Abstract
Objectives
Australia has made significant progress towards in achieving the UNAIDS’s 95–95–95 cascade targets including HIV viral suppression. To investigate the burden of HIV viremia, we assessed viral blips, low-level viremia (LLV) and virologic failure (VF) in an Australian cohort.
Methods
We studied the proportion of people with viral suppression, viral blips, LLV and VF in the Australian HIV Observational database (AHOD) between 2010–2021. The association between blips or LLV, and VF were investigated using Cox regression, and predictors of viral blips and LLV were assessed using repeated-measured logistic regression.
Results
Among 2544 AHOD participants who were in follow-up and on ART from 1 January 2010 (88.7% male), 444 had experienced VF (incidence rate: 2.45 [95%CI: 2.23, 2.69] per 100 person-years [PY]) during 18,125 PY of follow-up (a median of 7.6 years). The proportion of people with VF decreased over time while rates of blips and LLV remained stable. Participants with blips (hazard ratio [HR], 2.89, 95% CI: 2.31, 3.61) and LLV (4.46, 95% CI: 3.38, 5.89) were at increased risk of VF. Hepatitis B co-infection, longer documented treatment interruption duration, younger age and lower CD4 at antiretroviral therapy (ART) initiation, and protease inhibitors-based initial regimen were associated with an increased risk of VF. Common predictors of blips and LLV such as higher HIV-1 RNA and lower CD4 at ART initiation, longer treatment interruption, more VL testing and types of care settings (hospitals vs. sexual health services) were identified.
Conclusions
Blips and LLV predict subsequent VF development. We identified important predictors of HIV viremia including VF among individuals on INSTI-based regimens to help direct HIV management plans.
Keywords: Viral blip, Low-level viremia, Virological failure, Treatment failure, HIV
Background
HIV treatment and viral suppression prevents HIV-related illness, averts AIDS-related deaths, prevents onwards HIV transmission, and prevents development of drug resistance [1–3]. In recent years, Australia has made significant progresses towards achieving the UNAIDS’s 95–95–95 targets [4, 5]. A high proportion of people living with HIV have achieved virological suppression [5–8], with a nearly 98% suppression rate by the end of 2021 [5]. Despite these efforts, in Australia, a small percentage of people with HIV on treatment still experience viremia [5, 6, 8].
Detectable HIV viremia may be due to viral load blips which are transient and small increases in VL, low level viraemia (LLV) which is persistent but low levels of viremia between the detection limits of the assay used and 200 copies/ml, or virologic failure (VF) with confirmed levels of viremia >200 copies/ml. Even with highly efficacious antiretroviral treatment (ART) options, VF has implications for disease progression, transmission, and the need for treatment change [9, 10]. Data are conflicting regarding whether blips and LLV increase the risk of subsequent VF [10–15]. A recent study from a large European Multicenter Cohort, which grouped viremia level experienced by people with HIV on ART as suppression, blips, and LLV categories, found that both blips and LLV were associated with increased risks of subsequent VF [10]. However, others have demonstrated inconsistent findings on the associations of either blip or LLV with VF, especially when different VL cutoffs were used to define the viremia groups [9, 12, 13].
A comprehensive understanding of the factors associated with HIV viremia can inform clinician responses to virological blips and LLV in clinical HIV care and therefore to assist Australia in meeting the UNAIDS’s target of virological suppression. In this study, we investigate the proportions of individuals with VF, detectable viral loads due to blips and LLV in the AHOD cohort between 2010–2021. Further, we investigate whether blips and LLV are associated with development of subsequent VF and individual factors associated with people with HIV experiencing blips, LLV and VF.
Methods
Study population
AHOD cohort was established since 1999 and its primary goals are to evaluate the trends in ART use and HIV disease and treatment outcomes among people with HIV in Australia. Since its inception a total of 31 clinical sites around Australia, including general practice clinicals, hospitals and sexual health clinics, have contributed data to AHOD. A detailed description of the cohort had previously been published [16, 17]. All 31 AHOD sites contributed data in this analysis and all AHOD participants on ART and in follow-up from 1st January 2010 were included. Data from sites that are no longer contributing data were administratively censored at their last data transfer date. Additional criteria for inclusion were having had at least 6 months of treatment resulting in virological suppression based on a VL carried out between 6–12 months after initiation of treatment, followed by at least 1 further year of follow-up and at least one VL every year during ART.
Outcomes
We aimed to estimate the rates of HIV viremia and to describe factors associated with viral blips, LLV and VF among participants on at least 3-drug combination ART or national guideline-endorsed dual therapy since 2010. For each year between 2010 and 2021, we described the proportion of participants who have VF, detectable VLs due to blips, and LLV. Secondly, we investigated factors associated with VF, viral blip and LLV.
In the primary analysis, the proportion of people with detectable VLs for each year between 2010 and 2021 was identified and were classified as follows: a) ‘VF’ defined as 2 consecutive VLs of ≥200 copies/mL or a single VL of ≥1000 copies/mL while on ART and b) a single ‘blip’ defined as a single/isolated VL of between 51 copies/mL and 999 copies/mL immediately preceded and followed by a VL ≤50 copies/mL, and c) ‘LLV’ defined as ≥2 consecutive VLs of 51–200 copies/mL ≥30 days apart. Readings within 30 days of each other were considered a single blip. Blips that occurred within 6 months of a treatment switch due to treatment failure or during periods of loss to follow-up were excluded. Additionally, if a participant had one VL episode of 51–200 copies/mL with one VL episode of 201–1000 copies/mL, followed by <200 copies/mL, they were categorized as LLV in a sensitivity analysis since these episodes did not meet the definition for VF.
Statistical analysis
Outcomes for VF, blips and LLV are presented by key participant characteristics (demographics, clinical and HIV-related). Data are presented using medians with interquartile ranges (IQRs) for continuous data and frequencies and percentages for categorical data.
In calculating the proportions of viremia episodes (VF, blips and LLV) per calendar year between 2010 and 2021, the episodes that spanned December and January were categorized into the respective calendar years by applying appropriate weighting to account for the proportionate representation of each episode across the years.
For the VF outcome analysis, a univariable Cox proportional hazard regression was carried out to investigate the associations between VF and the following variables: age group, sex, HIV exposure (male to male sex (MSM), heterosexual sex and injection drug use), duration of known HIV infection, Australian vs overseas born, duration of ART, CD4 cell count and VL at ART initiation, initial ART regimen (NNRTI, PI, INSTI and Other), HBV/HCV coinfection status, patient care setting (general practice [GP], hospital, sexual health clinics [SHC]), number of VL measurements, duration of documented treatment interruption. The duration of treatment interruption of participants was determined based on the number of days during which they did not have a documented history of being dispensed for ART from clinics. The documented treatment interruption was modelled as time-varying variable in the regression models. In the analysis using VF as an outcome, we investigated whether individuals with or without viral blips and LLV subsequently predict VF.
Viremia groups (blip, LLV and VF) were also separately modelled as time-varying variables and we allowed the reclassification only to a higher viremia group (i.e., viral suppression < blip < LLV < VF). Therefore, the viremia category included in the analyses were the highest historical VL result for each participant post-ART initiation [10]. Kaplan-Meier methods were also used to estimate the incidence of VF depending on viremia category (viral suppression, blip or LLV). All potential confounding variables were included in multivariable Cox proportional hazard regression regardless of their significance in the univariable analysis results. All statistical tests were two-sided, and statistical significance was set at p-value < 0.05.
For the analyses of viral blip and LLV outcomes, only participants who had 4 or more VL measurements were included in these analyses since at least 3 VLs are needed to define blips and LLVs. Univariable and multivariable random-effects repeated-measure logistic regression was used to investigate the individual factors associated with viral blips and LLV. We censored follow-up at last visit or at time of VF if that occurred. All analyses were adjusted by site to account for the heterogeneity of healthcare systems. SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for data management and Stata software version 16.1 (StataCorp, College Station, TX, USA) was used to perform all analyses.
