Abstract
Cellular models are important tools in various research areas related to colorectal biology and associated diseases. Herein, we review the most widely used cell lines and the different techniques to grow them, either as cell monolayer, polarized two-dimensional epithelia on membrane filters, or as three-dimensional spheres in scaffold-free or matrix-supported culture conditions. Moreover, recent developments, such as gut-on-chip devices or the ex vivo growth of biopsy-derived organoids, are also discussed. We provide an overview on the potential applications but also on the limitations for each of these techniques, while evaluating their contribution to provide more reliable cellular models for research, diagnostic testing, or pharmacological validation related to colon physiology and pathophysiology.
Keywords: 3D culture, Cell culture model, Cell polarization, Cell spheroids, Colon, Colorectal cancer, Cystic fibrosis, Epithelial chloride transport, Inflammatory bowel disease, Organoids, Small intestine
Introduction
The intestine is a tubular structure extending between stomach and anus, which operates as a highly efficient and selective barrier, separating an external milieu composed of ingested food, microorganisms, or toxic waste from the internal body fluids and metabolism. Indeed, a single epithelial cell layer—composed of different cell types and covered by a mucus layer—forms the barrier that separates the gut lumen from the underlying sterile tissue [1].
Whereas the small intestine sections duodenum, jejunum, and ileum absorb nutrients released from digested food, the colon as the last intestinal section is mainly responsible for reabsorbing water, ions, vitamins, and organic acids of microbial origin, as well as for storing and expelling waste material.
The intestinal barrier function relies on an epithelial layer of highly differentiated columnar cells that are mainly characterized by an absorptive apical surface and a tight junction belt sealing the intercellular spaces. In this way, selective uptake of luminal contents is controlled via apical membrane transporters.
The ex vivo use of intestinal cells as model systems is of great importance in various areas of basic and translational science but also clinical research, namely, diagnosis prognosis and personalized therapeutics. These models are crucial for exploring the physiology or pathophysiology of intestinal diseases in the laboratory, including disease mechanisms involved in cancer, inflammatory bowel disease, or cystic fibrosis (CF), as well as for toxicological and bioavailability tests of newly developed food ingredients or drugs, epithelial interaction with gut microbes, and to perform diagnostic tests based on electrophysiological measurements of ion transport properties in patient-derived biological materials. The constant improvements of such models should also allow to progressively use them to replace some of the experiments currently still performed in laboratory animals.
Intestinal cell lines used as models
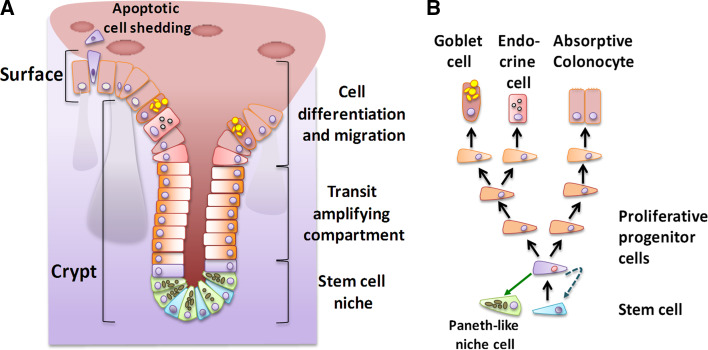
The entire intestinal epithelium contains crypt invaginations where adult stem cells continuously generate replacement cells, which then differentiate on their way to the epithelium surface into entero- or colonocytes (see Fig. 1). In the colon, this proliferative environment is susceptible to malignant transformation [2], so that most of the available cell line models were derived from human tumor samples. Their use became widespread following the successful establishment of the first immortalized human tumor cell line, HeLa, in 1952.
Fig. 1.
Cellular organization of the colon epithelium. a The epithelium is composed of crypt invaginations and the epithelial surface (villus elevations characterize the small intestine but not the colon). The scheme on the left depicts the crypt (with a stem cell niche at its basis and the transit-amplifying compartment) followed by a zone of cell migration and differentiation that ends in apoptotic cell loss at the surface. b Model of the cell lineage from a single stem cell to the various differentiated cell types of the colon epithelium and the Paneth cell-like secretory cells of the colon stem cell niche [134]
Caco-2 cells
The most widely used cellular model over the last 30 years has been the Caco-2 cell line, reaching over 13,590 references in the PubMed database in April 2016. Grown to a confluent culture these cells undergo differentiation with several morphological and functional characteristics of small intestine enterocytes, although cells were initially obtained from a human colon adenocarcinoma [3]. This coexistence of colonocyte and enterocyte characteristics has turned these cells very useful to explore absorptive and pathogen-defensive properties of the intestinal mucosa. When grown on filter membranes, these cells form a confluent cell layer with a polarized organization, in particular, the sealing of the lateral intercellular space through tight junctions [4, 5], which can be measured as transepithelial electrical resistance (TEER).
Caco-2 cells show a microsatellite-stable phenotype and harbor mutations in the tumor-suppressing proteins APC, p53, and SMAD4 but not in the oncogenes KRAS, BRAF, or PI3KCA [6, 7].
The parental Caco-2 cell line has revealed some morphological heterogeneity and a mosaic expression pattern of intestinal marker enzymes during the cellular differentiation process, which may be of interest to specific research questions [3, 8, 9]. This may indicate a heterogeneous differentiation potential or some intrinsic pattern of functional differentiation within the cell population. Alternatively, a proportion of the cells may retain stem-cell like properties or phenotypic plasticity and diverge in their features during differentiation. Interestingly, recent studies revealed that Caco-2 cells cultured under continuous flow conditions can form a crypt-villus organization and also differentiate into other cell types, such as goblet and enteroendocrine cells [10].
The observed morphological heterogeneity has motivated the selection of several clones, such as Caco-2/TC7, Caco-2/AQ, and Caco-2/15, with higher expression of the cell surface activities of taurocholic acid transport, alkaline phosphatase, or sucrase isomaltase, respectively [3, 11]. Another example is the Caco-2/C2BBe clone that was selected for a more homogeneous apical brush border morphology and exclusive apical villin localization [3, 12]. The use of these clones may explain conflicting results that appeared in the literature but also highlighted that cell culture conditions may modify the differentiation process of parental Caco-2 cells, so that these conditions need to be clearly defined [3].
The co-culture of Caco-2 cells separated by a filter membrane from B-cell lymphoma Raji cells induced about 20 % of them to adopt M-cell-like properties [13–15]. M-cells are microvilli-less specialized antigen-sampling cells that effectively bind, transport, and deliver non-nutritional macromolecules and microorganisms to the immune cells of the gut-associated lymphoid tissue that underlies the intestinal epithelium in Peyer’s patches [16, 17]. M-cells represent only about 1 % of the intestinal surface area but possess a high transcytotic capacity and ability to transport bacteria, viruses, or materials, such as nanoparticles. This cell model has been widely employed in the context of oral vaccination, nanomaterial-based delivery, or infection by pathogenic bacteria [16, 18, 19].
Other colorectal cell lines
SW480 are colorectal adenocarcinoma cells with a more mesenchymal phenotype and a high proliferation rate, which do not form a polarized monolayer. They are mostly used for research on Wnt-related oncogenic signaling pathways or assays validating anti-cancer drugs.
T84 cells were derived from a lung metastasis of a colon carcinoma and can spontaneously differentiate in culture forming apical microvilli and basolateral tight junctions. They have, thus, been frequently used, similar to Caco-2, as a model for studying a tight polarized enterocyte cell layer.
HT29 is a colorectal adenocarcinoma cell line with epithelial morphology but do not form a fully differentiated cell layer. They contain a small proportion of goblet cells with mucin secretion and carry a mutation in the BRAF oncogene.
Other commonly used cell lines are LS174T, INT-407, HT20-MTX, and NCM460. Properties and main applications of all these cell lines are summarized in Table 1. Not mentioned are many other colon cancer-derived cell lines [20, 21] that serve as models for specific genetic alterations found in colorectal cancer and are grown as standard monolayer cultures.
Table 1.
