Abstract
Purpose
To assess Gram-positive bacterial (GPB) bloodstream infection (BSI) in neonates, covering incidence, morbidity, mortality, antimicrobial resistance patterns and biomarkers in Region Stockholm, Sweden between 2006 and 2016.
Methods
A population-based retrospective epidemiological study including infants with GPB-BSI, admitted to the neonatal units at Karolinska University Hospital (KUH). Data were collected from patient records, the Swedish Neonatal Quality Register, the microbiological laboratory at KUH and the Swedish Public Health Agency.
Results
We identified 357 infants with GPB-BSI, representing an incidence of 1.47/1000 live births (LB). Group B streptococcus (GBS) was the most common pathogen causing BSI in full-term infants and early-onset sepsis (EOS) (0.20/1000 LB), while coagulase-negative staphylococci (CoNS) were predominant in infants born very preterm and in late-onset sepsis (LOS) (0.79/1000 LB). There were no fatal GBS BSI cases, but 10.2% developed meningitis. The GPB case fatality rate was 9.5% and the sepsis fatality rate 2.8%. In GPB-BSI, 1/10 did not have an elevated C-reactive protein level. Staphylococcus aureus (S. aureus) BSI increased during the study period, but no methicillin or vancomycin resistant strains were found. The antimicrobial resistance (AMR) rate was highest in CoNS isolates.
Conclusion
GPB-BSI was four times more common than Gram-negative BSI in neonates but resulted in lower mortality rate. GBS was the most common pathogen in full-term infants and in EOS. CoNS was the most common pathogen in LOS and infants born very preterm, and the AMR rate was high in these isolates. The increasing trend of S. aureus BSI indicates a need of further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-024-04809-8.
Keywords: Neonatal, Gram-positive, Sepsis, Group B streptococcus, Staphylococcus aureus, Coagulase-negative staphylococci
Introduction
Neonatal sepsis globally affects around 4/1000 live born (LB) infants and is associated with a high mortality and morbidity rate, longer hospital stays and increased healthcare costs [1, 2]. The clinical presentation of neonatal sepsis varies depending on time of onset, gestational age, and causative pathogen [1, 2]. A broad spectrum of pathogens (i.e., bacteria, viruses and fungi) can cause neonatal sepsis [1]. Signs can initially be few and subtle like temperature instability, feeding difficulties, change in skin color, tachy- or bradycardia, tachypnea, apnea, grunting, irritability, or lethargy [1]. A positive culture from blood or cerebrospinal fluid (CSF) is a strong indicator of sepsis [1]. However,certain pathogens, like coagulase-negative staphylococci (CoNS), can be contaminants and cause falsely positive cultures [3]. Polymerase Chain reaction (PCR) is a sensitive diagnostic method of bacterial infections, which require smaller samples than blood/CSF cultures. Nonetheless, the high sensitivity of PCR increases the risk of false positive samples [3, 4]. Biomarkers like C-reactive protein (CRP) and platelet (PLT) count may be unreliable for neonates [5]. Further, cytokines, e.g., interleukin-6 (IL-6) has been suggested as an indicator of neonatal sepsis [6]. However, both CRP and IL-6 could be difficult to use as they require various cut-off levels at different time-points after birth [7]. Despite several attempts, there is no consensus definition of neonatal sepsis [2, 8].
The likelihood of a certain bacteria being the pathogen in neonatal sepsis varies depending on time of symptom onset. In early-onset sepsis (EOS), often defined as start of infection symptoms < 72 h of age, the infection is vertical, with pathogens often being group B streptococci (GBS) and Escherichia coli (EC), the former being a GPB [3–6]. The risk of EOS increases with the occurrence of chorioamnionitis, prolonged rupture of membranes (i.e., > 18 h before birth) and maternal GBS colonization [9]. In late-onset sepsis (LOS), symptoms occur at > 72 h of age, the infection is usually hospital-acquired and caused by Gram-positive bacteria (GPB), such as CoNS and Staphylococcus aureus [10–12]. Low birth weight and gestational age increase the risk of LOS [10, 12].
Prematurity is an established risk factor for neonatal infections [1]. This has resulted in an excessive use of antibiotics in neonatal units, which is sometimes crucial for the infant’s survival. For example, in the US, almost 90% of all extremely low birth weight neonates (i.e., birth weight < 1000 g) receive antibiotics during their first days in life [13]. Likewise, a higher gestational age has been shown to be a protective factor against morbidity and mortality in neonatal sepsis [14]. A recent point prevalence study, The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC), examined antibiotic prescription in 240 neonatal intensive care units (NICUs) worldwide and found that 35% of all neonates received antibiotics [15]. Further, the study showed that the most prescribed antibiotics for nosocomial infections was vancomycin in several regions (including Europe), which has raised concerns, since it is classified as a ”watch” antibiotic in the WHO AWaRe classification [15, 16].
Simultaneously, an estimated 214,000 neonatal deaths from sepsis each year are caused by antimicrobial resistance (AMR) [17], and AMR in neonatal sepsis is increasing rapidly [18]. It has been estimated for late preterm and full-term neonates that around 50–100 infants are given antibiotics for every true EOS case in high income countries [19, 20]. This becomes further problematic as an association between antibiotic use in early life, dysbiosis of the neonatal intestinal microbiota and an increased risk of adverse short term- (e.g. NEC and LOS) and long term outcomes (e.g. asthma, inflammatory bowel disease and allergy) has been described [1, 21–24]. Further, there is a correlation between neonatal antibiotic exposure and an increase in antimicrobial resistance in the intestinal microbiome [25].
