Abstract
Background:
The Determine™ HIV-1/2 Ag/Ab Combo (DC) rapid test can identify HIV-1 infection earlier than rapid antibody-only tests in plasma specimens.
Objectives:
We compared the performance of DC with a laboratory-based antigen/antibody (Ag/Ab) combo assay in plasma and evaluated antigen reactivity in whole blood specimens.
Study design:
We tested by DC 508 plasma specimens collected in a prospective study and 107 sequential plasma and simulated whole blood specimens from 20 seroconversion panels. Previous results using the ARCHITECT (ARC) Ag/Ab combo assay were compared to DC results. In seroconversion panels, the days from the first HIV1 RNA-positive test to first DC-reactive in plasma and whole blood was compared. McNemar’s and Wilcoxon signed rank tests were used for statistical analysis.
Results:
Of 415 HIV-positive samples, ARC detected 396 (95.4%) and DC 337 (81.2%) (p < 0.0001). DC was reactive in 50.0% of ARC-reactive/MS-negative, 78.6% of ARC-reactive/MS-indeterminate, and 99.6% of ARC-reactive/MS-HIV-1-positive or −undifferentiated specimens. DC antigen reactivity was higher among ARC-reactive/MS-negative than MS-indeterminate samples. In 20 HIV-1 seroconversion panels, there was a significant difference between DC reactivity in plasma (91.1%) and whole blood (56.4%) (p < 0.0001). DC with whole blood showed a significant delay in reactivity compared to plasma (p = 0.008).
Conclusions:
In plasma, DC was significantly less sensitive than an instrumented laboratory-based Ag/Ab combo assay. DC in plasma was significantly more sensitive compared to whole blood in early HIV-1 infections. With the U.S. laboratory-based diagnostic algorithm, DC as the first step would likely miss a high proportion of HIV-1 infections in early stages of seroconversion.
Keywords: HIV diagnostics, Rapid test, Antigen-antibody detection
1. Background
In 2014 the Centers for Disease Control and Prevention (CDC) and the American Public Health Laboratories (APHL) published an updated HIV diagnostic algorithm for laboratory settings. The updated algorithm recommends screening with an HIV antigen/antibody (Ag/Ab) combination assay followed by a supplemental assay that differentiates between HIV-1 and HIV-2 antibodies. An HIV-1 RNA test is used to resolve discrepant results. With Food and Drug Administration (FDA) approval of new HIV diagnostic assays in the U.S., evaluation of the algorithm’s performance using these new assays is needed [1–4].
In the U.S., four laboratory-based immunoassays and one rapid test (RT) are approved by the FDA that can simultaneously detect HIV-1 p24 antigen and HIV-1/HIV-2 antibodies and potentially could be used as screening assays in the updated algorithm [5–11]. Of those assays, the Alere Determine™ HIV-1/2 Ag/Ab Combo (DC) RT is the only point-of-care (POC) assay and is one of only two assays that can distinguish HIV-1 antigen from antibody reactivity [12]. DC received FDA approval in 2013 and received a Clinical Laboratory Improvement Amendments (CLIA) waiver in 2014. Evaluation of DC for HIV diagnosis using serum/plasma during early stages of infection has been limited and most results showed poor performance of antigen detection [11,13–16]. Other DC evaluations using whole blood from within and outside of the U.S. also indicated limited ability of the test to detect p24 antigen although some of those evaluations were performed with the previous version of DC [17–22]. A laboratory study using plasma specimens revealed that DC detected early HIV-1 infections later than laboratory-based Ag/Ab combo assays, but before laboratory tests that detect HIV IgM antibodies and approximately 15.5 days before HIV-1 western blot positivity [11].
2. Objectives
We evaluated the performance of DC with plasma specimens from persons enrolled in a study to identify acute infections as the first step of the CDC/APHL HIV laboratory diagnostic algorithm. Additionally, we evaluated both antigen reactivity in simulated whole blood made from commercial seroconversion panels and overall test performance in plasma and simulated whole blood early after infection.
3. Study design
3.1. Sample sets
3.1.1. Plasma specimens from the STOP study
The Screening Targeted Populations to Interrupt On-going Chains of HIV Transmission with Enhanced Partner Notification (STOP) study was a prospective study to evaluate methods to detect acute HIV infection (AHI) among high-risk populations in New York City, North Carolina and San Francisco. The study provided an unique opportunity to evaluate improved detection of early HIV infections with the CDC/APHL HIV laboratory diagnostic testing algorithm using the instrumented Abbott ARCHITECT HIV Ag/Ab Combo Assay (ARC) and the Bio-Rad Multispot HIV-1/HIV-2 Rapid test (MS) [3,23–25]. The STOP study was approved by the Institutional Review Boards for the University of California at San Francisco, the University of North Carolina at Chapel Hill, and the New York City Department of Health & Mental Hygiene (HSR 6193). Study participants were screened for HIV antibody with RTs on whole blood. To identify AHIs at each site, plasma specimens collected between September 2011 and October 2013 from RT-antibody negative persons were further tested with ARC (screening assay) and MS (supplemental assay) at all sites and with an HIV-1 RNA assay (Gen-Probe APTIMA HIV-1 RNA; (APTIMA)) in NYC and NC, and with a validated Abbott m2000 HIV-1 viral load (VL) assay in SF [25,26]. Plasma specimens from individuals that screened RT antibody positive were also tested with ARC and MS (established HIV-1 infections). MS was discontinued in 2016. For this evaluation, 508 available frozen plasma specimens (including 415 HIV-positive and 93 ARC-false reactive −ARC-reactive/HIV-1 RNA-negative-) were sent to CDC for further testing. A subset of 87 ARC-false reactive plasma specimens were also tested with the Bio-Rad GS HIV Combo Ag/Ab EIA (BRC) and DC to assess concordance of false reactivity.
