Abstract
Background
Cardiovascular diseases (CVDs) are the leading causes of global mortality and disability. Several studies demonstrated that metabolic risk factors increase cardiovascular mortality. The aim of this study is to examine CVDs deaths and population attributable fractions (PAFs) of their metabolic risk factors in Iran.
Methods
This is a study on 8621 participants aged 45–75 years and older, recruited in the repeated measurement phase of the Golestan cohort study (GCS) in northeast of Iran. The Cox proportional hazards model was used to determine the adjusted hazard ratios (HRs). PAFs were calculated to enumerate CVDs mortality avoidable in the population if metabolic risk factors were eliminated.
Results
The mortality of CVDs was attributable to metabolic factors, including high waist circumference (PAF, 28 %, [95 % CI: 16%–38 %]), high fasting blood sugar (FBS) (20 %, [15%–24 %]), overweight and obesity (19 %, [8%–28 %]), high blood pressure (16 %, [11%–21 %]), high low-density lipoprotein cholesterol (LDL-C) (8 %, [1%–15 %]), and high triglyceride (TG) (7 %, [3%–11 %]). Collectively, these metabolic risk factors accounted for 50 % of CVDs deaths. Females (67 %, [50%–78 %]) had a higher joint PAF of metabolic risk factors compared to males (43 %, [27%–55 %]).
Conclusions
The pattern of CVDs mortality attributable to metabolic risk factors in this study is not the same as similar studies in other parts of the world and previous studies in Iran. It is imperative that CVDs risk factors be specifically evaluated and addressed in various populations due to variety in geographical and temporal patterns in contribution of metabolic risk factors to CVD mortality.
Keywords: Population attributable fraction, Metabolic risk factors, Cardiovascular diseases, Mortality
1. Introduction
Cardiovascular diseases (CVDs) are the leading causes of global mortality and disability. The overall prevalence of cardiovascular diseases nearly doubled from 271 million in 1990 to 523 million in 2019. Also, the number of deaths due to CVD has increased steadily and reached 18.6 million deaths in 2019, from 12.1 million deaths annually in 1990 [1]. In addition to high mortality and morbidity, CVDs are responsible for significant economic costs. In Iran, this disease is the leading cause of health loss. According to the results of the global burden of diseases study in 2019, 8026 per 100,000 people in Iran suffered from cardiovascular disease [1]. Also, cardiovascular diseases were the cause of 173,600 deaths in Iran in 2019, accounting for 44.4 % of the country's total casualties [1,2].
Metabolic risk factors are important modifiable factors for a variety of diseases, such as cardiovascular diseases [3]. The important metabolic risk factors include high blood pressure, blood lipid disorders such as increased triglyceride (TG) and increased low-density lipoprotein cholesterol (LDL-C)), high fasting blood sugar (FBS), and high waist circumference (WC) [3,4]. Metabolic risk factors tend to cluster in individuals and designate as metabolic syndrome. According to the meta analyses of 2021, the prevalence of metabolic syndrome has shown a range of 12.5 %–31.4 % worldwide [5]. In a systematic review conducted in 2018, in Iran, the prevalence of metabolic syndrome in people over 20 years old and under 20 years was 23.8 % and 11.0 %, respectively [6]. Several studies have shown that metabolic risk factors increase the cardiovascular risk and mortality [7,8].
The population attributable fraction (PAF) is defined as the proportion of diseases that will be prevented if the relevant risk factors were removed from the society [9]. The PAF can be utilized in policy-making for public health interventions, as it helps identify modifiable risk factors that their control is highly associated with reduction in the risk of disease. The purpose of this study is to examine CVD deaths and PAFs of the metabolic risk factors in a cohort study of Iranians recruited in the repeated measurement phase of the Golestan cohort study (GCS) in Northeast of Iran.
2. Methods
2.1. Study population
In 2004, the GCS was launched in Golestan province, northeastern Iran. The study recruited 50,045 participants aged 40–75 between 2004 and 2008 for a median follow-up of 15 years. From 2010 to 2012, 11,428 study participants aged 45–75 years and older were randomly selected and recruited for repeated measurement [10]. A total of 2807 participants who had received a fixed-dose combination therapy (Polypill) containing statin, aspirin, and anti-hypertensive agents were excluded from the current analyses [11]. Therefore, the exclusion criteria were: (i) unwillingness to participate at any stage of the study for any reason; (ii) being a temporary resident; and (iii) individuals undergoing drug treatment with a fixed-does combination therapy.
In Golestan province, participants aged 45–75 years were recruited from Gonbad city (20 %) and all of the 326 villages in the province (80 %). The baseline survey included demographic data, underlying diseases, medication history, lifestyle risk factors, blood pressure, and anthropometric measurements. However, in this phase, serum biomarkers were not measured as they were measured in the repeated measurement phase [10].
2.2. Data collection, definitions, and measurements
Demographic characteristics were recorded through face-to-face interview using a structured questionnaire. A wealth score was calculated using multiple correspondence analysis on household assets including house ownership, house structure, house size, having a bath in the residence, as well as possession of a personal automobile, a motorbike, a black-and-white television, a color television, a refrigerator, a freezer, a vacuum cleaner, and a washing machine. The score was then transformed to tertiles and was categorized as poor, intermediate, and rich [12]. Education level was divided into two levels: Illiterate and literate.
