Abstract
Objective Palivizumab is a humanized monoclonal antibody approved for the prevention of serious lower respiratory tract infection (LRTI) caused by respiratory syncytial virus (RSV) in infants and young children at high risk of RSV disease. This systematic review summarized evidence on the effectiveness and safety of palivizumab when used in approved populations.
Study Design A systematic review of Phase III trials and observational studies was conducted according to the population, intervention, comparator, outcome, timing, setting (PICOTS) approach (PROSPERO, CRD42021281380). Target populations consisted of infants with a history of premature birth (≤35-week gestational age) and children aged <2 years with bronchopulmonary dysplasia (BPD) or with hemodynamically significant congenital heart disease (hs-CHD). Outcomes of interest included RSV-related hospitalization, admission to intensive care unit (ICU), requirement for mechanical ventilation, treatment-related adverse events (AEs), and RSV-related deaths. Information sources were literature search (Ovid MEDLINE and Embase), pragmatic searches, and snowballing (covering the period up to 07 September 2021).
Results A total of 60 sources were included (5 Phase III trials and 55 observational studies). RSV-related hospitalization rates following palivizumab prophylaxis in Phase III trials were 1.8% in premature infants and 7.9% in children with BPD, which were significantly lower than rates in placebo arms. In the real-world setting, similar hospitalization rates were found (0.7–4.0% in premature infants [16 studies] and 0–5.5% in patients with BPD [10 studies]) with ICU admission reported in 0 to 33.3% of patients hospitalized for RSV. In Phase III trials, RSV-related mortality rates were 0.2 and 0.3%, while AEs occurred in 11% of premature and/or BPD patients and 7.2% of hs-CHD patients, consisting mainly of injection site reaction, fever, and diarrhea. Similar results were found in observational studies.
Conclusion This systematic review supports the effectiveness and safety of palivizumab in the indicated populations.
Key Points
Systematic review supports the positive benefit-risk profile of palivizumab in the indicated populations.
Real-world safety and effectiveness of palivizumab are consistent with Phase III trials results.
Palivizumab reduces RSV-related hospitalizations, ICU admissions, and need for mechanical ventilation.
Keywords: systematic review, respiratory syncytial virus, palivizumab, efficacy, effectiveness, safety
Respiratory syncytial virus (RSV) is a major cause of death globally in children below 5 years of age. 1 The clinical manifestations of RSV vary widely from an asymptomatic form to a mild, self-limiting upper respiratory tract infection, and to severe lower respiratory tract infection (LRTI), which may lead to hospitalization and death. 2 3 4 It is estimated that nearly 33.8 million new cases of RSV-associated LRTI occur worldwide every year in children less than 5 years old, leading to 3.4 million hospital admissions.
Palivizumab (SYNAGIS) is a humanized monoclonal antibody that was approved by the U.S. Food and Drug Administration (FDA) in 1998 for the prevention of serious LRTI caused by RSV in infants and young children at high risk of RSV disease. As per FDA approval, the indicated populations are the following: (1) patients with a history of premature birth (≤35-week gestational age [WGA]) and ≤ 6 months of age at the beginning of RSV season; (2) patients with bronchopulmonary dysplasia (BPD) who required medical treatment within the previous 6 months and ≤24 months of age at the beginning of RSV season; and (3) patients with hemodynamically significant congenital heart disease (CHD) and ≤24 months of age at the beginning of RSV season. 5 However, recommendations for RSV immunoprophylaxis have evolved over time and current clinical practice may not necessarily reflect original indications. 6 7
Despite these changes, the effectiveness and safety of palivizumab have been investigated in several studies, including a review of interventional and noninterventional studies (i.e., randomized controlled trials [RCTs], open-label non-comparative clinical trials, and prospective observational studies/registries) published in 2014, which showed heterogeneity of study populations and methods between studies. 8 The current systematic review aimed at summarizing and updating all available evidence on the efficacy, effectiveness, and safety of palivizumab from both the interventional and real-world clinical practice settings.
Materials and Methods
The systematic review, conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement, included Phase III trials and observational studies on palivizumab for the prevention of serious LRTI caused by RSV, published up to 07 September 2021 (PROSPERO, CRD42021281380). The review followed the PICOTS (population, intervention or exposure, comparator, outcomes, time period, setting) approach. Target populations consisted of the above-listed three indicated populations, and outcomes of interest were RSV-related hospitalization, RSV-related admission to intensive care unit (ICU), requirement for mechanical ventilation, long-term morbidity of RSV, treatment-related adverse events (AEs), and RSV-related death. No geographical restriction was applied.
Literature search was conducted in Ovid MEDLINE and Embase using free-text keywords and thesaurus terms (search strategies available in Supplementary Table S1 , available in the online version). Duplicate sources (i.e., same study and same citation) were eliminated using automated procedures. For additional sources, pragmatic searches of web sources, including websites of relevant learned or clinical societies and related conference proceedings, were conducted, along with a hand search of the reference list of retained studies (snowballing).
Study selection and data extraction were conducted independently by two assessors (with conflicts resolved by a third assessor). Eligibility criteria for the selection of relevant sources were based on the PICOTS and are listed in Table 1 . At stage 1, literature search outputs were screened based on titles and abstracts and percent agreement between assessors was determined. A review of full-text articles was conducted at stage 2 to confirm the eligibility of sources retained after screening. Reasons for the exclusion of sources at this stage were documented.
Table 1. Eligibility criteria for the selection of sources included in the systematic review on effectiveness and safety of palivizumab.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Studies conducted in humans • Studies that included, either as the study population or as a subgroup analysis, infants and children who received palivizumab (SYNAGIS) according to approved indications • Studies that reported on the safety, efficacy, and/or effectiveness of palivizumab • Full-text articles, conference proceedings including abstracts and posters, or reports • Original research articles, reviews, and meta-analyses (the latter two were used for snowballing only) •For studies with multiple publications, only the latest publication on each outcome of interest was retained |
• Off-label populations • Case reports • Letters to the editors and editorials • Opinions • Phase I and II clinical trials • Nonclinical and experimental studies • Studies reporting preliminary results (later published as full text) |
The methodological quality of retained full-text publications was assessed using the Cochrane Collaboration's tool for assessing the risk of bias (risk of bias in nonrandomized studies [ROBINS-I]) 9 for nonrandomized studies of interventions, the revised Cochrane risk-of-bias tool for randomized trials (RoB-2) 10 for RCTs, and the Joanna Briggs Institute (JBI) critical appraisal tools for observational studies. 11 To minimize the risk of bias, only studies deemed of moderate or good methodological quality were retained for the qualitative summary of findings. There was no attempt to contact study authors for supplementary data.