3.1.2. Plasma and whole blood specimens from seroconversion panels
Twenty well-characterized, frozen HIV-1 seroconversion plasma panels from U.S. donors (presumably subtype B) were purchased from Zeptometrix, Inc. (Buffalo, NY) and BBI-SeraCare Diagnostics (Milford, MA) [4,11,27]. Plasma specimens were tested with DC, ARC, MS and APTIMA. A subset of 107 sequential plasma specimens from the 20 seroconverters described above were used to prepare simulated whole blood. To assess antigen detection with DC, the collection points selected were around the previously documented seroconversion phase. Plasma specimens were thawed and mixed at 40% hematocrit with washed red blood cells (Group O) from a single donor to simulate HIV-1-infected whole blood. Fifty microliters of the prepared whole blood was tested with DC on the day of preparation. Whole blood specimens were not tested with other FDA-approved tests.
3.2. HIV serologic assays at CDC
The Alere DC RT (Orgenics, Ltd., Yavne, Israel) was used as indicated in the package insert for plasma and whole blood specimens [12]. All specimens were tested in singlet and repeated only if invalid results (absence of the control line) were obtained. Results were read by a single operator between 20 and 30 min after adding the sample at ambient temperature. In ARC-false reactive samples, the BRC assay was used following the package insert (Bio-Rad, CA, USA).
3.3. Analysis
For this study, HIV-1 infection status was defined following the recommendations of the CDC/APHL laboratory-based HIV diagnostic algorithm [2]: AHI as ARC-reactive/MS-negative or −indeterminate/RNA-positive and established infection as ARC-reactive/MS-HIV-1 positive or −positive undifferentiated. False reactive specimens (HIV-1-negative) were defined as ARC-reactive/MS-negative or −indeterminate/RNA-negative. The RNA test was either APTIMA or a validated VL assay as described earlier. The different proportions in DC reactivity between screening tests (DC and ARC) and between plasma and whole blood specimens were analyzed using the McNemar’s test. The number of days since the first plasma collection date with APTIMA-reactivity (first available indication of HIV-1 infection) to the first date where DC was reactive for plasma and simulated whole blood specimens was calculated and compared statistically using the Wilcoxon signed rank test. A p value < 0.05 was considered statistically significant.
4. Results
4.1. DC performance on plasma specimens from the STOP study
Of 508 STOP study plasma specimens available, 142 were from NYC, 37 from NC and 329 from SF. 277 SF specimens were unique and 52 had two or three follow-up plasma specimens that initially were RT-antibody negative. Table 1 shows the distribution of plasma specimens classified by previous testing at different sites.
Table 1.
Previously collected HIV-1 test results on plasma specimens from the STOP study.a
| Results of previous testing | Total | New York City | North Carolina | San Francisco |
|---|---|---|---|---|
| HIV−1 RNA-positive/ARC-nonreactive/MSb | 19 | 9 | 1 | 9 |
| HIV-1 RNA-positive/ARC-reactive/MS-negative | 110 | 26 | 10 | 74 |
| HIV-1 RNA-positive/ARC-reactive/MS-indeterminate | 14 | 3 | 0 | 11 |
| ARC-reactive/MS-HIV-1 positive | 271 | 67 | 2 | 202 |
| ARC-reactive/MS-positive undifferentiatedc | 1 | 0 | 0 | 1 |
| HIV-1 RNA-negative/ARC-reactive/MS-negative or −indeterminate | 93 | 37 | 24 | 32 |
| Total | 508 | 142 | 36 | 329 |
Plasma testing: HIV-1 RNA: GenProbe APTIMA or Abbott m2000 HIV-1 RNA viral load, ARC: Abbott ARCHITECT HIV Ag/Ab (screening assay), MS: Bio-Rad Multispot HIV-1/HIV-2 rapid test (supplemental assay). Tests were interpreted following the manufacturer’s package insert.
MS was not performed.
Specimens with MS-positive undifferentiated results are positive for HIV-1 and HIV-2 antibodies and require further testing to confirm the type of infection. Samples were defined as HIV-1 positive or HIV-1 negative following the recommendations of the CDC/APHL laboratory-based HIV diagnostic algorithm [2].