Sensitivity analysis
Sensitivity analyses for viral blips were conducted using VL cut-offs of 51–200 copies/mL to define blips. In the analyses that further explored whether individuals with viral blips or LLV subsequently developed VF, we conducted a sensitivity analysis using a single VL of ≥500 copies/mL as cut-off for virological failure (instead of ≥1000 copies/mL used in the primary analysis). Additional sensitivity analyses limiting only to participants who started ART after 1st January 2010 for all the outcomes mentioned in the above, analyses excluding those experienced treatment interruption and analyses limited to individuals started with only INSTI-based ART were conducted.
Ethics statement
Written informed consent was obtained from participating individuals. Ethics approval for the AHOD study was granted by St Vincent’s Human Research Ethics committee, Sydney (IRB 00002019)., and all other relevant institutional review boards.
Results
A total of 2544 people living with HIV who were on ART and in follow up from 1st January 2010 in AHOD were included in the analysis. Of them, 88.7% were male and 28.7% were born outside Australia/New Zealand. The median CD4 count at ART initiation was 320 (IQR: 200–489) cells/mm3 and the median age at ART initiation was 39 (IQR: 32–47) years. Hepatitis B and C co-infection was reported in 80 (3.1%) and 215 (8.5%) of AHOD participants, respectively. The median duration of known HIV infection and ART was 14.7 (8.5–21.6) and 11.3 years (6.6–17.5), respectively. Table 1 shows the detailed demographic characteristics of participants included.
Table 1.
Participant characteristics
| Number (%) or Median (IQR) | Total (N=2544, 100%) | No VF (N=2100, 82.5%) | VF (N=444, 17.5%) | |||
|---|---|---|---|---|---|---|
| Frequency or Median | Percentage or IQR | Frequency or Median | Percentage or IQR | Frequency or Median | Percentage or IQR | |
| Age at ART initiation | 39 | 32, 47 | 40 | 33, 48 | 37 | 30. 44 |
| <=30 | 449 | 17.7 | 338 | 16.1 | 111 | 25.0 |
| 31–40 | 907 | 35.7 | 739 | 35.2 | 168 | 37.8 |
| 41–50 | 717 | 28.2 | 598 | 28.5 | 119 | 26.8 |
| >50 | 471 | 18.5 | 425 | 20.2 | 46 | 10.4 |
| Sex | ||||||
| Male | 2,257 | 88.7 | 1,857 | 88.4 | 400 | 90.1 |
| Female | 287 | 11.3 | 243 | 11.6 | 44 | 9.9 |
| Country of birth | ||||||
| Australia & New Zealand | 1,532 | 60.2 | 1,245 | 59.3 | 287 | 64.6 |
| Overseas | 729 | 28.7 | 619 | 29.5 | 110 | 24.8 |
| Unknown | 283 | 11.1 | 236 | 11.2 | 47 | 10.6 |
| HIV mode of acquisition | ||||||
| MSM | 1,808 | 71.1 | 1,500 | 71.4 | 308 | 69.4 |
| Injecting drug use | 134 | 5.3 | 96 | 4.6 | 38 | 8.6 |
| Heterosexual | 516 | 20.3 | 435 | 20.7 | 81 | 18.2 |
| Other/Unknown | 86 | 3.4 | 69 | 3.3 | 17 | 3.8 |
| CD4 at ART initiation, cells/mm 3 | 320 | 200, 489 | 320 | 203, 490 | 290 | 168, 451 |
| <=200 | 497 | 19.5 | 392 | 18.7 | 105 | 23.7 |
| 201–350 | 564 | 22.2 | 480 | 22.9 | 84 | 18.9 |
| 351–500 | 402 | 15.8 | 349 | 16.6 | 53 | 11.9 |
| 500+ | 433 | 17.0 | 366 | 17.4 | 67 | 15.1 |
| Missing | 648 | 25.5 | 513 | 24.4 | 135 | 30.4 |
| HIV RNA at ART initiation, copies/mL | ||||||
| <=100,000 | 1,183 | 46.5 | 994 | 47.3 | 189 | 42.6 |
| >100,000 | 564 | 22.2 | 449 | 21.4 | 115 | 25.9 |
| Missing | 797 | 31.3 | 657 | 31.3 | 140 | 31.5 |
| Treatment interruption duration | ||||||
| No interruption | 1,568 | 61.6 | 1,420 | 67.6 | 148 | 33.3 |
| 1-<14 days | 267 | 10.5 | 238 | 11.3 | 29 | 6.5 |
| 14 days – 3 months | 128 | 5.0 | 101 | 4.8 | 27 | 6.1 |
| 3 months – 6 months | 62 | 2.4 | 33 | 1.6 | 29 | 6.5 |
| > 6 months | 519 | 20.4 | 308 | 14.7 | 211 | 47.5 |
| HBV surface antigen positivity | ||||||
| Negative | 2,055 | 80.8 | 1,676 | 79.8 | 379 | 85.4 |
| Positive | 80 | 3.1 | 60 | 2.9 | 20 | 4.5 |
| Unknown | 409 | 16.1 | 364 | 17.3 | 45 | 10.1 |
| HCV antibody positivity | ||||||
| Negative | 2,087 | 82.0 | 1,732 | 82.5 | 355 | 80.0 |
| Positive | 215 | 8.5 | 165 | 7.9 | 50 | 11.3 |
| Unknown | 242 | 9.5 | 203 | 9.7 | 39 | 8.8 |
| Number of VL measurement, median (IQR) | 14 | (8–23) | 13 | (7–22) | 18 | (10–26) |
| ART type commenced | ||||||
| NRTI+NNRTI | 1,251 | 49.2 | 1,058 | 50.4 | 193 | 43.5 |
| NRTI+PI | 691 | 27.2 | 522 | 24.9 | 169 | 38.1 |
| NRTI+INSTI | 436 | 17.1 | 396 | 18.9 | 40 | 9.0 |
| Other | 166 | 6.5 | 124 | 5.9 | 42 | 9.5 |
| Year of ART initiation | ||||||
| <=2005 | 955 | 37.5 | 733 | 34.9 | 222 | 50.0 |
| 2006–2010 | 696 | 27.4 | 567 | 27.0 | 129 | 29.1 |
| 2011–2015 | 701 | 27.6 | 619 | 29.5 | 82 | 18.5 |
| 2016–2022 | 192 | 7.6 | 181 | 8.6 | 11 | 2.5 |
| Participant care setting | ||||||
| Sexual health services | 1229 | 48.3 | 1003 | 47.8 | 226 | 50.9 |
| Genera Practice | 863 | 33.9 | 729 | 34.7 | 134 | 30.2 |
| Hospital/Tertiary referral settings | 452 | 17.8 | 368 | 17.5 | 84 | 18.9 |
| Duration of HIV (years), median (IQR) | 14.7 | 8.5 to 21.6 | 13.9 | 8.1 to 20.8 | 17.2 | 11.8 to 23.6 |
| Duration of ART (years), median (IQR) | 11.3 | 6.6 to 17.5 | 10.6 | 6.3 to 17.1 | 13.4 | 8.9 to 19.5 |
Abbreviations: ART, antiretroviral therapy; MSM, male to male sex; VL, viral load, IQR, interquartile range; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor.
Virological Failure
During 18,125 person-years of follow-up (PYFU) (median 7.6 [3.7–10.9] years), 444 participants experienced VF (incidence rate: 2.45 [95%CI 2.23–2.69] per 100 PYFU). As shown in Table 1, those who had VF were younger and had lower CD4 count at ART initiation (23.7% with VF and 18.7% without VF had CD4 count ≤200 cells/mm3). The proportion of people with viral suppression, blip, LLV, and VF during the years 2010–2021 is shown in Figure 1. Overall, the proportion of people with VF was lower in recent years, with a decrease from 9% in 2010 to 3% in 2021.
