary resorption (see “Ciliary disassembly during cell cycle progression”) [51, 53, 54]. Aurora-A is phosphorylated and activated at the basal body at both of these waves of ciliary disassembly. Inhibition of Aurora-A by siRNA or by Aurora-A specific kinase inhibitors suppresses ciliary disassembly. Conversely, microinjection of active Aurora-A into ciliated cells induces rapid ciliary loss. As α-tubulin deacetylation promotes MT instability [142], it was postulated that HDAC6, the MT deacetylase, may be the target of Aurora-A. As expected, inhibition of HDAC6 by chemical inhibitors or by siRNAi suppresses ciliary disassembly. HDAC6 interacts with Aurora-A in vivo and is activated by phosphorylation [54]. Thus, Aurora-A phosphorylates and activates HDAC6 to increase axonemal microtubule instability leading to ciliary disassembly.
The activation of Aurora-A during ciliary shortening can be mediated by several regulators including HEF1, Ca2+-CAM, Pitchfork (PIFO), the Dvl2 (Dishevelled 2)-Plk1 (Polo-like kinase 1) complex, and trichoplein. HEF1, a previous known activator of Aurora-A, is transiently expressed at the time when cilia undergo disassembly and accumulates at the basal body. Depletion of HEF1 impairs ciliary disassembly [54]. The Dvl2-Plk1 complex of the Wnt noncanonical pathway inhibits HEF1 degradation, leading to Aurora A activation and ciliary disassembly [143] (see “Plk1 and the noncanonical Wnt pathway”). In addition, Aurora A has a CAM binding domain. Mutants impaired for CAM binding prevents Aurora A activation. CAM is also implicated in stabilizing the interaction between Aurora A and HEF1 [129, 131].
Pitchfork is a protein that is only present in chordates and is only expressed in certain regions of the embryo. It accumulates at the basal body during ciliary disassembly [144]. PIFO physically interacts with and enhances Aurora-A activity. Overexpression of the PIFO mutant R80K abolishes Aurora-A activation and ciliary disassembly. Lastly, Trichoplein, which is a scaffold protein, activates Aurora-A by direct interaction [59]. Overexpression of Trichoplein results in ciliary loss in quiescent cells whereas its depletion promotes ciliary formation in proliferating cells. Because Trichoplein is expressed throughout G1, it was proposed that trichoplein functions in suppressing ciliary assembly in cycling cells [59].
Plk1 and the noncanonical Wnt pathway
The noncanonical Wnt signaling pathway has been linked to ciliary assembly [145, 146], it also participates in ciliary disassembly [143]. In response to the ligand Wnt5a, Dvl2 is phosphorylated by CK1ε and forms a complex with Plk1. Overexpression of Dvl2 induces formation of shorter cilia in RPE1 and NIH3T3 cells whereas its depletion results in longer cilia. Ablation of Plk1, overexpression of a Plk1 kinase dead mutant, or treatment with Plk1 inhibitor all delay serum induced ciliary disassembly. The Dvl2-Plk1 complex is required to stabilize HEF1. Thus, Wnt5a ligand stimulates a signaling cascade to stabilize HEF1 and activate Aurora-A to promote ciliary disassembly. Wang et al. showed that Plk1 is recruited to the pericentriolar matrix by PCM1 and physically interacts with and activates HDAC6 to promote ciliary disassembly [147]. These data suggests that Plk1 may have additional functions in the regulation of ciliary disassembly.
Tctex-1 and the IGF-1 mediated noncanonical Gβγ signaling pathway
In the developing neocortex, radial glia may differentiate into neurons while at the same time they proliferate to renew themselves. The fate of radial glia depends on the length of G1: shortening G1 accelerates cell cycle entry and proliferation whereas lengthening G1 promotes neuron differentiation [26]. It has been shown that phosphorylated Tctex-1 (a light chain of cytoplasmic dynein) stimulates ciliary disassembly and controls G1 length [57]. Mechanistically, phosphorylated Tctex-1 regulates actin dynamics (see “Actin dynamics”). The signaling cascade leading to Tctex-1 regulation was revealed in a subsequent study [148]. Insulin-like growth 1 (IGF-1) binds the ciliary localized receptor IGF-1R, triggers Gβγ signaling, and recruits phosphorylated Tctex-1 to the ciliary base during ciliary shortening.
