Summary
Background
Integration of paediatric health services across primary and secondary care holds great promise for the management of chronic conditions, yet limited evidence exists on its cost-effectiveness. This paper reports the results of the economic evaluation of the Children and Young People's Health Partnership (CYPHP) aimed at integrating care for children with common chronic conditions (asthma, eczema, and constipation).
Methods
Cost-effectiveness, cost-utility and cost-benefit analyses were conducted alongside a pragmatic cluster randomised controlled trial involving 97,970 children in 70 general practices in South London, including 1,731 participants with asthma, eczema and or constipation with self-reported health-related quality of life measures. Analyses considered the National Health Service (NHS)/Personal Social Service (PSS) and societal perspectives, and time horizons of 6 and 12-months. Costs included intervention delivery, health service use (primary and secondary care), referrals to social services, and time lost from work and school. Health outcomes were measured through the Paediatric Quality of Life Inventory, the Child Health Utility 9-Dimensions, and monetarised benefit combining Quality-Adjusted Life Years (QALYs) for children and parental mental well-being. Results present incremental cost-effectiveness ratios (ICERs), compared to a willingness to pay threshold (WTP) of £20,000–30,000/QALY, and net monetary benefit (NMB), with deterministic sensitivity analyses.
Findings
At 6 months, from the NHS/PSS perspective, CYPHP is not cost-effective (ICER = £721,000/QALY), and this result holds at 12 months (ICER = £45,586/QALY). However, under the societal perspective CYPHP falls within WTP thresholds (ICER = £22,966/QALY), with a probability of being cost-effective between 0.4 and 0.6 at £20,000/QALY and £30,000/QALY, respectively. The cost-benefit analysis yields a positive NMB of CYPHP at 12 months £109 under the societal perspective, with similar probabilistic results.
Interpretation
CYPHP was not cost-effective at 6 months or under the NHS/PSS perspective. Trends towards cost-effectiveness are observed once a longer time horizon and a more inclusive perspective on effects is considered. Further research beyond 12 months is needed as the model becomes firmly embedded into the paediatric healthcare delivery system.
Funding
This research was funded by Guy's and St Thomas' Charity, Lambeth and Southwark Clinical Commissioning Groups. The funders had no role in the writing of the manuscript, decision to submit it for publication, or any other process involved in the research.
Keywords: Cost-effectiveness, Integrated care, Child health, Chronic conditions, Quality-adjusted life years, Health care costs
Research in context.
Evidence before this study
The UK fares worse than other high-income countries in the clinical management and health outcomes of children with chronic conditions. Enhancing the integration of health services across primary and secondary care has been suggested as a way of improving quality of care and reducing secondary healthcare use. However, little is known about the cost-effectiveness of this approach. We performed a search on PubMed with (economic evaluation OR cost-effectiveness OR cost-utility OR cost-benefit) AND (integrat∗) AND (child∗) which retrieved 96 items as of November 8th, 2022. Seven studies were conducted in low- and middle-income countries (China, India, Colombia, Ethiopia, Indonesia, Kenya, Somalia, Rwanda), one observational study in the USA, a small RCT in Australia, and the remaining studies were not relevant (e.g., study protocols or not on integrated care for paediatric populations). No economic evaluation of integrated paediatric care in the UK was found. The scarce existing evidence weakly supports the cost-effectiveness of integrated care models; well-powered RCT studies are needed. Understanding whether paediatric care integration is an efficient strategy to improve health services for children with chronic conditions is particularly urgent in the current context of increasing constrained health budgets globally.
Added value of this study
The Children and Young People's Health Partnership (CYPHP) is a complex intervention aimed at advancing the integration of paediatric health services for children in the community with common chronic conditions (asthma, eczema, and constipation). The intervention was evaluated via a large, pragmatic, cluster randomised controlled trial in South London conducted between 2018 and 2021. This paper presents the results of the economic evaluation alongside the pragmatic cluster RCT, which involved 97,970 children including 1,731 participants with asthma, eczema or constipation with self-reported health-related quality of life measures. It includes cost-effectiveness, cost-utility, and cost-benefit analyses at 6 and 12 months, under both the NHS/Personal Social Service and societal perspectives, to comprehensively evaluate the possible diverse effects of CYPHP. This study contributes to the scarce literature on economic evaluations of complex service re-organisations.
Implications of all the available evidence
This paper concludes that CYPHP is not cost-effective at 6 months under the NHS/PSS perspective, but positive results begin to appear at 12 months once impacts on patients, families and schools are also considered under the societal perspective. These findings should be further explored via additional follow-ups or a decision analytic model.
Introduction
Chronic conditions account for an increasing share of the total disease burden in childhood, with a prevalence of 1.7 million children and young people (CYP) in England in 2018.1,2 The UK lags behind other high-income countries in chronic disease management. It has one of the highest asthma mortality rates in Europe, with 46% of these deaths linked to inadequate standards of asthma care.3 Suboptimal chronic disease management can lead to higher healthcare costs and poorer health-related quality of life (HRQoL) in childhood, with long-lasting consequences for CYP, families and society as a whole.4, 5, 6 The total cost to society of caring for preschool children in the year following a hospital attendance for asthma or wheeze is estimated at £14.53 m, 76% borne by the National Health Service (NHS). Children hospitalised with a chronic condition are also at higher risk of worse academic performance.7
Redesigning the paediatric healthcare system, so it can not only treat acute conditions through high-intensity specialist and inpatient services but also prevent and manage chronic conditions, aligns with the NHS Long-Term Plan8 and is a core part of health policy among high income countries. Integrated care connecting primary care, community services and specialised services has been proposed as a path forward to improve chronic disease management.8,9 Existing evidence on the effects of integrated care for children suggests it may yield gains in HRQoL, but results on health care quality, costs and cost-effectiveness are mixed.10, 11, 12 Limitations in current studies, involving intervention design, quality of data collection, and follow-up time, support the need for further research on the effectiveness and cost-effectiveness of integrated care systems for children.