Figure 1. HIV viremia group (viral suppression, blip, low-level viremia, and virological failure) in AHOD.
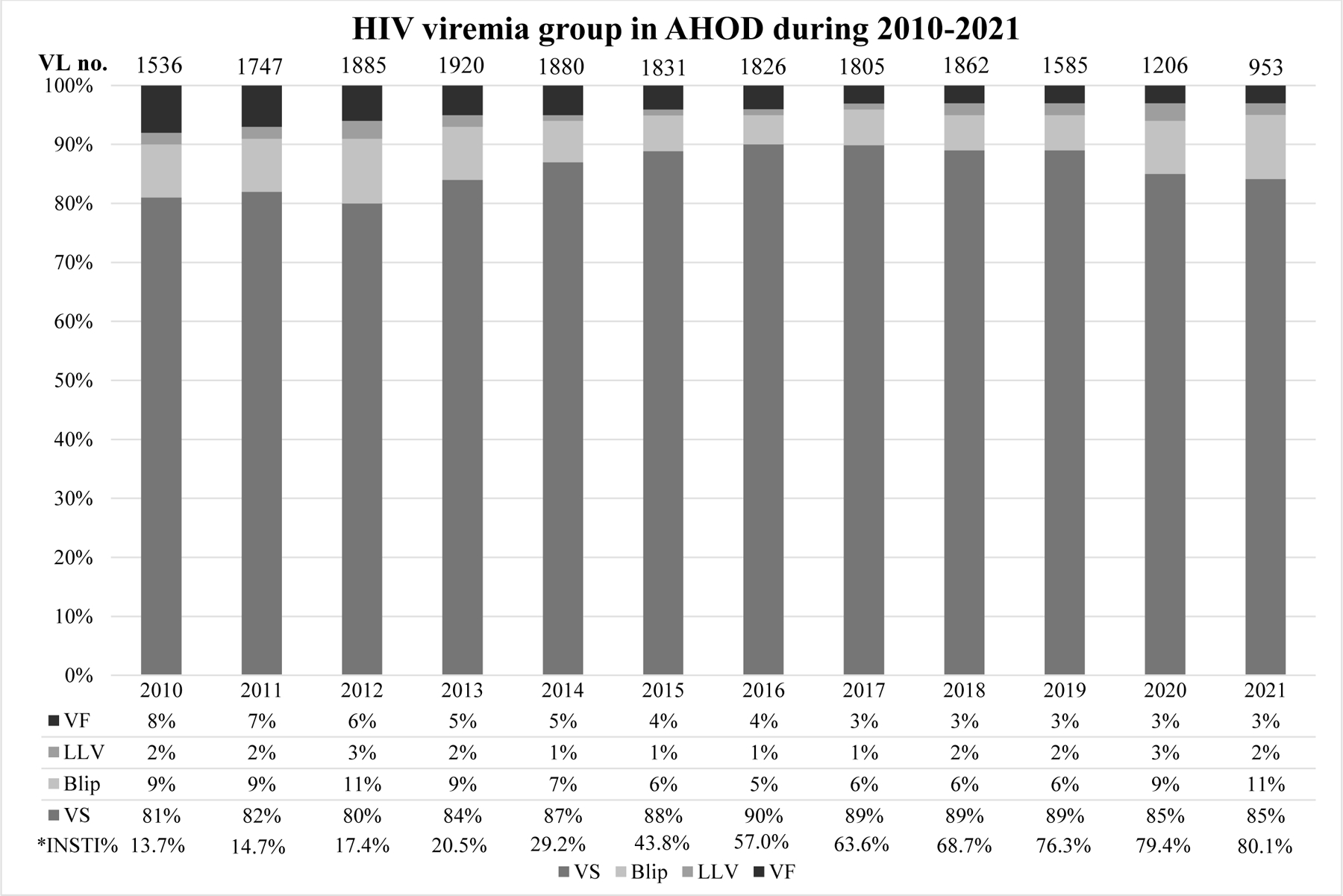
The proportion of VS, blip, LLV and VF are presented for each year between 2010 and 2021 in AHOD. The definitions of the viremia group are included in Methods. *The proportion of integrase strand transfer inhibitors (INSTI) use over time is presented for each year. Abbreviations: VS, viral suppression; LLV, low-level viremia; VF, virological failure.
Using Kaplan-Meier methods, the estimated probability of VF up to 12 years of ART initiation was 21% (95%CI: 19.2–22.9). Figure 2 shows the probability of virological failure by viremia group which was higher in participants with LLV and blip than in those with viral suppression, log rank p <0.001), respectively.
Figure 2.

Probability of virological failure by viremia group
In multivariable analysis, hepatitis B co-infection (adjusted hazard ratio [aHR]: 1.75, 95%CI 1.11–2.78), longer treatment interruption duration: 14 days to 3 months (2.42, 95%CI: 1.58–3.6), 3 months to 6 months (6.90, 95%CI: 4.47–10.64) and >6 months (6.23, 95%CI: 4.82–8.05) vs. no treatment interruption, higher number of VL measurements (per 5-times increase, 1.09, 95%CI: 1.03–1.16), NRTI+PI as initial ART regimen (1.34, 95%CI: 1.08–1.67; vs. NRTI+NNRTI), viral blip (2.78, 95%CI: 2.23–3.46) and LLV (1.69, 95%CI: 1.32–2.17) were associated with increased VF risk. Older participants, those with higher CD4 count at ART initiation and those who had longer duration of ART had reduced risk of VF (Multivariable model I, Table 2). The association between hepatitis B co-infection and VF remained significant in a multivariable model additionally adjusted for time-varying exposure to ARVs with anti-HBV activity (i.e., TDF- or TAF-containing regimen).
Table 2.