Summary of properties of the most commonly used colon-derived cell lines
| Cell line | Mutated genes | Citations | Origin | Main characteristics | Main applications | References |
|---|---|---|---|---|---|---|
| Caco-2 | APC, TP53, SMAD4 | 13592 | Colon adeno-carcinoma | Functional characteristics of small intestine enterocytes; can differentiate into a polarized monolayer | Electrophysiology; absorptive and pathogen-defensive properties of the intestinal barrier; innate immune response; drug resistance | [3, 11, 135] |
| SW480 | APC, TP53, KRAS, PIK3CA | 2333 | Duke’s type B colorectal adenocarcinoma | Do not form polarized monolayer; high proliferation rate; more mesenchymal phenotype | Research on Wnt and other oncogenic signaling pathways; drug resistance; toxicity of nanoparticles | [78, 136–140] |
| T84 | APC, KRAS, PI3KCA | 1395 | Lung metastasis of colon carcinoma | Differentiate into a polarized monolayer; mixed differentiation into both, chloride-secreting enterocytes and mucin-secreting goblet-like cells | Electrophysiology; pathogen-epithelium interactions; barrier function | [141–145] |
| LS174T | CTNNB1, KRAS | 982 | Duke’s type B colorectal adenocarcinoma | Goblet-like cells with secretion of mucins MUC2, MUC5A/C and MUC6; do not form polarized monolayer | Mucin expression studies; cancer research; drug resistance | [146–148] |
| INT-407 | Express E6-, E7 papilloma virus oncogenes; Myc overexpression | 199 | Originally from human embryonic intestinal epithelium; now HeLa cells (cervix adenocarcinoma) | Contaminated and overgrown by HeLa cells indistinguishable from HeLa by STR PCR DNA profiling; residual formation of polarized brush border | Attachment of Campylobacter jejuni and other pathogenic bacteria (also attach to HeLa cells) | [149–153] |
| HT29-MTX | APC, TP53, BRAF | 139 | Selected subclone of adenocarcinoma-derived HT29 cells | Display mucus producing goblet-cell properties; do not form polarized monolayer; HT29-MTX co-cultured with Caco-2 generate a confluent cell layer model covered by a protective mucus layer at the expense of reduced barrier function | Effect of food-borne nanomaterial additives on the epithelium; bioavailability of drugs in the presence of mucus; drug resistance | [154–162] |
| NCM460 | Cytogenetic changes; lack p16INK4a expression | 128 | Immortalized normal colonocytes | Metabolic characteristics of normal mucosa, with low glycolytic rate and pentose phosphate synthesis but high tricarboxylic acid cycle activity | Interaction with microbial pathogens; control cells for studying oncogenic signaling pathways | [163–173] |
Citation numbers were retrieved from the PubMed database in April 2016
Two-dimensional (2D) culture systems
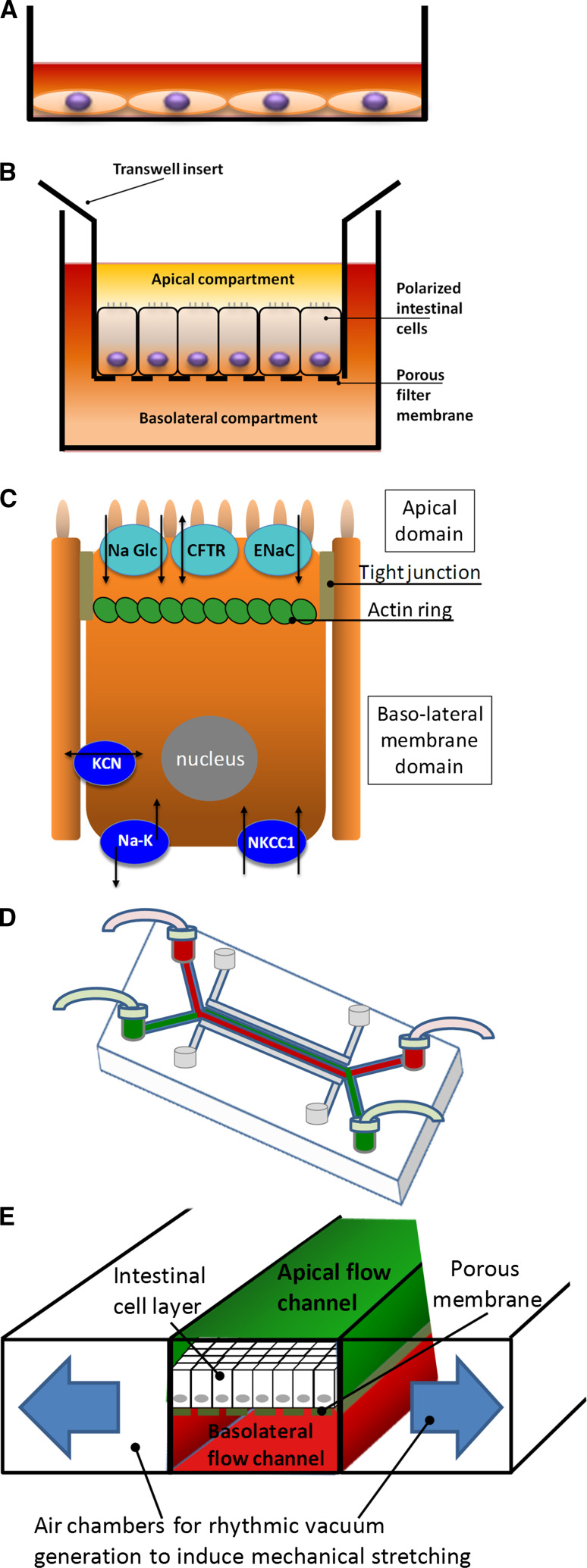
For many research applications, intestinal cells are cultivated as two-dimensional flat monolayers on plastic surfaces (Fig. 2a). This allows rapid growth under relatively cost-effective conditions, and the cells can be easily handled, manipulated or transfected, and harvested for analysis. Sufficiently large numbers of cells are obtained for monitoring cell viability, changes in gene expression, or biochemical determination of enzymatic activities underlying a given molecular response. These growth conditions and the size of spread colorectal cells are also well-suited for high-throughput drug or siRNA screenings, especially when using automated fluorescence image analyses. Thus, such monolayer cultures offer the advantage of a reductionist approach to study the intrinsic characteristics of colorectal tumor cells (Table 2).
Fig. 2.
Schematic representation of different colon cell cultivation techniques. a Two-dimensional monolayer of cells grown in a plastic culture dish. b Tightly sealed two-dimensional monolayer formed by cells grown on semi-permeable membrane filters that separate an upper and lower compartment, allowing the cells to assume a functionally polarized morphological organization. c Schematic representation of a polarized intestinal cell with tight junctions and an apical ring of actin filaments, functionally separating the plasma membrane into two domains: the microvilli-containing apical and the basolateral domain with concomitant differential sorting of transporter proteins, such as the apical sodium-glucose co-transporter (NaGlc), the chloride channel CFTR or the epithelial sodium channel (ENaC), as well as the basolateral sodium–potassium pump (Na–K), the sodium–potassium-chloride co-transporter (NKCC1) or potassium channels of the KCN family. d Schematic view of a flow cell used for organ-on-chip models. Channels for fluid perfusion (red or green color) and for air-suction (gray) are shown. e Magnified representation of the channel system from a gut-on-chip device shown in (d). Visible is the cell monolayer grown on the porous membrane with upper and lower fluid channels and lateral vacuum channels allowing to exert mechanical stretching forces on the cell monolayer
Table 2.
Summary of the different 2D and 3D culturing platforms
| Culture technique | Surface/materials | Advantages | Limitations | Key references | |
|---|---|---|---|---|---|
| 2D | |||||
| Undifferentiated flat cell monolayer | Stiff plastic surface (culture dish or flasks) |
Rapid and cost-effective cell growth Cells accessible for experimental manipulation and high-throughput screening |
Undifferentiated cells without tissue-specific organization Adhesion of cells to stiff substrate affects gene expression, drug response and pro-liferation rate |
[56, 75, 84] | |
| Polarized cells | Microporous filter membrane inserts |
Allows differentiation of apical and basolateral polarity Co-culture of different cell types in the two different filter compartments |
Cell polarization usually requires several days to weeks Filter membrane inserts or chip device represent additional costs Cell number is limited Cells grow on artificial membrane and lack a physiological ECM environment |
[3, 34] | |
| Gut-on-chip technique |
Cells growth under continuous flow of nutrients or signal molecules Cells exposed to apical flow-derived shear forces and rhythmic mechanical stretching |
[10, 47, 48] | |||
| 3D | |||||
| Scaffold-free techniques | Hanging drop | Culture medium without any artificial cell adhesion substrate |
Gravity-induced cell aggregation leads to formation of individual spheroids per drop Spheroids experience gradients of oxygen, nutrients and metabolism end products Special plates in 96 or 384-well format available for screening purposes |
Spheroids consist only of colon epithelial cells Cell number is limited Tonicity fluctuations in small volumes affect spheroid biology |
[69, 71] |
| Scaffold-based techniques | Hydrogels | Plant-derived polymers: methylcellulose, agarose, alginate |
Allow 3D self-organization into spheroids or polarized cysts Soft physiologic matrix-environment Allows 3D growth on natural ECM components |
Batch variability with lack of reproducibility Hydrogel can interfere with biochemical assays Hydrogel handling difficulties Cell number is limited |
[22, 80] |
| Animal-derived matrices: Collagen gel, Matrigel™ | [87, 88, 94, 98] | ||||
| Synthetic scaffolds: polyethylene glycol, Extracel™, QGel®, Puramatrix |
Biocompatible, reproducible matrix composition Amenable to large-scale use Allows chemical functionalization to model microenvironment stiffness and architecture |
Requires chemical modifications to allow cell attachment Residual synthetic compounds may have cytotoxic effects |
[111, 112] | ||
| Solid scaffolds | Polystyrene-based, fibrous or porous physical support |
Sterile, ready-to-use disposable material Highly reproducible manufacturing |
Awaits functional validation | [63, 114] | |
| Organoid cultures | Culture medium with specific growth factor composition and Matrigel |
High functional complexity Structure containing different colon cell types Genetic testing of patient-derived biopsies |
Lack fluid-flow conditions or mechanical stretching Apical surface not accessible to experimental manipulation |
[119, 120, 127] | |
Only key references for each technique are indicated
2D or 3D two- or three-dimensional, ECM extracellular matrix
However, when grown on plastic surface, many of the functionally important epithelial cell properties are not developed and may thus yield results of limited physiological relevance. For example, the undifferentiated state of cells without adherens and tight junctions that is observed in monolayer cell cultures does not exist in the tissue. In addition, growing various epithelial cell types, including Caco-2 and T84, on an ultra-stiff plastic matrix creates conditions that promote cell proliferation and mesenchymal-like malignant phenotypes [22–26]. In addition, the lack of signals contributed by other stroma cell types may affect the morphological organization or response of intestinal cells [27–29] or render sensitive tumor cell lines resistant to targeted drugs [30, 31].
Growth as polarized cells on microporous filter membranes
A significant improvement in physiological relevance of the two-dimensional (2D) cell models is achieved upon their growth on microporous membrane inserts that allow free access of ions and nutrients to either the apical or the basolateral sides of the cell monolayer (Fig. 2b). These conditions induce full cell polarization. This is a process requiring several days to weeks and implies a striking functional separation between the apical and basolateral plasma membrane domains. As a result, an apical domain (corresponding to the intestinal lumen) generates a multitude of actin bundle-supported microvilli forming the morphological structure known as brush border [32–34]. The microvilli projections massively increase the apical cell surface and guarantee the uptake of luminal nutrients through cell surface enzymes and transport proteins. In addition, a basolateral membrane domain establishes cell–cell adhesion complexes and cell–extracellular matrix (ECM) interactions, and exposes receptors to growth factors or hormones. Cell polarization also implies differential sorting of proteins to each membrane domain [35, 36], including specific sets of ion channels, receptors, and solute transporters. Another essential junctional hallmark is the sealing of the lateral intercellular space through tight junctions [4, 5] (Fig. 2c).
