Abstract
Vascular remodeling is a dynamic process of structural and functional changes in response to biochemical and biomechanical signals in a complex in vivo milieu. While inherently adaptive, dysregulation leads to maladaptive remodeling. Reactive oxygen species participate in homeostatic cell signaling in tightly regulated- and compartmentalized cellular circuits. It is well established that perturbations in oxidation–reduction (redox) homeostasis can lead to a state of oxidative-, and more recently, reductive stress. We provide an overview of the redox signaling in the vasculature and review the role of oxidative- and reductive stress in maladaptive vascular remodeling. Particular emphasis has been placed on essential processes that determine phenotype modulation, migration and fate of the main cell types in the vessel wall. Recent advances in systems biology and the translational opportunities they may provide to specifically target the redox pathways driving pathological vascular remodeling are discussed.
Keywords: Vascular remodeling, Reactive oxygen species, Redox stress, Signal transduction
Vascular remodeling
The vessel wall is a dynamic and integrated organ composed of endothelial cells (ECs), smooth muscle cells (SMCs), fibroblasts and perivascular tissue that interact with each other and the circulatory cells in a complex autocrine/paracrine manner [1]. The vasculature can sense changes within its milieu and integrate these signals to transduce intra- and intercellular communication that drive structural and functional changes in a process called vascular remodeling [1, 2]. Dynamic vascular remodeling involves alterations in many cellular processes including growth and proliferation, adhesion and migration, phenotypic changes, survival and death, as well as production or degradation of extracellular matrix (ECM). Remodeling is usually an adaptive process that occurs in response to long-term changes in hemodynamic conditions, but it may subsequently contribute to the pathophysiology of vascular diseases such as systemic- and pulmonary hypertension, atherosclerosis, restenosis following revascularization by balloon angioplasty and stenting (BAS) or venous bypass grafts [1, 2]. Complex interactions between growth factors, inflammatory cytokines, vasoactive substances and hemodynamic stimuli in the vessel wall determine physiologic- and pathophysiologic remodeling. Oxido-reductive or “redox” signaling via reactive oxygen species (ROS) plays an integral part in cellular effects of these stimuli and a key role in all aspects of the remodeling process [3].
Redox signaling in the vasculature
Cells are continuously exposed to ROS generated endogenously in what could be considered as a trade off for utilizing O2 for metabolism [4]. Several ROS including superoxide (), hydrogen peroxide (H2O2), peroxynitrite (OONO−), and the hydroxyl radical (HO·) are generated in biological systems [3]. To counter the damaging effects of ROS, a complex web of antioxidants, such as the abundant low molecular weight protein glutathione (GSH) and enzymatic antioxidant systems with specific subcellular distribution and reactivity maintain intracellular redox homeostasis [5]. In contrast to the historical view of ROS as purely harmful, extensive data indicate that some ROS, i.e. and H2O2, are generated as a regulated physiological process and function as signaling molecules in control of cell and tissue homeostasis [6]. Other ROS, such as OONO− and HO·, are not considered as signaling molecules because of their very reactive nature [3]. In the vasculature, ROS are produced by all cell types including ECs, SMCs, and adventitial cells. Another biologically generated free radical nitric oxide (NO) has many important effects in vessels, directly or via interaction with ROS. NO and ROS are generated by the membrane-bound NADPH-dependent enzymes, nitric oxide synthase (NOS) and NADPH oxidases (Nox), the expression of which is tightly controlled, compartmentalized and tissue-specific [6]. Other sources of ROS that are relevant to the vascular system are the mitochondrial respiration chain, xanthine oxidase, lipoxygensae and myeloperoxidase [3]. We provide an overview of the two NADPH-dependent sources of ROS/NO in the vasculature. It is important to note that ROS generation from each of these sources can lead to ROS release from other sources [3].
In addition to NO, hydrogen sulfide (H2S) is another gaseous signaling molecule [7] that is enzymatically generated in ECs and mediates vasorelaxation [8]. As an electron donor [9], H2S is a reductant [10] and can exert antioxidant effects via both direct- and indirect actions. The direct effect of H2S involves sulfhydration of target proteins, which is the conversion of cysteinyl thiolates (Cys-SH) to cysteinyl persulfide (Cys-S-SH) by the addition of H2S-derived sulfur [11]. Moreover, as shown in non-vascular tissues, H2S donors can protect against oxidative stress indirectly by increasing GSH levels [12] or directly by sulfhydration of two Cys residues in kelch-like ECH-associated protein 1 (Keap1), the cystoplasmic adaptor that represses the “master regulator” nuclear factor erythroid 2-related factor 2 (Nrf2), thus promoting Nrf2 localization to the nucleus and inducing the expression of multiple cellular antioxidants [13, 14]. As will be pointed out, emerging data indicate that H2S-induced redox signaling participates in EC fate processes that can determine vascular remodeling, a role that is the subject of ongoing basic- and clinical research [15].
Nox and “uncoupled” eNOS- major vascular sources of ROS
The Nox family is composed of 7 catalytic subunits termed Nox1-5, Duox1 and Duox2 (for Dual Oxidase) [16]. Several protein components form the classic NADPH oxidase complex, consisting of p22phox and gp91phox (the membrane-bound subunit, crucial for the activity) and p47phox, p67phox (regulatory cytosolic proteins) and the low-molecular weight G protein Rac. Nox1, 2, 4 and 5 isoenzymes are expressed in vascular tissues and regulate such diverse functions as differentiation, proliferation, apoptosis, senescence, inflammatory responses and O2 sensing [17]. The activity and expression of Nox can be regulated by cytokines [tumor necrosis factor-α (TNFα, transforming growth factor β (TGFβ), platelet-derived growth factor (PDGF)] and agonists like angiotensin II (AngII) and thrombin [18]. AngII is a potent stimulus of both Nox activity and expression, contributing to the association between activation of the renin–angiotensin system and ROS production in several vascular pathologies. When upregulated, Noxs have been implicated in diabetes-induced vascular disease, hypertension and atherosclerosis, but upregulation can be physiologically advantageous, as in angiogenesis and collateral formation [17].
eNOS, the predominant isoform of NOS in the vasculature, is critical in the regulation of vascular function and generates NO [19–21]. Under normal conditions, in the presence of Ca2+/calmodulin, eNOS catalyzes the generation of NO from l-arginine (l-Arg) by means of electron transfer from NADPH through a flavin-containing reductase domain to oxygen bound at the heme of an oxygenase domain, which also contains binding sites for tetrahydrobiopterin (BH4) and l-Arg [19, 21]. The oxidation of NADPH is tightly coupled to the production of NO by eNOS. However, when the oxidation of NADPH is uncoupled from the production of NO, eNOS generates and secondary ROS, in what is widely known as eNOS “uncoupling” [19]. eNOS uncoupling has been associated with many pathophysiologic conditions, such as hypertension, atherosclerosis and diabetes [21]. BH4 is crucial for eNOS function and is involved in stabilizing NOS protein structure and eNOS is uncoupled when BH4 is limiting. can oxidize the NOS-bound BH4 [22]. The in vivo source of the ROS that may lead to BH4 depletion has been attributed to pathways including Nox, xanthine oxidase and the mitochondrial electron transfer chain [23, 24]. ONOO− does rapidly oxidize BH4. However, it can also irreversibly inactivate the NOS enzymes, likely by a direct reaction with the NOS heme, producing an inactive enzyme rather than an uncoupled enzyme [20].
“Oxidative” and “reductive” stress
An extensive network of signaling cascades and effector proteins regulates elimination of ROS. Increased generation of ROS can disrupt the homeostasis in thiol-dependent redox circuits by changing the redox state of the GSH pool and thioredoxin (Trx) enzyme family that act as the critical control nodes in the cellular redox network, thus leading to a state of “oxidative stress” [25]. Oxidative stress results in changes in signaling, structural and regulatory proteins and in DNA damage that lead to altered cell growth, proliferation, differentiation and death. In addition to the control of redox potential by GSH and Trx redox circuits, antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (Gpx), catalyze rapid break down of ROS to less reactive or nonreactive products, and are key in preventing damage from oxidative stress [3].