Results
The flow of search results through the different stages of the selection process is presented in the PRISMA flow chart ( Fig. 1 ). The literature search yielded 2,250 sources of which 461 duplicates were removed. After stage 1 screening, 254 publications were retained (the agreement rate between assessors was 87.9%). At stage 2, 197 sources were further excluded, mainly because they did not report on outcomes of interest ( n = 63; 32.0%). Pragmatic searches and snowballing yielded 3 additional relevant sources. A total of 60 sources (5 Phase III trials and 55 observational studies) fulfilled the eligibility criteria and were thus included in the review. The characteristics, key findings, and methodological quality assessment (only for full-text publications) of retained studies are described in Supplementary Tables S2 (available in the online version; clinical trials) 12–16 and Supplementary Tables S3 (available in the online version; observational studies). 17–71
Fig. 1.
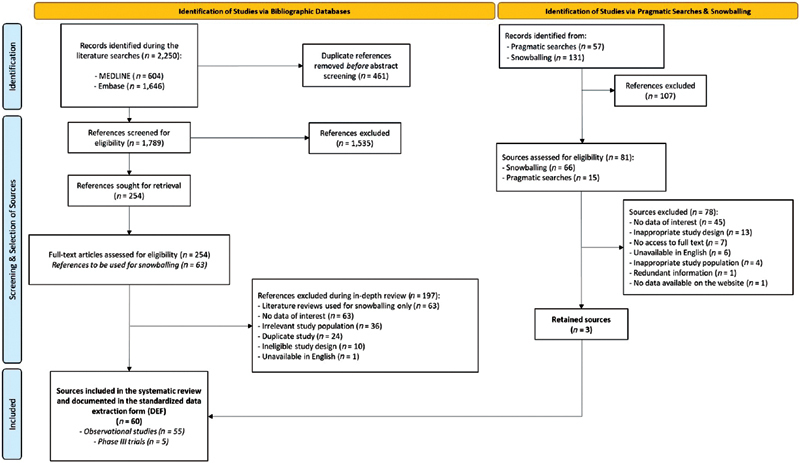
PRISMA flow chart of searches on safety and effectiveness of palivizumab. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Methodological Quality of Retained Studies
All clinical trials identified in this review were deemed to have a low risk of bias as measured with the RoB-2 and ROBINs-I tools. The methodological quality of observational studies published as full-text articles was assessed using the JBI critical appraisal tools for cohort studies ( n = 28), case series (i.e., noncomparative cohort studies; n = 9), cross-sectional studies ( n = 3), and case-control studies ( n = 1). Based on this evaluation, 23 (56.1%) of the 41 observational studies assessed were deemed of good methodological quality, and the remaining were considered to be of moderate methodological quality. Therefore, no studies were excluded from the narrative summary of findings due to the high risk of bias.
Rates of Respiratory Syncytial Virus-Related Hospitalization
According to two Phase III trials, RSV-related hospitalization rates were significantly lower in patients who received palivizumab prophylaxis compared with a placebo, for each of the indicated populations. Reported rates in the treated and placebo arms were, respectively, 1.8 and 8.1% in premature infants ( p < 0.001), 12 7.9 and 12.8% in patients with BPD ( p < 0.001), 12 and 5.3 and 9.7% in patients with hs-CHD ( p = 0.003). 14 In the IMpact trial which included both premature infants and patients with BPD, the hospital length of stay was significantly lower in patients who received palivizumab compared with placebo (36.4 and 62.6 days per 100 children, respectively, p < 0.001). 12 Similar results were reported in patients with hs-CHD, with a total number of days of hospitalization of 57.4 and 129.0 per 100 children, respectively, in the treated and placebo arms, corresponding to a statistically significant reduction of 56% associated with the use of palivizumab ( p = 0.003). 14
In the real-world setting, 28 studies reported rates of RSV-related hospitalization among patients who received palivizumab prophylaxis according to approved indications. Among these, four included patients who received palivizumab for any of the FDA-approved indications and reported estimates ranging from 0.3 to 2.1% with a higher estimate of 7.9% observed over a 7-month follow-up period in the United States. 17 43 58 66 Considering each indicated population individually, observed rates ranged between 0.7 and 4.0% in premature infants (16 studies), 18 26 27 37 41 42 47 49 51 52 57 61 64 66 69 71 between 0 and 5.5% in patients with BPD (10 studies), 18 33 37 47 50 55 56 66 69 71 and between 2.1 and 12.2% in patients with hs-CHD (4 studies), 28 33 66 68 as presented in Fig. 2 . Higher rates were found in extremely premature infants (5.0, 56 5.4, 71 and 6.7% 24 in children born ≤28 WGA, and 8.7% in those born <27 WGA 56 ), in patients with severe BPD (9.0%), 31 as well as in BPD patients with a history of premature birth and low birth weight (6.3 and 8.9%). 30 54 According to nine observational studies, palivizumab prophylaxis in premature infants and in BPD patients is associated with a significant decrease in RSV-related hospitalization compared with untreated patients 18 24 52 54 55 56 61 69 71 In a retrospective cohort study of 789 infants born at 29 through 32 WGA (262 received palivizumab and 527 did not), conducted in Austria over the period from 2004 to 2012, a risk reduction of 50% in RSV-related hospitalizations associated with the use of palivizumab was observed (odds ratio: 0.504 [95% confidence interval [CI], 0.259–0.981]). 61 In this study, patients receiving palivizumab may have concomitant BPD or CHD as the selection of patients for RSV prophylaxis was based on a risk score tool that considered these underlying diseases as the main risk factors for RSV, therefore requiring palivizumab. Similarly, in Spain, the IRIS study group reported that premature infants (≤32 WGA) with or without BPD who do not receive palivizumab had a higher risk of RSV-related hospitalization compared with those who received prophylaxis (odds ratio: 3.86 [95% CI, 2.83–5.25]). 71
Fig. 2.