None of the 508 plasma specimens tested invalid with DC. Of 415 HIV-positive samples, ARC detected 396 (95.4%) and DC 337 (81.2%) (Table 2). All 19 plasma specimens from AHIs that were positive in only the HIV-1 RNA assays were DC-negative. The difference in reactivity between the two screening assays was statistically significant (p < 0.0001). Overall DC detected 337 of 396 (85.1%) of HIV-1-positive plasma specimens that were ARC-repeatedly reactive and confirmed to be HIV-1 infections (Table 2). DC detected 50% (55/110) of ARC-reactive/MS-negative samples (p < 0.0001). There was no significant difference between the two screening assays when MS was either indeterminate, positive undifferentiated or HIV-1-positive. DC detected 78.6% (11/14) of ARC-reactive/MS-indeterminate specimens (p = 0.2482) and 99.6% (271/272) of ARC-reactive/MS-positive or −undifferentiated (p = 1). DC antigen detection was observed in 40.9% (45/110) of ARC-positive/MS-negative samples, 28.6% (4/14) of ARC-reactive/MS-indeterminate samples, and 2.9% (8/272) of ARC-reactive/MS-HIV-1-positive or −positive undifferentiated samples.
Table 2.
Reactivity of Determine Combo in plasma specimens from early and established HIV-1 infections from the STOP study.a
| Total | Determine Combo resultsb |
Overall DC positivity | ||||
|---|---|---|---|---|---|---|
| Ag-/Ab- | Ag+/Ab- | Ag+/Ab+ | Ag-/Ab+ | |||
| HIV−1 RNA-positive/ARC-nonreactive/MSc Total (%) | 19 (4.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HIV-1 RNA-positive/ARC-reactive/MS-negativeTotal (%) | 110 (26.5) | 55 (50.0) | 37 (33.6) | 8 (7.3) | 10 (9.1) | 55 (50.0) |
| HIV-1 RNA-positive/ARC-reactive/MS-indeterminate Total (%) | 14 (3.4) | 3 (21.4) | 1 (7.2) | 3 (21.4) | 7 (50.0) | 11 (78.6) |
| ARC-positive/MS-HIV-1 positive or −undifferentiated Total (%) | 272 (65.5) | 1 (0.4) | 0 (0) | 8 (2.9) | 263 (96.7) | 271 (99.6) |
| Total (%) | 415 (100) | 59 (14.2) | 38 (9.2) | 19 (4.6) | 280 (67.5) | 337 (81.2) |
Plasma testing: HIV-1 RNA: GenProbe APTIMA or Abbott m2000 HIV-1 RNA viral load, ARC: Abbott ARCHITECT HIV Ag/Ab (screening assay), MS: Bio-Rad Multispot HIV-1/HIV-2 (supplemental assay).
Ag: antigen, Ab: antibody, −: negative, +: positive.
MS was not performed.
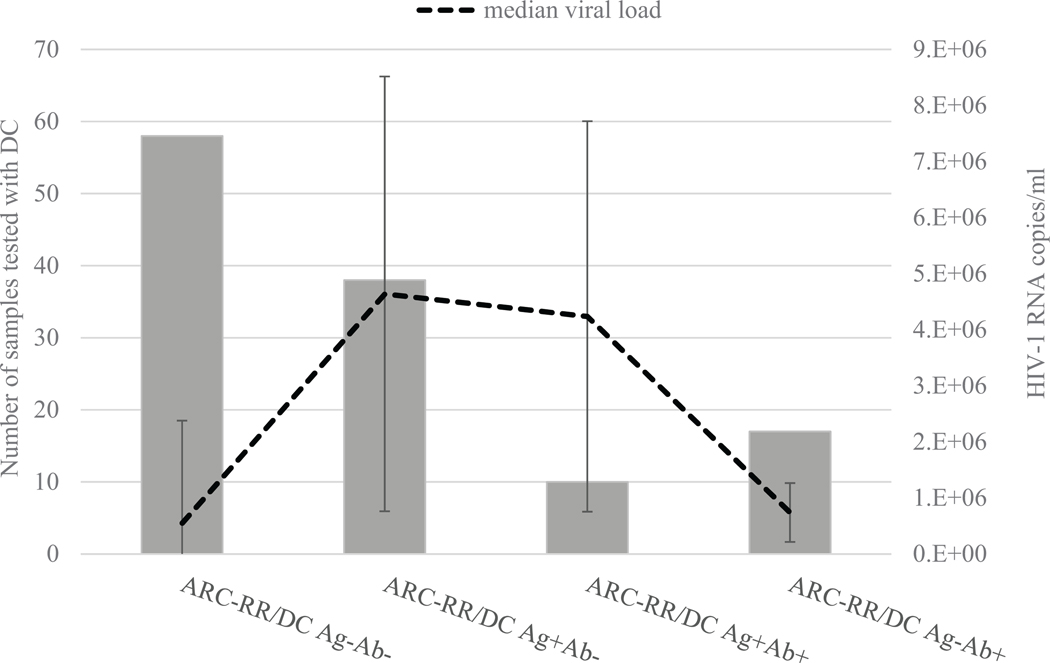
Of 124 ARC-reactive/MS-negative or −indeterminate samples, 123 had available VL results. Fig. 1 shows that detection of antigen by DC was mostly observed with high viremia in plasma specimens from early stages of HIV-1 infection. When the results were separated between ARC-reactive/MS-negative and ARC-reactive/MS-indeterminate (different stages of seroconversion), among 110 MS-negative samples, VL ranged from 3.1 × 103 to >107 copies/ml with a median of 107 copies/ml. Of 13 MS-indwhich one was DC antigen-reactiveeterminate samples, VL ranged from 3.9 × 104 to >107 copies/ml with a median 7.5 × 105 copies/ml (data not shown). Of 110 MS-negative, 45 samples were DC antigen-reactive with a median VL of 3.8 × 106 copies/ml, of which 37 were DC antigen-reactive/antibody-nonreactive and eight were DC antigen/antibody-reactive (data not shown). Of 13 MS-indeterminate specimens, three were antigen-reactive samples with VLs > 107 copies/ml, of which one was DC antigen-reactive/antibody-nonreactive and two were DC antigen/antibody-reactive (data not shown).