Factors associated with virological failure in AHOD using Cox regression
| Univariable | Multivariable model I | Multivariable model II | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years of follow-up (PYFU) | virological failure (n) | IR per 100 PYFU (95% CI) | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | |
| Overall (N=2544) | 18125.26 | 444 | 2.45 (2.23, 2.69) | ||||||
| Age at ART initiation | <0.001 | <0.001 | <0.001 | ||||||
| <=30 | 2655.35 | 111 | 4.18 (3.47, 5.03) | Ref | Ref | Ref | |||
| 31–40 | 6441.68 | 168 | 2.61 (2.24, 3.03) | 0.67 (0.53, 0.86) | 0.67 (0.53, 0.86) | 0.67 (0.52, 0.85) | |||
| 41–50 | 5366.04 | 119 | 2.22 (1.85, 2.65) | 0.58 (0.45, 0.75) | 0.59 (0.45, 0.77) | 0.58 (0.44, 0.76) | |||
| >50 | 3662.19 | 46 | 1.26 (0.94, 1.68) | 0.33 (0.24, 0.47) | 0.33 (0.23, 0.47) | 0.33 (0.23, 0.47) | |||
| Sex | |||||||||
| Male | 16144.74 | 400 | 2.48 (2.25, 2.73) | Ref | Ref | Ref | |||
| Female | 1980.51 | 44 | 2.22 (1.65, 2.99) | 0.88 (0.64, 1.20) | 0.408 | 0.73 (0.49, 1.1) | 0.13 | 0.72 (0.48, 1.08) | 0.11 |
| Country of birth | 0.298 | 0.46 | 0.46 | ||||||
| Australia & New Zealand | 11304.32 | 287 | 2.54 (2.26, 2.85) | Ref | Ref | Ref | |||
| Overseas | 4847.12 | 110 | 2.27 (1.88, 2.85) | 0.84 (0.68, 1.05) | 0.89 (0.69, 1.13) | 0.9 (0.71, 1.15) | |||
| Unknown | 1973.82 | 47 | 2.38 (1.79, 3.17) | 0.92 (0.67, 1.25) | 0.83 (0.59, 1.18) | 0.83 (0.59, 1.17) | |||
| HIV mode of acquisition | 0.008 | 0.88 | 0.88 | ||||||
| MSM | 13090.20 | 308 | 2.35 (2.10, 2.63) | Ref | Ref | Ref | |||
| Injecting drug use | 842.44 | 38 | 4.51 (3.28, 6.2) | 1.84 (1.31, 2.57) | 1.18 (0.81, 1.71) | 1.13 (0.78, 1.64) | |||
| Heterosexual | 3567.87 | 81 | 2.27 (1.83, 2.82) | 0.94 (0.73, 1.20) | 1.08 (0.78, 1.48) | 1.09 (0.79, 1.5) | |||
| Other/Unknown | 624.74 | 17 | 2.72 (1.69, 4.38) | 1.16 (0.71, 1.89) | 0.97 (0.59, 1.62) | 0.97 (0.59, 1.62) | |||
| CD4 at ART initiation, cells/mm 3 | 0.001 | 0.005 | 0.005 | ||||||
| <=200 | 3617.00 | 105 | 2.90 (2.40, 3.51) | Ref | Ref | Ref | |||
| 201–350 | 4277.51 | 84 | 1.96 (1.59, 2.43) | 0.68 (0.51, 0.91) | 0.74 (0.55, 0.99) | 0.74 (0.55, 0.99) | |||
| 351–500 | 2997.37 | 53 | 1.77 (1.35, 2.31) | 0.61 (0.44, 0.84) | 0.63 (0.44, 0.88) | 0.63 (0.44, 0.88) | |||
| 500+ | 2796.21 | 67 | 2.40 (1.89, 3.04) | 0.78 (0.57, 1.06) | 0.83 (0.6, 1.15) | 0.84 (0.6, 1.17) | |||
| Missing | 4437.17 | 135 | 3.04 (2.57, 3.6) | 1.02 (0.79, 1.31) | 1.16 (0.84, 1.62) | 1.18 (0.85, 1.63) | |||
| HIV RNA at ART initiation, copies/mL | 0.596 | 0.24 | 0.24 | ||||||
| <=100,000 | 8446.14 | 189 | 2.24 (1.94, 2.58) | Ref | Ref | Ref | |||
| >100,000 | 4010.69 | 115 | 2.87 (2.39, 3.44) | 1.29 (1.02, 1.62) | 1.08 (0.85, 1.38) | 1.1 (0.87, 1.4) | |||
| Missing | 5668.43 | 140 | 2.47 (2.09, 2.91) | 1.11 (0.89, 1.38) | 0.85 (0.63, 1.15) | 0.84 (0.62, 1.12) | |||
| Documented treatment interruption duration * | <0.001 | <0.001 | <0.001 | ||||||
| No interruption | 11065.04 | 148 | 1.34 (1.14, 1.57) | Ref | Ref | Ref | |||
| 1-<14 days | 2327.51 | 29 | 1.25 (0.87, 1.79) | 1.03 (0.69, 1.53) | 1.10 (0.73, 1.66) | 0.74 (1.67, 1.66) | |||
| 14 days – 3 months | 926.15 | 27 | 2.92 (2.00, 4.25) | 2.29 (1.52, 3.45) | 2.42 (1.58, 3.69) | 1.61 (3.76, 3.69) | |||
| 3 months – 6 months | 398.22 | 29 | 7.28 (5.06, 10.48) | 5.64 (3.79, 8.39) | 6.9 (4.47, 10.64) | 4.19 (9.98, 10.64) | |||
| > 6 months | 3408.34 | 211 | 6.19 (5.41, 7.08) | 4.88 (3.95, 6.03) | 6.23 (4.82, 8.05) | 4.81 (8.05, 8.05) | |||
| HBV surface antigen positivity | 0.013 | 0.001 | 0.001 | ||||||
| Negative | 14841.18 | 379 | 2.55 (2.31, 2.82) | Ref | Ref | Ref | |||
| Positive | 517.79 | 20 | 3.86 (2.49, 5.99) | 1.45 (1.02, 2.27) | 1.75 (1.11, 2.78) | 1.80 (1.13, 2.85) | |||
| Unknown | 2766.28 | 45 | 1.63 (1.21, 2.18) | 0.61 (0.45, 0.83) | 0.60 (0.4, 0.88) | 0.62 (0.42, 0.91) | |||
| HCV antibody positivity | 0.03 | 0.06 | 0.054 | ||||||
| Negative | 15035.41 | 355 | 2.36 (2.13, 2.62) | Ref | Ref | Ref | |||
| Positive | 1497.29 | 50 | 3.34 (2.53, 4.41) | 1.41 (1.05, 1.89) | 0.99 (0.72, 1.37) | 1.02 (0.74, 1.41) | |||
| Unknown | 1592.56 | 39 | 2.45 (1.79, 3.35) | 0.98 (0.71, 1.37) | 1.73 (1.14, 2.62) | 1.66 (1.1, 2.51) | |||
| Number of VL measurement (per 5-unit increase) | 1.11 (1.06, 1.16) | <0.001 | 1.09 (1.03, 1.16) | 0.004 | 1.1 (1.03, 1.17) | 0.004 | |||
| ART type commenced | <0.001 | 0.03 | 0.03 | ||||||
| NRTI+NNRTI | 9658.96 | 193 | 2 (1.74, 2.3) | Ref | Ref | Ref | |||
| NRTI+PI | 5028.11 | 169 | 3.36 (2.89, 3.91) | 1.67 (1.36, 2.06) | 1.34 (1.08, 1.67) | 1.35 (1.08, 1.68) | |||
| NRTI+INSTI | 2267.34 | 40 | 1.76 (1.29, 2.41) | 0.71 (0.50, 1.00) | 1.21 (0.8, 1.84) | 1.21 (0.80, 1.83) | |||
| Other | 1170.85 | 42 | 3.59 (2.65, 4.85) | 1.75 (1.25, 2.44) | 1.38 (0.97, 1.97) | 1.42 (1, 2.02) | |||
| Year of ART initiation | <0.001 | <0.001 | <0.001 | ||||||
| <=2005 | 7509.33 | 222 | 2.96 (2.59, 3.37) | Ref | Ref | Ref | |||
| 2006–2010 | 5663.17 | 129 | 2.28 (1.92, 2.71) | 0.76 (0.61, 0.95) | 0.54 (0.37, 0.77) | 0.56 (0.38, 0.8) | |||
| 2011–2015 | 4229.26 | 82 | 1.94 (1.56, 2.41) | 0.54 (0.42, 0.70) | 0.31 (0.19, 0.49) | 0.32 (0.2, 0.51) | |||
| 2016–2022 | 723.5 | 11 | 1.52 (0.84, 2.75) | 0.33 (0.18, 0.60) | 0.19 (0.08, 0.44) | 0.20 (0.09, 0.46) | |||
| Duration of ART (per 5-year increase) | 0.53 (0.43, 0.60) | <0.001 | 0.50 (0.42, 0.59) | <0.001 | 0.50 (0.43, 0.59) | <0.001 | |||
| Participant care setting | 0.08 | 0.014 | 0.013 | ||||||
| Sexual health services | 8395.00 | 226 | 2.69 (2.36, 3.07) | Ref | Ref | Ref | |||
| GP | 6526.71 | 134 | 2.05 (1.73, 2.43) | 0.79 (0.64, 0.98) | 0.71 (0.56, 0.89) | 0.71 (0.56, 0.90) | |||
| Hospital | 3203.55 | 84 | 2.62 (2.12, 3.25) | 0.99 (0.77, 1.27) | 0.98 (0.74, 1.29) | 0.98 (0.74, 1.29) | |||
| Viral blip * | |||||||||
| No | 11590.68 | 154 | 1.33 (1.13, 1.56) | Ref | Ref | ||||
| Yes | 6534.58 | 290 | 4.45 (3.97, 4.99) | 3.39 (2.79, 4.12) | <0.001 | 2.78 (2.23, 3.46) | <0.001 | ||
| Low level viremia * | |||||||||
| No | 16543.37 | 350 | 2.12 (1.91, 2.35) | Ref | Ref | ||||
| Yes | 1581.89 | 94 | 6.02 (4.92, 7.37) | 2.78;(2.21, 3.49) | <0.001 | 1.69 (1.32, 2.17) | <0.001 | ||
| Viremia group ** | <0.001 | <0.001 | |||||||
| Viral suppression | 11511.2 | 147 | 1.28 (1.09, 1.5) | Ref | Ref | ||||
| Blip | 5052.08 | 203 | 4.02 (3.5, 4.61) | 3.20 (2.59, 3.95) | 2.89 (2.31, 3.61) | ||||
| LLV | 1561.98 | 94 | 6.02 (4.92, 7.37) | 4.61 (3.56, 5.98) | 4.46 (3.38, 5.89) |
Time-updated variables
Viremia group was included as a time-updated covariate and reclassification was allowed only for a higher VL group.