It is crucial for every neonatal unit to know the current spectrum of bacterial pathogens, as well as their AMR pattern and impact of neonatal sepsis to provide a safe but effective antibiotic stewardship. Currently, this information is insufficient and treatment decisions risk of being based on outdated knowledge. The aim of this study was to describe incidence, morbidity, mortality, AMR patterns, and biomarkers of GPB sepsis in neonates during an 11-years period in Region Stockholm, Sweden. The overarching goal was to increase the knowledge support for risk assessment, diagnosis, and treatment of Gram-positive infections in newborns. This study complements a previous study of sepsis caused by Gram-negative bacteria (GNB) in Region Stockholm over the same 11-year period (2006–2016) [14].
Materials and methods
Study design
This was a retrospective epidemiological study over 11 years (1 January 2006- 31 December 2016).
Setting
Region Stockholm had 2,200,000 inhabitants in 2016. There were six delivery units and four hospitals that provide neonatal care. During March 2014-May 2016, a seventh delivery unit with a small level 1–2 neonatal unit, BB Sophia, operated. There are four neonatal intensive care units (NICUs), one at the South General Hospital and three at Karolinska University Hospital (i.e., Karolinska Solna, Karolinska Huddinge and Karolinska Danderyd). The three NICUs at Karolinska University Hospital are in different geographical areas in Stockholm and divided into one level 2- unit (i.e., for neonates born ≥ 32 gestational weeks (GW), whom need a high level of medical care) and two level 3-units (i.e., for all neonates who need the highest level of medical support for more than 48 h).
Study population
The study population consisted of infants admitted to the three NICUs at Karolinska University Hospital, the NICU at the South General Hospital and the NICU at BB Sophia, who had at least one positive blood or cerebrospinal fluid culture with growth of a GPB within 28 days of life or until 4 weeks after they reached full-term. All positive blood cultures with growth of Staphylococcus aureus (S. aureus) and GBS were considered sepsis cases. Blood cultures that were simultaneously positive for one or more GPB were classified as a mixed infection and reported in a separate group. CoNS-positive blood cultures were divided into two subgroups: sepsis and probable contamination. For the definition of sepsis, the following criteria should be met: (1) a positive blood culture (2) clinical symptoms of sepsis (i.e., at least two of the following symptoms: general malaise, fatigue, poor skin color, tachypnea, tachycardia, or bradycardia) and (3) intravenous antibiotic treatment for at least 5 days after a positive blood culture or until death, if death occurs within 5 days after a positive blood culture. Cases with probable contamination was defined as failure to meet the stated sepsis criteria and were excluded from further analysis. Further, cultures with growth of Bacillus cereus (n = 2) and Actinomyces naeslundii (n = 1) were considered contaminants and excluded from the study. In addition, blood cultures that were simultaneously positive for fungi or GNB were excluded. Sepsis fatality rate (SFR) was defined as death within 5 days after first GPB positive blood culture. Meningitis was defined as the presence of a GPB growth in cerebrospinal fluid. Septic shock was defined as sepsis caused by a GPB, where the child was given extra fluid and/or an inotropic drug according to guidelines for treatment of a circulatory shock.
Data collection
Both GPB and GNB isolates were identified. Data were collected from patient records from Stockholm’s five NICUs, the Swedish Neonatal Quality Register (SNQ), the microbiological laboratory at Karolinska University Hospital and from the Public Health Agency of Sweden. Data concerning neonatal sepsis with GNB is previously reported [14]. Due to lack of access to medical records, isolates from the South General Hospital and BB Sophia were thereafter excluded. Data from remaining three NICUs was retrieved from the electronic medical record system TakeCare and pseudonymized according to the General Data Protection Regulation.
Statistical analyses
This was a retrospective descriptive study with varying length of hospital stay and time between onset of symptoms and discharge. Data on all live born infants in the study region, during the study period was collected from Statistics Sweden, (www.scb.se).
Microbiological species identification and antimicrobial susceptibility testing
Species identification had been carried out using Matrix-Assisted Laser Desorption/Ionization Time Of Flight Mass Spectrometry (MALDI-TOF MS), and antimicrobial susceptibility testing using the disk diffusion method according to EUCAST, (www.eucast.org), except for vancomycin resistance which was investigated with the gradient test method (ETEST, bioMérieux, Askim, Sweden). In our region CoNS-isolates are not routinely subjected to identification at the species level and were therefore reported as a group in this study. All microbiological analyses were conducted at the clinical microbiology laboratory at Karolinska University Hospital.
Results
Incidence
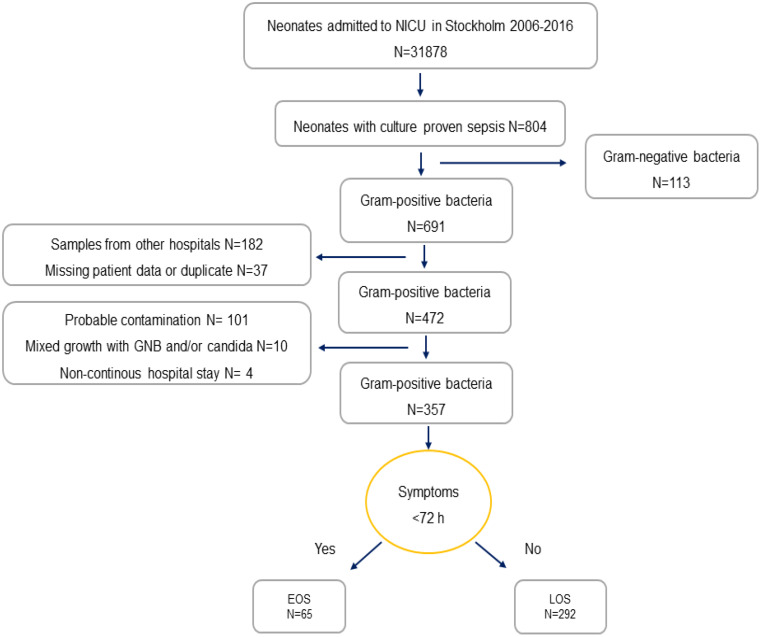
During the study period a total of 316,070 infants were born in Region Stockholm, of which 31,878 were admitted to a NICU; at Karolinska Solna (n = 5,928), Karolinska Huddinge (n = 6,904), Karolinska Danderyd (n = 10,418) and the South General Hospital (n = 8,728). In this group, 804 cases of culture proven sepsis were found, equivalent to an incidence of 2.5/1000 live born (LB), of which 691 cultures were GPB (85.9%), see Fig. 1. After excluding samples from which further information could not be gathered; samples from South General Hospital and other hospitals outside the region (n = 182) and missing data or duplicate (n = 37), a remaining 472 patient were screened for inclusion. Three hundred and fifty-seven infants with GPB sepsis were finally included in the study, equal to an incidence of 1.47/1000 LB, of which 65 were EOS (18.2%) and 292 were LOS (81.8%) (Table 1). Infants born at the South General Hospital (n = 71,999) were excluded for the incidence calculations. The general characteristics of the study population are showcased in Table 2.