The Children and Young People's Health Partnership (CYPHP) Evelina London model of care aims to provide timely, coordinated, biopsychosocial care in primary care and community setting to CYPHP with common chronic conditions, including asthma, eczema and constipation.13,14 CYPHP's staged implementation in South London allowed for a pragmatic cluster randomised controlled trial (cRCT) study design for evaluation. RCTs embedded in real clinical practice are rare and offer a unique opportunity to assess the effects of an intervention in real world circumstances. Between 2018 and 2021, general practices in Southwark and Lambeth were grouped into virtual clusters, consisting of 3–4 neighbouring GP practices, and randomised to CYPHP (intervention) or enhanced usual care (EUC, control). The CYPHP practices include local child health clinics (universal services or “in reach”), specialist nurse-led services, population health management, specialist team training and multidisciplinary team case planning in addition to the services of enhanced usual care.
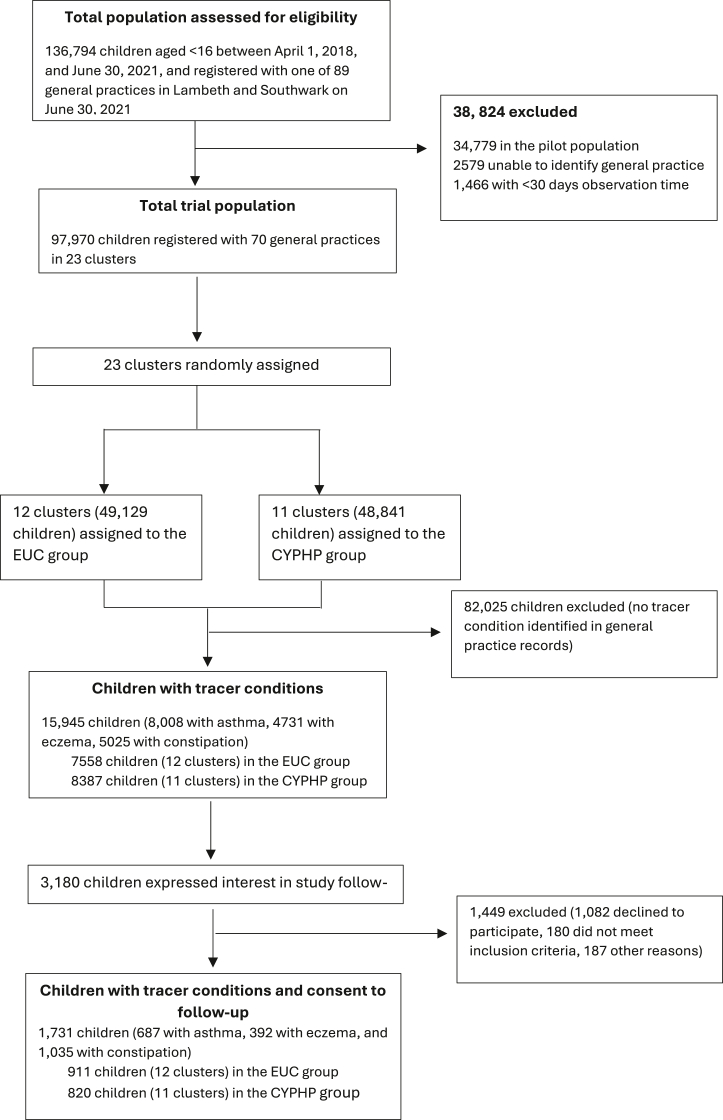
The aim of this paper is to conduct a within-trial economic evaluation of CYPHP compared to EUC for children with asthma, eczema, or constipation. A cost-effectiveness analysis using the Paediatric Quality of Life Inventory (PedsQL) was complemented with a cost-utility analysis based on the Quality-Adjusted Life Years (QALYs) from the Child Health Utility 9-Dimensions (CHU-9D). Analyses consider both the NHS/Personal Social Service (PSS) and societal perspectives and time horizons of 6 and 12-months. A cost-benefit analysis from a societal perspective brings together costs falling on CYP and parents by monetising health outcomes. The variety of economic evaluation types, perspectives, and time horizons responds to the possible diverse and unintended consequences of complex interventions, such as CYPHP, across sectors and allows this evaluation to be considered by government agencies using different types of results.15 This paper accompanies the trial outcomes paper by Wolfe et al.16 and focuses on one of the RCT subpopulations: children with tracer conditions who consented to follow up (Fig. 1). This population was selected for the economic evaluation to facilitate a patient level analysis of costs and health outcomes at baseline, 6 and 12 months.
Fig. 1.
CYPHP trial sample flow.
Methods
Study population and trial arms
Protocol papers for the trial and economic evaluation are published.13,17 In summary, this cRCT grouped 70 general practices in Southwark and Lambeth into 23 virtual clusters, 12 assigned to CYPHP (intervention) and 11 to EUC (control). The economic evaluation is focused on 1731 children below 16 years of age with at least one tracer condition (asthma, eczema, or constipation) and registered to a general practice in Southwark or Lambeth (Fig. 1). Besides EUC, CYPHP services for this population include specialist nurse-led services (community biopsychosocial care delivering health promotion and self-management advice), population health management (proactive case finding before conditions exacerbate), specialist team training (training on holistic and CYP-friendly care for healthcare professionals), and multidisciplinary team case planning. CYPHP, as part of its universal services, also includes in-reach clinics (integrated clinics delivered jointly by a paediatrician and a general practitioner) and lunch-and-learn sessions (gatherings to share knowledge and review cases). Children with tracer conditions may access the CYPHP service through universal services before being referred to the tracer service.
Healthcare costs
Costing followed the usual steps of identification, measurement, and valuation of resources. Total costs were generated at the patient level over two-time horizons, 6 and 12 months, and from both the NHS/PSS and societal perspectives, so costs on families and other sectors of the economy (educational sector as some CYPHP training components involved school staff) were also accounted for.
Identification
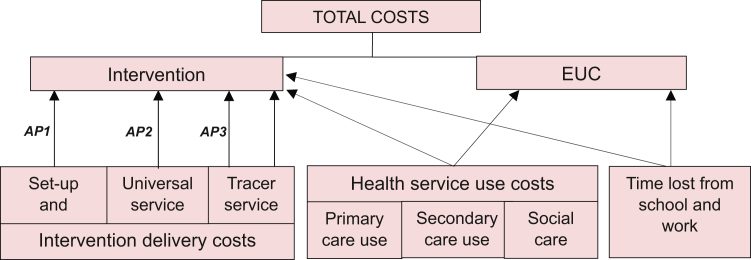
Three main cost components were considered, including intervention delivery costs, health service use costs, and costs of time lost from school and work; the last two contributed to the total costs of both study arms (Fig. 2). Intervention delivery costs included set-up and overhead costs, costs of universal service, and costs of the nurse-led tracer conditions service. Costs of the universal service comprised lunch-and-learn sessions, multidisciplinary team case-planning, visits, and triage. Costs of the nurse-led tracer conditions service included visits, specialist team case planning, and population health management. Health service use costs captured primary and secondary care (NHS perspective), and referrals to social services (PSS perspective). Hospital admissions, outpatient attendances, and emergency department visits were part of secondary care use. Medications were not included in health service use costs.