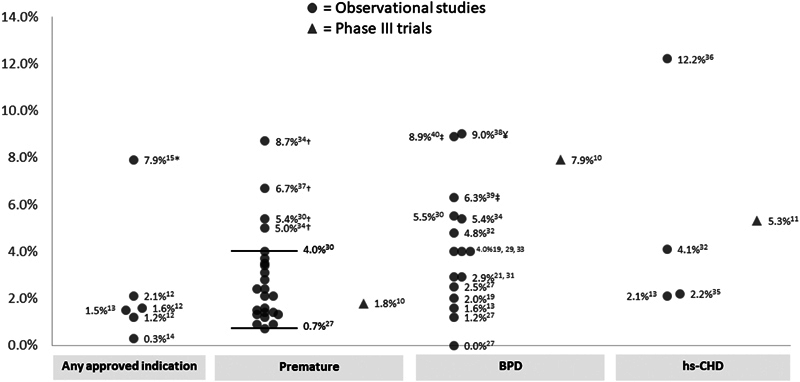
Rates of RSV-related hospitalizations among palivizumab-treated patients in observational studies and phase III trials. *Reported over a 7-month follow-up period in the United States. †Higher rates observed in extremely premature infants (≤28 WGA). ‡Higher rates observed in BPD patients with a history of premature birth and low birth weight. ¥Higher rate observed in patients with severe BPD. BPD, bronchopulmonary dysplasia; CHD, congenital heart disease; RSV, respiratory syncytial virus; WGA, week gestational age.
In a single-center retrospective cohort study conducted in Singapore on 407 infants born <32 WGA ( n = 109 palivizumab users and n = 306 untreated patients), the incidence rate of RSV-related hospitalizations within 6 months postinitial discharge from the neonatal service was significantly lower in treated than in untreated patients (18.3 vs. 143.9 per 1,000 patient-years, p = 0.01). 52 However, incidence rates did not differ significantly at 7 to 9 months (0 vs. 92.7 per 1,000 patient-years, p = 0.25) and at 10 to 12 months (81.6 vs. 41.8 per patient-years, p = 0.75).
In the hs-CHD population, a retrospective cohort study reported an incidence rate of 27.7 per 1,000 patient-years (95% CI, 17.5–44.2) in infants who received palivizumab prophylaxis ( n = 705) compared with 52.9 per 1,000 patient-years (95% CI, 38.0–74.1) in those who did not ( n = 705) ( p < 0.05). 48
Rates of Intensive Care Unit Admission and Intensive Care Unit Length of Stay
In a Phase III trial conducted in hs-CHD infants, there was no significant difference in the rate of ICU admission among patients hospitalized for RSV infection between the palivizumab and placebo arms (38.2 vs. 38.1%). 14 In this trial, the ICU length of stay related to RSV infection was 15.9 days per 100 children who received palivizumab ( n = 639) compared with 71.2 days per 100 children who did not receive palivizumab ( n = 648), corresponding to a reduction of 78% associated with the use of palivizumab ( p = 0.08). In the IMpact trial which included both premature infants and patients with BPD, the rate of ICU admission among children hospitalized for RSV was significantly lower in patients who received palivizumab compared with placebo (1.3 and 3.0%, respectively, p = 0.026). In this study, the ICU length of stay in the palivizumab and placebo arms were, respectively, 13.3 and 12.7 days per 100 children ( p = 0.023). 12
In the real-world setting, rates of ICU admission in premature infants who received prophylaxis with palivizumab and who were hospitalized for an RSV infection were reported in nine studies. 26 27 33 41 52 57 61 64 69 Of these, most (66.7%; n = 6) reported rates ranging from 16.6% to 33.3%. 26 27 33 41 61 64 A lower rate of 8.3% was found in a claims-based retrospective cohort study from Taiwan that included extremely premature infants (≤ 28 WGA). 69 Also, in a retrospective cohort study from Canada that included 87 palivizumab-treated infants born 29 to 32 WGA, only three patients were hospitalized due to RSV, of which two were admitted to the ICU (66.7%). 57 Finally, in a single center retrospective cohort study from Singapore, none of the three infants born <32 WGA who were hospitalized for RSV infection following prophylaxis with palivizumab required ICU admission. 27 In this study, 15.6% of the 32 infants who did not receive palivizumab and were hospitalized for RSV were admitted to the ICU. Similar to the population of premature infants, ICU admission rates of 23.7, 33 24.6, 68 and 33.3% 28 were found in infants with hs-CHD who received palivizumab prophylaxis and were hospitalized for an RSV infection. In infants with BPD, heterogeneous estimates were found in five studies due to differences in study populations and countries, 33 50 54 55 69 each described below.