Fig. 1.
Flow chart over the inclusion process and categorization into EOS and LOS. NICU neonatal intensive care unit, EOS early-onset sepsis, LOS late-onset sepsis
Table 1.
General characteristics of the study population
| Characteristics | No (%) | EOS (No tot) |
LOS (No tot) |
No tot |
|---|---|---|---|---|
| Gestation week | (65) | (292) | 357 | |
| Extremely pre-term (GW 22–27) | 216 (60.5) | 8 | 208 | |
| Very pre-term (GW 28–32) | 74 (20.8) | 10 | 64 | |
| Late pre-term (GW 33–36) | 20 (5,6) | 8 | 12 | |
| Full-term (GW 37–42) | 47 (13.1) | 39 | 8 | |
| Days in NICU (median) | 69.0 | 357 | ||
| Male sex | 217 (60.7) | 357 | ||
| Birth weight | (63) | (291) | 354 | |
| Extremely low birth weight (< 1000 g) | 210 (59.3) | 10 | 200 | |
| Very low birthweight (< 1500 g) | 66 (18.6) | 4 | 62 | |
| Low birthweight (< 2500 g) | 22 (6.2) | 8 | 14 | |
| Normal birthweight (≥ 2500 g) | 56 (15.8) | 41 | 15 | |
| APGAR-score at 5 min (median) | 7.0 | 332 | ||
| Vaginal delivery | 116 (33.4) | 37 (64) | 79 (283) | 347 |
| Prenatal factors | ||||
| Maternal risk of infection | 108 (31.4) | 26 (63) | 82 (281) | 344 |
| Antenatal steroids | 220 (73.6) | 15 (63) | 205 (236) | 299 |
| Postnatal inventions | ||||
| Intubationa | 255 (73.1) | 21 (64) | 234 (285) | 349 |
| Surfactant treatment | 213 (61.6) | 13 (64) | 200 (282) | 346 |
| Central catheter during NICU-stay | 288 (81.6) | 37 (64) | 251 (290) | 353 |
| UVC | 196 (58.7) | 21 (64) | 175 (270) | 334 |
| UAC | 255 (75.9) | 24 (64) | 231 (272) | 336 |
| pCVC | 222 (64.5) | 15 (63) | 206 (281) | 344 |
| Congenital condition | 356 | |||
| None | 320 (89.9) | 60 | 263 | |
| Heart disease | 13 (3.6) | 3 | 10 | |
| GI-anomaly | 6 (1.7) | 0 | 6 | |
| Kidney disease/UT-anomaly | 4 (1.1) | 0 | 4 | |
| CNS-anomaly | 1 (0.3) | 1 | 0 | |
| Chromosomal syndrome | 10 (2.8) | 1 | 9 | |
| Malignancy | 2 (0.6) | 0 | 2 |
aAll patients that were given surfactant are included in the group, regardless of later continuous intubation. GW gestation week, NICU neonatal intensive care unit, UVC umbilical venous catheter, UAC umbilical arterial catheter, pCVC peripheral central venous catheter, GI gastrointestinal, UT urinary tract, CNS central nervous system
Table 2.
Incidence of Gram-positive pathogen (total numbers and per 1000 LB)
| Pathogen | No (%) | Incidence sepsis | Incidence EOS (No) | Incidence LOS (No) |
|---|---|---|---|---|
| All GBS | 357 (100) | 1.47 | 0.27 (65) | 1.20 (292) |
| CoNS | 193 (53.8) | 0.79 | 0.02 (6) | 0.77 (187) |
| S. aureus | 88 (24.9) | 0.36 | 0.03 (8) | 0.33 (80) |
| GBS | 49 (13.7) | 0.20 | 0.18 (43) | 0.02 (6) |
| Othera | 13 (3.6) | 0.05 | 0.02 (6) | 0.03 (7) |
| Mixedb | 14 (3.9) | 0.06 | 0.01 (2) | 0.05 (12) |
aEnterococcus faecalis (n = 5), Streptococcus pneumoniae (n = 2), viridans group streptococci (n = 3), group A streptococci (n = 1), group C or G streptococci (n = 2).