Although oxidative stress is the established paradigm delineating an excess of ROS vis-à-vis the antioxidant capacity, “reductive stress” is also increasingly gaining recognition [26, 27]. A supra-physiological increase in the GSH pool can conceivably increase the reductive flux into the cellular thiol circuits and affect the redox-sensitive thiol elements, predominantly through formation of disulfide bonds with Cys residues in a reversible process called S-glutathiolation. For example, we have shown that reductive stress following vascular injury inflicted by BAS promotes S-glutathiolation of a regulatory protein to determine vascular remodeling [27]. In addition to thiol disulfide exchange, S-glutathiolation is promoted by ROS via thyil radical formation on numerous targets proteins, thus affecting virtually all aspects of the cellular processes (gene expression, cytoskeletal dynamics, signaling, ion channels and transporters function, cell death and survival) [28]—central processes in vascular remodeling in response to injury.
Effector cell types in redox-mediated vascular remodeling
Endothelial cells (ECs)
Redox signaling and endothelial dysfunction
ECs constitute the inner most layer of the vessel wall. Acting as the interface between blood circulation and multiple tissues, ECs are constantly exposed and are adapting to numerous biochemical and biomechanical stimuli. ECs have a remarkable ability for migration and proliferation from a quiescent state that is key in angiogenesis. It is therefore not surprising that perturbations in these critical EC functions are at the crux of several vascular pathologies [29].
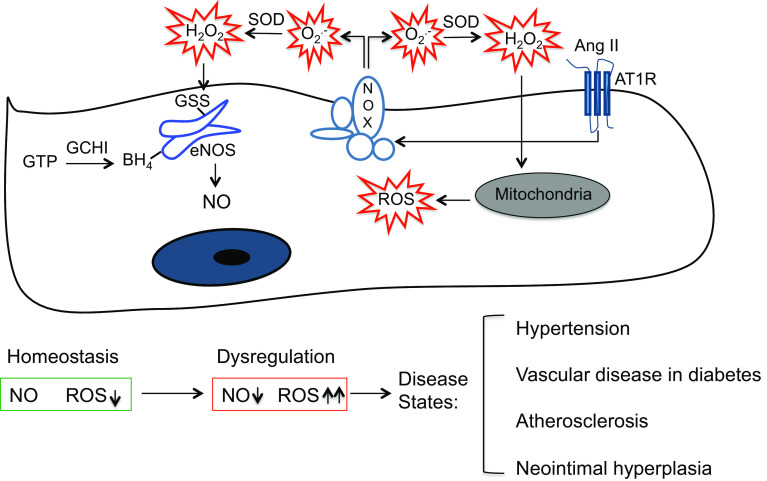
Redox signaling plays major regulatory roles in the maintenance of cellular homeostasis and in physiological adaptive responses in ECs. To this end, maintenance of a tight balance between NO- and ROS-dependent signaling is critical (Fig. 1). Endothelial dysfunction in the context of atherosclerosis [30] and diabetes [31] is characterized by a pathological decrease in NO- and an increase in redox-dependent signaling. eNOS-derived NO mediates endothelium-dependent vasodilation, required for normal vascular homeostasis, and it inhibits critical atherogenesis pathways such as platelet aggregation, SMC proliferation and migration and leukocyte adhesion [29]. eNOS uncoupling from NO- to ROS generation via BH4 depletion is an important pathobiological step in endothelial dysfunction. De novo biosynthesis of BH4 from guanosine triphosphate (GTP) is dependent on the rate limiting enzyme GTP cyclohydrolase (GCH) I (Fig. 1), and EC-specific knockout of this enzyme causes a loss of NO bioactivity and increase in production in ECs [32]. EC-targeted overexpression of GCH on the other hand increases BH4 and NO bioavailability, and reduces neointimal hyperplasia in vein grafts of atherosclerotic mice via accelerated EC repopulation and growth [33] and decreased inflammation [34]. Direct oxidation of eNOS via S-glutathiolation of specific Cys residues also mediates eNOS uncoupling [20], which is distinct from BH4 deficiency. Nonetheless, BH4 depletion and S-glutathiolation interact and exacerbate eNOS uncoupling [35]. Moreover, there is an intricate signaling cross-talk between various sources of ROS in ECs that promotes endothelial pathology. For instance, AngII-induced Nox2-derived induces ROS release from mitochondria and contributes to hypertension [16]. Furthermore, AngII-induced Nox-dependent generation is amplified by S-glutathiolation-mediated uncoupling of eNOS, akin to “kindling a bonfire”, and causes endothelial dysfunction [36] (Fig. 1).
Fig. 1.
Redox signaling and endothelial dysfunction. AngII angiotensin II, AT1R angiotensin II type 1 receptor, SOD superoxide dismutase, Nox NADPH oxidase, eNOS endothelial nitric oxide synthase, GSS S-glutathiolation, BH 4 tetrahydrobiopterin, GTP guanosine triphosphate, GCHI guanosine triphosphate cyclohydrolase I, NO nitric oxide, ROS reactive oxygen species
Mechano-sensitive redox pathways in ECs
Pulsatility of blood pressure and flow exposes the vessel wall to hemodynamic forces in the form of shear stress and cyclic stretch. The cytoskeleton and the integrins, transmembrane receptors that act as bridge between cell cytoskeleton and ECM [37], are the key structural framework for EC to transmit mechanical forces from its luminal, abluminal and junctional surfaces to its interior. ECs convert these mechanical stimuli into numerous intracellular signals that regulate a broad range of critical EC functions including migration, proliferation, permeability and apoptosis [38]. The response of ECs to physiologic levels of wall shear stress (WSS) serves a number of regulatory functions including modulation of hemostasis and thrombosis, control of inflammation through expression of chemotactic and adhesion molecules on the cell surface, and vascular SMC contraction through the release of vasoconstrictors and vasodilators [38]. Pulsatile flow induces WSS that varies temporally and spatially along the vascular bed [39]. In the straight part of the vessel, blood flow is undisturbed as opposed to disturbed blood flow on bends and bifurcations with very high WSS. Disruption or unsteady blood flow through these “atherosclerosis-prone” areas of the vessel can impair the physiological functions mentioned above leading to proatherogenic and/or prothrombotic states.
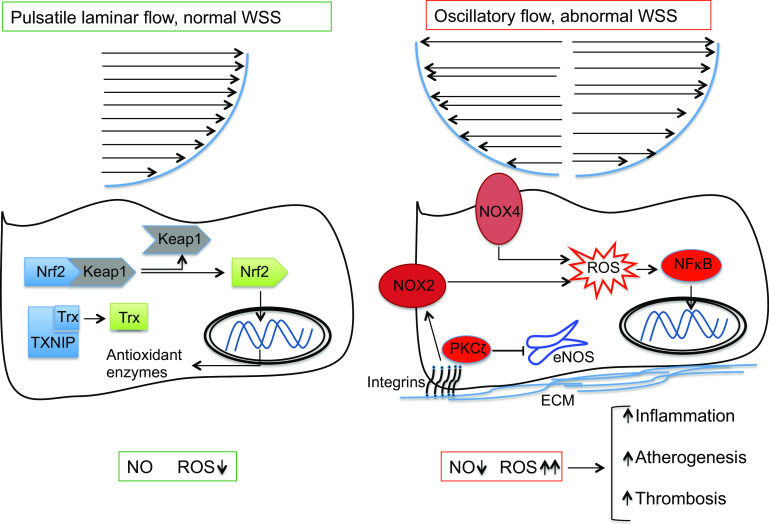
ROS have central role in physiological and pathophysiological WSS-induced vascular remodeling (Fig. 2). With normal pulsatile laminar flow, WSS-mediated generation of ROS at low levels by Nox regulates normal cell growth, proliferation and differentiation [40]. Several antioxidant pathways are induced and maintained by normal laminar WSS. Expression and activity of eNOS is enhanced by normal WSS through multiple mechanisms [39]. Normal WSS also activates Nrf2, a “master regulator” of numerous antioxidant enzymes, by releasing it from its cytoplasmic repressor Keap1, and it activates critical molecules involved in limiting inflammation [39]. Shear stress causes dissociation of cytoplasmic Nrf2 from Keap1 and Nrf2 translocation into the nucleus in a phosphoinositol 3-kinase (PI3K)/Akt-dependent pathway [41]. A mechano-sensitive switch has also been identified in the form of Trx-interacting protein, a scaffold protein that inactivates Trx [39]. By down-regulating Trx-interacting protein (TXNIP) expression hence activating Trx, physiological WSS inhibits pro-inflammatory signaling in ECs [42].