Growing cells on microporous membrane inserts allow monitoring the integrity of the monolayer through their TEER. TEER is measured in Ohm with an epithelial voltohmmeter and represents a quantitative technique to measure the integrity of the epithelial barrier or of tight junction dynamics in the cell culture model [37]. For electrical measurements, two small electrodes are used, one being placed in the upper apical and the other in the lower compartment underneath the porous membrane, so that the electrodes are separated by the cellular monolayer. Normally, monolayers with TEER values of over 1000 Ω cm2 are considered as “tight,” with values of 300–400 Ω cm2 as “intermediate,” and as “leaky” with values of 50–100 Ω cm2; however, TEER values ranging from 62 to 1290 Ω cm2 have been reported for Caco-2 cells and also depend on cell line variants and culture conditions [37, 38].
It should be recalled that Caco-2 cells alone do not fully represent the physiology of the intestinal mucosa, because other cell types, such as goblet and enteroendocrine cells exist. Including such cells in the cell layer grown on filter membranes will reduce the strength of the barrier function, so that these models are more permeable but can be more physiological, depending on the research question.
Because abnormal intestinal epithelial cell polarity and morphology are typical features of several human pathological conditions, TEER measurement under these growth conditions has physiologically or clinically relevant conclusions. One example is the CFTR-mediated chloride (Cl−) transport in colon biopsies of cystic fibrosis (CF) patients, because CFTR-mediated secretion can serve as a valuable biomarker for CF diagnosis and prognosis [39–41]. To this end, rectal biopsy specimens are generally mounted and analyzed in ringer solution-perfused micro-Ussing chambers under open-circuit conditions, although other options (non-perfused, short-circuit) have also been used [42]. The cAMP-dependent apical Cl− secretion is measured upon co-activation of calcium-dependent potassium channels in the basolateral membrane, which provide the driving force for luminal Cl− exit through CFTR and allow to distinguish functional from mutant CFTR [40].
The experimental limitations and disadvantages of growing an epithelial cell monolayer on filter membranes are first the long period required for establishment of a fully polarized organization (10–14 days) before experiments can be performed. Second, the required transwell filter inserts represent additional costs, and the cell number is limited, so that cell analyses using PCR, immunofluorescence and Western blot are possible, but fully differentiated cells have limitations regarding manipulation through transfection, unless a previously established stable cell line is seeded that was engineered to express a gene of interest after cell polarization, e.g., from an inducible gene promoter [43]. Furthermore, the microporous filter membrane is a structural surrogate for the basal membrane to which intestinal cells attach, and although it can be coated with different purified matrix proteins, the membrane does not functionally correspond to a physiological ECM.
Co-cultures using microporous filter membranes
Another important feature of this culture technique is the ability to grow co-cultures of different cell types in the two different filter compartments, so that they share soluble factors released into the medium but are not in a direct physical contact. This is important because the mucosa contains immune cells, the gut-associated lymphoid tissues, and myofibroblasts underneath the epithelial cell layer that are important players in the inflammatory bowel disease process. They may not only interact through the secretion of cytokines or growth factors and substantially modify drug response [30] but also induce differentiation of some epithelial cells into M-cells, as already described above [13–15].
An important consideration in this respect is the pore size of the chosen porous membrane filters. For studies concerning cell polarity, permeability or drug transport, tissue remodeling, or co-culture of different cell types, filters with pore sizes of 0.4, 1.0, or 3.0 µm are recommended to keep cell types and compartments separated. In contrast, pore sizes of 5.0 or 8.0 µm allow cells to cross the membrane and are required to study epithelial tumor cell invasion, transepithelial migration, chemotaxis, or phagocytosis. It should, however, be kept in mind that some cell types or their cell body protrusions may squeeze through 3-µm pores to the other membrane side and, thus, establish direct contact with cells present or grown in the opposite membrane compartment.
Deliberate promotion of cell–cell contact can be achieved using an inverted filter insert approach. Here, Caco-2 cells, for example, are first grown as a polarized monolayer on the filter membrane, which is then turned upside down to face the lower compartment. Then, the apical compartment can be loaded with lymphocytes that will sediment onto the membrane and contact Caco-2 cells from the physiologically relevant basolateral side [13].
Co-culture approaches are being used with increasing frequency to bridge the gap between simplistic single-lineage models and the dynamic biological processes that occur in vivo [44]. In the future, an increasing complexity of such co-culture models can be expected to uncover new and more accurate in vitro synergies to intestinal biology.
Gut-on-chip technique
A highly sophisticated improvement of filter membrane-based culture systems is microfluidic flow chambers [45–47]. In these devices, epithelial cells are grown on a porous membrane with one fluid-perfused channel underneath the membrane and another above the apical luminal face (see Fig. 2d). This allows the cells to experience nutrients or signal molecules from the basolateral side and flow-derived shear forces on the apical surface.
Several unprecedented technical improvements are achieved with this gut-on-chip technique [10, 48, 49]. First, it is possible to mimic the rhythmic peristaltic contraction of the intestine, because two adjacent hollow microchannels exist and serve to apply a vacuum through cyclic suction (Fig. 2e). In this way, the central porous membrane with its attached cell layer becomes deformed, and this exerts cyclic mechanical stretch forces. Because mechanical stimulation has now been recognized to affect not only cell morphology but also signaling transduction and gene expression [50–53], the properties of the seeded epithelial cell layer can be expected to be even closer to the in vivo situation. For example, under these conditions, Caco-2 cells were reported to spontaneously reorganize into 3D intestinal villi and reestablish basal proliferative crypt-like structures that give also rise to mucus-secreting, enteroendocrine, and Paneth cells [10]. These systems appear thus to reach an interface between 2D and 3D growth conditions.
Second, the continuous fluid flow of fresh culture medium in these microfluidic devices does not only constantly supply nutrients but also removes unbound residual bacteria as well as metabolic wastes. Thus, the kinetics of nanomaterial adsorption or bacterial growth on the epithelial cell layer differs from a static membrane filter assay and is further modulated by rhythmic cell stretching [54].
Third, a microenvironment in steady-state equilibrium can be created with stable co-culture conditions allowing to mimic in vitro the interaction between epithelial cells, underlying immune cells and probiotic or pathogenic gut microbes [54]. Moreover, this model system can be specifically manipulated over a time course regarding its biochemical and cellular composition or the presence of mechanical cell stretching. Microscale structures that generate a geometric landscape of villi on the chip [55] can further increase the dynamics of the intestinal microenvironment and provide novel insights into its role in pharmacokinetics and inflammatory bowel disease (IBD).
A limitation is that cells grow on an artificial porous membrane and lack the physiological ECM microenvironment.
Three-dimensional (3D) culture systems
Intestinal cells that grow as a 2D monolayer lack important aspects of tissue- and organ-specific microarchitecture. There is no doubt that immortalized colorectal tumor cell lines grown in 2D culture have contributed tremendously to the knowledge about the molecular pathways involved in malignant cell transformation, but they cannot represent adequate model systems for complex tumor biology, for example, [56]. Indeed, in drug development, only 5 % of the drugs found active in such cell culture models reach relevant clinical trials [57, 58]. There is now evidence that 3D culture models may better recapitulate the mechanisms of drug resistance found in tumors [59, 60]. The reason is that spatial and physical aspects exist in these cultures that alter signal transduction and influence the cellular response or behavior.
It is likely that 3D cell culture models may bridge the gap between in vitro models used in the discovery or screening phase of drug development and subsequent animal experiments for safety assessment prior to clinical trials. In particular, increasing predictive power of 3D culture models could contribute to reduce the number of animals used by the pharmaceutical industry for drug efficacy and toxicity tests. 3D cell cultures further revealed greater stability and longer life span than cell cultures in 2D and are thus more suitable for long-term studies on cell interactions or drug effects. Similarly, the growth of patient-derived cells in such 3D cultures may open unprecedented possibilities for personalized pharmacogenetic approaches that animal models cannot address.
Currently, there are several 3D culture methods, including the scaffold-free platforms for spheroid growth and the scaffold-based models (hydrogels or solid biomaterials) [61–64], described in the following.
Scaffold-free techniques
Scaffold-free methods in 3D culture do not contain added biomaterials or ECM and are based on the natural affinity shown by many cell types to establish cell–cell adhesion and generate their own ECM. When using established colorectal or intestinal cell lines, they primarily generate simple aggregates of cells, which do not develop any functional differentiation or polarized organization. When single isolated CD133+ colon cancer stem cells are cultivated, they generate a clonogenic 3D spheroid but do not further differentiate into different cell types [65]. HT29 cells may even become enriched in a population with stem-cell like properties [66], and primary CD133-positive colon cancer stem cells can be isolated through their ability to form spheroids in serum-free medium [67].
Spheroid formation can be induced by a variety of different techniques. The earliest method employed in cancer biology is spinner flask culture, where fluid turbulence prevents attachment and promotes cellular aggregation [28]. This technique has later been developed into bioreactors, allowing the culture of large populations of spheroids under perfusion-controlled parameters (oxygen, nutrient supply and pH, for example). A simpler method for spheroid production involves liquid overlay of a cell suspension over a non-adherent surface, such as agar-coated plates or special low-adherence culture dishes. Both methods can produce large numbers of relatively consistent spheroids, but commonly generate a large variation in size and number of the spheroids.
Recently, primary spheroid cultures obtained directly from colorectal cancer samples were isolated using low attachment culture plates. Following glass bead-mediated mechanical disruption of tumor material, crypt-like structures were isolated and grew into spheroids in the presence of a cell-death reducing RHO-associated kinase (ROCK1) inhibitor [68]. If viable in a clinical setting, such approaches should greatly improve the future design of a personalized cancer treatment.
A variation of low-attachment plates are dishes with micropatterned surfaces that create small square or honeycomb-shaped islands where cells can form adhesive contacts without allowing full cell spreading. Growth under such conditions also promotes 3D cell aggregation. Instead, precision micropatterns can be used to create small isolated compartments to which cell growth is confined, so that spheroids grow in regular patterns of defined size and in the same focal plane, thus suitable for automated analyses [69].