Other regulators of ciliary disassembly
The centrosomal protein Nde1 is highly expressed in mitosis. When Nde1 is depleted, cells grow longer cilia and have a delay in the G1-S transition, indicating that Nde1 promotes ciliary resorption at cell cycle re-entry [58]. Nde1 interacts with a dynein light chain, LC8, which is a subunit of the IFT motor cytoplasmic dynein 1b [149, 150]. Thus, the interaction of Nde1 with LC8 may sequester LC8 from IFT dynein and inhibit IFT mediated ciliary maintenance, subsequently leading to ciliary disassembly.
NIMA-related kinases (Nrks) are also implicated in ciliary resorption. NEK1 functions in ciliary stability and integrity [95]. It is a target of the anaphase-promoting complex (APC), an ubiquitin E3 ligase. It was proposed that NEK1 is ubiquitinated by APC and removed by protein degradation to allow ciliary disassembly. Null mutants of Nrks in Chlamydomonas including flagellar autotomy 2 (fa2) and cnk2 are defective in flagellar shortening [151, 152]. Overexpression of several Nrks in Tetrahymena causes rapid shortening of cilia [153].
In Chlamydomonas, mutations in several genes encoding protein kinases such as LF4 (a MAP kinase), LF2 (a CDK) and LF5 (a CDK-like kinase) result in formation of long flagella. This raises the possibility that these enzymes may also be involved in flagellar resorption as these kinases may inhibit flagellar disassembly activities [154–156]. However, lf4 mutants shorten their flagella normally after treatment with NaPPi [45]. It remains to be determined whether the other two kinases play any role in flagellar disassembly.
Concluding remarks
Alteration of the balance between ciliary assembly and disassembly will lead to changes of ciliary length. Thus, manipulation of related genes or proteins will lead to formation of short or long cilia. To determine whether a protein functions in ciliary shortening, one needs to show that its biochemical or cellular properties are regulated accordingly during the shortening process.
The regulation of ciliary shortening is complex. Ciliary shortening signaling cascades not only directly participate in disassembly but also suppress activities required for assembly, thus ensuring ciliary resorption. In addition, variations of cell type, cellular stage and cell growth condition studied also add to the complexity of our understanding of ciliary disassembly.
What is clear is that Aurora-A activation is a key event in ciliary resorption. HDAC6 is the only target of Aurora-A identified so far in the ciliary shortening pathway. As ciliary shortening involves participation of multiple cellular processes including IFT regulation, protein posttranslational modifications, timely ciliary targeting of disassembling effectors, actin dynamics, and reduction of the surface area of the ciliary membrane, activation of multiple parallel pathways along with Aurora-A activation is expected to occur. Great effort will be needed to identify these interacting regulatory pathways.
Many questions about ciliary disassembly remain to be answered. For example, how are IFT injection rate and IFT cargo loading and unloading regulated? How is the protein posttranslational modification machinery activated? What do these modifications contribute to ciliary disassembly? So far little is known about how the ciliary membrane is withdrawn. Recent studies also suggest that ciliary resorption serves as a checkpoint for S phase entry, but how ciliary resorption mediates this process remains elusive.
Research with Chlamydomonas has played a leading role in advancing our understanding of the mechanisms of ciliary disassembly, due in part to several advantages of this experimental system. Chlamydomonas flagella are long (average of 12 μm), thus, flagellar shortening kinetics can be easily followed. Flagellar disassembly can be scored in several situations including extracellular stress, zygote development and cell cycle progression. Experimentally induced flagellar disassembly is highly synchronous. Furthermore, Chlamydomonas flagella can be conveniently isolated and purified. These properties should allow the eventual elucidation of the biochemical and cellular changes that occur at different stages of ciliary shortening.
Acknowledgments
We thank Drs. Roger Sloboda and Kaiyao Huang for critical review and editing of this manuscript. This work was supported by awards from the National Basic Research Program of China (2012CB945000 and 2013CB910700) and the National Science Foundation of China (31330044) to J. P. Y. L is supported by a Postdoctoral Fellowship from Tsinghua-Peking Center for Life Sciences.