All analyses were adjusted by site to account for the heterogeneity of healthcare systems.
Global p-values are tested for heterogeneity excluding missing values.
Abbreviations: ART, antiretroviral therapy; MSM, male to male sex; VL, viral load, NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor; PYS, person-years follow up; IR, incidence rate; HR, hazard ratio.
In addition, when viremia group was included as a time-varying variable in the model which allowed the viremia reclassification only to a higher group, viral blip and LLV had significantly higher risk of VF compared to those with viral suppression (Multivariable model II, Table 2). The association of blips and LLV with increased VF risk was consistent in a multivariable Cox regression adjusting for time-varying ART regimens (aHR for blips: 2.94. 95%CI: 2.30–3.76; aHR for LLV: 4.50, 95%CI: 3.32–6.11 vs. viral suppression).
Sensitivity analyses:
The sensitivity analysis using a VF cutoff of 500 copies/mL, instead of 1000 copies/mL, is shown in Supplementary Table S1. Overall, 475 VF events were included (VF rate of 2.64, (95%CI: 2.42–2.89 per 100 PYFU), and results were consistent, including factors associated with VF. A total of 1078 participants were included in the second sensitivity analysis that was limited to participants who started ART from 2010. There were 128 VF events, resulting in an incidence rate of 1.98 (95% CI: 1.67–2.36) per 100 PYFU. The proportion of people with VF over the study period (2010–2021) was lower (ranging from 3–5%) than the proportion in the primary analysis (Supplementary Table S2). The factors associated with VF in this sensitivity analysis are presented in Supplementary Table S3. Viral blip and LLV were associated with VF in the multivariable Cox regression models.
In the sensitivity analysis where VL cutoff defining a blip was 51–200 copies/mL, the number of blips was reduced from 290 in the main analysis to 246 in the sensitivity analysis. Multivariable analysis shows that a viral blip remained a significant predictor for VF, although the magnitude of association was moderately reduced (aHR:2.19, 95% CI: 1.76–2.73).
There were 68 VF events which occurred during treatment interruption periods in the primary analysis. We conducted a sensitivity analysis excluding those who experienced treatment interruption, and blips and LLV remained associated with increased risks of VF (Supplementary table S4). Finally, when we limited the analysis only to participants who started with an INSTI regimen, blips and LLV still remained associated with higher subsequent VF risk (aHR for blip: 6.02, 95%CI: 2.29–15.87, and for LLV: 11.05, 95%CI: 3.06–43.21) (Supplementary table S5).
Viral blips
The proportion of people with a viral blip was stable at 6–11% during the years 2010–2021 (Figure 1). Using multivariable repeated measured logistic regression, factors associated with viral blips were age ≥50 years at ART initiation (adjusted odds ratio [aOR]: 0.75, 95%CI: 0.6–0.93; vs. ≤30 years), heterosexual (1.24, 95%CI: 1.02–1.51; vs. MSM), higher CD4 cell count ART initiation (201–350 cells/mm3: 0.68, 95%CI: 0.56–0.82, 351–500 cells/mm3: 0.57, 95% CI: 0.46–0.70, and >500 cells/mm3: 0.52, 95%CI: 0.41–0.65; vs. ≤200 cells/mm3), longer duration of treatment interruption (3–6 months: 1.53, 95%CI: 1.07–2.18 and >6 months: 1.62, 95%CI: 1.35–1.93), higher frequency of VL measurement (per 5-unit increase: 1.1, 95%CI: 1.06–1.15), longer duration of ART (per 5-year increase: 0.78, 95%CI: 0.69–0.88) and participants from hospitals 0.82, 95%CI: 0.68–0.98; vs. sexual health services (Table 3).
Table 3.
Factors associated with viral blip in AHOD using repeated measured logistic regression
| Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | ||
| Age at ART initiation | 0.12 | 0.049 | |||
| <=30 | Ref | Ref | |||
| 31–40 | 0.85 (0.71, 1.03) | 0.88 (0.73, 1.06) | |||
| 41–50 | 0.89 (0.74, 1.08) | 0.93 (0.77, 1.13) | |||
| >50 | 0.77 (0.62, 0.96) | 0.75 (0.6, 0.93) | |||
| Sex | |||||
| Male | Ref | Ref | |||
| Female | 1.02 (0.83, 1.26) | 0.85 | 0.82 (0.64, 1.06) | 0.14 | |
| Country of birth | 0.07 | 0.08 | |||
| Australia & New Zealand | Ref | Ref | |||
| Overseas | 1 (0.86, 1.15) | 0.91 (0.78, 1.06) | |||
| Unknown | 0.78 (0.63, 0.97) | 0.7 (0.56, 0.88) | |||
| HIV mode of acquisition | 0.018 | 0.07 | |||
| MSM | Ref | Ref | |||
| Injecting drug use | 1.41 (1.07, 1.86) | 1.31 (0.98, 1.75) | |||
| Heterosexual | 1.2 (1.03, 1.41) | 1.24 (1.02, 1.51) | |||
| Other/Unknown | 1.12 (0.8, 1.56) | 1.1 (0.78, 1.54) | |||
| CD4 at ART initiation, cells/mm 3 | <0.001 | <0.001 | |||
| <=200 | Ref | Ref | |||
| 201–350 | 0.68 (0.56, 0.82) | 0.68 (0.56, 0.82) | |||
| 351–500 | 0.59 (0.48, 0.74) | 0.57 (0.46, 0.7) | |||
| 500+ | 0.59 (0.47, 0.73) | 0.52 (0.41, 0.65) | |||
| Missing | 0.78 (0.65, 0.93) | 0.82 (0.66, 1.03) | |||
| HIV RNA at ART initiation, copies/mL | 0.03 | 0.37 | |||
| <=100,000 | Ref | Ref | |||
| >100,000 | 1.24 (1.05, 1.45) | 1.09 (0.93, 1.28) | |||
| Missing | 1.03 (0.89, 1.2) | 0.94 (0.78, 1.14) | |||
| Documented treatment interruption duration * | <0.001 | <0.001 | |||
| No interruption | Ref | Ref | |||
| 1-<14 days | 0.93 (0.75, 1.15) | 0.98 (0.79, 1.22) | |||
| 14 days – 3 months | 1.06 (0.8, 1.42) | 1.13 (0.85, 1.51) | |||
| 3 months – 6 months | 1.45 (1.02, 2.07) | 1.53 (1.07, 2.18) | |||
| > 6 months | 1.36 (1.17, 1.59) | 1.62 (1.35, 1.93) | |||
| HBV surface antigen positivity | 0.77 | 0.94 | |||
| Negative | Ref | Ref | |||
| Positive | 1.08 (0.76, 1.54) | 1.01 (0.71, 1.44) | |||
| Unknown | 0.95 (0.79, 1.14) | 0.96 (0.77, 1.2) | |||
| HCV antibody positivity | 0.38 | 0.98 | |||
| Negative | Ref | Ref | |||
| Positive | 1.11 (0.88, 1.38) | 1.02 (0.8, 1.3) | |||
| Unknown | 1.03 (0.81, 1.3) | 1.02 (0.78, 1.35) | |||
| Number of VL measurement (per 5-unit increase) | 1.03 (1, 1.07) | 0.05 | 1.1 (1.06, 1.15) | <0.001 | |
| ART type commenced | 0.30 | 0.57 | |||
| NRTI+NNRTI | Ref | Ref | |||
| NRTI+PI | 1.09 (0.94, 1.26) | 1.06 (0.91, 1.23) | |||
| NRTI+INSTI | 1.12 (0.92, 1.38) | 1.02 (0.79, 1.32) | |||
| Other | 0.88 (0.68, 1.15) | 0.87 (0.67, 1.14) | |||
| Year of ART initiation | 0.011 | <0.001 | |||
| <2005 | Ref | Ref | |||
| 2006–2010 | 0.87 (0.75, 1.02) | 0.68 (0.52, 0.88) | |||
| 2011–2015 | 1.03 (0.87, 1.21) | 0.84 (0.6, 1.16) | |||
| 2016–2022 | 1.45 (1.07, 1.98) | 1.2 (0.73, 1.96) | |||
| Duration of ART (per 5-year increase) | 0.97 (0.93, 1.02) | 0.28 | 0.78 (0.69, 0.88) | <0.001 | |
| Participant care setting | 0.36 | 0.015 | |||
| Sexual health services | Ref | Ref | |||
| GP | 1.02 (0.88, 1.18) | 1.09 (0.94, 1.27) | |||
| Hospital | 0.89 (0.75, 1.07) | 0.82 (0.68, 0.98) | |||
Time-updated variables
Global p-values are tested for heterogeneity excluding missing values.
