Abstract
Anti-dsDNA, anti-Sm, and anti-ribosomal-P autoantibodies are hallmarks of systemic lupus erythematosus (SLE), being anti-dsDNA and anti-Sm included in 2019-ACR/EULAR SLE-Classification Criteria. Enzyme-linked (ELISA) and chemiluminescence assays (CIA) are widely established in immunology laboratories, but new technologies, such as particle-based multi-analyte technology (PMAT), are nowadays available. The present study aimed to compare the presence of anti-dsDNA and anti-Sm autoantibodies measured by CIA and PMAT and analyze diagnostic and clinical SLE activity performance. Anti-ribosomal-P autoantibodies by PMAT were also included. Consequently, anti-dsDNA and anti-Sm detected by CIA showed substantial agreement with PMAT (Cohen's kappa = 0.662 and 0.671, respectively). Anti-dsDNA autoantibodies measured by PMAT showed a positive correlation with clinical SLEDAI-2K (p < 0.001) and a negative correlation with complement consumption (p < 0.001). Anti-Sm and anti-ribosomal-P autoantibodies showed a positive correlation with SLEDAI-2K (p < 0.001 and p = 0.001, respectively) and a negative correlation with complement consumption (p < 0.001 and p = 0.001, respectively). Finally, anti-Sm autoantibodies were associated with renal involvement (p < 0.05).
Keywords: Systemic lupus erythematosus, anti-dsDNA autoantibodies, Anti-Sm autoantibodies, Anti-ribosomal-, Chemiluminescence assays, Particle-based multi-analyte technology, Clinical SLEDAI-2K, SLEDAI-2K, SLE clinical manifestations
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease (AID) that could affect multiple organs. The loss of tolerance against self-antigens and the dysregulation of innate and adaptive immune responses contribute to the overproduction of inflammatory cytokines, autoantibodies, and immune complex deposition, which can fix complement and finally cause widespread organ damage [1]. The clinical manifestations of SLE are diverse, encompassing renal, cutaneous, mucosal, osteo-muscular, haematological, serosal, and neurological involvement [2]. Therefore, SLE diagnosis relies on the integration of different clinical manifestations, serological findings, and histopathological examination of the affected organs.
Hallmarks of SLE are autoantibodies, which serve as prominent markers of the disease and are considered crucial contributors to its not well-understood pathophysiology. Although more than 180 self-antigens have been reported as targets [3], only a minority are usually considered in clinical practice [1,4,5]. Among these, anti-double-stranded DNA (anti-dsDNA) and anti-Smith (anti-Sm) autoantibodies have demonstrated high specificity for SLE and have been included into the 2019 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) SLE Classification Criteria [6]. Anti-phospholipid autoantibodies are also included in this criterion. Additionally, complement levels and anti-dsDNA autoantibodies are components of the SLE Disease Activity Index 2000 (SLEDAI-2K) score [7]. Anti-ribosomal protein (anti-RibP) autoantibodies are not considered an immunological criterion, but their presence is a useful marker in the diagnosis of the disease because of their high specificity (96.1 %), comparable to that of anti-dsDNA and anti-Sm [8]. Several other autoantibodies have long been associated with SLE and may play a significant role. This subset includes anti-ribonucleoprotein, anti-Ro/SS-A, and anti-La/SS-B, which predominantly target nucleic acid-associated proteins. However, these autoantibodies are not included in the aforementioned classification criteria [9], and they do not exhibit high specificity or sensitivity.
Given the diagnostic potential associated with the presence of autoantibodies in serum samples, various techniques have been developed for their detection since the initial description of antinuclear autoantibodies (ANAs) in 1948 [10]. These include indirect immunofluorescence (IIF), immunodiffusion, hemagglutination, and complement fixation. Additionally, in the 1970s, counter-immunoelectrophoresis, Western blot, and enzyme-linked immunosorbent assay (ELISA) were introduced. However, it was not until the mid-1970s that the discovery of human epithelial type-2 (HEp-2) cells led to an unprecedented expansion in this field [11]. Nowadays, the gold standard approach for screening autoantibodies still entails utilizing indirect immunofluorescence (IIF) assays with HEp-2 cells, in the determination of ANAs, or Crithidia luciliae immunofluorescence test (CLIFT) for anti-dsDNA autoantibodies detection, as substrates. However, IIF is a laborious method that requires proficient operators for accurate interpretation and exhibits notable inter-observer variability as well as limited specificity [11,12]. Alternative and complementary approaches for studying ANAs and their specificities include antigen-specific methods such as ELISA and chemiluminescence assays (CIA), which are widely established in immunology laboratories. Nevertheless, emerging technologies, such as particle-based multi-analyte technology (PMAT), provide the ability to simultaneously detect multiple autoantibodies with different specificities [[13], [14], [15]].
Even though, ANA testing is an important tool widely used in diagnosing SLE and other AID due to its high sensitivity, its specificity is limited, leading to potential challenges in interpretation. While a positive ANA result is not diagnostic on its own, it serves as a valuable screening tool that, when considered alongside clinical symptoms and other tests, aids the in diagnosis and monitoring of disease activity. Moreover, all currently available methods differ, therefore clinicians and laboratory experts should be aware of the analytical variability among techniques when interpreting test results for SLE diagnosis, classification, and monitoring.
Thus, the aim of the present study was to evaluate the suitability of a new particle-based multi-analyte technology for measuring anti-dsDNA, anti-Sm, and anti-RibP autoantibodies in a large cohort of SLE patients and patients with other autoimmune diseases and to compare the results obtained with this new technology with chemiluminescence assays. Additionally, we aimed to establish the relationship between the presence of the aforementioned SLE-related autoantibodies with the clinical SLEDAI-2K (cSLEDAI-2K)/SLEDAI-2K score, complement consumption, and SLE clinical manifestations measured by the SLEDAI 2K and/or cSLEDAI-2K.