Fig. 2.
Total cost components for intervention and EUC. Notes: AP1, apportioning 1; AP2, apportioning 2; AP3, apportioning 3. See Box S1 for details on apportioning rules. Tracer service = specialist nurse-led service. Notice that the second arrow connecting the tracer service to the intervention was not apportioned, as it corresponded to the patient-level consultations delivered by the service. EUC, enhanced usual care.
Measurement
Data sources for intervention delivery costs included the study's accounting data, service caseloads, and nurse's personal caseload notes (Table 1). Health service use data were gathered from electronic medical records. Data were extracted from local primary care and secondary care patient administrative systems and linked using unique NHS numbers. All data were pseudonymised for confidentiality, extracted, and processed in accordance with data sharing and research ethics agreements. Time lost from school and work were self-reported by study participants and measured through the questions “How many days of work have you missed in the past three months due to your child's illness or healthcare appointments?” and “How many days of school has your child missed due to ill health or attending health-related appointments in the past three months”.
Table 1.
Overview of intervention and health service use costing.
| Resources used | Currency for valuation | Data sources | |
|---|---|---|---|
| Intervention costs | |||
| Set-up costs | Hiring costs, training, materials, IT purchases | Total annual costs | Study's accounting data, financial report |
| Overhead costs | Space, data access and storage, IT maintenance | Total annual costs | |
| In-reach | |||
| Lunch and learn | Time of presenters and attendants. Mostly GPs and nurses. | Unit cost of health professionals (£/hour) | Service Caseloads (quantity), PSSRU (unit costs)b |
| Multidisciplinary team case-planning | Time of presenters and attendants. Mostly GPs and nurses. | ||
| Visits | Time of healthcare providers. GPs and paediatricians. | ||
| Triage | Time of healthcare providers. GPs, paediatricians, and nurses | ||
| Specialist-nurse led servicea | |||
| Visits | Time of healthcare providers. Nurses, mental health professionals, pharmacists | Unit cost of health professionals (£/hour) | Service Caseloads (quantity), PSSRUb (unit costs) |
| Specialist team training | Time of healthcare providers. Nurses and mental health professionals | ||
| Multidisciplinary team case-planning | Time of presenters and attendants. Mostly GPs and nurses. | ||
| Population health management | Time of staff conducting call -recall and designing promotional materials | Total annual costs | Study's accounting data, financial report |
| Healthcare utilisation | |||
| Primary care | Primary care visits. Costing based on provider type (nurse, GP, etc.) and consultation type (face-to-face, telephone, home, or electronic) | Unit cost of health professionals (£/hour) | Primary care electronic records (quantity), PSSRUb (unit costs) |
| Secondary care | Hospital admissions, outpatient attendances, emergency department visits | HRGs and TFCs | Secondary care electronic records (quantity), National Tariffc (unit costs) |
| Referrals to social care services | Referrals from primary care to social care. | Cost of 1 episode of social services support for children | Primary care electronic records (quantity), PSSRUd (unit costs) |
The cost of 6 mobile phones (including device and data plan) was also included in the total costs of the specialist-nurse led service. HRGs, Healthcare Resource Groups; TFCs, Treatment Function Codes.
Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Personal Social Services Research Unit; 2020 | PSSRU.
NHS England. 2019/20 National Tariff Payment System.
Curtis LA, Burns A. Unit Costs of Health and Social Care 2014. Personal Social Services Research Unit; 2014 | PSSRU.
Valuation
Besides set-up and overhead costs, the remaining intervention delivery costs mostly included time spent by staff and medical professionals delivering CYPHP. Costs were obtained by multiplying the time each provider spent delivering a service or receiving training and their hourly cost. Providers’ hourly costs were gathered from the 2020 national unit costs for community- and hospital-based staff, publicly available from the Personal Social Services Research Unit (PSSRU)18 (Table 1). Apportioning rules were developed to properly allocate costs of intervention components that did not directly involve individuals with tracer conditions, and to obtain a per-patient cost for adding to the remaining patient-level costs (Box S1).
Provider type was used along with consultation type to identify relevant unit costs for primary care consultations from PSSRU.18 Secondary care use was valued using Health Related Cost Groups (HRGs) for hospital admissions and emergency department visits, while treatment function codes (TFCs) were applied to outpatient attendances. HRGs are standard groupings of clinically similar diagnoses and procedures with comparable levels of healthcare resource.19 TFCs capture activities requiring similar workforce resources.20 Units costs for each HRG and TFC were gathered from the 2019/20 National tariffs.21 To value referrals to social care services, the Unit Costs of Health and Social Care 2014 version was used to, which provided the most recent value of the cost of social services support for children.22 Time lost from school was valued at £13.25/day, which results from assuming a return to a year of schooling on income of 8%23 and a median UK wage of £31,461/year.24 Time lost from work is valued at £122.9/day, based on the aforementioned median (the preferred measure of average earning by the Office of National Statistics24) UK wage and 256 working days. A discount rate of 3.5% was used. All costs are presented in pounds sterling (£) for a base cost year 2019/2020. The NHS Cost Inflation Index (NHSCII) was used to adjust for inflation.22
Health outcomes
The PedsQL and the CHU-9D were part of the study questionnaires administered to study participants at baseline, 6, and 12 months after randomisation. The PedsQL (along with non-elective admissions) was the primary outcome of the trial. This generic health-related quality of life measure captures aspects of physical, emotional, and social health and wellbeing, along with physical symptoms, cognitive functioning, and school functioning25,26 using 23–45 questions depending on the age version applied. The PedsQL was used in the cost-effectiveness analysis with results reported as cost per unit improvement. To generate QALYs for the cost-utility analysis the CHU-9D27 was selected. This generic preference-based instrument uses nine items to measure mental and physical health, schoolwork, and social activities. Health states based on responses are assigned preference weights using general population tariff values, which yield utilities to compute QALYs. Results from the cost-utility analysis are presented as cost per QALY. Finally, monetary benefit was used to combine CYP and parental outcomes within a cost-benefit analysis. The Warwick–Edinburg Mental Well-being Scale (WEMWBS)28 was monetised using the wellbeing valuation of WEMWBS scores29 and added to QALYs from CYP using the government sector willingness to pay of £20,000 per QALY gained.30 Results from the cost-benefit analysis are reported as cost per monetarised unit of parental well-being and CYP's QALYs.