Fig. 1.
Distribution of 1 viral load and Determine Combo (DC) antigen results in plasma specimens from acute HIV-1 infections.
In plasma specimens that were repeatedly reactive with Abbott Architect (ARC-RR) and were either Multispot-negative or indeterminate, 123 had HIV-1 viral load results. For calculations of the median viral load and standard deviations, the values of >107 were considered as 107 copies/ml. DC Ag-Ab-: DC antigen-nonreactive/antibody-nonreactive, DC Ag+Ab-: antigen-reactive/antibody-nonreactive, DC Ag+Ab+: antigen-reactive/antibody-reactive, and DC Ag-Ab+: antigen-nonreactive/antibody-reactive.
Among 93 ARC-false reactive, HIV-1 RNA negative samples, DC testing showed antibody reactivity in three specimens (3.2%). Of 87 available plasma specimens tested with BRC, another laboratory-based Ag/Ab combo assay, six were repeatedly reactive (6.9%). Of those six, two specimens were also reactive with DC.
4.2. Comparison of DC performance on plasma and whole blood specimens from seroconversion panels
DC was nonreactive using plasma and whole blood in all eight specimens that were collected before HIV-1 RNA was detected. Of 14 plasma specimens that were HIV-1 RNA-positive and ARC/DC-nonreactive, one was antigen reactive and one was repeatedly invalid with whole blood.
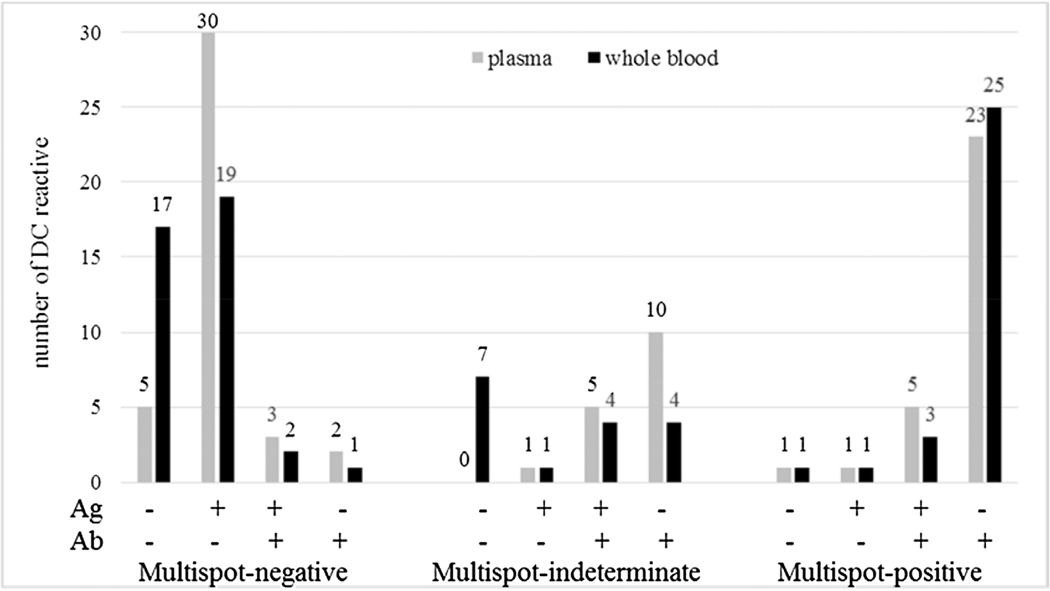
The reactivity of DC among ARC-reactive plasma and whole blood specimens is shown in Fig. 2. In plasma, DC detected 87.5% (35/40), 100% (16/16), and 96.7% (29/30) of MS-negative, MS-indeterminate and MS-HIV-1-positive samples, respectively. In whole blood, DC detected 56.4% (22/39), 56.3% (9/16), and 96.7% (29/30) of MS-negative, MS-indeterminate and MS-HIV-1 positive samples, respectively. One MS-negative whole blood sample was repeatedly invalid with DC. In plasma specimens, antigen detection (with or without antibody reactivity) was observed in 84.6% (33/39) and 37.7% (6/16) of MS-negative and MS-indeterminate samples, respectively. For whole blood, a marked reduction of antigen detection (53.8%, 21/39) was observed among MS-negative samples compared to plasma, whereas in MS-indeterminate samples, antigen detection was 31.3% (5/16). Overall, DC reactivity in AHIs (MS-negative or −indeterminate specimens) was significantly higher in plasma (91.1%) than in whole blood (56.4%) (p < 0.0001).
