Abstract
Since their discovery, SOCS have been characterised as regulatory cornerstones of intracellular signalling. While classically controlling the JAK/STAT pathway, their inhibitory effects are documented across several cascades, underpinning their essential role in homeostatic maintenance and disease. After 20 years of extensive research, SOCS3 has emerged as arguably the most important family member, through its regulation of both cytokine- and pathogen-induced cascades. In fact, low expression of SOCS3 is associated with autoimmunity and oncogenesis, while high expression is linked to diabetes and pathogenic immune evasion. The induction of SOCS3 by both viruses and bacteria and its impact upon inflammatory disorders, underscores this protein’s increasing clinical potential. Therefore, with the aim of highlighting SOCS3 as a therapeutic target for future development, this review revisits its multi-faceted immune regulatory functions and summarises its role in a broad ranges of diseases.
Keywords: Suppressor of cytokine signalling (SOCS), Janus kinase/signal transduction and activator of transcription (JAK/STAT), Rheumatoid arthritis, Cancer, Diabetes, Infection
Introduction
SOCS regulation of intracellular signalling
Inflammation represents a fundamental response to microbial, chemical and physical injury. Cytokine signalling regulates various pathophysiological processes and the generation of immune responses and inflammation [1]. Cytokines, such as interleukins (IL) and interferons (IFNs), activate the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [2], a critical intracellular cascade for the transduction of extracellular signals to the nucleus. The association of a ligand with its receptor results in receptor dimerisation, leading to JAK auto-phosphorylation. Activated JAKs phosphorylate cytoplasmic domains of the receptor, which provide docking sites for STATs. Phosphorylated STATs dissociate from the receptor, dimerise and translocate to the nucleus, where they interact with various regulatory elements that induce target gene expression [3–5]. Although cytokines are required to control infection, their overproduction can lead to local and/or systemic pathology. Several well-characterised mechanisms exist to prevent the overproduction of these mediators and down-regulate their signalling, including the upregulation of suppressor of cytokine signalling (SOCS) proteins [6, 7]. SOCS are intracellular, cytokine-inducible proteins that regulate the JAK/STAT pathway in numerous cell types, including those of the immune system [8, 9]. The SOCS family consists of 8 members, the cytokine-inducible Src homology 2 protein (CIS) and SOCS1-SOCS7 [10–12]. This group of proteins shares structural similarity: a central Src homology (SH)2 domain, a conserved C-terminal SOCS box and an amino-terminal domain of variable length and sequence [13]. SOCS1 and SOCS3 contain an additional kinase inhibitory region (KIR) [14] (Fig. 1). SOCS proteins can be induced by numerous cytokines, including IL-6 and TNF-α, growth factors, chemokines and pathogenic components (Fig. 2), including lipopolysaccharide (LPS) [11, 15–18]. Once upregulated, SOCS act via a negative feedback loop to inhibit further signal transduction. SOCS1 and SOCS3 proteins directly bind to JAKs through the SH2 domain and inhibit their activity. Babon et al. identified a new model of SOCS3 signalling inhibition. Once SOCS3 is recruited to receptors via high affinity binding sites, such as gp130, it binds to and inhibits the catalytic activity of JAK1, JAK2 and TYK2 [19]. This process is elegantly reviewed by Babon and Nicola [20]. Both SOCS1 and SOCS3 also compete for the acquisition of phosphorylated cytokine receptor tyrosine residues, thereby blocking STAT binding. SOCS use the ubiquitin proteasome system to degrade JAKs and other signalling molecules, via interaction with their SOCS box [21], which is also important for the stabilisation and/or degradation of SOCS1 and SOCS3 themselves [22] (Fig. 3). Interestingly, SOCS1 and SOCS3 retain some activity even after truncation of the SOCS box, highlighting that the SOCS box is not solely responsible for degradation of SOCS1 and SOCS3 target proteins [23]. Furthermore, compared to the other SOCS family members, the SOCS box of SOCS1 and SOCS3 binds with lower affinity to the E3 ligase protein, Cullin-5, revealing their differential mechanisms of action [24]. Among the SOCS family, SOCS1 and SOCS3 are the best characterised in their inhibition of JAK-STAT signalling. SOCS1 and SOCS3 also inhibit other signalling pathways, such as Ras/Extracellular Signal-Regulated Kinase (Ras/ERK), Phosphatidylinositide 3-kinases (PI3K) and focal adhesion kinase (FAK) signalling and the NF-κB cascades [25–29]. In recent years, increasing evidence detailing SOCS3’s broad-acting regulation of many biological processes has implicated it in several immune disorders, diabetes, infectious disease progression and oncogenesis, thus identifying SOCS3 as a key protein at the cross roads of numerous intracellular and pathological events.
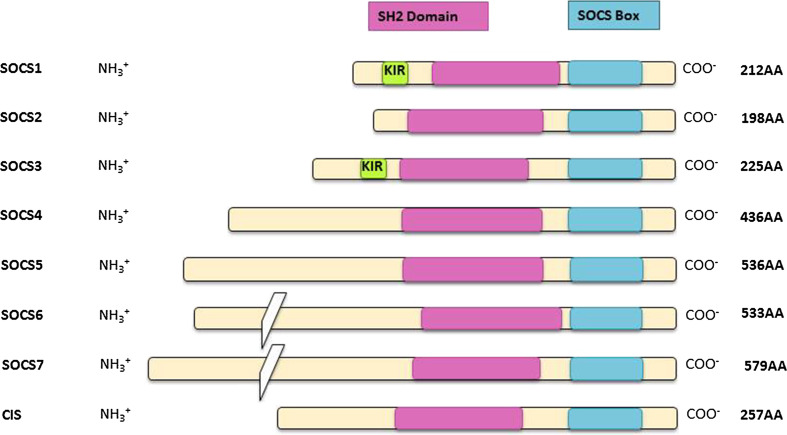
Fig. 1.
Suppressor of cytokine signalling (SOCS) protein family members; there are eight members of the SOCS family of proteins, with each member possessing a SOCS box domain (blue), an SH2 domain (pink) and an amino-terminal region. The highly conserved SOCS box domain, located at the carboxy-terminus, is 40 amino acids in length and is the site of recruitment for the components of the E3 ligase, used for protein degradation. The SH2 domain is centrally located and also exhibits a considerable level of homology between members of the SOCS family. However, the amino-terminal domain is more variable in terms of both length and sequence. In SOCS1 and SOCS3 only, there is a kinase inhibitory region (KIR) just upstream of the SH2 domain, which yields another method of inhibiting the catalytic activity of JAKs, in addition to E3 ligase assembly. The KIR is thought to bind to the activation loop of JAKs with high affinity and thereby act as a pseudo-substrate. The importance of this region to the suppressive activity of SOCS1 and SOCS3 is emphasised by the fact that point mutations in this region completely abrogate their capacity to regulate cytokine signalling
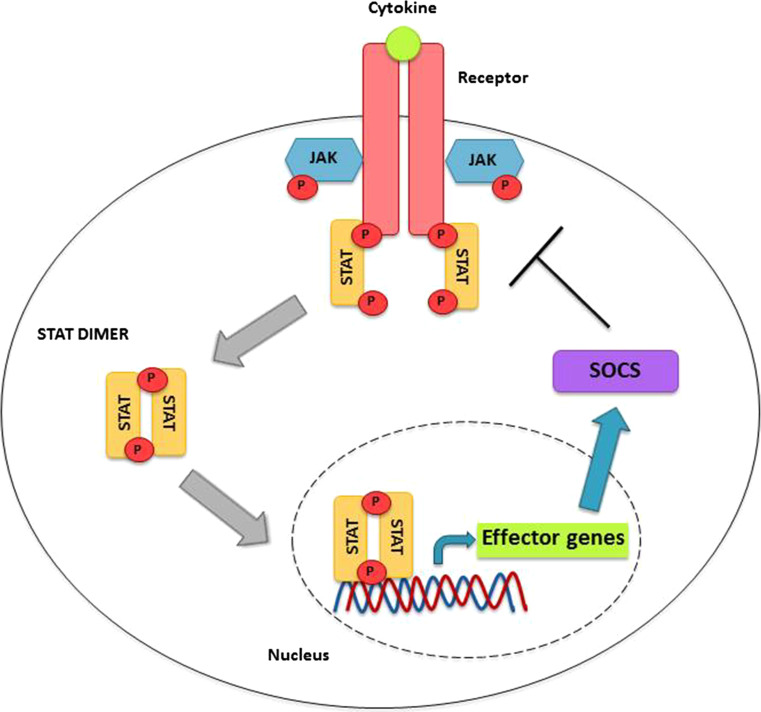
Fig. 2.