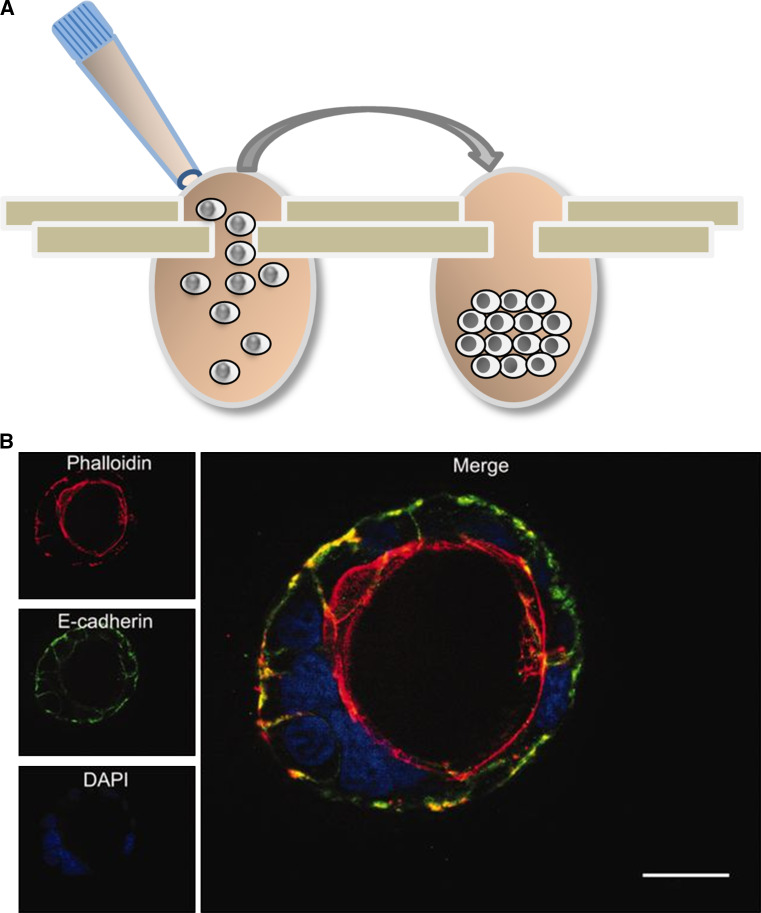
Alternatively, individual spheroids can be grown within small hanging drops, in which cells are first in suspension, then concentrate at the bottom of the drop by gravity and can subsequently form aggregates through cell–cell adhesion. This scaffold-free method is simple to use and generates spheroids with consistent sizes and shapes, so that testing series encounter comparable and controllable conditions. With this technique, spheroids can also be harvested and analyzed individually. Two approaches to the hanging drop are currently being used. One applies the cell suspension to the lid of a culture plate, which is then inverted to hang over the liquid-filled culture plate, which creates a humid atmosphere. A second approach employs culture plates specially designed to generate a hanging drop after applying a cell suspension. For example, the Perfecta3D® or GravityPlus™ hanging drop plates consist of the main culture plate with access holes and a complementary lid and tray (Fig. 3a). These operate in 96-well plates with small volumes of 30–50 µL and should contain only about 5000 cells in each drop. Some plates can also hold 384 drops of 25 µL with 2500 cells. Each day a small volume of fresh culture medium needs to be added to provide sufficient nutrients and prevent changes in osmolality, and this way reagents and drugs can also be added or removed from the drop cultures. Spheroids can be harvested from the top by aspirating with a pipette, or through the bottom side by centrifugation into a 96-well receiving plate or addition of excess medium until the drop falls. The addition of polymers to the culture medium can further standardize the formation of single spheres of equal size [70, 71], so that high-throughput assays become feasible. Spheroids can be analyzed using colorimetric, fluorescence, and luminescence assays measured with a plate reader. However, these hanging drop approaches require generally very careful and standardized conditions of medium or humidity supply to avoid volume and tonicity fluctuation within the drop that will damage the spheroids.
Fig. 3.
Three-dimensional (3D) techniques to culture colon cells. a 3D sphere formation using the hanging-drop technique. A cell suspension is applied to a specially designed plate (left) and cells accumulate and aggregate at the bottom of the drop. b 3D cyst formation by Caco-2 cells grown in Matrigel [101]. The confocal microscopy image shows DAPI-stained nuclei in blue and phalloidin-stained actin filaments in red, revealing the polarized cell organization and formation of a central apical lumen
From the biological point of view, spheres of colonic or intestinal cells are usually unstructured, high-density clumps of cells that experience gradients of oxygen, nutrients, and metabolism end products, so that cellular stress and apoptosis are created in their center, much alike the initial pre-angiogenic phase of tumor development. They are generally limited in size (400–600 µm) resulting from these gradients and develop a necrotic core surrounded by a rim of viable cells (100–300 µm) in the periphery They, thus, have their role in testing anti-cancer drugs, for instance, as described for the angiogenic and invasive behavior of HT29 or HCT116 tumor cells [69, 72, 73], or the induction of stemness properties in colorectal cells upon hypoxic stress conditions [74]. Spheroid biology is certainly closer to tumors than a thin monolayer of cells grown on a stiff plastic dish [56, 75].
Scaffold-based techniques
3D scaffolds can be manufactured from a range of natural or synthetic materials and can be divided into two approaches—hydrogels and solid scaffolds.
Through the use of hydrophilic polymer chains, highly hydrated scaffolds with water content above 30 % by weight can be formed and their mechanical and structural properties depend on chemical crosslinks or physical interactions between polymer chains, similar to or modified from tissue ECM [76]. The various available scaffold materials differ in their suitability for a given scientific question to be addressed, depending on their physical properties, such as pore size, or stiffness, their reluctance to diffusion of soluble molecules, and their biological effects, including cell adhesion, signaling, or biodegradability.
Plant-derived hydrogels
As plant-derived polymers methylcellulose, agarose or alginate is mainly used. The addition of up to 1 % methylcellulose to the culture medium generates a semi-solid medium with increased viscosity working as a biologically inert crowding agent, so that cell aggregation is promoted. Similarly, suspending cells in a 0.5 % agarose solution lead to the formation of a gel with a non-adhesive surface and scaffold-free environment for multicellular aggregates to form.
Alginate is linear polysaccharide derived from brown seaweed and a co-polymer with alternating regions of mannuronic acid and guluronic acid. It forms a gel in the presence of calcium ions and has been used as a scaffold for encapsulation of various types of cells. Cells are suspended in sodium alginate solution and gel-formation is then induced by addition of calcium ions, which crosslink the polysaccharide chains to entrap the cells. This approach has allowed 3D-culturing of microdissected primary colorectal adenomas without overgrowth by fibroblasts [77] and of colorectal cell lines used for drug response testing [78, 79]. Because cells have no receptors to adhere to alginate, increased adhesion has been achieved by coupling RGD peptides that represent the natural binding site for a subset of integrins in ECM proteins, including fibronectin, laminin, vitronectin, and collagen [80]. Alternatively, alginate gels can be interpenetrated with collagen-I fibers [22]. In both cases, the calcium concentration also allows adjusting the gel’s stiffness, and this is the major experimental advantage of this hydrogel type.
Animal-derived hydrogels
The animal-derived matrices collagen and Matrigel are described in the following paragraphs and widely used, allowing cell surface receptors, such as integrins to interact with natural matrix proteins. Pioneering work from the laboratory of Mina Bissell has demonstrated the importance of creating physiologically more relevant in vitro culturing models through 3D techniques that include the interaction of cultured cells with an appropriate ECM [81, 82].
Adhesion between cells and the ECM includes mainly focal adhesions (FAs) and focal complexes, where transmembrane integrin receptors link the ECM to the cytoskeleton. This activates sub-membrane signal transduction complexes, which regulate microenvironmental sensing and cell motility. In consequence, cell–ECM interaction modifies cell signaling networks and gene expression, and this in turn determines the cellular response to pharmaceutical compounds [83–85]. The ECM further establishes drug diffusion gradients similar to tissues and can include effector proteins like growth factors. Therefore, such natural ECM-based 3D approaches are unique in that they can promote the generation of self-organized multicellular structures, such as the acini observed with mammary cells, or hollow cyst-like structures of polarized Caco-2 cells.
Collagen was one of the earliest biomaterials with widespread use for 3D cell culture and is the most abundant ECM component. Several tissue-specific types exist, such as collagen type I from skin, tendon and bone, type II in cartilage, type IV in basal lamina, and type V in hair. They have been used for 3D in vitro pharmacological testing [86] or cell invasion [87] of HT29 and HCT116 colorectal cells, and can induce morphological differentiation in some colon cell lines [88]. Collagen gel cultures have also been used to study the role of matrix-degrading metalloproteinases that are expressed in colorectal cells [89].
More complex collagen gels can be engineered as interpenetrating polymer networks with other ECM components, such as hyaluronic acid (HA), a linear non-sulphated polysaccharide composed of a repeating glucuronic acid, and N-acetyl-glucosamine disaccharide units. The negative charge of glucuronic acid fixes cations and water to form a gel, but HA is also the ligand for the cell surface receptor CD44 [90]. The presence of HA can thus affect signaling pathways and determine the biological activity of cancer cells, such as drug response [91].
Novel bioengineering techniques have led to the development of microscale collagen structures that mirror the density and size of human intestinal villi [92], or of microwells that allow the formation of crypts below a surrounding surface [93]. These microscale supports can then be colonized with Caco-2 or crypt-derived primary cells and can be expected to add a physiologically more realistic geometry to the cell models in the near future.
Matrigel-based techniques
Matrigel™ is the most widely used hydrogel and a commercial product collected from the Engelbreth–Holm–Swarm (EHS) tumor grown in mice. As a natural biomaterial, it contains a complex mixture of multiple ECM proteins and associated molecules, providing a framework of signals from the microenvironment. Matrigel, also known as reconstituted basement membrane (rBM), is mainly composed of laminin, type IV collagen, and heparin sulphate [94–96], but its exact constituents or residual growth factors are not clearly defined and suffer batch-to-batch variation. Although different tissue types deposit varying amounts and types of basement membrane components, Matrigel is considered similar to a conserved developmental basement membrane and can thus not only facilitate 3D organization of cells from different tissue types but also allow stem cell propagation.