2. Patients and methods
2.1. Patient population and experimental design
The study cohort comprised of 335 individuals. Of these, 192 patients were diagnosed with SLE according to the 2019 ACR/EULAR SLE Classification Criteria [6], and the 143 remaining samples, which constituted the control group, were obtained from patients with autoimmune diseases other than SLE (AID no SLE; n = 121) and from healthy controls (HC, n = 22). Patients with AID no SLE were diagnosed with antiphospholipid syndrome (APS, n = 43), systemic sclerosis (SSc, n = 19), rheumatoid arthritis (RA, n = 26), autoimmune hepatitis (AIH, n = 25), and Sjögren's syndrome (SjS, n = 8). SLE and AID no SLE patients were followed up at the Department of Autoimmune Diseases of the Hospital Clínic, Barcelona, Catalonia, Spain. The SLEDAI-2K and clinical SLEDAI-2K (cSLEDAI-2K), considering only clinical variables from the SLEDAI-2K, were available for all of them, and a cut-off value of >6 was used to define active disease.
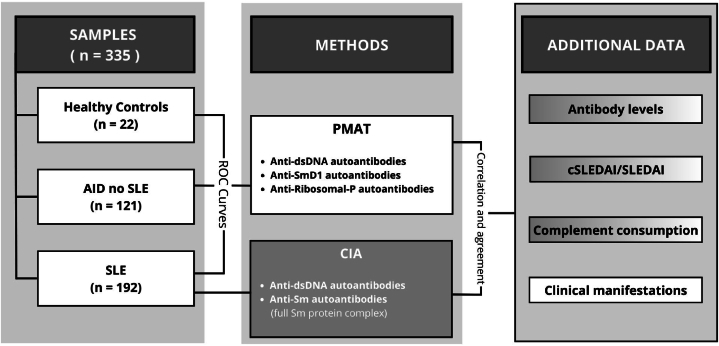
All individuals underwent the assessment of anti-dsDNA, anti-Sm, and anti-RibP autoantibodies levels using the PMAT on Aptiva® system (Fig. 1). For all SLE patients, autoantibodies titers were determined using the CIA on BIO-FLASH®. The results obtained from both methodologies were subjected to comparative analysis.
Fig. 1.
Study design. PMAT's ROC curves for anti-dsDNA, anti-SmD1 and anti-RibP autoantibodies were obtained with samples from healthy controls, AID no SLE and SLE patients. CIA's data proceed exclusively from SLE patients. Then, with samples from patients with SLE, an agreement between both techniques was performed, and additional data was correlated with antibody titers obtained from both technologies. Abbreviations: AID: autoimmune disease; SLE: systemic lupus erythematosus; PMAT: particle-based multi-analyte technology; CIA: chemiluminescence assay; SLEDAI; SLE Disease Activity Index 2000.
Ultimately, the data obtained from these two analytical technologies were correlated with various clinical parameters. The SLEDAI-2K was used to assess anti-Sm and anti-RibP autoantibodies, whereas its clinical counterpart, the cSLEDAI-2K, was employed to evaluate anti-dsDNA autoantibodies. Complement consumption was also evaluated. Furthermore, we analyzed the presence or absence of clinical manifestations, encompassing mucocutaneous, musculoskeletal, renal, haematological, serosal, and neurological features, as outlined in the SLEDAI-2K, with the positivity of anti-dsDNA, anti-SmD1, and anti-RibP autoantibodies detected via PMAT in our cohort of SLE patients.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of the Hospital Clínic de Barcelona (HCB/2022/0923).
2.2. Anti-dsDNA, anti-Sm and anti-RibP autoantibodies detection
The anti-dsDNA autoantibodies in serum samples were measured using two different commercial methods: CIA (QUANTA Flash®, Werfen, USA) on the BIO-FLASH® instrument (Werfen, Spain), and PMAT (Aptiva® CTD Essential, Werfen, USA) on the Aptiva instrument (Werfen, USA). For both assays, a 35 IU/mL cut-off value was established by the manufacturer.
The quantification of anti-Sm autoantibodies was performed first by CIA (QUANTA Flash®, Werfen, USA) on the BIO-FLASH® instrument (Werfen, Spain) whom antigen target is constituted by the full Sm protein-complex, and secondly, using PMAT (Aptiva® CTD Essential, Werfen, USA) which exclusively employs the SmD1 peptide from this multiproteinic complex. The cut-off recommended by the manufacturer is ≥ 20 CU for CIA and ≥5 FLU for PMAT.
Finally, levels of anti-RibP autoantibodies were obtained only by PMAT (Aptiva® CTD Essential, Werfen, USA) and, as is determined by the manufacturer, all samples with a cut-off value ≥ 5 FLU were considered positive.
CIA technology has been widely described [16], but PMAT is based on the procedure is described below.
Aptiva functions as an advanced digital system that employ PMAT to measure multiple autoantibodies concurrently in a single streamlined process. This approach harnesses a blend of suspended paramagnetic particles, each color-coded and coated with a unique antigen for precise identification. These individual color codes facilitate seamless antigen recognition during the procedure. The particles are mixed and incubated with the diluted patient sera, and if the target analyte is present, it binds to the corresponding bead. After washing, the mixture (paramagnetic particles and patient sera) is treated with anti-human IgG conjugated to phycoerythrin. After a thorough washing cycle, particles are arranged into a monolayer for analysis using a digital imaging technology equipped with two LEDs. The first LED, emitting a distinct red hue, identifies the paramagnetic particle, while the second LED, emitting a green light, measures the fluorescence emitted. The reaction data are digitally captured via a sophisticated high-resolution charged coupled device (CCD) sensor and securely stored within the system's database for subsequent quantitative analysis. To ensure the flawless functionality of the instrument, quality control reagents containing antibodies specific to each analyte under scrutiny are methodically employed.