Body mass index (BMI) was calculated using measured height and weight values and was classified into BMI <18.5 (underweight), 18.5 ≤ BMI <25 (normal weight), 25 ≤ BMI <30 (overweight), and BMI ≥30 (obese) based on the national institutes of health (NIH) definition. Also, high WC was defined as a waist circumference of ≥90 cm in males and females based on recommended Iranian cut-off definition [13]. Blood pressure was measured twice at baseline using Richter auscultator sphygmomanometers in the sitting position, with a 10-min interval between measurements. The mean of the two measurements was used in analyses. In our study, high blood pressure was defined as a systolic blood pressure equal to or greater than 140 mmHg or a baseline diastolic blood pressure equal to or greater than 90 mmHg [14]. High FBS was defined as having a FBS greater than 126 mg/dL. LDL-C and TG were included in the analyses, with high LDL-C defined as levels above 130 mg/dL and high TG defined as levels above 200 mg/dL.
Life style determinants were defined as current smoking and current routine alcohol or opium use during the preceding 6 months. Other comorbidities were recorded based on self-report.
2.3. Outcome ascertainment
Follow-up in GCS is accomplished through annual phone calls by the follow-up team. Deaths in GCS are assessed using various methods. In instances where deaths are reported via telephone or through monthly reports from local health workers or the provincial death registry, a general practitioner from the follow-up team visits the household of the deceased and completes a validated verbal autopsy questionnaire. Additionally, all relevant medical records, such as medical charts, radiographs, pathology reports, hospital discharge records, etc., are collected from all hospitals or pathology centers all over the province. Two internists independently review all collected records, including verbal autopsy records and medical data, and causes of death are recorded using International Classification of Diseases (ICD-10) codes. If the results are consistent, the diagnosis is made. However, in cases of inconsistency, a more experienced internist reviews all the documents and makes the final diagnosis [15]. CVD mortality cases were ascertained based on ICD. The following ICD codes have been included, as ischemic heart diseases (IHD) (ICD-10 codes I21.0, I21.1, I21.9 and I24.9) and cerebrovascular diseases (ICD-10 codes I60.0, I60.4, I60.9, I61.0, I61.5, I61.6, I61.9, I62.9, I63.0, I63.3, I63.9, I64.0, I67.9 and I69.8).
2.4. Statistical analyses
In this study, quantitative variables were presented as mean ± Standard Deviation (SD), while qualitative variables were presented as numbers and percentages. We estimated the relation between high blood pressure, high FBS, high WC, BMI, high blood TG, high LDL-C, and CVDs mortality among eligible participants. The Cox proportional hazards model was used to calculate hazard ratios (HRs) with 95 % confidence intervals (CIs). In this study, a segment of the target population had to be excluded due to drug intervention. This exclusion specifically pertained to individuals aged 50 years and above, leading to a disruption in the balance of the population under examination in GCS. Consequently, the HRs in the remaining population involved in this study were sex and age standardized based on the population of Golestan province. Indeed, we employed inverse probability weighting in our analyses. In order to present accurate and reliable findings, adjustments were made in all analyses to account for variables, such as age, sex, education level, urbanization, and wealth score. As a way to estimate the association between BMI and CVD mortality, the models were further adjusted for alcohol use, opium use, and smoking. We considered p-value <0.05 to be statistically significant. The PAF was calculated based on the proportions of participants who had each risk factor and its association with each outcome using the Levin's formula:
Where P is the prevalence of the risk factor, and HR is the hazard ratio of the disease risk [9,16]. All analyses were performed in STATA software V.12 (StataCorp, College Station, TX, USA).
3. Results
A total number of 8621 participants had valid laboratory measurements. The age of participants at enrolment ranged between 45 and 75 years and older. Out of the total sample, 58.4 % were between 45 and 55 years and 41.6 % were 55 years and older. Among modifiable risk factors, being overweight or obese (59.9 %) was the most prevalent metabolic factor in the 45–55 years category, followed by high TG (57.9 %) and high WC (57.6 %). In the 55 years and older age category, the most prevalent metabolic factor was high blood pressure (53.2 %), followed by high FBS (49.3 %) and high LDL-C (44. 7 %). Participants were followed till the end of December 2022, for a median of 10 years and a total of 87,933.5 person-years. During the follow-up, we identified 530 deaths from CVDs (251 IHDs and 170 cerebrovascular diseases, and 109 other CVDs). Mortality rates for all CVDs, IHDs, cerebrovascular disease, and other CVDs were thus: 602.7, 285.4, 193.3, and 123.9 per 100,000 person-years, respectively. More details of the demographics of all participants are provided in Table 1.
Table 1.
Demographic characteristics and risk factors at the repeated measurement phase of the Golestan Cohort Study participants.