Many epithelial cell types respond to Matrigel by ceasing proliferation and developing a polarized tissue-specific morphology, such as acinar structures from glands [97, 98] or capillary-like tube structure from the endothelium [99]. These structures allowed the identification of biological agents that repress the tumor phenotype and restore differentiated tissue architecture of malignant cell cultures, despite the presence of oncogenic mutations in their genomes [82, 98]. These studies elucidated that the ECM conveys important information that cannot be explained by cells being solitary entity defined only by their genome. Regarding the intestinal epithelium, some colorectal cell lines, such as HT29, form mere aggregates when cultured in Matrigel [100], whereas the other like Caco-2 is induced to grow as polarized cells and form cyst-like structures with a central lumen [101] (see Fig. 3b). The formation of the apical luminal space can involve hollowing through directional vesicle trafficking and cell–cell repulsion, or cavitation via luminal cell death [102].
With regard to colon cells, Matrigel has been used to enrich cell lines for cancer stem cells [103], to validate pharmacological inhibition of oncogenic signaling [104] or of spheroid growth [105], or to study the nutritional impact on intestinal crypt cell perturbation [106]. Caco-2 cells can be grown as 3D Matrigel cultures on cover slips or chamber slides and, subsequently, analyzed with most standard protocols, such as immunofluorescence analysis in formaldehyde-fixed preparations, Western blot analysis of protein expression levels after lysis in denaturing or non-denaturing sample buffers, or transcriptomics following total RNA extraction.
Limitations of Matrigel-based 3D cultures are related to batch variability and, therefore, some lack of reproducibility. In addition, the fact that it is generated by tumor cells may affect the response of cells grown in Matrigel. Other drawbacks concern handling difficulties. Matrigel must be kept on ice to keep its viscosity low enough for pipetting and prevent the gel formation that occurs at room temperature. Thus, to deposit Matrigel, for example, as 25-µL drops on cover slips or chamber slide wells, all involved materials need to be pre-chilled. Then, about 10,000 cells are seeded on top after polymerization at 37 °C giving rise to many 3D cysts in the same cushion. Once polymerized, the matrix is not suitable for long-term storage of samples, because it can collapse, especially when stored cooled. The dense gel structure does not allow easy retrieval of the individual 3D cell structures that form, and presents a diffusion barrier that can interfere with certain biochemical assays. From the biological perspective, Caco-2 cyst structures are well-suited to study effects from the basolateral side of the polarized epithelium, but the apical side is not easily accessible for testing the effect of microbes or luminal toxins. In addition, in co-culture approaches with fibroblasts, one needs to bear in mind that Matrigel components may suffer significant modification through MMP activity or secretion of additional ECM proteins [107].
Other scaffold material
Another development in the field is the use of decellularized extracellular matrix ‘ghosts’ that not only represent a biomechanical scaffold for cell–ECM adhesion, but also contain deposited signaling cues, such as growth factors and cytokines [108]. Such tissue-derived matrices (TDMs) have been prepared from rodent intestine [109] or human colon tumor biopsies [110] following decellularization with detergents and enzymes. When TDMs are then repopulated with specific cell types, they can help to elucidate how changes in the ECM, for example, during cancer or IBD, contribute to inflammation and the disease process. TDMs may further help to engineer a prosthetic seed structure to promote intestinal regeneration of damaged regions in vivo, or generate in vitro an intestinal tissue inoculum containing intestinal crypt or pluripotent stem cells, which can subsequently be introduced into the patient.
Other more recent types of hydrogels rely on fully synthetic scaffolds, such as polyethylene glycol (PEG) or repeating peptide sequences, and can mimic the nanofiber structure of an ECM while enabling chemical control over physical and adhesive properties [111–113]. Examples are Extracel™ combining HA, gelatin, and the crosslinker polyethylene glycol diacrylate, QGel® as a PEG-based matrix modified with Arg–Gly–Asp (RGD) sites for intergin binding, 3-DLife hydrogels as a two component polyvinyl alcohol-PEG gel, and PuraMatrix based on RADA-peptides that self-assemble into nanofiber structures. When compared with natural scaffolds, they are mechanically stiffer and, therefore, suitable for modeling the denser tumor microenvironment. However, they require surface modifications with RGD or other integrin-binding peptide sequences to allow cell attachment. In addition, some of the chemical reactions involved or the presence of toxic unpolymerized components (e.g., acrylamide) may have cytotoxic effects. Their suitability for intestinal or colorectal 3D models remains to be demonstrated.
Research on biomaterials has also generated solid scaffolds that provide a fibrous or porous physical support for seeding specific cell types. Such products are synthetic (for example polystyrene-based), supplied sterile and ready to use, and manufactured in highly reproducible technical processes [63, 114]. Their use with intestinal cells awaits functional validation.
Organoid cultures
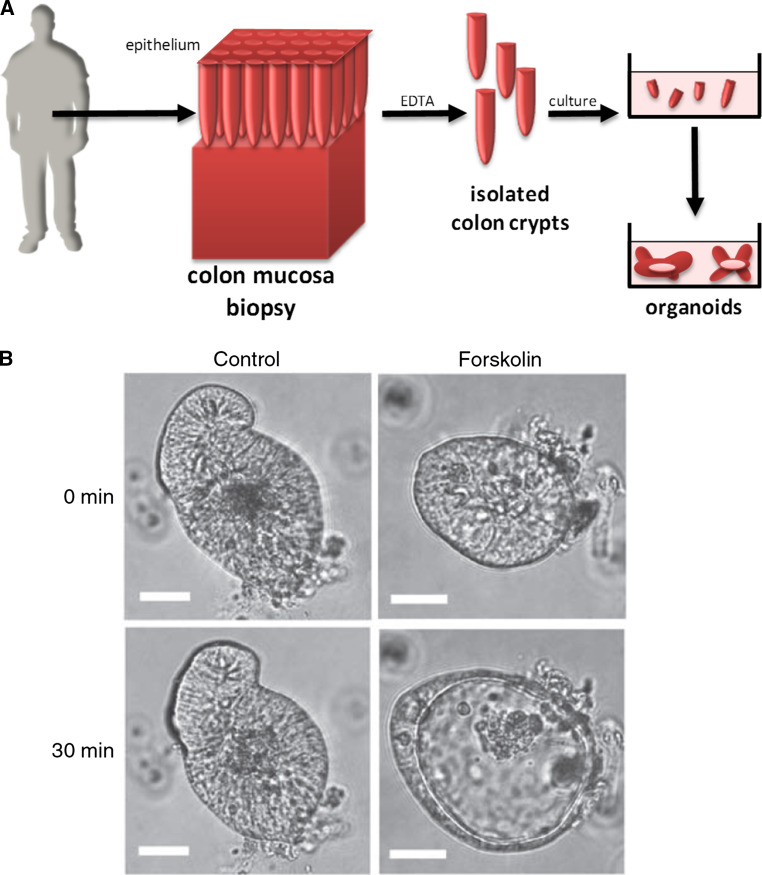
The epithelia of both the small and large intestines are constantly being regenerated through an adult stem cell population located at the basis of crypts. These stem cells generate highly proliferative cells that form the transit-amplifying compartment and from these the mature lineages of the surface epithelium progressively differentiate into goblet cells, entero- or colonocytes, enteroendocrine cells, or tuft cells [115]. Following the molecular identification of stem cell marker Lgr5, the corresponding mouse intestinal stem cells were isolated [116] and culture conditions developed allowing them to self-organize in vitro into a crypt-villus structure known as intestinal organoids or ‘mini-guts’ [117, 118] (Fig. 4a). These mini-guts contain the distinct cell types that are normally found in the gut besides enterocytes, including goblet and Paneth cells.
Fig. 4.
Patient-derived colon organoids. a Schematic representation of organoid generation from colon crypts obtained by patient biopsy. b Forskolin-induced swelling assay in colon organoids [120]. Phase contrast image of organoids from a colon biopsy grown in culture and treated with DMSO or forskolin to stimulate cAMP-dependent activation of CFTR. Intestinal CFTR is predominantly expressed at the apical membrane of colon cells, so that its activation drives the secretion of chloride and fluid into the central organoid lumen. Scale bar 30 μm
Later, single crypts, which can be readily isolated from mouse or human intestine biopsies by EDTA-based Ca2+/Mg2+ chelation, were found to grow with much higher efficiency into 3D organoids, because the association of stem cells with a Paneth cell retains essential information to form organoids in vitro [119]. In Matrigel, these mini-guts grow as cysts with a central lumen to which the enterocyte brush borders are located and the secretion by Paneth and goblet cells is directed. In the absence of Matrigel, however, they acquire the opposite conformation (i.e., apical side toward the outside of the spheroids). Crypt-like structures emanate at the basal side facing the Matrigel-containing medium. Thus, they represent a closed epithelial structure with a polarized topology comparable with that of physiological tissue.
Isolated intestinal crypts require Matrigel, and a cocktail of the Lgr-5-ligand R-spondin, EGF, and Noggin as minimal, essential stem cell maintenance factors. For colon crypt culture, Wnt3a is an additional factor required to maintain the stemness of Lgr5+ cells. Organoids grown from human colon are amenable to several standard experimental manipulations used for cell lines, including long-term storage by freezing, analysis by immunofluorescence microscopy, transcriptomic and proteomic analyses, and ion transport measurements [120–124]. For transfection or infection with recombinant retro- and lentiviruses, isolated crypts structures need to be first dissociated and then regrown into organoids [123, 125, 126].