Fig. 2.
Biomechanical stimuli and redox signaling in endothelial cells. WSS wall shear stress, Nrf2 nuclear factor erythroid 2-related factor 2, Keap1 kelch-like ECH-associated protein 1, TXNIP thioredoxin-interacting protein, ROS reactive oxygen species, eNOS endothelial nitric oxide synthase, PKCζ protein kinase Cζ, NF-κB nuclear factor κB, ECM extracellular matrix
Acute cessation of the laminar flow [43] or presence of oscillatory flow on the other hand acutely increases cellular ROS formation [44], which subsequently remains elevated for the duration of WSS exposure [45] (Fig. 2). Nox2 and Nox4 isoforms of NADPH oxidase generate ROS in ECs, with Nox2 more abundantly expressed [46]. Although these Nox isoforms exist in differential subcellular compartments in ECs and their response to stimuli such as AngII differs, expression of both isoforms is upregulated by oscillatory flow and downregulated by pulsatile laminar flow in vitro [39]. Although contribution of Nox2-derived ROS in atherogenesis is shown [47], the exact role of Nox4 remains controversial. The shear responsive protein kinase Cζ (PKCζ) negatively regulates WSS-induced eNOS expression [48], induces Nox-mediated ROS generation [49] and is highly activated in atheroprone vascular regions [50]. Finally, abnormal WSS can induce activation of nuclear factor κB (NF-κB), the prototypical transcription factor for pro-inflammatory pathways, through integrin-mediated signaling in a process that is also dependent on Rac1-induced ROS generation [51] (Fig. 2).
Redox signaling in EC growth, proliferation and migration
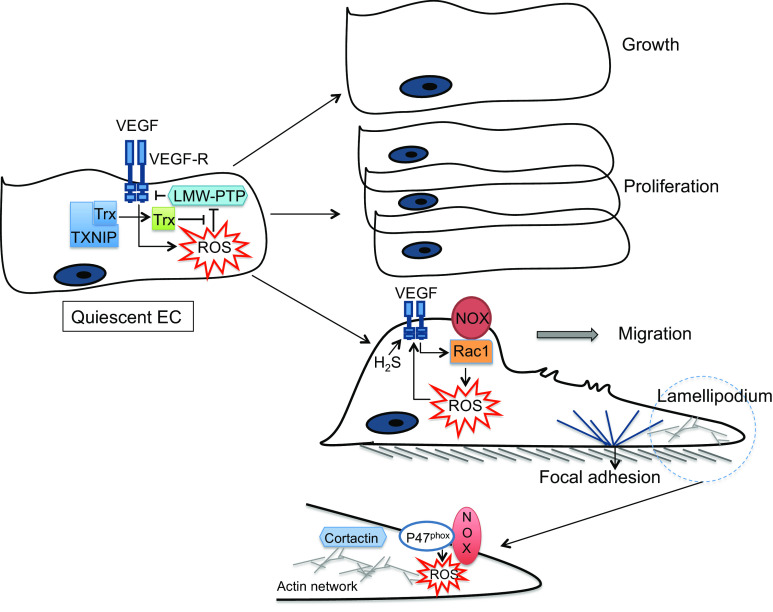
In ECs, ROS modulate many processes determining cell fate (e.g. growth, proliferation and survival), cytoskeletal reorganization and inflammatory responses that are central in EC-mediated vascular remodeling [52]. EC growth and survival are dependent on several factors, such as vascular endothelial growth factor (VEGF), which are coupled to the intracellular production of ROS [53] (Fig. 3). While the physiological ROS-dependent signaling is critical for induction of proliferative pathways in ECs [54], dysregulated generation of ROS impairs EC proliferation and promotes apoptosis [54] and induces vascular hypertrophy when ROS diffuse into the subjacent SMCs [55].
Fig. 3.
Redox signaling and cell fate in endothelial cells. VEGF vascular endothelial growth factor, VEGF-R vascular endothelial growth factor receptor, Trx thioredoxin, TXNIP thioredoxin-interacting protein, LMW-PTP low molecular weight protein tyrosine phosphatase, EC endothelial cell, ROS reactive oxygen species
Migration of ECs is essential for morphogenesis, wound healing and angiogenesis [56]. The migratory process primarily involves sensing a gradient by ECs and establishing polarity. The dynamic, integrated, cyclic process that ensues is facilitated by highly coordinated cytoskeletal changes and includes extracellular adhesion, plasma membrane protrusion at the leading edge (“lamellipodia” formation), formation of new adhesion sites under the protrusion called focal adhesions (FAs), disruption of the older adhesions at the rear of the cell, followed by cell body contraction that draws the cell forward [56]. ROS play a key role in multiple steps of this process (Fig. 3). The amount and location of ROS generation are critical since successful migration is dependent on several biomolecules the actions of which are spatiotemporally regulated in subcellular compartments in front and rear of migrating ECs. The effects of VEGF in initiating EC migration are mediated by Nox-derived ROS production in a Rac1-dependent mechanism [57]. Similarly, a role for Nox2- and Nox4-derived ROS in EC migration has been demonstrated [58]. VEGF-induced EC migration is suppressed by overexpressing mitochondrial catalase or mitochondrial DNA depletion [59], implicating a role for mitochondria-derived ROS in promoting EC migration. The lamellipodia are characterized by a dense network of short, branched actin and cortactin filaments [60]. These cytoskeletal proteins play a role in activation of Nox [61], and cortactin co-localization with p47phox subunit of Nox is important in the assembly of Nox components with the actin cytoskeleton during agonists-induced ROS generation in ECs [62]. Targeting Nox components to focal complexes in lamellipodia may therefore facilitate ROS generation at specialized sites in the leading edge of migrating ECs, a requirement for stimulus-induced migration [63]. Accordingly, a role for recruitment of cortactin, Rac1 and p47phox Nox subunit and localized ROS production in the formation of the lamellipodia in pulmonary ECs has recently been shown [64].
ROS mediate numerous effects in initiation and promotion of angiogenesis, another major function of ECs. Both physiological angiogenesis (wound healing, vessel damage and ischemic repair) and pathological angiogenesis (cancer, diabetic retinopathy and macular degeneration) involve the same initial signaling cascades, all of which involve ROS. VEGF plays a key role in EC activation from a quiescent state in a process that is, in part, ROS-dependent [65]. ROS upregulate VEGF expression and VEGF binding to VEGF receptor 2 (VEGFR2) induces ROS production that is critical for angiogenesis [66]. This is mediated by VEGFR2-induced localized ROS generation that promotes junctional detachment of the EC monolayer [67] and initiation of EC migration. During angiogenesis, ECs need to rapidly proliferate, and proliferating ECs have increased ROS production as compared with quiescent cells [68]. ROS generated by VEGF signaling induces S-glutathiolation-mediated inhibition of low-molecular weight protein tyrosine phosphatase (LMW-PTP), which dephosphorlyates and inhibits VEGFR2 signaling [69]. Downregulation of TXNIP results in deglutathiolation-mediated activation of LMW-PTP and thereby inhibition of VEGFR2 signaling [69] (Fig. 3). As discussed, EC proliferation in response to growth factors and activation of downstream kinases is ROS-dependent. Migration and proliferation of ECs result in tube formation, the earliest stage of new vessel formation. Autophagy, subcellular degradation that is critical for cell survival under nutrient-deprived conditions, plays a key role in phenotypic responses of ECs in tube formation in a process that is also driven by ROS generation [70]. Lastly, H2S, at low physiological concentrations, has been shown to stimulate EC proliferation and migration [71, 72] and to participate in VEGF signaling through breaking an intrinsic inhibitory disulfide bond in VEGFR2 thus promoting EC migration (Fig. 3) [73]. H2S exerts potent proangiogenic effect in ECs in the setting of chronic ischemia by activating extracellular kinase pathways that promote vessel growth [74].