Statistical analyses
Statistical analyses started with univariate analyses to assess sample mean differences between treatment and control groups for each outcome, including the PedsQL, the CHU-9D, monetary benefit (£ corresponding to QALYs from CYP and parental WEBWMS scores together), and total costs. To adjust for treatment group imbalances and clustering, four multilevel (or hierarchical) Generalised Linear Models (GLM)31 were estimated; one each for total costs, PedsQL, CHU-9D, and monetary benefits. Each model adjusted for participation in intervention or control arms and variables that, despite randomization, may still be unequally distributed between intervention and control groups including age, gender, deprivation level (IMD 2015 and IDACI 2015 quintiles), and borough for the patient-level models. For the regression model predicting the difference in CHU-9D between trial arms, baseline CHU-9D values were also included.32 PedsQL, CHU-9D and monetarised benefits were estimated using a multilevel GLM model with the normal distribution and the identify link (ordinary least squares33,34), and costs with a GLM model with a gamma distribution and a log-link. All models were defined at the individual level, clustering by randomised GP cluster. Missing values were replaced by multiple imputation via chained equations (MICE),35 as data were missing at random (analyses available upon request). The main strength of the MICE method is that it enhances the accuracy of data prediction for a variable of interest by using all other available variables (or a chosen subset) in the dataset, while allowing for variable-specific distributional assumptions rather than a large joint model as in other MI techniques.36 SAS (version 9.4) was used for all analyses.
Study results are presented through incremental cost-effectiveness ratios (ICER) for both the cost-effectiveness and the cost-utility analyses, and net monetary benefit (NMB) for the cost-benefit analysis. Indicators under the NHS/PSS and societal perspectives, at 6 and 12 months, are given for each summary measure. The cost-effectiveness thresholds were set at £144/PedsQL unit up to a maximum of £600/unit based on previous literature37,38 and at £20,000/QALY following NICE recommendations.30 To characterise uncertainty around deterministic results, confidence intervals for ICERs and NMBs based on the non-parametric bootstrap method (10,000 repetitions) were generated, along with cost-effectiveness planes and acceptability curves.30 Deterministic one-way sensitivity analyses (varying one parameter at a time keeping the rest constant) were conducted to identify key drivers of results and to isolate the impact of certain assumptions made in analyses. Analyses included varying the discount rate used for set-up and overhead cost calculations (from 3.5% to 1.5%), changing the assumed frequency and duration of some intervention components, excluding individuals with missing values (complete case analysis) to assess the impact of missing data imputation, and using the £30,000/QALY threshold value. Subgroup analyses were conducted for study participants in the most deprived IMD quintile, those with asthma as set out in the protocol,17 and individuals with severe symptoms related to their tracer condition. The latter group is likely to have more healthcare needs and be frequent users of services.
Role of the funding source
This research was funded by Guy's and St Thomas' Charity, Lambeth and Southwark Clinical Commissioning Groups. The funders had no role in the writing of the manuscript, decision to submit it for publication, or any other process involved in the research.
Results
Total intervention delivery costs were £1,014,473 over 6 years, including set-up costs two years before the actual intervention started in April 2018 (Table S1). The average annual cost during the actual delivery of CYPHP was £249,800/year. Overall, the majority of the costs corresponded to the universal service (in-reach clinics, 55%), followed by the tracer service (23%), and set-up and overhead costs (21%). The apportioned, per study participant, cost to deliver the intervention was £60.52, (Box S1). This value was added to the patient-level tracer service visits, health service use costs, and costs of time lost from school and work.
Costs of health service use and time lost from school and work were similar between intervention and EUC groups, both at 6- and 12-months follow-up (Table 2). Total costs both under the NHS/PSS and societal perspectives were higher in the intervention arm.
Table 2.
Unadjusted mean costs of health service use, time lost from school and work, and total costs.