| Variables | 45–55 years |
55+ years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cerebrovascular diseases death |
IHDs death |
CVDs death |
Total |
Cerebrovascular diseases death |
IHDs death |
CVDs death |
Total |
||
| 44 (0.87 %) | 83 (1.65 %) | 154 (3.06 %) | 5032 (58.37 %) | 126 (3.51 %) | 168 (4.68 %) | 376 (10.84 %) | 3589 (41.63 %) | ||
| Sex | Female | 21 (0.75 %) | 34 (1.21 %) | 64 (2.28 %) | 2803 (61.48 %) | 44 (2.51 %) | 64 (3.65 %) | 139 (7.92 %) | 1756 (38.52 %) |
| Male | 23 (1.03 %) | 49 (2.20 %) | 90 (4.04 %) | 2229 (54.87 %) | 82 (4.47 %) | 104 (5.67 %) | 237 (12.93 %) | 1833 (45.13 %) | |
| Residence | Urban | 6 (0.63 %) | 11 (1.16 %) | 21 (2.21 %) | 949 (47.93 %) | 27 (2.62 %) | 38 (3.69 %) | 305 (11.93 %) | 1031 (52.07 %) |
| Rural | 38 (0.93 %) | 72 (1.76 %) | 133 (3.26 %) | 4083 (61.48 %) | 99 (3.87 %) | 130 (5.08 %) | 71 (6.89 %) | 2558 (38.52 %) | |
| Education | Illiterate | 31 (1.09 %) | 49 (1.73 %) | 98 (3.45 %) | 2839 (51.84 %) | 99 (3.76 %) | 134 (5.08 %) | 69 (7.25 %) | 2637 (48.16 %) |
| Educated | 13 (0.59 %) | 34 (1.55 %) | 56 (2.55 %) | 2193 (69.73 %) | 27 (2.84 %) | 34 (3.57 %) | 307 (11.56 %) | 952 (30.27 %) | |
| Wealth score | Poor | 18 (1.22 %) | 30 (2.04 %) | 60 (4.07 %) | 1473 (55.27 %) | 50 (4.19 %) | 69 (5.79 %) | 158 (13.26 %) | 1192 (44.73 %) |
| Intermediate | 14 (0.88 %) | 32 (2.01 %) | 55 (3.45 %) | 1593 (60.89 %) | 41 (4.01 %) | 50 (4.89 %) | 115 (11.25 %) | 1023 (39.11 %) | |
| Rich | 12 (0.61) | 21 (1.07 %) | 39 (1.98 %) | 1966 (58.86 %) | 35 (2.55 %) | 49 (3.57 %) | 103 (7.50 %) | 1374 (41.14 %) | |
| Body mass index (kg/m2) | Normal | 18 (1.07 %) | 23 (1.36 %) | 48 (2.84 %) | 1690 (56.41 %) | 43 (3.30 %) | 56 (4.29 %) | 131 (10.04 %) | 1306 (43.59 %) |
| Underweight | 2 (1.24 %) | 5 (3.11 %) | 7 (4.35 %) | 161 (51.11 %) | 5 (3.25 %) | 9 (5.84 %) | 24 (15.58 %) | 154 (48.89 %) | |
| Overweight and obese | 24 (0.75 %) | 55 (1.73 %) | 99 (3.11 %) | 3180 (59.90 %) | 78 (3.66 %) | 103 (4.84 %) | 221 (10.38 %) | 2129 (40.10 %) | |
| High FBS | Yes | 14 (2.92 %) | 24 (5.01 %) | 50 (10.44 %) | 479 (50.69 %) | 19 (4.08 %) | 46 (9.87 %) | 83 (17.81 %) | 466 (49.31 %) |
| No | 30 (0.666 %) | 59 (1.30 %) | 104 (2.29 %) | 4532 (59.72 %) | 105 (3.43 %) | 119 (3.89 %) | 286 (9.36 %) | 3057 (40.28 %) | |
| High WC (cut-off: 90 cm) | Yes | 34 (1.01 %) | 62 (1.84 %) | 120 (3.56 %) | 3367 (57.57 %) | 96 (3.87 %) | 127 (5.12 %) | 273 (11.00 %) | 2482 (42.43 %) |
| No | 10 (0.60 %) | 21 (1.26 %) | 34 (2.04 %) | 1663 (60.08 %) | 30 (2.72 %) | 41 (3.71 %) | 103 (9.33 %) | 1105 (39.92 %) | |
| High TG | Yes | 5 (0.71 %) | 21 (2.97 %) | 33 (4.67 %) | 706 (57.87 %) | 19 (3.70 %) | 30 (5.84 %) | 64 (12.45 %) | 514 (42.13 %) |
| No | 39 (0.90 %) | 62 (1.44 %) | 121 (2.81 %) | 4310 (58.89 %) | 105 (3.49 %) | 135 (4.49 %) | 305 (10.14 %) | 3009 (41.11 %) | |
| High LDL-C | Yes | 16 (1.04 %) | 29 (1.88 %) | 54 (3.50 %) | 1541 (55.29 %) | 43 (3.45 %) | 68 (5.46 %) | 138 (11.08 %) | 1246 (44.71 %) |
| No | 27 (0.81 %) | 48 (1.43 %) | 89 (2.66 %) | 3350 (60.25 %) | 77 (3.48 %) | 91 (4.12 %) | 217 (9.82 %) | 2210 (39.75 %) | |
| High blood pressure | Yes | 26 (0.59 %) | 23 (3.88 %) | 46 (7.76 %) | 593 (46.80 %) | 86 (2.95 %) | 43 (6.38 %) | 109 (16.17 %) | 674 (53.20 %) |
| No | 18 (3.04) | 60 (1.35 %) | 108 (2.43 %) | 4439 (60.36 %) | 40 (5.93 %) | 125 (4.29 %) | 267 (9.16 %) | 2915 (39.64 %) | |
A significant association was found between the following risk factors and CVDs mortality: high FBS (weighted HR, 3.35 [95 % CI: 2.71–4.15]), high blood pressure (2.25 [1.83–2.76]), high WC (1.62 [1.31–2.00]), high TG (1.58 [1.25–1.99]), overweight and obesity (1.43 [1.17–1.76]), and high LDL-C (1.29 [1.06–1.57]). The HR for individuals classified as underweight compared to those with a normal BMI was determined to lack statistical significance in our study. Table 2 shows the HRs for the association between metabolic risk factors and mortality from CVDs, presented in details.
Table 2.