bTwo or more GPB simultaneously; CoNS + S. aureus (n = 4), CoNS + Enterococcus faecalis (n = 3), S. aureus + Enterococcus faecalis (n = 3), CoNS + GBS (n = 2), S. aureus + GBS (n = 1) and CoNS + S. aureus + Enterococcus faecalis (n = 1). CoNS Coagulase-negative staphylococci, GBS Group B streptococci, GPB Gram-positive bacteria
Etiology of sepsis
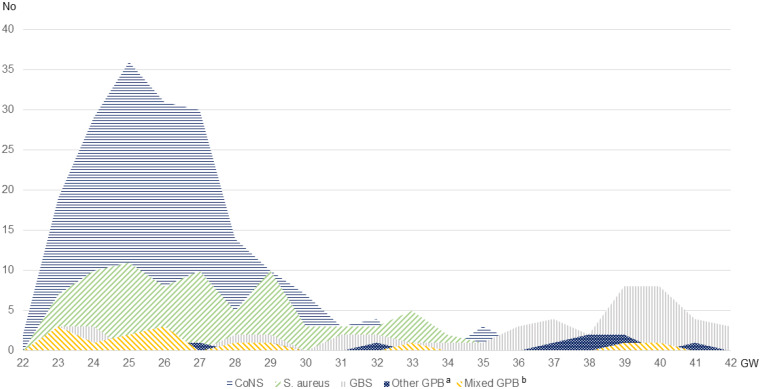
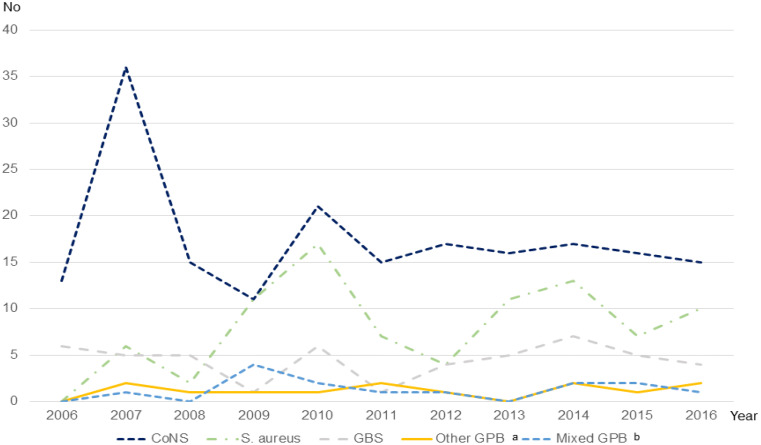
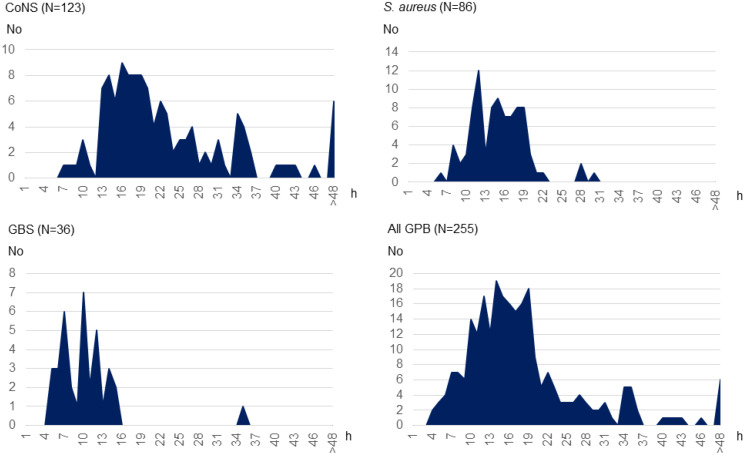
The most common pathogen was CoNS (n = 193, 53.8%), followed by S. aureus (n = 88, 24.9%) and GBS (n = 49, 13.7%). Figure 2 showcases an overlap in the distribution of pathogen in accordance with gestational age at birth, with a dominance of CoNS sepsis in the extremely (< 28 gestational weeks (GW) (n = 147, 69.0%) and very preterm born (< 32 GW) (n = 38, 51.6%) infants changing to S. aureus and GBS being the most common pathogens in the moderately preterm born (32 − 26 GW) (n = 6, 30.0%) and GBS in the full-term (n = 29, 61.7%) groups. Further, the number of S. aureus sepsis increased, while the number of infections from other GPB remained steady over the study period (Fig. 3). Microbiological data showed that time to growth differed depending on pathogen. Most cultures were positive within the first 24 h (n = 214, 83.9%), but almost 1/3 of CoNS cultures were positive only later (n = 40, 32.5%) (Fig. 4).
Fig. 2.
Total number of cases, according to pathogen and gestational age at birth. aEnterococcus faecalis (n = 5), Streptoococcus pneumoniae (n = 2), viridans group streptococci (n = 3), group A streptococci (n = 1), group C or G streptococci (n = 2). bTwo or more GPB simultaneously; CoNS + S. aureus (n = 4), CoNS + Enterococcus faecalis (n = 3), S. aureus + Enterococcus faecalis (n = 3), CoNS + GBS (n = 2), S. aureus + GBS (n = 1) and CoNS + S. aureus + Enterococcus faecalis (n = 1). EOS early-onset sepsis, LOS late-onset sepsis, CoNS Coagulase-negative staphylococci, GBS Group B streptococci, GPB Gram-positive bacteria , GW gestational weeks
Fig. 3.
Incidence according to pathogen over the study period. aEnterococcus faecalis (n = 5), Streptococcus pneumoniae (n = 2), viridans group streptococci (n = 3), group A streptococci (n = 1), group C or G streptococci (n = 2). bTwo or more GPB simultaneously; CoNS + S. aureus (n = 4), CoNS + Enterococcus faecalis (n = 3), S. aureus + Enterococcus faecalis (n = 3), CoNS + GBS (n = 2), S. aureus + GBS (n = 1) and CoNS + S. aureus + Enterococcus faecalis (n = 1). EOS early-onset sepsis, LOS late-onset sepsis, CoNS Coagulase-negative staphylococci, GBS Group B streptococci, GPB Gram-positive bacteria
Fig. 4.