| 6 months follow-up |
12 months follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
| Intervention (N=820) | EUC (N=911) | Intervention (N=820) | EUC (N=911) | |||||
| Primary care | ||||||||
| Mean | £39.42 | £41.46 | £70.12 | £75.78 | ||||
| 95% CI | £34.38 | £44.45 | £36.59 | £46.33 | £62.33 | £77.92 | £68.29 | £83.27 |
| p-value | 0.2482 | 0.087 | ||||||
| Non-elective admissions | ||||||||
| Mean | £20.32 | £21.57 | £32.05 | £43.41 | ||||
| 95% CI | £8.08 | £32.56 | £9.80 | £33.34 | £15.80 | £48.29 | £27.70 | £59.12 |
| p-value | 0.3424 | 0.0784 | ||||||
| Outpatient consultations | ||||||||
| Mean | £100.80 | £105.50 | £187.50 | £198.90 | ||||
| 95% CI | £83.81 | £117.80 | £87.75 | £123.30 | £159.10 | £216.00 | £169.90 | £227.80 |
| p-value | 0.3903 | 0.3851 | ||||||
| Emergency room visits | ||||||||
| Mean | £29.62 | £28.56 | £52.32 | £48.68 | ||||
| 95% CI | £23.47 | £35.76 | £23.39 | £33.73 | £43.33 | £61.32 | £41.33 | £56.03 |
| p-value | 0.2552 | 0.4397 | ||||||
| NHS Total health service use | ||||||||
| Mean | £190.20 | £197.1 | £342.00 | £366.8 | ||||
| 95% CI | 163.7 | 216.7 | 170.4 | 223.8 | 298.9 | 385.1 | 325.8 | 407.7 |
| p-value | 0.203 | 0.1835 | ||||||
| NHS/PSS Total health service use | ||||||||
| Mean | £192.40 | £203.2 | £351.00 | £370.8 | ||||
| 95% CI | 165.6 | 219.2 | 174.1 | 232.2 | 307.3 | 394.7 | 329.6 | 412 |
| p-value | 0.205 | 0.2245 | ||||||
| Time lost from school | ||||||||
| Mean | £42.1 | £38.0 | £61.3 | £60.5 | ||||
| 95% CI | £30.2 | £54.1 | £28.6 | £47.3 | £48.6 | £74.0 | £45.6 | £75.3 |
| p-value | 0.359 | 0.4326 | ||||||
| Time lost from work | ||||||||
| Mean | £530.80 | £529.50 | £753.8 | £803.2 | ||||
| 95% CI | £356.10 | £705.50 | £361.80 | £697.20 | £494.6 | £1013.0 | £547.4 | £1059.1 |
| p-value | 0.4691 | 0.3039 | ||||||
| NHS/PSS Costs | ||||||||
| Mean | £339.6 | £203.2 | £498.1 | £370.8 | ||||
| 95% CI | £310.6 | £368.6 | £174.1 | £232.2 | £452.7 | £543.5 | £329.6 | £412 |
| p-value | <0.0001 | <0.0001 | ||||||
| Societal Costs | ||||||||
| Mean | £912.6 | £770.7 | £1312.4 | £1252.2 | ||||
| 95% CI | £729.1 | £1096.1 | £595.0 | £946.4 | £1039.2 | £1585.6 | £980.4 | £1523.9 |
| p-value | <0.0001 | <0.0001 | ||||||
Notes: PSS, Personal Social Service. P-values correspond to the Wilcoxon-Mann-Whitney test, used to compare means of a non-normally distributed variables between two groups. EUC, enhanced usual care. NHS total health service use costs include primary care consultations, non-elective admissions, outpatient consultations, and emergency room visits. NHS/PSS total health service use results from adding referrals to social services to NHS total health service use costs. NHS/PSS costs correspond to NHS/PSS total health service use costs plus apportioned intervention costs. Societal costs include NHS/PSS costs plus time lost from school and work.
Our results indicate that at 6 months CYPHP is not a cost-effective intervention compared to EUC, as the ICER is −£5442 and −£8645 per unit improvement in the PedsQL, under the NHS/PSS and societal perspectives, respectively (Table 3). The negative ICERs result from a slightly lower effectiveness in the intervention group (not statistically significant). However, when the time window of analyses is extended to 12 months, CYPHP begins to approach the cost-effectiveness threshold, with an ICER under the societal perspective of £229/PedsQL unit. Similar results are observed in the cost-utility analysis. At 6-months, CYPHP is not a cost-effective intervention, with ICERs far above the cost-effectiveness threshold of £20,000–£30,000/QALY. At 12 months, CYPHP approaches the cost-effectiveness thresholds (£45,586/QALY) and becomes a cost-effective intervention under the societal perspective (£22,966/QALY). The cost-benefit analyses coincide, showing a net positive benefit of CYPHP at 12 months under the societal perspective (Table 3).
Table 3.
Cost-effectiveness, cost-utility, and cost-benefit results, at 6 and 12 months, NHS/PSS and societal perspectives.
| Intervention (N=820) | EUC (N=911) | ICER or NMB | ||||
|---|---|---|---|---|---|---|
| At 6 months | ||||||
| Costs NHS/PSS | ICER | −£5441.51/ | ||||
| Mean | £345.30 | £201.10 | NHS/PPS | PedsQL unit | ||
| 95% CI | £340.90 | £349.80 | £198.60 | £203.60 | ICER | £721,000/ |
| Cost difference | £144.20 | – | NHS/PPS | QALY | ||
| Costs Societal | ICER | −£8,645.28/ | ||||
| Mean | £964.10 | £735.00 | Societal | PedsQL unit | ||
| 95% CI | £935.80 | £992.50 | £714.20 | £755.80 | ICER | £1,145,500/ |
| Cost difference | £229.10 | – | Societal | QALY | ||
| PedsQL | NMB | −£714 | ||||
| Mean | 79.56 | 79.59 | Societal | |||
| 95% CI | 78.82 | 80.29 | 78.84 | 80.33 | ||
| Effect difference | −0.03 | |||||
| CHU-9D | ||||||
| Mean | 0.8807 | 0.8805 | ||||
| 95% CI | 0.8773 | 0.884 | 0.8771 | 0.8839 | ||
| Effect difference | 0.0002 | |||||
| Benefit | ||||||
| Mean | £39,133.3 | £39,618.1 | ||||
| 95% CI | £38,921.7 | £39,344.9 | £39,410.5 | £39,825.7 | ||
| Benefit difference | −£484.80 | |||||
| At 12 months | ||||||
| Costs NHS/PSS | ICER | £455.08/ | ||||
| Mean | £501.90 | £369.70 | NHS/PPS | PedsQL unit | ||
| 95% CI | £494.90 | £508.80 | £364.60 | £374.90 | ICER | £45,586/ |
| Cost difference | £132.20 | NHS/PPS | QALY | |||
| Costs Societal | ICER | £229.26/ | ||||
| Mean | £1301.30 | £1,234.70 | Societal | PedsQL unit | ||
| 95% CI | £1266.20 | £1336.40 | £1200.60 | £1268.70 | ICER | £22,966/ |
| Cost difference | £66.60 | Societal | QALY | |||
| PedsQL | NMB | £109 | ||||
| Mean | 79.5 | 79.21 | Societal | |||
| 95% CI | 78.74 | 80.26 | 78.43 | 79.99 | ||
| Effect difference | 0.29 | |||||
| CHU-9D | ||||||
| Mean | 0.8826 | 0.8797 | ||||
| 95% CI | 0.8793 | 0.8859 | 0.8763 | 0.8831 | ||
| Effect difference | 0.0029 | |||||
| Benefit | ||||||
| Mean | £39,385 | £39,209 | ||||
| 95% CI | £39,162 | £39,608 | £ 38,987.50 | £ 39,431.1 | ||
| Benefit difference | £175.50 | |||||
Notes: Mean costs, PedsQL and CHU9D are adjusted for confounders. The deal with missing data, multiple imputation was used. Complete case analysis results are presented in Box S4. EUC, enhanced usual care. NMB, Net Monetary Benefit. NMB is obtained by subtracting incremental costs from incremental total benefit.