PAFs and HRs for associations between demographic and metabolic risk factors and CVDs mortality at the repeated measurement phase of the Golestan Cohort Study.
| Variables | Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusted aHR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | ||||||||
| Body mass index (kg/m2)b | Normal weight | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Under weight | 1.45 (0.95–2.21) | 0.08 | 5 % (−1%–11 %) | 1.23 (0.71–2.11) | 0.46 | 2 % (−4%–8%) | 1.78 (0.89–3.56) | 0.10 | 10 % (−5%–24 %) | |
| Over weight and obese | 1.43 (1.17–1.76) | <0.001 | 19 % (8 %–28 %) | 1.37 (1.07–1.77) | 0.01 | 15 % (2 %–26 %) | 1.55 (1.09–2.21) | 0.01 | 27 % (5 %–44 %) | |
| High WC (cut-off: 90 cm)b | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.62 (1.31–2.00) | <0.001 | 28 % (16 %–38 %) | 1.44 (1.11–1.88) | 0.00 | 21 % (6 %–34 %) | 2.02 (1.40–2.92) | <0.001 | 41 % (21 %–56 %) | |
| High blood pressure | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.25 (1.83–2.76) | <0.001 | 16 % (11%–21 %) | 1.92 (1.46–2.53) | <0.001 | 12 % (6 %–17 %) | 2.73 (2.00–3.71) | <0.001 | 24 % (15 %–33 %) | |
| High TG | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.58 (1.25–1.99) | <0.001 | 7 % (3 %–11 %) | 1.34 (0.97–1.84) | 0.07 | 4 % (−1%–8%) | 1.96 (1.39–2.76) | <0.001 | 12 % (4 %–19 %) | |
| High LDL-C | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.29 (1.06–1.57) | 0.01 | 8 % (1 %–15 %) | 1.19 (0.92–1.54) | 0.17 | 5 % (−2%–12 %) | 1.44 (1.06–1.96) | 0.02 | 14 % (1 %–25 %) | |
| High FBS | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 3.35 (2.71–4.15) | <0.001 | 20 % (15 %–24 %) | 3.42 (2.60–4.51) | <0.001 | 19 % (13 %–25 %) | 3.25 (2.33–4.54) | <0.001 | 21 % (11 %–28 %) | |
| Joint PAF | 50 % (38%–60 %) | 43 % (27%–55 %) | 67 % (50%–78 %) | |||||||
Adjusted for age, education, urbanization, and wealth status.
For BMI and WC, the model was adjusted for alcohol, opium use and tobacco smoking in addition to age, education, urbanization and wealth status.
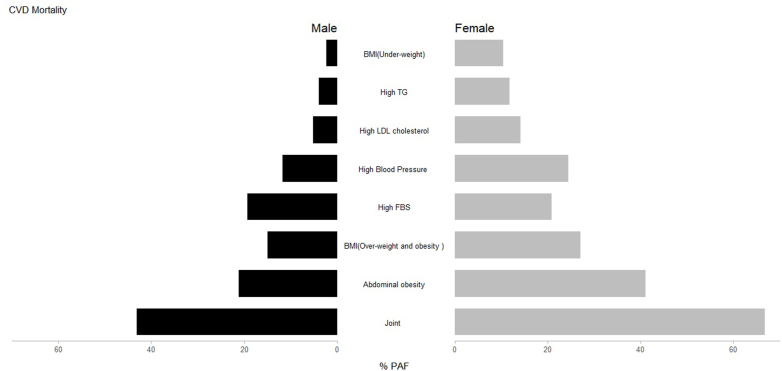
The PAFs for each of the modifiable risk factors was evaluated for both sexes combined. High WC (28 %, [16%–38 %]) had the highest PAF, followed by high FBS (20 %, [15%–24 %]), overweight and obesity (19 %, [8%–28 %]), high blood pressure (16 %, [11%–21 %]), high LDL-C (8 %, [1%–15 %]), and high TG (7 %, [3%–11 %]). However, there was not significant association between underweight and CVDs mortality.
In females, the highest PAFs was observed in participants with high WC (41 %, [95 % CI,21%–56 %]) that followed by overweight and obesity (27 %, [5%–44 %]), high blood pressure (24 %, [15%–33 %]), high FBS (21 %, [13%–28 %]), high LDL-C (14 %, [1%–25 %]), and high TG (12 %, [4%–19 %]). Though, no significant association was found between being underweight and CVDs mortality in women. Also, the highest estimated PAF in males was observed in participants with high WC (21 %, [6%–34 %]) that followed by high FBS (19 %, [12%–21 %]), and being overweight or obese (15 %, [2%–26 %]), and high blood pressure (12 %, [6%–17 %]). However, high LDL-C as well as high TG, and underweight was not significantly associated with CVDs mortality in men.
Collectively, 50 % of the PAFs for cardiovascular diseases mortality were attributable to the all-measured risk factors in both sexes combined, 43 % in males and 67 % in females. The PAF comparison for each sex-specific metabolic risk factor is presented in Fig. 1, which demonstrates how the PAFs differ in the CVDs associated risk in males and females. More details of the PAFs of CVDs mortality are provided in Table 2.
Fig. 1.
PAFs of metabolic risk factors attributable CVDs mortality at the repeated measurement phase of the Golestan Cohort Study by sex.