Aptiva instrument utilizes ready-to-use cartridges containing all the specific reagents for the analytical reaction. Specifically, the cartridge used in this study was the CTD IgG Essential™ panel, which allows for the simultaneous detection of anti-dsDNA, DFS70, U1-RNP, SmD1, Ro60/SSA, Ro52, La/SSB, Scl70, Jo1, CENP-B, and RibP autoantibodies.
2.3. Complement detection
Complement (C3, C4) serum levels and activity (CH50) were assessed using the turbidimetric immunoassay method (Atellica CH C3 and Atellica CH C4; Siemens, New York, NY, USA and Autokit CH50; Fujifilm Wako Chemicals, Neuss, Germany, respectively). All were tested in Atellica CH Solution, New York, NY, USA. The reference values for the measurements were as follows: 0.870–1.700 g/L for C3, 0.110–0.540 g/L for C4, and 28–60 U/mL for CH50. Hypocomplementemia was defined as the condition when either CH50 activity or C3 or C4 levels were below the respective reference values.
2.4. Statistical analysis
Statistical analyses were conducted using the IBM SPSS Statistics PC package (Version 22). Differences were considered statistically significant at p < 0.05. Cut-off values were determined through receiver operating characteristic (ROC) curve analysis, with the highest Youden Index value being selected. Descriptive statistics for continuous data included mean and standard deviation (SD) or median and 25th-75th interquartile range (IQR), depending on its distribution. Categorical variables were presented as absolute numbers and percentages. The normality of the data was assessed using the Shapiro-Wilk test or Kolmogorov-Smirnov test depending on the sample size. Differences in proportions were evaluated using the χ2 test or Fisher's exact test when the expected counts were ≤5. Differences in means of continuous variables were analyzed using the parametric Student t-test or the nonparametric Mann-Whitney U test when the variable distribution was not normal. Correlations were calculated using Pearson's R, Spearman's coefficient, or Kendall's tau b, based on the type of variable. Additionally, the odds ratio (OR) and 95 % confidence interval (95 % CI) were calculated.
3. Results
3.1. Study cohort
Most of the participants were females. There were significant differences between control group (HC and AID controls) and SLE group in gender (76.1 % vs 90.1 % females) and age (54.3 ± 17 vs 47 ± 13.8 years), respectively. From 192 SLE patients, 172 (89.5 %) were under treatment, 22 (11.4 %) or 19 (9.8 %) had active disease at the time of inclusion measured by SLEDAI-2K or cSLEDAI-2K, respectively, and 33 (17.1 %) had major organ involvement. Table 1 resumes the main demographic, clinical and laboratory characteristics of the cohort.
Table 1.
Demographic and clinical characteristics from study cohort.
| Control Group |
SLE Group |
||
|---|---|---|---|
| Healthy Controls (n = 22) | AID Controls (n = 121) | SLE (n = 192) | |
| Sex: female, n (%)a | 18 (81.8 %) | 90 (74.3 %) | 173 (90.1 %) |
| Age, mean ± SD (years)b | 35.1 ± 11.8 | 57.9 ± 15.4 | 47 ± 13.8 |
| Age at diagnosis, mean ± SD (years) | – | – | 32.8 ± 13.8 |
| Disease duration, mean ± SD (years) | – | – | 15.2 ± 10.3 |
| Treatment, n (%) | – | – | 172 (89.5 %) |
| Hypocomplementemia, n (%) | – | – | 66 (34.3 %) |
Active disease:
|
– | – | 22 (11.4 %) 19 (9.8 %) |
| Major organ involvement (at sampling), n (%) | – | – | 33 (17.1 %) |
| Mucocutaneous involvement, n (%) | – | – | 16 (8.3 %) |
| Musculoskeletal involvement, n (%) | – | – | 12 (6.2 %) |
| Renal involvement, n (%) | – | – | 6 (3.1 %) |
| Haematological involvement, n (%) | – | – | 5 (2.6 %) |
| Neurological involvement, n (%) | – | – | 0 |
| Serosal involvement, n (%) | – | – | 0 |
p = 0.001 Control group vs SLE group.
p < 0.001 Control group vs SLE group.
3.2. PMAT's clinical performance
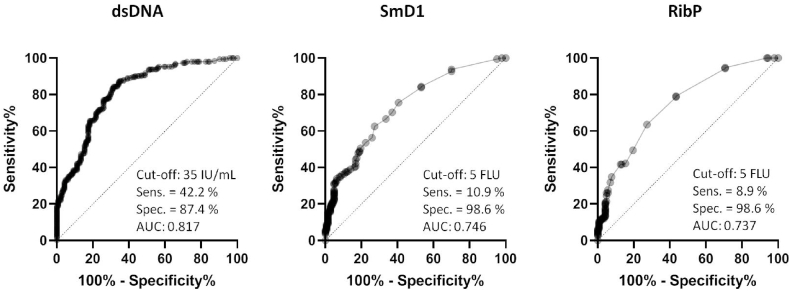
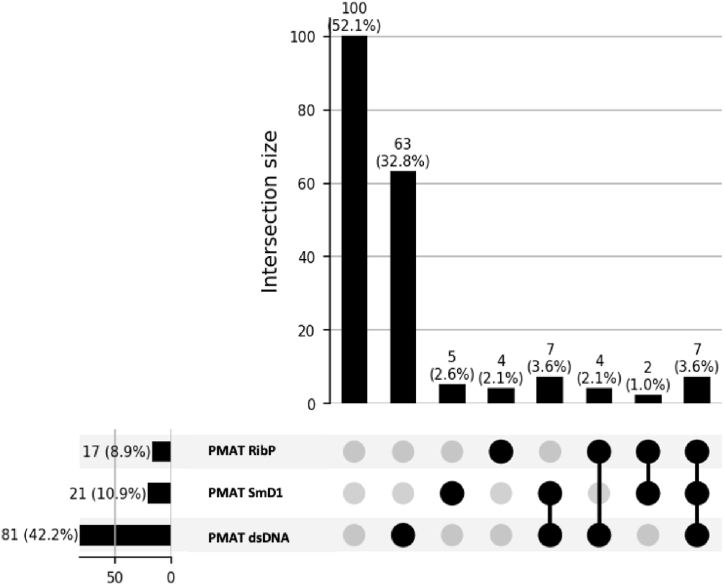
Anti-dsDNA determination by PMAT demonstrated a clinical sensitivity of 42.2 % (95 % CI: 35.4 %–49.3 %) for the diagnosis of SLE and 87.4 % (95 % CI: 81.0 %–91.9 %) specificity in the control group. Regarding anti-SmD1 autoantibodies, PMAT showed a clinical sensitivity of 10.9 % (95 % CI: 7.3 %–16.1 %) and 98.6 % (95 % CI: 95.0 %–99.8 %) specificity. Lastly, in the case of anti-RibP autoantibodies, PMAT showed a clinical sensitivity of 8.9 % (95 % CI: 5.6 %–13.7 %) and 98.6 % (95 % CI: 95.0 %–99.8 %) specificity. The ROC curve analysis among SLE patients and control group demonstrated an area under the curve (AUC) value of 0.817 for anti-dsDNA, 0.746 for anti-SmD1, and 0.737 for anti-RibP autoantibodies detection (Fig. 2). Among the patients enrolled, 36.7 % exhibited autoantibodies against a single antigen, 6.7 % demonstrated antibodies against two specificities, and 3.6 % displayed reactivity towards all three antigens analyzed (Fig. 3).