Abbreviations
- IFT
Intraflagellar transport
- MT
Microtubule
- NaPPi
Sodium pyrophosphate
- Plk1
Polo-like kinase 1
- Dvl2
Dishevelled 2
References
- 1.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 4.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JM, Witman GB. Cilia and diseases. Bioscience. 2014;64:1126–1137. doi: 10.1093/biosci/biu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 9.Ounjai P, Kim KD, Liu H, Dong M, Tauscher AN, Witkowska HE, Downing KH. Architectural insights into a ciliary partition. Curr Biol. 2013;23:339–344. doi: 10.1016/j.cub.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 2012;14:431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 12.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson PK. Do cilia put brakes on the cell cycle? Nat Cell Biol. 2011;13:340–342. doi: 10.1038/ncb0411-340. [DOI] [PubMed] [Google Scholar]
- 14.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Bloodgood RA. Resorption of organelles containing microtubules. Cytobios. 1974;9:142–161. [PubMed] [Google Scholar]
- 16.Sanders MA, Salisbury JL. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii . J Cell Biol. 1989;108:1751–1760. doi: 10.1083/jcb.108.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum JJ. Existence of a breaking point in cilia and flagella. J Theor Biol. 1971;33:257–263. doi: 10.1016/0022-5193(71)90065-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967;34:345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall SJ, Cavalier-Smith T, McVittie A, Warr JR, Hopkins JM. Developmental and control processes in the basal bodies and flagella of Chlamydomonas reinhardtii . Dev Biol Suppl. 1967;1:43–83. [Google Scholar]
- 20.Goto H, Inoko A, Inagaki M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci. 2013;70:3893–3905. doi: 10.1007/s00018-013-1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011;10:2683–2690. doi: 10.4161/cc.10.16.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan J, Seeger-Nukpezah T, Golemis EA. The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cell Mol Life Sci. 2013;70:1849–1874. doi: 10.1007/s00018-012-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao M, Li G, Pan J. Regulation of cilia assembly, disassembly, and length by protein phosphorylation. Methods Cell Biol. 2009;94:333–346. doi: 10.1016/S0091-679X(08)94017-6. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre PA, Rosenbaum JL. Regulation of the synthesis and assembly of ciliary and flagellar proteins during regeneration. Annu Rev Cell Biol. 1986;2:517–546. doi: 10.1146/annurev.cb.02.110186.002505. [DOI] [PubMed] [Google Scholar]
- 25.Seeger-Nukpezah T, Little JL, Serzhanova V, Golemis EA. Cilia and cilia-associated proteins in cancer. Drug Discov Today Dis Mech. 2013;10:e135–e142. doi: 10.1016/j.ddmec.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung CH, Li A. Ciliary resorption modulates G1 length and cell cycle progression. Cell Cycle. 2011;10:2825–2826. doi: 10.4161/cc.10.17.16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prodromou NV, Thompson CL, Osborn DP, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP. Heat shock induces rapid resorption of primary cilia. J Cell Sci. 2012;125:4297–4305. doi: 10.1242/jcs.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- 29.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, et al. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci USA. 2014;111:12871–12876. doi: 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solter KM, Gibor A. The relationship between tonicity and flagellar length. Nature. 1978;275:651–652. doi: 10.1038/275651a0. [DOI] [PubMed] [Google Scholar]
- 32.Telser A. The inhibition of flagellar regeneration in Chlamydomonas reinhardii by inhalational anesthetic halothane. Exp Cell Res. 1977;107:247–252. doi: 10.1016/0014-4827(77)90406-2. [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre PA, Nordstrom SA, Moulder JE, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978;78:8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969;41:600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rannestad J. The regeneration of cilia in partially deciliated Tetrahymena. J Cell Biol. 1974;63:1009–1017. doi: 10.1083/jcb.63.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi K, Hilding D. The development of the organ of Corti in the mouse. Acta Otolaryngol. 1965;60:207–222. doi: 10.3109/00016486509127003. [DOI] [PubMed] [Google Scholar]