The first was an administrative claims-based study conducted in Taiwan, in which no ICU admission was observed in patients who received palivizumab. 69 According to a multinational prospective cohort study based on the CARESS and Torino-Verona Northern Italy Network registries, 7.0% of the 57 infants with BPD hospitalized with RSV during the RSV season required care in the ICU following prophylaxis with palivizumab. 33 Also, based on the CARESS registry between 2005 and 2015, 22.3% of BPD patients treated with palivizumab during their first year of life and hospitalized for RSV were admitted to the ICU. 50 Two studies reported a higher estimate of ICU admission (33.3% each) in patients with BPD who were hospitalized for RSV. The former consisted of a retrospective cohort study conducted over the period from 2004 to 2009 in South Korea, 55 while the latter included BPD infants who were also premature and had a very low birth weight. 54
Rates and Duration of Mechanical Ventilation/Intubation
In a Phase III trial that included young children with hs-CHD, no statistically significant differences in the proportion of patients hospitalized for an RSV infection who required mechanical ventilation were found between the palivizumab and placebo arms (23.5 and 22.2%, respectively). 14 In this trial, the use of palivizumab decreased the duration of mechanical ventilation by 88% compared with placebo (6.5 vs. 54.7 days per 100 children, respectively, p = 0.224). In the IMpact trial which included both premature infants and patients with BPD, the duration of use of mechanical ventilation among children hospitalized for RSV in the palivizumab and placebo groups was, respectively, 8.4 and 1.7 days per 100 children ( p = 0.224). 12
According to six observational studies, the need for mechanical ventilation or intubation in premature infants hospitalized with RSV following palivizumab prophylaxis ranged between 5.6 and 14% 27 33 41 61 64 69 and no significant difference was observed between palivizumab users and nonusers. 61 69 A higher rate of 33.3% was reported in two studies including children born at 29 to 32 WGA. 26 57 In infants with BPD, reported rates varied across five studies, ranging from 0% in BPD patients born at 29 to 35 WGA in Taiwan 69 to 7.0% in BPD patients in Canada or Italy, 33 and even as high as 25% in BPD patients born at ≤35 WGA with low birth weight (≤1,500 g), 30 or 33.3% in patients with BPD born prematurely with very low birth weight. 54 Among patients with hs-CHD (three studies), the rates were 8.8, 68 22.0, 33 and 25.9%. 28
Oxygen Requirement among Respiratory Syncytial Virus
Hospitalized Patients
According to two Phase III trials, palivizumab prophylaxis was associated with a statistically significant reduction in the duration of supplemental oxygen requirement in patients hospitalized with RSV infection. 12 14 In young children with hs-CHD, total days of oxygen requirement in the palivizumab and placebo groups were, respectively, 27.9 and 101.5 days per 100 children ( p = 0.014). 14 Similarly, in a combined population of premature infants and patients with BPD (IMpact trial), the duration of supplemental oxygen requirement in patients hospitalized for RSV was 30.3 and 50.6 days per 100 children, respectively, in those who received palivizumab and placebo ( p < 0.001). 12
In the real-world setting, based on four studies, the proportion of premature infants hospitalized for an RSV infection who required supplemental oxygen following palivizumab prophylaxis were heterogenous, ranging from 14 to 33.3%. 26 27 41 61 In patients with BPD and hs-CHD, reported rates of oxygen therapy requirement among those who were hospitalized for an RSV infection following palivizumab prophylaxis were 25.0 and 14.0%, respectively. 30 68
Long-Term Morbidity in Patients who Received Palivizumab Prophylaxis
Based on five observational studies conducted in premature infants, wheezing was the most commonly investigated long-term morbidity in those treated with palivizumab. 24 29 38 40 51 Rates of physician-diagnosed recurrent wheezing in the palivizumab group ranged from 6.4 to 12.8%. 29 38 40 Another study reported a higher estimate of 56.3% after 11 years of follow-up. 51 However, no details on the method for case identification and/or diagnostic ascertainment were reported. There was a significant risk reduction of physician-diagnosed recurrent wheezing associated with the use of palivizumab compared with untreated patients (risk ratio [RR], 0.34, 95% CI, 0.19–0.60 29 ; RR, 0.49, 95% CI, 0.27–0.88 40 ). When considering caregiver-reported recurrent wheezing, similar results were found. More specifically, comparing patients treated with palivizumab to untreated infants, a multicenter prospective cohort study conducted in Canada, Germany, The Netherlands, Poland, Spain, and Sweden reported an RR of 0.51 (95% CI, 0.33–0.78). 40 In extremely premature infants (<29 WGA), the rate of parent-reported recurrent wheezing following RSV infection in the palivizumab group was significantly lower than that in untreated patients during the first 2 years of life (26.7 vs. 69.7%, p = 0.008), 24 but no statistically significant difference was observed at age 7 to 10 years (13.3 vs. 18.2%). 24 One study evaluated the occurrence of atopic asthma following RSV infection and found no statistically significant effect of palivizumab (OR, 1.27, 95% CI, 0.60–2.70). 38 In a study conducted in children aged 7 to 10 years, lower rates of upper respiratory tract infection were observed in patients who received prophylaxis with palivizumab as infants versus nonusers (30.0 vs. 69.7%, p < 0.05). 24 No statistically significant difference in lung function parameters was observed among adolescents (13–18 years old) born <29 WGA who received palivizumab as prophylaxis or not. 34
Treatment-Related Adverse Events
The frequency of AEs associated with the use of palivizumab was low in both the clinical trial (0–12% of patients) and real-world settings (0–7% of patients). In clinical trials, no serious AEs (SAEs) attributable to palivizumab were reported and rates of AEs were not statistically different between patients who received palivizumab or placebo for all indications: 11 versus 10% in patients with a history of premature birth (<35 WGA) and/or BPD, 12 and 7.2 versus 6.9% in patients with hs-CHD. 14 Overall, the most common AEs were fever, injection site reaction, diarrhea, and rash.
In the real-world setting, rates of AEs associated with palivizumab were 2.1, 26 4.0, 55 6.4, 18 and 7% 46 and the rates of SAEs were 0.3 18 and 1.4%. 26 The most common events were local erythema, systemic fever, local pain, irritability, vomiting, and diarrhea. 18 26 55 Based on one study, serious events, defined as any AE resulting in death, life-threatening situation, inpatient hospitalizations, persistent or significant disability or incapacity, congenital anomaly or birth defect, or other medically important events, that were possibly related to palivizumab were reported in two patients and included infection and bronchiolitis reported in one patient and pneumonia and conjunctivitis reported in the other patient. 26 In a retrospective cohort study of 2,018 children aged ≤2 years with hs-CHD, there was no increased risk of serious infection or serious arrhythmia associated with the use of palivizumab compared with unmatched controls (OR of 0.95 and 1.64, respectively, for each SAE). 67
Respiratory Syncytial Virus-Related Mortality in Patients who Received Palivizumab Prophylaxis
In two Phase III trials, RSV-related mortality in the palivizumab arm was as low as 0.2% in premature infants 12 and 0.3% in infants with hs-CHD. 14 In both trials, no significant differences were observed between palivizumab and placebo recipients.