SOCS protein induction by JAK/STAT signalling; when a cytokine or growth factor binds to its cognate receptor, the two chains of the receptor become more closely associated, which in turn brings the JAKs closer. This allows the JAK proteins to phosphorylate and activate each other and subsequently phosphorylate specific sites on the receptor chains. This leads to STAT recruitment to the phosphorylated sites on the receptor, through specific interactions via their SH2 domains. The STATs themselves are phosphorylated and activated by the JAKs, which enables their dimerisation and translocation to the nucleus, where they induce the transcription of their target genes. These genes include the SOCS family members. In this manner, a negative feedback loop is established, as SOCS proteins will go on to attenuate cytokine signalling by targeting JAKs and STATs, as represented in Fig. 3
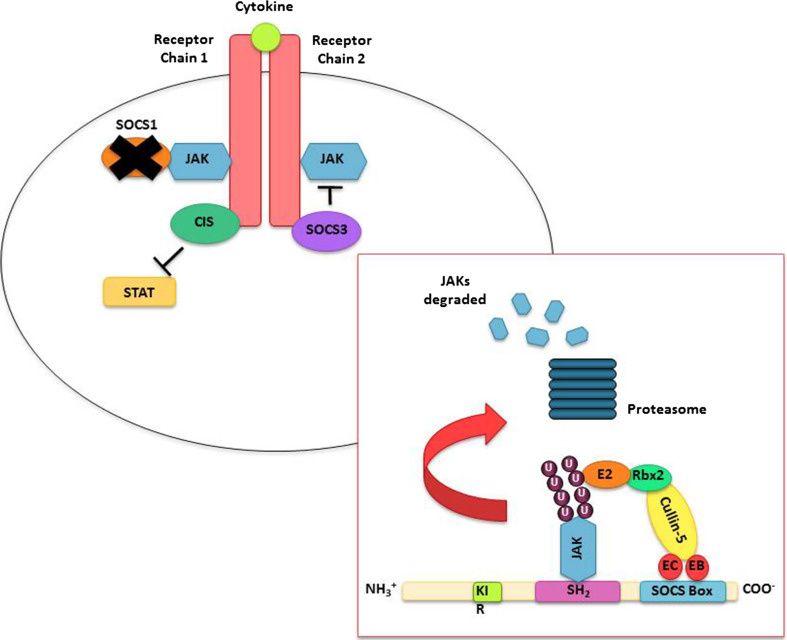
Fig. 3.
Mechanisms of SOCS-mediated inhibition of the JAK/STAT pathway; SOCS1 can inhibit the kinase activity of JAKs by directly binding to them, while it is thought that SOCS3 first binds to the receptor to hinder the activity of JAKs. It is believed that CIS also binds to the cytokine receptor chains, but in doing so obstructs the recruitment and therefore activation of the STAT proteins. SOCS proteins can also mediate the degradation of JAKs via the ubiquitin–proteasome system (box overlay). The highly conserved SOCS box domain directly interacts with Elongin B and C, two components of an E3 ligase complex, which then interact with Cullin-5 and RING-box 2 (Rbx2), as well as an E2 ubiquitin conjugating enzyme. The assembly of this complex allows the polyubiquitination of JAK proteins to occur, which labels them for degradation by the proteasome
SOCS3 signalling regulation
SOCS3 is a well characterised regulator of STAT3 activation in response to several cytokines, including those in the gp130-containing IL-6 receptor family [30–36], but has also been documented to inhibit STAT1 [37], STAT4 [38], STAT5 [39] and STAT6 [40]. Moreover, SOCS3’s broad regulation of several immune pathways is clearly demonstrated through its inhibition of IL-1-TRAF6 and TNF-α-TRAF2 signalling [29, 41], and its enhancement of FAK-mediated CCL11 signal transduction [28]. SOCS3’s important regulatory role during infection is evidenced by its rapid induction upon detection of TNF-α, IL-6 and several pathogen-associated molecular patterns (PAMPs), such as LPS and CPG-containing DNA [1, 42, 43]. While SOCS3 is mainly characterised for its role in negative feedback inhibition [44–46], silencing of SOCS3 decreases LPS-induced production of TNF-α and IL-6 in macrophages, revealing its “alternative” role in positively regulating TLR4-induced macrophage activation [47]. While these seemingly contradictory roles may be cell-type specific, they highlight the multi-functional and versatile effects of this molecule. Along with innate immune regulation, SOCS3 is an important regulator of adaptive immunity and plays a crucial role in T cell activation and polarisation. Differentiation of naive T-helper (Th) cells into the mature antigen-specific Th2 phenotype is associated with SOCS3 expression [48], with Egwuagu et al., finding 23-fold higher SOCS3 levels in Th2 cells, compared with CD4+ naive T cells [49]. In addition, SOCS3 is important for the onset and maintenance of Th2-mediated allergic immune disease, with SOCS3 transgenic mice displaying amplified Th2 responses and features characteristic of asthma [50]. Furthermore, SOCS3 inhibits Th1 differentiation via regulation of IL-12-induced STAT4 activation [48, 49, 51]. SOCS3 also plays a role in restricting Th17 cell generation, by inhibiting IL-23 signalling [52]. This SOCS3-mediated skewing towards Th2 differentiation has implications for asthma onset and development, with Veenbergen et al. showing that in SOCS3-transduced antigen-presenting cells (APCs), splenic CD3+ T cells had decreased antigen-specific proliferation and a significant reduction in IFN-γ (−43 %), IL-4 (−41 %), and IL-17 (−70 %) production [53]. In order to highlight these broad regulatory functions of SOCS3, Table 1 summarises several of its key roles.
Table 1.
The functions of SOCS3
| Role | Mechanism |
|---|---|
| Inhibits JAK/STAT signalling |
Binds to JAKs via SH2 domain and inhibits activity Competes for phosphorylation sites on cytokine receptors and inhibits STAT activation Ubiquitinates and degrades JAKs via SOCS box |
| Inhibits Ras/extracellular signal-regulated kinase (Ras/ERK) signalling |
Interacts with the Ras inhibitor; p120 RasGAP Maintains activation of ERK Ensures cell survival and proliferation |
| Inhibits phosphatidylinositide 3-kinases (PI3K) signalling | Prevents PI3K p85 activation |
| Inhibits focal adhesion kinase (FAK) signalling |
Interacts with FAK (Y397) via SH2 and KIR domains Inhibits kinase activity and phosphorylation of FAK Ubiquitinates and degrades FAK via SOCS box Inhibits cell motility on fibronectin |
| Inhibits NF-κB pathway | IL-1β-induced NF-κB-dependent pro-apoptotic early response genes are inhibited by SOCS-3, e.g. iNOS, ICAM, complement C3, Mob-1, MIP-1, CX3C, NF-κB-p105, IRF-1 and fibrinogen |
| T helper (Th) cell polarisation |
Highly expressed in Th2 cells Prevents differentiation into Th1 cells Restricts IL-17 induction |
Abnormal levels or dysfunction of SOCS3 have been linked to the onset and/or development of several human diseases, including rheumatoid arthritis (RA), hepatitis C virus (HCV) and Human Immunodeficiency Virus (HIV) infection, diabetes and cancer [29, 54–56]. Therefore, this review documents the role of SOCS3 in these disorders, with the aim of encouraging discussion around the therapeutic potential of SOCS3 and development of novel treatments.
SOCS3 and rheumatoid arthritis
RA is a common autoimmune disease characterised by chronic inflammation of multiple joints, resulting in mononuclear cell infiltration and progressive cartilage destruction [57–59]. The exact trigger for RA remains unknown, although pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6 and IL-17, have been shown to play an important role in its pathology [60–63]. Deregulation of TNF-α expression in transgenic mice is sufficient to cause chronic inflammatory polyarthritis [64]. Furthermore, blocking TNF-α with a monoclonal antibody or soluble receptor significantly improves the clinical status of patients [65–67]. IL-6 strongly signals via the JAK/STAT pathway and accumulating evidence suggests that STAT and SOCS proteins play important roles in RA pathogenesis [53, 54, 68]. In 1995, Wang et al. reported activated STAT3, but not STAT1, in cells isolated from the synovial fluid (SF) of patients with inflammatory arthritis [69, 70]. Moreover, the SF from RA patients has been shown to induce STAT3 activation in monocytes [71]; while hyper-activation of STAT3, as well as increased SOCS3, was reported in synovial tissue from an arthritis murine model [54, 61]. In 2001, Shouda et al., found that adenoviral delivery of SOCS3 or a dominant negative STAT3 in synovial tissue of mice with antigen-induced arthritis (AIA) and collagen-induced arthritis (CIA) significantly reduced the severity of arthritis and joint swelling, compared to control groups. SOCS3 expression suppressed bone destruction and reduced joint inflammation, which subsequently resulted in decreased IL-6 production. SOCS3 was found to be more effective than the dominant negative STAT3 in the CIA model, suggesting that SOCS3 induction in synovial cells could represent an effective therapeutic strategy for treating RA [61]. Additionally, high expression of SOCS3 in splenic APCs led to decreased production of IL-6 and TNF-α, but high production of the anti-inflammatory cytokine, IL-10. These altered splenic cellular responses were accompanied by a profound protective effect against the development of CIA [53]. Furthermore, deletion of SOCS3 in hematopoietic and endothelial cells was associated with severe IL-1-dependent inflammatory arthritis, characterised by a prominent neutrophil synovial infiltrate and increased bone destruction [58]. This absence of SOCS3 enhanced T lymphocyte and macrophage activation, resulting in upregulation of IL-17 and IL-6, respectively, most likely feeding uncontrolled, detrimental STAT3 signal transduction [58]. A mutation in the gp130 receptor chain (Y757F) of mice blocked SOCS3 binding and thus led to the development of a spontaneous RA-like phenotype associated with autoantibody production and T cell abnormalities with advanced age [72]. In addition, Van de Loo et al., showed that SOCS3 mRNA and protein are increased in human pathological chondrocytes, suggesting that SOCS3 dysregulates normal chondrocyte function, thereby playing a major role in the development of cartilage pathology observed in RA patients [54].