Time to bacterial growth in blood or cerebrospinal fluid (CSF) culture for different pathogens. CoNS Coagulase-negative staphylococci, GBS Group B streptococci, GPB Gram-positive bacteria
Morbidity and mortality
The sepsis fatality rate (SFR) was 2.8% (n = 10), whereof 0% (n = 0) for EOS and 3.4% (n = 10) for LOS (Table 3). Of the SFR cases, 9/10 were born extremely premature; GW 22 (n = 1), GW 23 (n = 5), GW 24 (n = 2) and GW 26 (n = 1). One infant the SFR group was born in GW 35. The total case fatality rate (CFR) was 9.5% (n = 34), whereof 0.3% (n = 1) was EOS and 97.1% (n = 33) LOS. Infections with two or more GPB at the same time had a high SFR (n = 2, 14.3%) and CFR (n = 4, 28.6%). There were no mortality cases of GBS sepsis in this study, despite 10.2% of the GBS infections developed meningitis (n = 5), whereof four had seizures and one had multiple abscesses in the brain. The median age at death was 46 days. Of all neonates with GPB sepsis, 200 had an adverse outcome during their stay at NICU (56.0%) (Table 3). Forty infants had a GPB sepsis leading to septic shock (11.3%).
Table 3.
Outcome of GPB-sepsis
| Outcome | No (%) | EOS | LOS | SFRa (%) | SFRa EOS (%) |
SFRa LOS (%) |
CFRb (%) | CFRb EOS (%) |
CFRb LOS (%) |
No tot |
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality all GBS | 357 (100) | 65 | 292 | 10 (2.8) | 0 (0) | 10 (3.4) | 34 (9.5) | 1 (0.3) | 33 (11.3) | 357 |
| CoNS | 193 (54.0) | 5 | 188 | 6 (3.1) | 0 (0) | 6 (3.2) | 22 (11.4) | 0 (0) | 22 (11.7) | 193 |
| S. aureus | 88 (24.6) | 9 | 79 | 1 (1.1) | 0 (0) | 1 (1.3) | 5 (5.7) | 1 (11.1) | 5 (6.3) | 88 |
| GBS | 49 (13.7) | 43 | 6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 49 |
| Otherc | 13 (3.6) | 6 | 7 | 1 (7.7) | 0 (0) | 1 (14.3) | 3 (23.1) | 0 (0) | 3 (42.9) | 13 |
| Mixede | 14 (3.9) | 2 | 12 | 2 (14.3) | 0 (0) | 2 (16.7) | 4 (28.6) | 0 (0) | 4 (33.3) | 14 |
| Morbidity | ||||||||||
| All GBS | 200 (56.0) | 357 | ||||||||
| NEC | 84 (23.5) | 357 | ||||||||
| - Medically treated | 69 (19.3) | 0 | 69 | |||||||
| - Surgically treated | 15 (4.2) | 0 | 15 | |||||||
| IVH 3–4 discharge | 34 (10.3) | 5 | 29 | 330 | ||||||
| ROP 3–4 discharge | 46 (18.6) | 4 | 42 | 247 | ||||||
| BPD | 144 (50.9) | 8 | 136 | 283 | ||||||
| Septic chock | 40 (11.3) | 8 | 32 | 354 |
aDeath within 5 days after GPB-positive culture
bDeath before discharge from the neonatal unit
cEnterococcus faecalis (n = 5), Streptococcus pneumoniae (n = 2), viridans group streptococci (n = 3), Group A streptococci (n = 1), Group C/G streptococci (n = 2). Two or more GPB simultaneously; CoNS + S. aureus (n = 4), CoNS + Enterococcus faecalis (n = 3), S. aureus + Enterococcus faecalis (n = 3), CoNS + GBS (n = 2), S. aureus + GBS (n = 1) and CoNS + S. aureus + Enterococcus faecalis (n = 1). NEC necrotizing enterocolitis, IVH intraventricular haemorrhage, ROP retinopathy of the newborn, BPD bronchopulmonary dysplasia, CoNS Coagulase-negative staphylococci and GBS Group B streptococci
Antimicrobial resistance patterns and biomarkers
The percentage of AMR in the GPB isolates in the study material was notably high (n = 205, 60.0%). This high percentage was primarily driven by the elevated AMR-prevalence observed in CoNS where 95.3% (n = 184) of isolates demonstrated AMR as indicated in Table 4. Most CoNS isolates showed resistance to both isoxazolyl penicillin (n = 177, 91.7%) and gentamicin (n = 172, 89.1%) and more than half were displaying resistance to fusidic acid (n = 99, 51.3%). S. aureus had the lowest percentage of AMR, with only 8% AMR (n = 7). Of these 5/7 were resistant to clindamycin. However, only mirroring 5.7% of all cultures positive for S. aureus in the study. There was no methicillin-resistant S. aureus (MRSA) or isolates with resistance to vancomycin. An increased concentration of C-reactive protein (CRP) in response to GPB sepsis was observed in most patients (n = 312, 90.2%) (Supplementary Table S1). However, 34 patients did not have an elevated CRP, despite an ongoing GPB sepsis (9.8%), of which most were caused by CoNS (n = 28) in infants born pre-term. In the group extremely premature neonates (Table 3). One hundred and forty out of 350 (40.0%) infants had a platelet (PLT) count < 100 × 109/l related to GPB sepsis (Supplementary Figure S1 and S2).
Table 4.