In the real-world setting, 10 observational studies including a nonselected population of infants treated with palivizumab for either of the three approved indications reported no deaths attributable to RSV. 18 25 26 31 36 43 55 56 57 70 In two studies restricted to BPD or hs-CHD patients, RSV-related deaths occurred in 1.1% of children treated with palivizumab. 30 68
Discussion
This systematic review included 5 Phase III clinical trials and 55 observational studies on palivizumab as prophylaxis for RSV infection in children at high risk for severe RSV disease. Across studies, efficacy, effectiveness, and safety of palivizumab assessed using outcomes of RSV-related hospitalization (rates and length of stay), ICU admission (rates and length of stay), need for mechanical ventilation, treatment-related AEs, and RSV-related mortality were well documented in both the interventional and noninterventional settings. In contrast, the effect of palivizumab on the need for supplemental oxygen and on the long-term morbidity associated with RSV infection was scarcely documented.
In the clinical trial setting, rates of RSV-related hospitalization following prophylaxis with palivizumab varied according to indication, with lower rates observed in premature infants than in the BPD or hs-CHD population and were significantly lower compared with placebo. Similarly, in observational studies for all approved indications, the use of palivizumab was found to significantly reduce RSV-related hospitalizations compared with untreated patients. For all subpopulations of interest, RSV-related hospitalization rates in patients treated with palivizumab were similar to those reported in the 2014 systematic review. 8 These findings support the effectiveness of palivizumab in preventing RSV infection and associated complications in patients at high risk for severe RSV disease and are consistent with findings from the 2014 systematic review. 8 However, assessment of the feasibility of conducting a meta-analysis highlighted important clinical and methodological heterogeneity across studies, therefore pooling data from retained studies would not be recommended according to Cochrane guidelines. 72
Palivizumab was also found to significantly reduce ICU admission and ICU length of stay. However, its impact on the reduction of the duration of mechanical ventilation compared with placebo was not statistically significant. 12 14
In the previous review conducted by Wegzyn et al in 2014, evidence suggested that palivizumab is associated with reduced infant mortality but that more research was needed to confirm the results. 8 The current review, therefore, adds to the data presented in 2014 by summarizing the available data on RSV-related mortality rates in infants treated with palivizumab as well as long-term morbidity following palivizumab prophylaxis. RSV-related mortality rates were low and did not vary across settings (i.e., clinical trials and observational studies) or across indicated populations. Long-term morbidity following palivizumab prophylaxis was only documented in observational studies conducted in patients with a history of premature birth. In most studies, the use of palivizumab was associated with a significant decrease in recurrent wheezing.
The frequency of AEs, including SAEs, associated with the use of palivizumab was low in both the clinical trials and real-world settings, and results were similar across all indicated populations. Injection site reaction, fever, and diarrhea were the most frequent AEs observed in clinical trials, whereas fever, rhinitis, and pain at the injection site were the most frequent in observational studies.
Limitations and Strengths
A limitation of this review is that included studies were mainly those that led to publications in the scientific literature and indexed in bibliographical databases such as MEDLINE or Embase using predefined MeSH and Emtree terms and keywords included in the search strategy. To mitigate publication bias and to enhance the scope of the search, pragmatic searches as well as snowballing were conducted. Another limitation consisted of the absence of information in nearly half of the publications on the criteria used to ascertain RSV cases, which limits the interpretation of findings regarding RSV-related hospitalizations, ICU admissions, and need for mechanical ventilation.
The strengths of the review were the following. The methodological quality of studies was evaluated using validated assessment tools based on expert critical review. Based on this assessment, all studies retained in this review were considered to be of moderate or good methodological quality with a low risk of bias. The screening and data extraction processes were performed independently by two reviewers with discrepancies resolved by a third independent assessor. A major strength of this review is that findings on the use of palivizumab published worldwide from over more than two decades (1998–2021) were summarized, yet results were overall consistent, therefore supporting its positive and consistent benefit-risk profile across all indicated populations in both the clinical trial and real-world practice settings.
Conclusion
The effectiveness of palivizumab for the prevention of RSV-related serious LRTI was well-documented in the literature, and findings from the real-world clinical practice setting are consistent with those reported in Phase III trials with regard to reduced RSV-related hospitalizations, ICU admission, and need for mechanical ventilation. The frequency of AEs associated with the use of palivizumab was low, and the safety profile was consistent with the safety information provided in the palivizumab product label. Results from this systematic review thus support the positive benefit-risk profile of palivizumab in the indicated populations.
Funding Statement
Funding This review was funded by Sobi.
Footnotes
Conflict of Interest A.B., T.C., C.G., and Y.M. are employees of YOLARX Consultants that received funding from Sobi for the conduct of this review. T.G., A.O., J.S., J.Y., and M.W. are employees of Sobi.