Together these findings reveal the significant role SOCS3 plays in RA progression and emphasise that signal inhibition of STATs, especially STAT3, by SOCS3 could be an effective strategy in the treatment of RA.
SOCS3 and diabetes
Diabetes is a significant metabolic disorder characterised by impaired insulin activity [73]. Pro-inflammatory cytokines, such as IL-6 and TNF-α, have been shown to play a critical role in insulin resistance and are associated with type 2 diabetes [74]. Several studies have shown that SOCS3 participates in the regulation of insulin signalling [75–78]. SOCS3 expression is elevated in the adipose tissue of insulin-resistant obese mice, while SOCS3 is induced transiently by insulin in the liver, muscle and white adipose tissue [75]. Even though insulin sensitivity was enhanced in the liver of hepatocyte-specific SOCS3-deficient mice, they exhibited obesity and systemic insulin resistance with age, suggesting that deletion of the SOCS3 gene in the liver can even modulate insulin sensitivity in other organs [76]. However, a separate study showed that in mice exposed to IL-6, increased hepatic SOCS3 inhibited both insulin receptor auto-phosphorylation and insulin receptor substrate 1 (IRS1) phosphorylation [79]. Furthermore, Jorgensen et al., reported that mice lacking SOCS3 in skeletal muscle were protected against the development of hyper-insulinemia and insulin resistance. This protection was thought to be mediated through increased glucose uptake as a result of enhanced IRS1 and protein kinase B (Akt) phosphorylation in the skeletal muscle [77]. In addition, overexpression of SOCS3 in adipocytes causes local adipocyte insulin resistance in mice. Shi et al., found that overexpression of SOCS3 in adipocytes decreased both total and phosphorylated IRS1 protein levels, limited p85 binding to IRS-1 and attenuated glucose uptake in adipocytes. This impaired insulin signalling in the adipose tissue of transgenic mice overexpressing SOCS3, decreased lipogenesis and blocked insulin’s anti-lipolytic activity [81]. SOCS3 was also shown to inhibit insulin action by binding to IRS1 and IRS2 and targeting them for proteasomal degradation [82].
These studies clearly demonstrate a crucial role for SOCS3 in regulating insulin signalling and highlight the significant impact clinical regulation of SOCS3 might have in therapeutically controlling insulin activity in patients with diabetes.
SOCS3 and viral infection
The interferon (IFN) response represents an early host defence mechanism against viral infection. Viruses evade immune responses using a variety of strategic interventions. DNA and RNA viruses often inhibit IFN-induced anti-viral responses by blocking the JAK/STAT pathway [83–86]. Induction of SOCS3 by viruses such as herpes simplex virus type 1 (HSV-1), HCV and HIV-1, suggests a key role for SOCS3 in suppressing anti-viral signal transduction [87–89].
HSV - 1 is estimated to infect ~3.7 billion people under 50 years of age (WHO, 2012). The virus rapidly induces SOCS3 expression via STAT3 activation, which consequently attenuates anti-viral IFN JAK/STAT signalling, thus enhancing HSV-1 replication [89, 90].
HCV infection represents another global health problem, with ~180 million of the world’s population currently infected. ~70–80 % of these patients develop chronic infection, with a risk for progressive liver fibrosis and hepatocellular carcinoma [91]. HCV core protein is thought to inhibit IFN-mediated STAT1 activation via increased SOCS3 expression, which may, at least in part, explain the lack of therapeutic responsiveness to IFN-α treatment [88]. In fact, hepatic SOCS3 expression is strongly associated with resistance to IFN-α therapy [87, 91]. Furthermore, Zhu et al., showed that IFN-α resistant HCV replicons produced higher levels of SOCS3 than their IFN-sensitive counterparts [92]. Recently, we reported that peripheral blood mononuclear cells from HCV-infected patients have elevated SOCS3 expression, compared to healthy controls, and that HCV overexpression in Huh7 hepatocytes induced SOCS3, which inhibited TNF-α signalling [29]. Interestingly, while Shao et al., also showed overexpression of SOCS3 inhibited IFN-induced STAT1 phosphorylation, it reduced HCV replication, suggesting that in this context, the anti-viral actions of SOCS3 are mediated through a JAK/STAT-independent pathway [93].
HIV is also a major health problem, infecting ~34 million individuals worldwide (WHO, 2013). SOCS1 and SOCS3 have been found to be increased upon HIV-1 infection and responsible for reduced IFN responsiveness and, in the case of SOCS1, regulation of HIV-1 Gag trafficking and assembly [94, 95]. IFN-β transiently suppresses viral replication within macrophages of the central nervous system (CNS) upon HIV-1 infection [96], but the virus overcomes this protective innate immune response via the induction of SOCS3, which inhibits IFN-β-mediated JAK/STAT signalling [96]. In contrast to these studies, Miller et al., reported that HIV-1 downregulates SOCS3 and SOCS1, which results in sustained activation of STAT proteins. The authors conclude that SOCS3- and SOCS1-mediated interference of HIV infection drives immune activation, thereby favouring HIV replication [97].
Influenza A virus triggers contagious acute respiratory disease that infects 5–10 % of the adult population each year (WHO, 2016) [98]. Overexpression of Influenza NS1 protein in HeLa cells upregulated SOCS1 and SOCS3 and inhibited STAT1-3 signalling, demonstrating an immune evasion strategy that ensures anti-viral responses to IFNs are blocked [99]. Pauli et al., also reported that Influenza A virus inhibited type I IFN signalling through induction of SOCS3, in an NF-κB-dependent manner. Additionally, SOCS3-deficient murine embryonic fibroblasts (MEFs) or SOCS3 knockdown cells showed sustained phosphorylation of STAT1, correlating with elevated expression of type I IFN-dependent genes and reduced viral titres [100].
RSV causes severe respiratory tract illness in infants and the elderly. RSV regulates IFN signalling via SOCS1, SOCS3 and CIS induction [101–103]. RSV also interferes with type I IFN signalling by mediating proteasomal degradation of STAT2, which demonstrates the broad anti-viral immune evasion strategies of this virus [104].
These findings collectively demonstrate the important role for SOCS3 in regulating type I IFN responses during viral infection and show how a number of viruses, including HSV-1, HCV, Influenza and RSV, all induce SOCS3 expression in several cell types. This immune evasion strategy has been shown to actively dampen host anti-viral responses, thus promoting the ability of the virus to replicate. Together these reports may suggest that, as with inflammatory disorders, therapeutic manipulation of SOCS3 expression could be a useful tool in restoring the anti-viral immune responses.
SOCS3 and bacterial infection
SOCS3 plays a critical role in restraining inflammation and, in doing so, generates optimal levels of protective immune responses against bacterial infection. Induction of SOCS3 by LPS indicates its important role in immune responses against bacteria [105], and has paved the way for analysis into SOCS3-induction via a plethora of specific bacterial species including Anaplasma phagocytophilum, the causative agent of tick-borne human granulocytic anaplasmosis (HGA) [106], Brucella species (B. melitensis, B. neotomae and B. ovis) [107], Lactobacillus rhamnosus GG and Streptococcus thermophilus [108].