Smooth muscle cells (SMCs)
Phenotypic plasticity of SMCs
Smooth muscle cells are highly plastic cell types that play key roles in normal vascular physiology and in pathophysiology. Biological responses in the SMCs are complex due to impressive ability of the SMCs to undergo phenotypic switching, heterogeneity of SMC origin in the vasculature, and the presence of SMC progenitor cells [75]. SMCs exist in different phenotypic states [76], with the switch from a quiescent contractile phenotype to a synthetic proliferative type playing an important role in pathologic vascular remodeling, particularly in atherosclerotic plaque progression and vascular injury-induced intimal proliferation [75, 76]. Due to the presence of SMC progenitor cells in the vessel wall and their potential contribution to vascular remodeling [77, 78], in vivo SMC lineage tracing studies are needed to elucidate the exact origin of the cell types that are found in intima lesions [75, 78]. This is further complicated by the diverse developmental origins of SMCs in the vascular system [79]. Although phenotypic switching seems to occur in all SMCs regardless of their origin, the responses of these SMCs to different stimuli vary [79].
Redox signaling and phenotypic modulation in SMCs
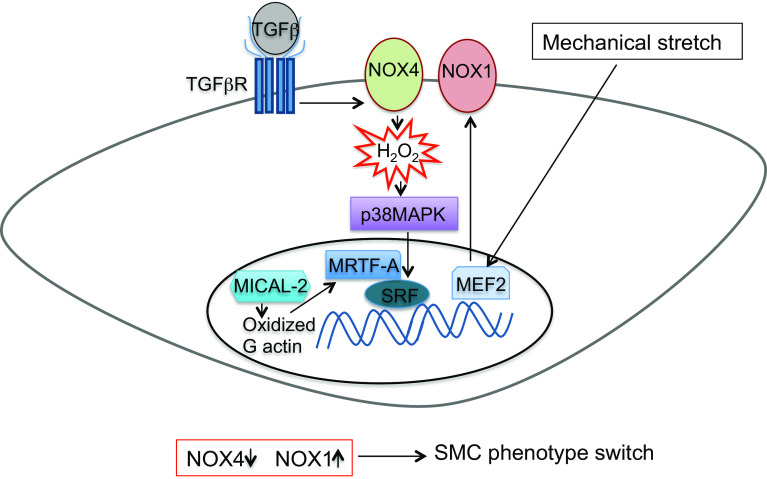
Intracellular regulation of SMC phenotype depends on several kinases and downstream transcription regulation of contractile proteins and proteins that are associated with the cytoskeleton [75]. ROS play an essential role in this process (Fig. 4). Specificity of the intracellular ROS-mediated effects is dependent on subcellular compartmentalization. For instance, Nox1 and Nox4 isoforms of NADPH oxidase have differential signaling roles in phenotypic regulation of SMCs, which correlate with their differential compartmentalization in the membrane and leading edge of migratory SMCs [3]. Nox4 mainly produces H2O2 and Nox4 expression and activity is critical in the maintenance of the differentiated phenotype of SMCs isolated from aorta in vitro [80]. Nox4 modulates effects of TGFβ in aortic SMCs via p38 mitogen-activated protein kinase (MAPK)-dependent regulation of several transcription factors that mediate gene transcription elicited by diverse signaling pathways (e.g. serum response factor (SRF) and myocardin-related transcription factor A (MRTF-A) [81]). In contrast to the homeostatic role in systemic arteries, Nox4 mediates hypoxia-induced proliferation of SMCs in the pulmonary artery [82]. Unlike Nox4, Nox1 expression and activity are associated with a reduction in differentiation markers and increase in migratory, synthetic and proliferative SMC type. Through these modulations, Nox1 plays a key role in neointima formation after vascular injury [83]. Of direct relevance, cyclophilin A, a secreted growth factor from SMCs under oxidative stress, induces Nox activation by translocation of the cytosolic p47phox subunit to membrane lipid rafts or caveolae [84] and promotes neointima formation [85].
Fig. 4.
Redox-dependent phenotype switch in smooth muscle cells. TGFβ transforming growth factor β, p38 MAPK p38 mitogen-activated protein kinase, MRTA-F myocardin-related transcription factor A, SRF serum response factor, MEF2 myocyte-enhanced factor 2, SMC smooth muscle cell
Redox signaling also regulates numerous aspects of cytoskeletal dynamics [86] and thereby modulates SMC differentiation. For example, carbonylation and subsequent degradation of annexin A1, a member of the annexin family of proteins that bind or “annex” to phospholipid membranes, promotes the growth of pulmonary artery SMCs [87]. In the nucleus, oxidation of actin by the oxidoreductase MICAL-2, an atypical actin regulatory protein, promotes actin disassembly and increases nuclear retention of MRTF-A and subsequent activation of SRF/MRTF-A-dependent gene transcription [88]. Finally, biomechanical forces can also modulate the SMC phenotype via redox-dependent mechanisms. Mechanical stretch for instance potentiates Nox1-mediated ROS production that causes vascular SMC switch to synthetic phenotype via myocyte-enhanced factor 2 (MEF2), a transcription factor that plays a key role in cell fate in response to extracellular signals [89].
Redox signaling and SMC adhesion and migration
In vascular remodeling associated with disease, a phenotypic switch in the SMCs to a synthetic type enables them to migrate and proliferate in response to a variety of extracellular stimuli. Migratory steps in the SMCs are similar to ECs, with ROS regulating multiple phases in the process (Fig. 5).
Fig. 5.
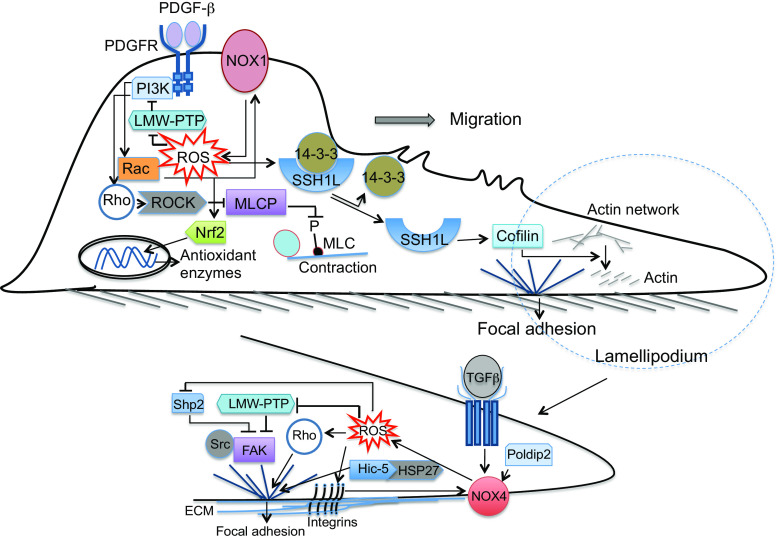
Redox-mediated smooth muscle cell migration. PDGFβ platelet-derived growth factor β, PDGFR platelet-derived growth factor receptor, PI3K phosphoinositol 3-kinase, LMW-PTP low molecular weight protein tyrosine phosphatase, ROS reactive oxygen species, ROCK rho-kinase, MLCP myosin light chain phosphatase, MLC myosin light chain, Nrf2 nuclear factor erythroid 2-related factor 2, SSH1L slingshot1L phosphatase, TGFβ transforming growth factor β, FAK focal adhesion kinase, Hic5 H2O2-inducible clone-5, HSP27 heat shock protein 27, Poldip2 polymerase [DNA-directed] delta-interacting protein 2, ECM extracellular matrix
Vascular SMC migration is modulated by numerous stimuli, in particular PDGF is the major promigratory factor [90]. The effects of PDGF occur mainly via PDGF-β receptor activation [91]. Although the PDGF receptors have very low abundance in normal vessels, PDGF-β receptor expression is induced during initial response to injury or the phenotypic transformation of SMCs [90]. Since Nox inhibition blocks PDGF-induced PDGF-β-receptor phosphorylation [92], ROS may be involved as early as the initial activation of the receptor. LMW-PTP limits PDGF receptor activation and its activity is inhibited by ROS through formation of an inactivating disulfide bond between two vicinal Cys in its catalytic pocket [93]. Upon activation, PDGF receptor provides binding sites for phospholipase C, Src kinase and PI3K. PI3K activates Rhoguanine nucleotide exchange factors to stimulate Rho-GTPase family members (such as Rho, Rac and cdc42) [3]. Rac activates several Nox family members, mainly Nox1 and Nox2 [3], and increases ROS, which in turn induce generation of other pro-migratory factors and thus amplify the migratory cell response [90]. The redox-sensitive transcription factor Nrf2 limits PDGF-stimulated vascular SMC migration by decreasing ROS, and is protective against neointimal hyperplasia after vascular injury [94] (Fig. 5). Mechano-responsive signaling can also initiate SMC migration, but the underlying mechanisms are not fully elucidated. It has recently been shown that cyclic mechanical stretch can induce Nox4-dependent activation of cofilin, which is required for cytoskeletal reorganization and SMC reorientation after mechanical stimulation [95]. Activation of cofilin increases depolymerization of actin filaments, a necessary step in the formation of new actin filaments, thereby playing an essential role in maintaining and protruding lamellipodia at the leading edge of migrating cells [90] (Fig. 5). In PDGF-stimulated SMCs, cofilin is activated through dephosphorylation by Slingshot1L (SSH1L) phosphatase via Nox1-dependent oxidation of 14-3-3, which results in disruption of its inhibitory association with SSH1L [96].