Supplementary Material
References
- 1.Meng J, Stobart C C, Hotard A L, Moore M L. An overview of respiratory syncytial virus. PLoS Pathog. 2014;10(04):e1004016. doi: 10.1371/journal.ppat.1004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisman L.Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: infants with predisposing conditionsPediatr Infect Dis J 2003;22(2, suppl):S33–S37, discussion S37–S39 [DOI] [PubMed]
- 3.Nair H, Nokes D J, Gessner B Det al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis Lancet 2010375(9725):1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira L P, Watanabe A SA, Camargo C N, Melchior T B, Granato C, Bellei N. Respiratory syncytial virus evaluation among asymptomatic and symptomatic subjects in a university hospital in Sao Paulo, Brazil, in the period of 2009-2013. Influenza Other Respir Viruses. 2018;12(03):326–330. doi: 10.1111/irv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Synagis is the first and only FDA-approved monoclonal antibody for the prevention of severe RSV DISEASE Synagis (palivizumab) Efficacy and SafetyAccessed October 10, 2022 at:https://synagishcp.com/synagis-palivizumab-efficacy.html
- 6.American Academy of Pediatrics Committee on Infectious Diseases ; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(02):e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein M, Krilov L R, Fergie J et al. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics Guidance on Immunoprophylaxis: 2012-2016. Am J Perinatol. 2018;35(14):1433–1442. doi: 10.1055/s-0038-1660466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegzyn C, Toh L K, Notario G et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: a systematic review. Infect Dis Ther. 2014;3(02):133–158. doi: 10.1007/s40121-014-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne J A, Hernán M A, Reeves B C et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne J A, Savović J, Page M Jet al. RoB 2: a revised tool for assessing risk of bias in randomised trialsBMJ [Online]. 2019. Accessed on December 04, 2021 at:https://methods.cochrane.org/risk-bias-2 [DOI] [PubMed]
- 11.Johanna Briggs Institute Critical Appraisal Tools[Online]. Accessed on December 09, 2021 at:http://joannabriggs.org/research/critical-appraisal-tools.html
- 12.IMpact-RSV Study Group . Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(03):531–537. [PubMed] [Google Scholar]
- 13.Motavizumab Study Group . Carbonell-Estrany X, Simões E A, Dagan R et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(01):e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 14.Cardiac Synagis Study Group . Feltes T F, Cabalka A K, Meissner H C et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(04):532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 15.Turti T V, Baibarina E N, Degtiareva E A et al. A prospective, open-label, non-comparative study of palivizumab prophylaxis in children at high risk of serious respiratory syncytial virus disease in the Russian Federation. BMC Res Notes. 2012;5(01):484. doi: 10.1186/1756-0500-5-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makari D, Jensen K M, Harris B, Jafri H S. Randomized, double-blind study of the safety of the liquid versus lyophilized formulation of palivizumab in premature infants and children with chronic lung disease of prematurity. Infect Dis Ther. 2014;3(02):339–347. doi: 10.1007/s40121-014-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abushahin A, Janahi I, Tuffaha A. Effectiveness of palivizumab immunoprophylaxis in preterm infants against respiratory syncytial virus disease in Qatar. Int J Gen Med. 2018;11:41–46. doi: 10.2147/IJGM.S156078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi H, Hsu C H, Chang J H et al. A novel six consecutive monthly doses of palivizumab prophylaxis protocol for the prevention of respiratory syncytial virus infection in high-risk preterm infants in Taiwan. PLoS One. 2014;9(06):e100981. doi: 10.1371/journal.pone.0100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resch B, Gusenleitner W, Müller W D, Haas J. Observational study of respiratory syncytial virus-associated hospitalizations and use of palivizumab in premature infants aged 29-32 weeks. Eur J Clin Microbiol Infect Dis. 2006;25(02):120–122. doi: 10.1007/s10096-005-0082-y. [DOI] [PubMed] [Google Scholar]
- 20.Viguria N, Navascués A, Juanbeltz R, Echeverría A, Ezpeleta C, Castilla J. Effectiveness of palivizumab in preventing respiratory syncytial virus infection in high-risk children. Hum Vaccin Immunother. 2021;17(06):1867–1872. doi: 10.1080/21645515.2020.1843336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza R P, Ribeiro A LR, de Menezes S AF, Machado L FA. Incidence of respiratory syncytial virus infection in children with congenital heart disease undergoing immunoprophylaxis with palivizumab in Pará state, north region of Brazil. BMC Pediatr. 2019;19(01):299. doi: 10.1186/s12887-019-1681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Yoseph R, Haddad J, Hanna M et al. Long term follow-up of palivizumab administration in children born at 29-32 weeks of gestation. Respir Med. 2019;150:149–153. doi: 10.1016/j.rmed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Kusuda S, Koizumi T, Sakai T, Fujimura M, Nishida H, Togari H. Results of clinical surveillance during the Japanese first palivizumab season in 2002-2003. Pediatr Int. 2006;48(04):362–368. doi: 10.1111/j.1442-200X.2006.02222.x. [DOI] [PubMed] [Google Scholar]
- 24.Prais D, Kaplan E, Klinger G et al. Short- and long-term pulmonary outcome of palivizumab in children born extremely prematurely. Chest. 2016;149(03):801–808. doi: 10.1378/chest.15-0328. [DOI] [PubMed] [Google Scholar]
- 25.Northern Hemisphere Expanded Access Study Group . Groothuis J R. Safety and tolerance of palivizumab administration in a large Northern Hemisphere trial. Pediatr Infect Dis J. 2001;20(06):628–630. doi: 10.1097/00006454-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Groothuis J R. Safety of palivizumab in preterm infants 29 to 32 weeks' gestational age without chronic lung disease to prevent serious respiratory syncytial virus infection. Eur J Clin Microbiol Infect Dis. 2003;22(07):414–417. doi: 10.1007/s10096-003-0961-z. [DOI] [PubMed] [Google Scholar]