Antimicrobial resistance
| Pathogen and antibiotic | No AMR (%) | No total |
|---|---|---|
| All GPB | 205 (60.0) | 342 |
| CoNS | 184 (95.3) | 193 |
| Penicillin | ||
| Isoxazolylpenicillin | 177 (91.7) | |
| Aminoglycosides | ||
| Gentamicin | 172 (89.1) | |
| Amikacin | 27 (14.0) | |
| Glycopeptides | ||
| Teicoplanin | 17 (8.8) | |
| Lincosamines | ||
| Clindamycin | 75 (38.9) | |
| Oxazolidinones | ||
| Linezolid | 1 (0.5) | |
| Others | ||
| Fusidic acid | 99 (51.3) | |
| Trimethoprim/sulfamethoxazole | 92 (47.7) | |
| Rifampicin | 8 (4.1) | |
| S. aureus | 7 (8.0) | 88 |
| Penicillin | ||
| Isoxazolylpenicillin | 0 (0) | |
| Lincosamines | ||
| Clindamycin | 5 (5.7) | |
| Others | ||
| Fusidic acid | 2 (2.3) | |
| GBS | 11 (22.4) | 49 |
| Macrolides and lincosamines | ||
| Clindamycin | 3 (6.1) | |
| Erythromycin | 3 (6.1) | |
| Others | ||
| Tetracycline | 2 (4.1) | |
| Other GPB a | 4 (25.0) | 13 |
| Aminoglycosides | ||
| Gentamicin | 2 (12.5) | |
| Lincosamines | ||
| Clindamycin | 1 (6.3) |
aEnterococcus faecalis (n = 5), Streptoococcus pneumoniae (n = 2), viridans group streptococci (n = 3), group A streptococci (n = 1), group C or G streptococci (n = 2). AMR Antimicrobial resistance, CoNS Coagulase-negative staphylococci, GBS Group B streptococci, GPB Gram-positive bacteria and No Number
Discussion
Neonatal sepsis is a feared condition, at the same time as consensus for diagnostics criteria are missing, the potential negative effects of unnecessary antibiotic treatment plentiful, and AMR is an upcoming crisis [2, 8, 16]. A cornerstone in a factful approach to AMR is knowledge of current spectrum of bacterial pathogens, as well as their AMR patterns. In this study, we have described incidence, morbidity, mortality, AMR patterns, and biomarkers of GPB sepsis in neonates to increase the knowledgebase for clinicians to use in diagnostics and treatment of neonatal sepsis.
The incidence of GPB sepsis in this study was 1.47/1000 LB, which is more than 4 times the incidence for GNB sepsis (0.35/1000 LB) in our area, but with a lower SFR (2.8% versus 16.8%) and CFR (9.5% versus 28.0%) [14]. This result is in line with previous studies showing a higher mortality rate for GNB sepsis compared to GPB sepsis [10, 26, 27]. Few studies have reported on GPB-sepsis separately, but our GPB-SFR in LOS is like that of a recent Norwegian study [27]. In EOS, most affected infants were born full-term and GBS was the most common pathogen, concordant to previous international and Swedish studies [1, 28–30]. However, the GBS-EOS incidence (0.18/1000 LB) in our study was lower than both earlier Swedish and international reports [28–30], but higher than a recent report from Poland [31]. Interestingly, there were no fatal cases of GBS sepsis in this study, something that contradicts an often-described mortality in neonatal GBS sepsis of 7–9% [1, 28]. This is presumably a result of an effective infection control and the Swedish risk factor based intrapartum antibiotic prophylactic treatment regimen, implemented in 2008. A decrease of GBS-EOS has been reported during the same timeframe [32].
Noticeable was that all SFR cases in this study were LOS and that 9/10 of them were born extremely preterm, the latter in line with previous findings [26]. CoNS, where most isolates came from infants born extremely- or very preterm, was the most frequent causing pathogen in LOS. This is consistent with previous studies [10, 26, 27]. The CFR for CoNS seen in our study was in line with previous studies [10, 33]. CoNS are commonly presented as a group, despite the individual bacteria’s varying virulence [3]. A distinction of individual CoNS could be of interest for future studies, to see any potential difference in outcome in neonatal sepsis caused by different bacteria within this group. It is important to consider the vulnerability of this extremely preterm born patient group, many with more than one serious condition, which could explain that we found a SFR significantly lower than the CFR for CoNS (3.1% vs. 11.4%).
Further, it is thought-provoking that we found an increasing trend in S. aureus sepsis over the study period (Fig. 3), similar to findings from the USA (neonatal) [34] and Europe (adults and children) [35], but in contrast to finding in Australia (neonatal) [36], Korea (neonatal) [26] and a recent international systematic review in adult patients [37]. The stable CoNS incidence indicates that the increasing numbers of S. aureus infections is unlikely to be related to breaches in infection control measures.
None of the S. aureus isolates in our study were MRSA in contrast to results from studies in the USA (28%) [38], Australia (26%) [36] and a study covering several low-and middle income countries (61%) [39]. However, an increase of MRSA-positive isolates (all sample types) has been seen in our region over the study period, according to the Public Health Institute of Sweden [40], though mostly from asymptomatic colonization of the bacteria. Besides, a high percentage of CoNS in this study were AMR (95.3%), which highlights the groups tendency to acquire AMR genes. Therefore, the risk of both current AMR as well as the risk of causing an increase in the antibiotic resistant gene-load in infants’ intestinal microbiota, should be taken into consideration when treating CoNS. For instance, 14% of the CoNS isolates were resistant to amikacin, a first line antibiotic commonly used in combination with cephalosporins or cloxacillin in LOS of unknown cause in our setting. Still, none of the CoNS isolates showed resistance to vancomycin, something that has been described from several countries [41, 42], which establishes vancomycin as first line treatment for culture-verified CoNS sepsis in neonates in our area. However, it is important to emphasize the limitation of vancomycin use to confirmed CoNS sepsis, to avoid contributing to the further development of AMR. Further, a recent Norwegian study found a wide variation in amounts of prescribed vancomycin in between sites, with no clear relation to the sepsis related mortality rates [43], which emphasis the need to use vancomycin restrictively. In addition, the WHO has classified vancomycin as “watch” in the AwaRe category. This classification indicates that vancomycin belongs to a group of antibiotics that should be used only for specific infections [16].