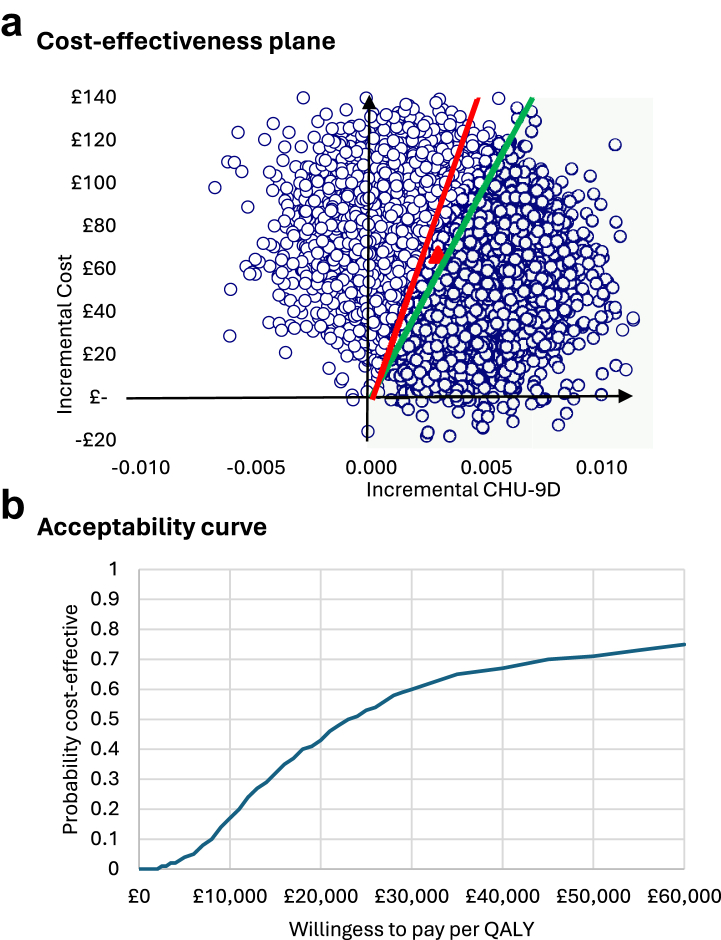
Probabilistic results indicate considerable uncertainty around the 12 months under the societal perspective (Fig. 3). For the cost-utility analysis, the 10,000 bootstrapped replications are distributed across the four quadrants, mostly in the north-east quadrant (suggesting that CYPHP yields more effectiveness and more costs compared to EUC) (Fig. 3, Panel a). Considering the £30,000/QALY threshold (red line) 60% of the estimates fall to the right of it (i.e., are cost-effective), while using the £20,000/QALY threshold (green line) 43.9% of the estimates are cost-effective (Fig. 3, Panel b). The mean and 95% CI from the probabilistic analysis are £56,596/QALY with a 95% CI £20,049 to £93,142. Thus, the probabilistic results provide a more conservative estimate of the cost-effectiveness of CYPHP at 12 months under the societal perspective, underscoring the uncertainty around the deterministic results.
Fig. 3.
Probabilistic results of the cost-utility analysis, societal perspective at 12 months. Notes: At the £30,000/QALY threshold (red line), 60% of the estimates fall to the right of it (i.e., are cost-effective), while at the £20,000/QALY threshold (green line) 43.9% of the estimates are cost-effective.
The degree of uncertainty is considerably lower in the cost-benefit analyses at 12 months (Box S2), with positive mean point estimates and 95% confidence intervals from the bootstrapped results. This finding suggests that at 12 months, when both CYP QALYs and parental wellbeing are jointly considered, CYPHP may yield positive net benefits compared to EUC.
Subgroup analyses suggest that CYPHP may be cost-effective for those with more severe symptoms- ICERs are consistently under the willingness to pay (WTP) thresholds across measures of effectiveness, study perspectives, and time horizons, including the results from probabilistic analyses (Table S2). For the most deprived and asthma groups, at 6 months CYPHP is not cost-effective under the societal perspective due to higher time lost from school and work in the intervention group, but these results change at 12 months with ICERs below the WTP thresholds.
Finally, sensitivity analyses support the stability of the main study results to changes in assumptions related to the discount rate, frequency of intervention components, imputation of missing data, and choice of wiliness to pay threshold for monetarisation of QALYs (Boxes S3, S4 and Table S3).
Discussion
This study aimed to evaluate the cost-effectiveness of CYPHP for individuals with asthma, constipation, or eczema from both the NHS/PSS and societal perspectives, at 6- and 12-months follow-ups. Findings indicate that at 6 months, CYPHP is not a cost-effective intervention. At 12 months, this result also holds from the NHS/PSS perspective. Under the societal perspective (which accounted for time lost from school and work), CYPHP shows trends towards cost-effectiveness, with an ICER of £22,966/QALY and a probability of being cost-effective between 0.4 and 0.6 for a WTP of £20,000/QALY and £30,000/QALY, respectively. Findings from the cost-benefit analysis (which considered both CYP QALYs and parental wellbeing) also are indicative of positive net monetary benefit of CYPHP at 12 months.
The main trial evaluation found no statistically significant difference in health service use and health related quality of life between CYPHP and EUC. The economic evaluation, which is not bound by statistical significance and combines costs and health outcomes, found slightly more optimistic results on the efficiency of CYPHP, yet several points warrant caution. The cost-utility findings at 12 months were characterised by a high degree of uncertainty, with 40% and 12% probabilities of CYPHP not being cost-effective or dominated, respectively, at the £30,000/QALY threshold. Under the NHS perspective at 12 months, both deterministic and probabilistic results provided further evidence against the cost-effectiveness of CYPHP (ICER = £45,586/QALY and a probability of cost-effectiveness of 25%).