Protocols have been developed to grow human epithelial mini-guts from biopsies [127] (Fig. 4a) and were applied to CF patients, namely, to assess the function of their CFTR protein [120, 128]. For example, five or six superficial rectal mucosa specimens (3–4 mm in diameter) can be recovered with colon forceps or suction and immediately placed into culture medium. Then, the rectal mucosa samples are EDTA-treated for 45–90 min to isolate the crypts. These are then cultured in Matrigel surrounded by medium, enriched with a cocktail of specific growth factors that maintains “stemness” of the epithelial stem cell compartment, including riboflavin (vitamin B2) and neuronal supplement N27, nicotinamide, N-acetyl-l-cystein (NAC), A83-01 (potent inhibitor of TGF-β type I receptor), and a p38 MAPK inhibition to stimulate stem cells to develop closed epithelial structures with an internal lumen.
After 2–3 weeks of culture mini-guts have grown and can be analyzed as primary patient-derived cell material. For example, Matrigel-grown organoids are closed epithelial structures that contain the internal lumen lined by the apical membrane and thus allow performing swelling assays. In particular, the cAMP-stimulating drug forskolin has been used to activate CFTR at the apical membrane, resulting in salt and fluid secretion into the organoid lumen and its corresponding rapid swelling (see Fig. 4b). This swelling event is greatly reduced in organoids derived from CF patients [120] and can be monitored using the fluorescent cell-permeable dye calcein green. Pharmacological compounds targeting CFTR can be screened with this assay in a primary human cell model to determine their ability to restore activity of mutant CFTR at the plasma membrane. Moreover, patient-derived organoids can be used to test for their individual response to different existing drugs. This personalized therapy approach is of particularly great value for patients bearing very rare mutations.
Organoids grown from patient-derived biopsies are also of major interest for cancer research to overcome the limitations of established cancer cell lines, which were generally derived by subjecting primary tumor cells to a challenging in vitro adaptation process. This leads to the selection of characteristics that favor metastatic and fast growing tumor cells, which, therefore, may not represent adequate models. Indeed, many promising candidate drugs to treat cancer perform well in preclinical cell line models but later fail to deliver a clinical response in animal or patient trials [58, 129]. With the high success rate of establishing organoid cultures from individual patient biopsies and their ability to expand in vitro, this technique may allow a novel approach to personalized medicine in oncology on the basis of cancer genetics and patient-cell drug sensitivity [130, 131]. It should be noted that tumor biopsies are usually polyclonal due to genetic heterogeneity and, therefore, is the corresponding organoid culture. The presence of sub-clonal organoid populations has interesting implications for predicting patient responses to therapy. A limitation is that crypts from normal adjacent mucosa were shown to overgrow the tumor organoids unless essential factors like Wnt or EGF are omitted from the culture medium [131]. Many tumor cells carry mutations in the corresponding signal transduction pathways, e.g., in the APC or KRAS genes, that turn them independent of the above stimuli; however, genetic subtypes of tumors without these mutations may be lost under these selective culture conditions.
Further limitations of organoids exist: despite the high level of intestinal differentiation of 3D organoid cultures, they do not experience physical stretching resulting from peristaltic contractions, in contrast to the gut-on-a-chip approach. They also consist largely of epithelial cells without any interaction with mesenchymal cell types or blood vessels. This aspect may be overcome by using embryonic or induced pluripotent stem cells (PSC) that can form organoids able to include sub-epithelial myofibroblasts, enteric nerves and immune cells [132, 133]. RNA-sequencing data demonstrated that organoids most closely resemble human foetal intestine and may be immature with respect to some metabolic and host-defence functions [121]. In addition, organoids form a sealed structure that cannot be cultured with living microbes at the luminal side. Developing these aspects of the model would be of special interest to research on IBD development or mucosa regeneration.
Final remarks
Significant advances in cell culture techniques have been described in the past 15 years and brought intestinal or colorectal cell models much closer to physiologically relevant systems. It is important to note, however, that the systems outlined above cannot provide all the required insights needed to understand complex biological questions related to the mucosa and some still lack functional validation in animal experiments. Therefore, for each technique, one should be aware of the limitations and, thus, the biological value of the obtained results. Ideally, a combination of different model systems with complementary properties should be employed to consolidate experimental data.
Acknowledgments
Work in the authors’ laboratories is supported by Fundação para a Ciência e Tecnologia (FCT) through center Grant UID/MULTI/04046/2013 (to BioISI), by research grants from FCT, Portugal (PTDC/BIM-MEC/2131/2014), CFF-Cystic Fibrosis Foundation, USA (AMARAL15XX0, AMARAL15XX1), Gilead GÉNESE-Portugal Programme (PGG/008/2015); CF Trust, UK (SRC 003) to MDA, and from Portuguese association for inflammatory bowel disease (GEDII 2013), Portuguese association Maratona da Saúde (Cancro 2014), Portugal to PJ. J.F.P. was supported by fellowships BRJ-DGH 2012_oncologia from Instituto Nacional de Saúde Doutor Ricardo Jorge (Lisbon, Portugal) and BD/109162/2015 from FCT.
Abbreviations
- CF
Cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- IBD
Inflammatory bowel disease
- ECM
Extracellular matrix
- TEER
Transepithelial electrical resistance
- TJ
Tight junction
- 2D or 3D
Two- or three-dimensional
References
- 1.Zhang K, Hornef MW, Dupont A. The intestinal epithelium as guardian of gut barrier integrity: the epithelium as a barrier to infection. Cell Microbiol. 2015;17:1561–1569. doi: 10.1111/cmi.12501. [DOI] [PubMed] [Google Scholar]
- 2.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 3.Sambuy Y, De Angelis I, Ranaldi G, et al. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 4.Liang GH, Weber CR. Molecular aspects of tight junction barrier function. Curr Opin Pharmacol. 2014;19:84–89. doi: 10.1016/j.coph.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci CMLS. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bosscher K, Hill CS, Nicolás FJ. Molecular and functional consequences of Smad4 C-terminal missense mutations in colorectal tumour cells. Biochem J. 2004;379:209–216. doi: 10.1042/bj20031886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992;103:414–423. doi: 10.1016/0016-5085(92)90829-N. [DOI] [PubMed] [Google Scholar]
- 9.Vachon PH, Perreault N, Magny P, Beaulieu JF. Uncoordinated, transient mosaic patterns of intestinal hydrolase expression in differentiating human enterocytes. J Cell Physiol. 1996;166:198–207. doi: 10.1002/(SICI)1097-4652(199601)166:1<198::AID-JCP21>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Ingber DE. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol Quant Biosci Nano Macro. 2013;5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 11.Meunier V, Bourrié M, Berger Y, Fabre G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol Toxicol. 1995;11:187–194. doi: 10.1007/BF00756522. [DOI] [PubMed] [Google Scholar]
- 12.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102(Pt 3):581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 13.des Rieux A, Fievez V, Théate I, et al. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2007;30:380–391. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Gullberg E, Leonard M, Karlsson J, et al. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun. 2000;279:808–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 15.Kerneis S. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 16.Corr SC, Gahan CCGM, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52:2–12. doi: 10.1111/j.1574-695X.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 17.Niedergang F, Kraehenbuhl JP. Much ado about M cells. Trends Cell Biol. 2000;10:137–141. doi: 10.1016/S0962-8924(00)01731-1. [DOI] [PubMed] [Google Scholar]
- 18.Brayden DJ, Jepson MA, Baird AW. Keynote review: intestinal Peyer’s patch M cells and oral vaccine targeting. Drug Discov Today. 2005;10:1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- 19.Lai YH, D’Souza MJ. Microparticle transport in the human intestinal M cell model. J Drug Target. 2008;16:36–42. doi: 10.1080/10611860701639848. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed D, Eide PW, Eilertsen IA, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouradov D, Sloggett C, Jorissen RN, et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014;74:3238–3247. doi: 10.1158/0008-5472.CAN-14-0013. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri O, Koshy ST, Branco da Cunha C, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 23.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tung JC, Barnes JM, Desai SR, et al. Tumor mechanics and metabolic dysfunction. Free Radic Biol Med. 2015;79:269–280. doi: 10.1016/j.freeradbiomed.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen KA, Abshire MY, Tilghman RW, et al. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS One. 2011;6:e23123. doi: 10.1371/journal.pone.0023123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nukuda A, Sasaki C, Ishihara S, et al. Stiff substrates increase YAP-signaling-mediated matrix metalloproteinase-7 expression. Oncogenesis. 2015;4:e165. doi: 10.1038/oncsis.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolznig H, Rupp C, Puri C, et al. Modeling colon adenocarcinomas in vitro a 3D co-culture system induces cancer-relevant pathways upon tumor cell and stromal fibroblast interaction. Am J Pathol. 2011;179:487–501. doi: 10.1016/j.ajpath.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin TJ, Jessup JM, Wolf DA. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. Vitro Cell Dev Biol J Tissue Cult Assoc. 1992;28A:47–60. doi: 10.1007/BF02631079. [DOI] [PubMed] [Google Scholar]
- 29.Paduch R, Kandefer-Szerszeń M, Piersiak T. The importance of release of proinflammatory cytokines, ROS, and NO in different stages of colon carcinoma growth and metastasis after treatment with cytotoxic drugs. Oncol Res. 2010;18:419–436. doi: 10.3727/096504010X12671222663593. [DOI] [PubMed] [Google Scholar]
- 30.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fath KR, Mamajiwalla SN, Burgess DR. The cytoskeleton in development of epithelial cell polarity. J Cell Sci. 1993;1993:65–73. doi: 10.1242/jcs.1993.Supplement_17.10. [DOI] [PubMed] [Google Scholar]
- 33.Massey-Harroche D. Epithelial cell polarity as reflected in enterocytes. Microsc Res Tech. 2000;49:353–362. doi: 10.1002/(SICI)1097-0029(20000515)49:4<353::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. doi: 10.1016/S0016-5085(89)80072-1. [DOI] [PubMed] [Google Scholar]