Fig. 2.
Reactivity of Determine Combo using whole blood specimens from commercial seroconversion panels.
The selected bleeds were repeatedly reactive with the Abbott Architect test and were Multispot negative, indeterminate or HIV-1-positive in plasma specimens. The reactivity of Determine Combo for the antigen (Ag) or the antibody (Ab) line is indicated as nonreactive (−) or reactive (+) under the x axis. The number of total results is indicated by each bar for plasma and whole blood specimens.
An initial invalid DC result showing absence of the control line occurred in 19 (17.8%) of whole blood specimens. Two invalid DC result scenarios were observed: (1) the whole blood specimen did not flow through the pad to the control line and (2) the specimen had a very reactive antibody line but no control line. Seventeen samples were resolved and 2 specimens remained invalid after repeat testing (data not shown).
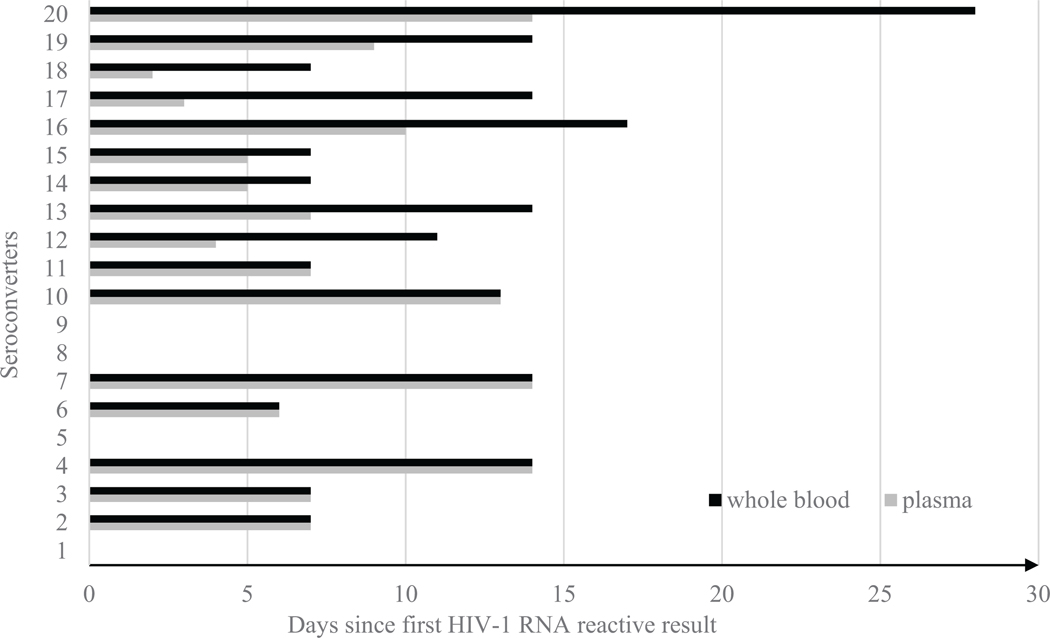
DC reactivity in plasma and whole blood was measured in days since the first available plasma APTIMA-reactive result (Fig. 3). Delay between specimen types was not observed in 11 of 20 seroconverters. Nine showed a delay in reactivity in simulated whole blood specimens, but one seroconverter had two repeatedly invalid DC results and never become DC-positive up to 28 days after the first available APTIMA-reactive result. The remaining 8 seroconverters had a median delay of six days between plasma and whole blood specimens. One seroconverter had delayed reactivity and a second negative DC phase over three consecutive bleeds in whole blood for a period of seven days after becoming antigen positive. The second negative phase was not observed in plasma with DC and ARC. Excluding the negative seroconverter whole blood specimen, DC reactivity with whole blood was observed significantly later, relative to the first available APTIMA-reactive result, than with plasma (p = 0.008, Wilcoxon signed rank test).
Fig. 3.
Reactivity of Determine Combo since first HIV-1 RNA positive result on plasma and whole blood specimens from commercial seroconversion panels.
The x-axis represents the number of days since the first available APTIMA HIV-1 RNA reactive result for each seroconverter (SC1-SC20) shown on the y-axis. SC20 was capped at 28 days because later time points were not available for whole blood specimens. SC1, SC5, SC8 and SC9 showed reactivity at the same time as the first available APTIMA HIV-1 RNA reactive results with both plasma and whole blood.