- 37.Kimura RS. Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol. 1966;61:55–72. doi: 10.3109/00016486609127043. [DOI] [PubMed] [Google Scholar]
- 38.Rozycki M, Lodyga M, Lam J, Miranda MZ, Fatyol K, Speight P, Kapus A. The fate of the primary cilium during myofibroblast transition. Mol Biol Cell. 2014;25:643–657. doi: 10.1091/mbc.E13-07-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Goetz SC, Ocbina PJ, Anderson KV. The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol. 2009;94:199–222. doi: 10.1016/S0091-679X(08)94010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009;27:703–713. doi: 10.1634/stemcells.2008-0888. [DOI] [PubMed] [Google Scholar]
- 42.Forcioli-Conti N, Lacas-Gervais S, Dani C, Peraldi P. The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem Biophys Res Commun. 2015;458:117–122. doi: 10.1016/j.bbrc.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 43.Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci USA. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii . J Cell Sci. 1974;16:529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- 45.Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- 47.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 48.Dingemans KP. The relation between cilia and mitoses in the mouse adenohypophysis. J Cell Biol. 1969;43:361–367. doi: 10.1083/jcb.43.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rash JE, Shay JW, Biesele JJ. Cilia in cardiac differentiation. J Ultrastruct Res. 1969;29:470–484. doi: 10.1016/S0022-5320(69)90067-7. [DOI] [PubMed] [Google Scholar]
- 50.Fonte VG, Searls RL, Hilfer SR. The relationship of cilia with cell division and differentiation. J Cell Biol. 1971;49:226–229. doi: 10.1083/jcb.49.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- 52.Archer FL, Wheatley DN. Cilia in cell-cultured fibroblasts. II. Incidence in mitotic and post-mitotic BHK 21-C13 fibroblasts. J Anat. 1971;109:277–292. [PMC free article] [PubMed] [Google Scholar]
- 53.Spalluto C, Wilson DI, Hearn T. Evidence for reciliation of RPE1 cells in late G1 phase, and ciliary localisation of cyclin B1. FEBS Open Bio. 2013;3:334–340. doi: 10.1016/j.fob.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/S0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- 56.Tucker RW, Scher CD, Stiles CD. Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell. 1979;18:1065–1072. doi: 10.1016/0092-8674(79)90219-8. [DOI] [PubMed] [Google Scholar]
- 57.Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T, et al. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol. 2012;197:391–405. doi: 10.1083/jcb.201106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Z, Liang Y, He W, Pan J. Cilia disassembly with two distinct phases of regulation. Cell Rep. 2015;10:1803–1810. doi: 10.1016/j.celrep.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 61.Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langousis G, Hill KL. Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol. 2014;12:505–518. doi: 10.1038/nrmicro3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedlander M, Wahrman J. The spindle as a basal body distributor. A study in the meiosis of the male silkworm moth, Bombyx mori. J Cell Sci. 1970;7:65–89. doi: 10.1242/jcs.7.1.65. [DOI] [PubMed] [Google Scholar]
- 64.Riparbelli MG, Callaini G, Megraw TL. Assembly and persistence of primary cilia in dividing Drosophila spermatocytes. Dev Cell. 2012;23:425–432. doi: 10.1016/j.devcel.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.L’Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983;97:258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.L’Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 68.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 70.Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25:125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vashishtha M, Walther Z, Hall JL. The kinesin-homologous protein encoded by the Chlamydomonas FLA10 gene is associated with basal bodies and centrioles. J Cell Sci. 1996;109(Pt 3):541–549. doi: 10.1242/jcs.109.3.541. [DOI] [PubMed] [Google Scholar]
- 73.Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157:1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kozminski KG, Diener DR, Rosenbaum JL. High level expression of nonacetylatable alpha-tubulin in Chlamydomonas reinhardtii . Cell Motil Cytoskeleton. 1993;25:158–170. doi: 10.1002/cm.970250205. [DOI] [PubMed] [Google Scholar]