Fig. 2.
ROC curves for anti-dsDNA, anti-Sm and anti-RibP autoantibodies obtained by PMAT. Area under curve (AUC), cut-off values, sensitivity (Sens.) and specificity (Spec.) are shown for each graph.
Fig. 3.
UpSet Plot of autoantibodies in SLE patients who have been tested by PMAT.
3.3. Agreement between methods: PMAT vs. CIA for anti-dsDNA antibodies determination
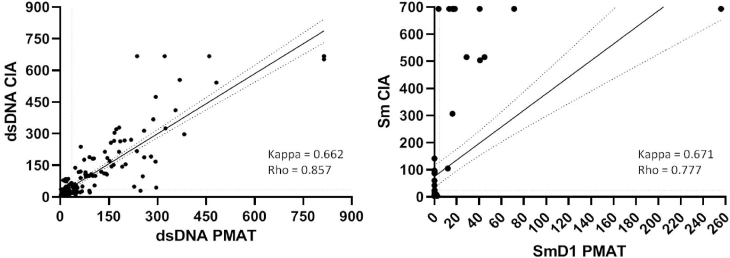
Regarding anti-dsDNA autoantibodies, overall, in the 192 SLE patients tested by both methods, the CIA and PMAT revealed a substantial agreement (Cohen's kappa = 0.662) and a Rho′s correlation coefficient of 0.857. Similarly, for the detection of anti-Sm/anti-SmD1 autoantibodies, substantial agreement (Cohen's kappa = 0.671) and a Rho′s coefficient of 0.777 were observed (Fig. 4). This comparison could not be performed for anti-RibP autoantibodies because of the low number of patients in our cohort who had undergone CIA testing for these autoantibodies.
Fig. 4.
Correlation and agreement from the comparison of anti-dsDNA and anti-Sm autoantibodies detection by PMAT and CIA. Anti-RibP data is not represented owing to the low number of patients with CIA results. Cohen's Kappa and Rho are indicated.
3.4. Correlation between titers of anti-dsDNA, anti-Sm and anti-RibP autoantibodies with cSLEDAI-2K/SLEDAI-2K
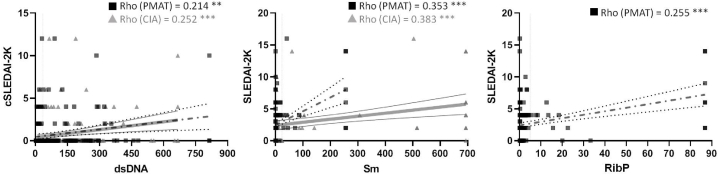
Subsequently, an analysis was conducted to assess the potential relationship between the titers of anti-dsDNA, anti-Sm, and anti-RibP autoantibodies obtained from 192 patients with SLE with cSLEDAI-2K or SLEDAI-2K indices (Fig. 5), where higher scores indicate more severe disease activity. Specifically, a positive Rho′s correlation coefficient was observed between anti-dsDNA autoantibody's titers and cSLEDAI-2K (Rho = 0.214; p = 0.003 vs. Rho = 0.252; p < 0.001, for PMAT and CIA, respectively). The same trend was observed for anti-Sm and anti-RibP autoantibodies concerning SLEDAI-2K (Rho = 0.353; p < 0.001 vs. Rho = 0.383; p < 0.001; Rho = 0.255; p < 0.001, respectively). Due to the limited number of patients in our cohort with anti-RibP autoantibodies determined by CIA, it was not possible to calculate the correlation of their titers measured by this assay with SLEDAI-2K.
Fig. 5.
Correlation between titers of anti-dsDNA, anti-Sm and anti-RibP autoantibodies with cSLEDAI/SLEDAI-2K. PMAT (black squares) and CIA (grey triangles) data are shown, and Rho is specified. **p < 0.01. ***p < 0.001.