5. Discussion
This evaluation describes the comparison of DC with plasma specimens collected during a multi-site study in high-risk populations to a laboratory-based Ag/Ab combo assay used as a first step in the CDC/APHL HIV diagnostic algorithm for laboratory settings used in the U.S. [2]. We also describe the performance of DC in plasma and simulated whole blood during early stages of HIV-1 infection.
For the study, sequential plasma specimens were analyzed independently to assess DC reactivity in the context of the diagnostic algorithm. The difference between screening plasma specimens with ARC and DC was significant. These findings confirm prior results using U.S. seroconversion panels that were tested with another laboratory-based Ag/Ab combo assay, BRC, and DC [11]. Although DC detected significantly fewer AHIs than the laboratory-based assays, the antigen line was reactive (with or without antibody reactivity) for 40.9% of MS-negative samples and 28.6% of MS-indeterminate samples. These results indicate better antigen detection by DC using plasma/serum than previously published studies from outside of the U.S. [13–15]. Using DC with plasma/serum as the initial test in the laboratory algorithm might offer a fast turnaround time and antigen detection, especially in laboratories that lack an instrumented HIV Ag/Ab combo assay. However, in absence of antigen detection DC antibody reactivity may not be equivalent to that of a laboratory-based IgG/IgM antibody only assay [11] but is similar to that of an IgG/IgM antibody only RT [13].
Not unexpectedly DC detected more antigen reactive specimens early during HIV-1 infection with peak viremia and when potentially more unbound p24 antigen is present for detection. In MS-indeterminate specimens, when IgG antibodies are likely complexed with p24 antigens, DC antigen detection decreases.
Other studies have also shown that DC has a high proportion of antigen false reactivity [15,19,28]. Because different assays may have different false reactivity causes, we also tested 87 available ARC-false reactive (HIV-1 RNA-negative) plasma specimens with another Ag/Ab combo assay, BRC, and six were repeatedly reactive. ARC and BRC cannot distinguish between Ag and Ab reactivity. DC was antibody-reactive in three of the ARC-false reactive and two of those were also BRC-reactive. Interestingly, two of those three STOP study plasma specimens tested in North Carolina were also reactive with Bio-Rad GS HIV-1/2 Plus O, an IgG/IgM immunoassay, with very high signal to cutoff ratios, but had undetectable HIV-1 RNA. One of those two specimens had a MS-indeterminate result. Information collected during enrollment in the STOP study indicates the two participants were unaware of their HIV status, but the reactivity observed with different HIV tests described above may reflect that the use of antiretroviral therapy and/or HIV status may have been misreported, subject to recall bias, or some other factor [29]. These test results might be interpreted as the participants misreporting their status and having false-negative HIV-1 RNA results [30] or showing false reactivity in the different assays. DC did not show any reactivity in plasma or simulated whole blood specimens with samples collected before infection was detected by HIV-1 RNA tests. However, antigen false reactivity was observed in one simulated whole blood specimen from a plasma specimen that was HIV-1 RNA-positive but ARC/DC-nonreactive. In this laboratory study performed by trained staff, the proportion of false reactive results was low.
A limitation of this study is the use of simulated whole blood and the lack of comparative data between fingerstick and simulated whole blood. Although an evaluation with fingerstick whole blood is optimal, this method is an alternative to setting up costly field studies to identify persons during early stages of seroconversion. Our results may not reflect the true performance of DC in point-of-care settings [17,18], but they are similar to reports showing that DC testing with whole blood detects significantly less early HIV-1 infection than with plasma specimens [21]. In our study, a decreased sensitivity (and antigen detection) and a significant delay in reactivity between plasma and whole blood specimens was observed. The lower sensitivity of DC with whole blood supports the findings of poor p24 antigen detection by DC in cross-sectional surveys [17,19,20]. Using simulated whole blood we found a high proportion (17.8%) of initially invalid results. However, only two specimens remained invalid after repeat testing although this could be a limitation of the study design.
In this laboratory analysis, DC detected AHI in plasma specimens, but significantly fewer than with an instrumented laboratory-based Ag/Ab combo assay. Use of DC with plasma specimens may be beneficial in settings where there is no access to complex laboratory-based assays. However, despite the ease of use and fast turnaround time, routine use of DC in the CDC/APHL HIV diagnostic algorithm will require careful consideration of the potential usage circumstances and reported limitations.
Acknowledgments
We are thankful for contributions of the STOP teams and study participants in New York City, North Carolina and San Francisco. We also acknowledge William M. Switzer, for kindly reviewing this manuscript.
Fundings
This research was supported by a cooperative agreement between the Centers for Disease Control and Prevention (CDC) and the San Francisco Department of Public Health (5U01PS001564), New York City Department of Health and Mental Hygiene (5U01PS001561), and the University of North Carolina at Chapel Hill (5U01PS001559) and CDC intramural funding.
Footnotes
Ethical approval
The STOP study was approved by the Institutional Review Boards for the University of California at San Francisco, the University of North Carolina at Chapel Hill, and the New York City Department of Health & Mental Hygiene (HSR 6193).
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
Competing interest
No financial disclosures were reported by the authors of this paper.