As noted, diagnostics of neonatal sepsis is challenging. Biomarkers are helpful, but our study showed that 9.8% of all GPB sepsis had no elevated CRP concentration, specifically apparent for infants in the lower GWs. This, together with a well described delayed increase in CRP-levels at the occurrence of an infection, makes CRP as a biomarker unreliable for early detection of neonatal sepsis [44]. It has been suggested that a combination of CRP and procalcitonin (PCT) testing could improve the diagnostic sensitivity as PCT-levels increase after just 6-hours [44, 45]. However, neither an increased CRP- nor an elevated PCT-level are specific for infections, as they can both be elevated by non-infectious factors as well [45]. Most infants with GPB born before GW 32 had a lowered PLT count (< 100). The variation in virulence levels among GPB, including CoNS, presents a complex scenario. Despite being GPB, CoNS exhibit properties that results in comparatively less inflammatory response than other GPB (Supplementary Figure S1 and S2). This distinction can be challenging to attribute solely to host factors, such as the immaturity of the host´s immune system in premature infants.
The retrospective design of this study is a limitation, due to the lack of possibility to control missing data or to re-test cultures for accuracy. Further, since we have not accounted for infants seeking care after being discharged to home, our incidence calculations could be falsely slightly underestimated, especially regarding GBS sepsis. Moreover, the vulnerability and co-morbidity of the study population cause for several possible confounders when it comes to interpreting symptoms of sepsis. Lastly, the high SFR and CFR that we found for mixed infections are difficult to interpret due to the low numbers, the fact that one of the bacteria could be a possible contaminant, and the unusual occurrence of several bloodstream bacteria at once. The population-based design and the availability to extract data from several different systems (patient records, the Swedish Neonatal Quality Register (SNQ), the microbiological laboratory at Karolinska University Hospital and from the Public Health Agency of Sweden) is a strength as it is less likely that we have missed culture positive GPB sepsis cases.
Conclusion
Neonatal GPB sepsis is four times more prevalent than GNB sepsis in the Stockholm region. Though the mortality is only a third of that for GNB sepsis, 1/10 patients with GPB sepsis still experience fatal outcomes, and of these all were LOS and 9/10 born extremely preterm. Moreover, a significant number of patients suffer from various sequelae. In EOS and infants born full-term, GBS was the most common causative agent, but with no fatal outcomes. CoNS was the predominant pathogen in LOS and among infants born before gestational week 32. CoNS also featured the highest percentage of AMR, which should be considered when determining treatment strategies. Lastly, the increasing trend of S. aureus in our area indicates a need to understand underlaying reasons.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the planning of this study. Material preparation and the first part of data collection was performed by Viveka Nordberg. The second part of data collection and analysis were performed by Frida Oldendorff with support from all authors. The first draft of the manuscript was written by Frida Oldendorff. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Author F.O. has received research support from the Samariten foundation for paediatric research and the Barnforskningen Foundation.
Open access funding provided by Karolinska Institute.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to data protection regulations but are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethical approval
This study is approved by the Regional Ethics Review board in Stockholm (Dnr:2016/202 − 31/2) and the Swedish Ethical Review Authority (Dnr 2023-05349-01) and performed in accordance with the General Data Protection Regulation (GDPR).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 2.Molloy EJ, Wynn JL, Bliss J, et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr Res. 2020;88(1):2–4. doi: 10.1038/s41390-020-0850-5. [DOI] [PubMed] [Google Scholar]
- 3.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27(4):870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeser C, Pond M, Butcher P, et al. PCR for the detection of pathogens in neonatal early onset sepsis. PLoS ONE. 2020;15(1):e0226817. doi: 10.1371/journal.pone.0226817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirico G, Loda C. Laboratory aid to the diagnosis and therapy of infection in the neonate. Pediatr Rep. 2011;3(1):e1. doi: 10.4081/pr.2011.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal YA, Álvarez-Nemegyei J, Lavadores-May AI, et al. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern Fetal Neonatal Med. 2019;32(17):2830–2836. doi: 10.1080/14767058.2018.1449828. [DOI] [PubMed] [Google Scholar]
- 7.Chiesa C, Pellegrini G, Panero A, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem. 2003;49(1):60–68. doi: 10.1373/49.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–140. doi: 10.1097/MOP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 11.Tsai MH, Hsu JF, Chu SM, et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. 2014;33(1):e7–e13. doi: 10.1097/INF.0b013e3182a72ee0. [DOI] [PubMed] [Google Scholar]
- 12.Gudjonsdottir MJ, Hentz E, Adlerberth I, et al. Late-onset neonatal infections 1997 to 2017 within a Cohort in Western Sweden-the last 21 years of a 43-Year surveillance. Pediatr Infect Dis J. 2021;40(4):359–364. doi: 10.1097/INF.0000000000002987. [DOI] [PubMed] [Google Scholar]
- 13.Flannery DD, Ross RK, Mukhopadhyay S, et al. Temporal trends and Center Variation in early antibiotic use among premature infants. JAMA Netw Open. 2018;1(1):e180164. doi: 10.1001/jamanetworkopen.2018.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordberg V, Iversen A, Tidell A, et al. A decade of neonatal sepsis caused by gram-negative bacilli-a retrospective matched cohort study. Eur J Clin Microbiol Infect Dis. 2021;40(9):1803–1813. doi: 10.1007/s10096-021-04211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versporten A, Bielicki J, Drapier N, et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71(4):1106–1117. doi: 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 16.WHO AWaRe classification of antibiotics for evaluation and monitoring of use, 2023 2023 [cited 2023]. Available from: www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04