3.5. Correlation between titers of anti-dsDNA, anti-Sm and anti-RibP autoantibodies with complement consumption
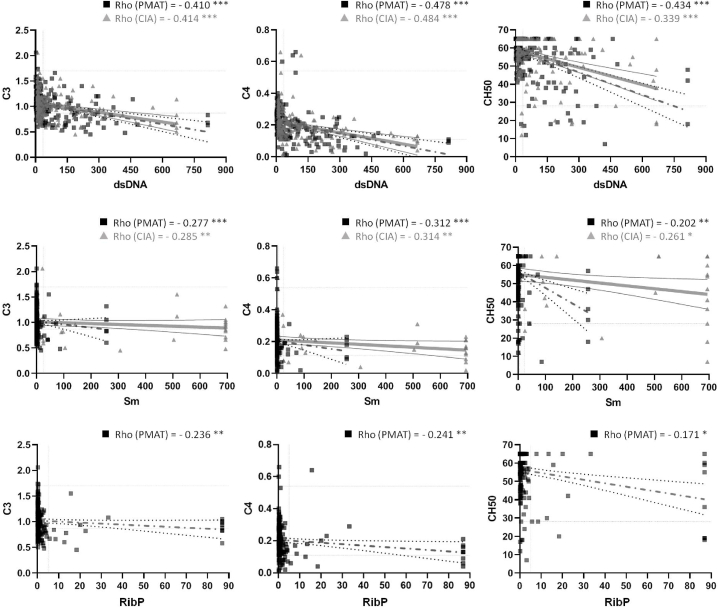
We also analyzed the relationship between the autoantibodies studied and complement consumption in patients with SLE. In all instances, the correlation observed between both technologies for each autoantibody was consistent and negative (Fig. 6). Precisely, anti-dsDNA autoantibody's titers exhibited a negative Rho′s correlation coefficient with C3 and C4 levels as well as CH50 (p < 0.001). Similarly, anti-Sm autoantibodies also displayed a negative Rho′s coefficient with C3, C4 and CH50 (PMAT: p < 0.001, p < 0.001 and p < 0.01; CIA: p < 0.01, p < 0.01 and p < 0.05, respectively). Finally, anti-RibP autoantibodies, measured only by PMAT, demonstrated a negative correlation with C3, C4 and CH50 (p < 0.01, p < 0.01 and p < 0.05, respectively).
Fig. 6.
Correlation between titers of anti-dsDNA, anti-Sm and anti-RibP autoantibodies with complement consumption. PMAT (black squares) and CIA (grey triangles) data are shown, and Rho is specified. *p < 0.05. **p < 0.01. ***p < 0.001.
3.6. Correlation between presence and titers of anti-dsDNA, anti-Sm and anti-RibP autoantibodies with clinical manifestations
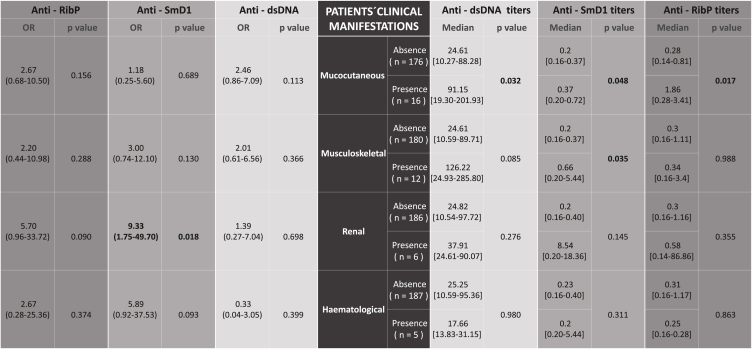
Ultimately, the presence of anti-dsDNA, anti-SmD1, and anti-RibP autoantibodies detected by PMAT in the cohort of SLE patients who exhibited clinical manifestations documented in the SLEDAI-2K index was investigated (Table 2). Out of the 192 SLE patients, a total of 33 (17.2 %) presented one of the following clinical manifestations at the time of inclusion to the study: 16/33 (48.4 %) mucocutaneous, 12/33 (36.3 %) musculoskeletal, 6/33 (18.1 %) renal and 5/33 (15.1 %) haematological. Six patients from the cohort presented concomitantly two manifestations: four mucocutaneous and musculoskeletal, one renal and haematological, and one musculoskeletal and haematological. None of the patients exhibited serosal or neurological abnormalities. Furthermore, we examined the median autoantibody titers among patients who presented the clinical manifestations compared to those whose manifestation was absent.
Table 2.
Odds ratio (left side) and median titers (right side) of anti-dsDNA, anti-Sm and anti-RibP autoantibodies in relation to SLE clinical manifestations included in SLEDAI-2K. Of 192 SLE patients, 33 presented clinical manifestations. Six of them presented two manifestations concurrently: 4 mucocutaneous and musculoskeletal, 1 renal and haematological, and 1 musculoskeletal and haematological. Neurological and serosal manifestations are not present in our cohort. The statistically significant results are indicated in bold typeface.
Among the findings, it is worth noting a higher prevalence of anti-SmD1 antibodies in patients with renal involvement (OR = 9.33; 95 % CI 1.75–49.70; p = 0.018), with no statistically significant differences found in the median antibody titers between the two groups. No other association was observed.
Regarding the relationship between the median antibody titers in patients with clinical manifestations and those without, we observed elevated titers for all the antibodies analyzed in patients with mucocutaneous manifestations (anti-dsDNA: p = 0.032, anti-SmD1: p = 0.048, and anti-RibP: p = 0.017). Additionally, it was noted that patients with musculoskeletal manifestations, as opposed to those without, exhibited higher titers of anti-SmD1 antibodies (p = 0.035).
4. Discussion
The first part of the study aimed to evaluate the suitability of the new PMAT on Aptiva® for measuring anti-dsDNA, anti-Sm and anti-RibP autoantibodies and compare it with the other diagnostic technology available in our laboratory, CIA on BIO-FLASH®.