- 35.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst. 1997;14:221–286. doi: 10.1615/CritRevTherDrugCarrierSyst.v14.i3.20. [DOI] [PubMed] [Google Scholar]
- 39.Hirtz S, Gonska T, Seydewitz HH, et al. CFTR Cl− channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology. 2004;127:1085–1095. doi: 10.1053/j.gastro.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Mall M, Wissner A, Seydewitz HH, et al. Defective cholinergic Cl(−) secretion and detection of K(+) secretion in rectal biopsies from cystic fibrosis patients. Am J Physiol Gastrointest Liver Physiol. 2000;278:G617–G624. doi: 10.1152/ajpgi.2000.278.4.G617. [DOI] [PubMed] [Google Scholar]
- 41.Sousa M, Servidoni MF, Vinagre AM, et al. Measurements of CFTR-mediated Cl- secretion in human rectal biopsies constitute a robust biomarker for Cystic Fibrosis diagnosis and prognosis. PLoS One. 2012;7:e47708. doi: 10.1371/journal.pone.0047708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beekman JM, Sermet-Gaudelus I, de Boeck K, et al. CFTR functional measurements in human models for diagnosis, prognosis and personalized therapy. J Cyst Fibros. 2014;13:363–372. doi: 10.1016/j.jcf.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Botelho HM, Uliyakina I, Awatade NT, et al. Protein traffic disorders: an effective high-throughput fluorescence microscopy pipeline for drug discovery. Sci Rep. 2015;5:9038. doi: 10.1038/srep09038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duell BL, Cripps AW, Schembri MA, Ulett GC. Epithelial cell coculture models for studying infectious diseases: benefits and limitations. J Biomed Biotechnol. 2011;2011:852419. doi: 10.1155/2011/852419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models. Annu Rev Pathol Mech Dis. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- 46.Huh D, Kim HJ, Fraser JP, et al. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 47.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 49.Sung JH, Esch MB, Prot J-M, et al. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellas E, Chen CS. Forms, forces, and stem cell fate. Curr Opin Cell Biol. 2014;31:92–97. doi: 10.1016/j.ceb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasparski AN, Beningo KA. Mechanoreception at the cell membrane: more than the integrins. Arch Biochem Biophys. 2015;586:20–26. doi: 10.1016/j.abb.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen KA, Donato DM, Balcioglu HE, et al. A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci. 2016;113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SH, Lee JW, Choi I, et al. A microfluidic device with 3-d hydrogel villi scaffold to simulate intestinal absorption. J Nanosci Nanotechnol. 2013;13:7220–7228. doi: 10.1166/jnn.2013.8088. [DOI] [PubMed] [Google Scholar]
- 56.Hickman JA, Graeser R, de Hoogt R, et al. Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J. 2014;9:1115–1128. doi: 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- 57.Hay M, Thomas DW, Craighead JL, et al. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 58.Hutchinson L, Kirk R. High drug attrition rates—where are we going wrong? Nat Rev Clin Oncol. 2011;8:189–190. doi: 10.1038/nrclinonc.2011.34. [DOI] [PubMed] [Google Scholar]
- 59.Longati P, Jia X, Eimer J, et al. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer. 2013;13:95. doi: 10.1186/1471-2407-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thoma CR, Zimmermann M, Agarkova I, et al. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev. 2014;69–70:29–41. doi: 10.1016/j.addr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Achilli T-M, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12:1347–1360. doi: 10.1517/14712598.2012.707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burdett E, Kasper FK, Mikos AG, Ludwig JA. Engineering tumors: a tissue engineering perspective in cancer biology. Tissue Eng Part B Rev. 2010;16:351–359. doi: 10.1089/ten.teb.2009.0676. [DOI] [PubMed] [Google Scholar]
- 63.Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 2014;227:746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Tang Z, Zhao Y, et al. Three-dimensional in vitro cancer models: a short review. Biofabrication. 2014;6:022001. doi: 10.1088/1758-5082/6/2/022001. [DOI] [PubMed] [Google Scholar]
- 65.Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan X, Ouyang N, Teng H, Yao H. Isolation and characterization of spheroid cells from the HT29 colon cancer cell line. Int J Colorectal Dis. 2011;26:1279–1285. doi: 10.1007/s00384-011-1248-y. [DOI] [PubMed] [Google Scholar]
- 67.Fang DD, Kim YJ, Lee CN, et al. Expansion of CD133+ colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br J Cancer. 2010;102:1265–1275. doi: 10.1038/sj.bjc.6605610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashley N, Jones M, Ouaret D, et al. Rapidly derived colorectal cancer cultures recapitulate parental cancer characteristics and enable personalized therapeutic assays. J Pathol. 2014;234:34–45. doi: 10.1002/path.4371. [DOI] [PubMed] [Google Scholar]
- 69.Hirschhaeuser F, Menne H, Dittfeld C, et al. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Ham SL, Atefi E, Fyffe D, Tavana H. Robotic production of cancer cell spheroids with an aqueous two-phase system for drug testing. J Vis Exp JoVE. 2015 doi: 10.3791/52754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leung BM, Lesher-Perez SC, Matsuoka T, et al. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci. 2015;3:336–344. doi: 10.1039/C4BM00319E. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich J, Eder W, Castaneda J, et al. A reliable tool to determine cell viability in complex 3-D culture: the acid phosphatase assay. J Biomol Screen. 2007;12:925–937. doi: 10.1177/1087057107306839. [DOI] [PubMed] [Google Scholar]
- 73.Howes AL, Chiang GG, Lang ES, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6:2505–2514. doi: 10.1158/1535-7163.MCT-06-0698. [DOI] [PubMed] [Google Scholar]
- 74.Wenzel C, Riefke B, Gründemann S, et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp Cell Res. 2014;323:131–143. doi: 10.1016/j.yexcr.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Keane JC, Kupchik HZ, Schroy PC, et al. A three-dimensional system for long-term culture of human colorectal adenomas. Am J Pathol. 1990;137:1539–1547. [PMC free article] [PubMed] [Google Scholar]
- 78.Buhrmann C, Shayan P, Kraehe P, et al. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- 79.Shakibaei M, Kraehe P, Popper B, et al. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15:250. doi: 10.1186/s12885-015-1291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 81.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bellis AD, Bernabé BP, Weiss MS, et al. Dynamic transcription factor activity profiling in 2D and 3D cell cultures. Biotechnol Bioeng. 2013;110:563–572. doi: 10.1002/bit.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubashkin MG, Ou G, Weaver VM. Deconstructing signaling in three dimensions. Biochemistry (Mosc) 2014;53:2078–2090. doi: 10.1021/bi401710d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magdeldin T, López-Dávila V, Villemant C, et al. The efficacy of cetuximab in a tissue-engineered three-dimensional in vitro model of colorectal cancer. J Tissue Eng. 2014;5:2041731414544183. doi: 10.1177/2041731414544183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nyga A, Loizidou M, Emberton M, Cheema U. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013;9:7917–7926. doi: 10.1016/j.actbio.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Buono R, Pignatelli M, Bodmer WF, Wright NA. The role of the arginine-glycine-aspartic acid-directed cellular binding to type I collagen and rat mesenchymal cells in colorectal tumour differentiation. Differ Res Biol Divers. 1991;46:97–103. doi: 10.1111/j.1432-0436.1991.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto Overexpression of MT1-MMP is insufficient to increase experimental liver metastasis of human colon cancer cells. Int J Mol Med. 1998;22:757–761. [PubMed] [Google Scholar]
- 90.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 91.Kassim YL, Tawil EAL, Lecerf D, Couteau J, Simon T, Buquet C, Vannier JP, Demange E. Biomimetic three dimensional cell culturing: colorectal cancer micro-tissue engineering. J Clin Exp Oncol. 2014;3:2. [Google Scholar]
- 92.Sung JH, Yu J, Luo D, et al. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389–392. doi: 10.1039/C0LC00273A. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Ahmad AA, Sims CE, et al. In vitro generation of colonic epithelium from primary cells guided by microstructures. Lab Chip. 2014;14:1622–1631. doi: 10.1039/C3LC51353J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benton G, Arnaoutova I, George J, et al. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79–80:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 95.Benton G, George J, Kleinman HK, Arnaoutova IP. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009;221:18–25. doi: 10.1002/jcp.21832. [DOI] [PubMed] [Google Scholar]
- 96.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 97.Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG) J Cell Sci. 1996;109(Pt 8):2013–2021. doi: 10.1242/jcs.109.8.2013. [DOI] [PubMed] [Google Scholar]
- 98.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ludwig K, Tse ES, Wang JY. Colon cancer cells adopt an invasive phenotype without mesenchymal transition in 3-D but not 2-D culture upon combined stimulation with EGF and crypt growth factors. BMC Cancer. 2013;13:221. doi: 10.1186/1471-2407-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pereira B, Sousa S, Barros R, et al. CDX2 regulation by the RNA-binding protein MEX3A: impact on intestinal differentiation and stemness. Nucleic Acids Res. 2013;41:3986–3999. doi: 10.1093/nar/gkt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol CB. 2011;21:R126–R136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeung TM, Gandhi SC, Wilding JL, et al. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci USA. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luca AC, Mersch S, Deenen R, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One. 2013;8:e59689. doi: 10.1371/journal.pone.0059689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guruswamy S, Swamy MV, Choi C-I, et al. S-Adenosyl l-methionine inhibits azoxymethane-induced colonic aberrant crypt foci in F344 rats and suppresses human colon cancer Caco-2 cell growth in 3D culture. Int J Cancer J Int Cancer. 2008;122:25–30. doi: 10.1002/ijc.23031. [DOI] [PubMed] [Google Scholar]
- 106.Mah AT, Van Landeghem L, Gavin HE, et al. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology. 2014;155:3302–3314. doi: 10.1210/en.2014-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pereira C, Araújo F, Barrias CC, et al. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials. 2015;56:36–45. doi: 10.1016/j.biomaterials.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 108.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 109.Totonelli G, Maghsoudlou P, Garriboli M, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401–3410. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genovese L, Zawada L, Tosoni A, et al. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng Part A. 2014;20:2005–2018. doi: 10.1089/ten.tea.2013.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lowe SB, Tan VTG, Soeriyadi AH, et al. Synthesis and high-throughput processing of polymeric hydrogels for 3D cell culture. Bioconjug Chem. 2014;25:1581–1601. doi: 10.1021/bc500310v. [DOI] [PubMed] [Google Scholar]