Borrelia burgdorferi or its lipidated outer surface protein A (L-OspA) amplified IL-10-induced SOCS1 and SOCS3 mRNA and protein expression in murine J774 macrophages [109]; Helicobacter pylori in a Korean isolate (HP99), induced the expression of SOCS3 in rat gastric mucosal cells (RGM-1) [110]; Mycobacterium bovis Bacille Calmette-Guérin (M. bovis BCG) up-regulated and activated NOTCH1 signalling, leading to the expression of SOCS3 [111], all suggesting a conserved bacterial immune evasion strategy. Salmonella typhimurium increased TLR4-mediated SOCS3 expression in draining lymph nodes (DLNs) and blocked Smad3 (small mothers against decapentaplegic homolog-3)-mediated production of CCL21. The reduction in CCL21 is thought to disrupt lymph node architecture and cell trafficking and thus enhance S. typhimurium virulence [112].
There is also an increasing body of evidence implicating SOCS3 as having a crucial role in Mycobacterium tuberculosis (TB) infection and disease severity. Using DNA array and RT-PCR technology, Mistry et al., found elevated SOCS3 expression in whole blood from TB patients as well as patients with recurrent TB, when compared to healthy donors with latent M. tuberculosis infection (LTBIs) [113]. Given that SOCS3 overexpression in mice leads to immune polarisation, promoting generation of Th2 cells [51] and suppression of Th17 responses [52], the modulation of SOCS3 in T cells is an important immune polarising process during M. tuberculosis infection.
A later study by Nair et al., showed that the PPE18 protein of M. tuberculosis upregulates the expression, as well as tyrosine phosphorylation, of SOCS3, which directly leads to inhibition of LPS-induced IL-12 and TNF-α production by blocking nuclear translocation of p50, p65 NF-κB, and c-rel transcription factors [114]. A separate study found that SOCS3 expression in either lymphoid or myeloid cells generates resistance to M. tuberculosis via SOCS3’s regulation of IL-6/STAT3 signalling, which prevented IL-6-mediated inhibition of TNF and IL-12 secretion. In this way, SOCS3 contributed to IFN-γ expression in CD4+ T cells and attenuated the secretion of IL-17 by γδ T cells, in response to infection [115]. Further studies have demonstrated that SOCS3 expression correlates with severity of disease, with SOCS3 mRNA accumulation significantly reduced in advanced pulmonary TB, compared with endemic controls [116, 117].
This data highlights the broad spectrum of bacterial pathogens that harness our immune responses via SOCS3 and further identify it as a target to improve the control of infection, or enhance the efficiency of novel vaccination strategies against bacteria.
SOCS3 and cancer
SOCS3 exhibits clear tumour suppressor activity, which is thought to be mediated via both the JAK/STAT pathway and focal adhesion kinase (FAK) signalling [80, 118–124]. FAK is a ubiquitously expressed, non-receptor, protein tyrosine kinase that plays a crucial role in many cellular processes including cell survival, proliferation and motility [125–127]. SOCS3 binds to FAK, inhibits its kinase activity and induces its degradation via the proteasome, thereby effecting cell migration and tumour invasion [118]. Aberrant methylation in the promoter region of the SOCS3 gene frequently occurs in several types of human malignancy, and its transcriptional silencing is associated with malignant tumour behaviour [80, 128]. Decreased SOCS3 expression was found in adenocarcinoma human alveolar epithelial cells (A549), induced by SOCS3 methylation. Reactivation of SOCS3, using a demethylation agent, attenuated proline-rich tyrosine kinase 2 (PYK2) expression and phosphorylation, resulting in reduced cell migration [129]. In addition, SOCS3 methylation has been reported in hepatocellular carcinoma (HCC) cells, with restoration of SOCS3 via demethylation, leading to suppressed STAT3 phosphorylation and cell growth in HCC cells [121]. Constitutive activation (by tyrosine phosphorylation) of STAT3 and STAT5, both of which are SOCS3-regulated, has been connected to cancer development [130, 131]. STAT3 is a substrate for the breast tumour kinase (Brk), a tyrosine kinase expressed in breast carcinoma, which has been linked to tumour progression [132]. Knockdown of Brk in breast cancer cells (T47D and BT474), decreased the phosphorylation of STAT3 and inhibited T47D cell migration, indicating that blocking Brk activity could have a profound effect in treating breast cancer [132]. Recently, Gao et al., demonstrated that SOCS3 binds to Brk and inhibits its kinase activity. SOCS3 associates with Brk via its SH2 domain, but its main inhibitory effect is mediated by the KIR domain [133]. In addition, the C-terminal SOCS box domain of SOCS3 has a modest effect on promoting Brk degradation [133]. The authors reported that, as SOCS3 is the only known inhibitor of Brk, it is a potential therapeutic target for blocking Brk activity and inhibiting cancer progression [133]. In T47D breast cancer cells, SOCS3 also suppressed STAT3 expression and abrogated STAT5 phosphorylation, decreasing cell proliferation [134]. Overexpression of SOCS3 in the head and neck squamous cell carcinoma (HNSCC) cell line again inhibited proliferation, migration and invasion, clearly identifying SOCS3 as an effective tumour suppressor gene [135].
Th17 cells produce pro-inflammatory mediators such as IL-17A, IL-17F, IL-21, and TNF-α, but their overproduction is linked to autoimmunity and cancer [136, 137]. The fish oil, docosahexaenoic acid (DHA), is suggested to be an effective adjuvant for anti-cancer drugs, with several intracellular targets, including NF-κB and peroxisome proliferator-activated receptor gamma (PPARγ) [138]. Interestingly, DHA reduces STAT3 phosphorylation, thereby interfering with Th17 cell differentiation. This effect was associated with DHA-induced SOCS3 expression, in a PPARγ-dependent manner. Silencing of SOCS3 in T cells blunted the capacity of DHA to restrain IL-17 expression. In addition, DHA prevented tumour outgrowth in an IL-17-dependent manner, as measured by cell viability assay [139]. Leptin JAK/STAT signalling is involved in gastric cancer [140]. Murine gastrointestinal epithelial cells, containing a SOCS3 deletion, developed gastric tumours, with mice demonstrating an increase in the cell damage-, cell cycle- and apoptosis-related molecules, p53, p21 and Bcl-xL. Furthermore, enhanced STAT3 phosphorylation, induced by deletion of SOCS3, led to increased leptin production, possibly acting through the zinc finger transcription factor, specificity protein 1 (Sp1). These SOCS3-deficient mice developed tumours in the stomach within 2 months and died within 6 months, demonstrating SOCS3’s role in regulating this tumourigenic pathway [140]. Sp1 and STAT3 regulate both distinct and overlapping groups of genes during tumourigenesis. These two transcription factors function in cooperation to activate target genes in cancer progression [141, 142].
While SOCS3 can inhibit activation of STAT3 of several cytokine receptors, including gp130 [143, 144], granulocyte-colony stimulating factor receptor [145], leptin receptor [146] and IL-12Rβ [38], it does not inhibit STAT3 activation in the IL-10 receptor pathway, suggesting a broad regulatory capacity for IL-10-induced SOCS3 [46, 147].
Altogether, these data explicitly illustrate the anti-tumour activity of SOCS3, which acts by limiting the production of a plethora of genes involved in cell survival, proliferation and motility, culminating in limited tumour growth. Indeed, new immune-related anti-cancer therapies may benefit by exploring SOCS3 as a potential target for treatment of specific malignancies.
Therapeutic implications of SOCS3
The broad regulatory properties of SOCS3 and its direct involvement in inflammatory disorders, diabetes, cancer and both bacterial and viral infection, highlights it as a strong therapeutic target. On the other hand, SOCS3 is induced by a number of cytokines with both pro- and anti-inflammatory functions, including IL-6 [46] and IL-10 [148], respectively. Furthermore, SOCS3 differentially regulates inflammation depending on the cell type, presenting obvious therapeutic challenges that must be addressed through specific cell targeting [8, 149]. For this reason, the transient nature of SOCS3 expression is being addressed to ensure therapeutic effectiveness [150]. For example, cell-penetrating (CP) forms of SOCS3 have been established in cell cultures and mice, effectively blocking signal transduction and protecting against inflammation and organ failure during bacterial challenge [151]. Also, overexpression of SOCS3, using a recombinant adenovirus cDNA, reduced inflammatory RA development in mice [61], further demonstrating the therapeutic potential of SOCS3.
Interestingly, HCV upregulates SOCS3 in both hepatocytes [93] and immune cells [29], and since SOCS3 regulates the IFN-α pathway, it makes its suppression an obvious target for enhancing the response to therapeutic IFN-α. However, as with artificial induction of SOCS3, its reduction may also be challenging. MicroRNAs are increasingly being identified as regulators of SOCS expression [152]. This is demonstrated in the repression of microRNA-122, which inhibits SOCS3 expression (via enhanced promoter methylation) and was postulated as a promising alternative to treat HCV [153–155]. We have also shown miR19a expression to suppress SOCS3, both at the mRNA and protein level [156], identifying another possible method of silencing SOCS3.