CIA consists of paramagnetic beads coated with a single autoantigen. This way, if autoantibodies are present in the patient's serum, they recognize the antigen and link to it. Then, the isoluminol-conjugated antibody detection attaches to it and emits a signal that is detected by the luminometer [17]. However, this technology only allows the detection of a single type of autoantibody per assay. Hence, for patients in whom it is necessary to determine multiple autoantibodies with varying specificities, conducting a separate assay for each autoantibody becomes imperative. This entails a significant allocation of resources and time. PMAT represents a significant step forward because it enables the simultaneous detection of multiple autoantibodies within a single assay. This capability enhances the comprehensive interpretation of the results and streamlines the process, offering valuable time savings for both patients and clinicians alike. PMAT uses different synthetic antigens to coat various paramagnetic particles. Each particle has a unique color code corresponding to the antigen on the surface. In this way, once the samples are processed, paramagnetic particles are illuminated by one LED, and color codes from the beads are identified. The positive and/or negative values, and the quantity of patient autoantibodies bound to the particle surface can then be assessed using the second LED [11].
The outcomes derived from the evaluation of anti-dsDNA, anti-Sm, and anti-RibP autoantibodies utilizing this novel technological approach demonstrated a limited sensitivity, notably in the case of anti-Sm and anti-RibP autoantibodies (anti-dsDNA: 42.2 %; anti-SmD1: 10.9 %; RibP: 8.9 %). Nevertheless, they concurrently manifested a commendable level of specificity (anti-dsDNA: 87.4 %; anti-SmD1: 98.6 %; and RibP: 98.6 %). It is noteworthy that this heightened specificity remains consistent, even when considering that the control group primarily comprised individuals with various non-SLE autoimmune conditions.
Upon thorough examination of PMAT's sensitivity and specificity, we proceeded to compare the outcomes achieved with this novel technology to those determined by CIA. The comparative analysis revealed a significant level of agreement and a commendable correlation coefficient between the two technologies in assessing anti-dsDNA autoantibodies (Cohen's kappa = 0.662; Rho = 0.857) and anti-Sm/anti-SmD1 autoantibodies (Cohen's kappa = 0.671; Rho = 0.777).
The dsDNA antigen employed by each of the analyzed technologies is the same. Nevertheless, it is important to highlight that the Sm antigens used by both assays differ significantly. PMAT technology exclusively employs the SmD1 peptide, whereas CIA utilizes a full Sm protein complex, consisting of the D1 polypeptide and six additional peptides [18]. Therefore, the different antigens utilized can impact the binding of the autoantibodies and influence the sensitivity, specificity, and correlation of both detection methods.
Although it is known that each of the different polypeptides of Sm has antigenicity against anti-Sm antibodies, the major target Sm antigens are the D1 polypeptide [19]. Moreover, it is also known that the sensitivity of anti-SmD1 is significantly higher than that of anti-Sm [20]. This phenomenon is because the Sm full proteins interact with ribonucleoproteins (RNP), forming a large spliceosome [21]. Consequently, a full Sm protein complex could have some parts from RNP. As a result, patients who have anti-RNP autoantibodies but not Sm ones could give false anti-Sm autoantibody positives on CIA but not on PMAT.
In the second part of the study, we examined whether there existed a correlation between the levels of autoantibodies, detected by PMAT and CIA, and disease activity in the SLE patients under study. The SLEDAI-2K was developed and validated as a clinical index for the measurement of disease activity in SLE and has been used as a global measure of disease activity in SLE since its introduction in 1951 [7]. Its clinical counterpart cSLEDAI-2K, is an adaptation of SLEDAI-2K excluding the points coming from anti-dsDNA and complement status, that allow a more accurate confirmation. Active SLE disease was established by a cSLEDAI-2K > 6 at the moment of inclusion. Thus, we analyzed the correlation between the scores obtained on the SLEDAI-2K index, with anti-Sm and anti-RibP autoantibodies levels, and cSLEDAI-2K, with anti-dsDNA autoantibodies levels. Additionally, we investigated any potential associations with complement consumption and the clinical manifestations of SLE as included in the aforementioned index.
The findings derived from our analysis revealed that the levels of anti-dsDNA autoantibodies, assessed by both PMAT and CIA methodologies, exhibited a weak positive correlation with the cSLEDAI-2K scale (Rho = 0.214; Rho = 0.252, respectively). Likewise, anti-Sm and anti-RibP autoantibodies demonstrated a similar weak correlation with the SLEDAI-2K scale (PMAT = 0.353 vs. CIA = 0.383; PMAT = 0.255, respectively). This may be deemed unexpected because, according to some literature, anti-dsDNA autoantibodies titers are associated with recurrent disease flares [22]. Nevertheless, in other research studies, it was noted that a mere 17.4 % of the flares were distinguished by an elevated level of anti-double-stranded DNA autoantibodies [23]. Moreover, it is also well known that autoantibodies at high titers can be also observed in patients in remission [24]. Therefore, results obtained suggest and support that higher autoantibody titers may potentially influence increased disease activity and a higher score on this scale, but they do not appear to be a definitive determining factor. This implies that there might be existing other qualitative attributes of autoantibodies, such as complement fixation capability, avidity, dissociation constant, or even immunoglobulin class, which represent more pivotal determinants and could exhibit a more robust correlation with disease activity [25].
In terms of how these autoantibodies contribute to tissue damage in SLE, it is well-established that one of the principal pathogenic mechanisms involves the formation, and subsequent deposition, of immune complexes, followed by the activation of the complement system and cytokine production. This is reflected in the decrease, due to their consumption, of the levels of C3 and C4, both of which are integral components of this system [26,27], and/or in their activity, represented by CH50. We observed that levels of anti-dsDNA autoantibodies in our cohort displayed a moderate negative correlation with C3 levels (PMAT = −0.410 vs. CIA = −0.414) and C4 levels (PMAT: −0.478 vs. CIA = −0.484) and CH50 (PMAT: −0.434 vs. CIA = −0.339). Conversely, anti-Sm and anti-RibP autoantibodies exhibited a notably weaker correlation with C3 levels (PMAT = −0.277 vs. CIA = −0.285; PMAT = −0.236), C4 levels (PMAT = −0.312 vs. CIA = −0.314; PMAT = −0.241) and CH50 (PMAT = −0.202 vs. CIA = −0.261; PMAT = −0.171), respectively.