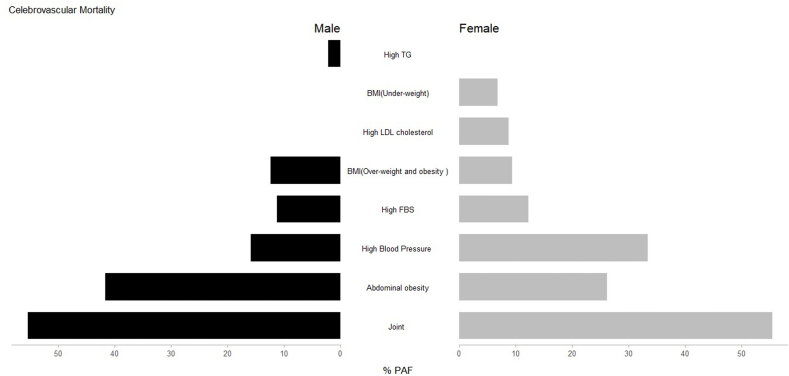
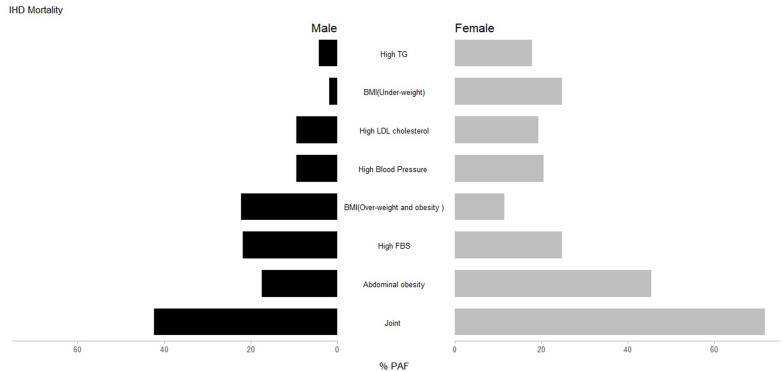
Also, we assessed the HRs and PAFs due to metabolic risk factors separately for cerebrovascular diseases and IHDs mortality. The highest PAF of cerebrovascular mortality was due to high WC, followed by high blood pressure and high FBS. The highest PAF of IHD mortality was due to high WC, followed by overweight and obesity and high FBS. The details are presented in Table 3, Table 4 and the PAFs of metabolic risk factors by sex for cerebrovascular disease and IHD are demonstrated in Fig. 2, Fig. 3.
Table 3.
PAFs and HRs for associations between demographic and metabolic risk factors and cerebrovascular disease mortality at the repeated measurement phase of the Golestan Cohort Study.
| Variables | Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusted aHR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | ||||||||
| Body mass index (kg/m2)b | Normal | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Underweight | 0.90 (0.39–2.07) | 0.81 | 0 % (−8%–6%) | 0.51 (0.13–1.92) | 0.32 | −4% (−12%–2%) | 1.59 (0.52–4.91) | 0.81 | 6 % (−14%–24 %) | |
| Overweight and obese | 1.23 (0.86–1.76) | 0.26 | 11 % (−10%–28 %) | 1.29 (0.83–2.02) | 0.26 | 12 % (−11%–31 %) | 1.15 (0.63–2.09) | 0.65 | 9 % (−39%–41 %) | |
| High WC (cut-off: 90 cm)b | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.90 (1.29–2.79) | <0.001 | 36 % (15%–52 %) | 2.21 (1.35–3.61) | <0.001 | 41 % (15%–60 %) | 1.51 (0.82–2.77) | 0.18 | 26 % (−18%–54 %) | |
| High blood pressure | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.79 (1.98–3.94) | <0.001 | 22 % (13%–31 %) | 2.27 (1.44–3.57) | <0.001 | 16 % (4%–26 %) | 3.71 (2.18–6.30) | <0.001 | 33 % (15%–47 %) | |
| High TG | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.00 (0.63–1.59) | 0.99 | 0 % (−6%–6%) | 1.19 (0.66–2.14) | 0.57 | 2 % (−5%–95 %) | 0.76 (0.36–1.62) | 0.48 | −3% (−12%–4%) | |
| High LDL-C | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.05 (0.75–1.49) | 0.75 | 2 % (−9%–12 %) | 0.95 (0.60–1.51) | 0.83 | −1% (−15%–10 %) | 1.25 (0.73–2.15) | 0.41 | 9 % (−15%–28 %) | |
| High FBS | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.31 (1.46–3.40) | <0.001 | 11 % (3%–19 %) | 2.27 (1.31–3.93) | 0.004 | 11 % (9%–20 %) | 2.18 (1.13–4.21) | <0.001 | 12 % (−1%–24 %) | |
| Joint PAF | 54 % (33%–68 %) | 55 % (28%–72 %) | 55 % (19%–75 %) | |||||||
Adjusted for age, education, urbanization, and wealth status.
For BMI and WC, the model was adjusted for alcohol, opium use and tobacco smoking in addition to age, education, urbanization and wealth status.
Table 4.
PAFs and HRs for associations between demographic and metabolic risk factors and IHD mortality at the repeated measurement phase of the Golestan Cohort Study.
| Variables | Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusted aHR (95%CI) |
P-Value |
PAF (95%CI) |
Adjusteda HR (95%CI) |
P-Value |
PAF (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | ||||||||
| Body mass index (kg/m2)** | Normal | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Under weight | 1.49 (0.80-0.2.77) | 0.21 | 5 % (−4%–14 %) | 1.18 (0.51–2.72) | 0.70 | 2 % (−8%–11 %) | 1.83 (0.67–4.97) | 0.23 | 11 % (−12%–30 %) | |
| Over weight and obese | 1.57 (1.17–2.12) | 0.003 | 24 % (8%–37 %) | 1.60 (1.11–2.30) | 0.01 | 22 % (4%–37 %) | 1.49 (0.90–2.47) | 0.12 | 25 % (−9%–49 %) | |
| High WC (cut-off: 90 cm)** | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.62 (1.18–2.21) | 0.003 | 28 % (10%–43 %) | 1.34 (0.91–1.98) | 0.14 | 17 % (−8%–37 %) | 2.22 (1.27–3.88) | 0.005 | 45 % (14%–65 %) | |
| High blood pressure | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.04 (1.49–2.75) | <0.001 | 14 % (6%–21 %) | 1.73 (1.14–2.63) | 0.01 | 9 % (1%–17 %) | 2.39 (1.51–3.80) | <0.001 | 20 % (7%–32 %) | |
| High TG | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.80 (1.31–2.49) | <0.001 | 9 % (3%–15 %) | 1.35 (0.86–2.11) | 0.19 | 4 % (−3%–11 %) | 2.56 (1.60–4.10) | <0.001 | 18 % (6%–28 %) | |
| High LDL-C | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.48 (1.12–1.95) | 0.006 | 13 % (3%–22 %) | 1.37 (0.95–1.97) | 0.09 | 9 % (−3%–20 %) | 1.64 (1.05–2.56) | 0.03 | 19 % (−1%–35 %) | |
| High FBS | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 3.79 (2.83–5.08) | <0.001 | 23 % (15%–29 %) | 3.77 (2.57–5.54) | <0.001 | 22 % (12%–30 %) | 3.85 (2.44–6.07) | <0.001 | 25 % (13%–35 %) | |
| Joint PAF | 52 % (35%–65 %) | 42 % (17%–60 %) | 72 % (44%–85 %) | |||||||
Adjusted for age, education, urbanization, and wealth status ** For BMI the model was adjusted for alcohol, opium use and tobacco smoking in addition to age, education, urbanization and wealth status.