Abbreviations: ART, antiretroviral therapy; MSM, male to male sex; VL, viral load, NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor; OR, odds ratio.
In the sensitivity analysis which we limited to participants who started ART from 2010, the proportion of viral blip over the study period was comparable to the primary analysis (Supplementary Table S2). We also found that higher HIV-1 RNA at ART initiation (≥100,000 copies/mL, aOR: 1.3, 95%CI: 1.03–1.63; vs. <100,000 copies/mL) was found to be associated with increased risk of viral blip in addition to the predictors in the primary analysis (i.e., lower CD4 cell count, treatment interruption duration >6 months, number of VL measurements, duration of ART). Among participants who started ART after 2010 in the sensitivity analysis (Supplementary Table S4), GP clinic attendees were more likely to have viral blips compared to sexual health service attendees.
Similar factors were identified when the cutoff of 51–200 copies/mL was used to define blips in the sensitivity analysis. Higher HIV-1 RNA (≥100,000 copies/mL) at ART initiation remained associated with blips.
Low-level viremia
The proportion of people with LLV was lower than those with viral blips and stable at 1–4% during the years 2010–2021 (Figure 1). In multivariable analysis, factors associated with LLV were lower CD4 cell count, longer duration of treatment interruption, longer duration on ART, higher number of VL measurements and those who were clients of hospitals compared to sexual health services (Table 4). When we limited the analysis to participants starting ART after 1 January 2010, treatment interruption duration and patient care setting were no longer associated with LLV compared to the results from the primary analysis. However, high level of HIV-1 RNA at ART initiation was associated with LLV among those who started ART after 2010 (Supplementary Table S4).
Table 4.
Factors associated with low-level viremia in AHOD using repeated measured logistic regression
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR | P-value | Adjusted OR | P-value | |
| Age at ART initiation | 0.06 | 0.21 | ||
| <=30 | Ref | Ref | ||
| 31–40 | 0.65 (0.38, 1.12) | 0.66 (0.38, 1.15) | ||
| 41–50 | 1.16 (0.68, 1.99) | 1.07 (0.61, 1.87) | ||
| >50 | 1.15 (0.64, 2.07) | 0.98 (0.53, 1.8) | ||
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.9 (0.5, 1.61) | 0.72 | 0.79 (0.38, 1.63) | 0.52 |
| Country of birth | 0.87 | 0.22 | ||
| Australia | Ref | Ref | ||
| Overseas | 0.89 (0.59, 1.35) | 0.75 (0.48, 1.18) | ||
| Unknown | 0.95 (0.54, 1.69) | 0.62 (0.33, 1.16) | ||
| HIV mode of acquisition | 0.49 | 0.39 | ||
| MSM | Ref | Ref | ||
| Injecting drug use | 0.98 (0.43, 2.22) | 0.9 (0.36, 2.24) | ||
| Heterosexual | 1.29 (0.83, 2) | 1.32 (0.76, 2.3) | ||
| Other/Unknown | 0.6 (0.21, 1.74) | 0.5 (0.16, 1.54) | ||
| CD4 at ART initiation, cells/mm 3 | 0.008 | 0.006 | ||
| <=200 | Ref | Ref | ||
| 201–350 | 0.54 (0.32, 0.92) | 0.54 (0.31, 0.93) | ||
| 351–500 | 0.39 (0.21, 0.72) | 0.37 (0.19, 0.71) | ||
| 500+ | 0.48 (0.26, 0.87) | 0.41 (0.22, 0.79) | ||
| Missing | 0.85 (0.51, 1.39) | 0.9 (0.48, 1.68) | ||
| HIV RNA at ART initiation, copies/mL | 0.07 | 0.44 | ||
| <=100,000 | Ref | Ref | ||
| >100,000 | 1.65 (1.05, 2.59) | 1.35 (0.85, 2.14) | ||
| Missing | 1.41 (0.93, 2.15) | 1.14 (0.66, 1.97) | ||
| Document treatment interruption duration * | 0.76 | 0.28 | ||
| No interruption | Ref | Ref | ||
| 1-<14 days | 1.02 (0.56, 1.84) | 1.1 (0.58, 2.06) | ||
| 14 days – 3 months | 1.51 (0.71, 3.25) | 1.59 (0.72, 3.5) | ||
| 3 months – 6 months | 1.09 (0.37, 3.28) | 1.23 (0.4, 3.74) | ||
| > 6 months | 1.26 (0.81, 1.96) | 1.76 (1.04, 2.98) | ||
| HBV surface antigen positivity | 0.62 | 0.64 | ||
| Negative | Ref | Ref | ||
| Positive | 1.54 (0.6, 3.95) | 1.54 (0.6, 3.96) | ||
| Unknown | 0.93 (0.55, 1.55) | 0.93 (0.49, 1.74) | ||
| HCV antibody positivity | 0.96 | 0.77 | ||
| Negative | Ref | Ref | ||
| Positive | 1.09 (0.58, 2.06) | 1.29 (0.65, 2.58) | ||
| Unknown | 1 (0.53, 1.9) | 1.04 (0.48, 2.28) | ||
| Number of VL measurement (per 5-unit increase) | 1.18 (1.08, 1.29) | <0.001 | 1.38 (1.23, 1.56) | <0.001 |
| ART type commenced | 0.87 | 0.64 | ||
| NRTI+NNRTI | Ref | Ref | ||
| NRTI+PI | 0.99 (0.65, 1.51) | 1.03 (0.66, 1.61) | ||
| NRTI+INSTI | 1.2 (0.7, 2.05) | 1.33 (0.67, 2.64) | ||
| Other | 0.87 (0.41, 1.8) | 0.7 (0.32, 1.51) | ||
| Year of ART initiation | 0.84 | 0.06 | ||
| <2005 | Ref | Ref | ||
| 2006–2010 | 1.08 (0.7, 1.67) | 0.37 (0.18, 0.78) | ||
| 2011–2015 | 1.12 (0.71, 1.77) | 0.46 (0.18, 1.15) | ||
| 2016–2022 | 1.44 (0.63, 3.28) | 0.49 (0.13, 1.9) | ||
| Duration of ART (per 5-year increase) | 0.93 (0.81, 1.06) | 0.27 | 0.51 (0.37, 0.72) | <0.001 |
| Participant care setting | 0.08 | 0.013 | ||
| Sexual health services | Ref | Ref | ||
| GP | 1.32 (0.89, 1.96) | 1.32 (0.86, 2.03) | ||
| Hospital | 0.73 (0.43, 1.23) | 0.55 (0.31, 0.97) | ||
Time-updated variables
Global p-values are tested for heterogeneity excluding missing values.