By suppressing the tumour-promoting activity of STATs, SOCS3 could also be a useful tool in the treatment of cancer; in fact, SOCS3 overexpression has already been shown to inhibit growth of non-small lung cancer cells and adenoviral transfer of SOCS3 enhanced the radio-sensitivity of non-small lung cancer cells [157]. Infection of liver tumour cells with oncolytic adenovirus CN305 (AdCN305)-SOCS3 and AdCN305-cell-penetrating peptides-SOCS3 resulted in dramatic cytotoxicity of liver tumour cells. However, the cytotoxic effects were not observed in normal cells infected with these vectors. Infection of liver tumour cells with AdCN305-SOCS3 and AdCN305-cpp-SOCS3 resulted in almost complete inhibition of STAT3 phosphorylation, demonstrating that the transfer of SOCS3 via an oncolytic adenovirus represents a useful approach to be explored further in the treatment of cancer [158, 159]. Furthermore, SOCS3 overexpression suppressed the growth of the malignant fibrous histiocytoma (MFH) cell line by inhibiting STAT3 and IL-6 production, adding to the growing body of evidence pointing towards SOCS3 as an effective tumour suppressor [160]. Upregulation of SOCS3 by platelet factor 4 (PF4) may also be a therapeutic target for cancer. PF4 is an angiostatic chemokine that suppresses tumour growth and metastasis [161]. Recently, PF4 was found to induce SOCS3, thereby inhibiting STAT3 activation, angiogenesis, growth and induced apoptosis in myeloma cells [161]. Silencing of SOCS3 abolished PF4's ability to inhibit STAT3 activation, suggesting a critical role of SOCS3 in PF4-induced STAT3 inhibition and indicating that PF4 may be a potential new targeting agent for the treatment of myeloma [161].
Expression of SOCS3 is also closely associated with the severity of allergic asthma and dermatitis, making it a target for therapeutic intervention in allergic disease [50, 162]. Heterozygous deletion of SOCS3 and overexpression of a dominant negative form of SOCS3 were both proven to be effective in prevention of early and late-phase responses of allergic conjunctivitis, a common allergic eye disease [162]. However, another study showed that defective SOCS3 expression causes inflammatory skin disease [163]. Keratinocyte-specific deletion of SOCS3 caused severe skin inflammation, with inflamed skin showing constitutive STAT3 activation and upregulation of IL-6 [163]. This SOCS3-mediated homeostatic function in skin inflammation is supported by other reports showing that a specific microRNA, miR203, is highly expressed in human psoriatic skin and inhibits the expression of SOCS3 [164]. Together, these studies confirm the therapeutic potential of SOCS3 in development, diagnoses and treatment of human disorders; thus manipulation of SOCS3 could be a novel therapeutic approach and methods of artificially regulating its expression may be a solution for many diseases.
Conclusion
The discovery of SOCS proteins has provided new insight into cytokine regulation and immune responses. SOCS3 plays a significant role in regulating different signal transduction pathways, with the classical JAK/STAT regulation being the target of its effects. SOCS3 expression is shown to be associated with many inflammatory, immunological, infectious and oncogenic disorders. The vast body of evidence which has accumulated over the past two decades indicates that the regulation of SOCS3 expression may be a powerful therapeutic tool to treat various human diseases such as RA, pathogenic infection, diabetes and cancer. The role of SOCS3 in several signalling pathways is becoming evident and, therefore, further investigation into its regulation of cascades, such as MAPK and NF-κB, may reveal even more intricate roles for SOCS3 in human disease. However, it is already clear that regulatory peptides, overexpression constructs or microRNA may be useful tools in enhancing or suppressing SOCS3 levels and could represent new and exciting approaches in treating disease.
References
- 1.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 2.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 3.Darnell J, Kerr I, Stark G. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 4.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 5.O’Shea JJ, Notarangelo LD, Johnston JA, Candotti F. Advances in the understanding of cytokine signal transduction: the role of Jaks and STATs in immunoregulation and the pathogenesis of immunodeficiency. J Clin Immunol. 1997;17:431–447. doi: 10.1023/A:1027388508570. [DOI] [PubMed] [Google Scholar]
- 6.Nicola NA, Greenhalgh CJ. The suppressors of cytokine signaling (SOCS) proteins: important feedback inhibitors of cytokine action. Exp Hematol. 2000;28:1105–1112. doi: 10.1016/S0301-472X(00)00525-7. [DOI] [PubMed] [Google Scholar]
- 7.Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–235. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piessevaux J, Lavens D, Peelman F, Tavernier J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008;19:371–381. doi: 10.1016/j.cytogfr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- 15.Sass G, Shembade ND, Tiegs G. Tumour necrosis factor alpha (TNF)-TNF receptor 1-inducible cytoprotective proteins in the mouse liver: relevance of suppressors of cytokine signalling. Biochem J. 2005;385:537–544. doi: 10.1042/BJ20040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson NJ, Haan S, McClurg AE, McGrattan MJ, Armstrong MA, Heinrich PC, Johnston JA. The chemoattractants, IL-8 and formyl-methionyl-leucyl-phenylalanine, regulate granulocyte colony-stimulating factor signaling by inducing suppressor of cytokine signaling-1 expression. J Immunol. 2004;173:3243–3249. doi: 10.4049/jimmunol.173.5.3243. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson NJ, Addley MR, Ryan EJ, Boyd CR, Carroll HP, Paunovic V, Bursill CA, Miller HC, Channon KM, McClurg AE, Armstrong MA, Coulter WA, Greaves DR, Johnston JA. CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic cells and hinders dendritic cell differentiation via suppressor of cytokine signaling expression. J Leukoc Biol. 2009;85:289–297. doi: 10.1189/jlb.0708394. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh CJ, Alexander WS. Suppressors of cytokine signalling and regulation of growth hormone action. Growth Horm IGF Res. 2004;14:200–206. doi: 10.1016/j.ghir.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, Lucet IS, Norton RS, Nicola NA. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–250. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babon JJ, Nicola NA. The biology and mechanism of action of suppressor of cytokine signaling 3. Growth Factors. 2012;30:207–219. doi: 10.3109/08977194.2012.687375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linossi EM, Nicholson SE. The SOCS box-adapting proteins for ubiquitination and proteasomal degradation. IUBMB Life. 2012;64:316–323. doi: 10.1002/iub.1011. [DOI] [PubMed] [Google Scholar]
- 22.Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr, Conaway RC, Conaway JW. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SH, Kim KE, Hwang HY, Kim TY. Regulatory effect of SOCS on NF-kappaB activity in murine monocytes/macrophages. DNA Cell Biol. 2003;22:131–139. doi: 10.1089/104454903321515931. [DOI] [PubMed] [Google Scholar]
- 24.Babon JJ, Sabo JK, Zhang J-G, Nicola NA, Norton RS. The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. J Mol Biol. 2009;387:162–174. doi: 10.1016/j.jmb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. SOCS, inflammation, and autoimmunity. Front Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madonna S, Scarponi C, De Pita O, Albanesi C. Suppressor of cytokine signaling 1 inhibits IFN-gamma inflammatory signaling in human keratinocytes by sustaining ERK1/2 activation. FASEB J. 2008;22:3287–3297. doi: 10.1096/fj.08-106831. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Fukuyama S, Yoshida R, Kobayashi T, Saeki K, Shiraishi H, Yoshimura A, Takaesu G. Loss of SOCS3 gene expression converts STAT3 function from anti-apoptotic to pro-apoptotic. J Biol Chem. 2006;281:36683–36690. doi: 10.1074/jbc.M607374200. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson NJ, McFarlane C, Ong ST, Nahlik K, Kelvin A, Addley MR, Long A, Greaves DR, O’Farrelly C, Johnston JA. Suppressor of cytokine signalling (SOCS) 1 and 3 enhance cell adhesion and inhibit migration towards the chemokine eotaxin/CCL11. FEBS Lett. 2010;584:4469–4474. doi: 10.1016/j.febslet.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Collins AS, Ahmed S, Napoletano S, Schroeder M, Johnston JA, Hegarty JE, O’Farrelly C, Stevenson NJ. Hepatitis C virus (HCV)-induced suppressor of cytokine signaling (SOCS) 3 regulates proinflammatory TNF-alpha responses. J Leukoc Biol. 2014;96:255–263. doi: 10.1189/jlb.2A1211-608RRRR. [DOI] [PubMed] [Google Scholar]