Extensive evidence has substantiated the intimate relationship between immunoglobulin deposition and DNA binding. Nephritogenic anti-DNA autoantibodies have the capacity to form immune complexes, which can subsequently be deposited on the glomerular basement membrane, thereby activating the complement system [28]. This activation leads to tissue damage and the recruitment of inflammatory cells, ultimately resulting in the onset of lupus nephritis [27]. Moreover, diminishing the affinity of these autoantibodies for DNA can culminate in the abrogation of glomerular deposition and nephritis [29]. However, it is worth noting that this mechanistic framework has not been as comprehensively elucidated in the context of anti-Sm and anti-RibP autoantibodies. Consequently, the observed distinctions in this association may be ascribed to the distinctive pathogenic mechanisms inherent to these autoantibodies. While, in the case of anti-dsDNA autoantibodies, the previously delineated mechanism appears to represent the principal pathogenic avenue, in the case of anti-Sm and anti-RibP antibodies it does not appear to be the predominant modus operandi or, at the very least, not as efficacious.
Lastly, our ultimate objective was to ascertain whether the presence and/or absence, as well as the titers of the autoantibodies under investigation obtained through PMAT in the 192 SLE patients, could be related to the clinical manifestations documented in the SLEDAI-2K scale (mucocutaneous, musculoskeletal, renal, haematological, serosal, and neurological). Many of the patients were enrolled in the study following the onset of the disease, and most of them (172 out of 192, 89.5 %) were receiving immunosuppressive treatment. Consequently, the cohort with clinical manifestations during the course of this study is limited.
Among the diverse clinical manifestations, a distinct association was observed between anti-Sm autoantibodies and the presence of renal involvement (OR = 9.33; 95 % CI 1.75–49.70; p = 0.018), although statistically significant differences were not found in the median titers. Remarkably, we did not observe a heightened presence of anti-dsDNA autoantibodies in patients with renal involvement, an association that has been traditionally documented, perhaps attributable to the limited size of the patient cohort displaying this clinical manifestation, as previously discussed. The presence of anti-Sm has been previously reported to be related to renal disease [30] considering anti-Sm to be an important factor in the development of nephritis [31]. This association was more common when anti-Sm was found together with anti-dsDNA [32]. In our study, however, we did not identify this correlation. Out of the total of six patients with renal involvement, three had anti-dsDNA autoantibodies, while the remaining three had anti-Sm autoantibodies. Notably, none of these patients exhibited both types of autoantibodies concurrently.
Nonetheless, our study exhibits certain limitations. Firstly, patients were not recruited at the onset of SLE, and a significant portion of them were undergoing immunosuppressive treatment (89.5 %). Consequently, we were unable to assess the real potential repercussions of autoantibody levels on disease activity (SLEDAI-2K), complement consumption and clinical manifestations at disease onset, where autoantibodies could exert a more pronounced impact and show a stronger correlation with these factors. In fact, this work is based on consecutive clinical and laboratory monitoring SLE patients' samples and reflect the daily practice in a clinical laboratory. Second, regarding the analysis of anti-Sm autoantibodies, it is important to highlight that the antigens used by PMAT and CIA technologies differ significantly. Therefore, it could influence on the sensitivity and specificity of both detection methods and, consequently, in our correlation between assays. Third, within our cohort, there were limited instances of patients with anti-RibP autoantibodies determined through CIA. Consequently, we were unable to establish a correlation with the PMAT technology, as was achieved with anti-dsDNA and anti-Sm autoantibodies. Finally, owing to the cohort's characteristics, including immunosuppression and the absence of SLE-onset cases, the pool of patients with available clinical manifestations was notably restricted. Consequently, certain clinical manifestations traditionally linked to the presence of specific autoantibodies were not observed in the scope of this study.
In summary, based on our findings, PMAT represents a reliable laboratory technology demonstrating a notably high specificity in the detection of anti-dsDNA, anti-SmD1, and anti-RibP autoantibodies. Furthermore, it exhibits substantial concordance with CIA, the other widely used technique at immunology laboratories, particularly in the detection of anti-dsDNA and anti-Sm autoantibodies. Additionally, the results reveal that anti-dsDNA autoantibodies display a positive, albeit weak, correlation with cSLEDAI-2K, along with a moderately negative correlation with C3 and C4 levels and CH50. Conversely, anti-Sm and anti-RibP autoantibodies show a positive weak correlation with SLEDAI-2K and a negative weak correlation with C3 and C4 levels and CH50. Furthermore, we observed that positive results for anti-SmD1 autoantibodies are associated with renal involvement, although no statistically significant differences in mean titers were observed. Finally, larger prospective studies are required to confirm the associations observed in this study and ascertain whether the technique can identify early changes, enabling the prediction of disease flares or the detection of newly emerging autoantibodies in patients as the disease evolves and progresses. Moreover, further studies using this technology could be important to investigate numerous other antigens classically linked to SLE, however these were not included in this study.
Data availability
Data and additional information will be accessible under request.