After formation of lamellipodia, integrins mediate the formation of focal adhesions (FA). The FAs act as organizers of the SMC contractile proteins and incorporate and integrate multiple signaling molecules such as focal adhesion kinase (FAK), integrin-linked kinase and Src kinase [97]. These kinases link integrins to the actin cytoskeleton and coordinate the formation and strengthening of FAs in the lamellipodium, as well as their recycling from the rear of the cell [90]. ROS are critically involved in many aspects of FA formation and turnover in SMCs (Fig. 5). Integrin activity is modulated by oxidation of redox-sensitive motifs when cells attach to surface [98]. Integrin signaling itself involves ROS generation [45]. ROS production via this mechanism can inhibit LMW-PTP [99] that, in addition to modulating PDGFR activation discussed earlier, associates with and inactivates FAK [100]. Moreover, redox-mediated inhibition of the phosphatase Shp2 can lead to activation of FAK [101]. ROS are critically involved in FA turnover, which is key in successful cell motility. Rho mediates actin polymerization and FA formation and ROS can directly activate Rho by oxidation of a redox-sensitive motif [102]. Moreover, Nox4 is key in FA turnover and polymerase [DNA-directed] delta-interacting protein 2 (poldip2), an activator of Nox4-mediated ROS production in vascular SMCs, affects FA turnover and inhibits SMC migration in a RhoA/FAK-dependent manner [103]. Nox4 expression is increased by TGFβ and TGFβ in turn increases the number of FAs. Downstream mediators of TGFβ-induced Nox4-dependent FA formation have recently been shown to include the FA resident protein H2O2-inducible clone-5 (Hic-5) and its binding partner the heat shock protein 27 [104].
Following FA formation, cell body contraction generates the force that is needed to move the SMC forward. FAs are connected to actin and ROS can influence actin dynamics directly or indirectly during specific phases of migration [90]. Actin polymerization is affected by ROS, with the direction of effects depending on the type and amount of ROS that are generated [3]. Interaction of actin and myosin to generate contractile force is modulated by the redox-sensitive GTPase Rho [105]. Activation of the Rho/Rho-kinase (ROCK) pathway promotes myosin light chain phosphorylation by inhibiting the regulatory subunit of myosin light chain phosphatase (MLCP), thus promoting contraction in pulmonary SMCs [106]. Consistent with this, ROCK2 isoform of Rho-kinase promotes migratory and proliferative phenotype in pulmonary SMCs in mice, and its expression is increased in patients with PAH [107]. Further studies are needed to clarify the specific roles and functional differences between ROCK1 and ROCK2 isoforms [108].
Redox signaling and SMC hypertrophy and proliferation
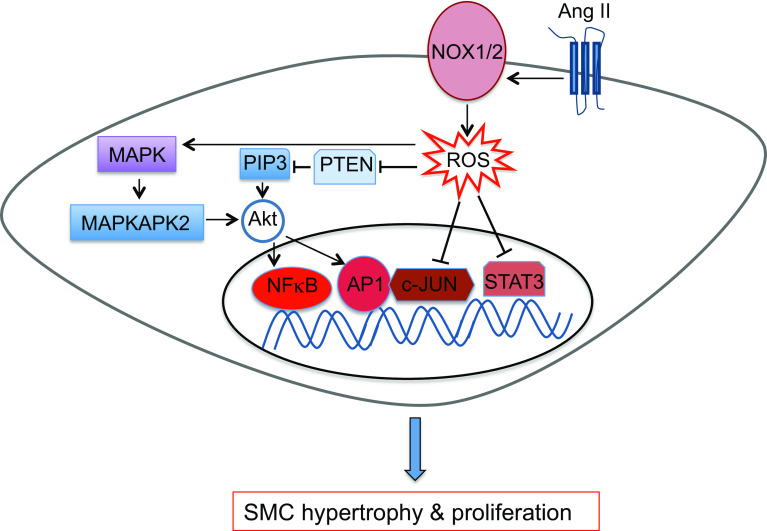
SMC proliferation is an essential part of biological development and contributes to adaptive responses to injury—i.e. vascular repair. Similar to the migratory process, dysregulation of SMC proliferation perpetuates pathology (e.g. progression of atherosclerosis or neointima formation postBAS). Several migratory and proliferative pathways overlap in the SMCs and critical promigratory factors such as PDGF also promote proliferation [3]. AngII mainly mediates SMC hypertrophy [109] rather than migration or proliferation. SMC proliferation is tightly coupled with cellular redox state, and ROS have key regulatory roles by modulating the function of many growth factors and kinases that are essential in proliferative signaling cascades (Fig. 6), largely by oxidative modification of Cys residues [110]. These regulatory roles are perturbed under conditions associated with increased ROS. High levels of ROS generally inactivate downstream effectors of growth factor signaling [3]. For example, many signaling elements and effectors in growth factor-mediated PI3K signal transduction are redox-sensitive. While PI3K itself is susceptible to sulfenation [111], the functional significance of this modification or the presence of other oxidative Cys modifications is yet to be determined. Oxidative modification of one or two Cys on Src, depending on the context and cell type, leads to its activation [112], and Src in turn can activate PI3K. Growth factors potentiate the accumulation of phosphatidylinositol 3,4,5 trisphosphate (PIP3), both through a PI3K-dependent increase in synthesis as well as oxidative inactivation of phosphatase and tensin homolog (PTEN) that catalyzes the removal of PIP3 [113]. PIP3 then activates the redox-sensitive Akt, activity of which is affected by sulfenation of a Cys residue [114]. Moreover, AngII-induced ROS generation promotes association of the redox-sensitive p38 mitogen-activated protein kinases (MAPK) and MAPK-activated protein kinase-2 with Akt [115]. Akt activates the transcription factors activator protein 1 (AP1) and NF-κB to promote cell cycle progression [3]. Finally, many transcription factors in proliferative pathway are directly regulated by redox modifications, for instance signal transducer and activator of transcription 3 (STAT3) and c-Jun are both negatively regulated by S-glutathiolation [116, 117] (Fig. 6).
Fig. 6.