- 77.Ran J, Yang Y, Li D, Liu M, Zhou J. Deacetylation of alpha-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci Rep. 2015;5:12917. doi: 10.1038/srep12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walczak CE, Gayek S, Ohi R. Microtubule-depolymerizing kinesins. Annu Rev Cell Dev Biol. 2013;29:417–441. doi: 10.1146/annurev-cellbio-101512-122345. [DOI] [PubMed] [Google Scholar]
- 79.Vasudevan KK, Jiang YY, Lechtreck KF, Kushida Y, Alford LM, Sale WS, Hennessey T, Gaertig J. Kinesin-13 regulates the quantity and quality of tubulin inside cilia. Mol Biol Cell. 2015;26:478–494. doi: 10.1091/mbc.E14-09-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu Z, Liang Y, Meng D, Wang L, Pan J. Microtubule-depolymerizing kinesins in the regulation of assembly, disassembly, and length of cilia and flagella. Int Rev Cell Mol Biol. 2015;317:241–265. doi: 10.1016/bs.ircmb.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Niwa S, Nakajima K, Miki H, Minato Y, Wang D, Hirokawa N. KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev Cell. 2012;23:1167–1175. doi: 10.1016/j.devcel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 82.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in giardia intestinalis. Eukaryot Cell. 2007;6:2354–2364. doi: 10.1128/EC.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–782. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 84.Chan KY, Ersfeld K. The role of the Kinesin-13 family protein TbKif13-2 in flagellar length control of Trypanosoma brucei. Mol Biochem Parasitol. 2010;174:137–140. doi: 10.1016/j.molbiopara.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA. 2009;106:4713–4718. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto T, Hosoba K, Ochiai H, Royba E, Izumi H, Sakuma T, Yamamoto T, Dynlacht BD, Matsuura S. The microtubule-depolymerizing activity of a mitotic kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Rep. 2015;10:664–673. doi: 10.1016/j.celrep.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Werner-Peterson R, Sloboda RD. Methylation of structural components of the axoneme occurs during flagellar disassembly. Biochemistry. 2013;52:8501–8509. doi: 10.1021/bi4011623. [DOI] [PubMed] [Google Scholar]
- 89.Sloboda RD, Howard L. Protein methylation in full length Chlamydomonas flagella. Cell Motil Cytoskeleton. 2009;66:650–660. doi: 10.1002/cm.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schneider MJ, Ulland M, Sloboda RD. A protein methylation pathway in Chlamydomonas flagella is active during flagellar resorption. Mol Biol Cell. 2008;19:4319–4327. doi: 10.1091/mbc.E08-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 92.Kirkin V, Dikic I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr Opin Cell Biol. 2007;19:199–205. doi: 10.1016/j.ceb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol. 2009;186:601–613. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Wang W, Wu T, Kirschner MW. The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. Elife. 2014;3:e03083. doi: 10.7554/eLife.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/S1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 98.Pan J, Naumann-Busch B, Wang L, Specht M, Scholz M, Trompelt K, Hippler M. Protein phosphorylation is a key event of flagellar disassembly revealed by analysis of flagellar phosphoproteins during flagellar shortening in Chlamydomonas. J Proteome Res. 2011;10:3830–3839. doi: 10.1021/pr200428n. [DOI] [PubMed] [Google Scholar]
- 99.Brown DL, Rogers KA. Hydrostatic pressure-induced internalization of flagellar axonemes, disassembly, and reutilization during flagellar regeneration in Polytomella. Exp Cell Res. 1978;117:313–324. doi: 10.1016/0014-4827(78)90145-3. [DOI] [PubMed] [Google Scholar]
- 100.Liang Y, Pang Y, Wu Q, Hu Z, Han X, Xu Y, Deng H, Pan J. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev Cell. 2014;30:585–597. doi: 10.1016/j.devcel.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 101.Mc LR, Laurendi CJ, Brown RM., Jr The relationship of gamone to the mating reaction in Chlamydomonas moewusii. Proc Natl Acad Sci USA. 1974;71:2610–2613. doi: 10.1073/pnas.71.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood CR, Rosenbaum JL. Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol. 2015;25:276–285. doi: 10.1016/j.tcb.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wood CR, Rosenbaum JL. Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr Biol. 2014;24:1114–1120. doi: 10.1016/j.cub.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014;24:519–525. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yan X, Zhu X. Branched F-actin as a negative regulator of cilia formation. Exp Cell Res. 2013;319:147–151. doi: 10.1016/j.yexcr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, Zhu X. miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol. 2012;14:697–706. doi: 10.1038/ncb2512. [DOI] [PubMed] [Google Scholar]