Fig. 2.
PAFs of metabolic risk factors attributable cerebrovascular mortality at the repeated measurement phase of the Golestan Cohort Study by sex.
Fig. 3.
PAFs of metabolic risk factors attributable IHD mortality at the repeated measurement phase of the Golestan Cohort Study by sex.
4. Discussion
The present study reports data from a large cohort in Iran and estimated the disease-specific mortality attributable to metabolic risk factors. Results showed that the most significant proportions of PAFs for CVDs mortality were attributable to high WC, overweight and obesity, high FBS, and high blood pressure, respectively. In females, the estimated PAFs resulting from high LDL-C and high TG levels were nearly three times higher, and the PAFs for high blood pressure were found to be twice as high compared to males. The findings indicated a significant proportion of approximately half of the CVD mortality to be attributable to metabolic risk factors (joint PAF = 50 %). Females presented a higher joint PAF of metabolic risk factors compared with males. To the best of our knowledge, this is the first study to estimate the PAFs for CVD death and its subgroups due to the highly prevalent metabolic risk factors in an Iranian population with a median follow-up of 10 years. Patterns of CVDs attributable to metabolic risk factors in this study are not the same as those in other parts of the world and even previous studies in Iran.
High WC and obesity were significantly associated with an increased risk of CVD-related mortality in males, females, and both sexes combined with cut-off point of 90 cm. WC is one of the most important predictors of CVDs risk. Various studies have been conducted regarding the relationship between high WC and CVD mortality, which reported this association in both sexes. Similarly, the PAF of 17 % was reported for central obesity related to CVD event in a study was conducted among Iranian population in 2016 [17]. Additionally, a study by Pirani et al. indicated that overweight and obesity had PAF of 5.9 % [18]. Our results report a much higher PAF for high WC and overweight and obesity than previous studies in Iran, which shows there is an urgent need for interventions to control and reduce the prevalence of overweight and obesity in Iran, even pharmacologically in addition to non-pharmacological routine life style advice [19,20]. Recent evidence has demonstrated that WC, as a measure of abdominal obesity, is strongly associated with higher mortality independent of BMI [21]. High WC has been linked to increased CVD mortality, even among individuals with normal BMI, emphasizing the importance of WC as a risk factor for mortality in older adults [22]. We didn't detect significant collinearity between WC and BMI.
High FBS contributed to 20 % of CVD mortality in our study. High FBS is widely recognized as the primary metabolic risk factor associated with CVDs mortality [23]. The study by Pirani et al., in 2017 estimated that 7 %, of CVD mortality was due to diabetes in Iran [18]. According to the Global burden of disease study in 2019, estimated PAF of high FBS regarding CVD mortality in Iran was 12.2 % [1]. The higher estimations of PAF in our study may reflect the growing trend of prevalence of diabetes and high FBS in Iran [24]. Prevention programs for diabetes have been developed for years now and should be implemented in national policy making to control and reverse the increasing trend in diabetes in Iran [25].
In our study, we observed that high blood pressure predicts a relatively low proportion of mortality for total and subcomponents of CVDs (16 %), and particularly cerebrovascular diseases (22 %) compared to studies in other parts of the world and other previous studies in Iran. The study by Pirani et al., in 2017 estimated the proportion of CVDs attributable to hypertension to be 11.4 % in Iran [18]. Also, based on the GBD results the estimated PAF in Iran was 24.4 % in 2019 [1]. The most substantial finding, a PAF of 22 % for cerebrovascular disease mortality associated with HTN, was present among participants. In the given statement, it is mentioned that a study conducted by Donald Clark III et al. found a population-attributable risk of 32.5 % for CVDs and 38.9 % for stroke [26]. The lower PAF for hypertension in our study may reflect the success of the hypertension surveillance in rural regions of Iran [27].
In the present study the estimated PAF due to high blood pressure was much higher in females compared with males. Currently, there is no consensus in this regard. A previous longitudinal cohort study showed a stronger association between HTN and stroke in females compared with males [28]. There are several possible explanations for these results. Hormonal and biological mechanisms (preeclampsia, autoimmune diseases, alternation in left ventricular (LV) systolic and diastolic function), sex specific synergistic effects between HTN and other risk factors such as diabetes, and different efficacy of the antihypertensive drug are some of the particular determinants among females which may contribute to higher CVDs mortality than males [28].
Generally, our results showed sex difference in the PAFs of CVDs risk factors. Joint PAF in females was higher than males. In a study that was conducted in Iran (2011), the joint PAFs for the effects of high systolic blood pressure, fasting plasma glucose, total cholesterol, and BMI on IHD and stroke were higher in females than in males due to more prominent exposure [29]. These sex differences may be due to social, psychological, biological, and genetic reasons [30]. Also, this finding may be partly explained by the fact that the participants in this study were over 45 years old, with a majority of menopausal females, and is in line with previous research demonstrating that menopause has been associated with an increased CVDs mortality risk in females [31,32]. It has been shown that females tend to have higher rates of obesity, HTN, hyperglycemia and dyslipidemia [[33], [34], [35]]. It is important for healthcare officials to address sex differences and possible inequities and inequalities in provided care.