Trends towards cost-effectiveness were observed once the NHS/PSS perspective was expanded to societal and effects on children and their families were considered, including time loss from school and work and parental wellbeing. The positive results from the cost-benefit analysis had low levels of uncertainty. CYPHP also showed signs of cost-effectiveness among individuals with severe symptoms, with ICERs below £20,000/QALY across analytic perspectives and time horizons. The benefits of CYPHP may have been underestimated in this economic evaluation. The positive change in study findings between 6- and 12-months indicate that CYPHP may have a longer-term, rather than immediate, effect on health outcomes and costs beyond 12 months. The observed delay in the embedment of CYPHP into real clinical practice may also explain these results and justify further evaluation. New health care models and complex interventions need time to become consolidated and normalised as usual clinical care. For example, the Sure Start programme in the UK required around 10 years to fully embed, which further suggests that the CYPHP trial may have been conducted too early in implementation.39 Previous cost-effectiveness analyses of integrated paediatric care in the UK are non-existent, and outside of the UK very scarce.10 A study of 60 children with diabetes type 1 in Sweeden also detected positive cost-effectiveness signs of integrated care in the longer term driven by improvements in family's daily living.40
CYPHP may have also had positive equity impacts, by addressing unmet need among a particularly deprived population. CYPHP may have yielded some positive externalities, such as enhanced workforce capabilities that are applicable to care delivery beyond CYPHP, that, if considered, could have contributed to more positive efficiency results.
The strengths of this study include a rigorous, pragmatic, cluster RCT study design aimed at minimising the effect of confounders while assessing the intervention effects in real clinical practice. Three economic evaluation techniques (cost-effectiveness, cost-utility, and cost-benefit) and two analytic perspectives (NHS/PSS and societal) were used to comprehensively capture the manifold effects that CYPHP, a complex intervention, may have had not only on the national health service, but also on social services, schools, CYP and their families.
The study has several limitations. Firstly, the study sample was confined to South London, an urban, ethnically diverse, and deprived geographical area, which may make findings difficult to transfer to other health systems, settings or countries. The generalisability of the result across the UK may depend on the overlap in sociodemographic characteristics with South London. Secondly, missing data due to loss to follow-up may have also influenced study findings. However, multiple imputation and a complete case sensitivity analyses supported the stability of our results. Thirdly, the economic evaluation was focused on children with tracer conditions, who only represent a portion of the total population of CYPHP recipients. Analyses considered intervention components beyond the tracer conditions service (universal services), along with overhead and set-up costs. These costs were apportioned to avoid a disproportionate allocation to the subpopulation of interest. It is unclear if the cost-effectiveness results reported in this study would hold if the tracer conditions service was assessed in complete isolation from the additional CYPHP components that may have facilitated its implementation. Further research as CYPHP is rolled out for asthma is needed. Fourthly, the CYPHP implementation partially overlapped with the COVID-19 pandemic, which caused disruptions in the types of services offered. A suboptimal tailoring of CYPHP services may have resulted in an underestimation of the potential true intervention effects on health outcomes that could have been observed under normal circumstances. Finally, two changes to the study protocol17 should be acknowledged. The comparison between protocolised and actual duration of certain intervention components, such as the lunch-and-learn sessions or some training components, could not be conducted due to lack of data recording. This paper also presents results on a third subgroup, CYP with high symptoms, that was not originally listed in the protocol and, thus, these results should be considered exploratory and worth further investigation.41
In conclusion, CYPHP was not a cost-effective intervention compared to EUC at 6-months follow-up. However, at 12 months, it shows trends towards cost-effectiveness, particularly under the societal perspective and among children with severe chronic conditions. These initial results, 12 months post enrolment into the service, need to be further explored as the model becomes firmly embedded into the paediatric healthcare delivery system.
Contributors
MSB as Trial health economist conceptualised the economic evaluation, curated the data, developed the methodology, conducted the formal analyses (including statistical software use and visualisation), interpreted the data, wrote, reviewed, and edited the manuscript. JRF as Trial statistician curated and validated data, reviewed and edited the manuscript. LC as Trial coordinator curated and validated data, contributed to, and reviewed the manuscript. RL as External Evaluation lead contributed to funding acquisition and resources, reviewed and edited the manuscript. IW as Principal Investigator acquired funding and resources, reviewed and edited the manuscript. JFR oversaw the economic evaluation (including conceptualisation, development of methodology, and data interpretation), and reviewed and edited the manuscript.
All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Trial-related data are available on reasonable request to the principal investigator (IW).
Declaration of interests
We declare no competing interests.
Acknowledgements
We thank the CYPHP team and partnership, and the children and families of Lambeth and Southwark. We also acknowledge our funder, the Guy's and St Thomas Charity, Lambeth and Southwark Clinical Commissioning Groups.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100917.
Appendix A. Supplementary data
References
- 1.Wolfe I., Thompson M., Gill P., et al. Health services for children in Western Europe. Lancet. 2013;381(9873):1224–1234. doi: 10.1016/S0140-6736(12)62085-6. [DOI] [PubMed] [Google Scholar]
- 2.Perrin J.M., Anderson L.E., Van Cleave J. The rise in chronic conditions among infants, children, and youth can Be met with continued health system innovations. Health Aff (Millwood) 2014;33(12):2099–2105. doi: 10.1377/hlthaff.2014.0832. [DOI] [PubMed] [Google Scholar]
- 3.RCPCH . 2021. State of child health.https://stateofchildhealth.rcpch.ac.uk/evidence/long-term-conditions/asthma/ [cited 2022 Sep 26]. Available from: [Google Scholar]
- 4.Sundbom F., Malinovschi A., Lindberg E., et al. Effects of poor asthma control, insomnia, anxiety and depression on quality of life in young asthmatics. J Asthma. 2016;53(4):398–403. doi: 10.3109/02770903.2015.1126846. [DOI] [PubMed] [Google Scholar]
- 5.Roddy Á. Income and conversion handicaps: estimating the impact of child chronic illness/disability on family income and the extra cost of child chronic illness/child disability in Ireland using a standard of living approach. Eur J Health Econ. 2022;23(3):467–483. doi: 10.1007/s10198-021-01371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belli P.C., Bustreo F., Preker A. Investing in children's health: what are the economic benefits? Bull World Health Organ. 2005;83:777–784. [PMC free article] [PubMed] [Google Scholar]