- 17.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 18.Wattal C, Kler N, Oberoi JK, et al. Neonatal Sepsis: mortality and morbidity in neonatal Sepsis due to Multidrug-Resistant (MDR) organisms: part 1. Indian J Pediatr. 2020;87(2):117–121. doi: 10.1007/s12098-019-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocker M, Klingenberg C, Navér L, et al. Less is more: antibiotics at the beginning of life. Nat Commun. 2023;14(1):2423. doi: 10.1038/s41467-023-38156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannoni E, Dimopoulou V, Klingenberg C, et al. Analysis of antibiotic exposure and early-onset neonatal Sepsis in Europe, North America, and Australia. JAMA Netw Open. 2022;5(11):e2243691. doi: 10.1001/jamanetworkopen.2022.43691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrieta MC, Arévalo A, Stiemsma L, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142(2):424–434e10. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 23.Simonyte Sjödin K, Vidman L, Rydén P, et al. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr Opin Allergy Clin Immunol. 2016;16(4):390–395. doi: 10.1097/ACI.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 24.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparrini AJ, Wang B, Sun X, et al. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat Microbiol. 2019;4(12):2285–2297. doi: 10.1038/s41564-019-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song WS, Park HW, Oh MY, et al. Neonatal sepsis-causing bacterial pathogens and outcome of trends of their antimicrobial susceptibility a 20-year period at a neonatal intensive care unit. Clin Exp Pediatr. 2022;65(7):350–357. doi: 10.3345/cep.2021.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huncikova Z, Vatne A, Stensvold HJ, et al. Late-onset sepsis in very preterm infants in Norway in 2009–2018: a population-based study. Arch Dis Child Fetal Neonatal Ed. 2023;108(5):478–484. doi: 10.1136/archdischild-2022-324977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson Gudjónsdóttir M, Elfvin A, Hentz E, et al. Changes in incidence and etiology of early-onset neonatal infections 1997–2017 - a retrospective cohort study in western Sweden. BMC Pediatr. 2019;19(1):490. doi: 10.1186/s12887-019-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madrid L, Seale AC, Kohli-Lynch M, et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: systematic review and Meta-analyses. Clin Infect Dis. 2017;65(suppl2):S160–s172. doi: 10.1093/cid/cix656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. Coli disease continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golińska E, Kozień Ł, Tomusiak-Plebanek A, et al. Epidemiology of neonatal sepsis in two neonatal intensive care units in Krakow, Poland in 2016–2017 years. BMC Infect Dis. 2023;23(1):827. doi: 10.1186/s12879-023-08836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Håkansson S, Lilja M, Jacobsson B, et al. Reduced incidence of neonatal early-onset group B streptococcal infection after promulgation of guidelines for risk-based intrapartum antibiotic prophylaxis in Sweden: analysis of a national population-based cohort. Acta Obstet Gynecol Scand. 2017;96(12):1475–1483. doi: 10.1111/aogs.13211. [DOI] [PubMed] [Google Scholar]
- 33.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickel N, Brooks S, Mize C, et al. Reducing Staphylococcus aureus infections in the neonatal intensive care unit. J Perinatol. 2022;42(11):1540–1545. doi: 10.1038/s41372-022-01407-4. [DOI] [PubMed] [Google Scholar]
- 35.Gagliotti C, Högberg LD, Billström H et al (2021) Staphylococcus aureus bloodstream infections: diverging trends of meticillin-resistant and meticillin-susceptible isolates, EU/EEA, 2005 to 2018. Euro Surveill. ;26(46) [DOI] [PMC free article] [PubMed]
- 36.Shadbolt R, We MLS, Kohan R, et al. Neonatal Staphylococcus Aureus Sepsis: a 20-year western Australian experience. J Perinatol. 2022;42(11):1440–1445. doi: 10.1038/s41372-022-01440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hindy JR, Quintero-Martinez JA, Lee AT, et al. Incidence trends and Epidemiology of Staphylococcus aureus Bacteremia: a systematic review of Population-Based studies. Cureus. 2022;14(5):e25460. doi: 10.7759/cureus.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ericson JE, Popoola VO, Smith PB, et al. Burden of Invasive Staphylococcus aureus infections in hospitalized infants. JAMA Pediatr. 2015;169(12):1105–1111. doi: 10.1001/jamapediatrics.2015.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell NJ, Stöhr W, Plakkal N, et al. Patterns of antibiotic use, pathogens, and prediction of mortality in hospitalized neonates and young infants with sepsis: a global neonatal sepsis observational cohort study (NeoOBS) PLoS Med. 2023;20(6):e1004179. doi: 10.1371/journal.pmed.1004179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkhalsomyndigheten Epidemiologisk årsrapport 2015 2015 [cited 2023]. Available from: www.folkhalsomyndigheten.se/contentassets/d4bb6d59921c423290c6b5b628c142b0/epidemiologisk_arsrapport_2015.pdf
- 41.Rasigade JP, Raulin O, Picaud JC, et al. Methicillin-resistant Staphylococcus capitis with reduced Vancomycin susceptibility causes late-onset sepsis in intensive care neonates. PLoS ONE. 2012;7(2):e31548. doi: 10.1371/journal.pone.0031548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peixoto PB, Massinhani FH, Netto Dos Santos KR, et al. Methicillin-resistant Staphylococcus epidermidis isolates with reduced Vancomycin susceptibility from bloodstream infections in a neonatal intensive care unit. J Med Microbiol. 2020;69(1):41–45. doi: 10.1099/jmm.0.001117. [DOI] [PubMed] [Google Scholar]
- 43.Huncikova Z, Stensvold HJ, Øymar KAA et al (2023) Variation in antibiotic consumption in very preterm infants-a 10 year population-based study. J Antimicrob Chemother. Nov 21 [DOI] [PMC free article] [PubMed]
- 44.Hofer N, Zacharias E, Müller W, et al. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 45.Eichberger J, Resch E, Resch B. Diagnosis of neonatal Sepsis: the role of inflammatory markers. Front Pediatr. 2022;10:840288. doi: 10.3389/fped.2022.840288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to data protection regulations but are available from the corresponding author on reasonable request.
Not applicable.