Abbreviations: ART, antiretroviral therapy; MSM, men who have sex with men; VL, viral load, NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor; OR, odds ratio.
Discussion
Among AHOD participants who were on treatment and in follow up from 1st January 2010, VF was frequent, yet steadily declined over the years 2010–2021. In this study, we found that participants with viral blips, as well as those with LLV had increased risks of subsequent VF. Our study has also identified important factors associated with VF development, blips and LLV. Younger age at ART initiation, longer duration of treatment interruption, hepatitis B co-infection, lower CD4 count, and those who started ART in the earlier years of the study were associated with increased risk of VF. Although the rates of VF in our cohort were lower than those from other settings, such as in low to middle-income settings [18–20], it is still essential to continue monitor those with low-level viremia or those who are at risk of VF to navigate the path to the UNAIDS’s 95–95–95 targets and for the benefits of “Undetectable=Untransmittable”.
Our study results are robust to different VL cutoffs for the definitions of VF, blips or LLV. Previous studies which used different definitions of VF such as VL ≥500 or ≥1000 copies/mL did not find an association of LLV with increased risk of VF [12, 13]. In line with a recent study from a large European cohort of people living with HIV [10], our study which also defined VF as 2 consecutive VLs of ≥200 copies/mL or a single VL of ≥1000 copies/mL while on ART, found that both viral blips and LLV predicted VF development. In the sensitivity analysis, in which we defined VF using 2 consecutive VLs of ≥200 copies/mL or a single VL of ≥500 copies/mL, blips and LLV remained associated with increased risks of VF. Moreover, similar to several recent studies [9, 10, 21] in which a VL cutoff of <200 copies/mL was used to define blips, as in our sensitivity analysis, we found that individuals with blips had significantly elevated risks of subsequent VF. We also confirmed the findings from recent studies, which were conducted among individuals starting with INSTI regimens [9, 21], that there are associations between viral blips and LLV and subsequent VF, despite the very high potency of new generation INSTI regimens. This suggests that viral blips and LLV are important risk factors to monitor regardless of the ART regimen used.
Interestingly, compared to participants with viral suppression, those with LLV had a higher risk of VF than those with viral blips. This suggests that blips, which are more transient in nature than LLV, may have less impact on VF. LLV is more likely to reflect the residual HIV viremia that could be the result of several mechanisms such as ongoing viral replication, suboptimal drug adherence, treatment resistance and other underlying causes. In the analysis which was limited to individuals who started ART after 2010, lower CD4 counts and VL >100,000 copies/mL at ART-initiation were associated with higher risks of LLV development. This finding suggests that those who initiated ART with more advanced HIV disease and/or possibly with a large viral reservoir were at higher risk of LLV and subsequently, higher VF risk. The association of younger age with elevated risks for VF development has also been reported in other studies [19, 22]. The finding suggests that younger individuals may face greater challenges for ART adherence compared to older participants. Several social and environmental factors, such as stigma and fear of disclosure, may contribute to poorer ART adherence among younger individuals [23]. In addition, PI-based ART, but not INSTI-based ART, has been shown to be associated with increased risk of VF in a recent large multicohort study [10], also shown in our study.
The proportion of people with viral blips and LLV remained stable over the study period even in the era of INSTI use despite decreasing proportion of VF. Our study has also identified other important factors associated with blips and LLVs. For example, when the analysis was limited to those who started ART after 2010 high HIV-1 RNA (≥100,000 copies/mL) at ART initiation, was associated with later blips and LLV. Consistent with the recent RESPOND analysis [21],higher HIV-1 RNA levels at ART initiation were not associated with VF but lower CD4 cell count was. As reported previously [10, 24], the extent of reservoirs established and CD4+ T-cell depletion before initiation of ART, especially among those with advanced HIV infection, could impact the subsequent treatment outcomes after ART initiation.
Other important factors for VF, blips and LLV identified in the study include hepatitis B co-infection and number of VL measurements. The association of hepatitis B co-infection and VF remains significant in a multivariable model additionally adjusted for time-varying exposure to ARVs with anti-HBV activity (i.e., TDF- or TAF-containing regimen) (data not shown). There is also ongoing debate on whether the frequency of VL monitoring for people with HIV on long-term ART could be reduced and optimized in HIV care settings [25]. For example, point-of-care VL monitoring may provide additional benefits for VL testing cascade, especially in resource-limited settings [26–28]. Our findings support the recommendation that the frequency of viral load testing could be tailored to individual risk of VF. Individuals with longer duration of treatment interruption could benefit from more frequent VL testing that could identify blips or LLV episodes and offer interventions such as adherence counselling and support. While our study and other recent reports [10, 15] have identified an association between blips or LLV and subsequent VF, iťs important to recognize that there is no definitive evidence linking individuals with LLV to HIV sexual transmission [31].
Several limitations of this study need to be acknowledged. First, as an observational study, we cannot account for the unknown biases due to uncontrolled confounders. Second, we did not have genotypic resistance data in our cohort to evaluate the impacts of LLVs and blips on the development of resistant mutations. The emergence of drug-resistant mutations following LLV has previously been reported, although mostly occur when LLV is categorized above 200 copies/mL [10, 32–34]. Third, the low number of VF events among those who started with INSTI provided wider confidence intervals than for the primary analysis, and thus we lacked power to investigate any effects of different INSTI agents. In addition, we did not undertake a trend analysis to evaluate the statistical significance of the observed viremia trends. It is also important to note that our results may not be readily generalizable to all settings, particularly in resource-limited settings with limited access to HIV VL testing. Finally, we could not rule out the impact of different VL assays on our results. The potential influence of assay variability on the interpretation of our findings, especially within the LLV range, even when the limit of quantification is set at or above 50 copies/mL should also be considered. Nonetheless, our study has a number of strengths including a long duration of follow-up to investigate VF and consistent findings with various sensitivity analyses using different cut-offs of VF, viral blips and LLV.
In conclusion, we found that viral blips and LLV were strongly associated with increased risk of subsequent VF, and that the important predictors such as high HIV-1 RNA and low CD4 counts at ART initiation were associated with elevated risks of blips and LLV. Further studies are needed to explore whether newer ART regimens including new INSTI drugs, dual therapies and long-acting ART could lead to fewer blips and LLV and whether blips and LLV from these regimens have impact on subsequent virological outcomes.