References
- [1].Branson BM, Mermin J, Establishing the diagnosis of HIV infection: new tests and a new algorithm for the United States, J. Clin. Virol. 52 (Suppl. 1) (2011) S3–S4, 10.1016/j.jcv.2011.09.024. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention and Association of Public Health Laboratories, Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations, 2014. [Google Scholar]
- [3].Geren KME, Tomlinson C, Hobohm D, et al. , Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm-United States, 2011–2013, MMWR 62 (24) (2013). [PMC free article] [PubMed] [Google Scholar]
- [4].Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM, Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections, J. Clin. Virol. 52 (Suppl. 1) (2011) S17–S22, 10.1016/j.jcv.2011.09.011, PubMed PMID: 21981983. [DOI] [PubMed] [Google Scholar]
- [5].Bentsen C, McLaughlin L, Mitchell E, Ferrera C, Liska S, Myers R, et al. , Performance evaluation of the Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA, a 4th generation HIV assay for the simultaneous detection of HIV p24 antigen and antibodies to HIV-1 (groups M and O) and HIV-2 in human serum or plasma, J. Clin. Virol. 52 (Suppl. 1) (2011) S57–S61, 10.1016/j.jcv.2011.09.023. [DOI] [PubMed] [Google Scholar]
- [6].Chavez P, Wesolowski L, Patel P, Delaney K, Owen SM, Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo assay, J. Clin. Virol. 52 (Suppl. 1) (2011) S51–S55, 10.1016/j.jcv.2011.09.010. [DOI] [PubMed] [Google Scholar]
- [7].Brennan CA, Yamaguchi J, Vallari A, Swanson P, Hackett JR Jr., ARCHITECT(R) HIV Ag/Ab Combo assay: correlation of HIV-1 p24 antigen sensitivity and RNA viral load using genetically diverse virus isolates, J. Clin. Virol. 57 (2) (2013) 169–172, 10.1016/j.jcv.2013.01.017, PubMed PMID: 23485348. [DOI] [PubMed] [Google Scholar]
- [8].Vallefuoco L, Aden Abdi F, Sorrentino R, Spalletti-Cernia D, Mazzarella C, Barbato S, et al. , Evaluation of the siemens HIV antigen-antibody immunoassay, Intervirology 57 (2) (2014) 106–111, 10.1159/000358879, PubMed PMID: 24557036. [DOI] [PubMed] [Google Scholar]
- [9].Lee K, Park HD, Kang ES, Reduction of the HIV seroconversion window period and false positive rate by using ADVIA Centaur HIV antigen/antibody combo assay, Ann. Lab. Med. 33 (6) (2013) 420–425, 10.3343/alm.2013.33.6.420, PubMed PMID: 24205491; PubMed Central PMCID: PMC3819441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salmona M, Delarue S, Delaugerre C, Simon F, Maylin S, Clinical evaluation of BioPlex 2200 HIV Ag-Ab, an automated screening method providing discrete detection of HIV-1 p24 antigen HIV-1 antibody, and HIV-2 antibody, J. Clin. Microbiol. 52 (1) (2014) 103–107, 10.1128/JCM.02460-13, PubMed PMID: 24153130; PubMed Central PMCID: PMC3911434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Masciotra S, Luo W, Youngpairoj AS, Kennedy MS, Wells S, Ambrose K, et al. , Performance of the Alere Determine HIV-1/2 Ag/Ab combo rapid test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast, J. Clin. Virol. 58 (Suppl. 1) (2013) e54–e58, 10.1016/j.jcv.2013.07.002, PubMed PMID: 23911678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Orgenics L, Alere DetermineTM HIV-1/2 Ag/Ab Combo Package Insert, 2014. [Google Scholar]
- [13].Chetty V, Moodley D, Chuturgoon A, Evaluation of a 4th generation rapid HIV test for earlier and reliable detection of HIV infection in pregnancy, J. Clin. Virol. 54 (2) (2012) 180–184, 10.1016/j.jcv.2012.02.021, PubMed PMID: 22445263. [DOI] [PubMed] [Google Scholar]
- [14].Beelaert G, Fransen K, Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2, J. Virol. Methods 168 (1–2) (2010) 218–222, 10.1016/j.jviromet.2010.06.002, PubMed PMID: 20561542. [DOI] [PubMed] [Google Scholar]
- [15].Kilembe W, Keeling M, Karita E, Lakhi S, Chetty P, Price MA, et al. , Failure of a novel, rapid antigen and antibody combination test to detect antigen-positive HIV infection in African adults with early HIV infection, PLoS One 7 (6) (2012) e37154, 10.1371/journal.pone.0037154, PubMed PMID: 22715363; PubMed Central PMCID: PMC3371037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fox J, Dunn H, O’Shea S, Low rates of p24 antigen detection using a fourth-generation point of care HIV test, Sex. Transm. Infect. 87 (2) (2011) 178–179, 10.1136/sti.2010.042564, PubMed PMID: 21084439. [DOI] [PubMed] [Google Scholar]