- 27.CARESS Investigators . Paes B, Mitchell I, Li A, Lanctôt K L. A comparative study of respiratory syncytial virus (RSV) prophylaxis in premature infants within the Canadian Registry of Palivizumab (CARESS) Eur J Clin Microbiol Infect Dis. 2012;31(10):2703–2711. doi: 10.1007/s10096-012-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CARESS Investigators . Li A, Wang D Y, Lanctôt K L, Mitchell I, Paes B A. Comparing first- and second-year palivizumab prophylaxis in patients with hemodynamically significant congenital heart disease in the CARESS Database (2005-2015) Pediatr Infect Dis J. 2017;36(05):445–450. doi: 10.1097/INF.0000000000001357. [DOI] [PubMed] [Google Scholar]
- 29.C-CREW Investigators . Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões E A. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132(05):811–818. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 30.Han Y M, Seo H J, Choi S H et al. Effect of prophylactic palivizumab on admission due to respiratory syncytial virus infection in former very low birth weight infants with bronchopulmonary dysplasia. J Korean Med Sci. 2015;30(07):924–931. doi: 10.3346/jkms.2015.30.7.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French Pediatricians' Group of Sunagis Patients' Name-Based Programs . Lacaze-Masmonteil T, Rozé J C, Fauroux B. Incidence of respiratory syncytial virus-related hospitalizations in high-risk children: follow-up of a national cohort of infants treated with palivizumab as RSV prophylaxis. Pediatr Pulmonol. 2002;34(03):181–188. doi: 10.1002/ppul.10175. [DOI] [PubMed] [Google Scholar]
- 32.Charkaluk M L, Rousseau J, Dehouck-Vallois M et al. Occurrence and severity of acute respiratory infections during the first year among very preterm infants: an Epipage-2 cohort analysis. Eur J Pediatr. 2021;180(06):1833–1840. doi: 10.1007/s00431-021-03956-w. [DOI] [PubMed] [Google Scholar]
- 33.Manzoni P, Paes B, Lanctôt K L et al. Outcomes of infants receiving palivizumab prophylaxis for respiratory syncytial virus in Canada and Italy: an international, prospective cohort study. Pediatr Infect Dis J. 2017;36(01):2–8. doi: 10.1097/INF.0000000000001340. [DOI] [PubMed] [Google Scholar]
- 34.Amitai N, Stafler P, Blau H et al. Palivizumab following extremely premature birth does not affect pulmonary outcomes in adolescence. Chest. 2020;158(02):660–669. doi: 10.1016/j.chest.2020.02.075. [DOI] [PubMed] [Google Scholar]
- 35.Athiraman N, Agarwal R. Palivizumab immunoprophylaxis (PIP) for infants with chronic lung disease (CLD) of prematurity—a prospective observational study. Arch Dis Child. 2012;97 01:A28–A28. [Google Scholar]
- 36.Soraiz M G, Andres S B, Castro S B et al. Palivizumab in infants less than 1 year with hemodynamically significant congenital heart disease in Argentina a comparative study with historical control group. Cardiol Young. 2017;27(04):S165–S6. [Google Scholar]
- 37.Composs Investigators . Oh P I, Lanctôt K L, Yoon A et al. Palivizumab prophylaxis for respiratory syncytial virus in Canada: utilization and outcomes. Pediatr Infect Dis J. 2002;21(06):512–518. doi: 10.1097/00006454-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Scientific Committee for Elucidation of Infantile Asthma . Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simões E AF. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med. 2017;196(01):29–38. doi: 10.1164/rccm.201609-1812OC. [DOI] [PubMed] [Google Scholar]
- 39.Palivizumab Outcomes Registry Study Group . Parnes C, Guillermin J, Habersang R et al. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry. Pediatr Pulmonol. 2003;35(06):484–489. doi: 10.1002/ppul.10288. [DOI] [PubMed] [Google Scholar]
- 40.Palivizumab Long-Term Respiratory Outcomes Study Group Simoes E A, Groothuis J R, Carbonell-Estrany Xet al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing J Pediatr 20071510134–42., 42.e1 [DOI] [PubMed] [Google Scholar]
- 41.Paes B, Mitchell I, Li A, Lanctôt K L. Respiratory hospitalizations and respiratory syncytial virus prophylaxis in special populations. Eur J Pediatr. 2012;171(05):833–841. doi: 10.1007/s00431-011-1654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oncel M Y, Arayici S, Simsek G K et al. Risk factors for hospitalization due to lower respiratory tract infection in preterm infants on palivizumab prophylaxis. Iran J Pediatr. 2013;23(06):693–700. [PMC free article] [PubMed] [Google Scholar]
- 43.AbbVie Observational program to assess respiratory syncytial virus (RSV) hospitalization rate in population of children at high-risk of serious RSV illness who received palivizumab immunoprophylaxis (SUNRISE)ClinicalTrials.gov. [Online] 2014. Accessed on November 13, 2021 at:https://clinicaltrials.gov/ct2/show/NCT02282982
- 44.Winterstein A G, Hampp C, Kubilis P, Saidi A. Age-dependent effectiveness of RSV immunoprophylaxis. Pharmacoepidemiol Drug Saf. 2011;20:S322–S323. [Google Scholar]
- 45.Buckley B C, Roylance D, Mitchell M P, Patel S M, Cannon H E, Dunn J D. Description of the outcomes of prior authorization of palivizumab for prevention of respiratory syncytial virus infection in a managed care organization. J Manag Care Pharm. 2010;16(01):15–22. doi: 10.18553/jmcp.2010.16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmasoudi A, Robson T, Badran H, Khalil A. The effect of palivizumab in the incidence rate of hospitalisation for respiratory syncytial virus infection in highrisk preterm infants in Doha, Qatar: three-year retrospective. Pharmacotherapy. 2015;35(11):e255. [Google Scholar]
- 47.The Palivizumab Outcomes Study Group . Sorrentino M, Powers T. Effectiveness of palivizumab: evaluation of outcomes from the 1998 to 1999 respiratory syncytial virus season. Pediatr Infect Dis J. 2000;19(11):1068–1071. doi: 10.1097/00006454-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Chiu S N, Wang J N, Fu Y C et al. Efficacy of a novel palivizumab prophylaxis protocol for respiratory syncytial virus infection in congenital heart disease: a multicenter study. J Pediatr. 2018;195:108–1140. doi: 10.1016/j.jpeds.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 49.Blake S M, Tanaka D, Bendz L M, Staebler S, Brandon D. Evaluation of the financial and health burden of infants at risk for respiratory syncytial virus. Adv Neonatal Care. 2017;17(04):292–298. doi: 10.1097/ANC.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 50.CARESS Investigators . Wang D Y, Li A, Paes B, Mitchell I, Lanctôt K L. First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015) Eur J Pediatr. 2017;176(03):413–422. doi: 10.1007/s00431-017-2849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Socher R, Bhardwaj A, Graw-Panzer K, Wadowski S.Incidence of wheezing in premature infants who received Synagis (Palivizumab) prophylaxis: ten-year follow-upAmerican Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS 2010;181