- 109.Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee JK, Heo WD, Kim J. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun. 2015;6:6781. doi: 10.1038/ncomms7781. [DOI] [PubMed] [Google Scholar]
- 110.Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell. 2010;19:270–283. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lua BL, Low BC. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 2005;579:577–585. doi: 10.1016/j.febslet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75–86. doi: 10.1016/j.devcel.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Avasthi P, Onishi M, Karpiak J, Yamamoto R, Mackinder L, Jonikas MC, Sale WS, Shoichet B, Pringle JR, Marshall WF. Actin is required for IFT regulation in Chlamydomonas reinhardtii . Curr Biol. 2014;24:2025–2032. doi: 10.1016/j.cub.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dentler WL, Adams C. Flagellar microtubule dynamics in Chlamydomonas: cytochalasin D induces periods of microtubule shortening and elongation; and colchicine induces disassembly of the distal, but not proximal, half of the flagellum. J Cell Biol. 1992;117:1289–1298. doi: 10.1083/jcb.117.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kato-Minoura T, Uryu S, Hirono M, Kamiya R. Highly divergent actin expressed in a Chlamydomonas mutant lacking the conventional actin gene. Biochem Biophys Res Commun. 1998;251:71–76. doi: 10.1006/bbrc.1998.9373. [DOI] [PubMed] [Google Scholar]
- 118.Hirono M, Uryu S, Ohara A, Kato-Minoura T, Kamiya R. Expression of conventional and unconventional actins in Chlamydomonas reinhardtii upon deflagellation and sexual adhesion. Eukaryot Cell. 2003;2:486–493. doi: 10.1128/EC.2.3.486-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim S, Lee K, Choi JH, Ringstad N, Dynlacht BD. Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun. 2015;6:8087. doi: 10.1038/ncomms9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang B, Rifkin MR, Luck DJ. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii . J Cell Biol. 1977;72:67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adams GM, Huang B, Luck DJ. Temperature-sensitive, assembly-defective flagella mutants of Chlamydomonas reinhardtii . Genetics. 1982;100:579–586. doi: 10.1093/genetics/100.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller MS, Esparza JM, Lippa AM, Lux FG, Cole DG, Dutcher SK. Mutant kinesin-2 motor subunits increase chromosome loss. Mol Biol Cell. 2005;16:3810–3820. doi: 10.1091/mbc.E05-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang Y, Pan J. Regulation of flagellar biogenesis by a calcium dependent protein kinase in Chlamydomonas reinhardtii . PLoS One. 2013;8:e69902. doi: 10.1371/journal.pone.0069902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheshire JL, Evans JH, Keller LR. Ca2+ signaling in the Chlamydomonas flagellar regeneration system: cellular and molecular responses. J Cell Sci. 1994;107(Pt 9):2491–2498. doi: 10.1242/jcs.107.9.2491. [DOI] [PubMed] [Google Scholar]
- 126.Cheshire JL, Keller LR. Uncoupling of Chlamydomonas flagellar gene expression and outgrowth from flagellar excision by manipulation of Ca2+ J Cell Biol. 1991;115:1651–1659. doi: 10.1083/jcb.115.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Quader H, Cherniack J, Filner P. Participation of calcium in flagellar shortening and regeneration in Chlamydomonas reinhardii . Exp Cell Res. 1978;113:295–301. doi: 10.1016/0014-4827(78)90369-5. [DOI] [PubMed] [Google Scholar]
- 128.Tucker RW, Meade-Cobun KS, Jayaraman S, More NS. Centrioles, primary cilia and calcium in the growth of Balb/c 3T3 cells. J Submicrosc Cytol. 1983;15:139–143. [PubMed] [Google Scholar]