- [17].Stekler JD, Ure G, O’Neal JD, Lane A, Swanson F, Maenza J, et al. , Performance of Determine Combo and other point-of-care HIV tests among Seattle MSM, J. Clin. Virol. 76 (2016) 8–13, 10.1016/j.jcv.2015.12.011, PubMed PMID: 26774543; PubMed Central PMCID: PMCPMC4762745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Conway DP, Holt M, McNulty A, Couldwell DL, Smith DE, Davies SC, et al. , Multi-centre evaluation of the Determine HIV Combo assay when used for point of care testing in a high risk clinic-based population, PLoS One 9 (4) (2014) e94062, 10.1371/journal.pone.0094062, PubMed PMID: 24714441; PubMed Central PMCID: PMC3979750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, et al. , Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a National Household Survey in Swaziland, J. Clin. Microbiol. 52 (10) (2014) 3743–3748, 10.1128/JCM.01989-14, PubMed PMID: 25122853; PubMed Central PMCID: PMC4187782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, Rutstein SE, et al. , Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test, J. Infect. Dis. 205 (4) (2012) 528–534, 10.1093/infdis/jir789, PubMed PMID: 22207651; PubMed Central PMCID: PMC3318673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pavie J, Rachline A, Loze B, Niedbalski L, Delaugerre C, Laforgerie E, et al. , Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting, PLoS One 5 (7) (2010) e11581, 10.1371/journal.pone.0011581, PubMed PMID: 20657834; PubMed Central PMCID: PMCPMC2906506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laperche S, Leballais L, Ly TD, Plantier JC, Failures in the detection of HIV p24 antigen with the determine HIV-1/2 Ag/Ab combo rapid test, J. Infect. Dis. 206 (12) (2012) 1946–1947, 10.1093/infdis/jis616, author reply 9–50, PubMed PMID: 23045630. [DOI] [PubMed] [Google Scholar]
- [23].Westheimer E, Fu J, Radix A, Giancotti FR, Hall L, Daskalakis DC, et al. , An HIV-1 RNA test following a reactive fourth-generation antigen/antibody combination assay confirms a high proportion of HIV infections, J. Clin. Virol. 61 (4) (2014) 623–624, 10.1016/j.jcv.2014.10.010, PubMed PMID: 25453336. [DOI] [PubMed] [Google Scholar]
- [24].Pandori MW, Westheimer E, Gay C, Moss N, Fu J, Hightow-Weidman LB, et al. , The Multispot rapid HIV-1/HIV-2 differentiation assay is comparable with the Western blot and an immunofluorescence assay at confirming HIV infection in a prospective study in three regions of the United States, J. Clin. Virol. 58 (Suppl. 1) (2013) e92–e96, 10.1016/j.jcv.2013.10.006, PubMed PMID: 24342485. [DOI] [PubMed] [Google Scholar]
- [25].Peters PJ, Westheimer E, Cohen S, Hightow-Weidman LB, Moss N, Tsoi B, et al. , Screening yield of HIV antigen/antibody combination and pooled HIV RNA testing for acute HIV infection in a high-prevalence population, JAMA 315 (7) (2016) 682–690, 10.1001/jama.2016.0286, PubMed PMID: 26881371. [DOI] [PubMed] [Google Scholar]
- [26].Ren A, Louie B, Rauch L, Castro L, Liska S, Klausner JD, et al. , Screening and confirmation of human immunodeficiency virus type 1 infection solely by detection of RNA, J. Med. Microbiol. 57 (Pt. 10) (2008) 1228–1233, 10.1099/jmm.0.2008/002386-0, PubMed PMID: 18809550. [DOI] [PubMed] [Google Scholar]
- [27].Nasrullah M, Wesolowski LG, Meyer WA 3rd, Owen SM, Masciotra S, Vorwald C, et al. , Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm, Aids 27 (5) (2013) 731–737, 10.1097/QAD.0b013e32835bc535, PubMed PMID: 23135170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taegtmeyer M, MacPherson P, Jones K, Hopkins M, Moorcroft J, Lalloo DG, et al. , Programmatic evaluation of a combined antigen and antibody test for rapid HIV diagnosis in a community and sexual health clinic screening programme, PLoS One 6 (11) (2011) e28019, 10.1371/journal.pone.0028019, PubMed PMID: 22132195; PubMed Central PMCID: PMC3222669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sanchez TH, Kelley CF, Rosenberg E, Luisi N, O’Hara B, Lambert R, et al. , Lack of awareness of Human Immunodeficiency Virus (HIV) infection: problems and solutions with self-reported HIV serostatus of men who have sex with men, Open Forum Infect. Dis. 1 (2) (2014) ofu084, 10.1093/ofid/ofu08, PubMed PMID: 25734150; PubMed Central PMCID: PMC4281805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ethridge SF, Wesolowski LG, Nasrullah M, Kennedy MS, Delaney KP, Candal D, et al. , Comparative evaluation of Aptima HIV-1 Qualitative RNA assay performance using plasma and serum specimens from persons with established HIV-1 infection, J. Clin. Virol. 52 (Suppl. 1) (2011) S63–S66, 10.1016/j.jcv.2011.09.019. [DOI] [PubMed] [Google Scholar]