- 52.Yeo K T, Yung C F, Khoo P C et al. Effectiveness of palivizumab against respiratory syncytial virus hospitalization among preterm infants in a setting with year-round circulation. J Infect Dis. 2021;224(02):279–287. doi: 10.1093/infdis/jiaa749. [DOI] [PubMed] [Google Scholar]
- 53.Diehl J L, Daw J R, Coley K C, Rayburg R. Medical utilization associated with palivizumab compliance in a commercial and managed Medicaid health plan. J Manag Care Pharm. 2010;16(01):23–31. doi: 10.18553/jmcp.2010.16.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quek B H, Khoo P C, Tan P L.Outcomes of palivizumab prophylaxis for respiratory syncytial virus (RSV) infection in preterm children with bronchopulmonary dysplasia in a country with no seasonal RSV peaksJournal of Perinatal Medicine Conference: 12th World Congress of Perinatal Medicine 2015;43:Suppl.1
- 55.Chang S G, Park M S, Yu J E. Outcomes of palivizumab prophylaxis for respiratory syncytial virus infection in preterm children with bronchopulmonary dysplasia at a single hospital in Korea from 2005 to 2009. J Korean Med Sci. 2010;25(02):251–256. doi: 10.3346/jkms.2010.25.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S R, Kwok K L, Ng D KK, Hon K L. Palivizumab for infants < 29 weeks in Hong Kong without a clear-cut season for respiratory syncytial virus infection—a cost-effectiveness analysis. J Trop Pediatr. 2018;64(05):418–425. doi: 10.1093/tropej/fmx086. [DOI] [PubMed] [Google Scholar]
- 57.Taylor R S, Baker M H. Palivizumab prophylaxis for infants 29 to 32 weeks gestation at birth: a 10-year audit from Vancouver Island using BC Guidelines. Paediatr Child Health. 2020;26(02):e110–e114. doi: 10.1093/pch/pxz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krilov L R, Masaquel A S, Weiner L B, Smith D M, Wade S W, Mahadevia P J. Partial palivizumab prophylaxis and increased risk of hospitalization due to respiratory syncytial virus in a Medicaid population: a retrospective cohort analysis. BMC Pediatr. 2014;14(01):261. doi: 10.1186/1471-2431-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell I, Paes B, Li A, Chen J, Lanctôt K L. Respiratory illness and respiratory syncytial virus hospitalizations in the Canadian Registry for Synagis (CARESS) over eight seasons (2005–2013) Paediatr Child Health. 2014;19(06):e49. [Google Scholar]
- 60.Mitchell I, Abraha H, Li A, Paes B, Lanctot K.Respiratory related hospitalizations in premature infants prophylaxed with palivizumab In the Canadian registry of palivizumab (CARESS)European Respiratory Journal Conference 2015;46
- 61.Resch B, Bramreiter V S, Kurath-Koller S, Freidl T, Urlesberger B. Respiratory syncytial virus associated hospitalizations in preterm infants of 29 to 32 weeks gestational age using a risk score tool for palivizumab prophylaxis. Eur J Clin Microbiol Infect Dis. 2017;36(06):1057–1062. doi: 10.1007/s10096-016-2891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang L, Wang J. Respiratory syncytial virus immunoprophylaxis and asthma symptoms development in prematurity with bronchopulmonary dysplasia. Allergy. 2018;73:310–311. doi: 10.1016/j.jacig.2023.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chadha A D, Bao W, Holloway J, Mann J, Rye A K, Brown D E., III Respiratory syncytial virus morbidity and outpatient palivizumab dosing in South Carolina, 2004-2009. South Med J. 2012;105(08):399–404. doi: 10.1097/SMJ.0b013e31825ea57d. [DOI] [PubMed] [Google Scholar]
- 64.and the CARESS investigators . Paes B, Kim D, Saleem M, Wong S, Mitchell I, Lanctot K L. Respiratory syncytial virus prophylaxis in infants with congenital airway anomalies compared to standard indications and complex medical disorders. Eur J Pediatr. 2019;178(03):377–385. doi: 10.1007/s00431-018-03308-1. [DOI] [PubMed] [Google Scholar]
- 65.Lavoie P, Claydon J, Popescu C et al. Respiratory syncytial virus-related outcomes from an abbreviated palivizumab dose regimen in children with congenital heart disease. Paediatr Child Health. 2019;24 02:e16–e17. [Google Scholar]
- 66.Paes B, Mitchell I, Li A, Harimoto T, Lanctôt K L. Respiratory-related hospitalizations following prophylaxis in the Canadian registry for palivizumab (2005-2012) compared to other international registries. Clin Dev Immunol. 2013;2013:917068. doi: 10.1155/2013/917068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonnet D, Prahl R, Fredrick L M, Schulz G A, Campbell A L, Notario G.Retrospective analysis suggests palivizumab prophylaxis is not associated with an increased risk of serious infection, serious arrhythmia, or death in pediatric patients <24 months of age with hemodynamically significant congenital heart disease (HSCHD) Acta Paediatr 20111005921143292 [Google Scholar]
- 68.Kim A Y, Jung S Y, Choi J Y et al. Retrospective multicenter study of respiratory syncytial virus prophylaxis in Korean children with congenital heart diseases. Korean Circ J. 2016;46(05):719–726. doi: 10.4070/kcj.2016.46.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin Y J, Chung C H, Chi H, Lin C H. Six-monthly palivizumab prophylaxis effectively reduced RSV-associated hospitalization rates of preterm infants in a subtropical area: a population-based cohort study. Pediatr Res. 2019;86(05):628–634. doi: 10.1038/s41390-019-0492-7. [DOI] [PubMed] [Google Scholar]
- 70.Claydon J, Popescu C R, Shaiba L et al. Outcomes related to respiratory syncytial virus with an abbreviated palivizumab regimen in children with congenital heart disease: a descriptive analysis. CMAJ Open. 2019;7(01):E88–E93. doi: 10.9778/cmajo.20180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.IRIS Study Group . Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22(09):823–827. doi: 10.1097/01.inf.0000086403.50417.7c. [DOI] [PubMed] [Google Scholar]
- 72.Ryan R.Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stageCochrane Consumers and Communication Review Group. December 2016. Accessed 28 June 2019 at:http://cccrg.cochrane.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