- 112.Trappmann B, Chen CS. How cells sense extracellular matrix stiffness: a material’s perspective. Curr Opin Biotechnol. 2013;24:948–953. doi: 10.1016/j.copbio.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels—versatile matrices for 3D cell culture in cancer medicine. Front Oncol. 2015;5:92. doi: 10.3389/fonc.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 115.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 116.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 117.Jung P, Sato T, Merlos-Suárez A, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 118.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 119.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 120.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 121.Finkbeiner SR, Hill DR, Altheim CH, et al. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Rep. 2015;4:1140–1155. doi: 10.1016/j.stemcr.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Foulke-Abel J, In J, Yin J, et al. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology. 2016 doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Onuma K, Ochiai M, Orihashi K, et al. Genetic reconstitution of tumorigenesis in primary intestinal cells. Proc Natl Acad Sci. 2013;110:11127–11132. doi: 10.1073/pnas.1221926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walker NM, Liu J, Stein SR, et al. Cellular chloride and bicarbonate retention alters intracellular pH regulation in Cftr KO crypt epithelium. Am J Physiol Gastrointest Liver Physiol. 2015;310:G70–G80. doi: 10.1152/ajpgi.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drost J, van Jaarsveld RH, Ponsioen B, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 126.Fujii M, Matano M, Nanki K, Sato T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc. 2015;10:1474–1485. doi: 10.1038/nprot.2015.088. [DOI] [PubMed] [Google Scholar]
- 127.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 128.Schwank G, Koo B-K, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 129.Caponigro G, Sellers WR. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat Rev Drug Discov. 2011;10:179–187. doi: 10.1038/nrd3385. [DOI] [PubMed] [Google Scholar]
- 130.Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 131.van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zachos NC, Kovbasnjuk O, Foulke-Abel J, et al. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem. 2016 doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stange DE, Clevers H. Concise review: the Yin and Yang of intestinal (cancer) stem cells and their progenitors: intestinal (Cancer) stem cells and their progenitors. Stem Cells. 2013;31:2287–2295. doi: 10.1002/stem.1475. [DOI] [PubMed] [Google Scholar]
- 135.Kuo W-T, Lee T-C, Yang H-Y, et al. LPS receptor subunits have antagonistic roles in epithelial apoptosis and colonic carcinogenesis. Cell Death Differ. 2015;22:1590–1604. doi: 10.1038/cdd.2014.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abbott Chalew TE, Schwab KJ. Toxicity of commercially available engineered nanoparticles to Caco-2 and SW480 human intestinal epithelial cells. Cell Biol Toxicol. 2013;29:101–116. doi: 10.1007/s10565-013-9241-6. [DOI] [PubMed] [Google Scholar]
- 137.Freeman TJ, Smith JJ, Chen X, et al. Smad4-mediated signaling inhibits intestinal neoplasia by Inhibiting expression of β-catenin. Gastroenterology. 2012;142(562–571):e2. doi: 10.1053/j.gastro.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirsch D, Barker N, McNeil N, et al. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis. 2014;35:849–858. doi: 10.1093/carcin/bgt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elimrani I, Dionne S, Saragosti D, et al. Acetylcarnitine potentiates the anticarcinogenic effects of butyrate on SW480 colon cancer cells. Int J Oncol. 2015 doi: 10.3892/ijo.2015.3029. [DOI] [PubMed] [Google Scholar]
- 140.Matsuda Y, Miura K, Yamane J, et al. SERPINI1 regulates the epithelial-mesenchymal transition in an orthotopic implantation model of colorectal cancer. Cancer Sci. 2016 doi: 10.1111/cas.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993;265:C859–C868. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- 142.Dharmsathaphorn K, McRoberts JA, Mandel KG, et al. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 143.Lee WY, Chin AC, Voss S, Parkos CA. In vitro neutrophil transepithelial migration. Methods Mol Biol Clifton NJ. 2006;341:205–215. doi: 10.1385/1-59745-113-4:205. [DOI] [PubMed] [Google Scholar]
- 144.McCool DJ, Marcon MA, Forstner JF, Forstner GG. The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J. 1990;267:491–500. doi: 10.1042/bj2670491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nataro JP, Hicks S, Phillips AD, et al. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bu X-D, Li N, Tian X-Q, Huang P-L. Caco-2 and LS174T cell lines provide different models for studying mucin expression in colon cancer. Tissue Cell. 2011;43:201–206. doi: 10.1016/j.tice.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Mologni L, Brussolo S, Ceccon M, Gambacorti-Passerini C. Synergistic effects of combined Wnt/KRAS inhibition in colorectal cancer cells. PLoS One. 2012;7:e51449. doi: 10.1371/journal.pone.0051449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Klinken BJ, Oussoren E, Weenink JJ, et al. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj J. 1996;13:757–768. doi: 10.1007/BF00702340. [DOI] [PubMed] [Google Scholar]
- 149.Basu I, Mitra R, Saha PK, et al. Morphological and cytoskeletal changes caused by non-membrane damaging cytotoxin of Vibrio cholerae on int 407 and HeLa cells. FEMS Microbiol Lett. 1999;179:255–263. doi: 10.1111/j.1574-6968.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 150.Canonico B, Campana R, Luchetti F, et al. Campylobacter jejuni cell lysates differently target mitochondria and lysosomes on HeLa cells. Apoptosis Int J Program Cell Death. 2014;19:1225–1242. doi: 10.1007/s10495-014-1005-0. [DOI] [PubMed] [Google Scholar]
- 151.Henle G, Deinhardt F. The establishment of strains of human cells in tissue culture. J Immunol Baltim Md 1950. 1957;79:54–59. [PubMed] [Google Scholar]
- 152.Lacroix M. Persistent use of “false” cell lines. Int J Cancer. 2008;122:1–4. doi: 10.1002/ijc.23233. [DOI] [PubMed] [Google Scholar]
- 153.Sarem F, Sarem-Damerdji LO, Nicolas JP. Comparison of the adherence of three Lactobacillus strains to Caco-2 and Int-407 human intestinal cell lines. Lett Appl Microbiol. 1996;22:439–442. doi: 10.1111/j.1472-765X.1996.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 154.Antunes F, Andrade F, Araújo F, et al. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm. 2013;83:427–435. doi: 10.1016/j.ejpb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 155.Béduneau A, Tempesta C, Fimbel S, et al. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur J Pharm Biopharm. 2014;87:290–298. doi: 10.1016/j.ejpb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 156.Chen X-M, Elisia I, Kitts DD. Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J Pharmacol Toxicol Methods. 2010;61:334–342. doi: 10.1016/j.vascn.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 157.Hilgendorf C, Spahn-Langguth H, Regårdh CG, et al. Caco-2 versus caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci. 2000;89:63–75. doi: 10.1002/(SICI)1520-6017(200001)89:1<63::AID-JPS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 158.Johansson MEV, Ambort D, Pelaseyed T, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Navabi N, McGuckin MA, Lindén SK. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS One. 2013;8:e68761. doi: 10.1371/journal.pone.0068761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pontier C, Pachot J, Botham R, et al. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J Pharm Sci. 2001;90:1608–1619. doi: 10.1002/jps.1111. [DOI] [PubMed] [Google Scholar]
- 161.Walter E, Janich S, Roessler BJ, et al. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: in vitro-in vivo correlation with permeability data from rats and humans. J Pharm Sci. 1996;85:1070–1076. doi: 10.1021/js960110x. [DOI] [PubMed] [Google Scholar]
- 162.Lesuffleur T, Porchet N, Aubert JP, et al. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993;106(Pt 3):771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- 163.Alcarraz-Vizán G, Sánchez-Tena S, Moyer MP, Cascante M. Validation of NCM460 cell model as control in antitumor strategies targeting colon adenocarcinoma metabolic reprogramming: trichostatin A as a case study. Biochim Biophys Acta BBA Gen Subj. 2014;1840:1634–1639. doi: 10.1016/j.bbagen.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 164.Henriques A, Barros P, Moyer MP, et al. Expression of tumour-related Rac1b antagonizes B-Raf-induced senescence in colorectal cells. Cancer Lett. 2015;369:368–375. doi: 10.1016/j.canlet.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 165.Lea MA, Ibeh C, Shah N, Moyer MP. Induction of differentiation of colon cancer cells by combined inhibition of kinases and histone deacetylase. Anticancer Res. 2007;27:741–748. [PubMed] [Google Scholar]
- 166.Liu Z, Kang L, Li C, et al. Knockout of MIMP protein in lactobacillus plantarum lost its regulation of intestinal permeability on NCM460 epithelial cells through the zonulin pathway. BMC Gastroenterol. 2014;14:171. doi: 10.1186/1471-230X-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Z, Shen T, Chen H, et al. Functional characterization of MIMP for its adhesion to the intestinal epithelium. Front Biosci Landmark Ed. 2011;16:2106–2127. doi: 10.2741/3842. [DOI] [PubMed] [Google Scholar]
- 168.Matos P, Kotelevets L, Goncalves V, et al. Ibuprofen inhibits colitis-induced overexpression of tumor-related Rac1b. Neoplasia. 2013;15:102–111. doi: 10.1593/neo.121890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Minoo P, Moyer MP, Jass JR. Role of BRAF-V600E in the serrated pathway of colorectal tumourigenesis. J Pathol. 2007;212:124–133. doi: 10.1002/path.2160. [DOI] [PubMed] [Google Scholar]
- 170.Moyer MP, Manzano LA, Merriman RL, et al. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 171.Sahi J, Nataraja SG, Layden TJ, et al. Cl- transport in an immortalized human epithelial cell line (NCM460) derived from the normal transverse colon. Am J Physiol. 1998;275:C1048–C1057. doi: 10.1152/ajpcell.1998.275.4.C1048. [DOI] [PubMed] [Google Scholar]
- 172.Schäfer H, Struck B, Feldmann E-M, et al. TGF-β1-dependent L1CAM expression has an essential role in macrophage-induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene. 2013;32:180–189. doi: 10.1038/onc.2012.44. [DOI] [PubMed] [Google Scholar]
- 173.Zhao D, Keates AC, Kuhnt-Moore S, et al. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–44471. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]