Supplementary Material
Acknowledgements
Australian HIV Observational Database
New South Wales: D Ellis, Plaza Medical Centre, Coffs Harbour^; M Bloch, Holdsworth House Medical Practice, Sydney; D Allen, Holden Street Clinic, Gosford^; L Burton, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, R Mousavi, H Farlow, E Byrne, East Sydney Doctors, Surry Hills; DJ Templeton*, L Garton, T Doyle, RPA Sexual Health, Camperdown; Eva Jackson, Nepean and Blue Mountains Sexual Health and HIV Clinic, Penritĥ; N Ryder, G Sweeney, B Moran, Clinic 468, HNE Sexual Health, Tamworth; A Carr, K Hesse, A Hawkes, St Vincent’s Hospital, Darlinghurst; R Finlayson, M Shields, R Burdon, P Calleia, Taylor Square Private Clinic, Darlinghurst; K Brown, Illawarra Sexual Health Service, Warrawonĝ; R Varma, Sydney Sexual Health Centrê, Sydney; R Bopage, J Walsh, S Varghese, C Chung, Western Sydney Sexual Health Clinic; DE Smith, Albion Street Centrê; A Cogle*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, J Hutchinson*, N Rose, T Dougherty, D Byonanebye, A Han, D Rupasinghe, The Kirby Institute, University of NSW. Northern Territory: M Gunathilake*, S Hall, Centre for Disease Control, Darwin. Queensland: C Thng*, Gold Coast Sexual Health Clinic, Southport; D Russell*, M Rodriguez, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, J Broom, S Dennien, Clinic 87, Sunshine Coast Hospital and Health Service, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill^; E Priscott, S Benn, E Griggs, Sexual Health and HIV Service in Metro North, Brisbane. South Australia: W Donohue, O’Brien Street General Practice, Adelaidê. Victoria: R Moore, Northside Clinic, North Fitzroŷ; NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; R Teague, J Silvers, W Zeng, A Levey, Melbourne Sexual Health Centre, Melbourne; J Hoy*, M Giles, M Bryant, S Price, P Rawson Harris*, The Alfred Hospital, Melbourne; I Woolley*, T Korman, J O’Bryan*, K Cisera, Monash Medical Centre, Clayton. Western Australia: D Nolan, Department of Clinical Immunology, Royal Perth Hospital, Pertĥ. New Zealand: G Mills, Waikato District Hospital Hamilton^; N Raymond, Wellington Hospital, Wellington^.
*Indicates steering committee members in 2023
Îndicates no longer participating in the AHOD study
Funding statement
AHOD is a component of the IeDEA Asia-Pacific Research Collaboration, a constituent project of the International Epidemiology Databases to Evaluate AIDS (IeDEA). IeDEA, a research program of the U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID).Including support from NIAID, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Drug Abuse (NIDA), National Heart, Lung, and Blood Institute (NHLBI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), Fogarty International Center (FIC), the National Cancer Institute (NCI), and the National Institute of Mental Health (NIMH). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. AHOD is further supported by grant No. U01-AI069907 from the US National Institutes of Health and GNT2023845 from the National Health and Medical Research Council, Australia.
Footnotes
Conflicts of interest statement
The authors declare no conflicts of interest.
References
- 1.Young B, Zuniga JM, Montaner J, Mayer KH. Controlling the HIV epidemic with antiretrovirals: moving from consensus to implementation. Clin Infect Dis 2014; 59 Suppl 1: S1–2. [DOI] [PubMed] [Google Scholar]
- 2.Pennings PS. HIV Drug Resistance: Problems and Perspectives. Infect Dis Rep 2013; 5(Suppl 1): e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373(9): 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frescura L, Godfrey-Faussett P, Feizzadeh AA, El-Sadr W, Syarif O, Ghys PD. Achieving the 95 95 95 targets for all: A pathway to ending AIDS. PLoS One 2022; 17(8): e0272405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abstract Supplement Abstracts from IAS 2023, the 12th IAS Conference on HIV Science, 23 – 26 July, Brisbane, Australia & Virtual. J Int AIDS Soc 2023; 26 Suppl 3(Suppl 3): e26134.37485598 [Google Scholar]
- 6.Callander D, McManus H, Gray RT, et al. HIV treatment-as-prevention and its effect on incidence of HIV among cisgender gay, bisexual, and other men who have sex with men in Australia: a 10-year longitudinal cohort study. Lancet HIV 2023. [DOI] [PubMed] [Google Scholar]
- 7.Callander D, Moreira C, El-Hayek C, et al. Monitoring the Control of Sexually Transmissible Infections and Blood-Borne Viruses: Protocol for the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS). JMIR Res Protoc 2018; 7(11): e11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National update on HIV, viral hepatitis and sexually transmissible infections in Australia 2009–2018. Available at: https://kirby.unsw.edu.au/report/national-update-hiv-viral-hepatitis-and-sexually-transmissible-infections-australia-2009-2018. [Google Scholar]
- 9.Cuzin L, Flandre P, Allavena C, et al. Low-level viral loads and virological failure in the integrase strand transfer era. J Antimicrob Chemother 2023; 78(4): 1111–6. [DOI] [PubMed] [Google Scholar]
- 10.Elvstam O, Malmborn K, Elén S, et al. Virologic Failure Following Low-level Viremia and Viral Blips During Antiretroviral Therapy: Results From a European Multicenter Cohort. Clin Infect Dis 2023; 76(1): 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joya C, Won SH, Schofield C, et al. Persistent Low-level Viremia While on Antiretroviral Therapy Is an Independent Risk Factor for Virologic Failure. Clin Infect Dis 2019; 69(12): 2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenhende MA, Ingle S, May M, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. Aids 2015; 29(3): 373–83. [DOI] [PubMed] [Google Scholar]
- 13.Bernal E, Gómez JM, Jarrín I, et al. Low-Level Viremia Is Associated With Clinical Progression in HIV-Infected Patients Receiving Antiretroviral Treatment. J Acquir Immune Defic Syndr 2018; 78(3): 329–37. [DOI] [PubMed] [Google Scholar]
- 14.Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18(2): 188–97. [DOI] [PubMed] [Google Scholar]
- 15.Fleming J, Mathews WC, Rutstein RM, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. Aids 2019; 33(13): 2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med 2002; 3(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 17.Templeton DJ, Wright ST, McManus H, et al. Antiretroviral treatment use, co-morbidities and clinical outcomes among Aboriginal participants in the Australian HIV Observational Database (AHOD). BMC Infect Dis 2015; 15: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MP, Cutsem GV, Giddy J, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012; 60(4): 428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta-Wright A, Fielding K, van Oosterhout JJ, et al. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. Lancet HIV 2020; 7(9): e620–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han WM, Law MG, Egger M, et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV 2021; 8(12): e766–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Álvarez H, Mocroft A, Ryom L, et al. Plasma HIV-1 RNA and CD4+ T-cell counts are determinants of virological non-suppression outcomes with initial integrase inhibitor-based regimens: A prospective RESPOND cohort study. Clin Infect Dis 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors Associated with Virological Non-suppression among HIV-Positive Patients on Antiretroviral Therapy in Uganda, August 2014-July 2015. BMC Infect Dis 2017; 17(1): 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16(3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirden M, Todesco E, Valantin MA, et al. Low-level HIV-1 viraemia in patients on HAART: risk factors and management in clinical practice. J Antimicrob Chemother 2015; 70(8): 2347–53. [DOI] [PubMed] [Google Scholar]
- 25.Lecher SL, Fonjungo P, Ellenberger D, et al. HIV Viral Load Monitoring Among Patients Receiving Antiretroviral Therapy - Eight Sub-Saharan Africa Countries, 2013–2018. MMWR Morb Mortal Wkly Rep 2021; 70(21): 775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drain PK, Dorward J, Bender A, et al. Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response. Clin Microbiol Rev 2019; 32(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV 2020; 7(4): e229–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh P, Heller T, Chione B, et al. Near Point-of-Care HIV Viral Load: Targeted Testing at Large Facilities. J Acquir Immune Defic Syndr 2021; 86(2): 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 2006; 80(13): 6441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmann N, von Siebenthal C, Vongrad V, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 2019; 10(1): 3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet 2023; 402(10400): 464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis 2011; 204(4): 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One 2012; 7(5): e36673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vancoillie L, Mortier V, Demecheleer E, et al. Drug resistance is rarely the cause or consequence of long-term persistent low-level viraemia in HIV-1-infected patients on ART. Antivir Ther 2015; 20(8): 789–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