- 7.Hu N., Fardell J., Wakefield C.E., et al. School academic performance of children hospitalised with a chronic condition. Arch Dis Child. 2022;107(3):289–296. doi: 10.1136/archdischild-2020-321285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NHS . 2019. The NHS long term plan. [Google Scholar]
- 9.Wolfe I., Mandeville K., Harrison K., Lingam R. Child survival in England: strengthening governance for health. Health Policy Amst Neth. 2017;121(11):1131–1138. doi: 10.1016/j.healthpol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe I., Satherley R.M., Scotney E., et al. Integrated care models and child health: A meta-analysis. Pediatrics. 2020;145 doi: 10.1542/peds.2018-3747. [DOI] [PubMed] [Google Scholar]
- 11.Rocks S., Berntson D., Gil-Salmerón A., et al. Cost and effects of integrated care: a systematic literature review and meta-analysis. Eur J Health Econ. 2020;21(8):1211–1221. doi: 10.1007/s10198-020-01217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter S., Johnson M., Chambers D., et al. The effects of integrated care: a systematic review of UK and international evidence. BMC Health Serv Res. 2018;18(1):350. doi: 10.1186/s12913-018-3161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newham J.J., Forman J., Heys M., et al. Children and young people's health partnership (CYPHP) Evelina London model of care: protocol for an opportunistic cluster randomised controlled trial (cRCT) to assess child health outcomes, healthcare quality and health service use. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-027301. https://bmjopen.bmj.com/content/9/8/e027301 [cited 2020 Feb 13] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satherley R.M., Green J., Sevdalis N., et al. The children and young people's health partnership Evelina London model of care: process evaluation protocol. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-027302. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6731816/ [cited 2020 Feb 13] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiell A., Hawe P., Gold L. Complex interventions or complex systems? Implications for health economic evaluation. BMJ. 2008;336(7656):1281–1283. doi: 10.1136/bmj.39569.510521.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe I., Forman J., Cecil E., et al. Effect of the Children and Young People’s Health Partnership model of paediatric integrated care on health service use and child health outcomes: a pragmatic two-arm cluster randomised controlled trial. Lancet Child Adolesc Health. 2023;7(12):830–843. doi: 10.1016/S2352-4642(23)00216-X. [DOI] [PubMed] [Google Scholar]
- 17.Soley-Bori M., Lingam R., Satherley R.M., et al. Children and young people's health partnership evelina London model of care: economic evaluation protocol of a complex system change. BMJ Open. 2021;11(11) doi: 10.1136/bmjopen-2020-047085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis L., Burns A. Unit costs of health and social care 2020 | PSSRU. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/ [cited 2021 Mar 30] Available from:
- 19.NHS Digital ISB 0070: healthcare resource groups (HRGs) https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb-0070-healthcare-resource-groups-hrgs [cited 2024 Mar 14] Available from:
- 20.NHS Digital [cited 2024 Mar 14] Treatment function codes. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/treatment-function-codes Available from:
- 21.NHS England and NHS Improvement 2019/20 national tariff payment system. 2019. https://www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/ Available from:
- 22.Curtis L.A., Burns A. Personal Social Services Research Unit; 2014. Unit costs of health and social care 2014. [Google Scholar]
- 23.Harmon C., Oosterbeek H., Walker I. 2000. The returns to education: a review of evidence, issues and deficiencies in the literature. Centre for the Economics of Education, London School of Economics and …. [Google Scholar]
- 24.Employee earnings in the UK - Office for national statistics. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2020 [cited 2022 May 11] Available from:
- 25.Varni J. Mapi Research Trust; 2017. Scaling and scoring of the pediatric quality of life inventory, PedsQL. [Google Scholar]
- 26.Seid M., Limbers C.A., Driscoll K.A., et al. Reliability, validity, and responsiveness of the pediatric quality of life inventory (PedsQL) generic core scales and asthma symptoms scale in vulnerable children with asthma. J Asthma. 2010;47(2):170–177. doi: 10.3109/02770900903533966. [DOI] [PubMed] [Google Scholar]
- 27.Furber G., Segal L. The validity of the Child Health Utility instrument (CHU9D) as a routine outcome measure for use in child and adolescent mental health services. Health Qual Life Outcomes. 2015;13(1):22. doi: 10.1186/s12955-015-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennant R., Hiller L., Fishwick R., et al. The warwick-edinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5(1):63. doi: 10.1186/1477-7525-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotter L., Rallings Adams M.-K. HACT; 2017. Valuing improvements in mental health: applying the wellbeing valuation method to WEMWBS. [Google Scholar]
- 30.NICE . 2013. Methods for the development of NICE public health guidance (third edition) [Google Scholar]
- 31.Lee Y., Nelder J.A. Hierarchical generalized linear models. J R Stat Soc Ser B Methodol. 1996;58(4):619–678. [Google Scholar]
- 32.Manca A., Hawkins N., Sculpher M.J. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 33.Pullenayegum E.M., Wong H.S., Childs A. Generalized additive models for the analysis of EQ-5D utility data. Med Decis Making. 2013;33(2):244–251. doi: 10.1177/0272989X12465354. [DOI] [PubMed] [Google Scholar]
- 34.Kelly C.B., Soley-Bori M., Lingam R., et al. Mapping PedsQLTM scores to CHU9D utility weights for children with chronic conditions in a multi-ethnic and deprived metropolitan population. Qual Life Res. 2023;32(7):1909–1923. doi: 10.1007/s11136-023-03359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azur M.J., Stuart E.A., Frangakis C., et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 37.Sampaio F., Bonnert M., Olén O., et al. Cost-effectiveness of internet-delivered cognitive–behavioural therapy for adolescents with irritable bowel syndrome. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023881. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6347900/ [cited 2020 Apr 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards R.T., Neal R.D., Linck P., et al. Enhancing ventilation in homes of children with asthma: cost-effectiveness study alongside randomised controlled trial. Br J Gen Pract J R Coll Gen Pract. 2011;61(592):e733–e741. doi: 10.3399/bjgp11X606645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melhuish E., Belsky J., Leyland A.H., et al. Effects of fully-established Sure Start Local Programmes on 3-year-old children and their families living in England: a quasi-experimental observational study. Lancet. 2008;372(9650):1641–1647. doi: 10.1016/S0140-6736(08)61687-6. [DOI] [PubMed] [Google Scholar]
- 40.Tiberg I., Lindgren B., Carlsson A., et al. Cost-effectiveness and cost-utility analyses of hospital-based home care compared to hospital-based care for children diagnosed with type 1 diabetes; a randomised controlled trial; results after two years’ follow-up. BMC Pediatr. 2016;16:94. doi: 10.1186/s12887-016-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NICE Guide to the methods of technology appraisal 2013. 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword Available from: [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.