- 129.Plotnikova OV, Pugacheva EN, Dunbrack RL, Golemis EA. Rapid calcium-dependent activation of Aurora-A kinase. Nat Commun. 2010;1:64. doi: 10.1038/ncomms1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell. 2012;23:2658–2670. doi: 10.1091/mbc.E11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tuxhorn J, Daise T, Dentler WL. Regulation of flagellar length in Chlamydomonas. Cell Motil Cytoskeleton. 1998;40:133–146. doi: 10.1002/(SICI)1097-0169(1998)40:2<133::AID-CM3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 133.Lefebvre PA, Silflow CD, Wieben ED, Rosenbaum JL. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980;20:469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- 134.Pasquale SM, Goodenough UW. Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii . J Cell Biol. 1987;105:2279–2292. doi: 10.1083/jcb.105.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci. 2012;69:165–173. doi: 10.1007/s00018-011-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pan J, Snell WJ. Regulated targeting of a protein kinase into an intact flagellum. An aurora/Ipl1p-like protein kinase translocates from the cell body into the flagella during gamete activation in Chlamydomonas. J Biol Chem. 2000;275:24106–24114. doi: 10.1074/jbc.M002686200. [DOI] [PubMed] [Google Scholar]
- 138.Cao M, Meng D, Wang L, Bei S, Snell WJ, Pan J. Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length. Proc Natl Acad Sci USA. 2013;110:12337–12342. doi: 10.1073/pnas.1302364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhogaraju S, Weber K, Engel BD, Lechtreck KF, Lorentzen E. Getting tubulin to the tip of the cilium: one IFT train, many different tubulin cargo-binding sites? BioEssays. 2014;36:463–467. doi: 10.1002/bies.201400007. [DOI] [PubMed] [Google Scholar]
- 140.Pan J, Snell WJ. Organelle size: a cilium length signal regulates IFT cargo loading. Curr Biol. 2014;24:R75–R78. doi: 10.1016/j.cub.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 141.Wang L, Piao T, Cao M, Qin T, Huang L, Deng H, Mao T, Pan J. Flagellar regeneration requires cytoplasmic microtubule depolymerization and kinesin-13. J Cell Sci. 2013;126:1531–1540. doi: 10.1242/jcs.124255. [DOI] [PubMed] [Google Scholar]
- 142.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, Bang JK, Zhou M, Veenstra TD, Yeon Kim B, Lee KS. Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 2012;31:3104–3117. doi: 10.1038/emboj.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinzel D, Boldt K, Davis EE, Burtscher I, Trumbach D, Diplas B, Attie-Bitach T, Wurst W, Katsanis N, Ueffing M, et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell. 2010;19:66–77. doi: 10.1016/j.devcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation. 2012;83:S49–S55. doi: 10.1016/j.diff.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 146.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G, Chen Q, Zhang X, Zhang B, Zhuo X, Liu J, Jiang Q, Zhang C. PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. J Cell Sci. 2013;126:1355–1365. doi: 10.1242/jcs.114918. [DOI] [PubMed] [Google Scholar]
- 148.Yeh C, Li A, Chuang JZ, Saito M, Caceres A, Sung CH. IGF-1 activates a cilium-localized noncanonical Gbetagamma signaling pathway that regulates cell-cycle progression. Dev Cell. 2013;26:358–368. doi: 10.1016/j.devcel.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hou Y, Witman GB. Dynein and intraflagellar transport. Exp Cell Res. 2015;334:26–34. doi: 10.1016/j.yexcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stehman SA, Chen Y, McKenney RJ, Vallee RB. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol. 2007;178:583–594. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM. The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr Biol. 2013;23:2208–2214. doi: 10.1016/j.cub.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 152.Parker JD, Quarmby LM. Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biol. 2003;4:1471–2121. doi: 10.1186/1471-2121-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M, Gaertig J. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell. 2006;17:2799–2810. doi: 10.1091/mbc.E05-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol. 2003;13:1145–1149. doi: 10.1016/S0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 155.Tam LW, Ranum PT, Lefebvre PA. CDKL5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas. Mol Biol Cell. 2013;24:588–600. doi: 10.1091/mbc.E12-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol. 2007;176:819–829. doi: 10.1083/jcb.200610022. [DOI] [PMC free article] [PubMed] [Google Scholar]