- 30.Auernhammer CJ, Chesnokova V, Bousquet C, Melmed S. Pituitary corticotroph SOCS-3: novel intracellular regulation of leukemia-inhibitory factor-mediated proopiomelanocortin gene expression and adrenocorticotropin secretion. Mol Endocrinol. 1998;12:954–961. doi: 10.1210/mend.12.7.0140. [DOI] [PubMed] [Google Scholar]
- 31.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 32.Demoulin JB, Van Snick J, Renauld JC. Interleukin-9 (IL-9) induces cell growth arrest associated with sustained signal transducer and activator of transcription activation in lymphoma cells overexpressing the IL-9 receptor. Cell Growth Differ. 2001;12:169–174. [PubMed] [Google Scholar]
- 33.Bode JG, Ludwig S, Freitas CA, Schaper F, Ruhl M, Melmed S, Heinrich PC, Haussinger D. The MKK6/p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biol Chem. 2001;382:1447–1453. doi: 10.1515/BC.2001.178. [DOI] [PubMed] [Google Scholar]
- 34.Ronn SG, Hansen JA, Lindberg K, Karlsen AE, Billestrup N. The effect of suppressor of cytokine signaling 3 on GH signaling in beta-cells. Mol Endocrinol. 2002;16:2124–2134. doi: 10.1210/me.2002-0082. [DOI] [PubMed] [Google Scholar]
- 35.Denson LA, Held MA, Menon RK, Frank SJ, Parlow AF, Arnold DL. Interleukin-6 inhibits hepatic growth hormone signaling via upregulation of Cis and Socs-3. Am J Physiol Gastrointest Liver Physiol. 2003;284:2. doi: 10.1152/ajpgi.00178.2002. [DOI] [PubMed] [Google Scholar]
- 36.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 37.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Yamaguchi M, Miyasaka N, Miura O. SOCS-3 inhibits IL-12-induced STAT4 activation by binding through its SH2 domain to the STAT4 docking site in the IL-12 receptor beta2 subunit. Biochem Biophys Res Commun. 2003;310:1188–1193. doi: 10.1016/j.bbrc.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 39.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, Johnston JA. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980–4988. doi: 10.1128/MCB.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebenstreit D, Luft P, Schmiedlechner A, Duschl A, Horejs-Hoeck J. SOCS-1 and SOCS-3 inhibit IL-4 and IL-13 induced activation of Eotaxin-3/CCL26 gene expression in HEK293 cells. Mol Immunol. 2005;42:295–303. doi: 10.1016/j.molimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Frobose H, Ronn SG, Heding PE, Mendoza H, Cohen P, Mandrup-Poulsen T, Billestrup N. Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol Endocrinol. 2006;20:1587–1596. doi: 10.1210/me.2005-0301. [DOI] [PubMed] [Google Scholar]
- 42.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 43.Ramgolam VS, Markovic-Plese S. Regulation of suppressors of cytokine signaling as a therapeutic approach in autoimmune diseases, with an emphasis on multiple sclerosis. J Signal Transduct. 2011;2011:635721. doi: 10.1155/2011/635721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, Murray PJ, Hunter CA. A critical role for SOCS3 in innate resistance to Toxoplasma gondii . Cell Host Microbe. 2011;10:224–236. doi: 10.1016/j.chom.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan C, Ward PA, Wang X, Gao H. Myeloid depletion of SOCS3 enhances LPS-induced acute lung injury through CCAAT/enhancer binding protein delta pathway. FASEB J. 2013 doi: 10.1096/fj.12-225797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Zhang Y, Yu Y, Yang X, Cao X. SOCS3 promotes TLR4 response in macrophages by feedback inhibiting TGF-beta1/Smad3 signaling. Mol Immunol. 2008;45:1405–1413. doi: 10.1016/j.molimm.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–29759. doi: 10.1074/jbc.M300489200. [DOI] [PubMed] [Google Scholar]
- 49.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 50.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, Cacalano N, O’Garra A, Oshida T, Saito H, Johnston JA, Yoshimura A, Kubo M. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 51.Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021–1031. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veenbergen S, Bennink MB, de Hooge AS, Arntz OJ, Smeets RL, van den Berg WB, van de Loo FA. Splenic suppressor of cytokine signaling 3 transgene expression affects T cell responses and prevents development of collagen-induced arthritis. Arthritis Rheum. 2008;58:3742–3752. doi: 10.1002/art.24072. [DOI] [PubMed] [Google Scholar]
- 54.van de Loo FA, Veenbergen S, van den Brand B, Bennink MB, Blaney-Davidson E, Arntz OJ, van Beuningen HM, van der Kraan PM, van den Berg WB. Enhanced suppressor of cytokine signaling 3 in arthritic cartilage dysregulates human chondrocyte function. Arthritis Rheum. 2012;64:3313–3323. doi: 10.1002/art.34529. [DOI] [PubMed] [Google Scholar]
- 55.Akhtar LN, Benveniste EN. Viral exploitation of host SOCS protein functions. J Virol. 2011;85:1912–1921. doi: 10.1128/JVI.01857-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu WP, Li WD. SOCS3: a potential therapeutic target for many human diseases. Yao Xue Xue Bao. 2011;46:747–752. [PubMed] [Google Scholar]
- 57.Fujii K, Tsuji M, Tajima M. Rheumatoid arthritis: a synovial disease? Ann Rheum Dis. 1999;58:727–730. doi: 10.1136/ard.58.12.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, Wicks IP. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 2006;116:1571–1581. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 60.Paradowska A, Masliniski W, Grzybowska-Kowalczyk A, Lacki J. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2007;55:329–334. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 61.Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 63.Tsao JT, Kuo CC, Lin SC. The analysis of CIS, SOCS1, SOSC2 and SOCS3 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Clin Exp Med. 2008;8:179–185. doi: 10.1007/s10238-008-0006-0. [DOI] [PubMed] [Google Scholar]
- 64.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 66.Rottapel R. Putting the brakes on arthritis: can suppressors of cytokine signaling (SOCS) suppress rheumatoid arthritis? J Clin Invest. 2001;108:1745–1747. doi: 10.1172/JCI200114661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S, Koopman WJ, Mohler K, Widmer MB, Blosch CM. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 68.Wiland P, Sokalska-Jurkiewicz M, Madej M. The role of Jak/STAT signaling in rheumatoid arthritis. Adv Clin Exp Med. 2008;17:447–452. [Google Scholar]
- 69.Wang F, Sengupta TK, Zhong Z, Ivashkiv LB. Regulation of the balance of cytokine production and the signal transducer and activator of transcription (STAT) transcription factor activity by cytokines and inflammatory synovial fluids. J Exp Med. 1995;182:1825–1831. doi: 10.1084/jem.182.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32:1650–1653. [PubMed] [Google Scholar]
- 71.de Hooge AS, van de Loo FA, Koenders MI, Bennink MB, Arntz OJ, Kolbe T, van den Berg WB. Local activation of STAT-1 and STAT-3 in the inflamed synovium during zymosan-induced arthritis: exacerbation of joint inflammation in STAT-1 gene-knockout mice. Arthritis Rheum. 2004;50:2014–2023. doi: 10.1002/art.20302. [DOI] [PubMed] [Google Scholar]
- 72.Atsumi T, Ishihara K, Kamimura D, Ikushima H, Ohtani T, Hirota S, Kobayashi H, Park SJ, Saeki Y, Kitamura Y, Hirano T. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–990. doi: 10.1084/jem.20020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53:2232–2241. doi: 10.2337/diabetes.53.9.2232. [DOI] [PubMed] [Google Scholar]
- 74.Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012;57:136–142. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M008700200. [DOI] [PubMed] [Google Scholar]
- 76.Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells. 2007;12:143–154. doi: 10.1111/j.1365-2443.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 77.Jorgensen SB, O’Neill HM, Sylow L, Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Oberg L, Balendran A, Galic S, van der Poel C, Trounce IA, Lynch GS, Schertzer JD, Steinberg GR. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes. 2013;62:56–64. doi: 10.2337/db12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Z, Hulver M, McMillan RP, Cai L, Kershaw EE, Yu L, Xue B, Shi H. Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3) PLoS One. 2012;7:e47493. doi: 10.1371/journal.pone.0047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 80.Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, Masuda T. Methylation status of the SOCS3 gene in human malignant melanomas. Int J Oncol. 2007;30:689–694. [PubMed] [Google Scholar]
- 81.Shi H, Cave B, Inouye K, Bjorbaek C, Flier JS. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes. 2006;55:699–707. doi: 10.2337/diabetes.55.03.06.db05-0841. [DOI] [PubMed] [Google Scholar]