Redox signaling and smooth muscle cell hypertrophy and proliferation. PDGFβ platelet-derived growth factor β, PDGFR platelet-derived growth factor receptor, AngII angiotensin II, AT1R angiotensin II type 1 receptor, ROS reactive oxygen species, MAPK mitogen-activated protein kinase, MAPKAPK2 mitogen-activated protein kinase-activated protein kinase 2, PIP3 phosphatidylinositol 3,4,5 trisphosphate, PTEN phosphatase and tensin homolog, AP1 activator protein 1, NF-κB nuclear factor κB, STAT3 signal transducer and activator of transcription 3
In contrast to growth factor signaling, functional effects of oxidative modification of phosphatases are varied. S-glutathiolation of a reactive Cys in protein tyrosine phosphatase 1B reversibly inhibits its activity and promotes proliferative signaling [110]. Similarly, reversible oxidation of Shp1/2 inhibits their function through a different mechanism. Of the critical Cys residues in the enzymes’ active site, when the catalytic Cys is re-reduced, two conserved “backdoor” Cys form an intramolecular disulfide. Formation of this backdoor–backdoor disulfide is dependent on the presence of the active site Cys and can proceed via either active site Cys-backdoor Cys intermediate [118]. These two backdoor Cys are necessary and sufficient to ensure reversible oxidation of the Shps because removal of both Cys leads to irreversible oxidative inactivation [27, 118]. This regulatory mechanism has recently been shown to be critical in neointima formation postBAS [27].
Perivascular tissue
Perivascular tissue, in particular perivascular adipose tissue (PVAT) is increasingly recognized to play important physiological roles in vascular homeostasis [119]. PVAT generates numerous cytokines (pro-inflammatory such as IL6 and anti-inflammatory like adiponectin [120]) as well as ROS that affect the adjacent vascular layers in a paracrine manner and play an integral role in vascular remodeling [121]. PVAT actively participates in the inflammatory response to BAS [122]. Vessel injury downregulates the anti-inflammatory adiponectin in PVAT and promotes SMC growth [123] and PVAT-released leptin contributes on neointima formation after vascular injury [124]. PVAT also exerts effects on vascular remodeling via redox pathways. For example, 4-hydroxynonenal (4-HNE), a product of lipid peroxidation generated in the vascular wall, mediates paracrine activation of peroxisome proliferator-activated receptor-γ signaling in the PVAT, thus promoting the release of adiponectin, which exerts a paracrine effect back to the vascular wall to reduce Nox activity [120] and to promote eNOS coupling [125]. Moreover, a reduction in the activity of the anti-inflammatory mammalian target of rapamycin complex 2 (mTORC2) in the PVAT leads to inducible NOS-mediated increase in ONOO−, which impairs endothelium-mediated vasorelaxation [126]. Further mechanistic studies are needed to elucidate the role of redox signaling in PAVT-mediated vascular remodeling, particularly in the context of obesity and metabolic syndrome.
Pericytes in the microvasculature
Pericytes are contractile cells on capillaries that may have a role in regulating local blood flow in addition to stabilizing newly formed capillaries [127]. Pericytes can be constricted and dilated by signaling molecules in vitro and capillary blood flow heterogeneity might reflect differences in pericyte tone. These properties of pericytes are increasingly recognized in physiology and in remodeling of microvasculature in pathology. Pericyte contraction in response to ischemia–reperfusion contributes to “no-reflow” phenomenon in the brain that is mediated by oxidative-nitrosative stress [128], nonetheless, pericyte death in rigor, which majorly contributes to no-reflow phenomenon, does not change with ROS scavenging [127]. A role for pericytes in myocardial no-reflow has been implicated [129] but remains to be established. A marked increase in the capillary pericyte coverage and a switch in phenotype to contractile SMC have been shown to contribute to distal vascular remodeling in human PAH [130]. Since receptor tyrosine kinases have been shown to reduce pericyte density in solid tumor models, it is plausible that these agents might be useful in treatment of PAH. Given these recent studies, it is clear that the physiological regulatory role of pericytes in the microvasculature and putative involvement of redox-dependent mechanisms in these cell types require further elucidation.
Therapeutic implications and future directions
Like most biological processes, vascular remodeling and redox signaling are extremely complex. The complexity of the biology mandates sophisticated approaches to tackle dysregulation of biosystems. As can be seen from our overview, enormous efforts have been made for gaining in-depth insights into the pathobiology of vascular remodeling and the role that ROS play in this phenomenon. Bearing this in mind, it is no surprise that general antioxidants that primarily aimed to scavenge ROS failed to improve redox-dependent cardiovascular pathologies [131].
The advent of systems biology, with high dimensional “omics” tools, provides a unique opportunity for more sophisticated, unbiased understanding of the biological processes. This approach promises to provide most biologically relevant therapeutic targets. A relevant example of using this method in vascular remodeling is transcriptomic characterization of in-stent restenosis by our group. We performed near genome wide analysis in de novo atherosclerosis and in-stent restenosis in atherectomy samples from patients. Independently, we generated networks of gene–gene interactions using text mining of the entire abstracted literature. By overlaying gene expression from atherectomy tissue on these networks and scoring individual networks according to the average differential significance of network members, we found the network with Gpx1 as its hub to be the most significantly down-regulated of all gene networks in in-stent restenosis [27]. In mechanistic studies, we found that loss of Gpx1 leads to increased SMC proliferation, migration and apoptosis, and that this is attenuated by inhibition of the orphan receptor tyrosine kinase ROS1 through cell-fate regulation. Sustained ROS1 activation that triggered SMC proliferation and neointimal hyperplasia was mediated by the reductive stress associated with Gpx1 deficiency, which lead to inhibition of the regulatory phosphatase Shp2 by S-glutathiolation of 2 backdoor Cys residues. Importantly, we determined that pharmacological inhibition of ROS1 attenuated in-stent restenosis without affecting reendothelialization. This differential effect on SMCs and ECs is critical since the current anti-proliferative drugs used in stents, whilst very effective, indiscriminately affect ECs and SMCs leading to delayed reendothelialization [132] and higher risk of late stent thrombosis [133].
As our understanding of the mechanisms governing phenotype switching and cell fate in ECs and SMCs deepens, differential targeting of these cells will be more attainable. Similar to ROS1 inhibitors, targeting pyruvate dehydrogenase kinase isoform 2, which governs mitochondrial hyperpolarization in SMCs after BAS [134] or cytidine triphosphate synthase 1 that catalyzes generation of the energy-rich nucleotide cytidine triphosphate in proliferating SMCs after balloon injury [135], reduce neointima formation without affecting reendothelialization. It is high time for development of similarly tailored therapies that are directed at redox pathways to slow or halt pathological vascular remodeling.
Acknowledgments
KKG is supported by a Columbia University Research Grant and Lucy Falkiner Fellowship from Sydney Medical School Foundation. ZAA is supported by NIH Grant R00HL109256.