CRediT authorship contribution statement
Daniel Lorca-Arce: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Albert Pérez-Isidro: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Investigation, Formal analysis. Judit Becerra: Methodology, Investigation. Maria José Martínez: Methodology, Investigation. Noemí De Moner: Methodology, Investigation. Roberto Ríos-Garcés: Writing – review & editing, Investigation. Sergio Prieto-González: Writing – review & editing, Investigation, Data curation. Gerard Espinosa: Writing – review & editing, Methodology, Investigation, Data curation, Conceptualization. Ricard Cervera: Writing – review & editing, Project administration, Investigation, Data curation, Conceptualization. Carmen Andalucía: Writing – review & editing, Software, Resources, Project administration, Funding acquisition, Formal analysis, Conceptualization. Odette Viñas-Gomis: Writing – review & editing, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Estibaliz Ruiz-Ortiz: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Han S., Zhuang H., Shumyak S., Yang L., Reeves W.H. Mechanisms of autoantibody production in systemic lupus erythematosus. Front. Immunol. 2015;6 doi: 10.3389/fimmu.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul A., et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 3.Yaniv G., et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun. Rev. 2015;14:75–79. doi: 10.1016/j.autrev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Dema B., Charles N. Autoantibodies in SLE: specificities, isotypes and receptors. Antibodies. 2016;5:2. doi: 10.3390/antib5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzani E., Drosera M., Gasparini G., Parodi A. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis. 2014:1–13. doi: 10.1155/2014/321359. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aringer M., et al. European League against rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400–1412. doi: 10.1002/art.40930. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladman D.D., Ibañez D., Urowitz M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 8.Viana V.T., Durcan L., Bonfa E., Elkon K.B. Ribosomal P antibody: 30 years on the road. Lupus. 2017;26:453–462. doi: 10.1177/0961203317690243. [DOI] [PubMed] [Google Scholar]
- 9.Artim-Esen B., et al. Cluster analysis of autoantibodies in 852 patients with systemic lupus erythematosus from a single center. J. Rheumatol. 2014;41:1304–1310. doi: 10.3899/jrheum.130984. [DOI] [PubMed] [Google Scholar]
- 10.Classic Papers in Rheumatology 48–49. CRC Press; 2001. Presentation of two bone marrow elements: the ‘tart cell’ and the ‘LE’ cell. [DOI] [Google Scholar]
- 11.Irure-Ventura J., López-Hoyos M. The past, present, and future in antinuclear antibodies (ANA) Diagnostics. 2022;12:647. doi: 10.3390/diagnostics12030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossuyt X., De Langhe E., Borghi M.O., Meroni P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020;16:715–726. doi: 10.1038/s41584-020-00522-w. [DOI] [PubMed] [Google Scholar]
- 13.Villalta D., et al. Evaluation of a novel extended automated particle-based multi-analyte assay for the detection of autoantibodies in the diagnosis of primary biliary cholangitis. Clin. Chem. Lab. Med. 2020;58:1499–1507. doi: 10.1515/cclm-2020-0122. [DOI] [PubMed] [Google Scholar]
- 14.Mahler M., et al. Evaluation of a novel particle-based multi-analyte technology for the detection of anti-fibrillarin antibodies. Immunol. Res. 2021;69:239–248. doi: 10.1007/s12026-021-09197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bizzaro N., et al. Multiparametric autoantibody analysis: a new paradigm for the diagnosis of connective tissue diseases. Arthritis Res. Ther. 2022;24:278. doi: 10.1186/s13075-022-02980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentow C., et al. International multi-center evaluation of a novel chemiluminescence assay for the detection of anti-dsDNA antibodies. Lupus. 2016;25:864–872. doi: 10.1177/0961203316640917. [DOI] [PubMed] [Google Scholar]
- 17.Cinquanta L., Fontana D.E., Bizzaro N. Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Autoimmun Highlights. 2017;8:9. doi: 10.1007/s13317-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urlaub H. Sm protein-Sm site RNA interactions within the inner ring of the spliceosomal snRNP core structure. EMBO J. 2001;20:187–196. doi: 10.1093/emboj/20.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou Y., Sun D., Sharp G.C., Hoch S.O. Screening of SLE sera using purified recombinant Sm-D1 protein from a baculovirus expression system. Clin. Immunol. Immunopathol. 1997;83:310–317. doi: 10.1006/clin.1997.4355. [DOI] [PubMed] [Google Scholar]
- 20.Hu C., et al. Anti-SmD1 antibodies are associated with renal disorder, seizures, and pulmonary arterial hypertension in Chinese patients with active SLE. Sci. Rep. 2017;7:7617. doi: 10.1038/s41598-017-08099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamond A.I. Running rings around RNA. Nature. 1999;397:655–656. doi: 10.1038/17697. [DOI] [PubMed] [Google Scholar]
- 22.Yu F., Haas M., Glassock R., Zhao M.-H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017;13:483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 23.Petri M., Genovese M., Engle E., Hochberg M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991;34:937–944. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- 24.Yurasov S., et al. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 2006;203:2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayanan K., Marwaha V., Shanmuganandan K., Shankar S. Correlation between systemic lupus erythematosus disease activity index, C3, C4 and anti-dsDNA antibodies. Med. J. Armed Forces India. 2010;66:102–107. doi: 10.1016/S0377-1237(10)80118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan C., Zhang T., Putterman C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat. Rev. Nephrol. 2023;19:491–508. doi: 10.1038/s41581-023-00722-z. [DOI] [PubMed] [Google Scholar]
- 27.Pisetsky D.S., Lipsky P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020;16:565–579. doi: 10.1038/s41584-020-0480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisetsky D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023;19:509–524. doi: 10.1038/s41581-023-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz J.B., Limpanasithikul W., Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J. Exp. Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arroyo-Ávila M., et al. Clinical associations of anti-Smith antibodies in PROFILE: a multi-ethnic lupus cohort. Clin. Rheumatol. 2015;34:1217–1223. doi: 10.1007/s10067-015-2941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alba P. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann. Rheum. Dis. 2003;62:556–560. doi: 10.1136/ard.62.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janwityanuchit S., Verasertniyom O., Vanichapuntu M., Vatanasuk M. Anti-Sm: its predictive value in systemic lupus erythematosus. Clin. Rheumatol. 1993;12:350–353. doi: 10.1007/BF02231577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and additional information will be accessible under request.