- 82.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Sastre A. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology. 2001;279:375–384. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- 84.Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunol Today. 2000;21:447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith GL, Benfield CT, Maluquer de Motes C, Mazzon M, Ember SW, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94:2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 86.Stevenson NJ, Bourke NM, Ryan EJ, Binder M, Fanning L, Johnston JA, Hegarty JE, Long A, O’Farrelly C. Hepatitis C virus targets the interferon-alpha JAK/STAT pathway by promoting proteasomal degradation in immune cells and hepatocytes. FEBS Lett. 2013;587:1571–1578. doi: 10.1016/j.febslet.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y, Feld JJ, Sapp RK, Nanda S, Lin JH, Blatt LM, Fried MW, Murthy K, Liang TJ. Defective hepatic response to interferon and activation of suppressor of cytokine signaling 3 in chronic hepatitis C. Gastroenterology. 2007;132:733–744. doi: 10.1053/j.gastro.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Haussinger D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 89.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology. 2005;338:173–181. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 90.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim KA, Lin W, Tai AW, Shao RX, Weinberg E, De Sa Borges CB, Bhan AK, Zheng H, Kamegaya Y, Chung RT. Hepatic SOCS3 expression is strongly associated with non-response to therapy and race in HCV and HCV/HIV infection. J Hepatol. 2009;50:705–711. doi: 10.1016/j.jhep.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu H, Nelson DR, Crawford JM, Liu C. Defective Jak-Stat activation in hepatoma cells is associated with hepatitis C viral IFN-α resistance. J Interferon Cytokine Res. 2005;25:528–539. doi: 10.1089/jir.2005.25.528. [DOI] [PubMed] [Google Scholar]
- 93.Shao RX, Zhang L, Peng LF, Sun E, Chung WJ, Jang JY, Tsai WL, Hyppolite G, Chung RT. Suppressor of cytokine signaling 3 suppresses hepatitis C virus replication in an mTOR-dependent manner. J Virol. 2010;84:6060–6069. doi: 10.1128/JVI.02484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moutsopoulos NM, Vazquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol. 2006;80:1145–1155. doi: 10.1189/jlb.0306142. [DOI] [PubMed] [Google Scholar]
- 95.Ryo A, Tsurutani N, Ohba K, Kimura R, Komano J, Nishi M, Soeda H, Hattori S, Perrem K, Yamamoto M, Chiba J, Mimaya J, Yoshimura K, Matsushita S, Honda M, Yoshimura A, Sawasaki T, Aoki I, Morikawa Y, Yamamoto N. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc Natl Acad Sci USA. 2008;105:294–299. doi: 10.1073/pnas.0704831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, Clements JE, Benveniste EN. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J Immunol. 2010;185:2393–2404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller RC, Schlaepfer E, Baenziger S, Crameri R, Zeller S, Byland R, Audige A, Nadal D, Speck RF. HIV interferes with SOCS-1 and -3 expression levels driving immune activation. Eur J Immunol. 2011;41:1058–1069. doi: 10.1002/eji.201041198. [DOI] [PubMed] [Google Scholar]
- 98.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 99.Jia D, Rahbar R, Chan RW, Lee SM, Chan MC, Wang BX, Baker DP, Sun B, Peiris JS, Nicholls JM, Fish EN. Influenza virus non-structural protein 1 (NS1) disrupts interferon signaling. PLoS One. 2010;5:e13927. doi: 10.1371/journal.pone.0013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, Ludwig S. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashimoto K, Ishibashi K, Ishioka K, Zhao D, Sato M, Ohara S, Abe Y, Kawasaki Y, Sato Y, Yokota S, Fujii N, Peebles RS, Jr, Hosoya M, Suzutani T. RSV replication is attenuated by counteracting expression of the suppressor of cytokine signaling (SOCS) molecules. Virology. 2009;391:162–170. doi: 10.1016/j.virol.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 102.Moore EC, Barber J, Tripp RA. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15) Virol J. 2008;5:116. doi: 10.1186/1743-422X-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol. 2009;22:147–161. doi: 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- 104.Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M, Power UF, Johnston JA. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol. 2007;81:3428–3436. doi: 10.1128/JVI.02303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- 106.Bussmeyer U, Sarkar A, Broszat K, Ludemann T, Moller S, van Zandbergen G, Bogdan C, Behnen M, Dumler JS, von Loewenich FD, Solbach W, Laskay T. Impairment of gamma interferon signaling in human neutrophils infected with Anaplasma phagocytophilum. Infect Immun. 2010;78:358–363. doi: 10.1128/IAI.01005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Covert J, Mathison AJ, Eskra L, Banai M, Splitter G. Brucella melitensis, B. neotomae and B. ovis elicit common and distinctive macrophage defense transcriptional responses. Exp Biol Med. 2009;234:1450–1467. doi: 10.3181/0904-RM-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Latvala S, Miettinen M, Kekkonen RA, Korpela R, Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin Exp Immunol. 2011;165:94–103. doi: 10.1111/j.1365-2249.2011.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dennis VA, Jefferson A, Singh SR, Ganapamo F, Philipp MT. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect Immun. 2006;74:5780–5789. doi: 10.1128/IAI.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cha B, Kim KH, Matsui H, Kim H. Expression of suppressors of cytokine signaling-3 in Helicobacter pylori-infected rat gastric mucosal RGM-1cells. Ann N Y Acad Sci. 2007;1096:24–28. doi: 10.1196/annals.1397.066. [DOI] [PubMed] [Google Scholar]
- 111.Narayana Y, Balaji KN. NOTCH1 up-regulation and signaling involved in Mycobacterium bovis BCG-induced SOCS3 expression in macrophages. J Biol Chem. 2008;283:12501–12511. doi: 10.1074/jbc.M709960200. [DOI] [PubMed] [Google Scholar]
- 112.St John AL, Abraham SN. Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med. 2009;15:1259–1265. doi: 10.1038/nm.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mistry R, Cliff JM, Clayton CL, Beyers N, Mohamed YS, Wilson PA, Dockrell HM, Wallace DM, van Helden PD, Duncan K, Lukey PT. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis. 2007;195:357–365. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 114.Nair S, Pandey AD, Mukhopadhyay S. The PPE18 protein of Mycobacterium tuberculosis inhibits NF-kappaB/rel-mediated proinflammatory cytokine production by upregulating and phosphorylating suppressor of cytokine signaling 3 protein. J Immunol. 2011;186:5413–5424. doi: 10.4049/jimmunol.1000773. [DOI] [PubMed] [Google Scholar]
- 115.Carow B, Reuschl AK, Gavier-Widen D, Jenkins BJ, Ernst M, Yoshimura A, Chambers BJ, Rottenberg ME. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis . PLoS Pathog. 2013;9:4. doi: 10.1371/journal.ppat.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B, Islam M, Hussain R, Hasan Z. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC Infect Dis. 2013;13:1471–2334. doi: 10.1186/1471-2334-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis . Semin Immunol. 2014;26:518–532. doi: 10.1016/j.smim.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Liu E, Cote JF, Vuori K. Negative regulation of FAK signaling by SOCS proteins. EMBO J. 2003;22:5036–5046. doi: 10.1093/emboj/cdg503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C, Tannapfel A. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24:6699–6708. doi: 10.1038/sj.onc.1208818. [DOI] [PubMed] [Google Scholar]
- 121.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 122.Ying M, Li D, Yang L, Wang M, Wang N, Chen Y, He M, Wang Y. Loss of SOCS3 expression is associated with an increased risk of recurrent disease in breast carcinoma. J Cancer Res Clin Oncol. 2010;136:1617–1626. doi: 10.1007/s00432-010-0819-6. [DOI] [PubMed] [Google Scholar]
- 123.Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011;122:241–251. doi: 10.1007/s00401-011-0832-0. [DOI] [PubMed] [Google Scholar]
- 124.Iwahori K, Serada S, Fujimoto M, Nomura S, Osaki T, Lee CM, Mizuguchi H, Takahashi T, Ripley B, Okumura M, Kawase I, Kishimoto T, Naka T. Overexpression of SOCS3 exhibits preclinical antitumor activity against malignant pleural mesothelioma. Int J Cancer. 2011;129:1005–1017. doi: 10.1002/ijc.25716. [DOI] [PubMed] [Google Scholar]