Almost 50 % of CVD mortality was attributable to joint metabolic risk factors. Study results suggest that the joint effect of these metabolic factors may be more relevant to CVDs mortality than their independent effects. A holistic approach to simultaneously address several metabolic risk factors may be more effective than strategies addressing each risk factor separately. In this regard, the package of essential non-communicable (PEN) disease intervention for primary care in low-resource settings was launched in 2010 by the WHO to deliver care with adequate quality and consequently, reduce the burden of non-communicable diseases in developing countries. In Iran, the IraPEN program has been launched based on the WHO framework. The cost-effectiveness of the program needs to be evaluated and the program needs to be updated based on diverse trends of CVD risk factors among Iranians [36,37].
The strengths of our study lie in several aspects. Firstly, we utilized a large population-based sample of Iranian adults, which enhances the generalizability of our findings. Additionally, we implemented a relatively long follow-up time to accurately assess CVDs mortality rates. This allowed us to estimate hazard ratios with a low degree of uncertainty, thereby revealing the association between metabolic risk factors and CVDs mortality. One notable contribution of our research lies in the meticulous exclusion of participants exhibiting pre-existing cardiovascular disease at baseline. This deliberate selection enabled us to enhance our comprehension of the impacts that different metabolic risk factors have on the likelihood of mortality due to CVDs.
Nonetheless, our study had some limitations. The exposure data did not include individuals younger than 45 years of age. However, it is worth mentioning that previous studies have demonstrated that the prevalence of metabolic risk factors is lowest in those younger than 40 years. Additionally, we have measured the exposure to risk factors at one point in time (the first repeated measurement) and have not explored their trends across the cohort. This topic will be addressed in future analyses as data from next repeated measurements will be collected in GCS. Generally speaking, our results pertain to a localized setting. We have found results that are not quite compatible with other similar results at other time points. This provides a call for action aimed to identify the CVD risk factors at local scales and in different countries. The different distribution of CVD risk factors in our study may be due to differences in life style and culture between communities. These may involve increase in prevalence of obesity in recent years in Iran [38] and decrease in the CVD mortality attributable to high blood pressure as a result of implementing national surveillance system for blood pressure control. These highlight the necessity of exploring the contribution of specific risk factors to CVD across communities and time periods.
5. Conclusions
In conclusion, using attributable fractions offers a valuable method for quantifying the impact of metabolic risk factors on CVDs morbidity and mortality. The findings of this study reveal that the contribution of various metabolic risk factors to CVD mortality may have different geographical and temporal patterns across various populations. Additionally, the design of studies, whether cross-sectional or longitudinal, may affect estimates for PAF of various metabolic risk factors. Our results showed relatively higher PAFs for overweight and obesity and high FBS and lower PAFs for hypertension compared with previous reports for Iran and other parts of the world. This highlights the need for updated data gathering and meticulous policy making in different settings. The IraPEN can be a good beginning for surveillance and control of metabolic risk factors in Iran and similar approaches in other low-resource countries.
Patient consent statement
The authors confirm that patient consent forms have been obtained for this article.
Funding
None.
Ethical approval and consent to participate
The ethics committee of Tehran University of Medical Sciences, International Agency for Research on cancer, and National Cancer Institute approved the study, and written informed consent was signed by all participants in the study.
Acknowledgement of grant support
The authors state no funding involved.
CRediT authorship contribution statement
Fatemeh Gorgani: Writing – original draft. Maryam Sharafkhah: Validation, Methodology, Formal analysis, Data curation. Sahar Masoudi: Methodology, Formal analysis. Hossein Poustchi: Project administration. Alireza Delavari: Project administration. Alireza Sadjadi: Project administration. Gholamreza Roshandel: Project administration. Masoud Khoshnia: Project administration. Layli Eslami: Project administration. Negar Rezaei: Writing – review & editing, Supervision. Sadaf G. Sepanlou: Writing – review & editing, Supervision, Project administration, Methodology.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgments
The authors express their gratitude to the Digestive Disease Research Institute (DDRI) of Tehran University of Medical Sciences. We also thank all study participants for their cooperation.