Abbreviations
- AngII
Angiotensin II
- AP1
Activator protein 1
- AT1R
Angiotensin II type 1 receptor
- BH4
Tetrahydrobiopterin
- Cys
Cysteine
- Cys-SH
Cysteinyl thiolates
- Cys-S-SH
Cysteinyl persulfide
- EC
Endothelial cell
- ECM
Extracellular matrix
- eNOS
Endothelial nitric oxide synthase
- FAK
Focal adhesion kinase
- GCHI
Guanosine triphosphate cyclohydrolase I
- GSS
S-Glutathiolation
- GTP
Guanosine triphosphate
- Hic5
H2O2-inducible clone-5
- HSP27
Heat shock protein 27
- Nox
NADPH oxidase
- Keap1
Kelch-like ECH-associated protein 1
- LMW-PTP
Low molecular weight protein tyrosine phosphatase
- MAPKAPK2
Mitogen-activated protein kinase-activated protein kinase 2
- MEF2
Myocyte-enhanced factor 2
- MLC
Myosin light chain
- MLCP
Myosin light chain phosphatase
- MRTF-A
Myocardin-related transcription factor A
- NF-κB
Nuclear factor κB
- Nrf2
Nuclear factor erythroid 2-related factor 2
- p38 MAPK
p38 mitogen-activated protein kinase
- PDGFβ
Platelet-derived growth factor β
- PDGFR
Platelet-derived growth factor receptor
- PI3K
Phosphoinositol 3-kinase
- PIP3
Phosphatidylinositol 3,4,5 trisphosphate
- PKCζ
Protein kinase Cζ
- Poldip2
Polymerase [DNA-directed] delta-interacting protein 2
- PTEN
Phosphatase and tensin homolog
- ROCK
Rho-kinase
- ROS
Reactive oxygen species
- SMC
Smooth muscle cell
- SOD
Superoxide dismutase
- SRF
Serum response factor
- SSH1L
Slingshot1L phosphatase,
- TAT3
Signal transducer and activator of transcription 3
- TGFβ
Transforming growth factor β
- TXNIP
Thioredoxin-interacting protein
- VEGF
Vascular endothelial growth factor
- VEGF-R
Vascular endothelial growth factor receptor
- WSS
Wall shear stress
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 2.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27(8):1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 3.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116(3):531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4(5):278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 5.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27(2):601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 10.Keefe AD, Miller SL, McDonald G, Bada J. Investigation of the prebiotic synthesis of amino acids and RNA bases from CO2 using FeS/H2S as a reducing agent. Proc Natl Acad Sci USA. 1995;92(25):11904–11906. doi: 10.1073/pnas.92.25.11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13(8):499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18(10):1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 13.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35(6):995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 14.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122(1):11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song P, Zou MH. Redox regulation of endothelial cell fate. Cell Mol Life Sci. 2014;71(17):3219–3239. doi: 10.1007/s00018-014-1598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20(2):281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10(6):453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 20.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14(10):1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA. 2007;104(38):15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 24.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8(3):132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Limphong P, Pieper J, Liu Q, Rodesch CK, Christians E, Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26(4):1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali ZA, de Jesus Perez V, Yuan K, Orcholski M, Pan S, Qi W, Chopra G, Adams C, Kojima Y, Leeper NJ, Qu X, Zaleta-Rivera K, Kato K, Yamada Y, Oguri M, Kuchinsky A, Hazen SL, Jukema JW, Ganesh SK, Nabel EG, Channon K, Leon MB, Charest A, Quertermous T, Ashley EA. Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J Clin Invest. 2014;124(12):5159–5174. doi: 10.1172/JCI77484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7(4):381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res. 2015;116(7):1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuaiphichai S, McNeill E, Douglas G, Crabtree MJ, Bendall JK, Hale AB, Alp NJ, Channon KM. Cell-autonomous role of endothelial GTP cyclohydrolase 1 and tetrahydrobiopterin in blood pressure regulation. Hypertension. 2014;64(3):530–540. doi: 10.1161/HYPERTENSIONAHA.114.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali ZA, Rinze R, Douglas G, Hu Y, Xiao Q, Qi W, McNeill E, Bursill C, George I, Greaves DR, Xu Q, Channon KM. Tetrahydrobiopterin determines vascular remodeling through enhanced endothelial cell survival and regeneration. Circulation. 2013;128(11 Suppl 1):S50–S58. doi: 10.1161/CIRCULATIONAHA.112.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali ZA, Bursill CA, Douglas G, McNeill E, Papaspyridonos M, Tatham AL, Bendall JK, Akhtar AM, Alp NJ, Greaves DR, Channon KM. CCR2-mediated antiinflammatory effects of endothelial tetrahydrobiopterin inhibit vascular injury-induced accelerated atherosclerosis. Circulation. 2008;118(14 Suppl):S71–S77. doi: 10.1161/CIRCULATIONAHA.107.753558. [DOI] [PubMed] [Google Scholar]
- 35.Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem. 2013;288(1):561–569. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galougahi KK, Liu CC, Gentile C, Kok C, Nunez A, Garcia A, Fry NA, Davies MJ, Hawkins CL, Rasmussen HH, Figtree GA. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc. 2014;3(2):e000731. doi: 10.1161/JAHA.113.000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475(7356):316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryan MT, Duckles H, Feng S, Hsiao ST, Kim HR, Serbanovic-Canic J, Evans PC. Mechanoresponsive networks controlling vascular inflammation. Arterioscler Thromb Vasc Biol. 2014;34(10):2199–2205. doi: 10.1161/ATVBAHA.114.303424. [DOI] [PubMed] [Google Scholar]
- 40.Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011;15(5):1389–1403. doi: 10.1089/ars.2010.3361. [DOI] [PubMed] [Google Scholar]
- 41.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 42.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115(3):733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Browning EA, Chatterjee S, Fisher AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annu Rev Physiol. 2012;74:403–424. doi: 10.1146/annurev-physiol-020911-153324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiai TK, Hwang J, Barr ML, Correa A, Hamilton R, Alavi M, Rouhanizadeh M, Cadenas E, Hazen SL. Hemodynamics influences vascular peroxynitrite formation: implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol Med. 2007;42(4):519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases in mechano-transduction: mechanisms and consequences. Antioxid Redox Signal. 2014;20(6):887–898. doi: 10.1089/ars.2013.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7(3–4):308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 47.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100(7):1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 48.Nigro P, Abe J, Woo CH, Satoh K, McClain C, O’Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, Berk BC. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010;116(11):1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-α-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90(9):1012–1019. doi: 10.1161/01.RES.0000017631.28815.8E. [DOI] [PubMed] [Google Scholar]
- 50.Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ Res. 2005;97(5):443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J Cell Biol. 2013;201(6):863–873. doi: 10.1083/jcb.201207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breton-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486(3):252–256. doi: 10.1016/S0014-5793(00)02305-X. [DOI] [PubMed] [Google Scholar]
- 54.Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 55.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32(3):488–495. doi: 10.1161/01.HYP.32.3.488. [DOI] [PubMed] [Google Scholar]
- 56.Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell. 2012;23(2):310–323. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal. 2012;16(11):1229–1247. doi: 10.1089/ars.2011.4489. [DOI] [PubMed] [Google Scholar]
- 58.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11(4):747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Zang QS, Liu Z, Wu Q, Maass D, Dulan G, Shaul PW, Melito L, Frantz DE, Kilgore JA, Williams NS, Terada LS, Nwariaku FE. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol. 2011;301(3):C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung BH, Zhu X, Kaverina I, Weaver AM. Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Curr Biol. 2011;21(17):1460–1469. doi: 10.1016/j.cub.2011.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11(4):841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usatyuk PV, Singleton PA, Pendyala S, Kalari SK, He D, Gorshkova IA, Camp SM, Moitra J, Dudek SM, Garcia JG, Natarajan V. Novel role for non-muscle myosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation, and reactive oxygen species generation in lung endothelium. J Biol Chem. 2012;287(12):9360–9375. doi: 10.1074/jbc.M111.294546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishina NM, Tyurin-Kuzmin PA, Markvicheva KN, Vorotnikov AV, Tkachuk VA, Laketa V, Schultz C, Lukyanov S, Belousov VV. Does cellular hydrogen peroxide diffuse or act locally? Antioxid Redox Signal. 2011;14(1):1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 64.Usatyuk PV, Fu P, Mohan V, Epshtein Y, Jacobson JR, Gomez-Cambronero J, Wary KK, Bindokas V, Dudek SM, Salgia R, Garcia JG, Natarajan V. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J Biol Chem. 2014;289(19):13476–13491. doi: 10.1074/jbc.M113.527556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99(2):715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266(1):37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 68.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380(2):193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 69.Abdelsaid MA, Matragoon S, El-Remessy AB. Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells. Antioxid Redox Signal. 2013;19(18):2199–2212. doi: 10.1089/ars.2012.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du J, Teng RJ, Guan T, Eis A, Kaul S, Konduri GG, Shi Y. Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol. 2012;302(2):C383–C391. doi: 10.1152/ajpcell.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA. 2012;109(23):9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys 1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013;19(5):448–464. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szabo G, Veres G, Radovits T, Gero D, Modis K, Miesel-Groschel C, Horkay F, Karck M, Szabo C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide. 2011;25(2):201–210. doi: 10.1016/j.niox.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wall VZ, Bornfeldt KE. Arterial smooth muscle. Arterioscler Thromb Vasc Biol. 2014;34(10):2175–2179. doi: 10.1161/ATVBAHA.114.304441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95(2):156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, Giachelli CM, Parmacek MS, Raines EW, Rusch NJ, Speer MY, Sturek M, Thyberg J, Towler DA, Weiser-Evans MC, Yan C, Miano JM, Owens GK. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112(1):17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 80.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27(1):42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK. NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic Biol Med. 2011;50(2):354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29(4):480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soe NN, Sowden M, Baskaran P, Smolock EM, Kim Y, Nigro P, Berk BC. Cyclophilin A is required for angiotensin II-induced p47phox translocation to caveolae in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2013;33(9):2147–2153. doi: 10.1161/ATVBAHA.113.301894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satoh K, Matoba T, Suzuki J, O’Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117(24):3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gellert M, Hanschmann EM, Lepka K, Berndt C, Lillig CH. Redox regulation of cytoskeletal dynamics during differentiation and de-differentiation. Biochim Biophys Acta. 2015;1850(8):1575–1587. doi: 10.1016/j.bbagen.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 87.Wong CM, Marcocci L, Liu L, Suzuki YJ. Cell signaling by protein carbonylation and decarbonylation. Antioxid Redox Signal. 2010;12(3):393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lundquist MR, Storaska AJ, Liu TC, Larsen SD, Evans T, Neubig RR, Jaffrey SR. Redox modification of nuclear actin by MICAL-2 regulates SRF signaling. Cell. 2014;156(3):563–576. doi: 10.1016/j.cell.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez AI, Csanyi G, Ranayhossaini DJ, Feck DM, Blose KJ, Assatourian L, Vorp DA, Pagano PJ. MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2015;35(2):430–438. doi: 10.1161/ATVBAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.San Martin A, Griendling KK. Redox control of vascular smooth muscle migration. Antioxid Redox Signal. 2010;12(5):625–640. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buetow BS, Tappan KA, Crosby JR, Seifert RA, Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163(3):979–984. doi: 10.1016/S0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009;104(2):219–227. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 93.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276(36):33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 94.Ashino T, Yamamoto M, Yoshida T, Numazawa S. Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33(4):760–768. doi: 10.1161/ATVBAHA.112.300614. [DOI] [PubMed] [Google Scholar]
- 95.Montenegro MF, Valdivia A, Smolensky A, Verma K, Robert Taylor W, San Martin A. Nox4-dependent activation of cofilin mediates VSMC reorientation in response to cyclic stretching. Free Radic Biol Med. 2015;85:288–294. doi: 10.1016/j.freeradbiomed.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, San Martin A. Platelet-derived growth factor (PDGF) regulates Slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem. 2011;286(41):35430–35437. doi: 10.1074/jbc.M111.268284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116(Pt 22):4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 98.de Rezende FF, Martins Lima A, Niland S, Wittig I, Heide H, Schroder K, Eble JA. Integrin α7β1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic Biol Med. 2012;53(3):521–531. doi: 10.1016/j.freeradbiomed.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161(5):933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rigacci S, Rovida E, Dello Sbarba P, Berti A. Low Mr phosphotyrosine protein phosphatase associates and dephosphorylates p125 focal adhesion kinase, interfering with cell motility and spreading. J Biol Chem. 2002;277(44):41631–41636. doi: 10.1074/jbc.M201709200. [DOI] [PubMed] [Google Scholar]
- 101.Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell-matrix adhesion. J Biol Chem. 2006;281(23):15593–15596. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- 102.Mitchell L, Hobbs GA, Aghajanian A, Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid Redox Signal. 2013;18(3):250–258. doi: 10.1089/ars.2012.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307(7):H945–H957. doi: 10.1152/ajpheart.00918.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandez I, Martin-Garrido A, Zhou DW, Clempus RE, Seidel-Rogol B, Valdivia A, Lassegue B, Garcia AJ, Griendling KK, San Martin A. Hic-5 mediates TGFβ-induced adhesion in vascular smooth muscle cells by a Nox4-dependent mechanism. Arterioscler Thromb Vasc Biol. 2015;35(5):1198–1206. doi: 10.1161/ATVBAHA.114.305185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heo J, Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem. 2005;280(35):31003–31010. doi: 10.1074/jbc.M504768200. [DOI] [PubMed] [Google Scholar]
- 106.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shimizu T, Fukumoto Y, Tanaka S, Satoh K, Ikeda S, Shimokawa H. Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arterioscler Thromb Vasc Biol. 2013;33(12):2780–2791. doi: 10.1161/ATVBAHA.113.301357. [DOI] [PubMed] [Google Scholar]
- 108.Shimokawa H, Satoh K. Vascular function. Arterioscler Thromb Vasc Biol. 2014;34(11):2359–2362. doi: 10.1161/ATVBAHA.114.304119. [DOI] [PubMed] [Google Scholar]
- 109.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 110.Chiu J, Dawes IW. Redox control of cell proliferation. Trends Cell Biol. 2012;22(11):592–601. doi: 10.1016/j.tcb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol. 2009;4(9):783–799. doi: 10.1021/cb900105q. [DOI] [PubMed] [Google Scholar]
- 112.Giannoni E, Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal. 2014;20(13):2011–2025. doi: 10.1089/ars.2013.5525. [DOI] [PubMed] [Google Scholar]
- 113.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101(47):16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antico Arciuch VG, Galli S, Franco MC, Lam PY, Cadenas E, Carreras MC, Poderoso JJ. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS ONE. 2009;4(10):e7523. doi: 10.1371/journal.pone.0007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23(22):8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Butturini E, Darra E, Chiavegato G, Cellini B, Cozzolino F, Monti M, Pucci P, Dell’Orco D, Mariotto S. S-glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem Biol. 2014;9(8):1885–1893. doi: 10.1021/cb500407d. [DOI] [PubMed] [Google Scholar]
- 117.Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13(12):1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 118.Chen CY, Willard D, Rudolph J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48(6):1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 119.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3(2):e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64(6):2207–2219. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 121.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34(8):1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104(18):2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 123.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30(8):1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 124.Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch FS, Chalikias G, Konstantinides S, Schafer K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol. 2013;33(5):980–987. doi: 10.1161/ATVBAHA.113.301393. [DOI] [PubMed] [Google Scholar]
- 125.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127(22):2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 126.Bhattacharya I, Dragert K, Albert V, Contassot E, Damjanovic M, Hagiwara A, Zimmerli L, Humar R, Hall MN, Battegay EJ, Haas E. Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression. Arterioscler Thromb Vasc Biol. 2013;33(9):2105–2111. doi: 10.1161/ATVBAHA.112.301001. [DOI] [PubMed] [Google Scholar]
- 127.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15(9):1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 129.O’Farrell FM, Attwell D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol. 2014;11(7):427–432. doi: 10.1038/nrcardio.2014.58. [DOI] [PubMed] [Google Scholar]
- 130.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmuller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 131.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111(8):1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 132.Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS, Nagata S. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47(10):2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 133.Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Juni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125(9):1110–1121. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 134.Deuse T, Hua X, Wang D, Maegdefessel L, Heeren J, Scheja L, Bolanos JP, Rakovic A, Spin JM, Stubbendorff M, Ikeno F, Langer F, Zeller T, Schulte-Uentrop L, Stoehr A, Itagaki R, Haddad F, Eschenhagen T, Blankenberg S, Kiefmann R, Reichenspurner H, Velden J, Klein C, Yeung A, Robbins RC, Tsao PS, Schrepfer S. Dichloroacetate prevents restenosis in preclinical animal models of vessel injury. Nature. 2014;509(7502):641–644. doi: 10.1038/nature13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang R, Cui XB, Wang JN, Chen SY. CTP synthase 1, a smooth muscle-sensitive therapeutic target for effective vascular repair. Arterioscler Thromb Vasc Biol. 2013;33(10):2336–2344. doi: 10.1161/ATVBAHA.113.301561. [DOI] [PMC free article] [PubMed] [Google Scholar]