- 125.Villa-Moruzzi E. Tyrosine phosphatases in the HER2-directed motility of ovarian cancer cells: involvement of PTPN12, ERK5 and FAK. Anal Cell Pathol. 2011;34:101–112. doi: 10.1155/2011/870459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geng D, Zhao W, Feng Y, Liu J. Overexpression of Rab5a promotes hepatocellular carcinoma cell proliferation and invasion via FAK signaling pathway. Tumour Biol. 2015;6:6. doi: 10.1007/s13277-015-4124-5. [DOI] [PubMed] [Google Scholar]
- 127.Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K, Kuwano H. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89:140–145. doi: 10.1038/sj.bjc.6601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hiwatashi K, Tamiya T, Hasegawa E, Fukaya T, Hashimoto M, Kakoi K, Kashiwagi I, Kimura A, Inoue N, Morita R, Yasukawa H, Yoshimura A. Suppression of SOCS3 in macrophages prevents cancer metastasis by modifying macrophage phase and MCP2/CCL8 induction. Cancer Lett. 2011;308:172–180. doi: 10.1016/j.canlet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 129.Zhang S, Guo D, Jiang L, Zhang Q, Qiu X, Wang E. SOCS3 inhibiting migration of A549 cells correlates with PYK2 signaling in vitro. BMC Cancer. 2008;8:150. doi: 10.1186/1471-2407-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 131.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ludyga N, Anastasov N, Gonzalez-Vasconcellos I, Ram M, Hofler H, Aubele M. Impact of protein tyrosine kinase 6 (PTK6) on human epidermal growth factor receptor (HER) signalling in breast cancer. Mol BioSyst. 2011;7:1603–1612. doi: 10.1039/c0mb00286k. [DOI] [PubMed] [Google Scholar]
- 133.Gao Y, Cimica V, Reich NC. Suppressor of cytokine signaling 3 inhibits breast tumor kinase activation of STAT3. J Biol Chem. 2012;287:20904–20912. doi: 10.1074/jbc.M111.334144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. SOCS3 as a tumor suppressor in breast cancer cells, and its regulation by PRL. Int J Cancer. 2009;124:1756–1766. doi: 10.1002/ijc.24172. [DOI] [PubMed] [Google Scholar]
- 135.Rossa C, Jr, Sommer G, Spolidorio LC, Rosenzweig SA, Watson DK, Kirkwood KL. Loss of expression and function of SOCS3 is an early event in HNSCC: altered subcellular localization as a possible mechanism involved in proliferation, migration and invasion. PLoS One. 2012;7:e45197. doi: 10.1371/journal.pone.0045197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 137.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. BioFactors. 2011;37:399–412. doi: 10.1002/biof.181. [DOI] [PubMed] [Google Scholar]
- 139.Berger H, Vegran F, Chikh M, Gilardi F, Ladoire S, Bugaut H, Mignot G, Chalmin F, Bruchard M, Derangere V, Chevriaux A, Rebe C, Ryffel B, Pot C, Hichami A, Desvergne B, Ghiringhelli F, Apetoh L. SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4018. [DOI] [PubMed] [Google Scholar]
- 140.Inagaki-Ohara K, Mayuzumi H, Kato S, Minokoshi Y, Otsubo T, Kawamura YI, Dohi T, Matsuzaki G, Yoshimura A. Enhancement of leptin receptor signaling by SOCS3 deficiency induces development of gastric tumors in mice. Oncogene. 2012 doi: 10.1038/onc.2012.540. [DOI] [PubMed] [Google Scholar]
- 141.Gibadulinova A, Oveckova I, Parkkila S, Pastorekova S, Pastorek J. Key promoter elements involved in transcriptional activation of the cancer-related gene coding for S100P calcium-binding protein. Oncol Rep. 2008;20:391–396. [PubMed] [Google Scholar]
- 142.Cantwell CA, Sterneck E, Johnson PF. Interleukin-6-specific activation of the C/EBPdelta gene in hepatocytes is mediated by Stat3 and Sp1. Mol Cell Biol. 1998;18:2108–2117. doi: 10.1128/MCB.18.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem. 2000;275:12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 144.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, Metcalf D, Hilton DJ, Nicola NA, Baca M. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hortner M, Nielsch U, Mayr LM, Johnston JA, Heinrich PC, Haan S. Suppressor of cytokine signaling-3 is recruited to the activated granulocyte-colony stimulating factor receptor and modulates its signal transduction. J Immunol. 2002;169:1219–1227. doi: 10.4049/jimmunol.169.3.1219. [DOI] [PubMed] [Google Scholar]
- 146.De Souza D, Fabri LJ, Nash A, Hilton DJ, Nicola NA, Baca M. SH2 domains from suppressor of cytokine signaling-3 and protein tyrosine phosphatase SHP-2 have similar binding specificities. Biochemistry. 2002;41:9229–9236. doi: 10.1021/bi0259507. [DOI] [PubMed] [Google Scholar]
- 147.El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, Smith AM, Rutschman R, Kaushal D, Shen Y, Suda T, Donnelly RP, Myers MG, Jr, Alexander W, Vignali DA, Watowich SS, Ernst M, Hilton DJ, Murray PJ. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880–7888. doi: 10.4049/jimmunol.177.11.7880. [DOI] [PubMed] [Google Scholar]
- 148.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 149.Matsumura Y, Kobayashi T, Ichiyama K, Yoshida R, Hashimoto M, Takimoto T, Tanaka K, Chinen T, Shichita T, Wyss-Coray T, Sato K, Yoshimura A. Selective expansion of Foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J Immunol. 2007;179:2170–2179. doi: 10.4049/jimmunol.179.4.2170. [DOI] [PubMed] [Google Scholar]
- 150.Fletcher TC, DiGiandomenico A, Hawiger J. Extended anti-inflammatory action of a degradation-resistant mutant of cell-penetrating suppressor of cytokine signaling 3. J Biol Chem. 2010;285:18727–18736. doi: 10.1074/jbc.M109.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 152.Lui PY, Jin DY, Stevenson NJ. MicroRNA: master controllers of intracellular signaling pathways. Cell Mol Life Sci. 2015;72:3531–3542. doi: 10.1007/s00018-015-1940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yoshikawa T, Takata A, Otsuka M, Kishikawa T, Kojima K, Yoshida H, Koike K. Silencing of microRNA-122 enhances interferon-alpha signaling in the liver through regulating SOCS3 promoter methylation. Sci Rep. 2012;2:637. doi: 10.1038/srep00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Croce L, La Mura V, Moschella F, Masutti F, Torella R, Tiribelli C, Iolascon A. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009–1015. doi: 10.1002/hep.21782. [DOI] [PubMed] [Google Scholar]
- 155.El-Saadany S, Ziada DH, El Bassat H, Farrag W, El-Serogy H, Eid M, Abdallah M, Ghazy M, Salem HA. The role of hepatic expression of STAT1, SOCS3 and PIAS1 in the response of chronic hepatitis C patients to therapy. Can J Gastroenterol. 2013;27:e13–e17. doi: 10.1155/2013/562765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Collins AS, McCoy CE, Lloyd AT, O’Farrelly C, Stevenson NJ. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling. PLoS One. 2013;8:e69090. doi: 10.1371/journal.pone.0069090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin YC, Lin CK, Tsai YH, Weng HH, Li YC, You L, Chen JK, Jablons DM, Yang CT. Adenovirus-mediated SOCS3 gene transfer inhibits the growth and enhances the radiosensitivity of human non-small cell lung cancer cells. Oncol Rep. 2010;24:1605–1612. doi: 10.3892/or_00001024. [DOI] [PubMed] [Google Scholar]
- 158.Cui Q, Jiang W, Wang Y, Lv C, Luo J, Zhang W, Lin F, Yin Y, Cai R, Wei P, Qian C. Transfer of suppressor of cytokine signaling 3 by an oncolytic adenovirus induces potential antitumor activities in hepatocellular carcinoma. Hepatology. 2008;47:105–112. doi: 10.1002/hep.21951. [DOI] [PubMed] [Google Scholar]
- 159.Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. SOCS, inflammation, and cancer. JAKSTAT. 2013;2:e24053. doi: 10.4161/jkst.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shouda T, Hiraoka K, Komiya S, Hamada T, Zenmyo M, Iwasaki H, Isayama T, Fukushima N, Nagata K, Yoshimura A. Suppression of IL-6 production and proliferation by blocking STAT3 activation in malignant soft tissue tumor cells. Cancer Lett. 2006;231:176–184. doi: 10.1016/j.canlet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 161.Liang P, Cheng SH, Cheng CK, Lau KM, Lin SY, Chow EY, Chan NP, Ip RK, Wong RS, Ng MH. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98:288–295. doi: 10.3324/haematol.2012.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ozaki A, Seki Y, Fukushima A, Kubo M. The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol. 2005;175:5489–5497. doi: 10.4049/jimmunol.175.8.5489. [DOI] [PubMed] [Google Scholar]
- 163.Uto-Konomi A, Miyauchi K, Ozaki N, Motomura Y, Suzuki Y, Yoshimura A, Suzuki S, Cua D, Kubo M. Dysregulation of suppressor of cytokine signaling 3 in keratinocytes causes skin inflammation mediated by interleukin-20 receptor-related cytokines. PLoS One. 2012;7:e40343. doi: 10.1371/journal.pone.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bostjancic E, Glavac D. Importance of microRNAs in skin morphogenesis and diseases. Acta Dermatovenerol Alp Panonica Adriat. 2008;17:95–102. [PubMed] [Google Scholar]