Handling Editor: Dr D Levy
Footnotes
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Abbreviations
- BMI
Body mass index
- CVD
Cardiovascular disease
- DDRI
Digestive disease research institute
- FBS
Fasting blood sugar
- GCS
Golestan cohort study
- HDL:
High-density lipoprotein cholesterol
- HTN
Hypertension
- IHD
Ischemic heart disease
- LDL:
Low-density lipoprotein
- HR
Hazard ratio
- PAF
Population attributable fraction
- TG
Triglycerides
- WC
Waist circumference
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tadbiri H., Moradi-Lakeh M., Naghavi M. Release of civil registry causes of death data in Iran (2015 to 2019) - expectations and doubts. Arch. Iran. Med. 2021;24:741–746. doi: 10.34172/aim.2021.109. [DOI] [PubMed] [Google Scholar]
- 3.Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendrick D.L., Diehl A.M., Topor L.S., et al. Metabolic syndrome and associated diseases: from the bench to the clinic. Toxicol. Sci. 2018;162:36–42. doi: 10.1093/toxsci/kfx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noubiap J.J., Nansseu J.R., Lontchi-Yimagou E., et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022;188 doi: 10.1016/j.diabres.2022.109924. [DOI] [PubMed] [Google Scholar]
- 6.Mazloomzadeh S., Rashidi Khazaghi Z., Mousavinasab N. The prevalence of metabolic syndrome in Iran: a systematic review and meta-analysis. Iran. J. Public Health. 2018;47:473–480. [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Zhai Y., Zhao J., et al. Impact of metabolic syndrome and it's components on prognosis in patients with cardiovascular diseases: a meta-analysis. Frontiers in Cardiovascular Medicine. 2021;8 doi: 10.3389/fcvm.2021.704145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao Q., Gao W., Zhang L., Nyamdorj R., Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann. Clin. Biochem. 2007;44:232–263. doi: 10.1258/000456307780480963. [DOI] [PubMed] [Google Scholar]
- 9.Mansournia M.A., Altman D.G. Population attributable fraction. Bmj. 2018;360:k757. doi: 10.1136/bmj.k757. [DOI] [PubMed] [Google Scholar]
- 10.Pourshams A., Khademi H., Malekshah A.F., et al. Cohort Profile: the Golestan Cohort Study—a prospective study of oesophageal cancer in northern Iran. Int. J. Epidemiol. 2009;39:52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roshandel G., Khoshnia M., Poustchi H., et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394:672–683. doi: 10.1016/S0140-6736(19)31791-X. [DOI] [PubMed] [Google Scholar]
- 12.Islami F., Kamangar F., Nasrollahzadeh D., et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int. J. Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteghamati A., Abbasi M., Rashidi A., et al. Optimal waist circumference cut-offs for the diagnosis of metabolic syndrome in Iranian adults: results of the third national survey of risk factors of non-communicable diseases (SuRFNCD-2007) Diabet. Med. 2009;26:745–746. doi: 10.1111/j.1464-5491.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian A., Bakris G., Black H., et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA, J. Am. Med. Assoc. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Khademi H., Etemadi A., Kamangar F., et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miettinen O.S. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am. J. Epidemiol. 1974;99:325–332. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- 17.Sardarinia M., Akbarpour S., Lotfaliany M., et al. Risk factors for incidence of cardiovascular diseases and all-cause mortality in a middle eastern population over a decade follow-up: tehran lipid and glucose study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirani N., Khiavi F.F. Population attributable fraction for cardiovascular diseases risk factors in selected countries: a comparative study. Mater Sociomed. 2017;29:35–39. doi: 10.5455/msm.2017.29.35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shadmani F.K., Karami M. Joint effect of modifying selected risk factors on attributable burden of cardiovascular diseases. Int. J. Prev. Med. 2013;4:1461–1467. [PMC free article] [PubMed] [Google Scholar]
- 20.Burini R. Behavioral factors of abdominal obesity and effects of lifestyle changes with fiber adequacy. New Insights in Obesity: Genetics. 2017;1:14–22. [Google Scholar]
- 21.Jacobs E.J., Newton C.C., Wang Y., et al. Waist circumference and all-cause mortality in a large US cohort. Arch. Intern. Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C., Rexrode K.M., Dam R.M.v., Li T.Y., Hu F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan S., Vassy J.L., Ho Y.L., et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteghamati A., Larijani B., Aghajani M.H., et al. Diabetes in Iran: prospective analysis from first nationwide diabetes report of national program for prevention and control of diabetes (NPPCD-2016) Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark D., III, Colantonio L.D., Min Y.-I., et al. Population-attributable risk for cardiovascular disease associated with hypertension in black adults. JAMA Cardiology. 2019;4:1194–1202. doi: 10.1001/jamacardio.2019.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panahi M.H., Mahdavi Hezaveh A.R., Samavat T., Hodjatzadeh A., Yousefi E. Hypertension surveillance in rural regions of Iran. Iran. J. Public Health. 2019;48:2313–2314. [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen T.E., Howard G., Kleindorfer D.O., et al. Sex differences in hypertension and stroke risk in the regards study: a longitudinal cohort study. Hypertension. 2019;74:749–755. doi: 10.1161/HYPERTENSIONAHA.119.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzadfar F., Danaei G., Namdaritabar H., et al. National and subnational mortality effects of metabolic risk factors and smoking in Iran: a comparative risk assessment. Popul. Health Metrics. 2011;9:55. doi: 10.1186/1478-7954-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regensteiner J.G., Golden S., Huebschmann A.G., et al. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2015;132:2424–2447. doi: 10.1161/CIR.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 31.Prabakaran S., Schwartz A., Lundberg G. Cardiovascular risk in menopausal women and our evolving understanding of menopausal hormone therapy: risks, benefits, and current guidelines for use. Ther Adv Endocrinol Metab. 2021;12 doi: 10.1177/20420188211013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Khoudary S.R., Aggarwal B., Beckie T.M., et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 33.Wright A.K., Kontopantelis E., Emsley R., et al. Cardiovascular risk and risk factor management in type 2 diabetes mellitus: a population-based cohort study assessing sex disparities. Circulation. 2019;139:2742–2753. doi: 10.1161/CIRCULATIONAHA.118.039100. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., O'Neil A., Jiao Y., et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17:136. doi: 10.1186/s12916-019-1355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azadnajafabad S., Karimian M., Roshani S., et al. Population attributable fraction estimates of cardiovascular diseases in different levels of plasma total cholesterol in a large-scale cross-sectional study: a focus on prevention strategies and treatment coverage. J. Diabetes Metab. Disord. 2020;19:1453–1463. doi: 10.1007/s40200-020-00673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamshidi A., Daroudi R., Aas E., Khalili D. A cost-effectiveness analysis of risk-based intervention for prevention of cardiovascular diseases in IraPEN program: a modeling study. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1075277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokhtari M., Khalil D., Farzadfar F., Daroudi R., Asadi-Lari M. The burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of IraPEN program in the general population of Iran. Med. J. Islam. Repub. Iran. 2022;36:73. doi: 10.47176/mjiri.36.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abiri B., Ahmadi A.R., Amini S., et al. Prevalence of overweight and obesity among Iranian population: a systematic review and meta-analysis. J. Health Popul. Nutr. 2023;42:70. doi: 10.1186/s41043-023-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]